Abstract
Objectives
To investigate effects of platelet‐rich plasma on tendon cell proliferation and the underlying molecular mechanisms.
Materials and methods
Platelet‐rich plasma was prepared manually by two‐step centrifugation. Proliferation was evaluated in cultured rat tendon cells by the 3‐[4,5‐dimethylthiazol‐2‐yl]‐2,5‐diphenyltetrazolium bromide assay. Cell cycle progression was assessed by flow cytometry. Messenger RNA expression of proliferating cell nuclear antigen (PCNA), cyclin E1, A2 and B1, and cyclin‐dependent kinases (Cdks) 1 and 2 was assessed by real‐time polymerase chain reaction. Protein expression of the above cyclins and Cdks and of signal transducer and activator of transcription (Stat) 3 and p27 was evaluated by western blotting.
Results
Platelet‐rich plasma used in the present study had concentrations of platelets, TGF‐β1 and PDGF over 3‐fold higher than normal whole blood. Platelet‐rich plasma enhanced tendon cell proliferation (P = 0.008) by promoting G1/S phase transition in the cell cycle, and increased expression of PCNA, cyclin E1, A2 and B1, Cdks1 and 2, and phosphorylated Stat3, while inhibiting p27 expression.
Conclusions
Platelet‐rich plasma contains high concentrations of TGF‐β1 and PDGF that increase tendon cell proliferation by modulating Stat3/p27Kip1, which enhances expression of cyclin–Cdk complexes that promote cell cycle progression. These results provide molecular evidence for positive effects of platelet‐rich plasma on tendon cell proliferation, which can be useful in clinical applications of tendon injury.
Introduction
Tendon damage and tendinopathy are common injuries that occur during athletic activity. These injuries can lead to loss of function and may interfere with both sporting and normal daily pursuits. In 24–45.5% of patients with Achilles tendinopathy, conservative management is unsuccessful and surgery must be considered 1. There is clear need for improved conservative therapy. Recent application of platelet‐rich plasma (PRP) to treat tendon injury has raised high expectations for both clinicians and patients 2. In tendon healing, proliferating tendon cells produce collagen for tissue repair; various growth factors such as insulin‐like growth factor (IGF), transforming growth factor (TGF) and platelet‐derived growth factor (PDGF) have been implicated in aspects of tendon cell proliferation 3, 4. It is thus thought that exogenous growth factor application can increase tendon cell proliferation. PRP, by definition, has supra‐physiological levels of platelets, which can be activated to produce high concentrations of growth factors 5, 6. These may then act synergistically to promote tendon cell proliferation. However, to date, available clinical research has shown only inconsistent results with the use of PRP 7, 8, 9, 10, 11, and large and carefully designed randomized clinical trials are needed to draw definitive conclusions on potential benefits of platelet‐rich plasma. In basic science, in addition to fundamental knowledge of differences in preparation of PRP and principles of tissue healing, an understanding of mechanisms of action of PRP is essential to successful application of this blood preparation.
Cell proliferation is governed by the cell cycle, comprised of G0/G1, S, G2 and M phases 12. These are regulated precisely by sequential activation of cyclin‐dependent kinases (Cdks) that form complexes with various cyclins 13 and phosphorylate downstream proteins such as retinoblastoma protein (Rb). Accumulation of cyclins and their activating Cdks has previously been thought to be the main regulator of passage through G1/S and G2/M transitions, and a number of families of growth factors have been identified as positive regulators of cell cycle progression 14. One study that provided evidence for epidermal growth factor (EGF)‐induced DNA synthesis suggested a role for EGF ligands in regulating G1 progression 15. Over‐expression of TGF in transgenic mice leads to hyperplasia and up‐regulation of cyclin 16, and treatment of normal mammary epithelial cells with IGF induces increase in mRNA levels of cyclins E1, A2, and B1, thereby promoting cell cycle entry to quiescent cells 17. Given that PRP contains high concentrations of these growth factors, it has been hypothesized that it could regulate cyclin and Cdk expression to stimulate cell cycle progression and cell proliferation.
Many cytokines and growth factors signal through the Janus kinase (JAK)/signal transducer and activator of transcription (Stat) pathway 18 and certain growth factor receptors (for example, those of EGF and PDGF), possess intrinsic tyrosine kinase activity that is stimulated by ligand binding, which directly phosphorylates and activates Stat protein 19. Members of the Stat family moderate expression of a variety of gene products that promote cell proliferation 20. Cdk inhibitor p27Kip1 plays a critical role in regulating cell proliferation in response to the extracellular growth environment and evidence suggests that suppression of p27 is essential for cell proliferation 21. One of the mechanisms that suppresses p27 has been postulated to involve the JAK/Stat3 signalling pathway. It has been reported that blocking JAK/Stat3 signalling leads to up‐regulation of p27 and p21 expression, which inhibits Cdk‐induced cell proliferation 22. Therefore, transcriptional regulator Stat3, may contribute to action of PRP on tendon cell proliferation by modulating cell cycle progression by down‐regulation of p27.
The aim of this study was to investigate effects of PRP on cultured tendon cells, focusing on molecular mechanisms underlying induction of cell proliferation by PRP.
Materials and methods
All experimental procedures were approved by the Institutional Review Board of our hospital prior to initiation of this study. All assays were performed in at least triplicate.
Primary culture of rat Achilles tendon cells
Tendon cells were obtained from Sprague–Dawley rats (weighing 200–250 g) as previously described 23. Cells were cultured and sampled from passages 2 to 4 having appropriate growth rate and normal fibroblast shape, to be used for the experiments.
Collection and preparation of PRP
PRP was obtained from adult Sprague–Dawley rats (weighing 250–300 g). Animals were anesthetized and 9 ml blood was drawn from the heart of each using a 21 gauge needle. It was collected into 10 ml syringes containing 1 ml anticoagulant (acid citrate dextrose solution) to total volume of 10 ml. Both PRP and platelet‐poor plasma (PPP) were manually isolated by a two‐step platelet concentration method modified from a published protocol 24. Anticoagulant‐treated blood was centrifuged at 800 × g for 30 min for separation into plasma and erythrocyte fractions, and the plasma was further centrifuged at 3000 × g for 30 min to separate PRP (containing a high number of platelets) from PPP (containing few platelets). A total of 2 ml PRP was obtained from each 10 ml whole blood. PRP was clotted by adding thrombin solution (500 U/ml in 100 mm CaCl2) for 1 h. After centrifugation at 5500 × g for 15 min, soluble PRP releasate was isolated from the clotted preparation. Final releasate was cleared by ultra‐filtration (0.22 μm) and frozen at −80 °C until used.
Determination of platelet and white blood cell count, TGF‐β1 and PDGF‐BB levels
Platelet concentration and white blood cell count (WBC) were assessed in samples of whole blood and PRP before clotting, by using a Hemavet 950 hematology analyzer (Drew Scientific, Waterbury, CT, USA). Serum (whole blood) and PRP releasate concentrations of TGF‐β1 and PDGF‐BB were determined using specific enzyme‐linked immunosorbent assay kits (R & D system, Minneapolis, MN, USA) according to the manufacturers' instructions. Absorbance was measured using a VICTOR X3 multi‐well plate reader (PerkinElmer Inc., Waltham, MA, USA) at 450 nm. Growth factor concentrations were determined using a standard curve.
Cell culture
Trypsinized rat tendon cells were pre‐cultured for 24 h in Dulbecco's Modified Eagle's Medium containing 10% FBS and supplements. Cells were then cultured in serum‐free medium under five different conditions: medium only (control), or 0.1%, 0.5%, 1% or 2% PRP, and incubated at 37 °C in a humidified atmosphere of 5% CO2/95% air for 24 h.
Determination of cell viability using 3‐[4,5‐dimethyl‐thiazol‐2‐yl]‐2,5‐diphenyltetrazolium bromide (MTT) assay
Tendon cell viability was examined 24 h after treatment by adding 50 μg/ml MTT and incubating at 37 °C for 1 h. Solutions were discarded and 1 ml dimethyl sulphoxide was added to dissolve formazan crystals. Aliquots were transferred to a 96‐well plate and absorbance was measured at 570 nm using a scanning multi‐well plate reader (VICTOR X3, PerkinElmer Inc.).
Cell cycle analysis
Tendon cells subjected to various treatments were washed twice in PBS and fixed by resuspension in 1 ml of 70% methanol in PBS for 1 h at −20 °C. They were centrifuged at 3000 × g for 5 min, resuspended in 1 ml of 0.5% Triton X‐100 with 0.05% RNase A in PBS, and incubated at 37 °C for 1 h. Suspensions were centrifuged, washed and resuspended in 1 ml 50 μg/ml propidium iodide in PBS. Cells were stored overnight at 4 °C and were then analysed on a FACScan flow cytometer (Becton Dickinson, San Francisco, CA, USA).
RNA isolation, reverse transcription and quantitative real‐time polymerase chain reaction (PCR)
Total cell RNA from tendon cells was isolated by lysing cells in a guanidine‐isothiocyanate solution (UltraPure, Life Technologies Inc.) followed by phenol–chloroform–isoamyl alcohol extraction, as previously described 23. Total RNA (1 μg) was reverse‐transcribed into cDNA by incubation with 200 U reverse transcriptase in 20 μl reaction buffer containing 0.25 μg random primers and 0.8 mm dNTPs at 42 °C for 1 h. Quantitative real‐time PCR was performed using 20 ng cDNA and the SYBR Green and Mx3000P QPCR system (Stratagene, La Jolla, CA, USA), with each reaction run in triplicate. Oligonucleotide sequences for specific primers used were as follows: for 18S rRNA, sense 5′ CCATAAACGATGCCGACTGG 3′ and antisense 5′ TCAAATTAAGCCGCAGGCTC 3′; for PCNA, sense 5′ CCGGGACCTTAGCCATATTG 3′ and antisense 5′ GCTGAACTGGCTCATTCATCTC 3′; for Cdk 1, sense 5′ TGGCCAGTTCATGGATTC 3′ and antisense 5′ GCCGAAATCTGCCAGTTTG 3′; for Cdk 2, sense 5′ CACTTAACCCGACTTCCAG 3′ and antisense 5′ TTCCCTCAACACGGTAAC 3′; for cyclin E1 sense 5′ GCATCACAACAGAATATCATAA 3′ and antisense 5′ AAGCACCATCAGTAACATAA 3′; for cyclin A2, sense 5′ CACGTACCTTAGGGAAATGG 3′ and antisense 5′ CCAAATGCAGGGTCTCATTC 3′; and for cyclin B1 sense 5′ TGAGCCTGAACCTGTTATGG 3′ and antisense 5′ CCACCATCGTCTGCATCTAC 3′. Relative gene expression between experimental groups was determined using MxPro software (Stratagene), with 18S rRNA used as an internal control.
Western blot analysis
Cell extracts were prepared as previously described 23. Samples with identical protein quantities were separated by 10% sodium dodecyl sulphate polyacrylamide gel electrophoresis and transferred to a polyvinylidene difluoride membrane. Membranes were incubated at room temperature in blocking solution (1% bovine serum albumin and 1% goat serum in PBS) for 1 h, followed by 2‐h incubation in blocking solution containing one of the following primary antibodies: anti‐ tubulin, PCNA (NeoMarkers, Fremont, CA, USA), Cdk 1 or 2, cyclin E1, A2 or B1 (ABclonal Biotechnology Co. Ltd., Wuhan, China), p27 or phosphorylated Stat3 (Cell Signaling Technology Inc., Beverly, MA, USA). After washing, membranes were incubated in PBS containing horseradish peroxidase‐conjugated secondary antibody (Sigma, St. Louis, MO, USA) for 1 h. Membranes were washed and positive signals were developed using enhanced chemiluminescence reagent (Amershan Pharmacia Biotech, Buckinghamshire, UK). For quantification of protein expression relative to tubulin, densitometric analysis was performed using a BioSpectrum 500 automated imaging system (UVP Inc., Upland, CA, USA).
Statistical analysis
All data are expressed as mean ± standard error of the mean. Comparisons of tendon cell viability and gene and protein expression under the chosen conditions were carried out using the Kruskal–Wallis test. Mann–Whitney testing was used to identify where differences occurred. Level of statistical significance was set at P < 0.05.
Results
The PRP had high concentrations of platelets, TGF‐β1 and PDGF‐BB
Platelet and WBC counts were performed on whole blood and PRP preparations from rats. Number of platelets in acid citrate dextrose‐treated whole blood was 937 ± 55 × 106/ml; the count in PRP being 3.81‐fold higher at 3584 ± 322 × 106/ml. Number of WBCs was comparable for these two preparations (5.404 ± 0.646 × 103/ml for whole blood and 6.568 ± 1.029 × 103/ml for PRP; P = 0.465). TGF‐β1 and PDGF‐BB levels in whole blood and in PRP releasates were quantified in the first three samples. Mean concentrations of the two growth factors in PRP releasate were 138.88 and 23.26 ng/ml, respectively, which were 3.19 and 4.26 times higher, respectively, than in whole blood releasates. These results indicate that our PRP preparations had significantly higher concentration of platelets and TGF‐β1 and PDGF‐BB than whole blood (P = 0.009, 0.034, and 0.019 respectively) (Fig. 1).
Figure 1.
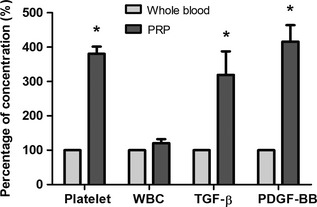
Concentrations of platelet and WBC counts and levels of TGF ‐β1 and PDGF ‐ BB in PRP compared to whole blood ( n = 4). *P < 0.05.
Tendon cell proliferation was enhanced by PRP
To assess effects of PRP on cell proliferation, cultures were treated with medium alone as a control, or 0.1%, 0.5%, 1% or 2% PRP releasate for 24 h. PRP increased tendon cell proliferation relative to controls in a dose‐dependent manner (120.3 ± 2.84%, 145.5 ± 4.11%, 157.1 ± 2.48% and 181.4 ± 1.04% increases for cultures treated with 0.1%, 0.5%, 1% and 2% of PRP releasate respectively; P = 0.008) (Fig. 2), indicating that the PRP enhanced proliferation of our cultured tendon cells.
Figure 2.
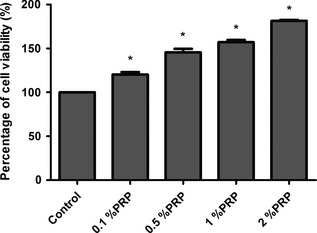
Viability of PRP ‐treated and untreated (control) tendon cells as evaluated by MTT assay ( n = 4). *P < 0.05.
PRP increased S phase fraction of tendon cells
Fraction of PRP‐treated cells in each phase of the cell cycle was assessed by flow cytometry. Percentage of cells in G0/G1 markedly decreased after PRP treatment, in a dose‐dependent manner (91.00 ± 1.05%, 89.77 ± 1.74%, 84.87 ± 3.12% and 74.68 ± 3.47% for cultures treated with 0.1%, 0.5%, 1% and 2% PRP releasate, respectively, versus 90.07 ± 1.54% control). Concomitant increase in number of S phase cells was observed in treated cells (2.05 ± 1.10%, 3.29 ± 1.13%, 6.53 ± 1.35% and 16.02 ± 1.29% for cultures treated with 0.1%, 0.5%, 1% and 2% PRP releasate, respectively, versus 2.10 ± 1.34% control; P = 0.006) (Fig. 3). These data provide evidence of increased cell cycle progression of our tendon cells treated with the PRP, which lead more tendon cells into division.
Figure 3.
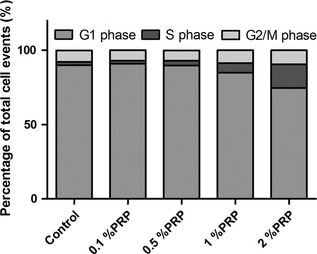
Flow cytometry analysis of untreated or PRP ‐treated rat tendon cells ( n = 4).
Expression of genes involved in cell cycle regulation was up‐regulated by PRP treatment
Quantitative real‐time PCR analysis of cell cycle‐related gene expression was carried out. Cycling of sub‐confluent tendon cells was synchronized by culturing in low serum medium (0.2% FBS) for 48 h; medium alone was added to control cells, while treatment groups received 0.1%, 0.5%, 1% or 2% PRP, with gene expression measured 18 h later. In tendon cells treated with PRP, mRNA levels of PCNA, Cdks 1 and 2, and cyclin E1, A2 and B1 increased dose‐dependently with PRP treatment (Fig. 4). These findings indicate that critical factors required for cell cycle progression were up‐regulated by the PRP treatment, accounting for its positive effect on tendon cell proliferation.
Figure 4.
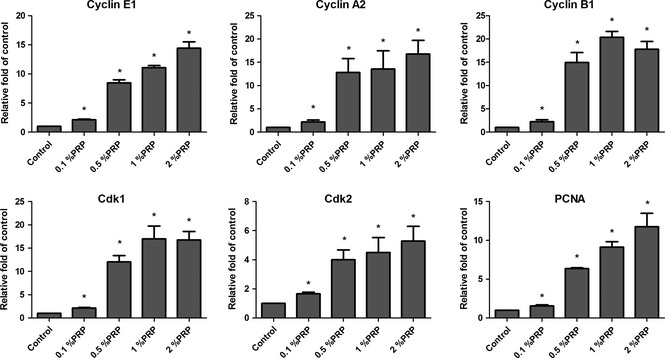
Transcript expression of PCNA , cyclin E1 , A2 and B1 , and Cdks 1 and 2 in untreated or PRP ‐treated tendon cells, as assessed by real‐time quantitative PCR , with 18S rRNA used as an internal control ( n = 3). *P < 0.05.
PRP acted through Stat3 signalling to increase tendon cell proliferation
Level of protein expression in PRP‐treated tendon cells was evaluated in cells synchronized by treatment with low concentration FBS (0.2%) for 48 h, which was followed by addition of 0%, 0.1%, 0.5%, 1% or 5% PRP to culture media and western blotting of cell lysates 18 h later. Addition of more than 0.5% PRP induced increase in PCNA expression (Fig. 5). Expression levels of cell cycle regulatory proteins Cdks 1 and 2 and cyclin E1, A2 and B1 showed PRP dose‐dependent increases (Fig. 5). Moreover, PRP treatment increased phosphorylated Stat3 and reduced p27 expression (Fig. 6), suggesting that the proliferative effect of PRP on tendon cells was exerted via cell cycle‐mediated activation of Stat3/p27 signalling.
Figure 5.
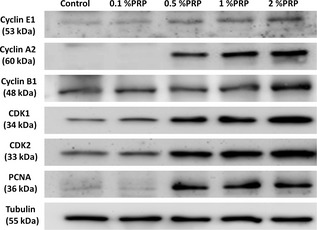
Protein expression of PCNA , cyclin E1, A2 and B1, Cdks 1 and 2 in untreated and PRP ‐treated tendon cells. Tubulin was used as a loading control (n = 3).
Figure 6.
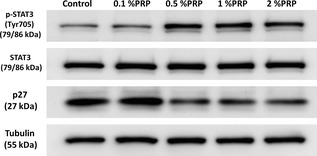
Protein expression of Stat 3, phosphorylated Stat3 and p27 in untreated and PRP ‐treated tendon cells. Tubulin was used as a loading control (n = 3).
Discussion
Activation of platelets and platelet counts of PRP or growth factor concentration in its releasate, are two critical parameters that determine the effects of PRP 25. It is widely presumed that PRP enhances platelet and growth factor concentrations 3‐ to 5‐fold over baseline 6, 26. Platelets are known to release many kinds of growth factor, such as PDGF, TGF‐β1, epidermal growth factor (EGF) and vascular endothelial growth factor (VEGF) 5, 6; of these, PDGF and TGF‐β are present at the highest concentrations, and are known to stimulate cell proliferation (6,27), and are used to quantify PRP preparations (6,28‐30). PRP used in the present study had concentrations of platelets, PDGF and TGF‐β over 3‐fold higher than whole blood that are required to achieve the biological effects observed in most studies.
It has been shown that PRP has potential to stimulate bone regeneration by inducing mitogenic activity of cultured trabecular bone‐derived cells 31 or proliferation of osteoblasts 32. It has also been shown to promote proliferation of gingival fibroblasts, epithelial cells and articular chondrocytes 27, 33. Data presented here indicate that PRP also has a proliferative effect on cultured rat tendon cells.
PCNA acts as a clamp for DNA polymerase‐δ during DNA replication and is widely used as a marker of cell proliferation 34. In addition, during DNA synthesis, PCNA interacts with proteins involved in cell cycle control 35. This study showed increase in PCNA mRNA and protein expression associated with PRP‐induced tendon cell proliferation. This finding suggests that the observed increase in tendon cell proliferation after PRP treatment acted through a PCNA‐related mechanism.
In unpublished data from our laboratory, cell extracts have been prepared from cells taken at different time points, and levels of cyclin E1 was induced by PRP stimulation with maximum expression at 18 h. Together with the result from flow cytometry, cells were harvested 18 h after treatment and used in the experiments described above. The cell cycle is comprised of a set of events that lead to cell division 36, and progression through it is regulated by coordinated activities of cyclin–Cdk complexes. Here, PRP‐treated tendon cells entered S phase at higher rates than untreated control cells, suggesting that PRP modulated the formation or activity of cyclin–Cdk complexes. PRP treatment also induced up‐regulation of Cdks 1 and 2 and cyclins E1, A2 and B1 in the tendon cells. Formation and activation of cyclin E–Cdk2 complexes results in Rb phosphorylation, which leads to activation of S phase genes via the aid of transcription factor E2F 14. In addition, activation of cyclin A–Cdk2/1 and cyclin B–Cdk1 is important for progression of cells through S and G2/M phases 37. Thus, the observed increases in cyclin and Cdk levels indicate that PRP exerted positive effects on tendon cell proliferation by promoting their progression through the cell cycle.
Cyclin–Cdk complexes are regulated in part by their binding to Cdk inhibitor proteins. Members of the Cip/Kip family, including p21Cip1, p27Kip1 and p57Kip2, bind both cyclin and Cdk subunits and modulate activities of cyclin D–, E–, A– and B–Cdk complexes 38. p27 inhibits not only cyclin E– and cyclin A–Cdk2, which are kinases that promote DNA synthesis, but also cyclin B– and cyclin A–Cdk1, which allow entry into mitosis 39. Thus, down‐regulation of p27 expression and accompanying release from inhibition of cyclin–Cdk complexes, may account for the enhanced proliferation of our PRP‐treated tendon cells.
Stat3 belongs to a family of latent cytoplasmic transcription factors that play key roles in a variety of biological activities such as cell differentiation and proliferation 40. Stat3 is recruited to the plasma membrane upon ligand–receptor binding, where it is activated via phosphorylation by its own receptor tyrosine kinase, or by JAK 19. In the present study, it was found that up‐regulation of expression of phosphorylated Stat3 and concomitant down‐regulation of p27 accompanied increase in G1 to S phase transition in PRP‐treated tendon cells. This finding provides evidence that PRP promoted tendon cell proliferation via Stat3 activation, which suppressed p27, resulting in cyclin E–Cdk2, cyclin A–Cdk2/1 and cyclin B–Cdk1 complex activity. In this way, PRP promoted cell cycle progression and thus, tendon cell proliferation.
In conclusion, PRP, which is enriched in PDGF and TGF‐β, can potentially enhance cell proliferation. In this study, we showed that PRP promoted progression of the cell cycle and proliferation of tendon cells, by modulating expression of Stat3, p27 and cyclin–Cdk complexes. These findings provide novel insights into the action of PRP and a basis for successful therapeutic strategies to treat tendon injuries.
Acknowledgements
The authors would like to thank the Chang Gung Memorial Hospital for financially supporting this research.
References
- 1. Maffulli N, Sharma P, Luscombe KL (2004) Achilles tendinopathy: aetiology and management. J. R. Soc. Med. 97, 472–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mei‐Dan O, Lippi G, Sanchez M, Andia I, Maffulli N (2010) Autologous platelet‐rich plasma: a revolution in soft tissue sports injury management? Phys. Sportsmed. 38, 127–135. [DOI] [PubMed] [Google Scholar]
- 3. Molloy T, Wang Y, Murrell G (2003) The roles of growth factors in tendon and ligament healing. Sports Med. 33, 381–394. [DOI] [PubMed] [Google Scholar]
- 4. Hsu C, Chang J (2004) Clinical implications of growth factors in flexor tendon wound healing. J. Hand Surg. Am. 29, 551–563. [DOI] [PubMed] [Google Scholar]
- 5. Eppley BL, Woodell JE, Higgins J (2004) Platelet quantification and growth factor analysis from platelet‐rich plasma: implications for wound healing. Plast. Reconstr. Surg. 114, 1502–1508. [DOI] [PubMed] [Google Scholar]
- 6. van den Dolder J, Mooren R, Vloon AP, Stoelinga PJ, Jansen JA (2006) Platelet‐rich plasma: quantification of growth factor levels and the effect on growth and differentiation of rat bone marrow cells. Tissue Eng. 12, 3067–3073. [DOI] [PubMed] [Google Scholar]
- 7. Peerbooms JC, Sluimer J, Bruijn DJ, Gosens T (2010) Positive effect of an autologous platelet concentrate in lateral epicondylitis in a double‐blind randomized controlled trial: platelet‐rich plasma versus corticosteroid injection with a 1‐year follow‐up. Am. J. Sports Med. 38, 255–262. [DOI] [PubMed] [Google Scholar]
- 8. Mishra A, Pavelko T (2006) Treatment of chronic elbow tendinosis with buffered platelet‐rich plasma. Am. J. Sports Med. 34, 1774–1778. [DOI] [PubMed] [Google Scholar]
- 9. Sanchez M, Anitua E, Azofra J, Andia I, Padilla S, Mujika I (2007) Comparison of surgically repaired Achilles tendon tears using platelet‐rich fibrin matrices. Am. J. Sports Med. 35, 245–251. [DOI] [PubMed] [Google Scholar]
- 10. de Vos RJ, Weir A, van Schie HT, Bierma‐Zeinstra SM, Verhaar JA, Weinans H et al (2010) Platelet‐rich plasma injection for chronic Achilles tendinopathy: a randomized controlled trial. JAMA 303, 144–149. [DOI] [PubMed] [Google Scholar]
- 11. Krogh TP, Bartels EM, Ellingsen T, Stengaard‐Pedersen K, Buchbinder R, Fredberg U et al (2013) Comparative effectiveness of injection therapies in lateral epicondylitis: a systematic review and network meta‐analysis of randomized controlled trials. Am. J. Sports Med. 41, 1435–1446. [DOI] [PubMed] [Google Scholar]
- 12. Kapinas K, Grandy R, Ghule P, Medina R, Becker K, Pardee A et al (2013) The abbreviated pluripotent cell cycle. J. Cell. Physiol. 228, 9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Reddy GP (1994) Cell cycle: regulatory events in G1–>S transition of mammalian cells. J. Cell. Biochem. 54, 379–386. [DOI] [PubMed] [Google Scholar]
- 14. Stull MA, Rowzee AM, Loladze AV, Wood TL (2004) Growth factor regulation of cell cycle progression in mammary epithelial cells. J. Mammary Gland Biol. Neoplasia 9, 15–26. [DOI] [PubMed] [Google Scholar]
- 15. Gabelman BM, Emerman JT (1992) Effects of estrogen, epidermal growth factor, and transforming growth factor‐alpha on the growth of human breast epithelial cells in primary culture. Exp. Cell Res. 201, 113–118. [DOI] [PubMed] [Google Scholar]
- 16. Timms JF, White SL, O'Hare MJ, Waterfield MD (2002) Effects of ErbB‐2 overexpression on mitogenic signalling and cell cycle progression in human breast luminal epithelial cells. Oncogene 21, 6573–6586. [DOI] [PubMed] [Google Scholar]
- 17. Stull MA, Richert MM, Loladze AV, Wood TL (2002) Requirement for IGF‐I in epidermal growth factor‐mediated cell cycle progression of mammary epithelial cells. Endocrinology 143, 1872–1879. [DOI] [PubMed] [Google Scholar]
- 18. Park OK, Schaefer TS, Nathans D (1996) In vitro activation of Stat3 by epidermal growth factor receptor kinase. Proc. Natl Acad. Sci. USA 93, 13704–13708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ullrich A, Schlessinger J (1990) Signal transduction by receptors with tyrosine kinase activity. Cell 61, 203–212. [DOI] [PubMed] [Google Scholar]
- 20. Wang YZ, Wharton W, Garcia R, Kraker A, Jove R, Pledger WJ (2000) Activation of Stat3 preassembled with platelet‐derived growth factor beta receptors requires Src kinase activity. Oncogene 19, 2075–2085. [DOI] [PubMed] [Google Scholar]
- 21. Sa G, Stacey DW (2004) P27 expression is regulated by separate signaling pathways, downstream of Ras, in each cell cycle phase. Exp. Cell Res. 300, 427–439. [DOI] [PubMed] [Google Scholar]
- 22. Xiong H, Zhang ZG, Tian XQ, Sun DF, Liang QC, Zhang YJ et al (2008) Inhibition of JAK1, 2/STAT3 signaling induces apoptosis, cell cycle arrest, and reduces tumor cell invasion in colorectal cancer cells. Neoplasia 10, 287–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tsai WC, Hsu CC, Chen CP, Chen MJ, Lin MS, Pang JH (2006) Ibuprofen inhibition of tendon cell migration and down‐regulation of paxillin expression. J. Orthop. Res. 24, 551–558. [DOI] [PubMed] [Google Scholar]
- 24. Landesberg R, Roy M, Glickman RS (2000) Quantification of growth factor levels using a simplified method of platelet‐rich plasma gel preparation. J. Oral Maxillofac. Surg. 58, 297–300; discussion 300–291. [DOI] [PubMed] [Google Scholar]
- 25. Eppley BL, Pietrzak WS, Blanton M (2006) Platelet‐rich plasma: a review of biology and applications in plastic surgery. Plast. Reconstr. Surg. 118, 147e–159e. [DOI] [PubMed] [Google Scholar]
- 26. Marx RE (2004) Platelet‐rich plasma: evidence to support its use. J. Oral Maxillofac. Surg. 62, 489–496. [DOI] [PubMed] [Google Scholar]
- 27. Okuda K, Kawase T, Momose M, Murata M, Saito Y, Suzuki H et al (2003) Platelet‐rich plasma contains high levels of platelet‐derived growth factor and transforming growth factor‐beta and modulates the proliferation of periodontally related cells in vitro. J. Periodontol. 74, 849–857. [DOI] [PubMed] [Google Scholar]
- 28. Weibrich G, Kleis WK, Hafner G, Hitzler WE (2002) Growth factor levels in platelet‐rich plasma and correlations with donor age, sex, and platelet count. J. Craniomaxillofac. Surg. 30, 97–102. [DOI] [PubMed] [Google Scholar]
- 29. Weibrich G, Kleis WK, Hafner G (2002) Growth factor levels in the platelet‐rich plasma produced by 2 different methods: curasan‐type PRP kit versus PCCS PRP system. Int. J. Oral Maxillofac. Implants 17, 184–190. [PubMed] [Google Scholar]
- 30. He L, Lin Y, Hu X, Zhang Y, Wu H (2009) A comparative study of platelet‐rich fibrin (PRF) and platelet‐rich plasma (PRP) on the effect of proliferation and differentiation of rat osteoblasts in vitro. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 108, 707–713. [DOI] [PubMed] [Google Scholar]
- 31. Gruber R, Varga F, Fischer MB, Watzek G (2002) Platelets stimulate proliferation of bone cells: involvement of platelet‐derived growth factor, microparticles and membranes. Clin. Oral Implants Res. 13, 529–535. [DOI] [PubMed] [Google Scholar]
- 32. Celotti F, Colciago A, Negri‐Cesi P, Pravettoni A, Zaninetti R, Sacchi MC (2006) Effect of platelet‐rich plasma on migration and proliferation of SaOS‐2 osteoblasts: role of platelet‐derived growth factor and transforming growth factor‐beta. Wound Repair Regen. 14, 195–202. [DOI] [PubMed] [Google Scholar]
- 33. Akeda K, An HS, Okuma M, Attawia M, Miyamoto K, Thonar EJ et al (2006) Platelet‐rich plasma stimulates porcine articular chondrocyte proliferation and matrix biosynthesis. Osteoarthritis Cartilage 14, 1272–1280. [DOI] [PubMed] [Google Scholar]
- 34. Prosperi E (1997) Multiple roles of the proliferating cell nuclear antigen: DNA replication, repair and cell cycle control. Prog. Cell Cycle Res. 3, 193–210. [DOI] [PubMed] [Google Scholar]
- 35. Xiong Y, Zhang H, Beach D (1992) D type cyclins associate with multiple protein kinases and the DNA replication and repair factor PCNA. Cell 71, 505–514. [DOI] [PubMed] [Google Scholar]
- 36. MacLachlan TK, Sang N, Giordano A (1995) Cyclins, cyclin‐dependent kinases and cdk inhibitors: implications in cell cycle control and cancer. Crit. Rev. Eukaryot. Gene Expr. 5, 127–156. [DOI] [PubMed] [Google Scholar]
- 37. Pucci B, Kasten M, Giordano A (2000) Cell cycle and apoptosis. Neoplasia 2, 291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Besson A, Dowdy SF, Roberts JM (2008) CDK inhibitors: cell cycle regulators and beyond. Dev. Cell 14, 159–169. [DOI] [PubMed] [Google Scholar]
- 39. Toyoshima H, Hunter T (1994) p27, a novel inhibitor of G1 cyclin‐Cdk protein kinase activity, is related to p21. Cell 78, 67–74. [DOI] [PubMed] [Google Scholar]
- 40. Pedranzini L, Leitch A, Bromberg J (2004) Stat3 is required for the development of skin cancer. J. Clin. Invest. 114, 619–622. [DOI] [PMC free article] [PubMed] [Google Scholar]


