Abstract
Objectives
Hyperforin, a phloroglucinol derivative of St. John's Wort, has been identified as the major molecule responsible for this plant's products anti‐depressant effects. It can be expected that exposure to St. John's Wort during pregnancy occurs with some frequency although embryotoxic or teratogenic effects of St. John's Wort and hyperforin have not yet been experimentally examined in detail. In this study, to determine any embryotoxic effects of hyperforin, we have attempted to determine whether hyperforin affects growth and survival processes of employing mouse embryonic stem (mES) cells (representing embryonic tissue) and fibroblasts (representing adult tissues).
Materials and methods
We used a modified embryonic stem cell test, which has been validated as an in vitro developmental toxicity protocol, mES cells, to assess embryotoxic potential of chemicals under investigation.
Results
We have identified that high concentrations of hyperforin inhibited mouse ES cell population growth and induced apoptosis in fibroblasts. Under our cell culture conditions, ES cells mainly differentiated into cardiomyocytes, although various other cell types were also produced. In this condition, hyperforin affected ES cell differentiation into cardiomyocytes in a dose‐dependent manner. Analysis of tissue‐specific marker expression also revealed that hyperforin at high concentrations partially inhibited ES cell differentiation into mesodermal and endodermal lineages.
Conclusions
Hyperforin is currently used in the clinic as a safe and effective antidepressant. Our data indicate that at typical dosages it has only a low risk of embryotoxicity; ingestion of large amounts of hyperforin by pregnant women, however, may pose embryotoxic and teratogenic risks.
Introduction
Previous studies have demonstrated that many natural products with anti‐bacterial, anti‐inflammatory, and anti‐tumour qualities, especially those related to chemoprevention and chemotherapy of various diseases, represent potential sources for new drug development 1, 2, 3. Extracts of St. John's Wort, Hypericum perforatum, have been used for centuries in traditional medicine, most notably for treatment of depression 4, 5, 6 and a number of its biologically active compounds have been isolated and characterized. These include naphthodianthrones, flavonoids and phloroglucinols such as hyperforin 6. Hyperforin has been identified as the major molecule responsible for the plant's anti‐depressant effects. Its neurobiological consequences include neurotransmitter re‐uptake inhibition, ability to increase intracellular sodium and calcium levels, recognized transient receptor potential activation and N‐methyl‐d‐aspartic acid receptor antagonism 4, 5, 6. Hyperforin also displays several other biological properties of potential pharmacological interest, including anti‐bacterial and anti‐oxidant properties and an inhibitory effect on inflammatory mediators 5, 6. In addition, hyperforin effectively inhibits proliferation of a number of mammalian cancer cell lines in vitro 4, 5; it also induces apoptosis in K562 (chronic myeloid leukaemia) and U937 (acute myeloid leukaemia) cell lines through a caspase‐dependent pathway 7, 8.
St. John's Wort is an herbal medicine that has been shown to be effective in treating mild‐to‐moderate depression, which is common in women of childbearing years. Given significant numbers of unplanned pregnancies, it is expected that exposure to St. John's Wort during pregnancy occurs with some frequency. Despite fears related to maternal and foetal safety, embryotoxic or teratogenic effects of St. John's Wort and hyperforin have not, up to now, been experimentally examined in detail.
One powerful tool for studying these risks is in embryonic stem (ES) cell research. As ES cells have the ability to develop into differentiated cell types of endodermal, ectodermal and mesodermal lineages, ES cell lines are highly valuable for analyses of mutagenic, cytotoxic and embryotoxic effects of chemical compounds, in vitro. The ES cell test (EST), which employs mouse ES (mES) cells to assess embryotoxic potential of the tested chemicals, has been validated as an in vitro developmental toxicity test 9, 10, 11. In our laboratory, we developed an assay system based on the EST in our previous studies, and tested this in vitro system by evaluating embryotoxicity of known in vivo teratogens valproic acid, carbamazepine and fluoxetine 12, 13, 14. In the study described here, to experimentally estimate embryotoxic effect of hyperforin, we have attempted to determine whether hyperforin would affect prolferation and survival processes of mES cells (representing embryonic tissue) and fibroblasts (representing adult tissues) using this system. In addition, we sought to characterize embryotoxicity of hyperforin.
Materials and methods
Cell culture and differentiation
Mouse ES cells (R1; SCRC‐1011) and NIH/3T3 cells (CRL‐1658) were purchased from American Type Culture Collection (Manassas, VA, USA). NIH/3T3 cells were maintained at 37 °C in an atmosphere of 95% air and 5% CO2 in Dulbecco's modified Eagle's medium (DMEM) containing 10% foetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin. mES cells were maintained as previously described 12, 13, 14. ES cells were grown on gelatin‐coated tissue culture dishes in standard ES cell culture medium [DMEM supplemented with 10% FCS, 2 mm glutamine, 0.1 mm non‐essential amino acids, 0.1 mm βmercaptoethanol, 1000 U/ml LIF (Chemicon, Temecula, CA, USA), 50 U/ml penicillin G and 50 μg/ml streptomycin].
mES cell differentiation
R1 ES cell differentiation was carried out as described previously 12, 13, 14. In brief, ES cells were suspended in ES cell differentiation medium (DMEM supplemented with 20% FCS, 2 mm glutamine, 0.1 mm non‐essential amino acids, 0.1 mm βmercaptoethanol, 50 U/ml penicillin G, and 50 μg/ml streptomycin) containing the appropriate dilution of hyperforin (Sigma‐Aldrich, St. Louis, MO, USA), and cultured in hanging drops (500 cells/drop) as aggregates embryoid bodies, for 3 days. Embryoid bodies were then transferred to suspension culture dishes (Sumitomo Bakelite, Tokyo, Japan) and cultured for 2 days. These (n = 1/well) were then plated into 24‐well tissue culture plates on day 5 and incubated for five additional days. To estimate efficiency of differentiation of ES cells into cardiomyocytes, cultures were analysed under an inverted phase‐contrast microscope (Olympus, Tokyo, Japan) to examine distinctive beating movements of newly differentiated cardiomyocytes.
Cytotoxicity assay
To study for cytotoxic effects of hyperforin we followed the EST method 10 with some modifications. ES cells and NIH/3T3 fibroblasts in 100 μl culture medium containing appropriate dilution of hyperforin were seeded into 96‐well flat‐bottomed tissue culture microtitre plates and incubated in a humidified atmosphere with 5% CO2 at 37 °C. On days 3 and 5, culture medium was removed. Subsequently, 100 μl of the same concentration test substance used on day 0 was added to the microtitre plates. After 24 h, 72 h or 7 days incubation with reagents, cell viability was determined using a CellTiter‐Glo luminescent cell viability assay (Promega, Tokyo, Japan). Control cells cultured according to the same procedure, but without hyperforin, were considered to be 100% viable. Cell viability of each drug‐treated sample was presented as percentage viability of control cells cultured without hyperforin.
Analysis of apoptosis
Quantification of apoptotic cells was performed using a cell death detection ELISAPLUS (Roche Diagnostics, Tokyo, Japan). This assay allows specific determination of mono‐ and oligonucleosomes in the cytoplasmatic fraction of cell lysates. Enrichment of mono‐ and oligonucleosomes in the cytoplasm occurs due to DNA degradation in apoptotic cells before plasma membrane breakdown. After 24 or 72 h incubation with reagents, cells were lysed in lysis buffer (included in the kit) and the assay was performed according to the manufacturer's instructions. Absorbance values were measured at 405 nm using a microplate reader (ARVO; PerkinElmer Japan, Kanagawa, Japan). Apoptotic ratio of cells for each drug‐treated sample is presented as fold‐change from that of untreated samples. All samples were run five times per assay.
5‐bromo‐2′‐deoxy‐uridine (BrdU) incorporation assay
DNA synthesis levels were determined by measuring BrdU incorporation using the commercial Cell Proliferation ELISA System (Roche Diagnostics). After 24 or 72 h incubation with reagents, cells were incubated for 3 h in BrdU labelling solution containing 10 μm BrdU (included in the kit); the assay was performed according to the manufacturer's instructions. Absorbance values were measured at 405 nm using a microplate reader. All samples were run five times per assay.
Caspase assays
Caspases ‐3, ‐8, and ‐9 are important mediators of apoptosis; caspases ‐8 and ‐9 are initiators and caspase‐3 is the executioner enzyme 15. Caspase‐3/7, ‐8, and ‐9 activities were assayed with Caspase‐Glo Assays (Promega) according to the manufacturer's respective standard cell‐based assay protocol. Luminescence of each sample was measured using a plate‐reading luminometer. Comparison of luminescence of a treated sample with that of a control sample enabled relative increase in caspase activity to be determined. All samples were run five times per same assay.
RNA isolation, cDNA synthesis and quantitative RT‐polymerase chain reaction
Total RNA was extracted from samples on day 10 of the differentiation assay, and from undifferentiated samples, using the RNeasy RNA extraction kit (Qiagen, Tokyo, Japan). cDNA was synthesized using RNA and PrimeScript II 1st strand cDNA Synthesis Kit (Takara, Shiga, Japan). To analyse relative expression levels of various mRNAs, amount of cDNA was normalized on the basis of signals from ubiquitously expressed GAPDH mRNA. Real‐time polymerase chain reaction was carried out using an SYBR Premix Ex Taq II (Takara) and a thermal cycler dice real time system (Takara) according to the manufacturer's standard instructions, to final volume of 25 μl. Primer sequences are summarized in Table 1.
Table 1.
PCR primers used in this study for the detection of tissue‐specific marker gene expression
| Gene symbol | Official full name (NCBI reference sequence) | |
|---|---|---|
| Forward (5′→3′) | Reverse (5′→3′) | |
| POU5F1 (Oct3/4) | POU domain, class 5, transcription factor 1 (NM_013633.3) | |
| GGTGGAGGAAGCCGACAAC | TTCGGGCACTTCAGAAACATG | |
| SOX2 | SRY‐box containing gene 2 (NM_011443.3) | |
| AGATGCACAACTCGGAGATCAG | CCGCGGCCGGTATTTATAAT | |
| GATA6 | GATA binding protein 6 (NM_010258.3) | |
| CGGTCATTACCTGTGCAATG | GCATTTCTACGCCATAAGGTA | |
| TTR | transthyretin (NM_013697) | |
| GTCCTCTGATGGTCAAAGTC | TCCAGTTCTACTCTGTACAC | |
| BMP4 | bone morphogenetic protein 4 (NM_007554.2) | |
| CTGCCGTCGCCATTCACTAT | TGGCATGGTTGGTTGAGTTG | |
| NPPA (ANF) | natriuretic peptide type A (NM_008725.2) | |
| CGGTGTCCAACACAGATCTG | TCTCTCAGAGGTGGGTTGAC | |
| NES | nestin (NM_016701.3) | |
| TGCATTTCCTTGGGATACCAG | CTTCAGAAAGGCTGTCACAGGAG | |
| GFAP | glial fibrillary acidic protein (NM_001131020.1) | |
| TGCCACGCTTCTCCTTGTCT | GCTAGCAAAGCGGTCATTGAG | |
| GAPDH | glyceraldehyde‐3‐phosphate dehydrogenase (NM_008084.2) | |
| TGCACCACCAACTGCTTAG | GGATGCAGGGATGATGTTC | |
Statistical analysis
Values are expressed as mean ± SEM. Statistical analyses were performed using unpaired Student's t‐test or two‐way analysis of variance (ANOVA) followed by Fisher's protected least significant difference test post‐hoc. Probability values (P) of less than 0.05 were considered statistically significant.
Results
Effects of hyperforin on ES cell and fibroblast viability
In both ES cells and NIH/3T3 fibroblasts, hyperforin at high concentrations inhibited cell survival in a dose‐dependent manner, after 7 days of hyperforin treatment (Fig. 1). However, there were some differences between ES cells (Fig. 1a) and NIH/3T3 fibroblasts (Fig. 1b) in cytotoxic sensitivity to hyperforin. IC50 values (inhibitory concentration of 50% cell viability) were 2.38 and 5.89 μm for NIH/3T3 fibroblasts and ES cells, respectively. Proportions of viable cells after treatment with 10 μm hyperforin were 0.32 ± 0.07 and 29.35 ± 0.34% for NIH/3T3 fibroblasts and ES cells, respectively.
Figure 1.
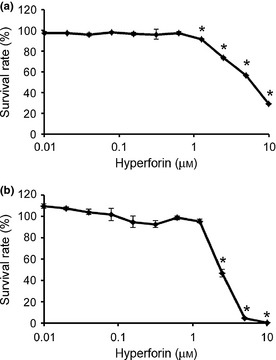
Effect of hyperforin on embryonic stem ( ES ) cell and fibroblast viability. Employing mouse ES cells (a) and NIH/3T3 cells (b) were treated with hyperforin at indicated concentrations for 7 days. Cell viability was measured by CellTiter‐Glo luminescent cell viability assay. Cell cultures exposed to 0 μm drug were considered to be 100% viable. Cell viability of each drug‐treated sample was presented as a percentage of viability of cultures treated with 0 μm drug. Data are mean ± SEM of results from at least three independent experiments. *P < 0.05, compared to 0 μm.
Hyperforin inhibited proliferation of ES cells
To further examine effects of hyperforin on proliferation and viability of ES cells, cells were cultured for 24 or 72 h in the absence or presence of increasing concentrations of hyperforin. ES cell population growth was reduced in samples treated with high concentrations of hyperforin compared to untreated control samples after 24 and 72 h, respectively (Fig. 2a,b). Proportions of viable cells after treatment with 10 μm hyperforin were 37.38 ± 1.42 and 16.46 ± 3.13% after 24 and 72 h. To determine whether hyperforin induced death in ES cells, we measured apoptosis after 24 or 72 h hyperforin treatment. The cell death detection ELISA assay indicated that hyperforin could not induce apoptosis, characterized by DNA fragmentation, in ES cells after 24 h (Fig. 2c) and 72 h (Fig. 2d). In addition, to determine whether hyperforin could inhibit proliferation of ES cells, we conducted a BrdU incorporation assay after 24 or 72 h hyperforin treatment. BrdU incorporation assay showed that uptake of BrdU by ES cells was reduced after exposure to hyperforin (Fig. 2e,f). These results indicate that high concentrations of hyperforin inhibited ES cell proliferation.
Figure 2.
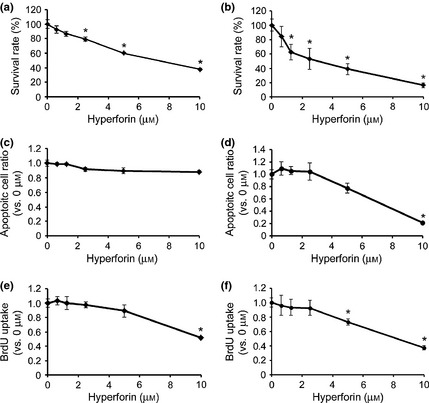
Effects of hyperforin on embryonic stem ( ES ) cell apoptosis and proliferation. (a, b) Employing mouse ES (mES) cells were treated with hyperforin at indicated concentrations for 24 h (a) or 72 h (b). Cell viability was measured by CellTiter‐Glo luminescent cell viability assay. Cell cultures exposed to 0 μm drug were considered to be 100% viable. Cell viability of each drug‐treated sample was presented as percentage of that of cultures treated with 0 μm drug. (c, d) mES cells were treated with hyperforin at indicated concentrations for 24 h (c) or 72 h (d). Apoptosis was measured by cell death detection ELISA assay. Apoptotic level in each drug‐treated sample was presented as fold‐change compared to that in cultures treated with 0 μm drug. (e, f) mES cells were treated with hyperforin at indicated concentrations for 24 h (e) or 72 h (f). Uptake of BrdU was measured by ELISA. BrdU incorporation in each drug‐treated sample was presented as fold‐change compared to that in cultures treated with 0 μm drug. Data are the mean ± SEM of results from at least three independent experiments. *P < 0.05, compared to 0 μm.
Hyperforin induced apoptosis of fibroblasts
To further examine effects of hyperforin on proliferation and viability of NIH/3T3 cells, cells were cultured for 72 h in the absence or presence of increasing concentrations of hyperforin. NIH/3T3 cell proliferation was markedly lower in samples treated with high concentrations of hyperforin compared to untreated control samples (Fig. 3a); proportion of viable cells after treatment with 10 μm hyperforin was 6.52 ± 0.25%. To determine whether hyperforin induced apoptosis in NIH/3T3 cells, we measured it after 72 h hyperforin treatment. The cell death detection ELISA assay indicated that hyperforin induced apoptotic cell death in NIH/3T3 cells (Fig. 3b). In addition, activity of caspase‐3/7 was significantly increased at growth‐suppressive concentration of hyperforin (Fig. 3c), as were those of caspases ‐8 (Fig. 3d) and ‐9 (Fig. 3e).
Figure 3.
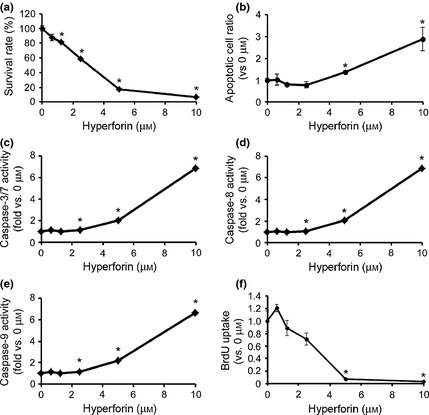
Effects of hyperforin on fibroblast apoptosis. (a) NIH/3T3 cells were treated with hyperforin at indicated concentrations for 72 h. Cell viability was measured by CellTiter‐Glo luminescent cell viability assay. Cell cultures exposed to 0 μm drug were considered to be 100% viable. Cell viability of each drug‐treated sample was presented as percentage of that of cultures treated with 0 μm drug. (b) NIH/3T3 cells were treated with hyperforin at indicated concentrations for 72 h. Apoptosis was measured by cell death detection ELISA assay. Apoptotic level in each drug‐treated sample was presented as fold‐change compared to that in cultures treated with 0 μm drug. (c–d) NIH/3T3 cells were treated with hyperforin at indicated concentrations for 72 h. Caspase‐3/7 (c), ‐8 (d) and ‐9 (e) activities were determined using Caspase‐Glo Assays. Data are expressed as fold‐increases relative to respective untreated samples (RLU/60 min/μg protein). (f) NIH/3T3 cells were treated with hyperforin at indicated concentrations for 72 h. Uptake of BrdU was measured by ELISA. BrdU incorporation in each drug‐treated sample was presented as fold‐change compared to that in cultures treated with 0 μm drug. Data are expressed as mean ± SEM of results from at least four independent experiments. *P < 0.05, compared to 0 μm.
Furthermore, to determine whether hyperforin could inhibit proliferation of NIH/3T3 cells, we conducted BrdU incorporation assay after 72 h hyperforin treatment. This indicated that uptake of BrdU by NIH/3T3 cells was lower after exposure to hyperforin (Fig. 3f).
Effects of hyperforin on tissue‐specific marker gene expression in ES cells
To characterize effects of hyperforin in the ES cell differentiation system, we performed quantitative expression analysis of tissue‐specific genes (thus of their products) in samples on day 10 of the differentiation assay. Under our cell culture conditions, ES cells mainly differentiated into cardiomyocytes, although various other cell types were also produced. We demonstrated that expression levels of Oct4 and Sox2 (undifferentiated markers) were markedly lower under differentiating conditions compared to non‐differentiating conditions, and that these expression levels were enhanced after treatment with 10 μm hyperforin, although they remained below their initial undifferentiated levels (Fig. 4a,b). For endodermal lineages, expression levels of GATA6 and TTR were markedly higher under differentiating conditions compared to non‐differentiating conditions, and high concentrations of hyperforin reduced these expression levels (Fig. 4c,d). Expression of mesodermal markers BMP4 and ANF were also markedly higher under differentiating conditions compared to non‐differentiating conditions, and high concentrations of hyperforin reduced these expression levels (Fig. 4e,f). For ectodermal lineages, expression levels of neuron‐specific markers such as nestin and Glial markers such as GFAP were not significantly affected by hyperforin (Fig. 4g,h). Proportion of induced cardiomyocytes was determined based on their autonomous contractile motions which was shown to reduce in a hyperforin concentration‐dependent manner (Fig. 4i).
Figure 4.
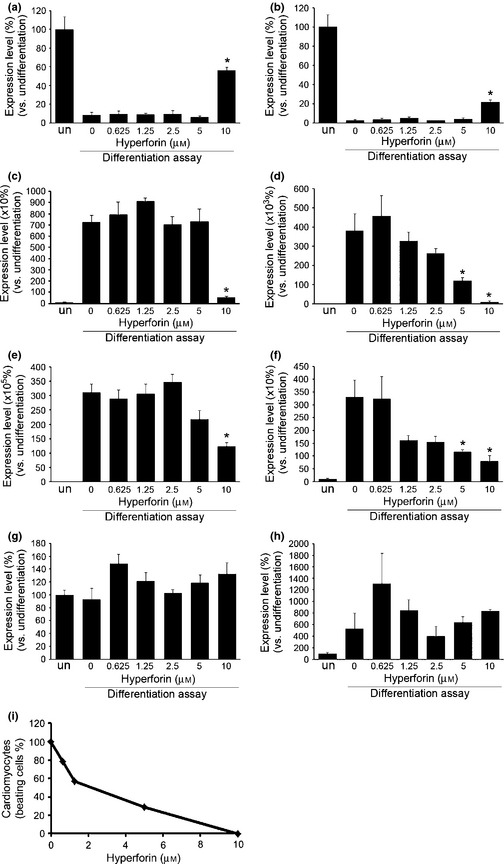
Effect of hyperforin on embryonic stem ( ES ) cell differentiation. Analysis of expression levels for undifferentiated state and tissue‐specific differentiation markers. Expression levels of undifferentiated markers Oct3/4 (a) and Sox2 (b), endodermal markers GATA6 (c) and TTR (d), mesodermal markers BMP4 (e) and ANF (f) and ectodermal markers nestin (g) and GFAP (h) were quantified at each concentration of hyperforin with real‐time RT‐PCR. Each experiment was performed in triplicate. Data are expressed as mean ± SEM of results from at least three independent experiments. *P < 0.05, compared to 0 μm. un, undifferentiated ES cell. (e) The frequencies of cardiomyocytes, identified by their distinctive beating movement, derived from ES cells were quantified at each concentration of hyperforin.
Discussion
In this study, we demonstrated that high concentrations of hyperforin significantly reduced viability of ES cells (an embryonic tissue cell model) and NIH/3T3 fibroblasts (an adult tissue cell model).
Data we obtained from our various analyses suggest that hyperforin at high concentrations inhibits ES cell proliferation. Proliferating somatic cells spend much of their mitotic cycle in the G1 phase, and their progression to S phase is largely controlled by cyclin‐dependent kinases (CDK), whose activity is regulated by various cyclins. Although they are expressed in ES cells, in some cases several major CDK‐cyclin control complexes, including Cdk4/cyclin D, appear to exhibit little or no regulatory activity. Instead, division of ES cells is driven by the Cdk2/cyclin A/E pathway, constitutively active throughout the cell cycle 16. Given that hyperforin inhibits proliferation of ES cells, it may affect the function of cell cycle regulators such as the Cdk2/cyclin A/E pathway.
On the other hand, it is acknowledged that there are several types of cell death, including apoptosis, necrosis and autophagic cell death. Cells are archetypically known to disassemble in two morphologically and biologically distinct processes, namely, programmed cell death (PCD) and necrosis. PCD, referring to apoptosis, autophagy and programmed necrosis, is proposed to be death of a cell in any pathological format, when mediated by an intracellular program. These three forms of PCD may jointly decide the fate of cells; apoptosis and programmed necrosis invariably contribute to cell death, whereas autophagy can play either a pro‐survival role or a pro‐death role 17. Cells undergoing apoptosis show a series of well‐characterized physical changes such as plasma membrane blebbing, permeabilization of mitochondrial outer membranes, DNA fragmentation, nuclear disintegration and eventually cell disintegration into apoptotic bodies that are then engulfed and degraded by phagocytes 18. On the other hand, autophagy (macroautophagy) occurs by formation of autophagosomes, double‐membraned vesicles that sequester organelles, proteins, or portions of cytoplasm, which then fuse with lysosomes. As a result of this process, sequestered contents are degraded by lysosomal enzymes and recycled for future re‐use 19. Autophagy does not involve DNA fragmentation 20. Our results here show that hyperforin did not induce apoptosis in ES cells. It is not known, however, whether other types of cell death such as necrosis or autophagy contribute to ES cell growth inhibition.
The molecular mechanisms that account for the hyperforin‐induced cell viability reduction of ES cells remain to be investigated.
Our data also suggest that NIH/3T3 fibroblast cytotoxicity to hyperforin at high concentrations induced apoptosis. During apoptosis, caspases are essential for initiation and execution of cell death in a self‐amplifying cascade in response to various stimuli 21. Two major apoptotic pathways have been identified, one extrinsic and one intrinsic. The extrinsic pathway is activated by death receptors, which recruit initiator caspases ‐2, ‐8, or ‐10 through adaptor molecules, whereas intrinsic signals result in activation of caspase‐9. Initiator caspases can sequentially cleave and activate effector caspases (caspases ‐3, ‐6, and ‐7), which play an important role in mediating cell destruction 22. Our results show that hyperforin increased mono‐ and oligonucleosomes and activities of caspases ‐3/7, ‐8 and ‐9 in NIH/3T3 fibroblasts, indicating that it induced not necrosis nor autophagy, but apoptosis of NIH/3T3 fibroblasts, via both the intrinsic pathway (as shown by activation of caspase‐9) and the extrinsic pathway (as shown by activation of caspase‐8), and also led to caspase‐3 activation.
Thus, our results suggest that hyperforin affected cytotoxicity in a cell‐dependent manner; it inhibited proliferation of ES cells (representing embryonic tissue) and induced apoptosis of fibroblasts (representing adult tissues). Effects and the molecular mechanisms of hyperforin must therefore be examined further, in individual cells and tissues.
It must be noted that concentrations of hyperforin taken into the body through consumption of St. John's Wort are very low. Agrosi et al. 23 observed peak plasma hyperforin level of 168.35 ng/ml (about 30 nm) after consumption of a soft gelatin formulation and 84.25 ng/ml (about 15 nm) after consumption of hard gelatin capsules. Vitiello et al. 24 were unable to detect any hyperforin in 17 of 97 volunteers who took St. John's Wort. Hyperforin had an IC50 value of around 2.4 μm for NIH/3T3 fibroblasts and around 5.9 μm for ES cells as measured for this study; these levels are approximately 80–200 times plasma hyperforin level observed by Agrosi et al. These results suggest that hyperforin can be expected to have few embryotoxic effects in general use.
In a previous study, subjects taking St. John's Wort were prospectively identified, followed and compared with a matched group of pregnant women taking other forms of pharmacological therapy for depression, and a third group of healthy women not exposed to any known teratogens. It was demonstrated that levels of major malformations were similar across the three groups: they were 5%, 4% and 0% in St. John's Wort, disease comparator and healthy groups, respectively. These levels are not significantly different from the 3–5% risk expected in the general population. Levels of live birth and prematurity also did not differ among the three groups 25. In this study, using an assay system based on the EST developed in our previous studies 12, 13, 14, we have demonstrated that high concentration (10 μm) of hyperforin increased expression level of undifferentiated marker genes and reduced expression level of mesodermal and endodermal marker genes under differentiating conditions. In addition, hyperforin inhibited differentiation of ES cells into cardiomyocytes. We found, however, that undifferentiated marker expression levels were lower in ES cells that had been subjected to 10 μm hyperforin treatment under differentiating conditions than they were in undifferentiated ES cells, while mesodermal and endodermal marker expression levels were higher in ES cells that had been subjected to 10 μm hyperforin treatment under differentiating conditions than they were in undifferentiated ES cells. These results suggest that high concentrations of hyperforin could partially inhibit ES cell differentiation into mesoderm and endoderm lineages, and that ingestion of large amounts of hyperforin could pose embryotoxic and teratogenic risks.
Hyperforin is currently in used in the clinic as a safe and effective antidepressant. Our experimental data indicate that it could be expected to have few embryotoxic and teratogenic effects in general use, although ingestion of large amounts of hyperforin may incur risks.
Acknowledgements
We thank Dr. Shinji Kusakawa of the Foundation for Biomedical Research and Innovation for his valuable support. This work was supported in part by research grants from the Mishima Kaiun Memorial Foundation, the Scientific Fund of the Ministry of Human Health and Welfare of Japan (H24‐Shokuhin‐Wakate‐019) and the Grant of National Center for Child Health and Development (22A‐6).
References
- 1. Calixto JB, Campos MM, Otuki MF, Santos AR (2004) Anti‐inflammatory compounds of plant origin. Part II. modulation of pro‐inflammatory cytokines, chemokines and adhesion molecules. Planta Med. 70, 93–103. [DOI] [PubMed] [Google Scholar]
- 2. Calixto JB, Otuki MF, Santos AR (2003) Anti‐inflammatory compounds of plant origin. Part I. Action on arachidonic acid pathway, nitric oxide and nuclear factor kappa B (NF‐kappaB). Planta Med. 69, 973–983. [DOI] [PubMed] [Google Scholar]
- 3. Bailly C (2009) Ready for a comeback of natural products in oncology. Biochem. Pharmacol. 77, 1447–1457. [DOI] [PubMed] [Google Scholar]
- 4. Medina MA, Martinez‐Poveda B, Amores‐Sanchez MI, Quesada AR (2006) Hyperforin: more than an antidepressant bioactive compound? Life Sci. 79, 105–111. [DOI] [PubMed] [Google Scholar]
- 5. Quiney C, Billard C, Salanoubat C, Fourneron JD, Kolb JP (2006) Hyperforin, a new lead compound against the progression of cancer and leukemia? Leukemia 20, 1519–1525. [DOI] [PubMed] [Google Scholar]
- 6. Borrelli F, Izzo AA (2009) Herb‐drug interactions with St John's wort (Hypericum perforatum): an update on clinical observations. AAPS J. 11, 710–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hostanska K, Reichling J, Bommer S, Weber M, Saller R (2003) Hyperforin a constituent of St John's wort (Hypericum perforatum L.) extract induces apoptosis by triggering activation of caspases and with hypericin synergistically exerts cytotoxicity towards human malignant cell lines. Eur. J. Pharm. Biopharm. 56, 121–132. [DOI] [PubMed] [Google Scholar]
- 8. Liu JY, Liu Z, Wang DM, Li MM, Wang SX, Wang R et al (2011) Induction of apoptosis in K562 cells by dicyclohexylammonium salt of hyperforin through a mitochondrial‐related pathway. Chem. Biol. Interact. 190, 91–101. [DOI] [PubMed] [Google Scholar]
- 9. Genschow E, Spielmann H, Scholz G, Pohl I, Seiler A, Clemann N et al (2004) Validation of the embryonic stem cell test in the international ECVAM validation study on three in vitro embryotoxicity tests. Altern. Lab. Anim. 32, 209–244. [DOI] [PubMed] [Google Scholar]
- 10. Spielmann H, Pohl I, Droing B, Liebsch M, Moldenhauser F (1997) The embryonic stem cell test, in vitro embryo toxicity test using two permanent mouse cell line: 3T3 fibroblast and embryonic stem cells. In Vitro Toxicol. 10, 119–127. [Google Scholar]
- 11. Seiler AE, Spielmann H (2011) The validated embryonic stem cell test to predict embryotoxicity in vitro. Nat. Protoc. 6, 961–978. [DOI] [PubMed] [Google Scholar]
- 12. Murabe M, Yamauchi J, Fujiwara Y, Hiroyama M, Sanbe A, Tanoue A (2007) A novel embryotoxic estimation method of VPA using ES cells differentiation system. Biochem. Biophys. Res. Commun. 352, 164–169. [DOI] [PubMed] [Google Scholar]
- 13. Murabe M, Yamauchi J, Fujiwara Y, Miyamoto Y, Hiroyama M, Sanbe A et al (2007) Estimation of the embryotoxic effect of CBZ using an ES cell differentiation system. Biochem. Biophys. Res. Commun. 356, 739–744. [DOI] [PubMed] [Google Scholar]
- 14. Kusakawa S, Yamauchi J, Miyamoto Y, Sanbe A, Tanoue A (2008) Estimation of embryotoxic effect of fluoxetine using embryonic stem cell differentiation system. Life Sci. 83, 871–877. [DOI] [PubMed] [Google Scholar]
- 15. Wang ZB, Liu YQ, Cui YF (2005) Pathways to caspase activation. Cell Biol. Int. 29, 489–496. [DOI] [PubMed] [Google Scholar]
- 16. Miura T, Mattson MP, Rao MS (2004) Cellular lifespan and senescence signaling in embryonic stem cells. Aging Cell 3, 333–343. [DOI] [PubMed] [Google Scholar]
- 17. Ouyang L, Shi Z, Zhao S, Wang FT, Zhou TT, Liu B et al (2012) Programmed cell death pathways in cancer: a review of apoptosis, autophagy and programmed necrosis. Cell Prolif. 45, 487–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Suzanne M, Steller H (2013) Shaping organisms with apoptosis. Cell Death Differ. 20, 669–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Green DR, Galluzzi L, Kroemer G (2011) Mitochondria and the autophagy‐inflammation‐cell death axis in organismal aging. Science 333, 1109–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wlodkowic D, Skommer J, Darzynkiewicz Z (2010) Cytometry in cell necrobiology revisited. Recent advances and new vistas. Cytometry A 77, 591–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Leist M, Gantner F, Bohlinger I, Germann PG, Tiegs G, Wendel A (1994) Murine hepatocyte apoptosis induced in vitro and in vivo by TNF‐alpha requires transcriptional arrest. J. Immunol. 153, 1778–1788. [PubMed] [Google Scholar]
- 22. Liedtke C, Plumpe J, Kubicka S, Bradham CA, Manns MP, Brenner DA et al (2002) Jun kinase modulates tumor necrosis factor‐dependent apoptosis in liver cells. Hepatology 36, 315–325. [DOI] [PubMed] [Google Scholar]
- 23. Agrosi M, Mischiatti S, Harrasser PC, Savio D (2000) Oral bioavailability of active principles from herbal products in humans. A study on Hypericum perforatum extracts using the soft gelatin capsule technology. Phytomedicine 7, 455–462. [DOI] [PubMed] [Google Scholar]
- 24. Vitiello B, Shader RI, Parker CB, Ritz L, Harlan W, Greenblatt DJ et al (2005) Hyperforin plasma level as a marker of treatment adherence in the National Institutes of Health Hypericum Depression Trial. J. Clin. Psychopharmacol. 25, 243–249. [DOI] [PubMed] [Google Scholar]
- 25. Moretti ME, Maxson A, Hanna F, Koren G (2009) Evaluating the safety of St. John's Wort in human pregnancy. Reprod. Toxicol. 28, 96–99. [DOI] [PubMed] [Google Scholar]


