Abstract
Introduction
Pregnancy losses may be associated with increased risks of dementia.
Methods
We conducted a register-based cohort study in 1,243,957 women with ≥1 pregnancy in Denmark in the period 1977–2015. Using Cox regression, we estimated hazard ratios (HRs) comparing risks of dementia in women with and without pregnancy losses.
Results
During 21,672,433 person-years of follow-up, 261,279 women experienced a pregnancy loss, and 2188 women were diagnosed with dementia. Stillbirth was associated with an 86% increased risk of dementia overall (HR 1.86, 95% confidence interval [CI] 1.28–2.71). By contrast, miscarriage was not associated with later risk of dementia overall (single miscarriage, HR 0.99, 95% CI 0.87–1.12; recurrent miscarriages, HR 1.06, 95% CI 0.84–1.35). Adjustment for cardiovascular disease, hypertension, and diabetes did not meaningfully alter the association magnitudes.
Discussion
Stillbirth and dementia may share underlying mechanisms, suggesting that a history of stillbirth should be considered when assessing dementia risk in women.
Keywords: Dementia, Alzheimer's disease, Vascular dementia, Pregnancy loss, Miscarriage, Stillbirth
1. Introduction
As the average lifespan has increased, so have the prevalence of dementia and its economic impact. In 2018, the annual health care and social costs associated with dementia worldwide were projected to reach the trillion-dollar mark [1]. Pharmacologic interventions to slow down dementia progression have not performed as well as anticipated, and research focus has shifted to dementia prevention strategies and a search for modifiable risk factors [2]. Many candidate risk factors have been proposed, but the Alzheimer's Association concluded in 2014 that significant uncertainty remained regarding the relationship between individual risk factors and dementia and that further studies were needed to provide a solid foundation for disease prevention [3].
Evidence suggests that biological differences between the sexes may contribute to differences in dementia susceptibility [4], [5], although sex-specific risk factors for dementia are not well understood [6]. The biological changes and events surrounding pregnancy are an obvious example of lifecourse events to which only women are exposed, suggesting that the impact of a woman's reproductive history on her risk of later dementia ought to be explored. In fact, a recent study found that a history of preeclampsia was associated with an increased risk of dementia, vascular dementia in particular, later in life [7], and results from a second study suggested that incomplete pregnancies might be associated with a decreased risk of Alzheimer's disease [8].
A history of pregnancy loss (miscarriage and stillbirth) is associated with increased risks of atherosclerotic disease and diabetes later in life [9], [10]. As both atherosclerotic disease and diabetes are predictors of dementia [11], [12], [13], we hypothesized that pregnancy losses might also be markers of increased dementia risk. In a nationwide cohort of more than 1.2 million women, we compared the risks of dementia, overall and by subtype and timing of onset, in women with and without a history of pregnancy losses. We also explored the role played by cardiovascular disease, hypertension, and diabetes in these associations.
2. Methods
2.1. Data sources and study cohort
The Danish Civil Registration System registers all Danish residents using unique personal identification numbers and updates information on demographics and vital status daily [14]. The Medical Birth Register contains information on all live births and stillbirths in Denmark since 1973 [15]. The National Patient Register contains information on all hospital discharge diagnoses assigned since 1977 and all outpatient diagnoses assigned since 1995, registered using International Classification of Diseases (ICD) codes [16]. The Danish Causes of Death Register includes information on the causes (underlying and contributing) of deaths in Denmark since 1970 [17]. Finally, the National Prescription Register contains individual-level information on all prescriptions filled in Denmark since 1994, recorded using Anatomic Therapeutic Chemical codes [18].
Using information from the Civil Registration System, Medical Birth Register, and National Patient Register, we constructed a study cohort consisting of all women in Denmark aged ≥15 years with at least one pregnancy ending in live birth, miscarriage, or stillbirth between 1977 and 2015 (Fig. 1). Women were followed from the end of their first such pregnancy in the study period or 1 January 1994 (when ICD-10 codes were introduced in Denmark, allowing more reliable subclassification of dementia), whichever came later, until the first of (1) dementia; (2) death; (3) emigration; (4) designated “missing” in the Civil Registration System; or (5) 31 May 2017 (end of follow-up). Women with eligible pregnancies who died or emigrated before 1994 were excluded from the cohort, as were women with a diagnosis of dementia before the start of follow-up.
Fig. 1.
Study cohort assembly and exclusions.
2.2. Exposure: Miscarriages and stillbirths
Miscarriage was defined as a missed abortion or spontaneous abortion registered in the National Patient Register as follows: 1977–1994, ICD-8 codes 634.61, 643.00-643.99, or 645.10-645.19; 1995–2015, ICD-10 codes O02.1, O02.1A, or O03.0-O03.9 registered between 7 and 22 completed weeks' gestation. Miscarriages registered within 8 weeks of molar pregnancies, induced abortions (surgical or medical), extra-uterine pregnancies, and other abnormal products of conception were ignored. Stillbirth was defined based on registration of a stillbirth in the Medical Birth Register (where stillbirth was defined as a pregnancy loss occurring after 28 weeks in the period 1977–2003 and after 22 weeks in the period 2004–2015) or a miscarriage in gestational weeks 23–28 in the National Patient Register (in the period 1995–2003). Information on gestational week at loss was not available before 1995, making it impossible to reclassify miscarriages in weeks 23–28 registered before then; therefore, losses before 1995 that were classified as miscarriages inevitably include losses that would have been classified as stillbirths after 1995.
History of pregnancy loss was considered as a hierarchical time-dependent variable, where stillbirth was considered a more severe loss than miscarriage. A woman could contribute with person-time in more than one exposure category during follow-up, with her exposure status at any given time reflecting her most severe pregnancy loss to date. If she had a miscarriage followed by a live birth, she would be classified as having a history of one miscarriage until the end of follow-up, unless she later had a second miscarriage or a stillbirth. In the case of a second miscarriage, she contributed all subsequent person-time to the “≥ 2 miscarriages” group. However, if she had a stillbirth, she was classified as having a history of stillbirth for the rest of the follow-up period, regardless of later events (subsequent miscarriages or live births).
2.3. Outcome: Dementia
Dementia was defined as registration of any dementia code (ICD-10 codes F00.0-F02.0, F03.9, G30.0, G30.1, G30.8, G30.9) in the National Patient Register during follow-up. Dementia was further classified as Alzheimer's disease (ICD-10 codes F00.0-F00.9, G30.0, G30.1, G30.8, G30.9), vascular dementia (ICD-10 codes F01.0-F01.9), and other/unspecified dementia (ICD-10 codes F02.0, F03.9). (The ICD-8 codes ICD-8 codes 290.00, 290.10, 290.11, 290.19, and 299.99 were also used when excluding women diagnosed with dementia before the start of follow-up).
2.4. Covariates
We considered parity (number of live births and/or stillbirths, 1, 2, and ≥3), maternal birth year (5-year intervals), and maternal age as potential confounders; parity and age were treated as time-dependent variables. We also evaluated the influence of cardiovascular disease, hypertension, diabetes, major depression, psychosis, and a history of preeclampsia in any pregnancy, all treated as time-dependent variables (binary yes/no variables). Women with cardiovascular disease, diabetes, depression, psychosis, or preeclampsia were identified using the National Patient Register and the Causes of Death Register, based on the following ICD-8 and ICD-10 codes: myocardial infarction, 410.09-410.99, I21.0-I23.9; ischemic heart disease, 411.09-414.99, 420.00-429.09, I20.0-I20.9, I24.0-I24.9, I25.0-I25.9; stroke, 433.09-433.99, 436.00-436.99, I63.0-I63.9; heart failure, 427.09-427.19, 427.99, 428.99, 782.49, I50.0-I50.9; diabetes, 249.00-250.09, E10.0-E14.9; major depressive disorders, 296.09, 296.29, 298.09, 300.49, F32.0-F33.9; schizophrenia spectrum disorder, 295.x9, 296.89, 297.x9, 298.29–298.99, 299.04, 299.05, 299.09, 301.83, F20.0-F29.9; preeclampsia, 637.03, 637.04, 637.09, 637.19, 637.99, 762.19, 762.29, 762.39, O14.0-O15.9. Hypertension (prevalent hypertension already being treated in 1994, as well as new cases of hypertension with treatment initiated after 1994) was identified based on the filling of two prescriptions for antihypertensive medication (Anatomic Therapeutic Chemical codes C02-03, C07-09 registered in the National Prescription Register) within a 6-month period.
2.5. Statistical analysis
Using Cox regression with age as the underlying time, we estimated hazard ratios for dementia comparing women with and without a history of pregnancy loss. All analyses were adjusted for maternal age, maternal birth year, and parity; further analyses also evaluated potential confounding by cardiovascular disease, hypertension, and diabetes. We used competing risk methodology [19] when analyzing associations with dementia subtypes, censoring on dementia subtypes other than the subtype of interest. We checked the proportional hazards assumption by plotting cumulative martingale residuals against maternal age [20]. All analyses were performed using SAS statistical software, version 9.4 (SAS Institute, Inc., Cary, NC).
2.6. Sensitivity analyses
We performed a number of sensitivity analyses to evaluate the influence of additional covariates, some of which were well registered and others that were incompletely measured, on the observed associations. The former group included major depressive disorders, psychosis, and preeclampsia (not necessarily in the same pregnancy as any pregnancy loss), for which we additionally adjusted in our analyses. The latter category included smoking and obesity (body mass index ≥30), which were only registered from 1991 and 2004, respectively, and then only in connection with pregnancy; consequently, we could not adjust our analyses for these potential confounders. To determine the potential impact of these variables on the observed associations, we performed sensitivity analyses using the array approach for testing the impact of an unmeasured or incompletely measured confounder [21]. We based these analyses on the prevalence of obesity and smoking in Danish women [22] with histories of stillbirth, recurrent miscarriage, or no pregnancy loss, and a range of magnitudes for the associations between obesity, smoking, and dementia obtained from the literature [23], [24], [25], [26].
3. Results
Using the Danish national registers, we followed 1,243,957 women for 21,672,433 person-years, with a median follow-up time of 21.6 years per woman (interquartile range: 11.6–23.4 years). Table 1 shows the characteristics of the cohort at entry into the study. The cohort consisted predominantly of younger women; 90% of the women were 42 years of age or less at the start of follow-up. The median age at the end of follow-up was 49 years; only 10% of women were >65 years of age at the end of follow-up.
Table 1.
Characteristics at the start of follow-up∗ for a cohort of women with ≥1 miscarriages, live births, or stillbirths in Denmark in the period 1977–2015
| Characteristic at the start of follow-up | History of pregnancy loss at start of follow-up† |
Total |
|||
|---|---|---|---|---|---|
| Stillbirth |
1 miscarriage |
≥2 miscarriages |
No loss |
||
| N (%) | N (%) | N (%) | N (%) | N (%) | |
| Total | 7154 (0.6) | 156,812 (12.6) | 15,508 (1.2) | 1,064,483 (85.6) | 1,243,957 (100) |
| Age (years) | |||||
| <25 | 801 (11.2) | 23,069 (14.7) | 454 (2.9) | 154,866 (14.6) | 179,190 (14.4) |
| 25–29 | 1618 (22.6) | 38,059 (24.3) | 2045 (13.2) | 322,247 (30.3) | 363,969 (29.3) |
| 30–34 | 1879 (26.3) | 36,713 (23.4) | 4078 (26.3) | 260,958 (24.5) | 303,628 (24.4) |
| 35–39 | 1503 (21.0) | 29,039 (18.5) | 4317 (27.8) | 161,609 (15.2) | 196,468 (15.8) |
| 40–44 | 885 (12.4) | 17,290 (11.0) | 2904 (18.7) | 102,562 (9.6) | 123,641 (9.9) |
| 45–49 | 359 (5.0) | 8464 (5.4) | 1293 (8.3) | 49,326 (4.6) | 59,442 (4.8) |
| ≥50 | 109 (1.5) | 4178 (2.7) | 417 (2.7) | 12,915 (1.2) | 17,619 (1.4) |
| Birth year | |||||
| ≤1944 | 153 (2.1) | 5312 (3.4) | 558 (3.6) | 18,457 (1.7) | 24,480 (2.0) |
| 1945–1949 | 418 (5.8) | 9445 (6.0) | 1617 (10.4) | 58,027 (5.5) | 69,507 (5.6) |
| 1950–1954 | 977 (13.7) | 16,666 (10.6) | 3189 (20.6) | 104,269 (9.8) | 125,101 (10.1) |
| 1955–1959 | 1301 (18.2) | 23,261 (14.8) | 4454 (28.7) | 123,893 (11.6) | 152,909 (12.3) |
| 1960–1964 | 1266 (17.7) | 24,755 (15.8) | 3778 (24.4) | 139,744 (13.1) | 169,543 (13.6) |
| 1965–1969 | 964 (13.5) | 22,152 (14.1) | 1618 (10.4) | 153,353 (14.4) | 178,087 (14.3) |
| 1970–1974 | 750 (10.5) | 18,660 (11.9) | 283 (1.8) | 150,663 (14.2) | 170,356 (13.7) |
| ≥1975 | 1325 (18.5) | 36,561 (23.3) | 11 (0.1) | 316,077 (29.7) | 353,974 (28.5) |
| Parity | |||||
| 0 | - | 83,817 (53.4) | 2026 (13.1) | - | 86,018 (6.9) |
| 1 | 3201 (44.8) | 23,488 (15.0) | 4176 (26.9) | 750,231 (70.5) | 780,921 (62.8) |
| 2 | 1370 (19.1) | 34,472 (22.0) | 6096 (39.3) | 240,588 (22.6) | 282,526 (22.7) |
| ≥3 | 2583 (36.1) | 15,035 (9.6) | 3210 (20.7) | 73,664 (6.9) | 94,492 (7.6) |
Follow-up began on 1 January 1994 for women with births or miscarriages before 1994 and on the date of first delivery for women whose first birth occurred in or after 1994.
Note that these totals reflect the number of women with pregnancy losses in pregnancies occurring before the start of follow-up. Because pregnancy loss was a time-dependent variable, additional women experienced pregnancy loss during follow-up.
By the end of follow-up, 10,440 women had a history of stillbirth, 203,654 women had had a single miscarriage, and 47,185 women had a history of ≥2 miscarriages. Dementia was diagnosed in 2188 women, with an average age at diagnosis of 60.2 years. In our cohort, 39.3% of women (n = 860) were specifically diagnosed with Alzheimer's disease and 6.7% were diagnosed with vascular dementia (n = 148); 54% of women (n = 1180) were registered with other/unspecified dementia.
Women with a history of stillbirth had an 86% (95% confidence interval [CI] 28%–171%) increase in overall dementia risk compared with women with no history of pregnancy loss (Table 2). By contrast, a history of miscarriage was not associated with overall dementia risk, either for women with one miscarriage (hazard ratio [HR] 0.99, 95% CI 0.87–1.12) or for those with two or more miscarriages (HR 1.06, 95% CI 0.84–1.35), compared with women with no history of pregnancy loss (Table 2). A history of stillbirth was equally strongly associated with early-onset (<65 years) and late-onset (≥65 years) dementia (P = .49) (Table 2). Conversely, it appeared that a history of ≥2 miscarriages might be associated with a modest increase in risk of early-onset dementia but not with the risk of late-onset dementia (P = .02) (Table 2).
Table 2.
Hazard ratios for dementia overall and by timing of onset, according to history of pregnancy loss, in a cohort of women with ≥1 live births, stillbirths, or miscarriages in Denmark in the period 1977–2015
| History of pregnancy loss | Dementia overall |
Dementia with onset <65 years |
Dementia with onset ≥65 years |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Person-years (× 103) | No. of events | HR∗ (95% CI) | Person-years (× 103) | No. of events | HR∗ (95% CI) | Person-years (× 103) | No. of events | HR∗ (95% CI) | |
| Stillbirth | 180 | 28 | 1.86 (1.28–2.71) | 177 | 18 | 1.70 (1.06–2.72) | 3 | 10 | 2.25 (1.20–4.22) |
| ≥2 miscarriages | 764 | 74 | 1.06 (0.84–1.35)† | 751 | 61 | 1.25 (0.97–1.63) | 13 | 13 | 0.61 (0.34–1.08) |
| 1 miscarriage | 3462 | 402 | 0.99 (0.87–1.12) | 3365 | 215 | 0.99 (0.79–1.13) | 10 | 187 | 0.98 (0.78–1.24) |
| No pregnancy loss | 17,266 | 1684 | 1 (Ref) | 16,817 | 1109 | 1 (Ref) | 449 | 575 | 1 (Ref) |
Abbreviations: CI, confidence interval; HR, hazard ratio.
Hazard ratios are adjusted for birth year (5-year intervals), parity (1, 2, ≥3), and age (the underlying time scale in the Cox model).
For 2 miscarriages, the HR was 1.05 (95% CI 0.81–1.37); for ≥ 3 miscarriages, the HR was 1.10 (95% CI 0.66–1.84).
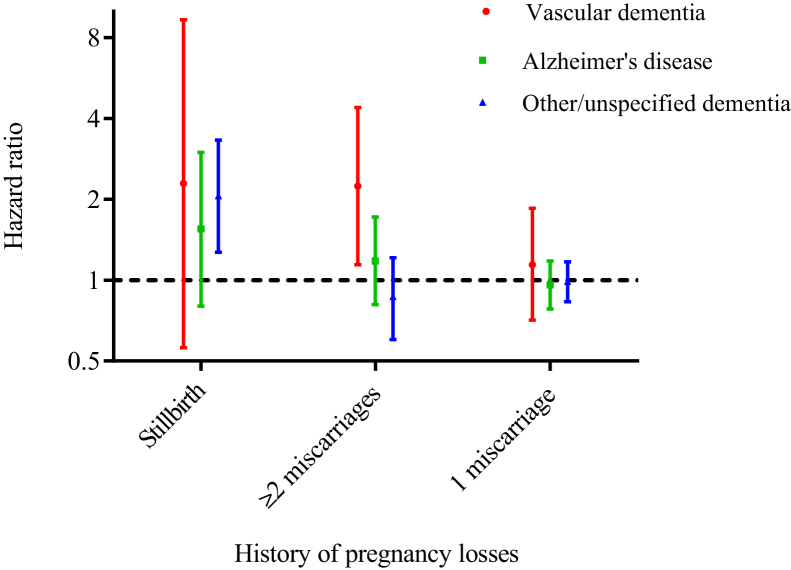
Compared with no history of pregnancy loss, a history of stillbirth was associated with a doubling of the risk of other/unspecified dementia and also appeared to be associated with an increased risk of vascular dementia. However, the confidence interval around the latter estimate was too wide to allow for firm conclusions and statistical testing showed that the estimates for vascular dementia, Alzheimer's disease, and unspecified dementia did not differ from one another (P = .91) (Fig. 2; Supplementary Table 1). Women with a history of two or more miscarriages had twice the risk of vascular dementia compared with women with no history of pregnancy loss, but no increased risk of Alzheimer's disease or unspecified dementia (Pdiff = 0.04) (Fig. 2; Supplementary Table 1). A single miscarriage was not associated with any dementia subtype (Fig. 2). The associations with a history of stillbirth seemed most pronounced for late-onset vascular dementia (HR 7.40, 95% CI 1.77–33.0) and other/unspecified dementia (HR 2.94, 95% CI 1.21–7.15), whereas a history of two or more miscarriages was most strongly associated with early-onset vascular dementia (HR 2.44, 95% CI 1.11–5.37) (Supplementary Table 1).
Fig. 2.
Hazard ratios for vascular dementia (red), Alzheimer's disease (green), and other/unspecified dementia (blue) in women with a history of stillbirth, ≥2 miscarriages, and 1 miscarriage, compared to women with no history of pregnancy loss (reference group). Hazard ratios were estimated in a cohort of women with ≥1 pregnancies ending in live birth, stillbirth, or miscarriage in Denmark in the period 1977–2015 and are adjusted for birth year (5-year intervals) and parity (1, 2, ≥3); age was the underlying time scale in the Cox model.
Adjustment of the overall dementia estimates for cardiovascular disease, hypertension, and diabetes did not change these estimates meaningfully (Supplementary Table 2a) for either miscarriage or stillbirth. Adjustment of dementia subtype–specific estimates attenuated the results for the association of recurrent miscarriage with vascular dementia in particular, but the associations remained strong and statistically significant nonetheless (Supplementary Table 2b).
Because dementia diagnoses are refined over time, we conducted a sensitivity analysis based on the last dementia diagnosis (if any) a woman received during the study period (Supplementary Table 3). After this refinement, a history of stillbirth was associated with more than a four-fold increase in the risk of vascular dementia, while the association with other/unspecified dementia decreased somewhat. A history of ≥2 miscarriages remained strongly associated with vascular dementia risk but not with the risk of Alzheimer's disease or other/unspecified dementia.
The results of sensitivity analyses suggested that major depression and psychosis could not explain the observed associations. Adjustment for these comorbidities did not affect the association between a history of stillbirth and dementia overall (HR 1.86, 95% CI 1.28–2.71) and the association between a history of ≥2 miscarriages and vascular dementia was only slightly attenuated (HR 2.15, 95% CI 1.09–4.21). Similarly, adjustment for preeclampsia in any pregnancy did not change hazard ratio magnitudes meaningfully (association between stillbirth and overall dementia, HR 1.83, 95% CI 1.25–2.67; association between ≥2 miscarriages and vascular dementia, HR 2.21, 95% CI 1.12–4.34).
Sensitivity analyses also suggested that obesity was unlikely to be a strong enough confounder to explain the associations observed between stillbirth and dementia overall (Supplementary Table 4a) and between recurrent miscarriage and vascular dementia (Supplementary Table 4b). Assuming that obesity at most doubles the risk of dementia overall [24], uncontrolled confounding by obesity would only have biased the observed hazard ratio for stillbirth and dementia overall by 6.21%, resulting in an adjusted estimate of 1.75 (95% CI 1.21–2.55). Similarly, even under the extreme assumption that obese women are five times as likely as normal weight women to develop vascular dementia [23], uncontrolled confounding by obesity would only have biased the observed hazard ratio for recurrent miscarriage and vascular dementia by 13.1%, leading to an adjusted estimate of 1.98 (95% CI 1.01–3.88).
Confounding by smoking could not explain the observed association between stillbirth and dementia overall (Supplementary Table 5). Furthermore, because there is little evidence to suggest that smoking is associated with miscarriage, at least in the Danish population [27], smoking was unlikely to be a confounder of the association between recurrent miscarriage and dementia.
4. Discussion
In this large nationwide cohort study, we found that a history of stillbirth was associated with an almost two-fold increase in the overall risk of dementia. The strength of the association did not appear to depend on age of dementia onset, and the magnitudes of the associations between stillbirth and specific dementia subtypes were not statistically significantly different from one another. The association between history of stillbirth and dementia overall remained pronounced even after adjustment for cardiovascular disease, hypertension, diabetes, major depression, psychosis, smoking, and obesity. By contrast, no association was observed between a history of miscarriage, either isolated or recurrent, and later risk of dementia overall.
The lack of association observed for miscarriage and dementia overall persisted when we looked at Alzheimer's disease separately: we found no hint of any association between a history of miscarriage, either isolated or recurrent, and risk of later Alzheimer's disease. This is in contrast to recently published results suggesting that incomplete pregnancies might be associated with a substantially reduced risk of later Alzheimer's disease [8]. However, the two studies are difficult to compare. The recent study by Jang and colleagues included not only miscarriages but also induced abortions in its definition of “incomplete pregnancy,” and women who had never been pregnant were included in the reference group [8]. We only considered the association of spontaneous abortions with later dementia among women who had ever been pregnant, to separate the potential contribution of pregnancy loss to dementia risk from the contribution of pregnancy itself. Interestingly, although we found no association between miscarriage and Alzheimer's disease, our analyses of dementia subtypes hinted that recurrent miscarriage might potentially be associated with a doubling of the risk of later vascular dementia, a connection that has not previously been reported. However, owing to the small numbers of exposed women in these analyses, these results should be interpreted with caution and confirmed by others.
Our finding that stillbirths were associated with an increased risk of dementia provides little support for the hypothesis promoted by Jang and colleagues that exposure to pregnancy-induced increases in estrogen levels might protect women against dementia [8]. Instead, stillbirth, and potentially also miscarriage, may be linked with dementia through shared mechanisms involving vascular pathology and endothelial dysfunction. Such mechanisms could produce both poor placental implantation during pregnancy, leading to pregnancy losses, and ideal conditions for dementia later in life. Previous studies linking pregnancy loss and vascular diseases [9], [28], [29] indicate that associations between pregnancy complications involving vascular pathology and dementia, vascular dementia in particular, are plausible. Moreover, data from pathology and epidemiologic studies suggest considerable overlap between cerebrovascular disease and Alzheimer's disease [30]. Alternatively, high homocysteine levels in early pregnancy are also a risk factor for pregnancy loss [31], and increased total homocysteine levels have been associated with vascular dementia [32]. Abnormal maternal immune responses could also plausibly link pregnancy loss with later dementia; a recent study found that autoimmune diseases had a strong impact on the risk of vascular dementia [33]. Finally, apolipoprotein E (APOE) gene polymorphisms have been associated with recurrent pregnancy loss, with variants in APOE2 and APOE4 associated with increased risk of recurrent losses, whereas a variant in APOE3 might be associated with a reduction in risk [34]. APOE4 variants are strongly associated with an increased risk of Alzheimer's disease, and there is evidence to suggest that they might also be associated, albeit not as strongly, with an increase in vascular disease risk [35]. Consequently, a woman's APOE status might also contribute to the associations we observed.
Hypertension, obesity, and diabetes might also link stillbirth and dementia. However, adjusting for cardiovascular disease, hypertension, and diabetes had little effect on the observed associations between stillbirth and dementia in our study, suggesting that the association is likely not mediated by other vascular conditions but instead may be a consequence of shared underlying etiologic factors. Similarly, a proportion of women who have experienced a stillbirth develop depression and other psychiatric comorbidities [36], [37], and depression early in life may be a risk factor for dementia [38], [39], [40], suggesting that depression might play a role in the observed association between stillbirth and dementia. However, adjustment for major depression and psychosis did not change the magnitude of the association between stillbirth and dementia, suggesting that these psychiatric comorbidities were neither confounders nor mediators of the observed association.
4.1. Strengths and potential limitations
Our register-based design allowed us to construct a nationwide cohort of all registered births and pregnancy losses over a 38-year period, with ascertainment of exposure and outcome that did not depend on personal recall of events. The population-based nature of the registers also minimized selection bias. Validation studies have stated a near-complete (>99.5%) recording of births in the Medical Birth Register [15].
Not all dementia is diagnosed, and registration of dementia is probably also incomplete or at least delayed, as general practitioners handle a certain proportion of the milder cases of dementia [41]. However, a study of dementia diagnoses registered in the National Patient Register found that 88% of persons with a registered dementia diagnosis did in fact have dementia according to their medical records, and registered diagnoses of Alzheimer's disease, vascular dementia, and other/unspecified dementia agreed with the diagnosis noted in the medical record for 97%, 96%, and 81% of patients, respectively [42]. Whether dementia in our study population was diagnosed or registered was unlikely to have depended on pregnancy history, such that any misclassification of persons with dementia as healthy would have been nondifferential.
Because the women in our cohort were followed from the end of their first pregnancy in the study period, as a group they were still relatively young at the end of follow-up (only 10% were >65 years of age). As a result, the rates of dementia we report here are much lower than those one would expect from a study that followed women into their 70s and 80s [43], and we appeared to have an excess of early-onset dementia (64% of our dementia cases were diagnosed before 65 years of age). However, the observed rates of dementia compare favorably with incidences reported in studies of early-onset dementia (incidence among persons 45–64 years of age, 2.4 to 11.9 per 100,000 persons [44]). There were also relatively few women diagnosed with Alzheimer's disease (39% of the dementia diagnoses in our cohort), compared with the proportion (54%) who were registered with a diagnosis of other/unspecified dementia. Again, this imbalance likely reflects both the clinical challenges involved in classifying dementia into its subtypes, particularly when many patients exhibit “mixed” forms of dementia, and the relative youth of our cohort. Dementia diagnoses are often refined over time and our relatively young women may not have been followed long enough to allow assignment of a more specific diagnosis; furthermore, the prevalence of Alzheimer's disease increases with age.
We did not have information on several variables potentially relevant to dementia risk, including socioeconomic status, education, employment, ethnicity, smoking, and obesity. However, while socioeconomic factors, including education, are associated with dementia risk [45], there is little evidence to suggest that they are associated with stillbirths in Danish women; for example, a study found no relationship between maternal education and risk of stillbirth [46]. The impact of any potential confounding by educational level and other indices of socioeconomic status were also likely reduced by Denmark's free, universal health care and education systems. Our cohort was comprised overwhelmingly of women of Scandinavian descent, such that any confounding by ethnicity was also likely to have been minimal; this ethnic homogeneity may, however, have limited our study's generalizability to other populations.
Smoking during pregnancy has been linked with a slightly increased risk of stillbirth [47]. Studies relating smoking to the risk of dementia have produced inconsistent results [45], [48], and a recent meta-analysis showed that only current smoking, and not former smoking, increased the risk of both Alzheimer's disease and vascular dementia [26]. When we evaluated smoking as a potential unmeasured confounder, we found little evidence that confounding by smoking could have accounted for the observed associations.
A recent meta-analysis suggested that obesity might be modestly associated with dementia overall [24], although effect magnitudes appeared to depend both on whether the comparison group only consisted of normal weight women or whether overweight women were also included, and on when weight was evaluated (mid-life vs. late-life). Evidence for a relationship between obesity and vascular dementia risk is even more sparse and inconsistent [23], [24], [25]. However, when we conducted sensitivity analyses of the effect of unmeasured confounding by obesity, we found that obesity could account for only a small percentage of our observed associations, even when we assumed the most extreme degree of association between obesity and dementia published in the literature [23].
4.2. Public health impact
Examining the relationships between elements of a woman's reproductive history and dementia risk may shed new light on mechanisms involved in dementia pathophysiology and help to identify pathways to dementia that are unique to women. Pregnancy history could also potentially be incorporated into a screening tool to identify women at increased risk of dementia at a relatively young age, allowing interventions targeting modifiable risk factors to be implemented and increasing the chance that dementia could be prevented or at least delayed.
Research in context.
-
1.
Systematic review: We searched PubMed for literature on dementia risk factors related to pregnancy. Only one previous study examined the link between incomplete pregnancies and dementia, finding that incomplete pregnancies were associated with a decreased risk of Alzheimer's disease.
-
2.
Interpretation: Our nationwide cohort study found that a history of stillbirth was associated with an increased risk of dementia overall. A history of miscarriage was not associated with the overall risk of dementia. Recurrent miscarriage might be associated with an increased risk of vascular dementia, but there were few exposed events. Confounding by cardiovascular disease, hypertension, diabetes, obesity, and smoking could not account for the observed associations, suggesting that pregnancy losses and dementia may share etiologic pathways.
-
3.
Future directions: Future studies should evaluate 1) potential shared mechanisms for pregnancy loss and dementia subtypes, and 2) whether asking about pregnancy losses might improve screening tools to identify women at risk of dementia.
Acknowledgments
This work was supported by the Danish Council for Independent Research (DFF-4092-00213). The research council played no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Footnotes
The authors have declared that no conflict of interest exists.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.trci.2019.02.006.
Supplementary Data
References
- 1.Prince M., Wimo A., Guerchet M., Gemma-Claire A., Wu Y.-T., Prina M. World Alzheimer Report 2015: The global impact of dementia - An analysis of prevalence, incidence, cost and trends. Alzheimer’s Dis Int. 2015:84. [Google Scholar]
- 2.Olanrewaju O., Clare L., Barnes L., Brayne C. A multimodal approach to dementia prevention: A report from the Cambridge Institute of Public Health. Alzheimer’s Dement Transl Res Clin Interv. 2015;1:151–156. doi: 10.1016/j.trci.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baumgart B., Snyder H.M., Carrillo M.C., Fazio S., Kim H., Johns H. Summary of the evidence on modifiable risk factors for cognitive decline and dementia: A population-based perspective. Alzheimer’s Dement. 2015;11:718–726. doi: 10.1016/j.jalz.2015.05.016. [DOI] [PubMed] [Google Scholar]
- 4.Rocca W.A., Mielke M.M., Vemuri P., Miller V.M. Sex and gender differences in the causes of dementia: A narrative review. Maturitas. 2014;79:196–201. doi: 10.1016/j.maturitas.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carter C.L., Resnick E.M., Mallampalli M., Kalbarczyk A. Sex and gender differences in Alzheimer’s disease: recommendations for future research. J Women’s Health. 2012;21:1018–1023. doi: 10.1089/jwh.2012.3789. [DOI] [PubMed] [Google Scholar]
- 6.Podcasy J.L., Epperson C.N. Considering sex and gender in Alzheimer disease and other dementias. Dialogues Clin Neurosci. 2016;18:437–446. doi: 10.31887/DCNS.2016.18.4/cepperson. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Basit S., Wohlfahrt J., Boyd H.A. Preeclampsia and the risk of dementia later in life -- a nationwide cohort study. BMJ. 2018;363:k4109. doi: 10.1136/bmj.k4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jang H., Bae J.B., Dardiotis E., Scarmeas N., Sachdev P.S., Lipnicki D.M. Differential effects of completed and incomplete pregnancies on the risk of Alzheimer disease. Neurology. 2018;91:e643–e651. doi: 10.1212/WNL.0000000000006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ranthe M.F., Andersen E.A.W., Wohlfahrt J., Bundgaard H., Melbye M., Boyd H.A. Pregnancy loss and later risk of atherosclerotic disease. Circulation. 2013;127:1775–1782. doi: 10.1161/CIRCULATIONAHA.112.000285. [DOI] [PubMed] [Google Scholar]
- 10.Kharazmi E., Lukanova A., Teucher B., Groß M.L., Kaaks R. Does pregnancy or pregnancy loss increase later maternal risk of diabetes? Eur J Epidemiol. 2012;27:357–366. doi: 10.1007/s10654-012-9683-9. [DOI] [PubMed] [Google Scholar]
- 11.Meyer J.S., Rauch G.M., Rauch R.A., Haque A., Crawford K. Cardiovascular and other risk factors for Alzheimer’s disease and vascular dementia. Vasc Factors Alzheimer’s Dis. 2000;903:411–423. doi: 10.1111/j.1749-6632.2000.tb06393.x. [DOI] [PubMed] [Google Scholar]
- 12.Bos D., Vernooij M.W., De Bruijn R.F.A.G., Koudstaal P.J., Hofman A., Franco O.H. Atherosclerotic calcification is related to a higher risk of dementia and cognitive decline. Alzheimer’s Dement. 2015;11:639–647.e1. doi: 10.1016/j.jalz.2014.05.1758. [DOI] [PubMed] [Google Scholar]
- 13.Gudala K., Bansal D., Schifano F., Bhansali A. Diabetes mellitus and risk of dementia: A meta-analysis of prospective observational studies. J Diabetes Investig. 2013;4:640–650. doi: 10.1111/jdi.12087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmidt M., Pedersen L., Sørensen H.T. The Danish Civil Registration System as a tool in epidemiology. Eur J Epidemiol. 2014;29:541–549. doi: 10.1007/s10654-014-9930-3. [DOI] [PubMed] [Google Scholar]
- 15.Knudsen L.B., Olsen J. The Danish Medical Birth Registry. Dan Med Bull. 1998;45:320–323. [PubMed] [Google Scholar]
- 16.Lynge E., Sandegaard J.L., Rebolj M. The Danish National Patient Register. Scand J Public Health. 2011;39:30–33. doi: 10.1177/1403494811401482. [DOI] [PubMed] [Google Scholar]
- 17.Helweg-Larsen K. The Danish Register of Causes of Death. Scand J Public Health. 2011;39:26–29. doi: 10.1177/1403494811399958. [DOI] [PubMed] [Google Scholar]
- 18.Kildemoes H.W., Sørensen H.T., Hallas J. The Danish National Prescription Registry. Scand J Public Health. 2011;39:38–41. doi: 10.1177/1403494810394717. [DOI] [PubMed] [Google Scholar]
- 19.Lau B., Cole S.R., Gange S.J. Competing risk regression models for epidemiologic data. Am J Epidemiol. 2009;170:244–256. doi: 10.1093/aje/kwp107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin D.Y., Wei L.J., Ying Z. Checking the Cox model with cumulative sums of martingale-based residuals. Biometrika. 1993;80:557–572. [Google Scholar]
- 21.Schneeweiss S. Sensitivity analysis and external adjustment for unmeasured confounders in epidemiologic database studies of therapeutics. Pharmacoepidemiol Drug Saf. 2006;15:291–303. doi: 10.1002/pds.1200. [DOI] [PubMed] [Google Scholar]
- 22.Hahn K.A., Hatch E.E., Rothman K.J., Mikkelsen E.M., Brogly S.B., Sørensen H.T. Body size and risk of spontaneous abortion among Danish pregnancy planners. Paediatr Perinat Epidemiol. 2014;28:412–423. doi: 10.1111/ppe.12142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Whitmer R.A., Gunderson E.P., Quesenberry C.P., Zhou J., Yaffe K. Body mass index in midlife and risk of Alzheimer disease and vascular dementia. Curr Alzheimer Res. 2007;4:103–109. doi: 10.2174/156720507780362047. [DOI] [PubMed] [Google Scholar]
- 24.Anstey K.J., Cherbuin N., Budge M., Young J. Body mass index in midlife and late-life as a risk factor for dementia: A meta-analysis of prospective studies. Obes Rev. 2011;12:e426–e437. doi: 10.1111/j.1467-789X.2010.00825.x. [DOI] [PubMed] [Google Scholar]
- 25.Fitzpatrick A.L., Kuller L.H., Lopez O.L., Diehr P., O’Meara E.S., Longstreth W.T. Mid- and Late-Life Obesity: Risk of Dementia in the Cardiovascular Health Cognition Study. Arch Neurol. 2009;66:336–342. doi: 10.1001/archneurol.2008.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhong G., Wang Y., Zhang Y., Guo J.J., Zhao Y. Smoking is associated with an increased risk of dementia: A meta-analysis of prospective cohort studies with investigation of potential effect modifiers. PLoS One. 2015;10:e0118333. doi: 10.1371/journal.pone.0118333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feodor Nilsson S., Andersen P.K., Strandberg-Larsen K., Nybo Andersen A.M. Risk factors for miscarriage from a prevention perspective: A nationwide follow-up study. BJOG An Int J Obstet Gynaecol. 2014;121:1375–1384. doi: 10.1111/1471-0528.12694. [DOI] [PubMed] [Google Scholar]
- 28.Parker D.R., Lu B., Sands-Lincoln M., Kroenke C.H., Lee C.C., O’Sullivan M. Risk of cardiovascular disease among postmenopausal women with prior pregnancy loss: The women’s health initiative. Ann Fam Med. 2014;12:302–309. doi: 10.1370/afm.1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oliver-Williams C.T., Heydon E.E., Smith G.C.S., Wood A.M. Miscarriage and future maternal cardiovascular disease: a systematic review and meta-analysis. Heart. 2013;99:1636–1644. doi: 10.1136/heartjnl-2012-303237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Attems J., Jellinger K.A. The overlap between vascular disease and Alzheimer’s disease - lessons from pathology. BMC Med. 2014;12:206. doi: 10.1186/s12916-014-0206-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dodds L., Fell D.B., Dooley K.C., Armson B.A., Allen A.C., Nassar B.A. Effect of homocysteine concentration in early pregnancy on gestational hypertensive disorders and other pregnancy outcomes. Clin Chem. 2008;54:326–334. doi: 10.1373/clinchem.2007.097469. [DOI] [PubMed] [Google Scholar]
- 32.Clarke R., Smith A.D., Jobst K.A., Refsum H., Sutton L., Ueland P.M. Folate, vitamin B12, and serum total homocysteine levels in confirmed Alzheimer disease. Arch Neurol. 1998;55:1449–1455. doi: 10.1001/archneur.55.11.1449. [DOI] [PubMed] [Google Scholar]
- 33.Goldacre M.J., Wotton C.J. Associations between specific autoimmune diseases and subsequent dementia: Retrospective record-linkage cohort study, UK. J Epidemiol Community Health. 2017;71:576–583. doi: 10.1136/jech-2016-207809. [DOI] [PubMed] [Google Scholar]
- 34.Li J., Chen Y., Wu H., Li L. Apolipoprotein E (Apo E) gene polymorphisms and recurrent pregnancy loss: A meta-analysis. J Assist Reprod Genet. 2014;31:139–148. doi: 10.1007/s10815-013-0128-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rohn T.T. Is apolipoprotein E4 an important risk factor for vascular dementia? Int J Clin Exp Pathol. 2014;7:3504–3511. [PMC free article] [PubMed] [Google Scholar]
- 36.Hogue C.J., Parker C.B., Willinger M., Temple J.R., Bann C.M., Silver R.M. The association of stillbirth with depressive symptoms 6-36 months post-delivery. Paediatr Perinat Epidemiol. 2015;29:131–143. doi: 10.1111/ppe.12176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heazell A.E., Siassakos D., Blencowe H., Burden C., Bhutta Z.A., Cacciatore J. Stillbirths: economic and psychosocial consequences. Lancet. 2016;387:604–616. doi: 10.1016/S0140-6736(15)00836-3. [DOI] [PubMed] [Google Scholar]
- 38.Byers A.L., Yaffe K. Depression and risk of developing dementia. Nat Rev Neurol. 2011;7:323–331. doi: 10.1038/nrneurol.2011.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bennett S., Thomas A.J. Depression and dementia: Cause, consequence or coincidence? Maturitas. 2014;79:184–190. doi: 10.1016/j.maturitas.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 40.Bellou V., Belbasis L., Tzoulaki I., Middleton L.T., Ioannidis J.P.A., Evangelou E. Systematic evaluation of the associations between environmental risk factors and dementia: An umbrella review of systematic reviews and meta-analyses. Alzheimers Dement. 2017;13:406–418. doi: 10.1016/j.jalz.2016.07.152. [DOI] [PubMed] [Google Scholar]
- 41.Phung T.K.T., Waltoft B.L., Kessing L.V., Mortensen P.B., Waldemar G. Time trend in diagnosing dementia in secondary care. Dement Geriatr Cogn Disord. 2010;29:146–153. doi: 10.1159/000269933. [DOI] [PubMed] [Google Scholar]
- 42.Phung T.K.T., Andersen B.B., Høgh P., Kessing L.V., Mortensen P.B., Waldemar G. Validity of dementia diagnoses in the Danish hospital registers. Dement Geriatr Cogn Disord. 2007;24:220–228. doi: 10.1159/000107084. [DOI] [PubMed] [Google Scholar]
- 43.Fiest K.M., Jetté N., Roberts J.I., Maxwell C.J., Smith E.E., Black S.E. The prevalence and incidence of dementia: a systematic review and meta-analysis. Can J Neurol Sci. 2016;43:S3–S50. doi: 10.1017/cjn.2016.18. [DOI] [PubMed] [Google Scholar]
- 44.Vieira R.T., Caixeta L., Machado S., Silva A.C., Nardi A.E., Arias-Carrión O. Epidemiology of early-onset dementia: a review of the literature. Clin Pract Epidemiol Ment Health. 2013;9:88–95. doi: 10.2174/1745017901309010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beydoun M.A., Beydoun H.A., Gamaldo A.A., Teel A., Zonderman A.B., Wang Y. Epidemiologic studies of modifiable factors associated with cognition and dementia: Systematic review and meta-analysis. BMC Public Health. 2014;14:643. doi: 10.1186/1471-2458-14-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Olsen O., Madsen M. Effects of maternal education on infant mortality and stillbirths in Denmark. Scand J Public Health. 1999;27:128–136. [PubMed] [Google Scholar]
- 47.Morales-Suárez-Varela M., Nohr E.A., Bech B.H., Wu C., Olsen J. Smoking, physical exercise, BMI and late foetal death: a study within the Danish National Birth Cohort. Eur J Epidemiol. 2016;31:999–1009. doi: 10.1007/s10654-016-0190-2. [DOI] [PubMed] [Google Scholar]
- 48.Peters R., Beckett N., Geneva M., Tzekova M., Lu F.H., Poulter R. Sociodemographic and lifestyle risk factors for incident dementia and cognitive decline in the HYVET. Age Ageing. 2009;38:521–527. doi: 10.1093/ageing/afp094. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.