Biofilm control is a critical approach to the better management of dental caries. Antimicrobial small molecules have shown their potential in the disruption of oral biofilm and control of dental caries.
KEYWORDS: oral biofilm, Streptococcus mutans, ZY354, antimicrobial small molecule, caries management, cytotoxicity, dental caries
ABSTRACT
Biofilm control is a critical approach to the better management of dental caries. Antimicrobial small molecules have shown their potential in the disruption of oral biofilm and control of dental caries. The objectives of this study were to examine the antimicrobial activity and cytotoxicity of a newly designed small-molecule compound, ZY354. ZY354 was synthesized, and its cytotoxicity was evaluated in human oral keratinocytes (HOK), human gingival epithelial cells (HGE), and macrophages (RAW) by CCK-8 assays. Minimal inhibitory concentrations (MICs), minimum bactericidal concentrations (MBCs), minimum biofilm inhibition concentrations (MBICs), and minimum biofilm reduction concentrations (MBRCs) of ZY354 against common oral streptococci (i.e., Streptococcus mutans, Streptococcus gordonii, and Streptococcus sanguinis) were determined by microdilution method. The exopolysaccharide (EPS)/bacterium ratio and the dead/live bacterium ratio in the ZY354-treated multispecies biofilms were determined by confocal laser scanning microscopy, and the microbial composition was visualized and quantified by fluorescent in situ hybridization and quantitative PCR (qPCR). The demineralizing activity of ZY354-treated biofilms was evaluated by transverse microradiography. The results showed that ZY354 exhibited low cytotoxicity in HOK, HGE, and RAW cells and exhibited potent antimicrobial activity against common oral streptococci. The EPS and the abundance of S. mutans were significantly reduced after ZY354 treatment, along with an increased dead/live microbial ratio in multispecies biofilms compared to the level with the nontreated control. The ZY354-treated multispecies biofilms exhibited reduced demineralizing activity at the biofilm/enamel interface. In conclusion, the small-molecule compound ZY354 exhibits low cytotoxicity and remarkable antimicrobial activity against oral streptococci, and it may have a great potential in anticaries clinical applications.
TEXT
Dental caries is a multifactorial infectious disease dependent on diet, nutrition, resident oral flora, and host response (1–3). Mechanical removal of dental plaque by tooth brushing is the most important measure to control dental caries. However, difficulties in maintaining adequate plaque control, particularly at interproximal sites, necessitate the use of chemotherapeutic agents for plaque control (4).
Among the chemotherapeutic agents used in mouthwashes, chlorhexidine (CHX) is the gold standard or positive control in comparison with other substances due to its proven efficiency (5–8). Though effective, it has certain side effects, including tooth discoloration, oral mucosal erosion, and bitter taste. One possible drawback of CHX is its cytotoxicity on alveolar bone cells and gingival epithelial cells (9, 10). Hence, there is a need for an alternative mouth rinse that could negate the side effects of chlorhexidine but yet exhibit equivalent effectiveness.
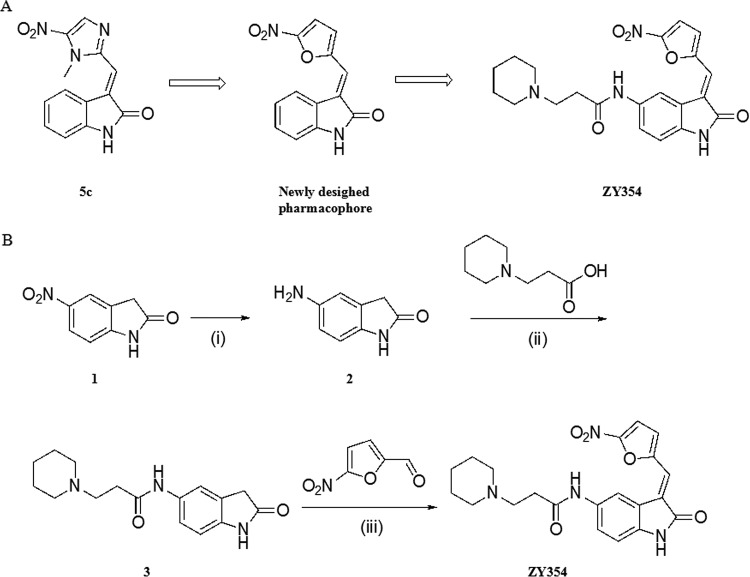
A novel strategy to control oral biofilm is to disrupt its formation (11). Small molecules are promising for controlling biofilm formation due to their good stability, activity at low concentrations, and low toxicity (12). Previous studies showed that molecules with a nitroimidazole pharmacophore possessed biological activities, especially antibacterial potency, against various infectious diseases (13). Our previous work has shown that 3-(substituted methylene)indolin-2-ones, such as compound 5c (Fig. 1), exhibited excellent bactericidal activity against both clinically related Gram-positive and Gram-negative bacteria, including methicillin-resistant Staphylococcus aureus (MRSA), methicillin-sensitive Staphylococcus aureus (MSSA), Escherichia coli, and Pseudomonas aeruginosa (14). Antibiotics featuring nitrofuran, another antibacterial pharmacophore with a mode of action similar to that of nitroimidazole (13, 15), have shown inhibitory activities against oral bacteria such as S. mutans and Enterococcus faecalis (16–18), indicating the good potential of this motif in the treatment of oral diseases such as dental caries. Hence, we combined indole-2-one and nitrofuran motifs to get a potent pharmacophore against oral bacteria (Fig. 1). However, this type of hybrid usually has poor aqueous solubility, which may limit its pharmaceutical characterization. Thus, in this study, we designed and synthesized a novel water-soluble hybrid of indolin-2-one and nitrofuran, ZY354 (Fig. 1), in which a hydrophilic side chain, 3-(piperidin-1-yl) propenamide, was introduced at the C-5 position.
FIG 1.
(A) Chemical structures of compound 5c and ZY354. (B) Synthesis of compound ZY354. Reagents and conditions are as follows: (i) 80% aqueous solution of hydrazine hydrate, FeCl3, activated carbon, EtOH, 78°C, 91.9%; (ii) HATU, Et3N, DCM, 25°C, 33.4%; (iii) piperidine, MeOH, 25°C, 48.6%.
The objectives of this study were to examine the cytotoxicity and antimicrobial effects of a newly designed small-molecule compound, ZY354, against oral microbial biofilms. We hypothesize that ZY354 exhibits low cytotoxicity but good antimicrobial activity against oral streptococcal biofilms. In addition, ZY354 can alter the microbial composition and consequently suppress the demineralizing activity of the oral biofilms.
RESULTS
Chemical synthesis of compound ZY354.
As shown in Fig. 1, the synthesis of the target compound ZY354 was accomplished in three steps: (i) a reduction of the nitro group of the starting material, 5-nitroindolin-2-one, was performed to yield intermediate 2; (ii) 3-(piperidin-1-yl)propanoic acid was then attached to intermediate 2 through amide condensation to yield intermediate 3; (iii) finally, the target compound ZY354 was obtained from an aldol condensation of intermediate 3 with 5-nitrofuran-2-carbaldehyde (Fig. 1). Compound ZY354 exists as an E isomer exclusively (see Fig. S1 in the supplemental material).
ZY354 exhibits good antimicrobial activity against oral streptococci.
ZY354 was bactericidal against S. mutans, Streptococcus gordonii, and Streptococcus sanguinis planktonic cultures, with MICs ranging from 0.12 to 0.49 μg/ml and minimal bactericidal concentrations (MBCs) ranging from 0.24 to 1.95 μg/ml (Table 1). More importantly, ZY354 exhibited good antibiofilm activity against oral streptococcal biofilms, with minimum biofilm inhibition concentrations (MBICs) against S. mutans, S. gordonii, and S. sanguinis biofilms ranging from 0.24 to 0.49 μg/ml, and minimum biofilm reduction concentrations (MBRCs) ranging from 0.12 to 31.25 μg/ml (Table 1).
TABLE 1.
Antimicrobial effect of ZY354 against S. mutans, S. gordonii, and S. sanguinis planktonic cultures and biofilms in BHI medium
| Bacterial species |
Planktonic cells |
Biofilmb |
||
|---|---|---|---|---|
| MIC (μg/ml) | MBCa (μg/ml) | MBIC (μg/ml) | MBRC (μg/ml) | |
| S. mutans | 0.24 | 1.95 | 0.24 | 31.25 |
| S. gordonii | 0.49 | 0.98 | 0.24 | 15.63 |
| S. sanguinis | 0.12 | 0.24 | 0.49 | 0.12 |
MBC, minimum bactericidal concentration.
MBIC, minimum biofilm inhibition concentration; MBRC, minimum biofilm reduction concentration.
ZY354 exhibits low cytotoxicity against human oral cells.
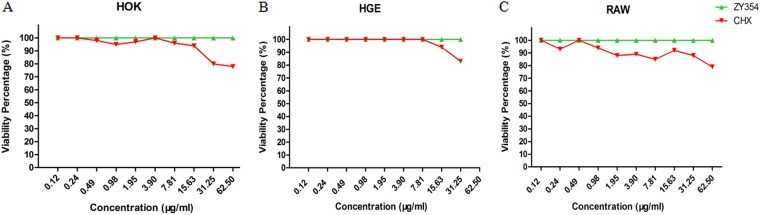
The cytotoxicity of ZY354 was evaluated by testing the viability of human oral keratinocytes (HOK), human gingival epithelial (HGE) cells, and RAW cells after treatment. Both ZY354 and CHX exhibited low cytotoxicity against the tested cells in an exposure duration of 5 min (50% inhibitory concentration [IC50], >62.5 μg/ml) (Fig. 2A to C). It is noticeable that ZY354 was even less cytotoxic than CHX as the viability of ZY354-treated cells was significantly higher at doses of 31.25 μg/ml and 62.50 μg/ml for HOK cells, 62.50 μg/ml for HGE cells, and ≥1.95 μg/ml for RAW cells than that of the CHX-treated cells.
FIG 2.
Cytotoxicity of ZY354 on human oral keratinocytes, human gingival epithelial cells, and macrophages. (A to C) Viability of HOK, HGE, and RAW cells, as indicated, treated with ZY354 was evaluated by CCK-8 assay. Data are represented as means ± standard deviations. HOK, human oral keratinocytes; HGE, human gingival epithelial cells; RAW, macrophages; CHX, chlorhexidine.
ZY354 suppresses the development of oral streptococcal biofilms.
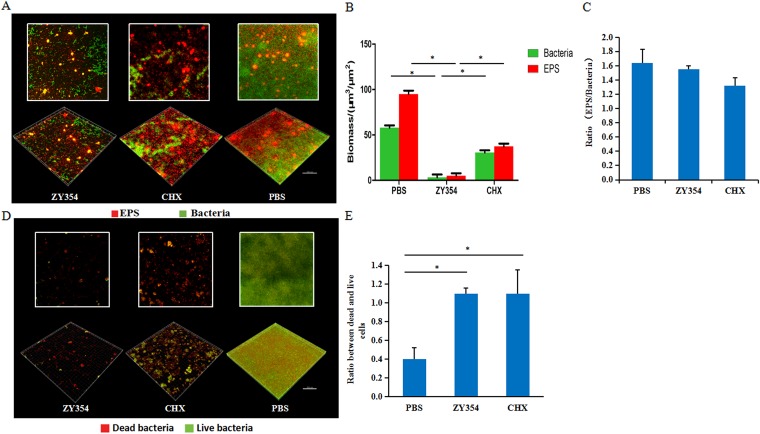
The development of oral streptococcal biofilm is a concerted process of bacterial accumulation and exopolysaccharide (EPS) generation. We further examined the effect of compound ZY354 on bacterial cell viability and EPS production in a multispecies consortium. The microarchitecture of the multispecies biofilms treated with ZY354 and CHX was significantly disrupted compared with that of the negative control (Fig. 3A). Either bacterial viability or EPS production of the biofilms was significantly reduced after ZY354 treatment compared to levels with the other two groups (P < 0.05) (Fig. 3B). Specifically, the dead/live fluorescent staining of the multispecies biofilms showed an inhibitory effect of ZY354 against the development of multispecies biofilms comparable to that of the nontreated controls (Fig. 3D and E), and neither ZY354 nor CHX treatment altered the EPS/bacterium ratio of the biofilms compared to that of the negative control (Fig. 3C), indicating that the reduction in EPS is parallel to the elimination of bacteria within the biofilms.
FIG 3.
The antimicrobial effects of ZY354 against oral streptococcal multispecies biofilms. (A) Representative images of multispecies biofilms treated with ZY354. Green, bacteria (SYTO 9); red, extracellular polysaccharides (EPS). (B) Quantitative analysis of EPS and bacteria within the biofilms. (C) The ratio of EPS/bacteria within the biofilms. (D) Representative images of dead/live bacteria within the multispecies biofilms after treatment. Green, live bacteria; red, dead bacteria. (E) Quantitative ratio of dead to live bacteria after treatment. Data are presented as means ± standard deviations. *, P < 0.05.
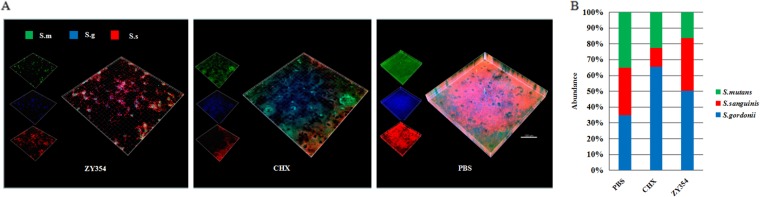
The effects of ZY354 on the microbial composition of multispecies biofilms were further investigated with species-specific fluorescent in situ hybridization (FISH) and quantitative PCR (qPCR). Both ZY354 and CHX altered the microbial composition of the oral streptococcal biofilms. The abundance of S. mutans was significantly reduced after ZY354 treatment (Fig. 4A and B) compared to the level in the nontreated negative control. Conversely, the abundances of S. sanguinis and S. gordonii increased after ZY354 treatment (Fig. 4A and B). Of note, although CHX treatment suppressed S. mutans in the multispecies biofilms, it also depleted the commensal S. sanguinis in the biofilms.
FIG 4.
The composition shift of multispecies biofilms. (A) Representative fluorescent in situ hybridization images of multispecies biofilms: S. m., S. mutans; S. g., S. gordonii; S. s., S. sanguinis. (B) The ratios of S. mutans, S. gordonii, and S. sanguinis in multispecies biofilms quantified by qPCR. Data are presented as means ± standard deviations.
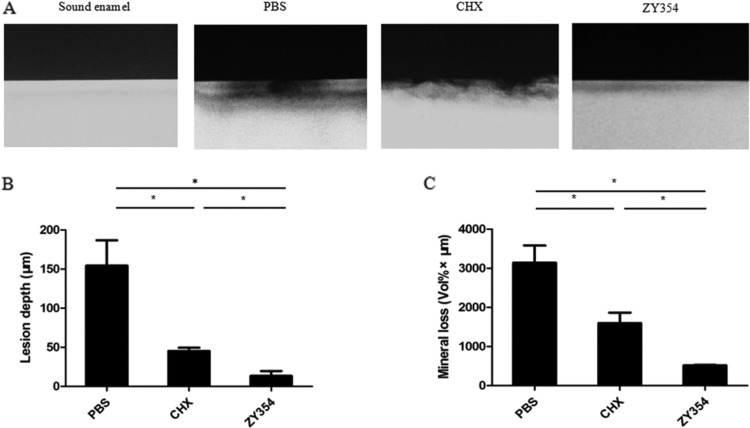
ZY354 halts the biofilm-mediated demineralization process of tooth enamel.
The demineralizing activity of oral biofilm is closely related to its cariogenicity. Hence, we developed saliva-derived biofilm on the tooth enamel and used transverse microradiography to further evaluate the effect of ZY354 on the biofilm-mediated demineralization process of tooth enamel. Either the depth of biofilm-induced lesion or the mineral loss of the tooth enamel was significantly reduced after ZY354 treatment compared to the levels with the negative control. More importantly, the ZY354 treatment showed a superior inhibitory effect against biofilm-mediated demineralization compared to that with CHX treatment (Fig. 5A to C).
FIG 5.
The anti-demineralization effect of ZY354 against multispecies biofilms. (A) Representative transverse microradiography images of human enamel discs exposed to 5-day biofilm-induced experimental demineralization. The high-density regions represent the sound enamel tissues, while the low-density shadows indicate the caries-like lesions. (B and C) Lesion depth and mineral loss were calculated. Data are presented as means ± standard deviations. *, P < 0.05.
DISCUSSION
Dental caries has a polymicrobial infection etiology (3). Effective plaque biofilm control is the most important measure for the management of dental caries. Along with mechanical removal, a chemotherapeutic is an indispensable supplement to dental plaque control (4, 19). Here, we developed a small-molecule compound, ZY354, which showed potent antimicrobial activity against streptococcal biofilms and low cytotoxicity against human oral keratinocytes, human gingival epithelial cells, and macrophages, indicating its great translational potential in the clinical control of dental caries.
Our previous data have demonstrated that small molecules with a nitroimidazole pharmacophore (14) possess biological activities, with good antibacterial potency, against various infectious diseases (13). Antibiotics with nitrofuran (13, 15) also show inhibitory activities against oral bacteria such as S. mutans and E. faecalis (16–18). Here, we designed and synthesized compound ZY354, a hybrid of indolin-2-one and nitrofuran. We observed good antimicrobial activity of this compound against oral streptococci in both planktonic cultures and biofilms.
Oral biofilm formation is a well-concerted process of bacterial adhesion, EPS production, and biomass accumulation. EPS produced by oral streptococci, particularly by the cariogenic S. mutans, functions as a scaffold for biofilm growth and maturation, and it is also closely associated with biofilm properties, including surface adhesion, spatial and chemical heterogeneities, and synergistic/competitive interactions (20–24). Hence, EPS has been well recognized as the key virulence factor of oral biofilm in respect to caries development, and inhibiting EPS generation is a promising approach to the disruption of oral biofilms (20, 25, 26). In the current study, we found that compound ZY354 could significantly reduce EPS production by oral biofilms, and this is likely attributed to the great bactericidal effect of ZY354 against oral streptococci. The reduction in live bacterial cells within the oral biofilms after ZY354 treatment further led to decreased production of EPS and ultimately disrupted the microarchitecture and formation of mature biofilms.
Microbial equilibrium is a major determinant of biofilm cariogenicity (1–3, 27). An oral biofilm with well-balanced acidogenic streptococci such as S. mutans and peroxidase- and alkaline-generating competitors such as S. sanguinis is not cariogenic (1, 2, 28). The emergence of S. mutans in the multispecies biofilms usually implies enhanced acidogenicity and may consequently result in dental caries (29–31). Here, we developed a three-species biofilm consisting of S. mutans, S. gordonii, and S. sanguinis, and we found that ZY354 treatment could selectively inhibit S. mutans but enrich competing streptococci within the biofilms. Interestingly, although the exact mechanism still needs further investigation, we found that, compared to CHX, which suppressed both S. mutans and alkaline-generating S. sanguinis, ZY354 treatment kept the abundance of S. sanguinis intact; in the meantime ZY354 enriched the peroxidase-generating S. gordonii within the multispecies biofilms, leading to a microbial consortium with a better competitive edge against the opportunistic overgrowth of S. mutans and, thus, being beneficial to caries management.
The biocompatibility of newly synthesized material is the bottleneck for its clinical translation. This study tested the cytotoxicity of ZY354 against common human oral cells that might be exposed to a mouth rinse, including HOK, HGE, and RAW cells. Intriguingly, although its exact activities on human cells are still unclear, the compound ZY354 exhibited lower cytotoxicity at comparable test concentrations than CHX, one of the most commonly used antimicrobial mouth rinses in the dental clinic (5–8). These data demonstrate that short-duration exposure to ZY354 is not cytotoxic to human oral cells, and this compound may have great clinical translational potential with antiplaque efficacy comparable to that of CHX.
In conclusion, this study investigated the antimicrobial effects and cytotoxicity of a novel small molecule, ZY354, against selected oral streptococci in planktonic cultures and multispecies biofilms for the first time. ZY354 has low cytotoxicity against common human oral cells and possesses a potent antimicrobial effect against oral biofilms. It can also inhibit the key cariogenic factor of oral biofilm and alter its microbial composition, leading to a biofilm with reduced demineralizing activity. The small molecule ZY354 may have great potential in the development of novel antiplaque and anticaries oral hygiene products.
MATERIALS AND METHODS
Synthesis of ZY354 and specimen preparation.
(i) Synthesis of intermediate 2. Activated carbon (1.0 g) and FeCl3 (1.0 g) were added to a suspension of 5-nitroindolin-2-one (5.0 g, 1.0 eq) in ethanol (EtOH; 50 ml). The mixture was heated to 78°C and stirred for 10 min. Then, an 80% aqueous solution of hydrazine hydrate (8.0 eq) was added dropwise into the reaction mixture in 5 min; the resulting mixture was stirred at 78°C for 8 to 10 h and then cooled to room temperature. The mixture was filtered to remove the residue of activated carbon, and the filtrate was concentrated under vacuum to yield a crude product that was purified by recrystallization from EtOH (about 15 ml) to give 5-aminoindolin-2-one (intermediate 2) as a pale yellow solid (yield, 91.9%). (ii) Synthesis of intermediate 3.
(ii) Synthesis of intermediate 3. Triethylamine (1.4 eq) and 1-[bis(dimethylamino)methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium 3-oxide hexafluorophosphate (HATU) (1.3 eq) were added to a solution of 3-(piperidin-1-yl)propanoic acid (600 mg; 1.0 eq) in dichloromethane (DCM) (7 ml). The reaction mixture was stirred at room temperature for 20 to 30 min, and then 5-aminoindolin-2-one (1.1 eq) was added into the reaction mixture. The resulting mixture was stirred at room temperature for 6 h. After the reaction was finished, the solvent was evaporated under vacuum to give a crude product which was purified by column chromatography to afford intermediate 3 as a colorless oil (yield, 33.4%).
(iii) Synthesis of the target compound ZY354. For synthesis of ZY354, (E)-N-(3-((5-nitrofuran-2-yl)methylene)-2-oxoindolin-5-yl)-3-(piperidin-1-yl) propenamide, piperidine (1.5 eq) and 5-nitrofuran-2-carbaldehyde (1.2 eq) were added to a solution of intermediate 3 (200 mg, 1.0 eq) in MeOH (5 ml). The reaction mixture was stirred at room temperature for 30 min. After the reaction was finished, water (15 ml) was added to quench the reaction. The resulting mixture was extracted with DCM (10 ml) three times. The combined organic layer was evaporated under vacuum and then purified by column chromatography to afford compound ZY354 as a dark brown solid. The product exists as an E isomer (yield, 48.6%). 1H nuclear magnetic resonance (NMR) (400 MHz, Pyr-d5) δ 12.03 (s, 1H), 10.86 (s, 1H), 8.98 (s, 1H), 7.84 (d, J = 6.0 Hz, 1H), 7.63 (s, 1H), 7.56 (m, 1H), 7.10 (s, 1H), 7.02 (d, J = 7.6 Hz, 1H), 2.79 (m, 4H), 2.39 (m, 4H), 1.48 (m, 4H), 1.28 (m, 2H) (see Fig. S2 in the supplemental material). 13 C NMR (101 MHz, Pyr-d5) δ 170.89, 169.70, 152.92, 152.56, 141.57, 133.90, 130.05, 126.04, 121.55, 120.72, 119.40, 117.19, 113.98, 110.20, 55.01, 53.98 (2C), 34.26, 26.14 (2C), 24.39 (Fig. S3). High-resolution mass spectrometry (HRMS) quadrupole-time of flight (Q-TOF), calculated for C21H22N4O5 [M]: 410.1590. Found [M+H]+: 411.1665 (Fig. S4).
Bacterial strains and growth medium.
Streptococcus mutans UA159, Streptococcus gordonii DL1, and Streptococcus sanguinis ATCC 10556 were kindly provided by the State Key Laboratory of Oral Diseases (Sichuan University, Chengdu, China). S. mutans, S. gordonii, and S. sanguinis were routinely grown at 37°C under aerobic condition (5% CO2) in brain heart infusion (BHI) broth (Difco, Sparks, MD).
Bacteria inoculation and biofilm formation.
Inoculum for the experiment was adjusted to 1 × 107 CFU/ml for S. mutans, S. gordonii, and S. sanguinis based on the optical density at 600 nm (OD600) versus a graph of each bacterium in terms of the number of CFU/milliliter. When needed, medium was supplemented with 1% sucrose (designated BHIS broth) or with 1% sucrose and ZY354 at the same time, and the pH value was adjusted to 7.0 before the experiment. For multispecies biofilm formation, 300 μl of bacterial inoculum consisting of S. mutans (1 × 105 CFU/ml), S. gordonii (1 × 105 CFU/ml), and S. sanguinis (1 × 105 CFU/ml) in BHIS broth was added in the chemotaxis chamber (μ-slide 8-well, 80826; Ibidi) as described by Arthur et al. (32). For single-species biofilm formation, bacteria were inoculated at a concentration of 1 × 107 CFU/ml in 100 μl of BHIS broth in a 96-well plate. The bacterial culture medium was changed every 24 h.
Bacterial susceptibility assays.
The MIC and minimum bactericidal concentration (MBC) of ZY354 against S. mutans, S. gordonii, and S. sanguinis were determined by a microdilution method as described previously (31) and by Nudera et al. (33). Microtiter plates contained ZY354, their respective solvent controls, chlorhexidine (CHX) as a positive control, a cell control (test bacteria and growth medium) as a negative control, a blank well (sterile double-distilled H2O [ddH2O]), and blank medium (containing sterile ddH2O and growth medium). All plates were then placed under aerobic conditions (5% CO2) at 37.0°C for 24 h for S. mutans, S. gordonii, and S. sanguinis. OD600 values were obtained with a microplate reader (Power Wave 200 Microplate Scanning Spectrophotometer; Bio-TeK Instruments Inc., Winooski, VT) and the Windows-based computer program KC4 Data Analysis Software (Bio-TeK Instruments, Inc.). The MIC for test solutions was the concentration of test solution allowing an A600 of <0.05. The MBC of the test solutions was determined by inoculating the solution mixture in wells representing the MIC and the next three higher-concentration wells onto BHI agar plates (1.5% agar; Difco, Sparks, MD). The inoculated agar plates were placed under aerobic conditions (5% CO2) at 37.0°C for 24 h, and the MBC was determined as the lowest concentration of test solution that exhibited no growth.
Biofilm susceptibility assays.
The effects of ZY354 on S. mutans, S. gordonii, and S. sanguinis biofilm formation were examined by the microdilution method as described by Wei et al. (34). S. mutans UA159, S. gordonii, or S. sanguinis (1 × 107 CFU/ml) was grown in BHIS broth and ZY354 (0.10 to 125 μg/ml) at 37°C for 24 h. The culture supernatant from each well was then decanted, and the adherent biofilm was washed three times with phosphate-buffered saline (PBS), fixed with methanol for 15 min, and stained with 0.1% (wt/vol) crystal violet (Sigma) for 5 min. Subsequently, the wells were rinsed with deionized water until the blank wells appeared colorless; 200 μl of 95% ethanol was added. The plates were shaken at room temperature for 30 min, and the absorbance at 595 nm was recorded. The minimum biofilm inhibition concentration (MBIC) was defined as the lowest ZY354 concentration that resulted in at least 90% (MBIC90) inhibition of the formation of biofilms compared with that in the untreated control. A parallel study was also performed with BHIS broth.
The effect of ZY354 on the removal of S. mutans, S. gordonii, and S. sanguinis biofilms was determined by the microdilution method modified from that of Ramage et al. (35). A 200-μl bacterial cell suspension (1 × 107 CFU/ml) in BHIS broth was added to the wells of a 96-well microtiter plate for biofilm formation. After anaerobic incubation at 37°C for 24 h, the growth medium was removed without disrupting the integrity of the biofilms. The formed biofilms were then washed three times with PBS to remove nonadherent cells. BHIS broth supplemented with ZY354 (0.10 to 125 μg/ml) was added to wells containing biofilm and incubated at 37°C for 24 h. The control wells contained BHIS broth without ZY354. The treated biofilms were then stained and quantified according to the method described above, and the minimum biofilm reduction concentration (MBRC) was defined as the lowest ZY354 concentration that resulted in at least 90% (MBRC90) reduction of biofilms compared with that in the untreated control.
In vitro cytotoxicity/viability assay.
Cell viability was determined colorimetrically with a CCK-8 assay as described by Diab et al. (36) and Tsukatani et al. (37). Cytotoxicity was evaluated in human oral keratinocytes (HOK), human gingival epithelial cells (HGE), and macrophages (RAW). HOK, HGE, and RAW cells (50 × 103 cell/100 μl/well in a 96-well plate) were grown in medium for 24 h and then treated with medium containing different concentrations of ZY354 (0.12 to 62.5 μg/ml) for 5 min, and the positive control was treated with CHX at the same time. After incubation, a volume of 10 μl of CCK-8 was added per well, and the plate was incubated in a CO2 incubator for 3 h. The sample absorbance was measured at the wavelength of 450 nm against a blank which contained medium only. Cell viability was calculated according to the following formula: percent viability = (OD of sample − OD of blank)/(OD of control − OD of blank) × 100%.
Biofilm imaging.
Oral multispecies biofilms were cultured in accordance with a previous study (32). Biofilms were then exposed to PBS, 62.5 μg/ml ZY354, and 0.2% CHX for 3 days (5 min three times per day).
For EPS staining, 2.5 μM Alexa Fluor 647-labeled dextran conjugate (Molecular Probes) was added at the beginning of biofilm formation, and the bacteria were stained with 2.5 μM SYTO9 (Molecular Probes) for 15 min after biofilms formed (38). The biofilms were imaged with a Leica DMIRE2 confocal laser scanning microscope equipped with a 60× oil immersion lens objective for dead/live imaging.
For dead/live imaging, biofilms were stained according to the manufacturer’s instructions (Invitrogen, Carlsbad, CA, USA). Briefly, the biofilms were stained with 2.5 μM SYTO9 (Molecular Probes, Invitrogen) and propidium iodide (Molecular Probes) for 15 min. The labeled biofilms were imaged with a Leica DMIRE2 confocal laser scanning microscope (Leica, Wetzlar, Germany) equipped with a 60× oil immersion lens objective (38).
For fluorescent in situ hybridization imaging, biofilms were fixed in 4% paraformaldehyde overnight and investigated by species-specific probes (38). The biofilms were imaged with a Leica DMIRE2 confocal laser scanning microscope (Leica, Wetzlar, Germany) equipped with a 60× oil immersion lens objective.
All three-dimensional reconstructions of the biofilms were performed with Imaris, version 7.0.0 (Bitplane, Zürich, Switzerland), and the quantification of dead/live bacteria and EPS/bacterial volume ratios was performed with Image-Pro Plus (Media Cybernetics, Silver Spring, MD, USA) and COMSTAT (https://www.imageanalysis.dk) (21), respectively.
DNA isolation and real-time PCR.
Total DNA of biofilms was isolated and purified using a TIANamp Bacteria DNA kit (Tiangen, Beijing, China) according to the manufacturer’s instructions. The bacteria were lysed using enzymatic lysis buffer (20 mM Tris-HCl, pH 8.0, 2 mM sodium EDTA, and 1.2% Triton X-100) containing 25 mg/ml of lysozyme at 37°C for 1.5 h. The purity and concentration of DNA were detected by NanoDrop 2000 spectrophotometer (Thermo Scientific, Waltham, MA, USA). The extracts were stored at −20°C until use. TaqMan real-time PCR (Life Technologies, Carlsbad, CA, USA) was used to quantify the absolute number of S. mutans, S. gordonii, and S. sanguinis bacteria as described by the manufacturer (TaKaRa, Dalian, China).
Transverse microradiography.
Transverse microradiography was performed on extracted human teeth. Collection of extracted human teeth was approved by the Ethics Committee of West China Hospital of Stomatology, Sichuan University. All efforts were made to minimize suffering and ensure the highest ethical and humane standards.
The labial dental crown of extracted tooth was cut into sections measuring 5 mm by 5 mm by 2 mm by using a diamond-coated band saw with continuous water cooling (Struers Minitom; Struers, Copenhagen, Denmark). Enamel blocks were embedded in polymethylmethacrylate and painted with two layers of acid-resistant nail varnish, leaving a 4-mm by 4-mm window exposed on the labial enamel surface. These surfaces were then ground flat with water-cooled carborundum discs of waterproof silicon carbide paper (Struers) of various grits (1,000, 1,200, 2,400, 3,000, and 4,000). All of the polished samples were individually sonicated in distilled water for 5 min to remove the residual abrasives.
A 2-ml bacterial inoculum consisting of S. mutans, S. gordonii, and S. sanguinis (1 × 107 CFU/ml for each bacterium) in BHIS broth was added to each well with an enamel disc and then was cultured anaerobically in BHIS broth at 37°C (5% CO2). Then discs with biofilms were exposed to PBS, 62.5 μg/ml ZY354, and 0.2% CHX for 5 min three times per day. To minimize the variation in baseline mineral levels, enamel discs obtained from the same tooth were evenly distributed to each test group. The pH of all experimental solutions was adjusted to 7.0 prior to treatment. After exposure, specimens were washed with PBS and repositioned in the plate. BHIS broth was refreshed after the third exposure every day. After a 5-day incubation, biofilms were detached by sonification, and discs were repeatedly washed by PBS. The resulting enamel discs were prepared as described by Eversole et al. (39). X-ray films of experimental lesions were acquired by an X-ray generator (Softex, Japan) equipped with a microradiography camera and then were further examined using a Zeiss Axio Imager A2 microscope (Carl Zeiss, Germany). Quantitative data were acquired by the calibrated analysis system TMR2006 (Inspektor Research Systems BV, Netherlands). Data were obtained as the means of 10 separate samples.
Statistical analysis.
All of the experiments were repeated at least three times independently. One-way analysis of variance was performed to detect the significant effects of variables, followed by a Student-Newman-Keuls test. Differences were considered significant at a P value of < 0.05. Statistical analysis was performed with SPSS software, version 16.0 (SPSS, Inc., Chicago, IL, USA).
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China (81670978 to X.Z., 81771099 to X.X., and 81602956 to T.Y.) and a research grant from the Science and Technology Department of Sichuan Province (2018SZ0121 to X.X.).
We declare that we have no competing financial interests.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.02414-18.
REFERENCES
- 1.Marsh PD. 1994. Microbial ecology of dental plaque and its significance in health and disease. Adv Dent Res 8:263–271. doi: 10.1177/08959374940080022001. [DOI] [PubMed] [Google Scholar]
- 2.Marsh PD. 2003. Are dental diseases examples of ecological catastrophes? Microbiology 149:279–294. doi: 10.1099/mic.0.26082-0. [DOI] [PubMed] [Google Scholar]
- 3.Takahashi N, Nyvad B. 2011. The role of bacteria in the caries process: ecological perspectives. J Dent Res 90:294–303. doi: 10.1177/0022034510379602. [DOI] [PubMed] [Google Scholar]
- 4.Fine DH. 1995. Chemical agents to prevent and regulate plaque development. Periodontol 2000 8:87–107. doi: 10.1111/j.1600-0757.1995.tb00047.x. [DOI] [PubMed] [Google Scholar]
- 5.Jenkins S, Addy M, Newcombe RG. 1994. A comparison of cetylpyridinium chloride, triclosan and chlorhexidine mouthrinse formulations for effects on plaque regrowth. J Clin Periodontol 21:441–444. doi: 10.1111/j.1600-051X.1994.tb00743.x. [DOI] [PubMed] [Google Scholar]
- 6.Pires JR, Rossa Junior C, Pizzolitto AC. 2007. In vitro antimicrobial efficiency of a mouthwash containing triclosan/gantrez and sodium bicarbonate. Braz Oral Res 21:342–347. doi: 10.1590/S1806-83242007000400011. [DOI] [PubMed] [Google Scholar]
- 7.Kim BH, Seo HS, Jung SC, Ohk SH, Kim KH, Cho DL, Kol YM. 2011. Study in bactericidal properties of chlorhexidine grafting on the modified titanium. J Nanosci Nanotechnol 11:1530–1533. doi: 10.1166/jnn.2011.3314. [DOI] [PubMed] [Google Scholar]
- 8.Kozlovsky A, Artzi Z, Moses O, Kamin-Belsky N, Greenstein RB. 2006. Interaction of chlorhexidine with smooth and rough types of titanium surfaces. J Periodontol 77:1194–1200. doi: 10.1902/jop.2006.050401. [DOI] [PubMed] [Google Scholar]
- 9.Cabral CT, Fernandes MH. 2007. In vitro comparison of chlorhexidine and povidone-iodine on the long-term proliferation and functional activity of human alveolar bone cells. Clin Oral Investig 11:155–164. doi: 10.1007/s00784-006-0094-8. [DOI] [PubMed] [Google Scholar]
- 10.Babich H, Tipton DA. 2002. In vitro response of human gingival epithelioid S-G cells to minocycline. Toxicol In Vitro 16:11–21. doi: 10.1016/S0887-2333(01)00103-5. [DOI] [PubMed] [Google Scholar]
- 11.Koo H. 2008. Strategies to enhance the biological effects of fluoride on dental biofilms. Adv Dent Res 20:17–21. doi: 10.1177/154407370802000105. [DOI] [PubMed] [Google Scholar]
- 12.Worthington RJ, Richards JJ, Melander C. 2012. Small molecule control of bacterial biofilms. Org Biomol Chem 10:7457–7474. doi: 10.1039/c2ob25835h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ang CW, Jarrad AM, Cooper MA, Mat B. 2017. Nitroimidazoles: molecular fireworks that combat a broad spectrum of infectious diseases. J Med Chem 60:7636–7657. doi: 10.1021/acs.jmedchem.7b00143. [DOI] [PubMed] [Google Scholar]
- 14.Zhou Y, Ju Y, Yang Y, Sang Z, Wang Z, He G, Yang T, Luo Y. 2018. Discovery of hybrids of indolin-2-one and nitroimidazole as potent inhibitors against drug-resistant bacteria. J Antibiot (Tokyo) 71:887–897. doi: 10.1038/s41429-018-0076-5. [DOI] [PubMed] [Google Scholar]
- 15.Sisson G, Goodwin A, Raudonikiene A, Hughes NJ, Mukhopadhyay AK, Berg DE, Hoffman PS. 2002. Enzymes associated with reductive activation and action of nitazoxanide, nitrofurans, and metronidazole in Helicobacter pylori. Antimicrob Agents Chemother 46:2116–2123. doi: 10.1128/AAC.46.7.2116-2123.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Silva AR, Santos EB, Pinto SC, Gomes JC, Vaz IP, Carvalho MF. 2014. Antimicrobial effect and transdentinal diffusion of new intracanal formulations containing nitrofurantoin or doxycycline. Braz Dent J 25:425–429. doi: 10.1590/0103-6440201302338. [DOI] [PubMed] [Google Scholar]
- 17.Jimenez E, Delgado S, Maldonado A, Arroyo R, Albujar M, Garcia N, Jariod M, Fernandez L, Gomez A, Rodriguez JM. 2008. Staphylococcus epidermidis: a differential trait of the fecal microbiota of breast-fed infants. BMC Microbiol 8:143. doi: 10.1186/1471-2180-8-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ashraf SH, Afrah AA. 2015. In vitro antimicrobial activity of Allium sativum (garlic) and Syzygium aromaticum (green cardamom) against Streptococcus mutans and Lactococcus raffinolactis isolating from dental caries in Baghdadcity. J Chem Eng Chem Res 2:845–854. [Google Scholar]
- 19.Baehni PC, Takeuchi Y. 2003. Anti-plaque agents in the prevention of biofilm-associated oral diseases. Oral Dis 9(Suppl 1):23–29. doi: 10.1034/j.1601-0825.9.s1.5.x. [DOI] [PubMed] [Google Scholar]
- 20.Koo H, Xiao J, Klein MI. 2009. Extracellular polysaccharides matrix—an often forgotten virulence factor in oral biofilm research. Int J Oral Sci 1:229–234. doi: 10.4248/IJOS.09086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiao J, Klein MI, Falsetta ML, Lu B, Delahunty CM, Yates JR III, Heydorn A, Koo H. 2012. The exopolysaccharide matrix modulates the interaction between 3D architecture and virulence of a mixed-species oral biofilm. PLoS Pathog 8:e1002623. doi: 10.1371/journal.ppat.1002623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bowen WH, Burne RA, Wu H, Koo H. 2018. Oral biofilms: pathogens, matrix, and polymicrobial interactions in microenvironments. Trends Microbiol 26:229–242. doi: 10.1016/j.tim.2017.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klein MI, Hwang G, Santos PH, Campanella OH, Koo H. 2015. Streptococcus mutans-derived extracellular matrix in cariogenic oral biofilms. Front Cell Infect Microbiol 5:10. doi: 10.3389/fcimb.2015.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lamont RJ, Koo H, Hajishengallis G. 2018. The oral microbiota: dynamic communities and host interactions. Nat Rev Microbiol 16:745–759. doi: 10.1038/s41579-018-0089-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lebeaux D, Ghigo JM, Beloin C. 2014. Biofilm-related infections: bridging the gap between clinical management and fundamental aspects of recalcitrance toward antibiotics. Microbiol Mol Biol Rev 78:510–543. doi: 10.1128/MMBR.00013-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koo H, Allan RN, Howlin RP, Stoodley P, Hall-Stoodley L. 2017. Targeting microbial biofilms: current and prospective therapeutic strategies. Nat Rev Microbiol 15:740–755. doi: 10.1038/nrmicro.2017.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kleinberg I. 2002. A mixed-bacteria ecological approach to understanding the role of the oral bacteria in dental caries causation: an alternative to Streptococcus mutans and the specific-plaque hypothesis. Crit Rev Oral Biol Med 13:108–125. doi: 10.1177/154411130201300202. [DOI] [PubMed] [Google Scholar]
- 28.Ge Y, Caufield PW, Fisch GS, Li Y. 2008. Streptococcus mutans and Streptococcus sanguinis colonization correlated with caries experience in children. Caries Res 42:444–448. doi: 10.1159/000159608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giacaman RA, Torres S, Gomez Y, Munoz-Sandoval C, Kreth J. 2015. Correlation of Streptococcus mutans and Streptococcus sanguinis colonization and ex vivo hydrogen peroxide production in carious lesion-free and high caries adults. Arch Oral Biol 60:154–159. doi: 10.1016/j.archoralbio.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 30.Becker MR, Paster BJ, Leys EJ, Moeschberger ML, Kenyon SG, Galvin JL, Boches SK, Dewhirst FE, Griffen AL. 2002. Molecular analysis of bacterial species associated with childhood caries. J Clin Microbiol 40:1001–1009. doi: 10.1128/JCM.40.3.1001-1009.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu X, Zhou XD, Wu CD. 2011. The tea catechin epigallocatechin gallate suppresses cariogenic virulence factors of Streptococcus mutans. Antimicrob Agents Chemother 55:1229–1236. doi: 10.1128/AAC.01016-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arthur RA, Waeiss RA, Hara AT, Lippert F, Eckert GJ, Zero DT. 2013. A defined-multispecies microbial model for studying enamel caries development. Caries Res 47:318–324. doi: 10.1159/000347050. [DOI] [PubMed] [Google Scholar]
- 33.Nudera WJ, Fayad MI, Johnson BR, Zhu M, Wenckus CS, Begole EA, Wu CD. 2007. Antimicrobial effect of triclosan and triclosan with Gantrez on five common endodontic pathogens. J Endod 33:1239–1242. doi: 10.1016/j.joen.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 34.Wei GX, Campagna AN, Bobek LA. 2006. Effect of MUC7 peptides on the growth of bacteria and on Streptococcus mutans biofilm. J Antimicrob Chemother 57:1100–1109. doi: 10.1093/jac/dkl120. [DOI] [PubMed] [Google Scholar]
- 35.Ramage G, Vande Walle K, Wickes BL, López-Ribot JL. 2001. Standardized method for in vitro antifungal susceptibility testing of Candida albicans biofilms. Antimicrob Agents Chemother 45:2475–2479. doi: 10.1128/AAC.45.9.2475-2479.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Diab KA, Shafik RE, Yasuda S. 2015. In vitro antioxidant and antiproliferative activities of novel orange peel extract and it's fractions on leukemia HL-60 cells. Asian Pac J Cancer Prev 16:7053–7060. doi: 10.7314/APJCP.2015.16.16.7053. [DOI] [PubMed] [Google Scholar]
- 37.Tsukatani T, Suenaga H, Ishiyama M, Ezoe T, Matsumoto K. 2011. Determination of water-soluble vitamins using a colorimetric microbial viability assay based on the reduction of water-soluble tetrazolium salts. Food Chem 127:711–715. doi: 10.1016/j.foodchem.2010.12.130. [DOI] [PubMed] [Google Scholar]
- 38.Zheng X, Zhang K, Zhou X, Liu C, Li M, Li Y, Wang R, Li Y, Li J, Shi W, Xu X. 2013. Involvement of gshAB in the interspecies competition within oral biofilm. J Dent Res 92:819–824. doi: 10.1177/0022034513498598. [DOI] [PubMed] [Google Scholar]
- 39.Eversole SL, Saunders-Burkhardt K, Faller RV. 2015. Erosion prevention potential of an over-the-counter stabilized SnF2 dentifrice compared to 5000 ppm F prescription-strength products. J Clin Dent 26:44–49. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.