Abstract
Abstract. We have shown that the kinetics of conversion of intestinal crypt cell populations to a partially or wholly mutant phenotype are consistent with a model in which each crypt contains an infrequently dividing ‘deep’ stem cell that is the progenitor of several more frequently dividing ‘proximate’ stem cells. An assumption of our model is that each deep stem cell exists in a growth inhibitory niche. We have used information from the literature to develop a model for a quiescent intestinal stem cell niche. This niche is postulated to be primarily defined by an enteroendocrine cell type that maintains stem cell quiescence by secretion of growth inhibitory peptides such as somatostatin and guanylin/uroguanylin. Consistent with this model, there is evidence that the proteins postulated as defining a growth‐inhibitory stem cell niche can act as intestinal tumour suppressors. Confirmation that a growth‐inhibitory niche does exist would have important implications for our understanding of intestinal homeostasis and tumorigenesis.
INTRODUCTION
Adult mammalian stem cells are thought to exist in specific niches that provide a microenvironment necessary for their maintenance and regulation. Recently, significant progress has been made in defining niches such as those occupied by haemopoietic and neural stem cells (Doetsch 2003; Zhang et al. 2003). A common feature of such niches is that the stem cells exist in close association with a differentiated cell type that acts as a source of regulatory signals.
The extensive proliferation that occurs in the intestinal epithelium is thought to be ultimately dependent on stem cells located in the crypts of Lieberkuhn (Marshman et al. 2002). Comparatively, little is known about the intestinal epithelium stem cell niche, although it has been postulated to be defined by the mesenchymal cells of the underlying lamina propria and by the intervening basement membrane (by Brittan & Wright 2004).
In the accompanying paper (Lobachevsky & Radford 2006), we have demonstrated that the kinetics of conversion of intestinal crypt cell populations to a partially or wholly mutant phenotype are consistent with the presence in each crypt of an infrequently dividing ‘deep’ stem cell that is the progenitor of several more frequently dividing ‘proximate’ stem cells. An assumption of our model is that each deep stem cell exists in a growth inhibitory niche. Quiescent niches for haemopoietic (Arai et al. 2004) and skin (Blanpain et al. 2004) stem cells have been identified; however, their existence in the intestinal epithelium has not been conclusively demonstrated. Indeed, it has generally been assumed that all intestinal epithelium stem cells are continuously in a multiplicative state (Potten et al. 2003). As we have discussed previously, such a state of continuous replication would result in intestinal stem cells greatly exceeding the limits normally imposed on the number of divisions that can be completed by adult mammalian cells (Lobachevsky & Radford 2006). In addition, it has been shown that the progeny of embryonic stem cells that over‐express a LEF‐1/β‐catenin fusion gene (β‐catenin can combine with a member of the TCF/LEF protein family to form a transcriptional activator that is an important mediator of proliferation in intestinal epithelium) are eliminated from the intestine of chimeric mice during development (Wong et al. 2002), supporting an absolute requirement for intestinal stem cells to be convertible to a quiescent state.
Here we present a model for a quiescent intestinal stem cell niche along with supportive evidence. We have not tried to consider/review all possible aspects of intestinal stem cell niche regulation, as this is not the purpose of this study; rather, we have examined those aspects that we believe are relevant to our model.
Searching for a niche
The intestinal crypt epithelium is a single layer of cells resting on a basement membrane and surrounded by a lamina propria that contains myofibroblasts, inflammatory cells, capillaries, lymphatics and neural processes (Madara & Trier 1987). Although some differences in basement membrane composition between crypt regions have been described (Teller & Beaulieu 2001), such differences do not appear relevant to defining the location of stem cells (Simon‐Assmann et al. 1998), and it is not obvious why one region of the crypt should be preferred to another as the locale for stem cells. Indeed, there appear to be region‐dependent differences in their placement along the rodent colon, with stem cells found at the base of crypts in the mid and distal regions but towards the centre of crypts in the proximal colon (Sato & Ahnen 1992; Potten & Grant 1998). Based on our hypothesis of a quiescent intestinal stem cell niche and on the deduction that the niche was unlikely to be solely or largely defined by elements external to the epithelium (that is, we were unable to identify components of the lamina propria that might be responsible for a highly localized growth inhibitory signal), we became interested in the distribution of cell types within the crypt epithelium.
The progeny of intestinal stem cells include both enterocytes and a variety of enteroendocrine cell types. The latter is characterized by differences in peptide hormone and biogenic amine output (Jenny et al. 2002; Rindi et al. 2004). The production of these differentiated cell types is generally assumed to follow a hierarchical model involving cell division and ultimate restriction of differentiatative and replicative ability (Jenny et al. 2002; Marshman et al. 2002). Whilst these cells are dividing and differentiating, they move up the crypt wall and are ultimately discarded (Kaur & Potten 1986). This suggests that an additional property of the intestinal stem cell niche is inhibition of migration and/or increased cellular adhesion. Indeed, studies with model systems have demonstrated the essential role of niche adhesion in stem cell maintenance and function (Song & Xie 2002).
Various studies have described the presence of enteroendocrine cells at the base of crypts from human or rodent large and small intestine (Cheng & Leblond 1974; Tsubouchi & Leblond 1979; Evans & Potten 1988; Satoh et al. 1988; Krantis et al. 1994; Nakajima et al. 1997). The presence of enteroendocrine cells in crypt bases led Evans & Potten (1988) to comment that these cells are ‘an obvious candidate of interest’ regarding niche formation. We have gone a step further and now suggest that a type of enteroendocrine cell is the critical definer of the quiescent intestinal stem cell niche and, in the remainder of this study, we present a model for how it may perform this role.
Functions of a quiescent niche
Important functions of a stem cell niche include regulation of proliferation and adhesion/migration, as well as suppression of differentiation and facilitation of asymmetric division (Spradling et al. 2001). In this section, we examine whether there is evidence to support the possible performance of these functions by an enteroendocrine cell‐defined niche.
The quiescence of haemopoietic and skin stem cell niches has been associated with the production of growth inhibitory ligands by niche‐forming cells (Arai et al. 2004; Blanpain et al. 2004). Hence, if an enteroendocrine cell type is primarily responsible for the creation of a quiescent intestinal stem cell niche, it might be expected to similarly produce growth inhibitory molecules. Consistent with this prediction, enteroendocrine cells can produce potent growth‐inhibitory peptides such as somatostatin (Patel 1999) and guanylin and its close relative, uroguanylin (Beltowski 2001). Indeed, somatostatin‐positive enteroendocrine cells were found predominantly at the base of human colonic crypts (Krantis et al. 1998), and costorage of guanylin and somatostatin has been demonstrated in enteroendocrine cells (Ieda et al. 1998; Magert et al. 1998). Accordingly, we suggest that somatostatin and guanylin/uroguanylin are responsible for maintaining intestinal stem cell quiescence. Such a postulate is consistent with the finding that multiple signalling pathways are commonly used to regulate important cellular activities (e.g. Oe et al. 2004).
The actions of guanylin/uroguanylin and somatostatin on target cells result from the activation of membrane‐bound receptors. Somatostatin receptor activation can lead to a variety of growth‐inhibitory events including: increased levels of the cyclin‐dependent kinase inhibitors 1 A (p21, Cip1) and 1B (p27, Kip1) (Alderton et al. 2001; Charland et al. 2001) and of the hypophosphorylated form of retinoblastoma‐1 protein (Sharma et al. 1999); increased production of somatostatin potentially resulting in a negative autocrine loop (Delesque et al. 1997); and activation of the tyrosine phosphatase SHP‐1, which is a negative regulator of various activated growth factor tyrosine kinase receptors, an inhibitor of β‐catenin/TCF transcriptional activity (Duchesne et al. 2003), and an activator of neuronal nitric oxide synthase, which in turn can elevate cGMP levels (via production of nitric oxide and activation of cytoplasmic guanylate cyclase) leading to inhibition of cell growth (Lopez et al. 2001). Guanylin and uroguanylin bind and activate guanylate cyclase 2C resulting in increased intracellular cGMP levels (Beltowski 2001). Increased cGMP levels may have various growth inhibitory effects including activation of a cyclic nucleotide‐gated channel that allows entry of calcium ions (Pitari et al. 2003).
Mice that are homozygous for loss of the somatostatin, guanylate cyclase 2C, uroguanylin or guanylin gene develop normally, although guanylin loss leads to an increase in colonic crypt depth that reflects an increase in crypt cell number (Schulz et al. 1997; Low et al. 2001; Steinbrecher et al. 2002; Lorenz et al. 2003). These nullizygous mouse results do not invalidate our postulate that somatostatin and guanylin/uroguanylin are mediators of niche quiescence for the following reasons: gunaylin and uroguanylin are both expressed in the intestine and have similar functions (Beltowski 2001); in addition to guanylate cyclase 2C, there appear to be other uncharacterized receptors for guanylin and uroguanylin (Carrithers et al. 1999); there may be an uncharacterized somatostatin‐related gene that is up‐regulated in intestinal tissue of mice lacking somatostatin (Ramirez et al. 2002); and, as already evidenced, the growth inhibitory effects of somatostatin and guanylin/uroguanylin may partially overlap.
As well as having growth inhibitory actions, somatostatin has been shown to increase cellular adhesion to basement membrane components (Levite et al. 1998; Talme et al. 2001) and to inhibit cell migration (Pola et al. 2003). Guanylin also appears to have a role in inhibiting intestinal crypt cell movement (Steinbrecher et al. 2002). Hence, guanylin and somatostatin can potentially perform at least two important quiescent‐niche‐defining functions (that is, inhibition of both cell proliferation and migration). γ‐Aminobutyric acid (GABA) has been found in enteroendocrine cells near the base of rat colonic crypts (Krantis et al. 1994), and has been shown to inhibit the migration of colonic carcinoma cells (Joseph et al. 2002). Hence, GABA might also contribute to the postulated retention of the deep stem cell within its niche.
An important role of the stem cell niche is to prevent differentiation. In other stem cell systems, cell contact‐dependent activation of a member of the Notch family of transmembrane receptors by a delta‐like or a jagged ligand, present on a neighbouring differentiated cell, has been associated with inhibition or induction of differentiation. Examples of notch signalling apparently facilitating proliferation and inhibiting differentiation (‘lateral inhibition’) include the haemopoietic stem cell niche, where niche‐defining osteoblasts express high levels of jagged1 (Calvi et al. 2003), and the neural stem cell niche where endothelial cells appear to perform the same function (Shen et al. 2004). We suggest that enteroendocrine cells play a similar role in the putative intestinal stem cell niche. Schonhoff et al. (2004) have previously suggested that endocrine cells can mediate lateral inhibition. In support of a role for notch signalling in the intestinal stem cell niche, it has been shown that members of the notch and jagged families are expressed in the proliferative region of intestinal crypts (Sander & Powell 2004), that Hes‐1 (a transcriptional repressor activated by notch signalling) is expressed in proliferating intestinal crypt cells but is absent from enteroendocrine cells, and that delta‐like ligands are up‐regulated in the intestine of mice lacking Hes‐1 (Jensen et al. 2000).
Another important niche property is the facilitation of asymmetric division in which a stem cell reproduces itself and also generates a second cell capable of producing differentiated progeny. An unequal distribution in daughter cells of notch signalling pathway components may, in some stem cell systems, be an important determinant of this type of division (Roegiers & January 2004). We suggest, however, that asymmetric division and the postulated presence of one deep stem cell per intestinal crypt may reflect an inability of the niche‐defining enteroendocrine cell to interact with more than one stem cell.
Regulating and maintaining a quiescent niche
The postulated existence of a quiescent intestinal niche in turn raises questions of niche regulation and maintenance, such as, how is peptide hormone release from the niche‐defining enteroendocrine cell regulated and directed to the deep stem cell? How is the deep stem cell stimulated to divide? What happens when the niche‐defining enteroendocrine cell dies or is displaced? Possible answers to these questions are presented in succeeding discussions.
Interactions between neural processes and enteroendocrine cells control crucial intestinal functions such as the peristaltic reflex (Wade et al. 1996). In addition, the results of chemical or surgical denervation suggest that the enteric nervous system has an inhibitory effect on intestinal epithelium proliferation, although the basis for this action is unclear (See et al. 1990; Hadzijahic et al. 1993). Consistent with such functional interactions, morphological studies have shown that intestinal crypts are surrounded by a network of nerve fibres (Bjerknes & Cheng 2001) and that there are close contacts between enteroendocrine cells and neural processes (Wade & Westfall 1985). Accordingly, we postulate that the niche‐defining enteroendocrine cell be regulated by interaction with neural processes.
Enteroendocrine cells share some features with neural cells, such as the uptake, synthesis, storage and release of various neurotransmitters and neuromodulators, and the presence of neural synapse‐like storage vesicles (Rindi et al. 2004). The neural cell products, GABA and nitric oxide, are potential regulators of the release from enteroendocrine cells of growth‐inhibitory peptides. GABA is an activator of cell membrane ion channels, and through GABAA receptors can produce cell type‐dependent membrane depolarization or hyperpolarization (Glassmeier et al. 1998). The depolarization of enteroendocrine cells has been shown to be coupled to the release of peptides such as somatostatin (Glassmeier et al. 1998; Patel 1999). Nitric oxide has also been linked to the release of somatostatin by endocrine cells (Burrell et al. 1996; Arebi et al. 2002). The nitric oxide may derive from nitrergic processes, which are abundant in the mucosal lamina propria (Krantis et al. 1998; Chino et al. 2002). Alternatively, nitric oxide may be produced within the enteroendocrine cell by activation of neuronal nitric oxide synthase following an increase in the intracellular calcium level (Mayer et al. 1992) produced by GABA‐induced depolarization resulting in the opening of voltage‐gated calcium channels (Mantelas et al. 2003) or by the binding of a Wnt protein of the 5a class produced by a neighbouring myofibroblast (Lickert et al. 2001). (The functioning of intestinal myofibroblasts is known to be regulated by interaction with neural processes (Powell et al. 1999)). In support of this regulatory scheme, it has been shown that a GABAergic fibre network underlies the base of rat colonic crypts (Krantis & Clark 1991), that enteroendocrine cells at the base of rat colonic crypts take up GABA (Krantis et al. 1994) and that neuronal nitric oxide synthase is found in close association with the secretory granules of somatostatin‐producing endocrine cells (Burrell et al. 1996). In view of the possible involvement of nitric oxide in both the release of growth inhibitory peptides from the niche‐forming cell and the quiescence of the putative intestinal deep stem cell, it is of interest that studies using inhibitors of nitric oxide synthase suggested that nitric oxide is a negative regulator of haemopoietic stem cell division (Michurina et al. 2004).
The postulated existence of a niche containing a single quiescent deep stem cell, which must exist in close proximity to actively dividing daughter cells, suggests a requirement for a mechanism of directed communication between the niche‐forming enteroendocrine cell and the deep stem cell. Various studies have described highly polarized somatostatin‐containing enteroendocrine cells with basal axon‐like processes that contain secretory granules and extend along the basement membrane and beneath neighbouring cells (Larsson et al. 1979; Tsubouchi & Leblond 1979; Magney et al. 1986; Satoh et al. 1988; Krantis et al. 1994). Although there is disagreement between these reports as regards the frequency in different regions of the gastrointestinal tract of enteroendocrine cells with cytoplasmic processes, it is, nevertheless, clear that such cells can be found close to the presumed location of intestinal stem cells (Satoh et al. 1988; Krantis et al. 1994). It has been suggested that differences in the length of basal processes reflect the distance between the enteroendocrine cell and its target, and that this distance may increase with the age of the cell (Satoh et al. 1988). Hence, cytoplasmic processes, or other methods of directed communication, may provide the means for paracrine signalling by the niche‐forming enteroendocrine cell.
Studies of crypt lifespan (Bjerknes 1986; Li et al. 1994) and recovery after irradiation (Martin et al. 1998) suggest that the postulated intestinal crypt niche must be both resilient and stable. How then to explain the niche's regeneration after possible loss of the niche‐forming enteroendocrine cell through death or displacement? The presence of enteroendocrine cells close to the presumed location of intestinal stem cells is difficult to explain based on classical hierarchical models of stem cell differentiation and given the continual upward movement of most differentiating crypt cells. We suggest that the niche‐forming enteroendocrine cell can, when necessary, be derived by direct differentiation of a stem cell or its daughter. Indeed, recent studies of stem cell differentiation suggest the lack of true hierarchies and that there can be considerable plasticity in stem cell differentiatative ability (Colvin et al. 2004; Zipori 2005).
An important issue is determining how a normally quiescent deep stem cell might occasionally be stimulated to divide. In the accompanying paper, we have suggested that intestinal deep stem cells have a finite replicative ability and that, in order to both provide sufficient cells to populate the crypt over a lifetime and to stay within their cell division limit, they only divide in response to loss (following apoptosis or differentiation) of a proximate stem cell (Lobachevsky & Radford 2006). That is, we are suggesting that a vacancy amongst the neighbouring proximate stem cells in some way stimulates division of the deep stem cell. Consistent with this postulate, studies of radiation effects have suggested that mouse intestinal crypt stem cells undergoing apoptosis release signals (the molecular nature of which is obscure) that initiate the replication of surviving stem cells (Tsubouchi & Potten 1985; Potten & Grant 1998).
A schematic outline of our postulated intestinal deep stem cell niche is shown in Fig. 1.
Figure 1.
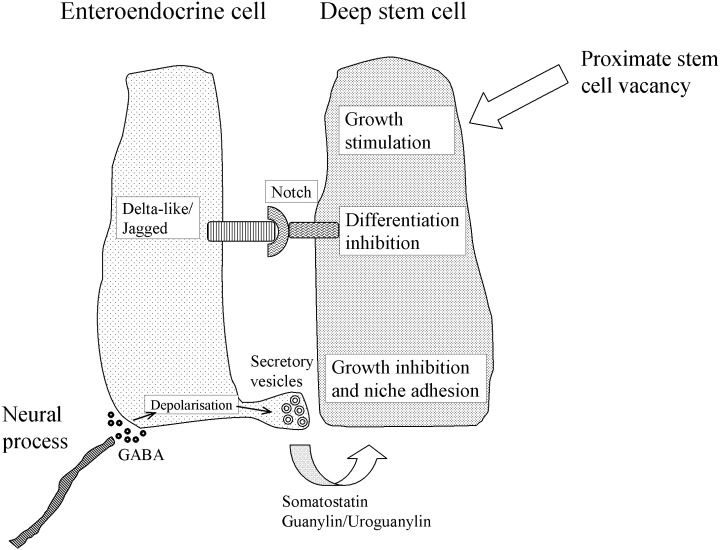
Basic features of the proposed intestinal deep stem cell niche.
Predictions of the model and further supportive evidence
The model presented in previous discussions makes a number of testable predictions. Some of these predictions are outlined in succeeding paragraphs and, where possible, compared with the available evidence.
It is commonly assumed that tumour‐initiating genetic alterations arise in stem cells (Cairns 1975). Accordingly, changes in the regulation of stem cells could potentially alter the risk of tumorigenesis. Indeed, there is evidence that the proteins postulated previously as defining a growth‐inhibitory stem cell niche can act as intestinal tumour suppressors. Several studies have linked loss of somatostatin‐ or guanylin/uroguanylin‐initiated cellular pathways to intestinal tumorigenesis. Lack of expression of somatostatin receptor 2 (an important cytostatic receptor that is expressed in colonic crypts (Warhurst et al. 1996; Pages et al. 1999)) was found to be common in human colorectal tumours (Buscail et al. 1996), and it is perhaps also relevant that the transforming growth factor‐β signalling pathway (elements of which are often mutated in human colorectal tumours (Woodford‐Richens et al. 2001)) mediates transcriptional up‐regulation of the somatostatin receptor 2 gene (Puente et al. 2001). Similarly, a frequent lack of expression of guanylin and uroguanylin in intestinal tumours has been reported (Zhang et al. 1997; Cohen et al. 1998; Shailubhai et al. 2000; Steinbrecher et al. 2000), and it is of interest that guanylate cyclase 2F receptor (which is expressed in colonic epithelium (Zhang et al. 1997), but whose ligand is currently undetermined) shows a significant frequency of mutations in human colorectal tumours (Bardelli et al. 2003).
An inverse correlation between the incidences of infectious diarrhoea and colorectal cancer, led to the suggestion that bacterial heat‐stable enterotoxin protects against gastrointestinal tumours (Pitari et al. 2003). Heat‐stable enterotoxin is a structural and functional homologue of guanylin/uroguanylin (Beltowski 2001), and its putative anticancer action has been linked to potent stimulation of the growth inhibitory guanylate cyclase 2C receptor on intestinal tumour cells (Pitari et al. 2003). An additional/alternative explanation for the link between diarrhoea and suppression of colorectal cancer, based on the intestinal stem cell niche model and the role of guanylin in intestinal cell adhesion is that chronic exposure to heat‐stable enterotoxin increases cell adhesion and thereby helps to retain precancerous stem cells within the niche, where their multiplication is more easily regulated. Two pieces of evidence are supportive of the latter suggestion. First, oral administration of uroguanylin was shown to approximately halve the incidence of intestinal polyps in genetically predisposed mice (Shailubhai et al. 2000), suggesting that uroguanylin suppresses the formation and not just the growth of polyps. Second, inactivating mutations of the adenomatous polyposis coli gene are a common early event in the development of human colorectal cancer (Rowan et al. 2000), and loss of the encoded protein decreases cell adhesion (Bienz & Hamada 2004; Faux et al. 2004). We suggest that mutation of the adenomatous polyposis coli gene be such a frequent and central event in colorectal tumorigenesis because, in addition to its well‐described effects on β‐catenin transcriptional signalling, it can lead to a decrease in cell adhesion that allows the precancerous stem cell to escape its quiescent niche.
We acknowledge that different explanations of the link between intestinal tumorigenesis and the previously mentioned growth‐suppressive molecules can be offered. Nevertheless, we suggest that our stem cell niche model provides an integrated explanation for a variety of observations.
During development and following injury, intestinal crypts have the capacity to increase their number by fission (Park et al. 1995; Cheng et al. 2000). Our model predicts that a necessary precursor to crypt fission be a doubling of the number of niche‐forming enteroendocrine cells.
A further prediction of our stem cell niche model is that there is a defined sequence of developmental events, including interaction between the enteric nervous system and a stem cell, resulting in the appearance of a niche‐forming enteroendocrine cell and culminating in the production of a monoclonal intestinal crypt. That is, we are suggesting that the stem cell selection process derives from the formation of a quiescent niche and that monoclonality reflects the size of this niche. The available evidence is limited but appears broadly consistent with this prediction. Enteroendocrine cells first appear in the mouse foetal colon at around day 15.5, with somatostatin‐containing cells found at day 18.5 (Upchurch et al. 1996). During this same period of foetal development, neuroblasts of various ultimate phenotype populate the enteric submucosal ganglia (Pham et al. 1991). Mouse intestinal crypts begin to form at around the time of birth and assume adult appearance around 14 days later (Vidrich et al. 2003), by which time the cells of most crypts are monoclonal in origin (Schmidt et al. 1988).
The ultimate defining characteristic of a niche is its ability to support stem cell activity of competent exogenous stem cells (Spradling et al. 2001). Accordingly, we predict that a proximate intestinal stem cell would function as a deep stem cell when placed in an enteroendocrine cell‐defined niche. We are not aware of evidence that supports such a prediction.
Studies of intestinal radiation response are potentially supportive of an interrelationship between enteroendocrine cells and crypt stem cells. Prostaglandin E2, synthesized via cyclooxygenase‐1 or ‐2, is known to be an important radioprotector of mouse intestinal crypt clonogens (Houchen et al. 2000; Anant et al. 2004) and has been shown to up‐regulate anti‐apoptotic proteins in target cells (Lin et al. 2001; Tessner et al. 2004). It has been reported that cyclooxygenase‐1 (Muller‐Decker et al. 1999) and ‐2 (Nakajima et al. 1997; Soslow et al. 2000) are expressed in enteroendocrine cells at or near the base of human colonic crypts, and it has been shown that mouse colonic crypt clonogenic cells are relatively radioresistant and appear to express elevated levels of the anti‐apoptotic Bcl‐2 protein (Merritt et al. 1995).
Concluding comments
We have only considered the possible form of the niche occupied by a putative intestinal deep stem cell; however, such a niche is presumed to include or exist in close inter‐relationship with the proximate stem cells. A variety of growth stimulatory proteins, which may act on proximate stem cells, has been identified, and includes members of the epidermal growth factor and Wnt families (Dignass & Sturm 2001; Brittan & Wright 2004). Recently, an important role for bone morphogenetic protein 4 (produced by mesenchymal cells in the intestinal lamina propria) in inhibiting Wnt‐β‐catenin signalling‐induced proliferation of stem cells in mouse small intestine has been identified (Haramis et al. 2004; He et al. 2004). We suggest that the latter effect acts as a brake on proximate stem cell division (for which β‐catenin signalling is a crucial initiator), rather than defining the quiescence of the deep stem cell niche. Experimental confirmation that a growth‐inhibitory niche containing a deep stem cell does exist would be an important starting point for a deeper understanding of crypt cell proliferation and its relationship to intestinal tumorigenesis.
REFERENCES
- Alderton F, Humphrey PPA, Sellers LA (2001) High‐intensity p38 kinase activity is critical for p21cip1 induction and the antiproliferative function of Gi protein‐coupled receptors. Mol. Pharmacol. 59, 1119–1128. [DOI] [PubMed] [Google Scholar]
- Anant S, Murmu N, Houchen CW, Mukhopadhyay D, Riehl TE, Young SG, Morrison AR, Stenson WF, Davidson NO (2004) Apobec‐1 protects intestine from radiation injury through posttranscriptional regulation of cyclooxygenase‐2 expression. Gastroenterology 127, 1139–1149. [DOI] [PubMed] [Google Scholar]
- Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, Takubo K, Ito K, Koh GY, Suda T (2004) Tie2/Angiopoietin‐1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell 118, 149–161. [DOI] [PubMed] [Google Scholar]
- Arebi N, Healey ZV, Bliss PW, Ghatei M, Van Noorden S, Playford RJ, Calam J (2002) Nitric oxide regulates the release of somatostatin from cultured gastric rabbit primary D‐cells. Gastroenterology 123, 566–576. [DOI] [PubMed] [Google Scholar]
- Bardelli A, Parsons DW, Silliman N, Ptak J, Szabo S, Saha S, Markowitz. S, Willson JK, Parmigiani G, Kinzler KW, Vogelstein B, Velculescu VE (2003) Mutational analysis of the tyrosine kinome in colorectal cancers. Science 300, 949. [DOI] [PubMed] [Google Scholar]
- Beltowski J (2001) Guanylin and related peptides. J. Physiol. Pharmacol. 52, 351–375. [PubMed] [Google Scholar]
- Bienz M, Hamada F (2004) Adenomatous polyposis coli proteins and cell adhesion. Curr. Opin. Cell Biol. 16, 528–535. [DOI] [PubMed] [Google Scholar]
- Bjerknes M (1986) A test of the stochastic theory of stem cell differentiation. Biophys. J. 49, 1223–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjerknes M, Cheng H (2001) Modulation of specific intestinal epithelial progenitors by enteric neurons. Proc. Natl. Acad. Sci. USA 98, 12497–12502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanpain C, Lowry WE, Geoghegan A, Polak L, Fuchs E (2004) Self‐renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell 118, 635–648. [DOI] [PubMed] [Google Scholar]
- Brittan M, Wright NA (2004) The gastrointestinal stem cell. Cell Prolif. 37, 35–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrell MA, Montuenga LM, Garcia M, Villaro AC (1996) Detection of nitric oxide synthase (NOS) in somatostatin‐producing cells of human and murine stomach and pancreas. J. Histochem. Cytochem. 44, 339–346. [DOI] [PubMed] [Google Scholar]
- Buscail L, Saint‐Laurent N, Chastre E, Vaillant JC, Gespach C, Capella G, Kalthoff H, Lluis F, Vaysse N, Susini C (1996) Loss of sst2 somatostatin receptor gene expression in human pancreatic and colorectal cancer. Cancer Res. 56, 1823–1827. [PubMed] [Google Scholar]
- Cairns J (1975) Mutation selection and the natural history of cancer. Nature 255, 197–200. [DOI] [PubMed] [Google Scholar]
- Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Bringhurst FR, Milner LA, Kronenberg HM, Scadden DT (2003) Osteoblastic cells regulate the haematopoietic stem cell niche. Nature 425, 841–846. [DOI] [PubMed] [Google Scholar]
- Carrithers SL, Hill MJ, Johnson BR, O’Hara SM, Jackson BA, Ott CE, Lorenz. J, Mann EA, Giannella RA, Forte LR, Greenberg RN (1999) Renal effects of uroguanylin and guanylin in vivo . Braz. J. Med. Biol. Res. 32, 1337–1344. [DOI] [PubMed] [Google Scholar]
- Charland S, Boucher MJ, Houde M, Rivard N (2001) Somatostatin inhibits Akt phosphorylation and cell cycle entry, but not p42/p44 mitogen‐activated protein (MAP) kinase activation in normal and tumoral pancreatic acinar cells. Endocrinology 142, 121–128. [DOI] [PubMed] [Google Scholar]
- Cheng H, Leblond CP (1974) Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. III. Enteroendocrine cells. Am. J. Anat. 141, 503–519. [DOI] [PubMed] [Google Scholar]
- Cheng L, Araki K, Furuya Y, Matsuoka T, Mashima K, Kobayashi M, Matsuura K (2000) Morphological study of the regeneration mechanism of acetic acid‐injured colon crypts in the rat. Med. Electron Microsc. 33, 165–171. [DOI] [PubMed] [Google Scholar]
- Chino Y, Fujimura M, Kitahama K, Fujimiya M (2002) Colocalization of NO and VIP in neurons of the submucous plexus in the rat intestine. Peptides 23, 2245–2250. [DOI] [PubMed] [Google Scholar]
- Cohen MB, Hawkins JA, Witte DP (1998) Guanylin mRNA expression in human intestine and colorectal adenocarcinoma. Laboratory Invest. 78, 101–108. [PubMed] [Google Scholar]
- Colvin GA, Lambert JF, Abedi M, Dooner MS, Demers D, Moore BE, Greer D, Aliotta JM, Pimentel J, Cerny J, Lum LG, Quesenberry PJ (2004) Differentiation hotspots: the deterioration of hierarchy and stochasm. Blood Cells Mol. Dis. 32, 34–41. [DOI] [PubMed] [Google Scholar]
- Delesque N, Buscail L, Esteve JP, Saint‐Laurent N, Muller C, Weckbecker G, Bruns C, Vaysse N, Susini C (1997) sst2 Somatostatin receptor expression reverses tumorigenicity of human pancreatic cancer cells. Cancer Res. 57, 956–962. [PubMed] [Google Scholar]
- Dignass AU, Sturm A (2001) Peptide growth factors in the intestine. Eur. J. Gastroenterol. Hepatol. 13, 763–770. [DOI] [PubMed] [Google Scholar]
- Doetsch F (2003) A niche for adult neural stem cells. Curr. Opin. Genet. Dev. 13, 543–550. [DOI] [PubMed] [Google Scholar]
- Duchesne C, Charland S, Asselin C, Nahmias C, Rivard N (2003) Negative regulation of β‐catenin signaling by tyrosine phosphatase SHP‐1 in intestinal epithelial cells. J. Biol. Chem. 278, 14274–14283. [DOI] [PubMed] [Google Scholar]
- Evans GS, Potten CS (1988) The distribution of endocrine cells along the mouse intestine: a quantitative immunocytochemical study. Virchows Arch. B Cell Pathol. 56, 191–199. [DOI] [PubMed] [Google Scholar]
- Faux MC, Ross JL, Meeker C, Johns T, Ji H, Simpson RJ, Layton MJ, Burgess AW (2004) Restoration of full‐length adenomatous polyposis coli (APC) protein in a colon cancer cell line enhances cell adhesion. J. Cell Sci. 117, 427–439. [DOI] [PubMed] [Google Scholar]
- Glassmeier G, Herzig KH, Hopfner M, Lemmer K, Jansen A, Scherubl H (1998) Expression of functional GABAA receptors in cholecystokinin‐secreting gut neuroendocrine murine STC‐1 cells. J. Physiol. 510, 805–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadzijahic N, Renehan WE, Ma CK, Zhang X, Fogel R (1993) Myenteric plexus destruction alters morphology of rat intestine. Gastroenterology 105, 1017–1028. [DOI] [PubMed] [Google Scholar]
- Haramis APG, Begthel H, Van Den Born M, Van Es J, Jonkheer S, Offerhaus GJA, Clevers H (2004) De novo crypt formation and juvenile polyposis on BMP inhibition in mouse intestine. Science 303, 1684–1686. [DOI] [PubMed] [Google Scholar]
- He XC, Zhang J, Tong WG, Tawfik O, Ross J, Scoville DH, Tian Q, Zeng X, He X, Wiedemann LM, Mishina Y, Li L (2004) BMP signaling inhibits intestinal stem cell self‐renewal through suppression of Wnt‐β‐catenin signaling. Nat. Genet. 36, 1117–1121. [DOI] [PubMed] [Google Scholar]
- Houchen CW, Stenson WF, Cohn SM (2000) Disruption of cyclooxygenase‐1 gene results in an impaired response to radiation injury. Am. J. Physiol. Gastrointest. Liver Physiol. 279, G858–G865. [DOI] [PubMed] [Google Scholar]
- Ieda H, Naruse S, Furuya S, Ozaki T, Ando E, Nokihara K, Hori S, Kitagawa M, Hayakawa T (1998) Coexistence of proguanylin (1–15) and somatostatin in the gastrointestinal tract. J. Gastroenterol. Hepatol. 13, 1225–1233. [PubMed] [Google Scholar]
- Jenny M, Uhl C, Roche C, Duluc I, Guillermin V, Guillemot F, Jensen J, Kedinger M, Gradwohl G (2002) Neurogenin3 is differentially required for endocrine cell fate specification in the intestinal and gastric epithelium. EMBO J. 21, 6338–6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen J, Pedersen EE, Galante P, Hald J, Heller RS, Ishibashi M, Kageyama R, Guillemot F, Serup P, Madsen OD (2000) Control of endodermal endocrine development by Hes‐1. Nat. Genet. 24, 36–44. [DOI] [PubMed] [Google Scholar]
- Joseph J, Niggemann B, Zaenker KS, Entschladen F (2002) The neurotransmitter γ‐aminobutyric acid is an inhibitory regulator for the migration of SW 480 colon carcinoma cells. Cancer Res. 62, 6467–6469. [PubMed] [Google Scholar]
- Kaur P, Potten CS (1986) Cell migration velocities in the crypts of the small intestine after cytotoxic insult are not dependent on mitotic activity. Cell Tissue Kinet. 19, 601–610. [DOI] [PubMed] [Google Scholar]
- Krantis A, Clark D (1991) Localization of [3H]GABA‐labelled nerve fibre networks in the rat intestinal mucosa. J. Auton. Nerv. Syst. 34, 195–200. [DOI] [PubMed] [Google Scholar]
- Krantis A, Tufts K, Nichols K, Morris GP (1994) [3H]GABA uptake and GABA localization in mucosal endocrine cells of the rat stomach and colon. J. Auton. Nerv. Syst. 47, 225–232. [DOI] [PubMed] [Google Scholar]
- Krantis A, Nichols K, Staines W (1998) Neurochemical characterization and distribution of enteric GABAergic neurons and nerve fibres in the human colon. J. Auton. Nerv. Syst. 68, 33–42. [DOI] [PubMed] [Google Scholar]
- Larsson LI, Goltermann N, De Magistris L, Rehfeld JF, Schwartz. TW (1979) Somatostatin cell processes as pathways for paracrine secretion. Science 205, 1393–1395. [DOI] [PubMed] [Google Scholar]
- Levite M, Cahalon L, Hershkoviz. R, Steinman L, Lider O (1998) Neuropeptides, via specific receptors, regulate T cell adhesion to fibronectin. J. Immunol. 160, 993–1000. [PubMed] [Google Scholar]
- Li YQ, Roberts SA, Paulus U, Loeffler M, Potten CS (1994) The crypt cycle in mouse small intestinal epithelium. J. Cell Sci. 107, 3271–3279. [DOI] [PubMed] [Google Scholar]
- Lickert H, Kispert A, Kutsch S, Kemler R (2001) Expression patterns of Wnt genes in mouse gut development. Mech. Dev. 105, 181–184. [DOI] [PubMed] [Google Scholar]
- Lin MT, Lee RC, Yang PC, Ho FM, Kuo ML (2001) Cyclooxygenase‐2 inducing Mcl‐1‐dependent survival mechanism in human lung adenocarcinoma CL1.0 cells. Involvement of phosphatidylinositol 3‐kinase/Akt pathway. J. Biol. Chem. 276, 48997–49002. [DOI] [PubMed] [Google Scholar]
- Lobachevsky PN, Radford IR (2006) Intestinal crypt properties fit a model that incorporates replicative ageing and deep and proximate stem cells. Cell Prolif submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez. F, Ferjoux G, Cordelier P, Saint‐Laurent N, Esteve JP, Vaysse N, Buscail L, Susini C (2001) Neuronal nitric oxide synthase: a substrate for SHP‐1 involved in sst2 somatostatin receptor growth inhibitory signaling. FASEB J. 15, 2300–2302. [DOI] [PubMed] [Google Scholar]
- Lorenz. JN, Nieman M, Sabo J, Sanford LP, Hawkins JA, Elitsur N, Gawenis LR, Clarke LL, Cohen MB (2003) Uroguanylin knockout mice have increased blood pressure and impaired natriuretic response to enteral NaCl load. J. Clin. Invest. 112, 1244–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low MJ, Otero‐Corchon V, Parlow AF, Ramirez. JL, Kumar U, Patel YC, Rubinstein M (2001) Somatostatin is required for masculinization of growth hormone‐regulated hepatic gene expression but not of somatic growth. J. Clin. Invest. 107, 1571–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madara JL, Trier JS (1987) Functional morphology of the mucosa of the small intestine In: Johnson LR, ed. Physiology of the Gastrointestinal Tract, 2nd edn New York: Raven Press, 1209–1249. [Google Scholar]
- Magert HJ, Reinecke M, David I, Raab HR, Adermann K, Zucht HD, Hill O, Hess R, Forssmann WG (1998) Uroguanylin: gene structure, expression, processing as a peptide hormone, and co‐storage with somatostatin in gastrointestinal D‐cells. Regul. Pept. 73, 165–176. [DOI] [PubMed] [Google Scholar]
- Magney JE, Erlandsen SL, Bjerknes ML, Cheng H (1986) Scanning electron microscopy of isolated epithelium of the murine gastrointestinal tract: morphology of the basal surface and evidence for paracrine‐like cells. Am. J. Anat. 177, 43–53. [DOI] [PubMed] [Google Scholar]
- Mantelas A, Stamatakis A, Kazanis I, Philippidis H, Stylianopoulou F (2003) Control of neuronal nitric oxide synthase and brain‐derived neurotrophic factor levels by GABA‐A receptors in the developing rat cortex. Brain Res. Dev. Brain Res. 145, 185–195. [DOI] [PubMed] [Google Scholar]
- Marshman E, Booth C, Potten CS (2002) The intestinal epithelial stem cell. Bioessays 24, 91–98. [DOI] [PubMed] [Google Scholar]
- Martin K, Potten CS, Roberts SA, Kirkwood TBL (1998) Altered stem cell regeneration in irradiated intestinal crypts of senescent mice. J. Cell Sci. 111, 2297–2303. [DOI] [PubMed] [Google Scholar]
- Mayer B, Klatt P, Bohme E, Schmidt K (1992) Regulation of neuronal nitric oxide and cyclic GMP formation by Ca2+ . J. Neurochem. 59, 2024–2029. [DOI] [PubMed] [Google Scholar]
- Merritt AJ, Potten CS, Watson AJM, Loh DY, Nakayama KI, Nakayama K, Hickman JA (1995) Differential expression of bcl‐2 in intestinal epithelia. Correlation with attenuation of apoptosis in colonic crypts and the incidence of colonic neoplasia. J. Cell Sci. 108, 2261–2271. [DOI] [PubMed] [Google Scholar]
- Michurina T, Krasnov P, Balazs A, Nakaya N, Vasilieva T, Kuzin B, Khrushchov N, Mulligan RC, Enikolopov G (2004) Nitric oxide is a regulator of hematopoietic stem cell activity. Mol. Ther. 10, 241–248. [DOI] [PubMed] [Google Scholar]
- Muller‐Decker K, Albert C, Lukanov T, Winde G, Marks F, Furstenberger G (1999) Cellular localization of cyclo‐oxygenase isozymes in Crohn's disease and colorectal cancer. Int. J. Colorectal Dis. 14, 212–218. [DOI] [PubMed] [Google Scholar]
- Nakajima T, Hamanaka K, Fukuda T, Oyama T, Kashiwabara K, Sano T (1997) Why is cyclooxygenase‐2 expressed in neuroendocrine cells of the human alimentary tract? Pathol. Int. 47, 889–891. [DOI] [PubMed] [Google Scholar]
- Oe S, Lemmer ER, Conner EA, Factor VM, Leveen P, Larsson J, Karlsson S, Thorgeirsson SS (2004) Intact signaling by transforming growth factor β is not required for termination of liver regeneration in mice. Hepatology 40, 1098–1105. [DOI] [PubMed] [Google Scholar]
- Pages P, Benali N, Saint‐Laurent N, Esteve JP, Schally AV, Tkaczuk J, Vaysse N, Susini C, Buscail L (1999) sst2 Somatostatin receptor mediates cell cycle arrest and induction of p27Kip1. Evidence for the role of SHP‐1. J. Biol. Chem. 274, 15186–15193. [DOI] [PubMed] [Google Scholar]
- Park HS, Goodlad RA, Wright NA (1995) Crypt fission in the small intestine and colon. A mechanism for the emergence of G6PD locus‐mutated crypts after treatment with mutagens. Am. J. Pathol. 147, 1416–1427. [PMC free article] [PubMed] [Google Scholar]
- Patel YC (1999) Somatostatin and its receptor family. Front. Neuroendocrinol. 20, 157–198. [DOI] [PubMed] [Google Scholar]
- Pham TD, Gershon MD, Rothman TP (1991) Time of origin of neurons in the murine enteric nervous system: sequence in relation to phenotype. J. Comp. Neurol. 314, 789–798. [DOI] [PubMed] [Google Scholar]
- Pitari GM, Zingman LV, Hodgson DM, Alekseev AE, Kazerounian S, Bienengraeber M, Hajnoczky G, Terzic A, Waldman SA (2003) Bacterial enterotoxins are associated with resistance to colon cancer. Proc. Natl. Acad. Sci. USA 100, 2695–2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pola S, Cattaneo MG, Vicentini LM (2003) Anti‐migratory and anti‐invasive effect of somatostatin in human neuroblastoma cells: involvement of Rac and MAP kinase activity. J. Biol. Chem. 278, 40601–40606. [DOI] [PubMed] [Google Scholar]
- Potten CS, Grant HK (1998) The relationship between ionizing radiation‐induced apoptosis and stem cells in the small and large intestine. Br. J. Cancer 78, 993–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potten CS, Booth C, Hargreaves D (2003) The small intestine as a model for evaluating adult tissue stem cell drug targets. Cell Prolif. 36, 115–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell DW, Mifflin RC, Valentich JD, Crowe SE, Saada JI, West AB (1999) Myofibroblasts. II. Intestinal subepithelial myofibroblasts. Am. J. Physiol. Cell Physiol. 277, C183–C201. [DOI] [PubMed] [Google Scholar]
- Puente E, Saint‐Laurent N, Torrisani J, Furet C, Schally AV, Vaysse N, Buscail L, Susini C (2001) Transcriptional activation of mouse sst2 somatostatin receptor promoter by transforming growth factor‐β. Involvement of Smad4. J. Biol. Chem. 276, 13461–13468. [DOI] [PubMed] [Google Scholar]
- Ramirez. JL, Mouchantaf R, Kumar U, Otero Corchon V, Rubinstein M, Low MJ, Patel YC (2002) Brain somatostatin receptors are up‐regulated in somatostatin‐deficient mice. Mol. Endocrinol. 16, 1951–1963. [DOI] [PubMed] [Google Scholar]
- Rindi G, Leiter AB, Kopin AS, Bordi C, Solcia E (2004) The ‘normal’ endocrine cell of the gut: changing concepts and new evidences. Ann. N. Y. Acad. Sci. 1014, 1–12. [DOI] [PubMed] [Google Scholar]
- Roegiers F, Jan YN (2004) Asymmetric cell division. Curr. Opin. Cell Biol. 16, 195–205. [DOI] [PubMed] [Google Scholar]
- Rowan AJ, Lamlum H, Ilyas M, Wheeler J, Straub J, Papadopoulou A, Bicknell D, Bodmer WF, Tomlinson IPM (2000) APC mutations in sporadic colorectal tumors: a mutational ‘hotspot’ and interdependence of the ‘two hits’. Proc. Natl. Acad. Sci. USA 97, 3352–3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander GR, Powell BC (2004) Expression of notch receptors and ligands in the adult gut. J. Histochem. Cytochem. 52, 509–516. [DOI] [PubMed] [Google Scholar]
- Sato M, Ahnen DJ (1992) Regional variability of colonocyte growth and differentiation in the rat. Anat. Record 233, 409–414. [DOI] [PubMed] [Google Scholar]
- Satoh Y, Oomori Y, Ishikawa K, Satoh T, Ono K (1988) Application of immunohistochemistry to the isolated mucosa of the mouse gastrointestinal tract, with special reference to somatostatin cells. Acta Anat. 133, 229–233. [DOI] [PubMed] [Google Scholar]
- Schmidt GH, Winton DJ, Ponder BA (1988) Development of the pattern of cell renewal in the crypt‐villus unit of chimaeric mouse small intestine. Development 103, 785–790. [DOI] [PubMed] [Google Scholar]
- Schonhoff SE, Giel‐Moloney M, Leiter AB (2004) Minireview: development and differentiation of gut endocrine cells. Endocrinology 145, 2639–2644. [DOI] [PubMed] [Google Scholar]
- Schulz. S, Lopez. MJ, Kuhn M, Garbers DL (1997) Disruption of the guanylyl cyclase‐C gene leads to a paradoxical phenotype of viable but heat‐stable enterotoxin‐resistant mice. J. Clin. Invest. 100, 1590–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See NA, Epstein ML, Dahl JL, Bass P (1990) The myenteric plexus regulates cell growth in rat jejunum. J. Auton. Nerv. Syst. 31, 219–229. [DOI] [PubMed] [Google Scholar]
- Shailubhai K, Yu HH, Karunanandaa K, Wang JY, Eber SL, Wang Y, Joo NS, Kim HD, Miedema BW, Abbas SZ, Boddupalli SS, Currie MG, Forte LR (2000) Uroguanylin treatment suppresses polyp formation in the Apc Min/+ mouse and induces apoptosis in human colon adenocarcinoma cells via cyclic GMP. Cancer Res. 60, 5151–5157. [PubMed] [Google Scholar]
- Sharma K, Patel YC, Srikant CB (1999) C‐terminal region of human somatostatin receptor 5 is required for induction of Rb and G1 cell cycle arrest. Mol. Endocrinol. 13, 82–90. [DOI] [PubMed] [Google Scholar]
- Shen Q, Goderie SK, Jin L, Karanth N, Sun Y, Abramova N, Vincent P, Pumiglia K, Temple S (2004) Endothelial cells stimulate self‐renewal and expand neurogenesis of neural stem cells. Science 304, 1338–1340. [DOI] [PubMed] [Google Scholar]
- Simon‐Assmann P, Lefebvre O, Bellissent‐Waydelich A, Olsen J, Orian‐Rousseau V, De Arcangelis A (1998) The laminins: role in intestinal morphogenesis and differentiation. Ann. N. Y. Acad. Sci. 859, 46–64. [DOI] [PubMed] [Google Scholar]
- Song X, Xie T (2002) DE‐cadherin‐mediated cell adhesion is essential for maintaining somatic stem cells in the Drosophila ovary. Proc. Natl. Acad. Sci. USA 99, 14813–14818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soslow RA, Dannenberg AJ, Rush D, Woerner BM, Khan KN, Masferrer J, Koki AT (2000) COX‐2 is expressed in human pulmonary, colonic, and mammary tumors. Cancer 89, 2637. [DOI] [PubMed] [Google Scholar]
- Spradling A, Drummond‐Barbosa D, Kai T (2001) Stem cells find their niche. Nature 414, 98–104. [DOI] [PubMed] [Google Scholar]
- Steinbrecher KA, Tuohy TM, Heppner Goss K, Scott MC, Witte DP, Groden J, Cohen MB (2000) Expression of guanylin is downregulated in mouse and human intestinal adenomas. Biochem. Biophys. Res. Commun. 273, 225–230. [DOI] [PubMed] [Google Scholar]
- Steinbrecher KA, Wowk SA, Rudolph JA, Witte DP, Cohen MB (2002) Targeted inactivation of the mouse guanylin gene results in altered dynamics of colonic epithelial proliferation. Am. J. Pathol. 161, 2169–2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talme T, Ivanoff J, Hagglund M, Van Neerven RJJ, Ivanoff A, Sundqvist KG (2001) Somatostatin receptor (SSTR) expression and function in normal and leukaemic T‐cells. Evidence for selective effects on adhesion to extracellular matrix components via SSTR2 and/or 3. Clin. Exp. Immunol. 125, 71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teller IC, Beaulieu JF (2001) Interactions between laminin and epithelial cells in intestinal health and disease. Expert Rev. Mol. Medical 28 September, http://www‐ermm.cbcu.cam.ac.uk/01003623h.htm . [DOI] [PubMed] [Google Scholar]
- Tessner TG, Muhale F, Riehl TE, Anant S, Stenson WF (2004) Prostaglandin E2 reduces radiation‐induced epithelial apoptosis through a mechanism involving AKT activation and bax translocation. J. Clin. Invest. 114, 1676–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsubouchi S, Leblond CP (1979) Migration and turnover of enteroendocrine and caveolated cells in the epithelium of the descending colon, as shown by radioautography after continuous infusion of 3H‐thymidine into mice. Am. J. Anat. 156, 431–451. [DOI] [PubMed] [Google Scholar]
- Tsubouchi S, Potten CS (1985) Recruitment of cells in the small intestine into rapid cell cycle by small doses of external gamma or internal beta‐radiation. Int. J. Radiat. Biol. 48, 361–369. [DOI] [PubMed] [Google Scholar]
- Upchurch BH, Fung BP, Rindi G, Ronco A, Leiter AB (1996) Peptide YY expression is an early event in colonic endocrine cell differentiation: evidence from normal and transgenic mice. Development 122, 1157–1163. [DOI] [PubMed] [Google Scholar]
- Vidrich A, Buzan JM, Cohn SM (2003) Intestinal stem cells and mucosal gut development. Curr. Opin. Gastroenterol. 19, 583–590. [DOI] [PubMed] [Google Scholar]
- Wade PR, Westfall JA (1985) Ultrastructure of enterochromaffin cells and associated neural and vascular elements in the mouse duodenum. Cell Tissue Res. 241, 557–563. [DOI] [PubMed] [Google Scholar]
- Wade PR, Chen J, Jaffe B, Kassem IS, Blakely RD, Gershon MD (1996) Localization and function of a 5‐HT transporter in crypt epithelia of the gastrointestinal tract. J. Neurosci. 16, 2352–2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warhurst G, Higgs NB, Fakhoury H, Warhurst AC, Garde J, Coy DH (1996) Somatostatin receptor subtype 2 mediates somatostatin inhibition of ion secretion in rat distal colon. Gastroenterology 111, 325–333. [DOI] [PubMed] [Google Scholar]
- Wong MH, Huelsken J, Birchmeier W, Gordon JI (2002) Selection of multipotent stem cells during morphogenesis of small intestinal crypts of Lieberkuhn is perturbed by stimulation of Lef‐1/β‐catenin signaling. J. Biol. Chem. 277, 15843–15850. [DOI] [PubMed] [Google Scholar]
- Woodford‐Richens KL, Rowan AJ, Gorman P, Halford S, Bicknell DC, Wasan HS, Roylance RR, Bodmer WF, Tomlinson IP (2001) SMAD4 mutations in colorectal cancer probably occur before chromosomal instability, but after divergence of the microsatellite instability pathway. Proc. Natl. Acad. Sci. USA 98, 9719–9723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Zhou W, Velculescu VE, Kern SE, Hruban RH, Hamilton SR, Vogelstein B, Kinzler KW (1997) Gene expression profiles in normal and cancer cells. Science 276, 1268–1272. [DOI] [PubMed] [Google Scholar]
- Zhang J, Niu C, Ye L, Huang H, He X, Tong WG, Ross J, Haug J, Johnson T, Feng JQ, Harris S, Wiedemann LM, Mishina Y, Li L (2003) Identification of the haematopoietic stem cell niche and control of the niche size. Nature 425, 836–841. [DOI] [PubMed] [Google Scholar]
- Zipori D (2005) The stem state: plasticity is essential, whereas self‐renewal and hierarchy are optional. Stem Cells 23, 719–726. [DOI] [PubMed] [Google Scholar]


