LETTER
Amid the global crisis of increasing gonococcal antimicrobial resistance, we report the first case of ceftriaxone-resistant multidrug-resistant Neisseria gonorrhoeae in Singapore. Specimen 18DG342 was isolated from a throat swab taken from a female sex worker during a routine screening for sexually transmitted infections. The patient was empirically treated with intramuscular ceftriaxone 500 mg and azithromycin 1 g orally, based on local management guidelines (1). Repeated throat swab was culture negative for N. gonorrhoeae 1 week later. Two subsequent N. gonorrhoeae nucleic acid amplification tests (cobas 4800 CT/NG; Roche Diagnostics) were negative. It is possible that she was in contact with international clients as part of her sex work. Her travel history was unknown.
The isolate was cultured on GC-Lect agar (BD BBL) and was confirmed to be N. gonorrhoeae by matrix-assisted laser desorption ionization–time of flight mass spectrometry (Bruker), API NH (bioMérieux), and whole-genome sequencing. MIC values for ceftriaxone and 13 other antimicrobials were determined using Etests (bioMérieux) according to the manufacturer’s instructions, and results were interpreted in accordance with Clinical and Laboratory Standards Institute (CLSI) breakpoints (2). Isolate 18DG342 was nonsusceptible to ceftriaxone (MIC, 1 mg/liter); resistant to cefixime (MIC, 4 mg/liter), penicillin (MIC, >32 mg/liter), and ciprofloxacin (MIC, >256 mg/liter); and of intermediate resistance to tetracycline (MIC, 1 mg/liter). It remained susceptible to azithromycin (MIC, 0.25 mg/liter) and spectinomycin (MIC, 16 mg/liter). Isolate 18DG342 was a β-lactamase-producing strain based on the positive Cefinase paper disc (BD BBL) test. Based on Australian Gonococcal Surveillance Program (AGSP) (http://cdstest.net/manual/neisseria-gonorrhoeae/) interpretative criteria (3–5), 18DG342 was less sensitive to gentamicin (MIC, 8 mg/liter).
We performed molecular typing in silico using whole-genome sequence data (BioProject accession no. PRJNA508549). We sequenced the isolate with the Illumina MiSeq platform (Illumina) and used the genomic quality, assembly, and phylogenetic analysis pipeline as described previously (6). Sequence data were submitted to the Neisseria multilocus sequence typing (MLST) website (https://pubmlst.org/neisseria/) (7) and were assigned the novel sequence type ST13871. While most previously reported ceftriaxone-resistant N. gonorrhoeae isolates belong to MLST ST1903 (fumC allele 157), 18DG342 differed in the fumC locus (fumC allele 987). The N. gonorrhoeae multiantigen sequence type (NG-MAST) (http://www.ng-mast.net/) (8) was ST1086 (porB allele number 581 and tbpB allele number 21). N. gonorrhoeae sequence typing for the antimicrobial resistance (NG-STAR) profile was 233 (https://ngstar.canada.ca/pages/welcome?lang=en) (9). NG-STAR profile 233 contains a mosaic penA-60.001 allele, an mtrR-35A deletion, and porB G120K, ponA L421P, gyrA S91F/D95A, and parC S87R mutations. 23S rRNA mutations were not detected. blaTEM-135 was detected in the whole-genome sequence data. The molecular antimicrobial resistance profile corresponds to the MICs determined phenotypically.
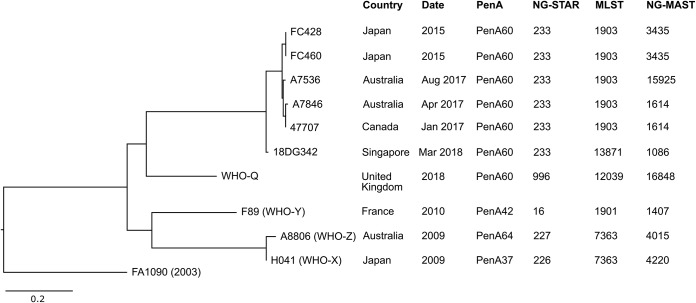
The 18DG342 genome sequence was compared with those of previously genome-sequenced ceftriaxone-resistant isolates FC428 (10), F460 (10), 47707 (11), A7536 (12), A7846 (12), H041 (13), A8806 (13), F89 (13), and WHO-Q (G97687/G7944) (14). A single nucleotide variation (SNV) phylogenetic tree was created by mapping reads to the reference sequence FA1090 (GenBank accession no. NC_002946.2) (Fig. 1). By core genome SNV analysis (12, 15), 18DG342 was distinct from the previously described H041 (WHO-X), F89 (WHO-Y), and A8806 (WHO-Z) ceftriaxone resistance strains and was more closely related to the internationally spreading Japanese FC428 clone. Isolate 18DG342 was most closely related to the MLST ST1903 strains (FC428, FC460, A7536, A7846, and 47707), with a difference of 35 to 39 SNVs between the core genomes of 18DG342 and the ST1903 strains included in this analysis.
FIG 1.
Core genome single nucleotide variation (SNV) phylogenetic tree of the ceftriaxone-resistant multidrug-resistant Neisseria gonorrhoeae isolate 18DG342 and a panel of previously reported ceftriaxone-resistant N. gonorrhoeae isolates (10–15). The complete genome of N. gonorrhoeae FA1090 (GenBank accession no. NC_002946.2) was included as the reference sequence. Scale bar indicates nucleotide substitution per site. PenA, penicillin-binding protein 2; NG-STAR, Neisseria gonorrhoeae sequence type for antimicrobial resistance; MLST, multilocus sequence type; NG-MAST, Neisseria gonorrhoeae multiantigen sequence type.
Situated in Southeast Asia with a large transient population of foreign visitors, the importation and dissemination of antimicrobial resistance is a constant public health threat to Singapore. Ceftriaxone-resistant multidrug-resistant N. gonorrhoeae undermines the effectiveness of currently recommended first-line dual therapy. Enhanced surveillance of antimicrobial susceptibilities is necessary to detect and monitor the resistance trends so as to safeguard the effectiveness of the remaining therapeutic options. The emergence of ceftriaxone-resistant gonococcal infection had been anticipated (1), and effective control measures (e.g., mandatory regular screening of sex workers, safe sex practice advice to sex workers) are in place to safeguard public health (https://sso.agc.gov.sg/Act/IDA1976). The detection of this gonococcal isolate serves as a stark reminder of the continual spread of multidrug-resistant N. gonorrhoeae globally and calls for even greater vigilance to limit the potential of dissemination.
Accession number(s).
Raw sequencing reads have been deposited at GenBank under BioProject accession no. PRJNA508549.
ACKNOWLEDGMENTS
The sequencing was funded by a SingHealth Pathology Academic Clinical Program Clinical Innovation Support Program grant.
We declare no conflicts of interest.
REFERENCES
- 1.Sen P, Tan HE. 2013. Transmitted infection management guidelines. DSC Clinic, National Skin Centre, Singapore, Singapore. [Google Scholar]
- 2.Clinical and Laboratory Standards Institute. 2018. Performance standards for antimicrobial susceptibility testing; 28th informational supplement. CLSI M100-S28. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 3.Bell SM, Pham JN, Fisher GT. 2018. Antibiotic susceptibility testing by the CDS method. A manual for medical and veterinary laboratories 2018, 9th ed The CDS Reference Laboratory, Kogarah, Australia: http://cdstest.net/. [Google Scholar]
- 4.Lahra MM, Enriquez RP. 2017. Australian gonococcal surveillance programme, 1 July to 30 September 2016. Commun Dis Intell Q Rep 41:109–110. [PubMed] [Google Scholar]
- 5.Bala M, Singh V, Philipova I, Bhargava A, Joshi C, Unemo M. 2016. Gentamicin in vitro activity and tentative gentamicin interpretation criteria for the CLSI and calibrated dichotomous sensitivity disc diffusion methods for Neisseria gonorrhoeae. J Antimicrob Chemother 71:1856–1859. doi: 10.1093/jac/dkw102. [DOI] [PubMed] [Google Scholar]
- 6.Faksri K, Tan JH, Disratthakit A, Xia E, Prammananan T, Suriyaphol P, Khor CC, Teo YY, Ong RTH, Chaiprasert A. 2016. Whole-genome sequencing analysis of serially isolated multi-drug and extensively drug resistant Mycobacterium tuberculosis from Thai patients. PLoS One 11:e0160992. doi: 10.1371/journal.pone.0160992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jolley KA, Bray JE, Maiden M. 2018. Open-access bacterial population genomics: BIGSdb software, the PubMLST.org website and their applications. Wellcome Open Res 3:124. doi: 10.12688/wellcomeopenres.14826.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martin IM, Ison CA, Aanensen DM, Fenton KA, Spratt BG. 2004. Rapid sequence-based identification of gonococcal transmission clusters in a large metropolitan area. J Infect DIS 189:1497–1505. doi: 10.1086/383047. [DOI] [PubMed] [Google Scholar]
- 9.Demczuk W, Sidhu S, Unemo M, Whiley DM, Allen VG, Dillon JR, Cole M, Seah C, Trembizki E, Trees DL, Kersh EN, Abrams AJ, de Vries HJC, van Dam AP, Medina I, Bharat A, Mulvey MR, Van Domselaar G, Martin I. 2017. Neisseria gonorrhoeae sequence typing for antimicrobial resistance, a novel antimicrobial resistance multilocus typing scheme for tracking global dissemination of N. gonorrhoeae strains. J Clin Microbiol 55:1454–1468. doi: 10.1128/JCM.00100-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakayama S, Shimuta K, Furubayashi K, Kawahata T, Unemo M, Ohnishi M. 2016. New ceftriaxone- and multidrug-resistant Neisseria gonorrhoeae strain with a novel mosaic penA gene isolated in Japan. Antimicrob Agents Chemother 60:4339–4341. doi: 10.1128/AAC.00504-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lefebvre B, Martin I, Demczuk W, Deshaies L, Michaud S, Labbe AC, Beaudoin MC, Longtin J. 2018. Ceftriaxone-resistant Neisseria gonorrhoeae, Canada. Emerg Infect Dis 24:381–383. doi: 10.3201/eid2402.171756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lahra MM, Martin I, Demczuk W, Jennison AV, Lee K, Nakayama S, Lefebvre B, Longtin J, Ward A, Mulvey MR, Wi T, Ohnishi M, Whiley D. 2018. Cooperative recognition of internationally disseminated ceftriaxone-resistant Neisseria gonorrhoeae strain. Emerg Infect Dis 24:735–743. doi: 10.3201/eid2404.171873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Unemo M, Golparian D, Sánchez-Busó L, Grad Y, Jacobsson S, Ohnishi M, Lahra MM, Limnios A, Sikora AE, Wi T, Harris SR. 2016. The novel 2016 WHO Neisseria gonorrhoeae reference strains for global quality assurance of laboratory investigations: phenotypic, genetic and reference genome characterization. J Antimicrob Chemother 71:3096–3108. doi: 10.1093/jac/dkw288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eyre DW, Sanderson ND, Lord E, Regisford-Reimmer N, Chau K, Barker L, Morgan M, Newnham R, Golparian D, Unemo M, Crook DW, Peto TEA, Hughes G, Cole MJ, Fifer H, Edwards A, Andersson MI. 2018. Gonorrhoea treatment failure caused by a Neisseria gonorrhoeae strain with combined ceftriaxone and high-level azithromycin resistance, England, February 2018. Euro Surveill 23:pii=1800323 https://www.eurosurveillance.org/content/10.2807/1560-7917.ES.2018.23.27.1800323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petkau A, Mabon P, Sieffert C, Knox NC, Cabral J, Iskander M, Iskander M, Weedmark K, Zaheer R, Katz LS, Nadon C, Reimer A, Taboada E, Beiko RG, Hsiao W, Brinkman F, Graham M, Domselaar GV. 2017. SNVPhyl: a single nucleotide variant phylogenomics pipeline for microbial genomic epidemiology. Microb Genom 3:e000116. doi: 10.1099/mgen.0.000116. [DOI] [PMC free article] [PubMed] [Google Scholar]