Abstract
Objectives: Neovascularization represents a major challenge in tissue engineering applications since implantation of voluminous grafts without sufficient vascularity results in hypoxic cell death of implanted cells. An attractive therapeutic approach to overcome this is based on co‐implantation of endothelial cells to create vascular networks. We have investigated the potential of human endothelial progenitor cells (EPC) to form functional blood vessels in vivo in direct comparison to vascular‐derived endothelial cells, represented by human umbilical vein endothelial cells (HUVEC).
Materials and methods: EPCs were isolated from human peripheral blood, expanded in vitro and analysed in vitro for phenotypical and functional parameters. In vivo vasculogenic potential of EPCs and HUVECs was evaluated in a xenograft model where spheroidal endothelial aggregates were implanted subcutaneously into immunodeficient mice.
Results: EPCs were indistinguishable from HUVECs in terms of expression of classical endothelial markers CD31, von Willebrand factor, VE‐cadherin and vascular endothelial growth factor‐R2, and in their ability to endocytose acetylated low‐density lipoprotein. Moreover, EPCs and HUVECs displayed almost identical angiogenic potential in vitro, as assessed by in vitro Matrigel sprouting assay. However in vivo, a striking and unexpected difference between EPCs and HUVECs was detected. Whereas implanted HUVEC spheroids gave rise to formation of a stable network of perfused microvessels, implanted EPC spheroids showed significantly impaired ability to form vascular structures under identical experimental conditions.
Conclusion: Our results indicate that vascular‐derived endothelial cells, such as HUVECs are superior to EPCs in terms of promoting in vivo vascularization of engineered tissues.
Introduction
Tissue vascularization is one of the major challenges to be addressed for clinical success of tissue engineering applications. Without rapid and high level of vascularization of transplanted grafts, the majority of cells fail to survive the early post‐transplantational phase (1). Amongst therapeutic approaches to overcome the problem of insufficient vascularization are use of angiogenic growth factors embedded into appropriate scaffolds to promote ingrowth of microvessels (2, 3, 4), and cell‐based concepts to enhance vasculogenesis (5, 6). Using Bcl‐2‐transduced human umbilical vein endothelial cells (HUVEC), microvascular networks in collagen‐fibronectin gels have been formed within 4 weeks of implantation into immunodeficient mice (5). Similar results have been reported with HUVECs co‐implanted with mesenchymal precursor cells, which led to formation of functional, long‐lasting microvessels upon implantation into immunocompromised mice (6). However, this effect was strictly dependent on co‐seeding of mesenchymal precursor cells having the potential to differentiate in vivo into mural cells, suggesting that stabilization of newly formed vessels by mural cells represents a critical step in generating stable vasculature. We have recently reported the development of a spheroid‐based endothelial cell transplantation technique for formation of durable and perfused blood vessels in vivo (7). In this model, Matrigel/fibrin‐immobilized spheroidal HUVECs were able to form a network of stable and perfused blood vessels in mice without the need of co‐seeding mural cells.
Endothelial progenitor cells (EPC) represent a very interesting alternative cell source for mature endothelial cells in tissue engineering applications. EPCs can be found in peripheral blood as well as in the bone marrow (8), from which they can be mobilized by action of various growth factors and cytokines (9, 10). EPCs have already been used as a cell source for enhancing angiogenesis in the field of regenerative medicine, and have been proven to be effective in regenerating infarcted myocardium by supporting neovascularization (11, 12).
In the present study, we investigated whether human EPCs would have the potential to form functional blood vessels in vivo. To test this, we analysed in vivo vasculogenic potential of EPCs in direct comparison to vascular‐derived endothelial cells (HUVECs), in a xenograft model where human spheroidal endothelial aggregates were mixed in a Matrigel/fibrin matrix and implanted subcutaneously into SCID mice. Whereas implanted HUVEC spheroids gave rise to complex and stable three‐dimensional networks of perfused human neovessels, which maturated by recruiting mouse mural cells, implanted EPC spheroids showed significantly impaired ability to form blood vessels in vivo. Moreover, the few vessels derived upon EPC spheroid implantation were not able to recruit mouse perivascular cells and were not perfused.
Although HUVECs and EPCs have almost identical phenotypical and functional properties in vitro, in vivo data clearly demonstrate an important difference between vascular‐derived endothelial cells and EPCs regarding their in vivo vasculogenic potential.
Materials and methods
Isolation of human EPCs and cell culture
Human EPCs from five different donors were isolated and expanded as previously described (13, 14). In brief, peripheral blood mononuclear cells were isolated from 50 ml of peripheral blood from human volunteers or from human blood‐derived buffy coats, with slight modification by density gradient centrifugation with biocoll separation solution (Biochrom, Berlin, Germany). Cells from individual volunteers were plated in 25‐cm2 culture flasks coated with rat type I collagen (50 µg/ml) in EGM‐2 (Lonza, Cologne, Germany) with supplement mix (human epidermal growth factor, vascular endothelial growth factor (VEGF), human fibroblast growth factor‐B, R3‐insulin‐like growth factor‐1, ascorbic acid, heparin, gentamicin/amphotericin‐B) and 10% foetal calf serum (FCS), and were cultured at 37 °C with 5% CO2, in a humidified atmosphere. After 4 days in culture, non‐adherent cells were removed by washing with phosphate‐buffered saline (PBS), new media were applied, and cultures were maintained for another 4–5 weeks. Media were changed every 3 days. After reaching approximately 80% confluence, cells were trypsinized then seeded into 75‐cm2 culture flasks, coated with human fibronectin (50 µg/ml) (Sigma, Deisenhofen, Germany) for expansion. EPCs from passages 2–5 were used for experiments.
HUVECs were purchased from Promocell (Heidelberg, Germany) and were cultured in endothelial cell growth medium (ECGM, Promocell) supplemented with 10% FCS and supplement mix (epidermal growth factor, hydrocortisone, basic fibroblast growth factor (bFGF)) at 37 °C and 5% CO2 in 75‐cm2 tissue culture flasks. HUVECs from passages 2 to 5 were used for experiments.
Cell staining
For acetylated low‐density lipoprotein (acLDL) uptake experiments, adherent cells were incubated in EGM‐2 (Lonza, Cologne, Germany) supplemented with single aliquots and 10% FCS with 20 µg/ml (Dil)‐labelled acLDL (Invitrogen, Karlsruhe, Germany) for 4 h at 37 °C in 5% CO2 in a humidified atmosphere. After washing with PBS, cells were fixed with 2% paraformaldehyde for 10 min at 37 °C. After fluorescence staining, intensity was measured using an inverted fluorescence microscope using the rhodamine filter setting.
For immunostaining, cells were fixed in methanol and were incubated for 30 min with blocking solution (5% goat serum, Sigma) followed by incubation with the respective primary antibodies, monoclonal mouse anti‐CD31 antibody (diluted 1 : 25, Dako, Hamburg, Germany), monoclonal mouse anti‐VEGF‐R2 antibody (antibody 3G2, diluted 1 : 200) (15), monoclonal mouse anti‐VE‐cadherin antibody (diluted 1 : 100, Santa Cruz Biotechnology, Santa Cruz, CA, USA), monoclonal mouse anti‐CD45 antibody FITC‐conjugated (diluted 1 : 100, Becton Dickinson, Heidelberg, Germany), monoclonal mouse anti‐CD14 antibody FITC‐conjugated (diluted 1 : 100, Becton Dickinson) and monoclonal mouse anti‐von Willebrand factor antibody (diluted 1 : 200, Dako), for 1 h at room temperature. After washing with PBS, corresponding secondary antibodies (ready‐to‐use horseradish peroxidase‐conjugated goat anti‐mouse immunoglobulin; Dako) were applied and incubated for another 30 min. Thereafter, cells were exposed to AEC chromogen substrate (ready‐to‐use, Dako) and were weakly counterstained with haematoxylin. For FITC‐fluorescence detection, cells were photographed using an inverted fluorescence microscope.
For histochemical examination of in vivo blood vessel formation, Matrigel/fibrin implants were removed 3 weeks after xenografting, fixed in buffered formalin for 24 h, embedded in paraffin wax and sectioned at 5 µm. Sections were stained with haematoxylin and eosin (H&E) and examined for presence of luminal structures. Immunohistochemical analyses were performed on deparaffinised, rehydrated sections. Sections for immunoperoxidase staining were treated with 3% H2O2 to inhibit activity of endogenous peroxidase. After washing in PBS, sections were incubated for 30 min with blocking solution (goat serum, ready‐to‐use, Zymed, San Francisco, CA, USA) followed by incubation with corresponding mouse monoclonal primary antibodies: anti‐human CD34 (diluted 1 : 75, Novocastra, Newcastle upon Tyne, UK) or anti‐human CD31, (diluted 1 : 25, Dako) in a humidity chamber at room temperature for 1 h. For immunofluorescence, sections were incubated with secondary goat anti‐mouse/Alexa 488 antibody (diluted 1 : 200, Invitrogen, Karlsruhe, Germany) and DAPI (diluted 1 : 1000, Sigma). Afterwards, sections were incubated for 1 h with anti‐α smooth muscle actin‐Cy3 (diluted 1 : 100, Sigma). For immunoperoxidase staining sections were incubated with horseradish peroxidase‐labelled ready‐to‐use goat anti‐mouse immunoglobulin antibody (diluted 1 : 200, Dako), developed with AEC substrate (Dako) and weakly counterstained with haematoxylin.
Matrigel assay
Matrigel® (Becton Dickinson, Heidelberg, Germany) was thawed on ice overnight and spread evenly over each well (30 µl) of a 24‐well plate. Plates were incubated for 30 min at 37 °C to allow the Matrigel to solidify. Thirty thousand HUVECs or EPCs were seeded in triplicate into each well in 500 µl of ECGM (Promocell) supplemented with 10% FCS and supplement mix. Plates were then incubated at 37 °C and 5% CO2 in a humidified atmosphere for 17 h. Then, cells were fixed in 4% formalin. Images were captured at ×100 magnification and analysed using freeware image analysis program Image J (http://rsb.info.nih.gov). For quantification, representative fields from each group (n = 3) were taken and total length of resultant tube per field was calculated, as well as number of branch points. Results for different experimental groups were expressed as mean ± standard deviation from three independent experiments.
Generation of endothelial spheroids
HUVEC and EPC spheroids (500 cells/spheroid) were generated as previously described (16). Cells were suspended in culture medium containing 0.25% (w/v) methylcellulose and were seeded on plastic dishes as hanging drops to allow overnight spheroid aggregation. Under these conditions, all suspended cells contributed to formation of a single spheroid per drop, of defined size and cell number.
Implantation of spheroids into SCID mice
Four‐ to 6‐week‐old SCID mice (C.B.‐17‐SCID, Harlan Winkelmann, Borchen, Germany) served as recipients of cell‐seeded matrices. German regulations for care and use of laboratory animals were observed at all times. All experiments were approved by the animal care committee of the University of Freiburg. Animals were housed in the veterinary care facility of the University of Freiburg Medical Centre. Implantation of spheroids was performed as previously described (7). In brief, spheroids were harvested, washed in endothelial cell basal medium (Promocell), centrifuged and mixed in 500 µl Matrigel (growth factor reduced; BD Biosciences, Heidelberg, Germany) and fibrinogen (final concentration 2 mg/ml; Merck, Darmstadt, Germany) containing angiogenic growth factors VEGF and bFGF (500 ng each; R&D Systems, Wiesbaden, Germany). Thrombin (0.4 U, Merck) was added to the mixture before subcutaneous injection on each side, lateral to the abdominal midline of SCID mice. One thousand spheroids were used per implant. EPC and HUVEC spheroids were either implanted individually in different animals or in the same animal on opposite sides lateral to the abdominal midline. In total, nine mice were used for the implantation studies. Twenty‐one days after implantation, mice were killed and implants were retrieved.
Quantification of blood vessels
Each explant was entirely sectioned and three samples were taken from the front, the middle and the back of the scaffold. The selected sections were stained for human CD31 (Dako). Microscopic pictures were taken at ×100 magnification of three randomized areas per section. CD31‐positive structures were manually counted in the matrix area and calculated as vessel number per square millimetre.
Results
Immunological and functional characterization of EPCs
Human EPCs were isolated from the mononuclear cell fraction of peripheral blood samples and cultured on collagen type I‐coated culture dishes. After 2–4 weeks, so‐called late EPC‐, also referred to by other authors as outgrowth endothelial cell colonies (17), were visible with typical cobblestone morphology (Fig. 1a). Cells were expanded from few in number to a monolayer, and showed multiple population doublings without senescence. Endothelial phenotype of expanded EPCs was confirmed by immunohistochemistry, demonstrating expression of endothelial markers CD31, von Willebrand factor, VE‐cadherin and VEGF‐R2 (Fig. 1a), whereas expression of haematopoietic‐specific surface antigen CD45 and monocyte/macrophage cell surface antigen CD14 were not detectable (data not shown). These results are in agreement with previous studies where EPCs have been analysed for these endothelial markers, by means of flow cytometry, reverse transcription–polymerase chain reaction and immunofluorescence (13). Moreover, expanded EPCs were also able to incorporate acLDL, which is also a hallmark of endothelial lineage cells (Fig. 1b). Of importance, EPCs were indistinguishable from HUVECs in terms of cell morphology, endothelial marker expression or acLDL uptake, demonstrating that cells isolated and expanded from adult peripheral blood were, indeed endothelial cells (Fig. 1). In addition to phenotypical characterization, we tested in vitro angiogenic potential of EPCs, in direct comparison to HUVECs, in the Matrigel sprouting assay (Fig. 2). Tube formation was quantified by measuring two parameters: total tube lengths (Fig. 2a) and number of branch points (Fig. 2b). No significant difference could be detected between EPCs and HUVECs in their ability to form capillary‐like sprouts in vitro.
Figure 1.
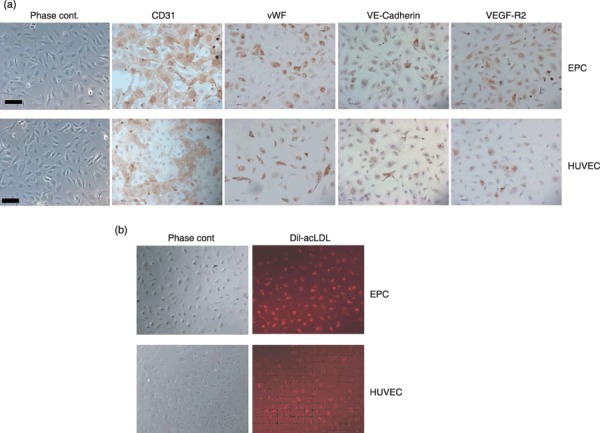
Morphology and immunohistochemical characterization of endothelial progenitor cells (EPC) in comparison to human umbilical vein endothelial cells (HUVEC). (a) Phase contrast images and immunohistochemical analysis of late EPCs and HUVECs for CD31, von Willebrand factor, VE‐cadherin and VEGF‐R2 (magnification ×200, scale bar 100 µm). (b) Uptake of Dil‐acLDL (red fluorescence) by late EPCs and HUVECs (magnification ×100).
Figure 2.
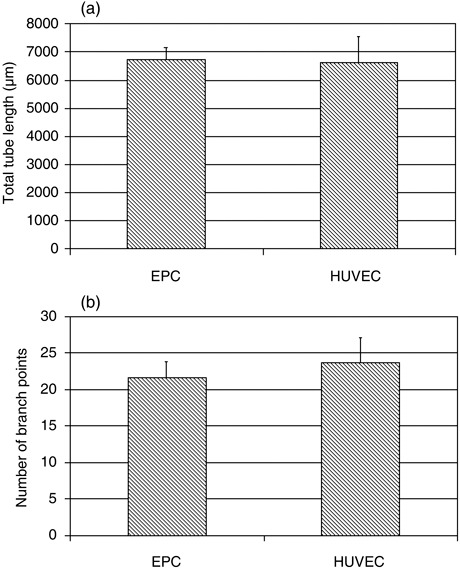
Quantification of in vitro tube formation by endothelial progenitor cells (EPC) and human umbilical vein endothelial cells (HUVEC) in Matrigel assay. Total tube length (a) and number of branch points (b) of EPCs and HUVECs grown on Matrigel for 17 h. Images used for quantification were captured at ×100 magnification. Mean values ± standard deviation from three experiments are shown.
In vivo vasculogenic potential of EPCs and HUVECs implanted subcutaneously into SCID mice
We have previously shown that upon implantation, HUVEC spheroids are able to form a network of stable and perfused blood vessels in immunodeficient mice without the need of co‐seeding supporting mural cells (7).
In order to investigate whether EPCs would be able to form functional blood vessels in vivo, we implanted EPC spheroids in a Matrigel/fibrin matrix, containing angiogenic growth factors VEGF and bFGF, on one side lateral to the dorsal midline region of SCID mice. On the opposite side, HUVEC spheroids were implanted. This experimental design allows direct comparison of vasculogenic potential of EPCs and HUVECs in the same animal. Implants were dissected after 3 weeks and analysed for formation of human endothelial cell‐derived vasculature. H&E staining revealed presence of vascular structures in high density in HUVEC implants, whereas very few microvessels were detectable in EPC implants (Fig. 3a).
Figure 3.
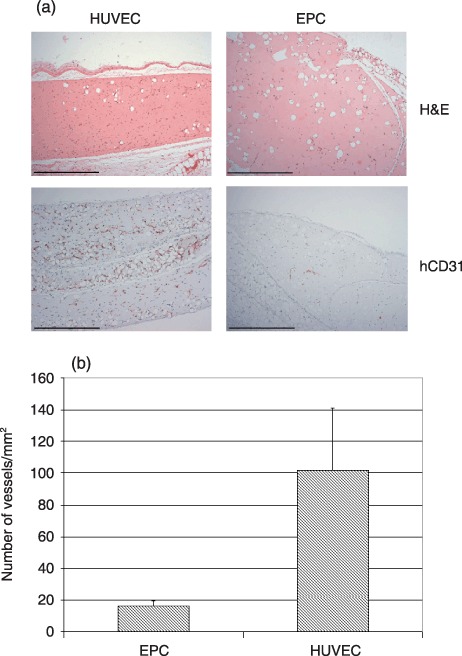
In vivo vasculogenic potential of endothelial progenitor cell (EPC) spheroids in direct comparison to human umbilical vein endothelial cell (HUVEC) spheroids. Matrigel/fibrin constructs containing EPC or HUVEC spheroids were implanted subcutaneously into SCID mice and retrieved after 3 weeks. (a) Haematoxylin and eosin (H&E) and hCD31 staining of cross‐sections (magnification ×100, scale bar 500 µm). (b) Microvessel density in implants was quantified by counting hCD31‐positive vessel structures. Values are expressed as number of vessels per square millimetre. Means + standard deviations form three different constructs per experimental group are shown.
Sections of implants were also immunohistochemically stained using human‐specific anti‐CD31 antibody, to visualize transplanted endothelial cells. This staining revealed dense networks of human CD31‐positive neovessels in HUVEC implants, whereas upon implantation of EPC spheroids, very few CD31‐positive microvessels could be detected (Fig. 3a). Similar results were obtained with EPC preparations from four different donors. Quantification of hCD31‐positive vessel structures revealed vascular densities of 101.5 ± 39.5 microvessels/mm2 for HUVEC implants and 15.9 ± 3.2 microvessels/mm2 in EPC spheroids (Fig. 3b). Of importance, vessel densities were significantly lower in EPC implants in comparison to HUVEC implants. The immunohistochemical experiments, carried out with human‐specific anti‐CD31 antibody, clearly revealed that newly formed vasculature in the implants was of human origin and not formed by angiogenic ingrowth of mouse blood vessels, from surrounding mouse tissue. Human vasculature that emerged from implanted HUVEC spheroids formed anastomoses with mouse vasculature and perfused blood vessels, as evidenced by presence of intraluminal mouse erythrocytes, could be detected at higher magnification (Fig. 4). In contrast, no intraluminal mouse erythrocytes were detectable in EPC‐derived vessels.
Figure 4.
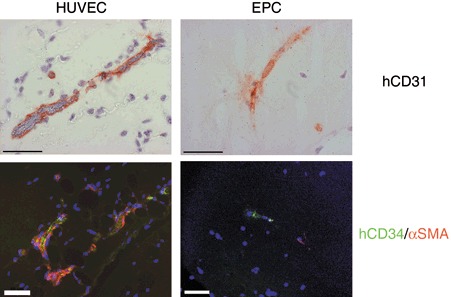
In vivo analysis of vessel maturation and perfusion. Sections from human umbilical vein endothelial cell (HUVEC) and endothelial progenitor cell (EPC) implants were stained for hCD31 and analysed for the presence of mouse red blood cells, at high magnification (magnification ×630, scale bar 50 µm). Coverage of human blood vessels by mouse perivascular cells was analysed by immunofluorescence staining with antibodies against hCD34 (human‐specific, green) and α‐smooth muscle actin (αSMA, red). Nuclei stained with DAPI (blue) (magnification ×400, scale bar 50 µm). Note α‐smooth muscle actin‐positive mouse cells (red) recruited to the HUVEC‐derived blood vessels (green) but not to EPC‐derived vessels.
We also performed double staining for human CD34 and α‐smooth muscle actin (αSMA) on sections of implants seeded with HUVEC or EPC spheroids, in order to investigate maturation of newly formed vessels. As shown in Fig. 4, newly formed HUVEC‐derived blood vessels were covered with α‐smooth muscle actin‐positive cells, indicating that vessels were stabilized by murine pericytes or smooth muscle cells, recruited from surrounding mouse tissue. In contrast, no covering of EPC‐derived neovessels by mouse perivascular cells could be detected.
In summary, our experiments showed that although EPCs and HUVECs display similar phenotypical and functional characteristics in vitro, they clearly differ in in vivo vasculogenic potential, with EPCs displaying dramatically lower ability to form stable and perfused vascular networks. These results suggest that vascular‐derived endothelial cells, such as HUVECs, are superior to EPCs in terms of promoting in vivo vascularization of engineered tissues.
Discussion
For applications in tissue engineering, construction of stable and perfusable blood vessels is crucial for graft survival after transplantation. Without generation of a functional vascular network, implanted cells undergo cell death due to limitation in oxygen and nutrient supply. It is known from the literature that tissue vascularization can be promoted by cell‐based approaches using vascular‐derived endothelial cells (5, 6, 18). HUVECs seeded into collagen/fibronectin gels have been shown to be able to form functional blood vessels after implantation into immunodeficient mice (5). However, this process was only effective when cells were transduced with caspase‐resistant Bcl‐2, which leads to delay in apoptosis and to recruitment of perivascular α‐smooth muscle actin‐expressing mouse cells, and hence, to stabilization of the newly formed vessels. Recent work from other groups suggests that stable blood vessels can only be formed in vivo when HUVECs are co‐implanted with other cell types having potential to stabilize the neovessels. This has been shown in co‐implantation studies of HUVECs with mesenchymal precursor cells (6) or embryonic fibroblasts (18). In both cases, co‐implanted cells differentiated in vivo into supporting mural cells and contributed to maturation and stabilization of the engineered blood vessels. These results suggest that durable blood vessels, in a tissue engineering setting, can only be formed when implanted endothelial cells undergo the complete angiogenic cascade, consisting of vessel assembly, maturation and stabilization (19, 20, 21). We have recently reported an implantation technique based on HUVECs grown in three‐dimensional spheroidal configuration (7); this model was originally developed as an in vitro assay for studying endothelial cell differentiation and maturation (16, 22). The finding that spheroid aggregation stabilized endothelial cells and rendered them responsive to survival factors, led to the assumption that endothelial cell spheroids could be used as focal starting points for sprouting of capillaries in vivo. Indeed, upon subcutaneous implantation of HUVEC spheroids immobilized in a Matrigel/fibrin matrix supplemented with angiogenic growth factors VEGF and bFGF into SCID mice, development of a complex network of perfused human neovessels could be observed (7). Of importance, in vivo vascularization emerging from implanted HUVEC spheroids was evident in the absence of co‐implanted supporting perivascular cells.
We therefore intended to investigate whether EPCs isolated from peripheral blood and implanted in spheroidal conformation would have similar vasculogenic in vivo potential as HUVECs. In this context, we used the HUVECs as a kind of positive control in our xenotransplantation assay. However, whereas implanted HUVEC spheroids gave rise to formation of stable and dense networks of perfused microvessels, implanted EPC spheroids showed a significantly lower ability to form vascular sprouts in vivo. Moreover, the few vascular structures emerging from implanted EPC spheroids were neither perfused with erythrocytes, nor covered by mouse mural cells.
Absence of perivascular mural cell coverage of EPC‐derived neovessels was of particular interest. It is well established that endothelial cells produce factors, such as PDGF‐BB (23, 24), hepatocyte growth factor (25) and transforming growth factor‐beta (19), which play important roles in recruitment of pericytes or smooth muscle cells during vascular maturation. It may be interesting in future experiments to investigate whether absence of mural cell coverage of implanted EPCs may be related to reduced expression of these chemoattractant molecules.
Our result on the impaired ability of implanted EPCs to form functional vessels is in agreement with recent reports from other groups, where peripheral blood‐ or cord blood‐derived EPCs were tested for their in vivo vasculogenic potential. Au et al. (26) implanted EPCs in collagen/fibronectin gel onto the pial surface in cranial windows of SCID mice and saw that implantation of EPCs alone was not effective in forming stable vasculature. Similarly, cord‐derived EPCs dispersed in Matrigel matrix and implanted subcutaneously into immunodeficient mice, failed to develop microvessels (27). However, the same authors have shown that upon co‐implantation of 10T1/2 cells (26) or human saphenous vein smooth muscle cells (27) EPCs were able to develop stable vascular networks in vivo. These results indicate that, as previously demonstrated for HUVECs implanted in single cell configuration, in vivo formation of durable vessels from implanted EPCs is strongly dependent on co‐seeding of supporting perivascular cells.
In our study, we observed impaired vasculogenic potential of EPCs after 3 weeks of in vivo growth. This raises the question of whether the lower ability of EPCs to form vascular structures is the result of impaired vessel assembly or of enhanced disintegration of neovessels. Results from Au et al. strongly support the hypothesis that low vessel density in EPC implants is the consequence of reduced vessel‐forming potential of EPCs (26).
The observed difference in in vivo vasculogenic potential between spheroidal HUVECs and EPCs reported here emphasizes an important difference of in vivo function between vascular‐derived endothelial cells and EPCs. However, it should be noted that our experiments were performed in a xenogenic transplantation model and cannot be completely transferred to an autologous setting. Therefore, it is difficult to predict usefulness of this approach for direct human application. This also raises questions concerning physiological function of EPCs in neovascularization of ischaemic tissues. It is still a matter of debate to what extent EPCs contribute to new vessel formation in the adult. Several studies have shown a significant contribution of bone marrow‐derived EPC to human tumour endothelium (9, 28), while others were not able to define a critical role of EPCs in tumour neovascularization (29, 30). Therefore in the human setting, integration of EPCs into neovessels of ischaemic tissues has not been convincingly shown. On the other hand, EPCs clearly show therapeutic potential to improve angiogenesis in patients with cardiovascular disease (31). This effect might be attributed to secretion of high amounts of angiogenic growth factors by transplanted EPCs, thereby promoting angiogenesis in previously ischaemic tissues (32, 33).
In summary, our experiments support the hypothesis that EPCs are not very effective in forming stable vasculature in vivo. Although EPCs and vascular‐derived endothelial cells, represented by HUVECs, display similar phenotypical and functional characteristics in vitro, EPCs clearly show less ability to form vascular networks in vivo upon implantation into immunodeficient mice.
Acknowledgements
We thank Beate vom Hoevel for excellent technical assistance and Arash Momeni for critical reading of the manuscript. This work was supported by funding through the Deutsche Forschungsgemeinschaft (STA‐472/1‐1).
This work was performed at the Department of Plastic and Hand Surgery, University of Freiburg Medical Center, Freiburg, Germany.
References
- 1. Shieh SJ, Vacanti JP (2005) State‐of‐the‐art tissue engineering: from tissue engineering to organ building. Surgery 137, 1–7. [DOI] [PubMed] [Google Scholar]
- 2. Baumgartner I, Pieczek A, Manor O, Blair R, Kearney M, Walsh K et al (1998) Constitutive expression of phVEGF165 after intramuscular gene transfer promotes collateral vessel development in patients with critical limb ischemia. Circulation 97, 1114–1123. [DOI] [PubMed] [Google Scholar]
- 3. Schumacher B, Pecher P, Von Specht BU, Stegmann T (1998) Induction of neoangiogenesis in ischemic myocardium by human growth factors: first clinical results of a new treatment of coronary heart disease. Circulation 97, 645–650. [DOI] [PubMed] [Google Scholar]
- 4. Henry TD, Rocha‐Singh K, Isner JM, Kereiakes DJ, Giordano FJ, Simons M et al (2001) Intracoronary administration of recombinant human vascular endothelial growth factor to patients with coronary artery disease. Am. Heart J. 142, 872–880. [DOI] [PubMed] [Google Scholar]
- 5. Schechner JS, Nath AK, Zheng L, Kluger MS, Hughes CC, Sierra‐Honigmann MR et al (2000) In vivo formation of complex microvessels lined by human endothelial cells in an immunodeficient mouse. Proc. Natl. Acad. Sci. USA 97, 9191–9196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Koike N, Fukumura D, Gralla O, Au P, Schechner JS, Jain RK (2004) Tissue engineering: creation of long‐lasting blood vessels. Nature 428, 138–139. [DOI] [PubMed] [Google Scholar]
- 7. Alajati A, Laib AM, Weber H, Boos AM, Bartol A, Ikenberg K et al (2008) Spheroid‐based engineering of a human vasculature in mice. Nat. Methods 5, 439–445. [DOI] [PubMed] [Google Scholar]
- 8. Asahara T, Murohara T, Sullivan A, Silver M, Van Der Zee R, Li T et al (1997) Isolation of putative progenitor endothelial cells for angiogenesis. Science 275, 964–967. [DOI] [PubMed] [Google Scholar]
- 9. Hattori K, Dias S, Heissig B, Hackett NR, Lyden D, Tateno M et al (2001) Vascular endothelial growth factor and angiopoietin‐1 stimulate postnatal hematopoiesis by recruitment of vasculogenic and hematopoietic stem cells. J. Exp. Med. 193, 1005–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Heeschen C, Aicher A, Lehmann R, Fichtlscherer S, Vasa M, Urbich C et al (2003) Erythropoietin is a potent physiologic stimulus for endothelial progenitor cell mobilization. Blood 102, 1340–1346. [DOI] [PubMed] [Google Scholar]
- 11. Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B et al (2001) Bone marrow cells regenerate infarcted myocardium. Nature 410, 701–705. [DOI] [PubMed] [Google Scholar]
- 12. Kawamoto A, Gwon HC, Iwaguro H, Yamaguchi JI, Uchida S, Masuda H et al (2001) Therapeutic potential of ex vivo expanded endothelial progenitor cells for myocardial ischemia. Circulation 103, 634–637. [DOI] [PubMed] [Google Scholar]
- 13. Fuchs S, Hermanns MI, Kirkpatrick CJ (2006) Retention of a differentiated endothelial phenotype by outgrowth endothelial cells isolated from human peripheral blood and expanded in long‐term cultures. Cell Tissue Res. 326, 79–92. [DOI] [PubMed] [Google Scholar]
- 14. Finkenzeller G, Torio‐Padron N, Momeni A, Mehlhorn AT, Stark GB (2007) In vitro angiogenesis properties of endothelial progenitor cells: a promising tool for vascularization of ex vivo engineered tissues. Tissue Eng. 13, 1413–1420. [DOI] [PubMed] [Google Scholar]
- 15. Siemeister G, Schirner M, Reusch P, Barleon B, Marme D, Martiny‐Baron G (1998) An antagonistic vascular endothelial growth factor (VEGF) variant inhibits VEGF‐stimulated receptor autophosphorylation and proliferation of human endothelial cells. Proc. Natl. Acad. Sci. USA 95, 4625–4629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Korff T, Augustin HG (1998) Integration of endothelial cells in multicellular spheroids prevents apoptosis and induces differentiation. J. Cell Biol. 143, 1341–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sieminski AL, Hebbel RP, Gooch KJ (2005) Improved microvascular network in vitro by human blood outgrowth endothelial cells relative to vessel‐derived endothelial cells. Tissue Eng. 11, 1332–1345. [DOI] [PubMed] [Google Scholar]
- 18. Levenberg S, Rouwkema J, Macdonald M, Garfein ES, Kohane DS, Darland DC et al (2005) Engineering vascularized skeletal muscle tissue. Nat. Biotechnol. 23, 879–884. [DOI] [PubMed] [Google Scholar]
- 19. Darland DC, D’Amore PA (2001) TGFβ is required for the formation of capillary‐like structures in three‐dimensional cocultures of 10T1/2 and endothelial cells. Angiogenesis 4, 11–20. [DOI] [PubMed] [Google Scholar]
- 20. Carmeliet P (2003) Angiogenesis in health and disease. Nat. Med. 9, 653–660. [DOI] [PubMed] [Google Scholar]
- 21. Jain RK (2003) Molecular regulation of vessel maturation. Nat. Med. 9, 685–693. [DOI] [PubMed] [Google Scholar]
- 22. Korff T, Augustin HG (1999) Tensional forces in fibrillar extracellular matrices control directional capillary sprouting. J. Cell Sci. 112, 3249–3258. [DOI] [PubMed] [Google Scholar]
- 23. Abramsson A, Lindblom P, Betsholtz C (2003) Endothelial and nonendothelial sources of PDGF‐B regulate pericyte recruitment and influence vascular pattern formation in tumors. J. Clin. Invest. 112, 1142–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hellström M, Kalén M, Lindahl P, Abramsson A, Betsholtz C (1999) Role of PDGF‐B and PDGFR‐β in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development 126, 3047–3055. [DOI] [PubMed] [Google Scholar]
- 25. Kobayashi H, DeBusk LM, Babichev YO, Dumont DJ, Lin PC (2006) Hepatocyte growth factor mediates angiopoietin‐induced smooth muscle cell recruitment. Blood 108, 1260–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Au P, Daheron LM, Duda DG, Cohen KS, Tyrrell JA, Lanning RM et al (2008) Differential in vivo potential of endothelial progenitor cells from human umbilical cord blood and adult peripheral blood to form functional long‐lasting vessels. Blood 111, 1302–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Melero‐Martin JM, Khan ZA, Picard A, Wu X, Paruchuri S, Bischoff J (2007) In vivo vasculogenic potential of human blood‐derived endothelial progenitor cells. Blood 109, 4761–4768. [DOI] [PubMed] [Google Scholar]
- 28. Lyden D, Hattori K, Dias S, Costa C, Blaikie P, Butros L et al (2001) Impaired recruitment of bone‐marrow‐derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat. Methods 7, 1194–1201. [DOI] [PubMed] [Google Scholar]
- 29. Göthert JR, Gustin SE, Van Eekelen JA, Schmidt U, Hall MA, Jane SM et al (2004) Genetically tagging endothelial cells in vivo: bone marrow‐derived cells do not contribute to tumor endothelium. Blood 104, 1769–1777. [DOI] [PubMed] [Google Scholar]
- 30. De Palma M, Venneri MA, Roca C, Naldini L (2003) Targeting exogenous genes to tumor angiogenesis by transplantation of genetically modified hematopoietic stem cells. Nat. Med. 9, 789–795. [DOI] [PubMed] [Google Scholar]
- 31. Assmus B, Schächinger V, Teupe C, Britten M, Lehmann R, Döbert N et al (2002) Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction (TOPCARE‐AMI). Circulation 106, 3009–3017. [DOI] [PubMed] [Google Scholar]
- 32. Hur J, Yoon CH, Kim HS, Choi JH, Kang HJ, Hwang KK et al (2004) Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arterioscler. Thromb. Vasc. Biol. 24, 288–293. [DOI] [PubMed] [Google Scholar]
- 33. Rehman J, Li J, Orschell CM, March KL (2003) Peripheral blood ‘endothelial progenitor cells’ are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation 107, 1164–1169. [DOI] [PubMed] [Google Scholar]


