Summary
Aims
Although compelling evidence suggests that human hypothalamic hamartoma (HH) is intrinsically epileptogenic for gelastic seizures, the molecular mechanisms responsible for epileptogenesis within HH remain to be elucidated. The aim of this study was to test the hypothesis that hyperactivation of BDNF‐TrkB signaling pathways in surgically resected HH tissue is a possible mechanism for downregulation of KCC2 expression, which in turn underlies GABA‐mediated excitation within HH.
Methods
Activation of three major BDNF‐TrkB signaling pathways including MAPKs, Akt, and PLC γ1 were evaluated in surgically resected HH tissue (n = 14) versus human hypothalamic control tissue (n = 8) using combined methodologies of biochemistry, molecular biology, cell biology, and electrophysiology.
Results
Our data show that compared with hypothalamic control tissue, in HH tissue, (i) activation of TrkB and expression of mature BDNF are elevated; (ii) MAPKs (including ERK1/2, p38, and JNK), Akt, and PLC γ1 are highly activated; (iii) KCC2 expression is downregulated; and (iv) pharmacological manipulation of TrkB signaling alters HH neuronal firing rate.
Conclusion
Our findings suggest that multiple BDNF‐TrkB signaling pathways are activated in HH. They act independently or collaboratively to downregulate KCC2 expression, which is the key component for GABA‐mediated excitation associated with gelastic seizures.
Keywords: Brain‐derived neurotrophic factor, Epileptogenesis, Human hypothalamic hamartoma, Signal transduction, Tropomysin‐related kinase B
Introduction
Human hypothalamic hamartoma (HH) is a developmental malformation that occurs in the region of the tuber cinereum and inferior hypothalamus. HH is associated with diverse neurological and endocrine disorders, including epilepsy, cognitive impairment, behavioral disturbances, and central precocious puberty 1, 2. The epileptic syndrome in HH patients is often characterized by gelastic (laughing) seizures beginning in early infancy, followed by the development of additional seizure types, which are usually refractory to available anti‐epilepsy drugs (AEDs) and alternative therapies 2. HH is a human model of subcortical epilepsy 3. There is now compelling evidence that HH is intrinsically epileptogenic for gelastic seizures, including seizure recordings with surgically implanted electrodes and positive results for seizure control with surgical resection 4, 5. However, the molecular and cellular mechanisms responsible for epileptogenesis within HH are largely unknown.
Electrophysiological features of HH neurons suggest that gamma‐aminobutyric acid (GABA)‐mediated excitation plays an important role in epileptogenesis 6, 7, 8. Binding of GABA to its ionotropic GABAA receptor, a ligand‐gated Cl− channel, usually leads to the hyperpolarization of neuronal membranes via the influx of Cl− ions 9. However, GABA can exert an excitatory role in immature brain and in mature brain under certain pathophysiological conditions including epilepsy 10. The key mechanism underlying GABA‐induced neuronal excitation relates to the intracellular Cl− concentration and the transmembrane Cl− gradient. Maintenance of the Cl− gradient required for hyperpolarizing ionotropic responses (relatively low intracellular Cl− concentration) is attributable to the functional expression of the mature neuron‐specific K+‐Cl− cotransporter, KCC2 11. We have observed that most large HH neurons, which appear to be excitatory projection‐type pyramidal neurons, demonstrate depolarization and increased firing in response to GABA, in association with relatively low levels of KCC2 expression and elevated intracellular Cl− concentrations 6, 7, 12, 13. As a result, GABA exerts an excitatory role in these neurons, potentially contributing to seizure genesis. Here, we hypothesized that downregulation of KCC2 expression related to alterations in key intracellular signal transduction pathways in HH tissue that have not been previously investigated.
Brain‐derived neurotrophic factor (BDNF), a member of the family of neurotrophins, is essential for neuronal development and is implicated in the modulation of synaptic function and plasticity 14, 15. BDNF exerts its biological effects via its high‐affinity receptor, TrkB, a member of the tropomysin‐related kinase (Trk) family of the receptor tyrosine kinase (RTK) superfamily. BDNF binding leads to TrkB dimerization and intrinsic kinase activation. This results in trans‐autophosphorylation on multiple tyrosine residues within the intracellular domain of TrkB, which potentiates the receptor tyrosine kinase activity and creates docking sites for adaptor proteins, such as Shc and phospholipase Cγ (PLCγ). These adaptor proteins couple the receptor to several important intracellular signaling cascades, including the Ras‐mitogen‐activated protein kinase (MAPK), phosphoinositide 3‐kinase (PI3K)‐Akt, and PLCγ‐Ca2+ pathways 16, 17. These pathways regulate gene expression and protein translation 18.
It has been previously reported that TrkB activation can reduce GABAA receptor‐mediated responses of CA1 pyramidal cells 19, 20. One possible mechanism by which TrkB impairs GABAergic inhibition is through downregulation of KCC2 expression. This is supported by the following findings: (i) BDNF‐mediated TrkB activation resulted in reduced KCC2 expression and impaired neuronal Cl− extrusion in rat hippocampal slice cultures; (ii) in kindled mice, KCC2 expression was downregulated in hippocampus with a spatio‐temporal profile complementary to the upregulation of BDNF and TrkB; (iii) hippocampal slices derived from transgenic trkB mutant mice (trkB SHC/SHC and trkB PLC/PLC) suggested that activation of both Shc and PLCγ signaling cascades is required for TrkB‐mediated downregulation of KCC2 11, 21, 22.
In this study, we aimed to test the hypothesis that hyperactivation of BDNF‐TrkB signaling pathways in surgically resected HH tissue is a possible mechanism for downregulation of KCC2 expression, which in turn underlies GABA‐mediated excitation within HH.
Materials and Methods
Clinical Profiles of Study Subjects
The use of surgically resected tissue in this study was approved by the Institutional Research Board (IRB) of St. Joseph's Hospital and Medical Center. HH tissue was extracted from 14 patients (mean age 8.5 years, range 0.7 to 40.8 years; 7 [50%] were female). All patients had treatment‐resistant epilepsy and had failed at least three AEDs. The mean age for seizure onset was 5.6 months (range 1 to 18 months; 7 [50%] had first seizure activity before one month of age). At the time of surgery, 6 (43%) had only gelastic seizures, while 8 (57%) had multiple seizure types. All patients had gelastic seizures at some point during their clinical course. Twelve patients (86%) experienced at least one seizure every day, while two patients (14%) had at least one seizure every month. At surgery, nine patients (64%) were taking at least 2 AEDs (range 0 to 4 AEDs). Prior treatment for epilepsy had included gamma knife radiosurgery in three patients (21%). Seven patients (50%) had a history of intellectual disability (intelligence quotient [IQ] or developmental quotient [DQ] <50). Four patients (29%) had a history of central precocious puberty. None of the patients in this cohort had Pallister‐Hall syndrome or other identified syndromes. The mean HH lesion volume was 3.2 cm3 (range 0.2 to 20 cm3). All HH tissue used in Western blot analysis was snap frozen on dry ice immediately after surgical resection and stored at −80°C until assay.
Human Control Tissue
Postmortem (frozen) hypothalamic tissue derived from 8 normal human donors (two females and six males) was obtained from the Harvard Brain Tissue Resource Center, the Human Brain & Spinal Fluid Resource Center (West Los Angles Healthcare Center), and the University of Maryland. Brain tissue was collected within 4 h after death. The mean age was 39 years, range 12.7–78 years.
Antibodies and Reagents
Primary antibodies used are detailed in Table S1. The Trk kinase inhibitor K252a was from EMD Millipore.
RNA Extraction and Real‐Time RT‐PCR
Total RNAs were extracted from tissue using QIAzol Lysis Reagent (QIAGEN, Valencia, CA, USA). cDNA synthesis and quantitative PCR were performed as previously described 6, 7, 23.
Protein Extraction, SDS‐PAGE, and Immunoblotting
Tissue samples (~50 mg) were dissected from frozen sections, weighed, thawed on ice, and lysed in ice‐cold RIPA buffer (25 mM Tris‐HCl (pH 7.6), 150 mM NaCl, 1% NP‐40, 1% sodium deoxycholate, and 0.1% SDS) containing protease and phosphatase inhibitors 24. Following homogenization with plastic pestles that tightly fit microtubes, the homogenates were sheared 5 times by passing through single‐use needles (0.4 × 13 mm) (Becton Dickinson, Franklin Lakes, NJ, USA) and solubilized for 1 h on ice. After centrifugation, the supernatants (protein extracts) were collected, quantitated and resolved on SDS‐PAGE 23, 24. Immunoblotting with antibodies (Table S1) was performed using standard procedures. Immunoblotting signals were detected with SuperSignal chemiluminescent substrate (Pierce) and images were captured using a Kodak 4000 MM molecular imager 23, 25, 26.
Immunohistochemical Staining
Tissue sections were prepared and immunostaining with a goat anti‐KCC2 antibody (R‐14) (Santa Cruz Biotechnology) was performed as described previously 6, 8.
Patch‐Clamp Recordings in HH Slices
Acute HH slices were prepared as previously described 6, 7, 8. The conventional whole‐cell recordings were made using a patch‐clamp amplifier (Multiclamp 700A, Axon Instruments, Molecular Devices, Sunnyvale, CA, USA) under infrared‐DIC (differential interference contrast) microscopy. The patch‐pipette solution contained (in mM): 140 K+‐gluconate, 5 KCl, 10 HEPES, 0.2 EGTA, 2 MgCl2, 4 MgATP, 0.3 Na2GTP and 10 Na2‐phosphocreatine (pH7.3 with KOH). HH neuron action potential was recorded in current‐clamp mode. Whole‐cell access resistance <20 MΩ was accepted for experiments. The series resistance was not compensated in this study. Typically, data were acquired at 10 kHz, filtered at 2 kHz, displayed and digitized online (Digidata 1322 series A/D board, Axon Instruments), and stored to a hard drive. Data acquisition and analyses of action potential firing using patch‐clamp whole‐cell recording in current‐clamp mode were done using Clampex 9.2 and Clampfit 9.2 (Axon Instruments), respectively, and results were plotted using Origin 5.0 (Microcal, OriginLab, Northampton, MA, USA). The standard external solution contained (in mM): 150 NaCl, 5 KCl, 1 MgCl2, 2 CaCl2, 10 glucose and 10 HEPES (pH adjusted to 7.4 with Tris‐base). All experiments were performed at room temperature (22 ± 1°C).
Densitometry and Statistical Analysis
Densitometric quantification of digital immunoblotting images was performed using Kodak Molecular Imaging Software (Version 4.0). Data were given as mean ± SEM. The significance (P value) was calculated with analysis of variance (ANOVA) or paired or unpaired t‐tests when applicable. Pearson correlation analysis was performed using GraphPad Prism (Version 5.0) to evaluate the relationships among the variables of interest. P < 0.05 was considered statistically significant.
Results
Enhanced Overall Tyrosine Phosphorylation Signals in HH Tissue
Tyrosine phosphorylation is one of the key steps in intracellular signal transduction and regulation of enzymatic activity in eukaryotic cells, and perturbations in tyrosine phosphorylation underlie many human diseases 27, 28. We sought to assess the overall tyrosine phosphorylation levels in both HH tissue and normal human hypothalamus (control) by immunoblotting analysis.
Proteins extracted from HH (n = 14) and control (n = 8) tissue were resolved on SDS‐PAGE and further analyzed by immunoblotting with a mouse monoclonal anti‐phosphotyrosine antibody (pTyr, 4G10). This antibody is very sensitive and reliable for detection of tyrosine phosphorylation and has been successfully used in our previous studies 24, 25, 26. Our results indicated that the overall phosphorylation level in HH tissue was markedly higher than in control tissue (Fig. S1).
This experimental approach utilizes quantitative assessment of phosphorylated protein levels relative to total protein levels by densitometric analysis of immunoblotting results. This technique has been successfully employed in our previous studies 23, 24, 25, 26. However, the impact of postmortem interval (PMI) on protein phosphorylation and total protein levels could be a concern given the nature of tissue collection. To address this, we performed a set of immunoblotting experiments using rat brain tissues collected 1–4 h after death of the animals at room temperature (22 ± 1°C). Representative images of immunoblotting with anti‐active‐ERK1/2 (pERK1/2) and anti‐total ERK1/2 blots are shown in Fig. S2. Both antibodies have been well documented in our previous studies 23, 24, 26. Our results consistently demonstrated that PMI had very little effect on both protein phosphorylation and total protein levels at least within the initial 4 h after death (Fig. S2). This ensures the accuracy of our immunoblotting data throughout the study.
Elevated Phosphorylation of TrkB and Expression of BDNF in HH Tissue
Increased overall phosphorylation in HH versus control tissue prompted us to further examine BDNF‐TrkB signaling. We first assessed TrkB phosphorylation by immunoblotting with an anti‐phospho‐TrkB (Tyr816) (pTrkB) antibody, and detected enhanced immunoreactive signals in HH versus control group (Figure 1A). In contrast, the expression of TrkB was detected by anti‐TrkB antibody in all samples (Figure 1A). The levels of pTrkB and total TrkB were further estimated by densitomeric analyses (Figure 1B,C). Our results showed that TrkB phosphorylation was significantly enhanced in HH compared with the control (P < 0.05) (Figure 1B).
Figure 1.

Enhanced BDNF‐TrkB signaling in HH. (A–D) Elevated phosphorylation of TrkB in HH. Total proteins were extracted from HH and normal human hypothalamic control tissue specimens and equal amounts of proteins were subjected to SDS‐PAGE and analyzed by immunoblotting with anti‐phospho‐TrkB (Tyr816) (pTrkB) and anti‐TrkB, respectively (A). Densitometric analysis results of immunoblotting data of anti‐pTrkB (B) and anti‐TrkB (C), respectively, from 14 HH and 8 controls are shown. (D) Elevated BNDF expression (mRNA) in HH. Total RNAs were extracted from HH (n = 16) and control (n = 3) tissue specimens and used for real‐time RT‐PCR analysis to measure the mRNA level of mature BDNF relative to that of human GAPDH. *P < 0.05; **P < 0.01.
The elevated phosphorylation of TrkB could be due to excessive expression of BDNF (the specific ligand for TrkB) in HH tissue. To test this, we compared the mRNA levels of mature BDNF between HH and control groups by real‐time RT‐PCR. As in Figure 1D, when normalized to the mRNA level of a housekeeping gene (GAPDH), the expression level of BDNF in HH was significantly higher than that in the control group (P < 0.01).
Hyperactivation of MAPKs in HH Tissue
We next sought to assess the TrkB downstream signaling cascades. MAPKs are a family of serine/threonine kinases that play important roles in signal transduction initiated by RTKs including Trks 29. MAPKs mainly include ERK1/2 (p44/p42 MAPKs), c‐Jun N‐terminal kinases (JNKs), and p38 MAPK 29. Thus, we examined the activation status of these three major MAPKs. Immunoblotting with anti‐active p38 (p‐p38), anti‐active JNK1 (pJNK1), and anti‐active ERK1/2 (pERK1/2), respectively, revealed that each antibody detected corresponding active forms of MAPKs present in HH tissue (Figure 2A). In contrast, the signals were almost negligible in all controls. Immunoblotting with anti‐ERK antibody confirmed the equal protein loading (Figure 2A). Densitometric analyses further revealed that the respective pJNK1, p‐p38, and pERK (but not ERK) levels were significantly higher in HH versus control tissue (p‐p38, P < 0.05; pJNK1, P < 0.01; pERK1/2, P < 0.05) (Figure 2B–E). These findings suggest that all three major MAPK pathways were highly activated in HH tissue.
Figure 2.
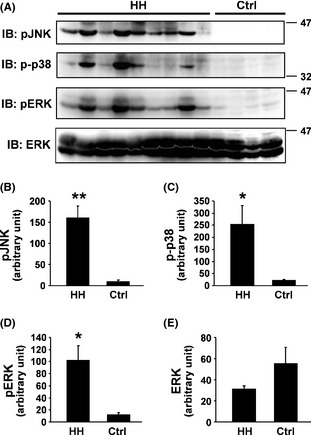
Hyperactivation of MAPKs in HH. Proteins extracted from HH and control tissue specimens were analyzed by immunoblotting with anti‐active JNK (pJNK), anti‐active p38 MAPK (p‐p38), anti‐active ERK (pERK), and anti‐total ERK, respectively (A). Densitometric analysis results of immunoblotting data of anti‐pJNK (B), anti‐p‐p38 (C), anti‐pERK (D), and anti‐ERK (E), respectively, from 14 HH and eight controls are shown. *P < 0.05; **P < 0.01.
Interrelationship Between Active Akt and PTEN in HH Tissue
In parallel with the MAPK pathways, Akt is another key signaling molecule downstream of RTKs 30. Akt is a serine/threonine kinase and its activity can be impacted by the tumor suppressor PTEN (phosphatase and tensin homolog deleted on chromosome 10), which is upstream of Akt. PTEN is considered as the primary negative regulator of Akt 31. Loss of PTEN or PTEN mutation is the most common cause of hyperactivation of Akt signaling in many human diseases 32. Here, we assessed the Akt activation, PTEN expression, and their interrelationship by immunoblotting with anti‐phospho‐Akt (pAkt), anti‐Akt, and anti‐PTEN, respectively, combined with densitometric analyses (Figure 3). Our results showed that the pAkt (but not Akt) level was significantly higher in HH than that in the control group (P < 0.05) (Figure 3A–C). Surprisingly, the PTEN level in HH was also higher than that in the control (P < 0.05) (Figure 3A,D).
Figure 3.
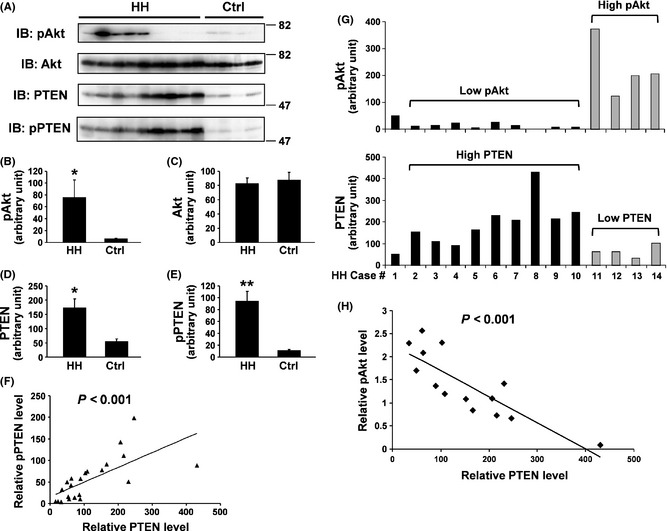
Profiles of Akt activation and PTEN expression in HH. (A) Proteins extracted from HH and control tissue specimens were analyzed by immunoblotting with anti‐phospho‐Akt (pAkt), anti‐total Akt, anti‐total PTEN, and anti‐phospho‐PTEN (pPTEN), respectively. (B–E) Densitometric analysis results of immunoblotting data of anti‐pAkt (B), anti‐Akt (C), anti‐PTEN (D), and anti‐pPTEN (E), respectively, from 14 HH and eight controls are shown. *P < 0.05; **P < 0.01. (F) Correlation analysis indicates a positive relationship between pPTEN and total PTEN levels. (G) Within HH group, pAkt levels show a negative relationship with PTEN levels. In this display, the HH subject No. 1 was used as a standard for the purpose of densitometric analyses with its mass set as 50. The HH subjects No. 2–10 had relatively lower pAkt but higher PTEN. Conversely, the HH subjects No. 11–14 had relatively higher pAkt but lower PTEN. (H) Correlation analysis confirms the negative relationship between pAkt and PTEN within HH group.
It is known that PTEN possesses a C‐terminal, noncatalytic regulatory domain with three phosphorylation sites (Ser380, Thr382, and Thr383) that regulate PTEN stability and may affect its biological activity 33. To confirm our findings of PTEN expression herein, we performed immunoblotting with an anti‐phospho‐PTEN (Ser380/Thr382/Thr383) (pPTEN) antibody (Figure 3A) and subsequent quantification (Figure 3E). The results indicated that the pPTEN level was indeed higher in HH tissue compared with the control (Figure 3E). Further, pPTEN positively correlated with PTEN (P < 0.001) (Figure 3F). Collectively, our data suggested that for the comparison between the two groups (HH versus control), the pAkt level did not exhibit a negative relationship with the PTEN level as expected. Rather, both were higher in the HH group than those in the control group. However, when comparing the pAkt and PTEN levels within the HH group (n = 14), we uncovered that pAkt displayed a negative relationship to PTEN. It seemed that the HH group could be classified into two subgroups, Low pAkt‐High PTEN and High Akt‐Low PTEN (Figure 3G). Correlation analysis further confirmed such an inverse interrelationship (P < 0.001) (Figure 3H).
Increased PLCγ1 Phosphorylation and CaMKII Expression in HH Tissue
The PLCγ/CaMKII (Ca2+/calmodulin‐dependent kinase II) pathway is one of the major pathways that are activated upon BDNF binding to TrkB 16, 17. Functional expression of CaMKII is regulated by Ca2+/calmodulin complex downstream of PLCγ 16. The increased TrkB phosphorylation at Tyr816 (the docking site for PLCγ) in HH versus control tissue (Figure 1) prompted us to further assess this pathway by immunoblotting followed by desitometric analyses. The results indicated that PLCγ1 phosphorylation was significantly enhanced in HH versus control tissue (P < 0.001) (Figure 4A,B). Our results also revealed that the anti‐CaMKII (pan) antibody detected two isoforms of CaMKII, including CaMKII‐α (~50Kd) and CaMKII‐β (~60Kd), and both were significantly upregulated in HH versus control tissue (P < 0.01) (Figure 4C,D). As expected, correlation analysis confirmed the positive correlation between PLCγ1 phosphorylation and CaMKII expression (P < 0.01) (Figure 4E).
Figure 4.

Enhanced PLCγ1‐CaMKII signaling in HH. (A and B) Proteins extracted from HH and control tissue specimens were analyzed by immunoblotting with anti‐phospho‐PLC γ1 (pPLC γ1) and anti‐β‐actin, respectively (A). Densitometric analysis results of immunoblotting data of anti‐pPLC γ1 from 14 HH and eight controls are shown (B). (C and D) Proteins extracted from HH and control tissue specimens were analyzed by immunoblotting with anti‐CaMKII and anti‐ERK, respectively (C). Densitometric analysis results of immunoblotting data of anti‐CaMKII from 14 HH and eight controls are shown (D). **P < 0.01. (E) Correlation analysis indicates a positive relationship between CaMKII and pPLC γ1 levels.
Downregulation of KCC2 in HH Tissue
It has been previously reported that the expression of the K+‐Cl− cotransporter KCC2 in adult rat neurons is downregulated following epileptiform activity and/or neuronal damage by activation of BDNF‐TrkB signaling 21, 22. Here, we asked whether KCC2 expression was altered in HH in comparison with control tissue. The immunoblotting and densitometric analysis results indicated that the KCC2 level in HH tissue was significantly lower than that in the control (P < 0.01) (Figure 5A,B). Likewise, immunostaining in tissue section slices demonstrated strong anti‐KCC2 immunoreactivity in controls versus weak signals in HH tissue (Figure 5C, arrows).
Figure 5.
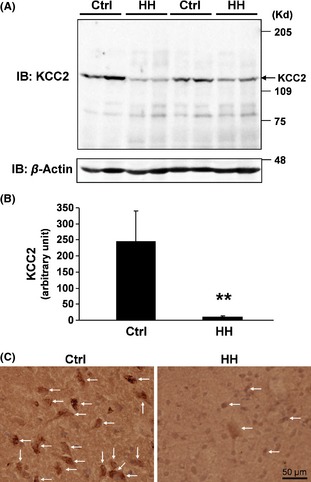
Downregulation of KCC2 in HH. (A and B) Proteins extracted from HH and control tissue specimens were analyzed by immunoblotting with anti‐KCC2 and anti‐β‐actin, respectively (A). Densitometric analysis results of immunoblotting data of anti‐KCC2 from 14 HH and eight controls are shown (B). **P < 0.01. (C) Immunostaining with an anti‐KCC2 antibody in tissue sections (arrows) indicates downregulated KCC2 expression in HH versus control tissue. Bar, 50 μm.
Pharmacological Manipulation of TrkB Signaling Alters HH Neuronal Firing Rate
As above, we have demonstrated that the BDNF‐TrkB signaling is upregulated, and concomitantly, KCC2 expression is downregulated in HH compared with the control tissue. These findings suggest that activation of BDNF‐TrkB signaling may underlie the KCC2 downregulation and thereby GABA‐mediated excitation in HH. If this is true, HH neuronal firing would be altered if the BDNF‐TrkB signaling is activated or blocked.
We tested this possibility using patch‐clamp recordings in freshly prepared HH slices. As shown in Figure 6A, patch‐clamp current‐clamp recording showed that a large‐sized HH neuron exhibited spontaneous, irregular action potential (AP) firing at a low firing rate (<3 Hz). Bath application of BDNF (10 ng/mL) increased the HH neuronal firing (Figure 6B). In five large HH cells tested (from five HH patients), 2 min exposure to BDNF increased AP firing rate from 2.02 ± 0.75 Hz to 5.06 ± 0.89 Hz (P < 0.01, paired t‐test). Then, we tested the effect of blockade of BDNF‐TrkB signaling on HH AP firing. K252a is a selective inhibitor of Trk kinase activity 34, and has been widely used to verify BDNF/TrkB‐mediated effects 15, 35. Our results showed that bath application of K252a (10 μM) reduced the AP firing rate in a large (Figure 6C), but not in a small HH neuron (Figure 6D). Statistical analysis showed that exposure to K252a for 2 min reduced the AP firing from 3.34 ± 0.49 Hz to 1.98 ± 0.33 Hz (P < 0.01, paired t‐test, n = 5 from five patients). Together, these results suggest that the BDNF‐TrkB signaling plays an important role in modulation of HH neuronal function.
Figure 6.
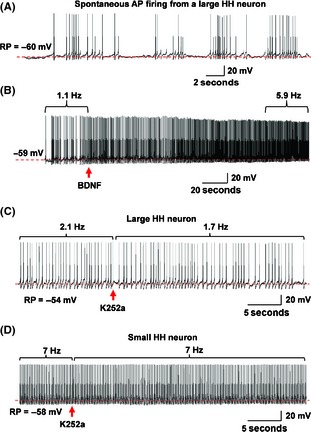
Alteration of HH neuronal firing by pharmacological manipulation of BDNF‐TrkB signaling. (A) Typical action potential (AP) firing from a large HH neuron showing spontaneous and irregular firing at low frequency as we previously reported 8. (B) Bath application of BDNF (10 ng/mL) enhanced AP firing. (A and B) were recorded from the same neuron. (C, D) Blockade of TrkB signaling by K252a (10 μM) reduced neuronal firing rate in a large (C), but not in a small (D) HH neuron. (A–D are representative traces from 10 HH neuron recordings (from 10 HH patients).
Discussion
The goal of our research is to develop a cellular and molecular model for seizure genesis within HH. Earlier work on surgically resected HH tissue focused on characterizing the phenotype, including electrophysiological properties, of the neurons within HH tissue 6, 7, 8, 12, 36. We uncovered that most large HH neurons have the functionally immature property of depolarizing and firing in response to GABA agonists as a result of elevated intracellular Cl− concentration 6, 12, 13. These neurons also show decreased KCC2 expression (relative to small HH neurons) at both mRNA and protein levels 6, 12. These data suggest that downregulated KCC2 expression may be causative for depolarizing and excitatory GABA responses in HH. In the present study, we explore intracellular signal transduction pathways potentially related to KCC2 downregulation in HH tissue. Our data show that compared with normal human hypothalamic control tissue, in HH tissue, (i) activation of TrkB and expression of mature BDNF are elevated; (ii) three major BDNF‐TrkB signaling pathways, including MAPKs, Akt, and PLCγ1, are highly activated; and (iii) KCC2 expression is downregulated.
There is no animal model for HH, and consequently research on the cellular mechanisms resulting in epileptogenesis of HH relies upon the use of surgically resected human tissue. Obtaining control material is problematic, as there is a very high surgical premium on avoiding resection of adjacent tissue when resecting lesions within the hypothalamus. We make use of human autopsy control material as the best alternative under these circumstances. Our data suggest that protein expression and phosphorylation levels are stable during the postmortem time interval necessarily encountered for control tissue of this nature.
To our knowledge, this is the first report of comprehensive profiles of the BDNF‐TrkB signaling system in HH, from the expression level of the ligand (BDNF) to phosphorylation/activation status of the receptor (TrkB) and its downstream signaling cascades. Our findings of increased BDNF mRNA levels and enhanced TrkB phosphorylation in HH tissue are consistent with other epilepsy models. It has been previously shown that BDNF is upregulated in multiple brain regions implicated in epileptogenesis, including hippocampus, neocortex, and ventromedial hypothalamus 37, 38, 39. Similarly, seizures induced by hippocampal kindling lead to increased expression of TrkB, but not TrkA and TrkC, in the hippocampus 40. Conditional neuron‐specific deletion of TrkB prevents chronic seizures in the kindling model 41.
We have determined that multiple signaling pathways downstream to BDNF‐TrkB binding are highly activated in HH tissue. TrkB‐mediated cellular changes are likely to occur via redundant activation of multiple pathways during normal development 42. The details of the link between TrkB activation and downregulation of KCC2 expression are incompletely understood. It is now generally believed that BDNF binding to TrkB activates three major intracellular signaling pathways, including PLCγ‐CaMKII, Shc‐Ras‐MAPK, and Shc‐PI3K‐Akt cascades 16, 17 (Figure S3). Rivera et al. reported that both PLCγ‐ and Shc‐mediated signaling cascades were required for BDNF‐induced KCC2 downregulation 22. Surprisingly, they showed that in trkB PLC/PLC mice with deletion of the PLCγ‐coupled pathway, BDNF rather caused upregulation of KCC2 22. The discrepancy of results from these studies could be related to the status of each or both of PLCγ‐ and Shc‐coupled signaling pathways in the context of the actual experimental settings. Rivera et al. suggested that downregulation of KCC2 seemed to take place if the Shc pathway was activated in conjunction with the PLCγ pathway, whereas in the absence of the PLCγ signaling KCC2 upregulation was initiated by the Shc signaling 22. In the current study, we demonstrate that compared with the control tissue, the Shc‐mediated cascades (both Ras‐MAPK and PI3K‐Akt) and the PLCγ‐CAMKII cascade are highly activated in HH tissue. Concomitantly, KCC2 expression is downregulated. Although at this stage we cannot dissect these pathways in individual neurons or specific cell types within the tissue, our results mainly favor the opinion that both Shc‐ and PLCγ‐coupled pathways are involved in regulation of KCC2 expression.
We have hypothesized that BDNF‐TrkB activation and downregulation of KCC2 expression contributes to epileptogenesis in HH tissue. However, other consequences of activating TrkB deserve consideration, since each of the activated intracellular signaling pathways has been associated with seizures and epilepsy. The ERK MAPK signaling pathway is a highly conserved kinase cascade that has been linked to epileptogenesis in several animal models 43, 44, 45, 46, 47, 48. In this study, we show that ERK1 and EKR2 are hyperactivated in HH tissue compared with the normal control. Concomitantly, both BDNF expression and TrkB phosphorylation are enhanced in the HH tissue. Our results are in agreement with those from several previous studies. Nateri et al. reported that active ERKs are predominantly localized to the stratum lucidum in the hippocampus 48. Immunhistochemical evidence indicated that although ubiquitously expressed throughout the brain, during epileptogenesis, TrkB undergoes phosphorylation at the mossy fiber synapse, and phosphorylated TrkB is specifically detected in the stratum lucidum 49, 50. The concomitant activation of TrkB and ERKs and their localization in the same anatomical region of the brain suggest that activation of the TrkB‐Ras‐ERK cascade is probably a key pro‐epileptogenic signaling event.
The p38 and JNKs are also well‐known MAPKs. Both p38‐ and JNK‐directed signaling have been involved in pathological conditions including epilepsy, possibly via regulation of neuronal cell death 51, 52, 53. However, compared with the BDNF‐TrkB‐Ras‐ERK cascade, little is known about the roles of p38 and JNKs as downstream effectors of BDNF/TrkB in neuronal signal transduction 16. We find increased activation of ERKs, p38 and JNK1 in HH versus control hypothalamus, suggesting that MAPK‐regulated intracellular signals may participate in HH pathogenesis in some as‐yet unknown manner.
The PI3K‐Akt signaling pathway plays a vital role in determining cell fate and contributes to the onset and progression of many neurological disorders, including seizures and epilepsy 54, 55. Akt is a serine/threonine kinase that is constitutively inhibited by PTEN and has downstream effectors that include mammalian target of rapamycin (mTOR) 55, 56, 57. Chronic hippocampal infusion of rapamycin (the mTOR kinase inhibitor) blocks mossy fiber sprouting in a rodent pilocarpine model of temporal lobe epilepsy 57. In this study, we find that HH tissue levels of active Akt (pAkt) and total PTEN display a negative interrelationship (as expected). This suggests that a further classification of HH cases into two subgroups (Low pAkt‐High PTEN and High pAkt‐Low PTEN) is possible. The significance of this difference between different patients is unclear, but is noteworthy as other anatomical or physiological features which differentiate HH tissue have been elusive.
Although the detailed mechanisms are still unclear, our data at least offer an explanation of how BDNF‐TrkB signaling is linked to KCC2 downregulation and GABAA receptor‐mediated neuronal excitation within the HH lesion. Based on our recent findings 6, 7, 8, 36 and the new data presented in this study, we hypothesize that within the HH tissue, excessive BDNF activates TrkB, causing hyperactivation of multiple downstream signaling pathways, including the PLCγ‐CaMKII, the Shc‐Ras‐MAPK (ERKs, p38 and JNK), and the Shc‐PI3K‐Akt cascades (Fig. S3). These pathways may act independently or collaboratively to downregulate KCC2 expression, which is the key component for GABA‐mediated excitation associated with gelastic seizures. Furthermore, we demonstrate that activation of BDNF‐TrkB signaling (by BDNF) increase large HH neuron firing rate, while pharmacological inhibition of TrkB kinase activity markedly reduces the large, but not small, HH neuronal firing rate. Considering that large HH neurons have lower KCC2 expression and higher intracellular Cl− concentration 6, the BDNF‐TrkB signaling appears to more specifically modulate large HH neuronal function, which supports our hypothesis.
Conflict of Interest
The authors declare no conflict of interests.
Supporting information
Figure S1. Enhanced tyrosine phosphorylation in HH.
Figure S2. Postmortem interval (PMI) has no significant impact on protein phosphorylation and expression.
Figure S3. Proposed model of how BDNF‐TrkB signaling contributes to KCC2 downregulation and GABA‐mediated excitation and epileptogenesis.
Table S1. Lists of primary antibodies used in the study.
Acknowledgments
This work was supported by an Arizona Biomedical Research Commission Award (ADHS13‐031259) and partially by a National Institutes of Health Award (R01GM085237). We thank the Harvard Brain Tissue Resource Center, the Human Brain & Spinal Fluid Resource Center (West Los Angles Healthcare Center), and the University of Maryland for providing normal human hypothalamic tissue. We are grateful to our HH patients and their families for their willingness to allow scientific use of surgically resected tissue.
References
- 1. Deonna T, Ziegler AL. Hypothalamic hamartoma, precocious puberty and gelastic seizures: A special model of “epileptic” developmental disorder. Epileptic Disord 2000;2:33–37. [PubMed] [Google Scholar]
- 2. Berkovic SF, Andermann F, Melanson D, Ethier RE, Feindel W, Gloor P. Hypothalamic hamartomas and ictal laughter: Evolution of a characteristic epileptic syndrome and diagnostic value of magnetic resonance imaging. Ann Neurol 1988;23:429–439. [DOI] [PubMed] [Google Scholar]
- 3. Kerrigan JF, Ng YT, Chung S, Rekate HL. The hypothalamic hamartoma: A model of subcortical epileptogenesis and encephalopathy. Semin Pediatr Neurol 2005;12:119–131. [DOI] [PubMed] [Google Scholar]
- 4. Kuzniecky R, Guthrie B, Mountz J, et al. Intrinsic epileptogenesis of hypothalamic hamartomas in gelastic epilepsy. Ann Neurol 1997;42:60–67. [DOI] [PubMed] [Google Scholar]
- 5. Ng YT, Rekate HL, Prenger EC, et al. Transcallosal resection of hypothalamic hamartoma for intractable epilepsy. Epilepsia 2006;47:1192–1202. [DOI] [PubMed] [Google Scholar]
- 6. Wu J, DeChon J, Xue F, et al. GABA(A) receptor‐mediated excitation in dissociated neurons from human hypothalamic hamartomas. Exp Neurol 2008;213:397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wu J, Chang Y, Li G, et al. Electrophysiological properties and subunit composition of GABAA receptors in patients with gelastic seizures and hypothalamic hamartoma. J Neurophysiol 2007;98:5–15. [DOI] [PubMed] [Google Scholar]
- 8. Wu J, Xu L, Kim DY, et al. Electrophysiological properties of human hypothalamic hamartomas. Ann Neurol 2005;58:371–382. [DOI] [PubMed] [Google Scholar]
- 9. Chang Y, Wang R, Barot S, Weiss DS. Stoichiometry of a recombinant GABAA receptor. J Neurosci 1996;16:5415–5424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nardou R, Ferrari DC, Ben‐Ari Y. Mechanisms and effects of seizures in the immature brain. Semin Fetal Neonatal Med 2013;18:175–184. [DOI] [PubMed] [Google Scholar]
- 11. Rivera C, Voipio J, Payne JA, et al. The K+/Cl‐ co‐transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature 1999;397:251–255. [DOI] [PubMed] [Google Scholar]
- 12. Kim DY, Fenoglio KA, Simeone TA, et al. GABAA receptor‐mediated activation of L‐type calcium channels induces neuronal excitation in surgically resected human hypothalamic hamartomas. Epilepsia 2008;49:861–871. [DOI] [PubMed] [Google Scholar]
- 13. Kim DY, Fenoglio KA, Kerrigan JF, Rho JM. Bicarbonate contributes to GABAA receptor‐mediated neuronal excitation in surgically resected human hypothalamic hamartomas. Epilepsy Res 2009;83:89–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Binder DK, Scharfman HE. Brain‐derived neurotrophic factor. Growth Factors 2004;22:123–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bramham CR, Messaoudi E. BDNF function in adult synaptic plasticity: The synaptic consolidation hypothesis. Prog Neurobiol 2005;76:99–125. [DOI] [PubMed] [Google Scholar]
- 16. Huang EJ, Reichardt LF. Trk receptors: Roles in neuronal signal transduction. Annu Rev Biochem 2003;72:609–642. [DOI] [PubMed] [Google Scholar]
- 17. McNamara JO, Huang YZ, Leonard AS. Molecular signaling mechanisms underlying epileptogenesis. Sci STKE 2006;2006:re12. [DOI] [PubMed] [Google Scholar]
- 18. Cunha C, Brambilla R, Thomas KL. A simple role for BDNF in learning and memory? Front Mol Neurosci 2010;3:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kim HG, Wang T, Olafsson P, Lu B. Neurotrophin 3 potentiates neuronal activity and inhibits gamma‐aminobutyratergic synaptic transmission in cortical neurons. Proc Natl Acad Sci U S A 1994;91:12341–12345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tanaka T, Saito H, Matsuki N. Inhibition of GABAA synaptic responses by brain‐derived neurotrophic factor (BDNF) in rat hippocampus. J Neurosci 1997;17:2959–2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rivera C, Li H, Thomas‐Crusells J, et al. BDNF‐induced TrkB activation down‐regulates the K+–Cl− cotransporter KCC2 and impairs neuronal Cl− extrusion. J Cell Biol 2002;159:747–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rivera C, Voipio J, Thomas‐Crusells J, et al. Mechanism of activity‐dependent downregulation of the neuron‐specific K‐Cl cotransporter KCC2. J Neurosci 2004;24:4683–4691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shi C, Lu J, Wu W, et al. Endothelial cell‐specific molecule 2 (ECSM2) localizes to cell‐cell junctions and modulates bFGF‐directed cell migration via the ERK‐FAK pathway. PLoS ONE 2011;6:e21482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Huang Y, Li X, Jiang J, Frank SJ. Prolactin modulates phosphorylation, signaling and trafficking of epidermal growth factor receptor in human T47D breast cancer cells. Oncogene 2006;25:7565–7576. [DOI] [PubMed] [Google Scholar]
- 25. Ma F, Wei Z, Shi C, et al. Signaling cross talk between growth hormone (GH) and insulin‐like growth factor‐I (IGF‐I) in pancreatic islet β‐cells. Mol Endocrinol 2011;25:2119–2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gan Y, Shi C, Inge L, Hibner M, Balducci J, Huang Y. Differential roles of ERK and Akt pathways in regulation of EGFR‐mediated signaling and motility in prostate cancer cells. Oncogene 2010;29:4947–4958. [DOI] [PubMed] [Google Scholar]
- 27. Schemarova IV. The role of tyrosine phosphorylation in regulation of signal transduction pathways in unicellular eukaryotes. Curr Issues Mol Biol 2006;8:27–49. [PubMed] [Google Scholar]
- 28. Cohen P. The regulation of protein function by multisite phosphorylation–a 25 year update. Trends Biochem Sci 2000;25:596–601. [DOI] [PubMed] [Google Scholar]
- 29. Krishna M, Narang H. The complexity of mitogen‐activated protein kinases (MAPKs) made simple. Cell Mol Life Sci 2008;65:3525–3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Manning BD, Cantley LC. AKT/PKB signaling: Navigating downstream. Cell 2007;129:1261–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wu X, Senechal K, Neshat MS, Whang YE, Sawyers CL. The PTEN/MMAC1 tumor suppressor phosphatase functions as a negative regulator of the phosphoinositide 3‐kinase/Akt pathway. Proc Natl Acad Sci U S A 1998;95:15587–15591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cantley LC, Neel BG. New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3‐kinase/AKT pathway. Proc Natl Acad Sci U S A 1999;96:4240–4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vazquez F, Ramaswamy S, Nakamura N, Sellers WR. Phosphorylation of the PTEN tail regulates protein stability and function. Mol Cell Biol 2000;20:5010–5018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tapley P, Lamballe F, Barbacid M. K252a is a selective inhibitor of the tyrosine protein kinase activity of the trk family of oncogenes and neurotrophin receptors. Oncogene 1992;7:371–381. [PubMed] [Google Scholar]
- 35. Rattiner LM, Davis M, French CT, Ressler KJ. Brain‐derived neurotrophic factor and tyrosine kinase receptor B involvement in amygdala‐dependent fear conditioning. J Neurosci 2004;24:4796–4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Li G, Yang K, Zheng C, et al. Functional rundown of gamma‐aminobutyric acid(A) receptors in human hypothalamic hamartomas. Ann Neurol 2011;69:664–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Binder DK, Croll SD, Gall CM, Scharfman HE. BDNF and epilepsy: Too much of a good thing? Trends Neurosci 2001;24:47–53. [DOI] [PubMed] [Google Scholar]
- 38. Ernfors P, Bengzon J, Kokaia Z, Persson H, Lindvall O. Increased levels of messenger RNAs for neurotrophic factors in the brain during kindling epileptogenesis. Neuron 1991;7:165–176. [DOI] [PubMed] [Google Scholar]
- 39. Isackson PJ, Huntsman MM, Murray KD, Gall CM. BDNF mRNA expression is increased in adult rat forebrain after limbic seizures: Temporal patterns of induction distinct from NGF. Neuron 1991;6:937–948. [DOI] [PubMed] [Google Scholar]
- 40. Merlio JP, Ernfors P, Kokaia Z, et al. Increased production of the TrkB protein tyrosine kinase receptor after brain insults. Neuron 1993;10:151–164. [DOI] [PubMed] [Google Scholar]
- 41. He XP, Kotloski R, Nef S, Luikart BW, Parada LF, McNamara JO. Conditional deletion of TrkB but not BDNF prevents epileptogenesis in the kindling model. Neuron 2004;43:31–42. [DOI] [PubMed] [Google Scholar]
- 42. Stephens RM, Loeb DM, Copeland TD, Pawson T, Greene LA, Kaplan DR. Trk receptors use redundant signal transduction pathways involving SHC and PLC‐gamma 1 to mediate NGF responses. Neuron 1994;12:691–705. [DOI] [PubMed] [Google Scholar]
- 43. Hetman M, Gozdz A. Role of extracellular signal regulated kinases 1 and 2 in neuronal survival. Eur J Biochem 2004;271:2050–2055. [DOI] [PubMed] [Google Scholar]
- 44. Thomas GM, Huganir RL. MAPK cascade signalling and synaptic plasticity. Nat Rev Neurosci 2004;5:173–183. [DOI] [PubMed] [Google Scholar]
- 45. Xi ZQ, Wang XF, He RQ, et al. Extracellular signal‐regulated protein kinase in human intractable epilepsy. Eur J Neurol 2007;14:865–872. [DOI] [PubMed] [Google Scholar]
- 46. Park SA, Kim TS, Choi KS, Park HJ, Heo K, Lee BI. Chronic activation of CREB and p90RSK in human epileptic hippocampus. Exp Mol Med 2003;35:365–370. [DOI] [PubMed] [Google Scholar]
- 47. Merlo D, Cifelli P, Cicconi S, Tancredi V, Avoli M. 4‐Aminopyridine‐induced epileptogenesis depends on activation of mitogen‐activated protein kinase ERK. J Neurochem 2004;89:654–659. [DOI] [PubMed] [Google Scholar]
- 48. Nateri AS, Raivich G, Gebhardt C, et al. ERK activation causes epilepsy by stimulating NMDA receptor activity. EMBO J 2007;26:4891–4901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Binder DK, Routbort MJ, McNamara JO. Immunohistochemical evidence of seizure‐induced activation of trk receptors in the mossy fiber pathway of adult rat hippocampus. J Neurosci 1999;19:4616–4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. He XP, Minichiello L, Klein R, McNamara JO. Immunohistochemical evidence of seizure‐induced activation of trkB receptors in the mossy fiber pathway of adult mouse hippocampus. J Neurosci 2002;22:7502–7508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cole‐Edwards KK, Musto AE, Bazan NG. c‐Jun N‐terminal kinase activation responses induced by hippocampal kindling are mediated by reactive astrocytes. J Neurosci 2006;26:8295–8304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Jeon SH, Kim YS, Bae CD, Park JB. Activation of JNK and p38 in rat hippocampus after kainic acid induced seizure. Exp Mol Med 2000;32:227–230. [DOI] [PubMed] [Google Scholar]
- 53. Kyriakis JM, Avruch J. Mammalian MAPK signal transduction pathways activated by stress and inflammation: A 10‐year update. Physiol Rev 2012;92:689–737. [DOI] [PubMed] [Google Scholar]
- 54. Chong ZZ, Shang YC, Wang S, Maiese K. A critical kinase cascade in neurological disorders: PI 3‐K, Akt, and mTOR. Future Neurol 2012;7:733–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Dunleavy M, Provenzano G, Henshall DC, Bozzi Y. Kainic acid‐induced seizures modulate Akt (SER473) phosphorylation in the hippocampus of dopamine D2 receptor knockout mice. J Mol Neurosci 2013;49:202–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zeng LH, Rensing NR, Wong M. The mammalian target of rapamycin signaling pathway mediates epileptogenesis in a model of temporal lobe epilepsy. J Neurosci 2009;29:6964–6972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Buckmaster PS, Ingram EA, Wen X. Inhibition of the mammalian target of rapamycin signaling pathway suppresses dentate granule cell axon sprouting in a rodent model of temporal lobe epilepsy. J Neurosci 2009;29:8259–8269. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Enhanced tyrosine phosphorylation in HH.
Figure S2. Postmortem interval (PMI) has no significant impact on protein phosphorylation and expression.
Figure S3. Proposed model of how BDNF‐TrkB signaling contributes to KCC2 downregulation and GABA‐mediated excitation and epileptogenesis.
Table S1. Lists of primary antibodies used in the study.


