Abstract
Background
Crush injury of nerves is a common condition but the biomechanical integrity of the human peripheral nerve after crushing is unknown. This study aims to investigate the impact of crush injury on human digital nerves based on different compressive forces.
Materials and methods
Twenty digital nerves were harvested from three fresh-frozen cadaver hands. The original diameters of proximal, middle and distal end of nerve segment were measured. The midst of each digital nerve was compressed by a customized mechanical system, at 1N, 3N and 5N for 30sec. The diameters were measured again within 1 minute after the nerve crush test was performed. The digital nerve was then subjected to biomechanical test to measure its ultimate tensile strength, stiffness, maximum stress and strain. Deformity of digital nerve was computed based on the diameter of middle nerve segment before and after crush test.
Results
No significant difference was found in between groups for ultimate tensile strength (p=0.598), stiffness (p=0.593), maximum stress (p=0.7) and strain (p=0.666). The deformity of nerves under the compression of 1N, 3N and 5N was computed at 72.1%, 54.2% and 45.9%. The effect of compression on the deformity of nerves was statistically significant (p<0.001).
Conclusions
It was found that the compressive forces have no impact on the biomechanical integrity of peripheral nerves but the deformity of nerves could be severely caused by low compressive force. It is suggested that the management of nerve crush injury shall be taken immediately and focus on neurophysiological function and degeneration of nerves for a crush with low compressive force and short duration.
Keywords: Biomedical engineering, Neurology, Mechanical engineering, Bioengineering
1. Introduction
Nerve crush injuries occur through mechanical compression of the nerves. Nerve crush injuries, may induce impairment of impulse conduction, loss of functions of the nerve and result in long term damage [1, 2, 3].
Dahlin et al. [2] reported that the degree of nerve impairment could be affected by two major factors: pressure and duration of crush. Nerve conductivity was reduced to 70.6% over a period of 15 minutes when a pressure of 400 mmHg was applied, or nerve conductivity was reduced to 86.5% over a period of 2 hours when a pressure of 80mmHg was applied. They concluded that the compressive forces applied onto nerves for a specific duration had an additive effect on top of the pressure, which caused edema and nerve fiber damage.
Biomechanical properties of nerves after laceration, stretch-related, and crush injuries have been studied in order to understand the complete neurophysiological behavior of nerves after injury [4, 5, 6]. Mechanical properties such as ultimate failure, stiffness, stress, strain, and position of failure have been evaluated for both intact and repaired nerves based on rodent or rabbit model. However, fewer studies were done to investigate the impact of crush injury on human peripheral nerves.
The objective of this study is to investigate the biomechanical integrity of human digital nerves based on different compressive forces. This study compares the mechanical properties of intact and crushed digital nerves, mainly ultimate tensile strength (UTS), stiffness, maximum stress and strain, as well as deformity. It provides an insight of human peripheral nerve biomechanical behavior after crush injury.
2. Methods
Fresh-frozen cadaver hands are commercially purchased for this study. Cadaveric hands are considered legacy tissue according our Centralized Institutional Review Board (CIRB) and ethics review for use of legacy tissues are not required.
2.1. Specimen preparation
Three fresh-frozen cadaver hands (Cadaver age, 76 year, left and right hand, female; 77 year; left hand, 1 male) were thawed at room temperature and dissected. The digital nerves were carefully separated from the digital artery and nerve branches to the skin. Each digital nerve was dissected from the palm, near to the metacarpophalangeal joint (MCP) and proximal interphalangeal joint (PIPJ). The nerve was transected further into 20 nerve segments with an average length of 15.4mm (±3.3). The digital nerves were then divided randomly into 4 groups equally for the experiments (n = 5).
The original diameter of each nerve was measured using a micrometer (Draper 46599, Hampshire, UK). Measurements were taken at three different location of the digital nerve: proximal, middle and distal nerve segment. Firstly, the predetermined location of digital nerve was placed freely in between the measuring probes. Next, the gap between the measuring probes was closed up until the digital nerve was held steadily by the probes. The reading of micrometer was recorded and the average diameter measured at three locations was calculated.
The diameter of digital nerve at its middle point where the nerve was crushed, was measured again within 1 minute after the nerve crush test was performed and compared with its original diameter.
2.2. Nerve crush testing
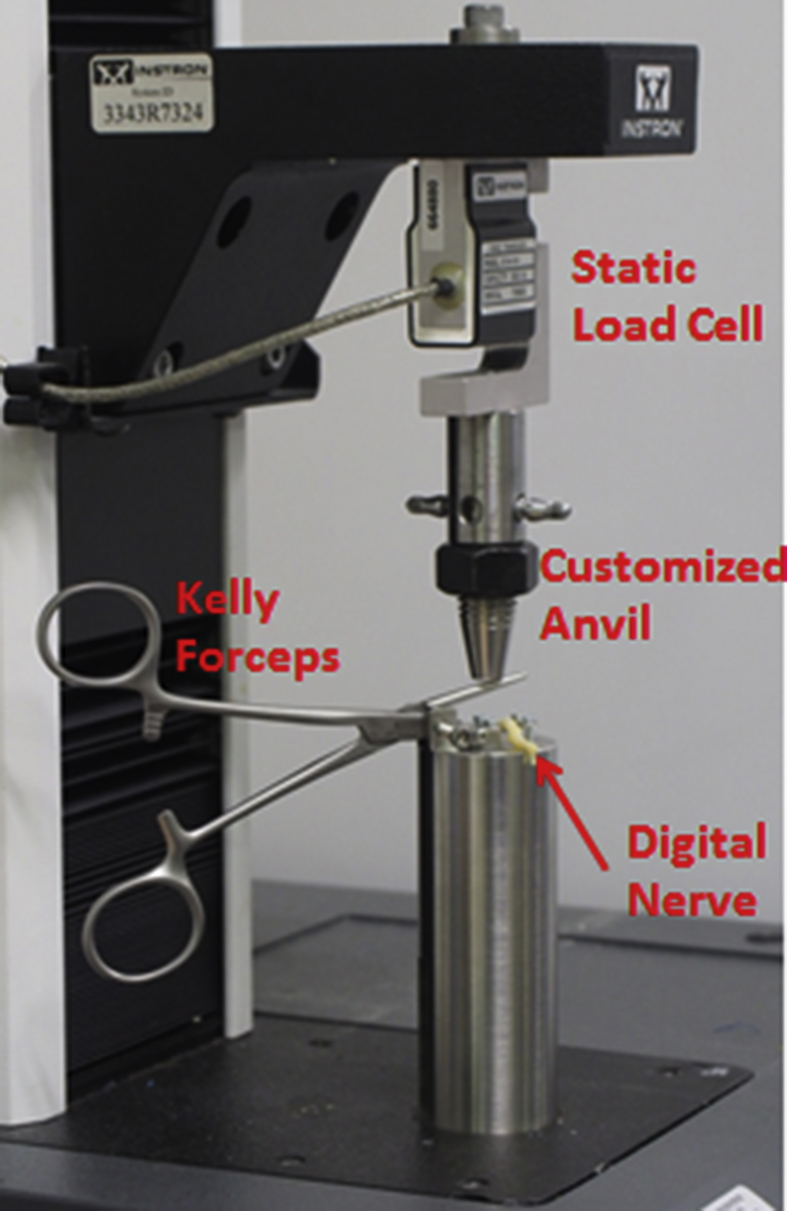
A nerve crush test method was developed in order to stimulate nerve crush injury. As shown in Fig. 1, a pair of standard Kelly forceps was mounted onto a customized jig attached to the biomechanical tester (Instron 3343, Instron Corp., Canton MA, USA). The upper gripping surface of Kelly forceps was originally jagged but the bottom gripping surface was flattened so that the jig can position the lower jaw of the Kelly forceps horizontally. A downward perpendicular compressive force was applied through its upper jaw. The compression force was set accurately by attaching the customized anvil onto a 100N static load cell. The middle of the digital nerve was placed in between the jaws, and the compression of digital nerve was achieved by lowering the anvil gradually. Three different compressive forces were applied: i) 1N; ii) 3N and iii) 5N, together with 1 control (without compression, 0N) group. Duration of the crush was standardized at 30 seconds so that a stable compression on the nerve could be achieved. After that, approximate 2mm of the crushing zone in length was incurred according to the width of the Kelly forceps. Biomechanical testing of all the digital nerves was performed immediately after their diameters at the clamped zone were measured.
Fig. 1.
Nerve crush test.
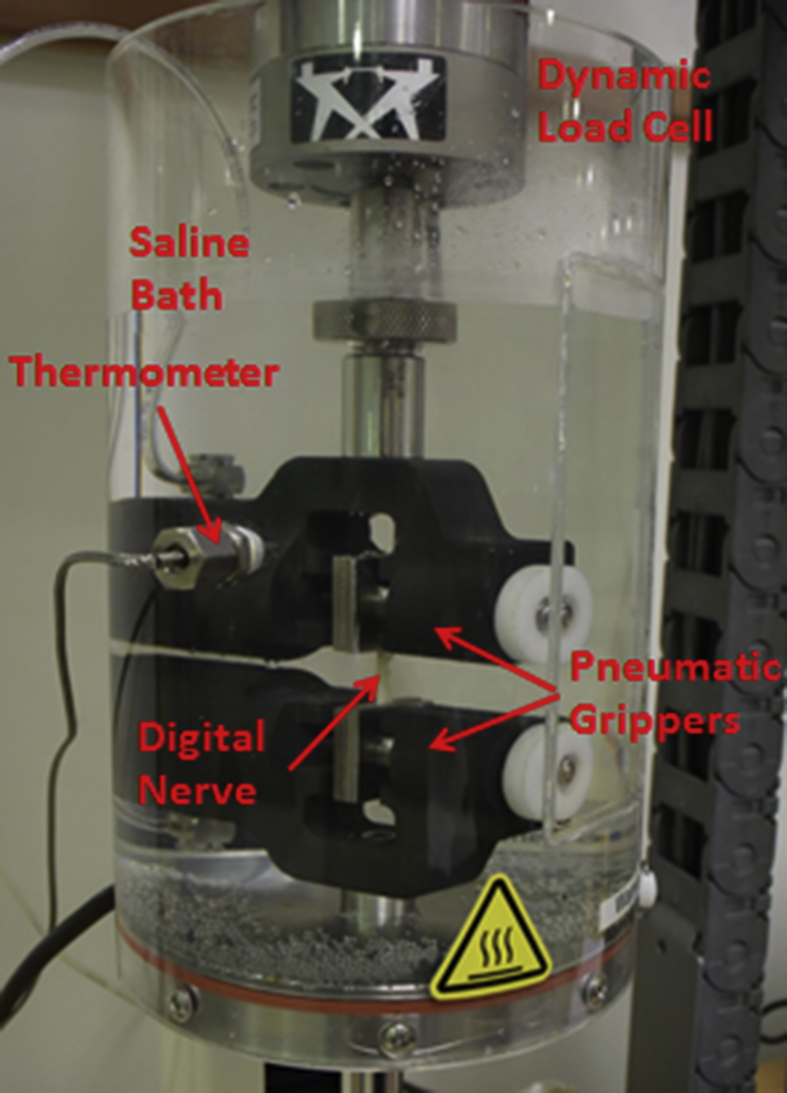
Biomechanical testing was conducted using an Instron Electropuls E1000 with 250N dynamic load cell attachment (Instron Corp., Canton MA, USA). The digital nerve was submerged into a BioPuls bath filled with saline to prevent dehydration (Fig. 2). A thermometer and heat controller was installed to maintain the temperature of the saline at 37 °C (±0.2), imitating the average human body temperature. Both ends of the digital nerves were secured using a pair of pneumatic grippers, with a gauge length of approximately 8mm. The digital nerves were then pulled to failure at a strain rate of 0.11 mm/sec [7]. The tensile load and displacement were recorded. A high resolution camera was used to record the failure mechanism of the digital nerves.
Fig. 2.
Experimental setup for biomechanical testing.
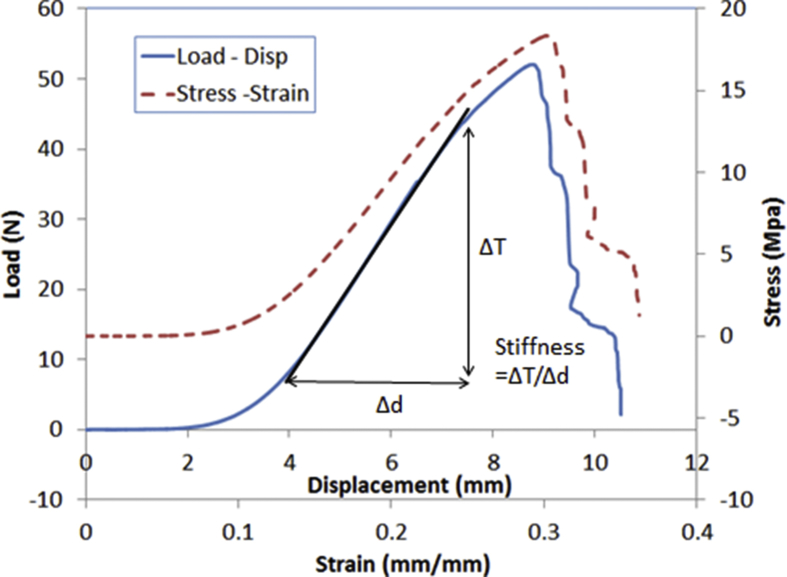
A typical load-displacement and stress-strain curve of digital nerve under tensile test is shown in Fig. 3. Stiffness was obtained by calculating the gradient of the load-displacement curve along the linear region. The stress was obtained by dividing the tensile load with the constant cross sectional area of the digital nerve based on its average diameter. The strain was calculated by taking the quotient of displacement or the elongation of digital nerve to its original length.
Fig. 3.
Typical load – displacement and stress – strain curve for one biomechanical test.
Deformity in percentage of the digital nerves was calculated as follows:
Where “Deformed diameter” is the diameter of the digital nerves measured at its middle point after the induced crush.
The nonparametric Kruskal–Wallis test was adopted to compute the statistical significance of differences between the groups. A p-value of less than 0.05 is considered to be statistically significant.
5. Results
Median and interquartile range (IQR) of ultimate tensile strength (UTS), stiffness, stress, strain and deformity were computed and presented in Figs. 4, 5, 6, 7, and 8. The detail of experimental results was tabulated in Table 1.
Fig. 4.
Ultimate tensile strength of the digital nerves to rupture. The plots showed the median values with the 25 percentile (Q1) and 75 percentile (Q3) values. The lower whiskers indicate Q1 +1.5 IQR and the upper whiskers Q3+1.5 IQR. Outliers are indicated by a star. No statistically significant difference was found within the groups (p = 0.638).
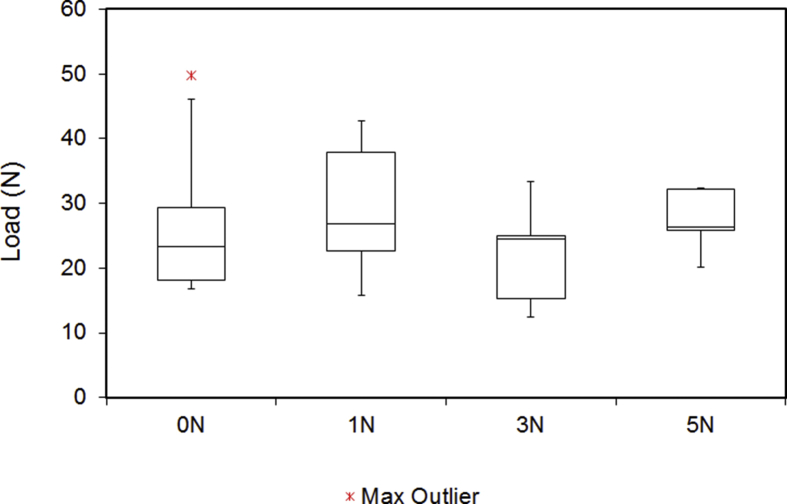
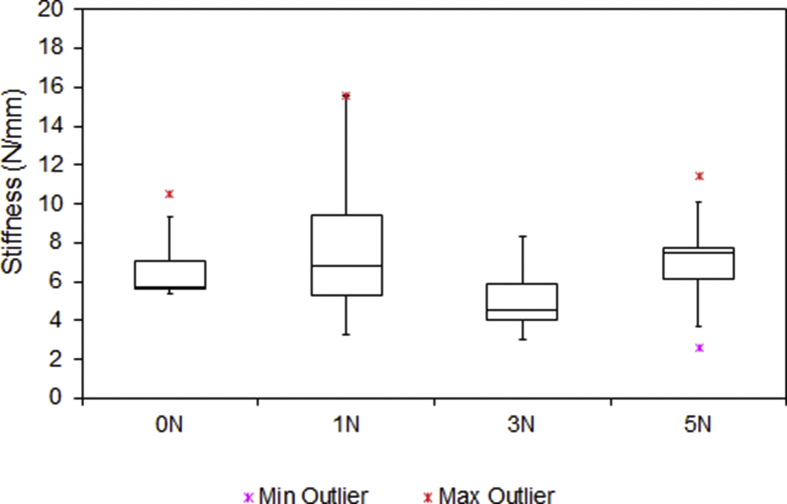
Fig. 5.
Stiffness of the digital nerves under tensile testing. Outliers are indicated by a red and purple star. No statistically significant difference was found within the groups (p = 0.593).
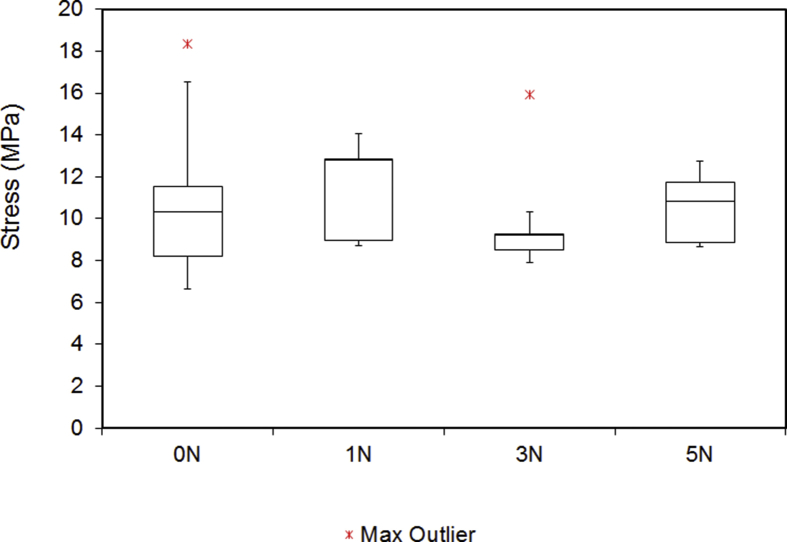
Fig. 6.
Stress of the digital nerves under tensile testing. Outliers are indicated by a red star. No statistically significant difference was found within the groups (p = 0.7).
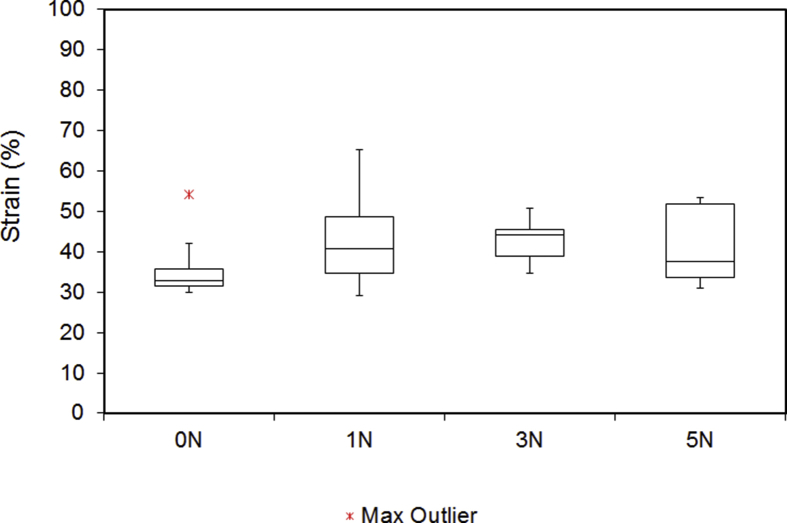
Fig. 7.
Strain of the digital nerves under tensile testing. Outliers are indicated by a red star. No statistically significant difference was found within the groups (p = 0.666).
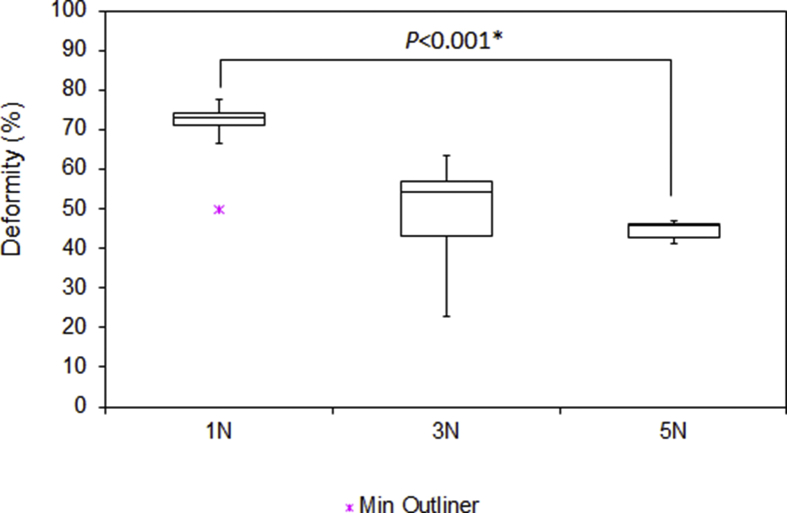
Fig. 8.
Deformity of the digital nerves after crush test. Outliers are indicated by a purple star. There is statistically significant difference within the groups (p < 0.001).
Table 1.
Digital nerves characteristics after crush test.
| Compression (N) | Ultimate Tensile Strength (N) | Stiffness (N/mm) | Maximum Stress (N/mm2) | Maximum Strain (%) | Deformity (%) |
|---|---|---|---|---|---|
| 0 | 23.3 | 5.7 | 10.34 | 32.9 | - |
| 1 | 26.8 | 6.8 | 12.79 | 40.6 | 72.9 |
| 3 | 24.4 | 4.5 | 9.21 | 44.1 | 54.2 |
| 5 | 26.3 | 7.5 | 10.84 | 37.6 | 45.9 |
The data are presented in median value.
The median UTS of digital nerves under different groups, ranged from 23.3 to 26.8N. There was no statistically significant difference in between the groups (p = 0.598). Similarly, applying compressive forces up to 5N had no significant effect on the stiffness (4.5–7.5N/mm) (p = 0.593), stress (9.21–12.79MPa) (p = 0.7) and strain (32.9–44.1%) (p = 0.666). These results showed that the biomechanical properties of digital nerves did not change as result of crush injury. However, it was noticed that the deformity of digital nerve was very sensitive to the compression. As shown in Fig. 8, the diameter of the digital nerve was reduced to 72.1% when the compressive force was set at 1N. As the compressive forces increased to 3N and 5N, the diameter of digital nerve further reduced to 54.2% and 45.9% respectively. The difference between groups were statistically significant (p < 0.001).
6. Discussion
Mechanical deformation of peripheral nerve has been classified as the primary mechanism in most of severe crush injury [1]. The neurophysiological function of nerve could be badly affected including the loss of both motor and sensory functions function for weeks with or without full recovery. In vivo studies conducted by Ochoa et al. [8] demonstrated that nerves degeneration occurred at the edges of the compressed area. Furthermore, ultrasonography images of nerves reveal that the axoplasm and myelin beneath the compressed area were pushed away from the point of greatest compression and toward the edges of compressed area. In this study, our findings showed that a low compressive force up to 5N for a short duration of 30sec is good enough to cause more than 50% deformity of the digital nerves. Therefore, it is suggested that the management of nerve crush injury shall be taken immediately and focus on neurophysiological function and degeneration of peripheral nerves for a crush with low compressive force and short duration.
In other biomechanical studies of nerve crushes, compressive forces of up to 150N were used to induce the crush injury of peripheral nerve [9, 10]. We are of the opinion that a compressive force above 5N is not necessary for nerve crust test because a deformity of digital nerve above 50% can be easily reproduced with a compressive force of 5N.
We are of the opinion that understanding the biomechanical properties of human digital nerve is important in order to provide a laboratory-based evidence for peripheral nerve repair studies. In this study, the median UTS of intact digital nerve was 23.3N. This is higher compared to the average failure load of 6N for human digital nerves (mean diameter, 1.82mm) previously reported by Goldberg et al. [11]; rabbit sciatic nerves with mean diameter of 2.52mm was 12.09N6. However, we are aware that the strain rate could affect the results of biomechanical strength and therefore it is suggested that the testing method shall be standardized and more experiments shall be carried out in order to evaluate the effect of strain rate.
Standardization of nerve crush test is important to evaluate the biomechanical integrity of peripheral nerve as a result of crush injury [12]. Beer et al. [10] listed the important parameters such as the duration and compressive force for conducting nerve crush test. They have developed a novel crushing device, in which the predetermined compressive force can be applied to the nerves. They also claimed that the device was capable of exerting different, standardized forces to crush a nerve within a scale of reproducing second-degree injuries. In our study, a pair of Kelly forceps and a customized anvil was developed in order to apply the compressive forces on the digital nerve accurately. We believe that our device will allow us to conduct more high quality experiments in order to evaluate the effect of crush duration.
The average diameter of digital nerves in this study was 1.7mm (±0.2), which was comparable to the average diameter of digital nerves (1.82mm (±0.3)) reported by Goldberg et al. [11]. They also found that there were no significant differences in terms of UTS and stiffness with respect to the intact radial or ulnar nerves within a finger or between fingers. Therefore, we conclude that the biomechanical properties of digital nerves were consistent among different fingers.
The major limitation in this study was the use of cadaveric digital nerves, which was unable to reflect the changes in neurophysiological function of digital nerve after compression. Future in vivo models including histology studies should aim to quantify the relationship between the deformity of the nerve and neurophysiological function. This study focused on the effect of different compressive forces on human digital nerves. More experiments shall be carried out in order to evaluate the duration of crush.
Our findings are limited to bare nerve crush injuries, in which the digital nerves were not exposed and freed from the surrounding soft tissue. However, a clinical cause of nerve crush injury could be the nerve damaged by a bone spike displaced at a severe fracture event. This compound fractures of the humerus can cause the radial nerve anatomically spirals around the bone. Under such circumstances, the nerve is effectively exposed from its anatomical coverings at the time of the crush [13, 14]. We believe that our study is still relevant to this clinical condition although the digital nerves were harvested solely from cadaveric hands.
7. Conclusions
Based on our results, we conclude that the nerve compression has insignificant effect on the UTS, stiffness, stress, and strain of digital nerves but its effect on the deformity of digital nerve was statistically significant. We also found that a compressive force less than 5N is sufficient to reproduce large mechanical deformation of digital nerve. For clinical practice, we suggest that the management of nerve crush injury shall be taken immediately and focus on neurophysiological function and degeneration of peripheral nerves for a crush with low compressive force and short duration.
Declarations
Author contribution statement
Yoke Rung Wong: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Xin Pang: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Zeus Yiwei Lim: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Hejun Du, Shian-Chao Tay: Contributed reagents, materials, analysis tools or data.
Duncan Angus McGrouther: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was funded by the Nanyang Technological University, facilitated by the NTU Institute for Health Technologies and the SingHealth Duke-NUS Academic Medical Centre, facilitated by the Joint Office of Academic Medicine; the Singapore Ministry of Health’s National Medical Research Council under its Centre Grant (NMRC/CG/M011/2017); the Surgery Academic Clinical Program grant (Biomechanics Lab Programme).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Burnett M.G., Zager E.L. Pathophysiology of peripheral nerve injury: a brief review. Neurosurg. Focus. 2004;16:1–7. doi: 10.3171/foc.2004.16.5.2. [DOI] [PubMed] [Google Scholar]
- 2.Dahlin L.B., Danielsen N., Ehira T., Lundborg G., Rydevik B. Mechanical effects of compression of peripheral nerves. J. Biomech. Eng. 1986;108:120–122. doi: 10.1115/1.3138590. [DOI] [PubMed] [Google Scholar]
- 3.Rydevik B., Nordborg C. Changes in nerve function and nerve fibre structure induced by acute, graded compression. J. Neurol. Neurosurg. Psychiatry. 1980;3:1070–1082. doi: 10.1136/jnnp.43.12.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beel J.A., Groswald D.E., Luttges M.W. Alterations in the mechanical properties of peripheral nerve following crush injury. J. Biomech. 1984;17:185–193. doi: 10.1016/0021-9290(84)90009-5. [DOI] [PubMed] [Google Scholar]
- 5.Abrams R.A., Butler J.M., Bodine-Fowler S., Botte M.J. Tensile properties of the neurorrhaphy site in the rat sciatic nerve. J. Hand Surg. 1998;23:465–470. doi: 10.1016/S0363-5023(05)80464-2. [DOI] [PubMed] [Google Scholar]
- 6.Mekaj A.Y., Morina A.A., Lajqi S., Manxhuka-Kerliu S., Kelmendi F.M., Duci S.B. Biomechanical properties of the sciatic nerve following repair: effects of topical application of hyaluronic acid or tacrolimus. Int. J. Clin. Exp. Med. 2015;8:20218. [PMC free article] [PubMed] [Google Scholar]
- 7.Borschel G.H., Kia K.F., Kuzon W.M., Dennis R.G. Mechanical properties of acellular peripheral nerve. J. Surg. Res. 2003;14:133–139. doi: 10.1016/s0022-4804(03)00255-5. [DOI] [PubMed] [Google Scholar]
- 8.Ochoa J., Danta G., Fowler T.J. Nature of the nerve lesion caused by a pneumatic tourniquet. Nature. 1971;233:265–266. doi: 10.1038/233265a0. [DOI] [PubMed] [Google Scholar]
- 9.Chen L.E., Seaber A.V., Glisson R.R. The functional recovery of peripheral nerves following defined acute crush injuries. J. Orthop. Res. 1992;10:657. doi: 10.1002/jor.1100100508. [DOI] [PubMed] [Google Scholar]
- 10.Beer G.M., Steurer J., Meyer V.E. Standardizing nerve crushes with a non-serrated clamp. J. Reconstr. Microsurg. 2001;17:531–534. doi: 10.1055/s-2001-17755. [DOI] [PubMed] [Google Scholar]
- 11.Goldberg S.H., Jobin C.M., Hayes A.G., Gardner T., Rosenwasser M.P., Strauch R.J. Biomechanics and histology of intact and repaired digital nerves: an in vitro study. J. Hand Surg. 2007;32:474–482. doi: 10.1016/j.jhsa.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 12.Bridge P.M., Ball D.J., Mackinnon S.E., Nakao Y., Brandt K., Hunter D.A., Hertl C. Nerve crush injuries—a model for axonotmesis. Exp. Neurol. 1994;27:284–290. doi: 10.1006/exnr.1994.1104. [DOI] [PubMed] [Google Scholar]
- 13.Livani B., Belangero W.D., Castro de Medeiros R. Fractures of the distal third of the humerous with palsy of the radial nerve: management using minimally-invasive percutaneous plate oesteosynthesis. J Bone Joint Surg Br. 2006;88:1625–1628. doi: 10.1302/0301-620X.88B12.17924. [DOI] [PubMed] [Google Scholar]
- 14.Runner Robert, Whicker Emily, De Sayan. Delayed radial nerve palsy after closed reduction of a pediatric humeral shaft fracture. Case Rep Orthop. 2017:9723497. doi: 10.1155/2017/9723497. [DOI] [PMC free article] [PubMed] [Google Scholar]