Abstract
Objectives: The fate choice of neural progenitor cells could be dictated by local cellular environment of the adult CNS. The aim of our study was to investigate the effect of hippocampal tissue on differentiation and maturation of oligodendrocyte NG2 precursor cells.
Materials and methods: Hippocampal slice culture was established from the brains of 7‐day‐old rats. NG2 precursor cells, obtained from a 12‐day‐old mixed primary culture of neonatal rat cerebral hemispheres, were labelled with chloromethyl‐fluorescein‐diacetete and seeded on the hippocampal slices. After 7–14 days in co‐culture, cells were stained with neural markers.
Results: NG2 cells differentiated predominantly into oligodendrocytes, presenting various stages of maturation: progenitors (NG2), pre‐oligodendrocytes (O4) and finally mature GalC‐positive cells. However, except for a few cells with astrocyte‐specific S100b staining, a considerable number of these cells differentiated into neurons: TUJ+ and even MAP‐2+ cells were frequently observed. Moreover, a certain population of these cells preserved proliferative properties of primary precursor cells, as revealed by Ki67 expression.
Conclusions: The neuronal micro‐environment provided by the culture of hippocampal slices is potent for induction of neurogenesis from oligodendrocyte NG2+/PDGFRα+/CNP+ progenitor cells and promotes their differentiation not only into macroglia but also into neurons. It also sustains their proliferative capacity. The results indicate the crucial role of the local cellular environment in fate decision of primary NG2+ multipotent neural progenitor cells, which may affect their behaviour after transplantation into the central nervous system.
Introduction
In recent years, the biology of neural stem cells and progenitors has been extensively investigated. Chondroitin sulphate proteoglycan (NG2) progenitor cells, committed to the oligodendrocyte lineage, have also drawn much attention with respect to their multipotency (1, 2, 3). This highly conserved proteoglycan is expressed by a variety of tissues during development and adulthood (4). NG2s comprise the largest population of dividing cells in the central nervous system (5–6%) and constitute approximately 20% of white matter cells (5). Both in vivo and in vitro, they are oligodendrocyte progenitors. A2B5/NG2 precursors (also called O2‐A cells) are able to differentiate either into oligodendrocytes (in serum‐free medium) or into type 2 astrocytes (when cultured with serum) in vitro (6, 7). NG2 progenitors have been reported to differentiate into all the three main types of neural cells in vitro, suggesting that these progenitors are not likely to be restricted to the progenitor lineage (8). In vivo evidence shows that NG2‐expressing cells divide in response to different acute and chronic conditions (9, 10, 11, 12), presumably for cell replacement purposes (13), holding out the hope of repairing neuronal damage.
Proliferative capacity and motility of NG2 cells, retained even in the adult brain, along with their mobilization observed after the injury, indicate their precursor characteristics (14, 15, 16, 17). Presence of multipotential progenitors may be of great therapeutic significance for a wide variety of clinical disorders, mainly for cell‐replacement strategies based on transplantation, or on endogenous mobilization of stem cells (18, 19, 20, 21), as well as for their possible anti‐inflammatory effects (22, 23). However, to qualify the NG2 progenitors as neural stem cells, they should display multipotentiality in adopting their phenotypes in response to various micro‐environmental stimulating factors. They should also retain the ability for long‐term proliferation, since self‐renewal is also attributed to stemness. Taking those into consideration, we decided to investigate the development of the isolated neonatal NG2 cells transplanted into the neuronal micro‐environment provided by the organotypic culture of hippocampal slices. The hippocampus is well known to be a neurogenic niche (24, 25, 26, 27) thus, local endogenous signals can be thought to influence the fate of cells with progenitor characteristics and to modulate their development.
Materials and methods
NG2 progenitor cell isolation
Brain cerebral hemispheres from neonatal Wistar rats, bred in the Animal Care Facility of the Medical Research Institute (Warsaw, Poland), were used to prepare mixed glial primary cell cultures. All procedures were approved by our Ethics Committee on Animal Care and Use. Briefly, dissected tissue was placed in Ca2+ and Mg2+‐free Hank's Buffered Salt Solution (Invitrogen, Carlsbad, CA), and dispersed mechanically by stirring with a Pasteur pipette and 22‐µm needle. A certain amount of isolated neural cells was labelled with chloromethyl‐fluorescein‐diacetete (CMFDA, Molecular Probes, Carlsbad, CA) cell tracker (30 min at 37 °C as control for marker distribution during the differentiation process). Cells were filtered using 41 µm Hydrophilic Nylon Net Filter, (Millipore, Bedford, MA), spun down (1000 ×g, 10 min) and plated into 75‐cm2 culture flasks precoated with 0.1 mg/ml poly‐l‐lysine. Half the volume of medium was replaced with fresh medium once every 2 days. After 12–14 days in Dulbecco's (Gibco) medium (high glucose) with 10% foetal bovine serum and supplementated with penicillin‐streptomycin, oligodendrocyte precursor cells were isolated according to the modified procedure of McCarthy and de Vellis (28), based on different adhesion properties of particular neural cell types. Cell cultures were rinsed with complete medium and shaken first for 1 h on an orbital shaker (180 r.p.m.) at 37 °C to remove the microglial fraction, then (after medium replacement) for additional 15–18 h, with the aim of gently detaching the oligodendrocytes. Progenitors obtained by this sequential dislodging method were spun down (1000 × g, 10 min), mechanically dispersed with the 22‐µm needle in F12/DMEM medium supplemented with Insulin‐Transferrin‐Selenium‐A Solution (Invitrogen), then filtered through 41‐µm Millipore membranes. The population of single NG2 progenitors, suspended in 10 ml of culture medium, was placed in a 75‐ml Falcon flask for 4 h in order to eliminate potentially contaminating cells (glia and neurons from the primary culture). The supernatant was gently collected and, finally, the purified NG2 population was labelled with CMFDA (30 min at 37 °C) and seeded on the hippocampal slices. With the aim of controlling culture homogeneity and differentiation process, cells were seeded at 2 × 105/cm2 density on poly‐l‐lysine‐coated coverslips placed in 24‐well plates (NUNC, Naperville, IL). After 2 h of adhesion, cultures were either fixed for 20 min in 4% paraformaldehyde/phosphate‐buffered saline (PBS) for immunocytochemistry or further cultured for 7 days in serum‐free F12/DMEM medium with ITS supplement, then analysed immunocytochemically with neural markers.
Organotypic hippocampal culture
Hippocampal slices were prepared from 7‐ to 10‐day‐old Wistar rats according to the method of Stoppini (29), which was slightly modified in our laboratory. After brief anaesthesia with pentobarbital (Vetbutal, Sigma, St. Louis, MO, USA), ice‐cooled pups were plunged into 70% alcohol solution, decapitated with scissors, then brains were quickly removed and placed into ice‐cold HBSS (Gibco). Hippocampi were separated and cut into 400‐µm slices using a McIlwain tissue chopper. Slices were removed with cut tips and transposed to Millicell‐CM (Millipore) membranes, four slices on each. Millicell‐CM membranes in 6‐well plates were pre‐equilibrated with 1 ml of culture medium (pH 7.2; DMEM 50%, HEPES, HBSS 25%, horse serum 25% (Gibco), 2 mmol/l l‐glutamine, 5 mg/ml glucose, 1% amphotericin B and 0.4% penicillin‐streptomycin) (30, 31). Initially, the slices were cultured in horse serum‐containing medium, which was gradually replaced from 4th to 7 day culture, by serum‐free defined solution based on DMEM/F12 and including HEPES, HBSS 25%, 2 mmol/l l‐glutamine, 5 mg/ml glucose, 1% amphotericin B and 0.4% penicillin‐streptomycin, N2 (1 : 10; Gibco) and B27 (1 : 100; Gibco) supplements. Cultures were maintained in a moist atmosphere of air with 5% CO2, at 36 °C for 14–16 days.
Transplantation of labelled NG2 progenitors on organotypic hippocampal culture
Transplantation took place a week after the organotypic hippocampal culture (OHC) preparation, when medium had already been replaced by serum‐free equivalent. Subsequently, approximately 105 of CMFDA‐labelled NG2 progenitors were transplanted all over the whole surface of OHC. One day after transplantation, cells that did not adhere to the slices were washed away. Then, the slices/NG2 cells were cultured for up to 1 week at 36 °C in air + 5% CO2 atmosphere with 100% humidity, medium being changed every 2 days. After 7 days in co‐culture, slices were washed briefly with PBS, twice, and fixed for 30 min in 4% paraformaldehyde/PBS and subjected to immunohistochemistry.
NG2 cell culture in hippocampal‐conditioned medium
The purified population of the freshly isolated NG2 cells was seeded on 2 × 105/cm2 density on poly‐l‐lysine‐coated coverslips placed in 24‐well plates (NUNC) in differentiating culture media: fresh medium for standard OHC, serum‐free hippocampal‐conditioned OHC medium (collected every 2 days from OHC and diluted 1 : 1 with fresh medium) and DMEM/F12 + ITS as controls. Cells were cultured for 7 days, then fixed with 4% paraformaldehyde/phosphate‐buffered saline (PBS) and immunostained.
Immunostaining
Blocking solution containing 10% normal goat serum in PBS, was applied for 1 h at 25 °C (RT). For cytoskeletal markers, cells were permeabilized for 0.5 h in solution containing 0.01% Triton and 5% normal goat serum in PBS. Immunostaining with primary antibodies was carried out by overnight incubation at 4 °C. After rinsing with PBS, cells were incubated for 1 h at room temperature with appropriate secondary antibody conjugated to Alexa‐546 (1 : 1000, Molecular Probes). Controls for specific immunostaining were stained omitting either the primary or the secondary antibody. Markers for different stages of oligodendrocyte development were used: rabbit polyclonal anti‐NG2 (1 : 200, Chemicon, Temecula, CA) and monoclonal anti‐mouse against O4 (1 : 200, Sigma), CNP‐ase (Sigma, 1 : 500), GalC (1 : 200, Chemicon) and MBP (1 : 100, Sigma). To identify differentiating neurons, the following monoclonal anti‐mouse markers were applied: anti‐NF200 (1 : 200, Sigma), TUJ1 (β‐tubulin III) (1 : 500, Sigma) and anti‐MAP2 (1 : 500, Pharmingen, San Diego, CA). Rabbit polyclonal antibodies rinsed either against S100b (1 : 2000, Swant) or GFAP (1 : 500, Dako, Glostrup, Denmark) served as astrocyte markers. Additionally, Ki67 the indicator of proliferating cells (1 : 100, Novocastra, Newcastle Upon Tyne, UK) and nestin a marker of neural stem cells (1 : 250, R&D Systems) were used. Precursor characteristics of the cells was verified by application of rabbit polyclonal anti‐PDGFRα (1 : 100, Santa Cruz Biotechnology, Santa Cruz, CA, USA) and mouse mab anti‐A2B5 (1 : 1000, Chemicon). Cell nuclei were visualized by incubation with 5 µm Hoechst 33258 (Sigma). Labelled oligodendroglial cultures were examined using an Axiovert 25 fluorescence microscope. Images were captured by the Videotronic CCD‐4230 camera (Carl Zeiss, Jena, Germany) and processed by the Axiovision (Carl Zeiss) image analysis system. Confocal microscopy using an LSM 510 (Zeiss) was used for image analysis of the histochemical samples. The argon laser (488 nm) was used to identify CMFDA‐labelled transplants and helium‐neon laser (543 nm) enabled visualization of Alexa‐stained cells. After acquisition, images were processed using the LSM software package version 3.2.
Results
To investigate predicted influence of the local micro‐environment on differentiation of the progenitors, freshly isolated and green marker CMFDA‐labelled NG2 cells were transplanted on to the hippocampal slices (Fig. 1). The purified population of isolated progenitors was analysed by means of immunocytochemistry with spectrum of lineage‐ and stage‐specific antibodies. The simple protocol for isolated fraction purification, aimed at minimal manipulation of the cells before their seeding, allowed us to obtain a homogenous population (98 ± 3.31%) of NG2+ glia‐committed (95 ± 2.78% PDGFRα+; 96 ± 5.25% A2B5+; 79.48 ± 2.78% CNP+) progenitor cells (2, 3). A significant number of these NG2+/PDGFRα+/CNP+ cells (10.49 ± 2.5%) was proliferative, as indicated immunocytochemically by expression of Ki67 (2, 3). Additionally, NF200+ cells were rarely noticed (0.73 ± 0.32%) (2, 3). Interestingly, 100% of the sparse NF200+ cells was also positive for NG2. The isolated population was also characterized by transient, declining (lateral only) nestin expression (50.32 ± 11.3%).
Figure 1.

Experimental scheme for co‐culture experiments: freshly isolated and labelled NG2 progenitor cells were transplanted on to hippocampal slices and co‐cultured for the following week.
Figure 2.
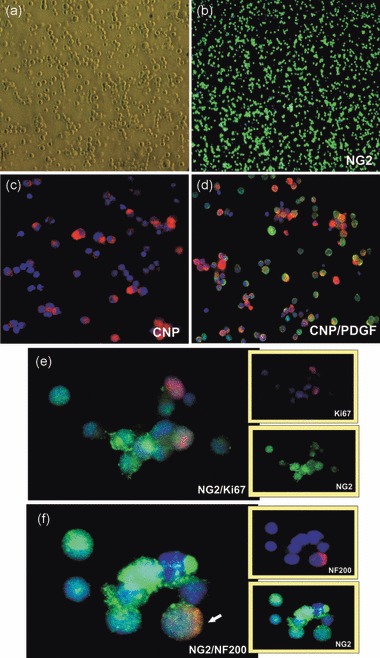
Purified population of oligodendroglial progenitors, used for co‐culture experiments with hippocampal slices. Cell nuclei are visualized by using Hoechst 33258 (blue). (a) Small, round progenitor cells (live), (b) NG2‐positive cells, (c) cells expressing CNP antigen, (d) co‐expression of CNP (red) and PDGFRα (green) antigens. (e) Proliferating (Ki67+) cells, (f) Co‐expression of NG2 (green) and NF200 (red) markers, rarely (< 1%) observed on the freshly isolated progenitors.
Figure 3.

Immunocytochemical description of glial‐committed NG2 population. Data from five to six independent experiments represent means ± standard deviation.
One portion of the fraction was placed on to the hippocampal slices. Transplants were co‐cultured for the following week to allow seeded cells to integrate and differentiate. The other portion was simultaneously cultured in standard conditions to obtain monoculture of mature oligodendrocytes in vitro. As expected cells differentiated, sequentially expressing the most characteristic markers of oligodendrocyte developmental stages (Fig. 4). After 1 week in culture, GalC‐ (99 ± 1.2%) and MBP‐positive mature oligodendrocytes were obtained (Fig. 4e,f). To control culture homogeneity, astrocyte and neuronal markers were also used. After careful examination, single TUJ‐positive neurons were observed (less than 1% of total cells). Astrocytes were absent from the culture.
Figure 4.

Monoculture of oligodendrocytes differentiated from NG2 progenitors over 7 days in vitro: (a) Multibranched oligodendrocytes (live), (b) NG2 immunostaining (3rd DIV), (c) O4‐positive cells, (d) double staining with O4 (red) and GalC (green), (e) GalC‐expressing oligodendrocytes, and (f) MBP‐immunostaining. Cell nuclei visualized with Hoechst (blue) dye.
Green labelling allowed us to observe localization and differentiation of the other portion of the same NG2 population, placed on to hippocampal slices. Just a few hours after transplantation, progenitor cell migration into the tissue had already begun, as revealed by confocal scanning microscopy. Initial amounts of CMFDA stored in small round cells appeared dispersed in cytoplasm during cell differentiation. This was fainter in flatter cells while more condensed in thin, long processes (specially of neurons). Control CMFDA‐labelled primary culture, was established each time to determine the pattern of CMFDA distribution during cell differentiation. This experimental protocol allowed us to verify double‐labelled cells within the slices.
For 1 week of culture, progenitor cells survived very efficiently, both on and in the slices (without any contact with culture medium). Immunohistochemical analysis showed that most of the NG2 progenitors differentiated, predominantly into oligodendrocytes, expressing typical developmental markers: precursor NG2 (showing however, more advanced morphology of the multiprocessed cells) (53.1 ± 6.7%) (Fig. 5a–f), O4 for so‐called pre‐ (or immature) oligodendrocytes (9.6 ± 3.7%) (not shown); and GalC for mature cells (7.1 ± 2.9%) (Fig. 5g–i). However, a considerable number (27.1 ± 2.8%) of the total grafted NG2 cell population differentiated into TUJ‐positive (Fig. 6a–f) and even into MAP‐2+ neurons (Fig. 6g–i). Net‐like structures of few TUJ/CMDFA cells were occasionally observed (Fig. 6d–f). In addition to those predominant phenotypes, clusters of S100b+ astrocytes were rarely seen (4.8 ± 1.87% of CMFDA‐positive cells) (Fig. 5j–l).
Figure 5.

Differentiation of CMFDA‐labelled (green) NG2 progenitors co‐cultured with hippocampal slices for 1 week: (a–f) NG2‐positive cells characterized by more advanced, complex morphology, (g–i) GalC‐expressing mature oligodendrocytes (arrow points to CMFDA+/GalC− cells); (j–l) S100b‐positive astrocytes (arrows: CMFDA‐labelled progenitor differentiated into S100b+ cells), (m–o) NG2‐derived astrocytes with characteristic fibrillar pattern, immunostained with anti‐GFAP antibody.
Figure 6.

Neuronal differentiation of CMFDA‐labelled (green) NG2 progenitors co‐cultured with hippocampal slices for 1 week: (a–c) CMFDA+/TUJ− (yellow) cells among numerous host neurons (red), (d–f) several CMFDA/TUJ‐positive neurons (arrows: host TUJ+ cells), (g–i) MAP‐2 expressing cells (arrows: host MAP‐2 neurons).
Addressing the question of progenitor cells retaining the ability to divide in the hippocampal micro‐environment, specific antibodies were also used to check their proliferative capacity. As a result, Ki67‐positive, dividing cells were observed (approximately 5% of total cell number) (Fig. 7a–f). A certain pool of the transplanted population (15.9 ± 2.8%) was also shown to express nestin (Fig. 7g–i).
Figure 7.

Proliferative properties of CMFDA‐labelled (green) NG2 progenitors co‐cultured with hippocampal slices for 1 week: (a–f) Dividing cells’ nuclei labelled with Ki67, (g–i) sparse nestin‐expressing cells.
Discussion
In the work presented here, we show that the neuronal micro‐environment is potent for induction of neurogenesis from oligodendroglial NG2 progenitor cells in vitro. The multistage protocol of progenitor cell isolation and purification, based on different adhesion properties of the distinct types of neural cells, allowed us to obtain a homogenous population of cells for co‐culture experiments. This time‐consuming procedure unfortunately limits size of the population obtained but greatly improves its homogeneity, which was the priority of this study. Triple control was used to verify the results. First, immunolabelling of the freshly isolated NG2 progenitor cells with the panel of neural markers was performed in order to check potential contamination with other cell types (glia and neurons from primary culture). Second, these progenitors were further spontaneously differentiated in vitro into mature oligodendroglia expressing the spectrum of markers characteristic for the sequential developmental stages (CNP, O4, GalC, MBP) (32). In this condition, the nearly pure (> 99%) oligodendrocyte monoculture was obtained each time. Finally, the progenitor cells were cultured in medium conditioned by the hippocampal slices to answer the question, of whether the tissue itself was able to promote multilineage cell differentiation. However, no neuronal differentiation was observed in the applied experimental conditions. This might point to a crucial role of direct contact in neuronal fate decision. Also, this could have been due to ths relatively short time (2 days) for which the medium was conditioned by the hippocampal slices and/or by dilution of potential active factors. The limited amount of stimulants might have simply been insufficient to promote neurogenesis.
NG2 progenitors are an abundant and widespread population of cycling cells, evenly distributed throughout neurogenic (subventricular and dentate subgranular zones) as well as non‐neurogenic regions in the adult central nervous system (33, 34). Over the last decade, their possible intrinsic stem cell potential has been discussed. Published data are contradictory, depending on experimental protocols, since NG2 cells have been shown to respond readily to both various well‐known and undefined signals (27, 35, 36), which modulate expression of transcription factors critical for the fate choice.
On the one hand, studies of NG2+ progenitors obtained from the CNP‐GFP transgenic mouse line have revealed their multipotentiality in vitro: besides oligodendrocytes and astrocytes, they generate electrically excitable neurons too (37). They formed neurospheres when cultured on non‐coated plastic surfaces and expressed nestin, the marker characteristic of neuro‐epithelium‐derived neural stem cells. The same transgenic mice were used to prove multipotentiality of hippocampal NG2 progenitor cells in vivo, confirmed by presence of GABAergic neurons. Purified early postnatal NG2+/EGFP+ cells from the subventicular zone, grafted into a wild‐type brain, were able to generate hippocampal interneurons (38).
On the other hand, recently published data concerning development of minimally manipulated NG2 progenitors (i.e. they had not been subjected to any in vitro propagation or priming treatment prior to transplantation) showed no bias towards differentiation into other than glial‐lineage cells after transplantation into neonatal and adult rats. They also failed to respond to extrinsic clues from an environment of injured hippocampus (39), and adult cells of NG2 lineage were shown to not form neurospheres (40).
Here we report that early postnatal and minimally manipulated NG2+/PDGFRα+/CNP+ cells obtained from brain cerebral hemispheres and placed on hippocampal slices were able to migrate and differentiate into all three main neural phenotypes. Oligodendrocyte lineage‐committed cells were shown to express platelet‐derived growth factor‐α receptor (PDGFRα) prior to NG2 antigen in embryonic and early postnatal animals (2, 41). Expression of the PDGFα receptor is known to be associated with oligodendroglial lineage (42, 43). Its activation has been shown to control most crucial biological functions, such as proliferation, differentiation, and survival and maturation, of cells although each of these mechanisms seems to be regulated by different signal transduction pathways (44, 45). For example, activation of the phosphoinositol 3′‐kinase pathway is required for PDGFRα‐induced migration, whereas activation of both phosphoinositol 3′‐kinase and phospholipase Cγ are required for PDGFRα‐induced proliferation (46). Cyclic nucleotide phosphodiesterase (CNP) is an additional useful marker characteristic for early stages of oligodendrocyte development (47). The combination of markers used confirms oligodendroglial‐lineage commitment of the progenitors used. Interestingly, a significant subpopulation of the NG2 progenitor cells also expressed nestin, a well‐known marker of neural stem cells. Characteristic, lateral localization of that antigen is consistent with its transient, declining expression during phenotype development. This leads to the conclusion that we could distinguish two subpopulations of neonatal NG2 cells, and the prevalence of one of them might contribute to controversial results coming as a result of other various experimental protocols.
Our studies on differentiation of NG2 progenitors carried out supported on hippocampal slices offer some significant advantages. The very restricted conditions (technical simplicity and short time taken to purify the isolated NG2 population, together with absence of any additional survival/morphogenic compounds, before transplantation on to the slices) were applied in order to eliminate potential in vitro promoting factors. Organotypic culture systems are half‐way between in vitro and in vivo studies and allow evaluation of results predicted in animal models. Tissue slices provide the specific micro‐environment and this allows for reduction in number of animals experiments, which is in accordance with current directions of the International Council for Laboratory Animal Science. Continuous observation of labelled cell status is possible as well. Thanks to this advantage, cell migration into the slices was observed as soon as a few hours after transplantation. Morphological changes, manifested by single process elongation, was recorded by confocal scanning the following day. Immunocytochemical analysis revealed that labelled progenitor cells had differentiated into glial, as well as into neuronal lineages.
Progenitor differentiation is governed by local micro‐environmental signals. Glia were shown to express receptors for many neurotransmitters (48, 49). And conversely during development, expression of metabotrophic and ionotrophic glutamate receptors is also regulated on both neurons and oligodendrocytes, most probably in order to match the final cell number of axons that require myelination (50, 51, 52). In the hippocampus, synaptic signalling between GABAergic interneurons and oligodendrocyte precursor cells was detected, presumably playing an additional role in oligodendrocyte development by regulating efficacy of glutamatergic signalling in oligodendrocyte precursor cells (53, 54). Hippocampal NG2 glia were shown to persistently express sodium channel currents during development, which could be associated with the regulation of cell proliferation (55, 56, 57).
Regulation of cell development by the local micro‐environment is reciprocal; that is, the glia also contribute to regulation of neighbouring cell fates. We have demonstrated that in the close vicinity of pure oligodendrocyte population are factors able to efficiently stimulate neurogenesis from neural stem cells derived from human cord blood cells (HUCB‐NSC line) (58), while the neuronal culture promotes glial differentiation (Markiewicz et al., submitted). Glia are known to control neuronal differentiation, survival, formation of nodes of Ranvier and synapses (e.g. 59, 60, 61, 62). NG2 cells are postulated to be involved in alignment and formation of specific glia‐neuron synapses (63, 64). The NG2 proteoglycan itself has been reported to increase extracellular matrix production (65). Extracellular matrix components are the factors best known to regulate many of such crucial cell functions as proliferation, migration, differentiation and survival (66, 67). Direct interactions of NG2‐syntenin1 seem to play a role in normal rate of precursor migration (68). Oligodendrocyte precursor cells have also been reported to express numerous neurotrophic factors that govern basic biological processes (69). Taken together, the presence of endogenous, or supplementation with developing progenitors can actively contribute to modulation of local micro‐environment composition, although in apparently a subtle rather than drastic manner (70).
In this piece of work, we show that the local neuronal micro‐environment provided by the organotypic culture of hippocampal slices is potent to promote neurogenesis from naive (not exposed to any active compounds) glial lineage‐committed, NG2+/PDGFRα+/CNP+ precursor cells. Hippocampal slice co‐culture is also conducive to preservation of non‐committed nestin‐positive NG2 cells and sustains their proliferative capacity, proved by Ki67 expression. In a primary population of NG2+ cells, co‐localization of NG2 and neuronal markers, although very marginal, points however to their intrinsic potency for neurogenesis. This is in accordance with the observation of doublecortin‐ and NG2‐co‐expressing cells in the adult rat neocortex in vivo (16).
Data coming from our observations strongly indicate multipotentiality of the NG2 progenitors. It allows us to hypothesize that the local micro‐environment could therefore modulate fate of transplanted NG2+ cells, which should be considered in the perspective of therapeutic applications.
Supporting information
Figure S1. Differentiation of CMFDA‐labelled (green) NG2 progenitors co‐cultured with hippocampal slices for 1 week into TUJ‐positive neurons.
Please note: Wiley‐Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item
Acknowledgement
This work is supported by Polish Ministry of Science and Higher Education grant (N40101832/0296).
References
- 1. Levine JM, Stallcup WB (1987) Plasticity of developing cerebellar cells in vitro studied with antibodies against the NG2 antigen. J. Neurosci. 7, 2721–2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nishiyama A, Lin XH, Giese N, Heldin CH, Stallcup WB (1996) Colocalization of NG2 proteoglycan and PDGF α‐receptor on O‐2A progenitor cells in the developing rat brain. J. Neurosci. Res. 43, 299–314. [DOI] [PubMed] [Google Scholar]
- 3. Nishiyama A, Watanabe M, Yang Z, Bu J (2002) Identity, distribution and development of polydendrocytes: NG2‐expressing glial cells. J. Neurocytol. 31, 437–455. [DOI] [PubMed] [Google Scholar]
- 4. Levine JM, Beasley L, Stallcup WB (1986) Localization of neuroectoderm‐associated cell surface antigen in the developing and adult rar. Brain Res. 392, 211–222. [DOI] [PubMed] [Google Scholar]
- 5. Horner PJ, Thallamir M, Gage FH (2002) Defining the NG2‐expressing cell in the adult CNS. J. Neurocytol. 31, 469–480. [DOI] [PubMed] [Google Scholar]
- 6. Raff MC, Miller RH, Noble M (1983) A glial progenitor cell that develops in vitro into an astrocyte or an oligodendrocyte depending on culture medium. Nature 303, 390–396. [DOI] [PubMed] [Google Scholar]
- 7. Trotter J, Schachner M (1989) Cells positive for O4 surface antigen isolated by cell sorting are able to differentiate into astrocytes or oligodendrocytes. Dev. Brain Res. 46, 115–122. [DOI] [PubMed] [Google Scholar]
- 8. Sellers DL, Horner PJ (2005) Instructive niches: environmental instructions that confound NG2 proteoglycan expression and the fate‐restriction of CNS progenitors. J. Anat. 207, 727–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Keirstead HS, Levine JM, Blakemore WF (1998) Response of the oligodendrocyte progenitor cell population (defined by NG2 labelling) to demyelination of the adult spinal cord. Glia 22, 161–170. [PubMed] [Google Scholar]
- 10. Reynolds R, Dawson M, Papadopoulos D, Polito A, Di Bello IC, Pham‐Dinh D, Levine J (2002) The response of NG2‐expressing oligodendrocyte progenitors to demyelination in MOG‐EAE and MS. J. Neurocytol. 31, 523–536. [DOI] [PubMed] [Google Scholar]
- 11. Watanabe M, Toyama Y, Nishiyama A (2002) Differentiation of proliferated NG2‐positive glial progenitor cells in a remyelinating lesion. J. Neurosci. Res. 69, 826–836. [DOI] [PubMed] [Google Scholar]
- 12. Givogri MI, Galbiati F, Fasano S, Amadio S, Perani L, Superchi D, Morana P, Del Carro U, Marchesini S, Brambilla R, Wrabetz L, Bongarzone E (2006) Oligodendroglial progenitor cell therapy limits central neurological deficits in mice with metachromatic leukodystrophy. J. Neurosci. 26, 3109–3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lytle J, Wrathall J (2007) Glial cell proliferation and replacement in the contused murine spinal cord. Eur. J. Neurosci. 25, 1711–1724. [DOI] [PubMed] [Google Scholar]
- 14. Nait‐Oumesmar B, Decker L, Lachapelle F, Avellana‐Adalid V, Bachelin C, Baron‐Van Evercooren A (1999) Progenitor cells of the adult mouse subventricular zone proliferate, migrate and differentiate into oligodendrocytes after demyelination. Eur. J. Neurosci. 11, 4357–4366. [DOI] [PubMed] [Google Scholar]
- 15. Chari DM, Blakemore WF (2002) Efficient recolonisation of progenitor‐depleted areas of the CNS by adult oligodendrocyte progenitor cells. Glia 37, 307–313. [PubMed] [Google Scholar]
- 16. Tamura Y, Katoka Y, Cui Y, Takamori Y, Watanabe Y, Yamada H (2007) Multi‐directional differentiation of doublecortin‐ and NG2‐immunopositive progenitor cells in the adult rat neocortex in vivo . Eur. J. Neurosc. 25, 3489–3498. [DOI] [PubMed] [Google Scholar]
- 17. Magnus T, Carmen J, Deleon J, Xue H, Pardo AC, Lepore AC, Mattson MP, Rao MS, Maragakis NJ (2008) Adult glial precursor proliferation in mutant SOD1G93A mice. Glia 56, 200–2008. [DOI] [PubMed] [Google Scholar]
- 18. Magavi SS, Macklis JD (2001) Manipulation of neural precursors in situ: induction of neurogenesis in the neocortex of adult mice. Neuropsychopharmocology. 25, 816–835. [DOI] [PubMed] [Google Scholar]
- 19. Wernig M, Benninger F, Schmandt T, Rade M, Tucker KL, Büssow H, Beck H, Brüstle O (2004) Functional integration of embryonic stem cell‐derived neurons in vivo . J. Neurosci. 24, 5258–5268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kim SU (2007) Genetically engeneered human neural stem cells for brain repair in neurological diseases. Brain. Dev. 29, 193–201. [DOI] [PubMed] [Google Scholar]
- 21. Comi AM, Cho E, Mulholland JD, Hooper A, Li Q, Qu Y, Gary DS, McDonald JW, Johnston MV (2008) Neural stem cells reduce brain injury after unilateral carotid ligation. Pediatr. Neurol. 38, 86–92. [DOI] [PubMed] [Google Scholar]
- 22. Vendrame M, Gemma C, Mesquita D, Collier L, Bickford PC, Sanberg CD, Pennypacker KR, Willing AE (2005) Anti‐inflammatory effects of human cord blood cells in a rat model of stroke. Stem Cells Dev. 14, 595–604. [DOI] [PubMed] [Google Scholar]
- 23. Lee ST, Chu K, Jung KH, Kim SJ, Kim DH, Kang KH, Hong NH, Kim JH, Ban JJ, Park HK, Kim SU, Park CG, Lee SK, Kim M, Roh JK (2008) Anti‐inflammatory mechanism of intravascular neural stem cell transplantation in haemorrhagic stroke. Brain 131, 616–629. [DOI] [PubMed] [Google Scholar]
- 24. Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ (1999) Learning enhances adult neurogenesis in the hippocampal formation. Nat. Neurosci. 2, 260–265. [DOI] [PubMed] [Google Scholar]
- 25. Nakatomi H, Kuriu T, Okabe S, Yamamoto S, Hatano O, Kawahara N, Tamura A, Kirino T, Nakafuku M (2002) Regeneration of hippocampal pyramidal neurons after ischemic brain injury by recruitment of endogenous neural progenitors. Cell 110, 429–441. [DOI] [PubMed] [Google Scholar]
- 26. Seaberg RM, Van Der Kooy D (2002) Adult rodent neurogenic regions: the ventricular subependyma contain neural stem cells but dentate gyrus contains restricted progenitors. J. Neurosci. 22, 1784–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gaughwin PM, Caldwell MA, Anderson JM, Schwienning CJ, Fawcett JW, Compston DA, Chadran S (2006) Astrocytes promote neurogenesis from oligodendrocyte precursor cells. Eur. J. Neurosci. 23, 945–956. [DOI] [PubMed] [Google Scholar]
- 28. McCarthy KD, De Vellis J (1980) Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J. Cell Biol. 85, 890–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stoppini L, Buchs PA, Muller D (1991) A simple method for organotypic cultures of nervous tissue. J. Neurosci. Methods 37, 173–182. [DOI] [PubMed] [Google Scholar]
- 30. Aitken PG, Breese GR, Dudek FF, Edwards F, Espanol MT, Larkman PM, Lipton P, Newman GC, Nowak TS Jr, Panizzon KL, Raley‐Susman KM, Reid KH, Rice ME, Sarvey JM, Schoepp DD, Segal M, Taylor CP, Teyler TJ, Voulalas PJ (1995) Preparative methods for brain slices: a discussion. J. Neurosci. Methods 59, 139–149. [DOI] [PubMed] [Google Scholar]
- 31. Gähwiler BH, Capogna M, Debanne D, McKinney RA, Thompson SM (1997) Organotypic slice cultures: a technique has come of age. Trends Neurosci. 20, 471–477. [DOI] [PubMed] [Google Scholar]
- 32. Pfeiffer SE, Warrington AE, Bansal R (1993) The oligodendrocyte and its many cellular processes. Trends Cell Biol. 3, 191–197. [DOI] [PubMed] [Google Scholar]
- 33. Dawson MR, Levine JM, Reynolds R (2000) NG2‐expressing cells in the central nervous system: are they oligodendroglial progenitors? J. Neurosci. Res. 61, 471–479. [DOI] [PubMed] [Google Scholar]
- 34. Dawson MR, Polito A, Levine JM, Reynolds R (2003) NG2‐expressing glial progenitor cells: an abundant and widespread population of cycling cells in the adult rat CNS. Mol. Cell. Neurosci. 24, 476–488. [DOI] [PubMed] [Google Scholar]
- 35. Kondo T, Raff M (2000) Oligodendrocyte precursor cells reprogrammed to become multipotential CNS stem cells. Science 289, 1754–1757. [DOI] [PubMed] [Google Scholar]
- 36. Liu A, Han YR, Li J, Sun D, Plummer M, Casaccia‐Bonnefil (2007) The glial or neuronal fate choice of the oligodendrocyte progenitors is modulated by their ability to acquire an epigenetic memory. J. Nueurosci. 27, 7339–7343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Belachew S, Chittajallu R, Aguirre A, Yuan X, Kirby M, Anderson S, Gallo V (2003) Postnatal NG2 proteoglycan‐expressing progenitor cells are intrinsically multipotent and generate functional neurons. J. Cell Biol. 161, 169–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Aguirre A, Gallo V (2004) Postnatal neurogenesis and gliogenesis In the olfactory bulb from NG2‐expressing progenitors of the subventricular zone. J. Neurosci. 24, 10530–10541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Webber D, Compston A, Chandran S (2007) Minimally manipulated oligodendrocyte precursor cells retain exclusive commitment to the oligodendrocyte lineage following transplantation into intact and injured hippocampus. Eur. J. Neurosci. 26, 1791–1800. [DOI] [PubMed] [Google Scholar]
- 40. Buffo A, Rite I, Tripathi P, Lepier A, Colak D, Horn AP, Mori T, Götz M (2008) Origin and progeny of reactive gliosis: a source of multipotent cells in the injured brain. Proc. Natl. Acad. Sci. USA 195, 3581–3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Grinspan JB, Franceschini B (1995) Platelet‐derived growth factor is a survival factor for PSA‐NCAM+ oligodendrocyte pre‐progenitor cells. J. Neurosci. Res. 41, 540–551. [DOI] [PubMed] [Google Scholar]
- 42. Calver AR, Hall AC, Yu WP, Walsh FS, Heath JK, Betzholtz C, Richardson WD (1998) Oligodendrocyte population dynamics and the role of PDGF in vivo . Neuron 20, 869–882. [DOI] [PubMed] [Google Scholar]
- 43. Finzsch M, Stolt CC, Lommes P, Wegner M (2008) Sox9 and Sox10 influence survival and migration of oligodendrocyte precursors in the spinal cord by regulating PDGF receptor alpha expression. Development 135, 637–646. [DOI] [PubMed] [Google Scholar]
- 44. Chittajallu R, Aguirre A, Gallo V (2005) Downregulation of platelet‐derived growth factor‐α receptor‐mediated tyrosine kinase activity as a cellular mechanism for K+‐channel regulation during oligodendrocyte development in situ. J. Neurosci. 25, 8601–8610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hu JG, Fu SL, Li Y, Jiang XY, Wang XF, Qiu MS, Lu PH, Xu XM (2008) Platelet‐derived growth factor‐AA mediates oligodendrocyte lineage differentiation through activation of extracellular signal‐regulated kinase signaling pathway. Neuroscience 151, 138–147. [DOI] [PubMed] [Google Scholar]
- 46. McKinnon RD, Waldron S, Kiel ME (2005) PDGF‐α Receptor signal strength controls an RTK rheostat that integrates phosphoinositol 3′‐kinase and phospholipase Cγ pathways during oligodendrocyte maturation. J. Neurosci. 25, 3499–3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Scherer SS, Braun PE, Grinspan J, Collarini E, Wang DY, Kamholz J (1994) Different regulation of the 2′,3′‐cyclic nucloetide 3′‐phosphodiesterase gene during oligodendrocyte development. Neuron 12, 1363–1375. [DOI] [PubMed] [Google Scholar]
- 48. Barres BA, Koroshetz WJ, Swartz KJ, Corey DP (1990) Ion chanel expression by white matter glia: the O‐2A glial progenitor cell. Neuron 4, 507–524. [DOI] [PubMed] [Google Scholar]
- 49. Yuan X, Eisen AM, McBain CJ, Gallo VA (1998) A role for glutamate and its receptors in the regulation of the oligodendrocyte development in cerebellar tissue slices. Development 125, 2901–2914. [DOI] [PubMed] [Google Scholar]
- 50. Deng W, Wang H, Rosenberg PA, Volpe JJ, Jensen FE (2004) Role of metabotropic glutamate receptors in oligodendrocyte excitotoxicity and oxidative stress. Proc. Natl. Acad. Sci. USA 101, 7751–7756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Karadottir R, Cavelier P, Bergersen LH, Attwell D (2005) NMDA receptors are expressed in oligodendrocytes and activated in ischaemia. Nature 22, 1162–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Luyt K, Varadi A, Durant CF, Molnar E (2006) Oligodendroglial metabotropic glutamate receptors are developmentally regulated and involved in the prevention of apoptosis. J. Neurochem. 99, 641–656. [DOI] [PubMed] [Google Scholar]
- 53. Lin S, Bergles D (2004) Synaptic signalling between GABAergic interneurons and oligodendrocyte precursor cells in the hippocampus. Nature Neurosci. 7, 24–32. [DOI] [PubMed] [Google Scholar]
- 54. Jabs R, Pivnera T, Hutmann K, Wyczynski A, Notte C, Kettenmann H, Steinhäuser C (2005) Synaptic transmission onto hippocampal glial cells with hGFAP promoter activity. J. Cell Sci. 118, 3791–3803. [DOI] [PubMed] [Google Scholar]
- 55. Pardo LA (2004) Voltage‐gated potassium channels in cell proliferation. Physiology 19, 285–292. [DOI] [PubMed] [Google Scholar]
- 56. Zhou M, Schools GP, Kimelberg HK (2006) Development of GLASt (+) astrocytes and NG2 glia in rat hippocampus CA1: mature astrocytes are electrophysiologically passive. J. Neurophysiol. 95, 134–143. [DOI] [PubMed] [Google Scholar]
- 57. Xie M, Lynch DT, Schools GP, Feustel PJ, Kimelberg HK, Zhou M (2007) Sodium channel currents in rat hippocampal NG2 glia: characterization and contribution to resting membrane potential. Neuroscience 150, 853–862. [DOI] [PubMed] [Google Scholar]
- 58. Bużańska L, Jurga M, Stachowiak E, Stachowiak M, Domańska‐Janik K (2006) Neural stem‐like cell line derived from a nonhematopoietic population of human umbilical cord blood. Stem Cells Dev. 15, 391–406. [DOI] [PubMed] [Google Scholar]
- 59. Lim DA, Alvarez‐Buylla A (1999) Interaction between astrocytes and adult subventricular zone precursors stimulates neurogenesis. Proc. Natl. Acad. Sci. USA 96, 7526–7531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Allen NJ, Barres BA (2005) Signaling between glia and neurons: focus on synaptic plasticity. Curr. Opin. Neurobiol. 15, 542–548. [DOI] [PubMed] [Google Scholar]
- 61. Bhat MA, Rios JC, Lu Y, Garcia‐Gresco GP, Ching W, St Martin M, Li J, Einheber S, Chesler M, Rosenbluth J, Salzer JL, Bellen HJ (2001) Axon‐glia interactions and the domain organization of myelinated axons requires neurexin IV/Caspr/Paranodin. Neuron 30, 369–383. [DOI] [PubMed] [Google Scholar]
- 62. Göritz C, Thiebaut R, Tessier LH, Nieweg K, Moehle C, Buard I, Dupont JL, Schurgers LJ, Schmitz G, Pfrieger FW (2007) Glia‐induced neuronal differentiation by transcriptional regulation. Glia 55, 1108–1122. [DOI] [PubMed] [Google Scholar]
- 63. Stegmüller J, Werner H, Nave KA, Trotter J (2003) The proteoglycan NG2 is complexed with alpha‐amino‐3‐hydroxy‐5‐methyl‐4‐isoxazolepropionic acid (AMPA) receptors by the PDZ glutamate receptor interaction protein (GRIP) in glial progenitor cells. Implications for glial‐neuronal signaling. J. Biol. Chem. 278, 3590–3598. [DOI] [PubMed] [Google Scholar]
- 64. Ge WP, Yang XJ, Zhang Z, Wang HJ, Shen W, Deng QD, Duan S (2006) Long‐term potentiation of neuron‐glia synapses mediated by Ca2+‐permeable AMPA receptors. Science 312, 1533–1537. [DOI] [PubMed] [Google Scholar]
- 65. Xiong J, Wang Y, Zhu Z, Liu J, Wang Y, Zhang C, Hammes HP, Lang F, Feng Y (2007) NG2 proteoglycan increases mesangial cell proliferation and extracellular matrix production. Biochem. Biophys. Res. Commun. 361, 960–967. [DOI] [PubMed] [Google Scholar]
- 66. Baron W, Shattil SJ, Ffrench‐Constant C (2002) The oligodendrocyte precursor mitogen PDGF stimulates proliferation by activation of αvβ3 integrins. EMBO J. 21, 1957–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Watkins TA, Barres BA (2002) Integrins as developmental switches. Nat. Cell Biol. 4, 253–255. [DOI] [PubMed] [Google Scholar]
- 68. Chatterjee N, Stegmüller J, Schätzle P, Karram K, Koroll M, Werner HB, Nave KA, Trotter J (2008) Interaction of syntenin‐1 and the NG2 proteoglycan in migratory oligodendrocyte precursor cells. J. Biol. Chem. 283, 8310–8317. [DOI] [PubMed] [Google Scholar]
- 69. Zhang Y, Denham J, Thies R (2006) Oligodendrocyte progenitor cells derived from human embryonic stem cells express neurotrophic factors. Stem Cells Dev. 15, 943–952. [DOI] [PubMed] [Google Scholar]
- 70. Tallmair M, Ray J, Stallcup WB, Gage FH (2006) Functional and morphological effects of NG2 proteoglycan deletion on hippocampal neurogenesis. Exp. Neurol. 202, 167–178. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Differentiation of CMFDA‐labelled (green) NG2 progenitors co‐cultured with hippocampal slices for 1 week into TUJ‐positive neurons.
Please note: Wiley‐Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item


