Abstract
Purpose:
To identify deregulated and inhibitory microRNAs (miRNA, miR), and generate novel mimics for replacement nanomedicine for head and neck squamous cell carcinomas (HNSCC).
Experimental Design:
We integrated miRNA and mRNA expression, copy number variation, and DNA methylation results from The Cancer Genome Atlas (TCGA), with a functional genome-wide screen.
Results:
We reveal that the miR-30 family is commonly repressed, and all 5 members sharing these seed sequence similarly inhibit HNSCC proliferation in vitro. We uncover a previously unrecognized inverse relationship with overexpression of a network of important predicted target mRNAs deregulated in HNSCC, that includes key molecules involved in proliferation (EGFR, MET, IGF1R, IRS1, E2F7), differentiation (WNT7B, FZD2), adhesion, and invasion (ITGA6, SERPINE1). Re-expression of the most differentially repressed family member, miR-30a-5p, suppressed this mRNA program, selected signaling proteins and pathways, and inhibited cell proliferation, migration, and invasion in vitro. Further, a novel miR-30a-5p mimic formulated into a targeted nanomedicine significantly inhibited HNSCC xenograft tumor growth and target growth receptors EGFR and MET in vivo. Significantly decreased miR-30a/e family expression was related to DNA promoter hypermethylation and/or copy loss in TCGA data, and clinically with decreased disease-specific survival in a validation dataset. Strikingly, decreased miR-30e-5p distinguished oropharyngeal HNSCC with poor prognosis in TCGA (p=0.002) and validation (p=0.007) datasets, identifying a novel candidate biomarker and target for this HNSCC subset.
Conclusions:
We identify the miR-30 family as an important regulator of signal networks and tumor suppressor in a subset of HNSCC patients, that may benefit from miR replacement nanomedicine therapy.
Keywords: microRNA-30, genomic analysis, tumor suppressor, microRNA, nanoparticles, head, neck cancer
Introduction
Deregulation of microRNA (miRNA, miR) expression contributes to the aberrant expression of mRNAs that mediate the complex malignant phenotypes of cancers (1). Recent efforts support the concept of replacing deficient miRNA expression using synthetic miRNA mimics (2). Since a single miRNA can target multiple mRNAs, miRNA-based therapeutics could help mitigate intrinsic or acquired resistance observed using more selective small molecule or biologic therapies that target a single oncogene.
Genome-wide expression profiling studies have demonstrated broad deregulation and heterogeneity in miRNA and mRNA expression in primary tumors. This underscores the complexity and challenge in identifying miRNAs and mRNAs of critical importance to the malignant phenotype and therapeutic resistance from among hundreds of candidates. Deregulation of mRNAs and miRNAs has been observed in head and neck squamous cell carcinomas (HNSCC) (3). Insights gained through genome-wide analyses have uncovered candidate transcription factors and miRNAs that regulate broader mRNA programs implicated in cancer (4, 5). However, until the recent publication of the head and neck and pan-cancer analyses from The Cancer Genome Atlas (TCGA) (6, 7), comprehensive data from multiple platforms has not been available to compare and identify the most significantly altered miRNAs, inversely expressed mRNA targets, and the contributions of genomic alterations driving their expression.
While functional screens have provided an alternative means to identify miRNAs of interest in vitro (8), prioritization is difficult, as many candidate miRNAs do not translate to therapeutic activity in vivo. Few studies on the tumor suppressor function of miRNAs have comprehensively linked genomic alterations to changes in miRNA expression, regulation of a broad pathway of oncogenic mRNAs, and their functional relationship to the cancer phenotype and clinical features. Contributing factors include a prior lack of comprehensive multiplatform data from large tumor datasets, tumor heterogeneity in miRNA and mRNA expression and function, and differences in conditions affecting cancer cell growth in the tumor microenvironment versus culture 9, 10).
To identify miRNAs of potential regulatory, biologic and therapeutic importance in HNSCC, we integrated analysis of miRNA expression with functional screening for anti-proliferative miRNAs, and inversely correlated predicted target mRNAs from TCGA and a validation data set of HNSCC tumors. Intriguingly, this approach uncovered overlap of 9 under-expressed and antiproliferative miRNAs, of which four were members of the miR-30 family. Remarkably, decreased miR-30a-5p was inversely related to overexpression of a program of growth, differentiation, and metastasis-related mRNAs that are implicated in the biology and clinical features of HNSCC. We confirmed the role of miR-30 family in regulation of several classical oncogenes centering on cell growth, differentiation and adhesion molecules. We developed a novel synthetic miR-30a-5p mimic nanomedicine which delayed tumor growth and inhibited expression of growth receptors when delivered into xenograft tumor models of HNSCC. Further, decreased miR-30 family expression was linked with DNA copy loss and promoter hypermethylation, and patient outcome, identifying the miR-30–5p family as a tumor suppressor and potential therapeutic target in HNSCC subsets.
Materials and Methods
Integrative Analysis of TCGA miRNA and mRNA Expression Data
TCGA HNSCC tumor and control specimens were collected under IRB approved protocols with informed consent (6,7). The characteristics and miRNA, mRNA, data for 279 HNSCC and 16 normal mucosa previously reported were accessed from Level 3 data (REF=TCGA Network, supplementary information S7.43). Reads were aligned to NCBI GRCh37-lite reference genome, and annotated based on miRBase v16. Although all identified miRNAs (mature strand, star strand, precursor miRNA, etc.) were counted and normalized to RPM, only mature miR-5p and 3p strands were used for analysis. The miRNAs were ranked by RPM variance across the samples, and the most variable 50% with a minimum expression of at least 50 RPM were used for integrated analysis. mRNA expression was calculated from RNA-Seq data with RSEM v1.1.132 (11). The most-variant 50% of genes were used for integrated analysis. Both miRNA and mRNA expression data were log2 transformed. To generate a high confidence dataset of global miRNA-mRNA interactions, we identified pair-wise negative correlations of miRNA with target mRNA expression, using linear regression in conjunction with available prediction tools from miRNA target databases.
miRNA Expression Validation in HNSCC Samples
An independent validation set of 13 HNSCC tissue and 9 mucosa samples were collected by the University of Michigan Medical Center as part of an IRB approved protocol UM2002–0691, with informed consent. The collected tissues were snap frozen and mounted in OCT freezing media (Fischer), cut into 7 micrometer sections, and stained by H & E standard methods. The stained slides were scanned using a Scanscope (Aperio), to ensure the presence of tumor or mucosa squamous epithelium. The stained slides were used to macrodissect tissue blocks to attain a minimum of 70% desired squamous tumor or epithelium cells in each sample. Small RNA were purified using a mirVana miRNA isolation Kit (Life Technologies). Small RNA sequencing libraries were constructed using the SOLiD™ Total RNA-Seq Kit (Life Technologies). Sequencing was performed on the SOLiD™ 5500 next generation sequencer and the SOLiD™ Small RNA SP Kit (Life Technologies), using manufacturer’s protocols. miRNA expression was analyzed as described above.
HNSCC Cell Lines
A panel of 10 HNSCC cell lines was obtained from the University of Michigan squamous cell carcinoma (UM-SCC) series from Dr. T.E. Carey. The origin of these UM-SCC cell lines was authenticated in 2010 by genotyping with 9 markers as described in Supplemental Methods, and frozen stocks verified to be free of mycoplasma by RT-PCR were established. Preserved frozen stocks of lines were used within 3 months of culture. UM-SCC cell lines and Human primary oral keratinocytes (HOK) from oral gingival mucosa purchased from Lonza (Walkersville, MD), were cultured in medium as detailed in Supplemental Methods.
Functional microRNA Mimic Viability Screen
UM-SCC-1 cells were maintained in MEM containing 10% heat inactivated fetal bovine serum (FBS) supplemented with non-essential amino acids and sodium pyruvate. Transfections were performed in 384-well plates (Corning 3570). For transfections, 20 µL of serum free media containing Lipofectamine RNAiMax was added to wells containing miRNA mimic (0.8 pmol). Lipid and miRNA mimic were allowed to complex for 45 min at ambient temperature before addition of 1500 cells in MEM, 20% FBS to yield final transfection mixtures containing 20 nM miR mimic in MEM, 10% FBS. Screening was conducted with a miRNA mimic library (Qiagen) based on Sanger miRBase 13.0 and consisting of ~800 mimics. Viability (CellTiter Glo, Promega) was assayed 72 h post-transfection on a PerkinElmer Envision 2104 Multilabel plate reader. Ambion Silencer Select Negative Control #2 was incorporated on all screening plates for normalization (16 wells per plate; the median negative control value on each plate was used to normalize sample wells). All screen plates exhibited assay z’-factors greater than 0.6. Negative control normalized viability data was converted into robust z-scores by median absolute deviation (MAD) (12).
RT-PCR, Western Blot, and Luciferase Reporter Validation of mRNA Targets
These standard assays are detailed in Supplementary Methods.
Migration, Matrigel and Colony Formation Assays
These standard assays are detailed in Supplementary Methods.
Development of miR-30a-5p Nanocomplex Targeted to Transferrin Receptor Expressed by Tumor Cells
Fluorescent siRNA to test nanoparticle delivery, a modified mir-30a-5-p mimetic, and control miRNA mimetic was synthesized by Trilink Biotechnologies. The miR-30a double-stranded mimic contained a guide strand sequence of 5’UGUAAACAUCCUCGACUGGAAGCU 3’ and a passenger strand sequence of 5’ AGCUUCCAGUCGGAUGUUUACACG 3’, chemically capped on the 5’end to prevent phosphorylation and loading via RISC, and mutated to enhance duplex formation, thereby favoring stability and function of the 5-p strand. The annealed and nanoparticle complexed miR-30a mimic is referred to as miR-30a-scL. The control miRNA mimic had a guide strand sequence of 5’ UUGUACUACACAAAAGUACUG 3’ and a passenger strand sequence of 5’ CAGUACUUUUGUGUAGUACAA 3’, which is based on C. elegans cel-miR-239b, a miRNA that has minimal sequence identity with miRNAs in human and mouse. The formulation of oligonucleotides into a liposomal nanodelivery system (scL) targeted by anti-transferrin Receptor Single-chain antibody Fragment was performed as previously described (13), and detailed in Supplementary Methods.
In Vivo Tumor Growth and Target Immunofluorescence Assays
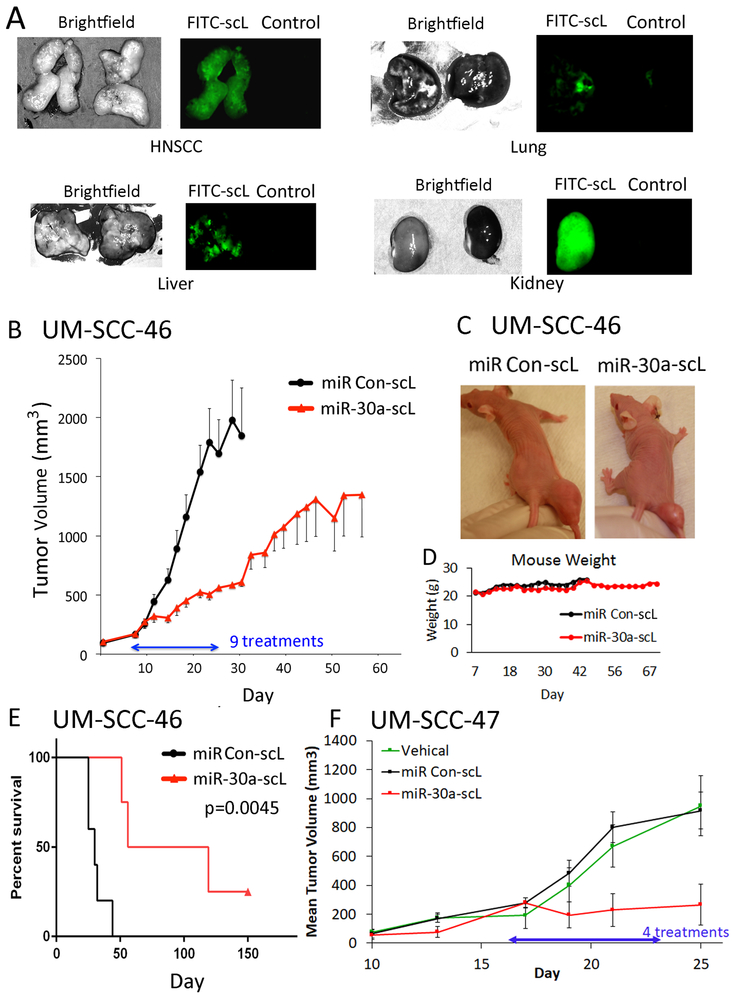
All animal experiments were carried out under protocols approved by the Animal Care and Use Committee of the NIDCD, and were in compliance with the Guide for the Care and Use of Laboratory Animal Resource (14). Six-to-eight week-old athymic nu/nu female mice (obtained from the Frederick Cancer Research and Development Center, NCI) were injected subcutaneously (s.c.) with 2.5 × 106 UM-SCC-46 or UM-SCC-47 cells in 100 µL of 30% Type 3 BME Cultrex(Trevigen)/MEM media on the right leg. Once tumors reached ~150 mm3, mice were randomized into treatment groups of (n=5 mice each); control 5% sucrose (vehicle), control miR-scL, and miR-30a-scL. Nine doses of 3 mg/kg miR-30a-scL was administered via tail vain injection on MWF over three weeks for a total of nine doses. Tumor size was measured on MWF with external calipers and volume calculated with the formula V = ½ L*W2. Tumor growth was reported as mean volume with standard error of the mean. Kaplan-Meier survival analysis was performed in Graphpad PRISM software (v6.05). Survival statistics were performed using the Log-rank (Mantel-Cox) test, and Hazard ratio calculated via Log-rank test.
An expanded description of all methods is included in the supplementary information.
Validation Study of mir-30a/e-5p Expression with Disease Free Survival
The Einstein study was previously described (15) and included global miRNA expression profiling, clinicopathologic, and long-term disease specific survival data from 148 prospectively enrolled patients with histologically confirmed primary HNSCC undergoing curative treatment at Montefiore Medical Center since 2002. The study protocol was approved by the IRB, and all patients provided written informed consent. The survival analyses were performed with R package (16). The overall survival curves were obtained using Kaplan-Meier method and were compared using the log-rank test. The Cox proportional hazards model was used to estimate Hazard Ratios (HRs) with 95% Confidence Intervals (CIs). Subjects were dichotomized as low miRNA expression (< median) and high miRNA expression (≥ median), using the median expression of each miRNA as a cutoff. To compare overall survival time by CNV, subjects were categorized as having MIR30E/A deletion if their GISTIC copy number value was less than −0.1, otherwise they were considered to have no deletion.
Results
Genome-Wide miRNA Expression Analysis Identifies Decreased Expression of miR-30-5p Family Members in HNSCC Tissues
To identify miRNAs of potential therapeutic importance in HNSCC, we employed an integrated approach that combined structural and functional genomic analyses (Supplementary Fig. S1). To identify miRNAs that were differentially expressed in HNSCC tissues, we analyzed TCGA miRNA sequencing data for 279 HNSCC with 16 squamous control specimens (6). This analysis identified 129 significantly deregulated miRNAs, including 77 increased and 53 decreased miRNAs (the cutoff for significance is an FDR adjusted q-value of 0.2, as listed in Supplementary Table S1A, Supplementary Fig. S2A). We validated these observations by miRNA sequencing and expression analysis of an independent panel of 13 HNSCC specimens from the oral cavity and 9 matched mucosa samples from the University of Michigan (UMSC) (Supplementary Table S2, Supplementary Fig. S2). Pair-wise comparison of significantly altered miRNAs in the smaller dataset (cutoff q-value of 0.2) supported the novel finding of broad repression of several members of the miR-30 family, as well as other miRNAs identified in prior studies of HNSCC (Fig. 1A and 1B; Supplementary Table S1). Notably, miR-30a and −30e family members exhibited at least a 2-fold decreased expression in >70% of the specimens in both cohorts.
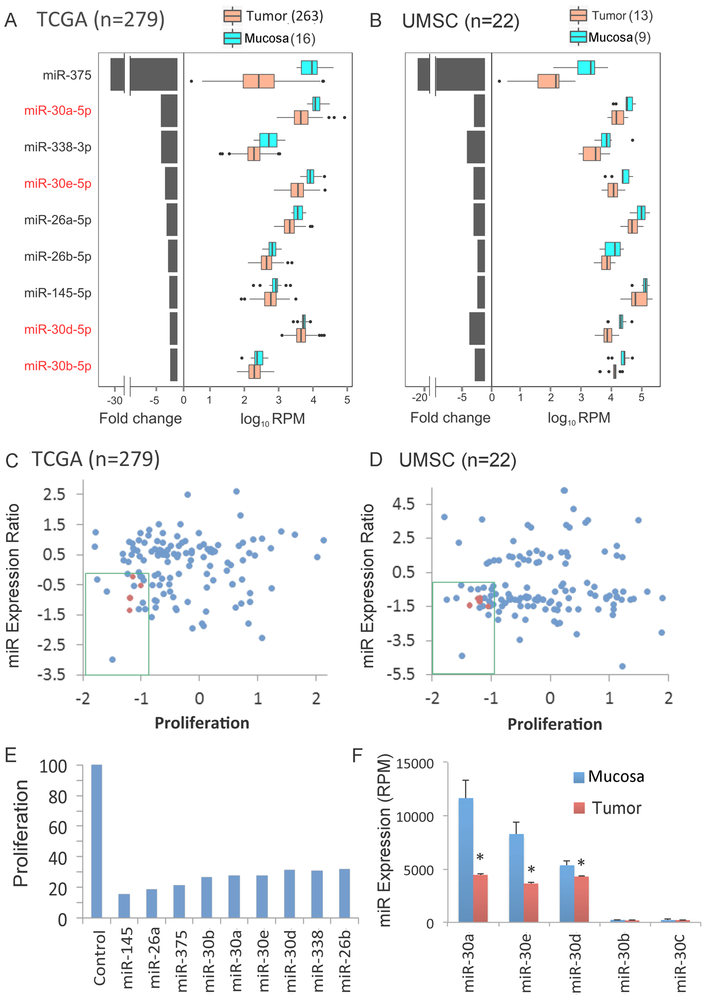
Fig. 1. Integrative genome-wide analysis identified decreased expression of miR-30 family members with anti-proliferative activity in HNSCC tissues and cell lines.
Nine miRNAs that were differentially abundant between tumor and normal mucosa control samples (q-value <0.2) in in TCGA (A) and independent UMSC (B) HNSCC tumor cohorts. Genome-wide mature microRNA profiling was performed using high throughput sequencing of human HNSCC tissues. TCGA: tumor=279, mucosa=16; samples from UMSC: tumor=13, mucosa=9. Left: median fold change between tumor and mucosa, presented on a linear scale. Right: expression distribution of mucosa and tumor presented as log10 RPM (reads per million base pairs). Medians, thick black lines; bars, 25th and 75th percentiles; outliers are displayed as individual points. Relationship of proliferation score to log2 miRNA tumor-mucosa expression ratio of TCGA (C) and UMSC (D) differentially expressed miRNAs (log2 tumor compared to mucosa in y axis) vs. proliferation score [Median Absolute Deviation (MAD)]. Anti-proliferative activity was determined in an in vitro genome wide RNAi screening in the HNSCC cell line UM-SCC-1 as described in Methods. The green box denotes miRNAs that are repressed in tumor tissue and exhibit anti-proliferative activity. miR-30-5p family members are highlighted in red. (E) Anti-proliferative effect of miRNA mimics, 96 hours after transfection into UM-SCC-1, as percentage of control miRNA mimic. (F) Expression of miR-30 family members in mucosa and tumor specimens from the TCGA cohort. Bars represent SEM and * denotes q < 0.2 as reported by SAMseq. miR-30a-5p and miR-30e-5p display the highest expression in mucosa specimens and the greatest reduction in tumor specimens.
Integrative Functional Genomics Screening Reveals that miR-30 Family Members Inhibit HNSCC Proliferation
To identify candidate miRNAs that inhibit proliferation, we performed a functional genomic screen after transfecting a library of ~800 miRNAs into the human HNSCC line UM-SCC-1 (Supplementary Table S3). To enrich screening hits for miRNAs with relevance to disease biology, miRNAs that displayed high anti-proliferative activity (MAD score < −1) were filtered against miRNAs that display reduced expression in both TCGA and the UMSC validation datasets. We identified 9 miRNAs with decreased expression in tumor specimens (Fig. 1C and D) and display significant inhibitory activity when re-expressed (Fig. 1E). Strikingly, several members of the miR-30 family were again present among this highly selected class of miRNAs, supporting the biologic and functional importance of miR-30 family members in HNSCC. Next we validated the anti-proliferative screening results for each miR-30–5p family member in UM-SCC-1 and an additional HNSCC cell line UM-SCC-46 (Fig. S3A), which all displayed similar activity in both lines. Among these, miR-30a-5p followed by miR-30e-5p were the most highly expressed in mucosa samples and strongly decreased across the tumor specimens (Fig. 1F). In two HNSCC cell line models and primary Human Oral Keratinocytes, miR-30a-5p displays higher relative expression (Fig. S3B). Thus, as these and other miR-30 family members share the same seed sequences for targets, we included the most differentially expressed miR-30-a/e-5p family members in selected genomic, functional and clinical outcome studies below.
miR-30a-5p is Inversely Correlated with Target Transcripts Implicated in Cancer Growth Signaling and Metastasis
To identify the network of target mRNAs regulated by miR-30 in HNSCC that underlie its function(s), we explored whether the reduced expression of the most differentially expressed family member, miR-30a-5p, is anti-correlated with mRNAs of potential biologic importance in cancer. Linear regression analysis was performed between miR-30a-5p and genome-wide mRNA expression levels obtained from RNA-seq tumor specimens in the TCGA dataset. 91 mRNAs were inversely expressed to miR-30a-5p with FDR ≤ 0.05, and also contained predicted or verified binding sites for miR-30a-5p in the 3’ UTR, based on the Ingenuity Pathway Analysis (IPA) microRNA target filter (Supplementary Table S4). The significant anti-correlations of miR-30a-5p with several representative candidate cancer-related target genes are presented in Supplementary Fig. S3C. Interestingly, miR-30a-5p expression displayed an inverse relationship to several oncogenes previously shown to be overexpressed in HNSCC, including EGFR, MET, IGF1R, ITGA6, and SERPINE1 (17–19). The two most statistically significant cancer disease functions identified by functional pathway analysis of inversely expressed target genes were cell proliferation (21 mRNAs, p=8.95×10−10) and metastasis (23 mRNAs, p=9.54×10−12 Supplementary Table S5). Remarkably, these networks harbor a diverse repertoire of molecules critically implicated in cancer growth (EGFR, MET, IGF1R, PDGFRB, IRS1, SOCS1, CCNA1); adhesion, migration and invasion (MET, ITGA6, NT5E, SERPINE1); and differentiation (WNT7B/5A, FZD2, CELSR3, CTHRC1).
Most of the mRNAs identified above are novel targets of miR-30 family and not previously characterized. To functionally validate miR-30a-5p regulation of inversely expressed mRNAs, we first examined the effects of ectopic expression of miR-30a-5p or antisense-miR30a-5p on potentially targeted mRNAs in two HNSCC cell lines, HPV(–) UM-SCC-46 (Fig. 2A, left panel), and HPV(+) UM-SCC-47 (Fig. S4A). After transfection of miR-30a-5p, a reduction in mRNA expression was observed for 11 selected mRNAs by qRT-PCR, involved in growth and proliferation (Fig. 2A, left panel), and differentiation, adhesion and invasion (Fig 2A, right panel). Thus, both bioinformatic analyses and experimental data support the hypothesis that miR-30a-5p contributes to regulation of several target mRNAs implicated in pathogenesis of HNSCC.
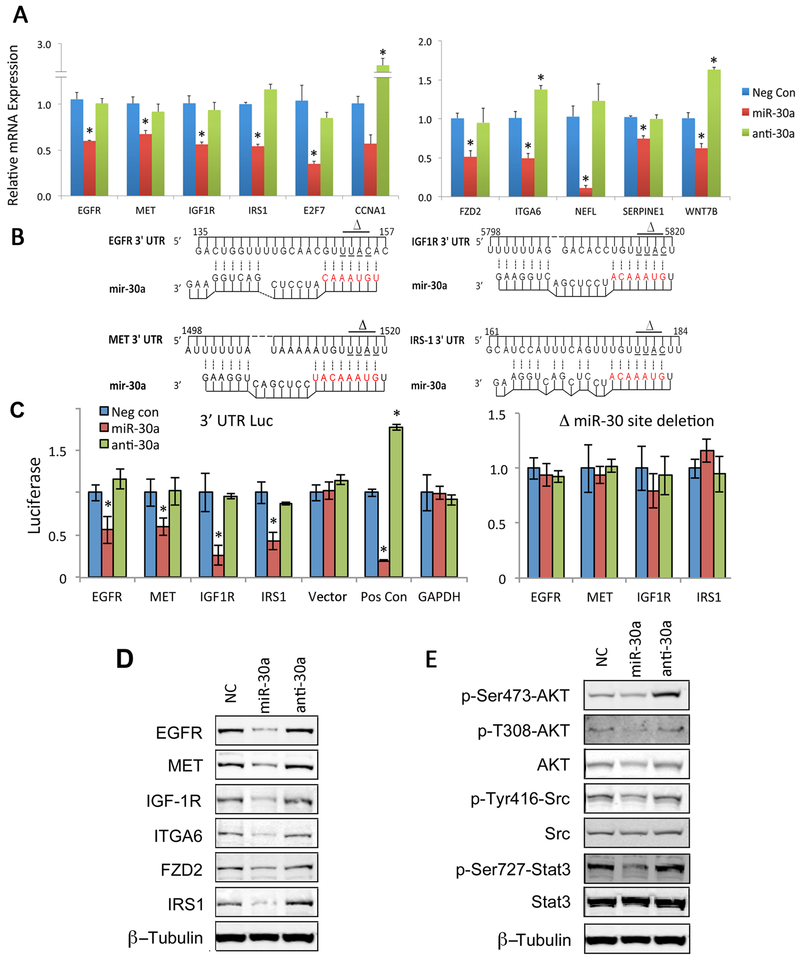
Fig. 2. Validation of miR-30a-5p predicted targets in an HNSCC line.
(A) Selected miR-30 target genes were validated by qRT-PCR measurement in UM-SCC-46 cells transfected with miRNA negative control (neg Con), miR-30a, or anti-miR-30a-5p oligonucleotide for 72 hrs. The mean of three independent experiments, ±SEM; * denotes p< 0.05 by Student’s t-test. (B) Base pairing of miR-30a-5p with 3’ UTR of target mRNAs was predicted by Mfold (http://unafold.rna.albany.edu/?q=mfold). Bases in red depict binding of miR-30a-5p seed sequence. Horizontal lines with delta symbols mark bases in the mRNA 3’ UTR that were deleted in cloned 3’-mutant UTR vectors to ablate miR-30-5p regulation. (C) Relative luciferase activity after co-transfection of UM-SCC-46 cells with miR-30a-5p or anti-miR30a-5p and vectors containing wild-type 3’-UTR (left) or 3’-mutant UTR (right) cloned behind a renilla luciferase reporter gene. Results for positive control vector (Pos Con) containing 5x miR-30-5p binding sites and a negative GAPDH 3’-UTR control are also displayed. All data represent the mean of three independent experiments and error bars represent SEM. * denotes p< 0.05 by Student’s t-test. (D) Protein expression for selected miR-30 targets and (E) downstream phospho- and total signal protein expression examined by Western blot using whole cell lysates isolated from Primary Human Oral Keratinocytes (HOK) and UM-SCC-46 cells 72 h after transfection with miR-30a-5p or anti-miR30a-5p oligonucleotides. β-tubulin is a loading control.
Functional Validation of miR-30a-5p Direct Regulation of Target Gene Expression
We further functionally validated direct regulation of a subset of 4 selected growth signaling related target genes by miR-30 family members, utilizing luciferase constructs containing the 3’ UTRs of EGFR, MET, IGF1R and IRS-1, which contain the predicted target binding sites for miR-30 family members (Fig. 2B). We also constructed ΔmiR-30 vectors in which the sequence that is complementary to the seed sequence of miR-30a-5p was deleted. miR-30a-5p but not anti-miR30a-5p suppressed reporter activity, and this was abrogated by deletion of the complimentary sequence (Fig. 2B, C). The inhibitory effects of miR-30a-5p on protein expression of a subset of the molecules implicated in growth signaling (EGFR, MET, IGF1R, IRS1), adhesion (ITGA6) and differentiation (FZD2) were also confirmed by Western blots in two cell lines (Fig. 2D and Supplementary Fig. S4B). As these growth factor receptors stimulate several oncogenic signaling pathways, we examined the functional effect of miR-30a-5p on signal phosphorylation on PI3K/mTOR-AKT (19), SRC (20), and STAT3 signaling (21). miR-30a-5p decreased downstream phosphorylation of these signaling molecules (Fig. 2E and S4C). Our data support the direct regulatory effects of miR-30a-5p on the several overexpressed molecular targets implicated in aberrant signaling of HNSCC.
miR-30a-5p Inhibits Cell Proliferation, Motility and Invasion by HNSCC Cells
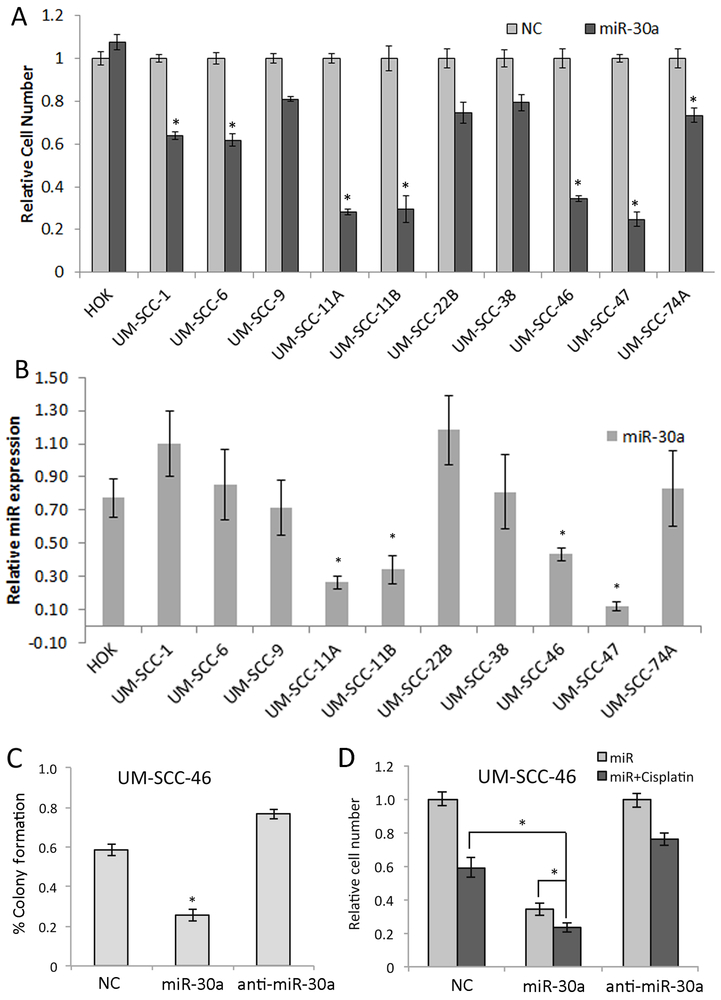
As several of the miR-30a-5p targets identified can modulate cell growth, we next surveyed the anti-proliferative effects of miR-30a-5p and potential correspondence with mRNA expression in normal primary oral keratinocytes (HOK), and a panel of HNSCC cell lines that includes UM-SCC lines derived from 9 primary tumors and 2 paired recurrences. Four HNSCC cells lines (UM-SCC-11A, 11B, 46, 47) treated with miR-30a-5p displayed significantly decreased cell density of <50% when compared to normal controls, while non-malignant HOKs displayed smaller or no inhibitory effects (Fig. 3A). This corresponded with significantly lower expression of miR-30a-5p observed in the HPV(–) lines UM-SCC-11A, B, UM-SCC-46, and the HPV(+) line UM-SCC-47 (Fig. 3B, p<0.05). Both miR-30a-5p and miR-30e-5p were expressed at lower levels in the relatively more responsive UM-SCC-46 line, compared with HOK and the less responsive UM-SCC-1 line (Supplementary Fig. S3B), similar to the pattern of decreased expression in a subset of TCGA tumors relative to normal epithelia. Further, we confirmed that miR30a/e-5p, and other family members sharing the same seed sequence, had similar inhibitory effects on proliferation in these two lines (Supplementary Fig. S3A), further supporting studies using the more abundant miR-30a-5p. Further studies confirmed that miR-30a-5p suppressed proliferation, colony formation, and exhibited additive activity with chemotherapy drug cisplatin used for treatment of HNSCC, in UM-SCC-46 and/or UM-SCC-1 cells (Fig. 3C,D and Supplementary Fig. S5A-D). To confirm the potential of a miR-30a-5p target to partially rescue HNSCC from the anti-proliferative effect of miR-30a-5p, we examined the effect of stably expressing EGFR without its regulatory 3’UTR in UM-SCC-46. Forced EGFR expression significantly attenuated and rescued cells from the anti-proliferative effect of miR-30a-5p (Supplementary Fig. S5E,F).
Fig. 3. A miR-30a-5p mimic inhibited HNSCC cell proliferation, colony formation, and enhanced cisplatin sensitivity in vitro.
(A) Proliferation measured by XTT assay in 6 replicates on day 5 following transfection with control or miR-30a-5p mimic across primary Human Oral kerytinocytes (HOK) and ten HNSCC cell lines. (B) Basal level of miR-30a-5p expression measured by qRT-PCR in HOK and ten HNSCC cell lines in log-growth phase. Relative miR-30a-5p expression was normalized to the mean expression of the cell lines. * denotes p< 0.05 by a Student’s t-test compared to HOK cells. (C) Colony formation assay of UM-SCC-46 cells was performed following 48 h transfection with miR-30a-5p or anti-miR30a-5p oligonucleotides. Colonies were counted in three wells and repeated in three independent experiments. (D) UM-SCC-46 cells were transfected with miR-30a-5p mimic for 48 hrs, and treated with 2µM cisplatin for 3 h and then washed with warm media and cell density was measured by XTT assay 72 h after cisplatin treatment. Values represent the mean of at least three experiments ±SEM, * denotes p< 0.05 by a Student’s t-test.
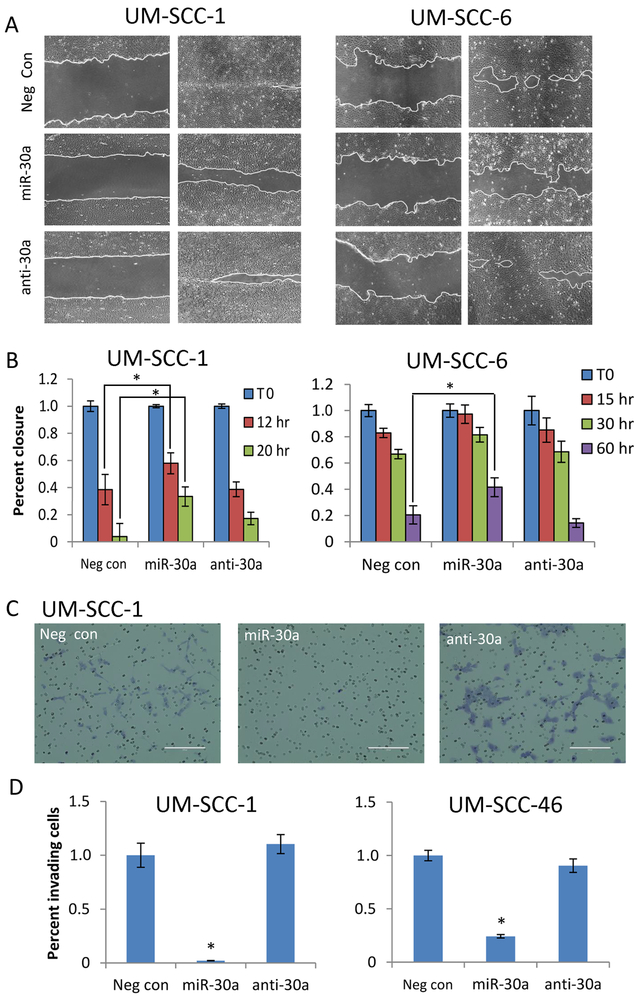
Several of the mir-30–5p family targets in HNSCC are also implicated in cell motility and invasiveness, including EGFR (19), MET (22, 23), ITGA6 (17, 24), and Serpine1 (25). Ectopic expression of miR-30a-5p significantly slowed cell motility in migration assays in two HNSCC cell lines that spread as monolayers and display wound closure in controls (Fig. 4A and B). miR-30a-5p also significantly reduced EGF-stimulated invasiveness in transwell migration assays (Fig. 4C and D). In summary, increased expression of miR-30a-5p significantly inhibited cell proliferation, colony formation, migration, and invasion, as well as enhanced chemo-sensitivity of HNSCC.
Fig. 4. Ectopic expression of miR-30a-5p reduces HNSCC cell migration and invasion.
(A) Representative light microscopy images (100x) for wound closure in a cell migration assay. UM-SCC-1 (left) and UM-SCC-6 cells (right) were transfected with miR-30a-5p or anti-miR-30a-5p oligonucleotides for 48 h before wound scratch creation. Cell migration was observed until wound closure in controls. UM-SCC-1, left, time 0; right, time 20hrs. UM-SCC-6, left, time 0; right, time 60hrs. (B) Cell invasion assay. UM-SCC-1 and UM-SCC-46 cells were transfected with neg control, miR-30a-5p, or anti-miR-30a-5p oligonucleotides for 48 h, then trypsinized and placed in a matrigel coated trans-well migration chambers. 50 ng/mL rEGF was placed in the bottom wells as a chemoattractant. After a 20 h incubation, invasion membranes were fixed and stained to quantify invading cells. (C) Representative light microscopy images (100x) of invasion membranes are shown for UM-SCC-1. (D) Relative quantitation of invading cells for UM-SCC-1 (left) and UM-SCC-46 (right). All data represents the mean of at least three experiments and error bars represent SEM. * denotes p < 0.05 by a Student’s T-test for statistical comparison with either negative control or anti-miR30a.
A Novel miR-30-5p Mimic Delivered by Targeted Nanoparticles Suppresses Tumor Growth
To examine the therapeutic potential of miR-30a-5p in HNSCC in vivo, we formulated a miR-30a-5p mimic with novel modifications into a nanodelivery system (scL) bearing an antibody fragment (TfRscFv) that targets transferrin receptor on tumor cells (26, 27). We confirmed that the scL carrier containing FITC conjugated control oligonucleotide undergoes preferential uptake in HNSCC xenografts, when compared to lung or liver (Fig. 5A). We next examined the therapeutic effect and general systemic toxicity of scL particles complexed with a miR-30a-5p mimic (miR-30a-scL) or with a control miR-scL (60µg or ~3 mg/kg) given in 9 doses intravenously on MWF for 3 weeks in mice bearing UM-SCC-46 xenograft tumors (Fig. 5B-E). A significant tumor growth delay and prolongation of survival was observed with miR-30a-scL treatment (Fig. 5B, C and E). Treatment with miR-30a-scL did not cause any visible toxicity, or significant reduction in weight to indicate decreased intake, mucosal, or general systemic toxicity (Fig. 5C and D). In addition, we tested miR-30a-scL nanoparticles in a second HPV+ HNSCC xenograft model, UM-SCC-47. We observed a similar inhibitory effect on tumor growth in vivo (Fig. 5F). We performed quantitative RT-PCR of six miR-30a-5p target genes and observed substantially decreased gene expression after treatment by four doses of miR-30a-scL nanoparticles (Supplementary Fig. S6A). Confirming miR-30a-5p family’s anti-proliferative effect we also observe a decrease in ki-67 staining (Supplementary Fig. S6B, C). We also observed decreased expression of EGFR and MET by immunofluorescence staining in frozen sections harvested from UM-SCC-46 and 47 xenograft tumors after treatment in vivo (Supplementary Fig. S7A-C). With confirmation both in vitro and in vivo of several target genes of miR-30a-5p, a pathway model predicted by Ingenuity Pathway Analysis was constructed connecting reported interactions and functions of anti-correlated targets identified above in relation to proliferation and migration (Supplementary Fig. S8).
Fig. 5. Anti-tumor activity of miR-30a-5p mimic delivered via nanoparticles in HNSCC xenograft tumors in vivo.
(A) Detection of FITC-labeled-scL in tumor and selected organ tissues. UM-SCC-46 cells were subcutaneously injected into athymic nu/nu female mice, and mice were randomized when tumors reached ~300 mm3. Mice were administered 100 μg (~5 mg/kg) of complexed FITC-labeled control oligonucleotide or control vehicle intravenously (IV). 24 h later, mice were sacrificed for tumor and organ harvest. Dissected organs were then imaged. Left panels, bright field; right panels, fluorescence microscopy, where tissues with FITC nanoparticles are on the left with green fluorescence and control vehicle on the right. (B) Effect of miR-30a-5p mimic on tumor xenogafts. Mice bearing ~150 mm3 UM-SCC-46 xenograft tumors were randomized into groups of 5 animals and injected IV with nine doses of 60 µg (~3 mg/kg) of miR-30a-5p mimic nanoparticles (miR-30a-scL) or control nanoparticles on Monday, Wednesday, and Friday (MWF) for 3 weeks. Mean tumor volume for each group ±SEM; statistically significant size difference between the two groups was achieved by day 16 (p<0.05, student’s t-test). (C) Representative images of tumor size at the end of treatment on day 24. (D) Mouse weight (grams) over course of the experiment. (E) Kaplan-Meier analysis plot showing significant improvement in survival between mice treated with miR-30a-scL vs. control. (F) Mice bearing HPV+ UM-SCC-47 xenograft tumors grown to ~150 mm3, were randomized into groups of 4, and then the mice were injected IV with 60 µg miR-30a-scL or control on an MWF schedule for 25 days. Tissue was collected 36 hrs after the final dose. Mean tumor volume ± SEM, * p < 0.05, student’s T-test on day 25 after tumor implant.
Genetic Alterations of miR-30 Family Members are Associated with Clinical Features of HNSCC
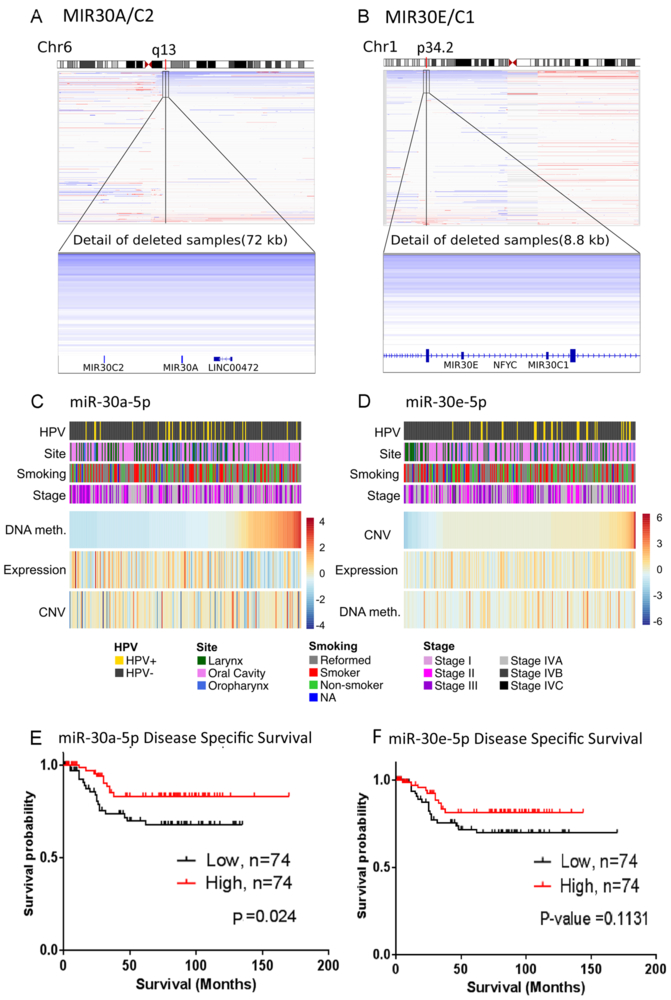
We hypothesized that if loss of expression of miR-30 family members is important in pathogenesis of HNSCC, there may be selective pressure for deletion or epigenetic silencing. We analyzed copy number variation of miR-30 family members from the HNSCC TCGA datasets. For the MIR30A and MIR30C2 genes on chromosome 6, and the MIR30E and MIR30C1 on chromosome 1, 19.7 % and 14.7% display at least heterozygous copy number loss at these genetic loci, with infrequent overlap (Fig. 6A and B). Integrative analysis supported a trend or significant correlation of heterozygous loss with decreased expression for miR-30a-5p (p=0.15, Fig. 6A and C), and miR-30e (p-value=0.0006, Fig. 6B and D). We further analyzed if the broader decreased expression of miR-30a/e observed is associated with methylation of putative promoters, along the MIR30A/C2 promoter and coding regions (Supplementary Table S7). We observed a correlation between increasing DNA methylation of MIR30A promoter and lower expression in a subset of tumor specimens (Spearman correlation = −0.22 and p-value = 0.00057, Fig. 6C). We observed that a high percentage of oral cavity tumors (n=87) display a significant correlation with hypermethylation of CpG sites in the MIR30A promoter, and with reduced miR-30a-5p expression (Fig. 6C; Supplementary Table S8–1, p-values 6.15e-07 and 0.0082, respectively). Reduced expression of miR-30e-5p was significantly correlated with current smoking among a subset of oral cancers (Supplementary Table S8–2, p=0.022), but did not reach significance for oropharyngeal cancers or HNSCC overall. Copy number deletion and reduced expression of miR-30e-5p is correlated with HPV-negative HNSCC (Fig. 6D; Supplementary Table S8, p-values 0.0053 and 0.0001, respectively), including laryngeal subsites.
Fig. 6. Genomic deletion, methylation, and expression of miR-30 family members, and associated clinical features.
DNA copy number variation (CNV) for MIR30A/C2 (A) and MIR30E/C1 (B). Integrated Genome Viewer plots for chromosomes 6 and 1 shown on the top, with CNV for MIR30 genes at 6q13 and 1p34.2 shown on the bottom for 279 HNSCC tumors from TCGA. Blue indicates reduced copy number and red indicates increased copy number. (C and D) Relation of miRNA expression with copy number and methylation for 244 HNSCC samples from TCGA. Samples are ordered by DNA methylation for MIR30A and by CNV for MIR30E. Samples are annotated with clinical characteristics (Site, Smoking and Stage) and HPV status. Discrete gene copy number values are obtained from GISTIC. Clinical features (colored bars, top four rows) and genetic characteristics (heat maps, bottom three rows) are assorted accordingly. Survival analysis for miR-30a-5p (E) and miR-30e-5p (F) segregated into high and low by median expression. Kaplan-Meier plots and log rank test p-values comparing disease specific survival are plotted for low (solid lines) versus high (dashed lines) tumor expression based on a Median cut point. Censored subjects are indicated by vertical hash marks.
Next, we examined if miR-30a/e-5p expression is associated with differences in prognosis. As overall survival (OS) data for TCGA is incomplete and showed no or borderline significance (miR30a-5p, p=0.36; miR-30e-5p, p=0.046; Supplementary Fig. S9A and B), we analyzed disease-specific survival data for miR-30a/e-5p available from a miRNA profiling study of 123 primary HNSCC tumors from patients with over 10 years of clinical follow-up (30). This dataset displays a significant correlation between low miR-30a-5p expression with poorer disease specific survival (p-value 0.024, Fig. 6E), and a similar trend for miR-30e-5p (p-value 0.113, Fig. 6F). A trend towards reduced OS was also observed in the subset of patients that display CN loss of the MIR30E loci, supporting the contribution of genomic copy alteration to decreased miR-30e-5p expression in a subset of tumors (Supplementary Fig. S9B). Strikingly, OS and disease specific survival (DSS) for tumor anatomical sub-sites revealed that low expression of miR-30e-5p is most significantly associated with worse prognosis for oropharyngeal carcinomas in both the TCGA and Einstein datasets (Supplementary Fig. S9B and C). Moreover, patients showing low expression for miR-30e-5p and displaying worse prognosis were both HPV+ and HPV- (Supplementary Table S8), providing a candidate marker for prognosis not previously available for this site that warrants further investigation. These data suggest that reduced miR-30a/e-5p expression may be associated with genetic or epigenetic alterations, HNSCC tumor sub-sites, HPV status, and prognosis, which are of clinical relevance in HNSCC.
Discussion
To identify miRNAs of potential therapeutic importance in HNSCC, we employed an integrated approach that combined structural and functional genomic analyses. We observed that several miR-30 family members display at least a 2-fold decreased expression in >70% of specimens in TCGA, and among a validation set of tumors. While the miR-30 family has not been recognized nor extensively studied in the context of HNSCC, analysis of supplementary data files from previous studies profiling miRNA expression independently support our finding of miR-30–5p repression in HNSCC (15, 28–30). We also observed deregulated expression of several miRNAs that have previously been reported as altered in HNSCC, including increased expression of miR-21–5p (31), miR-31–5p (32), and reduced expression of miR-375 (15, 29, 30) and the miR-100 family (33). Integrating miRNA expression results with a functional anti-proliferation screen in vitro, identified nine miRs that are frequently repressed in tumors and inhibit HNSCC cell proliferation. Strikingly, this analysis also identified four members of the miR-30 family, miR-30a/b/d/e-5p, as well as miR-26a/b-5p, miR-145–5p, miR-375, and miR-338–3p. This overlap in structural and functional analyses identified the miR-30a/e-5p to be of potential biologic and clinical significance in HNSCC.
To identify which mRNAs are targeted by miR-30 in HNSCC and underlie its function as a putative tumor suppressor, we performed linear regression analysis of mRNA expression data against the highest expressed miR-30 family member, miR-30a-5p, across the TCGA dataset. This unexpectedly revealed a network of candidate miR-30a-5p targets of established biologic and clinical importance in HNSCC. Significant among these predicted miR-30a-5p targets are several receptor tyrosine kinase genes EGFR, MET, IGF1R, and the previously validated miR-30b target PDGFRβ (34). These receptors orchestrate growth and migration of HNSCC in response to diverse autocrine or paracrine signal ligands in tumors (19, 23, 35). We also identified other cell signaling receptors such as ITGA6, SERPINE1 and FZD2, which have been implicated in proliferation, adhesion, migration and differentiation of HNSCC both in vitro and in vivo (17, 36, 37).
Interestingly, overexpression of EGFR, MET, ITGA6 and several other of these receptors is reported in HNSCC, but is inadequately explained by regulation via gene amplification or transcriptional activation alone (6, 38). We also show that ectopic miR-30a-5p suppresses multiple mRNAs and proteins implicated in growth signaling networks in cancer, including IRS-1, which potentially enhances activation of PI3K-AKT-mTOR signal pathways downstream of these receptors. Our findings for several of these proteins are consistent with data from a study that used pSILAC to study global changes in protein expression when HeLa cervical squamous cell carcinoma cells were transfected with a miR-30a-5p mimic. Analysis of primary data files (http://psilac.mdc-berlin.de/) indicated that transfection of miR-30a-5p reduced EGFR, MET, IGF1R, ITGA6, and WNT5A protein expression, although these data were not recognized or further validated. Together, our data suggest decreased miR-30a-5p is associated with the overexpression of a diverse set of mRNAs and proteins that are implicated in promoting the malignant phenotype in HNSCC.
We report ectopic expression of miR-30a-5p reduces proliferation and colony formation in HNSCC cell lines in vitro. Regulation of these targets both in vitro and in vivo likely underlies this effect. This is supported by partial rescue of miR-30a-5p anti-proliferative effect by stable over-expression of EGFR. Prior TCGA analysis suggested that clusters with lower miR-30a/e also display higher EMT scores and are associated with mesenchymal and basal mRNA clusters. RTK signaling through EGFR (18, 39) and MET (22, 23) have been implicated in an aggressive migratory phenotype in HNSCC. Here we show that miR-30a-5p slows HNSCC cell migration and suppresses EGF-induced invasion in vitro. miR-30a-5p target ITGA6 encodes the integrin α6 subunit, which we previously established as a marker of poor prognosis, and mediator of adhesion and migration in HNSCC (17). SERPINE1 is downstream of TGF-β signaling and has been implicated in an aggressive migratory phenotype in HNSCC (40). Others have reported that miR-30 family members can inhibit additional molecules that mediate cell migration and invasion in other cancers, including MMP19 in NSCLC (41), and VIM and Metadherin in breast cancer (42, 43). Furthermore, repression of miR-30e-5p has been shown to induce EMT in pancreatic cell development (44). As our studies were limited to effects on migration, we believe further in vivo studies are needed to examine the role and potential therapeutic significance of miR-30 in metastatic models of HNSCC. In conjunction with our observation of miR-30a-5p targeting of WNT7B and FRZD2 in HNSCC, miR-30 family members have previously been shown to repress WNT pathway activation through regulation of BCL9 in multiple myeloma cells (45). We believe that interaction of miR-30 with the WNT pathway regulation of cancer stemness deserves further study.
The significant response and prolonged survival that we report for treatment of HNSCC xenograft tumor bearing mice by IV administration of nanocomplex containing a therapeutic miR-30a-5p mimic validates our findings and highlights is potential in cancer therapy. The broader significance and therapeutic potential of our novel nanomedicine is supported by previous experimental studies reporting inhibitory activity of miR-30 in other cancer types. Stable overexpression of miR-30 reduced tumor take and slowed tumor growth in breast cancer models through targeting UBC9, ITGA3 (46) and AVEN (47), and prostate cancer models through targeting the TMPRSS2-ERG fusion gene (48). In multiple myeloma, interparental administration of liposomal miR-30c-5p mimic displayed pre-clinical anti-cancer activity (45).
The potential of miRNA-based therapeutics to simultaneously target multiple mRNAs could help mitigate intrinsic or acquired resistance that has been observed using small molecule or biologic therapies that target a single oncogene or pathway in cancer. Interestingly, resistance to EGFR signal inhibition in HNSCC has been shown to involve overexpression and/or co-activation of other growth factor receptor tyrosine kinases (RTKs), such as the oncogene c-MET, IGFR1 (22, 23), and IRS-1, shown here to be deregulated targets of miR-30. These and other signal molecules can cross-activate the mitogen activated protein kinase (MAPK), phosphatidyl-inositol 3-kinase (PI3K), and Signal and Transcription Factor (STAT) pathways, to promote cancer cell growth, survival, and therapeutic resistance. Although these miR-30 family targets are also transcriptionally regulated and incompletely inhibited by miR-30a-5p alone, our findings support the capability of miR-30a-5p to inhibit the expression of key growth signaling molecules, phosphorylation of signal network components, and phenotypic features upon which they converge. miR-30a-5p may hold greatest potential for activity when combined with inhibitors targeting these pathways, downstream transcription factors, and with standard chemo and radiation therapy.
Of the miR-30 family members we found, miR-30a-5p and miR-30e-5p are the most highly expressed in normal specimens and most frequently repressed in tumors. To understand mechanisms that drive decreased expression, we analyzed copy number variation and DNA methylation results from TCGA. ~20% tumors exhibited genetic deletion of MIR30A/C2 on chromosome 6, which has been reported as a significantly deleted site in HNSCC (6), while ~15% tumors exhibited deletion of MIR30E/C1 on chromosome 1p, another region of recurrent copy number alterations in HNSCC. In addition, increased DNA methylation at the MIR30A/MIR30C promoter was significantly correlated with decreased expression in ~24 % of samples. The observed deletion and methylation of MIR30A/E genes suggests selective pressure for their loss by genetic or epigenetic mechanisms. Further, a subset of samples that display reduced expression were not deleted or methylated, suggesting that other transcriptional mechanism(s) may underlie repression. Indeed, previous studies have implicated EGF/SRC signaling (48), and TGFβ signaling (49) in repression of miR-30 family member expression, suggesting these mechanisms deserve further study in HNSCC.
The association we identify between low expression of miR-30a/e-5p and sensitivity to a mir-30a-5p mimic in vitro raises the possibility of identifying patients who are likely to respond to miR-30a-5p therapy. Furthermore, the observation that hypermethylated or deleted MIR30A and MIR30E are associated with differences in tobacco or HPV-related sub-sites and prognosis in HNSCC, supports further investigation of these alterations as potential biomarkers for loss of expression and identification of patients that may benefit from treatment with a miR-30 family based therapy. The prognostic association we identified between MIR30E deletion and poor survival in oropharyngeal cancer patients the TCGA data further supports this possibility. In a previous report, the prognostic value of low miR-30 family expression predicted low survival and resistance to chemotherapy in breast cancer (50). Taken as a whole, our results support the role of the miR-30 family as a tumor suppressor and validate its further study as a therapeutic agent for cancer and HNSCC.
Supplementary Material
Translational Relevance: Through an integrated genomic profiling and functional drug screening approach, we successfully identified microRNA mimics as potential therapeutics for cancer. This analysis identified the miR-30 family as tumor suppressors in HNSCC, and a previously undefined network of mRNA targets that underlie its function. It also identified the subset of patients with genomic deletion, or methylation that correlate with repression of miR-30a/e-5p expression and prognostic outcome, who could most likely to benefit from treatment with miR-30-5p replacement therapy. We have demonstrated the proof of concept for a therapeutic miR-30-5p mimic-based nanomedicine in xenograft models of HNSCC with no observable toxic side effects.
Acknowledgments
This study is supported by NIDCD intramural Project nos. ZIADC000016, 73 and 74 (C. Van Waes), and Extramural grant NCI P30 CA006973 (L. Danilova). Con miR-scL and miR-30a-scL were provided by SynerGene Therapeutics. We thank Dr. Jianhong Chen and Clint Allen for reading of the manuscript.
Footnotes
Conflict of interest: Dr. Saleh is a shareholder in miRecule Inc., Dr. Chang and Dr. Rait are shareholders in SynerGene Therapeutics.
References
- 1.Stahlhut C, Slack FJ. MicroRNAs and the cancer phenotype: profiling, signatures and clinical implications. Genome Med 2013;5(12):111. Epub 2014/01/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Rooij E, Purcell AL, Levin AA. Developing microRNA therapeutics. Circ Res 2012;110(3):496–507. Epub 2012/02/04. [DOI] [PubMed] [Google Scholar]
- 3.Sethi N, Wright A, Wood H, Rabbitts P. MicroRNAs and head and neck cancer: reviewing the first decade of research. Eur J Cancer. 2014;50(15):2619–35. Epub 2014/08/12. [DOI] [PubMed] [Google Scholar]
- 4.Yan B, Li H, Yang X, Shao J, Jang M, Guan D, et al. Unraveling regulatory programs for NF-kappaB, p53 and microRNAs in head and neck squamous cell carcinoma. PLoS One. 2013;8(9):e73656. Epub 2013/09/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yan B, Yang X, Lee TL, Friedman J, Tang J, Van Waes C, et al. Genome-wide identification of novel expression signatures reveal distinct patterns and prevalence of binding motifs for p53, nuclear factor-kappaB and other signal transcription factors in head and neck squamous cell carcinoma. Genome Biol 2007;8(5):R78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cancer Genome Atlas N. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature. 2015;517(7536):576–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoadley KA, Yau C, Wolf DM, Cherniack AD, Tamborero D, Ng S, et al. Multiplatform analysis of 12 cancer types reveals molecular classification within and across tissues of origin. Cell. 2014;158(4):929–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lindenbergh-van der Plas M, Martens-de Kemp SR, de Maaker M, van Wieringen WN, Ylstra B, Agami R, et al. Identification of lethal microRNAs specific for head and neck cancer. Clin Cancer Res 2013;19(20):5647–57. Epub 2013/08/15. [DOI] [PubMed] [Google Scholar]
- 9.HogenEsch H, Nikitin AY. Challenges in pre-clinical testing of anti-cancer drugs in cell culture and in animal models. J Control Release. 2012;164(2):183–6. Epub 2012/03/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang D, Sun Y, Hu L, Zheng H, Ji P, Pecot CV, et al. Integrated analyses identify a master microRNA regulatory network for the mesenchymal subtype in serous ovarian cancer. Cancer Cell. 2013;23(2):186–99. Epub 2013/02/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li B, Dewey CN. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics. 2011;12:323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chung N, Zhang XD, Kreamer A, Locco L, Kuan PF, Bartz S, et al. Median absolute deviation to improve hit selection for genome-scale RNAi screens. J Biomol Screen. 2008;13(2):149–58. Epub 2008/01/25. [DOI] [PubMed] [Google Scholar]
- 13.Yu W, Pirollo KF, Yu B, Rait A, Xiang L, Huang W, et al. Enhanced transfection efficiency of a systemically delivered tumor-targeting immunolipoplex by inclusion of a pH-sensitive histidylated oligolysine peptide. Nucleic Acids Res 2004;32(5):e48. Epub 2004/03/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Council NR. Guide for the Care and Use of Laboratory Animals. Washington, DC: The National Academies Press; 1996. 140 p. [PubMed] [Google Scholar]
- 15.Harris T, Jimenez L, Kawachi N, Fan JB, Chen J, Belbin T, et al. Low-level expression of miR-375 correlates with poor outcome and metastasis while altering the invasive properties of head and neck squamous cell carcinomas. Am J Pathol. 2012;180(3):917–28. Epub 2012/01/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Therneau TM. A Package for Survival Analysis in S, R package version 2.38. R package version 2.38; 2015; Available from: http://CRAN.R-project.org/package=survival.
- 17.Van Waes C, Surh DM, Chen Z, Kirby M, Rhim JS, Brager R, et al. Increase in suprabasilar integrin adhesion molecule expression in human epidermal neoplasms accompanies increased proliferation occurring with immortalization and tumor progression. Cancer Res 1995;55(22):5434–44. Epub 1995/11/15. [PubMed] [Google Scholar]
- 18.Van Waes C, Allen CT, Citrin D, Gius D, Colevas AD, Harold NA, et al. Molecular and clinical responses in a pilot study of gefitinib with paclitaxel and radiation in locally advanced head-and-neck cancer. Int J Radiat Oncol Biol Phys 2010;77(2):447–54. Epub 2009/11/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Freudlsperger C, Burnett JR, Friedman JA, Kannabiran VR, Chen Z, Van Waes C. EGFR-PI3K-AKT-mTOR signaling in head and neck squamous cell carcinomas: attractive targets for molecular-oriented therapy. Expert Opin Ther Targets. 2011;15(1):63–74. Epub 2010/11/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Egloff AM, Grandis JR. Targeting epidermal growth factor receptor and SRC pathways in head and neck cancer. Semin Oncol 2008;35(3):286–97. Epub 2008/06/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mali SB. Review of STAT3 (Signal Transducers and Activators of Transcription) in head and neck cancer. Oral Oncol 2015;51(6):565–9. Epub 2015/03/31. [DOI] [PubMed] [Google Scholar]
- 22.Dong G, Chen Z, Li ZY, Yeh NT, Bancroft CC, Van Waes C. Hepatocyte growth factor/scatter factor-induced activation of MEK and PI3K signal pathways contributes to expression of proangiogenic cytokines interleukin-8 and vascular endothelial growth factor in head and neck squamous cell carcinoma. Cancer Res 2001;61(15):5911–8. Epub 2001/08/02. [PubMed] [Google Scholar]
- 23.Dong G, Lee TL, Yeh NT, Geoghegan J, Van Waes C, Chen Z. Metastatic squamous cell carcinoma cells that overexpress c-Met exhibit enhanced angiogenesis factor expression, scattering and metastasis in response to hepatocyte growth factor. Oncogene. 2004;23(37):6199–208. Epub 2004/06/29. [DOI] [PubMed] [Google Scholar]
- 24.Carey TE, Nair TS, Chern C, Liebert M, Grossman HB, Wolf GT, et al. Blood group antigens and integrins as biomarkers in head and neck cancer: is aberrant tyrosine phosphorylation the cause of altered alpha 6 beta 4 integrin expression? J Cell Biochem Suppl 1993;17F:223–32. Epub 1993/01/01. [DOI] [PubMed] [Google Scholar]
- 25.Karbiener M, Neuhold C, Opriessnig P, Prokesch A, Bogner-Strauss JG, Scheideler M. MicroRNA-30c promotes human adipocyte differentiation and co-represses PAI-1 and ALK2. RNA Biol 2011;8(5):850–60. Epub 2011/09/01. [DOI] [PubMed] [Google Scholar]
- 26.Pirollo KF, Zon G, Rait A, Zhou Q, Yu W, Hogrefe R, et al. Tumor-targeting nanoimmunoliposome complex for short interfering RNA delivery. Hum Gene Ther 2006;17(1):117–24. Epub 2006/01/18. [DOI] [PubMed] [Google Scholar]
- 27.Pirollo KF, Chang EH. Targeted delivery of small interfering RNA: approaching effective cancer therapies. Cancer Res 2008;68(5):1247–50. Epub 2008/03/05. [DOI] [PubMed] [Google Scholar]
- 28.Childs G, Fazzari M, Kung G, Kawachi N, Brandwein-Gensler M, McLemore M, et al. Low-level expression of microRNAs let-7d and miR-205 are prognostic markers of head and neck squamous cell carcinoma. Am J Pathol 2009;174(3):736–45. Epub 2009/01/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kong KL, Kwong DL, Chan TH, Law SY, Chen L, Li Y, et al. MicroRNA-375 inhibits tumour growth and metastasis in oesophageal squamous cell carcinoma through repressing insulin-like growth factor 1 receptor. Gut 2012;61(1):33–42. Epub 2011/08/05. [DOI] [PubMed] [Google Scholar]
- 30.Hui AB, Lenarduzzi M, Krushel T, Waldron L, Pintilie M, Shi W, et al. Comprehensive MicroRNA profiling for head and neck squamous cell carcinomas. Clin Cancer Res 2010;16(4):1129–39. Epub 2010/02/11. [DOI] [PubMed] [Google Scholar]
- 31.Bourguignon LY, Earle C, Wong G, Spevak CC, Krueger K. Stem cell marker (Nanog) and Stat-3 signaling promote MicroRNA-21 expression and chemoresistance in hyaluronan/CD44-activated head and neck squamous cell carcinoma cells. Oncogene 2012;31(2):149–60. Epub 2011/06/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu CJ, Tsai MM, Hung PS, Kao SY, Liu TY, Wu KJ, et al. miR-31 ablates expression of the HIF regulatory factor FIH to activate the HIF pathway in head and neck carcinoma. Cancer Res 2010;70(4):1635–44. Epub 2010/02/11. [DOI] [PubMed] [Google Scholar]
- 33.Chen Z, Jin Y, Yu D, Wang A, Mahjabeen I, Wang C, et al. Down-regulation of the microRNA-99 family members in head and neck squamous cell carcinoma. Oral Oncol 2012;48(8):686–91. Epub 2012/03/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tanaka S, Suto A, Ikeda K, Sanayama Y, Nakagomi D, Iwamoto T, et al. Alteration of circulating miRNAs in SSc: miR-30b regulates the expression of PDGF receptor beta. Rheumatology (Oxford). 2013;52(11):1963–72. Epub 2013/07/31. [DOI] [PubMed] [Google Scholar]
- 35.Schmitz S, Kaminsky-Forrett MC, Henry S, Zanetta S, Geoffrois L, Bompas E, et al. Phase II study of figitumumab in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck: clinical activity and molecular response (GORTEC 2008–02). Ann Oncol 2012;23(8):2153–61. Epub 2012/01/12. [DOI] [PubMed] [Google Scholar]
- 36.Zhang E, Li Z, Xu Z, Duan W, Sun C, Lu L. Frizzled2 mediates the migration and invasion of human oral squamous cell carcinoma cells through the regulation of the signal transducer and activator of transcription-3 signaling pathway. Oncol Rep 2015;34(6):3061–7. Epub 2015/09/24. [DOI] [PubMed] [Google Scholar]
- 37.Freytag J, Wilkins-Port CE, Higgins CE, Carlson JA, Noel A, Foidart JM, et al. PAI-1 Regulates the Invasive Phenotype in Human Cutaneous Squamous Cell Carcinoma. J Oncol 2009;2009:963209. Epub 2009/01/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.He Y, Zeng Q, Drenning SD, Melhem MF, Tweardy DJ, Huang L, et al. Inhibition of human squamous cell carcinoma growth in vivo by epidermal growth factor receptor antisense RNA transcribed from the U6 promoter. J Natl Cancer Inst 1998;90(14):1080–7. Epub 1998/07/22. [DOI] [PubMed] [Google Scholar]
- 39.Pectasides E, Rampias T, Sasaki C, Perisanidis C, Kouloulias V, Burtness B, et al. Markers of epithelial to mesenchymal transition in association with survival in head and neck squamous cell carcinoma (HNSCC). PLoS One. 2014;9(4):e94273. Epub 2014/04/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pavon MA, Arroyo-Solera I, Tellez-Gabriel M, Leon X, Viros D, Lopez M, et al. Enhanced cell migration and apoptosis resistance may underlie the association between high SERPINE1 expression and poor outcome in head and neck carcinoma patients. Oncotarget 2015;6(30):29016–33. Epub 2015/09/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu G, Herazo-Maya JD, Nukui T, Romkes M, Parwani A, Juan-Guardela BM, et al. Matrix metalloproteinase-19 promotes metastatic behavior in vitro and is associated with increased mortality in non-small cell lung cancer. Am J Respir Crit Care Med 2014;190(7):780–90. Epub 2014/09/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang N, Wang X, Huo Q, Sun M, Cai C, Liu Z, et al. MicroRNA-30a suppresses breast tumor growth and metastasis by targeting metadherin. Oncogene 2014;33(24):3119–28. Epub 2013/07/16. [DOI] [PubMed] [Google Scholar]
- 43.Cheng CW, Wang HW, Chang CW, Chu HW, Chen CY, Yu JC, et al. MicroRNA-30a inhibits cell migration and invasion by downregulating vimentin expression and is a potential prognostic marker in breast cancer. Breast Cancer Res Treat. 2012;134(3):1081–93. Epub 2012/04/06. [DOI] [PubMed] [Google Scholar]
- 44.Joglekar MV, Patil D, Joglekar VM, Rao GV, Reddy DN, Mitnala S, et al. The miR-30 family microRNAs confer epithelial phenotype to human pancreatic cells. Islets. 2009;1(2):137–47. Epub 2009/09/01. [DOI] [PubMed] [Google Scholar]
- 45.Zhao JJ, Lin J, Zhu D, Wang X, Brooks D, Chen M, et al. miR-30–5p functions as a tumor suppressor and novel therapeutic tool by targeting the oncogenic Wnt/beta-catenin/BCL9 pathway. Cancer Res 2014;74(6):1801–13. Epub 2014/03/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yu F, Deng H, Yao H, Liu Q, Su F, Song E. Mir-30 reduction maintains self-renewal and inhibits apoptosis in breast tumor-initiating cells. Oncogene 2010;29(29):4194–204. Epub 2010/05/26. [DOI] [PubMed] [Google Scholar]
- 47.Ouzounova M, Vuong T, Ancey PB, Ferrand M, Durand G, Le-Calvez Kelm F, et al. MicroRNA miR-30 family regulates non-attachment growth of breast cancer cells. BMC Genomics. 2013;14:139. Epub 2013/03/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kao CJ, Martiniez A, Shi XB, Yang J, Evans CP, Dobi A, et al. miR-30 as a tumor suppressor connects EGF/Src signal to ERG and EMT. Oncogene 2014;33(19):2495–503. Epub 2013/06/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu L, Lin W, Zhang Q, Cao W, Liu Z. TGF-beta induces miR-30d down-regulation and podocyte injury through Smad2/3 and HDAC3-associated transcriptional repression. J Mol Med (Berl). 2016;94(3):291–300. Epub 2015/10/04. [DOI] [PubMed] [Google Scholar]
- 50.Bockhorn J, Dalton R, Nwachukwu C, Huang S, Prat A, Yee K, et al. MicroRNA-30c inhibits human breast tumour chemotherapy resistance by regulating TWF1 and IL-11. Nat Commun 2013;4:1393. Epub 2013/01/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.