Abstract
Somatostatin analogs have been examined as a treatment for somatostatin receptor overexpressing tumors for years; specifically, octreotate (TATE) and octreotide (TOC). Several versions of these analogs coupled to beta or gamma nuclides are currently used as imaging agents, as treatments with peptide receptor radionuclide therapy (PRRT) for patients with neuroendocrine tumors or are being explored in preclinical and clinical settings. Our study describes the use of 212Pb-DOTAMTATE, the octreotate analog, in combination with 212Pb, the parent of an alpha emitter. Preclinical studies demonstrated tumor targeting of 212Pb-DOTAMTATE of >20% ID/g up to 24hrs post drug injection. The addition of kidney protection agents, including L-lysine and L-arginine decreases drug accumulation in the kidneys and the addition of ascorbic acid to the chelation mixture reduces oxidation of the drug product. 212Pb-DOTAMTATE displays a favorable toxicity profile with single dose injections of 20μCi showing 100% survival and with non-toxic cumulative doses up to 45μCi, when fractionated into three smaller doses of 15μCi. In an initial efficacy study, a single 10μCi of 212Pb-DOTAMTATE extended the mean survival 2.4-fold. Efficacy was enhanced by giving three treatment cycles of 212Pb-DOTAMTATE and reducing the time between injections to two weeks. Efficacy was optimized further by the addition of a chemo sensitizing agent, 5-fluorouracil, given in combination with three cycles of 10μCi 212Pb-DOTAMTATE. These conditions led to 79% of the animals being tumor free at the end of the 31-week study suggesting that 212Pb-DOTAMTATE alone or in combination with a chemotherapeutic may have positive clinical implications.
Keywords: 212Pb-DOTAMTATE, neuroendocrine tumor, cancer, PRRT, alpha-emitter therapy
Introduction:
Although great strides have been taken to increase the success of cancer treatments, new and more specific strategies are urgently needed to increase cancer cytotoxicity while minimizing damage to healthy tissue. One such strategy is to link peptides targeting tumor-associated receptors to radioisotopes, to direct the killing power of these isotopes to tumor cells. For successful targeted radiation, crucial considerations must be addressed regarding emission type, energy/range of emission and half-life. 212Pb provides a radiotherapeutic agent with short range cancer cell destruction (α-particles) and potential imaging (γ-ray) capabilities. The 212Pb half-life of 10.6 hours provides clinical feasibility and allows for its production and world-wide distribution.
Peptide receptor radiotherapy (PRRT), specifically with somatostatin analogs has been examined as a treatment for somatostatin overexpressing tumors for years. The SSTR binding Tyr3-octreotate (TATE) peptide used in this study has been extensively evaluated in clinical studies in the USA and worldwide. Octreotate based compounds are routinely used in clinical studies for diagnosis of patients with SSTR positive neuroendocrine tumors (NETs) using gamma-emitting isotopes such as 68Ga (US commercial name Netspot, Novartis) and 64Cu as well as other radiolabeled analogs 111ln-octreoscan and 99mTc-/EDDA/HYNIC-octreotide [1–4]. They have shown favorable results in therapy of NET patients using beta-emitting isotopes (177Lu and 90Y) [5, 6] and more recently with alpha particle emitting isotopes such as 225Ac and 213Bi [7]. The 177Lu-DOTATATE Phase 3 study NETTER-1 trial demonstrated a statistically significant and clinically meaningful risk reduction of 79% in disease progression or death versus a treatment with a double dose of Octreotide LAR versus standard of care in patients with progressed midgut carcinoid tumors [8]. This study also demonstrated a favorable safety profile of 177Lu-DOTATATE. The median progression-free-survival in the 177Lu-DOTATATE arm (NETTER-1) at 30 months has not yet been reached, while the median progression free survival in the Octreotide LAR 60 mg arm was only 8.4 months. While beta-emitter peptide receptor radiotherapy (PRRT) showed very promising results and 177Lu-DOTATATE (Lutathera) has recently been approved in US and Europe, it is known to be limited in some populations. Patients previously resistant to beta PRRT have responded favorably to alpha therapy [9]. Previous studies have demonstrated the low toxicity profile of alpha-emitter labeled SSTR targeting agents [7] but limited preclinical data is available. Further studies showed that PRRT could be combined with chemotherapeutics to enhance efficacy [10–14]. The studies presented here further support the use of alpha SSTR agents as treatment for NETs.
Extensive preclinical work, including relevant xenograft models of SSTR overexpressing tumors, has been accomplished showing tumor uptake > 20% ID/g at 1hr post injection and remaining for up to 24hrs, a reduction in kidney accumulation by the addition of positively charged amino acids and the reduction of drug oxidation by ascorbic acid added during the chelation step. Furthermore, 212Pb-DOTAMTATE showed a favorable toxicity profile with a Highest Non-Severely Toxic Dose (HNSTD) dose of 20μCi and efficacy which can be improved by decreasing the timing between drug injections from three weeks to two weeks. Efficacy data showed a 2.4-fold increase in median survival in mice treated with a single 10μCi dose of 212Pb-DOTAMTATE. This could be further enhanced by the addition of a chemo-sensitizing agent, 5-fluorouracil, which when given in combination of 212Pb-DOTAMTATE (at 2-week intervals) yielded 79% tumor free mice at the end of the 31-week study. These data suggest that there is therapeutic potential for 212Pb-DOTAMTATE alone or in combination with a chemotherapy as a treatment of SSTR positive NET’s and have supported the initiation of a phase I clinical study with 212Pb-DOTAMTATE (NCT03466216).
Materials and Methods:
Cell line and Mice:
AR42J rat pancreatic cell line was purchased from ATCC. The cells were tested for mycoplasma by Hoechst DNA stain, Agar culture and PCR-based assay by ATCC and were not detected as per certificate of analysis. The cells were maintained in F12K media (Gibco) containing 20% fetal bovine serum (Gibco). Athymic nude mice were purchased from Charles River or Envigo and CD-1 mice were purchased from Envigo. All studies were conducted using female mice unless otherwise mentioned. All studies were conducted under the approval of the institutional IACUC committee.
Manufacturing and Radiolabeling
GMP DOTAMTATE (C65H93N17O16S2, Figure 1) was manufactured by Macrocyclics using Fmoc solid phase peptide synthesis. DOTAMTATE was added to purified 212Pb at a ratio of 2.4μCi/ng and incubated at 50°C for 10 minutes with shaking at 300rpm. For studies using the ascorbic acid enriched formulation, metal-free L-ascorbic acid (Honeywell) was diluted in Optima water (Fisher) and added prior to the drug chelation to a final injection concentration of 10mM.
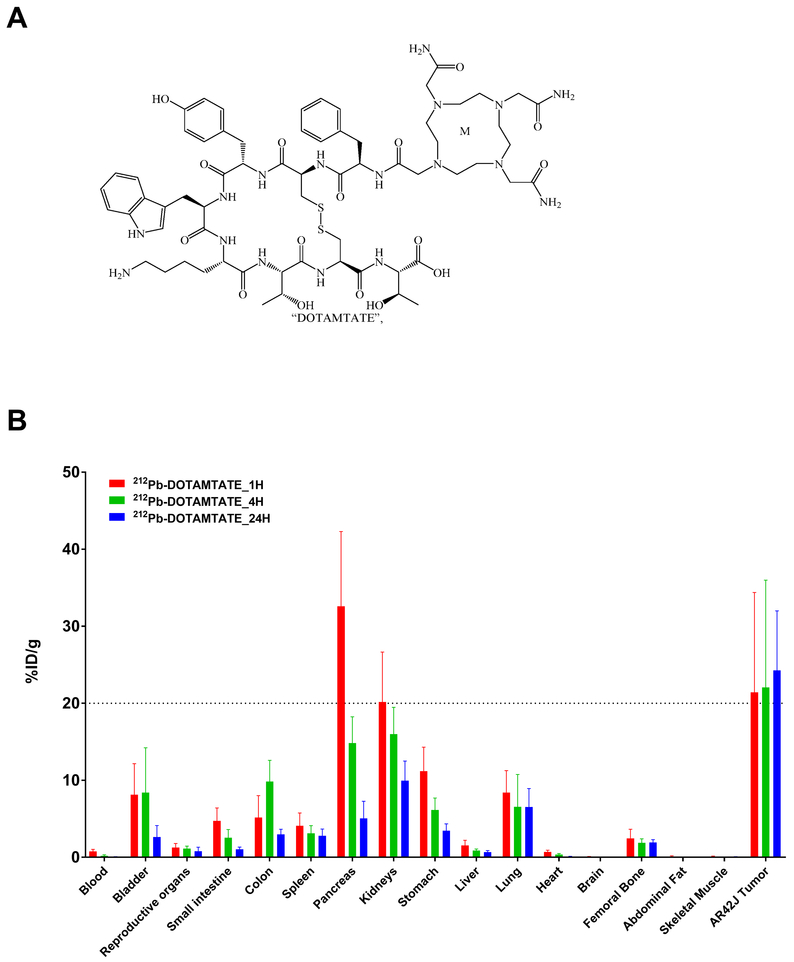
Figure 1. Drug Structure and Initial Biodistribution.
Figure 1 shows the chemical structure of DOTAMTATE and biodistribution of 212Pb-DOTAMTATE in athymic nude tumor bearing mice.
A. Chemical structure of DOTAMTATE (Formula: C65H93N17O16S2) B. Drug was administered, and organs were collected from 5 mice per timepoint: 1 hour post (red), 4 hours (green) and 24 hours (blue) post injection.
iTLC was used to confirm chelation was greater than 95%. Samples were diluted to appropriate activity in PBS or saline prior to injection.
Cell Binding Assay
Peptide binding to somatostatin receptors 2 (SSTR2) and Kd was evaluated in SSTR2 expressing AR42J cells by growing 250,000 cells into the wells of a 24-well plate for 48hrs. Concentrations from 0.5nM to 64nM of 212Pb-DOTAMTATE were incubated in the AR42J containing wells for 10 minutes at 37°C. Four replicates were performed for each concentration. Cells were then washed with PBS and cells from each well were counted for presence of radioactivity. Binding curves were then created and Kd calculated using GraphPad Prism software.
Cell killing Assay
30,000 AR42J cells were grown in a 96 well plate for 48hrs. Cells were then incubated for 4 hours with increasing 212Pb-DOTAMTATE ranging from 0nCi/ml to 800nCi/ml. Eight well per group were treated. Cells were washed with PBS to remove the unbound peptide fraction and then fresh media was introduced. Cells were allowed to incubate for 4 days at 37°C. Cells were then rinsed and incubated with fluorescein diacetate for 30 minutes and read with a fluorimeter at 485/535nm. Percentage of viable cells was calculated based on untreated cells as a control.
Tumor Models
For all tumor studies, two million (2×106) AR42J cells were implanted subcutaneously, in an equal volume mixture of GFR-Matrigel (Corning) and RPMI media (Gibco), into the right flank of each mouse and grown to a volume of ~200–300mm3.
Preparation of Kidney Protection Agents
200μL of L-Lysine-L-Arginine (35mg/mL of each) diluted in saline or 10% dextrose, 200μL of L-lysine (35mg/mL or 70mg/mL) in saline or 200μL of L-arginine (70mg/mL) in saline were given via intravenous injection five minutes prior to drug injection.
Biodistribution studies
Female mice were grown until an approximate tumor volume of 300mm3 was reached. 200μL of 212Pb-DOTAMTATE (5μCi) was administered to the mice via the tail vein and mice were euthanized at predetermined timepoints. The background was automatically subtracted from the counts. A standard is also used for decay correction. %ID/g was calculated for each organ collected.
Alpha Imaging
Ex vivo assessment of 212Pb-DOTAMTATE localization and microdosimetry was performed on frozen section (10–12 μm) of AR42J xenograft tumors placed on a phosphor sheet (Eljen Technology) and imaged using a high-sensitivity QHYCCD camera (Andor). Images were analyzed with Micromanager software (Image J)
Radio HPLC Studies
212Pb-DOTAMTATE was analyzed on an Agilent 1220 HPLC using a C18 reverse phase column (Restek) with an acetonitrile gradient. Fractions were collected off the column every 10 seconds for a total of 10 minutes and then analyzed for radiometric detection by auto gamma counter (Perkin Elmer).
Toxicity Studies
Female athymic nude mice received an injection of either 10μCi, 20μCi, 40μCi or 60μCi of 212Pb-DOTAMTATE or control PBS intravenously. Animals were weighed three times per week and monitored daily for signs of termination criteria over a four-week period. For fractionated toxicity, studies animals (n=10 per group) received a single injection of 40μCi 212Pb-DOTAMTATE, 2 × 20μCi of 212Pb-DOTAMTATE or 3 × 15μCi of 212Pb-DOTAMTATE. Repeat injections were given at three-week intervals. Control mice received PBS only. Blood was sampled via the retro-orbital plexus using potassium-EDTA capillaries and tubes (Greiner Bio-one) and complete cell blood count (CBC) was obtained using VETSCAN® HM5 hematology analyzer (Abaxis). Animals were euthanized when termination criteria were met.
Efficacy Study
Tumor bearing animals were injected with 100μL of 5μCi or 10μCi 212Pb-DOTAMTATE or control (PBS or cold peptide). After three weeks mice who received the 5μCi dose, received a second dose of 212Pb-DOTAMTATE. Animals were monitored daily and calipered three times per week to monitor tumor volume. Mice were sacrificed when termination criteria were met.
Combination Efficacy with 5-fluorouracil
All animals were grown with tumors as described above. Control groups were injected with saline alone or 15mg/kg 5-fluorouracil (Acros) once per week for nine weeks (5-fluorouracil alone). Radiotherapy only groups received 10μCi of 212Pb-DOTAMTATE at two-week or three-week intervals. Combination therapy groups received a treatment of 5-fluorouracil (15mg/kg) followed 24hrs later by 10μCi of 212Pb-DOTAMTATE. The 5-fluorouracil was continued weekly for a total of nine weeks for both treatment groups. 10μCi of 212Pb-DOTAMTATE was given 24hrs after the first 5-fluorouracil injection and then at two or three-week intervals for a total of three injections. Animals were monitored daily for signs of termination criteria and calipered three times per week to monitor tumor volume. Animals were euthanized when termination criteria were met.
Termination Criteria
Mice were sacrificed when tumor volumes reached 3000mm3 or other predetermined termination criteria were met (weight loss over 15% for two consecutive days or 20% weight loss from initial weight, serious bleeding, necrosis or ulceration of the tumor, scruffiness or lack of grooming over 5 days, lethargy over 3 days, weakness/balance issues over 5 days, hunchback appearance, diarrhea or hypothermia).
Statistical Analysis
Animals were randomly assigned to each group. An unpaired t-test was used for statistical analysis.
Patient Studies
The study was conducted in accordance with the Declaration of Helsinki ethical guidelines and upon signature of the IRB approved informed consent form. Studies were performed under FDA IND 130960.
Results:
In vitro Data
An in vitro binding study of 212Pb-DOTAMTATE to SSTR2 expressing AR42J cells yielded a Kd of 12.9nM (Supplementary Figure S1A), which is in line with other studies that have examined the binding of octreotate peptides to somatostatin expressing cell lines [15]. In addition, a cytotoxicity assay showed a dose dependent cytotoxic effect of 212Pb-DOTAMTATE for AR42J cells with complete death observed at 800nCi/mL and 50% viability observed between 12.5nCi/mL to 25nCi/mL (Supplementary Figure S1B). A 212Pb-chelate only negative control did not show a dose dependent cytotoxic effect with viability ranging from 47% to 156%.
Biodistribution Studies
All studies were conducted in female mice, as a biodistribution study showed that there was no significant difference in organ uptake between male and female mice (Supplementary Figure S2A) and the literature suggests that female mice may be more susceptible to toxicity and may provide a worst case scenario between the two sexes [16]. When animals were injected with a single dose of 5μCi 212Pb-DOTAMTATE, the average tumor uptake exceeded 20% ID/g one hour after drug administration and remained constant through 4 and 24 hours post drug administration (Figure 1). The pancreas and kidneys were the two organs with the highest non-target uptake but these organs also showed significantly less accumulation by 24 hours post-injection. In further examining AR42J tumors for 212Pb-DOTAMTATE distribution, no correlation between tumor volume and tumor uptake is visible in tumors up to 1500mm3 (Supplementary Figure S3) and alpha imaging of tumors treated with 212Pb-DOTAMTATE showed homogenous distribution of the drug at all tumor sizes up to 1500mm3 (Supplementary Figure S4A). Three specific activities of 4.1ng, 22ng or 110ng per 10μCi were also examined via biodistribution study (Supplementary Figure S2B). 10μCi per 4.1ng (2.4μCi/ng) has been primarily used in 212Pb-DOTAMTATE studies to date however a decrease in the specific activity does not appear to have a significant effect on tumor uptake. This suggests that receptor saturation is not occurring even at over 25-fold lower specific activity then what has been primarily used in these studies.
Reduction of Renal Retention of 212Pb-DOTAMTATE
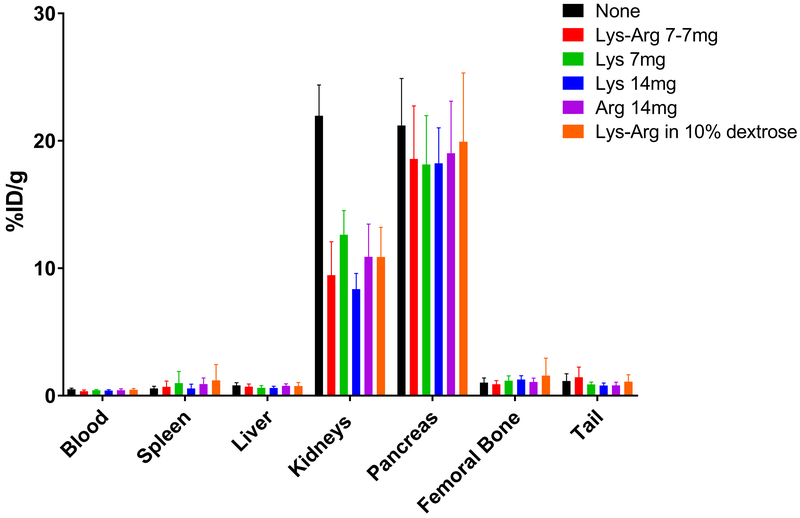
As kidney protection agents are often given with targeted radiotherapies to minimize nephrotoxicity, several kidney protection agents and diuretics were tested in combination with 212Pb-DOTAMTATE through biodistribution studies for their ability to minimize drug accumulation in the kidneys (Figure 2). Of the five versions of amino acid combinations/concentrations given, all were able to significantly reduce 212Pb-DOTAMTATE uptake in the kidneys (p=<0.0001) at 1hr post drug injection. Additional studies conducted with higher levels of L-lysine in tumor bearing mice at three timepoints showed that while kidney uptake is reduced no effect on drug accumulation in the tumor was observed (Supplementary Figure S4B).
Figure 2. 212Pb-DOTAMTATE in the Presence of Kidney Protection Agents.
Figure 2 shows a biodistribution of 212Pb-DOTAMTATE in CD-1 mice at 1-hour post drug injection with kidney protection agents. 200μL of kidney protections agents 7mg Arg-7mg Lys in saline (red), 7mg Lys in saline (green), 14mg Lys in saline (blue), 14mg Arg in saline (purple) or 7mg Arg-7mg Lys in 10% dextrose (orange) were given IP five minutes before drug injection. No kidney protection agent control shown in black. 5 mice per group. Average of two studies displayed.
Enhancing Stability of 212Pb-DOTAMTATE binding with Ascorbic Acid
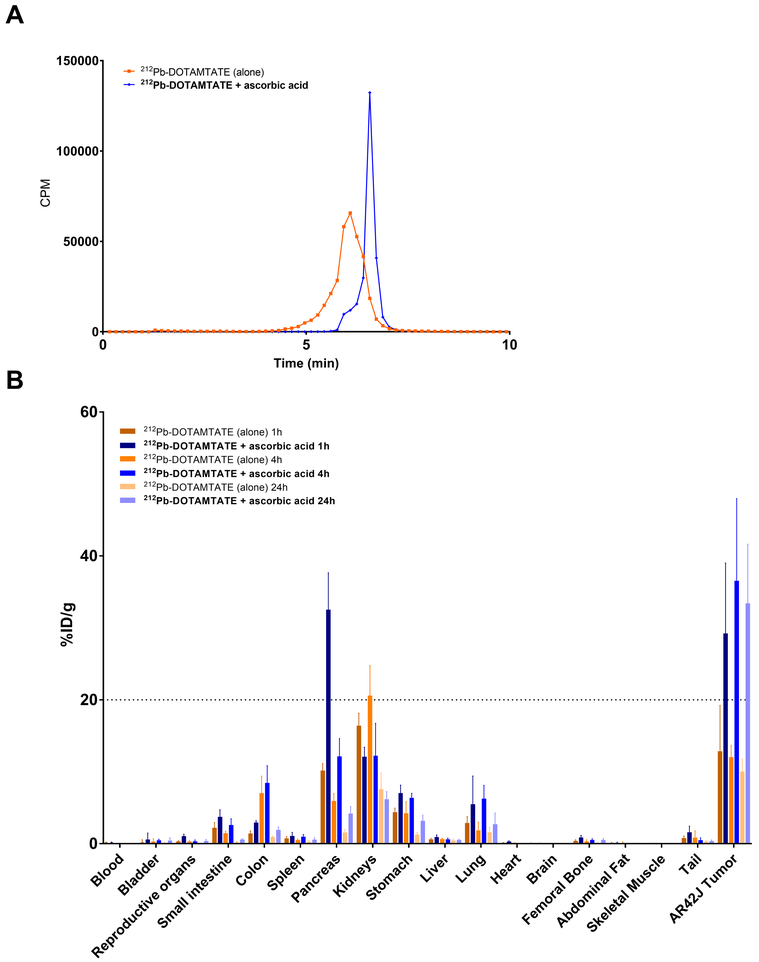
Oxidation of DOTATATE peptides, specifically on the indol ring of the tryptophan residue has been shown to occur when the peptide is labeled with radioisotopes and can be minimized by the addition of ascorbic acid [17]. The presence of an oxidized form of DOTAMTATE was also witnessed in our studies when the drug was prepared and not used immediately, however, it was not known if this oxidation influenced the drug binding to its SSTR targets. To test if the presence of oxidized DOTAMTATE influenced overall drug binding, a biodistribution study was conducted in AR42J tumor bearing mice. 212Pb-DOTAMTATE prepared with and without ascorbic acid present during chelation was left overnight (to obtain a worst-case scenario) and oxidation confirmed the following day by Radio-HPLC before the biodistribution was conducted (Figure 3). Drug binding to the tumor was significantly enhanced (p>0.01) in the presence of ascorbic acid during the chelation reaction at 1, 4 and 24hrs (33% ID/g 24hrs post drug injection) compared to the ascorbic acid-free formulation (10% ID/g 24hrs post drug injection) suggesting that oxidation was having a negative effect on the drug but could be minimized with the addition of the antioxidant.
Figure 3. Addition of Ascorbic Acid to Drug Preparation.
Figure 3 shows radio HPLC and biodistribution studies of 212Pb-DOTAMTATE with ascorbic acid. A. Radio-HPLC of 212Pb-DOTAMTATE without ascorbic acid present during chelation (orange) or with 10mM final concentration of ascorbic acid (blue) prior to biodistribution. B. Biodistribution of 212Pb-DOTAMTATE in AR42J tumor bearing mice (n=5 per group) at 1hr, 4hr and 24hrs post drug injection in the presence of 10mM ascorbic acid (blue) or none (orange).
212Pb-DOTAMTATE toxicity studies
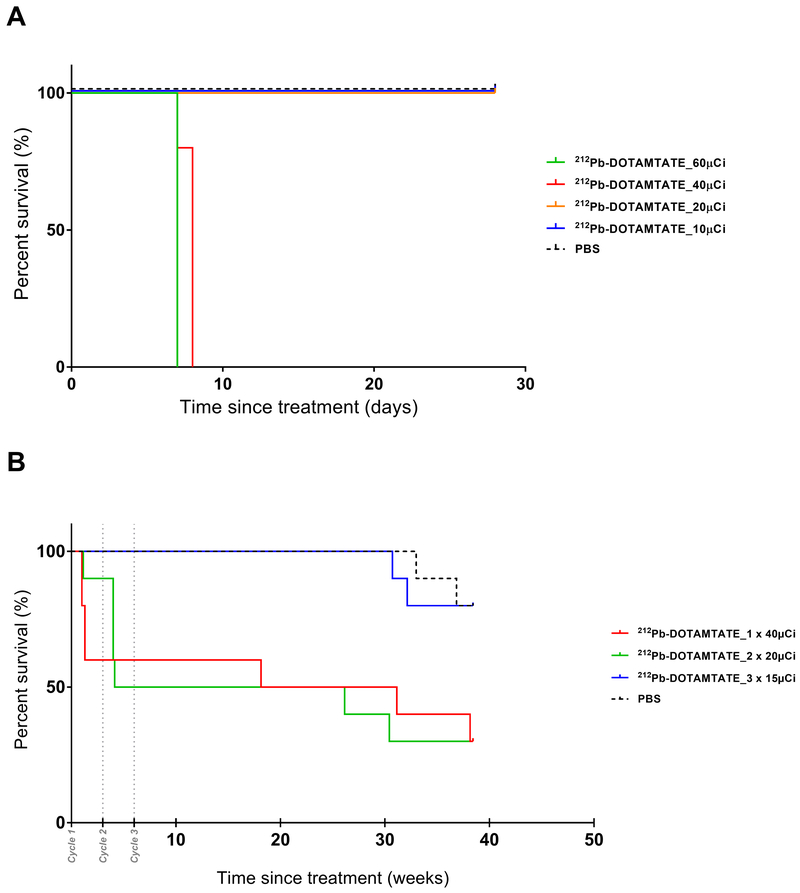
To assess the toxicity profile of 212Pb-DOTAMTATE, a dose range finding study in female, athymic nude mice was first conducted as a basis for dose selection for subsequent efficacy studies and for a GLP toxicity study. A maximum tolerated dose was determined to be between 20μCi and 40μCi (Figure 4A).
Figure 4. Dose Range Finding and Toxicity Studies.
Figure 4 shows initial dose range finding studies and fractionated dose toxicity studies with 212Pb-DOTAMTATE in athymic nude mice. A. Kaplan-Meier survival curve of 212Pb-DOTAMTATE treated athymic nude mice. Animals received a single dose of 10μCi (purple), 20μCi (blue), 40μCi (green), or 60μCi (red) of 212Pb-DOTAMTATE or PBS control (black). n=5 mice per group. Survival of the animals are shown in days post injection during the 4-week study. B. Kaplan-Meier curve of 212Pb-DOTAMTATE in CD-1 mice in a fractionated dose toxicity study. PBS alone, n=10 (black), 1 × 40μCi, n=10 (red), 2 × 20μCi, n=10 (green) and 3 ×15μCi, n=10 (blue) treatment groups. Drug cycles 1, 2 and 3 are shown with grey dots.
A single dose GLP toxicity study was conducted in female CD-1 non-tumor bearing mice at doses of 212Pb-DOTAMTATE ranging from 0μCi to 40μCi per mouse. Body weights, clinical chemistry and hematology parameters were examined throughout the nine-months duration of the study. The 2 and 10 μCi doses appeared to be reasonably well tolerated whereas administration of a single 40μCi intravenous dose of 212Pb-DOTAMTATE was associated with adverse findings including mortality, decreased body weight gain, leukocyte, erythrocyte, serum albumin and organ weights as well as histopathologic findings of bone marrow depletion and gastrointestinal lesions. At 20 μCi, there were relatively mild and reversible effects on weight gain and leukocyte counts along with chronic glomerular nephritis which appears late in the study due to a combination of aging and dosing. Based on the study findings, the dose of 10 μCi was considered a no-observable effect (NOEL) dose and an HNSTD of 20μCi was determined (Supplementary Figure S5).
To further examine if the HNSTD determined in the single dose toxicity study could be overcome through fractionation of the 212Pb-DOTAMTATE, a repeat dose toxicity study was conducted in non-tumor bearing CD-1 mice (Figure 4B). As hematological toxicity is routinely dose limiting for radiotherapeutics and is usually reversible with time at lower doses, fractionation was expected to overcome the lower HNSTD determined in the single dose study. Animals were given a single dose of 40μCi of 212Pb-DOTAMTATE, two cycles of 20μCi or three cycles of 15μCi 212Pb-DOTAMTATE every three weeks. Almost 40% of animals in the 1×40μCi group died nine days after injection but those that survived were able to survive through the remainder of the study. 50% of the animals in the 2 × 20μCi group died within four weeks of the study and one week after receiving the second dose. The animal group that received 3×15μCi of 212Pb-DOTAMTATE were consistent with the control group. Hematological toxicity appeared to be the reason for death in the first two groups. This was evident by the significantly low white blood cell counts (WBC) and platelets (PLT) in the 1 × 40μCi and 2 × 20μCi groups after drug injections (Supplementary S6). Animals who received 3 × 15μCi doses of 212Pb-DOTAMTATE also had a decrease in their WBC and PLT counts but were able to recover after each dose. This study suggests that a fractioned dose of drug is optimal as it allows the same cumulative dose but with recoverable hematological effects.
Efficacy Studies with 212Pb-DOTAMTATE
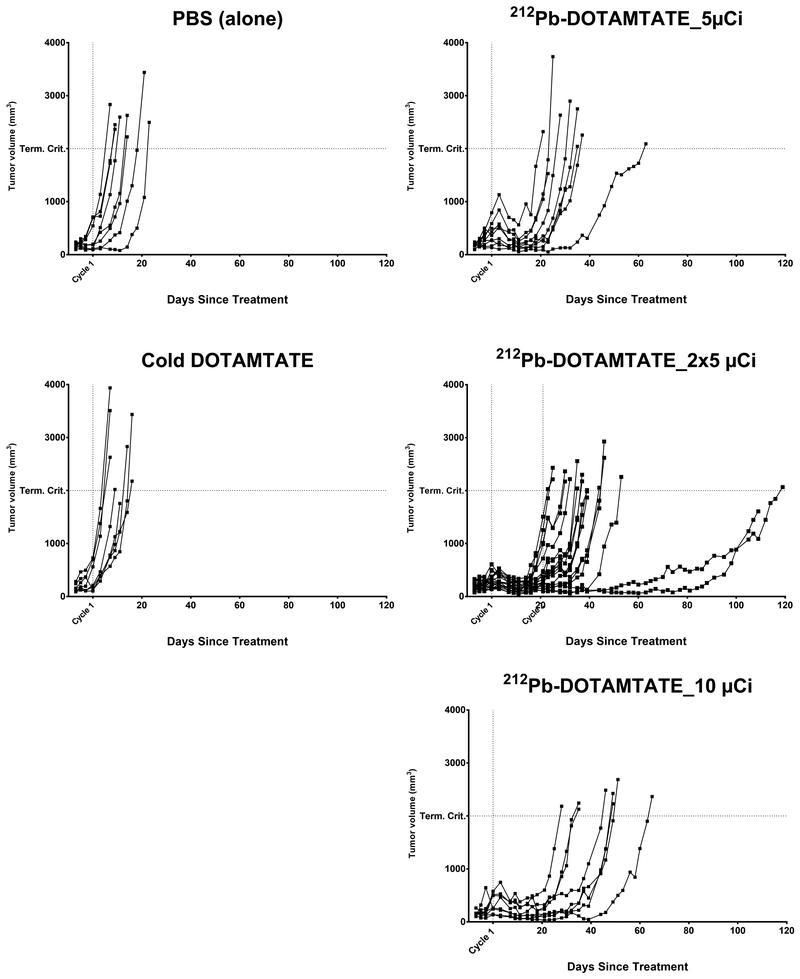
An initial low-dose efficacy study of 212Pb-DOTAMTATE was performed to examine the effectiveness of the drug in tumor bearing mice at 1/4 of the HNSTD. Animals were given one or two cycles of 5μCi 212Pb-DOTAMTATE or 10μCi 212Pb-DOTAMTATE. Control animals received cold-DOTAMTATE or PBS. Animals that were injected with cold-DOTAMTATE or PBS had similar median survival of 3.4 weeks and 3.5 weeks, respectively post injection. Mice that received one injection of 5μCi 212Pb-DOTAMTATE had a median survival of 6.3 weeks while mice who received one injection of 10μCi 212Pb-DOTAMTATE had a median survival of 8.5 weeks showing a dose dependent effect. Two injections of 5μCi 212Pb-DOTAMTATE led to a median survival of 7.1 weeks (Figure 5). The median survival time was similar between animals that received 1×10μCi vs 2×5μCi of drug suggesting that at low doses a fractionated dose does not appear to be beneficial. Overall however, the 212Pb-DOTAMTATE does show efficacy at these low doses but efficacy could likely be improved with higher treatment doses.
Figure 5. Single vs. multiple dose efficacy study.
Figure 5 shows the efficacy of mice treated with 212Pb-DOTAMTATE in a single and multi-injection dose setting. Groups of animals were injected with cold DOTAMTATE (n=8), PBS (n=8), 5μCi 212Pb-DOTAMTATE (n=9), 10μCi 212Pb-DOTAMTATE (n=8), 2 × 5μCi 212Pb-DOTAMTATE (n=18), Tumor volumes for individual mice per group are shown in mm3 over time.
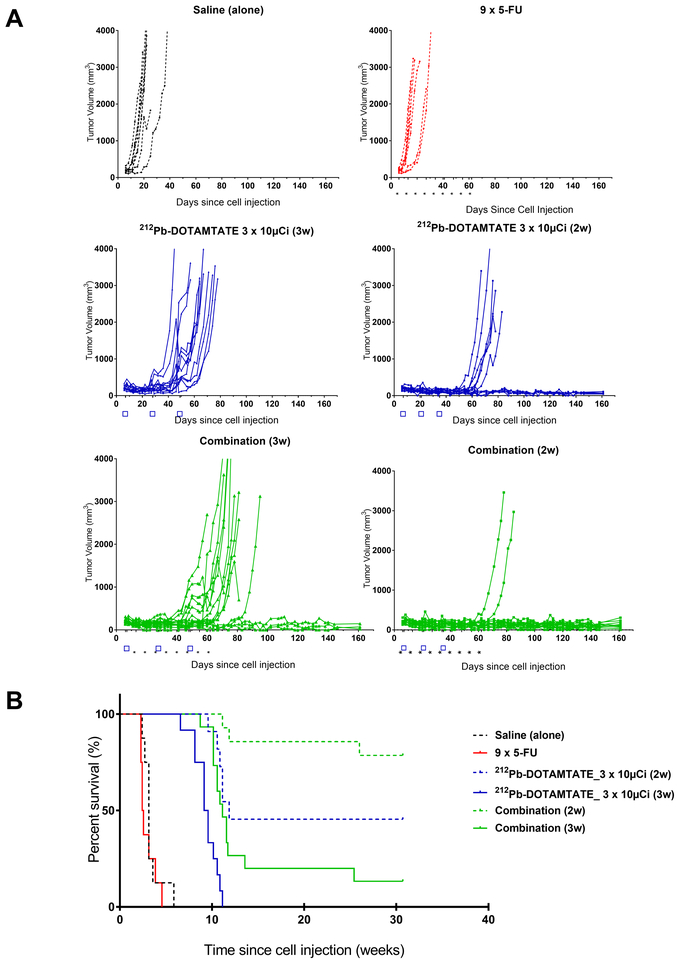
With this data, efficacy studies with 212Pb-DOTAMTATE were further optimized with a combination therapy and treatment cycle study. The aim of this study was to optimize the timing of treatment cycles and to combine the radiotherapeutic with a subtherapeutic (~40mg/m2 versus at least 400mg/m2 in human) chemotherapy dose of 5-fluorouracil (5-FU) to maximize tumor devastation. Animals received saline only, 5-FU only, 3 × 10μCi of 212Pb-DOTAMTATE at two-week or three-week intervals, or a combination of 5-FU and 3 × 10μCi 212Pb-DOTAMTATE at two-week or three-week intervals (Figure 6). Animals that were injected with 5-fluorouracil alone had a median survival of 2.4 weeks while the saline alone group had a median survival of 3.1 weeks post cell injection. Mice that received three injections of 212Pb-DOTAMTATE only at three-week intervals had a median survival rate of 9.4 weeks while combination therapy with 5-fluorouracil led to a longer median survival of 11.1 weeks with 20% of the mice alive and tumor free at the termination of the 31-week study. When 212Pb-DOTAMTATE was given at two-week intervals the median survival was 11.9 weeks and this was further improved by the addition of 5-FU where 79% of the animals survived to the end of the 31-week study. This suggests that the timing of the drug treatment is critical in maximizing its effectiveness. Furthermore, optimal timing of the radiotherapeutic combined with a radiosensitizer can significantly enhance efficacy versus the drug alone and lead to a significant group of tumor-free animals.
Figure 6. Combination Efficacy Study.
Figure 6 shows an efficacy study of AR42J tumor bearing mice treated with 212Pb-DOTAMTATE in combination with 5-fluorouracil. A. Individual mouse efficacy data showing tumor volumes of mice injected with saline (black), 5-FU only (red), 212Pb-DOTAMTATE only (blue) and in combination with 5-FU (green). B. Kaplan Meier of animals treated with saline (black), 5-FU only (red), 212Pb-DOTAMTATE only at 2-week intervals (blue dashed), 3-week intervals (blue solid), in combination with 5-FU at 2-week intervals (green dashed) or three-week intervals (green solid).
Discussion:
Extensive preclinical work and optimization has been accomplished to demonstrate the feasibility, safety and therapeutic potential of 212Pb-DOTAMTATE alone or in combination as a treatment for SSTR positive NETs. Specifically, in vitro assays have shown that the peptide binds to its SSTR receptor with an appropriate affinity for therapeutic use and has cytotoxic effects. Furthermore, in vivo tissue distribution studies in tumor bearing animals showed 212Pb-DOTAMTATE has a high uptake in the tumor relative to other organs. Although some drug uptake and retention was observed in the kidneys and pancreas of animals, it decreased significantly by 24hrs post drug injection. This uptake is not unexpected as these organs have also shown high uptake in other nonclinical rodent studies involving alpha emitters, which have not transliterated into adverse effects in human studies. [7, 18–21]. However, given the particularly high tumor uptake, the DOTAMTATE peptide has potential not only for therapeutic applications with 212Pb but also for imaging applications using longer-lived and gamma-emitting lead isotope such as 203Pb. Biodistribution studies conducted in our lab have shown that CD-1 mice given 203Pb-DOTAMTATE did not show significantly different tissue uptake compared to mice treated with 212Pb-DOTAMTATE in all critical organs (Supplementary Figure S7). This was confirmed by an exploratory IND (IND 102,590) conducted to examine the dosimetry and biodistribution of 203Pb-DOTAMTATE in patients with SSTR expressing NET’s as a surrogate for 212Pb-DOTAMTATE. 203Pb-DOTAMTATE showed similar PK properties to other commercially available octreotate drugs but with the advantage that the same metal could be used for imaging and therapeutic applications. This further confirms that the two isotopes have a similar physical property and pharmacokinetic profile and could therefore be used for theranostic purposes.
As with many PRRT treatments, the presence of radiolabeled somatostatin analogs in the kidneys is common due to their renal clearance and retention by megalin/cubulin receptors [20–22]. Kidney protection agents including L-lysine-L-arginine, mixtures of positively charged amino acids and amifostine are often given in combination with radiolabeled drugs [23–25]. With 212Pb-DOTAMTATE specifically, multiple kidney protection agents were tested, and all were found to significantly reduce drug uptake in the kidneys 1 hr post drug injection. It should be noted, however, that these were given as a bolus injection 5 minutes prior to drug injection rather than an IV over the course of four hours which is done with patients, due to animal model constraints; therefore, the data may not directly translate into a clinical setting.
In addition to kidney uptake, another factor that must be considered with PRRT is the oxidation of peptides in the proximity of radionuclides. The presence of an oxidized form of DOTAMTATE was detected in our studies by radio-HPLC and was shown to have a negative impact on tumor binding through biodistribution studies. Free radicals have been shown to form in solutions containing high energy β-particles and tryptophan residues, specifically, can become oxidized [26–28]. The addition of the antioxidant, ascorbic acid, during the chelation reaction significantly enhanced (3X vs. a mostly oxidized peptide) tumor binding presumably by minimizing this tryptophan oxidation within the 212Pb-DOTAMTATE peptide as confirmed by Radio-HPLC analysis.
To better characterize the safety profile of 212Pb-DOTAMTATE a dose range finding study in female, athymic nude mice was conducted as a basis for dose selection for subsequent efficacy studies and a single dose GLP toxicity study with 212Pb-DOTAMTATE. The dose range finding study led to a maximum tolerated dose between 20μCi and 40μCi and provided the preliminary information for a single dose GLP toxicity study, which included a 9-month follow-up to determine potential delayed toxicities in radiation-sensitive organs. Based on histopathology, body weights, hematology and clinical chemistry from this GLP toxicity study, an HNSTD of 20μCi was determined. An additional toxicity study showed that fractionating the dose was optimal and allowed for a cumulative dose that would be toxic if given as a single injection.
Efficacy studies showed that 212Pb-DOTAMTATE has therapeutic potential as it was able to extend median life span 2.4-fold with a single treatment at low doses. Furthermore, it was able to cure approximately 50% of the animals when the timing of the drug was optimized. The time between cycles must be sufficient to allow for acute hematological toxicity recovery without being too long of a duration that the tumor growth rate renders the drug less effective. Furthermore, combination therapy with PRRT can be used to enhance the efficacy of the drugs beyond the additive efficacy of each. By targeting multiple mechanisms involved in tumor cell proliferation and resistance, combination therapies using two or more drugs achieve efficacy with lower doses or toxicity than individual treatments. Several radiosensitizers have shown additive or synergistic effects when combined with PRRT [10–14]. Fluorouracil acts as an inhibitor of thymidylate synthase (TS), which is a nucleoside required for DNA replication and DNA repair [29–31]. Fluorouracil’s mechanism of action makes it an ideal candidate for combination therapy with PRRT as the main goal is to maximize irreversible DNA damage. 212Pb-DOTAMTATE and fluorouracil combination therapy showed a significant improvement in tumor regression. A three-cycle 212Pb-DOTAMTATE treatment combined with weekly subtherapeutic fluorouracil dosing was able to durably cure approximately 80% of the animals.
Overall, the non-clinical studies provide appropriate justification on the safety and efficacy of 212Pb-DOTAMTATE in animals and have provided sufficient data to warrant a clinical trial study. The rodent models showed a promising safety index with a 3.2-fold increase in median survival and one third of the animals being tumor free. Somatostatin analogs have long been studied and used in preclinical and clinical settings for the treatment of SSTR expressing neuroendocrine tumors, but a successful TAT treatment remains elusive. The preclinical data presented supports the further progression of 212Pb-DOTAMTATE into a clinical setting and was used to support the initiation of a Phase I study (NCT03466216, https://clinicaltrials.gov/ct2/show/NCT03466216?term=radiomedix&rank=2).
Supplementary Material
Acknowledgment:
The authors are grateful to the working group of Comparative Bioscience, INC for their contribution to the GLP-Toxicity analyses and reporting. RAPID is particularly acknowledged for their technical assistance in the dosimetry study of 203Pb-DOTAMTATE in patients. This work was supported, in part, by The National Cancer Institute (NCI SBIR Phase I Contract HHSN261201600015C awarded to I. Tworowska, RadioMedix Inc.).
Financial support: Izabela Tworowska (RadioMedix) has been awarded NCI SBIR Phase I Contract HHSN261201600015C (2016)”Targeted radionuclide therapy of neuroendocrine tumors using Pb212-octreotate analogs”
Footnotes
Conflict of Interest Disclosure:
Tania A Rozgaja Stallons, Amal Saidi and Julien J Torgue are Orano Med employees. Izabela Tworowska and Ebrahim S Delpassand are RadioMedix Inc. employees.
References:
- 1.Pfeifer A, Knigge U, Binderup T, Mortensen J, Oturai P, Loft A, et al. , 64Cu-DOTATATE PET for Neuroendocrine Tumors: A Prospective Head-to-Head Comparison with 111In-DTPA-Octreotide in 112 Patients. J Nucl Med, 2015. 56(6): p. 847–54. [DOI] [PubMed] [Google Scholar]
- 2.Maxwell JE and Howe JR, Imaging in neuroendocrine tumors: an update for the clinician. International journal of endocrine oncology, 2015. 2(2): p. 159–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olsen JO, Pozderac RV, Hinkle G, Hill T, O’Dorisio TM, Schirmer WJ, et al. , Somatostatin receptor imaging of neuroendocrine tumors with indium-111 pentetreotide (Octreoscan). Semin Nucl Med, 1995. 25(3): p. 251–61. [DOI] [PubMed] [Google Scholar]
- 4.Storch D, Behe M, Walter MA, Chen J, Powell P, Mikolajczak R, et al. , Evaluation of [99mTc/EDDA/HYNIC0]octreotide derivatives compared with [111In-DOTA0,Tyr3, Thr8]octreotide and [111In-DTPA0]octreotide: does tumor or pancreas uptake correlate with the rate of internalization? J Nucl Med, 2005. 46(9): p. 1561–9. [PubMed] [Google Scholar]
- 5.Bushnell D, Menda Y, O’Dorisio T, Madsen M, Miller S, Carlisle T, et al. , Effects of intravenous amino acid administration with Y-90 DOTA-Phe1-Tyr3-Octreotide (SMT487[OctreoTher) treatment. Cancer Biother Radiopharm, 2004. 19(1): p. 35–41. [DOI] [PubMed] [Google Scholar]
- 6.Kwekkeboom DJ, de Herder WW, Kam BL, van Eijck CH, van Essen M, Kooij PP, et al. , Treatment with the radiolabeled somatostatin analog [177 Lu-DOTA 0,Tyr3]octreotate: toxicity, efficacy, and survival. J Clin Oncol, 2008. 26(13): p. 2124–30. [DOI] [PubMed] [Google Scholar]
- 7.Kratochwil C, Giesel FL, Bruchertseifer F, Mier W, Apostolidis C, Boll R, et al. , (213)Bi-DOTATOC receptor-targeted alpha-radionuclide therapy induces remission in neuroendocrine tumours refractory to beta radiation: a first-in-human experience. Eur J Nucl Med Mol Imaging, 2014. 41(11): p. 2106–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strosberg J, El-Haddad G, Wolin E, Hendifar A, Yao J, Chasen B, et al. , Phase 3 Trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors. New England Journal of Medicine, 2017. 376(2): p. 125–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nayak TK, Norenberg JP, Anderson TL, Prossnitz ER, Stabin MG, and Atcher RW, Somatostatin-receptor-targeted alpha-emitting 213Bi is therapeutically more effective than beta(−)-emitting 177Lu in human pancreatic adenocarcinoma cells. Nucl Med Biol, 2007. 34(2): p. 185–93. [DOI] [PubMed] [Google Scholar]
- 10.Barber TW, Hofman MS, Thomson BNJ, and Hicks RJ, The potential for induction peptide receptor chemoradionuclide therapy to render inoperable pancreatic and duodenal neuroendocrine tumours resectable. European Journal of Surgical Oncology, 2012. 38(1): p. 64–71. [DOI] [PubMed] [Google Scholar]
- 11.Claringbold PG, Brayshaw PA, Price RA, and Turner JH, Phase II study of radiopeptide 177Lu-octreotate and capecitabine therapy of progressive disseminated neuroendocrine tumours. European Journal of Nuclear Medicine and Molecular Imaging, 2011. 38(2): p. 302–311. [DOI] [PubMed] [Google Scholar]
- 12.van Essen M, Krenning EP, Kam BL, de Herder WW, van Aken MO, and Kwekkeboom DJ, Report on short-term side effects of treatments with (177)Lu-octreotate in combination with capecitabine in seven patients with gastroenteropancreatic neuroendocrine tumours. European Journal of Nuclear Medicine and Molecular Imaging, 2008. 35(4): p. 743–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kong G, Johnston V, Ramdave S, Lau E, Rischin D, and Hicks RJ, High-Administered Activity In-111 Octreotide Therapy with Concomitant Radiosensitizing 5FU Chemotherapy for Treatment of Neuroendocrine Tumors: Preliminary Experience. Cancer Biotherapy and Radiopharmaceuticals, 2009. 24(5): p. 527–533. [DOI] [PubMed] [Google Scholar]
- 14.Ballal S, Yadav MP, Damle NA, Sahoo RK, and Bal C, Concomitant 177Lu-DOTATATE and Capecitabine Therapy in Patients With Advanced Neuroendocrine Tumors: A Long-term-Outcome, Toxicity, Survival, and Quality-of-Life Study. Clinical Nuclear Medicine, 2017. 42(11): p. e457–e466. [DOI] [PubMed] [Google Scholar]
- 15.Ullrich M, Bergmann R, Peitzsch M, Zenker EF, Cartellieri M, Bachmann M, et al. , Multimodal Somatostatin Receptor Theranostics Using [(64)Cu]Cu-/[(177)Lu]Lu-DOTA-(Tyr(3))octreotate and AN-238 in a Mouse Pheochromocytoma Model. Theranostics, 2016. 6(5): p. 650–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lipnick RL, Cotruvo JA, Hill RN, Bruce RD, Stitzel KA, Walker AP, et al. , Comparison of the up-and-down, conventional LD50, and fixed-dose acute toxicity procedures. Food Chem Toxicol, 1995. 33(3): p. 223–31. [DOI] [PubMed] [Google Scholar]
- 17.Mu L, Hesselmann R, Oezdemir U, Bertschi L, Blanc A, Dragic M, et al. , Identification, characterization and suppression of side-products formed during the synthesis of high dose (6)(8)Ga-DOTA-TATE. Appl Radiat Isot, 2013. 76: p. 63–9. [DOI] [PubMed] [Google Scholar]
- 18.Norenberg JP, Krenning BJ, Konings IR, Kusewitt DF, Nayak TK, Anderson TL, et al. , 213Bi-[DOTA0, Tyr3]octreotide peptide receptor radionuclide therapy of pancreatic tumors in a preclinical animal model. Clin Cancer Res, 2006. 12(3 Pt 1): p. 897–903. [DOI] [PubMed] [Google Scholar]
- 19.Kulaksiz H, Eissele R, Rossler D, Schulz S, Hollt V, Cetin Y, et al. , Identification of somatostatin receptor subtypes 1, 2A, 3, and 5 in neuroendocrine tumours with subtype specific antibodies. Gut, 2002. 50(1): p. 52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rolleman EJ, Valkema R, Melis M, Krenning EP, Visser TJ, and de Jong M, Cubilin and megalin in radiation-induced renal injury with labelled somatostatin analogues: are we just dealing with the kidney? European Journal of Nuclear Medicine and Molecular Imaging, 2006. 33(6): p. 749–750. [DOI] [PubMed] [Google Scholar]
- 21.Vegt E, Melis M, Eek A, de Visser M, Brom M, Oyen WJG, et al. , Renal uptake of different radiolabelled peptides is mediated by megalin: SPECT and biodistribution studies in megalin-deficient mice. European Journal of Nuclear Medicine and Molecular Imaging, 2011. 38(4): p. 623–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vegt E, de Jong M, Wetzels JF, Masereeuw R, Melis M, Oyen WJ, et al. , Renal toxicity of radiolabeled peptides and antibody fragments: mechanisms, impact on radionuclide therapy, and strategies for prevention. J Nucl Med, 2010. 51(7): p. 1049–58. [DOI] [PubMed] [Google Scholar]
- 23.Melis M, Valkema R, Krenning EP, and de Jong M, Reduction of renal uptake of radiolabeled octreotate by amifostine coadministration. J Nucl Med, 2012. 53(5): p. 749–53. [DOI] [PubMed] [Google Scholar]
- 24.Rolleman EJ, Valkema R, de Jong M, Kooij PP, and Krenning EP, Safe and effective inhibition of renal uptake of radiolabelled octreotide by a combination of lysine and arginine. European Journal of Nuclear Medicine and Molecular Imaging, 2003. 30(1): p. 9–15. [DOI] [PubMed] [Google Scholar]
- 25.Chan HS, Konijnenberg MW, de Blois E, Koelewijn S, Baum RP, Morgenstern A, et al. , Influence of tumour size on the efficacy of targeted alpha therapy with (213)Bi-[DOTA(0),Tyr(3)]-octreotate. EJNMMI Research, 2016. 6: p. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu S and Edwards DS, Stabilization of 90Y-Labeled DOTA-Biomolecule Conjugates Using Gentisic Acid and Ascorbic Acid. Bioconjugate Chemistry, 2001. 12(4): p. 554–558. [DOI] [PubMed] [Google Scholar]
- 27.Garrison WM, Reaction mechanisms in the radiolysis of peptides, polypeptides, and proteins. Chemical Reviews, 1987. 87(2): p. 381–398. [Google Scholar]
- 28.Simat TJ and Steinhart H, Oxidation of Free Tryptophan and Tryptophan Residues in Peptides and Proteins. Journal of Agricultural and Food Chemistry, 1998. 46(2): p. 490–498. [DOI] [PubMed] [Google Scholar]
- 29.Kjellström J, Kjellén E, and Johnsson A, In vitro radiosensitization by oxaliplatin and 5-fluorouracil in a human colon cancer cell line. Acta Oncologica, 2005. 44(7): p. 687–693. [DOI] [PubMed] [Google Scholar]
- 30.Ojima E, Inoue Y, Watanabe H, Hiro J, Toiyama Y, Miki C, et al. , The optimal schedule for 5-fluorouracil radiosensitization in colon cancer cell lines. Oncol Rep, 2006. 16(5): p. 1085–91. [PubMed] [Google Scholar]
- 31.Valdes G and Iwamoto KS, Re-evaluation of cellular radiosensitization by 5-fluorouracil: High-dose, pulsed administration is effective and preferable to conventional low-dose, chronic administration. International Journal of Radiation Biology, 2013. 89(10): p. 851–862. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.