Abstract
Islet replacement therapy is limited by shortage of donor islet cells. Usage of islet cells derived from porcine pancreatic stem cells (PSCs) is currently viewed as the most promising alternative for human islet transplantation. However, PSCs are rare and have a finite proliferative lifespan. In this study, we isolated and established an immortalized mesenchymal stem cell (MSC) line derived from foetal porcine pancreas, by transfecting human telomerase reverse transcriptase (hTERT) and called these immortalized pancreatic mesenchymal stem cells (iPMSCs). The iPMSCs have been cultured for more than 80 passages and have capacity to differentiate into neurons, cardiomyocytes, germ cells and islet‐like cells, analysed by morphology, RT‐PCR, western blotting, immunofluorescence, immunocytochemistry and transplantation assay. Islets derived from iPMSCs reversed hyperglycaemia in streptozotocin‐induced diabetic mice and secreted insulin and C‐peptide in vitro. These results demonstrated that iPMSCs might provide unlimited resources for islet replacement therapy and models for functional cell differentiation.
Introduction
Numbers of patients suffering from diabetes mellitus is growing at an alarming rate. Insulin‐secreting β cells of islets of Langerhans are damaged to different extents in diabetic patients, either through an autoimmune reaction as in type 1 diabetic patients or through inherent changes within β cells that affect their function, in type 2 diabetic patients (1). Type 1 diabetes mellitus is a common endocrine disorder that results in substantial morbidity and mortality, and leads to considerable financial costs to patients and health care systems. Treatment with insulin injections, while life‐saving, often does not provide sufficient control of blood glucose to prevent complications of this disease; this has provided the impetus for intensive research to discover better methods of sustaining normoglycaemia. Cell replacement strategies via islet transplantation offer potential therapeutic options for diabetic patients because islet replacement therapy can restore not only the insulin‐secreting unit, but also the precise fine tuning of insulin release in response to multiple neural and humoral signals, arising within and outside the islets of Langerhans. However, difference between limited number of donor islets and high numbers of patients who could benefit from such treatment reflects the need for renewable sources of high‐quality islet β cells (1). Human embryonic stem cells (hESCs) are capable of self‐renewal and can differentiate into mature cell types of all three germ layers (1, 2). Differentiation of hESCs into β cells highlights a promising strategy to resolve shortage of β cells. However, immunological rejection, ethical concern, low efficiency and sophisticated protocols have restricted application of hESCs (3).
The usage of porcine islet cells is currently viewed as the most promising alternative, as there is a plentiful supply of porcine islet cells; moreover, porcine and human insulins are highly conserved, and porcine normal physiological glucose levels are similar to those in humans (4). The rationale for xenotransplantation is that implanted porcine islets have the potential to mimic normal physiological insulin response in type 1 diabetics so that near‐normal blood glucose levels may be achievable without insulin administration or with reduced requirement for it (5). As a consequence, long‐term diabetes complications may be prevented and patients may experience less hypoglycaemia compared to currently recommended ‘intensive’ insulin regimens.
New islet cells can also be derived from adult pancreatic ductal cells of mice (6) and humans (7, 8). However, pancreatic stem cells (PSCs) are rare and have a finite proliferative lifespan, culminating in permanent population growth arrest – known as replicative senescence, resulting in inability to multiply, and to phenotypic instability (9). Studies have shown that this crisis can be overcome by introducing the catalytic subunit of human telomerase reverse transcriptase (hTERT) (10, 11). hTERT appears to be essential for immortalization, and some studies have reported that induction of telomerase activity alone is sufficient for immortalization of functional cells (12).
In this study, we transfected hTERT into cultured foetal porcine pancreatic MSCs and established an immortalized mesenchymal stem cell line derived from foetal porcine pancreas, and called them immortalized pancreatic mesenchymal stem cells (iPMSCs). These cells have been cultured for more than 80 passages, with capacity to differentiate into neurocytes, cardiomyocytes, germ cells and islet‐like cells analysed by morphology, RT‐PCR, western blotting, immunofluorescence or immunohistochemical staining and transplantation assay. These results demonstrated that iPMSCs might provide unlimited sources for islet replacement therapy and models for islet and other functional cell differentiation.
Materials and methods
Isolation, culture and transfection of foetal porcine pancreatic cells
2 months foetal porcine pancreases were removed, and minced into 1–2 mm3 pieces. After being rinsed with phosphate‐buffered saline (PBS), chopped tissue was digested with 0.1% collagenase at 37 °C for 25 min, with shaking at intervals. Digested tissue was then filtered, centrifuged at 20 g for 10 min and rinsed three times in PBS before being resuspended in RPMI 1640 supplemented with 10% foetal bovine serum (FBS; Hyclone, Logan, UT, USA), 1 mm sodium pyruvate (Invitrogen, Carlsbad, CA, USA), 0.1 mmβ‐mercaptoethanol (Sigma, St Louis, MO, USA), 100 mg/ml penicillin/streptomycin (Invitrogen), 20 ng/ml epidermal growth factor (EGF) (Millipore, Billenca, MA, CA, USA) and 20 ng/ml basic fibroblast growth factor (bFGF) (Millipore, Billenca, MA, CA, USA). Resultant suspensions were seeded in 60 mm culture dishes and incubated at 37 °C and 5% CO2. After 96 h incubation, suspended islets were physically manually picked out and transferred to six‐well plates (at 50–60 islets/well) in subculture medium (Low‐glucose DMEM [Dulbecco's Modified Eagle Medium], Invitrogen) containing 15% FBS, 0.1 mmβ‐mercaptoethanol (Sigma), 2 mm glutamine (Invitrogen) and 100 mg/ml penicillin/streptomycin. Specimens were subcultured at 70–80% confluency after dissociation with 0.25% (w/v) trypsin. The culture medium was replenished every 2–3 days (5).
For transfection, islet‐derived cells were seeded in six‐well plates at concentration of 1 × 105 cells/well. After 48 h culture, cells were transfected with pCI‐hTERT using Lipofectamine Plus™ (Invitrogen). They were then distributed between three wells after 24 h. Transfected cells were selected with 400 μg/ml G418 after 48 h culture for up to 30 days. Drug‐resistant colonies (mesenchymal stem cell‐like) were maintained in 400 μg/ml G418 and expanded for further experiments.
Characterization of iPMSCs
Growth curves. G418‐positive cells were seeded in 24‐well plates at 1 × 104 cells/well and their proliferative ability was assessed by construction of growth curves at intervals of 24 h. Cells were trypsinized and cell number was determined for seven consecutive days (n = 3). Cell population doubling times (PDT) were calculated using the formula, PDT = [log2/(logN t−logN 0)] × t, where N t = number of cells after t hours of culture, where N 0 = number of cells seeded.
Flow cytometry analysis. Passage 18 cells were used for cell‐surface antigen phenotyping. Cells were cultured to 85% confluence, trypsinized for 3–5 min, collected and incubated with fluorescein isothiocyanate (FITC) or phycoerythrin (PE)‐coupled antibodies. The following antibodies were used in this experiment including anti‐: common leucocyte antigen CD45 (PE, Becton Dickinson, Franklin, NJ, USA), CD71 (PE, Becton Dickinson), CD29 (PE, Becton Dickinson), CD166 (FITC, Becton Dickinson), CD34 (FITC, Becton Dickinson), CD44 (FITC, Becton Dickinson) and anti‐mouse IgG conjugate (Becton Dickinson), isotype‐specific FITC or PE‐conjugated goat anti‐mouse immunoglobulin G (IgG) F(ab′)2 antibodies (Becton Dickinson). Briefly, cells were trypsinized and aliquoted at a concentration of 1 × 106 cells/ml and stained for 30 min with either conjugated specific antibodies or isotype‐matched control mouse IgGs, at recommended concentrations. Labelled cells were washed twice, resuspended in PBS supplemented with 1% BSA and analysed using a Beckman Coulter flow cytometer (Beckman Coulter, Brea, CA, USA). Results were plotted as mean percentage of positive cells.
Immunofluorescence and immunocytochemistry. iPMSCs were fixed in 4% paraformaldehyde (PFA) for 10 min at room temperature. Cells were permeabilized with 0.1% Triton X‐100 for 10 min, blocked with 1% goat serum in PBS at room temperature for 1 h, and incubated with in primary antibodies overnight at 4 °C. Primary antibodies included anti‐: hTERT (1:200; Chemicon, Temecula, CA, USA), vimentin (Vim, 1:200; Chemicon), Nanog (1:200; Chemicon), Sox2 (1:200; Chemicon), Oct4 (1:500; Chemicon), Klf4 (1:200; Chemicon), C‐Myc (1:200; Chemicon), PCNA (1:200; Millipore), PC1/3 (1:200; Millipore), Glut2 (1:200; Millipore), glial fibrillary acidic protein (GFAP, 1:100; Boshide Biochemical Corporation, Wuhan, China), Islet1 (1:1000; DSHB), Nestin (1:1000; Chemicon), neuron‐specific enolase (NSE, 1:100; Chemicon), cardiac α‐actin (1:500; Sigma, Iowa City, Iowa, USA), CT3 (1:1000; DSHB), Vasa (1:200; Abcam, Cambridge, MA, USA), Scp3 (1:300; Santa Cruz, California, USA), PDX1 (1:200; Abcam), C‐peptide (1:200; Abcam) and insulin (1:200; Chemicon). After three washes in PBS, cells were incubated in secondary antibodies at room temperature for 1 h followed by three washes in PBS, then incubated in DAPI at room temperature for 5 min. Images were captured and analysed using a Leica fluorescence microscope (Hicksville, NY, USA).
For immunocytochemistry, cells were incubated in 3% H2O2 for 10 min to inhibit endogenous peroxidise activity and rinsed in PBS. Blocking was performed with 4% goat serum at room temperature for 20 min. Cells were then exposed to primary antibodies overnight at 4 °C or for 1–2 h at room temperature. Appropriate HRP‐conjugated secondary antibodies and chromogen solution 3,3‐diaminobenzidine (DAB) were used according to the manufacturer’s manual (Beijing Zhongshan Golden Bridge Biochemical Factory, Beijing, China). iPMSCs and induced iPMSCs were analysed respectively.
Reverse transcriptase‐polymerase chain reaction
Total RNA for reverse transcriptase‐polymerase chain reaction (RT‐PCR) analysis was extracted from typical iPMSCs, porcine foetal pancreas, ovary and induced iPMSCs using TRIzol (Invitrogen). cDNA was synthesized from 500 μg of RNA using a commercially available kit (TaKaRa; Biotech. Co. Ltd, Dalian, Liaoning, China). PCR steps included denaturation at 94 °C for 5 min, followed by 35 cycles at 95 °C for 30 s, 55–58 °C for 30 s and 72 °C for 30–60 s, and final extension at 72 °C for 10 min. Primers were designed based on sequences of the open reading frame, from NCBI GenBank, and synthesized by AuGCT Biotechnology (Beijing). PCR primers and length of the amplified products are shown in Table 1.
Table 1.
PCR primers and the length of the amplified products
| Primers | Forward | Reverse | Product size (bp) | T m (°C) |
|---|---|---|---|---|
| β‐actin | 5′‐gcggcatccacgaaactac‐3′ | 5′‐tgatctccttctgcatcctgtc‐3′ | 138 | 58 |
| Oct4 | 5′‐gaagctggacaaggagaagct‐3′ | 5′‐catgctctccaggttgcctc‐3′ | 247 | 58 |
| Sox2 | 5′‐ccagcgcatggacagtta‐3′ | 5′‐tggagtgggaggaagaggta‐3′ | 323 | 54 |
| Nanog | 5′‐gcgaatcttcaccaatg‐3′ | 5′‐tttctgccacctcttac‐3′ | 407 | 54 |
| c‐Myc | 5′‐ctggtgggcgagatcatca‐3′ | 5′‐cactgccatgaatgatgttcc3′ | 304 | 54 |
| Klf4 | 5′‐cactgtctcatcaggagtca‐3′ | 5′‐cgacggtgcacgaggagaca‐3′ | 525 | 55 |
| c‐Kit | 5′‐accgcactgccactgat‐3′ | 5′‐taagccctgcactccac‐3′ | 427 | 54 |
| PCNA | 5′‐agtggagaacttggaaatggaa‐3′ | 5′‐gagacagtggagtggcttttgt‐3′ | 154 | 58 |
| hTERT | 5′‐gtgtgctgcagctcccatttc‐3′ | 5′‐gctgcgtctgggctgtcc‐3′ | 264 | 58 |
| Tbx5 | 5′‐attgctgaaaccgagaatgg‐3′ | 5′‐gcgctccttgaggttgaaaag‐3′ | 250 | 55 |
| Nkx2.5 | 5′‐agcacttctccgctcacttc‐3′ | 5′‐ccgtgcacagagtggtactg‐3′ | 232 | 60 |
| Gata4 | 5′‐tccctcttccctcctcaaattc‐3′ | 5′‐tcagcgtgtaaaggcatctg‐3′ | 193 | 54 |
| Scp3 | 5′‐ctagaattgttcagagccagag‐3′ | 5′‐gttcaagttctttcttcaaag‐3′ | 247 | 58 |
| GDF9 | 5′‐tagtccacccacacacctga‐3′ | 5′‐ccagaagcctgagaaccaag‐3′ | 197 | 57 |
| ZP3 | 5′‐ggcatgtgacagaagaagca‐3′ | 5′‐agagtcagggacaccaccac‐3′ | 151 | 58 |
| Vim | 5′‐gtccaagtttgccgacctct‐3′ | 5′‐agcgcatccacttcacagg‐3′ | 123 | 58 |
| Ngn3 | 5′‐gcgagttggcactgagca‐3′ | 5′‐aagctgtggtccgctatgc‐3′ | 220 | 58 |
| Mafa | 5′‐ttcagcaaggaggaggtcat‐3′ | 5′‐acaggtcccgctctttgg‐3′ | 190 | 58 |
| NeuroD1 | 5′‐agctcccatgtcttccacgta‐3′ | 5′‐gaagttgccgttgatgctga‐3′ | 144 | 58 |
| PC1/3 | 5′‐aacaggggagacaaggaaagg‐3′ | 5′‐cgaggcactgctgatggag‐3′ | 128 | 58 |
| Glut2 | 5′‐ttgccttggatgagttatgtga‐3′ | 5′‐gcgtggtccttgactgaaaa‐3′ | 120 | 58 |
| Pdx1 | 5′‐gagcccgaggagaacaagc‐3′ | 5′‐tgacagccagctccaccc‐3′ | 121 | 58 |
| Insulin | 5′‐aagcgtggcatcgtggag‐3′ | 5′‐tcaggactttattgggttttgg‐3′ | 128 | 58 |
| Nestin | 5′‐cctcaagatgtccctcagcc‐3′ | 5′‐ccaacttagggtccaagatgc‐3′ | 125 | 58 |
| Brachyury | 5′‐aagaacggcaggaggatgg‐3′ | 5′‐ctctgggaagcagtggc‐3′ | 372 | 53 |
| βIII‐Tubulin | 5′‐cttttggccagatctttagacc‐3′ | 5′‐ctcgttgtcaatgcaataggtc‐3′ | 377 | 57 |
PCR products were analysed by 2% agarose (Invitrogen) gel electrophoresis, stained with ethidium bromide (Invitrogen) and visualized under UV illumination. Primary pancreatic islet cells derived from porcine foetuses and oocytes from ovaries were used as positive controls, respectively.
Quantitative reverse transcriptase‐polymerase chain reaction
Quantitative reverse transcriptase‐polymerase chain reaction (QRT‐PCR) was set up in 25 μl reaction mixtures containing 12.5 μl 1× SYBR@ PremixExTaqTM (TaKaRa; Biotech. Co. Ltd), 0.5 μl sense primer, 0.5 μl antisense primer, 11 μl distilled water and 0.5 μl template. Reaction conditions were as follows: 95 °C for 30 s, followed by 45 cycles at 95 °C for 5 s, and 58 °C for 20 s. All expression levels were normalized to β‐actin in each well. Expression was quantified as ratio of mRNA levels obtained from untreated iPMSCs to those of induced islets and foetal pancreas. QRT‐PCR primers including Vim, Ngn3, Mafa, NeuroD1, Glut2, PDX2 and insulin are shown in Table 1.
Western blotting
Total cell proteins of iPMSCs and islet‐derived iPMSCs were extracted in 1× SDS–PAGE sample loading buffer, resolved by SDS–PAGE and transferred to PVDF membranes, then probed with primary antibodies, including to β‐actin (1:1000; Beyotime, Haimen, Jiangsu, China), Vim (1:1000; Chemicon), PDX1 (1:1000; Abcam) and insulin (1:1000; Chemicon). Horseradish peroxidase‐conjugated anti‐rabbit or anti‐mouse IgG was used as secondary antibody (1:1000; Beyotime). Detection was performed followed by visualization using BM‐Chemiluminescence blotting substrate (Roche, Shanghai, China).
Tumorigenicity assay
To detect any tumorigenicity of iPMSCs, cells were collected, resuspended in PRMI 1640 medium, then injected subcutaneously into nude mice (4–6 weeks) at concentration of 1 × 107 cells per mouse (n = 4). Mice were monitored for up to 2 months.
Generation of neuronal cells from iPMSCs
iPMSCs were incubated in serum‐free high‐glucose DMEM (Sigma), containing 1 mmβ‐mercaptoethanol, for 24 h. Media were changed for further 8 h (13, 14). Induced cells were examined morphologically and by RT‐PCR. Immunohistochemistry was performed with a variety of primary antibodies including anti‐: Nestin (1:1000; Chemicon), NSE (1:100; Chemicon) and GFAP (1:100; Boshide Biochemical Corporation). Primer sequences for RT‐PCR are shown in Table 1 (Nestin, βIII‐Tubulin). Untreated iPMSCs were used as negative control.
Generation of cardiomyocytes from iPMSCs
iPMSCs were induced with basal medium containing 10 μm 5‐azacytidine (5‐AZA) (Sigma) or 10−7 M all trans‐retinoic acid (RA) (Sigma) in combination with 0.75% (w/v) DMSO (Sigma) for 48 h, and then induced in low‐glucose DMEM (15). Induced cardiomyocytes were identified by morphology, immunohistochemistry and RT‐PCR. Primary antibodies used in this experiment were anti‐: cardiac α‐actin (1:500; Sigma), CT3 (1:1000; DSHB) and islet1 (1:1000; DSHB). Primer sequences for RT‐PCR are shown in Table 1 (Tbx5, Nkx2.5 and Gata4). Untreated iPMSCs were used as negative control.
Generation of germ cells from iPMSCs
To detect spontaneous differentiation capacity, iPMSCs were cultured according to a modified method, which has been previously well established for ES cells (16). Six hundred cells were cultured in hanging drops of 20 μl of Low‐glucose DMEM containing 15% FBS, 0.1 mm non‐essential amino acids (Invitrogen), 2 mm l‐glutamine (Invitrogen), 0.1 mmβ‐mercaptoethanol (Sigma) and 100 mg/ml penicillin/streptomycin. After 2 days culture in hanging drops, cells aggregated and formed embryoid bodies (EBs), which were maintained in suspension culture in bacteriological dishes for another 3–5 days. For microscopic inspection, EBs were seeded in tissue culture dishes. RT‐PCR was used to investigate expression of genes related to differentiated cells of all three embryonic germ layers. Further cultivation of the EBs led to border cells shed and proliferation. Border cells were used to differentiate into germ cells using conditioned medium with 5% or 10% porcine follicular fluid (PFF) and 0.1 U/ml follicle‐stimulating hormone (FSH; Sigma) (17, 18). Large follicle‐like structures were formed and identified by morphology and immunohistochemistry respectively with anti‐: Oct4 (1:500; Chemicon), Vasa (1:200; Abcam) and Scp3 (1:300; Santa Cruz) antibodies, and RT‐PCR with Oct4, Scp3, GDF9 and ZP3, which are markers of germ cells, spermatozoa or oocytes (19, 20, 21, 22, 23, 24). Untreated iPMSCs and porcine ovary were used as negative control and positive control respectively.
Generation of islet‐like clusters derived from iPMSCs
We used a two‐step protocol to obtain islet‐like cells. First, iPMSCs were dissociated using 0.05% trypsin at 37 °C for 5 min and cultured in RPMI 1640/B27 medium supplemented with 1% BSA, 100 mm nicotinamide (NIC; Sigma), 10 ng/ml exendin‐4 (Sigma), 1 mm sodium pyruvate (Invitrogen), 2 mm glutamine (Invitrogen), 1 mmβ‐mercaptoethanol (Sigma), 100 mg/ml penicillin/streptomycin, 20 ng/ml EGF (Millipore) and 20 ng/ml bFGF (Millipore) for 7 days. Cells were then cultured for another 7 days in the same medium supplemented with 10−6 m RA (Sigma) and without EGF or bFGF. Cells were transferred to ultra‐low attachment plates and cultured in the same medium for 2 weeks. Medium was changed every 3 days.
Pancreatic islet‐like clusters were stained with dithizone (DTZ; Sigma) to rapidly assess presence of insulin‐producing cells, staining crimson (7). Clusters were also identified by immunofluorescence assay with anti‐: PDX1, C‐peptide, insulin and Nestin antibodies. Vim, Ngn3, Mafa, NeuroD1, Glut2, PDX1 and insulin were analysed by RT‐PCR and QRT‐PCR at days 7 and 14 after induction. Expression of Vim, PDX1 and insulin were analysed by western blotting at day 14 after induction. Untreated iPMSCs and foetal porcine pancreas were used as the negative control and the positive control respectively.
Glucose‐stimulated insulin release and insulin content analysis
After 14 days induction, iPMSC‐derived islet clusters were plated in six‐well plates in triplicate, approximately 200 clusters/well, and cultured overnight to ensure that clusters attached to plates. Then, clusters were washed three times in glucose‐free RPMI 1640 medium and incubated in RPMI 1640 containing 5.6 mm glucose at 37 °C for 30 min or 3.5 h. Then, clusters were further incubated in RPMI 1640 containing 25 and 5.6 mm glucose at 37 °C for another 30 min (25) or 3.5 h (26). In this experiment, subsequently different stages of clusters were incubated with 5.6 and 25 mm glucose for 30 min or 3.5 h to determine glucose‐stimulated insulin content and C‐peptide release. RPMI 1640 induction medium was determined to be basal level of insulin content and C‐peptide release. Cell supernatant of both low‐ and high‐glucose‐treated clusters, and non‐induced iPMSCs treated with low and high concentrations of glucose were collected and stored at −20 °C for further use. The insulin content released by islet clusters was detected by RIA (26). The results were analysed by t‐test analysis.
Transplantation of iPMSC‐derived islet clusters
Eighteen normal adult male mice (Kunbai strain), weighing 40 ± 2 g, were used for the transplantation assay. Fifteen mice were used to establish diabetic models by intraperitoneal injection with streptozotocin (STZ; Sigma), which was dissolved in 0.1 m citric acid and sodium citrate buffer. STZ injections were given intraperitoneally and the doses were determined according to the body weight of mice (100 mg/kg body weight, i.p.) for two consecutive days. Three mice injected with 0.1 m citric acid and sodium citrate buffer remained as the normal mice. Blood glucose levels of the experimental group of mice, at 3, 6 and 9 days after STZ injection, were measured. Mice with blood glucose levels above 16.65 mm after three measurements were regarded as suitable diabetic models.
Induced dithizone‐positive clusters and non‐induced iPMSCs were scraped, resuspended in 100 μl low‐glucose DMEM (approximately 1 × 106 cells) and transplanted into abdomens of nine and three diabetic mice respectively. Similarly, three diabetic mice were injected with 100 μl low‐glucose DMEM. Blood glucose levels of transplantation mice and normal mice were measured every 3 days. Death rates of experimental mice were recorded daily. This diabetic model and transplant experiment was approved by the animal committee of Shaanxi Centre of Stem Cells Engineering & Technology.
Results
Characterization of iPMSCs
Typical pancreatic stem cell colonies (Fig. 1a) and monolayer cells with fibroblast‐like morphology were obtained in our study (Fig. 1b) (27), and typical iPMSC colonies were formed 2–3 weeks after transfection and selection with 400 μg/ml G418, over five stable hTERT transfected iPMSC colonies were obtained which had similar fibroblast‐like morphology. Success rate of iPMSCs was in the range 12.5% (5/40). We expanded one of these, and maintained the typical spindle‐shape and fibroblastic morphology of MSC up to 12 months (80 passages). They formed fibroblast colonies and grew as confluent with typical triangles and long spindle cell morphology of normal MSCs (Fig. 1c, passage 35; Fig. 1d, passage 75). These typical iPMSCs had one nucleus each, and at mitotic features were revealed with Giemsa staining (Fig. 1e). iPMSCs expressed hTERT as determined by RT‐PCR, and maintained hTERT activity at passages 35 and 75. However, hTERT was not detected in untransfected primary porcine pancreatic cells (Fig. 1f). From different passages of transfected cells, growth curves of iPMSCs maintained 1–3 days lag phase, and by 4–7 days entered log (exponential) phase (Fig. 1g). PDT of untransfected cells was 68.9 h, and of iPMSCs was 48.2 h (passage 35) and 54.5 h (passage 75); this showed that iPMSCs were genetically stable, presented consistent phenotypes over time and had a strong proliferative capacity as confirmed by the growth curves (Fig. 1g). Immunofluorescence analysis showed that these cells expressed markers of PSCs including Vim, PC1/3, Glut2, PDX1 and hTERT (Fig. 2). At mRNA level, we also detected expression of pancreatic stem cell markers including Vim, Ngn3, Mafa, NeuroD1, PC1/3, Glut2 and PDX1 (Fig. 2). RT‐PCR analysis indicated that they expressed Vim, Ngn3, Mafa, NeuroD1, PC1/3, Glut2 and PDX1.
Figure 1.
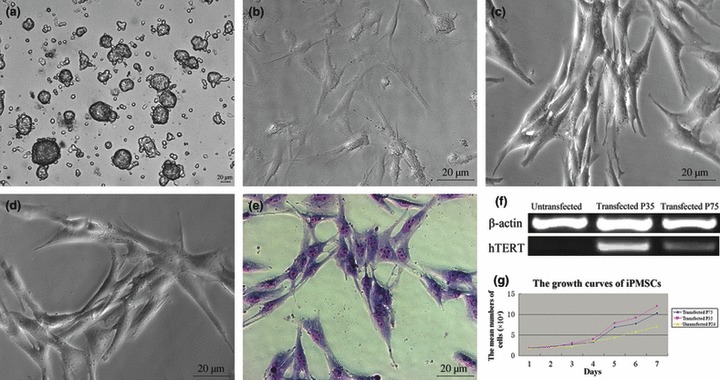
Morphology of iPMSCs, hTERT expression and growth curves of iPMSCs. (a) typical primary PSC colonies; (b) morphology of untransfected iPMSCs; (c, d) typical spindle fibroblast‐like iPMSCs; (e) Giemsa staining of iPMSCs; Bar = 20 μm; (f), hTERT expression of untransfected cells, and hTERT was present in iPMSCs and stably expressed in iPMSCs at passages 35 and 75, but absent in untransfected cells; (g) growth curves of iPMSCs. ♦ (P75 iPMSCs); (P35 iPMSCs); (Untransfected P24 PSCs).
Figure 2.
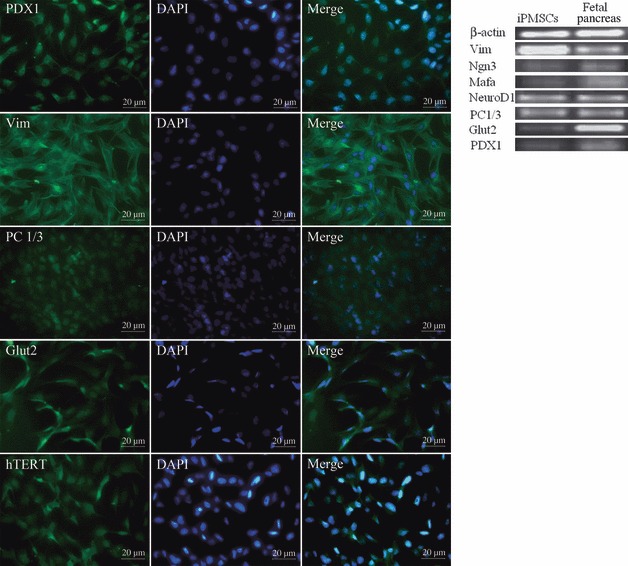
Characteristics of iPMSCs. Immunofluorescence analysis showed that iPMSCs were positive for PDX1, Vim, PC1/3, Glut2 and hTERT. Bar = 20 μm. RT‐PCR analysis showed that iPMSCs were positive for Vim, Ngn3, Mafa, NeuroD1, PC1/3, Glut2 and PDX1.
Immunophenotyping for surface antigens by flow cytometry was performed on iPMSCs at passages 15–35 to further characterize this cell population. Cells strongly expressed markers for mesenchymal progenitors such as β1 integrin (CD29) and CD44. They were negative for markers of haematopoietic lineage cells (CD71, CD45 and CD34) and CD166 (Fig. 3).
Figure 3.
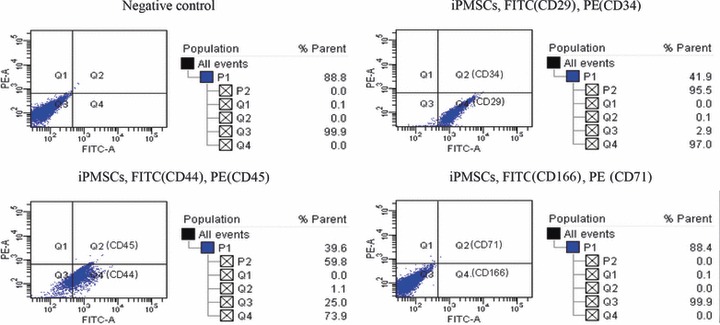
Detection of cell surface markers. Analysis of FACs showed that iPMSCs were positive for CD29 and CD44, and negative for CD34, CD45, CD71 and CD166.
In addition, our cells also expressed specific markers of embryonic stem cells including Oct4, Sox2, Nanog, C‐Myc, Klf4, C‐kit and PCNA, as analysed by immunohistochemistry (Fig. 4). Nanog, C‐Myc, Klf4, Oct3/4, Sox2 and PCNA were located in the nucleus of iPMSCs, although these proteins can also be found in cytoplasm (Fig. 4). These results were similar to previous ones described for adult stem cells (28). We also detected specific markers of embryonic stem cells at mRNA level including Oct4, Sox2, Nanog, C‐Myc, Klf4, C‐kit and PCNA. These results demonstrated that the iPMSCs possessed some properties of bone marrow mesenchymal stem cells and pluripotent embryonic stem cells (29, 30).
Figure 4.
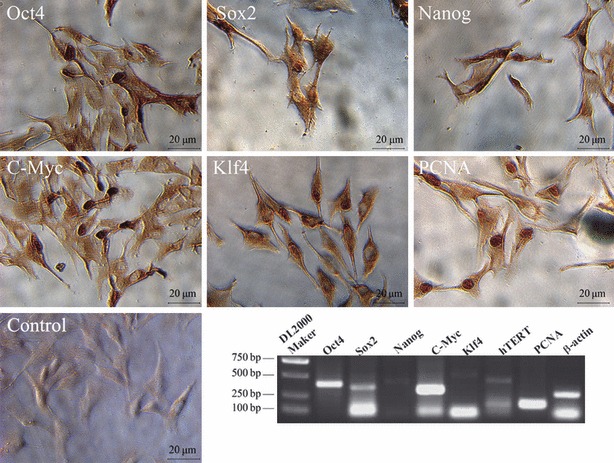
Pluripotent ESC markers were expressed in iPMSCs. Immunocytochemistry analysis showed that iPMSCs were positive for pluripotent ESC markers (Oct4, Sox2, Nanog, C‐Myc and Klf4) and PCNA, Bar = 20 μm; RT‐PCR analysis showed that iPMSCs were positive for Oct4, Sox2, Nanog, C‐Myc, Klf4 and PCNA.
These results demonstrate that iPMSCs retained some characteristics of PSCs (31), and shared some characteristics of bone marrow mesenchymal stem cells and pluripotent stem cells (1, 29, 30).
To determine functional tumorigenicity, iPMSCs subcultured for 35 passages had been suspended in PRMI 1640 and injected subcutaneously into flanks of four nude mice (4–6 weeks) at concentration of 1 × 107 cells in 0.3 ml per mouse. Mice were monitored and no tumour outgrowths were observed for up to 2 months after injection (Fig. S1).
Multipotential differentiation of iPMSCs
To determine whether iPMSCs were/resembled early uncommitted MSCs capable of differentiation into neural lineages, our cells were induced to differentiate in vitro based on the protocol of Mareschi et al. (14). Induced by β‐mercaptoethanol, iPMSCs gradually shrank, protruded, and became bipolar and/or multipolar cells (Fig. 5). Immunocytochemical analysis showed that astrocytes and neuron‐like cells expressed Nestin, NSE and GFAP protein (Fig. 5), and Nestin and β‐III tubulin were detected at mRNA level analysed by RT‐PCR; these are specific markers of neural stem cells (NSCs), astrocytes and neurocytes (14). Untreated cells expressed expressed Nestin weekly, not expressed β‐βIII Tubulin (Fig. 5).
Figure 5.
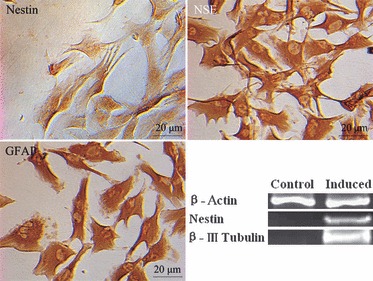
Neuron‐like cells were formed after induction. Nestin, NSE, GFAP staining were positive respectively after induction and analysed by immunohistology. Bar = 20 μm; and expression of Nestin and β III‐tubulin were up‐regulated in induced cells relative to untreated cells as analysed by RT‐PCR.
To investigate potential for cardiomyocyte differentiation, cells were induced by 5‐AZA or 10−7 m RA and 0.75% DMSO for 2–15 days. Cell morphology began to change after induction, and muscle tube‐like structures could be seen (Fig. 6). Immunocytochemical reactions showed that cardiomyocyte‐like cells expressed the cardiac α‐actin, CT3 and islet1 protein (15). We also detected specific markers of cardiomyocytes by RT‐PCR analysis indicated that they expressed Tbx5, Nkx2.5 and Gata4 (15). Compared to the untreated group, the expression of Tbx5 and Nkx2.5 showed an upward trend in the 5‐AZA induced cells, but there was no up‐regulation in the RA‐DMSO induced group. These results demonstrated that 5‐AZA was more beneficial for cardiac cell differentiation (Fig. 6).
Figure 6.
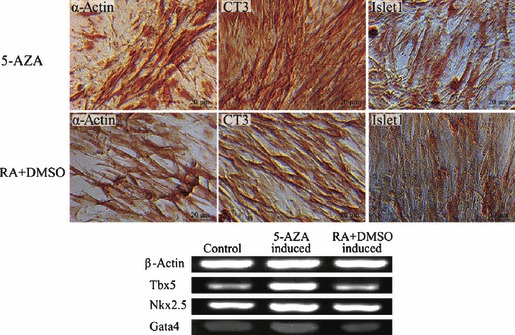
Cardiomyocyte‐like cells were formed after induction. Cardiac α‐actin, CT3, Islet1 staining were positive respectively after induction by 10 μm 5‐AZA or 10−7 m all trans‐retinoic acid in combination with 0.75% DMSO, Bar = 20 μm; cardiac specific markers analysed by RT‐PCR showed that cardiac α‐actin, Nkx2.5 were up‐regulated induced by 5‐AZA but there was no up‐regulation by or RA in combination with DMSO.
To test for spontaneous differentiation, iPMSCs were cultured according to a modified method, which has been well established for ES cells (19). Cells aggregated and formed EBs in suspension culture in bacteriological dishes, and there large round cells were detached from the periphery of EBs. RT‐PCR analysis indicated that EBs expressed Nestin (ectoderm), Brachuary (mesdoderm) and PDX1 (endoderm). Compared to the undifferentiated group, there was an upward trend of PDX1 expression, while undifferentiated iPMSCs did not express Nestin or Brachuary (Fig. 7). To confirm the potential of germ cell differentiation, detached cells were treated for 2–15 days with 0.1 U/ml FSH and PFF; we found that large round cells were formed after treatment and a percentage of large cells specifically expressed increased Vasa, Scp3 and Oct4 as shown by immunohistochemical analysis (Fig. 7). They were also positive for oocyte markers, Scp3, GDF9 and ZP3, and levels of expression were up‐regulated compared to cells cultured in control medium, as analysed by RT‐PCR analysis. However, expression pattern of Oct4 was not clearly up‐regulated (Fig. 7). All these markers are expressed specifically in PGCs and oocytes (18, 19, 22). Our results indicate that a small sub‐population of iPMSCs differentiated into germ cells and a few oocyte‐like cells. However, we have not yet determined whether these germ cells would be able to complete meiosis and form functional oocytes.
Figure 7.
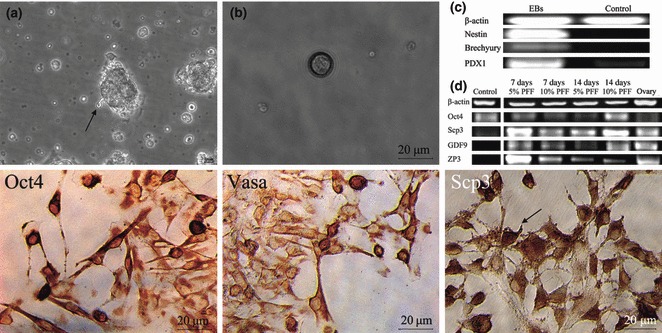
Germ cells were formed after induction. (a) EBs derived from iPMSCs; (b) Large round cells detached from the periphery of EBs; (c) EBs derived from iPMSCs expressed Nestin (ectoderm), Brachuary (mesdoderm) and PDX1 (endoderm) analysed by RT‐PCR. Follicle‐like cells were obtained from EBs derived iPMSCs and immunohistochemical analysis showed that large cells expressed Vasa, Scp3 and Oct4 (the markers of germ cells), bar = 20 μm; Germ cell‐specific markers showed that oocyte markers (Scp3, GDF9 and ZP3) were up‐regulated after induction as analysed by semi‐RT‐PCR, similar to that in porcine oocyte.
Potential differentiation of islet cells
To test potential for islet cell differentiation, cells were induced using a two‐step protocol. On the 14th day, most induced pancreatic islet‐like clusters became crimson with DTZ staining (Fig. 8a,b). Clusters stained deeply and single cells were also stained, albeit weakly. After 2 weeks, induced clusters were transferred to plates and stained for PDX1, C‐peptide, insulin and Nestin, all positive, as analysed by immunofluorescence (Fig. 8). which were markers of early and mature beta cells including Pdx1, C‐peptide and insulin (32). Nestin plays important roles as an intermediate regulator governing both stemness and differentiation of stem cells in the process of their differentiation into insulin‐secreting cells (33). Expression of PDX1 was located in the cytoplasm of iPMSCs, while in islets derived from iPMSCs induction was located in nuclei (34). These results demonstrate that the cells were able to differentiate into cell types capable of secreting endocrine hormones, not only islet cell clusters here (35). At the mRNA level, we detected specific markers of pancreatic islets including Vim, Ngn3, Mafa, NeuroD1, Glut2, PDX1 and insulin, expressed in the iPMSCs (Fig. 8c). After induction, expression levels of pancreatic islet‐specific markers, Ngn3, Mafa, NeuroD1, Glut2, PDX1 and insulin, were up‐regulated compared to untreated cells, while expression levels of primary pancreas‐specific marker, Vim, was down‐regulated compared to untreated cells, as analysed by QRT‐PCR (Fig. 8d). When compared to the 1‐week induced group, there was an upward trend in expression of Mafa, NeuroD1, Glut2, PDX1 and insulin in the 2‐week induced group, while expression of Ngn3 showed a weak downward trend, as Ngn3 is a short‐term protein formed during development of the pancreas, and is a directional factor in pancreatic endocrine cells, and does not exist in mature islets (36). These results demonstrate that the induced cells begun to differentiate into islet cells. Expression of Vim, PDX1 and insulin proteins showed upward trends compared to untreated cells as analysed by western blotting (Fig. 8e).
Figure 8.
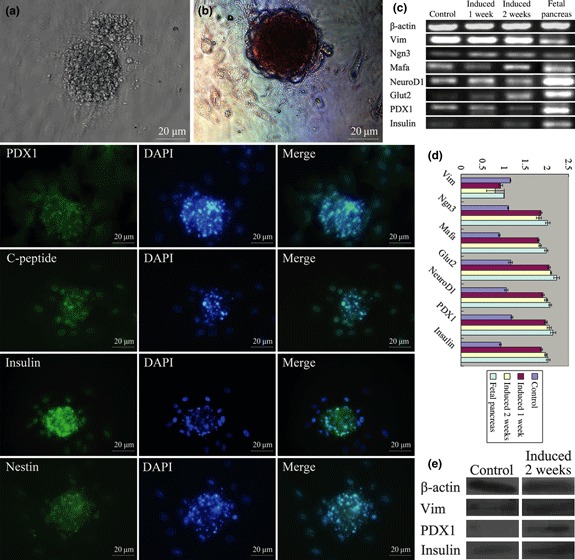
Potential differentiation of islet cells. (a) islet clusters derived from iPMSCs were formed at day 14; (b) islet clusters were positive for DTZ staining; islet clusters derived iPMSCs were positive for PDX1, C‐peptide, insulin and Nestin, Bar = 20 μm; (c) induced islets derived from iPMSCs and non‐induced iPMSCs expressed pancreatic stem cell markers including Vim, Ngn3, Mafa, NeuroD1, Glut2, PDX1 and insulin as analysed by RT‐PCR; (d) pancreatic islet‐specific markers (Ngn3, Mafa, NeuroD1, Glut2, PDX1 and insulin) in induced islets were up‐regulated relative to non‐induced iPMSCs after 1‐week induction, while expression levels of primary pancreatic specific marker (Vim) was down‐regulated relative to undifferentiated iPMSCs, and there was an upward trend expression of Mafa, NeuroD1, Glut2, PDX1 and insulin in the 2‐week induced group compared with 1‐week group as analysed by QRT‐PCR; (e) western blotting showed that expression of Vim, PDX1 and insulin in induced islets were up‐regulated relative to non‐induced iPMSCs.
To determine whether cell clusters derived from iPMSCs were capable of secreting insulin in response to glucose stimulation, we tested the ability of iPMSC‐derived islet clusters for C‐peptide production after sequential treatment with low and high concentrations of glucose in a static assay. Insulin and C‐peptide release of islet clusters in 25 and 5.6 mm glucose stimulated groups (Test groups) were significantly higher than those of non‐induced cells (Control) (P < 0.01), and there were significantly different levels of secreted insulin and C‐peptide between 25 and 5.6 mm glucose stimulation respectively (P < 0.05). However, there were no significant differences in secreted insulin and C‐peptide levels for 30 min or 3.5 h glucose stimulation (P > 0.05). However, levels of insulin and C‐peptide were higher for 3.5 h stimulation than for 30 min stimulation (Table 2). These results indicate that clusters generated by our protocol were able to secrete C‐peptide and insulin in response to high concentration of glucose, as measured by the static assay.
Table 2.
Insulin and C‐peptide release of islet‐like cells in response to glucose stimulation
| Group | Insulin (μIU/ml) | C‐peptide (ng/ml) |
|---|---|---|
| PRMI1640 (n = 1) | 0.14 | 0 |
| Control (n = 1) | ||
| 5.6 mm glucose | 0.33 | 0.01 |
| 25 mm glucose | 0.44 | 0.18 |
| Test group (0.5 h, n = 3) | ||
| 5.6 mm glucose | 227.89 ± 18.34 | 0.36 ± 0.04 |
| 25 mm glucose | 350.25 ± 30.27 | 0.52 ± 0.02 |
| Test group (3.5 h, n = 3) | ||
| 5.6 mm glucose | 273.15 ± 11.23 | 0.38 ± 0.04 |
| 25 mm glucose | 369.64 ± 19.10 | 0.55 ± 0.05 |
All three normal control mice remained alive with blood glucose levels of 3.54–6.5 mm over the experimental period. During 35‐day transplantation experiments, eight of the nine mice transplanted with induced pancreatic islets derived from iPMSCs were alive. The mouse that died did so 1 day after transplantation. Blood glucose levels of the eight induced pancreatic islet‐transplanted diabetic model mice gradually decreased to 4.4–16.3 mm after transplantation, and body weight increased to normal, that is, in the region of 32 g. However, lower blood glucose levels of transplanted mice only lasted for 21 days, and then increased to higher than 27 mm (Fig. 9). Blood glucose levels of three mice transplanted with non‐induced iPMSCs showed a downward trend, but did not attain normal levels and body weights were similar to those of diabetic model mice transplanted with the medium. Blood glucose levels of three diabetic model mice transplanted with medium remained higher than 27 mm, while body weights were maintained at around 27 g. Furthermore, we detected Nestin protein by immunofluorescence assay, and found that induced islets expressed Nestin weakly. This indicated that induced islets were not fully differentiated or only some cells had fully developed into mature pancreatic islet cells. These results indicate that induced islets derived from iPMSCs could reverse hyperglycaemia after transplantation into streptozotocin‐induced diabetic mice, but time that blood glucose levels were reduced, were short (Fig. 9). Reasons for this need to be elucidated further (37).
Figure 9.
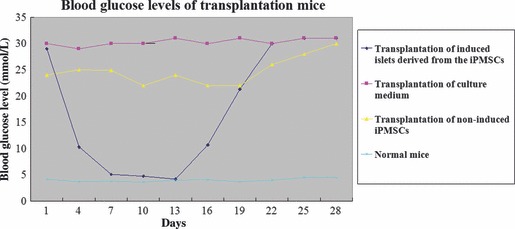
Blood glucose levels of transplantation mice. Blood glucose level of transplantated mice gradually decreased after transplantation with induced islets derived from iPMSCs, and this was maintained for around 3 weeks at low levels (<16.65 mm), then increased up to >27 mm (♦). Blood glucose level of all diabetic model mice transplanted with culture medium was higher than 27 mm (). All diabetic model mice transplanted with non‐induced iPMSCs maintained a higher level of glucose than those in islets transplantation, but lower than in culture medium transplantation (). All normal control mice were at 3.54–6.5 mm(×).
Discussion
Reports have shown that ESCs have the ability to differentiate into insulin‐producing cells and may provide an unlimited source of them for cell transplantation in diabetic patients (1, 38). However, immunological rejection, concern about ethics and low direct efficiency of differentiation restricts application of hESCs. Use of islet cells derived from porcine PSCs is currently viewed as a most promising alternative to supply cells as the can be readily expanded by optimizing supply of donor animals. However, PSCs proliferate only for a finite proliferative lifespan and an immortalized functional cell line is still a major issue. In this study, we investigated the possibility of establishing an immortalized porcine pancreatic mesenchymal stem cell line (27). The cells exhibited several characteristics of typical bone marrow‐derived MSCs, ESCs, PSCs and unlimited potential for population growth (29, 39, 40, 41, 42, 43). First, the cells exhibited typical morphology of MSCs and expressed surface proteins associated with MSCs, did not express haematopoietic stem cell markers, and also expressed typical markers of ESCs and PSCs (1, 29, 35); Second, they possessed the capacity for pluripotental differentiation and could be differentiated into other functional cell types including neural cells, cardiomyocytes, even germ and islet‐like cells as analysed by morphology, RT‐PCR, immunohistochemical analysis, western blotting and transplantation assay. According to our knowledge, this is the first study demonstrating that pancreatic MSCs are present in the porcine pancreas and can be immortalized by transfection with hTERT, and that these cells share characteristics of typical bone marrow‐derived MSCs, ESCs and PSCs and may differentiate into functional cells (1, 10, 11, 27, 29, 35). Long‐term survival of these cells supports the concept of presence of organ‐specific MSC populations that would be likely to serve as a reservoir of tissue cells of non‐haematopoietic organ systems (44, 45). These results have demonstrated that MSCs from porcine pancreas underwent multilineage differentiation in response to various differentiation‐inducing culture conditions (27, 46, 47).
There is a growing number of reports on putative MSC‐like cells isolated from various non‐haematopoietic organs, including the pancreas (48, 49, 50, 51). However, no human or animal study has definitively determined whether these cells are tissue residents or whether they require continual replenishment from the bone marrow, and no immortalized pancreatic stem cell lines have been reported (51). Usually, these MSCs cannot maintain the ability to proliferate for a long time. Therefore, they would not be able to provide a sufficient cell resource for transplantation and tissue engineering. Another main characteristic of MSCs is their ability to differentiate into multiple cell lineages (29). We found that our established MSC line was immortalized and survived for 12 months in vitro; also more importantly, that they had the capacity for self‐renewal for long‐term expansion and could differentiate into neural lineages as well as connective tissues including cardiomyocytes in vitro, and even germ cells (all three germ layers) and islet cells. These results suggest that the cells may provide an in vitro model to study plasticity and differentiation of stem cells and be a potential source of therapy for neural and heart dysfunction therapy (4, 9, 43, 52).
Furthermore, these cells can be induced to form islet cell clusters, as analysed by immunofluorescence staining and RT‐PCR analysis, and can secrete insulin and C‐peptide in response to high levels of glucose stimulation in vitro. In addition, transplantation assay demonstrated that these iPMSCs may have the potential to mimic the normal physiological insulin response in diabetic mice and that near‐normal blood glucose levels may be achieved without insulin administration or with reduced requirement for it. Although these cell functions were only maintained for a short time after transplantation, the results demonstrated that we have established efficient protocols for obtaining functional islets derived from our established iPMSCs and showed that this will provide a promising supply of alternative islet cells for diabetes (2).
Previous studies have shown that ESCs, foetal porcine skin stem cells, bone marrow and lung MSCs can differentiate into germ cells in vitro (17, 18, 19, 22, 23, 53, 54, 55). Stem cells may provide a new potential source of male and female germ cells that could be used for infertility and sterility treatments (21, 53, 54). However, the mechanisms remain unclear and efficiency of germ‐like cells derived from stem cells is still low and few have been shown to complete meiosis (21, 55, 56). In this study, a small number of follicle‐like large cells was formed in follicle fluid‐treated iPMSCs, and expressions of GDF9 and ZP3 were upward in induced cells. These results demonstrate that iPMSCs are pluripotent with the capacity to differentiate into germ cells with appropriate stimulation by growth factors and environment (17, 18, 22, 23, 53, 54). Dyce et al. (18) have shown that follicular fluid promotes induction of porcine stem cells to differentiate into oocytes. These results have demonstrated that follicular fluid might contain factors secreted from granulosa cells, theca cells and oocytes, within follicles. To our knowledge, this is the first report to study derivation of follicle‐like cells from porcine pancreas MSCs (17, 53, 54, 56).
In summary, this study has demonstrated isolation and characterization of a non‐haematopoietic MSC population from porcine foetal pancreas and established immortalized porcine pancreatic mesenchymal stem cells through transfected hTERT, named iPMSCs. Importantly, these cells shared characteristics of typical bone marrow‐derived MSCs, ESCs and PSCs and unlimited potential for population growth. The cells showed multipotential differentiation capacity and could differentiate into other functional cell types including neural cells, cardiomyocytes, and follicle‐like cells and islet‐like cells. These islet‐derived iPMSCs reversed hyperglycaemia in diabetic mouse model animals and secreted insulin and C‐peptide in vitro. Thus, this study has demonstrated that these iPMSC may provide an unlimited source of such cells for regenerative medicine, tissue engineering and basic research.
Supporting information
Fig. S1 No tumour outgrowths were observed for up to 2 months after injection.
Supporting info item
Acknowledgements
The authors thank Drs Methichit Chayosumrit and Mr Long Wang for careful reading and editing of the manuscript, and Jie Rong for careful editing of figures. We appreciate the editor and reviewer’s excellent work and suggestions. This study has been supported by grants from Program (30972097), National Natural Science Foundation of China, Key Program of State Education Ministry (109148), Program for New Century Excellent Talents in University (NCET‐09‐0654), The Scientific Research Program of Shaanxi Province (2008K02‐05), China Postdoctoral Science Foundation funded project (20080431253, 200801438), The Scientific Research Program of Yangling, and the Basic Technological and Research Programme of Jiangsu Province (BM2008146).
References
- 1. Guo T, Hebrok M (2009) Stem cells to pancreatic β‐cells: new sources for diabetes cell therapy. Endocr. Rev. 30, 214–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cai J, Yu C, Liu Y, Chen S, Guo Y, Yong Jun et al. (2010) Generation of homogeneous PDX1+ pancreatic progenitors from human es cell‐derived endoderm cells. J. Mol. Cell Biol. 2, 50–60. [DOI] [PubMed] [Google Scholar]
- 3. Steve KW, Andre BH (2006) Human Embryonic stem cells: technological challenges towards therapy. Clin. Exp. Pharmacol. Physiol. 33, 489–495. [DOI] [PubMed] [Google Scholar]
- 4. Rogers SA, Chen F, Talcott MR, Faulkner C, Thomas JM, Thevis M et al. (2007) Long‐term engraftment following transplantation of pig pancreatic primordia into non‐immunosuppressed diabetic rhesus macaques. Xenotransplantation 14, 591–560. [DOI] [PubMed] [Google Scholar]
- 5. Feng R, Zhang H, Wang Y, Qiao H, Zhao T, Shen W et al. (2007) Isolation, culture and induced differentiation of fetal porcine islet derived pancreatic stem cell. Agric. Sci. China 6, 742–748. [Google Scholar]
- 6. Ramiya VK, Maraist M, Arfors KE, Schatz DA, Peck AB, Cornelius JG (2000) Reversal of insulin‐dependent diabetes using islets generated in vitro from pancreatic stem cells. Nat. Med. 6, 278–282. [DOI] [PubMed] [Google Scholar]
- 7. Bonner‐Weir S, Taneja M, Weir GC, Tatarkiewicz K, Song KH, Sharma A et al. (2000) In vitro cultivation of human islets from expanded ductal tissue. Proc. Natl. Acad. Sci. USA 97, 79–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhao M, Amiel SA, Christie MR, Muiesan P, Srinivasan P, Littlejohn W et al. (2007) Evidence for the presence of stem cell‐like progenitor cells in human adult pancreas. J. Endocrinol. 195, 407–414. [DOI] [PubMed] [Google Scholar]
- 9. Suzuki A, Nakauchi H, Taniguchi H (2004) Prospective isolation of multipotent pancreatic progenitors using flow‐cytometric cell sorting. Diabetes 53, 2143–2152. [DOI] [PubMed] [Google Scholar]
- 10. Davies BR, Steele IA, Edmondson RJ, Zwolinski SA, Saretzki G, von Zglinicki T et al. (2003) Immortalisation of human ovarian surface epithelium with telomerase and temperature‐senstitive SV40 large T antigen. Exp. Cell Res. 288, 390–402. [DOI] [PubMed] [Google Scholar]
- 11. Hong HX, Zhang YM, Xu H, Su Zheng Y, Sun P (2006) Immortalization of swine umbilical vein endothelial cells with human telomerase reverse transcriptase. Mol. Cells 24, 358–363. [PubMed] [Google Scholar]
- 12. Bodnar AG, Ouellette M, Holt SE, Chiu CP, Morin GB, Harley CB et al. (1998) Extension of life‐span by introduction of telomerase into normal human cells. Science 279, 349–352. [DOI] [PubMed] [Google Scholar]
- 13. Woodbury D, Schwarz EJ, Prockop DJ, Black IB (2000) Adult rat and human bone marrow stromal cells differentiate into neurons. J. Neurosci. Res. 61, 364–370. [DOI] [PubMed] [Google Scholar]
- 14. Mareschi K, Novara M, Rustichelli D, Ferrero I, Guido D, Carbone E et al. (2006) Neural differentiation of human mesenchymal stem cells: evidence for expression of neural markers and K+ channel types. Exp. Hematol. 34, 1563–1572. [DOI] [PubMed] [Google Scholar]
- 15. Martin‐Rendon E, Sweeney D, Lu F, Girdlestone J, Navarrete C, Watt SM (2008) 5‐Azacytidine‐treated human mesenchymal stem/progenitor cells derived from umbilical cord, cord blood and bone marrow do not generate cardiomyocytes in vitro at high frequencies. Vox Sang. 95, 137–148. [DOI] [PubMed] [Google Scholar]
- 16. Wobus AM, Grosse R, Schöneich J (1988) Specific effects of nerve growth factor on the differentiation pattern of mouse embryonic stem cells in vitro. Biomed. Biochim. Acta 47, 965–973. [PubMed] [Google Scholar]
- 17. Danner S, Kajahn J, Geismann C, Klink E, Kruse C (2007) Derivation of oocyte‐like cells from a clonal pancreatic stem cell line. Mol. Hum. Reprod. 13, 11–20. [DOI] [PubMed] [Google Scholar]
- 18. Dyce PW, Wen L, Li J (2006) In vitro germline potential of stem cells derived from fetal porcine skin. Nat. Cell Biol. 8, 384–390. [DOI] [PubMed] [Google Scholar]
- 19. Clark AT, Bodnar MS, Fox M, Rodriquez RT, Abeyta MJ, Firpo MT et al. (2004) Spontaneous differentiation of germ cells from human embryonic stem cells in vitro. Hum. Mol. Genet. 13, 727–739. [DOI] [PubMed] [Google Scholar]
- 20. Geijsen N, Horoschak M, Kim K, Gribnau J, Eggan K, Daley GQ (2004) Derivation of embryonic germ cells and male gametes from embryonic stem cells. Nature 427, 148–154. [DOI] [PubMed] [Google Scholar]
- 21. Hua J, Sidhu KS (2008) Recent advances in the derivation of germ cells from the embryonic stem cells. Stem Cells Dev. 17, 399–411. [DOI] [PubMed] [Google Scholar]
- 22. Hübner K, Fuhrmann G, Christenson LK, Kehler J, Reinbold R, De La FR et al. (2003) Derivation of oocytes from mouse embryonic stem cells. Science 300, 1251–1256. [DOI] [PubMed] [Google Scholar]
- 23. Lacham‐kaplan O, Chy H, Trounson A (2006) Testicular cell conditioned medium supports differentiation of embryonic stem cells into ovarian structures containing oocytes. Stem Cells 24, 266–273. [DOI] [PubMed] [Google Scholar]
- 24. Toyooka Y, Tsunekawa N, Akasu R, Noce T (2003) Embryonic stem cells can form germ cells in vitro. Proc. Natl. Acad. Sci. USA 100, 11457–11462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Leibiger B, Moede T, Schwarz T, Brown GR, Köhler M, Leibiger IB et al. (1998) Short‐term regulation of insulin gene transcription by glucose. Proc. Natl. Acad. Sci. USA 95, 9307–9312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jiang J, Au M, Kuanghui L, Eshpeter A, Gregory K, Greg F et al. (2007) Generation of insulin‐producing islet‐like clusters from human embryonic stem cells. Stem Cells 25, 1940–1953. [DOI] [PubMed] [Google Scholar]
- 27. Noguchi H, Oishi K, Ueda M, Yukawa H, Hayashi S, Kobayashi N et al. (2009) Establishment of mouse pancreatic stem cell line. Cell Transplant. 18, 563–571. [DOI] [PubMed] [Google Scholar]
- 28. Zuk PA (2009) The intracellular distribution of the ES cell totipotent markers OCT4 and Sox2 in adult stem cells differs dramatically according to commercial antibody used. J. Cell. Biochem. 106, 867–877. [DOI] [PubMed] [Google Scholar]
- 29. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD et al. (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284, 143–147. [DOI] [PubMed] [Google Scholar]
- 30. Ohnuki M, Takahashi K, Yamanaka S (2009) Generation and characterization of human induced pluripotent stem cells. Curr. Protoc. Stem Cell Biol.. Chapter 4, Unit 4A.2. [DOI] [PubMed] [Google Scholar]
- 31. Bella AD, Regoli M, Nicoletti C, Ermini L, Fonzi L, Bertelli E (2009) An appraisal of intermediate filament expression in adult and developing pancreas: vimentin is expressed in α cells of rat and mouse embryos. J. Histochem. Cytochem. 57, 577–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kim S‐Y, Lee S, Hong S‐W, Min B‐H, Lee K‐U, Bendayan Moise et al. (2010) Nestin action during insulin‐secreting cell differentiation. J. Histochem. Cytochem. 58, 567–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Blyszczuk P, Asbrand C, Rozzo A, Kania G, Stonge L, Rupnik M et al. (2004) Embryonic stem cells differentiate into insulin‐producing cells without selection of nestin‐expressing cells. Int. J. Dev. Biol. 48, 1095–1104. [DOI] [PubMed] [Google Scholar]
- 34. Gao R, Ustinov J, Pulkkinen MA, Lundin K, Korsgren O, Otonkoski T (2003) Characterization of endocrine progenitor cells and critical factors for their differentiation in human adult pancreatic cell culture. Diabetes 52, 2007–2015. [DOI] [PubMed] [Google Scholar]
- 35. List JF, Habener JF (2004) Glucagon‐like peptide 1 agonists and the development and growth of pancreatic β‐cells. Am. J. Physiol. Endocrinol. Metab. 286, 875–881. [DOI] [PubMed] [Google Scholar]
- 36. Grapin‐Botton A, Majithia AR, Melton DA (2001) Key events of pancreas formation are triggered in gut endoderm by ectopic expression of pancreatic regulatory genes. Genes Dev. 15, 444–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kim MS, Kim JW, Sun C, Oh ST, Yoon KH, Lee SK (2008) Induction of efficient differentiation and survival of porcine neonatal pancreatic cell clusters using an EBV‐based plasmid expressing HGF. J. Biochem. 143, 497–503. [DOI] [PubMed] [Google Scholar]
- 38. Eshpeter A, Jiang J, Au M, Rajotte RV, Lu K, Lebkowski JS et al. (2008) In vivo characterization of transplanted human embryonic stem cell‐derived pancreatic endocrine islet cells. Cell Prolif. 41, 843–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lermen D, Gorjup E, Dyce PW, Von BH, Müller P (2010) Neuro‐muscular differentiation of adult porcine skin derived stem cell‐like cells. PLoS One 5, 8968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kajahn J, Gorjup E, Tiede S, von Briesen H, Paus R, Kruse C et al. (2008) Skin‐derived human adult stem cells surprisingly share many features with human pancreatic stem cells. Eur. J. Cell Biol. 87, 39–46. [DOI] [PubMed] [Google Scholar]
- 41. Kruse C, Birth M, Rohwedel J, Assmuth K, Goepel A, Wedel T (2004) Pluripotency of adult stem cells derived from human and rat pancreas. Appl. Phys. A Mater. Sci. Process 79, 1617–1624. [Google Scholar]
- 42. Majumdar MK, Thiede MA, Mosca JD, Moorman M, Gerson SL (1998) Phenotypic and functional comparison of cultures of marrow‐derived mesenchymal stem cells (MSCs) and stromal cells. J. Cell. Physiol. 176, 57–66. [DOI] [PubMed] [Google Scholar]
- 43. Da Silva Meirelles L, Caplan AI, Nardi NB (2008) In search of the in vivo identity of mesenchymal stem cells. Stem Cells 26, 2287–2299. [DOI] [PubMed] [Google Scholar]
- 44. Dazzi F, Ramasamy R, Glennie S, Jones SP, Roberts I (2006) The role of mesenchymal stem cells in haemopoiesis. Blood Rev. 20, 161–171. [DOI] [PubMed] [Google Scholar]
- 45. Jarvinen L, Badri L, Wettlaufer S, Ohtsuka T, Standiford TJ, Toews GB et al. (2008) Lung resident mesenchymal stem cells isolated from human lung allografts inhibit cell proliferation via a soluble mediator. J. Immunol. 181, 4389–4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hoogduijn MJ, Gorjup E, Genever PG (2006) Comparative characterization of hair follicle dermal stem cells and bone marrow mesenchymal stem cells. Stem Cells Dev. 15, 49–60. [DOI] [PubMed] [Google Scholar]
- 47. Weiss DJ, Berberich MA, Borok Z, Gail DB, Kolls JK, Penland C et al. (2006) Adult stem cells, lung biology, and lung disease. Proc. Am. Thorac. Soc. 3, 193–207. [DOI] [PubMed] [Google Scholar]
- 48. De Bari C, Dell Accio F, Tylzanowski P, Luyten FP (2001) Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum. 44, 1928–1942. [DOI] [PubMed] [Google Scholar]
- 49. Prockop DJ (1997) Marrow stromal cells as stem cells for nonhematopoietic tissues. Science 276, 71–74. [DOI] [PubMed] [Google Scholar]
- 50. Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ et al. (2001) Multilineage cells from human adipose tissue: implications for cell‐based therapies. Tissue Eng. 7, 211–228. [DOI] [PubMed] [Google Scholar]
- 51. Young HE, Steele TA, Bray RA, Hudson J, Floyd JA, Hawkins K et al. (2001) Human reserve pluripotent mesenchymal stem cells are present in the connective tissues of skeletal muscle and dermis derived from fetal, adult, and geriatric donors. Anat. Rec. 264, 51–62. [DOI] [PubMed] [Google Scholar]
- 52. Korbling M, Estrov Z (2003) Adult stem cells for tissue repair – a new therapeutic concept? N. Engl. J. Med. 349, 570–582. [DOI] [PubMed] [Google Scholar]
- 53. Hua J, Pan S, Yang C, Dong W, Dou Z, Sidhu KS (2009a) Derivation of male germ cell‐like lineage from human bone marrow stem cells. Reprod. Biomed. Online 19, 99–105. [DOI] [PubMed] [Google Scholar]
- 54. Hua J, Yu H, Dong W, Yang C, Gao Z, Lei A et al. (2009b) Characterization of mesenchymal stem cells (MSCs) from human fetal lung: potential differentiation of germ cells. Tissue Cell 41, 448–455. [DOI] [PubMed] [Google Scholar]
- 55. Nayernia K, Lee JH, Drusenheimer NN, Nolte J, Wulf G, Dressel R (2006) Derivation of male germ cells from bone marrow stem cells. Lab. Invest. 86, 654–663. [DOI] [PubMed] [Google Scholar]
- 56. Marques‐Mari AI, Lacham‐Kaplan O, Medrano JV, Pellicer A, Simon C (2009) Differentiation of germ cells and gametes from stem cells. Hum. Reprod. Update 15, 379–390. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 No tumour outgrowths were observed for up to 2 months after injection.
Supporting info item


