This study investigated the molecular epidemiology of carbapenem-resistant Acinetobacter nosocomialis and Acinetobacter pittii (ANAP). Clinical isolates of Acinetobacter spp.
KEYWORDS: Acinetobacter nosocomialis, Acinetobacter pittii, carbapenem resistance, mechanism
ABSTRACT
This study investigated the molecular epidemiology of carbapenem-resistant Acinetobacter nosocomialis and Acinetobacter pittii (ANAP). Clinical isolates of Acinetobacter spp. collected by the biennial nationwide Taiwan Surveillance of Antimicrobial Resistance program from 2010 to 2014 were subjected to species identification, antimicrobial susceptibility testing, and PCR for detection of carbapenemase genes. Whole-genome sequencing or PCR mapping was performed to study the genetic surroundings of the carbapenemase genes. Among 1,041 Acinetobacter isolates, the proportion of ANAP increased from 11% in 2010 to 22% in 2014. The rate of carbapenem resistance in these isolates increased from 7.5% (3/40) to 22% (14/64), with a concomitant increase in their resistance to other antibiotics. The blaOXA-72 and blaOXA-58 genes were highly prevalent in carbapenem-resistant ANAP. Various genetic structures were found upstream of blaOXA-58 in different plasmids. Among the plasmids found to contain blaOXA-72 flanked by XerC/XerD, pAB-NCGM253-like was identified in 8 of 10 isolates. Conjugations of plasmids carrying blaOXA-72 or blaOXA-58 to A. baumannii were successful. In addition, three isolates with chromosome-located blaOXA-23 embedded in AbGRI1-type structure with disruption of genes other than comM were detected. Two highly similar plasmids carrying class I integron containing blaIMP-1 and aminoglycoside resistance genes were also found. The universal presence of blaOXA-272/213-like on A. pittii chromosomes and their lack of contribution to carbapenem resistance indicate its potential to be a marker for species identification. The increase of ANAP, along with their diverse mechanisms of carbapenem resistance, may herald their further spread and warrants close monitoring.
INTRODUCTION
The Acinetobacter calcoaceticus-Acinetobacter baumannii (Acb) complex has emerged as an important nosocomial pathogen worldwide due to an increasing prevalence in intensive care units, rapid acquisition of various mechanisms of antimicrobial resistance, and association with poor patient outcomes. Among the phenotypically undifferentiated species in the Acb complex, A. baumannii, A. nosocomialis, and A. pittii are clinically relevant but differ in their resistance profiles, virulence, and pathogenicity (1).
Increasing carbapenem resistance in Acb complex, especially in A. baumannii, poses an enormous threat to health care costs and patient outcomes. The main mechanism of carbapenem resistance in the Acb complex is production of carbapenem-hydrolyzing class D β-lactamase (CHDL) and/or metallo-β-lactamase (MBL) (1, 2). Many cross-sectional and longitudinal epidemiological studies have revealed comprehensive portraits of the mechanism and evolution of resistance in A. baumannii (2–6). The most common carbapenemase genes in A. baumannii are blaOXA-23-like, blaOXA-24-like, and ISAba1-blaOXA-51-like. With the decreasing prevalence of ISAba1-blaOXA-51-like, the successful evolution of Acinetobacter transposons carrying blaOXA-23-like has lead to its worldwide spread (7), and the presence of blaOXA-24-like in different plasmids via XerC/XerD module (3) is associated with the high prevalence of blaOXA-24-like in certain areas.
A. baumannii has been the focus of most studies, and relatively few studies have been conducted on A. nosocomialis and A. pittii, perhaps due to their low prevalence and low rates of resistance in the past few decades. However, there is currently more interest in A. nosocomialis and A. pittii due to increases in carbapenem resistance and changes in resistance mechanisms (8–13). Previously, OXA-58-like and MBL were primarily responsible for carbapenem resistance in A. nosocomialis and A. pittii, but blaOXA-23-like and blaOXA-24-like have recently become more common in carbapenem-resistant A. nosocomialis and A. pittii (8–13). The flanking regions and mobile elements surrounding the blaOXA-23-like and blaOXA-24-like carbapenemase genes are similar to those found in A. baumannii.
Many studies have investigated the clinical impacts and resistance mechanisms of A. nosocomialis and A. pittii (8–13), but there are only few epidemiological studies. The Taiwan Surveillance of Antimicrobial Resistance (TSAR), a longitudinal multicenter surveillance program, collects clinical isolates in Taiwan and tests their susceptibility biennially. In this nationwide study, we used isolates from the 2010 to 2014 TSAR collection to analyze the prevalence, antibiograms, mechanisms of dissemination and resistance, and molecular epidemiology of carbapenem resistance in A. nosocomialis and A. pittii.
RESULTS AND DISCUSSION
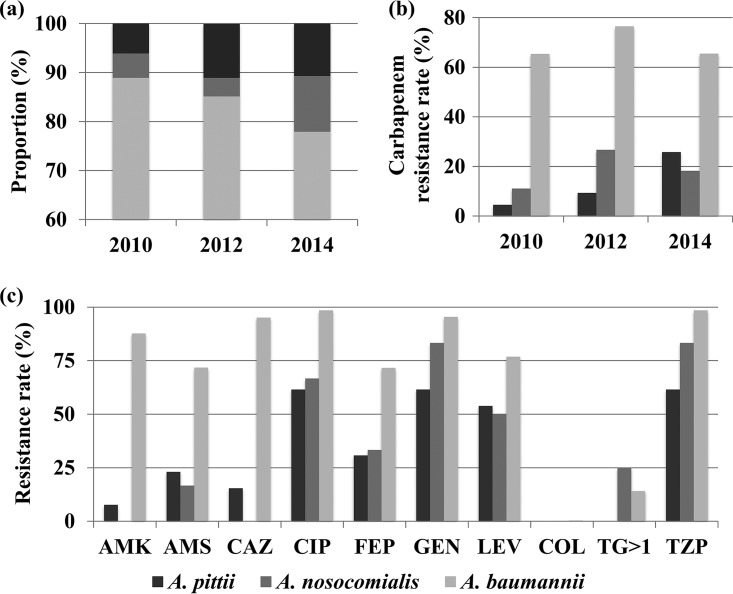
A total of 1,041 Acb complex isolates were collected between 2010 and 2014. The percentages of A. baumannii, A. nosocomialis, and A. pittii determined by gyrB typing, and their antibiograms are shown in Fig. 1a and Tables S1 and S2. A. calcoaceticus was not detected from these isolates. Between 2010 and 2014, the percentage of A. baumannii decreased by more than 11%, whereas the percentages of A. nosocomialis and A. pittii increased by 6.4 and 4.6%, respectively (Fig. 1a) and were frequently identified in medical centers in Northern Taiwan (Table S3). Like A. baumannii, hospitalized elderly and adult patients were more commonly affected by A. nosocomialis and A. pittii. A. baumannii was primarily recovered from respiratory specimens, whereas A. nosocomialis and A. pittii were recovered equally from blood and respiratory specimens.
FIG 1.
Species distribution and antimicrobial susceptibilities of A. baumannii, A. nosocomialis, and A. pittii from the 2010 to 2014 TSAR collection. The percentages of each Acinetobacter spp. based on gyrB typing (a), carbapenem resistance in each Acinetobacter species (b), and resistance to other antibiotics in carbapenem-resistant A. nosocomialis and A. pittii (c) are shown. AMK, amikacin; AMS, ampicillin/sulbactam; CAZ, ceftazidime; CIP, ciprofloxacin; FEP, cefepime; GEN, gentamicin; LEV, levofloxacin; COL, colistin; TG>1, tigecycline MIC > 1 μg/ml; TZP, piperacillin-tazobactam.
The distribution of A. baumannii, A. nosocomialis, and A. pittii varied among Acb complex isolates from clinical samples in different geographic regions. A. baumannii has been the most prevalent species in Asian countries (14), United Kingdom (15), and the United States (16), ranging from 53 to 79%. However, A. nosocomialis was reported to be more prevalent (46.9%) in Norway, and A. pittii comprised around 60% of the Acb complex in Ireland and Germany (17, 18). Our data indicated that although A. baumannii remained the predominant Acb complex species in Taiwan, its percentage has been decreasing.
In contrast to the persistent high rates of carbapenem resistance in A. baumannii, A. nosocomialis, and A. pittii had been generally more susceptible to carbapenems. The present study found that in 2010, 2012, and 2014, the rates of resistance to carbapenems (imipenem and meropenem) remained stable at 65.4, 76.5, and 65.5% in A. baumannii, but increased from 4.5 to 9.3% to 25.8% in A. pittii and from 11.1 to 26.7% to 18.2% in A. nosocomialis, respectively (Fig. 1b). Carbapenem resistance rates of up to 22% in A. pittii and 53% in A. nosocomialis have been reported in Ireland (17) and South Korea (19). Similar to carbapenem-resistant A. baumannii isolates, which are resistant to multiple antibiotics, our 25 carbapenem-resistant A. nosocomialis and A. pittii isolates also had high rates of resistance to fluoroquinolones, piperacillin-tazobactam, and gentamicin (Fig. 1c).
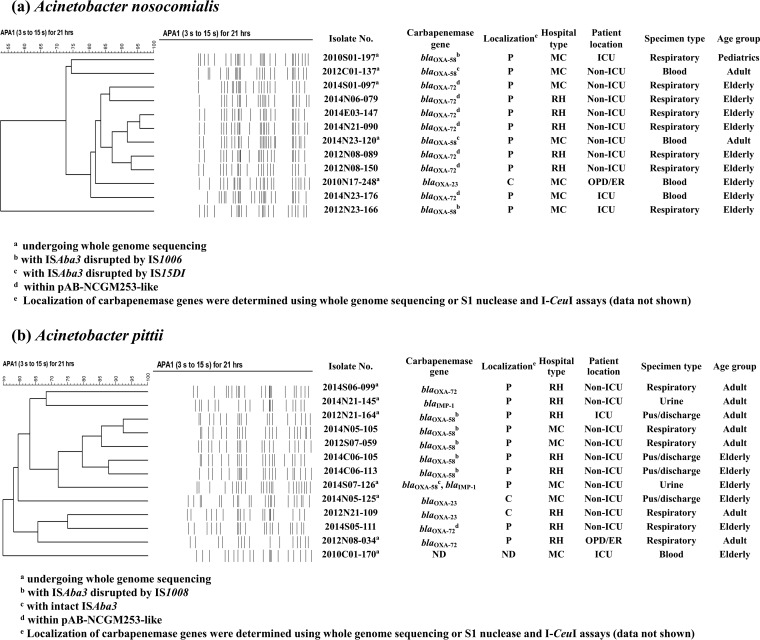
Pulsed-field gel electrophoresis (PFGE) dendrograms of the carbapenem-resistant A. nosocomialis and A. pittii isolates and their carbapenemase genes are shown in Fig. 2. Based on PFGE, the A. nosocomialis isolates from different hospitals were more closely related, whereas the A. pittii isolates were more diverse. PCR and subsequent sequencing showed that the most common carbapenemase genes were blaOXA-58 in A. pittii and blaOXA-72 in A. nosocomialis. To delineate their resistance mechanisms and genetic backgrounds, 12 isolates were chosen based on species, pulsotype, and type of carbapenemase genes for whole-genome sequencing (WGS) analysis.
FIG 2.
Patient and molecular characteristics of carbapenem-resistant A. nosocomialis (a) and A. pittii (b) in Taiwan. PFGE dendrograms are shown along with carbapenemase genes and their localization (P, plasmid; C, chromosome) and patient characteristics. MC, medical center; RH, regional hospital; ICU, intensive care unit; OPD/ER, outpatient department or emergency room; ND, not detected.
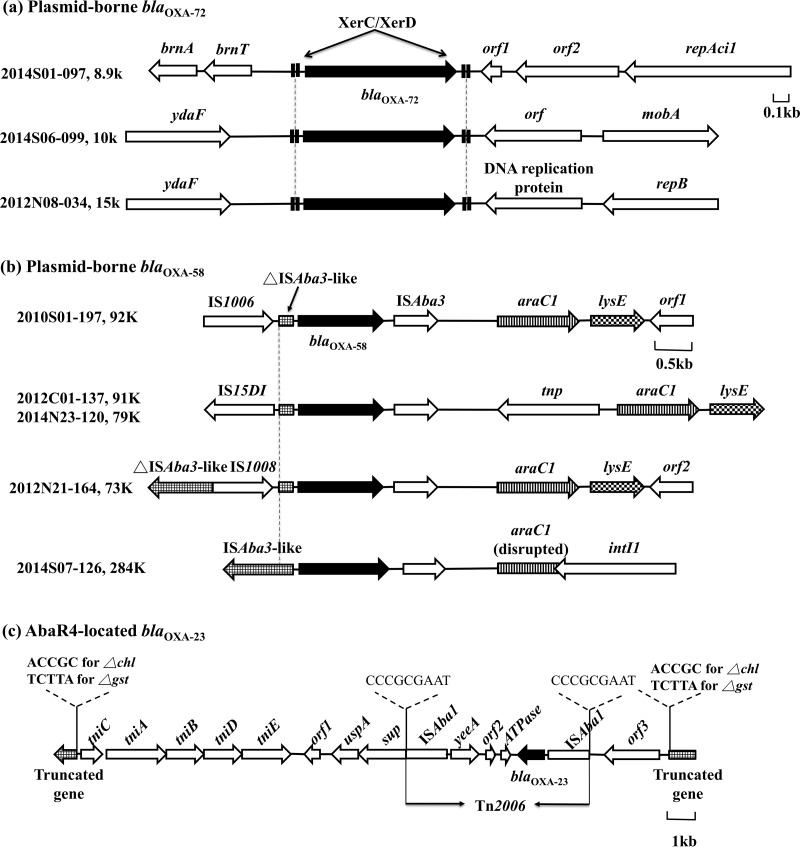
Plasmid-borne blaOXA-72 flanked by XerC/XerD-like binding sites.
Plasmid pAB-NCGM253, which carries blaOXA-72, was recently identified in carbapenem-resistant A. nosocomialis isolates in Taiwan (20). PCR mapping showed that all seven A. nosocomialis isolates and one of the three A. pittii isolates with blaOXA-72 in our collection harbored pAB-NCGM253-like. WGS performed on one of the seven A. nosocomialis (2014S01-097) isolates confirmed the presence of the 8.9-kb pAB-NCGM253 plasmid (accession number AB823544, 100% coverage, 99% identity, Fig. 3a). In addition, WGS confirmed the presence of pAB-NCGM253 in a randomly selected blaOXA-72-positive A. baumannii isolate (2008S11-069) from our previous collection (21). Transfer of the pAB-NCGM253 plasmid from A. nosocomialis to A. baumannii increased the imipenem MICs of transconjugants by >8-fold (Table 1 ).
FIG 3.
Genetic backgrounds of blaOXA-72 (a), blaOXA-58 (b), and blaOXA-23 (c) in carbapenem-resistant A. nosocomialis and/or A. pittii. The number designations of the isolates sent for WGS and the sizes of the plasmids carrying the carbapenemase genes are listed.
TABLE 1.
Contribution of different resistance determinants from A. nosocomialis and A. pittii to carbapenem resistance in A. baumannii
| Straina | Original strain | MIC (μg/ml)b |
|
|---|---|---|---|
| Imipenem | Meropenem | ||
| 2010N07-100 | 1 | 0.5 | |
| 2010N07-100 (pAB-NCGM253-like with blaOXA-72) | 2014E03-147 | >16 | >16 |
| 2010N07-100 (plasmid with IS1006-ΔISAba3-blaOXA-58) | 2010S01-197 | >16 | >16 |
| 2010N07-100 (plasmid with IS15DI-ΔISAba3-blaOXA-58) | 2014N23-120 | >16 | >16 |
| ATCC 17978 (pYMAb2) | ≤0.25 | ≤0.25 | |
| ATCC 17978 (pYMAb2::blaOXA-500)c | 2014S07-126 | ≤0.25 | ≤0.25 |
A carbapenem-susceptible A. baumannii clinical isolate (2010N07-100) was the recipient.
The MICs were determined using the broth microdilution method.
blaOXA-500 belongs to the blaOXA-272-like branch of blaOXA-213-like in A. pittii.
WGS on two A. pittii isolates (2014S06-099 and 2012N08-034) showed that one harbored a 10-kb and the other a 15-kb blaOXA-72-containing plasmid, neither of which has been previously identified. Conjugation experiments with both plasmids performed on three separate days failed, which may explain their low prevalence. The 10-kb, 15-kb, and pAB-NCGM253 plasmids all contain similar XerC/XerD-like binding sites flanking blaOXA-72 (Fig. 3a). The XerC/XerD-like binding site has been shown to be involved in the mobilization of blaOXA-72 from various plasmids in Acinetobacter spp. (3, 12, 13).
Plasmid-borne blaOXA-58 with different upstream insertion sequences.
WGS performed on three of the four blaOXA-58-positive A. nosocomialis isolates (2010S01-197, 2012C01-137, and 2014N23-120) and two of the six blaOXA-58-positive A. pittii isolates (2012N21-164 and 2014S07-126) revealed that blaOXA-58 exists within different genetic backgrounds (Fig. 3b) and is carried by various plasmids (Table S4). These results indicated a successful and diverse evolutionary history of blaOXA-58 in the non-baumannii Acb complex and its efficient dissemination by plasmids.
A PCR scheme was then designed to detect the upstream genetic structures of blaOXA-58 in the isolates on which WGS was not performed (Table S5). In all of the four A. nosocomialis isolates containing blaOXA-58, it was found that the preceding ISAba3 is disrupted by an additional insertion sequence. IS1006 was identified in 2010S01-197 by WGS and in 2012N23-166 by PCR and has been shown to create hybrid promoters for blaOXA-58 that enhance carbapenem resistance (22). Another insertion sequence, IS15DI, in 2012C01-137 and 2014N23-120 also provided an extra promoter, TTTGCA, in addition to TTTATA in ISAba3. In A. pittii, WGS of 2012N21-164 and PCR mapping showed that ISAba3 was disrupted by IS1008 in five of the six isolates containing blaOXA-58. In the remaining isolate, 2014S07-126, ISAba3-blaOXA-58 was intact and, since ISAba3-blaOXA-58 has been shown to confer only moderate resistance to carbapenems (22), the carbapenem resistance in 2014S07-126 may come primarily from the blaIMP-1 gene carried in the same plasmid (see below). Successful transfer of the plasmids carrying IS1006- or IS15DI-disrupted ISAba3-blaOXA-58 in A. nosocomialis to A. baumannii by conjugation revealed their comparable abilities to confer carbapenem resistance (Table 1). The plasmids carrying IS1008 failed to transfer by conjugation after three independent attempts.
AbaR4-located blaOXA-23.
Only one A. nosocomialis isolate and two A. pittii isolates were positive for blaOXA-23, and WGS was performed on the A. nosocomialis isolate (2010N17-248) and one of the A. pittii (2014N05-125). In 2010N17-248, the blaOXA-23 gene was carried on Tn2006 that was embedded in AbaR4, which is an AbGRI1-type genetic structure (7) (Fig. 3c). The AbaR4 interrupted a gene coding for magnesium chelatase (chl) with flanking direct repeats (ACCGC). This type of AbaR4 is prevalent in A. baumannii in Asia (4, 5) and has been found in A. nosocomialis in Korea and Thailand (8, 9).
Two copies of blaOXA-23 were detected 1.23 Mb apart in 2014N05-125, and both were carried in the same AbaR4 structure described above. One copy of blaOXA-23 interrupted the chl gene, and the other interrupted the gene that codes for glutathione S-transferase, gst, and was flanked by TCTTA direct repeats. PCR mapping showed the same AbaR4 region in the other blaOXA-23-positive A. pittii (2012N21-109). In A. baumannii, it has been shown that comM (ATPase gene) is commonly interrupted by AbaR4, and this interruption has also been found in A. nosocomialis (8). However, PCR targeting comM (6) and an in silico search showed that comM is not present in our three blaOXA-23-positive isolates.
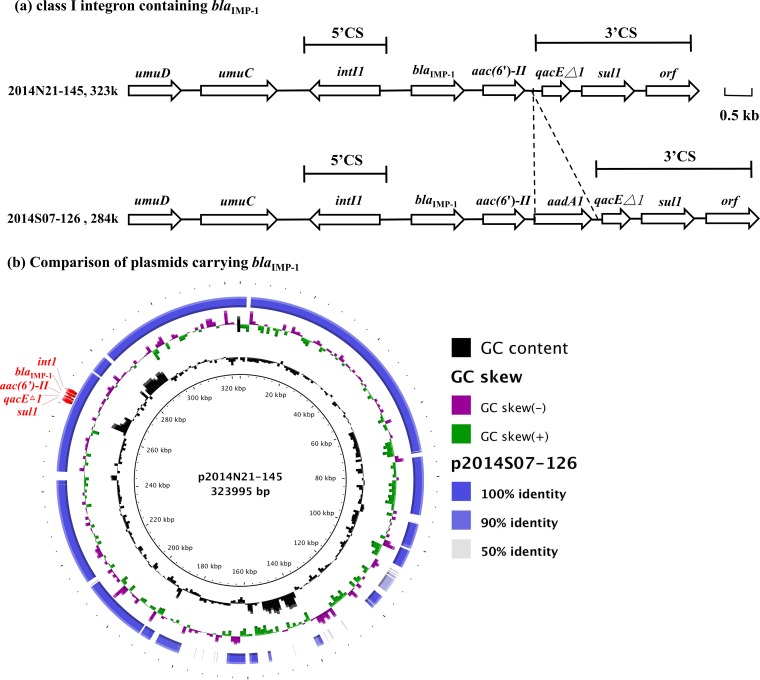
Plasmid-borne class I integron containing blaIMP-1.
While CHDLs are the most prevalent mechanisms of carbapenem resistance in A. baumannii, MBLs, such as Verona integron-encoded MBL (VIM), imipenemase (IMP), and Seoul imipenemase MBL (SIM), have also been found in Acinetobacter spp. In this study, two A. pittii isolates, 2014N21-145 and 2014S07-126, harbored blaIMP-1 embedded in class I integron. The integron incorporated additional aminoglycoside resistance determinants, aac(6′)-II and/or aadA1 (Fig. 4a). Plasmids p2014N21-145 (323 kb) and p2014S07-126 (284 kb) that contained the integron shared a 256-kb region with 99% similarity (Fig. 4b, accession numbers CP033569 and CP033531). An additional ISAba3-blaOXA-58 was located in p2014S07-126 in the region that shared little similarity with p2014N21-145.
FIG 4.
Comparison of flanking regions of blaIMP-1 (a) and the plasmids carrying blaIMP-1 (b). A BLAST ring image generator was used to generate an image of the compared plasmids. Plasmid p2014S07-126 was mapped to the reference sequence (p2014N21-145) by BLASTn.
The blaIMP-like gene was first discovered in Pseudomonas aeruginosa in 1991 and has since been reported in Enterobacteriaceae and Acinetobacter spp. (23–25). Although the prevalence of blaIMP-like has been relatively low, many variants of blaIMP-like have been identified worldwide in clinical Acinetobacter isolates (2). Similar to other MBL genes (26), the blaIMP-like gene is usually incorporated into class I integron in Acinetobacter spp. (25, 27). In this survey, the similarities of plasmids carrying blaIMP-1, which were identified in isolates from different geographic areas, suggest that plasmids may be responsible for transferring integron containing blaIMP-like. However, transfer of the p2014N21-145 and p2014S07-126 plasmids in A. pittii to A. baumannii by conjugation failed three independent times.
High prevalence of chromosomal blaOXA-272-like branch of blaOXA-213-like in A. pittii.
WGS revealed the presence of blaOXA-213-like on the chromosomes of A. pittii isolates in the TSAR collection. The amino acid sequences of OXA-213-like in our isolates, OXA-213-like in A. calcoaceticus from the NCBI database, and other common CHDLs were compared (Fig. S1a). The phylogenetic tree showed the difference of OXA-213-like in A. pittii from OXA-213-like in A. calcoaceticus and other common CHDL. Based on NCBI data, the 85 to 90% similarity between the OXA-213-like in our A. pittii and in A. calcoaceticus (Fig. S1b) may not justify the creation of an extra name of OXA. However, to differentiate our OXA-213-like in A. pittii and naturally occurring OXA-213-like in A. calcoaceticus (28), OXA-272-like branch of OXA-213-like, which is the oldest and founding member of the subgroup, is used in this article.
One previous study (29) using in silico search suggested blaOXA-272-like may be intrinsic to A. pittii. With more clinical isolates tested, our finding supports the suggestion. Since the primer set in the previous study (30) that identified the blaOXA-213-like in A. calcoaceticus was unable to identify blaOXA-272-like in our study (data not shown), new PCR primers were developed to identify blaOXA-272-like and to differentiate them from blaOXA-213-like in A. calcoaceticus. This PCR scheme was positive in all of the 13 imipenem-resistant A. pittii isolates in this study and in 13 imipenem-susceptible A. pittii isolates randomly selected from TSAR. Sequencing of the amplicons using extra-genetic primers confirmed them to be blaOXA-272-like. Furthermore, PCR of randomly selected 13 A. nosocomialis and 13 A. baumannii isolates from TSAR, and two reference strains of A. calcoaceticus (ATCC14987 and ATCC23055) were all negative for blaOXA-272-like.
Since this study included isolates from 2010 to 2014, it is possible that blaOXA-272-like was present in A. baumannii and A. nosocomialis isolates before 2010. Therefore, we collected bacteremic Acb complex isolates from 1997 to 2015 from four different hospitals (Table S6) and tested for carbapenem susceptibility and for carbapenem resistance genes in A. baumannii, A. nosocomialis, and A. pittii (A. calcoaceticus was not identified in the collection). PCR showed that all 20 of the carbapenem-susceptible A. pittii isolates contained blaOXA-272-like. In contrast, none of the A. baumannii or A. nosocomialis isolates contained blaOXA-272-like or blaOXA-213-like. Transformation of pYMAb2::blaOXA-272-like into A. baumannii clinical isolate 2010N07-100 did not confer resistance to imipenem or meropenem (Table 1). Thus, blaOXA-272-like may serve as a marker for A. pittii, just like blaOXA-51-like is for A. baumannii.
In conclusion, this longitudinal nationwide survey revealed an increase of A. nosocomialis and A. pittii among Acb complex isolates recovered from clinical samples in Taiwan. The prevalence of carbapenem resistance also increased in these two species. Various carbapenem resistance determinants with diverse surrounding genetic structures were identified, with plasmid-borne blaOXA-72 or blaOXA-58 being the main mechanisms, but AbaR4-located blaOXA-23 in the chromosome and plasmid-borne class I integron containing blaIMP-1 were also detected.
MATERIALS AND METHODS
Isolate collection, species identification, and antimicrobial susceptibility testing.
The Acinetobacter isolates were identified from the 2010–2014 TSAR collection of clinical isolates (corresponding to TSAR VII to IX). The isolate collection protocol and participating hospitals have been described previously (31). All of the clinical isolates identified initially as Acb complex by Vitek II GN card (bioMérieux, Marcy l’Etoile, France) were further identified to the species level using the gyrB PCR typing method (32). MICs were determined by the reference broth microdilution method using Sensititre custom-designed plates. Susceptibility was determined in accordance with the Clinical and Laboratory Standards Institute (CLSI) (33). Carbapenem resistance was defined as MICs of >4 μg/ml for both imipenem and meropenem.
Detection of carbapenemase genes.
The presence of genes encoding class A (blaNMC, blaSME, blaIMI, blaKPC, and blaGES), class B (blaIMP, blaVIM, blaGIM, blaSPM, blaSIM-1, and blaNDM-1), and class D (blaOXA-23-like, blaOXA-24-like, blaOXA-58-like, and blaOXA-51-like) β-lactamases with carbapenemase activity was detected by PCR in the carbapenem-resistant isolates (34–36). PCR to detect ISAba1 upstream of blaOXA-23-like or blaOXA-51-like was performed using the reverse ISAba1 primer and the reverse target gene primer (34–36). All detected carbapenemase genes were sent for sequencing.
Modified PCR scheme to detect the genetic background upstream of blaOXA-58.
Due to various forms of ISAba3 and/or insertion sequences upstream of blaOXA-58, a three-step PCR scheme (Fig. S2 and Table S5) was modified from previous published protocols (22, 37) to determine the presence of blaOXA-58 and its upstream genetic structure. The first step was to detect for blaOXA-58. The second step was to detect for truncated or complete ISAba3 using primers that targeted the conserved part of ISAba3. The third step was to detect for hybrid promoters upstream of blaOXA-58. Based on sequencing results from this study and on sequence data in the NCBI database, we determined that the sequences of the hybrid promoters are mostly conserved and have only minor differences (Fig. S2a). Three sets of forward primers targeting hybrid promoters were designed, and a mixture of two of the sets of forward primers successfully identified all of the hybrid promoter variants (Fig. S2b). To further identify the insertion sequences that interrupted ISAba3, an optional PCR using the primer sets listed in Table S5 was performed.
PCR for the detection of pAB-NCGM253-like and blaOXA-272-like.
Six sets of primers covering overlapping areas of pAB-NCGM253 and amplification programs are listed in Table S5. Two sets of primers targeting blaOXA-272-like and blaOXA-213-like, respectively, were designed (Table S5). The program included 30 cycles of denaturation at 94°C for 30 s, annealing at 50°C for 30 s, and elongation at 72°C for 30 s.
PFGE.
All of the carbapenem-resistant isolates were subjected to PFGE after digestion with ApaI as previously described (38). The stained gels were photographed and BioNumerics software (Applied Maths) was used to generate dendrograms to determine the relatedness of the isolates.
WGS using oxford nanopore technologies.
Isolates were then selected for WGS based on their species, resistance mechanism, and pulsotype. A Nanopore sequencing library was constructed using ligation methodology with a ligation sequencing kit 1D (SQK-LSK108) and a native barcoding kit (EXP-NBD103). Briefly, 10-kb genomic DNA fragments were generated by g-TUBE (Covaris). DNA fragments were repaired and dA tailed with a NEBNext FFPE DNA repair mix and a NEBNext Ultra II End Repair/dA-Tailing module (New England BioLabs). Individual barcodes were added to the dA-tailed DNA using NEB Blunt/TA Ligase Master Mix (New England BioLabs). Equimolar amounts of the barcoded DNAs were pooled and adaptors were attached using NEBNext Quick Ligation module (New England BioLabs). The library was loaded into a SpotON Flowcell R9.4 or 9.5 (FLO-MIN106 or FLO-MIN107), and the sequencing script NC_48Hr_Sequencing_FLO-MIN106 or 107_SQK_LSK108 was executed on MinKNOW (v1.7.14). Albacore (v2.1.7) was used for demultiplexing and base calling. The base-called sequencing reads were then de novo assembled by Canu (v1.6) (39) or minimap2 (v2.10)/miniasm (v0.2) (40, 41). The assembled contigs were subsequently polished with the sequencing reads using Nanopolish (v0.8.5) (https://github.com/jts/nanopolish) and Racon (v1.1.1) (42), respectively. Finally, redundant ends of the consensus sequences were trimmed, and the circular sequences were rearranged to begin at DnaA/RepA or a position with the minimum value of GC skew. These processes were all implemented in an in-house pipeline to produce the complete genomes of the sequenced strains.
Conjugation and transformation experiments.
Conjugation was performed following previously described protocols (43). Briefly, 500 μl of the donor culture in Luria-Bertani (LB) broth and 500 μl of the recipient culture in LB broth were mixed with 400 μl of 10 mM MgSO4, and the mixture was distributed evenly onto a filter. The filter was transferred to Mueller-Hinton agar and incubated at 37°C for 16 h. The mated cells were harvested by submerging the filter in 5 ml of LB broth and then vortexing the sample. The suspended bacteria were plated onto selective LB agar containing 2 μg/ml imipenem and another selective antibiotic and then incubated at 37°C overnight. Transconjugants were tested for target genes by PCR and for antibiotic susceptibility by using the Vitek II automated system and broth microdilution.
The blaOXA-272-like gene was PCR amplified with a proofreading DNA polymerase (Phusion high-fidelity DNA polymerase; Finnzymes, Espoo, Finland) and cloned into the pCRII-TOPO vector (Invitrogen, Carlsbad, CA). After confirmation of blaOXA-272-like by sequencing (Mission Biotech, Taipei, Taiwan), the pCRII-TOPO::blaOXA-272-like was digested with XbaI and XhoI, and the digested blaOXA-272-like fragment was cloned into the Escherichia coli-A. baumannii shuttle vector pYMAb2. The recombinant plasmid and a control plasmid (pYMAb2 without blaOXA-272-like) were transformed into kanamycin-susceptible A. baumannii ATCC17978 by electroporation with a gene pulser electroporator (Bio-Rad, Hercules, CA) using 2-mm electrode gap cuvettes (44). The pYMAb2 plasmid contains a kanamycin resistance gene, and kanamycin-resistant transformants were selected. Sequencing was performed to confirm the presence of blaOXA-272-like.
Data availability.
The nucleotide sequences of 2010N17-248, 2014N05-125, 2012N08-034, 2014S06-099, 2014S01-097, 2010S01-197, 2012C01-137, 2014N23-120, 2012N21-164, 2014S07-126, 2014N21-145, 2010C01-170, and 2008S11-069 (K069 in a previous study [21]) were deposited in GenBank under the accession numbers CP033572 to CP033573, CP033525 to CP033529, CP033520 to CP033524, CP033540 to CP033544, CP033550 to CP033556, CP033561 to CP033567, CP033557 to CP033560, CP033545 to CP033549, CP033535 to CP033539, CP033530 to CP033534, CP033568 to CP033571, CP029489, and CP033516 to CP033519, respectively.
Supplementary Material
ACKNOWLEDGMENTS
We express our sincere appreciation to the following 26 hospitals for their participation in the TSAR project: Buddhist Tzu Chi General Hospital, Cathay General Hospital, Changhua Christian Hospital, Cheng-Ching Hospital, Chung Shan Medical University Hospital, Ditmanson Medical Foundation Chia-Yi Christian Hospital, Far Eastern Memorial Hospital, Hua-Lien Hospital, Jen-Ai Hospital, Kaohsiung Armed Forces General Hospital, Kaohsiung Chang Gung Memorial Hospital of the Chang Gung Medical Foundation, Kaohsiung Medical University Chung-Ho Memorial Hospital, Kaohsiung Veterans General Hospital, Kuang Tien General Hospital, Lo-Hsu Foundation, Inc.-Lotung Poh-Ai Hospital, Mennonite Christian Hospital, Min-Sheng Healthcare, National Cheng Kung University Hospital, Saint Mary’s Hospital Luodong, Show Chwan Memorial Hospital, Tungs’ Taichung MetroHarbor Hospital, Taichung Veterans General Hospital, Tainan Sin-Lau Hospital-the Presbyterian Church in Taiwan, Taipei City Hospital Heping Fuyou Branch, Taipei City Hospital Zhongxiao Branch, and Tri-Service General Hospital. We also express our sincere appreciation to the ACTION study group for providing Acinetobacter spp.
This project was supported by intramural grants from the National Health Research Institutes (IV-106-PP-01, IV-107-PP-09, and IV-106-PP-09) and the Ministry of Science and Technology (105-2628-B-400-002-MY2 and 107-2320-B-400-010-MY3).
The bacterial isolates were recovered from clinical samples taken as part of standard care. This study was approved by the Research Ethics Committee of the National Health Research Institutes (EC960205, EC1010602-E, and EC1030406-E).
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.02007-18.
REFERENCES
- 1.Wong D, Nielsen TB, Bonomo RA, Pantapalangkoor P, Luna B, Spellberg B. 2017. Clinical and pathophysiological overview of Acinetobacter infections: a century of challenges. Clin Microbiol Rev 30:409–447. doi: 10.1128/CMR.00058-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Poirel L, Nordmann P. 2006. Carbapenem resistance in Acinetobacter baumannii: mechanisms and epidemiology. Clin Microbiol Infect 12:826–836. doi: 10.1111/j.1469-0691.2006.01456.x. [DOI] [PubMed] [Google Scholar]
- 3.Merino M, Acosta J, Poza M, Sanz F, Beceiro A, Chaves F, Bou G. 2010. OXA-24 carbapenemase gene flanked by XerC/XerD-like recombination sites in different plasmids from different Acinetobacter species isolated during a nosocomial outbreak. Antimicrob Agents Chemother 54:2724–2727. doi: 10.1128/AAC.01674-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamidian M, Hall RM. 2017. Origin of the AbGRI1 antibiotic resistance island found in the comM gene of Acinetobacter baumannii GC2 isolates. J Antimicrob Chemother 72:2944–2947. doi: 10.1093/jac/dkx206. [DOI] [PubMed] [Google Scholar]
- 5.Kim DH, Choi JY, Kim HW, Kim SH, Chung DR, Peck KR, Thamlikitkul V, So TM, Yasin RM, Hsueh PR, Carlos CC, Hsu LY, Buntaran L, Lalitha MK, Song JH, Ko KS. 2013. Spread of carbapenem-resistant Acinetobacter baumannii global clone 2 in Asia and AbaR-type resistance islands. Antimicrob Agents Chemother 57:5239–5246. doi: 10.1128/AAC.00633-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Post V, White PA, Hall RM. 2010. Evolution of AbaR-type genomic resistance islands in multiply antibiotic-resistant Acinetobacter baumannii. J Antimicrob Chemother 65:1162–1170. doi: 10.1093/jac/dkq095. [DOI] [PubMed] [Google Scholar]
- 7.Nigro SJ, Hall RM. 2016. Structure and context of Acinetobacter transposons carrying the oxa23 carbapenemase gene. J Antimicrob Chemother 71:1135–1147. doi: 10.1093/jac/dkv440. [DOI] [PubMed] [Google Scholar]
- 8.Kim DH, Ko KS. 2015. AbaR-type genomic islands in non-baumannii Acinetobacter species isolates from South Korea. Antimicrob Agents Chemother 59:5824–5826. doi: 10.1128/AAC.01175-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim DH, Choi JY, Jung SI, Thamlikitkul V, Song JH, Ko KS. 2012. AbaR4-type resistance island including the blaOXA-23 gene in Acinetobacter nosocomialis isolates. Antimicrob Agents Chemother 56:4548–4549. doi: 10.1128/AAC.00923-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu LL, Ji SJ, Ruan Z, Fu Y, Fu YQ, Wang YF, Yu YS. 2015. Dissemination of blaOXA-23 in Acinetobacter spp. in China: main roles of conjugative plasmid pAZJ221 and transposon Tn2009. Antimicrob Agents Chemother 59:1998–2005. doi: 10.1128/AAC.04574-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silva L, Mourao J, Grosso F, Peixe L. 2018. Uncommon carbapenemase-encoding plasmids in the clinically emergent Acinetobacter pittii. J Antimicrob Chemother 73:52–56. doi: 10.1093/jac/dkx364. [DOI] [PubMed] [Google Scholar]
- 12.Montealegre MC, Maya JJ, Correa A, Espinal P, Mojica MF, Ruiz SJ, Rosso F, Vila J, Quinn JP, Villegas MV. 2012. First identification of OXA-72 carbapenemase from Acinetobacter pittii in Colombia. Antimicrob Agents Chemother 56:3996–3998. doi: 10.1128/AAC.05628-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cayo R, Merino M, Ruiz Del Castillo B, Cano ME, Calvo J, Bou G, Martinez ML. 2014. OXA-207, a novel OXA-24 variant with reduced catalytic efficiency against carbapenems in Acinetobacter pittii from Spain. Antimicrob Agents Chemother 58:4944–4948. doi: 10.1128/AAC.02633-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang HZ, Zhang JS, Qiao L. 2013. The Acinetobacter baumannii group: a systemic review. World J Emerg Med 4:169–174. doi: 10.5847/wjem.j.issn.1920-8642.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turton JF, Shah J, Ozongwu C, Pike R. 2010. Incidence of Acinetobacter species other than A. baumannii among clinical isolates of Acinetobacter: evidence for emerging species. J Clin Microbiol 48:1445–1449. doi: 10.1128/JCM.02467-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wisplinghoff H, Paulus T, Lugenheim M, Stefanik D, Higgins PG, Edmond MB, Wenzel RP, Seifert H. 2012. Nosocomial bloodstream infections due to Acinetobacter baumannii, Acinetobacter pittii, and Acinetobacter nosocomialis in the United States. J Infect 64:282–290. doi: 10.1016/j.jinf.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 17.Boo TW, Walsh F, Crowley B. 2009. Molecular characterization of carbapenem-resistant Acinetobacter species in an Irish university hospital: predominance of Acinetobacter genomic species 3. J Medical Microbiology 58:209–216. doi: 10.1099/jmm.0.004911-0. [DOI] [PubMed] [Google Scholar]
- 18.Schleicher X, Higgins PG, Wisplinghoff H, Korber-Irrgang B, Kresken M, Seifert H. 2013. Molecular epidemiology of Acinetobacter baumannii and Acinetobacter nosocomialis in Germany over a 5-year period (2005-2009). Clin Microbiol Infect 19:737–742. doi: 10.1111/1469-0691.12026. [DOI] [PubMed] [Google Scholar]
- 19.Park YK, Jung SI, Park KH, Kim SH, Ko KS. 2012. Characteristics of carbapenem-resistant Acinetobacter spp. other than Acinetobacter baumannii in South Korea. Int J Antimicrob Agents 39:81–85. doi: 10.1016/j.ijantimicag.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 20.Chen HY, Yang YS, Hsu WJ, Chou YC, Huang LS, Wang YC, Chiueh TS, Sun JR. 2018. Emergence of carbapenem-resistant Acinetobacter nosocomialis strain ST410 harbouring plasmid-borne blaOXA-72 gene in Taiwan. Clin Microbiol Infect doi: 10.1016/j.cmi.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 21.Kuo SC, Huang WC, Huang TW, Wang HY, Lai JF, Chen TL, Lauderdale TL. 2018. Molecular epidemiology of emerging blaOXA-23-like- and blaOXA-24-like-carrying Acinetobacter baumannii in Taiwan. Antimicrob Agents Chemother 62 doi: 10.1128/AAC.01215-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen TL, Chang WC, Kuo SC, Lee YT, Chen CP, Siu LK, Cho WL, Fung CP. 2010. Contribution of a plasmid-borne blaOXA-58 gene with its hybrid promoter provided by IS1006 and an ISAba3-like element to beta-lactam resistance in Acinetobacter genomic species 13TU. Antimicrob Agents Chemother 54:3107–3112. doi: 10.1128/AAC.00128-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Watanabe M, Iyobe S, Inoue M, Mitsuhashi S. 1991. Transferable imipenem resistance in Pseudomonas aeruginosa. Antimicrob Agents Chemother 35:147–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Osano E, Arakawa Y, Wacharotayankun R, Ohta M, Horii T, Ito H, Yoshimura F, Kato N. 1994. Molecular characterization of an enterobacterial metallo beta-lactamase found in a clinical isolate of Serratia marcescens that shows imipenem resistance. Antimicrob Agents Chemother 38:71–78. doi: 10.1128/AAC.38.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bonomo RA, Szabo D. 2006. Mechanisms of multidrug resistance in Acinetobacter species and Pseudomonas aeruginosa. Clin Infect Dis 43:S49–S56. doi: 10.1086/504477. [DOI] [PubMed] [Google Scholar]
- 26.Laraki N, Galleni M, Thamm I, Riccio ML, Amicosante G, Frere JM, Rossolini GM. 1999. Structure of In31, a blaIMP-containing Pseudomonas aeruginosa integron phyletically related to In5, which carries an unusual array of gene cassettes. Antimicrob Agents Chemother 43:890–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamamoto M, Nagao M, Matsumura Y, Matsushima A, Ito Y, Takakura S, Ichiyama S. 2011. Interspecies dissemination of a novel class 1 integron carrying blaIMP-19 among Acinetobacter species in Japan. J Antimicrob Chemother 66:2480–2483. doi: 10.1093/jac/dkr336. [DOI] [PubMed] [Google Scholar]
- 28.Figueiredo S, Bonnin RA, Poirel L, Duranteau J, Nordmann P. 2012. Identification of the naturally occurring genes encoding carbapenem-hydrolysing oxacillinases from Acinetobacter haemolyticus, Acinetobacter johnsonii, and Acinetobacter calcoaceticus. Clin Microbiol Infect 18:907–913. doi: 10.1111/j.1469-0691.2011.03708.x. [DOI] [PubMed] [Google Scholar]
- 29.Perichon B, Goussard S, Walewski V, Krizova L, Cerqueira G, Murphy C, Feldgarden M, Wortman J, Clermont D, Nemec A, Courvalin P. 2014. Identification of 50 class D β-lactamases and 65 acinetobacter-derived cephalosporinases in Acinetobacter spp. Antimicrob Agents Chemother 58:936–949. doi: 10.1128/AAC.01261-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kamolvit W, Higgins PG, Paterson DL, Seifert H. 2014. Multiplex PCR to detect the genes encoding naturally occurring oxacillinases in Acinetobacter spp. J Antimicrob Chemother 69:959–963. doi: 10.1093/jac/dkt480. [DOI] [PubMed] [Google Scholar]
- 31.Kuo SC, Chang SC, Wang HY, Lai JF, Chen PC, Shiau YR, Huang IW, Lauderdale TL, Hospitals T. 2012. Emergence of extensively drug-resistant Acinetobacter baumannii complex over 10 years: nationwide data from the Taiwan Surveillance of Antimicrobial Resistance (TSAR) program. BMC Infect Dis 12:200. doi: 10.1186/1471-2334-12-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Higgins PG, Lehmann M, Wisplinghoff H, Seifert H. 2010. gyrB multiplex PCR to differentiate between Acinetobacter calcoaceticus and Acinetobacter genomic species 3. J Clin Microbiol 48:4592–4594. doi: 10.1128/JCM.01765-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clinical and Laboratory Standards Institute. 2017. Performance standards for antimicrobial susceptibility testing; 27th informational supplement. CLSI document M100. CLSI, Wayne, PA. [Google Scholar]
- 34.Ellington MJ, Kistler J, Livermore DM, Woodford N. 2006. Multiplex PCR for rapid detection of genes encoding acquired metallo-β-lactamases. J Antimicrob Chemother 59:321–322. doi: 10.1093/jac/dkl481. [DOI] [PubMed] [Google Scholar]
- 35.Queenan AM, Bush K. 2007. Carbapenemases: the versatile beta-lactamases. Clin Microbiol Rev 20:440–458. doi: 10.1128/CMR.00001-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee Y-T, Kuo S-C, Chiang M-C, Yang S-P, Chen C-P, Chen T-L, Fung C-P. 2012. Emergence of carbapenem-resistant non-baumannii species of Acinetobacter harboring a blaOXA-51-like gene that is intrinsic to A. baumannii. Antimicrob Agents Chemother 56:1124–1127. doi: 10.1128/AAC.00622-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Woodford N, Ellington MJ, Coelho JM, Turton JF, Ward ME, Brown S, Amyes SGB, Livermore DM. 2006. Multiplex PCR for genes encoding prevalent OXA carbapenemases in Acinetobacter spp. Int J Antimicrob Agents 27:351–353. doi: 10.1016/j.ijantimicag.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 38.Huang LY, Chen TL, Lu PL, Tsai CA, Cho WL, Chang FY, Fung CP, Siu LK. 2008. Dissemination of multidrug-resistant, class 1 integron-carrying Acinetobacter baumannii isolates in Taiwan. Clin Microbiol Infect 14:1010–1019. doi: 10.1111/j.1469-0691.2008.02077.x. [DOI] [PubMed] [Google Scholar]
- 39.Koren S, Walenz BP, Berlin K, Miller JR, Bergman NH, Phillippy AM. 2017. Canu: scalable and accurate long-read assembly via adaptive k-mer weighting and repeat separation. Genome Res 27:722–736. doi: 10.1101/gr.215087.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li H. 2016. Minimap and miniasm: fast mapping and de novo assembly for noisy long sequences. Bioinformatics 32:2103–2110. doi: 10.1093/bioinformatics/btw152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li H. 2018. Minimap2: pairwise alignment for nucleotide sequences. Bioinformatics 34:3094–3100. doi: 10.1093/bioinformatics/bty191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vaser R, Sovic I, Nagarajan N, Sikic M. 2017. Fast and accurate de novo genome assembly from long uncorrected reads. Genome Res 27:737–746. doi: 10.1101/gr.214270.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jacoby GA, Han P. 1996. Detection of extended-spectrum beta-lactamases in clinical isolates of Klebsiella pneumoniae and Escherichia coli. J Clin Microbiol 34:908–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kuo SC, Yang SP, Lee YT, Chuang HC, Chen CP, Chang CL, Chen TL, Lu PL, Hsueh PR, Fung CP. 2013. Dissemination of imipenem-resistant Acinetobacter baumannii with new plasmid-borne blaOXA-72 in Taiwan. BMC Infect Dis 13:319 10.1186/1471-2334-13-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The nucleotide sequences of 2010N17-248, 2014N05-125, 2012N08-034, 2014S06-099, 2014S01-097, 2010S01-197, 2012C01-137, 2014N23-120, 2012N21-164, 2014S07-126, 2014N21-145, 2010C01-170, and 2008S11-069 (K069 in a previous study [21]) were deposited in GenBank under the accession numbers CP033572 to CP033573, CP033525 to CP033529, CP033520 to CP033524, CP033540 to CP033544, CP033550 to CP033556, CP033561 to CP033567, CP033557 to CP033560, CP033545 to CP033549, CP033535 to CP033539, CP033530 to CP033534, CP033568 to CP033571, CP029489, and CP033516 to CP033519, respectively.