This cohort study uses US national Medicaid data to compare the effectiveness of different psychotropic medication strategies (antidepressant, benzodiazepine, mood stabilizer, or another antipsychotic) for adult patients with schizophrenia who are receiving antipsychotic treatment.
Key Points
Question
For patients with schizophrenia who are taking an antipsychotic medication but need a medication change, what is the comparative effectiveness of various psychotropic medication options?
Findings
In this comparative effectiveness study of 81 921 adult outpatients diagnosed with schizophrenia, compared with starting use of a new antipsychotic, adding an antidepressant was associated with a lower risk of psychiatric hospitalization and emergency department visits, whereas adding a benzodiazepine was associated with a higher risk of these outcomes.
Meaning
The findings suggest that in the treatment of schizophrenia, adjunctive antidepressants are associated with better outcomes compared with alternative psychotropic medication strategies.
Abstract
Importance
People with schizophrenia are commonly treated with psychotropic medications in addition to antipsychotics, but there is little evidence about the comparative effectiveness of these adjunctive treatment strategies.
Objective
To study the comparative real-world effectiveness of adjunctive psychotropic treatments for patients with schizophrenia.
Design, Setting, and Participants
This comparative effectiveness study used US national Medicaid data from January 1, 2001, to December 31, 2010, to examine the outcomes of initiating treatment with an antidepressant, a benzodiazepine, a mood stabilizer, or another antipsychotic among adult outpatients (aged 18-64 years) diagnosed with schizophrenia who were stably treated with a single antipsychotic. Data analysis was performed from January 1, 2017, to June 30, 2018. Multinomial logistic regression models were used to estimate propensity scores to balance covariates across the 4 medication groups. Weighted Cox proportional hazards regression models were used to compare treatment outcomes during 365 days on an intention-to-treat basis.
Main Outcomes and Measures
Risk of hospitalization for a mental disorder (primary), emergency department (ED) visits for a mental disorder, and all-cause mortality.
Results
The study cohort included 81 921 adult outpatients diagnosed with schizophrenia (mean [SD] age, 40.7 [12.4] years; 37 515 women [45.8%]) who were stably treated with a single antipsychotic and then initiated use of an antidepressant (n = 31 117), a benzodiazepine (n = 11 941), a mood stabilizer (n = 12 849), or another antipsychotic (n = 26 014) (reference treatment). Compared with initiating use of another antipsychotic, initiating use of an antidepressant was associated with a lower risk (hazard ratio [HR], 0.84; 95% CI, 0.80-0.88) of psychiatric hospitalization, whereas initiating use of a benzodiazepine was associated with a higher risk (HR, 1.08; 95% CI, 1.02-1.15); the risk associated with initiating use of a mood stabilizer (HR, 0.98; 95% CI, 0.94-1.03) was not significantly different from initiating use of another antipsychotic. A similar pattern of associations was observed in psychiatric ED visits for initiating use of an antidepressant (HR, 0.92; 95% CI, 0.88-0.96), a benzodiazepine (HR, 1.12; 95% CI, 1.07-1.19), and a mood stabilizer (HR, 0.99; 95% CI, 0.94-1.04). Initiating use of a mood stabilizer was associated with an increased risk of mortality (HR, 1.31; 95% CI, 1.04-1.66).
Conclusions and Relevance
In the treatment of schizophrenia, initiating adjunctive treatment with an antidepressant was associated with reduced risk of psychiatric hospitalization and ED visits compared with initiating use of alternative psychotropic medications. Associations of benzodiazepines and mood stabilizers with poorer outcomes warrant clinical caution and further investigation.
Introduction
Antipsychotic medications are the first-line treatment for schizophrenia.1 Their effectiveness is supported by strong evidence, but antipsychotics alone are often inadequate to address all the symptoms and functional limitations that occur in individuals with schizophrenia.1,2,3 Switches to other antipsychotics, the addition of other antipsychotics, and the addition of other classes of psychotropic medications are common treatment strategies, but little high-quality evidence is available concerning the effectiveness of these approaches.2,3
Previous evidence on adjunctive treatments in schizophrenia comes primarily from relatively small randomized clinical trials, meta-analyses of pooled results of these trials, and Scandinavian patient registries.4,5,6,7,8,9,10 Evidence that supports the effectiveness of adjunctive psychotropic medications for the treatment of schizophrenia has mostly been equivocal, resulting in calls for additional research to determine their clinical utility. Important exceptions to these mixed findings are recent meta-analyses11,12 that found antidepressants have beneficial, though small, effects. Considerably less information is available to support the effectiveness of benzodiazepines or mood stabilizers as adjunctive treatments in schizophrenia. The effectiveness of combinations of antipsychotic medications remains unclear; reviews13,14 of this treatment strategy agree on the need for further investigation.
Using a patient registry in Sweden, Tiihonen and colleagues4 examined associations between cumulative exposure to different classes of psychotropic medications and mortality in a national cohort of 21 492 individuals diagnosed with schizophrenia. In their comparisons of antidepressant vs no antidepressant use and benzodiazepine vs no benzodiazepine use, antidepressant treatment was associated with reduced mortality, whereas benzodiazepine treatment was associated with increased mortality.
Tiihonen and colleagues5 investigated associations of death with simultaneous use of multiple psychotropic medications (polypharmacy) among 2588 Finnish patients with schizophrenia. Compared with antipsychotic monotherapy, use of adjunctive benzodiazepines (but neither simultaneous use of multiple antipsychotics nor adjunctive antidepressants) was associated with increased risk of mortality.
The current investigation extends prior observational work by examining additional outcome measures, including psychiatric hospital admissions, psychiatric emergency department (ED) visits, self-injury, major adverse medical outcomes, and mortality. The analyses compare outcomes in cohorts that initiated new treatments (new user design), thereby reducing underascertainment of events that occur early in treatment and avoiding adjustment for risk factors that could have been altered by the medications under study.15 Compared with randomized clinical trials that have investigated adjunctive treatments for schizophrenia, the present study is larger and examines outcomes during a longer period.
We used 10 years of data from 44 state Medicaid programs in the United States to compare the effectiveness of initiating different classes of psychotropic medications with starting use of a new antipsychotic in a large cohort of individuals with schizophrenia. Medicaid is the medical insurance program for most individuals with schizophrenia in treatment in the United States.16 The focus was on people who were treated with a single antipsychotic and then initiated treatment with a new psychotropic. Our goal was to compare adjunctive psychotropic medication strategies; people who initiated use of a new antipsychotic were the reference group. We also examined heterogeneity of treatment effects among groups for whom prior evidence suggested there might be a difference in treatment outcomes—older vs younger patients1,17 and those with substance use disorders vs those without these disorders.18
Methods
Study Population and Design
This comparative effectiveness study used a retrospective cohort to examine outcomes among adult patients (aged 18-64 years) with schizophrenia who had been treated with a single second-generation antipsychotic medication and no other psychotropic for at least 90 days and then initiated use of a new psychotropic medication. Data were from US national (44 states) Medicaid Analytic eXtract files (January 1, 2001, to December 31, 2010). Data analysis was performed from January 1, 2017, to June 30, 2018. Schizophrenia was defined as 2 or more outpatient claims or 1 or more inpatient claims for schizophrenia (International Classification of Diseases, Ninth Revision, Clinical Modification code 295.X) during 365 days of consecutive Medicaid enrollment immediately before the date of starting use of a new psychotropic medication. Antipsychotic monotherapy was defined by filled prescriptions for only 1 second-generation antipsychotic (excluding clozapine) and no other psychotropics for 90 consecutive days or more immediately preceding and at the start of use of the study medication (index date). The inclusion criteria were designed to identify a population of patients with schizophrenia who were stably treated with antipsychotic monotherapy but who, because of clinical need, required a change in pharmacotherapy as evidenced by the initiation of a new psychotropic medication. This design takes advantage of a clinical decision point with several options, none of which have strong empirical support, to generate study groups for comparative evaluation. The New York State Psychiatric Institute’s Institutional Review Board reviewed this study and did not consider it to be research that involved human subjects. All data were deidentified.
We followed the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) reporting guideline in the following ways. We prepared and submitted our protocol to the Patient-Centered Outcomes Research Institute before beginning any analyses. The protocol included specification of the research question, the research design, and the analytic plan. Our cohort was defined by characteristics before beginning the new treatment. We used propensity scores to control for confounding by indication, and we acknowledge that residual confounding by unmeasured variables remains possible.
Interventions
All participants had active treatment with a single second-generation antipsychotic when use of the study medication was initiated. Study medications included initiation of an antidepressant, a benzodiazepine, a mood stabilizer, or another second-generation antipsychotic (reference treatment). For this study, mood stabilizers were defined as lithium and antiepileptic medications prescribed in the absence of a seizure disorder. Antipsychotics were included because initiation of use of another antipsychotic, either switching or adding, is common in the community management of schizophrenia.19 Because of clozapine’s unique indication for treatment-resistant schizophrenia, patients receiving clozapine, either as ongoing active treatment or as a new medication, were excluded from the study cohort because they were expected to have a more severe course of illness than individuals prescribed other antipsychotics. During the study period, most antipsychotics prescribed to this population were second-generation drugs. Because it was likely that some switches from first-generation antipsychotics to second-generation antipsychotics were driven by the changing practice patterns of practitioners rather than an individual patient’s clinical need, we only included patients taking or starting use of another second-generation medication.
Outcome Measures
The primary outcome measure was time to psychiatric hospitalization. Psychiatric hospitalization was defined as any hospital discharge with a first-listed mental disorder diagnosis. Secondary outcomes included psychiatric ED visits, self-injury, and death. Medical outcomes included admissions for cardiovascular conditions and incident cases of diabetes. For analyses of incident cases of diabetes, prevalent cases (evidence of diagnosis or treatment of diabetes before the index date) were excluded. eTable 1 in the Supplement gives the outcome definitions.
Statistical Analysis
Propensity score weighting was used to statistically balance the 4 medication treatment groups to be similar on all measured background characteristics.20 A separate propensity score for each psychotropic medication class (ie, the probability of receiving each of the 4 treatments) was estimated from multinomial logistic regression, including all demographic, clinical, and service use variables in eTable 2 in the Supplement.21 Balance on background characteristics after inverse probability of treatment weighting (IPTW) was assessed using the maximum standardized mean difference.22
All primary analyses examined outcomes on an intention-to-treat basis during the first 365 days after initiation of use of 1 of the study medications. Cox proportional hazards regression models with IPTW and robust variance estimation23 were used to estimate the comparative association of initiating use of the study medications (with initiation of use of another antipsychotic medication as the reference treatment) with the risk for each of the outcome measures. Follow-up began at initiation of use of the study medications (index date) and was censored at day 365, loss of Medicaid eligibility, or the end of follow-up data.
To study treatment effect heterogeneity, Cox proportional hazards regression models with IPTW were also fit, including a substance use disorder or age group (18-45 or 46-65 years of age) and its interaction with the treatments. The analyses were performed with R, version 3.4.1 (R Foundation for Statistical Computing). All CIs were calculated at the 2-sided 95% level.
Results
Background Characteristics
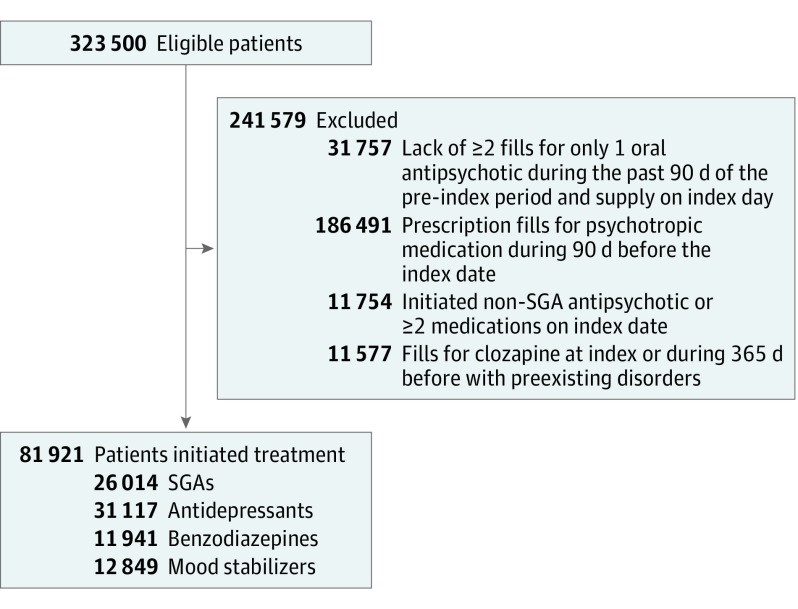
The cohort consisted of 81 921 individuals diagnosed with schizophrenia (mean [SD] age, 40.7 [12.4] years; 37 515 women [45.8%]), who after a period of stable treatment with a single antipsychotic, initiated use of a new psychotropic (Figure 1). Baseline demographic and clinical characteristics of the groups of patients who initiated each treatment after applying the IPTW are presented in Table 1, which shows that all standardized mean differences were less than 0.05. Because this is below the less than 0.10 criterion that is commonly recommended, good balance among the 4 groups was achieved.22 eTable 3 in the Supplement lists the medicines included in each of the psychotropic medication groups and their frequency of use.
Figure 1. Study Cohort Flowchart .
Study eligibility criteria are given in the Methods section. SGA indicates second-generation antipsychotic.
Table 1. Demographic and Clinical Characteristics of Antipsychotic-Treated Patients With Schizophrenia Who Initiated Use of New Psychotropic Medications .
| Characteristic | New Psychotropic Medication Usea | Standardized Mean Differenceb | |||
|---|---|---|---|---|---|
| Another Antipsychotic (n = 26 014) | Mood Stabilizer (n = 12 849) | Antidepressant (n = 31 117) | Benzodiazepine (n = 11 941) | ||
| Female sex | 45.8 | 46.5 | 45.9 | 46.5 | 0.0140 |
| Age, mean (SD), y | 40.5 (12.5) | 40.3 (12.5) | 40.5 (12.4) | 40.5 (12.5) | 0.0152 |
| Race/ethnicity | |||||
| White, non-Hispanic | 35.8 | 36.4 | 35.9 | 36.1 | 0.0124 |
| Black, non-Hispanic | 38.9 | 38.5 | 38.8 | 37.9 | 0.0215 |
| Hispanic or Latino | 2.1 | 2.1 | 2.1 | 2.0 | 0.0039 |
| Asian | 2.6 | 2.5 | 2.6 | 2.7 | 0.0121 |
| Hawaiian or Pacific Islander | 0.9 | 0.9 | 0.9 | 0.9 | 0.0028 |
| Native American or Alaskan Native | 0.2 | 0.2 | 0.2 | 0.2 | 0.0021 |
| >1 Race | 10.1 | 10.2 | 10.0 | 10.3 | 0.0106 |
| Unknown | 9.4 | 9.1 | 9.6 | 9.9 | 0.0265 |
| Medicaid eligibility | |||||
| Disability | 92.3 | 92.2 | 92.4 | 92.5 | 0.0139 |
| Low income | 3.1 | 3.2 | 3.0 | 3.1 | 0.0088 |
| Other | 4.6 | 4.7 | 4.6 | 4.4 | 0.0136 |
| Diagnostic history in the past year | |||||
| Depression | 29.4 | 31.1 | 29.1 | 30.2 | 0.0432 |
| Anxiety | 12.0 | 12.7 | 11.8 | 12.1 | 0.0271 |
| Bipolar | 18.7 | 19.2 | 18.6 | 19.2 | 0.0154 |
| Substance use disorder | 14.7 | 15.6 | 14.5 | 14.7 | 0.0307 |
| Schizophrenia subtype | |||||
| Schizoaffective | 41.7 | 42.5 | 41.1 | 42.1 | 0.0278 |
| Schizophreniform | 3.8 | 3.9 | 3.9 | 4.1 | 0.0113 |
| Psychotropic medication history in the past year | |||||
| Antidepressant | 27.2 | 28.4 | 26.6 | 27.6 | 0.0394 |
| Mood stabilizer | 15.9 | 15.8 | 15.7 | 16.3 | 0.0174 |
| Benzodiazepine | 12.8 | 14.0 | 12.9 | 12.5 | 0.0450 |
Data are presented as inverse probability of treatment weighted percentages unless otherwise indicated.
Standardized mean difference less than 0.05 indicates good balance of characteristics after propensity score weighting.
Psychiatric Hospitalization
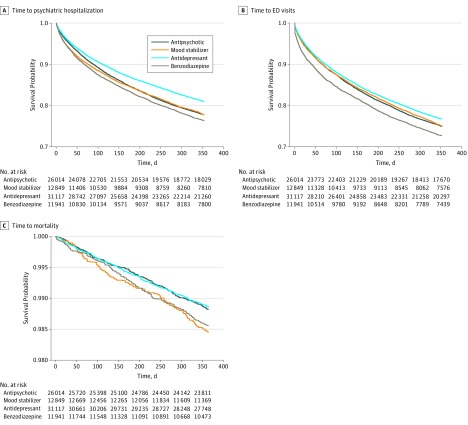
Table 2 contains the primary comparative effectiveness results. For the primary outcome of psychiatric hospitalization, patients initiating treatment with antidepressants had a lower risk of hospitalization compared with those initiating use of a new antipsychotic (hazard ratio [HR], 0.84; 95% CI, 0.80-0.88) (Table 2 and Figure 2A). Compared with patients initiating treatment with a new antipsychotic, patients initiating treatment with benzodiazepines had a higher risk of psychiatric hospitalization (HR, 1.08; 95% CI, 1.02-1.15).
Table 2. Effectiveness and Mortality Outcomes .
| Outcome | Another Antipsychotic (n = 26 014) | Antidepressant (n = 31 117) | Benzodiazepine (n = 11 941) | Mood Stabilizer (n = 12 849) | ||||
|---|---|---|---|---|---|---|---|---|
| No. (%) | Adjusted Hazard Ratio (95% CI)a | No. (%) | Adjusted Hazard Ratio (95% CI)a | No. (%) | Adjusted Hazard Ratio (95% CI)a | No. (%) | Adjusted Hazard Ratio (95% CI)a | |
| Inpatient psychiatric admission | 4965 (19.1) | 1 [Reference] | 5247 (16.9) | 0.84 (0.80-0.88) | 2385 (20.0) | 1.08 (1.02-1.15) | 3093 (24.1) | 0.98 (0.94-1.03) |
| Emergency department visit for mental health reason | 5505 (21.2) | 1 [Reference] | 6508 (20.9) | 0.92 (0.88-0.96) | 2930 (24.5) | 1.12 (1.07-1.19) | 3429 (26.7) | 0.99 (0.94-1.04) |
| Self-injurious behavior | 91 (0.4) | 1 [Reference] | 141 (0.5) | 0.92 (0.68-1.24) | 38 (0.3) | 0.80 (0.50-1.28) | 61 (0.5) | 0.97 (0.64-1.46) |
| Mortality | 259 (1.0) | 1 [Reference] | 327 (1.1) | 0.97 (0.81-1.17) | 204 (1.7) | 1.22 (0.98-1.52) | 161 (1.3) | 1.31 (1.04-1.66) |
| Admissions for cardiovascular disease | 67 (0.3) | 1 [Reference] | 102 (0.3) | 1.24 (0.88-1.75) | 43 (0.4) | 1.43 (0.97-2.09) | 41 (0.3) | 1.31 (0.79-2.18) |
| Diabetesb | 967 (4.5) | 1 [Reference] | 1004 (3.9) | 0.87 (0.79-0.96) | 424 (4.3) | 0.94 (0.82-1.08) | 423 (4.0) | 0.98 (0.85-1.12) |
Adjusted hazard ratios were obtained from propensity score–weighted Cox proportional hazards regression models.
Those with a diagnosis of diabetes during the baseline period were excluded. Therefore, sample sizes were 21 738 for the patients taking another antipsychotic, 26 062 for the antidepressant group, 9764 for the benzodiazepine group, and 10 564 for the mood stabilizer group.
Figure 2. Time to Psychiatric Hospitalization, Emergency Department (ED) Visits, and Mortality After Inverse Probability of Treatment Weighting.
The curves were defined after inverse probability weighting; the unweighted numbers at risk are provided for illustration.
Psychiatric ED Visits
For the psychiatric ED visit outcome, patients initiating treatment with antidepressants had a lower risk of ED visits compared with those who initiated treatment with a new antipsychotic (HR, 0.92; 95% CI, 0.89-0.96) (Table 2 and Figure 2B). Compared with patients initiating treatment with a new antipsychotic, patients initiating treatment with benzodiazepines had a higher risk of psychiatric ED visits (HR, 1.12; 95% CI, 1.07-1.19).
Mortality
Compared with those initiating treatment with a new antipsychotic, patients initiating treatment with mood stabilizers had a higher risk of mortality (HR, 1.31; 95% CI, 1.04-1.66) (Table 2 and Figure 2C). A higher death risk was found for benzodiazepines compared with a new antipsychotic (HR, 1.22; 95% CI, 0.98-1.52).
Because of the unexpected finding of increased risk of mortality associated with mood stabilizers, we investigated deaths according to specific medications. Gabapentin accounted for 1755 initiations (13.7%) of mood stabilizer use and was associated with 45 deaths (28.0%) (eTable 3 in the Supplement). No other mood stabilizer appeared to be associated with a higher rate of death than the others.
Self-injury
No significant differences were found among any of the treatments in risk of self-injury (Table 2).
Medical Outcomes
Use of antidepressants was associated with a lower risk of new-onset diabetes compared with initiating use of a different antipsychotic (HR, 0.87; 95% CI, 0.79-0.96). No significant differences were found in the association between the treatment strategies and risk of admission for a cardiovascular condition (Table 2).
Heterogeneity of Treatment Effects by Age Group and Substance Use Disorder
The presence of a substance use disorder affected the risk of hospitalization among those who initiated treatment with an antidepressant. For initiating antidepressant treatment vs treatment with another antipsychotic, patients without a substance use disorder had reduced psychiatric hospitalization rates (HR, 0.79; 95% CI, 0.75-0.83), but a similar reduction was not observed among those with substance use disorder (HR, 1.02; 95% CI, 0.93-1.11); the relative HR was 1.28 (95% CI, 1.16-1.42). Of note, substance use disorder was associated with a marked increase in risk of psychiatric hospitalization (HR, 2.05; 95% CI, 1.89-2.22).
The risk of hospitalizations associated with initiation of antidepressant and benzodiazepine use varied by age group. The benefit of initiating use of an antidepressant over initiating use of another antipsychotic in association with risk of hospitalization was greater for older (HR, 0.77; 95% CI, 0.71-0.83) than for younger patients (HR, 0.88; 95% CI, 0.83-0.93); the relative HR between older and younger patients was 0.87 (95% CI, 0.79-0.96). For initiating use of a benzodiazepine vs another antipsychotic, younger patients had elevated hospitalization rates (HR, 1.14; 95% CI, 1.06-1.22), but a similar increase was not observed for older patients (HR, 0.98; 95% CI, 0.89-1.08); the relative HR between older and younger patients was 0.86 (95% CI, 0.77-0.98).
Sensitivity Analyses
Multiple sensitivity analyses were performed; results were generally consistent with the main findings. When the intention-to-treat period was limited to 180 days, the findings were similar; as expected, there was a tendency for effects to be stronger during the shorter period. When individuals contributed multiple episodes (n = 103 858), analyses produced the same results (eTable 4 in the Supplement).
Discussion
A wide range of pharmacologic treatment options are available for individuals with schizophrenia that involve numerous individual psychotropic medications that can be used in almost endlessly permutable combinations. Figure 1 reveals the importance of psychotropic polypharmacy in this population by showing that more than half of eligible individuals were excluded because they were already taking multiple psychotropic medications. To generate clinically useful results in this investigation, we focused on a first step to more complicated pharmacotherapy regimens—the initiation of use of a new psychotropic medication among patients with schizophrenia already taking an antipsychotic medication. We focused on classes of psychotropic drugs rather than individual medications because of the large number of medications in each class.
The study examined the comparative effectiveness of different medication strategies among those for whom use of a new psychotropic medication was started. Among this group, adjunctive antidepressants were associated with significantly lower risks of psychiatric hospitalization and ED visits compared with other adjunctive psychotropic strategies. Adjunctive benzodiazepines were associated with increased risks of hospitalization and ED visits but no evidence of benefits compared with the other treatment strategies studied. The significant effects reported here are of similar magnitude to the effect sizes found in a meta-analysis of randomized clinical trials that compared the effectiveness of different antipsychotics24 and meta-analyses that found benefits for antidepressants.11,12 Reduction in the rates of hospitalization and ED visits by more judicious use of antidepressants and benzodiazepines may have important population health consequences considering the large number of people affected by schizophrenia and the considerable costs of these services.
The results of this investigation from the United States are consistent with and extend the findings from Scandinavia.4,5 In both the United States and Scandinavia, antidepressants were associated with reduced risk of mortality. In the United States, benzodiazepines were associated with a slight increase in the risk of death. The current investigation examined additional patient-oriented outcomes and found that antidepressants were also associated with reduced risk of hospitalizations and ED visits for psychiatric disorders and that benzodiazepines were associated with increased risks of psychiatric hospitalization and ED visits. The findings reported here are also consistent with recent meta-analyses that reported clinical benefits associated with use of adjunctive antidepressants.11,12 More specifically, this study and the meta-analysis by Galling and colleagues12 report benefits of adding adjunctive antidepressant medication to ongoing antipsychotic treatment.
Adjunctive mood stabilizers also had no evidence of benefits compared with other medication classes but were associated with increased risk of mortality. Meta-analyses6,7,8,9 of randomized clinical trials of mood stabilizers in the treatment of schizophrenia, both as adjunctive treatments and alone, have been inconclusive. The post hoc finding that gabapentin may be associated with a significant portion of this increased risk was unexpected. To our knowledge, there is no review by the Cochrane Collaboration of the use of gabapentin for schizophrenia, and we found no record of any randomized clinical trial studying it in this population. Although considered to be well tolerated, gabapentin is associated with somnolence and dizziness25 and has been associated with excess suicidal behaviors and suicide.26 In the absence of evidence that gabapentin has benefits in schizophrenia, clinical caution should be exercised if its use is considered.
The heterogeneity of treatment effect analyses, which found reduced hospitalization risk associated with antidepressant use only among people without substance use disorders, is consistent with previous research that found treatment outcome differences only among individuals without illicit substance use.18 This finding is also consistent with the existing literature, which provides little guidance regarding pharmacologic treatment of people with schizophrenia and co-occurring substance use disorders.27
Strengths and Limitations
The present investigation has many strengths and some limitations. The source of data, Medicaid, is the largest insurer of people in treatment for schizophrenia in the United States.16 The results of the study might best apply to individuals in the Medicaid program. Ten years of longitudinal data from 44 states allowed us to conduct, as far as we can determine, the largest comparative effectiveness study of treatments for schizophrenia to date. Because the data do not include information on what prompted the change in medications, it is possible that the treatments were selected for different reasons. For example, patients who started use of another antipsychotic may have had refractory illness, whereas those who started use of a benzodiazepine may have add agitation or anxiety. Propensity scores derived from a large number of covariates helped to control confounding by indication, although residual confounding by unmeasured variables remains possible. The data on medication use are based on prescriptions filled, which are known to be highly accurate,28 but no information was available on actual medication ingestion. The data also do not indicate what symptoms were targeted. For example, antidepressants may be prescribed for depression, anxiety, negative symptoms, and insomnia. In future analyses, we will explore possible explanations for the changes. In addition, we investigated multiple medication strategies and outcomes, increasing the probability of findings attributable to chance. To some extent, however, the consistency of results across outcomes and in sensitivity analyses mitigates this risk.
This study did not compare the association of starting use of a new psychotropic medication with making no change. In this observational data set, patients who made no medication change were likely significantly different—less severely ill or more clinically stable—than those who started taking a new medication. Whether use of a new psychotropic medication should be started is an important but different question that would be best addressed using a placebo-controlled randomized clinical trial.
Because this was an observational study, the results must be interpreted with caution. Additional investigations, including pragmatic randomized clinical trials if possible, are needed to confirm the results. Nevertheless, in the absence of evidence that benzodiazepines or mood stabilizers have benefits when added to antipsychotics in the treatment of schizophrenia, the current findings of poorer outcomes associated with their use compared with new antipsychotics and antidepressants indicate the need for caution regarding their use in this population. The possibility that adjunctive use of gabapentin is associated with increased risk of death raises a serious concern.
Conclusions
The findings suggest that initiating adjunctive treatment with an antidepressant is associated with reduced risk of psychiatric hospitalization and ED visits compared with initiating use of alternative psychotropic medications in patients with schizophrenia. Associations of benzodiazepines and mood stabilizers with poorer outcomes warrant clinical caution and further investigation. The increasing evidence base that supports adjunctive antidepressant treatment in schizophrenia suggests the need for increased clinical interest in this treatment strategy as well as further investigation into the specific situations for which antidepressants are indicated. Applied broadly, improved pharmacologic treatment of schizophrenia and consequent reduced need for hospitalization and ED visits associated with more antidepressant and less benzodiazepine use would represent a significant benefit for individuals and for public health.
eTable 1. Outcome Measures
eTable 2. Covariates Included in Propensity Score Calculation
eTable 3. Treatment Medications and Deaths
eTable 4. Effectiveness and Mortality Outcomes (Episodes*)
References
- 1.Buchanan RW, Kreyenbuhl J, Kelly DL, et al. ; Schizophrenia Patient Outcomes Research Team (PORT) . The 2009 schizophrenia PORT psychopharmacological treatment recommendations and summary statements. Schizophr Bull. 2010;36(1):71-93. doi: 10.1093/schbul/sbp116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Correll CU, Rubio JM, Inczedy-Farkas G, Birnbaum ML, Kane JM, Leucht S. Efficacy of 42 pharmacologic cotreatment strategies added to antipsychotic monotherapy in schizophrenia: systematic overview and quality appraisal of the meta-analytic evidence. JAMA Psychiatry. 2017;74(7):675-684. doi: 10.1001/jamapsychiatry.2017.0624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ballon J, Stroup TS. Polypharmacy for schizophrenia. Curr Opin Psychiatry. 2013;26(2):208-213. doi: 10.1097/YCO.0b013e32835d9efb [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tiihonen J, Mittendorfer-Rutz E, Torniainen M, Alexanderson K, Tanskanen A. Mortality and cumulative exposure to antipsychotics, antidepressants, and benzodiazepines in patients with schizophrenia: an observational follow-up study. Am J Psychiatry. 2016;173(6):600-606. doi: 10.1176/appi.ajp.2015.15050618 [DOI] [PubMed] [Google Scholar]
- 5.Tiihonen J, Suokas JT, Suvisaari JM, Haukka J, Korhonen P. Polypharmacy with antipsychotics, antidepressants, or benzodiazepines and mortality in schizophrenia. Arch Gen Psychiatry. 2012;69(5):476-483. doi: 10.1001/archgenpsychiatry.2011.1532 [DOI] [PubMed] [Google Scholar]
- 6.Leucht S, Helfer B, Dold M, Kissling W, McGrath JJ. Lithium for schizophrenia. Cochrane Database Syst Rev. 2015;(10):CD003834. doi: 10.1002/14651858.CD003834.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwarz C, Volz A, Li C, Leucht S. Valproate for schizophrenia. Cochrane Database Syst Rev. 2008;(3):CD004028. [DOI] [PubMed] [Google Scholar]
- 8.Leucht S, Helfer B, Dold M, Kissling W, McGrath J. Carbamazepine for schizophrenia. Cochrane Database Syst Rev. 2014;(5):CD001258. doi: 10.1002/14651858.CD001258.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Premkumar TS, Pick J. Lamotrigine for schizophrenia. Cochrane Database Syst Rev. 2006;(4):CD005962. Review. [DOI] [PubMed] [Google Scholar]
- 10.Volz A, Khorsand V, Gillies D, Leucht S. Benzodiazepines for schizophrenia. Cochrane Database Syst Rev. 2007;(1):CD006391. [DOI] [PubMed] [Google Scholar]
- 11.Helfer B, Samara MT, Huhn M, et al. Efficacy and safety of antidepressants added to antipsychotics for schizophrenia: a systematic review and meta-analysis. Am J Psychiatry. 2016;173(9):876-886. doi: 10.1176/appi.ajp.2016.15081035 [DOI] [PubMed] [Google Scholar]
- 12.Galling B, Vernon JA, Pagsberg AK, et al. Efficacy and safety of antidepressant augmentation of continued antipsychotic treatment in patients with schizophrenia. Acta Psychiatr Scand. 2018;137(3):187-205. doi: 10.1111/acps.12854 [DOI] [PubMed] [Google Scholar]
- 13.Fleischhacker WW, Uchida H. Critical review of antipsychotic polypharmacy in the treatment of schizophrenia. Int J Neuropsychopharmacol. 2014;17(7):1083-1093. doi: 10.1017/S1461145712000399 [DOI] [PubMed] [Google Scholar]
- 14.Correll CU, Rummel-Kluge C, Corves C, Kane JM, Leucht S. Antipsychotic combinations vs monotherapy in schizophrenia: a meta-analysis of randomized controlled trials. Schizophr Bull. 2009;35(2):443-457. doi: 10.1093/schbul/sbn018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ray WA. Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol. 2003;158(9):915-920. doi: 10.1093/aje/kwg231 [DOI] [PubMed] [Google Scholar]
- 16.Khaykin E, Eaton WW, Ford DE, Anthony CB, Daumit GL. Health insurance coverage among persons with schizophrenia in the United States. Psychiatr Serv. 2010;61(8):830-834. doi: 10.1176/ps.2010.61.8.830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kozma CM, Weiden PJ. Partial compliance with antipsychotics increases mental health hospitalizations in schizophrenic patients: analysis of a national managed care database. Am Health Drug Benefits. 2009;2(1):31-38. [PMC free article] [PubMed] [Google Scholar]
- 18.Swartz MS, Wagner HR, Swanson JW, et al. ; CATIE Investigators . The effectiveness of antipsychotic medications in patients who use or avoid illicit substances: results from the CATIE study. Schizophr Res. 2008;100(1-3):39-52. doi: 10.1016/j.schres.2007.11.034 [DOI] [PubMed] [Google Scholar]
- 19.Kreyenbuhl J, Marcus SC, West JC, Wilk J, Olfson M. Adding or switching antipsychotic medications in treatment-refractory schizophrenia. Psychiatr Serv. 2007;58(7):983-990. doi: 10.1176/ps.2007.58.7.983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70(1):41-55. doi: 10.1093/biomet/70.1.41 [DOI] [Google Scholar]
- 21.Tan Z. Bounded, efficient, and doubly robust estimation with inverse weighting. Biometrika. 2010;97:661-682. doi: 10.1093/biomet/asq035 [DOI] [Google Scholar]
- 22.Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34(28):3661-3679. doi: 10.1002/sim.6607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joffe MM, Ten Have TR, Feldman HI, Kimmel SE. Model selection, confounder control, and marginal structural models: review and new applications. Am Stat. 2004;58(4):272-279. doi: 10.1198/000313004X5824 [DOI] [Google Scholar]
- 24.Leucht S, Cipriani A, Spineli L, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382(9896):951-962. doi: 10.1016/S0140-6736(13)60733-3 [DOI] [PubMed] [Google Scholar]
- 25.Zaccara G, Gangemi PF, Cincotta M. Central nervous system adverse effects of new antiepileptic drugs: a meta-analysis of placebo-controlled studies. Seizure. 2008;17(5):405-421. doi: 10.1016/j.seizure.2007.12.003 [DOI] [PubMed] [Google Scholar]
- 26.Patorno E, Bohn RL, Wahl PM, et al. Anticonvulsant medications and the risk of suicide, attempted suicide, or violent death. JAMA. 2010;303(14):1401-1409. doi: 10.1001/jama.2010.410 [DOI] [PubMed] [Google Scholar]
- 27.Smelson DA, Dixon L, Craig T, et al. Pharmacological treatment of schizophrenia and co-occurring substance use disorders. CNS Drugs. 2008;22(11):903-916. doi: 10.2165/00023210-200822110-00002 [DOI] [PubMed] [Google Scholar]
- 28.West SL, Savitz DA, Koch G, Strom BL, Guess HA, Hartzema A. Recall accuracy for prescription medications: self-report compared with database information. Am J Epidemiol. 1995;142(10):1103-1112. doi: 10.1093/oxfordjournals.aje.a117563 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Outcome Measures
eTable 2. Covariates Included in Propensity Score Calculation
eTable 3. Treatment Medications and Deaths
eTable 4. Effectiveness and Mortality Outcomes (Episodes*)