Abstract
Vitamin D metabolite analysis possessed significant clinical value for the pediatric department. However, invasive venipuncture sampling and high blood consumption inflicted much suffering on patients. For alleviation, we carried out a LC-MS method for 25-hydroxyvitamin D quantification in only 3 μl of plasma from the considerably less invasive finger-prick blood samples. To improve sensitivity, acylation on C3-hydroxyl (by isonicotinoyl chloride) rather than Diels-Alder adduction on s-cis-diene structure was for the very first time introduced into vitamin D metabolite derivatization. Compared with the existing derivatization approaches, this novel strategy not only prevented isomer interference, but also exhibited higher reacting throughput. For certification, the methodology was systematically validated and showed satisfying consistency with SRM927a. During clinical application, we found a convincing correlation between 25-hydroxyvitamin D and indirect/total bilirubin in jaundiced newborns. Such an observation indicated that vitamin D supplementation may help to achieve optimal outcomes in neonatal jaundice.
Keywords: vitamin D, neonatal jaundice, liquid chromatography-mass spectrometry
It has been unanimously accepted that vitamin D plays a critical role in regulating human physiology. To date, numerous LC-MS/MS-based methods have been established (1–3), including ours (4), to quantify 25-hydroxyvitamin D2 [25(OH)D2], 25-hydroxyvitamin D3 [25(OH)D3], and the C-3 epimer of 25(OH)D3 [epi-25(OH)D3] for both nutritional and clinical purposes. However, after 2 years of application, we found that these methods, including the common venipuncture, were too invasive for pediatric patients, especially infants. In practice, there were two considerably less invasive sampling approaches, dry blood spot (DBS) and finger-prick blood (FPB). Although DBS has been regarded as an alternative medium for 25(OH)D validation, the absence of blank matrix Standard Reference Material (SRM) and consensus reference interval impeded us in introducing it into our hospital (5). In contrast, FPB was identical to conventional venous blood samples, which enabled us to easily match the results to our previously certified method. Consequently, it was decided that it would be beneficial to develop an FPB-based strategy for 25(OH)D evaluation. To this end, the most urgent issue was to improve the detection sensitivity, because the volume of FPB samples was much lower than the sample consumption required by the conventional direct detection methodologies (approximately 100 μl). Enlightened by the experience of DBS analysis, derivatization could be the most promising option.
Hitherto, numerous derivatization strategies have been designed for vitamin D metabolites, which were well summarized in recent reviews (6–8). In brief, the derivatization reagents could be divided into three categories, PTAD, 4-substituted-TAD (DAPTAD, DMEQ-TAD, Amplifex Diene, etc.), and PyrNO (9, 10). All of these derivatizations were directed to the s-cis-diene structure of vitamin D metabolites, forming Diels-Alder adducts. In spite of the outstanding performance in enhancing MS-response, there were still two obvious limitations to these methods. First, because the derivatization reagents could attack s-cis-diene from both α- and β-sides, the resulting diastereoisomer and even regioisomer products would inevitably increase difficulties of LC separation and peak identification. Second, constrained by the reaction kinetics, relatively long incubation time (more than 1 h) or heating process (up to 70°C) was required to achieve sufficient derivatization efficiency. In fact, other than s-cis-diene structure, the hydroxyl group on the C-3 position of vitamin D metabolites also possessed potential chemical activity. Unfortunately, it was untilled so far.
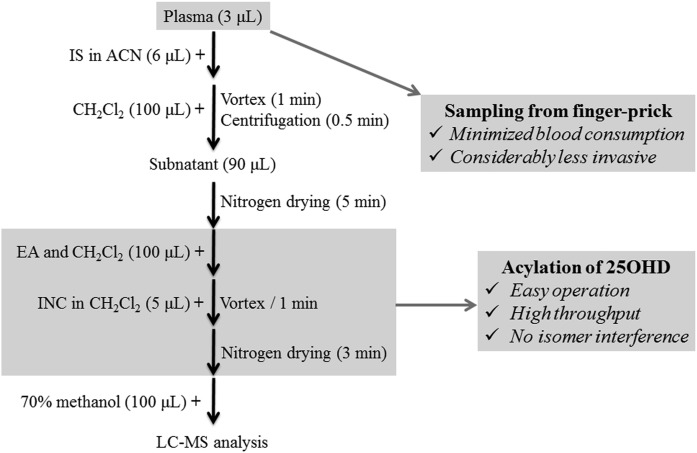
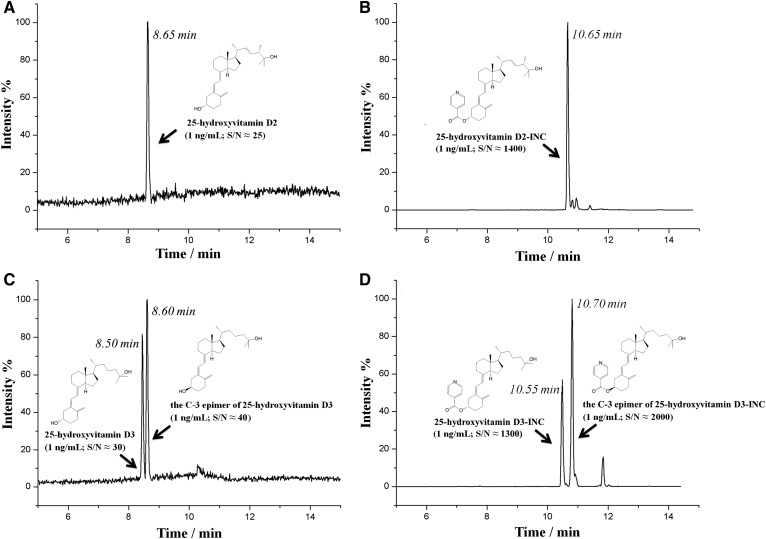
Here, for the first time, we exploited the acylation of vitamin D metabolites on the C-3 hydroxyl with isonicotinoyl chloride (INC). Such a derivatization approach circumvented the two drawbacks of the aforementioned strategies. First, no isomer interference was formed during the derivatization. Second, the reaction could be completed instantaneously at room temperature, with simple operation and high throughput (Fig. 1). Meanwhile, with the introduction of the positively charged isonicotinoyl group, the detection sensitivity was improved by 200- to 1,000-fold as compared with direct analysis of 25(OH)D2, 25(OH)D3, epi-25(OH)D3, 24,25-dihydroxyvitamin D2 [24,25(OH)2D2], 24,25-dihydroxyvitamin D3 [24,25(OH)2D3], 1α,25-dihydroxyvitamin D2 [1,25(OH)2D2], and 1α,25-dihydroxyvitamin D3 [1,25(OH)2D3] (Fig. 2, supplemental Fig. S1). Benefitting from these advantages, a highly applicable LC-MS method for quantification of 25(OH)D2, 25(OH)D3, and epi-25(OH)D3 for FPB samples was established. Low plasma consumption (3 μl per analysis), favorable throughput (less than 20 min for pretreatment), and enhanced sensitivity [lower limit of quantification (LLOQ), 1 ng/ml in 3 μl plasma] made this method especially suitable for routine 25(OH)D evaluation in the pediatric and neonatology departments.
Fig. 1.
The schematic procedures of the INC derivatization method for LC-MS analysis of 25(OH)D.
Fig. 2.
The typical LC-MS/MS chromatograms of 25(OH)D (1 ng/ml) before (A, C) and after (B, D) INC derivatization.
In 3 months of clinical practice, we observed that the plasma 25(OH)D level in jaundiced newborns seemed to be lower than in nonjaundiced ones. There were only a few reports regarding the relationship between 25(OH)D and neonatal jaundice (11–13). Besides, two issues made the previous data somewhat controversial. First, immunoassays or enzymatic analysis utilized in those studies were not effective in excluding epi-25(OH)D3, which may lead to erroneous estimations of 25(OH)D. Especially for infants, epi-25(OH)D3 concentration tended to be relatively high (11). Second, breastfeeding and vitamin D supplementation in pregnant women, which are important factors for neonatal jaundice, were rarely considered (12, 14). Therefore, the present work provided the first convincing evidence for the potential relationship between 25(OH)D and neonatal jaundice. We believed that the observed negative correlation between 25(OH)D and indirect bilirubin (IBIL)/total bilirubin (TBIL) in jaundiced newborns might indicate that vitamin D supplementation could help to achieve optimal outcomes for neonatal jaundice.
MATERIALS AND METHODS
Chemicals and reagents
The standards of 25(OH)D2 (H-073; 1 ml), 25(OH)D2-d3 (740071; 1 ml), 25(OH)D3 (739650; 1 ml), 25(OH)D3-d6 (H-074; 1 ml), epi-25(OH)D3 (739936; 1 ml), INC (228753; 25 g) with purity of 95%, and ethyl acetate (650528; 1 liter) were purchased from Sigma-Aldrich (Beijing, China). The standards of 24,25(OH)2D2 (HY-76801; 1 mg), 24,25(OH)2D3 (HY-32343; 1 mg), 1,25(OH)2D2 (HY-32350; 1 mg), and 1,25(OH)2D3 (HY-10002; 1 mg) were obtained from MedChem Express (Monmouth Junction, NJ). Formic acid, methanol, and acetonitrile (ACN) were purchased from Fischer Scientific. Dichloromethane (961203; 1 liter) was purchased from J&K Scientific (Beijing, China). All reagents were LC-MS grade unless indicated otherwise.
VD-DDC Mass Spect Gold from Golden West Diagnostics (Temecula, CA) was used as blank matrix. SRM972a was purchased from the National Institute of Standards and Technology. The water used throughout the study was purified by a Milli-Q apparatus (Millipore, Bedford, MA).
The INC saturated solution was prepared by dispersing INC (5 mg) in dichloromethane (1 ml). Before use, ultrasonic treatment and centrifugation (3,000 g, 30 s) was carried out; and then, the supernatant was used for derivatization.
Method development
LC-MS/MS.
The LC-MS/MS platform consisted of an Ekspert ultraLC 100-XL system and an AB SCIEX 4500 QTRAP mass spectrometer (Applied Biosystems, Foster City, CA) equipped with an ESI source operating in the positive mode. The ESI inlet parameters were curtain gas (25.0 psi), collision gas (medium), ion spray voltage (5,500 eV), temperature (500°C), ion source gas 1 (30.0 psi), and ion source gas 2 (30.0 psi). Data acquisition and processing were performed using AB SCIEX Analyst 1.6.2 software (Applied Biosystems). The targets were monitored by multiple reaction monitoring mode. The details are shown in supplemental Table S1.
The LC separation was manipulated on a Kinetex™ 2.6 μm PFP column (100 × 3 mm; Phenomenex, Germany) with a flow rate of 0.35 ml/min at 50°C. Formic acid in water (0.2%, v/v; solution A) and formic acid in methanol (0.2%, v/v; solution B) were wielded as mobile phases. The gradient was 0–3 min 70% B, 3–9 min 70–100% B, 9–11 min 100% B, 11–11.5 min 100–40% B, 11.5–15 min 40% B. To improve ionization efficiency and eliminate high matrix effect, the fluid (containing excessive derivatization reagents and other interference) from the first 2 min was discarded to waste by an exchange value.
Stock solutions, calibration, and quality control samples.
A certain amount of the standard solutions, as received, was nitrogen dried and then dissolved in ACN to prepare stock solutions. The final concentration was set as 2.0 μg/ml for 25(OH)D2, 25(OH)D3 and epi-25(OH)D3. Further dilution for calibration curve and quality control (QC) samples was operated using blank matrix. The spiked concentration range of plasma calibration was 1.0–100.0 ng/ml for all the targets (1.0, 2.5, 5.0, 10.0, 30.0, 60.0, and 100.0 ng/ml). The spiked concentrations in high-, medium-, low-, and LLOQ-level of QC samples were 100.0, 30.0, 10.0, and 1.0 ng/ml, respectively. Concentration of stable isotope-labeled internal standards (SIL-ISs), including 25(OH)D2-d3 and 25(OH)D3-d6, in ACN for protein precipitation was 15.0 ng/ml. All the stock solutions and calibration and QC samples were stored at −80°C before use.
VD-DDC Mass Spect Gold from Golden West Diagnostics was used as blank matrix. The established quantification protocol and our previous method (4) were both used to certify the absence of vitamin D in this matrix.
Study cohort.
The study cohort was conducted among 115 eligible jaundiced neonates and 32 healthy neonates. Sample collection was performed at the Department of Neonatology and the Department of Clinical Laboratory of Renmin Hospital of Wuhan University (Wuhan, China). Neonatal jaundice or neonatal hyperbilirubinemia was defined as a TBIL level in infant blood that exceeded 5 mg/dl (85 μmol/l) (15), and vitamin D deficiency was defined as a concentration of 25(OH)D less than 20 ng/ml (16). Healthy and jaundiced neonates with postnatal ages of 2–28 days and gestational ages of 37–40 weeks were recruited before any intervention (phototherapy or vitamin D supplementation). Gestational age at delivery was confirmed based on maternal last menstrual period or ultrasound scan findings. Cases were excluded when identified with pathological causes for neonatal jaundice, such as blood group incompatibility, sequestration or polycythemia, infection, glucose-6-phosphate dehydrogenase deficiency, cephalic hematoma, history of asphyxia, and congenital anomalies. Plasma and serum samples were collected respectively by finger-prick and venipuncture after overnight fasting (>12 h). Plasma and serum were immediately separated into a polypropylene conical centrifuge tube after blood collection (centrifuging at 1,000 g for 5 min at 4°C) and promptly frozen at −80°C until analysis. Qualified clinical laboratory technicians performed biochemical analysis and measurements based on the standard manufacturer’s instructions. Basic clinical information about the patients was obtained from computerized medical records and well-trained clinicians.
The whole study was supervised under the Ethics Committee of Renmin Hospital of Wuhan University. The consent procedure was based on the standard procedure. Written informed consent was obtained from each participant and from a parent or a representative of the newborn. All the samples and information were obtained after permission. The study abided by the Declaration of Helsinki principles and had no conflicts of interest.
Sample preparation and INC derivatization.
The schematic of sample preparation is illustrated in Fig. 1. Briefly, plasma (3 μl) from FPB was transferred into a polypropylene conical centrifuge tube. After mixing with SIL-IS (6 μl, ACN solution for protein precipitation), dichloromethane (100 μl) was added, vortexed (1 min), and centrifuged (3,000 g, 30 s) for liquid-liquid extraction. The resultant subnatant (90 μl) was then transferred into a 96-well plate, dried under nitrogen gas, and redissolved in a mixture (100 μl) of ethyl acetate and dichloromethane (1/3, v/v). In the end, INC solution (5 μl) was added. The final mixture was then dried under nitrogen gas again and reconstituted with the initial mobile phase (100 μl) for LC-MS/MS analysis. The injection volume was 10 μl.
Method validation
The calibration curves were determined using the peak ratios of the targets to the corresponding SIL-IS versus the nominal spiked concentrations by a linear least squares regression model. The lowest concentration of the calibration curve was accepted as the LLOQ. The ratio of signal to noise (S/N) was higher than 10 at various levels. The details are listed in supplemental Table S3.
To validate selectivity, we spiked a high level of cholesterol [10 μg/ml, analog for 25(OH)D] into low-QC and high-QC samples to fabricate interference-introduced plasma. The recoveries of the targets in these samples should remain at 85–115%. The carryover effects were evaluated by analyzing the background matrix after triple injection of the upper limit of calibration samples. The residues should be less than 15% of LLOQ.
Accuracy was calculated by dividing the measured target concentration by the nominal spiked value in the QC sample, which could also be regarded as the method recovery, and imprecision was reported as the coefficient of variation of the measurements. Both accuracy and imprecision were investigated for four levels (high-, medium-, low-, and LLOQ-level) of QC samples. The measurements were performed in 1 day (intraday) and in 10 consecutive days (interday). The values of accuracy should be 85–115% (80–120% for LLOQ-level). And imprecision should not be higher than 15% (20% for LLOQ-level).
The matrix effect was evaluated by comparing the results of two sample groups. For group-I, the targets and SIL-ISs were spiked into the pretreated blank matrix just before introducing INC. For group-II, the targets and SIL-ISs were spiked into a mixture (100 μl) of ethyl acetate and dichloromethane (1/3, v/v) for direct derivatization. Both groups were spiked at the medium-level of the targets. The matrix effect was evaluated by the ratio of the results from group-I to group-II, which should be within 85–115%.
The stability of 25(OH)D in plasma was comprehensively studied in our previous work (4). The stability of 25(OH)D-INC was tested with medium-level QC samples. Before injection, the pretreated samples were stored at 25°C for 1 week, 4°C for 1 month, or −80°C for one-half year. Freeze-thaw stability was tested after three cycles of freezing (−80°C) and thawing (25°C). During storage, no specific light-protection treatment was carried out.
Trueness of the established methodology was evaluated with SRM972a. We also compared the present methodology with the published one (4) through retesting the same sample pool (n = 30).
Laboratory analysis and statistical analysis
Siemens Advia 2400 automatic biochemistry analyzer (Siemens, Erlangen, Germany) was utilized to determine the levels of aminotransferase, aspartate aminotransferase, alkaline phosphatase, albumin, calcium, inorganic phosphorus, magnesium, direct bilirubin (DBIL), IBIL (calculated), TBIL, 25(OH)D2, 25(OH)D3, and epi-25(OH)D3 were determined by the newly established LC-MS/MS method. 25(OH)D was calculated as the sum of 25(OH)D2 and 25(OH)D3. Samples were measured in the same analytical batches. And laboratory technicians were blinded to the sample type and to any expected outcome.
Normally distributed parameters of laboratory variables were reported as the means ± standard deviation (x ± SD). Frequencies were expressed as number of cases/percentage (n, %). Skewed data are expressed as median and interquartile range (m, interquartile range). Normality of distribution was performed by Shapiro-Wilk test. If data was continuous and normally distributed, independent t-testing was employed to evaluate the comparison. Otherwise, the Mann-Whitney U-test was used. The Chi-square test was applied for the comparison of categorical variables. Correlations between 25(OH)D, biochemical markers, and demographic parameters were examined using linear regression analysis and the Pearson correlation coefficient. The statistical analyses were performed using IBM SPSS, version 22.0 (Chicago, IL). A two-tailed P < 0.05 was considered statistically significant.
RESULTS
Method validation
Linearity and sensitivity.
A linear range of 1.0–100.0 ng/ml was obtained for all the targets with R2 higher than 0.99 (supplemental Table S3). The S/N of the LLOQ-level sample was higher than 10 for all of these analytes. Obviously, after the derivatization, the assay sensitivity was increased approximately 200- to 1,000-fold, as compared with direct detection, and the LLOQ of the method reached 1.0 ng/ml when 3 μl plasma was used. Such sensitivity was comparable with the previous derivatization methods (6–8). Supplemental Fig. S2 displays the typical LC-MS/MS chromatograms at LLOQ-level.
Selectivity and carryover effect.
After introducing analog interference, the recoveries of the targets were all determined ranging within 85–115%, indicating satisfactory selectivity for the proposed method (details not shown). In addition, after successive analysis of upper limit of quantification samples, we identified no obvious residuals for analysis of blank matrix, suggesting that the carryover effect was negligible.
Accuracy and imprecision.
The accuracy was 89.9–103.6% for high-, medium-, and low-level and 86.5–102.5% for LLOQ-level (supplemental Table S4). The imprecision of the high-, medium-, and low-level was lower than 9.6%, while for the LLOQ-level, the imprecision was below 17.9%. Such results indicated that the proposed method possessed favorable accuracy and precision.
Matrix effect.
For all of the targets, the ratio of group-I to group-II ranged from 92.6% to 103.8%. Therefore, with the normalization of the SIL-IS, the performance of the present derivatization process was not compromised by the matrix.
Stability.
Under all the tested circumstances, little degradation was observed for all the derivatization products (recoveries 96.5–102.2%). And as no light-protection was carried out during experiments, photostability could be demonstrated satisfactorily. Such excellent stability expanded the feasibility for routine clinical usage.
Method certification.
We observed good consistency for SRM certification (supplemental Table S5) with the recoveries (measured values to the certified values) of 93.5–95.6% for all the targets (the imprecision lower than 8.1%). Additionally, by comparing the results of the same sample pool, the present method exhibited good correlation with our previous methodology (supplemental Fig. S3). All of these consequences demonstrated excellent reliability of the proposed method.
25(OH)D and jaundice in neonates
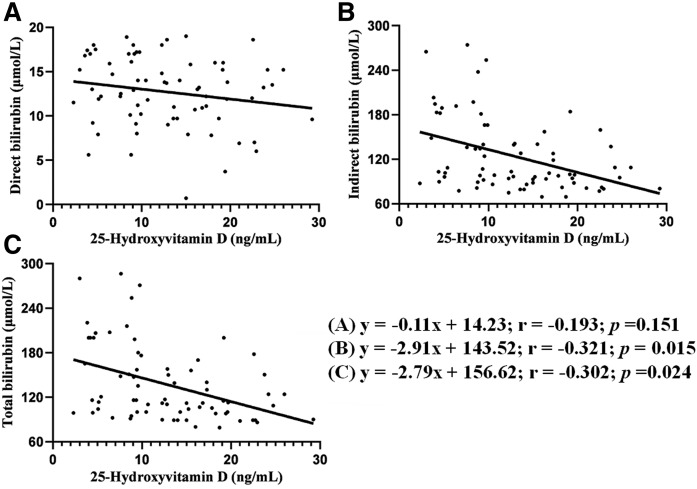
Table 1 summarizes the demographic variables and laboratory parameters in jaundiced and nonjaundiced neonates. Neither compliance nor baseline characteristics differed greatly between the two groups. Concentration differences were detected between the two groups for DBIL (13.02 ± 4.48 μmol/l vs. 4.96 ± 2.50 μmol/l, P < 0.001), IBIL (115.93 ± 69.37 μmol/l vs. 15.60 ± 4.12 μmol/l, P < 0.001), TBIL (128.95 ± 75.78 μmol/l vs.15.60 ± 4.12 μmol/l, P < 0.001), and 25(OH)D (13.21 ± 7.72 ng/ml vs. 20.23 ± 7.04 ng/ml, P < 0.001). We also found significant negative correlations between 25(OH)D and TBIL (r = −0.302, P = 0.024), 25(OH)D and IBIL (r = −0.321, P = 0.015), but not for 25(OH)D and DBIL (r = −0.193, P = 0.151), which is shown in Fig. 3.
TABLE 1.
A comparison of the demographic variables and laboratory parameters in jaundiced and nonjaundiced neonates
| Study Variables | Jaundiced Neonates (n = 115) | Non-Jaundiced Neonates (n = 32) | P |
| Maternal age (years) | 28.58 ± 5.01 | 28.03 ± 4.67 | 0.577 |
| Delivery type (Caesarean) | 59 (55.65%) | 14 (43.75%) | 0.317 |
| Gestational age (weeks) | 38.04 ± 1.44 | 37.74 ± 2.48 | 0.514 |
| Maternal BMI (kg/m2), | 26.07 ± 5.98 | 25.76 ± 4.37 | 0.751 |
| Mothers’ vitamin D use | 0.926 | ||
| Regularly | 25 (21.74%) | 8 (25.00%) | |
| Irregularly | 19 (16.52%) | 5 (22.72%) | |
| None | 71 (61.74%) | 19 (59.38%) | |
| Male neonatal | 67 (58.26%) | 15 (46.88%) | 0.315 |
| Birth weight (g) | 2,994.85 ± 369.06 | 3,059.28 ± 437.69 | 0.404 |
| Head circumference in newborn (cm) | 33.49 ± 1.33 | 33.7563 ± 1.17 | 0.323 |
| Body length of newborn (cm) | 49.94 ± 3.80 | 50.69 ± 2.95 | 0.299 |
| Breast feeding | 84 (73.04%) | 26 (81.25%) | 0.490 |
| ALT (U/l) | 23.00 (18.00–30.00) | 24.0 (14.00–33.00) | 0.785 |
| AST (U/l) | 22.00 (16.00–29.00) | 23.00 (18.00–34.00) | 0.868 |
| ALP (U/l) | 512.00(187.00–733.00) | 478.00 (213.00–827.00) | 0.597 |
| Albumin (g/l) | 31.42 ± 4.35 | 32.83 ± 3.49 | 0.095 |
| Calcium (mmol/l) | 2.29 ± 0.72 | 2.16 ± 0.49 | 0.312 |
| Inorganic phosphate (mmol/l) | 1.98 ± 0.73 | 1.86 ± 0.84 | 0.471 |
| Magnesium (mmol/l) | 0.70 ± 0.21 | 0.73 ± 0.29 | 0.635 |
| DBIL (μmol/l) | 13.02 ± 4.48 | 4.96 ± 2.50 | < 0.001 |
| IBIL (μmol/l) | 115.93 ± 69.37 | 10.64 ± 3.52 | < 0.001 |
| TBIL (μmol/l) | 128.95 ± 75.78 | 15.60 ± 4.12 | < 0.001 |
| 25(OH)D (ng/ml) | 13.21 ± 7.72 | 20.23 ± 7.04 | < 0.001 |
ALT, aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; 25(OH)D, 25-hydroxyvitamin D.
Fig. 3.
Correlation between neonatal 25(OH)D and different bilirubin levels (n = 115): DBIL (A), IBIL (B), and TBIL (C).
We observed that 78% (115/147) of newborns developed clinical neonatal jaundice (serum TBIL level >85 μmol/l) (15). Overall, 73% (125/147) of newborns had vitamin D deficiency, which was 47% in nonjaundiced neonates and 81% in jaundiced neonates (P < 0.001). Noteworthily, we have detected epi-25(OH)D3 in 63% (93/147) of neonatal plasma at concentrations ranging from 0% to 45% of 25(OH)D level.
DISCUSSION
Method development
We developed the proposed method to circumvent invasive venipuncture and large sample consumption (100 μl or more) during clinical evaluation of 25(OH)D for pediatric patients, especially infants. To this end, FPB was introduced instead of conventional blood sampling for plasma collection. However, frequently, there were only 20 μl or less of plasma available from the FPB samples. Therefore, it was of great importance to upgrade the detection sensitivity. As mentioned before, in the existing s-cis-diene-directed derivatization methods, the isomer interference and fair reacting throughput could compromise the practical applicability. For improvement, we pioneered the derivatization of 25(OH)D on the C-3 hydroxyl by INC.
Because this was the very first attempt for chemical modification of vitamin D metabolites other than Diels-Alder addiction, we carefully designed and optimized the derivatization process in four aspects: i) Reacting reagent. Recently, benzoyl chloride has been utilized in our laboratory for acylation derivatization (17). However, the absence of an ionizable group made it improper to derivatize vitamin D metabolites. In comparison, INC not only preserved the potential chemical activity toward the hydroxyl on the cyclohexanol part of vitamin D, but also possessed an isonicotinoyl group for protonation. Hence, INC was a preferable choice for vitamin D metabolite derivatization before MS analysis. ii) Reacting condition. To obtain satisfactory reaction efficiency, we investigated several parameters, including reacting solvent (hexane, ethyl acetate, dichloromethane, methylbenzene, diethyl ether, dimethylformamide), amount of INC (1–25 μl), incubation time (0–120 min), and reaction temperature (25–70°C). After optimization, the best performance was achieved in the mixture of ethyl acetate and dichloromethane (1/3, v/v), which may be ascribed to the suitable polarity. The optimal amount of INC was determined to be 2.5–10 μl. Noteworthily, the reaction exhibited a rapid and spontaneous nature at room temperature so that easy-operation and high-throughput were ensured. iii) Posttreatment. Being moisture sensitive, excess INC could be instantaneously hydrolyzed during the final reconstituting process. Moreover, because its hydrolysate (isonicotinic acid) was highly hydrophilic, it was easy to prevent its ion suppression by discarding the first segment of the mobile phase by value exchange after injection. iv) Reaction yield. High levels of QC samples were used to study the reaction yield (details are shown in the supplemental material). In consequence, all 25(OH)D species could be well detected in the control group (S/N >500), while after derivatization, no detectable signal could be observed for all the targets (S/N <3). Such results indicated the favorable reaction yield for INC derivatization.
Although our current work was mainly focused on the quantification of 25(OH)D species, we also investigated the derivatization of other vitamin D metabolites for proof-of-concept, including 24,25(OH)2D2, 24,25(OH)2D3, 1,25(OH)2D2, and 1,25(OH)2D3. As shown in supplemental Fig. S1, by comparing the S/N of direct analysis of these targets [10 ng/ml; supplemental Fig. S1(A1)–(D1)] and the S/N after derivatization [1 ng/ml; supplemental Fig. S1(A2)–(D2)], the detecting sensitivity was improved by two to three orders of magnitude, indicating its good potential applicability to quantify those targets.
25(OH)D and jaundice in neonates
Hyperbilirubinemia is one of the most common causes of neonatal morbidity and hospitalization (12, 18), which occurs as a result of rapid erythrocyte hemolysis and excessive bilirubin that cannot be eliminated properly by the immature liver of neonates (19, 20). Early diagnosis and effective treatment of neonatal jaundice are essential because high levels of bilirubin can lead to bilirubin encephalopathy or even kernicterus, with devastating permanent neurodevelopmental disorders (21). Based on the available sources of data, 78% (115/147) of newborns developed clinical neonatal jaundice, which was consistent with previous reports (13, 18, 19, 22). The relatively high level of epi-25(OH)D3 in neonates may explain the contradictory conclusions among previous studies, which failed to exclude epi-25(OH)D3. In the present work, we carried out the very first convincing study on the relationship between vitamin D and neonatal jaundice.
Generally, glucose-6-phosphate dehydrogenase deficiency, Rh incompatibility, and blood group incompatibility were considered to be the most common factors responsible for jaundice. However, in many cases, the pathogenesis remained unclear. Further research on the etiology of jaundice was undoubtedly beneficial to formulate prevention strategies and improve therapeutic effect for such a disease. As described above, we observed that vitamin D may also intimately relate to the development of jaundice. Such results may point to the possibility of decreasing the risk of neonatal jaundice through supplying vitamin D for expectant mothers. The specific mechanism was still not well understood. Nonetheless, as vitamin D could lower plasma erythropoietin levels, we hypothesized that the breakdown of fetal erythrocytes might be decelerated through this route so that the negative correlation between 25(OH)D and IBIL/TBIL was observed (23). On the other hand, evidence had shown that normal newborn erythrocytes were relatively sensitive to oxidative damage (24). Thereby, vitamin D might reduce bilirubin production by its antioxidant effect to prevent oxidative damage of erythrocytes (25, 26). Furthermore, phototherapy was known to be a simple and effective treatment for reducing IBIL. During phototherapy, we surmised that the UV light could penetrate the skin of the newborn and result in an elevated vitamin D level, allowing it to play a protective role. And this can be supported by the phenomenon that infants exposed to sunlight were less likely to develop jaundice (27). In conclusion, it was tempting to speculate that vitamin D supplementation may help to achieve optimal outcomes toward neonatal jaundice. Further specific studies on this topic are already in progress in our group.
Supplementary Material
Footnotes
Abbreviations:
- ACN
- acetonitrile
- DBIL
- direct bilirubin
- DBS
- dry blood spot
- epi-25(OH)D3
- C-3 epimer of 25-hydroxyvitamin D3
- FPB
- finger-prick blood
- IBIL
- indirect bilirubin
- INC
- isonicotinoyl chloride
- LLOQ
- lower limit of quantification
- 1
- 25(OH)2D2, 1α,25-dihydroxyvitamin D2
- 1
- 25(OH)2D3, 1α,25-dihydroxyvitamin D3
- 24
- 25(OH)2D2, 24,25-dihydroxyvitamin D2
- 24
- 25(OH)2D3, 24,25-dihydroxyvitamin D3
- 25(OH)D2
- 25-hydroxyvitamin D2
- 25(OH)D3
- 25-hydroxyvitamin D3
- QC
- quality control
- SIL-IS
- stable isotope-labeled internal standard
- S/N
- ratio of signal to noise
- SRM
- Standard Reference Material
- TBIL
- total bilirubin
This work was supported by National Natural Science Foundation of China Grants 21705121, 81572069, and 81501815.
The online version of this article (available at http://www.jlr.org) contains a supplement.
REFERENCES
- 1.Herrmann M., Farrell C. J. L., Pusceddu I., Fabregat-Cabello N., and Cavalier E.. 2017. Assessment of vitamin D status - a changing landscape. Clin. Chem. Lab. Med. 55: 3–26. [DOI] [PubMed] [Google Scholar]
- 2.Dirks N. F., Ackermans M. T., Lips P., de Jongh R. T., Vervloet M. G., de Jonge R., and Heijboer A. C.. 2018. The when, what & how of measuring vitamin D metabolism in clinical medicine. Nutrients. 10: 482–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaufmann M., Sepiashvili L., and Singh R. J.. 2018. Mass spectrometry assays of vitamin D metabolites. In Vitamin D. 4th edition. D. Feldman, editor. Academic Press, Salt Lake City, UT. 909–923. [Google Scholar]
- 4.Le J., Yuan T-F., Zhang Y., Wang S-T., and Li Y.. 2018. New LC-MS/MS method with single-step pretreatment analyzes fat-soluble vitamins in plasma and amniotic fluid. J. Lipid Res. 59: 1783–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoeller U., Baur M., Roos F. F., Brennan L., Daniel H., Fallaize R., Forster H., Gibney E. R., Gibney M., Godlewska M., et al. 2016. Application of dried blood spots to determine vitamin d status in a large nutritional study with unsupervised sampling: the Food4Me project. Br. J. Nutr. 115: 202–211. [DOI] [PubMed] [Google Scholar]
- 6.Higashi T., and Ogawa S.. 2016. Chemical derivatization for enhancing sensitivity during LC/ESI-MS/MS quantification of steroids in biological samples: a review. J. Steroid Biochem. Mol. Biol. 162: 57–69. [DOI] [PubMed] [Google Scholar]
- 7.van den Ouweland J. M. W. 2016. Analysis of vitamin D metabolites by liquid chromatography-tandem mass spectrometry. Trends Analyt. Chem. 84: 117–130. [Google Scholar]
- 8.Higashi T., and Shimada K.. 2017. Application of Cookson-type reagents for biomedical HPLC and LC/MS analyses: a brief overview. Biomed. Chromatogr. 31: e3808. [DOI] [PubMed] [Google Scholar]
- 9.Wan D., Yang J., Barnych B., Hwang S. H., Lee K. S. S., Cui Y., Niu J., Watsky M. A., and Hammock B. D.. 2017. A new sensitive LC/MS/MS analysis of vitamin D metabolites using a click derivatization reagent, 2-nitrosopyridine. J. Lipid Res. 58: 798–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ogawa S., Kittaka H., Nakata A., Komatsu K., Sugiura T., Satoh M., Nomura F., and Higashi T.. 2017. Enhancing analysis throughput, sensitivity and specificity in LC/ESI-MS/MS assay of plasma 25-hydroxyvitamin D3 by derivatization with triplex 4-(4-dimethylaminophenyl)-1,2,4-triazoline-3,5-dione (DAPTAD) isotopologues. J. Pharm. Biomed. Anal. 136: 126–133. [DOI] [PubMed] [Google Scholar]
- 11.Tuckey R. C., Cheng C. Y. S., and Slominski A. T.. 2019. The serum vitamin D metabolome: what we know and what is still to discover. J. Steroid Biochem. Mol. Biol. 186: 4–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gartner L. M. 2016. Studies of prolonged neonatal jaundice in the breast-fed infant. J. Pediatr. 168: 211. [DOI] [PubMed] [Google Scholar]
- 13.2010. Detection and treatment of neonatal jaundice. Lancet. 375: 1845. [DOI] [PubMed] [Google Scholar]
- 14.Wimalawansa S. J. 2018. Non-musculoskeletal benefits of vitamin D. J. Steroid Biochem. Mol. Biol. 175: 60–81. [DOI] [PubMed] [Google Scholar]
- 15.Aydın M., Hardalaç F., Ural B., and Karap S.. 2016. Neonatal jaundice detection system. J. Med. Syst. 40: 166. [DOI] [PubMed] [Google Scholar]
- 16.Holick M. F. 2007. Vitamin D deficiency. N. Engl. J. Med. 357: 266–281. [DOI] [PubMed] [Google Scholar]
- 17.Yuan T-F., Huang H-Q., Gao L., Wang S-T., and Li Y.. 2018. A novel and reliable method for tetrahydrobiopterin quantification: benzoyl chloride derivatization coupled with liquid chromatography-tandem mass spectrometry analysis. Free Radic. Biol. Med. 118: 119–125. [DOI] [PubMed] [Google Scholar]
- 18.Sarici S. U., Serdar M. A., Korkmaz A., Erdem G., Oran O., Tekinalp G., Yurdakök M., and Yigit S.. 2004. Incidence, course, and prediction of hyperbilirubinemia in near-term and term newborns. Pediatrics. 113: 775–780. [DOI] [PubMed] [Google Scholar]
- 19.Maisels M. J., and McDonagh A. F.. 2008. Phototherapy for neonatal jaundice. N. Engl. J. Med. 358: 920–928. [DOI] [PubMed] [Google Scholar]
- 20.Erdol S., and Ozgur T.. 2018. Vitamin B12 deficiency associated with hyperbilirubinemia and cholestasis in infants. Pak. J. Med. Sci. 34: 714–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuzniewicz M. W., Wickremasinghe A. C., Wu Y. W., McCulloch C. E., Walsh E. M., Wi S., and Newman T. B.. 2014. Incidence, etiology, and outcomes of hazardous hyperbilirubinemia in newborns. Pediatrics. 134: 504–509. [DOI] [PubMed] [Google Scholar]
- 22.Alkhotani A., Eldin E. E., Zaghloul A., and Mujahid S.. 2014. Evaluation of neonatal jaundice in the Makkah region. Sci. Rep. 4: 4802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pincikova T., Paquin-Proulx D., Sandberg J. K., Flodstrom-Tullberg M., and Hjelte L.. 2017. Vitamin D treatment modulates immune activation in cystic fibrosis. Clin. Exp. Immunol. 189: 359–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abdul-Razzak K. K., Nusier M. K., Obediat A. D., and Salim A. M.. 2007. Antioxidant vitamins and hyperbilirubinemia in neonates. Ger. Med. Sci. 5: Doc03. [PMC free article] [PubMed] [Google Scholar]
- 25.Bhat M., and Ismail A.. 2015. Vitamin D treatment protects against and reverses oxidative stress induced muscle proteolysis. J. Steroid Biochem. Mol. Biol. 152: 171–179. [DOI] [PubMed] [Google Scholar]
- 26.Haas M. J., Jafri M., Wehmeier K. R., Onstead-Haas L. M., and Mooradian A. D.. 2016. Inhibition of endoplasmic reticulum stress and oxidative stress by vitamin D in endothelial cells. Free Radic. Biol. Med. 99: 1–10. [DOI] [PubMed] [Google Scholar]
- 27.Salih F. M. 2001. Can sunlight replace phototherapy units in the treatment of neonatal jaundice? An in vitro study. Photodermatol. Photoimmunol. Photomed. 17: 272–277. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.