Abstract
Background
Sepsis occurs when an infection is complicated by organ failures as defined by a sequential organ failure assessment (SOFA) score of two or higher. Sepsis may be complicated by impaired corticosteroid metabolism. Giving corticosteroids may benefit patients. The original review was published in 2004 and was updated in 2010 and again in 2015.
Objectives
To examine the effects of corticosteroids on death at one month in patients with sepsis, and to examine whether dose and duration of corticosteroids influence patient response to this treatment.
Search methods
We searched the Central Register of Controlled Trials (CENTRAL; 2014, Issue 10), MEDLINE (October 2014), EMBASE (October 2014), Latin American Caribbean Health Sciences Literature (LILACS; October 2014) and reference lists of articles, and we contacted trial authors. The original searches were performed in August 2003 and in October 2009.
Selection criteria
We included randomized controlled trials of corticosteroids versus placebo or supportive treatment in patients with sepsis.
Data collection and analysis
All review authors agreed on the eligibility of trials. One review author extracted data, which were checked by the other review authors, and by the primary author of the paper when possible. We obtained some missing data from trial authors. We assessed the methodological quality of trials.
Main results
We identified nine additional studies since the last update, for a total of 33 eligible trials (n = 4268 participants). Twenty‐three of these 33 trials were at low risk of selection bias, 22 were at low risk of performance and detection bias, 27 were at low risk of attrition bias and 14 were at low risk of selective reporting.
Corticosteroids reduced 28‐day mortality (27 trials; n = 3176; risk ratio (RR) 0.87, 95% confidence interval (CI) 0.76 to 1.00; P value = 0.05, random‐effects model). The quality of evidence for this outcome was downgraded from high to low for imprecision (upper limit of 95% CI = 1) and for inconsistency (significant heterogeneity across trial results). Heterogeneity was related in part to the dosing strategy. Treatment with a long course of low‐dose corticosteroids significantly reduced 28‐day mortality (22 trials; RR 0.87, 95% CI 0.78 to 0.97; P value = 0.01, fixed‐effect model). The quality of evidence was downgraded from high to moderate for inconsistency (owing to non‐significant effects shown by one large trial). Corticosteroids also reduced mortality rate in the intensive care unit (13 trials; RR 0.82, 95% CI 0.68 to 1.00; P value = 0.04, random‐effects model) and at the hospital (17 trials; RR 0.85, 95% CI 0.73 to 0.98; P value = 0.03, random‐effects model). Quality of the evidence for in‐hospital mortality was downgraded from high to moderate for inconsistency and imprecision (upper limit of 95% CI for RR approaching 1). Corticosteroids increased the proportion of shock reversal by day seven (12 trials; RR 1.31, 95% CI 1.14 to 1.51; P value = 0.0001) and by day 28 (seven trials; n = 1013; RR 1.11, 95% CI 1.02 to 1.21; P value = 0.01) and reduced the SOFA score by day seven (eight trials; mean difference (MD) ‐1.53, 95% CI ‐2.04 to ‐1.03; P value < 0.00001, random‐effects model) and survivors' length of stay in the intensive care unit (10 trials; MD ‐2.19, 95% CI ‐3.93 to ‐0.46; P value = 0.01, fixed‐effect model) without inducing gastroduodenal bleeding (19 trials; RR 1.24, 95% CI 0. 92 to 1.67; P value = 0.15, fixed‐effect model), superinfection (19 trials; RR 1.02, 95% CI 0.87 to 1.20; P value = 0.81, fixed‐effect model) or neuromuscular weakness (three trials; RR 0.62, 95% CI 0.21 to 1.88; P value = 0.40, fixed‐effect model). Corticosteroid increased the risk of hyperglycaemia (13 trials; RR 1.26, 95% CI 1.16 to 1.37; P value < 0.00001, fixed‐effect model) and hypernatraemia (three trials; RR 1.64, 95% CI 1.28 to 2.09; P value < 0.0001, fixed‐effect model).
Authors' conclusions
Overall, low‐quality evidence indicates that corticosteroids reduce mortality among patients with sepsis. Moderate‐quality evidence suggests that a long course of low‐dose corticosteroids reduced 28‐day mortality without inducing major complications and led to an increase in metabolic disorders.
Keywords: Adult; Child; Humans; Adrenal Cortex Hormones; Adrenal Cortex Hormones/adverse effects; Adrenal Cortex Hormones/therapeutic use; Critical Care; Dexamethasone; Fludrocortisone; Fludrocortisone/therapeutic use; Hydrocortisone; Hydrocortisone/therapeutic use; Methylprednisolone; Methylprednisolone/therapeutic use; Organ Dysfunction Scores; Prednisolone; Prednisolone/therapeutic use; Randomized Controlled Trials as Topic; Sepsis; Sepsis/drug therapy; Sepsis/mortality; Shock, Septic; Shock, Septic/drug therapy; Shock, Septic/mortality; Time Factors
Corticosteroids for treating sepsis
Review question
We reviewed the evidence on effects on survival at one month and on tolerance of systemic corticosteroids in people with sepsis.
Background
Sepsis, the most severe form of infection, is present when a site of infection is apparent and evidence suggests body‐wide, systemic inflammation and organ failures. The person develops poor temperature control, an increase or decrease in white blood cells, an increase in heart rate and rapid breathing. Sepsis can interfere with effectiveness of the body’s own corticosteroids, which serve as key defence hormones against infection. Corticosteroids have been given for more than 60 years to people with severe infection resulting from various causes.
Search date
The evidence provided in this review was current to October 2014.
Study characteristics
This review included a total of 33 randomized controlled trials, accounting for 4268 hospitalized patients with sepsis. Three trials included children, and the remaining 30 trials included only adults. Corticosteroids were compared with placebo in all except five trials, in which they were compared with standard therapy alone.
Study funding sources
Ten out of 33 trials were funded by a drug company, 13 were funded by public organizations or received charitable funding and 10 stated no source of funding.
Key results
Corticosteroids reduced risk of death at 28 days by 13% (among 27 trials, 3176 participants). Both intensive care unit (ICU) and in‐hospital deaths were reduced (13 and 17 trials, respectively). The review found that survival benefits were dependent on the dose of corticosteroids ‐ the lower the dose (less than 400 mg of hydrocortisone or equivalent per day) for a longer duration of treatment (three or more days at the full dose) the better ‐ and on the severity of illness. Corticosteroids increased the chance of recovery from septic shock by day seven (when drugs are required to support blood pressure) by 31% (from 12 trials) and and decreased the number of organs that were not functioning properly (organ failure) (from eight trials). Length of stay in the ICU was reduced by more than two days (10 trials). Corticosteroids did not cause harm, except for a mild increase in blood glucose and salt (sodium) levels (13 and three trials, respectively). Gastrointestinal bleeding and infection (19 trials) and neuromuscular weakness (three trials) were not increased.
We found sparse data on effects of corticosteroids in children with sepsis.
Quality of evidence
We judged the quality of evidence for 28‐day mortality as low because of imprecision (the confidence interval of the statistical result approached no change) and inconsistency across trials. These findings were related in part to differences among study populations, type of corticosteroid given, dose and duration of treatment and use of additional interventions. The quality of evidence for 28‐day mortality in the subgroup given a long course of low‐dose corticosteroids was downgraded from high to moderate because one of the two largest trials on a long course of low‐dose corticosteroids reported no survival benefit.
Summary of findings
Summary of findings for the main comparison.
Steroids versus control for treating sepsis
| Steroids versus control for treating sepsis | ||||||
| Patient or population: patients with sepsis Settings: Intervention: steroids vs control | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Steroids vs control | |||||
| 28‐Day all‐cause mortality Follow‐up: 14 to 30 days | Study population | RR 0.87 (0.76 to 1) | 3176 (27 studies) | ⊕⊕⊝⊝ Lowa,b | Trials were conducted over a period from 1976 to 2015. Differences in participant management and in the definition of sepsis were substantial <BR/>18 trial | |
| 318 per 1000 | 276 per 1000 (241 to 318) | |||||
| 28‐Day all‐cause mortality by subgroups based on treatment dose/duration ‐ long course of low‐dose corticosteroids Follow‐up: 14 to 30 days | Study population | RR 0.87 (0.78 to 0.97) | 2266 (22 studies) | ⊕⊕⊕⊝ Moderatea | Meta‐regression analysis also showed evidence of a dose response: Low doses were associated with better treatment response | |
| 321 per 1000 | 279 per 1000 (250 to 311) | |||||
| Hospital mortality Follow‐up: 14 to 365 days | Study population | RR 0.85 (0.73 to 0.98) | 2014 (17 studies) | ⊕⊕⊕⊝ Moderatea,c | Low doses of corticosteroids were associated with better treatment response | |
| 413 per 1000 | 351 per 1000 (302 to 405) | |||||
| Number of participants with shock reversal at day 7 Follow‐up: 7 to 28 days | Study population | RR 1.31 (1.14 to 1.51) | 1561 (12 studies) | ⊕⊕⊕⊕ High | Low doses of corticosteroids were associated with better treatment response | |
| 523 per 1000 | 685 per 1000 (596 to 790) | |||||
| SOFA score at day 7 Follow‐up: 7 days | Mean SOFA score at day 7 in intervention groups was 1.53 lower (2.04 to 1.03 lower) | 1132 (8 studies) | ⊕⊕⊕⊕ High | Observed reduction in SOFA score was of a magnitude that exceeded any reduction seen with any other treatment for sepsis | ||
| Length of ICU stay for survivors Follow‐up: 14 to 365 days | Mean length of ICU stay for survivors in intervention groups was 2.19 lower (3.93 to 0.46 lower) | 778 (10 studies) | ⊕⊕⊕⊕ High | Observed reduction in length of ICU stay was of a magnitude that exceeded any reduction seen with any other treatment for sepsis | ||
| Length of hospital stay for survivors Follow‐up: 14 to 365 days | Mean length of hospital stay for survivors in intervention groups was 4.11 lower (8.5 lower to 0.28 higher) | 710 (9 studies) | ⊕⊕⊕⊝ Moderatec | Observed reduction in length of hospital stay was of a magnitude that exceeded any reduction seen with any other treatment for sepsis | ||
| Number of participants with adverse events ‐ superinfections Follow‐up: 14 to 90 days | Study population | RR 1.02 (0.87 to 1.2) | 2567 (19 studies) | ⊕⊕⊕⊕ High | One large trial suggested increased risk of new sepsis with corticosteroids | |
| 161 per 1000 | 164 per 1000 (140 to 193) | |||||
| Number of participants with adverse events ‐ hyperglycaemia Follow‐up: 14 to 90 days | Study population | RR 1.26 (1.16 to 1.37) | 2081 (13 studies) | ⊕⊕⊕⊕ High | One trial suggested that risk of hyperglycaemia was lower when corticosteroids were given as a continuous perfusion than when they were given as an intravenous bolus | |
| 348 per 1000 | 438 per 1000 (403 to 476) | |||||
| *The basis for the assumed risk is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: We are very uncertain about the estimate | ||||||
aQuality of evidence was downgraded by 1 point owing to some inconsistency; 1 of the 2 largest trials showed no survival benefit bQuality of evidence was downgraded by 1 point owing to potential publication bias; some asymmetry was noted in the funnel plot cQuality of evidence was downgraded by 1 point for imprecision, and the upper limit of the confidence interval approached 1
Background
Description of the condition
Sepsis occurs when a site of infection is apparent and evidence shows body‐wide, systemic inflammation. Systemic inflammation is usually defined by two or more criteria: fever or low body temperature, an increase or decrease in white blood cells, an increase in heart rate and rapid breathing (ACCP/SCCM 1992; Bone 1991). Severe sepsis is diagnosed when sepsis is associated with organ failure or hypoperfusion. Septic shock is reported when severe sepsis is combined with a fall in systemic blood pressure that does not improve even when healthcare staff give intravenous fluids. Discussion among the scientific community reveals the need for a more pragmatic definition, such as sepsis corresponding to infection combined with organ failure or hypoperfusion, or septic shock corresponding to vasopressor‐dependent sepsis (Vincent 2013). A task force set up by the Society of Critical Care Medicine and the European Society of Intensive Care Medicine recently introduced a new definition of sepsis and septic shock (Singer 2015). Sepsis is seen when an infection is complicated by organ failures, as defined by a sequential organ failure assessment (SOFA) score (Vincent 1996) of two or higher. Septic shock is noted when infection is complicated by hypotension that requires use of vasopressor therapy while lactate levels are increased to above 2 mmol/L. Current incidence of sepsis in industrialized countries ranges from 50 to 100 cases per 100,000 population, with short‐term mortality of 20% to 50% (Annane 2003; Finfer 2004; Martin 2003; Padkin 2003; The EPISPESIS Group 2004). People usually die from hypotension or from progressive multiple organ failure (Angus 2013; Annane 2005; Parrillo 1993).
Description of the intervention
Corticosteroids include the natural steroid hormones produced by adrenocortical cells and a broad variety of synthetic analogues. These substances have various effects that may be grossly classified into glucocorticoid and mineralocorticoid effects. Glucocorticoid effects include mainly regulation of carbohydrates, lipids and proteins metabolism, as well as regulation of inflammation. Mineralocorticoid effects include mainly regulation of electrolytes and water metabolism. At molecular levels, glucocorticoids have non‐genomic and genomic effects. Rapid (within minutes) non‐genomic effects of glucocorticoids include a decrease in platelet aggregation, in cell adhesion and in intracellular phosphotyrosine kinases, and they include an increase in annexin 1 externalization (Lowenberg 2005). These effects may result from interaction of glucocorticoids with specific membrane sites (Norman 2004). Glucocorticoids have indirect genomic effects, called transrepression (Rhen 2005). These occur within a few hours following exposure of cells to glucocorticoids. They result from physical interaction between the monomeric glucocorticoid‐glucocorticoid receptor (G‐GR) α complex and various nuclear transcription factors, such as nuclear factor (NF)‐κB and activator protein (AP)‐1. Subsequently, these nuclear transcription factors are sequestrated in the cytosol and cannot enter the nucleus, preventing expression of genes encoding for most if not all pro‐inflammatory mediators. Glucocorticoids also have direct genomic effects, called transactivation. They require only a few days of cell exposure to glucocorticoids. Indeed, conformational changes (i.e. dimerization of the G‐GRα complex) are needed before this complex can migrate to the nucleus to interact with glucocorticoid‐responsive elements, that is, parts of genes encoding for regulators of termination of inflammation. Then, key anti‐inflammatory factors are up‐regulated, leading to phagocytosis, chemokinesis and anti‐oxidative processes. The net effect of glucocorticoids involves reprogramming rather than inhibiting immune cell function (Erschen 2007). Glucocorticoids induce specific activated anti‐inflammatory monocyte subtypes that migrate quickly to inflamed tissues (Varga 2008). They prolonged survival of this subtype of monocytes via A3 adenosine receptor‐triggered anti‐apoptotic effects (Barczyk 2010). Obviously, these molecular mechanisms of action of glucocorticoids are appropriate for counteracting the uncontrolled inflammation that may characterize sepsis.
How the intervention might work
Researchers have explored the biological mechanisms of sepsis to explore potential interventions. Corticosteroids have been a topic of particular focus because of their influence on the immune response. In sepsis, the hypothalamic‐pituitary gland hormonal pathway to the adrenal glands stimulates corticosteroid production (Chrousos 1995; Cooper 2003). These hormones affect inflammation through production of white blood cells, cytokines (proteins that influence the immune response) and nitric oxide. In sepsis, cytokines may suppress adrenocorticotropin hormone synthesis (Polito 2011; Sharshar 2003) and the cortisol response to exogenous adrenocorticotropin hormone (Hotta 1986; Jaattela 1991). Likewise, sepsis may be associated with alterations in scavenger receptor B1‐mediated cholesterol delivery (Cai 2008). This causes poor adrenal activity in almost half of patients (Annane 2000; Lipiner 2007; Marik 2008; Rothwell 1991) and possible resistance of body tissues to corticosteroids (Meduri 1998a) due to fewer corticosteroid receptors or receptors with lower affinity (Barnes 1995; Huang 1987; Molijn 1995). Alteration in corticosteroid receptor numbers and in binding capacity may be related at least in part to nitric oxide (Duma 2004; Galigniana 1999). Recent works suggest that immune cells ‐ not steroid‐secreting cells ‐ are key regulators of the interaction between the immune sytem and the adrenals (Kanczkowski 2013). In addition, acute illness such as sepsis may be associated with decreased cortisol clearance from plasma (Boonen 2013; Melby 1958), likely resulting from altered hepatic and renal inactivation of cortisol (Boonen 2013), Early studies showed that a pharmacological dose of corticosteroids prolonged survival among animals with sepsis (Fabian 1982). More recent studies in rodents have demonstrated that lower doses of corticosteroids, for example, 0.1 mg/kg of dexamethasone, improved haemodynamic and organ function, favourably modulated the inflammatory response and prolonged survival (di Villa Bianca 2003; Heller 2003; Tsao 2004; Vachharajani 2006). Protective effects of these glucocorticoids against sepsis may be mediated in part by the endothelial glucocorticoid receptor (Goodwin 2013). In healthy volunteers challenged with endotoxin, a low dose of corticosteroids, for example, 10 mg of prednisolone, blocked the release of pro‐inflammatory cytokines, prevented endothelial cell and neutrophil activation and inhibited the acute phase response without altering coagulation and fibrinolysis balance (de Kruif 2007). Studies in patients with septic shock showed that a short course of corticosteroids may result in a rebound in the systemic inflammatory response (Briegel 1994; Keh 2003). In addition, it is now recognized that increased pro‐inflammatory cytokine release can be sustained for longer than a week in patients with sepsis (Kellum 2007). Likewise, timing of initiation of corticosteroids may be an important factor in response to treatment. Indeed, in observational studies, short‐term mortality increased with delayed initiation of hydrocortisone (Katsenos 2014; Park 2012). For these reasons, we would anticipate that corticosteroid treatment is beneficial for patients with sepsis, and that differences in dose, timing or duration of steroid treatment may differentially affect patient response to treatment.
Why it is important to do this review
Initially, researchers used high doses of corticosteroids, usually given as a single bolus, in an attempt to block potential bursts in pro‐inflammatory cytokines. Two systematic reviews and meta‐analyses of trials of corticosteroids in sepsis or in septic shock included 10 (Lefering 1995) and nine (Cronin 1995) randomized controlled trials, respectively. These systematic reviews showed no significant difference in relative risk of death, and no significant increase in risk of gastrointestinal bleeding or superinfection associated with corticosteroids.
Subsequently, most clinicians will not recommend use of high doses of corticosteroids in sepsis. However, this review covered a period from 1966 to 1993 and did not exclude potential benefits of a lower dose (≤ 300 mg of hydrocortisone or equivalent per day) and a longer duration at full dose (≥ three days) of treatment, as investigated in randomized controlled trials over the past two decades (Annane 2002; Arabi 2011; Bollaert 1998; Briegel 1999; Chawla 1999; Cicarelli 2007; Confalonieri 2005; Gordon 2014; Hu 2009; Huh 2007; Keh 2003; Liu 2012; Meduri 2007; Meijvis 2011; Mikami 2007; Oppert 2005; Rezk 2013; Rinaldi 2006; Sabry 2011; Snijders 2010; Tandan 2005; Torres 2015; Valoor 2009; Yildiz 2002; Yildiz 2011). Among these trials, the two largest yielded different findings (Annane 2002; Sprung 2008). In one study, the combination of hydrocortisone and fludrocortisone given at low doses for one week was associated with reduced duration of shock and of organ failure and reduced mortality among patients with septic shock, and a blunted response to corticotrophin was reported (Annane 2002). In the second trial, hydrocortisone was given alone for five days and was tapered off over six additional days (Sprung 2008). This trial found significant reduction in duration and intensity of shock and organ failure showed no survival benefit. In addition to differences in treatment modalities, major discrepancies between these trials (Annane 2002 vs Sprung 2008) included differences in routine practices at the time these studies were conducted (no vs broad use of corticosteroids in sepsis), differences in severity of illness (high vs low baseline risk of death) and differences in populations (mostly medical intensive care unit (ICU) patients with lung infection vs mostly surgical ICU patients with abdominal infection). Thereafter, international guidelines have suggested that corticosteroids should be used only in patients with septic shock who are poorly responsive to fluid replacement and vasopressor therapy (Dellinger 2008; Dellinger 2013).
We therefore aim to systematically review the effects of corticosteroids in patients with sepsis.
Objectives
To examine the effects of corticosteroids on death at one month in patients with sepsis, and to examine whether dose and duration of corticosteroids influence patient response to this treatment.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs) with or without blinding.
Types of participants
We included children and adults with sepsis defined by the following criteria (ACCP/SCCM 1992; Vincent 2013).
Documented infection defined as culture or Gram stain of blood, sputum, urine or normally sterile body fluid that is positive for a pathogenic micro‐organism; or a focus of infection identified by visual inspection (e.g. ruptured bowel with the presence of free air or bowel contents in the abdomen found at the time of surgery; wound with purulent drainage); and
At least two symptoms of a systemic inflammatory response syndrome, such as fever (body temperature > 38°C) or hypothermia (< 36°C), tachycardia (> 90 beats per minute), tachypnoea (> 20 breaths per minute) or hyperventilation (arterial carbon dioxide tension (PaCO2) < 32 mm Hg) and abnormal white blood cell count (> 12,000 cells/mL or < 4000 cells/mL) or more than 10% immature band of neutrophils; and
At least one sign of organ dysfunction, that is, metabolic acidosis, arterial hypoxaemia (arterial oxygen tension [PaO2]:fractional inspired oxygen [FiO2] < 250 mm Hg), oliguria (< 30 mL/h for ≥ three hours), coagulopathy or encephalopathy; and
Septic shock defined by a combination of these criteria and the presence of hypotension (persisting systolic arterial pressure < 90 mm Hg) that is refractory to fluid resuscitation and requires vasopressor support, that is, more than 5 µg/kg of body weight per minute of dopamine or any dose of epinephrine or norepinephrine.
We included data from trials of acute respiratory distress syndrome (ARDS) when separate data were available for participants with sepsis, or when contact with study authors resulted in provision of the data.
Types of interventions
Intervention
Systemic treatment with any type of corticosteroid preparation (e.g. cortisone, hydrocortisone, methylprednisolone, betamethasone, dexamethasone).
Low‐dose corticosteroid was defined by a total dose per day of 400 mg or less of hydrocortisone (or equivalent); otherwise the dose of corticosteroid would be considered as high. A long course for the intervention was defined by a full‐dose treatment duration of three or more days; otherwise treatment was considered as a short course.
Control
Standard therapy (which may have included antibiotics, fluid replacement, inotropic or vasopressor therapy, mechanical ventilation or renal replacement therapy) or placebo.
Types of outcome measures
Primary outcomes
28‐Day all‐cause mortality.
Indeed, this was the primary outcome measure in most of the RCTs on sepsis conducted since 1992 (Annane 2009b). Most studies performed before 1992 looked at 14‐day or hospital mortality rates. We used these data to compute the pooled analysis on 28‐day mortality, unless we could obtain actual 28‐day mortality rates from study authors.
Secondary outcomes
ICU mortality.
Hospital mortality.
Number of participants with shock reversal (as defined by stable haemodynamic status ≥ 24 hours after withdrawal of vasopressor therapy) at day seven and at day 28.
Number of organs affected and severity of organ dysfunction at day seven, as measured by the sequential organ failure assessment (SOFA) score (Vincent 1996).
Length of stay in the ICU (for all participants and for survivors only).
Length of hospital stay (for all participants and for survivors only).
Adverse events (i.e. gastrointestinal bleeding and superinfection or any other adverse effects or complications of corticosteroid treatment).
Search methods for identification of studies
We attempted to identify all relevant studies regardless of language or publication status (published, unpublished, in press, in progress).
Electronic searches
We originally searched the Cochrane Infectious Diseases Group Trials Register for relevant trials (to August 2003) using the search terms 'sepsis' and 'septic shock'. Full details on the methods of the Cochrane Infectious Diseases Group and of the journals handsearched are published in The Cochrane Library in the section on Cochrane Review Groups.
In this updated version, we searched the Cochrane Central Register of Controlled Trials (CENTRAL; 2014 Issue 10) using the search terms 'sepsis', 'septic shock', 'steroids' and 'corticosteroids' (for the detailed search strategy, see Appendix 1).
We also searched (up to October 2014) MEDLINE, EMBASE and Latin American Caribbean Health Sciences Literature (LILACS) using the topic search terms in combination with the search strategy for identifying trials developed by The Cochrane Collaboration (Higgins 2011). (For detailed search strategies, see Appendix 2 (MEDLINE), Appendix 3 (EMBASE) and Appendix 4 (LILACS)).
Searching other resources
We checked the reference lists of all trials identified by these methods, and we contacted study authors to request additional published or unpublished data. We also searched the proceedings of annual meetings of major critical care medicine symposia, that is, Society of Critical Care Medicine, American Thoracic Society, International Symposium on Intensive Care and Emergency Medicine, American College of Chest Physicians and European Society of Intensive Care Medicine (1998 to 2014).
Finally, we searched for ongoing RCTs (October 2014) in the metaRegister of Controlled Trials using the search terms 'septic shock', 'sepsis', steroids', 'corticosteroids', 'adrenal cortex hormones' and 'glucocorticoids' (www.controlled‐trials.com/mrct/active).
Data collection and analysis
Selection of studies
All review authors checked the titles and abstracts identified during the search. All review authors examined in full any trial that potentially met the inclusion criteria. Five review authors (Djillali Annane, Pierre Edouard Bollaert, Josef Briegel, Didier Keh and Yizhak Kupfer) evaluated all trials, except those in which they had participated. We decided which trials fitted the inclusion criteria and graded their methodological quality. We resolved disagreements between the five review authors by discussion with the sixth review author (Eric Bellissant) until consensus was reached. One review author (Djillali Annane) contacted study authors for clarification, when necessary.
Data extraction and management
One review author (DA) drew up a standard data extraction form, and four other review authors (PEB, JB, DK, YK) amended and validated the design of the form before data abstraction. Four review authors (DA, PEB, JB, DK) independently extracted data, except those from trials in which they had participated. One review author (DA) systematically contacted the authors of trials to request missing data when possible. One review author (DA) entered (DA secretary independently reentered all data to achieve a double entry) the data into the computer, and five review authors (EB, PEB, JB, DK, YK) checked the accuracy of data entered against the original articles.
Assessment of risk of bias in included studies
We assessed risk of bias within individual trials as recommended by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We considered the following domains: selection bias, performance bias, detection bias, attrition bias, reporting bias and any other bias. We judged selection bias on the basis of how the random sequence was generated and how allocation was concealed. We judged performance bias and detection bias on the basis of who was blinded and how, among participants, care‐givers, pharmacists, data collectors, outcome assessors and data analysts (Devereaux 2001). In judging attrition bias, we considered how many (and the reasons why) participants were lost to follow‐up or were not included in analyses. When available, we compared outcomes reported in trial protocols versus actual results reported, to identify potential selective reporting bias. We resolved disagreements between the five review authors by discussion with the sixth review author (EB) until consensus was reached. One review author (DA) contacted study authors for clarification, when necessary.
Measures of treatment effect
We performed intention‐to‐treat (ITT) analyses. We performed all statistical calculations using RevMan 5 or STATA/IC version 10.1 (Stata Corp, College Station, Texas) as appropriate.
We calculated a weighted treatment effect across trials. We expressed results as risk ratios (RRs) with 95% confidence intervals (CIs) for dichotomous outcomes, and as mean differences (MDs, 95% CIs) for continuous outcomes.
Unit of analysis issues
In this review, we used data from trials in which the unit of randomization was an individual, and in which parallel groups were designed. For events that may occur repeatedly, such as receiving vasopressor therapy, we used only the first occurrence of the event.
Dealing with missing data
We systematically tried to contact primary authors of original trials to obtain missing information and unpublished data.
Assessment of heterogeneity
We considered that evidence for significant heterogeneity was present when I2 > 30%.
Assessment of reporting biases
We sought evidence of publication bias by using the funnel plot method. We used STATA/IC version 10.1 (Stata Corp, College Station, Texas) to prepare a contour‐enhanced funnel plot (Peters 2008). This graphical analysis used the log of the RR and the standard error of the RR. We plotted contours illustrating the statistical significance of study effect estimates by using a two‐tailed test.
Data synthesis
We considered methods based on the random‐effects model for all analyses, except when we found no evidence for significant heterogeneity in the results. Indeed, we suspected that we would observe heterogeneity across studies, as they were conducted over a wide period of time (almost half a century between first and last trials) and the rationale on which studies were designed varied greatly over time, with marked differences in treatment strategies and in populations between studies conducted before and after the early 90s.
Subgroup analysis and investigation of heterogeneity
To identify potential sources of heterogeneity, we sought a priori to conduct a subgroup analysis based on 'dose and duration', that is, a long course (three or more days) of low‐dose (< 400 mg/d) hydrocortisone or equivalent. This subgroup analysis allowed evaluation of a strategy based on developments in our understanding of the role of corticosteroids in host response to sepsis, as tested in trials performed after 1992. Older trials used a short course (one to four bolus doses within 24 hours) of high‐dose corticosteroids (> 400 mg of hydrocortisone or equivalent) as an anti‐inflammatory approach, and trials conducted after 1992 used low‐dose corticosteroids at full dose over a longer period (≥ three days). To further explore the putative interaction between corticosteroid dose and duration and the magnitude of effect, we considered performing a meta‐regression analysis using 28‐day all‐cause mortality as the dependent variable, and dosage and duration of corticosteroids as predictors. We performed meta‐regression analyses using STATA/IC version 10.1 (Stata Corp, College Station, Texas). We also tested a priori the interaction between baseline severity of illness and magnitude of effect in a meta‐regression analysis using mortality rates in controls as predictors. Finally, we conducted a subgroup analysis based on targeted population, sepsis, only septic shock, sepsis with ARDS, community‐acquired pneumonia and sepsis with critical illness‐related corticosteroid insufficiency (Marik 2008).
We assessed the validity of subgroup analyses (based on treatment modalities, i.e. dose and duration, or on population) on the basis of the following criteria: (1) subgroup comparisons within studies rather than between studies; (2) hypothesis preceding the analysis; (3) one of very few hypotheses; (4) large and consistent differences across studies; and (5) external evidence supporting the results (Guyatt 2008b). When subgroup analyses met these criteria and were found to be statistically significant, we applied GRADE (Grades of Recommendation, Assessment, Development and Evaluation) criteria to evaluate the quality of evidence (Guyatt 2008a).
Sensitivity analysis
We conducted sensitivity analyses for generation of allocation sequence, concealment of allocation and blinding.
Assessment of quality of evidence using GRADE and selection of outcomes for 'Summary of findings' tables
For assessments of the overall quality of evidence for each outcome that included pooled data from RCTs only, we downgraded the evidence from 'high quality' by one level for serious (or by two for very serious) study limitations (according to risk of bias evaluation), indirectness of evidence, serious inconsistency (i.e. when I2 statistic > 30%), imprecision of effect estimates (large 95% confidence intervals or small treatment effects) or potential publication bias. Data from observational studies were first determined to be of low quality.
We exported data from Review Manager to GRADE profiler (GRADEpro version 3.6) to create 'Summary of findings' tables. We included the following patient‐centred outcomes in the 'Summary of findings' table.
28‐Day all‐cause mortality.
28‐Day all‐cause mortality for long courses of low‐dose corticosteroids.
In‐hospital all‐cause mortality.
Number of participants with shock reversal at day seven.
Sepsis‐related organ failure assessment score at day seven.
Length of stay in the ICU for survivors.
Number of participants with superinfection within 28 days.
Results
Description of studies
Results of the search
Our search results are detailed in Figure 1.
Figure 1.

Flow diagram.
The search strategy yielded 47 RCTs that evaluated corticosteroids in sepsis.
Included studies
Since the last update in 2010 (see Published notes), we have included nine additional trials for a total of 33 trials (n = 4268 participants) and have described them below (see Characteristics of included studies).
Source of information
In addition to the data extracted from these publications, we obtained unpublished information from 19 trials by contacting the primary authors (Annane 2002; Annane 2010; Arabi 2011; Bollaert 1998; Briegel 1999; Chawla 1999; Cicarelli 2007; Confalonieri 2005; Gordon 2014; Keh 2003; Meduri 2007; Meijvis 2011; Oppert 2005; Rinaldi 2006; Sprung 1984; Sprung 2008; Tandan 2005; Torres 2015; Yildiz 2002) (Appendix 5). In four cases, contact with study authors did not lead to provision of additional information (Luce 1988; Meijvis 2011; Rezk 2013; Snijders 2010).
Trial centres
Eleven trials were multi‐centre trials (i.e. > two centres) (Annane 2002; Annane 2010; Bone 1987; Confalonieri 2005; CSG 1963; Gordon 2014; Meduri 2007; Sabry 2011; Sprung 2008; Torres 2015; VASSCSG 1987).
Age of participants
One study enrolled both children and adults (CSG 1963). Two trials included only children (Slusher 1996; Valoor 2009). All remaining trials included only adults.
Description of participants
Seven trials included both participants with sepsis and individuals with septic shock (Bone 1987; Liu 2012; Luce 1988; Slusher 1996; VASSCSG 1987; Yildiz 2002; Yildiz 2011). One trial included participants with sepsis (Rinaldi 2006). Five trials targeted participants with community‐acquired pneumonia‐related sepsis (Confalonieri 2005; Meijvis 2011; Sabry 2011; Snijders 2010; Torres 2015). Three trials focused on participants with ARDS and sepsis (Liu 2012; Meduri 2007; Rezk 2013). The remaining trials focused only on participants with septic shock treated by a vasopressor (Annane 2002; Annane 2010; Arabi 2011; Bollaert 1998; Briegel 1999; Chawla 1999; Cicarelli 2007; CSG 1963; Gordon 2014; Hu 2009; Huh 2007; Keh 2003; Oppert 2005; Schumer 1976; Sprung 1984; Sprung 2008; Tandan 2005; Valoor 2009). Two trials included only participants with septic shock with adrenal insufficiency as defined by a cortisol increment less than 9 µg/dL after a corticotropin bolus (Huh 2007; Tandan 2005). In 10 trials, investigators systematically performed a short corticotropin test at baseline (Annane 2002; Annane 2010; Arabi 2011; Bollaert 1998; Huh 2007; Meduri 2007; Oppert 2005; Tandan 2005; Sprung 2008; Yildiz 2011).
Control
Two studies did not use a placebo and compared corticosteroid therapy versus standard therapy, that is, antibiotics, fluid resuscitation and vasopressor when needed (Hu 2009; Rinaldi 2006). In one study, only one centre used a placebo (Sprung 1984). One trial that compared hydrocortisone versus hydrocortisone plus fludrocortisone did not use a placebo of fludrocortisone for technical reasons (Annane 2010). Another trial compared duration of hydrocortisone treatment (i.e. three vs seven days) and did not use a placebo (Huh 2007). In the remaining trials, corticosteroid therapy was compared with placebo.
Corticosteroid dose and treatment course
Eighteen trials tested the effects of long‐course (three or more days at full dose) of low‐dose hydrocortisone (Annane 2002; Annane 2010; Arabi 2011; Bollaert 1998; Briegel 1999; Chawla 1999; Confalonieri 2005; Gordon 2014; Hu 2009; Huh 2007; Keh 2003; Liu 2012; Oppert 2005; Rinaldi 2006; Sabry 2011; Sprung 2008; Tandan 2005; Valoor 2009). In one trial (Huh 2007), investigators compared hydrocortisone 50 mg intravenously every six hours when given for three days versus seven days. Another trial compared seven‐day treatment with hydrocortisone versus seven‐day treatment with the combination of hydrocortisone plus fludrocortisone (Annane 2010). One trial compared a short course (two days at full dose) of low‐dose (300 mg on day one and 250 mg on day two) intravenous hydrocortisone (CSG 1963). Another study used a cross‐over design to compare a three‐day course of low‐dose hydrocortisone versus placebo (Keh 2003).
Three trials tested effects of a long course of low‐dose prednisolone (Snijders 2010; Yildiz 2002; Yildiz 2011).
Two trials tested effects of a long course of low‐dose dexamethasone (Cicarelli 2007; Meijvis 2011). Another trial tested effects of a short course of low‐dose dexamethasone (Slusher 1996).
Three studies tested effects of a long course of low‐dose intravenous methylprednisolone (Meduri 2007; Rezk 2013; Torres 2015).
Five trials tested effects of a short course of a large dose of methylprednisolone (Bone 1987; Luce 1988; Schumer 1976; Sprung 1984; VASSCSG 1987), and two tested effects of dexamethasone (Schumer 1976; Sprung 1984).
Outcomes
Nineteen trials explicitly reported 28‐day mortality rates (Annane 2002; Annane 2010; Arabi 2011; Bollaert 1998; Briegel 1999; Chawla 1999; Cicarelli 2007; Confalonieri 2005; Gordon 2014; Huh 2007; Liu 2012; Meijvis 2011; Oppert 2005; Snijders 2010; Sprung 2008; Tandan 2005; Valoor 2009; Yildiz 2002; Yildiz 2011). For three trials, contact with the primary author of the paper led to recording of 28‐day mortality rates (Meduri 2007; Rinaldi 2006; Sprung 1984). Two trials reported only 14‐day mortality rates (Bone 1987; VASSCSG 1987). Five trials reported only hospital mortality rates (CSG 1963; Luce 1988; Schumer 1976; Slusher 1996; Torres 2015). One trial did not report mortality rates (Keh 2003). For one trial, the time point for mortality rate was unclear and remained unclear after contact with the trial primary author (Rezk 2013).
Eleven trials explicitly reported ICU mortality rates (Annane 2002; Annane 2010; Arabi 2011; Bollaert 1998; Briegel 1999; Confalonieri 2005; Gordon 2014; Hu 2009; Meduri 2007; Sabry 2011; Sprung 2008), and the primary authors of three additional trials provided this outcome (Chawla 1999; Rinaldi 2006; Torres 2015).
Hospital mortality rates were available for 20 trials (Annane 2002; Annane 2010; Arabi 2011; Bollaert 1998; Briegel 1999; Chawla 1999; Confalonieri 2005; CSG 1963; Gordon 2014; Luce 1988; Meduri 2007; Meijvis 2011; Rinaldi 2006; Schumer 1976; Slusher 1996; Sprung 1984; Sprung 2008; Tandan 2005; Torres 2015; Yildiz 2002).
Twelve trials reported the rate of shock reversal at day seven (Annane 2002; Arabi 2011; Bone 1987; Bollaert 1998; Briegel 1999; Chawla 1999; Gordon 2014; Hu 2009; Oppert 2005; Sabry 2011; Sprung 1984; Sprung 2008), and eight trials (Annane 2002; Bollaert 1998; Briegel 1999; Chawla 1999; Gordon 2014; Huh 2007; Sprung 2008; Tandan 2005) the rate of shock reversal at day 28.
Nine trials reported the numbers of dysfunctional organs at seven days, that is, SOFA scores (Annane 2002; Annane 2010; Arabi 2011; Cicarelli 2007; Gordon 2014; Oppert 2005; Rinaldi 2006; Sabry 2011; Sprung 2008).
Fourteen trials reported length of ICU stay (Annane 2002; Annane 2010; Arabi 2011; Bollaert 1998; Briegel 1999; Chawla 1999; Confalonieri 2005; Gordon 2014; Hu 2009; Huh 2007; Meduri 2007; Rinaldi 2006; Sprung 2008; Torres 2015), and 14 reported length of hospital stay (Annane 2002; Annane 2010; Arabi 2011; Bollaert 1998; Chawla 1999; Confalonieri 2005; Gordon 2014; Meduri 2007; Meijvis 2011; Slusher 1996; Snijders 2010; Sprung 2008; Torres 2015; Yildiz 2002).
Excluded studies
We excluded 14 trials (see Characteristics of excluded studies).
Ongoing studies
We identified nine additional randomized trials of prolonged treatment with a low dose of corticosteroids from trials registries (but these nine studies were not included in the analysis; see Characteristics of ongoing studies). Three of these trials never recruited participants because they lacked funding (NCT00127985 2005; NCT00368381 2008; NCT00562835 2008). One trial was halted prematurely as the result of a too slow recruitment rate; no data are available at the time of this review update (NCT00732277 2008). Two trials are now completed, but no data are available at the time of this review update (Blum 2015; NCT00670254 2008). One trial was initiated with a 2 × 2 factorial design to also allow evaluation of the effects of drotrecogin alfa activated (DAA), a recombinant activated protein C (NCT00625209 2008). Owing to the withdrawal of DAA from the market on October 25, 2011, the trial committees (Steering Committee and Data Safety and Monitoring Board) decided, with the approval of French health authorities and of the Comité de Protection des Personnes de Saint Germain en Laye, to release the data on DAA and to continue the trial using a two‐parallel‐group design corresponding to hydrocortisone and fludrocortisone (experimental arm) and their placebos (control arm) (Annane 2013). Trials included only adults, except one that evaluated treatment with hydrocortisone in children with sepsis (NCT00732277 2008).
Risk of bias in included studies
The detailed methodological quality of individual trials is reported in the 'Risk of bias' tables, in Figure 2 and in Appendix 6.
Figure 2.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Randomization
In one trial, we considered that randomization (method of generation of allocation sequence) was inappropriate to minimize selection bias, that is, as based on a card system (Schumer 1976); we judged the method for generation of allocation sequence as having high risk of bias. In six trials, the method was unclear (CSG 1963; Hu 2009; Rezk 2013; Sabry 2011; Slusher 1996; Valoor 2009), and it was deemed to have low risk of bias in the remaining trials. We judged the method used for allocation concealment to be at low risk of bias in all but 10 trials. In one trial, assignment of treatment was based on the use of unsealed envelopes (Schumer 1976). In another trial, investigators enrolling participants at one of the two participating centres could have foreseen the upcoming assignment, as the local ethical committee refused to accept blind allocation (Sprung 1984). In eight trials, study authors did not report the method used for allocation concealment (CSG 1963; Hu 2009; Huh 2007; Liu 2012; Rezk 2013; Sabry 2011; Slusher 1996; Valoor 2009).
Blinding
In four trials, we judged the method used for blinding as having high risk of bias (Annane 2010; Rinaldi 2006; Sprung 1984; Valoor 2009). Four trials used open‐label treatments (Annane 2010; Huh 2007; Rinaldi 2006; Valoor 2009). In the fifth trial (Sprung 1984), the local ethical committee at one of the two centres did not permit double‐blind allocation and administration of treatment. Therefore, blinding was not possible for 40 of the 59 participants included in the trial.
Six additional trials did not report the method used to ensure blinding (CSG 1963; Hu 2009; Liu 2012; Rezk 2013; Sabry 2011; Schumer 1976).
The remaining trials were deemed appropriately double‐blinded.
Withdrawal
Fiftteen trials (Annane 2002; Annane 2010; Bollaert 1998; Briegel 1999; Chawla 1999; Gordon 2014; Keh 2003; Meduri 2007; Meijvis 2011; Oppert 2005; Rinaldi 2006; Snijders 2010; Sprung 2008; Torres 2015; VASSCSG 1987) explicitly provided the numbers of, and reasons for, withdrawals or losses to follow‐up. In one trial, only 500 of the 800 expected participants were recruited, mainly as the result of loss of equipoise among investigators (Sprung 2008). Another trial was halted prematurely for futility after enrolment of 75 of 150 foreseen participants (Arabi 2011).
Intention‐to‐treat analysis and adherence to the protocol
Nineteen trials explicitly reported use of intention‐to‐treat analysis (as the primary analysis) and numbers of, and reasons for, non‐adherence to the protocol (Annane 2002; Annane 2010; Arabi 2011; Bollaert 1998; Bone 1987; Briegel 1999; Chawla 1999; Confalonieri 2005; Gordon 2014; Keh 2003; Meduri 2007; Meijvis 2011; Oppert 2005; Rinaldi 2006; Sabry 2011; Snijders 2010; Sprung 2008; Torres 2015; VASSCSG 1987). One trial reported only use of intention‐to‐treat analysis (Luce 1988). The remaining trials provided no information about these criteria. However, the number of analysed participants matched the number of randomly assigned participants, except for five trials. In one trial, 191 participants were randomly assigned to the placebo group and 190 were analysed for the mortality outcome (Bone 1987). In two trials (Annane 2002; Sprung 2008), one participant withdrew his or her consent and 499 of 500 and 299 of 300 randomly assigned participants were analysed, respectively. In two trials, contact with the primary author allowed us to obtain information on participants who were dropped out from the analysis (Oppert 2005; Rinaldi 2006). In the first study, seven randomly assigned participants (five in the corticosteroid group and two in the placebo group) were not analysed (Oppert 2005). Four of these participants (two in the corticosteroid group and two in the placebo group) were discharged alive from the ICU and then were lost to follow up. The three remaining participants (in the corticosteroid group) died ‐ two before receiving hydrocortisone and the last at study day 17. In the second study, 12 of 52 participants were dropped out of the study ‐ six in the control group and six in the corticosteroid group (Rinaldi 2006). Nine participants (four in the control group) were excluded, as they developed renal failure. Two control participants died in the ICU at day five and day seven, respectively. Three of the corticosteroid‐treated participants died, at days five, six and 28, respectively. Three other participants (two control group) were excluded, as they developed septic shock. All died at days three, five and six, respectively. In two trials, additional open‐label corticosteroids were given to some participants (Gordon 2014; Snijders 2010). In the first trial, five participants in the placebo arm were given rescue corticosteroids for treatment of life‐threatening hypotension and were considered as cross‐overs (Gordon 2014). In the second trial, 37 (17.4%) participants did not complete the full course of study treatment as a consequence of premature death in 10 participants, consent withdrawal in five participants, post‐randomization exclusion in eight participants and additional open‐label corticosteroid treatment in 14 participants (Snijders 2010).
Explicit definition of septic shock
Seventeen trials provided an explicit definition of sepsis (as defined in the Methods section of this review) (Annane 2002; Annane 2010; Arabi 2011; Bollaert 1998; Briegel 1999; Chawla 1999; Cicarelli 2007; Gordon 2014; Hu 2009; Huh 2007; Keh 2003; Oppert 2005; Sprung 1984; Sprung 2008; Tandan 2005; Valoor 2009; Yildiz 2011). Seven trials provided a definition of septic shock without referring to the need for vasopressor agents (Bone 1987; Luce 1988; Rinaldi 2006; Schumer 1976; Slusher 1996; VASSCSG 1987; Yildiz 2002). One study did not explicitly provide the definition used for sepsis (CSG 1963). Six trials explicitly defined sepsis due to community‐acquired pneumonia (Confalonieri 2005; Meijvis 2011; Mikami 2007; Sabry 2011; Snijders 2010; Torres 2015). In two trials, participants were randomly assigned on the basis of the presence of ARDS, and data provided in papers confirmed the presence of sepsis (Liu 2012; Rezk 2013). In another trial on early ARDS, contact with the primary author confirmed that explicit definitions of sepsis were used (Meduri 2007).
Effects of interventions
See: Table 1
We did not pool the data from three trials that included children (CSG 1963; Slusher 1996; Valoor 2009), one cross‐over trial (Keh 2003), one trial that compared two durations of hydrocortisone treatment (Huh 2007) and one trial that compared hydrocortisone versus the combination of hydrocortisone plus fludrocortisone (Annane 2010).
28‐Day all‐cause mortality
Data for 28‐day mortality were available for 20 trials; among these, two trials had no corticosteroid‐free arm. In addition, we used data on 14‐day mortality (two trials), hospital mortality (four trials) or ICU mortality (two trials), or data on short‐term mortality (one trial). Thus, we computed data from 27 trials that accounted for 3176 participants. In the treated group, 474 of 1618 participants died by day 28 compared with 495 of 1558 participants in the control group. Significant heterogeneity was evident in the results (Chi2 test = 44.99, P value = 0.01, I2 statistic = 42%). The RR of dying at 28 days was 0.87 (95% CI 0.76 to 1.00, P value = 0.05, random‐effects model) (Analysis 1.1). We downgraded the quality of evidence for this outcome from high to low for imprecision (upper limit of 95% CI = 1) and for inconsistency (significant heterogeneity across trial results).
Analysis 1.1.
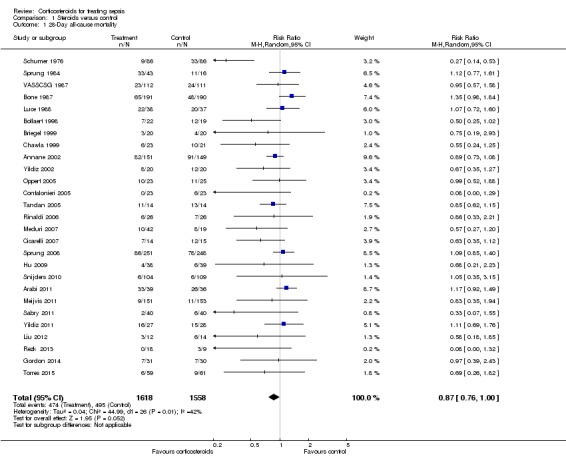
Comparison 1 Steroids versus control, Outcome 1 28‐Day all‐cause mortality.
We analysed separately the 18 studies for which 28‐day mortality data were available and the two studies reporting only 14‐day mortality (Analysis 1.2). A total of 327 of 1009 deaths occurred at 28 days in the corticosteroid‐treated group and 331 of 957 deaths in the control group (RR 0.90, 95% CI 0.80 to 1.00; P value = 0.06, fixed‐effect model), and mild heterogeneity was evident in the results (Chi2 test = 19.89, P value = 0.28, I2 statistic = 15%). Among studies reporting only 14‐day mortality rates, 88 of 303 deaths were reported in the corticosteroid‐treated group and 72 of 301 deaths in the control group (RR 1.21, 95% CI 0.93 to 1.59; P value = 0.15, random‐effects model), and moderate heterogeneity was noted in the results (Chi2 test = 1.32, P value = 0.25, I2 statistic = 24%). Subgroup differences were statistically significant (P value = 0.04).
Analysis 1.2.
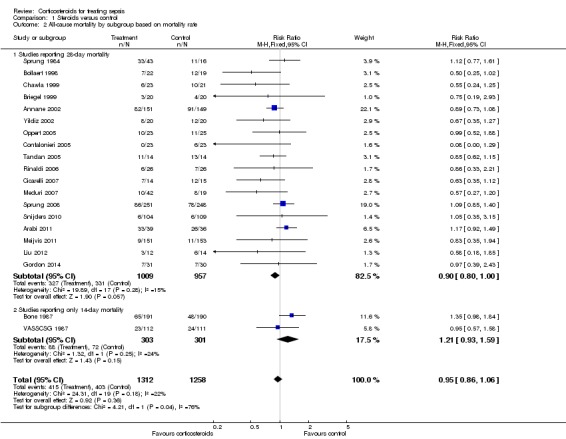
Comparison 1 Steroids versus control, Outcome 2 All‐cause mortality by subgroup based on mortality rate.
Differences in methodological quality across trials may have accounted for observed heterogeneity in the results. Subgroup analyses based on trials reporting an adequate method for generation of the allocation sequence showed an RR for dying at 28 days of 0.97 (95% CI 0.86 to 1.10) (Analysis 1.3). Similarly, subgroup analyses based on studies with adequate allocation concealment showed an RR for dying at 28 days of 0.96 (95% CI 0.84 to 1.09), and subgroup analyses on double‐blind trials showed an RR for dying at 28 days of 0.95 (95% CI 0.84 to 1.08) (Analysis 1.3).
Analysis 1.3.
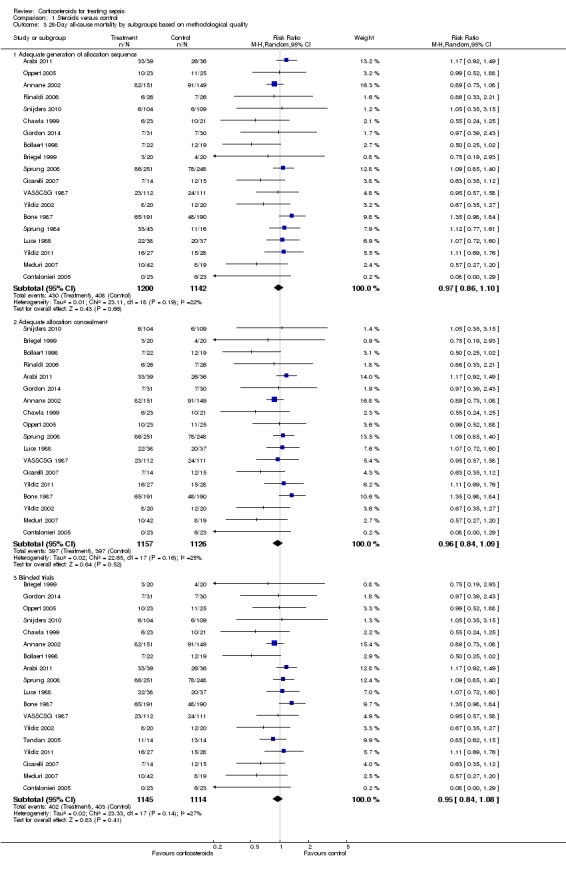
Comparison 1 Steroids versus control, Outcome 3 28‐Day all‐cause mortality by subgroups based on methodological quality.
Heterogeneity across trials may have been the result of different therapeutic regimens and different populations. Subgroup analysis on the 22 trials that tested a long course of low‐dose corticosteroids showed less heterogeneity across trials (Chi2 test = 25.09, P value = 0.240, I2 statistic = 16%) and an RR for dying at 28 days of 0.87 (95% CI 0.78 to 0.9; P value = 0.01, fixed‐effect model) in favour of the corticosteroid group (Analysis 1.4). We downgraded the quality of evidence from high to moderate for inconsistency (owing to one large trial showing non‐significant effects). Subgroup analyses on trials that tested a short course of high‐dose corticosteroids showed significant heterogeneity across trials (Chi2 test = 18.73, P value = 0.0009, I2 statistic = 79%) and an RR for dying at 28 days of 0.96 (95% CI 0.80 to 1.16; random‐effects model) (Analysis 1.4). Subgroup differences were not statistically significant (P value = 0.35).
Analysis 1.4.
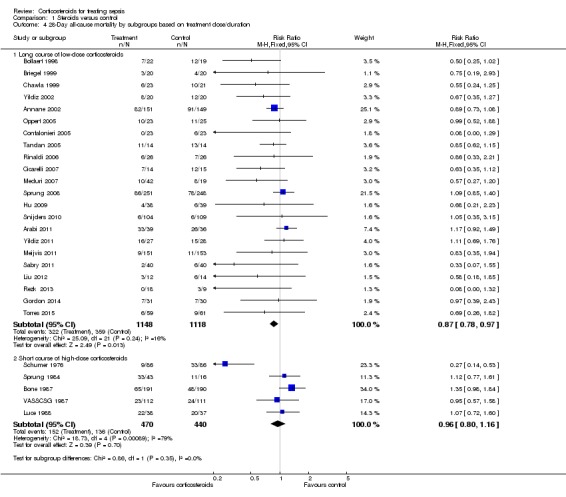
Comparison 1 Steroids versus control, Outcome 4 28‐Day all‐cause mortality by subgroups based on treatment dose/duration.
Meta‐regression analysis confirmed the positive interaction between the dose given at day one (regression coefficient 0.051; P value = 0.04) (Figure 3) and total dose (regression coefficient 0.072; P value = 0.05) (Figure 4) and the RR for dying at 28 days, that is, the lower the dose of treatment with corticosteroids, the lower was the risk of dying. No significant interaction was observed between duration of treatment (regression co‐efficient ‐0.057; P value = 0.15), mineralocorticoid activity of the experimental drug (regression co‐efficient ‐0.21; P value = 0.48) or mortality in the control arm (regression co‐efficient 0.0008; P value = 0.76) and the RR for dying.
Figure 3.
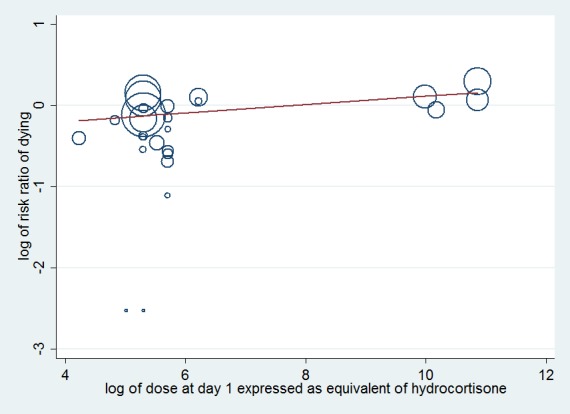
Figure represents the results from meta‐regression of log of risk ratio of dying and log of the dose of corticosteroids given at day 1 and expressed as equivalent mg of hydrocortisone. Estimates from each study are represented by circles. Circle sizes depend on the precision of each estimate (the inverse of its within‐study variance), which is the weight given to each study in the fixed‐effect model.
Meta‐regression included 26 trials. The trial by Schummer et al was not included.
REML estimate of between‐study variance tau2 = .01078. % residual variation due to heterogeneity: I2 res = 5.07% Proportion of between‐study variance explained Adj R2 = 11.16%
Figure 4.
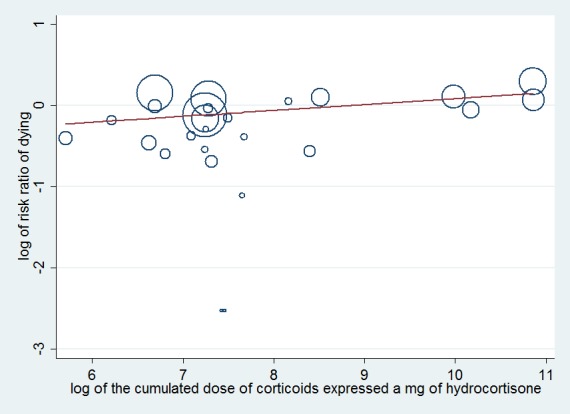
Figure represents results from meta‐regression of log of risk ratio of dying and log of cumulated dose of corticosteroids expressed as equivalent mg of hydrocortisone. Estimates from each study are represented by circles. Circle sizes depend on the precision of each estimate (the inverse of its within‐study variance), which is the weight given to each study in the fixed‐effect model.
Meta‐regression included 26 trials. The trial by Schummer et al was not included.
REML estimate of between‐study variance tau2 = .01183 % residual variation due to heterogeneity I2 res = 6.99% Proportion of between‐study variance explained Adj R2 = 2.49%
Subgroup analysis based on targeted populations found significant subgroup differences (P value = 0.01) (Analysis 1.5). In studies of heterogenous populations of participants with sepsis, the RR for dying at 28 days was 1.11 (95% CI 0.91 to 1.34; six trials; n = 826; I2 statistic = 0%). In studies of only participants with septic shock, the RR for dying at 28 days was 0.88 (95% CI 0.78 to 0.99; 12 trials; n = 1444; I2 statistic = 57%). In studies of participants with sepsis and ARDS, the RR was 0.46 (95% CI 0.25 to 0.85; three trials; n = 114; I2 = 0%), and in studies of participants with sepsis and community‐acquired pneumonia, the RR was 0.62 (95% CI 0.38 to 1.02; five trials; n = 763; I2 statistic = 2%).
Analysis 1.5.

Comparison 1 Steroids versus control, Outcome 5 28‐Day all‐cause mortality by subgroups based on targeted population.
Subgroup analysis of participants with adrenal insufficiency showed no heterogeneity in the results. Investigators reported 138 deaths among 294 participants in the treated group and 153 deaths among 289 in the placebo group. The RR for dying was 0.88 (95% CI 0.76 to 1.02; eight trials; n = 583; I2 statistic = 0%) (Analysis 1.6).
Analysis 1.6.
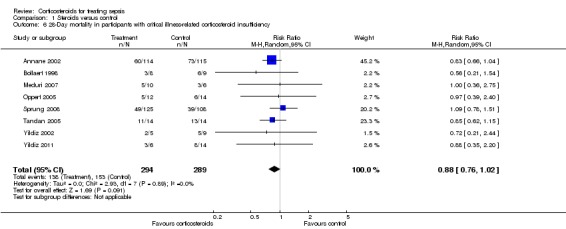
Comparison 1 Steroids versus control, Outcome 6 28‐Day mortality in participants with critical illness‐related corticosteroid insufficiency.
One trial of a large dose of corticosteroids was a statistical outlier and was excluded from the meta‐regression analysis (Schumer 1976).
Funnel plot analysis, including all trials, suggested some asymmetry (Figure 5). Contour‐enhanced funnel plot analysis including trials of a long course of low‐dose corticosteroids also suggested significant asymmetry (P value = 0.01) (Figure 6).
Figure 5.
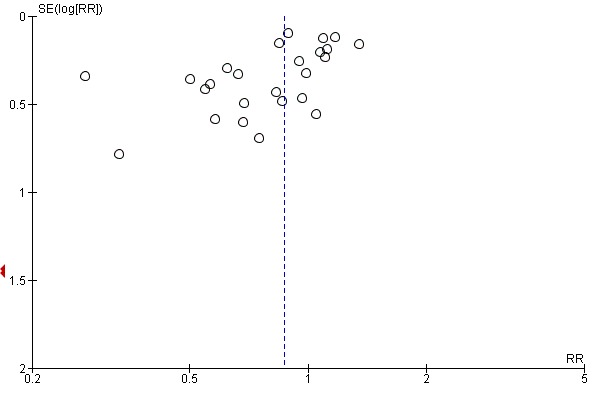
Funnel plot of comparison: 1 Steroids versus control, outcome: 1.1 28‐Day all‐cause mortality.
Figure 6.
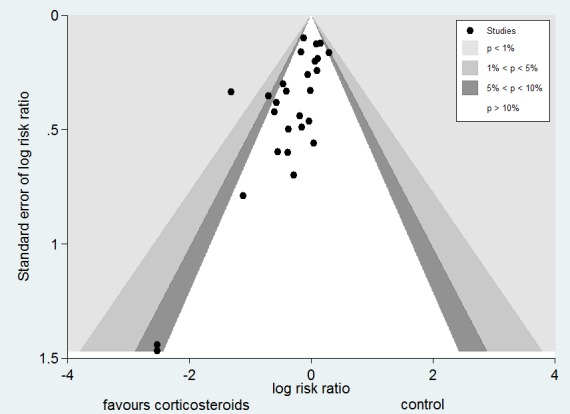
Contour‐enhanced funnel plot
Log of risk ratio for 28‐day mortality is plotted against its standard error
In one trial comparing hydrocortisone alone versus hydrocortisone plus fludrocortisone, the hazard ratio of death was 0.94 (95% CI 0.73 to 1.21) (Annane 2010).
Intensive care unit (ICU) mortality
Data were available from 13 trials, accounting for 1463 participants. All of these trials investigated a long course of low‐dose corticosteroids. A total of 264 of 748 participants in the treated group and 289 of 715 participants in the control group died in the ICU. Some heterogeneity was evident in the results (Chi2 test = 17.21, P value = 0.14, I2 statistic = 30%). The RR for dying in the ICU was 0.82 (95% CI, 0.68 to 1.00, P value = 0.045; random‐effects model) (Analysis 1.7).
Analysis 1.7.
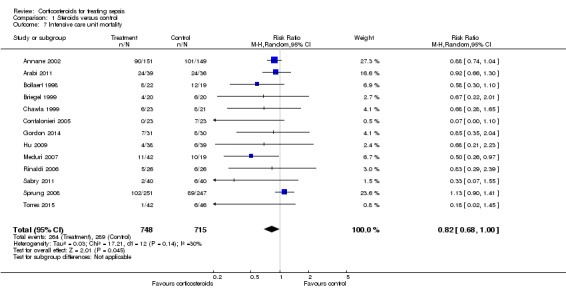
Comparison 1 Steroids versus control, Outcome 7 Intensive care unit mortality.
Hospital mortality
We could extract data on hospital mortality from 17 trials that accounted for 2014 participants. A total of 383 of 1041 participants in the treated group compared with 402 of 973 in the control group died in hospital. Heterogeneity in the results was significant (Chi2 test = 30.11, P value = 0.02, I2 statistic = 47%). The RR for dying in hospital was 0.85 (95% CI, 0.73 to 0.98; P value = 0.03, random‐effects model) (Analysis 1.8). The quality of evidence for this outcome was downgraded from high to moderate for inconsistency and imprecision (upper limit of 95% CI for RR approaching 1).
Analysis 1.8.
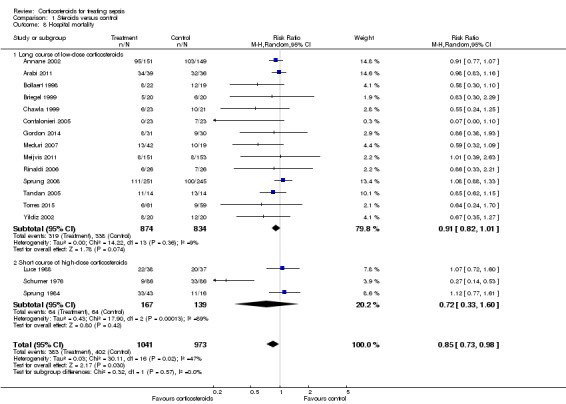
Comparison 1 Steroids versus control, Outcome 8 Hospital mortality.
In one trial comparing hydrocortisone alone versus hydrocortisone plus fludrocortisone, the RR for death was 0.94 (95% CI 0.77 to 1.14) (Annane 2010).
Shock reversal at day seven
We could extract data from 12 trials that accounted for 1561 participants. A total of 532 of 806 participants in the treated group and 395 of 755 in the control group had shock reversed at day seven. Significant heterogeneity was evident in the results (Chi2 test = 25.33, P value = 0.008, I2 statistic= 57%). The RR for having shock reversed at day seven was 1.31 (95% CI 1.14 to 1.51; P value = 0.0001, random‐effects model) in favour of the corticosteroid group (Analysis 1.9). We did not downgrade the quality of evidence for this outcome for inconsistency, as the direction of the effect was consistent across trials.
Analysis 1.9.
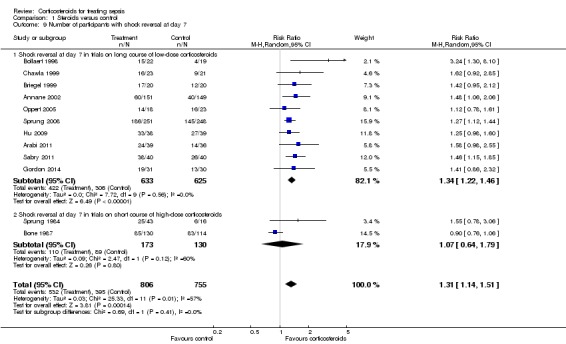
Comparison 1 Steroids versus control, Outcome 9 Number of participants with shock reversal at day 7.
Heterogeneity in the results could be explained by differences in treatment strategies used in the various trials. Two trials evaluated one or two boluses of high‐dose corticosteroids (Bone 1987; Sprung 1984), and the 10 remaining trials studied treatment with a long course of low‐dose corticosteroids. Analysis of these 10 trials (n = 1258) revealed no greater heterogeneity in the results (I2 statistic = 0%). Then, 422 of 633 participants in the treated group and 306 of 625 participants in the control group had shock reversed at day seven. The RR for having shock reversed was 1.34 (95% CI 1.22 to 1.46; P value < 0.00001) in favour of the corticosteroid group (Analysis 1.9).
In one cross‐over trial, hydrocortisone was given for three days at a dose of 240 mg per day (Keh 2003). Although this trial could not provide information on shock reversal at day seven, investigators showed that at day three, fewer hydrocortisone patients than placebo‐treated patients required norepinephrine treatment (6/20 vs 14/20; P value = 0.025).
Shock reversal at day 28
We could extract data from seven trials, accounting for 1013 participants. A total of 345 of 512 participants in the treated group had shock reversed at day 28, as did 297 of 501 in the placebo group. No heterogeneity was evident in the results (I2 statistic = 0%). The RR for having shock reversed was 1.11 (95% CI 1.02 to 1.21; P value = 0.01) in favour of the corticosteroid group (Analysis 1.10).
Analysis 1.10.
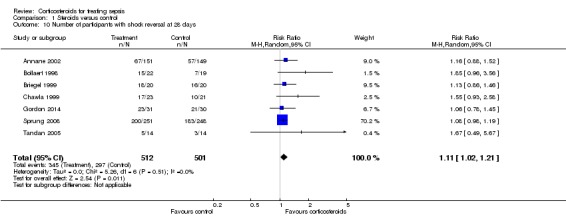
Comparison 1 Steroids versus control, Outcome 10 Number of participants with shock reversal at 28 days.
Number of organs affected and intensity of organ dysfunction according to SOFA score at day seven
In one study (Briegel 1999), corticosteroid treatment was associated with a non‐significant (P value = 0.18) trend toward earlier resolution of organ dysfunction. Eight studies (n = 1132) reported the SOFA score at seven days post randomization. The MD in the SOFA score at day seven was ‐1.53 (95% CI ‐2.04 to ‐1.03; P value < 0.00001, random‐effects model) in favour of corticosteroids. Moderate heterogeneity across studies was noted (Chi² test = 10.80, P value = 0.15, I² statistic = 35%) (Analysis 1.11). We did not downgrade the quality of evidence for this outcome, as the direction of the effect was consistent across trials.
Analysis 1.11.
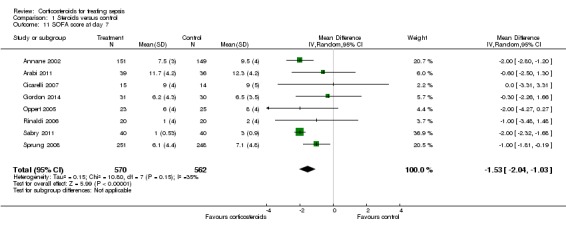
Comparison 1 Steroids versus control, Outcome 11 SOFA score at day 7.
Length of stay in the intensive care unit (ICU)
In 12 trials (n = 1384), the MD for ICU length of stay for all participants was ‐1.68 (95% CI ‐3.27 to ‐0.09; P value = 0.04, random‐effects model) with some heterogeneity evident across studies (Chi² test = 16.03, P value = 0.14, I² statistic = 31%) (Analysis 1.12). We could extract data from 10 trials on 778 ICU survivors. The mean difference for ICU length of stay among these survivors was ‐2.19 (95% CI ‐3.93 to ‐0.46; P value = 0.01, fixed‐effect model). No heterogeneity was evident across studies (Chi² test = 8.63, P value = 0.47, I² statistic = 0%) (Analysis 1.13). We judged the quality of evidence for this outcome as high.
Analysis 1.12.
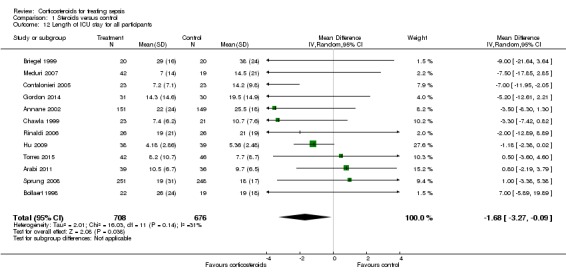
Comparison 1 Steroids versus control, Outcome 12 Length of ICU stay for all participants.
Analysis 1.13.
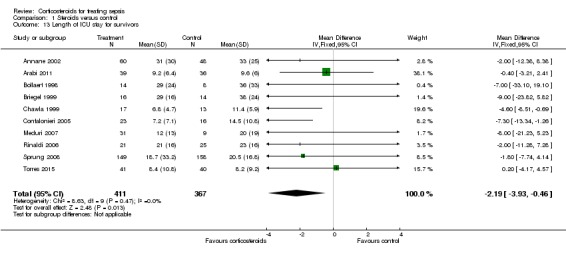
Comparison 1 Steroids versus control, Outcome 13 Length of ICU stay for survivors.
Length of hospital stay
From 12 trials (n = 1802), we could extract data on all participants. Some heterogeneity in the results was evident (I2 statistic = 22%). No evidence showed a difference between the two groups (MD ‐0.97, 95% CI ‐2.55 to 0.61, random‐effects model) (Analysis 1.14). We could extract data for hospital survivors from nine studies (n = 710). We noted some heterogeneity in the results (I2 statistic = 43%). No evidence suggested a difference between the two groups (MD ‐4.11, 95% CI ‐8.50 to 0.28) (Analysis 1.15).
Analysis 1.14.
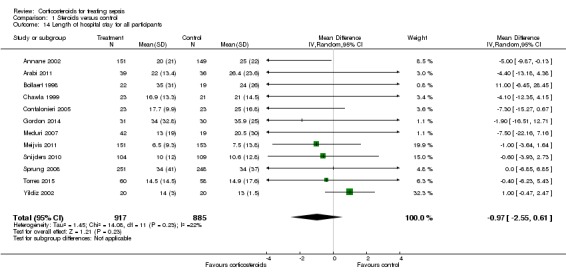
Comparison 1 Steroids versus control, Outcome 14 Length of hospital stay for all participants.
Analysis 1.15.
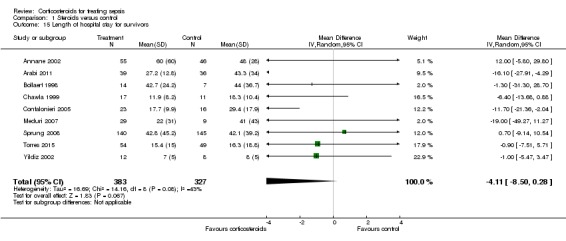
Comparison 1 Steroids versus control, Outcome 15 Length of hospital stay for survivors.
Adverse events
Gastroduodenal bleeding
We could extract data from 19 trials (n = 2382). A total of 81 of 1219 participants in the treated group and 62 of 1163 in the control group had an episode of gastroduodenal bleeding. We noted no heterogeneity in the results (I2 statistic = 0%). The RR for having gastroduodenal bleeding was 1.24 (95% CI 0. 92 to 1.67; P value = 0.15, fixed‐effect model) (Analysis 1.16).
Analysis 1.16.
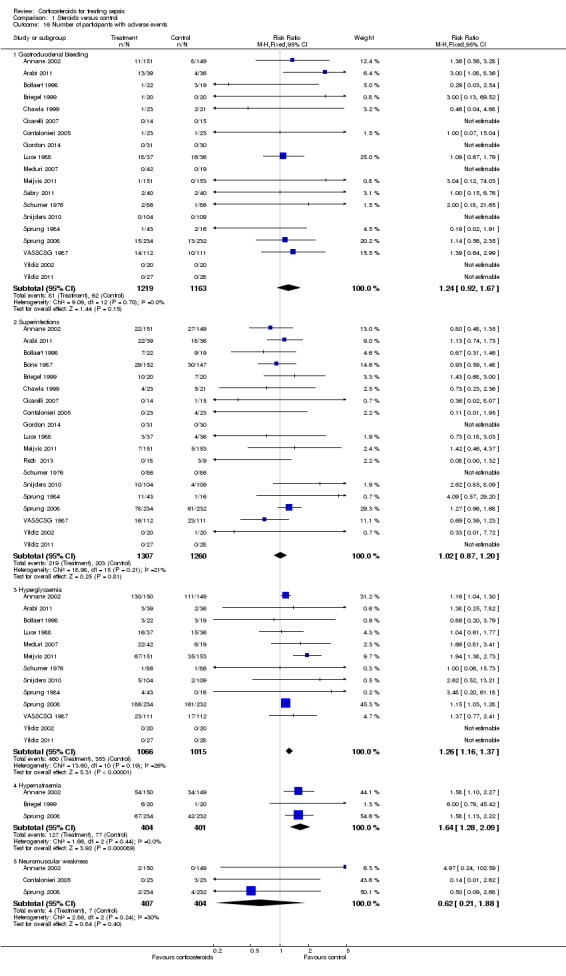
Comparison 1 Steroids versus control, Outcome 16 Number of participants with adverse events.
Superinfection
We could extract data from 19 trials (n = 2567). A total of 219 of 1307 participants in the treated group and 203 of 1260 participants in the control group had an episode of nosocomial infection. We noted moderate heterogeneity in the results (Chi² test = 18.98, P value = 0.21, I² statistic = 21%). The RR for superinfection was 1.02 (95% CI 0.87 to 1.20; P value = 0.81, fixed‐effect model) (Analysis 1.16).
Hyperglycaemia
The number of participants who presented with hyperglycaemia was reported for 13 trials (n = 2081). Moderate heterogeneity was noted in the results (Chi² test = 13.60, P value = 0.19; I² statistic = 26%). The RR for hyperglycaemia was 1.26 (95% CI 1.16 to 1.37; P value < 0.00001, fixed‐effect model) (Analysis 1.16).
One trial comparing tight glucose control versus standard care found no benefit in normalizing blood glucose levels among corticosteroid‐treated septic shock participants (Annane 2010).
Hypernatraemia
The number of participants who presented with hypernatraemia was reported for three trials (n = 805). We noted no heterogeneity in the results (I2 statistic = 0%). The RR for hypernatraemia was 1.64 (95% CI 1.28 to 2.09; P value < 0.00001, fixed‐effect model) (Analysis 1.16).
Neuromuscular weakness
The number of participants who presented with neuromuscular weakness was reported for three trials (n = 811). Moderate heterogeneity was evident in the results (I2 statistic = 30%). The RR for neuromuscular weakness was 0.62 (95% CI 0.21 to 1.88; P value = 0.24) (Analysis 1.16).
We have summarized the main results in Table 1.
Discussion
Summary of main results
Effects of corticosteroids on mortality
Overall, this review suggested that, in sepsis, corticosteroids reduced all‐cause 28‐day mortality, although the P value was 0.05, and significantly reduced intensive care unit (ICU) and in‐hospital mortality. For these outcomes, results showed strong heterogeneity.
Subgroup analysis based on treatment modalities showed that a long course of low‐dose corticosteroids significantly reduced 28‐day mortality with little heterogeneity in results. By contrast, a short course of high‐dose corticosteroids did not affect mortality, in keeping with previous reports (Cronin 1995; Lefering 1995).
Patients with more severe forms of sepsis, such as those with vasopressor‐dependent septic shock and those with acute respiratory distress syndrome (ARDS), may be more likely to derive a survival benefit from corticosteroids than patients with less severe sepsis. Patients whose sepsis is secondary to community‐acquired pneumonia also are more likely to benefit from corticosteroids. Indeed, analysis based on the targeted population showed statistically significant subgroup differences. Analysis of eight trials including patients with critical illness‐related corticosteroid insufficiency suggested a non‐significant reduction in the risk of death. However, studies did not use the same definition for adrenal insufficiency. Additional studies are needed to determine the best diagnostic tool for critical illness‐related corticosteroid insufficiency (Marik 2008).
Effects of corticosteroids on morbidity outcomes
The beneficial effects of corticosteroids on mortality may be related to the favourable effects of treatment on duration of shock. Indeed, this review showed that treatment with corticosteroids resulted in a substantial reduction in shock duration, with fewer patients remaining on vasopressor therapy by day seven and by day 28. Treatment with a long course of low‐dose corticosteroids may attenuate the severity of inflammation (Confalonieri 2005; Keh 2003; Mikami 2007a; Oppert 2005; Rinaldi 2006) and the intensity and duration of organ system failure (Briegel 1999; Confalonieri 2005; Keh 2003; Oppert 2005; Sprung 2008), as shown in this review by a marked decrease in sequential organ failure assessment (SOFA) score at day seven. In addition, subsequent to favourable effects on cardiovascular and other organ functions, corticosteroid therapy resulted in substantial shortening of ICU length of stay.
Tolerance of corticosteroids
Finally, this review also showed no evidence of effects of corticosteroids on rates of gastroduodenal bleeding or superinfection, or on the proportion of patients with acquired neuromuscular weakness. Corticosteroids were associated with increased risk for developing hyperglycaemia and hypernatraemia. One randomized controlled trial suggested that continuous infusion of hydrocortisone resulted in fewer episodes of hyperglycaemia than were seen with bolus administration (Loisa 2007). One trial on 509 corticosteroid‐treated patients with septic shock reported no benefit for normalizing blood glucose levels (Annane 2010).
Overall completeness and applicability of evidence
Although the subgroup analysis is a between‐study and not a within‐study hypothesis, we thought its validity was acceptable according to proposed criteria (Guyatt 2008b). First, we defined the hypothesis for an interaction between dose and duration and effects of corticosteroids on mortality a priori. Second, we conducted only three subgroup analyses (based on methodological quality of studies, dose and duration and targeted population). Third, treatment effect was seen as a 4% absolute difference in mortality and rather consistent findings in terms of 28‐day, ICU and hospital mortality (risk ratios (RRs) 0.87, 0.82 and 0.91, respectively). Meta‐regression analysis further confirmed the interaction of dose and duration with effects of corticosteroids on mortality. Fourth, strong external evidence supports these results. Experimental and human studies have shown that a dose of 400 mg or less of hydrocortisone or equivalent can reverse the systemic inflammatory response, endothelial activation and coagulation disorders secondary to infection (Annane 2005), thus arguing against the use of higher doses. Moreover, at these low doses, corticosteroids have been shown to improve rather than suppress innate immunity in patients with septic shock (Kaufman 2008). It is now established that sepsis results in a sustained pro‐inflammatory state, arguing against a short course of treatment (Kellum 2007).
Quality of the evidence
We judged the quality of evidence for 28‐day mortality as low rather than high because some imprecision and inconsistency across trials were related in part to differences in study populations and to differences in treatment dose and duration. We downgraded the quality of evidence for 28‐day mortality in the subgroup of long course of low‐dose corticosteroids from high to moderate because one of the two largest trials on a long course of low‐dose corticosteroids reported no survival benefit (Sprung 2008). Other differences between trials included differences in targeted populations, in control of co‐interventions or in type and dose of corticosteroids. In addition, ongoing trials (Gordon 2014a; NCT00625209 2008; Venkatesh 2013) may influence the direction and magnitude of treatment effects.
Potential biases in the review process
In this review, we performed a comprehensive search of the literature with no restriction on language, so we can assume that the risk of missing important trials was very limited. The asymmetrical funnel plot for the primary outcome of this review suggests some publication bias. However, potential sources of an asymmetrical funnel plot also include selection biases, poor methodological quality of smaller studies, true heterogeneity, artefacts and chance (Egger 1997). Visual inspection of the funnel plot suggests a small‐study effect (i.e. among small studies, the positive ones are more likely to be published). Nevertheless, our thorough search strategy and the need to enrol studies in public clinical trial registries may have decreased the risk of missing any randomized controlled trial. As discussed in this review, all studies on low‐dose corticosteroids described methods of acceptable quality. True heterogeneity seems to be a more plausible explanation for the observed asymmetrical funnel plot. Indeed, the effects of low‐dose corticosteroids on mortality may be proportional to the basal risk of death, and the Corticosteroid Therapy of Septic Shock trial (CORTICUS) included patients at lower risk of death (Sprung 2008). In addition, smaller intervention effects in the CORTICUS trial may have resulted from an improved standard of care introduced during the decade that separated most of the smaller trials from CORTICUS. Finally, the asymmetrical funnel plot may have been due to chance.
According to the primary objective of this systematic review, we included only trials that compared corticosteroids versus standard therapy alone or placebo. One trial used a cross‐over design (Keh 2003), and we could obtain none of the foreseen outcomes for this review. This trial concluded that prolonged treatment with a low dose of hydrocortisone improved haemodynamic and immune outcomes. Another trial compared three days versus seven days of hydrocortisone therapy and suggested no evidence for differences in outcomes between patients treated for three days or seven days (Huh 2007). However, this trial has some limitations, including lack of blinding and small sample size. Three other trials have included children (CSG 1963; Slusher 1996; Valoor 2009). We considered that pooling the results of remaining trials in a meta‐analysis was acceptable.
Two trials were published only as an abstract (Chawla 1999; Tandan 2005). Nevertheless, the primary investigators for these studies (Chawla 1999; Tandan 2005) provided sufficient unpublished data for review authors to compute the primary outcome and several secondary outcomes for this review, allowing us to include these trials in the meta‐analysis. Both published and unpublished data were available for 17 trials (Annane 2002; Annane 2010; Arabi 2011; Bollaert 1998; Briegel 1999; Chawla 1999; Cicarelli 2007; Confalonieri 2005; Gordon 2014; Keh 2003; Meduri 2007; Oppert 2005; Rinaldi 2006; Sprung 1984; Sprung 2008; Torres 2015; Yildiz 2002), and the primary author for each trial validated the data extraction form. For four studies, contact with the primary investigator yielded no additional data (Luce 1988; Meijvis 2011; Rezk 2013; Snijders 2010).
We chose to convert outcome measures that correspond to censored data into dichotomous variables, that is, the proportion of participants with a particular event after one week and after four weeks, or at ICU or hospital discharge.
Agreements and disagreements with other studies or reviews
Findings in this review that a short course of high‐dose corticosteroids provides no benefit for patients with sepsis are in line with reports from previous systematic reviews (Cronin 1995; Lefering 1995) and with current international guidelines (Dellinger 2013).
We found scarce data that could not allow conclusions on effects of corticosteroids in children with sepsis, in keeping with a recent systematic review (Menon 2013).
The beneficial effects of corticosteroids on shock reversal in patients with septic shock are consistent across recent systematic reviews (Kalil 2011; Moran 2010; Sherwin 2012). The survival benefit derived from corticosteroids for patients with sepsis was suggested by some previous authors (Moran 2010) but not by others (Kalil 2011; Sherwin 2012). Nevertheless, current systematic reviews have included trials that were not included in previous systematic reviews, as they were published only recently or were published in non‐English language. Current reviews have included non‐published information for a large number of trials after contact was made with original study authors, resulting in inclusion of qualitatively and quantitatively better data than were provided previously.
Authors' conclusions
Overall, corticosteroids may favourably impact all‐cause mortality at 28 days, and at ICU and hospital discharge, in patients with sepsis. Subgroup analyses have suggested that corticosteroids should be given at a low dose (of ≤ 400 mg per day, of hydrocortisone or equivalent) for three or more days at full dose, and preferably in patients with septic shock, sepsis and ARDS, community‐acquired pneumonia or critical illness‐related corticosteroid insufficiency. Evidence from this review is insufficient to support an abrupt or gradual interruption in treatment, or to support intravenous bolus or continuous infusion of treatment. Evidence accumulated from five trials uniformly does not support use of a short course of high‐dose corticosteroids in patients with sepsis.
The criteria for critical illness‐related corticosteroid insufficiency in septic shock remain to be defined.
Ongoing trials should clarify:
the role of a long course of low‐dose corticosteroids for treatment of septic shock in children;
the role of a long course of low‐dose corticosteroids for treatment of patients with sepsis without shock, or with a mild form of septic shock;
the role of mineralocorticoid replacement;
the optimal timing of initiation of treatment;
the optimal dose of hydrocortisone (or equivalent); and
the role of a long course of low‐dose corticosteroids for treatment of sepsis caused by different types of infections.
The optimal timing for starting treatment, the optimal dose of hydrocortisone (or equivalent) and the duration and modality of withdrawal of treatment require additional trials.
Feedback
Feedback, 9 May 2013
Summary
Annane et al (Annane 2004; updated 2010) in their systematic review of corticosteroids for treating severe sepsis and septic shock concluded “a long course of low dose corticosteroids reduced mortality without inducing major complications”. This was based on a subgroup analysis, as all doses/durations of corticosteroids for septic shock did not show a benefit in reduction of mortality.
Given that the two largest trials (Annane 2002; Sprung 2008) have contradicting results, we were interested in looking into these trials. Upon our review, we feel that the risk of bias assessment of these trials has not adequately addressed the issue of incomplete data. In one trial (Sprung 2008), authors used a per‐protocol analysis for their adverse event data; this was subsequently used in this review. Neither the trial nor the review addresses the reasons for this approach. Using per‐protocol data is not the preferred method of outcome reporting, as it does not allow for preservation of randomization. Section 14.6.1 of the Cochrane Handbook for Systematic Reviews of Interventions specifically asks whether any patients were excluded in reporting of adverse events. Participants who experience unfavourable adverse events may drop out of the trial. When the per‐protocol analysis is used, adverse effect results may therefore be biased in favour of steroids. The same scenario can be applied for the placebo group.
In another trial (Annane 2002), we are concerned with the way data were collected and reported for adverse events. Events are reported as being “possibly related to steroids” and “possibly related to vasopressors”. We are unsure as to how one would know whether or not an adverse event was related to the intervention. Neither the review nor the trial specifically outlines the adjusting procedure for determining whether or not adverse events were due to steroids. Patients in the intensive care unit have many risk factors for infection ‐ GI bleeding, psychiatric disorders (e.g. delirium) ‐ therefore it seems inappropriate to try to ascertain whether or not the event was secondary to steroids.Section 14.6.1 of the Cochrane Handbook for Systematic Reviews of Interventions explains how clinical trials may have a well‐designed method for collecting data for the primary outcome, but in fact may take a retrospective, unblinded approach to collecting adverse event data. An extension of the CONSORT statement for harm also echoes this, recommending that clinical trials should explicitly define how data were defined, collected and analysed for adverse events (Ioannidis 2004). As Annane 2002 was a randomized controlled trial, adverse events would not require assessment of whether or not they were thought to be due to treatment. Randomization should take care of confounding factors and thus should be able to show differences (if they truly exist) in adverse events. In our opinion, preference should be given to all‐cause adverse events for this reason. Lastly, we note that in Analysis 1.12 for ‘superinfections’, the percentage of participants with an event was used instead of the actual number of events in Annane 2002. For example, the number of events for the treatment arm should have been ‘22’, but ‘15’ was used instead. Given that these issues on selective reporting (Annane 2002) and incomplete data surrounding the two largest trials of this review have not been adequately addressed in the risk of bias assessments, we find it difficult to conclude at this time on the safety profile of corticosteroid use in treating severe sepsis and septic shock. We look forward to hearing your response to our concerns.
Reply
We are grateful to Dr. Harbin and colleagues for their comments on the Cochrane review on corticosteroids for severe sepsis and septic shock.
Dr. Harbin and colleagues questioned the validity of the concluding statement of this review that corticotherapy was overall well tolerated apart from inducing hyperglycaemia and hypernatraemia. Indeed, they pointed out that Sprung and colleagues reported serious adverse events as per‐protocol (Sprung 2008). In fact, in this trial, data for adverse events were reported only for 466 of 499 patients. We have now reported this information in the risk of bias table in a revised version of the review.
Annane and colleagues reported in their main paper the number of participants with any serious adverse events in each treatment arm as per intent‐to‐treat analysis (Annane 2002). All serious adverse events that were observed were reported in each treatment group. Serious adverse events were further classified according to what was known about complications of corticosteroids or catecholamines. For example, all superinfections, gastroduodenal bleeding, metabolic disorders and psychiatric disorders that occurred at any time from randomization were reported and further classified in a blinded manner as possibly related to corticosteroids. Thus, no manipulation of data occurred. All serious adverse events were carefully scrutinized and reported in the manuscript, and additional unpublished information (i.e. raw data) was available during preparation of the Cochrane review.
Finally, Dr. Harbin and colleagues highlighted an error in numbers used for Analysis 1.12 (number of participants with superinfection) that is now corrected in the revised version of the review. This modification did not significantly alter the direction and the magnitude of the pooled estimate for evaluation of serious adverse events. Thus, we believe that the conclusion statement ‐ that treatment with corticosteroids in patients with sepsis or septic shock is well tolerated apart from metabolic disorders ‐ is still valid.
Contributors
Megan Harbin, BSc Pharm Asal Taheri, BSc Pharm Wan‐Yun Polinna Tsai, BSc Pharm Gloria Su, BSc Pharm Aaron M Tejani, BSc Pharm, PharmD
Reply:
Djillali Annane
Feedback, 9 November 2017
Summary
Annane and colleagues in their systematic review (Annane 2015), concluded that “a long course of low dose corticosteroids reduced mortality without inducing major complications”. This was based on a subgroup analysis, analysis of outcome 1.4, so we pursued it in more detail. Upon our review, we found that the forest plot was created using a fixed‐effect model. However, the largest trial, Sprung 2008, only carried 21.5% weight while the second largest trial, Annane 2002, was allotted a larger weight of 25.1%. Although we acknowledge that given the I2 was 16%, a fixed‐effect model was chosen, we felt that because these two trials had conflicting results, suggesting clinical heterogeneity, a random‐effects model would have been a more conservative and more appropriate approach in this scenario. We took the initiative to recreate the forest plot using a random‐effects model, and found that the treatment effect became not statistically significant. This suggests that perhaps the conclusion is not as simple as “a long course of low dose corticosteroids reduced mortality” in severe sepsis and septic shock.
Furthermore, when we reviewed Table 1 we noticed that footnote “a” states that the quality of evidence for the primary outcome was downgraded due to “1 of the 2 largest trials [showing] no survival benefit”. We feel that this sentence implies that the other largest trial did show a survival benefit, and may mislead the readers to believe so. However, both of the largest trials (Annane 2002; Sprung 2008), did not show a statistical benefit for corticosteroids compared to placebo for mortality benefits in sepsis and septic shock. One trial simply showed that there was no increase in mortality, but did not show that it reduced mortality.
Based on our findings, we feel that there could be an alternative interpretation from the results of the trials. This review includes studies from 1976 to 2015, and when we look at the studies published before 2002, the studies as a group show a more convincing trend toward mortality benefit using corticosteroids. In contrast, the studies published after 2002 show more conflicting results. This may suggest that medical therapy today is better at treating sepsis and hence the benefits from corticosteroids are not as apparent as previously thought. In contrast to the conclusion stated by the authors, we feel that this review presents inconclusive evidence for mortality benefit with corticosteroid use in septic patients. We look forward to your response.
Reply
The planned analysis was to use fixed‐effect model unless heterogeneity across trials could be suspected (i.e. squared I statistic > 30%). We weighted studies by the amount of information they contribute (more specifically, by the inverse variances of their effect estimates). It is also important to highlight that CORTICUS (Sprung 2008), was terminated prematurely (after 500 participants were recruited out of 800 expected) owing to low recruitment rate and loss of equipoise among investigators. Changing from fixed‐ to random‐effects models did not change the magnitude nor the direction of the point estimate (RR 0.87 versus 0.88) and slightly enlarged the 95% CI 0.78 to 0.97 versus 0.77 to 1.00. Thus, we do not believe that the conclusion from our systematic review was not supported by data analysis, and we disagree about over‐interpreting the data.
In the trial Annane 2002, the primary outcome was time to death in non‐responders to ACTH test (modified intent‐to‐treat analysis). The primary analysis for the primary outcome in this trial found a statistically significant increase in survival time (P = 0.02). The CORTICUS trial (Sprung 2008), found no significant effect of treatment on mortality. Thus, we do not believe that we have misinterpreted (misreported) findings from either the Annane 2002 or Sprung 2008 trials.
The pooled RR of dying from trials published before 2002 was 0.90 ( 95% CI 0.75 to 1.07). The pooled RR of dying from trials published from 2002 was 0.89 ( 95% CI 0.80 to 1.00). Thus, there is no evidence that corticosteroids effect on mortality differed between trials published before 2002 versus those published since 2002. Finally, new trials have been published since the last update of this review including Keh et all JAMA 2016; Bi et al PLoSOne 2016; Menon et al Ped CCM 2017; El Nawawy The Pediatric Infectious Disease Journal 2016 ; Qing‐quan Lv et al Am J Emerg Med 2017; Tongyoo et al. Critical Care 2016 ; and two large trials are about to be published in the very next future (ADRENAl, n = 3800 and APROCCHSS, n = 1241). Thus, we believe that there is a need to update the review in light of these newly published studies.
Contributors
Summary
Candy Lee, Karen Ng, Shalini Singla, Marco Yeung
Pharmacy Residents
Lower Mainland Pharmacy Services, Pharmacy Association, Vancouver, Canada
We do not have any affiliation with or involvement in any organisation with a financial interest in the subject matter of my comment
Reply
Djillali Annane
Department of Critical Care, Hyperbaric Medicine and Home Respiratory Unit Center for Neuromuscular Diseases; Raymond Poincaré Hospital (AP‐HP) Faculty of Health Sciences Simone Veil, University of Versailles SQY‐ University of Paris Saclay 104 Boulevard Raymond Poincaré 92380 Garches France
Acknowledgements
We would like to thank Harald Herkner (Content Editor), Jing Xie (Statistical Editor), Philipp Schuetz and Mirjam Christ‐Crain (Peer Reviewers) and Janet Wale (Consumer Editor) for help and editorial advice provided during preparation of this 2015 updated systematic review.
We would like to thank Dr. R. DeGaudio, Dr. G.U. Meduri, Dr. M. Oppert, Dr. C. Sprung and Dr. S. Tandan for providing us with unpublished data. We also would like to thank Dr. Bin Du (Medical ICU, Peking Union Medical College Hospital, Beijing 100730, China) for helping with abstraction of information from papers in the Chinese language.
We would like to thank Prof. Harald Herkner (Content Editor); Marialena Trivella (Statistical Editor); Peter Minneci, Charles Natanson, Gordon Guyatt and Matthias Briel (Peer Reviewers) and Karen Hovhannisyan (Trials Search Co‐ordinator) for help and editorial advice provided during preparation of the 2010 updated review.
Appendices
Appendix 1. Search strategy for CENTRAL
#1 MeSH descriptor sepsis explode all trees #2 MeSH descriptor shock, septic explode all trees #3 steroid* in All Text #4 (sepsis in All Text or (septic in All Text and shock in All Text) ) #5 (#1 or #2 or #3 or #4) #6 MeSH descriptor Adrenal Cortex Hormones explode all trees #7 corticosteroid* in All Text #8 (#6 or #7) #9 (#5 and #8)
Appendix 2. Search strategy for MEDLINE (Ovid SP)
1. exp Sepsis/
2. exp Shock, Septic/
3. (sepsis or septic shock).mp.
4. 1 or 2 or 3
5. exp Adrenal Cortex Hormones/
6. (corticosteroid* or steroid*).mp.
7. 6 or 5
8. 4 and 7
9. ((randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or clinical trials as topic.sh. or randomly.ab. or trial.ti.) not (animals not (humans and animals)).sh.
10. 8 and 9
Appendix 3. Search strategy for EMBASE (Ovid SP)
1. exp sepsis/ 2.exp septic shock/ 3. (sepsis or septic shock).mp. 4.1 or 2 or 3 5.steroid/ 6. (corticosteroid* or steroid*).ti,ab. 7. 6 or 5 8. 4 and 7 9. (placebo.sh. or controlled study.ab. or random*.ti,ab. or trial*.ti,ab.) not (animals not (humans and animals)).sh. 10. 8 and 9
Appendix 4. Search strategy for LILACS (via BIREME)
("sepsis" or "septic$" or "SEPSIS" or "SEPTIC" or "SEPTIC SHOCK/" or "SEPTICEMIA") and ("corticosteroid$" or "steroid$" or "glucocorticoid$" or "CORTICOSTEROID" or "GLUCOCORTICOIDS/" or "STEROID")
Appendix 5. Unpublished data obtained from trial authors
| Studies | Type of unpublished data provided by primary authors |
| Annane 2002 | Full access to individual data, details for randomization and blinding procedures. |
| Annane 2010 | Full access to individual data, details for randomization and blinding procedures. |
| Arabi 2011 | Additional information on mortality data, length of stay, shock reversal and SOFA. |
| Bollaert 1998 | Details for randomization and blinding procedures. Additional information on adrenal function (data according to the review definition: delta cortisol ≤ 9 µg/dL. Additional information for ICU length of stay and adverse events. |
| Briegel 1999 | Details for randomization and blinding procedures. Additional information for ICU length of stay and adverse events. |
| Chawla 1999 | Details for randomization and blinding procedures. Additional information for mortality, shock reversal and ICU length of stay and adverse events. |
| Cicarelli 2007 | Details for randomization and blinding procedures. |
| Confalonieri 2005 | Full access to individual data, details for randomization and blinding procedures. |
| Gordon 2014 | Additional information on shock reversal, length of stay, SOFA and adverse events. |
| Keh 2003 | Details for randomization and blinding procedures. Additional information for adverse events. |
| Meduri 2007 | Details for randomization and blinding procedures. Additional information for subgroup of patients with sepsis or septic shock on mortality, ICU and hospital length of stay and adverse events. |
| Meijvis 2011 | Separate information for patients with sepsis. |
| Oppert 2005 | Details for randomization and blinding procedures. Additional information for mortality, for outcome of patients randomized and not analysed, shock reversal and adverse events. |
| Rinaldi 2006 | Details for randomization and blinding procedures. Additional information for mortality, for outcome of patients randomized and not analysed and adverse events. |
| Sprung 1984 | Additional information for 28. |
| Sprung 2008 | Full access to individual data, details for randomization and blinding procedures. |
| Tandan 2005 | Details for randomization and blinding procedures. |
| Torres 2015 | Details for randomization and blinding procedures. Additional information for mortality, shock reversal, SOFA, length of stay and adverse events. |
| Yildiz 2002 | Details for randomization and blinding procedures. Additional information for mortality, hospital length of stay and adverse events. |
Appendix 6. Methodological quality of studies
Data and analyses
Comparison 1.
Steroids versus control
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 28‐Day all‐cause mortality | 27 | 3176 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.76, 1.00] |
| 2 All‐cause mortality by subgroup based on mortality rate | 20 | 2570 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.86, 1.06] |
| 2.1 Studies reporting 28‐day mortality | 18 | 1966 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.80, 1.00] |
| 2.2 Studies reporting only 14‐day mortality | 2 | 604 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.93, 1.59] |
| 3 28‐Day all‐cause mortality by subgroups based on methodological quality | 20 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Adequate generation of allocation sequence | 19 | 2342 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.86, 1.10] |
| 3.2 Adequate allocation concealment | 18 | 2283 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.84, 1.09] |
| 3.3 Blinded trials | 18 | 2259 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.84, 1.08] |
| 4 28‐Day all‐cause mortality by subgroups based on treatment dose/duration | 27 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Long course of low‐dose corticosteroids | 22 | 2266 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.78, 0.97] |
| 4.2 Short course of high‐dose corticosteroids | 5 | 910 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.80, 1.16] |
| 5 28‐Day all‐cause mortality by subgroups based on targeted population | 26 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Sepsis | 6 | 826 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.91, 1.34] |
| 5.2 Septic shock only | 12 | 1444 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.78, 0.99] |
| 5.3 Sepsis and ARDS | 3 | 114 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.25, 0.85] |
| 5.4 Sepsis and community‐acquired pneumonia | 5 | 763 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.38, 1.02] |
| 6 28‐Day mortality in participants with critical illness‐related corticosteroid insufficiency | 8 | 583 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.76, 1.02] |
| 7 Intensive care unit mortality | 13 | 1463 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.68, 1.00] |
| 8 Hospital mortality | 17 | 2014 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.73, 0.98] |
| 8.1 Long course of low‐dose corticosteroids | 14 | 1708 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.82, 1.01] |
| 8.2 Short course of high‐dose corticosteroids | 3 | 306 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.33, 1.60] |
| 9 Number of participants with shock reversal at day 7 | 12 | 1561 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [1.14, 1.51] |
| 9.1 Shock reversal at day 7 in trials on long course of low‐dose corticosteroids | 10 | 1258 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [1.22, 1.46] |
| 9.2 Shock reversal at day 7 in trials on short course of high‐dose corticosteroids | 2 | 303 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.64, 1.79] |
| 10 Number of participants with shock reversal at 28 days | 7 | 1013 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [1.02, 1.21] |
| 11 SOFA score at day 7 | 8 | 1132 | Mean Difference (IV, Random, 95% CI) | ‐1.53 [‐2.04, ‐1.03] |
| 12 Length of ICU stay for all participants | 12 | 1384 | Mean Difference (IV, Random, 95% CI) | ‐1.68 [‐3.27, ‐0.09] |
| 13 Length of ICU stay for survivors | 10 | 778 | Mean Difference (IV, Fixed, 95% CI) | ‐2.19 [‐3.93, ‐0.46] |
| 14 Length of hospital stay for all participants | 12 | 1802 | Mean Difference (IV, Random, 95% CI) | ‐0.97 [‐2.55, 0.61] |
| 15 Length of hospital stay for survivors | 9 | 710 | Mean Difference (IV, Random, 95% CI) | ‐4.11 [‐8.50, 0.28] |
| 16 Number of participants with adverse events | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 16.1 Gastroduodenal bleeding | 19 | 2382 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.92, 1.67] |
| 16.2 Superinfections | 19 | 2567 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.87, 1.20] |
| 16.3 Hyperglycaemia | 13 | 2081 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [1.16, 1.37] |
| 16.4 Hypernatraemia | 3 | 805 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [1.28, 2.09] |
| 16.5 Neuromuscular weakness | 3 | 811 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.21, 1.88] |
What's new
| Date | Event | Description |
|---|---|---|
| 14 December 2018 | Amended | Editorial team changed to Cochrane Emergency and Critical Care |
History
Protocol first published: Issue 3, 2000 Review first published: Issue 1, 2004
| Date | Event | Description |
|---|---|---|
| 4 May 2018 | Feedback has been incorporated | New Feedback and reply posted in review |
| 18 January 2016 | Amended | Typo corrected in plain language summary. (It was made clear that Corticosteroids decreased the number of organs that were not functioning properly (organ failure).) |
| 30 November 2015 | New search has been performed | We reran the search from October 2009 to October 2014. |
| 30 November 2015 | New citation required and conclusions have changed | The new search of the literature identified nine additional trials. Cumulated evidence from 33 trials confirmed the direction and the magnitude of the point estimate for 28‐day mortality with narrow confidence interval limits. Thus, this update suggested moderate evidence for reduced 28‐day mortality with corticosteroids in the primary analysis. Evidence also confirmed significant interactions between the relative risk of dying at 28 days and treatment modalities (lower doses and longer durations yielded better chance of survival) and patient case mix (patients with septic shock, sepsis‐related acute respiratory distress syndrome (ARDS) or community‐acquired pneumonia may be more likely to benefit from corticosteroids). We decided to change the title to "Corticosteroids for treating sepsis" owing to recent changes in the definition of sepsis suggesting that the term "severe sepsis" should be avoided. We made several changes to the Methods section.
Sensitivity analyses based on methodological quality are now restricted to the primary outcome. We used random‐effects models only in cases of heterogeneity with an I2 statistic > 30%. Otherwise, we used fixed‐effect models. |
| 14 August 2013 | Feedback has been incorporated | Feedback was submitted and responded to. An error in numbers used for Analysis 1.12 (number of participants with superinfection) has been corrected in the amended version of this review. 'Risk of bias' tables and 'Summary of findings' tables have also been amended |
| 1 November 2010 | New search has been performed |
|
| 25 March 2008 | Amended | Converted to new review format. |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomized controlled trial with 2 parallel groups 19 centres |
|
| Participants | Adults (n = 300) with vasopressor‐ and ventilator‐dependent septic shock Stratification according to cortisol response to 250 µg Synacthene into non‐responders (delta cortisol ≤ 9 µg/dL) and responders (> 9 µg/dL) |
|
| Interventions |
Treatments have to be initiated within 8 hours from shock onset |
|
| Outcomes | PRIMARY
SECONDARY
|
|
| Notes | Study location: France | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization scheme |
| Allocation concealment (selection bias) | Low risk | Centralized randomization |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants: yes Care‐givers: yes Data collectors: yes Outcome assessors: yes Data analysts: yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Lost to follow‐up: none |
| Selective reporting (reporting bias) | Low risk | Access to study protocol excluding reporting bias |
| Other bias | Low risk | Full access to data excluding selection bias |
| Methods | Randomized controlled trial with 2 × 2 factorial design 11 centres |
|
| Participants | Adults (n = 509) with vasopressor‐dependent septic shock | |
| Interventions |
Treatments have to be initiated within 24 hours from shock onset |
|
| Outcomes | PRIMARY
SECONDARY
|
|
| Notes | Study location: France | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization scheme |
| Allocation concealment (selection bias) | Low risk | Centralized randomization through a secured website |
| Blinding (performance bias and detection bias) All outcomes | High risk | Participants: yes Care‐givers: no Data collectors: yes Outcome assessors: no Data analysts: yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Access to study protocol excluding reporting bias |
| Other bias | Low risk | Full access to data excluding selection bias |
| Methods | Randomized controlled trial 1 centre |
|
| Participants | Adult (n = 75) with liver cirrhosis and septic shock | |
| Interventions |
|
|
| Outcomes | Primary
Secondary
Outcomes were also analysed in relation to adrenal insufficiency |
|
| Notes | Study location: Saudi Arabia | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Low risk | Use of sealed envelopes by pharmacists |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Pharmacists: no Participants: yes Care‐givers: yes Data collectors: yes Outcome assessors: yes Data analysts: yes |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unexplained discrepancy between reported K‐M curves and number of deaths at 28 days in placebo arm |
| Selective reporting (reporting bias) | Low risk | Access to unpublished data |
| Other bias | High risk | Trial terminated prematurely after enrolment of 75 participants while planned sample size was 150 |
| Methods | Randomized controlled trial with 2 parallel groups 2 centres |
|
| Participants | Adults (n = 41) with vasopressor‐ and ventilator‐dependent septic shock Stratification according to cortisol response to 250 µg Synacthene into non‐responders (delta cortisol ≤ 6 µg/dL) and responders (> 6 µg/dL) |
|
| Interventions |
Treatments have to be initiated after 48 hours or longer from shock onset |
|
| Outcomes | PRIMARY
SECONDARY
|
|
| Notes | Study location: France | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization scheme |
| Allocation concealment (selection bias) | Low risk | Randomization list kept confidential by the pharmacist |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants: yes Care‐givers: yes Data collectors: yes Outcome assessors: yes Data analysts: yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Lost to follow‐up: none |
| Selective reporting (reporting bias) | Low risk | Access to study protocol excluding reporting bias |
| Other bias | Low risk | Full access to data excluding selection bias |
| Methods | Randomized controlled trial with 2 parallel groups 19 centres |
|
| Participants | Adults (n = 382) with sepsis (n = 234) or septic shock (n = 148) | |
| Interventions |
Treatments have to be initiated 2 hours from time entry criteria were met |
|
| Outcomes | PRIMARY
|
|
| Notes | Study location: USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization scheme |
| Allocation concealment (selection bias) | Low risk | Centralized randomization |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants: yes Care‐givers: yes Data collectors: yes Outcome assessors: yes Data analysts: yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Lost to follow‐up: none |
| Selective reporting (reporting bias) | Unclear risk | No access to study protocol to exclude reporting bias |
| Other bias | Low risk | No access to full data including screening log to exclude selection bias |
| Methods | Randomized controlled trial with 2 parallel groups 1 centre |
|
| Participants | Adults (n = 40) with vasopressor‐ and ventilator‐dependent septic shock | |
| Interventions |
Treatments have to be initiated within 72 hours from shock onset |
|
| Outcomes | PRIMARY
SECONDARY
|
|
| Notes | Study location: Germany | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization scheme |
| Allocation concealment (selection bias) | Low risk | Adequate randomization |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants: yes Care‐givers: yes Data collectors: yes Outcome assessors: yes Data analysts: yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Lost to follow‐up: none |
| Selective reporting (reporting bias) | Low risk | Access to study protocol excluding reporting bias |
| Other bias | Low risk | Access to full data including screening log |
| Methods | Randomized controlled trial with 2 parallel groups 1 centre |
|
| Participants | Adults (n = 44) with vasopressor‐dependent septic shock | |
| Interventions |
Treatments have to be initiated after 72 hours or longer from shock onset |
|
| Outcomes | PRIMARY
SECONDARY
|
|
| Notes | Study location: USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization scheme |
| Allocation concealment (selection bias) | Low risk | Randomization list was kept confidential by the pharmacist |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants: yes Care‐givers: yes Data collectors: yes Outcome assessors: yes Data analysts: yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Lost to follow‐up: none |
| Selective reporting (reporting bias) | Low risk | Access to study protocol excluding reporting bias |
| Other bias | Low risk | Access to full data including screening log |
| Methods | Randomized controlled trial with 2 parallel groups 1 centre |
|
| Participants | Adults (n = 29) with vasopressor‐dependent septic shock | |
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Study location: Brazil | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization scheme |
| Allocation concealment (selection bias) | Low risk | Randomization list kept confidential by the pharmacist |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants: yes Care‐givers: yes Data collectors: yes Outcome assessors: yes Data analysts: yes |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Lost to follow‐up: none; 3 participants were withdrawn after next of kin refused to consent |
| Selective reporting (reporting bias) | Unclear risk | No access to study protocol to rule out reporting bias |
| Other bias | Unclear risk | No access to data to rule out selection bias |
| Methods | Randomized controlled trial with 2 parallel groups 6 centres |
|
| Participants | Adults (n = 46) with severe community‐acquired pneumonia | |
| Interventions |
|
|
| Outcomes | PRIMARY
SECONDARY
|
|
| Notes | Study location: Italy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization scheme |
| Allocation concealment (selection bias) | Low risk | Centralized randomization |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants: yes Care‐givers: yes Data collectors: yes Outcome assessors: yes Data analysts: yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Lost to follow‐up: 2 at 60 days after randomization, all in the placebo group |
| Selective reporting (reporting bias) | High risk | Study was stopped prematurely for apparent benefit; no sample size was defined a priori, but study authors used the triangular test as a stopping rule, analysing the primary outcome after each 20 participants |
| Other bias | Low risk | Access to full data including screening log |
| Methods | Randomized controlled trial with 2 parallel groups 5 centres |
|
| Participants | Adults (n = 194) and children (n = 135) with vasopressor‐dependent septic shock | |
| Interventions |
|
|
| Outcomes | PRIMARY
SECONDARY
|
|
| Notes | Study location: USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not given |
| Allocation concealment (selection bias) | Unclear risk | Not given |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Participants: yes Care‐givers: yes Data collectors: unclear Outcome assessors: unclear Data analysts: unclear |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Lost to follow‐up: none |
| Selective reporting (reporting bias) | Unclear risk | No access to study protocol to exclude reporting bias |
| Other bias | Unclear risk | No access to data to exclude selection bias |
| Methods | Randomized controlled trial with 2 parallel groups 4 centres |
|
| Participants | Adults (n = 61) with septic shock on a maximal dose of vasopressin of up to 0.06 U/min | |
| Interventions |
|
|
| Outcomes | PRIMARY
SECONDARY
|
|
| Notes | Study location: United Kingdom | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers prepared by an independent statistician |
| Allocation concealment (selection bias) | Low risk | Randomization done via an online system |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Hydrocortisone and its placebo presented in indiscernible forms |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Lost to follow‐up: none |
| Selective reporting (reporting bias) | Low risk | Reported information matched published statistical plan |
| Other bias | Low risk | Access to unpublished information to exclude other risk of bias |
| Methods | Randomized controlled trial 1 centre |
|
| Participants | Adults (n = 77) with septic shock | |
| Interventions |
|
|
| Outcomes | PRIMARY
SECONDARY
|
|
| Notes | Study location: China | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated in the manuscript |
| Allocation concealment (selection bias) | Unclear risk | Not stated in the manuscript |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not stated in the manuscript |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Lost to follow‐up: none |
| Selective reporting (reporting bias) | Unclear risk | No information |
| Other bias | Unclear risk | No information |
| Methods | Randomized controlled trial with 2 parallel groups 1 centre |
|
| Participants | Adults (n = 82) with septic shock and adrenal insufficiency | |
| Interventions |
|
|
| Outcomes | PRIMARY
SECONDARY
|
|
| Notes | Study location: South Korea | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization scheme |
| Allocation concealment (selection bias) | Unclear risk | Not given |
| Blinding (performance bias and detection bias) All outcomes | High risk | Participants: no Care‐givers: no Data collectors: no Outcome assessors: no Data analysts: no |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Lost to follow‐up: none |
| Selective reporting (reporting bias) | Unclear risk | No explicit information on plan analysis |
| Other bias | Unclear risk | No information |
| Methods | Randomized controlled trial with cross‐over design 1 centre |
|
| Participants | Adults (n = 40) with vasopressor‐dependent septic shock | |
| Interventions |
All participants received hydrocortisone for 3 days preceded or followed by placebo for 3 days |
|
| Outcomes | PRIMARY
SECONDARY
|
|
| Notes | Study location: Germany | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization scheme |
| Allocation concealment (selection bias) | Low risk | Randomization list kept confidential by the pharmacist |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants: yes Care‐givers: yes Data collectors: yes Outcome assessors: yes Data analysts: yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Lost to follow‐up: none |
| Selective reporting (reporting bias) | Low risk | Access to study protocol |
| Other bias | Low risk | Full access to data including screening log |
| Methods | Randomized controlled trial with parallel groups 1 centre |
|
| Participants | Adults (n = 26) with ARDS and sepsis, including septic shock (n = 12) | |
| Interventions |
|
|
| Outcomes | PRIMARY
SECONDARY
|
|
| Notes | Study location: China | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization scheme |
| Allocation concealment (selection bias) | Unclear risk | No explicit information in the manuscript |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No explicit information in the manuscript |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No explicit information in the manuscript |
| Selective reporting (reporting bias) | Unclear risk | No information |
| Other bias | Unclear risk | No information |
| Methods | Randomized controlled trial 1 centre |
|
| Participants | Adults (n = 75) with sepsis and septic shock | |
| Interventions |
|
|
| Outcomes | PRIMARY
SECONDARY
|
|
| Notes | Study location: USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization scheme |
| Allocation concealment (selection bias) | Low risk | Randomization list kept confidential by the pharmacist |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants: yes Care‐givers: yes Data collectors: yes Outcome assessors: yes Data analysts: yes |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 12 out of 87 randomly assigned participants were not analysed, and their follow‐up was not given |
| Selective reporting (reporting bias) | Unclear risk | No access to study protocol |
| Other bias | Unclear risk | No access to data to exclude selection bias |
| Methods | Randomized controlled trial (2:1 scheme) 5 centres |
|
| Participants | Adults (n = 91) with early ARDS (≤ 72 hours from diagnosis of ARDS). 61 (67%) had sepsis or septic shock, and the primary author provided separate data for these participants Stratification according to cortisol response to 250 µg Synacthene into non‐responders (delta cortisol ≤ 9 µg/dL) and responders (> 9 µg/dL) |
|
| Interventions |
|
|
| Outcomes | PRIMARY
SECONDARY
|
|
| Notes | If participant failed to improve on Lung Injury Score between day 7 and day 9, he/she received open‐label methylprednisolone at 2 mg/kg/d for unresolving ARDS Study location: USA |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization scheme |
| Allocation concealment (selection bias) | Low risk | Centralized randomization |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants: yes Care‐givers: yes Data collectors: yes Outcome assessors: yes Data analysts: yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Full access to data excluding any attrition bias |
| Selective reporting (reporting bias) | High risk | Study was stopped prematurely for efficacy |
| Other bias | Low risk | Full access to data including screening log |
| Methods | Randomized controlled trial with 2 parallel groups 2 centres |
|
| Participants | Adults (n = 304) with confirmed community‐acquired pneumonia who presented to emergency departments | |
| Interventions |
|
|
| Outcomes | PRIMARY
SECONDARY
|
|
| Notes | Study location: The Netherlands | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization scheme |
| Allocation concealment (selection bias) | Low risk | Randomization list kept confidential by the pharmacist |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Pharmacist: no Participants: yes Care‐givers: yes Data collectors: yes Outcome assessors: yes Data analysts: yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Lost to follow‐up: none |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in the study protocol are reported in the final analysis |
| Other bias | Low risk | Full access to study protocol |
| Methods | Randomized controlled trial with 2 parallel groups 1 centre |
|
| Participants | Adults (n = 40) with vasopressor‐dependent septic shock | |
| Interventions |
|
|
| Outcomes | PRIMARY
SECONDARY
|
|
| Notes | Study location: Germany | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization scheme |
| Allocation concealment (selection bias) | Low risk | Randomization list kept confidential by the pharmacist |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants: yes Care‐givers: yes Data collectors: yes Outcome assessors: yes Data analysts: yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7 of 48 randomly assigned participants were not analysed: 5 in the corticosteroid group and 2 in the placebo group. 4 of these 7 participants were lost to follow‐up, and 3 died (all in the steroid group) |
| Selective reporting (reporting bias) | Low risk | Access to study protocol excluding reporting bias |
| Other bias | Unclear risk | Full access to data including screening log |
| Methods | Randomized controlled trial (2:1 scheme) with 2 parallel groups 1 centre |
|
| Participants | Adults (n = 27) with ARDS and hospital‐ or community‐acquired pneumonia | |
| Interventions |
|
|
| Outcomes | PRIMARY
SECONDARY
|
|
| Notes | Study location: Egypt | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No explicit information in the manuscript |
| Allocation concealment (selection bias) | Unclear risk | No explicit information in the manuscript |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No explicit information in the manuscript |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Lost to follow‐up: none |
| Selective reporting (reporting bias) | Unclear risk | No information |
| Other bias | Unclear risk | No information |
| Methods | Randomized controlled trial with 2 parallel groups 1 centre |
|
| Participants | Adults (n = 40) with sepsis and not receiving vasopressor support | |
| Interventions |
|
|
| Outcomes | PRIMARY
SECONDARY
|
|
| Notes | Study location: Italy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization list |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | High risk | Participants: no Care‐givers: no Data collectors: no Outcome assessors: no Data analysts: no |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 12 of 52 participants dropped out of the study: 6 in the control group and 6 in the corticosteroid group; contact with the primary author permitted completion of follow‐up for all 12 participants |
| Selective reporting (reporting bias) | Low risk | Access to study protocol excluding any reporting bias |
| Other bias | Low risk | Full access to data including screening log |
| Methods | Randomized controlled trial 3 centres |
|
| Participants | Adults (n = 80) admitted to ICU with community‐acquired pneumonia and sepsis | |
| Interventions |
|
|
| Outcomes | PRIMARY
SECONDARY
|
|
| Notes | Study location: Egypt | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information in the manuscript |
| Allocation concealment (selection bias) | Unclear risk | No information in the manuscript |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No information in the manuscript |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Lost to follow‐up: none |
| Selective reporting (reporting bias) | Unclear risk | No information in the manuscript |
| Other bias | Unclear risk | No information in the manuscript |
| Methods | Randomized controlled trial with 3 parallel groups 1 centre |
|
| Participants | Adults (n = 172) with septic shock with positive blood culture | |
| Interventions |
Treatments might have been repeated once after 4 hours and had to be initiated at the time of diagnosis |
|
| Outcomes | PRIMARY
SECONDARY
|
|
| Notes | Study location: USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomized card system |
| Allocation concealment (selection bias) | High risk | Unsealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Participants: yes Care‐givers: unclear Data collectors: unclear Outcome assessors: unclear Data analysts: unclear |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Lost to follow‐up: none |
| Selective reporting (reporting bias) | Unclear risk | No access to study protocol |
| Other bias | Unclear risk | No data to exclude selection bias |
| Methods | Randomized controlled trial 2 centres |
|
| Participants | African children (n = 72; 1 to 16 years of age) with sepsis or septic shock | |
| Interventions |
Treatments had to be initiated 5 to 10 minutes before first dose of antibiotic |
|
| Outcomes | PRIMARY
SECONDARY
|
|
| Notes | Study location: USA, Kenya and Nigeria | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not given |
| Allocation concealment (selection bias) | Unclear risk | Unclear; not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants: yes Care‐givers: yes Data collectors: yes Outcome assessors: yes Data analysts: yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Lost to follow‐up: none |
| Selective reporting (reporting bias) | Unclear risk | No access to study protocol |
| Other bias | Unclear risk | No data to exclude selection bias |
| Methods | Randomized controlled trial with 2 parallel groups 1 centre |
|
| Participants | Adults (n = 213) with severe community‐acquired pneumonia | |
| Interventions |
|
|
| Outcomes | PRIMARY
SECONDARY
|
|
| Notes | Study location: The Netherlands | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization scheme |
| Allocation concealment (selection bias) | Low risk | Randomization list kept confidential by the pharmacist |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Pharmacist: no Participants: yes Care‐givers: yes Data collectors: yes Outcome assessors: yes Data analysts: yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Lost to follow‐up: none |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in study protocol are reported in final analysis |
| Other bias | Unclear risk | No access to full protocol |
| Methods | Randomized controlled trial with 3 parallel groups 2 centres |
|
| Participants | Adults (n = 59) with vasopressor‐dependent septic shock | |
| Interventions |
Treatments might have been repeated once after 4 hours if shock persisted and had to be initiated at time of diagnosis |
|
| Outcomes | PRIMARY
SECONDARY
|
|
| Notes | Study location: USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization scheme |
| Allocation concealment (selection bias) | High risk | At 1 centre, not clear how randomization list was kept confidential |
| Blinding (performance bias and detection bias) All outcomes | High risk | Participants: yes at 1 centre, no at the other Care‐givers: yes at 1 centre, no at the other Data collectors: yes at 1 centre, no at the other Outcome assessors: yes at 1 centre, no at the other Data analysts: unclear University of Miami Research Committee did not allow study to be performed in a double‐blind manner, nor that participants received placebo |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Lost to follow‐up: none |
| Selective reporting (reporting bias) | Unclear risk | No access to study protocol |
| Other bias | Unclear risk | No data to exclude selection bias |
| Methods | Randomized controlled trial with 2 parallel groups 52 centres |
|
| Participants | Adults (n = 499) with septic shock | |
| Interventions |
|
|
| Outcomes | PRIMARY
SECONDARY
|
|
| Notes | Study locations: Europe and Israel | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization scheme |
| Allocation concealment (selection bias) | Low risk | Centralized randomization |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants: yes Care‐givers: yes Data collectors: yes Outcome assessors: yes Data analysts: yes |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Lost to follow‐up: none; 1 participant withdrew his consent Data for serious adverse events reported for only 466 of 499 participants, and analysis of these outcomes was performed per‐protocol, not by intent‐to‐treat |
| Selective reporting (reporting bias) | Low risk | Access to study protocol to confirm absence of reporting bias |
| Other bias | High risk | Only 500 participants included; expected sample size 800 participants |
| Methods | Randomized controlled trial with 2 parallel groups 1 centre |
|
| Participants | Adults (n = 28) with septic shock and adrenal insufficiency | |
| Interventions |
|
|
| Outcomes | PRIMARY
SECONDARY
|
|
| Notes | Study location: India | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization scheme |
| Allocation concealment (selection bias) | Low risk | Randomization list kept confidential by the local pharmacist |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants: yes Care‐givers: yes Data collectors: yes Outcome assessors: yes Data analysts: yes |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Lost to follow‐up: unknown |
| Selective reporting (reporting bias) | Unclear risk | No access to study protocol |
| Other bias | Unclear risk | No data to exclude selection bias |
| Methods | Randomized controlled trial with 2 parallel groups 3 centres |
|
| Participants | Adults (n = 61) with both severe CAP and high inflammatory response, defined as levels of C‐reactive protein > 15 mg/dL on admission | |
| Interventions |
|
|
| Outcomes | PRIMARY
SECONDARY
|
|
| Notes | Study location: Spain | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization scheme |
| Allocation concealment (selection bias) | Low risk | Randomization list kept confidential by the pharmacist |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants: yes Care‐givers: yes Data collectors: yes Outcome assessors: yes Data analysts: yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Lost to follow‐up: none |
| Selective reporting (reporting bias) | Low risk | Access to full protocol and unpublished information |
| Other bias | Low risk | Access to full protocol and unpublished information |
| Methods | Randomized controlled trial on 2 parallel groups 1 centre |
|
| Participants | Children (n = 38; 2 months to 12 years of age) with septic shock unresponsive to fluid therapy alone | |
| Interventions |
|
|
| Outcomes | PRIMARY
SECONDARY
|
|
| Notes | Study location: India | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No explicit information in the manuscript |
| Allocation concealment (selection bias) | Unclear risk | No explicit information in the manuscript |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open label |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Lost to follow‐up: none |
| Selective reporting (reporting bias) | Unclear risk | No information |
| Other bias | Unclear risk | No information |
| Methods | Randomized controlled trial 10 centres |
|
| Participants | Adults (n = 223) with sepsis or septic shock (n = 100) | |
| Interventions |
Treatment had to be initiated within 2 hours |
|
| Outcomes | PRIMARY
SECONDARY
|
|
| Notes | Study location: USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization scheme |
| Allocation concealment (selection bias) | Low risk | Centralized randomization |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants: yes Care‐givers: yes Data collectors: yes Outcome assessors: yes Data analysts: yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Lost to follow‐up: none |
| Selective reporting (reporting bias) | Unclear risk | No access to study protocol |
| Other bias | Unclear risk | No data to exclude selection bias |
| Methods | Randomized controlled trial 1 centre |
|
| Participants | Adults (n = 40) with sepsis (n = 14), severe sepsis (n = 17) and septic shock (n = 9) | |
| Interventions |
|
|
| Outcomes | PRIMARY
SECONDARY
|
|
| Notes | Study location: Turkey | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization scheme |
| Allocation concealment (selection bias) | Low risk | Randomization list kept confidential by the pharmacist |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants: yes Care‐givers: yes Data collectors: yes Outcome assessors: yes Data analysts: yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Lost to follow‐up: none |
| Selective reporting (reporting bias) | Unclear risk | No access to protocol |
| Other bias | Unclear risk | No data to exclude selection bias |
| Methods | Randomized controlled trial on 2 parallel groups 1 centre |
|
| Participants | Adults (n = 55) with sepsis or septic shock | |
| Interventions |
|
|
| Outcomes | PRIMARY
SECONDARY
Outcomes were also assessed in relation to adrenal insufficiency |
|
| Notes | Study location: Turkey | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers used |
| Allocation concealment (selection bias) | Low risk | Randomization list kept by the pharmacist |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Pharmacist: no Participants: yes Care‐givers: yes Data collectors: yes Outcome assessors: yes Data analysts: yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Lost to follow‐up: none |
| Selective reporting (reporting bias) | Unclear risk | No information |
| Other bias | Unclear risk | No information |
Abbreviations:
APACHE II: Acute Physiology and Chronic Health Evaluation II.
ARDS: acute respiratory distress syndrome.
CAP: community acquired pneumonia.
FiO2: fractional inspired oxygen.
ICU: intensive care unit.
LIS: Lung Injury Scale score.
MOD: multiple organ dysfunction.
PaO2: arterial oxygen tension.
SOFA: sequential organ failure assessment
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Cicarelli 2006 | Mixed population of critically ill patients; separate data on septic shock not available |
| Hahn 1951 | Patients with acute streptococcal infection This trial investigated effects of hydrocortisone on fever, anti‐streptolysin titers and onset of rheumatic fever. No data are reported for analysis of the various outcomes considered in this systematic review |
| Hughes 1984 | Only acute effects (within 1 hour) of methylprednisolone and/or naloxone on haemodynamic data were available; no data for any of the outcomes considered in this systematic review were reported |
| Kaufman 2008 | In this study, participants were randomly assigned to receive hydrocortisone or its placebo for 24 hours only. Then, treatment with open‐labelled hydrocortisone was given at physicians' discretion. This study was aimed at exploring effects of hydrocortisone on immune cell function |
| Klastersky 1971 | This study was not a randomized trial. Investigators did not describe how participants were allocated to experimental treatment |
| Lucas 1984 | This study was not a randomized trial. Participants were allocated to experimental treatment according to their hospital number |
| McKee 1983 | Mixed population of critically ill patients; separate data on septic shock not available |
| Meduri 1998b | This trial included participants with late acute respiratory distress syndrome phase ‐ not those with septic shock |
| Mikami 2007 | This study included participants with community‐acquired pneumonia and explicitly excluded patients with sepsis, those needing admission to the intensive care unit and those requiring mechanical ventilation |
| Rogers 1970 | Study published only as an abstract; no contact with study authors was possible; incomplete information on primary and secondary outcomes |
| Thompson 1976 | Study published only as an abstract; no contact with study authors was possible; incomplete information on primary and secondary outcomes |
| Venet 2015 | This study included severely burned patients without sepsis |
| Wagner 1955 | This study was not a randomized trial. Participants were allocated to experimental treatment according to their hospital numbers |
| Weigelt 1985 | Mixed population of critically ill patients Separate data on septic shock not available |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | STEP |
| Methods | Multi‐centre. randomized, placebo‐controlled, 2‐parallel‐group study |
| Participants | 800 adult patients hospitalized with community‐acquired pneumonia |
| Interventions | Prednisone 50 mg per day for 7 days Placebo |
| Outcomes | PRIMARY
SECONDARY
|
| Starting date | December 2009 |
| Contact information | Mirjam Christ‐Crain; Mirjam.Christ@usb.ch |
| Notes |
| Trial name or title | VANISH |
| Methods | Multi‐centre, factorial (2 × 2), randomized, double‐blind, placebo‐controlled trial |
| Participants | 412 adult patients who require vasopressors for management of sepsis despite fluid resuscitation. In this trial, hydrocortisone or its placebo will be initiated only when participants will require the maximum dose of vasopressin or norepinephrine as defined in the protocol |
| Interventions |
Hydrocrotisone phosphate (50 mg, i.e. 0.5 mL) will be administered by intravenous injection 6‐hourly for 5 days, then tapered to 0.5 mL every 12 hours for days 6 to 8, 0.5 mL every 24 hours for days 9 to 11, then stopped Placebo = 0.9% saline |
| Outcomes | PRIMARY
SECONDARY
|
| Starting date | February 2013 |
| Contact information | Anthony Gordon; anthony.gordon@imperial.ac.uk |
| Notes | EudraCT 2011‐005363‐24; ISRCTN20769191 |
| Trial name or title | 6‐Methylprednisolone for multiple organ dysfunction syndrome |
| Methods | Randomized, double‐blind, placebo‐controlled 2‐parallel‐group study |
| Participants | Adults with persistent multiple organ dysfunction |
| Interventions | Intravenous administration of 6‐methylprednisolone or placebo for 32 days Loading dose of 160 mg followed by IV bolus q6 of 40 mg from day 1 to 14, 20 mg from day 15 to 21, 10 mg from day 22 to 28, 5 mg on days 29 and 30 and 2.5 mg on days 31 and 32 |
| Outcomes | PRIMARY
|
| Starting date | 01/08/2005 |
| Contact information | Miguel Sanchez; miguelsanchez.areachip@wanadoo.es |
| Notes | This trial has been halted for low recruitment rate and lack of funding |
| Trial name or title | Hydrocortisone versus hydrocortisone plus fludrocortisone for treatment of adrenal insufficiency in sepsis |
| Methods | Treatment, randomized, single‐blind, placebo‐controlled, parallel‐assignment efficacy study |
| Participants | Adults with sepsis and positive corticotropin test (basal cortisol ≤ 34 µg/dL and delta cortisol ≤ 9 µg/dL |
| Interventions | Hydrocortisone vs hydrocortisone plus fludrocortisone |
| Outcomes | 28‐Day mortality |
| Starting date | September 2006 |
| Contact information | Contact: John A. Bethea, PharmD 304‐388‐6260 audis.bethea@camc.org Contact: Carol A. Morreale, PharmD 304‐388‐3767 carol.morreale@camc.org |
| Notes | This study has never started to recruit patients |
| Trial name or title | Steroids in patients with early ARDS |
| Methods | Randomized, double‐blind, placebo‐controlled, 2‐parallel‐group safety/efficacy study |
| Participants | Adults with ARDS < 72 hours |
| Interventions | Low‐dose methylprednisolone vs placebo |
| Outcomes | PRIMARY
|
| Starting date | February 2008 |
| Contact information | Massimo Antonelli; m.antonelli@rm.unicatt.it |
| Notes | This study has never started to recruit patients |
| Trial name or title | Activated protein C and corticosteroids for human septic shock (APROCCHS) |
| Methods | Randomized, double‐blind, placebo‐controlled trial ‐ 2 × 2 factorial design |
| Participants | Adults with septic shock |
| Interventions |
|
| Outcomes | 90‐Day mortality |
| Starting date | April 2008 |
| Contact information | Djillali Annane; telephone: 331 47 10 77 87; djillali.annane@rpc.aphp.f |
| Notes |
| Trial name or title | Hydrocortisone for prevention of septic shock |
| Methods | Randomized, double‐blind, placebo‐controlled, 2‐parallel‐group efficacy study |
| Participants | Sepsis |
| Interventions | Hydrocortisone vs placebo |
| Outcomes | PRIMARY
|
| Starting date | 01/06/2008 |
| Contact information | Didier Keh; didier.keh@charite.de |
| Notes |
| Trial name or title | Evaluation of corticosteroid therapy in childhood severe sepsis: a randomized pilot study |
| Methods | Randomized, open‐label, uncontrolled, 2‐parallel‐group study |
| Participants | Children with sepsis |
| Interventions | Hydrocortisone |
| Outcomes | PRIMARY
|
| Starting date | 01/04/2008 |
| Contact information | Saul N Faust; s.faust@soton.ac.uk |
| Notes |
| Trial name or title | ADRENAL |
| Methods | Multi‐centre, randomized, controlled, 2‐parallel‐group study |
| Participants | 3800 ICU adults with septic shock |
| Interventions | Hydrocortisone Placebo |
| Outcomes | PRIMARY
SECONDARY
|
| Starting date | February 2013 |
| Contact information | Bala Venkatesh; Bala_Venkatesh@health.qld.gov.au |
| Notes |
Contributions of authors
Conceiving of the review: Djillali Annane (DA), Eric Bellissant (EB), Pierre Edouard Bollaert (PEB), Josef Briegel (JB), Didier Keh (DK), Yizhak Kupfer (YK).
Co‐ordinating the review: DA.
Undertaking manual searches: DA, PEB, JB, DK.
Screening search results: DA, PEB, JB, DK, YK.
Organizing retrieval of papers: DA, PEB, JB, DK, YK.
Screening retrieved papers against inclusion criteria: DA, PEB, JB, DK, YK.
Appraising the quality of papers: DA, PEB, JB, DK, YK.
Abstracting data from papers: DA.
Writing to authors of papers to ask for additional information: DA.
Providing additional data about papers: DA, PEB, JB, DK, YK.
Obtaining and screening data on unpublished studies: DA, PEB, JB, DK, YK.
Managing data for the review: DA, EB.
Entering data into Review Manager (RevMan 5): DA.
Analysing RevMan statistical data: DA, EB.
Performing other statistical analyses not using RevMan: not applicable.
Performing double entry of data (data entered by person one: DA; data entered by person two: Laurenne Authelet).
Interpreting data: DA, EB, PEB, JB, DK, YK.
Making statistical inferences: EB.
Writing the review: DA, EB, PEB, JB, DK, YK.
Securing funding for the review: DA.
Performing previous work that served as the foundation of the present study: DA, EB, PEB, JB, DK, YK.
Serving as guarantor for the review (one review author): DA.
Taking responsibility for reading and checking the review before submission: DA.
Sources of support
Internal sources
Hopital Raymond Poincaré, Garches, France.
External sources
Department for International Development, UK.
Declarations of interest
The following review authors have been involved in randomized controlled trials of low‐dose hydrocortisone that are included in this updated review: Djillali Annane in Annane 2002 and Sprung 2008; Eric Bellissant in Annane 2002; Pierre Edouard Bollaert in Bollaert 1998 and Annane 2002; Josef Briegel in Briegel 1999 and Sprung 2008; Didier Keh in Keh 2003 and Sprung 2008; and Yizhak Kupfer in Chawla 1999.
Djillali Annane is involved with one ongoing study: NCT00625209 2008. This trial is funded by the French Ministry of Social Affairs, Health and Women Rights ‐ Programme Hospitalier de Recherche Clinique PHRC‐12‐002‐0030.
Didier Keh is involved with one ongoing study: NCT00670254 2008. This trial is funded by the Federal Ministry of Education and Research (01KG0701).
Yizhak Kupfer: I am a member of the Pfizer/BMS speakers' bureau for epixaban. This product has no relationship to steroids in sepsis.
Notes
This review was initially developed within the Infectious Diseases Group and was transferred to the Anaesthesia Group in May 2005.
Updated 2010
The review was updated in 2010 (Annane 2004). At that time, Cochrane updates did not earn a new citation unless they had new review authors or included a change to conclusions. Review authors found 21 new trials in 2010. Of those 21 trials, they included nine randomized controlled trials in the 2010 update. Additional included studies did not change the conclusions of this review. Therefore the 2010 update did not earn a new citation.
Updated 2015
The new search of the literature identified nine additional trials. Cumulated evidence from 33 trials confirmed the direction and magnitude of the point estimate for 28‐day mortality with narrow confidence interval limits. Thus, this update suggested moderate evidence for reduced 28‐day mortality with corticosteroids in the primary analysis and confirmed significant interactions between the relative risk of dying at 28 days and treatment modalities used (lower doses and longer duration yielded better chance of survival) and patient case mix included (patients with septic shock, sepsis‐related ARDS or community‐acquired pneumonia may be more likely to benefit from corticosteroids).
We decided to change the title to "Corticosteroids for treating sepsis" owing to recent changes in the definition of sepsis, suggesting that the term "severe sepsis" should be avoided.
Several changes were made to the Methods section.
We now exclude quasi‐randomized trials (three trials).
We changed the definition of "long course" from at least five days to at least three days. Indeed, and in keeping with the Surviving Sepsis Campaign recommendation, corticosteroids are often given at full dose until cessation of vasopressor therapy, which may occur sooner than five days. We changed the definition of "low dose" from 300 mg or less per day to 400 mg or less per day. Indeed, no consensus has been reached about what should be the optimal dose, and several RCTs testing so called "low‐dose" corticosteroids used variable doses up to 400 mg. According to findings from the meta‐regression analysis, changes in the definition of "low dose" and "long course" might have had a negative impact on observed survival benefits of corticosteroids. Indeed, we found that both longer duration and lower dose were associated with better survival rates.
We have incorporated information on how we used the GRADE system and how we selected outcomes for the 'Summary of findings' table.
Sensitivity analyses based on methodological quality are now restricted to the primary outcome.
Random‐effects models are used only in cases of heterogeneity, with I2 statistic > 30%. Otherwise, fixed‐effect models are used.
Edited (no change to conclusions)
References
References to studies included in this review
- Annane D, Sebille V, Charpentier C, Bollaert PE, François B, Korach JM, et al. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA 2002;288(7):862‐71. [PUBMED: 12186604] [DOI] [PubMed] [Google Scholar]
- COIITSS Study Investigators, Annane D, Cariou A, Maxime V, Azoulay E, D'honneur G, Timsit JF, et al. Corticosteroid treatment and intensive insulin therapy for septic shock in adults: a randomized controlled trial. JAMA 2010;303:341‐8. [DOI] [PubMed] [Google Scholar]
- Arabi YM, Aljumah A, Dabbagh O, Tamim HM, Rishu AH, Al‐Abdulkareem A, et al. Low‐dose hydrocortisone in patients with cirrhosis and septic shock: a randomized controlled trial. Canadian Medical Association Journal 2011;182:1971‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollaert PE, Charpentier C, Levy B, Debouverie M, Audibert G, Larcan A. Reversal of late septic shock with supraphysiologic doses of hydrocortisone. Critical Care Medicine 1998;26(4):645‐50. [PUBMED: 9559600] [DOI] [PubMed] [Google Scholar]
- Bone RG, Fisher CJ, Clemmer TP, Slotman GJ, Metz CA, Balk RA. A controlled clinical trial of high‐dose methylprednisolone in the treatment of severe sepsis and septic shock. New England Journal of Medicine 1987;317(11):653‐8. [DOI] [PubMed] [Google Scholar]
- Briegel J, Forst H, Haller M, Schelling G, Kilger E, Kuprat G, et al. Stress doses of hydrocortisone reverse hyperdynamic septic shock: a prospective, randomized, double‐blind, single‐center study. Critical Care Medicine 1999;27(4):723‐32. [PUBMED: 10321661] [DOI] [PubMed] [Google Scholar]
- Chawla K, Kupfer Y, Tessler S. Hydrocortisone reverses refractory septic shock. Critical Care Medicine 1999;27(1):A33. [Google Scholar]
- Cicarelli DD, Vieira JE, Benseñor FE. Early dexamethasone treatment for septic shock patients: a prospective randomized clinical trial. Sao Paulo Medical Journal 2007;125:237‐41. [PUBMED: 17992396 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Confalonieri M, Urbino R, Potena A, Piatella M, Parigi P, Giacomo P, et al. Hydrocortisone infusion for severe community acquired pneumonia: a preliminary randomized study. American Journal of Respiratory and Critical Care Medicine 2005;171:242‐8. [DOI] [PubMed] [Google Scholar]
- Cooperative Study Group. The effectiveness of hydrocortisone in the management of severe infections. JAMA 1963;183:462‐5. [Google Scholar]
- Gordon AC, Mason AJ, Perkins GD, Stotz M, Terblanche M, Ashby D, et al. The interaction of vasopressin and corticosteroids in septic shock: a pilot randomized controlled trial. Critical Care Medicine 2014;42:1325‐33. [DOI] [PubMed] [Google Scholar]
- Hu B, Li JG, Liang H, Zhou Q, Yu Z, Li L, et al. The effect of low‐dose hydrocortisone on requirement of norepinephrine and lactate clearance in patients with refractory septic shock. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue 2009;21:529‐31. [PubMed] [Google Scholar]
- Huh JW, Lim CM, Koh Y, Hong SB. Effect of low doses of hydrocortisone in patient with septic shock and relative adrenal insufficiency: 3 days versus 7 days treatment. Critical Care Medicine 2007;34 Suppl:A101. [Google Scholar]
- Keh D, Boehnke T, Weber‐Cartens S, Schulz C, Ahlers O, Bercker S, et al. Immunologic and hemodynamic effects of "low‐dose" hydrocortisone in septic shock: a double‐blind, randomized, placebo‐controlled, crossover study. American Journal of Respiratory and Critical Care Medicine 2003;167(4):512‐20. [PUBMED: 12426230 ] [DOI] [PubMed] [Google Scholar]
- Liu L, Li J, Huang YZ, Liu SQ, Yang CS, Guo FM, et al. The effect of stress dose glucocorticoid on patients with acute respiratory distress syndrome combined with critical illness‐related corticosteroid insufficiency. Zhonghua Nei Ke Za Zhi 2012;51:599‐603. [PubMed] [Google Scholar]
- Luce JM, Montgomery AB, Marks JD, Turner J, Metz CA, Murray JF. Ineffectiveness of high‐dose methylprednisolone in preventing parenchymal lung injury and improving mortality in patients with septic shock. American Review of Respiratory Diseases 1988;138(1):62‐8. [PUBMED: 3202402 ] [DOI] [PubMed] [Google Scholar]
- Meduri GU, Golden E, Freire AX, Taylor E, Zaman M, Carson SJ, et al. Methylprednislone infusion in early ARDS: result of a randomised controlled trial. Chest 2007;131(4):954‐63. [PUBMED: 17426195] [DOI] [PubMed] [Google Scholar]
- Meijvis SC, Hardeman H, Remmelts HH, Heijligenberg R, Rijkers GT, Velzen‐Blad H, et al. Dexamethasone and length of hospital stay in patients with community‐acquired pneumonia: a randomised, double‐blind, placebo‐controlled trial. The Lancet 2011;377:2023‐30. [DOI] [PubMed] [Google Scholar]
- Oppert M, Schindler R, Husung C, Offerman K, Graef KJ, Boenisch O, et al. Low‐dose hydrocortisone improves shock reversal and reduces cytokine levels in early hyperdynamic septic shock. Critical Care Medicine 2005;33:2457‐64. [PUBMED: 16276166 ] [DOI] [PubMed] [Google Scholar]
- Rezk NA, Ibrahim AM. Effects of methyl prednisolone in early ARDS. Egyptian Journal of Chest Diseases and Tuberculosis 2013;62(1):167–72. [Google Scholar]
- Rinaldi S, Adembri C, Grechi S, Gaudio R. Low‐dose hydrocortisone during severe sepsis: effects on microalbumineria. Critical Care Medicine 2006;34:2334‐9. [PUBMED: 16850006] [DOI] [PubMed] [Google Scholar]
- Sabry NA, El‐Din Omar E. Corticosteroids and ICU course of community acquired pneumonia in Egyptian settings. Pharmacology and Pharmacy 2011;2:73‐81. [Google Scholar]
- Schumer W. Steroids in the treatment of clinical septic shock. Annals of Surgery 1976;184(3):333‐41. [PUBMED: 786190 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slusher T, Gbadero D, Howard C, Lewinson L, Giroir B, Toro L, et al. Randomized, placebo‐controlled, double blinded trial of dexamethasone in African children with sepsis. Pediatric Infectious Diseases Journal 1996;15(7):579‐83. [PUBMED: 8823850] [DOI] [PubMed] [Google Scholar]
- Snijders D, Daniels JMA, Graaff CS, Werf TS, Boersma WG. Efficacy of corticosteroids in community‐acquired pneumonia – a randomized double blinded clinical trial. American Journal of Respiratory and Critical Care Medicine 2010;181:975‐82. [DOI] [PubMed] [Google Scholar]
- Sprung CL, Caralis PV, Marcial EH, Pierce M, Gelbard MA, Long WM, et al. The effects of high‐dose corticosteroids in patients with septic shock. A prospective, controlled study. New England Journal of Medicine 1984;311(18):1137‐43. [PUBMED: 6384785 ] [DOI] [PubMed] [Google Scholar]
- Sprung C, Annane D, Keh D, Moreno R, Singer M, Freivogel K, et al. The CORTICUS randomized, double‐blind, placebo‐controlled study of hydrocortisone therapy in patients with septic shock. New England Journal of Medicine 2008;358:111‐24. [PUBMED: 18184957] [DOI] [PubMed] [Google Scholar]
- Tandan SM, Guleria R, Gupta N. Low dose steroids and adrenocortical insufficiency in septic shock: a double‐blind randomised controlled trial from India. Proceedings of the American Thoracic Society Meeting. 2005:A24. [Google Scholar]
- Torres A, Sibila O, Ferrer M, Polverino E, Menendez R, Mensa J, et al. Effect of corticosteroids on treatment failure among hospitalized patients with severe community‐acquired pneumonia and high inflammatory response: a randomized clinical trial. JAMA. 2015; Vol. 313:677‐86. [DOI] [PubMed] [Google Scholar]
- Valoor HT, Singhi S, Jayashree M. Low‐dose hydrocortisone in paediatric septic shock: an exploratory study in a third world setting. Pediatric Critical Care Medicine 2009;10:121‐5. [DOI] [PubMed] [Google Scholar]
- The Veterans Administration Systemic Sepsis Cooperative Study Group. Effect of high‐dose glucocorticoid therapy on mortality in patients with clinical signs of systemic sepsis. New England Journal of Medicine 1987;317(11):659‐65. [PUBMED: 2888017] [DOI] [PubMed] [Google Scholar]
- Yildiz O, Doganay M, Aygen B, Guven M, Keleutimur F, Tutuu A. Physiologic‐dose steroid therapy in sepsis. Critical Care 2002;6(3):251‐9. [PUBMED: 12133187] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildiz O, Tanriverdi F, Simsek S, Aygen B, Kelestimur F. The effects of moderate‐dose steroid therapy in sepsis: a placebo‐controlled, randomized study. Journal of Research in Medical Sciences 2011;16:1410‐21. [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
- Cicarelli DD, Bensenor FEM, Vieira JE. Effects of single dose of dexamethasone on patients with systemic inflammatory response. Sao Paulo Medical Journal 2006;124:90‐5. [PUBMED: 16878192 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn EO, Houser HB, Rammelkamp CH, Denny FW, Wannamaker LW. Effect of cortisone on acute streptococcal infections and post‐streptococcal complications. Journal of Clinical Investigation 1951;30:274‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes GS Jr. Naloxone and methylprednisolone sodium succinate enhance sympathomedullary discharge in patients with septic shock. Life Sciences 1984;35(23):2319‐26. [PUBMED: 6390057] [DOI] [PubMed] [Google Scholar]
- Kaufmann I, Briegel J, Schliephake F, Hoelzl A, Chouker A, Hummel T, et al. Stress doses of hydrocortisone in septic shock: beneficial effects on opsonization‐dependent neutrophil functions. Intensive Care Medicine 2008;34:344‐9. [PUBMED: 17906853 ] [DOI] [PubMed] [Google Scholar]
- Klastersky J, Cappel R, Debusscher L. Effectiveness of betamethasone in management of severe infections. A double‐blind study. New England Journal of Medicine 1971;284(22):1248‐50. [PUBMED: 4929896] [DOI] [PubMed] [Google Scholar]
- Lucas C, Ledgerwood A. The cardiopulmonary response to massive doses of steroids in patients with septic shock. Archives of Surgery 1984;119(5):537‐41. [PUBMED: 6712466 ] [DOI] [PubMed] [Google Scholar]
- McKee JI, Finlay WE. Cortisol replacement in severely stressed patients. Lancet 1983;1(8322):484. [PUBMED: 6131207 ] [DOI] [PubMed] [Google Scholar]
- Meduri GU, Headley AS, Golden E, Carson SJ, Umberger RA, Kelso T, et al. Effect of prolonged methylprednisolone therapy in unresolving acute respiratory distress syndrome: a randomized controlled trial. JAMA 1998;280(2):159‐65. [PUBMED: 9669790] [DOI] [PubMed] [Google Scholar]
- Mikami K, Suzuki M, Kitagawa H, Kawakami M, Hirota N, Yamaguchi H, et al. Efficacy of corticosteroids in the treatment of community‐acquired pneumonia requiring hospitalization. Lung 2007;185:249‐55. [PUBMED: 17710485] [DOI] [PubMed] [Google Scholar]
- Rogers J. Large doses of steroids in septicaemic shock. British Journal of Urology 1970;42(6):742. [PUBMED: 4923652] [PubMed] [Google Scholar]
- Thompson WL, Gurley HT, Lutz BA, Jackson DL, Kvols LK, Morris IA. Inefficacy of glucocorticoids in shock (double‐blind study). Clinical Research 1976;24:258A. 1024008 [Google Scholar]
- Venet F, Plassais J, Textoris J, Cazalis MA, Pachot A, Bertin‐Maghit M, et al. Low‐dose hydrocortisone reduces norepinephrine duration in severe burn patients: a randomized clinical trial. Critical Care 2015;19:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner HN, Bennett IL, Lasagna L, Cluff LE, Rosenthal MB, Mirick GS. The effect of hydrocortisone upon the course of pneumococcal pneumonia treated with penicillin. Bulletin of Johns Hopkins Hospital 1955;98:197‐215. [PUBMED: 13304518] [PubMed] [Google Scholar]
- Weigelt JA, Norcross JF, Borman KR, Snyder WH 3rd. Early steroid therapy for respiratory failure. Archives of Surgery 1985;120(5):536‐40. [PUBMED: 3885915 ] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
- Blum CA, Nigro N, Briel M, Schuetz P, Ullmer E, Suter‐Widmer I, et al. Adjunct prednisone therapy for patients with community‐acquired pneumonia: a multicentre, double‐blind, randomised, placebo‐controlled trial.. Lancet 2015;385:1511‐8. [DOI] [PubMed] [Google Scholar]
- Gordon AC, Mason AJ, Perkins GD, Ashby D, Brett SJ. Protocol for a randomised controlled trial of VAsopressin versus Noradrenaline as Initial therapy in Septic sHock (VANISH). British Medical Journal Open 2014;4:e005866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6‐Methylprednisolone for multiple organ dysfunction syndrome. Ongoing study 01/08/2005.
- Hydrocortisone versus hydrocortisone plus fludrocortisone for treatment of adrenal insufficiency in sepsis. Ongoing studySeptember 2006.
- Steroids in patients with early ARDS. Ongoing studyFebruary 2008.
- Activated protein C and corticosteroids for human septic shock (APROCCHS). Ongoing studyApril 2008.
- Hydrocortisone for prevention of septic shock. Ongoing study 01/06/2008.
- Evaluation of corticosteroid therapy in childhood severe sepsis: a randomized pilot study. Ongoing study 01/04/2008.
- Venkatesh B, Myburgh J, Finfer S, Webb SA, Cohen J, Bellomo R, et al. ANZICS CTG Investigtors. The ADRENAL study protocol: adjunctive corticosteroid treatment in critically ill patients with septic shock. Critical Care resuscitation 2013;15:83‐8. [PubMed] [Google Scholar]
Additional references
- ACCP/SCCM Consensus Conference Panel. American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Critical Care Medicine 1992;20(6):864‐74. [PUBMED: 1597042 ] [PubMed] [Google Scholar]
- Angus DC, Poll T. Severe sepsis and septic shock. New England Journal of Medicine 2013;369:840‐51. [DOI] [PubMed] [Google Scholar]
- Annane D, Sébille V, Troché G, Raphael JC, Gajdos P, Bellissant E. A 3‐level prognostic classification in septic shock based on cortisol levels and cortisol response to corticotropin. JAMA 2000;283(8):1038‐45. [PUBMED: 10697064] [DOI] [PubMed] [Google Scholar]
- Annane D, Aegerter P, Jars‐Guincestre MC, Guidet B, CUB‐Rea Network. Current epidemiology of septic shock: the CUB‐Réa Network. American Journal of Respiratory and Critical Care Medicine 2003;168(2):165‐72. [PUBMED: 12851245] [DOI] [PubMed] [Google Scholar]
- Annane D, Bellissant E, Cavaillon JM. Septic shock. Lancet 2005;365:63‐78. [PUBMED: 15639681] [DOI] [PubMed] [Google Scholar]
- Annane D. Improving clinical trials in the critically ill: unique challenge: sepsis. Critical Care Medicine 2009;37 Suppl:117‐28. [PUBMED: 19104211] [DOI] [PubMed] [Google Scholar]
- Annane D, Timsit JF, Megarbane B, Martin C, Misset B, Mourvillier B, et al. on behalf of the APROCCHSS Trial Investigators. Recombinant human activated protein C for adults with septic shock: a randomised controlled trial. American Journal of Respiratory and Critical Care Medicine 2013;187:1091–7. [DOI] [PubMed] [Google Scholar]
- Barczyk K, Ehrchen J, Tenbrock K, Ahlmann M, Kneidl J, Viemann D, et al. Glucocorticoids promote survival of anti‐inflammatory macrophages via stimulation of adenosine receptor A3. Blood 2010;116:446‐55. [DOI] [PubMed] [Google Scholar]
- Barnes PJ, Greening AP, Crompton GK. Glucocorticoid resistance in asthma. American Journal of Respiratory and Critical Care Medicine 1995;152 Suppl(6 Pt 2):125‐40. [PUBMED: 7489120] [DOI] [PubMed] [Google Scholar]
- Bone RC. Sepsis, the sepsis syndrome, multi‐organ failure: a plea for comparable definitions. Annals of Internal Medicine 1991;114(4):332‐3. [PUBMED: 1987879] [DOI] [PubMed] [Google Scholar]
- Boonen E, Vervenne H, Meersseman P, Andrew R, Mortier L, Declercq PE, et al. Reduced cortisol metabolism during critical illness. New England Journal of Medicine 2013;388:1477‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briegel J, Kellermann W, Forst H, Haller M, Bittl M, Hoffmann GE, et al. Low‐dose hydrocortisone infusion attenuates the systemic inflammatory response syndrome. The Phospholipase A2 Study Group. Clinical Investigation 1994;72:782‐7. [PUBMED: 7865982] [DOI] [PubMed] [Google Scholar]
- Cai L, Ji A, Beer FC, Tannock LR, Westhuyzen DR. SR‐BI protects against endotoxemia in mice through its roles in glucocorticoid production and hepatic clearance. Journal of Clinical Investigation 2008;118:364–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrousos GP. The hypothalamic‐pituitary‐adrenal axis and immune‐mediated inflammation. New England Journal of Medicine 1995;322(20):1351‐62. [PUBMED: 7715646] [DOI] [PubMed] [Google Scholar]
- Cooper MS, Stewart PM. Corticosteroid insufficiency in acutely ill patients. New England Journal of Medicine 2003;348(8):727‐34. [PUBMED: 12594318 ] [DOI] [PubMed] [Google Scholar]
- Cronin L, Cook DJ, Carlet J, Heyland DK, King D, Lansang MAD, et al. Corticosteroid treatment for sepsis: a critical appraisal and meta‐analysis of the literature. Critical Care Medicine 1995;23(8):1430‐9. [PUBMED: 7634816 ] [DOI] [PubMed] [Google Scholar]
- Kruif MD, Lemaire LC, Giebelen IA, Zoelen MA, Pater JM, Pangaart PS, et al. Prednisolone dose‐dependently influences inflammation and coagulation during human endotoxemia. Journal of Immunology 2007;178:1845‐51. [PUBMED: 17237435] [DOI] [PubMed] [Google Scholar]
- Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Critical Care Medicine 2008;36:296‐327. [PUBMED: 20035219 ] [DOI] [PubMed] [Google Scholar]
- Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving Sepsis Campaign Guidelines Committee including The Pediatric Subgroup. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive care Medicine 2013;39:165‐228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereaux PJ, Manns BJ, Ghali WA, Quan H, Lacchetti C, Montori VM, et al. Physician interpretations and textbook definitions of blinding terminology in randomized controlled trials. JAMA 2001;285:2000‐3. [PUBMED: 11308438 ] [DOI] [PubMed] [Google Scholar]
- Villa Bianca RE, Lippolis L, Autore G, Popolo A, Marzocco S, Sorrentino L, et al. Dexamethasone improves vascular hyporeactivity induced by LPS in vivo by modulating ATP‐sensitive potassium channel activity. British Journal of Pharmacology 2003;140:91–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duma D, Silva‐Santos JE, Assreuy J. Inhibition of glucocorticoid receptor binding by nitric oxide in endotoxemic rats. Critical Care Medicine 2004;32:2304–10. [DOI] [PubMed] [Google Scholar]
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315:629‐34. [PUBMED: 9310563] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrchen J, Steinmüller L, Barczyk K, Tenbrock K, Nacken W, Eisenacher M, et al. Glucocorticoids induce differentiation of a specifically activated, anti‐inflammatory subtype of human monocytes. Blood 2007;109:1265‐74. [DOI] [PubMed] [Google Scholar]
- Fabian TC, Patterson R. Steroid therapy in septic shock. Survival studies in a laboratory model. American Surgeon 1982;48(12):614‐7. [PUBMED: 6760756] [PubMed] [Google Scholar]
- Finfer S, Bellomo R, Lipman J, French C, Dobb G, Myburgh J. Adult population incidence of severe sepsis in Australian and New Zealand intensive care units. Intensive Care Medicine 2004;30:589–96. [PUBMED: 14963646] [DOI] [PubMed] [Google Scholar]
- Galigniana MD, Piwien‐Pilipuk G, Assreuy J. Inhibition of glucocorticoid receptor binding by nitric oxide. Molecular Pharmacology 1999;55:317–23. [DOI] [PubMed] [Google Scholar]
- Goodwin JE, Feng Y, Velazquez H, Sessa WC. Endothelial glucocorticoid receptor is required for protection against sepsis. Proceeding of the National Academy of Science 2013;110:306–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE Working Group. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924‐6. [PUBMED: 18436948 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyatt GH, Wyer P, Ioannidis J. When to believe a subgroup analysis. In: Guyatt G, Rennie R, Meade M, Cook D editor(s). User’s Guides to the Medical Literature: A Manual for Evidence‐Based Clinical Practice. New York: McGraw‐Hill, 2008. [Google Scholar]
- Heller AR, Heller SC, Borkenstein A, Stehr SN, Koch T. Modulation of host defense by hydrocortisone in stress doses during endotoxaemia. Intensive Care Medicine 2003;29:1456‐63. [PUBMED: 12879235 [] [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. In: The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
- Hotta M, Baird A. Differential effects of transforming growth factor type beta on the growth and function of adrenocortical cells in vitro. Proceedings of the National Academy of Sciences of the United States of America 1986;83(20):7795‐9. [PUBMED: 3020557] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang ZH, Gao H, Xu RB. Study on glucocorticoid receptors during intestinal ischemia shock and septic shock. Circulatory Shock 1987;23(1):27‐36. [PUBMED: 3690811] [PubMed] [Google Scholar]
- Ioannidis JPA. Better reporting of harms in randomized trials: an extension of the CONSORT statement. Annals of Internal Medicine 2004;141:781‐8. [DOI] [PubMed] [Google Scholar]
- Jaattela M, Ilvesmaki V, Voutilainen R, Stenman UH, Saksela E. Tumor necrosis factor as a potent inhibitor of adrenocorticotropin‐induced cortisol production and steroidogenic P450 enzyme gene expression in cultured human fetal adrenal cells. Endocrinology 1991;128(1):623‐9. [PUBMED: 1702707] [DOI] [PubMed] [Google Scholar]
- Kalil AC, Sun J. Low‐dose steroids for septic shock and severe sepsis: the use of Bayesian statistics to resolve clinical trial controversies. Intensive Care Medicine 2011;37:420–9. [DOI] [PubMed] [Google Scholar]
- Kanczkowski W, Alexaki VI, Tran N, Großklaus S, Zacharowski K, Martinez A, et al. Hypothalamo‐pituitary and immune‐dependent adrenal regulation during systemic inflammation. Proceedings of the National Academy of Science 2013;110:14801–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsenos CS, Antonopoulou AN, Apostolidou EN, Ioakeimidou A, Kalpakou GT, Papanikolaou MN, et al. on behalf of the Hellenic Sepsis Study Group. Early administration of hydrocortisone replacement after the advent of septic shock: impact on survival and immune response. Critical Care Medicine 2014;42:1651‐7. [DOI] [PubMed] [Google Scholar]
- Kellum JA, Kong L, Fink MP, Weissfeld LA, Yealy DM, Pinsky MR, et al. Understanding the inflammatory cytokine response in pneumonia and sepsis: results of the Genetic and Inflammatory Markers of Sepsis (GenIMS) Study. Archives of Internal Medicine 2007;168:1468‐9. [PUBMED: 17698689] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefering R, Neugebauer EAM. Steroid controversy in sepsis and septic shock: a meta‐analysis. Critical Care Medicine 1995;23(7):1294‐303. [PUBMED: 7600840 ] [DOI] [PubMed] [Google Scholar]
- Lipiner D, Sprung CL, Laterre PF, Weiss Y, Goodman SV, Vogeser M, et al. Adrenal function in sepsis: the retrospective CORTICUS cohort study. Critical Care Medicine 2007;35:1012‐8. [DOI] [PubMed] [Google Scholar]
- Loisa P, Parviainen I, Tenhunen J, Hovilehto S, Ruokonen E. Effect of mode of hydrocortisone administration on glycemic control in patients with septic shock: a prospective randomized trial. Critical Care 2007;11:R21. [PUBMED: 17306016 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenberg M, Tuynman J, Bilderbeek J, Gaber T, Buttgereit F, Deventer S, et al. Rapid immunosuppressive effects of glucocorticoids mediated through Lck and Fyn. Blood 2005;106:1703‐10. [DOI] [PubMed] [Google Scholar]
- Marik PE, Pastores SM, Annane D, Meduri GU, Sprung CL, Arlt W, et al. Recommendations for the diagnosis and management of corticosteroid insufficiency in critically ill adult patients: consensus statements from an international task force by the American College of Critical Care Medicine. Critical Care Medicine 2008;36:1937–49. [DOI] [PubMed] [Google Scholar]
- Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. New England Journal of Medicine 2003;348:1546‐54. [PUBMED: 12700374 ] [DOI] [PubMed] [Google Scholar]
- Meduri GU, Chrousos GP. Duration of glucocorticoid treatment and outcome in sepsis: is the right drug used the wrong way?. Chest 1998;114(2):355‐60. [PUBMED: 9726712] [DOI] [PubMed] [Google Scholar]
- Melby JC, Spink WW. Comparative studies on adrenal cortical function and cortisol metabolism in healthy adults and in patients with shock due to infection. Journal of Clinical Investigation 1958;37:1791‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon K, McNally D, Choong K, Sampson M. A systematic review and meta‐analysis on the effect of steroids in pediatric shock. Pediatric Critical Care Medicine 2013;14:474‐80. [DOI] [PubMed] [Google Scholar]
- Molijn GJ, Koper JW, Uffelen CJ, Jong FH, Brinkmann AO, Bruining HA, et al. Temperature‐induced down‐regulation of the glucocorticoid receptor in peripheral blood mononuclear leucocyte in patients with sepsis or septic shock. Clinical Endocrinology 1995;43(2):197‐203. [PUBMED: 7554315 ] [DOI] [PubMed] [Google Scholar]
- Moran JL, Graham PL, Rockliff S, Bersten AD. Updating the evidence for the role of corticosteroids in severe sepsis and septic shock: a Bayesian meta‐analytic perspective. Critical Care 2010;14:R134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman AW, Mizwicki MT, Norman DP. Steroid‐hormone rapid actions, membrane receptors, and a conformational ensemble model. Nature Revue Drug Discovery 2004;3:27‐41. [DOI] [PubMed] [Google Scholar]
- Padkin A, Goldfrad C, Brady AR, Young D, Black N, Rowan K. Epidemiology of severe sepsis occurring in the first 24 hrs in intensive care units in England, Wales, and Northern Ireland. Critical Care Medicine 2003;31:2332–8. [PUBMED: 14501964 ] [DOI] [PubMed] [Google Scholar]
- Park HY, Suh GY, Song JU, Yoo H, Jo IJ, Shin TG, et al. Early initiation of low‐dose corticosteroid therapy in the management of septic shock: a retrospective observational study. Critical Care 2012;16:R3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrillo JE. Pathogenic mechanisms of septic shock. New England Journal of Medicine 1993;328(20):1471‐7. [DOI] [PubMed] [Google Scholar]
- Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Contour‐enhanced meta‐analysis funnel plots help distinguish publication bias from other causes of asymmetry. Journal of Clinical Epidemiology 2008;61:991‐6. [DOI] [PubMed] [Google Scholar]
- Polito A, Sonneville R, Guidoux C, Barrett L, Viltart O, Mattot V, et al. Changes in CRH and ACTH synthesis during experimental and human septic shock. PLoS One 2011;6:e25905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan) Version 5.0. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008.
- Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids: new mechanisms for old drugs. New England Journal of Medicine 2005;353:1711‐23. [DOI] [PubMed] [Google Scholar]
- Rothwell PM, Udwadia ZF, Lawler PG. Cortisol response to corticotropin and survival in septic shock. Lancet 1991;337(8741):582‐3. [DOI] [PubMed] [Google Scholar]
- Sharshar T1, Gray F, Lorin de la Grandmaison G, Hopkinson NS, Ross E, Dorandeu A, et al. Apoptosis of neurons in cardiovascular autonomic centres triggered by inducible nitric oxide synthase after death from septic shock. The Lancet 2003;362:1799‐805. [DOI] [PubMed] [Google Scholar]
- Sherwin RL, Garcia AJ, Bilkovski R. Do low‐dose corticosteroids improve mortality or shock reversal in patients with septic shock? A systematic review and position statement prepared for the American Academy of Emergency Medicine. Journal of Emergency Medicine 2012;43:7‐12. [DOI] [PubMed] [Google Scholar]
- Singer M, Deutschman CS, Seymour CW, Shankar Hari M, Angus DC, Annane D, et al. The Sepsis Definitions Task Force. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis‐3). JAMA 2015:in press. [Google Scholar]
- The EPISPESIS Group. EPISEPSIS: a reappraisal of the epidemiology and outcome of severe sepsis in French intensive care units. Intensive Care Medicine 2004;30:580‐8. [PUBMED: 14997295] [DOI] [PubMed] [Google Scholar]
- Tsao CM, Ho ST, Chen A, Wang JJ, Li CY, Tsai SK, et al. Low‐dose dexamethasone ameliorates circulatory failure and renal dysfunction in conscious rats with endotoxemia. Shock 2004;21::484‐91. [DOI] [PubMed] [Google Scholar]
- Vachharajani V, Vital S, Russell J, Scott LK, Granger DN. Glucocorticoids inhibit the cerebral microvascular dysfunction associated with sepsis in obese mice. Microcirculation 2006;13:477‐87. [PUBMED: 16864414 ] [DOI] [PubMed] [Google Scholar]
- Varga G, Ehrchen J, Tsianakas A, Tenbrock K, Rattenholl A, Seeliger S, et al. Glucocorticoids induce an activated, anti‐inflammatory monocyte subset in mice that resembles myeloid‐derived suppressor cells. Journal of Leukocyte Biology 2008;84:644‐50. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Moreno R, Takala J, Willatts S, Mendonça A, Bruining H, et al. The SOFA (Sepsis‐related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis‐Related Problems of the European Society of Intensive Care Medicine. Intensive Care Medicine 1996;22:707‐10. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Opal SM, Marshall JC, Tracey KJ. Sepsis definitions: time for change. Lancet 2013;381:774‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
- Annane D, Bellissant E, Bollaert PE, Briegel J, Keh D, Kupfer Y. Corticosteroids for treating severe sepsis and septic shock. Cochrane Database of Systematic Reviews 2004, Issue 1. [DOI: 10.1002/14651858.CD002243.pub2] [DOI] [PubMed] [Google Scholar]
- Annane D, Bellissant E, Bollaert PE, Briegel J, Confalonieri M, Gaudio R, et al. Corticosteroids in the treatment of severe sepsis and septic shock in adults: a systematic review. JAMA 2009;301:2362‐75. [PUBMED: 19509383 ] [DOI] [PubMed] [Google Scholar]
- Annane D, Bellissant E, Bollaert PE, Briegel J, Keh D, Kupfer Y. Corticosteroids for treating sepsis. Cochrane Database of Systematic Reviews 2015, Issue 12. [DOI: 10.1002/14651858.CD002243.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]


