The duration of antibiotic therapy for bacteremia due to Enterobacteriaceae is not well defined. We sought to evaluate the clinical outcomes with shorter- versus longer-course treatment.
KEYWORDS: Enterobacteriaceae, Gram negative, antibiotic treatment, antimicrobial therapy, bacteremia, bloodstream infection, optimal duration, sepsis, septicemia
ABSTRACT
The duration of antibiotic therapy for bacteremia due to Enterobacteriaceae is not well defined. We sought to evaluate the clinical outcomes with shorter- versus longer-course treatment. We performed a systematic search of the PubMed and EMBASE databases through May 2018. Studies presenting comparative outcomes between patients receiving antibiotic treatment for ≤10 days (“short-course”) and those treated for >10 days (“long-course”) were considered eligible. Four retrospective cohort studies and one randomized controlled trial comprising 2,865 patients met the inclusion criteria. The short- and long-course antibiotic treatments did not differ in 30-day all-cause mortality (1,374 patients; risk ratio [RR] = 0.99; 95% confidence interval [CI], 0.69 to 1.43), 90-day all-cause mortality (1,750 patients; RR = 1.16; 95% CI, 0.81 to 1.66), clinical cure (1,080 patients; RR = 1.02; 95% CI, 0.96 to 1.08), or relapse at 90 days (1,750 patients; RR = 1.08; 95% CI, 0.69 to 1.67). In patients with bacteremia due to Enterobacteriaceae, the short- and long-course antibiotic treatments did not differ significantly in terms of clinical outcomes. Further well-designed studies are needed before treatment for 10 days or less is adopted in clinical practice.
INTRODUCTION
Annually, nearly 2 million episodes of bloodstream infections (BSI) occur in North America and Europe, leading to around 250,000 deaths (1), and BSI is the 11th most common cause of death in the United States (2). BSI caused by Gram-negative bacteria account for approximately 45% of all cases of community-acquired and almost one-third of all health care-associated cases of bacteremia, with Escherichia coli being the most prevalent Gram-negative pathogen for both types of bacteremia (3). Timely administration of the appropriate antibiotic treatment remains the cornerstone for favorable clinical outcome in patients with BSI (4–6).
Determining the appropriate duration of therapy is included in the CDC Strategic Priorities for Combating Antimicrobial Resistance (7) and is part of the National Action Plan for Combating Antibiotic-Resistant Bacteria (8). However, the optimal duration of antibiotic therapy has yet to be defined. The current Infectious Diseases Society of America guidelines suggest that the duration of treatment for intravascular catheter-related Gram-negative bacteremia should be between 7 and 14 days (9), but there is no consensus on the optimal duration of the antimicrobial therapy for non-catheter-related Gram-negative bacteremia. Recently published studies on non-catheter-related bacteremia due to Enterobacteriaceae compared the outcomes of short (≤10 days) with longer (>10 days) treatment in terms of clinical outcomes in an attempt to define the optimal duration of therapy, but their findings are controversial (10, 11). The aim of the present study is to evaluate short versus longer courses of antibiotic treatment for bacteremia due to Enterobacteriaceae in clinical outcomes.
RESULTS
Study selection and patient characteristics.
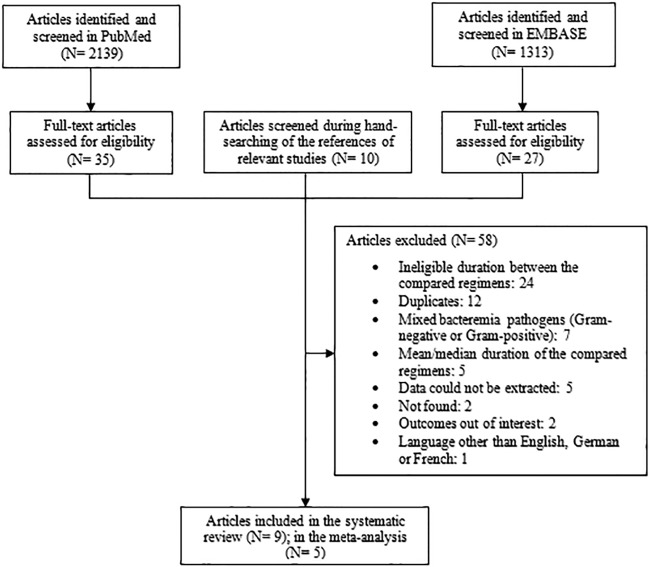
The literature search process retrieved 3,462 articles, with 2,139 articles from PubMed, 1,313 articles from EMBASE, and 10 articles from manual review searching of referenced papers. Among them, 67 studies were assessed in full text. Of these, 24 studies were excluded due to ineligible comparisons of treatment duration, and 5 studies were excluded because the mean/median instead of standard duration of treatment was provided. Other reasons of exclusion comprised duplicates (12 studies), data that could not be extracted (5 studies), study outcomes that were out of interest (2 studies), studies that were not found (2 studies), or ineligible language of publication (1 study). Finally, 7 studies included eligible comparisons of duration but were excluded because they referred to infections caused by Gram-negative or Gram-positive pathogens, where the authors did not distinguish the outcomes according to pathogen group (12–18). Among the remaining 9 studies, there was inconsistency in treatment duration comparisons among published studies, as follows: 5 studies compared ≤10 versus >10 days (10, 11, 19–21), 1 study (178 patients) compared ≤7 versus >7 days (22), 2 studies (56 patients) compared 7 versus 14 days (23, 24), and 1 study (92 patients) compared <14 versus ≥14 days (25). Also, 3 of those studies referred to bacteremia from specific sites of infections (acute pyelonephritis [23, 24] and acute cholangitis [25]). We decided to focus our analysis on studies that compared ≤10 versus >10 days of treatment and which included various sources of bacteremia. The remaining studies were evaluated and discussed as part of the systematic review, but they were not included in the meta-analysis (22–25). The detailed study selection process is depicted in Fig. 1.
FIG 1.
Flow diagram of the search process and study selection.
Five studies reporting on 2,865 patients met the inclusion criteria of the meta-analysis (4 studies that compared ≤10 versus >10 days and 1 study that compared <14 versus ≥14 days) (10, 11, 19–21). The characteristics and outcomes of the studies included in the meta-analysis are presented in Table 1. Four studies were based on retrospective cohort analyses (10, 11, 20, 21), and 1 study was an open-label noninferiority randomized controlled trial (19). On the Newcastle-Ottawa scale, 2 studies were assigned with 7 stars (10, 11) (4 for selection, 1 for comparability, and 2 for outcome [10]; 4 for selection and 3 for outcome [11]), 1 study was assigned with 8 stars (4 for selection, 1 for comparability, and 3 for outcome) (20), and another 1 was assigned with 5 stars (4 for selection and 1 for outcome) (21).
TABLE 1.
Studies comparing for ≤10 versus >10 days of antibiotic treatment for bacteremia due to Enterobacteriaceae
| Reference | Study design; study period; countrya | No. of patients who received antibiotics; causative pathogen; source(s) of bacteremia | Aggregate Newcastle-Ottawa scale scoreb | Outcome by duration, no. of patients/total no. of patientsc |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Clinical cure |
All-cause mortality |
Relapse |
|||||||
| Short-course | Long-course | Short-course | Long-course | Short-course | Long-course | ||||
|
Chotiprasitsakul et al. (10) |
Retrospective propensity score-matched cohort; 2008–2014; USA | 770; Enterobacteriaceae (46.9% Escherichia coli); uncomplicated, 36.1% urinary tract, 19.9% gastrointestinal tract, 16.2% biliary tract, 13.8% catheter-associated, 9% pneumonia, 4% skin and soft tissue | ******* | NR | NR | 30-day, 37/385 | 30-day, 39/385 | 30-day, 5/385 | 30-day, 9/385 |
| Giannella et al. (20) | Retrospective cohort; 2013–2016; Italy | 856; E. coli; 51.3% urinary tract, 15.4% primary, 13.7% biliary tract, 11.1% intra-abdominal, 3.9% lower respiratory tract, 2.1% CVCd, 1.1% skin and soft tissue | ******** | 90-day, 344/426 | 90-day, 341/430 | 90-day, 11/426 | 90-day, 13/430 | 90-day, 23/426 | 90-day, 19/430 |
| Yahav et al. (19) | MC open-label noninferiority RCT; Israel, Italy | 604; 90% Enterobacteriaceae (65% E. coli); 68% urinary tract | NA | NR | NR | 90-day, 36/306 | 90-day, 32/298 | 90-day, 8/306 | 90-day, 8/298 |
| Mercuro et al. (21) | Retrospective cohort; 2013–2016; USA | 224; Enterobacteriaceae (71.4% E. coli); 71% urinary tract, 21.4% intra-abdominal, skin and soft tissue, pneumonia, catheter-related, unknown source | ***** | 30-day, 45/51 | 30-day, 150/173 | NR | NR | NR | NR |
| Nelson et al. (11) | Retrospective cohort; 2010–2013; USA | 411; Enterobacteriaceae (66% E. coli); uncomplicated, 69% urinary tract | ******* | NR | NR | 90-day, 8/91e | 90-day, 7/199e | 90-day, 6/91e | 90-day, 15/199e |
MC, multicenter; RCT, randomized clinical trial.
NA, not applicable.
NR, not reported.
CVC, central venous catheter.
The data provided by the authors of the study after request were the following: 13 and 19 treatment failures (defined as mortality plus relapse) in the short- and long-course groups, and 26 and 95 patients lost to follow-up at day 90 in each group, respectively; 91 and 199 patients were evaluable at day 90 in the short- and long-course groups, respectively; treatment failures comprised 8 deaths plus 6 recurrences (1 patient died after recurrence) in the short-course group and 7 deaths plus 15 recurrences (3 patients died after recurrence) in the long-course group.
The source of bacteremia in the included studies accounted for urinary tract (54.8%), biliary/gastrointestinal tract infection (13.8%), intra-abdominal (5%), primary/central venous catheter-related bacteremia (4.8%), pneumonia (3.6%), soft tissue infection (1.4%), and other/unknown sources. Overall, the urinary tract was the most prevalent source of bacteremia in all 5 studies, comprising 71% (21), 69% (11), 68% (19), 51.3% (20), and 36.1% (10) of the included infections. E. coli was the only involved pathogen in 1 study (20) and the most common pathogen from the Enterobacteriaceae family in the other 4 studies, representing 71.4% (21), 66% (11), 65% (19), and 46.9% (10) of the pathogens. Overall, the majority of bacteremia cases in this meta-analysis came from urinary tract infections due to E. coli, and 41.2% (1,181 out of 2,865) of the patients were reported to have uncomplicated bacteremia. Klebsiella pneumoniae was the second most common cause of bacteremia in three studies, accounting for 32.6% (10), 17% (21), and 14.8% (11) of the included cases. The antibiotic treatment was not specified in any of these studies.
Studies included in the meta-analysis: treatment for ≤10 days versus >10 days.
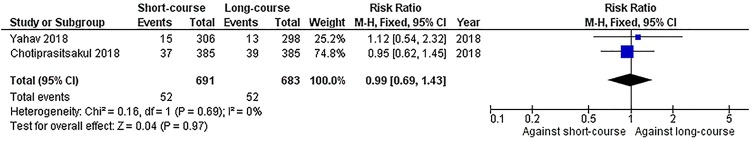
We pooled studies that assessed mortality at 30 days (10, 19) and 90 days (11, 19, 20) counting from the completion of the antibiotic therapy. Pooling of the studies that assessed 30-day mortality showed that there was no statistically significant difference in all-cause mortality between short- and long-course treatments (7.5% versus 7.6%, respectively) (Fig. 2; 1,374 patients; risk ratio [RR] = 0.99; 95% confidence interval [CI], 0.69 to 1.43), and no heterogeneity was detected (I2 = 0%). When we pooled data on 90-day mortality, again there was no significant difference between short- and long-course treatments (6.7% versus 5.6%, respectively; Fig. 3; 1,750 patients; RR = 1.16; 95% CI, 0.81 to 1.66). Mild statistical heterogeneity was detected in this analysis (I2 = 33%).
FIG 2.
Forest plot depicting the risk ratios of 30-day mortality among patients receiving antibiotic treatment for ≤10 days versus >10 days. Vertical line indicates “no difference” point between the two regimens; horizontal lines indicate the 95% confidence interval (CI). ■, risk ratios; ♦, pooled risk ratios for all studies. M-H, Mantel-Haenszel.
FIG 3.
Forest plot depicting the risk ratios of 90-day mortality among patients receiving antibiotic treatment for ≤10 days versus >10 days. The vertical line indicates the “no difference” point between the two regimens. The horizontal lines indicate the 95% CI. ■, risk ratios; ♦, pooled risk ratios for all studies.
Concerning clinical cure, no significant difference between the two treatments was detected when those studies were pooled (81.6% versus 81.4%, respectively; Fig. 4; 1,080 patients; RR = 1.02; 95% CI, 0.96 to 1.08), and no statistical heterogeneity was detected in this analysis (I2 = 0%). Moreover, 4 of the studies provided data on relapse of bacteremia (10, 11, 19, 20). Relapse was assessed within 30 days (10) or 90 days (11, 19, 20) from the completion of the antibiotic therapy. Pooling of the studies showed that relapse at 90 days (11, 19, 20) was not significantly different between short- and long-course treatment (4.5% versus 4.5%, respectively; Fig. 5; 1,750 patients; RR = 1.08; 95% CI, 0.69 to 1.67; I2 = 0%). The relapse rate was also similar between short- and long-course treatments in one study that assessed relapse at 30 days from the completion of treatment (1.2% versus 2.3%, respectively; RR = 0.56; 95% CI, 0.19 to 1.64) (10).
FIG 4.
Forest plot depicting the risk ratios of clinical cure of patients receiving antibiotic treatment for ≤10 days versus >10 days. The vertical line indicates the “no difference” point between the two regimens. The horizontal lines indicate the 95% CI. ■, risk ratios; ♦, pooled risk ratios for all studies.
FIG 5.
Forest plot depicting the risk ratios of relapse of patients receiving antibiotic treatment for ≤10 days versus >10 days. The vertical line indicates the “no difference” point between the two regimens. The horizontal lines indicate the 95% CI. ■, risk ratios; ♦, pooled risk ratios for all studies.
Studies comparing other treatment durations.
Table 2 presents four studies which used a cutoff other than the 10 days for the classification of treatment as “short” or “long” course. Two of them were double-blind randomized controlled trials (23, 24), 1 was a retrospective cohort study (25), and 1 was a retrospective case-control trial (22). Of those studies, 3 included patients with bacteremia secondary to a single source of infection. Among those 3 studies, 2 included patients with acute pyelonephritis (23, 24), and 1 included patients with acute cholangitis (25).
TABLE 2.
Studies comparing treatment durations among patients with bacteremia mostly due to Enterobacteriaceae using a cutoff other than 10 days
| Reference | Study design; study period; countrya | No. of patients who received antibiotics; causative pathogen; source(s) of bacteremia | Antibiotic course by duration |
No./total no. (%) with outcome by treatment durationb |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Clinical cure |
All-cause mortality |
Relapse |
||||||||
| Short-course | Long-course | Short-course | Long-course | Short-course | Long-course | Short-course | Long-course | |||
| Uno et al. (25) | Retrospective cohort; 2012–2014; Japan | 82; mostly Enterobacteriaceae; acute cholangitis | <14 days | ≥14 days | NR | NR | 0/47 (0) | 2/35 (5.7) | 0/37 (0) | 4/30 (13.3) |
| Swamy and Sharma (22) | Retrospective case-control; 2006–2013; USA | 178; mostly Enterobacteriaceae; urinary tract, catheter-related, intra-abdominal, respiratory tract, skin and soft tissue, other | ≤7 days | >7 days | 33/42 (78.6) | 118/136 (86.8) | NR | NR | NR | NR |
| Sandberg et al. (23) | Noninferiority double-blind RCT; 2006–2008; Sweden | 42; E. coli; acute pyelonephritis | Ciprofloxacin for 7 days | Ciprofloxacin for 14 days | 15/16 (93.8) | 25/26 (96.2) | NR | NR | NR | NR |
| Talan et al. (24) | Double-blind RCT; 1994-1997; USA | 14; E. coli; acute pyelonephritis | Ciprofloxacin for 7 days | Trimethoprim-sulfamethoxazole for 14 days | 4/4 (100) | 9/10 (90) | NR | NR | NR | NR |
RCT, randomized clinical trial.
NR, not reported.
In the studies referring to acute pyelonephritis, only a small subset of patients (56/503) had positive blood cultures (23, 24). The antibiotic treatment was ciprofloxacin for 7 days (short course) versus ciprofloxacin (23) or trimethoprim-sulfamethoxazole for 14 days (24) (long course). Clinical cure rates were not different between short- and long-course treatment in any of those studies (100% versus 90%, respectively, P = nonsignificant [NS] [24]; 94% versus 96%, respectively, P = NS [23]). Other clinical outcomes were not provided in those studies.
Another study evaluated treatment for ≤7 days versus >7 days for the treatment of bacteremia due to Enterobacteriaceae (22). The authors assessed clinical cure and bacteriological eradication among the compared treatment groups and found that there was no significant difference in either cure or eradication rates between short- and long-course treatment (78.6% versus 86.8%, respectively; P = NS; and 83.3% versus 89.7%, respectively; P = NS). Finally, 1 study referred to patients treated for bacteremia due to Enterobacteriaceae secondary to acute cholangitis for either <14 days or ≥14 days (25). No significant difference was found in mortality between short- and long-course treatment in that study (0% versus 5.7% for <14 days and ≥14 days, respectively; P = NS), but relapse was higher among patients who were treated for ≥14 days than those treated for <14 days (13.3% versus 0%, respectively; P < 0.05). The antibiotics administered were not determined in either of these two studies (22, 25).
DISCUSSION
Prolonged courses of antibiotics have been associated with increased adverse events (26) and the emergence of antibiotic-resistant strains (27–29), while inadequate courses lead to ineffective treatment and relapse of the infection (30). In this meta-analysis, we evaluated short versus longer courses of antibiotic for the treatment of bacteremia due to Enterobacteriaceae in terms of all-cause mortality, clinical cure, and relapse of bacteremia. We found that shorter courses of antibiotics (≤10 days) did not result in inferior clinical outcomes compared to longer courses of treatment. Notably, the included studies did not differ in terms of patient population and source of bacteremia and patient characteristics between the compared treatment groups. The lack of statistical heterogeneity from all analyses further strengthens these findings.
There is a scarcity of evidence regarding the optimal duration of antibiotic therapy for non-catheter-related Gram-negative bacteremia, and the limited studies that exist present controversial findings (10, 11). As a result, there is wide variation in treatment duration among clinicians, with a general tendency toward more prolonged courses (31). For example, according to a survey among infectious diseases and critical care physicians, the most common response was 14 days of treatment for BSI, irrespective of its source. However, in terms of duration of treatment, the majority of respondents recommended treatment for 7 to 10 days for all bacteremic syndromes, such as bacteremic urinary tract infection and bacteremic pneumonia (31). On the contrary, herein, we found that antibiotic treatment for 10 days or less for bacteremia due to Enterobacteriaceae was not associated with improved outcomes and cannot replace yet the standard of care with treatment for over 10 days.
The impact of treatment duration on antimicrobial resistance is another factor taken into account in the selection of the treatment regimen. Emergence of multidrug resistance during therapy was evaluated in one of the included studies, in which 4.4% versus 7.3% cases of multidrug-resistant bacteria occurred in the short- and long-course groups, respectively, without a significant difference between the compared arms (10). Likewise, no significant difference in the emergence of resistance was found between short- and long-course groups in the randomized controlled trial (10.8% versus 9.8%, respectively; P = NS) (19). On the other hand, antibiotic use is associated with Clostridioides (Clostridium) difficile infection (CDI) (32). Two of the included studies assessed the development of CDI between the short- and long-course groups, and both studies found that there was no significant difference between the compared treatments (10, 19).
Moreover, appropriateness of empirical therapy is another important factor that affects treatment outcome and should be assessed as a potential confounder in the results of the included studies (33). Two studies provided information on the empirical therapy, with one showing no difference in the occurrence of inappropriate empirical therapy between short- and long-course treatments (11) and the other showing more common inappropriate therapy in the short-course group (20). Besides the difference in the appropriateness of therapy noted in the latter study, clinical cure, relapse, and mortality did not differ significantly between short- and long-course treatments (20).
The present study bears certain limitations that should be considered in the interpretation of the findings. First, all but 1 included study were retrospective cohort, meaning that the quality of the data may be suboptimal or the data are prone to confounding factors. To eliminate confounding by indication, the authors of 1 study excluded patients in whom antimicrobial duration of treatment was dictated by outcome (in-hospital mortality) or clinical response to therapy (prolonged hospitalization) (11). In order to address this issue, another study included propensity score matching (10), while a third study utilized a propensity score of receiving short-course treatment using multivariate logistic regression (20). The randomized controlled trial did not bear confounding by indication due to randomization. However, it should be noted that the authors excluded hemodynamically unstable patients who tend to receive antibiotic treatment for longer periods (19). Moreover, in nonrandomized studies, survival bias might occur (34). In 3 out of 4 nonrandomized studies that were included in our meta-analysis, the authors addressed this concern. More specifically, in 1 study, the authors mentioned that in order to reduce the risk of survival bias, they excluded patients who did not survive initial hospitalization for bloodstream infection (11). Also, in 2 other studies, the authors excluded from the analyses all patients who died while receiving antibiotic treatment for bloodstream infection (10, 20). Second, mortality was assessed at either 30 days (10) or 90 days (11, 19, 20). Given that bacteremia is associated with long-term mortality (35), assessment of the effectiveness of the definitive treatment at 90 rather than 30 days may be preferable. In addition, clinical outcomes (such as the source or severity of bacteremia, the presence of comorbidities, and the antimicrobial resistance profile of the involved pathogens) were not available, and clearer conclusions could not be drawn. Third, the impact of treatment duration on outcomes may depend on the type of antibiotic used; however, data on the specific antibiotics used were not provided in the included studies. Interestingly, according to a previously published study which assessed the effectiveness of oral antibiotics in the treatment of Gram-negative bacteremia, clinical outcomes improve with oral antibiotics of high bioavailability compared to outcomes with antibiotics of moderate or low bioavailability (such as cephalosporins or penicillins) (36).
In conclusion, in patients with bacteremia due to Enterobacteriaceae, treatment for ≤10 days did not result in inferior clinical outcomes compared to treatment for >10 days. The current practice for the treatment of Gram-negative bacteremia varies widely (31). Further well-designed studies that will compare effectiveness, safety, and the emergence of resistance between short- and long-course treatments are necessary in order to assess whether shortening of treatment duration in specific sources of bacteremia would be beneficial. Also, future studies that include patients with bacteremia of urinary source should investigate whether clinical outcomes with short- versus long-course treatment differ based on sex. Last, cost-effectiveness analyses should be performed to evaluate whether differences in the duration of antibiotic treatment for bacteremia impact health care cost.
MATERIALS AND METHODS
Literature search.
We performed a systematic search of the available literature in the PubMed and EMBASE databases through May 2018. The following search terms were applied: “(“bloodstream infection” OR bacteremia OR sepsis OR septicemia) AND treatment AND (short-course OR long-course OR prolonged) AND (cure OR failure OR mortality).” A year limit to 1990 was set, and all articles published in English, German, or French were evaluated.
Study selection.
We defined short-course treatment as treatment for ≤10 days and long-course treatment as treatment for >10 days. As such, studies comparing the clinical outcomes between patients who received antibiotic treatment for ≤10 days and those who received treatment for >10 days were considered eligible for inclusion. Studies assessing clinical outcomes between different treatment duration groups using a cutoff other than 10 days were evaluated and discussed but were not included in the meta-analysis. Studies reporting only the mean or median data without a minimum duration of therapy were not eligible for inclusion. Also, studies reporting on antibiotics that are not currently approved by the Food and Drug Administration were excluded. Last, case reports and studies including pediatric patients were also excluded.
Data extraction and quality assessment.
Two investigators (G.S.T. and N.A.) independently performed the systematic search of databases, study selection, and data extraction. Any discrepancies between the investigators were resolved by consensus during meetings. The extracted data included the main characteristics of each study (first author name, year, and study design), number of patients with bacteremia who received antibiotic treatment, source of bacteremia, causative pathogen of bacteremia, aggregate Newcastle-Ottawa scale score, duration of antibiotic therapy in both arms, as well as the available clinical outcomes in each treatment group.
The methodological quality of the nonrandomized studies that were included in the meta-analysis was assessed with the “star system” of the Newcastle-Ottawa scale (37). The studies were evaluated according to the selection of study groups, comparability of groups, and ascertainment of the outcome of interest.
Definitions and outcomes.
The primary outcome of the meta-analysis was all-cause mortality. Clinical cure, as defined by the investigators of the individual studies, and relapse of bacteremia were the secondary outcomes.
Meta-analysis.
The meta-analysis was performed using Review Manager for Windows, version 5.3 (38). Pooled risk ratios and 95% confidence intervals were calculated regarding the outcomes of interest. We assessed statistical heterogeneity among studies by using a χ2 test (P < 0.10 was defined to indicate significant heterogeneity) and I2. When there was no significant statistical heterogeneity (<40%) (39) between the studies, the Mantel-Haenszel fixed-effect model was used (40). Otherwise, the random-effects model with the DerSimonian and Laird approach was used as appropriate (41). We assessed publication bias using a funnel plot (41).
REFERENCES
- 1.Goto M, Al-Hasan MN. 2013. Overall burden of bloodstream infection and nosocomial bloodstream infection in North America and Europe. Clin Microbiol Infect 19:501–509. doi: 10.1111/1469-0691.12195. [DOI] [PubMed] [Google Scholar]
- 2.National Center for Health Statistics. 2015. Deaths: final data for 2015. National Vital Statistics Reports; vol 6, no. 6. National Center for Health Statistics, Hyattsville, MD: https://www.cdc.gov/nchs/data/nvsr/nvsr66/nvsr66_06.pdf. [Google Scholar]
- 3.Diekema DJ, Beekmann SE, Chapin KC, Morel KA, Munson E, Doern GV. 2003. Epidemiology and outcome of nosocomial and community-onset bloodstream infection. J Clin Microbiol 41:3655–3660. doi: 10.1128/JCM.41.8.3655-3660.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ibrahim EH, Sherman G, Ward S, Fraser VJ, Kollef MH. 2000. The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting. Chest 118:146–155. doi: 10.1378/chest.118.1.146. [DOI] [PubMed] [Google Scholar]
- 5.MacArthur RD, Miller M, Albertson T, Panacek E, Johnson D, Teoh L, Barchuk W. 2004. Adequacy of early empiric antibiotic treatment and survival in severe sepsis: experience from the MONARCS trial. Clin Infect Dis 38:284–288. doi: 10.1086/379825. [DOI] [PubMed] [Google Scholar]
- 6.Timbrook TT, Morton JB, McConeghy KW, Caffrey AR, Mylonakis E, LaPlante KL. 2017. The effect of molecular rapid diagnostic testing on clinical outcomes in bloodstream infections: a systematic review and meta-analysis. Clin Infect Dis 64:15–23. doi: 10.1093/cid/ciw649. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. 2011. CDC strategic priorities for combating antimicrobial resistance. Centers for Disease Control and Prevention, Atlanta, GA: https://www.cdc.gov/drugresistance/pdf/cdc-priorities-combating-ar-infections-report-workshop.pdf. [Google Scholar]
- 8.Centers for Disease Control and Prevention. 2015. National action plan for combating antibiotic-resistant bacteria. Centers for Disease Control and Prevention, Atlanta, GA: https://www.cdc.gov/drugresistance/pdf/national_action_plan_for_combating_antibotic-resistant_bacteria.pdf. [Google Scholar]
- 9.Mermel LA, Allon M, Bouza E, Craven DE, Flynn P, O’Grady NP, Raad II, Rijnders BJA, Sherertz RJ, Warren DK. 2009. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis 49:1–45. doi: 10.1086/599376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chotiprasitsakul D, Han JH, Cosgrove SE, Harris AD, Lautenbach E, Conley AT, Tolomeo P, Wise J, Tamma PD, Antibacterial Resistance Leadership Group. 2018. Comparing the outcomes of adults with Enterobacteriaceae bacteremia receiving short-course versus prolonged-course antibiotic therapy in a multicenter, propensity score-matched cohort. Clin Infect Dis 66:172–177. doi: 10.1093/cid/cix767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nelson AN, Justo JA, Bookstaver PB, Kohn J, Albrecht H, Al-Hasan MN. 2017. Optimal duration of antimicrobial therapy for uncomplicated Gram-negative bloodstream infections. Infection 45:613–620. doi: 10.1007/s15010-017-1020-5. [DOI] [PubMed] [Google Scholar]
- 12.Daneman N, Rishu AH, Xiong W, Bagshaw SM, Dodek P, Hall R, Kumar A, Lamontagne F, Lauzier F, Marshall J, Martin CM, McIntyre L, Muscedere J, Reynolds S, Stelfox HT, Cook DJ, Fowler RA, Canadian Critical Care Trials Group. 2016. Duration of antimicrobial treatment for bacteremia in Canadian critically ill patients. Crit Care Med 44:256–264. doi: 10.1097/CCM.0000000000001393. [DOI] [PubMed] [Google Scholar]
- 13.Kollef MH, Chastre J, Clavel M, Restrepo MI, Michiels B, Kaniga K, Cirillo I, Kimko H, Redman R. 2012. A randomized trial of 7-day doripenem versus 10-day imipenem-cilastatin for ventilator-associated pneumonia. Crit Care 16:R218. doi: 10.1186/cc11862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doi A, Morimoto T, Iwata K. 2018. Shorter duration of antibiotic treatment for acute bacteraemic cholangitis with successful biliary drainage: a retrospective cohort study. Clin Microbiol Infect 24:1184–1189. doi: 10.1016/j.cmi.2018.01.021. [DOI] [PubMed] [Google Scholar]
- 15.Havey TC, Fowler RA, Pinto R, Elligsen M, Daneman N. 2013. Duration of antibiotic therapy for critically ill patients with bloodstream infections: a retrospective cohort study. Can J Infect Dis Med Microbiol 24:129–137. doi: 10.1155/2013/141989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pereira JM, Goncalves-Pereira J, Ribeiro O, Baptista JP, Froes F, Paiva JA. 2018. Impact of antibiotic therapy in severe community-acquired pneumonia: data from the Infauci study. J Crit Care 43:183–189. doi: 10.1016/j.jcrc.2017.08.048. [DOI] [PubMed] [Google Scholar]
- 17.Runyon BA, McHutchison JG, Antillon MR, Akriviadis EA, Montano AA. 1991. Short-course versus long-course antibiotic treatment of spontaneous bacterial peritonitis. A randomized controlled study of 100 patients. Gastroenterology 100:1737–1742. doi: 10.1016/0016-5085(91)90677-D. [DOI] [PubMed] [Google Scholar]
- 18.Siegel RE, Alicea M, Lee A, Blaiklock R. 1999. Comparison of 7 versus 10 days of antibiotic therapy for hospitalized patients with uncomplicated community-acquired pneumonia: a prospective, randomized, double-blind study. Am J Ther 6:217–222. doi: 10.1097/00045391-199907000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Yahav D, Franceschini E, Koppel F, Turjeman A, Babich T, Bitterman R, Neuberger A, Ghanem-Zoubi N, Santoro A, Eliakim-Raz N, Pertzov B, Steinmetz T, Stern A, Dickstein Y, Maroun E, Zayyad H, Bishara J, Alon D, Edel Y, Goldberg E, Venturelli C, Mussini C, Leibovici L, Paul M, Bacteremia Duration Study Group. 11 December 2018. Seven versus fourteen days of antibiotic therapy for uncomplicated Gram-negative bacteremia: a non-inferiority randomized controlled trial. Clin Infect Dis doi: 10.1093/cid/ciy1054. [DOI] [PubMed] [Google Scholar]
- 20.Giannella M, Pascale R, Toschi A, Ferraro G, Graziano E, Furii F, Bartoletti M, Tedeschi S, Ambretti S, Lewis RE, Viale P. 2018. Treatment duration for Escherichia coli bloodstream infection and outcomes: retrospective single-centre study. Clin Microbiol Infect 24:1077–1083. doi: 10.1016/j.cmi.2018.01.013. [DOI] [PubMed] [Google Scholar]
- 21.Mercuro NJ, Stogsdill P, Wungwattana M. 2018. Retrospective analysis comparing oral stepdown therapy for enterobacteriaceae bloodstream infections: fluoroquinolones versus beta-lactams. Int J Antimicrob Agents 51:687–692. doi: 10.1016/j.ijantimicag.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 22.Swamy S, Sharma R. 2016. Duration of treatment of Gram-negative bacteremia: are shorter courses of antimicrobial therapy feasible? Infect Dis Clin Pract 24:155–160. doi: 10.1097/IPC.0000000000000362. [DOI] [Google Scholar]
- 23.Sandberg T, Skoog G, Hermansson AB, Kahlmeter G, Kuylenstierna N, Lannergard A, Otto G, Settergren B, Ekman GS. 2012. Ciprofloxacin for 7 days versus 14 days in women with acute pyelonephritis: a randomised, open-label and double-blind, placebo-controlled, non-inferiority trial. Lancet 380:484–490. doi: 10.1016/S0140-6736(12)60608-4. [DOI] [PubMed] [Google Scholar]
- 24.Talan DA, Stamm WE, Hooton TM, Moran GJ, Burke T, Iravani A, Reuning-Scherer J, Church DA. 2000. Comparison of ciprofloxacin (7 days) and trimethoprim-sulfamethoxazole (14 days) for acute uncomplicated pyelonephritis pyelonephritis in women: a randomized trial. JAMA 283:1583–1590. doi: 10.1001/jama.283.12.1583. [DOI] [PubMed] [Google Scholar]
- 25.Uno S, Hase R, Kobayashi M, Shiratori T, Nakaji S, Hirata N, Hosokawa N. 2017. Short-course antimicrobial treatment for acute cholangitis with Gram-negative bacillary bacteremia. Int J Infect Dis 55:81–85. doi: 10.1016/j.ijid.2016.12.018. [DOI] [PubMed] [Google Scholar]
- 26.Meropol SB, Chan KA, Chen Z, Finkelstein JA, Hennessy S, Lautenbach E, Platt R, Schech SD, Shatin D, Metlay JP. 2008. Adverse events associated with prolonged antibiotic use. Pharmacoepidemiol Drug Saf 17:523–532. doi: 10.1002/pds.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barbosa TM, Levy SB. 2000. The impact of antibiotic use on resistance development and persistence. Drug Resist Updat 3:303–311. doi: 10.1054/drup.2000.0167. [DOI] [PubMed] [Google Scholar]
- 28.Cantón R, Morosini MI. 2011. Emergence and spread of antibiotic resistance following exposure to antibiotics. FEMS Microbiol Rev 35:977–991. doi: 10.1111/j.1574-6976.2011.00295.x. [DOI] [PubMed] [Google Scholar]
- 29.Ventola CL. 2015. The antibiotic resistance crisis: part 1: causes and threats. P T 40:277–283. [PMC free article] [PubMed] [Google Scholar]
- 30.Chong YP, Moon SM, Bang KM, Park HJ, Park SY, Kim MN, Park KH, Kim SH, Lee SO, Choi SH, Jeong JY, Woo JH, Kim YS. 2013. Treatment duration for uncomplicated Staphylococcus aureus bacteremia to prevent relapse: analysis of a prospective observational cohort study. Antimicrob Agents Chemother 57:1150–1156. doi: 10.1128/AAC.01021-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Daneman N, Shore K, Pinto R, Fowler R. 2011. Antibiotic treatment duration for bloodstream infections in critically ill patients: a national survey of Canadian infectious diseases and critical care specialists. Int J Antimicrob Agents 38:480–485. doi: 10.1016/j.ijantimicag.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 32.Hensgens MP, Goorhuis A, Dekkers OM, Kuijper EJ. 2012. Time interval of increased risk for Clostridium difficile infection after exposure to antibiotics. J Antimicrob Chemother 67:742–748. doi: 10.1093/jac/dkr508. [DOI] [PubMed] [Google Scholar]
- 33.Hanon FX, Monnet DL, Sorensen TL, Molbak K, Pedersen G, Schonheyder H. 2002. Survival of patients with bacteraemia in relation to initial empirical antimicrobial treatment. Scand J Infect Dis 34:520–528. doi: 10.1080/00365540110080827. [DOI] [PubMed] [Google Scholar]
- 34.van Walraven C, Davis D, Forster AJ, Wells GA. 2004. Time-dependent bias was common in survival analyses published in leading clinical journals. J Clin Epidemiol 57:672–682. doi: 10.1016/j.jclinepi.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 35.Nielsen SL, Lassen AT, Gradel KO, Jensen TG, Kolmos HJ, Hallas J, Pedersen C. 2015. Bacteremia is associated with excess long-term mortality: a 12-year population-based cohort study. J Infect 70:111–126. doi: 10.1016/j.jinf.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 36.Kutob LF, Justo JA, Bookstaver PB, Kohn J, Albrecht H, Al-Hasan MN. 2016. Effectiveness of oral antibiotics for definitive therapy of Gram-negative bloodstream infections. Int J Antimicrob Agents 48:498–503. doi: 10.1016/j.ijantimicag.2016.07.013. [DOI] [PubMed] [Google Scholar]
- 37.Wells G, Shea B, O’Connell J, Robertson J. 2011. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analysis. The Ottawa Hospital Research Institute, Ottawa, Ontario, Canada. [Google Scholar]
- 38.The Nordic Cochrane Centre. 2014. Review Manager (RevMan). Version 5.3. The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark. [Google Scholar]
- 39.Ryan R, Cochrane Consumers and Communication Review Group. 2016. Heterogeneity and subgroup analyses in Cochrane Consumers and Communication Group reviews: planning the analysis at protocol stage. Cochrane Consumers and Communication, London, United Kingdom: http://cccrg.cochrane.org/sites/cccrg.cochrane.org/files/public/uploads/heterogeneity_subgroup_analyses_revising_december_1st_2016.pdf. [Google Scholar]
- 40.Mantel N, Haenszel W. 1959. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 22:719–748. [PubMed] [Google Scholar]
- 41.DerSimonian R, Laird N. 1986. Meta-analysis in clinical trials. Control Clin Trials 7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]