To assess the potential for the induction of antimicrobial resistance following repeated subinhibitory exposures to the combination minocycline (MIN), rifampin (RIF), and chlorhexidine (CHX), a total of 29 clinical microbial pathogenic isolates were repeatedly exposed to subinhibitory concentrations of MIN, RIF, and CHX for 20 passages. MICs of the MIN, RIF, and CHX combination were assessed at each passage to evaluate the potential for resistance to have been induced.
KEYWORDS: chlorhexidine, intravascular devices, intravascular infections, minocycline, multidrug-resistant organisms, rifampin
ABSTRACT
To assess the potential for the induction of antimicrobial resistance following repeated subinhibitory exposures to the combination minocycline (MIN), rifampin (RIF), and chlorhexidine (CHX), a total of 29 clinical microbial pathogenic isolates were repeatedly exposed to subinhibitory concentrations of MIN, RIF, and CHX for 20 passages. MICs of the MIN, RIF, and CHX combination were assessed at each passage to evaluate the potential for resistance to have been induced. The combination of MIN, RIF, and CHX showed significant antimicrobial efficacy and synergy against organisms resistant to all 3 individual components (MIC of ≥16 μg/ml for MIN or MIC of ≥4 μg/ml for RIF or CHX). Among the organisms originally resistant to 2 or more individual components and the organisms originally susceptible to 2 or more individual components, there was no evidence that organisms became resistant following 20 repeated subinhibitory exposure cycles to the triple combination. The risk of resistance developing to the triple combination is extremely low because microbes are inhibited or killed before resistance can simultaneously emerge to all three agents. Surveillance studies monitoring the development of resistance should be conducted in a clinical setting.
INTRODUCTION
Minocycline (MIN) and rifampin (RIF) as well as chlorhexidine (CHX) are antibiotics and antiseptics used to coat catheters for reducing central line-associated bloodstream infections (CLABSIs) (1). Currently available antimicrobial vascular catheters based on CHX or the MIN-RIF double combination nevertheless present distinct risks for breakthrough infections. A randomized prospective trial comparing a CHX (single agent)-coated vascular catheter to uncoated catheters reported no significant reduction in infections in the CHX catheter group, and in fact, there were more infections (nonsignificant) reported for the CHX group than for the uncoated catheter group (2). Catheters treated with MIN and RIF have significantly reduced CLABSIs in several clinical trials (3–7), and several meta-analyses (8–11) have reinforced these conclusions. Despite the effectiveness of MIN and RIF in reducing CLABSIs, some breakthrough infections do occur with MIN and RIF catheters, particularly for selected Gram-negative organisms and fungi that have low innate susceptibilities to the MIN-RIF antibiotics (12, 13). Hence, while the overall number of infections is reduced, a limited but tangible number of breakthrough infections do occur with the use of MIN-RIF catheters due to gaps in antimicrobial coverage provided by the double combination of MIN and RIF. The combination of MIN, RIF, and CHX has been proposed as a new triple combination antimicrobial treatment for medical devices, such as vascular catheters, in order to prevent microbial colonization and CLABSI (12, 13). One risk associated with the prophylactic use of this new triple antimicrobial combination is the potential for the development of resistance by microbes as an adaptation resulting from exposure to this combination of antimicrobial agents. In order to assess the potential risk of inducing microbial resistance following exposure to this triple combination, we evaluated changes in MICs of various clinically virulent catheter-related bloodstream infection (CRBSI)-causing pathogens that were sequentially exposed to MIN, RIF, and CHX by repeatedly passaging them at subinhibitory concentrations.
RESULTS
MIC determination of MIN, RIF, and CHX and individual components.
MIC determination of individual agents resulted in 17 organisms classified as “low susceptibility,” while 12 organisms were classified as “high susceptibility” (Table 1). Of the low susceptibility organisms, 10 organisms, including Klebsiella pneumoniae, Enterobacter cloacae, and Pseudomonas aeruginosa, were resistant to all 3 individual components. In contrast, MICs to the MIN, RIF, and CHX combination were highly susceptible to all organisms tested (Table 1). This indicates that the MIN, RIF, and CHX combination demonstrates high efficacy in combination. Among high-susceptibility organisms, decreased rifampin susceptibilities were seen in Escherichia coli isolates (MICs, 8 or 16 μg/ml); however, all other isolates were highly susceptible to all individual agents. Staphylococcus aureus isolates (ATCC 25923 and ATCC 43300) and an Acinetobacter baumannii isolate (MB2767) isolate highly susceptible to the degree that their baseline MIC was less than the lower limit of testing. No subinhibitory concentrations could be achieved, and consequently, no passages were performed on these strains. Since a low rifampin MIC was driving the inability to achieve a subinhibitory concentration, 28 additional S. aureus and Staphylococcus epidermidis isolates were screened for rifampin susceptibility (data not shown); of those isolates, 4 were found to have lower susceptibility (S. aureus MDA 148 and 155; S. epidermidis MB 123 and 1915). Baseline MICs to the MIN, RIF, and CHX concentration were detectable at low concentrations (S. aureus MIC of 0.00024 μg/ml for both isolates; S. epidermidis MIC of 0.125 μg/ml for both isolates), and thus, a subinhibitory concentration could be established for further passage testing. This high susceptibility is attributed to a combination of high susceptibility to individual agents and synergy seen with the MIN, RIF, and CHX combination. Furthermore, any changes in the very small MICs for S. aureus and S. epidermidis are unlikely to have any practical clinical significance (i.e., the organisms remain highly susceptible to the triple combination).
TABLE 1.
MICs of baseline and after exposure to subinhibitory concentrations of MIN, RIF, and CHX
| Organism type and strain by susceptibility status | MIC (μg/ml) of individual agentsa |
MIC (μg/ml) of MIN, RIF, and CHX atb: |
|||
|---|---|---|---|---|---|
| Minocycline | Rifampin | Chlorhexidine | Baseline | Passage 20 | |
| Low-susceptibility organisms (n = 17) | |||||
| Vancomycin-resistant Enterococcus | |||||
| MDA 111 | 8 (I) | 16 (R) | 4 (R) | 2 | 1 |
| MDA 191 | 8 (I) | 4 (R) | 8 (R) | 2 | 2 |
| MDA 192 | 16 (R) | 16 (R) | 4 (R) | 2 | 2 |
| Carbapenem-resistant Enterobacteriaceae | |||||
| Klebsiella pneumoniae | |||||
| ATCC 1705 | 8 (I) | 32 (R) | 8 (R) | 1 | 1 |
| MDA 124 | 32 (R) | 32 (R) | 4 (R) | 2 | 1 |
| MDA 125 | 32 (R) | 32 (R) | 8 (R) | 1 | 1 |
| MDA 126 | 32 (R) | 32 (R) | 4 (R) | 1 | 2 |
| Escherichia coli | |||||
| MDA 122 | 8 (I) | 32 (R) | 1 (S) | 1 | 1 |
| Enterobacter cloacae | |||||
| MDA 166 | 8 (I) | >32 (R) | 16 (R) | 1 | 4 |
| MDA 167 | 32 (R) | 32 (R) | 16 (R) | 1 | 2 |
| MDA 121 | 4 (S) | 32 (R) | 8 (R) | <1 | 1 |
| Multidrug-resistant organisms | |||||
| Pseudomonas aeruginosa | |||||
| MDA 118 | 8 (I) | 8 (R) | 8 (R) | 2 | 1 |
| MDA 194 | 32 (R) | 32 (R) | 8 (R) | 4 | 2 |
| MDA 170 | 32 (R) | 32 (R) | 8 (R) | 1 | 1 |
| Other organisms | |||||
| Candida albicans | |||||
| MB 3655A | >16 (R) | >16 (R) | 4 (R) | 4 | 4 |
| Candida parapsilosis | |||||
| MB2247 | >16 (R) | >16 (R) | 4 (R) | 4 | 8 |
| Acinetobacter baumannii | |||||
| MB 2790 | 0.125 (S) | 4 (R) | 4 (R) | <1 | <1 |
| High-susceptibility organismsc (n = 12) | |||||
| Gram positive | |||||
| Staphylococcus aureus | |||||
| ATCC 25923 | 0.125 (S) | <0.031 (S) | 0.5 (S) | <0.031 | — |
| ATCC 43300 | 0.125 (S) | <0.031 (S) | 1 (S) | <0.031 | — |
| MDA 155 | <0.016 (S) | 1.0 (S) | 2 (S) | 0.0002 | — |
| MDA 148 | <0.016 (S) | >32 (R) | 2 (S) | 0.0002 | — |
| Staphylococcus epidermidis | |||||
| MB 123 | 0.125 (S) | >32 (R) | 1 (S) | 0.125 | — |
| MB 1915 | 0.125 (S) | >32 (R) | 1 (S) | 0.125 | — |
| Gram negative | |||||
| Acinetobacter baumannii | |||||
| MB 2875 | 0.125 (S) | 2 (I) | 2 (S) | <1 | <1 |
| MB 2767 | 0.031 (S) | 2 (I) | 2 (S) | <0.016 | — |
| Escherichia coli | |||||
| ATCC 25922 | 1 (S) | 8 (R) | 0.25 (S) | <1 | 1 |
| ATCC 35218 | 1 (S) | 8 (R) | 0.5 (S) | <1 | 1 |
| MDA 164 | 4 (S) | 16 (R) | 2 (S) | 1 | 2 |
| MDA 165 | 4 (S) | 16 (R) | 2 (S) | 1 | 1 |
Susceptibility testing for each component of MIN, RIF, and CHX before exposure. MICs to individual agents were then assigned susceptible (S), intermediate (I), or resistant (R) based on CLSI breakpoints for minocycline and rifampin (42) or EUCAST epidemiologic cutoffs for chlorhexidine (43). MIC breakpoints are defined as S ≤ 4, I = 8, R ≥ 16 for minocycline; S ≤ 1, I = 2, R ≥ 4 for rifampin; and S ≤ 2, R ≥ 4 for chlorhexidine.
MICs for triple combination MIN, RIF, and CHX in organisms at baseline and after 20 passages exposed to subinhibitory concentrations of MIN, RIF, and CHX.
For Staphylococcus aureus ATCC 25923 and ATCC 43300 and Acinetobacter baumannii MB 2767, the organisms were highly susceptible to the point that the MIC for MIN, RIF, and CHX was less that that of the testing limit; thus, no subinhibitory concentration could be achieved for passages to be conducted. For Staphylococcus aureus MDA 155 and MDA 148 and Staphylococcus epidermidis MB 123 and MB 1915, subinhibitory concentrations were established at baseline; however, organisms died after two passages and no further passages could be conducted. A dash indicates that a MIC at passage 20 could not be established.
MIC determination with subinhibitory exposure to MIN, RIF, and CHX.
Plots of median MIC values showing trends across 20 passages of subinhibitory exposure to MIN, RIF, and CHX are presented in Fig. S1 in the supplemental material for low-susceptibility organisms and Fig. S2 in the supplemental material for high-susceptibility organims. The overall change in MIC from baseline to passage 20 is shown in Table 1. Among low-susceptibility organims, MICs for the MIN, RIF, and CHX combination stayed within 2-fold of the baseline MIC across 20 passages for all organisms except Enterobacter cloacae (MDA 166), which displayed a 4-fold increase from baseline MIC (MIC 1 to MIC 4) by passage 4. This 4-fold increase was sustained throughout the remainder of the 20 passages. Among high-susceptibility organisms, all organisms tested remained within 2-fold of the baseline MIC after 20 passages. One E. coli isolate (MDA 164) showed a 4-fold increase for 4 of the 20 MIC passages; however, this increase returned to lower MICs at passage 20. An additional passage, 21, was conducted for organisms with an unanticipated result for passage 20. Passage 21 verified that the unanticipated result in passage 20 was within normal experimental MIC fluctuations (data not shown). None of the organisms tested demonstrated a steep vertical MIC trend (continually increasing MIC trending toward antimicrobial agent ineffectiveness) which would be indicative of induced resistance for a particular organism. Conversely, they all demonstrated horizontal MIC trends which would be indicative of the absence of induced resistance for a particular organism.
Among the S. aureus and S. epidermidis isolates for which we were able to achieve subinhibitory concentrations, organisms cultured on blood agar were visually stunted and exhibited unusal morphology, indicating they were not healthy organisms. Organisms were passed for 2 passages before all S. aureus and S. epidermidis organisms died. This testing was repeated twice with the same results.
Assessment of phenotypic adaptation.
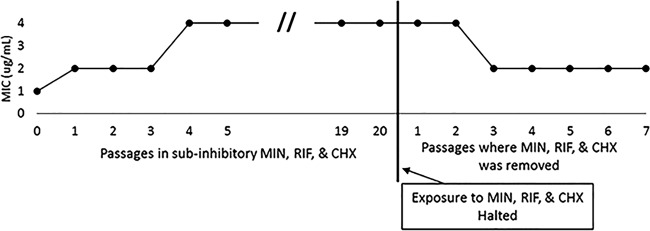
During the 20 sequential passages, Enterobacter cloacae (MDA 166) showed a sustained 4-fold increase in MIC. After passage 20, E. cloacae MDA 166 was grown in Mueller-Hinton broth (MHB) for 24 h, establishing normal growth without exposure to MIN, RIF, and CHX. After normal growth with MHB, the organism was exposed to the triple combination (MIN, RIF, and CHX) to evaluate changes in MIC values following removal of antimicrobial stress (Fig. 1).
FIG 1.
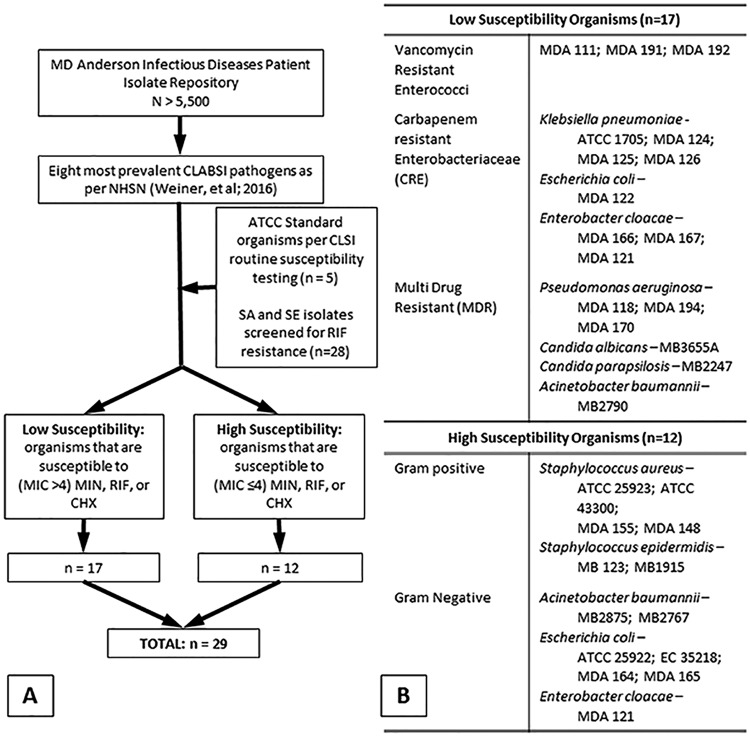
Organism identification and categorization. Organisms from the MD Anderson Patient Isolates bank as well as standard organisms used for CLSI testing were screened for high susceptibility or low susceptibility to individual antimicrobial agents of minocycline, rifampin, or chlorhexidine (A). From these banks, a total of 29 isolates were used for assessing the potential for inducing resistance to the triple combination of minocycline, rifampin, and chlorhexidine (B).
DISCUSSION
MIC measurements have experimental uncertainty associated with weighing, pipetting, and dilution variabilities. This leads to expected MIC variations within a factor of 2 during passages as not resulting from microbial susceptibility changes but rather a result of normal experimental variability. Sustained MIC increases of greater than 4-fold are required to attribute to true changes in microbial susceptibility (14).
Following this premise, experimental results on exhaustively passaging the comprehensive range of clinically relevant infectious pathogens at subinhibitory concentrations support that after 20 passages of microbial bloodstream isolates being exposed to subinhibitory concentrations of MIN, RIF, and CHX, there was no evidence of induced resistance in any of the organisms. Even among the already highly resistant organisms (resistant to 2 or more individual drugs), there was no evidence of organisms becoming more resistant. Furthermore, the triple combination possessed strong antimicrobial synergy, in that organisms that were resistant to all three drugs individually (MIC of ≥16 μg/ml for MIN; ≥4 μg/ml for RIF or CHX) showed MICs of 1 or 2 μg/ml for the combination.
One Enterobacter strain (MDA 166) showed an increase in MIC from 1 to 4 μg/ml following 4 passages but did not further increase through 20 passages. Furthermore, after continuing passages in Mueller-Hinton broth alone, the MIC decreased. When the tolerance of an organism toward bactericidal activity increases during the exposure of the triple combination, but it is lost after the bacteria are subcultured in the absence of MIN, RIF, and CHX, it is indicative of a phenotypic adaptation (i.e., activation of genes already present that were latent) and not induced resistance. Other studies also reported this type of adaptive response (15, 16). We hypothesize that any reduced susceptibility was likely induced by already present but latent resistance genes being activated by exposure to subinhibitory MIN, RIF, and CHX concentrations. Once the MIN, RIF, and CHX were removed, those genes likely gradually returned to latency, and the MIC for the triple combination decreased, indicating no new resistance was acquired from the repeated subinhibitory exposures.
These results can, in part, be explained by the three agents MIN, RIF, and CHX having three independent mechanisms of action that impact cells at very different structural levels. Rifampin inhibits RNA polymerase and, therefore, interferes with genetic transcription processes (17–20). Minocycline primarily inhibits protein synthesis by binding to the 30S ribosomal subunit (21, 22). Chlorhexidine interferes with cell membrane function by altering porosity and cell membrane integrity (23–26). This helps explain both the antimicrobial synergy as well as the absence of emergent resistance. In order for resistance to the triple combination to occur, three independent cellular modifications or thwarting adaptations would need to occur simultaneously at these very different cellular structural targets.
Resistance to rifampin has been reported by the occurrence of point mutations in the RNA polymerase (27). Minocycline resistance has been reported to occur through the expression of efflux pumps and less commonly through the expression of ribosomal protection proteins (21, 28). These types of resistance require the acquisition and expression of new genes which are less probable than the occurrence of point mutations. Two independent clinical studies at two different hospitals (29, 30) have demonstrated that when the rates of infections caused by resistant organisms for multiyear periods before the binary combination of MIN and RIF catheters were introduced are compared with rates after prolonged MIN and RIF catheter use, there was no increase in the incidence of staphylococcal infections caused by resistant organisms. Hence, the clinical evidence supports that the risk of resistance developing to the MIN and RIF binary combination catheters is already quite low. In vitro studies have further demonstrated that combining MIN with RIF protected against the development of RIF-resistant mutants (31, 32) and that resistant mutants retained susceptibility to clinically relevant concentrations of MIN and RIF in combination (33). Similarly, CHX resistance has been reported to be due to the presence of efflux pumps (34) which also require the acquisition and expression of new genes. Based on the independence of the mechanisms of action for each of the three agents, the overall probability of resistance to all three agents occurring in the same organism would be the multiplicative product of the probability of induction of each individual resistance. Mutational frequencies for bacteria ranging from 10−6 to 10−10 have been reported (35) with values of 10−7 to 10−8 common (36, 37) among CLABSI pathogens. Since each mutational probability is very small to begin with, the probability of having three independent resistance mechanisms emerging simultaneously as a results of exposure to the MIN, RIF, and CHX combination would be vanishingly and extremely small (ranging from 10−18 to 10−30). The lack of emergent resistance following the exhaustive subinhibitory passaging in this study is consistent with this theoretical mechanistic framework.
This experiment assessed the potential for a comprehensive range of clinically relevant infectious microbes to become resistant when repeatedly exposed to subinhibitory concentrations of the combination of three antimicrobial agents, namely, MIN, RIF, and CHX. The motivation for this testing was to determine the relative risk of developing more highly resistant pathogens from exposure to the MIN, RIF, and CHX combination when their concentrations on implanted medical devices (such as vascular catheters) become depleted through elution versus the risk of acquiring breakthrough infections from devices without antimicrobial protection or from devices with weaker protection from more limited antimicrobial agents. Surveillance studies monitoring the development of resistance should be conducted in a clinical setting.
Current CLABSI incidences in intensive care units (ICUs) in the United States are approximately 1 CLABSI per thousand catheter days (38) with much higher rates reported in high-risk patients (39) and other countries (40). Among the currently available antimicrobial catheter options, the use of MIN and RIF catheters has clinically demonstrated significant reductions in CLABSI rates; however, breakthrough infections by organisms with low susceptibility to the MIN and RIF combination occasionally occur. Therefore, adding CHX to the MIN and RIF combination promises to both reduce the risk of breakthrough CLABSIs occurring as well as reduce the risk of developing new antimicrobial resistance.
MATERIALS AND METHODS
Organism selection.
Isolates were chosen from an organism selection process as follows. First, from the historic library containing >5,500 deidentified isolates from cancer patients with bloodstream isolates collected at MD Anderson Cancer Center over a period of 10 years, 20 isolates from the 8 most prevalent CLABSI-related pathogens, as defined by from the National Healthcare Safety Network (NHSN) database, (41) were selected. Second, in order to encompass all organisms, rather than hospital-specific strains, 5 isolates from the American Type Culture Collection (ATCC) used in standardized MIC determination (42) were also chosen. (Fig. 2A) A total of 29 Gram-positive, Gram-negative, and yeast isolates were chosen for testing, including Staphylococcus aureus (strains MDA 148, 155, ATCC 25923, and 43300), Staphylococcus epidermidis (strains MDA 123 and MB1915), Enterococcus faecium (strains MDA 111, 191, and 192), Klebsiella pneumoniae (strains MDA 124, 125, 126, and ATCC 1705), Escherichia coli (strains MDA 122, 164, 165, ATCC 25922, and 35218), Enterobacter cloacae (strains MDA 121, 166, and 167), Pseudomonas aeruginosa (strains MDA 118, 170, and 194), Acinetobacter baumannii (strains MB2875, 2790, and 2767), Candida albicans (strain MB3655A), and Candida parapsilosis (strain MB2247). Susceptibilities to front-line antimicrobials for all organisms tested are presented in Table S1 in the supplemental material.
FIG 2.
Assessment of phenotypic adaptation. Enterobacter cloacae (MDA 166) showed a sustained 4-fold increase in MIC from passage 4 to 20. To determine whether the increase in MIC was due to induced resistance or phenotypic adaptation, the organism cultured in passage 20 was then exposed to Mueller-Hinton broth (MHB) without antimicrobial agents (no exposure to MIN, RIF, and CHX) and passaged for an additional 7 passages. MICs to MIN, RIF, and CHX were determined after each additional passage. After 3 passages without antimicrobial agents, the MIC returned to 2, indicating a phenotpyic adaptation to survival when exposed to MIN, RIF, and CHX.
At the initiation of the study, organisms were cultured from glycerol stocks stored at −80°C onto Trypticase soy agar and 5% sheep blood for bacteria and Sabouraud dextrose agar for yeast and incubated at 37°C for 24 hours. For testing, organisms were inoculated into 5 ml of Mueller-Hinton Broth (MHB) and incubated for approximately 1 hour and then further diluted in MHB to the appropriate inoculum concentration. Susceptibility profiles for all organisms are presented in the supplemental material.
MIC determination of individual agents.
The 29 isolates chosen for testing were further characterized into two categories based on susceptibility to individual antimicrobial agents minocycline (MIN), rifampin (RIF), or chlorhexidine (CHX), namely, (i) highly susceptible organisms are those susceptible to at least 2 of the individual antimicrobial agents and (ii) low-susceptible organisms are those susceptible to no more than 1 agent (Fig. 2B). The purpose for choosing these two categories of organisms is to assess the following: (i) whether organisms susceptible to individual antimicrobial agents and/or the triple combination would develop new resistance mechanisms as an adaptation to the presence of these agents and (ii) whether organisms that possessed prior low susceptibility to individual agents would become resistant to the triple combination as an adaptation to continuous exposures.
MICs of individual components (minocycline, rifampin, or chlorhexidine) were determined for each isolate using broth microdilution testing. All MIC determination procedures are based on CLSI guidelines M100 performance standards for antimicrobial susceptibility testing (1) and M07 methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically (14). Briefly, individual components of minocycline, rifampin, or chlorhexidine were dissolved in methanol, and solutions were then diluted >1000× in Mueller-Hinton Broth (MHB) for a final stock concentration of 32 μg/ml. From these stock solutions, 2-fold serial dilutions for each drug ranging in concentrations from 32 μg/ml to 0.031 μg/ml were made in a 96-well plate to create the testing panel. Additionally, a second MIC plate ranging in concentrations from 0.031 μg/ml to 0.000061 μg/ml was used to test highly susceptible isolates whose MICs were below the limit of detection in the original MIC plate. Isolates were inoculated at 5 × 104 CFU in each well and incubated at 37°C for 24 hours. After incubation, MICs were determined for each plate. The MIC for each drug is defined as the lowest concentration of drug where there is no visual growth of the microorganism. Each MIC was tested in triplicate and an MHB with no drug was used as a positive control. Resultant MICs were then assigned susceptible (S), intermediate (I), or resistant (R), for minocycline (42), rifampin (42), and chlorhexidine (43). MIC breakpoints are defined as S ≤ 4, I = 8, R ≥ 16 for minocycline; S ≤ 1, I = 2, R ≥ 4 for rifampin; and S ≤ 2, R ≥ 4 for chlorhexidine.
MIC determination and subinhibitory passages of minocycline/rifampin and chlorhexidine.
To examine the potential for developing resistance to the triple combination MIN, RIF, and CHX, MICs of the triple combination were assessed at baseline and through 20 passages by exposing organisms to subinhibitory concentrations of MIN, RIF, and CHX. Baseline MICs and MICs of subsequent passages were based on CLSI testing procedures (14, 42). To create MIC determination panels, minocycline, rifampin, and chlorhexidine were dissolved in methanol at a ratio of approximately 1:1:1. This ratio corresponds to the target ratio of drugs contained on the experimental MIN, RIF, and CHX central venous catheter. This triple combination was then diluted >1000× in MHB for a final stock concentration of 32 μg/ml (contains 32 μg/ml each of MIN, RIF, and CHX). From this stock, 2-fold serial dilutions from 32 μg/ml to 0.031 μg/ml were made in a 96-well plate to create the testing panel. Additionally, a second MIC plate ranging in concentrations from 0.031 μg/ml to 0.000061 μg/ml was used to test highly susceptible isolates whose MICs were below the limit of detection in the original MIC plate. Isolates were inoculated at 5 × 104 CFU in each well and incubated at 37°C for 24 hours. The number of organisms inoculated for the MIC studies is based on the CLIS standard; however, this concentration is also clinically relevant as it corresponds to the upper range of the number of organisms typically recovered in our hospital from cultures grown from catheter tips from patients with CRBSI. After incubation, MICs were visually determined for each plate. MIC is defined as the lowest concentration where there is no visible growth of microorganisms. Each MIC was determined in triplicate, and MHB with no drug was used as a positive control.
Passage studies are based well-defined methods in the literature (31, 44). Organisms from the first subinhibitory concentration (highest concentration of MIN, RIF, and CHX with visible growth) were cultured on Trypticase soy agar with 5% sheep blood for bacteria or Sabouraud dextrose agar for yeast and incubated at 37°C for 24 hours (passage 1). MIC determination and culture of organism exposed to subinhibitory drug concentrations was repeated for each passed organism for passages 1 to 20. MICs for MIN, RIF, and CHX were recorded for passages 1 to 20 of each organism. As an internal control, E. coli ATCC 35218 and Enterococcus faecium MDA 191 stock isolates were tested with passage. This was done to monitor any deviations that may have occurred during plate production. Internal control isolates must be within a 2-fold concentration of their baseline MIC (baseline MIN, RIF, and CHX E. coli 35218 MIC, <1; Enterococcus faecium 191 MIC, 2) in order for the test to be considered valid. Any test with a ≥4-fold increase is considered nonpassing, and the passage was repeated.
Assessment of phenotypic adaptation.
Organisms with sustained 4-fold increases in MIC following sequential passages were assessed to determine whether the increase in MIC was due to induced resistance or phenotypic adaptation. After passage 20, any organism with a 4-fold increase was grown in MHB for 24 h, establishing normal growth without exposure to MIN, RIF, and CHX. After normal growth with MHB, MICs were determined for MIN, RIF, and CHX as above to evaluate changes following the removal of antimicrobial stress. The following testing was repeated for multiple passages. When the tolerance of an organism toward bactericidal activity increases during the exposure of the triple combination, but it is lost after the bacteria are subcultured in the absence of MIN, RIF, and CHX, it is indicative of a phenotypic adaptation (i.e., activation of genes already present that were latent) and not induced resistance. Other studies reported an adaptive response (15, 16).
Supplementary Material
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.00040-19.
REFERENCES
- 1.O'Grady NP, Alexander M, Burns LA, Dellinger EP, Garland J, Heard SO, Lipsett PA, Masur H, Mermel LA, Pearson ML, Raad II, Randolph AG, Rupp ME, Saint S. 2011. Guidelines for the prevention of intravascular catheter-related infections. Clin Infect Dis 52:e162–e193. doi: 10.1093/cid/cir257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Storey S, Brown J, Foley A, Newkirk E, Powers J, Barger J, Paige K. 2016. A comparative evaluation of antimicrobial coated versus nonantimicrobial coated peripherally inserted central catheters on associated outcomes: a randomized controlled trial. Am J Infect Control 44:636–641. doi: 10.1016/j.ajic.2015.11.017. [DOI] [PubMed] [Google Scholar]
- 3.Darouiche RO, Berger DH, Khardori N, Robertson CS, Wall MJ Jr, Metzler MH, Shah S, Mansouri MD, Cerra-Stewart C, Versalovic J, Reardon MJ, Raad II. 2005. Comparison of antimicrobial impregnation with tunneling of long-term central venous catheters: a randomized controlled trial. Ann Surg 242:193–200. doi: 10.1097/01.sla.0000171874.29934.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raad I, Darouiche R, Dupuis J, Abi SD, Gabrielli A, Hachem R, Wall M, Harris R, Jones J, Buzaid A, Robertson C, Shenaq S, Curling P, Burke T, Ericsson C. 1997. Central venous catheters coated with minocycline and rifampin for the prevention of catheter-related colonization and bloodstream infections: a randomized, double-blind trial. Ann Intern Med 127:267–274. doi: 10.7326/0003-4819-127-4-199708150-00002. [DOI] [PubMed] [Google Scholar]
- 5.Hanna H, Benjamin R, Chatzinikolaou I, Alakech B, Richardson D, Mansfield P, Dvorak T, Munsell MF, Darouiche R, Kantarjian H, Raad I. 2004. Long-term silicone central venous catheters impregnated with minocycline and rifampin decrease rates of catheter-related bloodstream infection in cancer patients: a prospective randomized clinical trial. J Clin Oncol 22:3163–3171. doi: 10.1200/JCO.2004.04.124. [DOI] [PubMed] [Google Scholar]
- 6.Fraenkel D, Rickard C, Thomas P, Faoagali J, George N, Ware R. 2006. A prospective, randomized trial of rifampicin-minocycline-coated and silver-platinum-carbon-impregnated central venous catheters. Crit Care Med 34:668–675. doi: 10.1097/01.CCM.0000201404.05523.34. [DOI] [PubMed] [Google Scholar]
- 7.Chatzinikolaou I, Finkel K, Hanna H, Boktour M, Foringer J, Ho T, Raad I. 2003. Antibiotic-coated hemodialysis catheters for the prevention of vascular catheter-related infections: a prospective, randomized study. Am J Med 115:352–357. doi: 10.1016/S0002-9343(03)00367-X. [DOI] [PubMed] [Google Scholar]
- 8.Falagas ME, Fragoulis K, Bliziotis IA, Chatzinikolaou I. 2007. Rifampicin-impregnated central venous catheters: a meta-analysis of randomized controlled trials. J Antimicrob Chemother 59:359–369. doi: 10.1093/jac/dkl522. [DOI] [PubMed] [Google Scholar]
- 9.Chong HY, Lai NM, Apisarnthanarak A, Chaiyakunapruk N. 2017. Comparative efficacy of antimicrobial central venous catheters in reducing catheter-related bloodstream infections in adults: abridged Cochrane systematic review and network meta-analysis. Clin Infect Dis 64:S131–S140. doi: 10.1093/cid/cix019. [DOI] [PubMed] [Google Scholar]
- 10.Ramritu P, Halton K, Collignon P, Cook D, Fraenkel D, Battistutta D, Whitby M, Graves N. 2008. A systematic review comparing the relative effectiveness of antimicrobial-coated catheters in intensive care units. Am J Infect Control 36:104–117. doi: 10.1016/j.ajic.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 11.Wang H, Huang T, Jing J, Jin J, Wang P, Yang M, Cui W, Zheng Y, Shen H. 2010. Effectiveness of different central venous catheters for catheter-related infections: a network meta-analysis. J Hosp Infect 76:1–11. doi: 10.1016/j.jhin.2010.04.025. [DOI] [PubMed] [Google Scholar]
- 12.Raad I, Mohamed JA, Reitzel RA, Jiang Y, Raad S, Al Shuaibi M, Chaftari AM, Hachem RY. 2012. Improved antibiotic-impregnated catheters with extended-spectrum activity against resistant bacteria and fungi. Antimicrob Agents Chemother 56:935–941. doi: 10.1128/AAC.05836-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jamal MA, Rosenblatt JS, Hachem RY, Ying J, Pravinkumar E, Nates JL, Chaftari AM, Raad II. 2014. Prevention of biofilm colonization by Gram-negative bacteria on minocycline-rifampin-impregnated catheters sequentially coated with chlorhexidine. Antimicrob Agents Chemother 58:1179–1182. doi: 10.1128/AAC.01959-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.CLSI. 2015. M07-A10. Methods for dilution antimicrobial susceptibility tested for bacteria that grow aerobically; approved standard, 10th ed, vol 35 CLSI, Wayne, PA. [Google Scholar]
- 15.Meyer B, Cookson B. 2010. Does microbial resistance or adaptation to biocides create a hazard in infection prevention and control? J Hosp Infect 76:200–205. doi: 10.1016/j.jhin.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 16.Maillard JY. 2007. Bacterial resistance to biocides in the healthcare environment: should it be of genuine concern? J Hosp Infect 65:60–72. doi: 10.1016/S0195-6701(07)60018-8. [DOI] [PubMed] [Google Scholar]
- 17.Wehrli W, Knusel F, Schmid K, Staehelin M. 1968. Interaction of rifamycin with bacterial RNA polymerase. Proc Natl Acad Sci U S A 61:667–673. doi: 10.1073/pnas.61.2.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McClure WR, Cech CL. 1978. On the mechanism of rifampicin inhibition of RNA synthesis. J Biol Chem 253:8949–8956. [PubMed] [Google Scholar]
- 19.Campbell EA, Korzheva N, Mustaev A, Murakami K, Nair S, Goldfarb A, Darst SA. 2001. Structural mechanism for rifampicin inhibition of bacterial RNA polymerase. Cell 104:901–912. doi: 10.1016/S0092-8674(01)00286-0. [DOI] [PubMed] [Google Scholar]
- 20.Wehrli W. 1983. Rifampin: mechanisms of action and resistance. Rev Infect Dis 5:S407–S411. doi: 10.1093/clinids/5.Supplement_3.S407. [DOI] [PubMed] [Google Scholar]
- 21.Chopra I, Roberts M. 2001. Tetracycline antibiotics: mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol Mol Biol Rev 65:232–260. doi: 10.1128/MMBR.65.2.232-260.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olson MW, Ruzin A, Feyfant E, Rush TS III, O'Connell J, Bradford PA. 2006. Functional, biophysical, and structural bases for antibacterial activity of tigecycline. Antimicrob Agents Chemother 50:2156–2166. doi: 10.1128/AAC.01499-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chawner JA, Gilbert P. 1989. Adsorption of alexidine and chlorhexidine to Escherichia coli and membrane components. Int J Pharm 55:209–215. doi: 10.1016/0378-5173(89)90043-4. [DOI] [Google Scholar]
- 24.Sheppard FC, Mason DJ, Bloomfield SF, Gant VA. 1997. Flow cytometric analysis of chlorhexidine action. FEMS Microbiol Lett 154:283–288. doi: 10.1111/j.1574-6968.1997.tb12657.x. [DOI] [PubMed] [Google Scholar]
- 25.McDonnell G, Russell AD. 1999. Antiseptics and disinfectants: activity, action, and resistance. Clin Microbiol Rev 12:147–179. doi: 10.1128/CMR.12.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Russell AD. 1986. Chlorhexidine: antibacterial action and bacterial resistance. Infection 14:212–215. doi: 10.1007/BF01644264. [DOI] [PubMed] [Google Scholar]
- 27.Furustrand Tafin U, Aubin GG, Eich G, Trampuz A, Corvec S. 2015. Occurrence and new mutations involved in rifampicin-resistant Propionibacterium acnes strains isolated from biofilm or device-related infections. Anaerobe 34:116–119. doi: 10.1016/j.anaerobe.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 28.Draper MP, Weir S, Macone A, Donatelli J, Trieber CA, Tanaka SK, Levy SB. 2014. Mechanism of action of the novel aminomethylcycline antibiotic omadacycline. Antimicrob Agents Chemother 58:1279–1283. doi: 10.1128/AAC.01066-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramos ER, Reitzel R, Jiang Y, Hachem RY, Chaftari AM, Chemaly RF, Hackett B, Pravinkumar SE, Nates J, Tarrand JJ, Raad II. 2011. Clinical effectiveness and risk of emerging resistance associated with prolonged use of antibiotic-impregnated catheters: more than 0.5 million catheter days and 7 years of clinical experience. Crit Care Med 39:245–251. doi: 10.1097/CCM.0b013e3181feb83e. [DOI] [PubMed] [Google Scholar]
- 30.Turnbull IR, Buckman SA, Horn CB, Bochicchio GV, Mazuski JE. 2018. Antibiotic-impregnated central venous catheters do not change antibiotic resistance patterns. Surg Infect (Larchmt) 19:40–47. doi: 10.1089/sur.2017.087. [DOI] [PubMed] [Google Scholar]
- 31.Tambe SM, Sampath L, Modak SM. 2001. In vitro evaluation of the risk of developing bacterial resistance to antiseptics and antibiotics used in medical devices. J Antimicrob Chemother 47:589–598. doi: 10.1093/jac/47.5.589. [DOI] [PubMed] [Google Scholar]
- 32.Yourassowsky E, van der Linden MP, Lismont MJ, Crokaert F. 1981. Combination of minocycline and rifampicin against methicillin- and gentamicin-resistant Staphylococcus aureus. J Clin Pathol 34:559–563. doi: 10.1136/jcp.34.5.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sampath LA, Tambe SM, Modak SM. 2001. In vitro and in vivo efficacy of catheters impregnated with antiseptics or antibiotics: evaluation of the risk of bacterial resistance to the antimicrobials in the catheters. Infect Control Hosp Epidemiol 22:640–646. doi: 10.1086/501836. [DOI] [PubMed] [Google Scholar]
- 34.Wand ME, Bock LJ, Bonney LC, Sutton JM. 2017. Mechanisms of increased resistance to chlorhexidine and cross-resistance to colistin following exposure of Klebsiella pneumoniae clinical isolates to chlorhexidine. Antimicrob Agents Chemother 61:e01162-16. doi: 10.1128/AAC.01162-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martinez JL, Baquero F. 2000. Mutation frequencies and antibiotic resistance. Antimicrob Agents Chemother 44:1771–1777. doi: 10.1128/AAC.44.7.1771-1777.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kohler T, Michea-Hamzehpour M, Plesiat P, Kahr AL, Pechere JC. 1997. Differential selection of multidrug efflux systems by quinolones in Pseudomonas aeruginosa. Antimicrob Agents Chemother 41:2540–2543. doi: 10.1128/AAC.41.11.2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Galan JC, Tato M, Baquero MR, Turrientes C, Baquero F, Martinez JL. 2004. Fosfomycin and rifampin disk diffusion tests for detection of Escherichia coli mutator strains. J Clin Microbiol 42:4310–4312. doi: 10.1128/JCM.42.9.4310-4312.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Furuya EY, Dick AW, Herzig CT, Pogorzelska-Maziarz M, Larson EL, Stone PW. 2016. Central line-associated bloodstream infection reduction and bundle compliance in intensive care units: a national study. Infect Control Hosp Epidemiol 37:805–810. doi: 10.1017/ice.2016.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bundy DG, Gaur AH, Billett AL, He B, Colantuoni EA, Miller MR. 2014. Preventing CLABSIs among pediatric hematology/oncology inpatients: national collaborative results. Pediatrics 134:e1678–e1685. doi: 10.1542/peds.2014-0582. [DOI] [PubMed] [Google Scholar]
- 40.Rosenthal VD, Maki DG, Mehta Y, Leblebicioglu H, Memish ZA, Al-Mousa HH, Balkhy H, Hu B, Alvarez-Moreno C, Medeiros EA, Apisarnthanarak A, Raka L, Cuellar LE, Ahmed A, Navoa-Ng JA, El-Kholy AA, Kanj SS, Bat-Erdene I, Duszynska W, Van Truong N, Pazmino LN, See-Lum LC, Fernández-Hidalgo R, Di-Silvestre G, Zand F, Hlinkova S, Belskiy V, Al-Rahma H, Luque-Torres MT, Bayraktar N, Mitrev Z, Gurskis V, Fisher D, Abu-Khader IB, Berechid K, Rodríguez-Sánchez A, Horhat FG, Requejo-Pino O, Hadjieva N, Ben-Jaballah N, García-Mayorca E, Kushner-Dávalos L, Pasic S, Pedrozo-Ortiz LE, Apostolopoulou E, Mejía N, Gamar-Elanbya MO, Jayatilleke K, de Lourdes-Dueñas M, Aguirre-Avalos G. 2014. International Nosocomial Infection Control Consortium (INICC) report, data summary of 43 countries for 2007–2012. Device-associated module. Am J Infect Control 42:942–956. doi: 10.1016/j.ajic.2014.05.029. [DOI] [PubMed] [Google Scholar]
- 41.Weiner LM, Webb AK, Limbago B, Dudeck MA, Patel J, Kallen AJ, Edwards JR, Sievert DM. 2016. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2011–2014. Infect Control Hosp Epidemiol 37:1288–1301. doi: 10.1017/ice.2016.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.CLSI. 2015. M100S. Performance standards for antimicrobial susceptibility testing, 26th ed CLSI, Wayne, PA. [Google Scholar]
- 43.Morrissey I, Oggioni MR, Knight D, Curiao T, Coque T, Kalkanci A, Martinez JL. 2014. Evaluation of epidemiological cut-off values indicates that biocide resistant subpopulations are uncommon in natural isolates of clinically-relevant microorganisms. PLoS One 9:e86669. doi: 10.1371/journal.pone.0086669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.D'Lima L, Friedman L, Wang L, Xu P, Anderson M, Debabov D. 2012. No decrease in susceptibility to NVC-422 in multiple-passage studies with methicillin-resistant Staphylococcus aureus, S. aureus, Pseudomonas aeruginosa, and Escherichia coli. Antimicrob Agents Chemother 56:2753–2755. doi: 10.1128/AAC.05985-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.