Abstract
Calcium signaling in neurons as in other cell types can lead to varied changes in cellular function. Neuronal Ca2+ signaling processes have also become adapted to modulate the function of specific pathways over a wide variety of time domains and these can have effects on, for example, axon outgrowth, neuronal survival, and changes in synaptic strength. Ca2+ also plays a key role in synapses as the trigger for fast neurotransmitter release. Given its physiological importance, abnormalities in neuronal Ca2+ signaling potentially underlie many different neurological and neurodegenerative diseases. The mechanisms by which changes in intracellular Ca2+ concentration in neurons can bring about diverse responses is underpinned by the roles of ubiquitous or specialized neuronal Ca2+ sensors. It has been established that synaptotagmins have key functions in neurotransmitter release, and, in addition to calmodulin, other families of EF-hand-containing neuronal Ca2+ sensors, including the neuronal calcium sensor (NCS) and the calcium-binding protein (CaBP) families, play important physiological roles in neuronal Ca2+ signaling. It has become increasingly apparent that these various Ca2+ sensors may also be crucial for aspects of neuronal dysfunction and disease either indirectly or directly as a direct consequence of genetic variation or mutations. An understanding of the molecular basis for the regulation of the targets of the Ca2+ sensors and the physiological roles of each protein in identified neurons may contribute to future approaches to the development of treatments for a variety of human neuronal disorders.
Calcium signaling in many cell types can mediate a diverse range of changes in cellular function affecting gene expression, cell growth, development, survival, and cell death. In addition, neuronal calcium signaling processes have become adapted to modulate the function of other important pathways in the brain, including neuronal survival, axon outgrowth (Spitzer 2006), and changes in synaptic strength (Catterall and Few 2008; Catterall et al. 2013). Changes in the concentration of intracellular free Ca2+ ([Ca2+]i) are essential for the transmission of information through the nervous system as the trigger for neurotransmitter release at synapses. In addition, alterations in [Ca2+]i can lead to a wide variety of different physiological changes that can modify neuronal functions over a range of time domains of milliseconds through 10 sec, to minutes to days or longer (Berridge 1998). It has long been believed that the physiological outcome from a change in [Ca2+]i depends on its location, amplitude, and duration. The importance of location becomes even more pronounced in neurons because of their complex morphologies. Pathological changes in Ca2+ signaling pathways have been suggested to underlie various neuropathological disorders (Braunewell 2005; Berridge 2010, 2018; Brini et al. 2014, 2017), including neurological abnormalities and neurodegenerative disorders (Popugaeva and Bezprozvanny 2013; Egorova and Bezprozvanny 2018; Pchitskaya et al. 2018; Secondo et al. 2018; Wegierski and Kuznicki 2018). Such changes have implicated Ca2+ entry pathways and the release of Ca2+ from intracellular stores (Popugaeva and Bezprozvanny 2013; Schampel and Kuerten 2017; Egorova and Bezprozvanny 2018; Secondo et al. 2018; Wegierski and Kuznicki 2018).
The nature, magnitude, and location of the Ca2+ signal is crucial for the particular effect of neuronal physiology (Burgoyne 2007). Highly localized Ca2+ elevations because of Ca2+ entry though voltage-gated Ca2+ channels (VGCCs) lead to synaptic vesicle fusion with the presynaptic membrane for neurotransmitter release within less than a millisecond (Burgoyne and Morgan 1998; Barclay et al. 2005). Differently localized and timed Ca2+ signals can result in changes to the properties of the VGCCs themselves (Catterall and Few 2008), to alterations in synaptic plasticity (Catterall et al. 2013), or lead to changes in gene expression (Bito et al. 1997). Postsynaptic Ca2+ signals arising from activation of N-methyl-d-aspartate receptors (NMDARs) give rise to two important processes in synaptic plasticity, long-term potentiation (LTP) and long-term depression (LTD). Interestingly, the Ca2+ signals that bring about either LTP or LTD differ only in their amplitude and duration (Yang et al. 1999).
Specific neuronal Ca2+ signals are likely to be decoded by various Ca2+ sensor proteins (McCue et al. 2010b). These are proteins that undergo a conformational change on Ca2+ binding allowing them to interact with and regulate various target proteins (Ikuro and Ames 2006; Burgoyne and Haynes 2015). Among the Ca2+ sensors that are important for neuronal function are the synaptotagmins that control neurotransmitter release (Fernández-Chacón et al. 2001; Südhof 2013), the ubiquitous EF-hand-containing sensor calmodulin (Faas et al. 2011) that has many neuronal roles, and the more specific neuronal EF-hand-containing proteins, including the neuronal calcium sensor (NCS) proteins (Burgoyne and Weiss 2001; Burgoyne 2007; Burgoyne and Haynes 2012, 2015) and the calcium-binding protein (CaBP)/calneuron families (Haeseleer et al. 2002; Mikhaylova et al. 2006, 2011; McCue et al. 2010a; Haynes et al. 2012). The potential involvement of members of these protein families in neuronal disorders studied in both experimental models and in human subjects has become apparent in recent years. In this review, we assess the information available on the physiological roles of these various Ca2+ sensors and their modes of action, and also how they may contribute to neuronal dysfunction or be involved in disease-related processes in the nervous system.
SYNAPTOTAGMINS
The Physiology and Function of Synaptotagmins
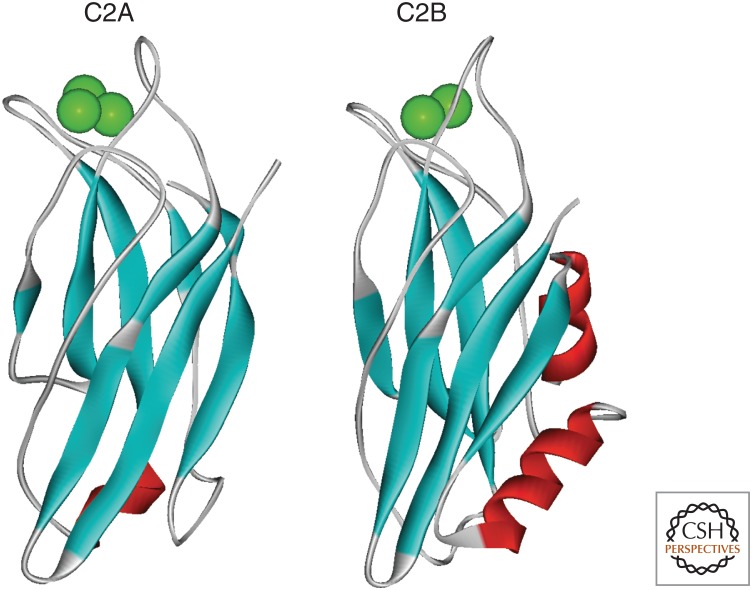
The synaptotagmins are transmembrane proteins predominantly associated with synaptic and secretory vesicles. There are multiple known isoforms of synaptotagmins (Craxton 2004), of which synaptotagmin 1 has been most widely studied. The role of synaptotagmins in neurotransmitter release has been the subject of intense investigations, which have been extensively reviewed (Chapman 2008; Rizo and Rosenmund 2008; Südhof and Rothman 2009). Synaptotagmins bind Ca2+ with relatively low affinity (Kd > 10 µM) through their two C2 domains (C2A and C2B) (Shao et al. 1998; Fernandez et al. 2001), which are functional in many but not all synaptotagmin isoforms. Ca2+ binding by C2 domains requires coordination of Ca2+ by both the protein and membrane lipids, and this lipid interaction is a key aspect for its function. In synaptotagmin 1, the C2A and C2B domains (Fig. 1) bind three and two Ca2+ ions, respectively (Shao et al. 1998; Fernandez et al. 2001). It is now well established that synaptotagmin 1 is the key sensor for evoked, synchronous neurotransmitter release in many classes of neurons (Fernández-Chacón et al. 2001). More recently, a key role for synaptotagmin 7 in neurotransmission has also been identified (Turecek and Regehr 2018) and synaptotagmin 2 has been shown to be a Ca2+ sensor in central inhibitory neurons (Chen et al. 2017a). Structure–function studies of synaptotagmin 1 based on expression of specific mutants have been carried out in mice, worms, and flies. For example, disruption of Ca2+ binding to the C2B domain of synaptotagmin 1 has been shown to have a more deleterious effect than disruption of Ca2+ binding to its C2A domain (Mackler et al. 2002; Robinson et al. 2002). The details of exactly how it triggers exocytosis and the function of other synaptotagmin isoforms remain to be fully resolved. Membrane fusion requires the pairing and interaction of so-called SNARE proteins on vesicle and target membranes (Söllner et al. 1993). These can assemble into a SNARE complex that may form the minimal fusion machinery. For synaptic vesicle and neuroendocrine exocytosis, the SNARE proteins are SNAP-25, syntaxin 1, and synaptobrevin. In the case of neurotransmitter release, vesicle fusion is tightly regulated and requires a Ca2+ signal for activation. Ca2+ entry through VGCCs leading to Ca2+ elevation in local microdomains close to the mouth of the Ca2+ channels is able to trigger rapid (<1 msec) fusion of synaptic vesicles. A synaptotagmin can bind to both syntaxin and SNAP-25, and fast neurotransmitter release requires synaptotagmin (Geppert et al. 1994), probably prebound to assembled or partially assembled SNARE complexes (Schiavo et al. 1997; Rickman et al. 2006), so that Ca2+-induced interaction with phospholipids can occur rapidly (Xue et al. 2008). It is still under debate how important synaptotagmin is in vesicle docking (de Wit et al. 2009; Chang et al. 2018) and how it acts at the plasma membrane in fusion itself (Fig. 2; Tang et al. 2006; Hui et al. 2009). A synaptotagmin could act as a brake on fusion that is relieved by Ca2+ binding or have a positive role in membrane fusion (Chicka et al. 2008). A recent focus has been on the combined role of synaptotagmin and another SNARE-interacting protein, complexin, in timing synaptic vesicle fusion (Südhof and Rothman 2009). The structure of a complex of synaptotagmin 1, complexin, and the SNAREs has been characterized (Zhou et al. 2017). It was suggested that this tripartite complex could be a primed structure at the site of vesicle docking that would then need to be disrupted to allow fusion to occur. It has also been suggested that oligomerization of synaptotagmin is essential to control spontaneous fusion (Bello et al. 2018), but much still remains to be learned about the molecular basis of its function (Bello et al. 2018; Kweon et al. 2018).
Figure 1.
Structures of the C2A and C2B domains of synaptotagmin 1. The structures show the isolated C2 domains in their Ca2+-loaded state with the bound Ca2+ ions shown in green. The coordinates for the structures for the C2A and C2B domains come from the Protein Data Bank (PDB) files 1BYN and 1K5W, respectively.
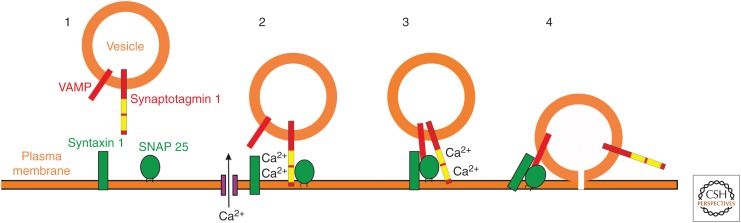
Figure 2.
Potential role of synaptotagmin 1 in synaptic vesicle exocytosis. Key components of the minimal fusion machinery are associated with the synaptic vesicle and the plasma membrane (1). Neurotransmitter release is triggered by Ca entry though voltage-gated calcium channels. Ca2+ binds to synaptotagmin 1, which may then lead to vesicle docking via interaction with phospholipids or with SNAP-25 on the plasma membrane (2). The SNARE complex assembles from the key components of VAMP, SNAP-25, and syntax and synaptotagmin associates with the complex (3). Through as-yet undefined steps synaptotagmin become dissociated from the SNARE complex and fusion of the vesicle with the plasma membrane occurs to allow release of neurotransmitter from the vesicle (4).
Synaptotagmins and Disease
The association of certain synaptotagmin isoforms with disease has been made. For example, synaptotagmin 7 has been implicated as a regulator of cancer cell proliferation (Wang et al. 2018b). In addition, synaptotagmin 11 has been identified as a Parkinson's disease risk gene and suggested to be involve in parkin-linked neurotoxicity in dopaminergic neurons (Wang et al. 2018a).
More significantly for central nervous system function and disease has been the discovery of de novo mutations in synaptotagmin 1 in patients associated with mental abnormalities. Synaptotagmin 1 has been shown to be essential for survival in model organisms but mutations that subtly change its function are not lethal (Chapman 2008). A rare variant in the gene SYT1 was identified in a subject with movement and cognitive disorders (Baker et al. 2015). Expression of a rat SYT1 with this mutation in hippocampal neurons in culture was found to impair exocytosis and endocytosis suggesting that it is indeed responsible for the mental abnormalities. A second missense mutation in SYT1 was later discovered (Cafiero et al. 2015). More recently, a series of further de novo mutations in SYT1 has been found in nine more patients with various neurodevelopmental and movement abnormalities (Baker et al. 2018). Five of the mutations were found in the C2B domain clustered around the Ca2+-binding pocket. The effect of these mutations was functionally characterized by expression of the mutated proteins in rat hippocampal cultures. While all the proteins were correctly targeted to synapses, one of these mutations impaired expression and the other four mutations resulted in differing defects in the rates of exocytosis and endocytosis that could in part be correlated with the disease phenotypes of the patients. These results support the idea that the SYT1 mutations were responsible for synaptic defects that resulted in the observed pathophysiology (Baker et al. 2018).
Interestingly, similar mutations to those found in the C2B domain of SYT1 had been identified in the C2B domain of SYT2 associated with Lambert–Eaton syndrome (Herrmann et al. 2014), which is the result of a defect at peripheral motor neurons. Electrophysiological analysis in patients indicated that the mutations resulted in a presynaptic defect (Whittaker et al. 2015). Further support for the significance of these mutations in SYT2 has come from functional studies in Drosophila (Shields et al. 2017). A potential linkage of SYT2 to defects of central nervous function comes from the observation that SYT2 protein levels were reduced in the brains of patients who had dementias (Bereczki et al. 2018).
CALMODULIN
The Physiology and Functions of Calmodulin
Calmodulin is a ubiquitously expressed 16.7 kDa Ca2+-binding protein playing a major role in regulating a wide variety of cellular events including motility, exocytosis, cytoskeletal assembly, muscle contraction, and modulation of intracellular Ca2+ concentrations. This protein has been highly conserved throughout evolution, is found in all eukaryotes and is 100% identical across all vertebrates at the amino acid level. Calmodulin can bind four Ca2+ ions through its four EF-hand structural motifs (Chattopadhyaya et al. 1992). The amino-terminal lobe of calmodulin is formed by the first two EF-hands, whereas the carboxy-terminal lobe is formed by the third and fourth EF-hands. The carboxy-terminal pair of EF-hands has a higher affinity for Ca2+ and slower binding kinetics than the amino-terminal pair, which allows the two domains to behave independently at varying Ca2+ concentrations (Tadross et al. 2008). The highly flexible linker between the two domains can alter confirmation dramatically upon binding to target proteins (Fig. 3) and is an essential property of calmodulin, which permits this protein to interact with a large and diverse array of partners. It has been recently demonstrated that calmodulin's bilobal architecture is essential for VGCC regulation (Banerjee et al. 2018). The significant conformational changes on binding to its targets (Fallon et al. 2005) can increase its affinity for Ca2+.
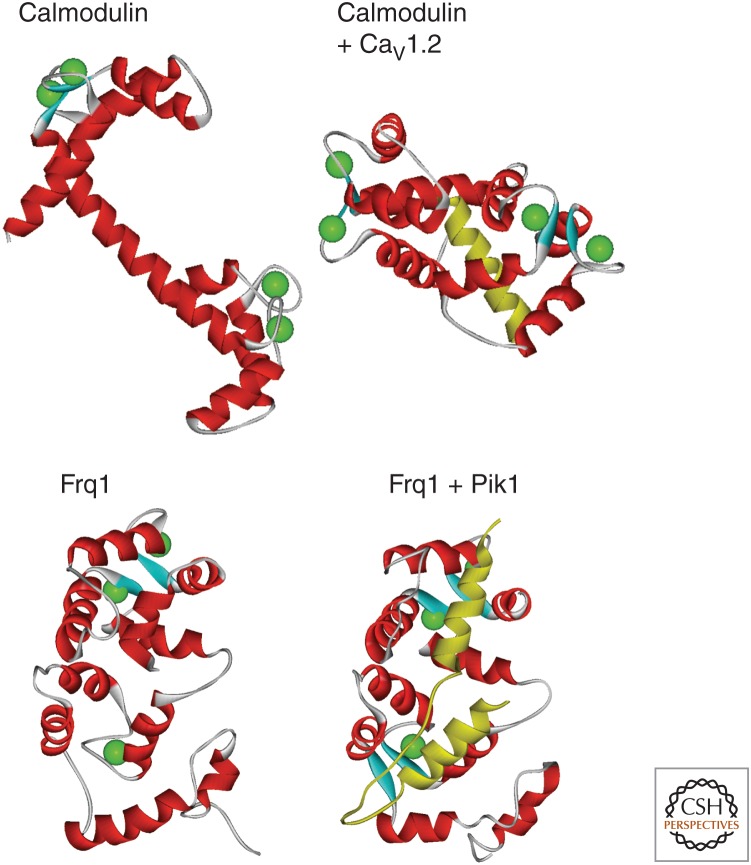
Figure 3.
Comparison of the structures of Ca2+-loaded calmodulin and yeast frequenin with and without bound target peptides. The structures at the top are of Ca2+-bound calmodulin alone (Protein Data Base [PDB] 1CLL) or in a complex with the IQ-like domain of the CaV1.2 Ca2+-channel α-subunit (PDB 2F3Z). The structures at the bottom are of the Ca2+-bound yeast frequenin (Frq1) alone (PDB 1FPW) or in a complex with the binding domain from Pik1 (PDB 2JU0). In each of the complexes the target peptide is shown in yellow.
Calmodulin is present in the brain at high concentrations (up to ∼100 µm). In addition to its more general functions, calmodulin also has a series of specific roles in transducing Ca2+ signals in neurons, including the regulation of glutamate receptors (O'Connor et al. 1999), ion channels (Saimi and Kung 2002), proteins in signaling pathways, such as neuronal nitric oxide synthase, and it can affect synaptic plasticity (Lisman et al. 2002; Xia and Storm 2005).
One key direct function of calmodulin is in regulating the activity of VGCCs by interacting with channel subunits (Catterall and Few 2008). As an example, Ca2+-free (apo) calmodulin can bind to the IQ domain of the α1 pore-forming subunit of the L-type Ca2+ channel CaV1.2 (Fig. 4; Erickson et al. 2001, 2003; Pitt et al. 2001). Prebound apo-calmodulin can then respond rapidly to Ca2+ elevation in local nanodomains and modulate the activity of the channel. Ca2+ binding to VGCC-associated calmodulin can have a range of effects on channel function, including mediating Ca2+-dependent facilitation (CDF) or Ca2+-dependent inactivation (CDI) (Peterson et al. 1999; Zühlke et al. 1999; Lee et al. 2000; DeMaria et al. 2001; Catterall and Few 2008; Liu et al. 2010). For CaV1.2 channels, CDI is mediated by the carboxy-terminal lobe of calmodulin (Peterson et al. 1999), whereas for CaV2.1 (DeMaria et al. 2001; Lee et al. 2003), CaV2.2 (Liang et al. 2003), and CaV2.3 (Liang et al. 2003), CDI is controlled by the amino-terminal lobe. Interestingly, for P/Q-type CaV2.1 channels, calmodulin is required for both CDI and CDF, with the amino-terminal lobe of calmodulin involved in CDI and the carboxy-terminal lobe underlying CDF (DeMaria et al. 2001; Lee et al. 2003). It is important to note that Ca2+-dependent regulation of VGCCs is complex and involves several Ca2+ sensor proteins as modulators. As an example, CaBP1 can elicit CDI, but also reduces CDF by displacing calmodulin from the IQ domain in CaV2.1 channels (Lee et al. 2002; Findeisen and Minor 2010; Christel and Lee 2012; Findeisen et al. 2013; Oz et al. 2013). In addition to calmodulin and CaBP1, it has been shown that visinin-like protein (VILIP)-2 inhibits calmodulin-mediated CDI and enhances CDF (Lautermilch et al. 2005; Nanou et al. 2012) through interaction with both the IQ and the calmodulin-binding domains. Calmodulin is also constitutively associated with, and regulates the opening of, Ca2+-activated potassium channels (Xia et al. 1998; Schumacher et al. 2001), and other types of potassium channels (Wen and Levitan 2002). SK and IK Ca2+-activated potassium channels lack Ca2+-binding sites but their intracellular carboxy-terminal region contains calmodulin-binding domains where calmodulin binds tightly and confers Ca2+-sensitivity to the channel. Two other major modes of action of calmodulin are exerted through Ca2+/calmodulin-dependent kinases (CaMKs) and calcineurin. CaMKs contribute to several regulatory pathways involving, for example, phosphorylation of AMPA receptors (Barria et al. 1997) and the nuclear transcription factor CREB (Deisseroth et al. 1998). Calmodulin also positively regulates presynaptic neurotransmitter vesicle release probability, which is mediated via activation of CaMKII (Pang et al. 2010). The Ca2+-activated phosphatase calcineurin can dephosphorylate a wide range of neuronal proteins leading to changes in gene transcription following activation of the transcription factor NFAT and its translocation into the nucleus. Calcineurin has also been implicated in synaptic plasticity (Malleret et al. 2001; Xia and Storm 2005).
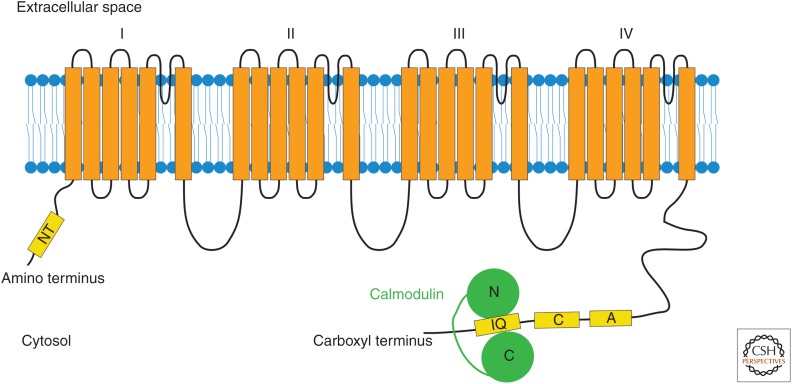
Figure 4.
Schematic illustration of the pore-forming a1 subunit of a CaV1.2 channel. The a1 subunit is composed of four domains (I–IV), each consisting of six putative transmembrane segments (orange). Several potential binding sites for Ca2+ sensors (yellow) have been identified in the amino-terminal (NT) and the carboxy-terminal region (A, C, and IQ) of CaV1.2 channels. Calmodulin (green), one of the major Ca2+ sensors, has been shown to be preassociated with the channel through its interaction with the IQ motif. Once calmodulin becomes Ca2+-loaded, it exerts its effects on channel function through either its amino or its carboxy lobe.
Calmodulin and Disease
The human genome contains three calmodulin genes (CALM1, CALM2, and CALM3) that encode for proteins with identical amino acid sequences. Despite the redundancy of calmodulin, single missense mutations, which change the way calmodulin functions, in any one of the six alleles are associated with disease phenotypes such as cardiac arrhythmia syndromes (Limpitikul et al. 2014; Makita et al. 2014; Yin et al. 2014; Boczek et al. 2016; Jiménez-Jáimez et al. 2016; Pipilas et al. 2016). In the brain, calmodulin dysfunction has also been suggested to be potentially linked to pathological conditions including epilepsy, memory loss, and intellectual disability. CaMKIIγ, a serine/threonine-specific protein kinase involved in long-term plasticity, learning, and memory, is a major target for calmodulin. A point mutation in γCaMKII (R292P) has been shown to interfere with calmodulin shuttling to the nucleus and therefore disrupted spatial learning, memory, and caused intellectual disability (de Ligt et al. 2012; Cohen et al. 2018). In another study, it has been shown that mutations in Kv7 potassium channels can decrease calmodulin binding, and thereby disrupt channel trafficking to the plasma membrane. As a result, neuronal excitability and firing frequency can be affected, leading to pathological conditions from mild epilepsy to early-onset encephalopathy (Alaimo et al. 2018). Similarly, mutations in NaV1.2 sodium channels can reduce calmodulin binding and lead to epilepsy (Yan et al. 2017). In addition, it has been experimentally verified that calmodulin is involved in the formation of amyloid-β plaques in Alzheimer's disease (O'Day et al. 2015). Altogether, these observations demonstrate the crucial role of calmodulin in regulating major signaling processes in neurons and show that mutations interfering with calmodulin binding or function can lead to serious neuropathological conditions.
NCS PROTEIN FAMILY
Although many aspects of neuronal function are known to be regulated by calmodulin, proteins related to calmodulin have been discovered in recent years, which are exclusively expressed or enriched in neurons. Duplication and diversification of the calmodulin gene family may have given rise to these NCS proteins, which are not all expressed in lower organisms, so that they can carry out neuronal functions specifically in higher organisms.
The Physiology and Function of NCS Proteins
Although calmodulin is ubiquitously expressed, the expression of other calcium-sensing proteins can be restricted to particular tissues and cell types. A good example of this is the NCS family of proteins, which are primarily expressed in neurons or retinal photoreceptors (Burgoyne 2007; Burgoyne and Haynes 2010). The NCS family of proteins are related in their protein sequence to calmodulin but have distinct properties, which allow them to carry out nonredundant roles that do not overlap with the functions of calmodulin (Fitzgerald et al. 2008). Members of the NCS protein family have been implicated, for example, in the regulation of neurotransmitter release, regulation of cell-surface receptors and ion channels, control of gene transcription (Carrión et al. 1999; Mellström and Naranjo 2001), cell growth and survival (Burgoyne 2007; Burgoyne and Haynes 2012), and specific retinal photoreceptor functions (Lim et al. 2014).
The NCS proteins are encoded by 14 genes in mammals, and with greater diversity from alternative splicing of transcripts from a number of the genes. All NCS gene products harbor four EF-hand motifs and display limited similarity (<20%) to calmodulin (Burgoyne 2004; Weiss et al. 2010). NCS-1 is the most widely expressed of the NCS proteins in and outside of the nervous system. The protein was first discovered as frequenin in Drosophila melanogaster (Pongs et al. 1993) where there are two very closely related genes known as frq1 and frq2 (Sanchez-Gracia et al. 2010). Although initially thought to be neuronal specific (Nef et al. 1995), an NCS-1 ortholog with 59% sequence identity and closely related structure has been identified in Saccharomyces cerevisiae (Hendricks et al. 1999), the lowest organism with an NCS-like sequence. After this first evolutionary appearance of NCS-1, there has been a steady increase in the diversity of the family throughout evolution, which roughly correlates with increasing organism complexity. Five classes of NCS proteins have now been identified in higher organisms (Braunewell and Gundelfinger 1999; Burgoyne 2007). Class A contains NCS-1, which is present in yeast and all higher organisms. Class B consists of the VILIPs, which appear first in Caenorhabditis elegans. Classes C and D evolved with the appearance of fish, and comprise recoverin and the guanylyl-cyclase-activating proteins (GCAPs), respectively. Finally, class E contains the K+ channel-interacting proteins (KChIPs), which are found in insects and evolutionary subsequent species (Burgoyne 2004). Mammals have a single NCS-1, five VILIPs proteins (hippocalcin, neurocalcin δ, and VILIPs1-3), a single recoverin, three GCAPs and four KChIPs. Expression of the recoverins and GCAPs is restricted to the retina, whereas the rest of the NCS family are found in varied neuronal populations (Burgoyne 2007). It has been established that certain neurons express several, or all, of the NCS proteins, but in general the expression profile for each of the NCS proteins is unique (Paterlini et al. 2000; Rhodes et al. 2004). This suggests that despite the high sequence homology between the proteins (∼35%–90% identity between each of the human family members, for example) (Burgoyne and Weiss 2001), each is likely to perform distinct functions in specific cell types (Burgoyne 2007).
Unlike calmodulin, not all EF-hands are functional in the NCS proteins, and the most amino-terminal EF-hand is unable to bind Ca2+ in any of the family members. In the case of recoverin and KChIP1, only two of its four EF-hand motifs are functional in Ca2+ binding (Burgoyne et al. 2004; Burgoyne 2007). Unlike the dumbbell structure of calmodulin, the NCS proteins are compact and globular when in their Ca2+-bound states, and they undergo limited conformational change following binding to their target proteins (Fig. 3; Ames et al. 2006; Pioletti et al. 2006; Strahl et al. 2007; Wang et al. 2007). NCS proteins also differ from calmodulin in that many have motifs that allow membrane association (McFerran et al. 1999; O'Callaghan and Burgoyne 2003, 2004; Haynes and Burgoyne 2008). KChIP1 and all the members of classes A–D are N-myristoylated, whereas certain KChIP2, KChIP3, and KChIP4 isoforms possess palmitoylation motifs. In some cases, the membrane association conferred by these moieties is dynamically regulated by Ca2+ binding when a sequestered myristoyl chain becomes exposed following a Ca2+-driven shift in conformation. This is known as the reversible Ca2+/myristoyl switch as originally described for recoverin (Ames et al. 1997). The VILIPs/neurocalcin/hippocalcin are also cytosolic at resting [Ca2+]i but localize to the plasma membrane or Golgi complex upon Ca2+ elevation (O'Callaghan et al. 2002, 2003b; Spilker et al. 2002). In contrast, NCS-1 does not show the Ca2+/myristoyl switch. Each of the NCS proteins displays distinct subcellular localizations, which are in part determined by additional interactions with specific phosphoinositides mediated by basic amino-terminal residues immediately proximal to the site of acylation (O'Callaghan et al. 2003a, 2005).
NCS-1 is a multifunctional regulator of various processes, and it has been intensively studied (Burgoyne 2004; Burgoyne and Haynes 2012). Mammalian NCS-1 is highly evolutionarily conserved, retaining 59% identity with its yeast ortholog. It displays a high Ca2+-binding affinity (Kd for Ca2+ ∼200–300 nm) and is able to respond to any fluctuations in [Ca2+]i above resting levels. NCS-1 is amino terminally myristoylated and is constitutively associated with membranes including plasma and Golgi membranes (O'Callaghan et al. 2002), although it is able to rapidly exchange between membrane and cytosolic pools (Handley et al. 2010). In contrast to all other NCS family members, NCS-1 is not neuron specific and is expressed in neuroendocrine cells (McFerran et al. 1998), and at low levels in several nonneuronal cell types (Gierke et al. 2004). NCS-1 has three functional EF-hand motifs, which have differing cation specificities for Ca2+ versus Mg2+. In the presence of elevated [Ca2+]i, EF2 and EF3 become Ca2+-occupied simultaneously followed by Ca2+ binding to EF4 (Aravind et al. 2008; Mikhaylova et al. 2009). Two variants of NCS-1 (frq1 and frq2) are expressed in Drosophila (Sanchez-Gracia et al. 2010) and may have distinct roles. A second human variant has been described but it is likely not to play any physiological role being expressed at only low levels (Wang et al. 2016).
Much of the current understanding concerning the function of NCS-1 derives from overexpression or knockout studies. Overexpression in Drosophila caused a frequency-dependent facilitation of neurotransmitter release (Pongs et al. 1993), and its importance for neurotransmissions has been confirmed by knockout of the two Drosophila frequenin genes (Dason et al. 2009). In Xenopus, overexpression caused enhanced spontaneous and evoked transmission at neuromuscular junctions (Olafsson et al. 1995). Consistent with a role of NCS-1 in neurotransmitter release, overexpression was found to increase Ca2+-dependent exocytosis of dense core granules in PC12 cells (McFerran et al. 1998), and to enhance associative learning and memory in C. elegans (Gomez et al. 2001).
Knockout of NCS-1 (frequenin) in the yeast S. cerevisiae is lethal because of its requirement for the activation of Pik1, one of the two yeast phosphatidylinositol-4 kinases (PI4Ks) (Hendricks et al. 1999). NCS-1 can also interact with the equivalent mammalian Golgi enzyme PI4KIIIβ and enhances its activity (Taverna et al. 2002; Haynes et al. 2005; de Barry et al. 2006). The interaction with Golgi-associated PI4KIIIβ suggests that it may regulate secretion through the modulation of phosphatidylinositol-dependent trafficking steps (Hendricks et al. 1999; Zhao et al. 2001; Haynes et al. 2005). In support of this, NCS-1 has also been demonstrated to associate with another PI4KIIIβ regulator ARF1, a small GTPase critical to multiple trafficking steps in mammalian cells (Haynes et al. 2005, 2007). The physiological significance of this interaction has been confirmed using genetic approaches in C. elegans. In this organism, knockout of NCS-1 impairs learning (Gomez et al. 2001) and affects temperature-dependent locomotion behavior (Martin et al. 2013). The role of NCS-1 in the control of temperature-dependent locomotion was shown to require interaction with ARF1.1 and also potentially pifk1 in the C. elegans ortholog of PI4KIIIβ (Todd et al. 2016).
Knockout of NCS-1 in organisms other than S. cerevisiae is not lethal but does generate specific developmental phenotypes. In Dictyostelium discoideum, loss of NCS-1 function alters developmental rate (Coukell et al. 2004), and in C. elegans results in impaired learning and memory (Gomez et al. 2001). Knockdown of one of the two NCS-1 genes in zebrafish, ncs-1, prevents formation of the semicircular canals of the inner ear (Blasiole et al. 2005). The signaling pathway involving NCS-1, ARF1, and PI4KIIIβ (Haynes et al. 2005) modulates the secretion of components important for the development of the vestibular apparatus of the inner ear (Petko et al. 2009). Knockdown of NCS-1, or expression of a dominant-negative inhibitor based on an EF-hand mutation (Weiss et al. 2000), disrupted the induction of LTD in rat cortical neurons (Jo et al. 2008). Overexpression of NCS-1 in adult mouse dentate gyrus promotes enhanced learning (Saab et al. 2009). Knockout of NCS-1 in mice was not lethal but caused behavioral changes and learning deficits (de Rezende et al. 2014; Nakamura et al. 2017).
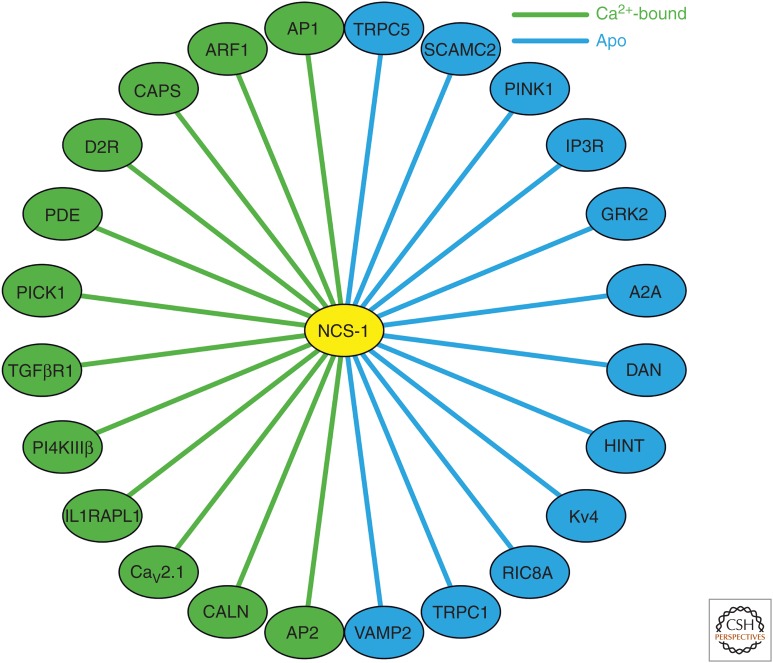
Many different specific binding partners (Fig. 5) have been identified for NCS-1, which interact with either the Ca2+-loaded or Ca2+-free (apo) forms of NCS-1 (Haynes et al. 2006, 2007). NCS-1 has a higher affinity for Ca2+ than calmodulin, and therefore may preferentially interact with certain Ca2+-dependent binding partners when the amplitude of a Ca2+ signal falls below the threshold for activation of calmodulin. For example, both calmodulin and NCS-1 have been shown to interact with, and desensitize, dopamine D2 receptors, but are likely to mediate their effects at different [Ca2+]i (Kabbani et al. 2002; Woods et al. 2008). Functional analyses have established that NCS-1 is a physiological regulator of D2 receptors (Saab et al. 2009; Dragicevic et al. 2014) (for clinical relevance of this regulation, see below). Other NCS-1 target proteins appear to be specific for NCS-1 (Haynes et al. 2006).
Figure 5.
Major known target proteins for neuronal calcium sensor (NCS)-1 indicating interactions that require either the Ca2+-bound or the apo form of NCS-1. The interactions shown include ones that are based on in vitro binding assays as well as interactions that have been substantiated and shown to have physiological relevance in functional studies.
Various studies have implicated NCS-1 in the regulation of VGCCs (Weiss et al. 2000; Weiss and Burgoyne 2001, 2002; Tsujimoto et al. 2002; Dason et al. 2009). In Drosophila, the effects of Frq1 on both neurotransmission and nerve-terminal growth can be explained by a functional interaction with the VGCC cacophony, which is related the mammalian P/Q-type VGCCs, but despite the physiological evidence a direct interaction of the proteins was not demonstrated (Dason et al. 2009). In contrast, a direct interaction of mammalian NCS-1 with the CaV2.1 of VGCCs was subsequently shown to occur (Lian et al. 2014), and this may be of physiological significance for the Ca2+-dependent regulation of these channels by NCS-1 underlying synaptic facilitation (Yan et al. 2014).
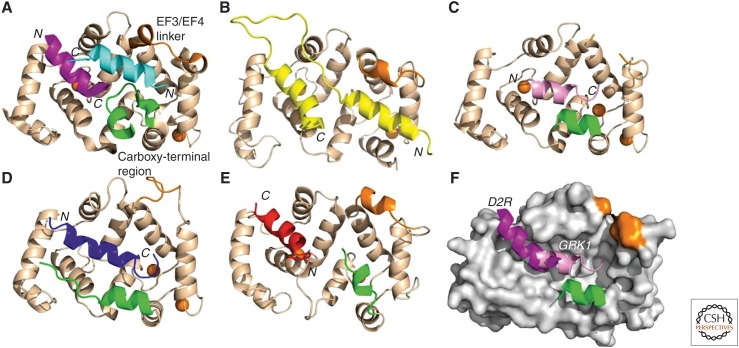
The structural basis of the interaction of NCS-1 with its target proteins has been well characterized. Key conserved residues within the hydrophobic groove that is exposed in the Ca2+-bound state have been shown to interact with target peptides in structural studies (Huttner et al. 2003; Ames and Lim 2012; Burgoyne and Haynes 2015). Moreover, functional mutagenesis studies in worms have confirmed the importance of these hydrophobic residues (Martin et al. 2013). Direct analysis has been made of NCS-1 interactions with two different target peptides based on structures solved in parallel by X-ray crystallography (Pandalaneni et al. 2015). Comparison to complexes involving other NCS proteins has shown structural differences in how these interactions occur. Figure 6 shows the structure of Ca2+-bound NCS-1 in complex with two peptides from the dopamine D2 receptor (Fig. 6A) or a single peptide from GRK1 (Fig. 6C; Pandalaneni et al. 2015). In this figure, NCS-1-target complexes are compared to earlier structures of S. cerevisiae frequenin in complex with two parts of a peptide from Pik1 (Fig. 6B; Strahl et al. 2007), of KChIP1 with a fragment of Kv4.3 (Fig. 6D; Pioletti et al. 2006), and of recoverin with one peptide from GRK1 (Fig. 6F; Ames et al. 2006). The two examined interactions for mammalian NCS-1, as in the other reported structures, involve residues within the exposed hydrophobic groove, although these differ for each target peptide. There are also differences in interaction with a mobile carboxy-terminal region of NCS-1, which can change its position in the complexes (Pandalaneni et al. 2015). The carboxy-terminal tail of NCS-1 appears to be crucial for the nature and regulation of target protein interactions being able to potentially occlude the hydrophobic groove for certain substrates (Handley et al. 2010; Lian et al. 2011; Heidarsson et al. 2012). There are also differences in how two NCS proteins (NCS-1 and recoverin) interact with the same target peptide from GRK1 (Ames et al. 2006; Pandalaneni et al. 2015).
Figure 6.
Comparison of the mode of binding of target peptides to neuronal calcium sensor (NCS)-1. Cartoon representation of the structures of (A) NCS-1 in complex with two molecules of D2R peptide (magenta and cyan) (PDB 5AER), (B) ScNcs1in complex with fragment of Pik1 (yellow) (2JU0) (45), (C) NCS-1 in complex with one molecule of GRK1 peptide (pink) (PDB 5AFP), (D) KChIP1 with a fragment of bound Kv4.3 (blue) (PDB 2I2R) (50), (E) recoverin bound to the amino terminus of GRK1 residues 1–25 with GRK1 peptide (red; PDB 2I94) (52), and (F) overlay of structures 5AER and 5AFP showing the locations of the D2R bound in the amino site and GRK1 peptides. The peptide orientations are indicated as N and C in italics and the orientations of the NCS protein are identical in all the structures. The EF3/EF4 linker is colored brown and the carboxy-terminal region green; for clarity, these regions are indicated only for the NCS-1-D2R peptide complex. In all the structures, Ca ions are shown as brown spheres. (From Pandalaneni et al. 2015; adapted, with permission, from the authors.)
Much less is known about the VILIP/neurocalcin/hippocalcin proteins, although the VILIPs themselves appear to modulate various signal transduction pathways such as cyclic nucleotide and MAPK signaling (Braunewell and Klein-Szanto 2009). VILIP-1 has been found to regulate a class of purinergic receptors (Chaumont et al. 2008). They have been shown to have effects on gene expression and are also involved in trafficking of proteins to the plasma membrane (Lin et al. 2002; Brackmann et al. 2005). VILIP-3 (HPCAL1) was found to control the differentiation of neuroblastoma cells through its interaction with the transcriptional regulator PHOX2B (Wang et al. 2014).
Hippocalcin has been suggested to be involved as a Ca2+ sensor in LTD in (Palmer et al. 2005; Jo et al. 2010). Consistent with this suggestion, it was observed that hippocalcin in cultured hippocampal neurons shows a rapid and reversible Ca2+/myristoyl switch for translocation in response to acute stimuli (Markova et al. 2008; Dovgan et al. 2010). Hippocalcin has also been implicated in protection from neuronal apoptosis (Mercer et al. 2000; Korhonen et al. 2005), and in promoting neuronal differentiation (Park et al. 2017).
Recoverin is expressed exclusively in the retina and is believed to have a role in light adaptation and can enhance visual sensitivity (Polans et al. 1996; Sampath et al. 2005; Morshedian et al. 2018). Recoverin is found primarily in rod and cone cells of the retina (Yamagata et al. 1990; Dizhoor et al. 1991). Recoverin was predicted to prolong the lifetime of photolyzed rhodopsin by inhibiting its phosphorylation by rhodopsin kinase to extend the light response (Chen et al. 1995; Klenchin et al. 1995). The function of recoverin has been controversial and this hypothesis may be oversimplified. Discrepancies have been noted regarding the [Ca2+]i required for rhodopsin kinase interaction, which may lie outside normal physiological limits, but analysis of recoverin knockout mice have shown changes in photoresponses consistent with a physiological role in inhibition of rhodopsin kinase (Makino et al. 2004).
The structure of recoverin has been extensively studied in its Ca2+-bound and Ca2+-free forms (Flaherty et al. 1993; Ames et al. 1995, 1997, 2002; Tanaka et al. 1995; Weiergräber et al. 2003). Recoverin is composed of two distinct domains connected through a linker and forms a compact structure in the absence of Ca2+. Unlike other NCS proteins, recoverin has just two functional EF-hand motifs. Upon binding of Ca2+, the amino-terminal domain comprising EF1 and EF2 rotates through 45° relative to the carboxy-terminal domain, driving extrusion of its buried myristoyl group. This permits recoverins to associate with membranes and reveals a hydrophobic surface, which can mediate interaction with the target protein rhodopsin kinase (Ames et al. 2006). The residues involved in the interaction of the myristoyl group with the hydrophobic pocket are also conserved in the other members of the NCS family. However, not all of the other family members display the Ca2+/myristoyl switch (O'Callaghan et al. 2002; Stephen et al. 2007). NCS-1 and KChIP1 expose a similar hydrophobic surface upon Ca2+ binding, which could be similarly important for target interactions (Bourne et al. 2001; Scannevin et al. 2004; Zhou et al. 2004b; Pioletti et al. 2006; Pandalaneni et al. 2015). In contrast, other NCS proteins are able to interact with certain binding proteins in their Ca2+-free state, therefore Ca2+-driven exposure of a hydrophobic surface cannot be the sole mechanism by which these proteins bind to effectors.
GCAPs are activators of retinal guanylyl cyclases (GCs) (Palczewski et al. 2004) and are known to be physiological regulators of light adaptation (Mendez et al. 2001; Burns et al. 2002; Howes et al. 2002; Pennesi et al. 2003; Vinberg et al. 2018). They show the unusual property of activating GCs when in their Ca2+-free form but become inhibitors of GCs at higher Ca2+ concentrations (Dizhoor and Hurley 1996). GCAP3 is expressed in cone cells, whereas GCAP1 and GCAP2 are expressed in rod cells. Although GCAP1 and GCAP2 have the same function in the same cell type, the two proteins have different Ca2+-binding affinities for GC activation. This means that both proteins are required for GC activation over the full physiological Ca2+ concentration range, maximizing the dynamic range of GC activity (Koch 2006). The GCAPs are an example of how Ca2+ sensors have become adapted to increase dynamic Ca2+ sensitivity of regulatory mechanisms (Palczewski et al. 2004; Lim et al. 2014; Koch and Dell'Orco 2015).
Four KChIP genes and a large number of splice variants are expressed in mammals (Pruunsild and Timmusk 2005). KChIPs were so named as they were found to associate with transient voltage-gated potassium channels of the Kv4 family (An et al. 2000; Bähring 2018). The majority of the KChIPs can stimulate the trafficking of Kv4 channels to the plasma membrane (O'Callaghan et al. 2003a; Shibata et al. 2003; Hasdemir et al. 2005; Prechtel et al. 2018). Certain KChIP isoforms, in contrast, inhibit the trafficking of Kv4 channels (Jerng and Pfaffinger 2008). In addition, expression of KChIPs regulates the gating kinetics of Kv4 channels while acting as channel subunits (An et al. 2000; Bähring 2018) and can do so in response to Ca2+ (Groen and Bähring 2017; Bähring 2018). Knockout of KChIP1 has revealed a potential role in the GABAergic inhibitory system (Xiong et al. 2009). The KChIPs are expressed predominantly in the brain but KChIP2 is also expressed in the heart and knockout of KChIP2 causes a complete loss of calcium-dependent transient outward potassium currents and susceptibility to ventricular tachycardia (Kuo et al. 2001). KChIP3 is also known as DREAM or calsenilin, and has documented roles in transcriptional regulation (Carrión et al. 1999; Mellström and Naranjo 2001) and in the processing of presenilins and amyloid precursor protein, which are important in the pathogenesis of Alzheimer's disease (Buxbaum et al. 1998; Jo et al. 2004). Although many of the KChIPs and their isoforms may have overlapping functions, some differences between them have emerged (Holmqvist et al. 2002; Venn et al. 2008).
Despite KChIP3 being implicated in three quite distinct functions, it is likely that they are all physiologically relevant. KChIP3 knockout mice show reduced responses in acute pain models because of changes in prodynorphin synthesis (Cheng et al. 2002), decreased β-amyloid production, and physiological defects consistent with changes to the Kv4 channels (Lilliehook et al. 2003). DREAM/KChIP3/calsenilin has been found to interact with a wide range of target proteins (Rivas et al. 2011). Recently, functional effects for DREAM/KChIP3/calsenilin have been reported for the regulation of ryanodine receptors (Grillo et al. 2019), and via an interaction with RhoA on neurite growth (Kim et al. 2018).
The NCS protein family has evolved to carry out specialized neuronal functions separate from those of calmodulin. Of relevance is their approximately 10-fold higher affinity for Ca2+ when compared to calmodulin. The higher affinity allows the NCS proteins to be activated at lower Ca2+ concentrations and, in combination with calmodulin, extends the dynamic range over which Ca2+ can regulate neuronal processes. In this way, the response of a cell to changes in [Ca2+]i of different amplitude or kinetics would depend on which populations of Ca2+-binding proteins are activated under particular conditions. The individual expression patterns and subcellular localization of each of the NCS proteins will also determine their specific roles in neuronal cell signaling. The characteristic amino-terminal myristoylation or palmitoylation modifications that allow these proteins to associate with membranes may spatially partition them to distinct subcellular sites within the cell, leading to a faster and more efficient response to particular Ca2+ signals. Specific physiological outcomes will also be determined by their distinct target proteins (Burgoyne and Haynes 2015). The various members of the NCS family arose at points in evolution corresponding to increasing neuronal sophistication in higher animals. As such, these proteins represent an example of how the properties of CaBPs have been fine-tuned to act in specific neuronal signaling pathways.
NCS Proteins and Disease
In support of key roles for the NCS family in higher organisms, a number of studies have implicated these proteins in the pathological progression of human neurological diseases. Some evidence suggests indirect links with Alzheimer's disease, but evidence has emerged for more direct involvement based on key physiological interactions and also on identification of human genetic variants.
NCS-1 has been suggested to interact directly with and enhance the activity of inositol 1,4,5-trisphosphate receptors (IP3Rs) (Schlecker et al. 2006; Nakamura et al. 2011), although this has not been seen in all studies (Haynes et al. 2004). It has been suggested that the regulation of IP3Rs by NCS-1 and changes in Ca2+ signaling (Boehmerle et al. 2006) may underlie a potential role of NCS-1 peripheral neuropathy (Mo et al. 2012), and also in tumor progression where it could be a therapeutic target (Moore et al. 2017; Boeckel and Ehrlich 2018).
Two other documented NCS-1 interactions are of possible significance for neuronal dysfunction. The importance of the regulation of dopamine D2 receptors by NCS-1, whereby NCS-1 inhibits receptor internalization (Kabbani et al. 2002), comes from the fact that dopamine is of key importance for signaling within the CNS and in addictive behavior (Koob 2006; Dagher and Robbins 2009). Regulation of D2 receptors by NCS-1 underlies the effect of overexpression of NCS-1 on spatial memory acquisition (Saab et al. 2009). Dopamine D2 receptors are the targets for all known effective antipsychotic drugs (Seeman 1992), and NCS-1 is up-regulated in patients with bipolar disorder or schizophrenia (Koh et al. 2003) and in response to antipsychotic drugs (Kabbani and Levenson 2006). NCS-1 is genetically associated with cocaine addiction (Multani et al. 2012), which is believed to be linked to the effects of cocaine on dopamine transporters (Ritz et al. 1987). It has also been suggested that NCS-1 may be linked to the effects of lithium on bipolar disorders (D'Onofrio et al. 2015, 2017a,b).
NCS-1 has been shown to be required for an adaptive response to dopaminergic agonists in substantia nigra neurons. Coupled with its up-regulation in the substantia nigra from Parkinson's disease patients, this suggests that it could be a target for modifying the vulnerability of neurons in the substantia nigra to neurodegeneration (Dragicevic et al. 2014; Poetschke et al. 2015; Duda et al. 2016). The binding of NCS-1 to the D2 receptor involves the short cytoplasmic carboxy-terminal domain of the receptor (Kabbani et al. 2002). This interaction has been partially characterized using structural approaches (Lian et al. 2011; Pandalaneni et al. 2015) and this may allow exploration of the interaction as a therapeutic drug target (Kabbani et al. 2012).
Another clinically important interaction is with the interleukin 1 receptor accessory protein-like 1 protein (IL1RAPL1), which appears to be specific for NCS-1 (Bahi et al. 2003). Mutations in IL1RAPL1 have been shown to result in X-linked mental retardation (Zhang et al. 2004; Tabolacci et al. 2006), and also have been linked to autism spectrum disorder (ASD) (Piton et al. 2008). Knockout of IL1RAPL1 in mice leads to neurodevelopmental and learning abnormalities (Montani et al. 2017). Effects of IL1RAPL1 on exocytosis (Bahi et al. 2003), channel regulation, and neurite growth (Gambino et al. 2007) appear to be mediated via NCS-1.
Interestingly, the study on IL1RAPL1 in ASD also identified a mutation (R120Q) within NCS-1 in an individual with ASD (Piton et al. 2008). This mutation was found to cause a functional deficit in NCS-1 (Handley et al. 2010) that appeared to be related to a change in the structural dynamics of the carboxyl terminus of the protein (Handley et al. 2010) and overall structural flexibility (Zhu et al. 2014). However, the physiological relevance of this mutation and its exact relationship to the disease phenotype remains to be established.
A second almost identical homolog of frequenin expressed in Drosophila (Frq2) and human NCS-1 are able to interact with the guanine nucleotide exchange factor Ric8a and this interaction was shown to be physiologically relevant for development and neurotransmission in flies (Romero-Pozuelo et al. 2014). Characterization of the structural basis for this interaction identified an interface that was used to examine potential therapeutic compounds that could prevent complex formation (Mansilla et al. 2017). As proof of principle, the authors showed that a potential therapeutic compound could alleviate the symptoms of fragile X syndrome in a fly model. These studies demonstrate the potential for such structural approaches to generate leads for new therapeutics that could be used in NCS-1-related pathologies (Roca et al. 2018).
The neurodegenerative disease known as Wolfram syndrome is caused by loss of function of the endoplasmic reticulum (ER) protein WF1. The molecular basis of the disorder appears to be because of a loss of coupling between mitochondria and the ER that is required for transfer of Ca2+ to the mitochondria (Angebault et al. 2018). This, in turn, leads to mitochondrial dysfunction and potential cell death. In exploring this mechanism further, it was discovered that WF1 intersects directly with NCS-1. Moreover, overexpression of NCS-1 was able to compensate for the loss of WF1 function in fibroblasts from Wolfram syndrome patients (Angebault et al. 2018), suggesting that NCS-1 may be a useful target for development of new therapeutic approaches.
VILIP1 has been suggested to have a role in Alzheimer's disease because of an association with amyloid plaques in diseased brains (Schnurra et al. 2001). It is not clear, however, whether there is any causal relationship, but VILIP1 in cerebrospinal fluid has been widely studied as an early-stage biomarker of Alzheimer's disease (Braunewell 2012; Groblewska et al. 2015; Babić Leko et al. 2016; Kirkwood et al. 2016).
A more direct involvement of neurocalcin δ in neuronal disease has come from a study (Riessland et al. 2017) showing that knockdown of this protein results in protective effects in various models of spinal muscular atrophy across a number of species. Neurocalcin δ had previously been found to interact with clathrin (Ivings et al. 2002), and the protective effects of neurocalcin δ were attributed to a consequence of the loss of neurocalcin δ as a negative regulator of endocytosis. Neurocalcin δ could, therefore, be a potential therapeutic target by inhibitors of its activity (Riessland et al. 2017).
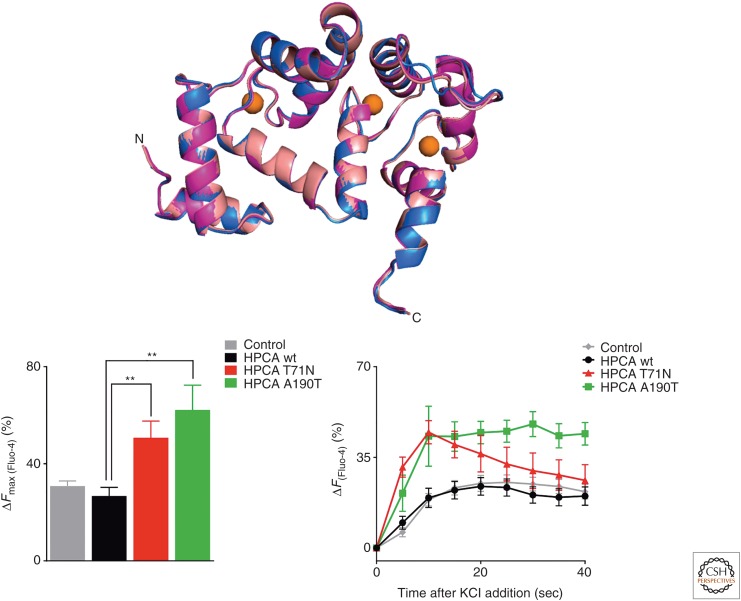
Dystonia is an early-onset movement disorder, which can be inherited in an autosomal-dominant manner linked to a defined set of genes or alternatively in an autosomal-recessive manner (the latter is classified as DYT2 dystonia). Missense mutations in hippocalcin were found in subjects with DYT2-like dystonia (Charlesworth et al. 2015). It was suggested that the mutations could have disrupted hippocalcin's role in neuronal calcium signaling. Direct examination of the effect of dystonia mutations on the physiological function of hippocalcin showed that the mutations did not affect the protein structure but resulted in an oligomerization defect (Helassa et al. 2017). It is noteworthy that for another NCS protein, namely GCAP1, dimerization has been suggested to be functionally important (Ames 2018; Lim et al. 2018). In addition, an increase in Ca2+ influx through VGCCs of the CaV2 type in cells expressing the mutants was observed compared to wild-type hippocalcin, thus suggesting a key role for perturbed Ca2+ homeostasis in DYT2 dystonia because of the missense mutations (Fig. 7). The existence of other mutations that would produce truncated proteins have subsequently been discovered in two dystonia families (Atasu et al. 2018) further substantiating the link between hippocalcin and DYT2 dystonia.
Figure 7.
Effect of dystonia mutations on the structure of hippocalcin and its effect on calcium entry. (Top) Alignment of hippocalcin crystal structure (magenta) with hippocalcin (T71N) (marine) and hippocalcin (A190T) (salmon) did not show any significant difference. Crystal structures were obtained for wild-type (wt) human hippocalcin (PDB 5G4P), hippocalcin (T71N) (PDB 5M6C), and hippocalcin (A190T) (PDB 5G58) at a resolution of the 2.42, 3.00, and 2.54 Å, respectively (Helassa et al. 2017). (Bottom) Dystonia-causing hippocalcin mutants increase depolarization-induced calcium influx. Differentiated SH-SY5Y cells transfected with hippocalcin-mCherry constructs were loaded with Fluo-4 to monitor calcium concentration changes. After KCl depolarization, live cells were imaged on a spinning-disk confocal microscope. Maximum intracellular calcium increase and time course after KCl stimulation, showing that both hippocalcin (T71N) and hippocalcin (A190T) increased calcium entry in response to depolarization. (From Helassa et al. 2017; adapted under the terms of the Creative Commons CC BY license, which permits unrestricted use, distribution, and reproduction in any medium.)
The retinally expressed NCS proteins, GCAPs, have known mutations that have been shown to result in retinal dystrophies and retinal degeneration (Behnen et al. 2010; Dell'Orco et al. 2010), consistent with their key functions in photoreceptors. For GCAP1, which is encoded by the human gene GUCA1A, nearly 20-point mutations have been identified in patients with autosomal-dominant retinal dystrophy, leading to the suggestion that photoreceptor death is linked to an abnormality in calcium signaling. A recently identified mutation in GCAP1 (E111V) has been characterized biochemically and shown to decrease GCAP1's affinity for calcium, and thereby shifts its regulation of GCs out of the physiological range of calcium concentration (Marino et al. 2018). In addition, many mutations are known in the photoreceptor guanylate cyclase GUCY2D, the target for GCAPs, which result in retinal dystrophies. Characterization of the effects of some of these mutations has indicated that their defects are in the Ca2+-dependent regulation of their catalytic activity by GCAPs (Wimberg et al. 2018).
The idea that KChIPs may contribute to neuronal disease first arose when calsenilin/KChIP3 was discovered as an interactor with presenilins, and to regulate the processing of presenilins, which suggested a link to the pathogenesis of Alzheimer's disease (Buxbaum et al. 1998; Jo et al. 2004). KChIP1 has been implicated in changes in behavioral anxiety in knockout mice (Xia et al. 2010) and a human genetic variant associated with attention deficit disorder (Yuan et al. 2017). KChIPs have also been shown to be involved in pain control (Jin et al. 2012; Kuo et al. 2017; Tian et al. 2018). More recently, DREAM has been shown to regulate the onset of cognitive decline in a mouse model of Huntington's disease (López-Hurtado et al. 2018). The KChIPs may become targets for therapeutics that could be used for several types of neurological disorders. A major challenge will to be develop drugs that target specific KChIP isoforms.
CaBP PROTEIN FAMILY
The Physiology and Function of CaBPs
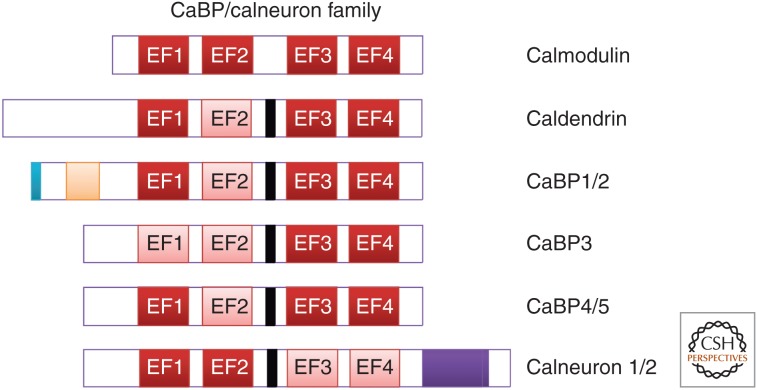
The CaBPs are a family of EF-hand-containing Ca2+-binding proteins, which are only found in vertebrates (Haeseleer et al. 2000), and appear to have arisen together in evolution as a family of genes (Fig. 8; McCue et al. 2010a). They represent another example of a diverse family of Ca2+-sensors capable of regulating discrete processes in the nervous systems of higher organisms. The CaBPs share sequence homology with calmodulin, and also display a similar structural arrangement of EF-hand motifs. Each of the CaBPs has four EF-hands although, like the NCS proteins, they display different patterns of EF-hand inactivation (Fig. 8). In CaBP1-5, the second EF-hand is inactive, with the exception of CaBP3, which also has an inactive EF-1 (although CaBP3 is believed to represent a pseudogene; Haeseleer et al. 2000). Two proteins were named CaBP7 and CaBP8 (Haeseleer et al. 2002), but bioinformatic analysis is more consistent with them being a conserved and distinct subfamily of CaBPs (McCue et al. 2010a). We will, therefore, refer to them by their alternative names calneuron 2 and calneuron 1, respectively (Wu et al. 2001; Mikhaylova et al. 2006). The calneurons, in contrast to the CaBPs, have a different pattern of EF-hand inactivation with active EF-hands 1 and 2 and inactive EF-hands 3 and 4 (Mikhaylova et al. 2006). The CaBPs also differ from calmodulin in that their central α helical linker domain connecting the carboxy- and amino-terminal EF-hand pairs is extended by four amino acid residues. This has been suggested to allow these proteins to interact with unique targets (Haeseleer et al. 2000). Calneuron 1 has been shown to be expressed in essentially all rat and human brain regions (Hradsky et al. 2015).
Figure 8.
Schematic diagram showing the domain structure of calmodulin and members of the CaBP/calneuron protein family. Active EF-hand motifs are shown in red and inactive EF-hand motifs are shown in pink. Compared to calmodulin the calcium-binding proteins (CaBPs) have an extended linker region between their first EF-hand pair and their second EF-hand pair (shown in black). CaBP1 and CaBP2 have an N-myristoylation site (shown in blue). CaBP1 and CaBP2 have alternative splice sites at their amino terminus, which give rise to long and short isoforms (shown in orange). Calneurons 1 and 2 possess a 38 amino acid extension at their carboxyl terminus (shown in purple).
A major difference compared with calmodulin is the ability of CaBP 1 CaBP2, calneurons 1 and calneuron 2 to target to specific cellular membranes (McCue et al. 2009). CaBP1 and CaBP2 are amino terminally myristoylated, which allows localization to the plasma membrane and Golgi apparatus (Haeseleer et al. 2000; Haynes et al. 2004). The precise amino-terminal sequence to which the myristoyl group is attached is also important in the targeting of these two proteins, as exemplified by the long and short splice isoforms, which show subtle differences in their localization. CaBP1-Long localizes predominantly to the Golgi and also displays some cytosolic localization, whereas CaBP1-Short localizes most prominently to the plasma membrane and to Golgi structures (Haeseleer et al. 2000; McCue et al. 2009). Alternative splicing of the CaBP1 gene generates a third protein product, caldendrin (Seidenbecher et al. 1998). This splice isoform is significantly larger than either CaBP1-Long or CaBP1-Short because of an amino-terminal extension, but caldendrin messenger RNA (mRNA) lacks the exon required for N-myristoylation and as a result the protein displays a markedly different subcellular localization to its shorter relatives.
Amino-terminal acylation is important in the localization of some CaBPs, but the calneurons appear to be targeted via a different mechanism. Like CaBP1 and CaBP2, calneuron 1 and calneuron 2 localize to internal membranes that colabel with Golgi specific markers and to vesicular structures (McCue et al. 2009; Mikhaylova et al. 2009). Calneuron 1 and calneuron 2 do not possess an amino-terminal myristoylation motif and differ from the rest of the CaBP family because of a 38 amino acid extension at their carboxyl terminus. Analysis of this sequence revealed a predicted carboxy-terminal transmembrane domain with a cytosolic amino terminus (McCue et al. 2011). The carboxy-terminal domain resembles tail-anchor motifs and directly localizes calneuron 1 and calneuron 2 to membranes particularly of the trans-Golgi network (TGN) (McCue et al. 2009; Hradsky et al. 2011).
To date, only a limited number of CaBP structures have been solved. These include CaBP1-Short (Wingard et al. 2005; Li et al. 2009; Findeisen and Minor 2010), CaBP4 (Park et al. 2014a,b; PDB codes: 2M28 [Ca2+-bound carboxy lobe]), 2M29 (Ca2+-bound amino lobe), and calneuron 2 (McCue et al. 2012). This information may provide insight into the structures of the rest of the CaBPs. Analogy to calmodulin would suggest that the CaBPs should adopt a dumbbell-like tertiary conformation consisting of an amino-terminal domain–containing EF-hand 1 and EF-hand 2, and a carboxy-terminal domain–containing EF-hand 3 and EF-hand 4 connected by a central linker. Nuclear magnetic resonance (NMR) analysis revealed that CaBP1 does indeed have two independent, noninteracting domains joined by a flexible linker (Wingard et al. 2005). The NMR structures of CaBP4 and calneuron 2 are of either the isolated amino- or carboxy-terminal domains, although in every instance these structures resemble compact, independently folded, helix-loop-helix arrangements very much like those observed in CaBP1 and calmodulin. Investigation into the effects of Mg2+ and Ca2+ binding has shown that, as predicted, the second EF-hand of CaBP1 is incapable of binding divalent cations. EF-hand 3 and EF-hand 4 bind to both Mg2+ and to Ca2+, whereas EF-hand 1 is thought to be constitutively occupied by Mg2+ (Wingard et al. 2005; Li et al. 2009). The Mg2+-bound form of CaBP1 is similar to that of apo-calmodulin but the Ca2+-bound form appears markedly different. This is perhaps unsurprising as neither of the amino-terminal EF-hands of CaBP1 bind to Ca2+ under saturating conditions and only EF-hand 1 binds to Mg2+. This results in a constitutively closed conformation of the amino-terminal domain, whereas the carboxy-terminal domain can switch to an open conformation upon Ca2+ binding to EF-hand 3 and EF-hand 4. Comparison of the carboxy-terminal domain with that of calmodulin reveals differences in exposed hydrophobic residues thought to mediate target interactions (Wingard et al. 2005).
The structural differences between calmodulin and CaBP1 may go some way to explaining how they mediate differing effects on the same target molecules. For instance, both CaBP1 and calmodulin bind to CaV1 VGCCs with calmodulin causing Ca2+-induced channel closure, but CaBP1 promotes channel opening (Zhou et al. 2004a, 2005).
Both calmodulin and CaBP1 also regulate IP3Rs (Yang et al. 2002; Haynes et al. 2004; Kasri et al. 2004) with CaBP1 binding the type I IP3R with 100-fold higher affinity than calmodulin. This high-affinity binding may result from the exposure of a distinct hydrophobic patch revealed in the carboxyl terminus of CaBP1 upon Ca2+ binding (Haynes et al. 2004; Li et al. 2009). This unique surface hydrophobicity profile is likely to be important for the specialization of the CaBP1 function in the brain and retina, and the existence of splice isoforms is also likely to further fine-tune the actions of this Ca2+ sensor. The higher affinity of CaBPs compared with calmodulin for the same target has led to the notion that the mechanism by which CaBPs differentially regulate such targets is through competition for binding to the same regulatory sites. It has been suggested that calmodulin, in spite of being present in a large molar excess over the various CaBPs, can be displaced from a target because of its lower affinity. Much of the experimental data leading to these conclusions has been derived from in vitro studies examining short binding motifs from a given effector protein (Kim et al. 2004; Zhou et al. 2005; Oz et al. 2011; Findeisen et al. 2013). To assess the situation in an intact system, an elegant study by Yang and coworkers (2014) tested Ca2+ channel regulation by calmodulin and CaBP4 in live cells. It was determined that in addition to a degree of competition for binding to the same target sites in CaV1.3 channels by calmodulin and CaBP4, an allosteric mechanism was also likely to exist whereby both proteins could simultaneously associate with the channel. This dual binding mode could potentially be favored in resting conditions where apo-calmodulin has high affinity for the channel IQ motif. CaBP4 would simultaneously be associated with a distinct binding motif on the channel. Upon an increase in [Ca2+]i, a competitive interaction is unmasked by the higher affinity of CaBP4 for the calmodulin-binding site on the channel. This model accurately predicts the observed experimental data and loss of Ca2+-dependent inactivation of CaV1.3 in the presence of CaBP4 and Ca2+ even when physiological levels of calmodulin are present (at least a 10-fold excess of calmodulin over CaBP4). It seems certain that for other effector proteins, including various VGCCs and ligand-gated Ca2+ channels, dual regulation by calmodulin and the CaBPs will represent a combination of allostery and direct competition.
In addition to novel modes of target interaction as discussed above, differing expression patterns, subcellular targeting mechanisms, and Ca2+-binding properties of the various members of the CaBP protein family likely bestow further specialization in the regulation of important Ca2+-channels in the central nervous system.
The majority of studies to date on CaBP1 have examined the functions of the longest splice isoform, caldendrin, and it is not yet clear whether the other splice isoforms of CaBP1 can carry out the same functions. Indeed, detection of CaBP1-Long and CaBP1-Short proteins in rodent brain has proven elusive (Kim et al. 2014; Reddy et al. 2014), and it would appear that caldendrin is expressed at significantly higher levels. In spite of this, in simplified experimental systems, CaBP1-Long and CaBP1-Short have been found to have roles in the regulation of various Ca2+channels, including P/Q-type (CaV2.1) channels (Lee et al. 2002), L-type (CaV1.2) channels (Zhou et al. 2005; Cui et al. 2007), TRPC5 channels (Kinoshita-Kawada et al. 2005), and IP3Rs (Yang et al. 2002), which they inhibit (Haynes et al. 2004; Kasri et al. 2004). A structural basis for the inhibition of IP3Rs has been determined, whereby CaBP1 binding locks the receptor and prevents the intersubunit motion required for initiation of channel opening (Li et al. 2013). The interaction of CaV2.1 with CaBP1 appears to rely acutely upon amino-terminal myristoylation. Wild-type, myristoylated, CaBP1-Long enhances channel inactivation and shifts the activation range to more depolarizing voltages (Lee et al. 2002). An N-myristoylation mutant, however, was unable to mediate these effects, and instead modulated channels in a similar fashion to calmodulin (Few et al. 2005). Differential modulation of L-type channels depending on the splice isoform of CaBP1 has also been observed. CaBP1-Short has been shown to completely inhibit inactivation of CaV1.2 channels (Zhou et al. 2005), but caldendrin causes a more modest suppression and signals through a different set of molecular determinants (Tippens and Lee 2007). This suggests that the subcellular localization of CaBP1 splice variants is important for their differing functions. In the auditory system, CaBP1 is expressed in spiral ganglion neurons (Yang et al. 2016), and CaBP1 knockout mice exhibit progressive hearing loss, albeit less severely than that observed in CaBP2 knockout animals (Yang et al. 2016). In the visual system, CaBP1 appears to exert similar functions to CaBP2 (Sinha et al. 2016), and loss of the CaBP1 proteins induces defects in transmission of light responses by the retina. It should be noted that a number of these studies have used knockout animals that do not express any of the CaBP1 isoforms and therefore assigning a function to a specific splice variant is not possible.
Interactions of caldendrin with other types of proteins have also been reported, such as its interaction with light chain 3 of MAP1A/B, a microtubule cytoskeletal protein (Seidenbecher et al. 2004), and with myo1c a member of the myosin-1 family of motor proteins (Tang et al. 2007). A role for caldendrin in NMDAR signaling has been reported involving an interaction with a novel neuronal protein, Jacob. Upon extrasynaptic NMDAR activation, Jacob translocates to the nucleus to influence CREB activity, resulting in the stripping of synaptic contacts and an associated simplification of dendritic architecture. Synaptic NMDAR-mediated synaptodendritic [Ca2+]i elevation induces caldendrin binding to Jacob, thereby inhibiting nuclear trafficking and maintaining dendritic organization. This interaction represents a novel mechanism of synapse to nucleus communication and highlights the important roles of CaBP family members in the mammalian central nervous system (Dieterich et al. 2008). Finally, caldendrin has recently been shown to control actin remodeling in dendritic spines in response to synaptic activity (Mikhaylova et al. 2018). Animals in which the caldendrin gene has been deleted exhibited impaired dendritic spine plasticity, defective LTP and impaired hippocampus-dependent learning (Mikhaylova et al. 2018). These findings are consistent with related studies highlighting a function for caldendrin in the hippocampus. It is required for efficient encoding of hippocampal-dependent spatial and fear-based memory (Yang et al. 2018a), and mice in which all CaBP1 isoforms are deleted, including caldendrin, exhibit defects in excitation/inhibition in hippocampal circuits (Nanou et al. 2018; Yang et al. 2018b).
Little information is available concerning the function of CaBP2. Although it was initially detected exclusively in the retina, it was also identified in auditory inner hair cells (Cui et al. 2007). CaBP5 was also detected in inner hair cells as well as in the retina, but in contrast to CaBP2 was found to have a modest inhibitory effect on the inactivation of CaV1.3 channels in transfected cells (Cui et al. 2007). Newer research points to an important role for CaBP2 in both the visual and auditory systems. Mice lacking CaBP2 have no gross morphological defects of the retina or retinal neuronal wiring; however, they do exhibit impaired transmission of retinal light responses (Sinha et al. 2016). CaBP2 is now known to be expressed in the cochlea in both inner and outer hair cells, and gene deletion of all CaBP2 splice variants leads to early-onset hearing loss (Yang et al. 2016, 2018c). Some of the functions of CaBP2 may stem from its ability to stimulate CaMK activity, although this has only been reported in vitro (Cui et al. 2007). Little is known about the functions of CaBP5 but knockout mice displayed reduced sensitivity of retinal ganglion cells to light responses implicating CaBP5 in phototransduction pathways. CaBP5 was also found to interact with, and suppress, calcium-dependent inactivation of CaV1.2 channels (Rieke et al. 2008). One report has detailed an interaction of CaBP5 with components of the exocytotic machinery, and showed that expression of CaBP5 in a neuroendocrine cell line enhanced secretory granule exocytosis (Sokal and Haeseleer 2011). These data implicate CaBP5 as a potential regulator of visual and auditory processing, perhaps through modulation of neurotransmitter release in special sensory neurons.
CaBP4 is the most extensively characterized of the CaBP family. It is expressed in the retina where it localizes to synaptic terminals and has also been detected in auditory inner hair cells. CaBP4 modulates VGCCs, and directly associates with the carboxyl terminus of the CaV1.4 α1 pore-forming subunit, shifting the activation range of the channel to more hyperpolarized voltages in transfected cells (Haeseleer et al. 2004; Shaltiel et al. 2012). A plausible structural basis for this regulation has now been presented, whereby CaBP4 is speculated (through molecular docking predictions) to relieve an inhibitory self-interaction of the channel through binding to the IQ motif (Park et al. 2014b). CaBP4 has also been shown to eliminate Ca2+-dependent inactivation of CaV1.3 channels, which is likely to be important in the modulation of these channels in inner hair cells where Ca2+-dependent inactivation is weak or absent probably allowing the audition of sustained sounds (Yang et al. 2006). A stronger inhibitory effect has been noted for CaBP1, however, suggesting that CaBP4 may not be the key Ca2+ sensor involved in this process (Cui et al. 2007). The function of CaBP4 is modulated by protein kinase Cζ in the retina, with increased CaBP4 phosphorylation in light-adapted tissue. Phosphorylation prolongs Ca2+ currents through CaV1.3 channels, which suggests that light-stimulated phosphorylation of CaBP4 might help to regulate presynaptic Ca2+ signals in photoreceptors (Lee et al. 2007). Conversely, dephosphorylation of CaBP4, studied in transfected HEK293T cells, by protein phosphatase 2A, inhibited its ability to modulate CaV1.3 activity (Haeseleer et al. 2013). CaBP4 has also been implicated in neurotransmitter release at synaptic terminals because of its interaction with unc119, a synaptic photoreceptor protein important for neurotransmitter release and maintenance of the nervous system (Haeseleer 2008). Knockout of CaBP4 results in mice with abnormalities in retinal function where rod bipolar responses are approximately 100 times lower than those observed in wild-type animals (Haeseleer et al. 2004).
The functions of calneuron 1 and calneuron 2 have only recently begun to be investigated in detail. Both have been found to and inhibit the activity of PI4KIIIβ at low, or resting, [Ca2+]i. Overexpression of the proteins was also found to inhibit Golgi-to-plasma membrane trafficking, caused enlargement of the TGN and reduced the number of Piccolo-Bassoon-positive transport vesicles. A molecular switch for the production of phosphoinositides at the TGN is thought to be created by the opposing roles of NCS-1 and calneuron 1 or calneuron 2. At elevated Ca2+ levels, NCS-1 preferentially binds to PI4KIIIβ displacing the calneurons thereby activating the enzyme to drive enhanced TGN-to-plasma membrane trafficking (Mikhaylova et al. 2009). Calneuron 2 was discovered in a genome-wide search for regulators of mitosis (Neumann et al. 2010). Analysis of the role of calneuron 2 during cell division has suggested that it plays a key role in cytokinesis through its inhibitory control of PI4KIIIβ (Rajamanoharan et al. 2015).
Patch clamping experiments have shown that overexpressed calneuron 1 can inhibit N-type Ca2+-channel currents in 293T cells, and this inhibition was not observed with a truncated calneuron lacking its hydrophobic carboxyl terminus, suggesting normal localization is important in carrying out this function (Shih et al. 2009). Calneuron 1 and NCS-1 differentially regulate adenosine receptor activity, an important molecular target for the treatment of numerous human neurological diseases (Navarro et al. 2014). Similar differential regulation by calneuron 1 and NCS-1 has recently been reported for the CB1 cannabinoid receptor, an important potential target in nociceptive signaling (Angelats et al. 2018).
CaBP Proteins and Disease
CaBPs have been directly or indirectly implicated in multiple neuronal diseases. The postmortem brains of chronic schizophrenics have lower numbers of caldendrin-immunoreactive neurons, which express the protein at a much higher level. This loss of caldendrin in some neurons (or loss of the neurons themselves) and up-regulation in others is likely to profoundly change synaptodendritic signaling in schizophrenic patients (Bernstein et al. 2007). Changes in the distribution of caldendrin have also been observed in kainate-induced epileptic seizures in rats. Caldendrin translocates to the postsynaptic density only in rats that suffered epileptic seizures, which may implicate the protein in the pathophysiology of the disease (Smalla et al. 2003). Mutations in CaBP2 have now been identified and linked with hearing impairments in humans (Schrauwen et al. 2012; Marková et al. 2016). One of these studies identified a splice-site mutation in three consanguineous Iranian families, which likely generates a truncated CaBP2 protein with lower affinity for CaV1.3, leading to moderate-to-severe hearing loss (Schrauwen et al. 2012). Calneuron 1 has recently been shown to be overexpressed in aldosterone-producing adenoma (Kobuke et al. 2018). The protein is required for stimulated aldosterone production and may therefore represent a therapeutic target for the control of excess hormone production in such tumors (Kobuke et al. 2018). CaBP4 function has been linked to disease, and mutations in this gene generate defects predominantly in retinal function. Knockout of CaBP4 was shown to cause a phenotype similar to that of incomplete congenital stationary night blindness in patients (Haeseleer et al. 2004), and mutations in CaBP4 can cause autosomal-recessive night blindness (Zeitz et al. 2006). Patients with mutations in the CaBP4 gene have been identified that display congenital stationary night blindness. However, some patients with mutations display different phenotypes (Zeitz et al. 2006). In particular, a novel homozygous nonsense mutation has been reported in two siblings that resulted in severely reduced cone function but only negligible effects on rod function (Littink et al. 2009). CaBP4 null mutations have been observed in a consanguineous family with four members affected by Leber's congenital amaurosis (Aldahmesh et al. 2010). This condition is relatively rare, affecting about 1:80,000 of the general population, but is the most common cause of inherited loss of vision in children. Numerous studies now point to genetic mutations in CaBP4 as causative factors in a set of related cone–rod retinopathies (Littink et al. 2009; Aldahmesh et al. 2010; Bijveld et al. 2013; Hendriks et al. 2017; Smirnov et al. 2018). Multimodal imaging of five genetically characterized patients affected by CaBP4-related retinopathy showed a variable amount of photoreceptor dysfunction but this remained stable and did not deteriorate over a period of years (Schatz et al. 2017). This has led to the hope that CaBP4-based retinopathies might be tractable to gene-therapy-mediated correction. More recently, a CaBP4 missense mutation (G155D) has been implicated in a rare inherited form of frontal lobe epilepsy (Chen et al. 2017b). This would suggest a wider central function for CaBP4 outside of the visual system, which requires further investigation in future studies.
CONCLUDING REMARKS
It has become increasing clear that a full understanding of how specific aspects of physiological neuronal function are regulated in response to spatially and temporally distinct Ca2+ signals will require a detailed knowledge of the Ca2+ sensors involved. In addition, many of these the Ca2+ sensors may be implicated indirectly because of abnormalities in Ca2+ signaling pathways in neurological or neurodegenerative disorders. In some cases, the Ca2+ sensors may be more directly implicated either because of their specific regulatory roles that impinge on key dysfunctional pathways. Alternatively, genetic mutations or variations of Ca2+ sensor activity may have a more direct effect on brain dysfunction. No matter how the Ca2+ sensors are involved, they are likely to be potential therapeutic targets for new drugs that can be used to treat human disorders. For further understanding of the normal roles of each of the Ca2+ sensors, further insight into the molecular basis for the regulation of their targets and more detailed dissection of the physiological roles of each protein in identified neurons is required. This knowledge will form the basis for future approaches to the development of treatments that could alleviate a variety of human neuronal disorders underpinned by defects in Ca2+ signaling.
ACKNOWLEDGMENTS
N.H. is supported by a British Heart Foundation Intermediate Basic Science Research fellowship (FS/17/56/32925). H.V.M was supported by a Biotechnology and Biosciences Research Council project grant to R.D.B. (BB/J005843/1).
Footnotes
Editors: Geert Bultynck, Martin D. Bootman, Michael J. Berridge, and Grace E. Stutzmann
Additional Perspectives on Calcium Signaling available at www.cshperspectives.org
REFERENCES
- Alaimo A, Etxeberria A, Gómez-Posada JC, Gomis-Perez C, Fernandez-Orth J, Malo C, Villarroel A. 2018. Lack of correlation between surface expression and currents in epileptogenic AB-calmodulin binding domain Kv7.2 potassium channel mutants. Channels (Austin) 12: 299–310. 10.1080/19336950.2018.1511512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldahmesh MA, Al-Owain M, Alqahtani F, Hazzaa S, Alkuraya FS. 2010. A null mutation in CABP4 causes Leber's congenital amaurosis-like phenotype. Mol Vis 16: 207–212. [PMC free article] [PubMed] [Google Scholar]
- Ames JB. 2018. Dimerization of neuronal calcium sensor proteins. Front Mol Neurosci 11: 397 10.3389/fnmol.2018.00397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames JB, Lim S. 2012. Molecular structure and target recognition of neuronal calcium sensor proteins. Biochim Biophys Acta 1820: 1205–1213. 10.1016/j.bbagen.2011.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames JB, Tanaka T, Ikura M, Stryer L. 1995. Nuclear magnetic resonance evidence for Ca-induced extrusion of the myristoyl group of recoverin. J Biol Chem 270: 30909–30913. 10.1074/jbc.270.52.30909 [DOI] [PubMed] [Google Scholar]
- Ames JB, Ishima R, Tanaka T, Gordon JI, Stryer L, Ikura M. 1997. Molecular mechanics of calcium-myristoyl switches. Nature 389: 198–202. 10.1038/38310 [DOI] [PubMed] [Google Scholar]
- Ames JB, Hamashima N, Molchanova T. 2002. Structure and calcium-binding studies of a recoverin mutant (E85Q) in an allosteric intermediate state. Biochemistry 41: 5776–5787. 10.1021/bi012153k [DOI] [PubMed] [Google Scholar]
- Ames JB, Levay K, Wingard JN, Lusin JD, Slepak VZ. 2006. Structural basis for calcium-induced inhibition of rhodopsin kinase by recoverin. J Biol Chem 281: 37237–37245. 10.1074/jbc.M606913200 [DOI] [PubMed] [Google Scholar]
- An WF, Bowlby MR, Bett M, Cao J, Ling HP, Mendoza G, Hinson JW, Mattsson KI, Strassle BW, Trimmer JS, et al. 2000. Modulation of A-type potassium channels by a family of calcium sensors. Nature 403: 553–556. 10.1038/35000592 [DOI] [PubMed] [Google Scholar]
- Angebault C, Fauconnier J, Patergnani S, Rieusset J, Danese A, Affortit CA, Jagodzinska J, Megy C, Quiles M, Cazevieille C, et al. 2018. ER-mitochondria cross-talk is regulated by the Ca2+ sensor NCS1 and is impaired in Wolfram syndrome. Sci Signal 11 10.1126/scisignal.aaq1380 [DOI] [PubMed] [Google Scholar]
- Angelats E, Requesens M, Aguinaga D, Kreutz MR, Franco R, Navarro G. 2018. Neuronal calcium and cAMP cross-talk mediated by cannabinoid CB1 receptor and EF-hand calcium sensor interactions. Front Cell Dev Biol 6: 67 10.3389/fcell.2018.00067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aravind P, Chandra K, Reddy PP, Jeromin A, Chary KV, Sharma Y. 2008. Regulatory and structural EF-hand motifs of neuronal calcium sensor-1: Mg2+ modulates Ca2+ binding, Ca2+-induced conformational changes, and equilibrium unfolding transitions. J Mol Biol 376: 1100–1115. 10.1016/j.jmb.2007.12.033 [DOI] [PubMed] [Google Scholar]
- Atasu B, Hanagasi H, Bilgic B, Pak M, Erginel-Unaltuna N, Hauser AK, Guven G, Simon-Sanchez J, Heutink P, Gasser T, et al. 2018. HPCA confirmed as a genetic cause of DYT2-like dystonia phenotype. Mov Disord 33: 1354–1358. 10.1002/mds.27442 [DOI] [PubMed] [Google Scholar]
- Babić Leko M, Borovecki F, Dejanovic N, Hof PR, Šimic G. 2016. Predictive value of cerebrospinal fluid visinin-like protein-1 levels for Alzheimer's disease early detection and differential diagnosis in patients with mild cognitive impairment. J Alzheimers Dis 50: 765–778. 10.3233/JAD-150705 [DOI] [PubMed] [Google Scholar]
- Bahi N, Friocourt G, Carrié A, Graham ME, Weiss JL, Chafey P, Fauchereau F, Burgoyne RD, Chelly J. 2003. IL1 receptor accessory protein like, a protein involved in X-linked mental retardation, interacts with neuronal calcium sensor-1 and regulates exocytosis. Hum Mol Genet 12: 1415–1425. 10.1093/hmg/ddg147 [DOI] [PubMed] [Google Scholar]
- Bähring R. 2018. Kv channel-interacting proteins as neuronal and non-neuronal calcium sensors. Channels (Austin) 12: 187–200. 10.1080/19336950.2018.1491243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker K, Gordon SL, Grozeva D, van Kogelenberg M, Roberts NY, Pike M, Blair E, Hurles ME, Chong WK, Baldeweg T, et al. 2015. Identification of a human synaptotagmin-1 mutation that perturbs synaptic vesicle cycling. J Clin Invest 125: 1670–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker K, Gordon SL, Melland H, Bumbak F, Scott DJ, Jiang TJ, Owen D, Turner BJ, Boyd SG, Rossi M, et al. 2018. SYT1-associated neurodevelopmental disorder: A case series. Brain 141: 2576–2591. 10.1093/brain/awy209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee R, Yoder JB, Yue DT, Amzel LM, Tomaselli GF, Gabelli SB, Ben-Johny M. 2018. Bilobal architecture is a requirement for calmodulin signaling to CaV1.3 channels. Proc Natl Acad Sci 115: E3026–E3035. 10.1073/pnas.1716381115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barclay JW, Morgan A, Burgoyne RD. 2005. Calcium-dependent regulation of exocytosis. Cell Calcium 38: 343–353. 10.1016/j.ceca.2005.06.012 [DOI] [PubMed] [Google Scholar]
- Barria A, Muller D, Derkach V, Griffith LC, Soderling TR. 1997. Regulatory phosphorylation of AMPA-type glutamate receptors by CaM-KII during long-term potentiation. Science 276: 2042–2045. 10.1126/science.276.5321.2042 [DOI] [PubMed] [Google Scholar]
- Behnen P, Dell'Orco D, Koch KW. 2010. Involvement of the calcium sensor GCAP1 in hereditary cone dystrophies. Biol Chem 391: 631–637. 10.1515/bc.2010.063 [DOI] [PubMed] [Google Scholar]
- Bello OD, Jouannot O, Chaudhuri A, Stroeva E, Coleman J, Volynski KE, Rothman JE, Krishnakumar SS. 2018. Synaptotagmin oligomerization is essential for calcium control of regulated exocytosis. Proc Natl Acad Sci 115: E7624–E7631. 10.1073/pnas.1808792115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bereczki E, Branca RM, Francis PT, Pereira JB, Baek JH, Hortobagyi T, Winblad B, Ballard C, Lehtio J, Aarsland D. 2018. Synaptic markers of cognitive decline in neurodegenerative diseases: A proteomic approach. Brain 141: 582–595. 10.1093/brain/awx352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein HG, Sahin J, Smalla KH, Gundelfinger ED, Bogerts B, Kreutz MR. 2007. A reduced number of cortical neurons show increased caldendrin protein levels in chronic schizophrenia. Schizophr Res 96: 246–256. 10.1016/j.schres.2007.05.038 [DOI] [PubMed] [Google Scholar]
- Berridge MJ. 1998. Neuronal calcium signalling. Neuron 21: 13–26. 10.1016/S0896-6273(00)80510-3 [DOI] [PubMed] [Google Scholar]
- Berridge MJ. 2010. Calcium hypothesis of Alzheimer's disease. Pflugers Arch 459: 441–449. 10.1007/s00424-009-0736-1 [DOI] [PubMed] [Google Scholar]
- Berridge MJ. 2018. Vitamin D deficiency: Infertility and neurodevelopmental diseases (attention deficit hyperactivity disorder, autism, and schizophrenia). Am J Physiol Cell Physiol 314: C135–C151. 10.1152/ajpcell.00188.2017 [DOI] [PubMed] [Google Scholar]
- Bijveld MM, Florijn RJ, Bergen AA, van den Born LI, Kamermans M, Prick L, Riemslag FC, van Schooneveld MJ, Kappers AM, van Genderen MM. 2013. Genotype and phenotype of 101 Dutch patients with congenital stationary night blindness. Ophthalmology 120: 2072–2081. 10.1016/j.ophtha.2013.03.002 [DOI] [PubMed] [Google Scholar]
- Bito H, Deisseroth K, Tsien RW. 1997. Ca2+-dependent regulation in neuronal gene expression. Curr Opin Neurobiol 7: 419–429. 10.1016/S0959-4388(97)80072-4 [DOI] [PubMed] [Google Scholar]
- Blasiole B, Kabbani N, Boehmler W, Thisse B, Thisse C, Canfield V, Levenson R. 2005. Neuronal calcium sensor-1 gene ncs-1a is essential for semicircular canal formation in zebrafish inner ear. J Neurobiol 64: 285–297. 10.1002/neu.20138 [DOI] [PubMed] [Google Scholar]
- Boczek NJ, Gomez-Hurtado N, Ye D, Calvert ML, Tester DJ, Kryshtal D, Hwang HS, Johnson CN, Chazin WJ, Loporcaro CG, et al. 2016. Spectrum and prevalence of CALM1-, CALM2-, and CALM3-encoded calmodulin variants in long QT syndrome and functional characterization of a novel long QT syndrome-associated calmodulin missense variant, E141G. Circ Cardiovasc Genet 9: 136–146. 10.1161/circgenetics.115.001323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeckel GR, Ehrlich BE. 2018. NCS-1 is a regulator of calcium signaling in health and disease. Biochim Biophys Acta 1865: 1660–1667. 10.1016/j.bbamcr.2018.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehmerle W, Splittgerber U, Lazarus MB, McKenzie KM, Johnston DG, Austin DJ, Ehrlich BE. 2006. Paclitaxel induces calcium oscillations via an inositol 1,4,5-trisphosphate receptor and neuronal calcium sensor 1-dependent mechanism. Proc Natl Acad Sci 103: 18356–18361. 10.1073/pnas.0607240103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne Y, Dannenberg J, Pollmann V, Marchot P, Pongs O. 2001. Immunocytochemical localization and crystal structure of human frequenin (neuronal calcium sensor 1). J Biol Chem 276: 11949–11955. 10.1074/jbc.M009373200 [DOI] [PubMed] [Google Scholar]
- Brackmann M, Schuchmann S, Anand R, Braunewell KH. 2005. Neuronal Ca2+ sensor protein VILIP-1 affects cGMP signalling of guanylyl cyclase B by regulating clathrin-dependent receptor recycling in hippocampal neurons. J Cell Sci 118: 2495–2505. 10.1242/jcs.02376 [DOI] [PubMed] [Google Scholar]
- Braunewell K-H. 2005. The darker side of Ca signaling by neuronal Ca-sensor proteins: From Alzheimer's disease to cancer. Trends Pharmacol Sci 26: 345–351. 10.1016/j.tips.2005.04.008 [DOI] [PubMed] [Google Scholar]
- Braunewell KH. 2012. The visinin-like proteins VILIP-1 and VILIP-3 in Alzheimer's disease-old wine in new bottles. Front Mol Neurosci 5: 20 10.3389/fnmol.2012.00020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braunewell KH, Gundelfinger ED. 1999. Intracellular neuronal calcium sensor proteins: A family of EF-hand calcium-binding proteins in search of a function. Cell Tissue Res 295: 1–12. 10.1007/s004410051207 [DOI] [PubMed] [Google Scholar]
- Braunewell KH, Klein-Szanto AJ. 2009. Visinin-like proteins (VSNLs): interaction partners and emerging functions in signal transduction of a subfamily of neuronal Ca2+-sensor proteins. Cell Tissue Res 335: 301–316. 10.1007/s00441-008-0716-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brini M, Calì T, Ottolini D, Carafoli E. 2014. Neuronal calcium signaling: Function and dysfunction. Cell Mol Life Sci 71: 2787–2814. 10.1007/s00018-013-1550-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brini M, Carafoli E, Calì T. 2017. The plasma membrane calcium pumps: Focus on the role in (neuro)pathology. Biochem Biophys Res Commun 483: 1116–1124. 10.1016/j.bbrc.2016.07.117 [DOI] [PubMed] [Google Scholar]
- Burgoyne RD. 2004. The neuronal calcium-sensor proteins. Biochim Biophys Acta 1742: 59–68. 10.1016/j.bbamcr.2004.08.008 [DOI] [PubMed] [Google Scholar]
- Burgoyne RD. 2007. Neuronal calcium sensor proteins: Generating diversity in neuronal Ca2+ signalling. Nat Rev Neurosci 8: 182–193. 10.1038/nrn2093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne RD, Haynes LP. 2010. Neuronal calcium sensor proteins: Emerging roles in membrane traffic and synaptic plasticity. F1000 Biol Rep 2: 5 10.3410/B2-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne RD, Haynes LP. 2012. Understanding the physiological roles of the neuronal calcium sensor proteins. Mol Brain 5: 2 10.1186/1756-6606-5-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne RD, Haynes LP. 2015. Sense and specificity in neuronal calcium signalling. Biochim Biophys Acta 1853: 1921–1932. 10.1016/j.bbamcr.2014.10.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne RD, Morgan A. 1998. Calcium sensors in regulated exocytosis. Cell Calcium 24: 367–376. 10.1016/S0143-4160(98)90060-4 [DOI] [PubMed] [Google Scholar]
- Burgoyne RD, Weiss JL. 2001. The neuronal calcium sensor family of Ca2+-binding proteins. Biochem J 353: 1–12. 10.1042/bj3530001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne RD, O'Callaghan DW, Hasdemir B, Haynes LP, Tepikin AV. 2004. Neuronal Ca2+-sensor proteins: Multitalented regulators of neuronal function. Trends Neurosci 27: 203–209. 10.1016/j.tins.2004.01.010 [DOI] [PubMed] [Google Scholar]
- Burns ME, Mendez A, Chen J, Baylor DA. 2002. Dynamics of cyclic GMP synthesis in retinal rods. Neuron 36: 81–91. 10.1016/S0896-6273(02)00911-X [DOI] [PubMed] [Google Scholar]
- Buxbaum JD, Choi EK, Luo Y, Lilliehook C, Crowley AC, Merriam DE, Wasco W. 1998. Calsenilin: A calcium-binding protein that interacts with the presenilins and regulates the levels of a presenilin fragment. Nat Med 4: 1177–1181. 10.1038/2673 [DOI] [PubMed] [Google Scholar]
- Cafiero C, Marangi G, Orteschi D, Ali M, Asaro A, Ponzi E, Moncada A, Ricciardi S, Murdolo M, Mancano G, et al. 2015. Novel de novo heterozygous loss-of-function variants in MED13L and further delineation of the MED13L haploinsufficiency syndrome. Eur J Hum Genet 23: 1499–1504. 10.1038/ejhg.2015.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrión AM, Link WA, Ledo F, Mellström B, Naranjo JR. 1999. DREAM is a Ca2+-regulated transcriptional repressor. Nature 398: 80–84. 10.1038/18044 [DOI] [PubMed] [Google Scholar]
- Catterall WA, Few AP. 2008. Calcium channel regulation and presynaptic plasticity. Neuron 59: 882–901. 10.1016/j.neuron.2008.09.005 [DOI] [PubMed] [Google Scholar]
- Catterall WA, Leal K, Nanou E. 2013. Calcium channels and short-term synaptic plasticity. J Biol Chem 288: 10742–10749. 10.1074/jbc.R112.411645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S, Trimbuch T, Rosenmund C. 2018. Synaptotagmin-1 drives synchronous Ca2+-triggered fusion by C2B-domain-mediated synaptic-vesicle-membrane attachment. Nat Neurosci 21: 33–40. 10.1038/s41593-017-0037-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman ER. 2008. How does synaptotagmin trigger neurotransmitter release? Annu Rev Biochem 77: 615–641. 10.1146/annurev.biochem.77.062005.101135 [DOI] [PubMed] [Google Scholar]
- Charlesworth G, Angelova PR, Bartolome-Robledo F, Ryten M, Trabzuni D, Stamelou M, Abramov AY, Bhatia KP, Wood NW. 2015. Mutations in HPCA cause autosomal-recessive primary isolated dystonia. Am J Hum Genet 96: 657–665. 10.1016/j.ajhg.2015.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyaya R, Meador WE, Means AR, Quiocho FA. 1992. Calmodulin structure refined at 1.7 Å resolution. J Mol Biol 228: 1177–1192. 10.1016/0022-2836(92)90324-D [DOI] [PubMed] [Google Scholar]
- Chaumont S, Compan V, Toulme E, Richler E, Housley GD, Rassendren F, Khakh BS. 2008. Regulation of P2X2 receptors by the neuronal calcium sensor VILIP1. Sci Signal 1: ra8 10.1126/scisignal.1162329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CK, Inglese J, Lefkowitz RJ, Hurley JB. 1995. Ca-dependent interaction of recoverin with rhodopsin kinase. J Biol Chem 270: 18060–18066. 10.1074/jbc.270.30.18060 [DOI] [PubMed] [Google Scholar]
- Chen C, Arai I, Satterfield R, Young SM Jr, Jonas P. 2017a. Synaptotagmin 2 is the fast Ca2+ sensor at a central inhibitory synapse. Cell Rep 18: 723–736. 10.1016/j.celrep.2016.12.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZH, Wang C, Zhuo MQ, Zhai QX, Chen Q, Guo YX, Zhang YX, Gui J, Tang ZH, Zeng XL. 2017b. Exome sequencing identified a novel missense mutation c.464G>A (p.G155D) in Ca2+-binding protein 4 (CABP4) in a Chinese pedigree with autosomal dominant nocturnal frontal lobe epilepsy. Oncotarget 8: 78940–78947. 10.18632/oncotarget.20694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng HYM, Pitcher GM, Laviolette SR, Whishaw IQ, Tong KI, Kockeritz LK, Wada T, Joza NA, Crackower M, Goncalves J, et al. 2002. DREAM is a critical transcriptional repressor for pain modulation. Cell 108: 31–43. 10.1016/S0092-8674(01)00629-8 [DOI] [PubMed] [Google Scholar]
- Chicka MC, Hui E, Liu H, Chapman ER. 2008. Synaptotagmin arrests the SNARE complex before triggering fast, efficient membrane fusion in response to Ca2+. Nat Struct Mol Biol 15: 827–835. 10.1038/nsmb.1463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christel C, Lee A. 2012. Ca2+-dependent modulation of voltage-gated Ca2+ channels. Biochim Biophys Acta 1820: 1243–1252. 10.1016/j.bbagen.2011.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SM, Suutari B, He X, Wang Y, Sanchez S, Tirko NN, Mandelberg NJ, Mullins C, Zhou G, Wang S, et al. 2018. Calmodulin shuttling mediates cytonuclear signaling to trigger experience-dependent transcription and memory. Nat Commun 9: 2451 10.1038/s41467-018-04705-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coukell B, Cameron A, Perusini S, Shim K. 2004. Disruption of the NCS-1/frequenin-related ncsA gene in Dictyostelium discoideum accelerates development. Dev Growth Differ 46: 449–458. 10.1111/j.1440-169x.2004.00761.x [DOI] [PubMed] [Google Scholar]
- Craxton M. 2004. Synaptotagmin gene content of the sequenced genomes. BMC Genomics 5: 43 10.1186/1471-2164-5-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui G, Meyer AC, Calin-Jageman I, Neef J, Haeseleer F, Moser T, Lee A. 2007. Ca2+-binding proteins tune Ca2+-feedback to Cav1.3 channels in mouse auditory hair cells. J Physiol 585: 791–803. 10.1113/jphysiol.2007.142307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagher A, Robbins TW. 2009. Personality, addiction, dopamine: Insights from Parkinson's disease. Neuron 61: 502–510. 10.1016/j.neuron.2009.01.031 [DOI] [PubMed] [Google Scholar]
- Dason JS, Romero-Pozuelo J, Marin L, Iyengar BG, Klose MK, Ferrus A, Atwood HL. 2009. Frequenin/NCS-1 and the Ca2+-channel α1-subunit co-regulate synaptic transmission and nerve-terminal growth. J Cell Sci 122: 4109–4121. 10.1242/jcs.055095 [DOI] [PubMed] [Google Scholar]
- de Barry J, Janoshazi A, Dupont JL, Procksch O, Chasserot-Golaz S, Jeromin A, Vitale N. 2006. Functional implication of neuronal calcium sensor-1 and phosphoinositol 4-kinase-β interaction in regulated exocytosis of PC12 cells. J Biol Chem 281: 18098–18111. 10.1074/jbc.M509842200 [DOI] [PubMed] [Google Scholar]
- Deisseroth K, Heist EK, Tsien RW. 1998. Translocation of calmodulin to the nucleus supports CREB phosphorylation in hippocampal neurons. Nature 392: 198–202. 10.1038/32448 [DOI] [PubMed] [Google Scholar]
- de Ligt J, Willemsen MH, van Bon BW, Kleefstra T, Yntema HG, Kroes T, Vulto-van Silfhout AT, Koolen DA, de Vries P, Gilissen C, et al. 2012. Diagnostic exome sequencing in persons with severe intellectual disability. N Engl J Med 367: 1921–1929. 10.1056/NEJMoa1206524 [DOI] [PubMed] [Google Scholar]
- Dell'Orco D, Behnen P, Linse S, Koch KW. 2010. Calcium binding, structural stability and guanylate cyclase activation in GCAP1 variants associated with human cone dystrophy. Cell Mol Life Sci 67: 973–984. 10.1007/s00018-009-0243-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMaria CD, Soong TW, Alseikhan BA, Alvania RS, Yue DT. 2001. Calmodulin bifurcates the local Ca2+ signal that modulates P/Q-type Ca2+ channels. Nature 411: 484–489. 10.1038/35078091 [DOI] [PubMed] [Google Scholar]
- de Rezende VB, Rosa DV, Comim CM, Magno LA, Rodrigues AL, Vidigal P, Jeromin A, Quevedo J, Romano-Silva MA. 2014. NCS-1 deficiency causes anxiety and depressive-like behavior with impaired non-aversive memory in mice. Physiol Behav 130: 91–98. 10.1016/j.physbeh.2014.03.005 [DOI] [PubMed] [Google Scholar]
- de Wit H, Walter AM, Milosevic I, Gulyás-Kovács A, Riedel D, Sørensen JB, Verhage M. 2009. Synaptotagmin-1 docks secretory vesicles to syntaxin-1/SNAP-25 acceptor complexes. Cell 138: 935–946. 10.1016/j.cell.2009.07.027 [DOI] [PubMed] [Google Scholar]
- Dieterich DC, Karpova A, Mikhaylova M, Zdobnova I, König I, Landwehr M, Kreutz M, Smalla KH, Richter K, Landgraf P, et al. 2008. Caldendrin-Jacob: A protein liaison that couples NMDA receptor signalling to the nucleus. PLoS Biol 6: e34 10.1371/journal.pbio.0060034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dizhoor AM, Hurley JB. 1996. Inactivation of EF-hands makes GCAP-2 (p24) a constitutive activator of photoreceptor guanylyl cyclase by preventing a Ca2+-induced “activator-to-inhibitor” transition. J Biol Chem 271: 19346–19350. 10.1074/jbc.271.32.19346 [DOI] [PubMed] [Google Scholar]
- Dizhoor AM, Ray S, Kumar S, Niemi G, Spencer M, Brolley D, Walsh KA, Philipov PP, Hurley JB, Stryer L. 1991. Recoverin: A calcium sensitive activator of retinal rod guanylate cyclase. Science 251: 915–918. 10.1126/science.1672047 [DOI] [PubMed] [Google Scholar]
- D'Onofrio S, Kezunovic N, Hyde JR, Luster B, Messias E, Urbano FJ, Garcia-Rill E. 2015. Modulation of gamma oscillations in the pedunculopontine nucleus by neuronal calcium sensor protein-1: Relevance to schizophrenia and bipolar disorder. J Neurophysiol 113: 709–719. 10.1152/jn.00828.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Onofrio S, Hyde J, Garcia-Rill E. 2017a. Interaction between neuronal calcium sensor protein 1 and lithium in pedunculopontine neurons. Physiol Rep 5: e13246 10.14814/phy2.13246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Onofrio S, Mahaffey S, Garcia-Rill E. 2017b. Role of calcium channels in bipolar disorder. Curr Psychopharmacol 6: 122–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dovgan AV, Cherkas VP, Stepanyuk AR, Fitzgerald DJ, Haynes LP, Tepikin AV, Burgoyne RD, Belan PV. 2010. Decoding glutamate receptor activation by the Ca2+ sensor protein hippocalcin in rat hippocampal neurons. Eur J Neurosci 32: 347–358. 10.1111/j.1460-9568.2010.07303.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragicevic E, Poetschke C, Duda J, Schlaudraff F, Lammel S, Schiemann J, Fauler M, Hetzel A, Watanabe M, Lujan R, et al. 2014. Cav1.3 channels control D2-autoreceptor responses via NCS-1 in substantia nigra dopamine neurons. Brain 137: 2287–2302. 10.1093/brain/awu131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duda J, Potschke C, Liss B. 2016. Converging roles of ion channels, calcium, metabolic stress, and activity pattern of Substantia nigra dopaminergic neurons in health and Parkinson's disease. J Neurochem 139: 156–178. 10.1111/jnc.13572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egorova PA, Bezprozvanny IB. 2018. Inositol 1,4,5-trisphosphate receptors and neurodegenerative disorders. FEBS J 285: 3547–3565. 10.1111/febs.14366 [DOI] [PubMed] [Google Scholar]
- Erickson MG, Alseikhan BA, Peterson BZ, Yue DT. 2001. Preassociation of calmodulin with voltage-gated Ca2+ channels revealed by FRET in single living cells. Neuron 31: 973–985. 10.1016/S0896-6273(01)00438-X [DOI] [PubMed] [Google Scholar]
- Erickson MG, Liang H, Mori MX, Yue DT. 2003. FRET two-hybrid mapping reveals function and location of L-type Ca2+ channel CaM preassociation. Neuron 39: 97–107. 10.1016/S0896-6273(03)00395-7 [DOI] [PubMed] [Google Scholar]
- Faas GC, Raghavachari S, Lisman JE, Mody I. 2011. Calmodulin as a direct detector of Ca2+ signals. Nat Neurosci 14: 301–304. 10.1038/nn.2746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon JL, Halling DB, Hamilton SL, Quiocho FA. 2005. Structure of calmodulin bound to the hydrophobic IQ domain of the cardiac Cav1.2 calcium channel. Structure 13: 1881–1886. 10.1016/j.str.2005.09.021 [DOI] [PubMed] [Google Scholar]
- Fernandez I, Arac D, Ubach J, Gerber SH, Shin OH, Gao Y, Anderson RGW, Sudhof TC, Rizo J. 2001. Three-dimensional structure of the synaptotagmin 1 C2B-domain: Synaptotagmin 1 as a phospholipid binding machine. Neuron 32: 1057–1069. 10.1016/S0896-6273(01)00548-7 [DOI] [PubMed] [Google Scholar]
- Fernández-Chacón R, Königstorfer A, Gerber SH, Garcia J, Matos MF, Stevens CF, Brose N, Rizo J, Rosenmund C, Sudhof TC. 2001. Synaptotagmin I functions as a calcium regulator of release probability. Nature 410: 41–49. 10.1038/35065004 [DOI] [PubMed] [Google Scholar]
- Few AP, Lautermilch NJ, Westenbroek RE, Scheuer T, Catterall WA. 2005. Differential regulation of Cav2.1 channels by calcium-binding protein 1 and visinin-like protein-2 requires N-terminal myristoylation. J Neurosci 25: 7071–7080. 10.1523/jneurosci.0452-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findeisen F, Minor DL Jr. 2010. Structural basis for the differential effects of CaBP1 and calmodulin on CaV1.2 calcium-dependent inactivation. Structure 18: 1617–1631. 10.1016/j.str.2010.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findeisen F, Rumpf CH, Minor DL Jr. 2013. Apo states of calmodulin and CaBP1 control CaV1 voltage-gated calcium channel function through direct competition for the IQ domain. J Mol Biol 425: 3217–3234. 10.1016/j.jmb.2013.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald DJ, Burgoyne RD, Haynes LP. 2008. Neuronal calcium sensor proteins are unable to modulate NFAT activation in mammalian cells. Biochim Biophys Acta 1780: 240–248. 10.1016/j.bbagen.2007.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaherty KM, Zoulya S, Stryer L, McKay DB. 1993. Three-dimensional structure of recoverin, a calcium sensor in vision. Cell 75: 709–716. 10.1016/0092-8674(93)90491-8 [DOI] [PubMed] [Google Scholar]
- Gambino F, Pavlowsky A, Begle A, Dupont JL, Bahi N, Courjaret R, Gardette R, Hadjkacem H, Skala H, Poulain B, et al. 2007. IL1-receptor accessory protein-like 1 (IL1RAPL1), a protein involved in cognitive functions, regulates N-type Ca2+-channel and neurite elongation. Proc Natl Acad Sci 104: 9063–9068. 10.1073/pnas.0701133104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geppert M, Goda Y, Hammer RE, Li C, Rosahl TW, Stevens CF, Südhof TC. 1994. Synaptotagmin I: A major Ca2+ sensor for transmitter release at a central synapse. Cell 79: 717–727. 10.1016/0092-8674(94)90556-8 [DOI] [PubMed] [Google Scholar]
- Gierke P, Zhao C, Brackmann M, Linke B, Heinemann U, Braunewell KH. 2004. Expression analysis of members of the neuronal calcium sensor protein family: Combining bioinformatics and Western Blot analysis. Biochem Biophys Res Commun 323: 38–43. 10.1016/j.bbrc.2004.08.055 [DOI] [PubMed] [Google Scholar]
- Gomez M, De Castro E, Guarin E, Sasakura H, Kuhara A, Mori I, Bartfai T, Bargmann CI, Nef P. 2001. Ca2+ signalling via the neuronal calcium sensor-1 regulates associative learning and memory in C. elegans. Neuron 30: 241–248. 10.1016/S0896-6273(01)00276-8 [DOI] [PubMed] [Google Scholar]
- Grillo MA, Grillo SL, Gerdes BC, Kraus JG, Koulen P. 2019. Control of neuronal ryanodine receptor-mediated calcium signaling by calsenilin. Mol Neurobiol 56: 525–534. 10.1007/s12035-018-1080-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groblewska M, Muszynski P, Wojtulewska-Supron A, Kulczynska-Przybik A, Mroczko B. 2015. The role of visinin-like protein-1 in the pathophysiology of Alzheimer's disease. J Alzheimers Dis 47: 17–32. 10.3233/JAD-150060 [DOI] [PubMed] [Google Scholar]
- Groen C, Bähring R. 2017. Modulation of human Kv4.3/KChIP2 channel inactivation kinetics by cytoplasmic Ca2+. Pflugers Arch 469: 1457–1470. 10.1007/s00424-017-2039-2 [DOI] [PubMed] [Google Scholar]
- Haeseleer F. 2008. Interaction and colocalization of CaBP4 and Unc119 (MRG4) in photoreceptors. Invest Ophthalmol Vis Sci 49: 2366–2375. 10.1167/iovs.07-1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeseleer F, Sokal I, Verlinde CLMJ, Erdjument-Bromage H, Tempst P, Pronin AN, Benovic JL, Fariss RN, Palczewski K. 2000. Five members of a novel Ca2+-binding protein (CABP) subfamily with similarity to calmodulin. J Biol Chem 275: 1247–1260. 10.1074/jbc.275.2.1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeseleer F, Imanishi Y, Sokal I, Filipek S, Palczewski K. 2002. Calcium-binding proteins: Intracellular sensors from the calmodulin superfamily. Biochem Biophys Res Commun 290: 615–623. 10.1006/bbrc.2001.6228 [DOI] [PubMed] [Google Scholar]
- Haeseleer F, Imanishi Y, Maeda T, Possin DE, Maeda A, Lee A, Rieke F, Palczewski K. 2004. Essential role of Ca2+-binding protein 4, a Cav1.4 channel regulator in photoreceptor synaptic function. Nat Neurosci 7: 1079–1087. 10.1038/nn1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeseleer F, Sokal I, Gregory FD, Lee A. 2013. Protein phosphatase 2A dephosphorylates CaBP4 and regulates CaBP4 function. Invest Ophthalmol Vis Sci 54: 1214–1226. 10.1167/iovs.12-11319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handley MT, Lian LY, Haynes LP, Burgoyne RD. 2010. Structural and functional deficits in a neuronal calcium sensor-1 mutant identified in a case of autistic spectrum disorder. PLoS ONE 5: e10534 10.1371/journal.pone.0010534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasdemir B, Fitzgerald DJ, Prior IA, Tepikin AV, Burgoyne RD. 2005. Traffic of Kv4 K+ channels mediated by KChIP1 is via a novel post-ER vesicular pathway. J Cell Biol 171: 459–469. 10.1083/jcb.200506005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes LP, Burgoyne RD. 2008. Unexpected tails of a Ca2+ sensor. Nat Chem Biol 4: 90–91. 10.1038/nchembio0208-90 [DOI] [PubMed] [Google Scholar]
- Haynes LP, Tepikin AV, Burgoyne RD. 2004. Calcium-binding protein 1 is an inhibitor of agonist-evoked, inositol 1,4,5-trisphosphate-mediated calcium signaling. J Biol Chem 279: 547–555. 10.1074/jbc.M309617200 [DOI] [PubMed] [Google Scholar]
- Haynes LP, Thomas GMH, Burgoyne RD. 2005. Interaction of neuronal calcium sensor-1 and ADP-ribosylation factor 1 allows bidirectional control of phosphatidylinositol 4-kinase β and trans-Golgi network-plasma membrane traffic. J Biol Chem 280: 6047–6054. 10.1074/jbc.M413090200 [DOI] [PubMed] [Google Scholar]
- Haynes LP, Fitzgerald DJ, Wareing B, O'Callaghan DW, Morgan A, Burgoyne RD. 2006. Analysis of the interacting partners of the neuronal calcium-binding proteins L-CaBP1, hippocalcin, NCS-1 and neurocalcin δ. Proteomics 6: 1822–1832. 10.1002/pmic.200500489 [DOI] [PubMed] [Google Scholar]
- Haynes LP, Sherwood MW, Dolman NJ, Burgoyne RD. 2007. Specificity, promiscuity and localization of ARF protein interactions with NCS-1 and phosphatidylinositol-4 kinase-IIIβ. Traffic 8: 1080–1092. 10.1111/j.1600-0854.2007.00594.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes LP, McCue HV, Burgoyne RD. 2012. Evolution and functional diversity of the calcium binding proteins (CaBPs). Front Mol Neurosci 5: 9 10.3389/fnmol.2012.00009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidarsson PO, Bjerrum-Bohr IJ, Jensen GA, Pongs O, Finn BE, Poulsen FM, Kragelund BB. 2012. The C-terminal tail of human neuronal calcium sensor 1 regulates the conformational stability of the Ca2+-activated state. J Mol Biol 417: 51–64. 10.1016/j.jmb.2011.12.049 [DOI] [PubMed] [Google Scholar]
- Helassa N, Antonyuk SV, Lian LY, Haynes LP, Burgoyne RD. 2017. Biophysical and functional characterization of hippocalcin mutants responsible for human dystonia. Hum Mol Genet 26: 2426–2435. 10.1093/hmg/ddx133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks KB, Wang BQ, Schnieders EA, Thorner J. 1999. Yeast homologue of neuronal frequenin is a regulator of phosphatidylinositol-4-OH kinase. Nat Cell Biol 1: 234–241. 10.1038/12058 [DOI] [PubMed] [Google Scholar]
- Hendriks M, Verhoeven VJM, Buitendijk GHS, Polling JR, Meester-Smoor MA, Hofman A, Consortium RD, Kamermans M, Ingeborgh van den Born L, Klaver CCW. 2017. Development of refractive errors-what can we learn from inherited retinal dystrophies? Am J Ophthalmol 182: 81–89. 10.1016/j.ajo.2017.07.008 [DOI] [PubMed] [Google Scholar]
- Herrmann DN, Horvath R, Sowden JE, Gonzalez M, Sanchez-Mejias A, Guan Z, Whittaker RG, Almodovar JL, Lane M, Bansagi B, et al. 2014. Synaptotagmin 2 mutations cause an autosomal-dominant form of Lambert-Eaton myasthenic syndrome and nonprogressive motor neuropathy. Am J Hum Genet 95: 332–339. 10.1016/j.ajhg.2014.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmqvist MH, Cao J, Hernandez-Pineda R, Jacobson MD, Carroll KI, Sung MA, Betty M, Ge P, Gilbride KJ, Brown ME, et al. 2002. Elimination of fast inactivation in Kv4 A-type potassium channels by an auxiliary subunit domain. Proc Natl Acad Sci 99: 1035–1040. 10.1073/pnas.022509299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes KA, Pennesi ME, Sokal I, Church-Kopish J, Schmidt B, Margolis D, Frederick JM, Rieke F, Palczewski K, Wu SM, et al. 2002. GCAP1 rescues rod photoreceptor response in GCAP1/GCAP2 knockout mice. EMBO J 21: 1545–1554. 10.1093/emboj/21.7.1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hradsky J, Raghuram V, Reddy PP, Navarro G, Hupe M, Casado V, McCormick PJ, Sharma Y, Kreutz MR, Mikhaylova M. 2011. Post-translational membrane insertion of tail-anchored transmembrane EF-hand Ca2+ sensor calneurons requires the TRC40/Asna1 protein chaperone. J Biol Chem 286: 36762–36776. 10.1074/jbc.M111.280339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hradsky J, Bernstein HG, Marunde M, Mikhaylova M, Kreutz MR. 2015. Alternative splicing, expression and cellular localization of Calneuron-1 in the rat and human brain. J Histochem Cytochem 63: 793–804. 10.1369/0022155415595841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui E, Johnson CP, Yao J, Dunning FM, Chapman ER. 2009. Synaptotagmin-mediated bending of the target membrane is a critical step in Ca2+-regulated fusion. Cell 138: 709–721. 10.1016/j.cell.2009.05.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttner IG, Strahl T, Osawa M, King DS, Ames JB, Thorner J. 2003. Molecular interactions of yeast frequenin (Frq1) with the phosphatidylinositol 4-kinase isoform, Pik1. J Biol Chem 278: 4862–4874. 10.1074/jbc.M207920200 [DOI] [PubMed] [Google Scholar]
- Ikuro M, Ames JB. 2006. Genetic polymorphism and protein conformational plasticity in the calmodulin superfamily: Two ways to promote multifunctionality. Proc Natl Acad Sci 103: 1159–1164. 10.1073/pnas.0508640103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivings L, Pennington SR, Jenkins R, Weiss JL, Burgoyne RD. 2002. Identification of Ca2+-dependent binding partners for the neuronal calcium sensor protein neurocalcin δ: Interaction with actin, clathrin and tubulin. Biochem J 363: 599–608. 10.1042/bj3630599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerng HH, Pfaffinger PJ. 2008. Multiple Kv channel-interacting proteins contain an N-terminal transmembrane domain that regulates Kv4 channel trafficking and gating. J Biol Chem 283: 36046–36059. 10.1074/jbc.M806852200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez-Jáimez J, Palomino Doza J, Ortega A, Macias-Ruiz R, Perin F, Rodriguez-Vázquez del Rey MM, Ortiz-Genga M, Monserrat L, Barriales-Villa R, Blanca E, et al. 2016. Calmodulin 2 mutation N98S is associated with unexplained cardiac arrest in infants due to low clinical penetrance electrical disorders. PLoS ONE 11: e0153851 10.1371/journal.pone.0153851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin HB, Yang YL, Song YL, Yang YB, Li YR. 2012. Nitric oxide modulated the expression of DREAM/Calsenilin/KChIP3 in inflammatory pain of rats. Inflammation 35: 1867–1871. 10.1007/s10753-012-9508-8 [DOI] [PubMed] [Google Scholar]
- Jo DG, Jang J, Kim BJ, Lundkvist J, Jung YK. 2004. Overexpression of calsenilin enhances γ-secretase activity. Neurosci Lett 378: 59–64. [DOI] [PubMed] [Google Scholar]
- Jo J, Heon S, Kim MJ, Son GH, Park Y, Henley JM, Weiss JL, Sheng M, Collingridge GL, Cho K. 2008. Metabotropic glutamate receptor-mediated LTD involves two interacting Ca2+ sensors, NCS-1 and PICK1. Neuron 60: 1095–1111. 10.1016/j.neuron.2008.10.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo J, Son GH, Winters BL, Kim MJ, Whitcomb DJ, Dickinson BA, Lee YB, Futai K, Amici M, Sheng M, et al. 2010. Muscarinic receptors induce LTD of NMDAR EPSCs via a mechanism involving hippocalcin, AP2 and PSD-95. Nat Neurosci 13: 1216–1224. 10.1038/nn.2636 [DOI] [PubMed] [Google Scholar]
- Kabbani N, Levenson R. 2006. Antipsychotic-induced alterations in D2 dopamine receptor interacting proteins within the cortex. Neuroreport 17: 299–301. 10.1097/01.wnr.0000199460.24412.04 [DOI] [PubMed] [Google Scholar]
- Kabbani N, Negyessy L, Lin R, Goldman-Rakic P, Levenson R. 2002. Interaction with neuronal calcium sensor NCS-1 mediates desensitization of the D2 dopamine receptor. J Neurosci 22: 8476–8486. 10.1523/jneurosci.22-19-08476.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabbani N, Woll MP, Nordman JC, Levenson R. 2012. Dopamine receptor interacting proteins: Targeting neuronal calcium sensor-1/D2 dopamine receptor interaction for antipsychotic drug development. Curr Drug Targets 13: 72–79. 10.2174/138945012798868515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasri NN, Holmes AM, Bultynck G, Parys JB, Bootman MD, Rietdorf K, Missiaen L, McDonald F, Smedt HD, Conway SJ, et al. 2004. Regulation of InsP3 receptor activity by neuronal Ca2+-binding proteins. EMBO J 23: 312–321. 10.1038/sj.emboj.7600037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Ghosh S, Nunziato DA, Pitt GS. 2004. Identification of the components controlling inactivation of voltage-gated Ca2+ channels. Neuron 41: 745–754. 10.1016/S0896-6273(04)00081-9 [DOI] [PubMed] [Google Scholar]
- Kim KY, Scholl ES, Liu X, Shepherd A, Haeseleer F, Lee A. 2014. Localization and expression of CaBP1/caldendrin in the mouse brain. Neuroscience 268: 33–47. 10.1016/j.neuroscience.2014.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Lee WH, Kim MJ, Shin S, Jang B, Park JB, Wasco W, Buxbaum JD, Kim YS, Choi EK. 2018. Calsenilin, a presenilin interactor, regulates RhoA signaling and neurite outgrowth. Int J Mol Sci 19: E1196 10.3390/ijms19041196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita-Kawada M, Tang J, Xiao R, Kaneko S, Foskett JK, Zhu MX. 2005. Inhibition of TRPC5 channels by Ca2+-binding protein 1 in Xenopus oocytes. Pflugers Arch 450: 345–354. 10.1007/s00424-005-1419-1 [DOI] [PubMed] [Google Scholar]
- Kirkwood CM, MacDonald ML, Schempf TA, Vatsavayi AV, Ikonomovic MD, Koppel JL, Ding Y, Sun M, Kofler JK, Lopez OL, et al. 2016. Altered levels of visinin-like protein 1 correspond to regional neuronal loss in Alzheimer disease and frontotemporal lobar degeneration. J Neuropathol Exp Neurol 75: 175–182. 10.1093/jnen/nlv018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenchin VA, Calvert PD, Bownds MD. 1995. Inhibition of rhodopsin kinase by recoverin. Further evidence for a negative feedback system in phototransduction. J Biol Chem 270: 16147–16152. 10.1074/jbc.270.27.16147 [DOI] [PubMed] [Google Scholar]
- Kobuke K, Oki K, Gomez-Sanchez CE, Gomez-Sanchez EP, Ohno H, Itcho K, Yoshii Y, Yoneda M, Hattori N. 2018. Calneuron 1 increased Ca2+ in the endoplasmic reticulum and aldosterone production in aldosterone-producing adenoma. Hypertension 71: 125–133. 10.1161/hypertensionaha.117.10205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch KW. 2006. GCAPs, the classical neuronal calcium sensors in the retina. Calcium Bind Proteins 1: 3–6. [Google Scholar]
- Koch KW, Dell'Orco D. 2015. Protein and signaling networks in vertebrate photoreceptor cells. Front Mol Neurosci 8: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh PO, Undie AS, Kabbani N, Levenson R, Goldman-Rakic PS, Lidow MS. 2003. Up-regulation of neuronal calcium sensor-1 (NCS-1) in the prefrontal cortex of schizophrenic and bipolar patients. Proc Natl Acad Sci 100: 313–317. 10.1073/pnas.232693499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. 2006. The neurobiology of addiction: A neuroadaptational view relevant for diagnosis. Addiction 101: 23–30. 10.1111/j.1360-0443.2006.01586.x [DOI] [PubMed] [Google Scholar]
- Korhonen L, Hansson I, Kukkonen JP, Brännvall K, Kobayashi M, Takamatsu K, Lindholm D. 2005. Hippocalcin protects against caspase-12-induced and age-dependent neuronal degeneration. Mol Cell Neurosci 28: 85–95. 10.1016/j.mcn.2004.08.015 [DOI] [PubMed] [Google Scholar]
- Kuo HC, Cheng CF, Clark RB, Lin JJC, Lin JLC, Hoshijima M, Nguyêñ-Trân VTB, Gu Y, Ikeda Y, Chu PH, et al. 2001. A defect in the Kv channel-interacting protein 2 (KChIp2) gene leads to a complete loss of Ito and confers susceptibility to ventricular tachycardia. Cell 107: 801–813. 10.1016/S0092-8674(01)00588-8 [DOI] [PubMed] [Google Scholar]
- Kuo YL, Cheng JK, Hou WH, Chang YC, Du PH, Jian JJ, Rau RH, Yang JH, Lien CC, Tsaur ML. 2017. K+ channel modulatory subunits KChIP and DPP participate in Kv4-mediated mechanical pain control. J Neurosci 37: 4391–4404. 10.1523/jneurosci.1619-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kweon DH, Kong B, Shin YK. 2018. Search for a minimal machinery for Ca2+-triggered millisecond neuroexocytosis. Neuroscience. 10.1016/j.neuroscience.2018.07.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lautermilch NJ, Few AP, Scheuer T, Catterall WA. 2005. Modulation of Cav2.1 channels by the neuronal calcium-binding protein visinin-like protein-2. J Neurosci 25: 7062–7070. 10.1523/jneurosci.0447-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A, Scheuer T, Catterall WA. 2000. Ca2+/calmodulin-dependent facilitation and inactivation of P/Q-type Ca2+ channels. J Neurosci 20: 6830–6838. 10.1523/jneurosci.20-18-06830.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A, Westenbroek RE, Haeseleer F, Palczewski K, Scheuer T, Catterall WA. 2002. Differential modulation of Cav2.1 channels by calmodulin and Ca2+-binding protein 1. Nat Neurosci 5: 210–217. 10.1038/nn805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A, Zhou H, Scheuer T, Catterall WA. 2003. Molecular determinants of Ca2+/calmodulin-dependent regulation of Cav2.1 channels. Proc Natl Acad Sci 100: 16059–16064. 10.1073/pnas.2237000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A, Jimenez A, Cui G, Haeseleer F. 2007. Phosphorylation of the Ca2+-binding protein CaBP4 by protein kinase C ζ in photoreceptors. J Neurosci 27: 12743–12754. 10.1523/jneurosci.4264-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Chan J, Haeseleer F, Mikoshiba K, Palczewski K, Ikura M, Ames JB. 2009. Structural insights into Ca2+-dependent regulation of inositol 1,4,5-trisphosphate receptors by CaBP1. J Biol Chem 284: 2472–2481. 10.1074/jbc.M806513200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Enomoto M, Rossi AM, Seo MD, Rahman T, Stathopulos PB, Taylor CW, Ikura M, Ames JB. 2013. CaBP1, a neuronal Ca2+ sensor protein, inhibits inositol trisphosphate receptors by clamping intersubunit interactions. Proc Natl Acad Sci 110: 8507–8512. 10.1073/pnas.1220847110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian LY, Pandalaneni SR, Patel P, McCue HV, Haynes LP, Burgoyne RD. 2011. Characterisation of the interaction of the C-terminus of the dopamine D2 receptor with neuronal calcium sensor-1. PLoS ONE 6: e27779 10.1371/journal.pone.0027779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian LY, Pandalaneni SR, Todd PA, Martin VM, Burgoyne RD, Haynes LP. 2014. Demonstration of binding of neuronal calcium sensor-1 to the Cav2.1 P/Q-type calcium channel. Biochemistry 53: 6052–6062. 10.1021/bi500568v [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang H, DeMaria CD, Erickson MG, Mori MX, Alseikhan BA, Yue DT. 2003. Unified mechanisms of Ca2+ regulation across the Ca2+ channel family. Neuron 39: 951–960. 10.1016/S0896-6273(03)00560-9 [DOI] [PubMed] [Google Scholar]
- Lilliehook C, Bozdagi O, Yao J, Gomez-Ramirez M, Zaidi NF, Wasco W, Gandy S, Santucci AC, Haroutunian V, Huntley GW, et al. 2003. Altered Aβ formation and long-term potentiation in a calsenilin knock-out. J Neurosci 23: 9097–9106. 10.1523/jneurosci.23-27-09097.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S, Dizhoor AM, Ames JB. 2014. Structural diversity of neuronal calcium sensor proteins and insights for activation of retinal guanylyl cyclase by GCAP1. Front Mol Neurosci 7: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S, Roseman G, Peshenko I, Manchala G, Cudia D, Dizhoor AM, Millhauser G, Ames JB. 2018. Retinal guanylyl cyclase activating protein 1 forms a functional dimer. PLoS ONE 13: e0193947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limpitikul WB, Dick IE, Joshi-Mukherjee R, Overgaard MT, George AL Jr, Yue DT. 2014. Calmodulin mutations associated with long QT syndrome prevent inactivation of cardiac L-type Ca2+ currents and promote proarrhythmic behavior in ventricular myocytes. J Mol Cell Cardiol 74: 115–124. 10.1016/j.yjmcc.2014.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L, Jeanclos EM, Treuil M, Braunewell K-H, Gundelfinger ED, Anand R. 2002. The calcium sensor protein visinin-like protein-1 modulates the surface expression and agonist sensitivity of the α4β2 nicotinic acetylcholine receptor. J Biol Chem 277: 41872–41878. 10.1074/jbc.M206857200 [DOI] [PubMed] [Google Scholar]
- Lisman J, Schulman H, Cline H. 2002. The molecular basis of CaMKII function in synaptic and behavioural memory. Nat Rev Neurosci 3: 175–190. 10.1038/nrn753 [DOI] [PubMed] [Google Scholar]
- Littink KW, van Genderen MM, Collin RW, Roosing S, de Brouwer AP, Riemslag FC, Venselaar H, Thiadens AA, Hoyng CB, Rohrschneider K, et al. 2009. A novel homozygous nonsense mutation in CABP4 causes congenital cone-rod synaptic disorder. Invest Ophthalmol Vis Sci 50: 2344–2350. 10.1167/iovs.08-2553 [DOI] [PubMed] [Google Scholar]
- Liu X, Yang PS, Yang W, Yue DT. 2010. Enzyme-inhibitor-like tuning of Ca2+ channel connectivity with calmodulin. Nature 463: 968–972. 10.1038/nature08766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Hurtado A, Burgos DF, González P, Dopazo XM, González V, Rábano A, Mellström B, Naranjo JR. 2018. Inhibition of DREAM-ATF6 interaction delays onset of cognition deficit in a mouse model of Huntington's disease. Mol Brain 11: 13 10.1186/s13041-018-0359-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackler JM, Drummond JA, Loewen CA, Robinson IM, Reist NE. 2002. The C2B Ca2+-binding motif of synaptotagmin is required for synaptic transmission in vivo. Nature 418: 340–344. 10.1038/nature00846 [DOI] [PubMed] [Google Scholar]
- Makino CL, Dodd RL, Chen J, Burns ME, Roca A, Simon MI, Baylor DA. 2004. Recoverin regulates light-dependent phosphodiesterase activity in retinal rods. J Gen Physiol 123: 729–741. 10.1085/jgp.200308994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makita N, Yagihara N, Crotti L, Johnson CN, Beckmann BM, Roh MS, Shigemizu D, Lichtner P, Ishikawa T, Aiba T, et al. 2014. Novel calmodulin mutations associated with congenital arrhythmia susceptibility. Circ Cardiovasc Genet 7: 466–474. 10.1161/circgenetics.113.000459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malleret G, Haditsch U, Genoux D, Jones MW, Bliss TV, Vanhoose AM, Weitlauf C, Kandel ER, Winder DG, Mansuy IM. 2001. Inducible and reversible enhancement of learning, memory, and long-term potentiation by genetic inhibition of calcineurin. Cell 104: 675–686. 10.1016/S0092-8674(01)00264-1 [DOI] [PubMed] [Google Scholar]
- Mansilla A, Chaves-Sanjuan A, Campillo NE, Semelidou O, Martinez-Gonzalez L, Infantes L, Gonzalez-Rubio JM, Gil C, Conde S, Skoulakis EM, et al. 2017. Interference of the complex between NCS-1 and Ric8a with phenothiazines regulates synaptic function and is an approach for fragile X syndrome. Proc Natl Acad Sci 114: E999–E1008. 10.1073/pnas.1611089114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino V, Dal Cortivo G, Oppici E, Maltese PE, D'Esposito F, Manara E, Ziccardi L, Falsini B, Magli A, Bertelli M, et al. 2018. A novel p.(Glu111Val) missense mutation in GUCA1A associated with cone-rod dystrophy leads to impaired calcium sensing and perturbed second messenger homeostasis in photoreceptors. Hum Mol Genet 27: 4204–4217. [DOI] [PubMed] [Google Scholar]
- Markova O, Fitzgerald D, Stepanyuk A, Dovgan A, Cherkas V, Tepikin A, Burgoyne RD, Belan P. 2008. Hippocalcin signaling via site-specific translocation in hippocampal neurons. Neurosci Lett 442: 152–157. 10.1016/j.neulet.2008.06.089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marková S, Šafka Brožková D, Mészárosová A, Neupauerová J, Groh D, Křečková G, Laššuthová P, Seeman P. 2016. Mutations in eight small DFNB genes are not a frequent cause of non-syndromic hereditary hearing loss in Czech patients. Int J Pediatr Otorhinolaryngol 86: 27–33. 10.1016/j.ijporl.2016.04.005 [DOI] [PubMed] [Google Scholar]
- Martin VM, Johnson JR, Haynes LP, Barclay JW, Burgoyne RD. 2013. Identification of key structural elements for neuronal calcium sensor-1 function in the regulation of the temperature-dependency of locomotion in C. elegans. Mol Brain 6: 39 10.1186/1756-6606-6-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCue HV, Burgoyne RD, Haynes LP. 2009. Membrane targeting of the EF-hand containing calcium-sensing proteins CaBP7 and CaBP8. Biochem Biophys Res Commun 380: 825–831. 10.1016/j.bbrc.2009.01.177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCue HV, Haynes LP, Burgoyne RD. 2010a. Bioinformatic analysis of CaBP/calneuron proteins reveals a family of highly conserved vertebrate Ca2+-binding proteins. BMC Res Notes 3: 118 10.1186/1756-0500-3-118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCue HV, Haynes LP, Burgoyne RD. 2010b. The diversity of calcium sensor proteins in the regulation of neuronal function. Cold Spring Harb Perspect Biol 2: a004085 10.1101/cshperspect.a004085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCue HV, Burgoyne RD, Haynes LP. 2011. Determination of the membrane topology of the small EF-hand Ca2+-sensing proteins CaBP7 and CaBP8. PLoS ONE 6: e17853 10.1371/journal.pone.0017853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCue HV, Patel P, Herbert AP, Lian LY, Burgoyne RD, Haynes LP. 2012. Solution NMR structure of the Ca2+-bound N-terminal domain of CaBP7: A regulator of golgi trafficking. J Biol Chem 287: 38231–38243. 10.1074/jbc.M112.402289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFerran BW, Graham ME, Burgoyne RD. 1998. Neuronal Ca2+ sensor 1, the mammalian homologue of frequenin, is expressed in chromaffin and PC12 cells and regulates neurosecretion from dense-core granules. J Biol Chem 273: 22768–22772. 10.1074/jbc.273.35.22768 [DOI] [PubMed] [Google Scholar]
- McFerran BW, Weiss JL, Burgoyne RD. 1999. Neuronal Ca2+ sensor 1. Characterization of the myristoylated protein, its cellular effects in permeabilized adrenal chromaffin cells, Ca2+-independent membrane association, and interaction with binding proteins, suggesting a role in rapid Ca2+ signal transduction. J Biol Chem 274: 30258–30265. 10.1074/jbc.274.42.30258 [DOI] [PubMed] [Google Scholar]
- Mellström B, Naranjo JR. 2001. Ca2+-dependent transcriptional repression and derepression: DREAM, a direct effector. Seminars Cell Develop Biol 12: 59–63. 10.1006/scdb.2000.0218 [DOI] [PubMed] [Google Scholar]
- Mendez A, Burns ME, Sokal I, Dizhoor AM, Baehr W, Palczewski K, Baylor DA, Chen J. 2001. Role of guanylate cyclase-activating proteins (GCAPs) in setting the flash sensitivity of rod photoreceptors. Proc Natl Acad Sci 98: 9948–9953. 10.1073/pnas.171308998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer WA, Korhonen L, Skoglosa Y, Olssen P-A, Kukknen JP, Lindholm D. 2000. NAIP interacts with hippocalcin and protects neurons against calcium-induced cell death through caspase-3-dependent and -independent pathways. EMBO J 19: 3597–3607. 10.1093/emboj/19.14.3597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikhaylova M, Sharma Y, Reissner C, Nagel F, Aravind P, Rajini B, Smalla KH, Gundelfinger ED, Kreutz MR. 2006. Neuronal Ca2+ signaling via caldendrin and calneurons. Biochim Biophys Acta 1763: 1229–1237. 10.1016/j.bbamcr.2006.08.047 [DOI] [PubMed] [Google Scholar]
- Mikhaylova M, Reddy PP, Munsch T, Landgraf P, Suman SK, Smalla KH, Gundelfinger ED, Sharma Y, Kreutz MR. 2009. Calneurons provide a calcium threshold for trans-Golgi network to plasma membrane trafficking. Proc Natl Acad Sci 106: 9093–9098. 10.1073/pnas.0903001106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikhaylova M, Hradsky J, Kreutz MR. 2011. Between promiscuity and specificity: Novel roles of EF-hand calcium sensors in neuronal Ca2+ signalling. J Neurochem 118: 695–713. 10.1111/j.1471-4159.2011.07372.x [DOI] [PubMed] [Google Scholar]
- Mikhaylova M, Bar J, van Bommel B, Schätzle P, YuanXiang P, Raman R, Hradsky J, Konietzny A, Loktionov EY, Reddy PP, et al. 2018. Caldendrin directly couples postsynaptic calcium signals to actin remodeling in dendritic spines. Neuron 97: 1110–1125.e14. 10.1016/j.neuron.2018.01.046 [DOI] [PubMed] [Google Scholar]
- Mo M, Erdelyi I, Szigeti-Buck K, Benbow JH, Ehrlich BE. 2012. Prevention of paclitaxel-induced peripheral neuropathy by lithium pretreatment. FASEB J 26: 4696–4709. 10.1096/fj.12-214643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montani C, Ramos-Brossier M, Ponzoni L, Gritti L, Cwetsch AW, Braida D, Saillour Y, Terragni B, Mantegazza M, Sala M, et al. 2017. The X-linked intellectual disability protein IL1RAPL1 regulates dendrite complexity. J Neurosci 37: 6606–6627. 10.1523/jneurosci.3775-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore LM, England A, Ehrlich BE, Rimm DL. 2017. Calcium sensor, NCS-1, promotes tumor aggressiveness and predicts patient survival. Mol Cancer Res 15: 942–952. 10.1158/1541-7786.MCR-16-0408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morshedian A, Woodruff ML, Fain GL. 2018. Role of recoverin in rod photoreceptor light adaptation. J Physiol 596: 1513–1526. 10.1113/JP275779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Multani PK, Clarke TK, Narasimhan S, Ambrose-Lanci L, Kampman KM, Pettinati HM, Oslin DW, O'Brien CP, Berrettini WH, Lohoff FW. 2012. Neuronal calcium sensor-1 and cocaine addiction: A genetic association study in African-Americans and European Americans. Neurosci Lett 531: 46–51. 10.1016/j.neulet.2012.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura TY, Jeromin A, Mikoshiba K, Wakabayashi S. 2011. Neuronal calcium sensor-1 promotes immature heart function and hypertrophy by enhancing Ca2+ signals. Circ Res 109: 512–523. 10.1161/circresaha.111.248864 [DOI] [PubMed] [Google Scholar]
- Nakamura TY, Nakao S, Nakajo Y, Takahashi JC, Wakabayashi S, Yanamoto H. 2017. Possible signaling pathways mediating neuronal calcium sensor-1-dependent spatial learning and memory in mice. PLoS ONE 12: e0170829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanou E, Martinez GQ, Scheuer T, Catterall WA. 2012. Molecular determinants of modulation of CaV2.1 channels by visinin-like protein 2. J Biol Chem 287: 504–513. 10.1074/jbc.M111.292581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanou E, Lee A, Catterall WA. 2018. Control of excitation/inhibition balance in a hippocampal circuit by calcium sensor protein regulation of presynaptic calcium channels. J Neurosci 38: 4430–4440. 10.1523/jneurosci.0022-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro G, Aguinaga D, Moreno E, Hradsky J, Reddy PP, Cortés A, Mallol J, Casadó V, Mikhaylova M, Kreutz MR, et al. 2014. Intracellular calcium levels determine differential modulation of allosteric interactions within G protein-coupled receptor heteromers. Chem Biol 21: 1546–1556. 10.1016/j.chembiol.2014.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nef S, Fiumelli H, de Castro E, Raes MB, Nef P. 1995. Identification of a neuronal calcium sensor (NCS-1) possibly involved in the regulation of receptor phosphorylation. J Receptor Signal Trans 15: 365–378. 10.3109/10799899509045227 [DOI] [PubMed] [Google Scholar]
- Neumann B, Walter T, Heriche JK, Bulkescher J, Erfle H, Conrad C, Rogers P, Poser I, Held M, Liebel U, et al. 2010. Phenotypic profiling of the human genome by time-lapse microscopy reveals cell division genes. Nature 464: 721–727. 10.1038/nature08869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan DW, Burgoyne RD. 2003. Role of myristoylation in the intracellular targeting of neuronal calcium sensor (NCS) proteins. Biochem Soc Trans 31: 963–965. 10.1042/bst0310963 [DOI] [PubMed] [Google Scholar]
- O'Callaghan DW, Burgoyne RD. 2004. Identification of residues that determine the absence of a Ca2+/myristoyl switch in neuronal calcium sensor-1. J Biol Chem 279: 14347–14354. 10.1074/jbc.M310152200 [DOI] [PubMed] [Google Scholar]
- O'Callaghan DW, Ivings L, Weiss JL, Ashby MC, Tepikin AV, Burgoyne RD. 2002. Differential use of myristoyl groups on neuronal calcium sensor proteins as a determinant of spatio-temporal aspects of Ca2+-signal transduction. J Biol Chem 277: 14227–14237. 10.1074/jbc.M111750200 [DOI] [PubMed] [Google Scholar]
- O'Callaghan DW, Hasdemir B, Leighton M, Burgoyne RD. 2003a. Residues within the myristoylation motif determine intracellular targeting of the neuronal Ca2+ sensor protein KChIP1 to post-ER transport vesicles and traffic of Kv4 K+ channels. J Cell Sci 116: 4833–4845. 10.1242/jcs.00803 [DOI] [PubMed] [Google Scholar]
- O'Callaghan DW, Tepikin AV, Burgoyne RD. 2003b. Dynamics and calcium sensitivity of the Ca2+-myristoyl switch protein hippocalcin in living cells. J Cell Biol 163: 715–721. 10.1083/jcb.200306042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan DW, Haynes LP, Burgoyne RD. 2005. High-affinity interaction of the N-terminal myristoylation motif of the neuronal calcium sensor protein hippocalcin with phosphatidylinositol 4,5-bisphosphate. Biochem J 391: 231–238. 10.1042/BJ20051001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor V, El Far O, Bofill-Cardona E, Nanoff C, Freissmuth M, Karschin A, Airas JM, Betz H, Boehm S. 1999. Calmodulin dependence of presynaptic metabotropic glutamate receptor signalling. Science 286: 1180–1184. 10.1126/science.286.5442.1180 [DOI] [PubMed] [Google Scholar]
- O'Day DH, Eshak K, Myre MA. 2015. Calmodulin binding proteins and Alzheimer's disease. J Alzheimers Dis 46: 553–569. 10.3233/JAD-142772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olafsson P, Wang T, Lu B. 1995. Molecular cloning and functional characterization of the Xenopus Ca2+ binding protein frequenin. Proc Natl Acad Sci 92: 8001–8005. 10.1073/pnas.92.17.8001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oz S, Tsemakhovich V, Christel CJ, Lee A, Dascal N. 2011. CaBP1 regulates voltage-dependent inactivation and activation of CaV1.2 (L-type) calcium channels. J Biol Chem 286: 13945–13953. 10.1074/jbc.M110.198424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oz S, Benmocha A, Sasson Y, Sachyani D, Almagor L, Lee A, Hirsch JA, Dascal N. 2013. Competitive and non-competitive regulation of calcium-dependent inactivation in CaV1.2 L-type Ca2+ channels by calmodulin and Ca2+-binding protein 1. J Biol Chem 288: 12680–12691. 10.1074/jbc.M113.460949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palczewski K, Sokal I, Baehr W. 2004. Guanylate cyclase-activating proteins: Structure, function and diversity. Biochem Biophys Res Commun 322: 1123–1130. 10.1016/j.bbrc.2004.07.122 [DOI] [PubMed] [Google Scholar]
- Palmer CL, Lim W, Hastie PG, Toward M, Korolchuk VI, Burbidge SA, Banting G, Collingridge GL, Isaac JT, Henley JM. 2005. Hippocalcin functions as a calcium sensor in hippocampal LTD. Neuron 47: 487–494. 10.1016/j.neuron.2005.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandalaneni S, Karuppiah V, Saleem M, Haynes LP, Burgoyne RD, Mayans O, Derrick JP, Lian LY. 2015. Neuronal calcium sensor-1 binds the D2 dopamine receptor and G-protein-coupled receptor kinase 1 (GRK1) peptides using different modes of interactions. J Biol Chem 290: 18744–18756. 10.1074/jbc.M114.627059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang ZP, Cao P, Xu W, Sudhof TC. 2010. Calmodulin controls synaptic strength via presynaptic activation of calmodulin kinase II. J Neurosci 30: 4132–4142. 10.1523/jneurosci.3129-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Li C, Ames JB. 2014a. 1H, 15N, and 13C chemical shift assignments of murine calcium-binding protein 4. Biomol NMR Assign 8: 361–364. 10.1007/s12104-013-9517-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Li C, Haeseleer F, Palczewski K, Ames JB. 2014b. Structural insights into activation of the retinal L-type Ca2+ channel (Cav1.4) by Ca2+-binding protein 4 (CaBP4). J Biol Chem 289: 31262–31273. 10.1074/jbc.M114.604439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SY, Yoon SN, Kang MJ, Lee Y, Jung SJ, Han JS. 2017. Hippocalcin promotes neuronal differentiation and inhibits astrocytic differentiation in neural stem cells. Stem Cell Rep 8: 95–111. 10.1016/j.stemcr.2016.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterlini M, Revilla V, Grant AL, Wisden W. 2000. Expression of the neuronal calcium sensor protein family in the rat brain. Neuroscience 99: 205–216. 10.1016/S0306-4522(00)00201-3 [DOI] [PubMed] [Google Scholar]
- Pchitskaya E, Popugaeva E, Bezprozvanny I. 2018. Calcium signaling and molecular mechanisms underlying neurodegenerative diseases. Cell Calcium 70: 87–94. 10.1016/j.ceca.2017.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennesi ME, Howes KA, Baehr W, Wu SM. 2003. Guanylate cyclase-activating protein (GCAP) 1 rescues cone recovery kinetics in GCAP1/GCAP2 knockout mice. Proc Natl Acad Sci 100: 6783–6788. 10.1073/pnas.1130102100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson BZ, DeMaria CD, Yue DT. 1999. Calmodulin is the Ca2+ sensor for Ca2+-dependent inactivation of L-type calcium channels. Neuron 22: 549–558. 10.1016/S0896-6273(00)80709-6 [DOI] [PubMed] [Google Scholar]
- Petko JA, Kabbani N, Frey C, Woll M, Hickey K, Craig M, Canfield VA, Levenson R. 2009. Proteomic and functional analysis of NCS-1 binding proteins reveals novel signaling pathways required for inner ear development in zebrafish. BMC Neurosci 10: 27 10.1186/1471-2202-10-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pioletti M, Findeisen F, Hura GL, Minor DL. 2006. Three-dimensional structure of the KChIP1–Kv4.3 T1 complex reveals a cross-shaped octamer. Nat Struct Mol Biol 13: 987–995. 10.1038/nsmb1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pipilas DC, Johnson CN, Webster G, Schlaepfer J, Fellmann F, Sekarski N, Wren LM, Ogorodnik KV, Chazin DM, Chazin WJ, et al. 2016. Novel calmodulin mutations associated with congenital long QT syndrome affect calcium current in human cardiomyocytes. Heart Rhythm 13: 2012–2019. 10.1016/j.hrthm.2016.06.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piton A, Michaud JL, Peng H, Aradhya S, Gauthier J, Mottron L, Champagne N, Lafreniere RG, Hamdan FF, Joober R, et al. 2008. Mutations in the calcium-related gene IL1RAPL1 are associated with autism. Hum Mol Genet 17: 3965–3974. 10.1093/hmg/ddn300 [DOI] [PubMed] [Google Scholar]
- Pitt GS, Zuhlke RD, Hudmon A, Schulman H, Reuter H, Tsien RW. 2001. Molecular basis of calmodulin tethering and Ca2+-dependent inactivation of L-type Ca2+ channels. J Biol Chem 276: 30794–30802. 10.1074/jbc.M104959200 [DOI] [PubMed] [Google Scholar]
- Poetschke C, Dragicevic E, Duda J, Benkert J, Dougalis A, DeZio R, Snutch TP, Striessnig J, Liss B. 2015. Compensatory T-type Ca2+ channel activity alters D2-autoreceptor responses of Substantia nigra dopamine neurons from Cav1.3 L-type Ca2+ channel KO mice. Sci Rep 5: 13688 10.1038/srep13688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polans A, Baehr W, Palczewski K. 1996. Turned on by Ca2+! The physiology and pathology of Ca2+-binding proteins in the retina. Trends Neurosci 19: 547–554. 10.1016/S0166-2236(96)10059-X [DOI] [PubMed] [Google Scholar]
- Pongs O, Lindemeier J, Zhu XR, Theil T, Endelkamp D, Krah-Jentgens I, Lambrecht H-G, Koch KW, Schwemer J, Rivosecchi R, et al. 1993. Frequenin—A novel calcium-binding protein that modulates synaptic efficacy in the Drosophila nervous system. Neuron 11: 15–28. 10.1016/0896-6273(93)90267-U [DOI] [PubMed] [Google Scholar]
- Popugaeva E, Bezprozvanny I. 2013. Role of endoplasmic reticulum Ca2+ signaling in the pathogenesis of Alzheimer disease. Front Mol Neurosci 6: 29 10.3389/fnmol.2013.00029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prechtel H, Hartmann S, Minge D, Bähring R. 2018. Somatodendritic surface expression of epitope-tagged and KChIP binding-deficient Kv4.2 channels in hippocampal neurons. PLoS ONE 13: e0191911 10.1371/journal.pone.0191911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruunsild P, Timmusk T. 2005. Structure, alternative splicing, and expression of the human and mouse KCNIP gene family. Genomics 86: 581–593. 10.1016/j.ygeno.2005.07.001 [DOI] [PubMed] [Google Scholar]
- Rajamanoharan D, McCue HV, Burgoyne RD, Haynes LP. 2015. Modulation of phosphatidylinositol 4-phosphate levels by CaBP7 controls cytokinesis in mammalian cells. Mol Biol Cell 26: 1428–1439. 10.1091/mbc.E14-07-1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy PP, Raghuram V, Hradsky J, Spilker C, Chakraborty A, Sharma Y, Mikhaylova M, Kreutz MR. 2014. Molecular dynamics of the neuronal EF-Hand Ca2+-sensor caldendrin. PLoS ONE 9: e103186 10.1371/journal.pone.0103186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes KJ, Carroll KI, Sung MA, Doliveira LC, Monaghan MM, Burke SL, Strassle BW, Buchwalder L, Menegola M, Cao J, et al. 2004. KChIPs and Kv4α subunits as integral components of A-type potassium channels in mammalian brain. J Neurosci 24: 7903–7915. 10.1523/jneurosci.0776-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickman C, Jiménez JL, Graham ME, Archer DA, Soloviev M, Burgoyne RD, Davletov B. 2006. Conserved pre-fusion protein assembly in regulated exocytosis. Mol Biol Cell 17: 283–294. 10.1091/mbc.e05-07-0620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieke F, Lee A, Haeseleer F. 2008. Characterization of Ca2+-binding protein 5 knockout mouse retina. Invest Ophthalmol Vis Sci 49: 5126–5135. 10.1167/iovs.08-2236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riessland M, Kaczmarek A, Schneider S, Swoboda KJ, Lohr H, Bradler C, Grysko V, Dimitriadi M, Hosseinibarkooie S, Torres-Benito L, et al. 2017. Neurocalcin δ suppression protects against spinal muscular atrophy in humans and across species by restoring impaired endocytosis. Am J Hum Genet 100: 297–315. 10.1016/j.ajhg.2017.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. 1987. Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science 237: 1219–1223. 10.1126/science.2820058 [DOI] [PubMed] [Google Scholar]
- Rivas M, Villar D, González P, Dopazo XM, Mellstrom B, Naranjo JR. 2011. Building the DREAM interactome. Sci China Life Sci 54: 786–792. 10.1007/s11427-011-4196-4 [DOI] [PubMed] [Google Scholar]
- Rizo J, Rosenmund C. 2008. Synaptic vesicle fusion. Nat Struct Mol Biol 15: 665–674. 10.1038/nsmb.1450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson IM, Ranjan R, Schwarz TL. 2002. Synaptotagmins I and IV promote transmitter release independently of Ca2+ binding in the C2A domain. Nature 418: 336–340. 10.1038/nature00915 [DOI] [PubMed] [Google Scholar]
- Roca C, Martinez-Gonzalez L, Daniel-Mozo M, Sastre J, Infantes L, Mansilla A, Chaves-Sanjuan A, Gonzalez-Rubio JM, Gil C, Canada FJ, et al. 2018. Deciphering the inhibition of the neuronal calcium sensor 1 and the guanine exchange factor Ric8a with a small phenothiazine molecule for the rational generation of therapeutic synapse function regulators. J Med Chem 61: 5910–5921. 10.1021/acs.jmedchem.8b00088 [DOI] [PubMed] [Google Scholar]
- Romero-Pozuelo J, Dason JS, Mansilla A, Banos-Mateos S, Sardina JL, Chaves-Sanjuan A, Jurado-Gomez J, Santana E, Atwood HL, Hernandez-Hernandez A, et al. 2014. The guanine-exchange factor Ric8a binds to the Ca2+ sensor NCS-1 to regulate synapse number and neurotransmitter release. J Cell Sci 127: 4246–4259. 10.1242/jcs.152603 [DOI] [PubMed] [Google Scholar]
- Saab BJ, Georgiou J, Nath A, Lee FJ, Wang M, Michalon A, Liu F, Mansuy IM, Roder JC. 2009. NCS-1 in the dentate gyrus promotes exploration, synaptic plasticity, and rapid acquisition of spatial memory. Neuron 63: 643–656. 10.1016/j.neuron.2009.08.014 [DOI] [PubMed] [Google Scholar]
- Saimi Y, Kung C. 2002. Calmodulin as an ion channel subunit. Annu Rev Physiol 64: 289–311. 10.1146/annurev.physiol.64.100301.111649 [DOI] [PubMed] [Google Scholar]
- Sampath A, Strissel KJ, Elias R, Arshavsky VY, McGinnis JF, Rieke F, Hurley JB. 2005. Recoverin improves rod-mediated vision by enhancing signal transmission in the mouse retina. Neuron 46: 413–420. 10.1016/j.neuron.2005.04.006 [DOI] [PubMed] [Google Scholar]
- Sanchez-Gracia A, Romero-Pozuelo J, Ferrus A. 2010. Two frequenins in Drosophila: Unveiling the evolutionary history of an unusual neuronal calcium sensor (NCS) duplication. BMC Evol Biol 10: 54 10.1186/1471-2148-10-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scannevin RH, Wang KW, Jow F, Megules J, Kospco DC, Edris W, Carroll KC, Lu Q, Xu W, Xu Z, et al. 2004. Two N-terminal domains of Kv4 K+ channels regulate binding to and modulation by KChIP1. Neuron 41: 587–598. 10.1016/S0896-6273(04)00049-2 [DOI] [PubMed] [Google Scholar]
- Schampel A, Kuerten S. 2017. Danger: High voltage-the role of voltage-gated calcium channels in central nervous system pathology. Cells 6: 43. 10.3390/cells6040043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz P, Abdalla Elsayed MEA, Khan AO. 2017. Multimodal imaging in CABP4-related retinopathy. Ophthalmic Genet 38: 459–464. 10.1080/13816810.2017.1289543 [DOI] [PubMed] [Google Scholar]
- Schiavo G, Stenbeck G, Rothman JE, Sollner TH. 1997. Binding of the synaptic vesicle v-SNARE, synaptotagmin, to the plasma membrane t-SNARE, SNAP-25 can explain docked vesicles at neurotoxin-treated synapses. Proc Natl Acad Sci 94: 997–1001. 10.1073/pnas.94.3.997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlecker C, Boehmerle W, Jeromin A, Degray B, Varshney A, Sharma Y, Szigeti-Buck K, Ehrlich BE. 2006. Neuronal calcium sensor-1 enhancement of InsP3 receptor activity is inhibited by therapeutic levels of lithium. J Clin Invest 116: 1668–1674. 10.1172/JCI22466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnurra I, Bernstein HG, Riederer P, Braunewell KH. 2001. The neuronal calcium sensor protein VILIP-1 is associated with amyloid plaques and extracellular tangles in Alzheimer's disease and promotes cell death and tau phosphorylation in vitro: A link between calcium sensors and Alzheimer's disease? Neurobiol Dis 8: 900–909. 10.1006/nbdi.2001.0432 [DOI] [PubMed] [Google Scholar]
- Schrauwen I, Helfmann S, Inagaki A, Predoehl F, Tabatabaiefar MA, Picher MM, Sommen M, Seco CZ, Oostrik J, Kremer H, et al. 2012. A mutation in CABP2, expressed in cochlear hair cells, causes autosomal-recessive hearing impairment. Am J Hum Genet 91: 636–645. 10.1016/j.ajhg.2012.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher MA, Rivard AF, Bächinger HP, Adelman JP. 2001. Structure of the gating domain of a Ca2+-activated K+ channel complexed with Ca2+/calmodulin. Nature 410: 1120–1124. 10.1038/35074145 [DOI] [PubMed] [Google Scholar]
- Secondo A, Bagetta G, Amantea D. 2018. On the role of store-operated calcium entry in acute and chronic neurodegenerative diseases. Front Mol Neurosci 11: 87 10.3389/fnmol.2018.00087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman P. 1992. Dopamine receptor sequences. Therapeutic levels of neuroleptics occupy D2 receptors, clozapine occupies D4. Neuropsychopharmacology 7: 261–284. [PubMed] [Google Scholar]
- Seidenbecher CI, Langnaese K, Sanmarti-Vila L, Boeckers TM, Smalla KH, Sabel BA, Garner CC, Gundelfinger ED, Kreutz MR. 1998. Caldendrin, a novel neuronal calcium-binding protein confined to the somato-dendritic compartment. J Biol Chem 273: 21324–21331. 10.1074/jbc.273.33.21324 [DOI] [PubMed] [Google Scholar]
- Seidenbecher CI, Landwehr M, Smalla KH, Kreutz M, Dieterich DC, Zuschratter W, Reissner C, Hammarback JA, Böckers TM, Gundelfinger ED, et al. 2004. Caldendrin but not calmodulin binds to light chain 3 of MAP1A/B: An association with the microtubule cytoskeleton highlighting exclusive binding partners for neuronal Ca2+-sensor proteins. J Mol Biol 336: 957–970. 10.1016/j.jmb.2003.12.054 [DOI] [PubMed] [Google Scholar]
- Shaltiel L, Paparizos C, Fenske S, Hassan S, Gruner C, Rotzer K, Biel M, Wahl-Schott CA. 2012. Complex regulation of voltage-dependent activation and inactivation properties of retinal voltage-gated Cav1.4 L-type Ca2+ channels by Ca2+-binding protein 4 (CaBP4). J Biol Chem 287: 36312–36321. 10.1074/jbc.M112.392811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao X, Fernandez I, Sudhof TC, Rizo J. 1998. Solution structures of the Ca2+-free and Ca2+-bound C2A domain of synaptotagmin I: Does Ca2+ induce a conformational change? Biochemistry 37: 16106–16115. 10.1021/bi981789h [DOI] [PubMed] [Google Scholar]
- Shibata R, Misonou H, Campomanes CR, Anderson AE, Schrader LA, Doliveira LC, Carroll KI, Sweatt JD, Rhodes KJ, Trimmer JS. 2003. A fundamental role for KChIPs in determining the molecular properties and trafficking of Kv4.2 potassium channels. J Biol Chem 278: 36445–36454. 10.1074/jbc.M306142200 [DOI] [PubMed] [Google Scholar]
- Shields MC, Bowers MR, Fulcer MM, Bollig MK, Rock PJ, Sutton BR, Vrailas-Mortimer AD, Lochmuller H, Whittaker RG, Horvath R, et al. 2017. Drosophila studies support a role for a presynaptic synaptotagmin mutation in a human congenital myasthenic syndrome. PLoS ONE 12: e0184817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih PY, Lin CL, Cheng PW, Liao JH, Pan CY. 2009. Calneuron I inhibits Ca2+ channel activity in bovine chromaffin cells. Biochem Biophys Res Commun 388: 549–553. 10.1016/j.bbrc.2009.08.046 [DOI] [PubMed] [Google Scholar]
- Sinha R, Lee A, Rieke F, Haeseleer F. 2016. Lack of CaBP1/Caldendrin or CaBP2 leads to altered ganglion cell responses. eNeuro 3 10.1523/eneuro.0099-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalla KH, Seidenbecher CI, Tischmeyer W, Schicknick H, Wyneken U, Böckers TM, Gundelfinger ED, Kreutz MR. 2003. Kainate-induced epileptic seizures induce a recruitment of caldendrin to the postsynaptic density in rat brain. Mol Brain Res 116: 159–162. 10.1016/S0169-328X(03)00235-3 [DOI] [PubMed] [Google Scholar]
- Smirnov VM, Zeitz C, Soumittra N, Audo I, Defoort-Dhellemmes S. 2018. Retinal findings in a patient of French ancestry with CABP4-related retinal disease. Doc Ophthalmol 136: 135–143. [DOI] [PubMed] [Google Scholar]
- Sokal I, Haeseleer F. 2011. Insight into the role of Ca2+-binding protein 5 in vesicle exocytosis. Invest Ophthalmol Vis Sci 52: 9131–9141. 10.1167/iovs.11-8246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söllner T, Whiteheart SW, Brunner M, Erdjument-Bromage H, Geromanos S, Tempst P, Rothman JE. 1993. SNAP receptors implicated in vesicle targeting and fusion. Nature 362: 318–324. 10.1038/362318a0 [DOI] [PubMed] [Google Scholar]
- Spilker C, Dresbach T, Braunewell KH. 2002. Reversible translocation and activity-dependent localization of the calcium-myristoyl switch protein VILIP-1 to different membrane compartments in living hippocampal neurons. J Neurosci 22: 7331–7339. 10.1523/jneurosci.22-17-07331.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer NC. 2006. Electrical activity in early neuronal development. Nature 444: 707–712. 10.1038/nature05300 [DOI] [PubMed] [Google Scholar]
- Stephen R, Bereta G, Golczak M, Palczewski K, Sousa MC. 2007. Stabilizing function for myristoyl group revealed by the crystal structure of a neuronal calcium sensor, guanylate cyclase-activating protein 1. Structure 15: 1392–1402. 10.1016/j.str.2007.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strahl T, Huttner IG, Lusin JD, Osawa M, King D, Thorner J, Ames JB. 2007. Structural insights into activation of phosphatidylinositol 4-kinase (pik1) by yeast frequenin (Frq1). J Biol Chem 282: 30949–30959. 10.1074/jbc.M705499200 [DOI] [PubMed] [Google Scholar]
- Südhof TC. 2013. Neurotransmitter release: The last millisecond in the life of a synaptic vesicle. Neuron 80: 675–690. 10.1016/j.neuron.2013.10.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Südhof TC, Rothman JE. 2009. Membrane fusion: Grappling with SNARE and SM proteins. Science 323: 474–477. 10.1126/science.1161748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabolacci E, Pomponi MG, Pietrobono R, Terracciano A, Chiurazzi P, Neri G. 2006. A truncating mutation in the IL1RAPL1 gene is responsible for X-linked mental retardation in the MRX21 family. Am J Med Genet A 140: 482–487. 10.1002/ajmg.a.31107 [DOI] [PubMed] [Google Scholar]
- Tadross MR, Dick IE, Yue DT. 2008. Mechanism of local and global Ca2+ sensing by calmodulin in complex with a Ca2+ channel. Cell 133: 1228–1240. 10.1016/j.cell.2008.05.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Ames JB, Harvey TS, Stryer L, Ikura M. 1995. Sequestration of the membrane-targeting myristoyl group of recoverin in the calcium-free state. Nature 376: 444–447. 10.1038/376444a0 [DOI] [PubMed] [Google Scholar]
- Tang J, Maximov A, Shin OH, Dai H, Rizo J, Südhof TC. 2006. A complexin/synaptotagmin 1 switch controls fast synaptic vesicle exocytosis. Cell 126: 1175–1187. 10.1016/j.cell.2006.08.030 [DOI] [PubMed] [Google Scholar]
- Tang N, Lin T, Yang J, Foskett JK, Ostap EM. 2007. CIB1 and CaBP1 bind to the myo1c regulatory domain. J Muscle Res Cell Motil 28: 285–291. 10.1007/s10974-007-9124-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taverna E, Francolini M, Jeromin A, Hilfiker S, Roder J, Rosa P. 2002. Neuronal calcium sensor 1 and phosphatidylinositol 4-OH kinase β interact in neuronal cells and are translocated to membranes during nucleotide-evoked exocytosis. J Cell Sci 115: 3909–3922. 10.1242/jcs.00072 [DOI] [PubMed] [Google Scholar]
- Tian NX, Xu Y, Yang JY, Li L, Sun XH, Wang Y, Zhang Y. 2018. KChIP3 N-terminal 31-50 fragment mediates its association with TRPV1 and alleviates inflammatory hyperalgesia in rats. J Neurosci 38: 1756–1773. 10.1523/jneurosci.2242-17.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tippens AL, Lee A. 2007. Caldendrin, a neuron-specific modulator of Cav/1.2 (L-type) Ca2+ channels. J Biol Chem 282: 8464–8473. 10.1074/jbc.M611384200 [DOI] [PubMed] [Google Scholar]
- Todd PA, McCue HV, Haynes LP, Barclay JW, Burgoyne RD. 2016. Interaction of ARF-1.1 and neuronal calcium sensor-1 in the control of the temperature-dependency of locomotion in Caenorhabditis elegans. Sci Rep 6: 30023 10.1038/srep30023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimoto T, Jeromin A, Satoh N, Roder JC, Takahashi T. 2002. Neuronal calcium sensor 1 and activity-dependent facilitation of P/Q-type calcium currents at presynaptic nerve terminals. Science 295: 2276–2279. 10.1126/science.1068278 [DOI] [PubMed] [Google Scholar]
- Turecek J, Regehr WG. 2018. Synaptotagmin 7 mediates both facilitation and asynchronous release at granule cell synapses. J Neurosci 38: 3240–3251. 10.1523/jneurosci.3207-17.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venn N, Haynes LP, Burgoyne RD. 2008. Specific effects of KChIP3/calsenilin/DREAM, but not KChIPs 1, 2 and 4, on calcium signalling and regulated secretion in PC12 cells. Biochem J 413: 71–80. 10.1042/BJ20080441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinberg F, Peshenko IV, Chen J, Dizhoor AM, Kefalov VJ. 2018. Guanylate cyclase-activating protein 2 contributes to phototransduction and light adaptation in mouse cone photoreceptors. J Biol Chem 293: 7457–7465. 10.1074/jbc.RA117.001574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Yan Y, Liu Q, Huang Y, Shen Y, Chen L, Chen Y, Yang Q, Hao Q, Wang K, et al. 2007. Structural basis for modulation of Kv4 K+ channels by auxiliary KChIP subunits. Nat Neurosci 10: 32–39. 10.1038/nn1822 [DOI] [PubMed] [Google Scholar]
- Wang W, Zhong Q, Teng L, Bhatnagar N, Sharma B, Zhang X, Luther W II, Haynes LP, Burgoyne RD, Vidal M, et al. 2014. Mutations that disrupt PHOXB interaction with the neuronal calcium sensor HPCAL1 impede cellular differentiation in neuroblastoma. Oncogene 33: 3316–3324. 10.1038/onc.2013.290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Boeckel GR, Huynh L, Nguyen L, Cao W, De La Cruz EM, Kaftan EJ, Ehrlich BE. 2016. Neuronal calcium sensor 1 has two variants with distinct calcium binding characteristics. PLoS ONE 11: e0161414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Kang X, Zhou L, Chai Z, Wu Q, Huang R, Xu H, Hu M, Sun X, Sun S, et al. 2018a. Synaptotagmin-11 is a critical mediator of parkin-linked neurotoxicity and Parkinson's disease-like pathology. Nat Commun 9: 81 10.1038/s41467-017-02593-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Xiao H, Zhang J, Zhu D. 2018b. Synaptotagmin7 is overexpressed in colorectal cancer and regulates colorectal cancer cell proliferation. J Cancer 9: 2349–2356. 10.7150/jca.25098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegierski T, Kuznicki J. 2018. Neuronal calcium signaling via store-operated channels in health and disease. Cell Calcium 74: 102–111. 10.1016/j.ceca.2018.07.001 [DOI] [PubMed] [Google Scholar]
- Weiergräber OH, Senin II, Philippov PP, Granzin J, Koch KW. 2003. Impact of N-terminal myristoylation on the Ca2+-dependent conformational transition in recoverin. J Biol Chem 278: 22972–22979. 10.1074/jbc.M300447200 [DOI] [PubMed] [Google Scholar]
- Weiss JL, Burgoyne RD. 2001. Voltage-independent inhibition of P/Q-type Ca2+ channels in adrenal chromaffin cells via a neuronal Ca2+ sensor-1-dependent pathway involves Src family tyrosine kinase. J Biol Chem 276: 44804–44811. 10.1074/jbc.M103262200 [DOI] [PubMed] [Google Scholar]
- Weiss JL, Burgoyne RD. 2002. Sense and sensibility in the regulation of voltage-gated Ca2+ channels. Trends Neurosci 25: 489–491. 10.1016/S0166-2236(02)02247-6 [DOI] [PubMed] [Google Scholar]
- Weiss JL, Archer DA, Burgoyne RD. 2000. Neuronal Ca2+ sensor-1/frequenin functions in an autocrine pathway regulating Ca2+ channels in bovine adrenal chromaffin cells. J Biol Chem 275: 40082–40087. 10.1074/jbc.M008603200 [DOI] [PubMed] [Google Scholar]
- Weiss JL, Hui H, Burgoyne RD. 2010. Neuronal calcium sensor-1 regulation of calcium channels, secretion, and neuronal outgrowth. Cell Mol Neurobiol 30: 1283–1292. 10.1007/s10571-010-9588-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen H, Levitan IB. 2002. Calmodulin is an auxiliary subunit of KCNQ2/3 potassium channels. J Neurosci 22: 7991–8001. 10.1523/jneurosci.22-18-07991.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker RG, Herrmann DN, Bansagi B, Hasan BA, Lofra RM, Logigian EL, Sowden JE, Almodovar JL, Littleton JT, Zuchner S, et al. 2015. Electrophysiologic features of SYT2 mutations causing a treatable neuromuscular syndrome. Neurology 85: 1964–1971. 10.1212/WNL.0000000000002185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimberg H, Lev D, Yosovich K, Namburi P, Banin E, Sharon D, Koch KW. 2018. Photoreceptor guanylate cyclase (GUCY2D) mutations cause retinal dystrophies by severe malfunction of Ca2+-dependent cyclic GMP synthesis. Front Mol Neurosci 11: 348 10.3389/fnmol.2018.00348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingard JN, Chan J, Bosanac I, Haeseleer F, Palczewski K, Ikura M, Ames JB. 2005. Structural analysis of Mg2+ and Ca2+ binding to CaBP1, a neuron-specific regulator of calcium channels. J BiolChem 280: 37461–37470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods AS, Marcellino D, Jackson SN, Franco R, Ferre S, Agnati LF, Fuxe K. 2008. How calmodulin interacts with the adenosine A2A and the dopamine D2 receptors. J Proteome Res 7: 3428–3434. 10.1021/pr8001782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YQ, Lin X, Liu CM, Jamrich M, Shaffer LG. 2001. Identification of a human brain-specific gene, calneuron 1, a new member of the calmodulin superfamily. Mol Genet Metab 72: 343–350. 10.1006/mgme.2001.3160 [DOI] [PubMed] [Google Scholar]
- Xia Z, Storm DR. 2005. The role of calmodulin as a signal integrator for synaptic plasticity. Nat Rev Neurosci 6: 267–276. 10.1038/nrn1647 [DOI] [PubMed] [Google Scholar]
- Xia XM, Fakler B, Rivard A, Wayman G, Johnson-Pais T, Keen JE, Ishii T, Hirschberg B, Bond CT, Lutsenko S, et al. 1998. Mechanism of calcium gating in small-conductance calcium-activated potassium channels. Nature 395: 503–507. 10.1038/26758 [DOI] [PubMed] [Google Scholar]
- Xia K, Xiong H, Shin Y, Wang D, Deerinck T, Takahashi H, Ellisman MH, Lipton SA, Tong G, Descalzi G, et al. 2010. Roles of KChIP1 in the regulation of GABA-mediated transmission and behavioral anxiety. Mol Brain 3: 23 10.1186/1756-6606-3-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong H, Xia K, Li B, Zhao G, Zhang Z. 2009. KChIP1: A potential modulator to GABAergic system. Acta Biochim Biophys Sin (Shanghai) 41: 295–300. 10.1093/abbs/gmp013 [DOI] [PubMed] [Google Scholar]
- Xue M, Ma C, Craig TK, Rosenmund C, Rizo J. 2008. The Janus-faced nature of the C2B domain is fundamental for synaptotagmin-1 function. Nat Struct Mol Biol 15: 1160–1168. 10.1038/nsmb.1508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata K, Goto K, Kuo CH, Kondo H, Miki N. 1990. Visinin: A novel calcium binding protein expressed in retinal cone cells. Neuron 4: 469–476. 10.1016/0896-6273(90)90059-O [DOI] [PubMed] [Google Scholar]
- Yan J, Leal K, Magupalli VG, Nanou E, Martinez GQ, Scheuer T, Catterall WA. 2014. Modulation of CaV2.1 channels by neuronal calcium sensor-1 induces short-term synaptic facilitation. Mol Cell Neurosci 63: 124–131. 10.1016/j.mcn.2014.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan H, Wang C, Marx SO, Pitt GS. 2017. Calmodulin limits pathogenic Na+ channel persistent current. J Gen Physiol 149: 277–293. 10.1085/jgp.201611721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SN, Tang YG, Zucker RS. 1999. Selective induction of LTP and LTD by postsynaptic [Ca2+]i elevation. J Neurophysiol 81: 781–787. 10.1152/jn.1999.81.2.781 [DOI] [PubMed] [Google Scholar]
- Yang J, McBride S, Mak DOD, Vardi N, Palczewski K, Haeseleer F, Foskett JK. 2002. Identification of a family of calcium sensors as protein ligands of inositol trisphosphate receptor Ca2+ release channels. Proc Natl Acad Sci 99: 7711–7716. 10.1073/pnas.102006299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang PS, Alseikhan BA, Hiel H, Grant L, Mori MX, Yang W, Fuchs PA, Yue DT. 2006. Switching of Ca2+-dependent inactivation of Cav1.3 channels by calcium binding proteins of auditory hair cells. J Neurosci 26: 10677–10689. 10.1523/jneurosci.3236-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang PS, Johny MB, Yue DT. 2014. Allostery in Ca2+ channel modulation by calcium-binding proteins. Nat Chem Biol 10: 231–238. 10.1038/nchembio.1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T, Scholl ES, Pan N, Fritzsch B, Haeseleer F, Lee A. 2016. Expression and localization of CaBP Ca2+ binding proteins in the mouse cochlea. PLoS ONE 11: e0147495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T, Britt JK, Cintron-Perez CJ, Vázquez-Rosa E, Tobin KV, Stalker G, Hardie J, Taugher RJ, Wemmie J, Pieper AA, et al. 2018a. Ca2+-binding protein 1 regulates hippocampal-dependent memory and synaptic plasticity. Neuroscience 380: 90–102. 10.1016/j.neuroscience.2018.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T, Choi JE, Soh D, Tobin K, Joiner ML, Hansen M, Lee A. 2018b. CaBP1 regulates Cav1 L-type Ca2+ channels and their coupling to neurite growth and gene transcription in mouse spiral ganglion neurons. Mol Cell Neurosci 88: 342–352. 10.1016/j.mcn.2018.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T, Hu N, Pangršič T, Green S, Hansen M, Lee A. 2018c. Functions of CaBP1 and CaBP2 in the peripheral auditory system. Hear Res 364: 48–58. 10.1016/j.heares.2018.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin G, Hassan F, Haroun AR, Murphy LL, Crotti L, Schwartz PJ, George AL, Satin J. 2014. Arrhythmogenic calmodulin mutations disrupt intracellular cardiomyocyte Ca2+ regulation by distinct mechanisms. J Am Heart Assoc 3: e000996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan FF, Gu X, Huang X, Hou YW, Zhong Y, Lin J, Wu J. 2017. Attention-deficit/hyperactivity disorder associated with KChIP1 rs1541665 in Kv channels accessory proteins. PLoS ONE 12: e0188678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitz C, Kloeckener-Gruissem B, Forster U, Kohl S, Magyar I, Wissinger B, Mátyás G, Borruat FX, Schorderet DF, Zrenner E, et al. 2006. Mutations in CABP4, the gene encoding the Ca2+-binding protein 4, cause autosomal recessive night blindness. Am J Hum Genet 79: 657–667. 10.1086/508067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YH, Huang BL, Niakan KK, McCabe LL, McCabe ER, Dipple KM. 2004. IL1RAPL1 is associated with mental retardation in patients with complex glycerol kinase deficiency who have deletions extending telomeric of DAX1. Hum Mutat 24: 273 10.1002/humu.9269 [DOI] [PubMed] [Google Scholar]
- Zhao X, Várnai P, Tuymetovna G, Balla A, Tóth ZE, Oker-Blom C, Roder J, Jeromin A, Balla T. 2001. Interaction of neuronal calcium sensor-1 (NCS-1) with phosphatidylinositol 4-kinase β stimulates lipid kinase activity and affects membrane trafficking in COS-7 cells. J Biol Chem 276: 40183–40189. 10.1074/jbc.M104048200 [DOI] [PubMed] [Google Scholar]
- Zhou H, Kim SA, Kirk EA, Tippens AL, Sun H, Haeseleer F, Lee A. 2004a. Ca2+-binding protein-1 facilitates and forms a postsynaptic complex with Cav1.2 (L-type) Ca2+ channels. J Neurosci 24: 4698–4708. 10.1523/jneurosci.5523-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Qian Y, Kunjilwar K, Pfaffinger PJ, Choe S. 2004b. Structural insights into the functional interaction of KChIP1 with shal-type K+ channels. Neuron 41: 573–586. 10.1016/S0896-6273(04)00045-5 [DOI] [PubMed] [Google Scholar]
- Zhou H, Yu K, McCoy KL, Lee A. 2005. Molecular mechanism for divergent regulation of Cav1.2 Ca2+ channels by calmodulin and Ca2+-binding protein-1. J Biol Chem 280: 29612–29619. 10.1074/jbc.M504167200 [DOI] [PubMed] [Google Scholar]
- Zhou Q, Zhou P, Wang AL, Wu D, Zhao M, Südhof TC, Brunger AT. 2017. The primed SNARE-complexin-synaptotagmin complex for neuronal exocytosis. Nature 548: 420–425. 10.1038/nature23484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Wu Y, Luo Y, Zou Y, Ma B, Zhang Q. 2014. R102Q mutation shifts the salt-bridge network and reduces the structural flexibility of human neuronal calcium sensor-1 protein. J Phys Chem B 118: 13112–13122. 10.1021/jp507936a [DOI] [PubMed] [Google Scholar]
- Zühlke RD, Pitt GS, Deisseroth K, Tsien RY, Reuter H. 1999. Calmodulin supports both inactivation and facilitation of L-type calcium channels. Nature 399: 159–162. 10.1038/20200 [DOI] [PubMed] [Google Scholar]