Abstract
Transgenic mice expressing MyrAkt from a proximal Lck promoter construct develop thymomas at an early age, whereas transgenic mice expressing constitutively active Lck-AktE40K develop primarily tumors of the peripheral lymphoid organs later in life. The thymus of 6- to 8-week-old MyrAkt transgenic mice is normal in size but contains fewer, larger cells than the thymus of nontransgenic control and AktE40K transgenic mice. Earlier studies had shown that cell size and cell cycle are coordinately regulated. On the basis of this finding, and our observations that the oncogenic potential of Akt correlates with its effect on cell size, we hypothesized that mechanisms aimed at maintaining the size of the thymus dissociate cell size and cell cycle regulation by blocking MyrAkt-promoted G1 progression and that failure of these mechanisms may promote cell proliferation resulting in an enlarged neoplastic thymus. To address this hypothesis, we examined the cell cycle distribution of freshly isolated and cultured thymocytes from transgenic and nontransgenic control mice. The results showed that although neither transgene alters cell cycle distribution in situ, the MyrAkt transgene promotes G1 progression in culture. Freshly isolated MyrAkt thymocytes express high levels of cyclins D2 and E and cdk4 but lower than normal levels of cyclin D3 and cdk2. Cultured thymocytes from MyrAkt transgenic mice, on the other hand, express high levels of cyclin D3, suggesting that the hypothesized organ size control mechanisms may down-regulate the expression of this molecule. Primary tumor cells, similar to MyrAkt thymocytes in culture, express high levels of cyclin D3. These findings support the hypothesis that tumor induction is caused by the failure of organ size control mechanisms to down-regulate cyclin D3 and to block MyrAkt-promoted G1 progression.
The Akt protooncogene encodes a serine/threonine protein kinase that is activated by a variety of signals by a phosphatidylinositol-3-kinase-dependent mechanism (for review, see ref. 1 and references therein). Akt1 (or c-akt) is the cellular homolog of the retrovirus-transduced oncogene v-akt. The virus carrying v-akt (AKT8) was isolated from an AKR mouse T cell lymphoma (1) and causes T cell lymphomas when inoculated into newborn AKR mice (1). Moreover, v-akt, but not c-akt, is highly oncogenic when expressed in the nononcogenic rat T cell lymphoma line 5675 (1). v-akt contains an amino-terminal myristoylation signal and is constitutively active. Given that c-akt with a Src-derived myristoylation signal (MyrAkt1, referred to as MyrAkt hereafter) is also constitutively active (1), we asked whether MyrAkt is also oncogenic. To address this question, we constructed MyrAkt and c-akt transgenic mice expressing the transgene in the thymus from a proximal Lck promoter construct. A constitutively active Akt mutant (AktE40K) was used as a control.
In this report, we show that MyrAkt induces thymic lymphomas with a short latency, whereas AktE40K induces lymphomas that arise primarily in peripheral lymphoid organs later in life. The fact that, despite the expression of constitutively active Akt, lymphomas develop later in life suggested that oncogenesis by the constitutively active Akt transgenes is a multiple-step process. The experiments presented here were designed to address the nature of subsequent events in tumor induction, focusing primarily on the MyrAkt transgenic mice. First we examined the size and cellularity of the transgenic and normal control thymus in young preleukemic mice. These results showed that thymocytes expressing the MyrAkt transgene are larger than normal control thymocytes or thymocytes expressing the AktE40K transgene. Despite the increase in cell size induced by the MyrAkt transgene, however, the size of the thymus of young MyrAkt transgenic mice was the same as that of young normal mice and AktE40K transgenic mice. This outcome is because the MyrAkt transgenic thymus contains fewer, larger cells. This led us to explore the correlation between cytomegaly and oncogenesis. The main questions we addressed were (i) what is the mechanism responsible for the low cellularity of the MyrAkt thymus, and (ii) is tumor induction caused by failure of the regulatory mechanisms aimed at preserving the size of the thymus?
Before cell division, cells synthesize macromolecules that will be equally distributed between the daughter cells. Accumulation of these molecules in the parental cells is responsible for a gradual increase in cell size. This increase is monitored by the dividing cells, which progress from the G1 phase to the S phase of the cell cycle only after they pass a minimal size checkpoint. Under normal conditions, cell growth and cell cycle progression are coordinately regulated (2–4). However, early genetic studies in the yeast Saccharomyces cerevisiae (5, 6) and more recent genetic studies in mammals (7–9) have shown that the two can be dissociated. The yeast studies were particularly informative in that they identified two classes of mutations, one that blocked cell cycle progression but allowed cell growth to continue, and a second one that coordinately blocked both cell growth and cell division. The first class of mutations affected cell cycle regulators, whereas the second class affected various biosynthetic pathways. These studies showed not only that cell growth and cell cycle progression can be dissociated but also that inhibition of cell growth exerts a dominant effect on the cell cycle (10).
The preceding description of the relationship between cell size and cell cycle progression applies to single cells. Multicellular organisms and their organs sense either the total cell mass (11, 12) or the total cell number (13, 14) to initiate homeostatic signals that are superimposed on the intrinsic cellular signals regulating cell cycle progression (15–17) or cell death (18–20). The purpose of these signals is to couple the total cell number or the total cell mass in a given organ or organism with the pathways that regulate cellular proliferation and death. The final outcome varies. Thus, tetraploid salamanders contain cells that are approximately double the size of the cells of diploid salamanders. These animals sense the larger size of the tetraploid cells and initiate signals aimed at maintaining the constancy of the total cell mass. As a result, the body size of tetraploid and diploid salamanders is the same despite the fact that tetraploid salamanders have only half the number of cells (21). On the other hand, changes in the size of the cells in the Drosophila wing and eye imaginal discs, induced by mutations of Dp70S6K (13), or Dakt (14), result in larger adult structures for these compartments, suggesting that the aim of signals resulting from sensing these changes is to maintain the total number of cells constant.
The signals that regulate organ size are either systemic or originate in the organ itself. This was demonstrated by thymus and spleen transplantation experiments in mice. All multiple fetal thymuses transplanted into developing mice grow to adult size (22), suggesting that their growth depends on control mechanisms intrinsic to the thymus. Multiple fetal spleens transplanted in developing mice, on the other hand, grow until their total mass attains the size of one adult spleen (23). Therefore, spleen growth differs from thymus growth in that it depends on systemic factors. These local and systemic mechanisms regulate organ size by initiating signals that are superimposed on intrinsic cellular controls that promote or inhibit cellular proliferation or apoptosis.
The cell cycle distribution of freshly isolated MyrAkt transgenic thymocytes was similar to that of normal control thymocytes and AktE40K thymocytes, suggesting a dissociation between the regulation of cell size and the regulation of cell cycle progression in the MyrAkt transgenic thymus. This dissociation was not observed in cultures of CD8 single-positive (SP) transgenic thymocytes from MyrAkt transgenic mice, which were shown to contain fewer cells in G1 and more cells in S by comparison with cultures of normal control and AktE40K thymocytes. The expression of cyclin D3 and cdk2 was lower in freshly isolated MyrAkt thymocytes than in freshly isolated normal control and AktE40K thymocytes. However, MyrAkt transgenic thymocytes in culture expressed cyclin D3 at levels comparable to or higher than those of cultured control and AktE40k thymocytes. These data collectively suggest that local mechanisms regulating the size of the thymus do so by down-regulating the expression of cyclin D3 and, perhaps, other cell cycle regulatory molecules such as cdk2. These mechanisms, which are not operating on CD8 SP cells in culture, may prolong the cell cycle by resetting the cell size checkpoint so that cells progress through the cell cycle only after they attain a larger size.
Developing tumors express high levels of cyclin D3 similar to cultured thymocytes from MyrAkt-expressing mice. These data, combined, support a model according to which genetic changes promoting cell cycle progression are not sufficient for tumor induction because they are neutralized by control mechanisms that regulate organ size. The balance established between the two opposing forces, however, is unstable. Tumor induction may result from disruption of this balance because of failure of the mechanisms that regulate organ size. The model presented here suggests a protective mechanism that explains why genetic changes that transform cells in culture may not be sufficient to induce tumors in animals.
Materials and Methods
Mice.
Hemagglutinin epitope (HA)-tagged wild-type Akt, MyrAkt, and AktE40K were cloned into the pLck-GH vector (24). The linearized constructs were microinjected into oocytes from (C57BL/6 × C3H/He)F2 mice (25). Nine c-akt, 18 AktE40K, and 21 MyrAkt transgene-positive founders were identified. Transgene-positive founders were crossed to C57BL/6 mice. Two of the AktE40K (E-3 and E-4) and three of the MyrAkt (M-1, M-9, and M-24) mice expressing high or low levels of the transgenes (Fig. 1A) were maintained as transgenic lines. The MyrAkt transgenic line M-24, which expressed the highest levels of the transgene, was lost because mice died of thymic lymphomas at a very young age. The c-akt transgenic mice were indistinguishable from the nontransgenic controls and they will not be discussed further in this report. The mice used in the oncogenicity studies were derived from the early backcross generations. Other experiments were carried out with mice from the 5th to the 7th backcross generations.
Figure 1.
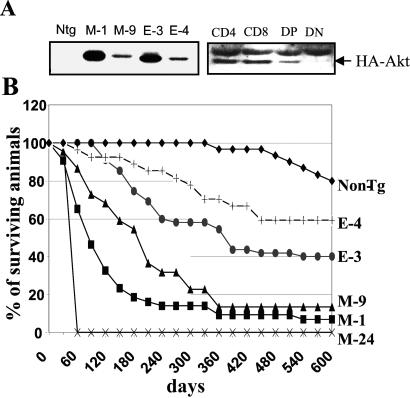
Biological consequences of constitutively active Akt transgenes in mouse thymocytes. (A) (Left) Expression of MyrAkt and AktE40K transgenes in the thymus of two Lck-MyrAkt HA (M-1 and M-9) and two Lck HA AktE40K (E-3 and E-4) transgenic mouse lines. Ntg, nontransgenic control. (Right) Western blot of cell lysates derived from sorted mouse thymocytes probed with the anti-HA antibody shows that the transgene is expressed in CD4+, CD8+, and CD4+/CD8+ double-positive (DP) but not CD4−/CD8− double-negative (DN) cells. (B) Mortality curves of transgenic mouse lines.
Single-Cell Suspension and Culture of Mouse Thymocytes.
Single-cell suspensions of thymocytes were washed twice in Dulbecco's minimal essential media (DMEM) (BRL/GIBCO), supplemented with nonessential amino acids (100 μM each), 2-mercaptoethanol (50 mM), penicillin (100 units/ml), streptomycin (100 μg/ml), and fetal bovine serum (FBS; 10%). Subsequently, single-cell suspensions of thymocytes in the cell wash media (0.5 × 106 cells per ml), were stimulated with concanavalin A (Con A) (50 μg/ml). Twenty-four hours later, the cells were washed and resuspended in the same medium supplemented with IL-2 (50 units/ml) and conditioned medium harvested from rat splenocytes stimulated with Con A for 24 h (Con A Sup) (10%).
Antibodies, Flow Cytometry, and Western Blotting.
FITC-labeled anti-mouse CD4 and Cy-chrome or phycoerythrin (PE)-labeled anti-mouse CD8 antibodies were purchased from PharMingen. With the exception of horseradish peroxidase-conjugated anti-mouse and anti-rabbit IgG, which were purchased from Life Sciences, the rest of the antibodies were purchased from Santa Cruz Biotechnology.
CD4 and/or CD8 staining was done following the protocols of labeled antibody suppliers. Flow cytometry was carried out on a Coulter EPICS XL-MCL flow cytometer. Data were analyzed by using the software program WIN MDI 2.8. To determine the cell cycle distribution of freshly isolated and cultured thymocytes, 106 cells were stained with ethidium bromide (EtdBr) in staining buffer (3.4 mM sodium citrate, pH 7/10 mM NaCl/0.1% Nonidet P-40/75 μM EtdBr) (26). The DNA content of stained nuclei was determined by flow cytometry.
Pellets of freshly isolated or cultured mouse thymocytes were lysed in 200 μl of Nonidet P-40 (20 mM Tris⋅HCl, pH 7.5/150 mM NaCl/1% Nonidet P-40/10% glycerol) or RIPA (50 mM Tris⋅HCl, pH 7.5/150 mM NaCl/1% Triton X-100/0.1% SDS/2 mM EDTA/0.5% sodium deoxycholate) lysis buffer. Western blotting was carried out by standard procedures.
Results
MyrAkt and AktE40K Transgenes Differ in Oncogenic Potential.
Thymocytes from AktE40K and MyrAkt transgenic mice were stained with antibodies recognizing a series of developmentally regulated membrane molecules. Flow-cytometric analysis of the stained cells showed that the AktE40K and the MyrAkt transgenes induce similar shifts in thymocyte subpopulations. One of these shifts involved the CD4 and CD8 SP cells, whose relative numbers were increased by both transgenes (data not shown). Despite the fact that the two transgenes exert similar effects on thymopoiesis, MyrAkt mice die of lymphomas at a significantly younger age than AktE40K mice (Fig. 1B). Lines expressing higher levels of the transgene died at a younger age than lines expressing lower levels of the transgene. Thus, M-1 mice died earlier than M-9 and E-3 mice died earlier than E-4. Ninety-seven percent of MyrAkt and 93% of AktE40K transgenic mice were autopsied at the time of death. The results (Table 1) revealed that, whereas MyrAkt transgenic mice die primarily of thymic lymphomas, AktE40K transgenic mice die primarily of lymphoid neoplasms arising in peripheral lymphoid organs. All tumors carried clonal rearrangements in the T cell receptor genes (data not shown). MyrAkt (M-9) transgenic mice, which express lower levels of the transgene, occupy an intermediate position between MyrAkt (M-1) and AktE40K transgenic mice regarding the frequency of lymphomas arising in peripheral lymphoid organs. This observation suggests that the phenotypic differences between the tumors arising in MyrAkt and AktE40K transgenic mice are likely to result from differences in specific kinase activity between the products of the two transgenes (1) rather than from qualitative differences between them. Histologically, all tumors were lymphoblastic lymphomas. The tumor surface phenotype was variable with some expressing a CD4 or CD8 SP, and some expressing a CD4/CD8 DP phenotype. Interestingly, an Lck-MyrAkt2 transgene is equally oncogenic with the Lck-MyrAkt1 transgene described here (27). To determine why the MyrAkt and AktE40K transgenes differ in oncogenic potential, we first examined whether they differ in their ability to inhibit apoptosis induced by growth factor (IL-2 and Con A Sup) withdrawal. The results showed that both transgenes protect cells from apoptosis as expected (1). Earlier studies using mice expressing v-akt transgene from a CD2 promoter construct had shown that the transgene did not prevent antigen-induced clonal deletion of thymocytes expressing P14, a class I MHC-restricted T cell receptor transgene (28). This finding suggests that the activated Akt transgenes are unlikely to promote tumor induction by inhibiting negative selection of DP thymocytes.
Table 1.
Incidence, latency, and spectrum of lymphomas induced by the MyrAkt and AktE40K transgenes
| Line | No. of mice | Deaths | Thymomas | Thymomas and splenomegaly | Splenomegaly | Not det* | Age of animals at time of death, days |
|---|---|---|---|---|---|---|---|
| MyrAkt (founders) | 21 | 15 | 7 | 3 | 1 | 4 | 146.6 ± 131.0 |
| MyrAkt (M-1) | 43 | 40 | 21 | 10 | 0 | 9 | 127.8 ± 77.0 |
| MyrAkt (M-9) | 22 | 19 | 6 | 10 | 2 | 1 | 193.0 ± 97.8 |
| MyrAkt (M-24) | 4 | 4 | 4 | 0 | 0 | 0 | 66.5 ± 0.9 |
| AktE40K (founders) | 18 | 3 | 1 | 0 | 1 | 1 | 525.7 ± 165.3 |
| AktE40K (E-3) | 55 | 33 | 5 | 8 | 6 | 13 | 264.0 ± 117.3 |
| AktE40K (E-4) | 27 | 11 | 0 | 3 | 5 | 3 | 297.3 ± 119.5 |
| Nontransgenic | 30 | 6 | 0 | 0 | 2 | 4 | 499.5 ± 133.3 |
Mice were monitored for a total of 600 days. Ages are mean ± SD.
This column includes mice that were discovered dead, and because of autolysis the cause of their death could not be determined objectively.
The v-akt transgenic mice described in the preceding paragraph exhibit T cell protection from Fas-induced apoptosis. However, these mice do not develop tumors. Instead, when they grow old, they develop an autoimmune syndrome that has been attributed to enhanced T cell survival (29). The low oncogenic potential of v-akt by comparison with MyrAkt in mice is reminiscent of the low oncogenic potential of v-akt in chickens (30). Other factors that may contribute to the inability of the v-akt transgene to induce tumors in mice may be the promoter used and the level of expression of the transgene.
MyrAkt Transgenic Thymocytes Are Larger than AktE40K Transgenic and Nontransgenic Control Thymocytes.
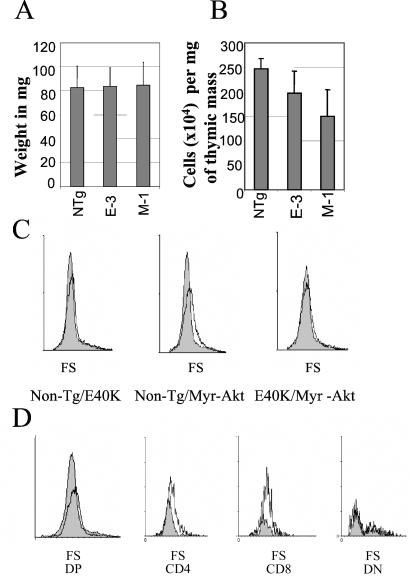
To determine whether expression of the MyrAkt or AktE40K transgenes affects the cellularity of the thymus, thymuses of six MyrAkt (line M-1), six AktE40K (line E-3), and six nontransgenic animals were weighed and processed to generate single-cell suspensions. The results showed that all thymuses were identical in size by weight (Fig. 2A). However, the thymuses of MyrAkt transgenic mice contained fewer cells (Fig. 2B). This result suggested that thymocytes of MyrAkt transgenic mice are larger. This prediction was confirmed by flow cytometry (Fig. 2C).
Figure 2.
MyrAkt transgenic thymocytes are larger than AktE40K transgenic and nontransgenic control thymocytes. (A) The weight of the thymus of AktE40K and MyrAkt transgenic and nontransgenic (NTg) mice is the same (six age- and sex-matched mice from each group). (B) Thymuses of MyrAkt1 transgenic mice contain fewer cells than thymuses of nontransgenic or AktE40K transgenic mice. (C) Cell size (forward scatter, FS) comparisons between nontransgenic (Non-Tg) control and AktE40K, nontransgenic control and MyrAkt, and AktE40K and MyrAkt thymocytes. MyrAkt thymocytes are larger. (D) The effect of MyrAkt on the cell size is cell autonomous. CD4 and CD8 SP and CD4/CD8 DP MyrAkt thymocytes are larger than their counterparts derived from nontransgenic control mice. DN, double-negative.
The size increase of the thymocytes derived from MyrAkt transgenic mice could be the direct result of MyrAkt expression. Alternatively, it may represent the cellular response to cytokines produced by the MyrAkt-expressing cells. To distinguish between these possibilities, we examined whether large size is a feature of the cells expressing the MyrAkt transgene. To this end, thymocytes were stained with anti-CD4 and anti-CD8 antibodies. Forward scatter analysis revealed that whereas the MyrAkt DP and SP cell populations were enlarged, the double-negative (DN) cells were not (Fig. 2D). Because the SP and the DP cells also express the transgene (Fig. 1A), we conclude that the effect of MyrAkt on the cell size is cell autonomous. Collectively, the data in Fig. 2 suggest that the thymus senses the large size of the cells expressing MyrAkt and initiates signals aimed at maintaining the total thymic mass constant (22). As a result, the increase in cell size is accommodated by a reciprocal decrease in cell number.
The MyrAkt and AktE40K Transgenes Do Not Alter the Cell Cycle Distribution of Thymocytes in Situ: Expression of Cell Cycle Regulatory Proteins in Freshly Isolated Thymocytes.
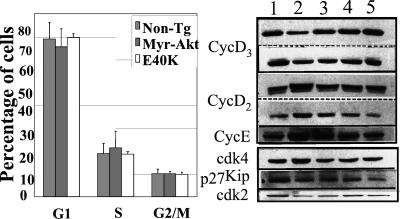
Given that cell growth and the cell cycle are normally coordinately regulated, we examined whether the MyrAkt transgene stimulates cell cycle progression. Single-cell suspensions of freshly isolated transgenic and nontransgenic thymocytes were stained with ethidium bromide and analyzed by flow cytometry. The results showed that the cell cycle distribution of thymocytes was similar in all mice (Fig. 3 Left). We conclude that cell size and cell cycle regulation are dissociated in the thymus of MyrAkt transgenic mice.
Figure 3.
Cell cycle distribution and expression of cell cycle regulators in freshly isolated transgenic and nontransgenic control thymocytes. (Left) Cell cycle distribution of freshly isolated thymocytes from nontransgenic, MyrAkt, and AktE40K transgenic mice. There were four 6- to 8-week-old, age- and sex-matched mice per group. (Right) Expression of cell cycle regulators in freshly isolated thymocytes from nontransgenic control and transgenic mouse lines. Lanes: 1, nontransgenic; 2, MyrAkt1 M-1; 3, MyrAkt1 M-9; 4, Akt1-E40K E-3; and 5, Akt1-E40K E-4. For cyclin D3 and cyclin D2, the results of two independent experiments are shown. The same blots were probed with the antibodies to cyclin D2 and cyclin D3. The intensity of individual bands was measured by densitometry. Band intensity ratios were as follows: cyclin D3, nontransgenic vs. MyrAkt M-1 transgenic thymocytes = 2.23 and 1.86 in the two experiments shown; cyclin D2, nontransgenic vs. MyrAkt M-1 transgenic thymocytes = 0.42 and 0.45; cyclin E, nontransgenic vs. MyrAkt M-1 transgenic thymocytes = 0.25; cdk2, nontransgenic vs. MyrAkt M-1 transgenic thymocytes = 2.89.
Previous studies had shown that constitutively active Akt up-regulates the expression of G1 cyclins by enhancing protein translation (31) and stability (32). These studies were carried out in cultured cells in which constitutively active MyrAkt promotes cell cycle progression (31, 32). Because constitutively active MyrAkt does not promote cell cycle progression in transgenic thymocytes in situ (Fig. 3 Left), we proceeded to also examine the expression of G1 cyclins in freshly isolated thymocytes. The results showed that cyclins D2 and E were up-regulated, whereas cyclin D3 and cdk2 were down-regulated, and that the down-regulation of these molecules was more pronounced in the MyrAkt transgenic line M-1, which expresses higher levels of the transgene (Fig. 3 Right). cdk4 expression was also marginally elevated in the M-1 MyrAkt transgenic mouse line. The unexpected down-regulation of cyclin D3 and cdk2 in the MyrAkt transgenic thymus suggests that these molecules may be the target of dominant inhibitory signals aimed at maintaining the normal size of the thymus.
The MyrAkt and AktE40K transgenic mouse lines differed with regard to the expression of G1 cyclins, cdk2, and perhaps cdk4. However, the expression of p27Kip did not distinguish between these lines in that it was down-regulated in all (Fig. 3 Right). Similarly, GSK3β phosphorylation and β-catenin expression were up-regulated in all transgenic lines (data not shown).
The MyrAkt Transgene Promotes G1 Progression in Culture.
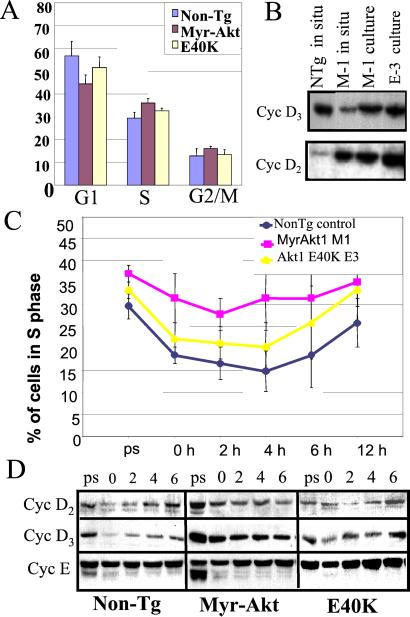
To determine whether the normal cell cycle distribution of MyrAkt transgenic thymocytes in situ is due to intrathymic inhibitory signals, we examined the cell cycle distribution of transgenic and normal control thymocytes in culture. The logic of this experiment was that, when in culture, the thymocytes would not be subject to the postulated inhibitory mechanisms. Thymocytes from MyrAkt and AktE40K transgenic mice and nontransgenic controls (four mice per group) were stimulated with Con A and they were cultured (two cultures per mouse) in DMEM supplemented with IL-2 and Con A Sup. Five days later, growing CD8 SP thymocyte cultures had been established (data not shown). Cells from these cultures were subcultured in the same medium at 0.5 × 106 cells per ml. Twenty-four hours later, 10% of the cells in each culture were stained with ethidium bromide and analyzed by flow cytometry to determine their cell cycle distribution. The results showed that cultures of MyrAkt transgenic thymocytes consistently contained a lower percentage of cells in G1 and a higher percentage of cells in S phase (Fig. 4A). Western blots of lysates from these cells revealed that not only cyclin D2, but also cyclin D3, was up-regulated (Fig. 4B). These findings suggest that elimination of the intrathymic inhibitory signals allows MyrAkt to up-regulate the expression of cyclin D3. Alternatively, up-regulation of cyclin D3 occurs as the cells mature to the SP stage, at which point they leave the thymus to populate the peripheral lymphoid organs.
Figure 4.
Cell cycle distribution and expression of cell cycle regulators in cultured thymocytes from nontransgenic control, MyrAkt, and AktE40K transgenic mice. (A) Cell cycle distribution of cultured thymocytes from nontransgenic, MyrAkt, and AktE40K transgenic mice. Cells were grown in complete media; eight independent cultures from four mice per group were compared. (B) Expression of cyclin D3 and cyclin D2 in freshly isolated nontransgenic and MyrAkt transgenic thymocytes, as well as in cultured MyrAkt and AktE40K thymocytes. The same blot was probed with both antibodies. (C) Percent of cells in S phase. Prestarved (ps) are cells growing exponentially in complete medium. Data are combined from cultures of thymocytes derived from four mice per group and two independent cultures per mouse. (D) Expression of cell cycle regulators in cultured thymocytes from nontransgenic, MyrAkt, and AktE40K transgenic mice.
To further explore the effects of MyrAkt on cell cycle progression in culture, thymocytes from MyrAkt, AktE40K, and normal control mice (six mice per group) growing in IL-2 and Con A Sup-supplemented media (two cultures per mouse) were plated at 0.5 × 106 cells per ml. Twenty-four hours later, the cells were starved of IL-2 and Con A Sup for 10 h, at which point they were analyzed for their cell cycle distribution. The remaining cells were restimulated and they were harvested and analyzed for their cell cycle distribution at 2, 4, 6, and 12 h after restimulation. The results showed that 10 h after starvation, a high percentage of MyrAkt-expressing cells continued to cycle. Restimulation promoted reentry of nontransgenic and AktE40K transgenic thymocytes into the cell cycle but had a lesser effect on the cell cycle distribution of MyrAkt transgenic thymocytes (Fig. 4C). Lysates of cells harvested before starvation and at 0, 2, 4, and 6 h after restimulation were analyzed for the expression of cyclins D2, D3, and E. The results showed that cyclins D2 and D3 were both expressed at higher levels in cultured MyrAkt transgenic thymocytes and that they were only partially down-regulated after starvation. We conclude that the MyrAkt transgene enhances the expression of G1 cyclins and cell cycle progression in culture.
Primary Thymomas from MyrAkt Transgenic Mice Express High Levels of Cyclin D3.
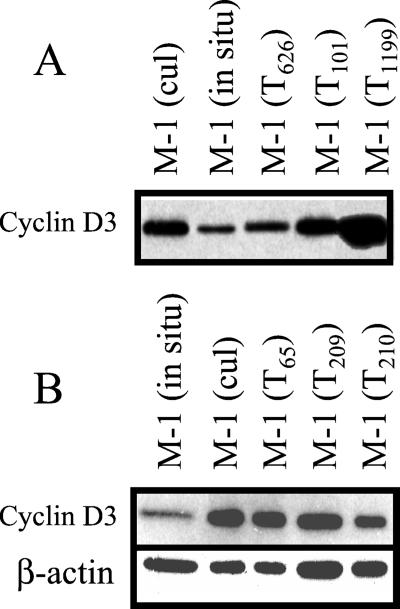
Despite the cell cycle stimulatory activity of the MyrAkt transgene, which can be detected early in life, tumors develop significantly later. On the basis of the preceding data, we propose that the delayed induction of thymic lymphomas in the transgenic mice may be caused by failure of cell cycle inhibitory mechanisms aimed at maintaining the normal size of the thymus. To test this hypothesis, we examined the expression of cyclin D3 in freshly isolated cells from six thymomas arising in MyrAkt transgenic mice. The results (Fig. 5) showed that tumor cells are similar to cultured thymocytes in that they express high levels of cyclin D3. We conclude that the tumor cells did escape the inhibitory signals down-regulating the expression of cyclin D3 in thymocytes in situ.
Figure 5.
Primary thymomas from MyrAkt transgenic mice express high levels of cyclin D3. (A) Western blots of cell lysates from freshly isolated and cultured MyrAkt transgenic thymocytes, as well as from freshly isolated tumor cells from six thymomas (three in each of two separate experiments) derived from MyrAkt transgenic mice were probed with an antibody to cyclin D3. (B) Equal loading was confirmed in the second experiment by reprobing with an anti β-actin antibody (Sigma).
Discussion
Evidence presented in this report shows that two constitutively active Akt transgenes, MyrAkt and AktE40K, expressed in the thymus from a proximal Lck promoter construct, are oncogenic. MyrAkt induces primarily thymic tumors with a short latency, whereas AktE40K exhibits long latency and induces primarily lymphomas of the peripheral lymphoid organs. The delayed appearance of MyrAkt and AktE40K-induced lymphomas suggests that tumor induction by the constitutively active Akt transgenes is a multiple-step process. The experiments presented here were designed to address the potential mechanism by which a second step may promote tumor induction in the MyrAkt transgenic mice.
Earlier studies had shown that the size of the thymus in mice is regulated by signals intrinsic to the thymus (22). In this report, we presented evidence that these signals monitor the total thymic cell mass. As a result, the increase in cell size induced by a MyrAkt transgene expressed in the thymus is accompanied by a decrease in the total cell number so that the total cell mass remains constant. The mammalian thymus clearly differs from the Drosophila wing and eye imaginal discs, where the aim of signals sensing changes in the cell size is to maintain the number of cells constant. As a result, changes in cell size in these compartments result in changes in organ size (13, 14). The differences between the mammalian thymus and the Drosophila wing and eye imaginal discs suggest that the regulation of organ size differs between organs or organisms.
During cell cycle progression, cells increase in size as they accumulate proteins and other macromolecules that are synthesized to be distributed between the daughter cells. To ensure that the daughter cells will contain a full complement of macromolecules, cells progress from G1 to S only after they cross a minimal size checkpoint (2–4). The coordinate regulation of the cell size and the cell cycle predicts that a primary cell size-enhancing signal induced by MyrAkt should speed up passage through the minimal size checkpoint. This prediction had been confirmed in MyrAkt-expressing cells in culture (33, 34). Stimulation of G1 progression in animal cells in situ if unchecked, however, would increase the size of the organ containing these cells. Data presented in this report suggest that this increase is prevented by intrathymic signals, which are triggered by the increased size of the cells and which elicit events aimed at maintaining the thymic cell mass constant. These signals may limit the number of thymocytes by any of three non-mutually exclusive mechanisms: (i) they may increase apoptosis (18–20); (ii) they may reset the minimal size checkpoint so that a larger cell size is required to permit the transition from G1 to S (7–11); or (iii) they may limit the number of cell divisions T cell precursors may undergo during maturation (35–38). MyrAkt exerts a strong anti-apoptotic effect (1) which makes the first possibility unlikely. On the other hand, MyrAkt stimulates G1 progression in primary cultures of CD8 SP T cells derived from the thymus, but not in thymocytes in situ, suggesting that the intrathymic signals limiting the number of enlarged thymocytes operate by inhibiting cell cycle progression.
Although we do not yet know the nature of the signals that maintain the total mass of thymocytes constant, we addressed the mechanism by which such signals inhibit cell cycle progression. Monitoring the expression levels of several cell cycle regulators revealed that cyclin D3 and cdk2 are down-regulated in MyrAkt thymocytes in situ. However, cyclin D3 is up-regulated in MyrAkt thymocytes in culture. This is specific for cyclin D3 and cdk2, because cyclin D2 and cdk4 are up-regulated in MyrAkt transgenic thymocytes in situ. The increase in cyclin D2 expression may result from the direct stimulatory effect of constitutively active Akt on protein translation or stability (31, 32).
On the basis of these findings, we hypothesized that the thymus of MyrAkt transgenic mice is the battleground of two opposing forces: one that is triggered by the MyrAkt transgene and promotes cell cycle progression and a second one that is triggered by the cell size increase induced by the transgene and inhibits the cell cycle. Failure of the cell cycle inhibitory signals will disrupt the unstable balance between these forces and will allow an increase in thymic cellularity and size. The correlation of cytomegaly with predisposition to neoplastic transformation in MyrAkt transgenic mice suggests that failure of the cell cycle inhibitory forces is the first step in tumor induction. This hypothesis is supported by data showing that primary thymomas arising in MyrAkt transgenic mice resemble cultured MyrAkt transgenic thymocytes in that they express high levels of cyclin D3. It remains to be determined whether cyclin D3 (and other cell cycle regulators) synergize with the MyrAkt transgene in oncogenesis.
In summary, the data presented here, support a hypothesis according to which tumor induction by a constitutively active Akt transgene is delayed by conservative signals intrinsic to the targeted organ and aimed at maintaining the normal size of the organ. According to this hypothesis, tumor induction results from failure of the unstable balance between the transgene and the intrinsic inhibitory signals. Exploring the nature of these inhibitory mechanisms may provide new insights into the prevention and treatment of cancer.
Acknowledgments
We thank Fuming Pan and Jugin Wang for technical assistance with the generation of the transgenic mice. This work was supported by National Institutes of Health Grant R01 CA57436. S.M. was supported by National Institutes of Health Grant 5-T32-CA09678.
Abbreviations
- SP
single-positive
- DP
double-positive
- HA
hemagglutinin epitope
Note Added in Proof.
The larger size of mammalian cells constitutively expressing Akt1 was also reported in a paper by Tuttle et al. (39) that was published while the present paper was in press.
References
- 1.Chan T O, Rittenhouse S E, Tsichlis P N. Annu Rev Biochem. 1999;68:965–1014. doi: 10.1146/annurev.biochem.68.1.965. [DOI] [PubMed] [Google Scholar]
- 2.Stocker H, Hafen E. Curr Opin Genet Dev. 2000;10:529–535. doi: 10.1016/s0959-437x(00)00123-4. [DOI] [PubMed] [Google Scholar]
- 3.Su T T, O'Farrell P H. Curr Biol. 1998;8:687–689. doi: 10.1016/s0960-9822(98)70436-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mitchison J M, Novak B, Sveiczer A. Cell Biol Int. 1997;21:461–463. doi: 10.1006/cbir.1997.0187. [DOI] [PubMed] [Google Scholar]
- 5.Hartwell L H. J Mol Biol. 1971;59:183–194. doi: 10.1016/0022-2836(71)90420-7. [DOI] [PubMed] [Google Scholar]
- 6.Nurse P, Thuriaux P, Nasmyth K. Mol Gen Genet. 1976;146:167–178. doi: 10.1007/BF00268085. [DOI] [PubMed] [Google Scholar]
- 7.Franch H A, Shay J W, Alpern R J, Preisig P A. J Cell Biol. 1995;129:245–254. doi: 10.1083/jcb.129.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hemerly A, Engler J d A, Bergounioux C, Van Montagu M, Engler G, Inze D, Ferreira P. EMBO J. 1995;14:3925–3936. doi: 10.1002/j.1460-2075.1995.tb00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sheikh M S, Rochefort H, Garcia M. Oncogene. 1995;11:1899–1905. [PubMed] [Google Scholar]
- 10.Johnston G C, Pringle J R, Hartwell L H. Exp Cell Res. 1977;105:79–98. doi: 10.1016/0014-4827(77)90154-9. [DOI] [PubMed] [Google Scholar]
- 11.Weigmann K, Cohen S M, Lehner C F. Development (Cambridge, UK) 1997;124:3555–3563. doi: 10.1242/dev.124.18.3555. [DOI] [PubMed] [Google Scholar]
- 12.Neufeld T P, de la Cruz A F, Johnston L A, Edgar B A. Cell. 1998;93:1183–1193. doi: 10.1016/s0092-8674(00)81462-2. [DOI] [PubMed] [Google Scholar]
- 13.Volarevic S, Stewart M J, Ledermann B, Zilberman F, Terracciano L, Montini E, Grompe M, Kozma S C, Thomas G. Science. 2000;288:2045–2047. doi: 10.1126/science.288.5473.2045. [DOI] [PubMed] [Google Scholar]
- 14.Verdu J, Buratovich M A, Wilder E L, Birnbaum M J. Nat Cell Biol. 1999;1:500–506. doi: 10.1038/70293. [DOI] [PubMed] [Google Scholar]
- 15.de Nooij J C, Letendre M A, Hariharan I K. Cell. 1996;87:1237–1247. doi: 10.1016/s0092-8674(00)81819-x. [DOI] [PubMed] [Google Scholar]
- 16.Gao F B, Durand B, Raff M. Curr Biol. 1997;7:152–155. doi: 10.1016/s0960-9822(06)00060-1. [DOI] [PubMed] [Google Scholar]
- 17.Henrique D, Hirsinger E, Adam J, Le Roux I, Pourquie O, Ish-Horowicz D, Lewis J. Curr Biol. 1997;7:661–670. doi: 10.1016/s0960-9822(06)00293-4. [DOI] [PubMed] [Google Scholar]
- 18.Raff M C. Nature (London) 1992;356:397–400. doi: 10.1038/356397a0. [DOI] [PubMed] [Google Scholar]
- 19.Calver A R, Hall A C, Yu W P, Walsh F S, Heath J K, Betsholtz C, Richardson W D. Neuron. 1998;20:869–882. doi: 10.1016/s0896-6273(00)80469-9. [DOI] [PubMed] [Google Scholar]
- 20.Bangs P, White K. Dev Dyn. 2000;218:68–79. doi: 10.1002/(SICI)1097-0177(200005)218:1<68::AID-DVDY6>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 21.Fankhauser G. Int Rev Cytol. 1952;1:165–193. [Google Scholar]
- 22.Metcalf D. Aust J Exp Med Sci. 1963;41:437–448. doi: 10.1038/icb.1963.4. [DOI] [PubMed] [Google Scholar]
- 23.Metcalf D. Transplantation. 1964;2:387–392. doi: 10.1097/00007890-196405000-00008. [DOI] [PubMed] [Google Scholar]
- 24.Perez P, Lira S A, Bravo R. Mol Cell Biol. 1995;15:3523–3530. doi: 10.1128/mcb.15.7.3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ceci J D, Kovatch R M, Swing D A, Jones J M, Snow C M, Rosenberg M P, Jenkins N A, Copeland N G, Meisler M H. Oncogene. 1991;6:323–332. [PubMed] [Google Scholar]
- 26.Grimes H L, Gilks C B, Chan T O, Porter S, Tsichlis P N. Proc Natl Acad Sci USA. 1996;93:14569–14573. doi: 10.1073/pnas.93.25.14569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mende I, Malstrom S, Tsichlis P N, Vogt P K, Aoki M. Oncogene. 2001;20:4419–4423. doi: 10.1038/sj.onc.1204486. [DOI] [PubMed] [Google Scholar]
- 28.Jones R G, Parsons M, Bonnard M, Chan V S, Yeh W C, Woodgett J R, Ohashi P A. J Exp Med. 2000;191:1721–1734. doi: 10.1084/jem.191.10.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parsons M J, Jones R G, Tsao M S, Odermatt B, Ohashi P S, Woodgett J R. J Immunol. 2001;167:42–48. doi: 10.4049/jimmunol.167.1.42. [DOI] [PubMed] [Google Scholar]
- 30.Aoki M, Batista O, Bellacosa A, Tsichlis P N, Vogt P K. Proc Natl Acad Sci USA. 1998;95:14950–14955. doi: 10.1073/pnas.95.25.14950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muise-Helmericks R C, Grimes H L, Bellacosa A, Malstrom S E, Tsichlis P N, Rosen N. J Biol Chem. 1998;273:29864–29872. doi: 10.1074/jbc.273.45.29864. [DOI] [PubMed] [Google Scholar]
- 32.Diehl J A, Cheng M, Roussel M F, Sherr C J. Genes Dev. 1998;12:3499–3511. doi: 10.1101/gad.12.22.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ahmed N N, Grimes H L, Bellacosa A, Chan T O, Tsichlis P N. Proc Natl Acad Sci USA. 1997;94:3627–3632. doi: 10.1073/pnas.94.8.3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brennan P, Babbage J W, Burgering B M, Groner B, Reif K, Cantrell D A. Immunity. 1997;7:679–689. doi: 10.1016/s1074-7613(00)80388-x. [DOI] [PubMed] [Google Scholar]
- 35.Kipreos E T, Lander L E, Wing J P, He W W, Hedgecock E M. Cell. 1996;85:829–839. doi: 10.1016/s0092-8674(00)81267-2. [DOI] [PubMed] [Google Scholar]
- 36.Knoblich J A, Sauer K, Jones L, Richardson H, Saint R, Lehner C F. Cell. 1994;77:107–120. doi: 10.1016/0092-8674(94)90239-9. [DOI] [PubMed] [Google Scholar]
- 37.Casaccia-Bonnefil P, Tikoo R, Kiyokawa H, Friedrich V, Jr, Chao M V, Koff A. Genes Dev. 1997;11:2335–2346. doi: 10.1101/gad.11.18.2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Durand B, Fero M L, Roberts J M, Raff M C. Curr Biol. 1998;8:431–440. doi: 10.1016/s0960-9822(98)70177-0. [DOI] [PubMed] [Google Scholar]
- 39.Tuttle R L, Gill N S, Pugh W, Lee J P, Koeberlein B, Furth E E, Polonsky K S, Naji A, Birnbaum M J. Nat Med. 2001;7:1133–1137. doi: 10.1038/nm1001-1133. [DOI] [PubMed] [Google Scholar]