Abstract
Delivering ions and molecules into living cells has become an important challenge in medical and biological fields. Conventional molecular delivery, however, has several issues such as physical and chemical damage to biological cells. Here, we present a method of directly delivering molecules into adhesive cells with an Au-based nanostraw membrane stamp that can physically inject a target molecule into the cytoplasm through a nanostraw duct. We successfully delivered calcein target molecules into adhesive cells with high efficiency (85%) and viability (90%). Furthermore, we modeled the molecular flow through Au nanostraws and then demonstrated the control of calcein flow by changing the concentration and geometry of Au nanostraws. Our Au membrane stamping provides a new way of accessing the cytoplasm to modulate cellular functions via injected molecules.
Subject terms: Permeation and transport, Electrophysiology, Nanoscale materials
Introduction
Gaining access to the cytoplasm of cells is now a significant challenge in biological research to analyze and modulate cellular functions1. Examples include gene injection to create induced pluripotent stem (iPS) cells2 or the inhibition of gene expression inside cells, which is called as RNA interference3.
A key challenge for intracellular access is to overcome the physical barrier of the outer membrane of cells without losing cellular functions4. Such research efforts have been classified as follows: (1) carrier-based delivery using viral vectors5, vesicles6, and nanocarriers including lipids7 and inorganic materials8 and (2) membrane-disruption delivery via mechanical9,10, electrical11, thermal12, and optical13 means. Each of these methods has specific advantages and disadvantages in terms of efficiency, invasiveness, safety, dosage control, and cost4. Alternatively, direct delivery using a solid nanoconduit is a noteworthy technique to deliver/extract molecules into/from living cells14. Carbon nanotube-15 and glass nanopipette-based endoscopes16 have also provided methods for minimally invasive insertion into cells, and controlled the flow of delivery and extraction of Ca2+ 17, proteins2, and mRNAs18. Examples of this using many nanomaterials have been reported, including vertical Si nanowires19, Si nanopillars20, Si3N411,21, and Au nanoelectrode9. Moreover, aluminum nanostraws14, which are arranged in a polycarbonate membrane, have been used as a powerful tool for delivering therapeutic DNA22 or drugs23 with improved transfection efficiency. However, even with the use of specific nanomaterials, molecular delivery into cells is difficult to control; therefore, this approach has been combined with other techniques such as electroporation24, optical stimulation25, and microfluidics14. In addition to these recent efforts focusing on molecular delivery using specific nanomaterials combination methods, we have developed a method of mechanical Au nanostraw membrane stamping with a height-adjustable stage controller (Fig. 1). Here, we investigate the optimal diameter and length of such Au nanostraws to penetrate and inject molecules into cells, and also evaluate the rate of delivery into adhesive cells and cellular viability. Furthermore we demonstrate the delivery using two types of molecules (small molecule: calcein dye (622.55 MW) and large molecule: fluorescein (FAM) labeled oligo DNA (6603.51 MW)) into two types of adhesive cells (NIH3T3 fibroblast and HeLa cancer cells) with high efficiency and viability. This is a simple and novel method compared with prior works using aluminum nanostraws26, Si27 and carbon nanotube28 nanomaterials that allows for direct molecular delivery through the nanostraws inserted mechanically into the cellular membrane.
Figure 1.
Nanostraw membrane stamping for direct intracellular molecular delivery.
Results
Au nanostraw membrane stamp
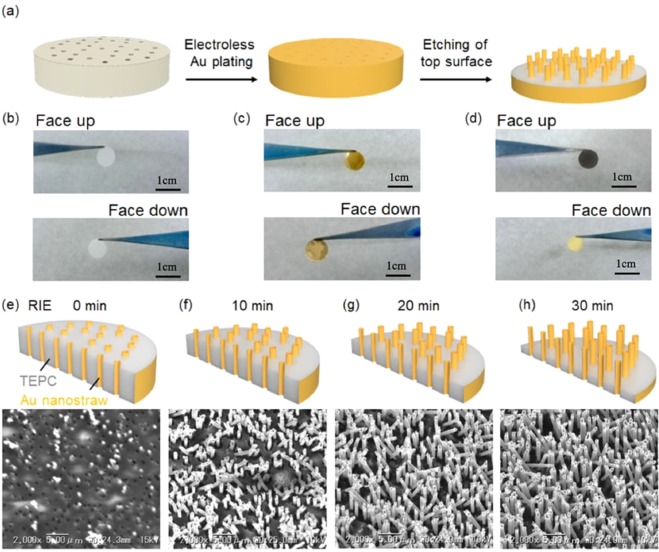
To make Au nanostraw membranes, we investigated electroless Au plating on track-etched polycarbonate (TEPC) templates and subsequent etching of the top surface (Fig. 2a). Electroless deposition of an Au nanolayer onto a porous membrane is a well-established plating process that consists of four steps: (1) sensitization, (2) activation, (3) displacement deposition, and (4) electroless deposition (Fig. S1a). To activate the surface of TEPC membranes (pore diameter: 600 nm, thickness: 23 µm), we immersed the TEPC membrane in 10.55 mM SnCl2 solution at 24 °C and then in 11.28 mM PdCl2 solution at 24 °C. After washing the membrane with water, the tin–palladium metallic layer was formed on the TEPC surface, and could be used as a catalyst for electroless gold plating. The amount of metallic catalyst on the TEPC surface was controlled by adjusting the immersion time and the number of activation cycles (Fig. S1b). After that, we immersed the catalyst-coated TEPC membrane in 2 g/L gold plating solution at 40 °C for 24 h. After coating Au nanolayer on the membrane, the original white color of the TEPC membrane (Fig. 2b) changed to gold on both the top and the bottom surfaces (Fig. 2c). Furthermore, we investigated the cross section of Au/TEPC membranes using a scanning electron microscope (SEM) to confirm that Au intrananotubes had formed after an immersion time of 5 min for one to four cycles (Fig. S1c-f, respectively). We confirmed the presence of Au intrananotubes in the TEPC membrane after more than three cycles (Fig. S1e,f) due to a sufficient metallic catalyst coating and subsequent gold plating onto the TEPC pore surface. When we performed immersion in SnCl2 and PdCl2 solutions for a longer time (10 and 20 min), we successfully formed Au intrananotubes upon treatment for two cycles. Eventually, we created a process map for electroless Au plating onto 600-nm-pore TEPC membranes (Fig. S1b).
Figure 2.
Nanostraw membrane fabrication. (a) Two steps for the nanostraw fabrication: (1) electroless Au plating to track-etched polycarbonate (TEPC) membrane and (2) etching of top surface. (b–d) Optical images of 8-mm-diameter samples at different stages of nanostraw fabrication. TEPC membrane included size-controlled pores. In our experiments, 0.4-, 0.6-, and 1.0-μm-diameter pores were used. (b) Images of the original TEPC membrane facing up and down. (c) Au is plated on the TEPC membrane surfaces as well as the pore surfaces. (d) Top surface of Au membrane was etched by two steps: (1) Au surface etching with aqua regia, including nitric acid and hydrochloric acid, and (2) PC surface etching with O2 RIE. (e–h) The Au/TEPC nanostraws after RIE at 0 (e), 10 (f), 20 (g), and 30 min (h).
After forming the Au/TEPC membrane, we performed etching of only the top surface of the Au deposition nanolayer with aqua regia, which was mixed with nitric acid and hydrochloric acid at a molar ratio of 1:3, to expose both the polycarbonate and the internal Au nanoducts on the membrane surface (Fig. 2a,e). After wet etching, we observed a change of color from gold to brown only on the etched surface (Fig. 2d). Furthermore, we confirmed the outer and inner diameters of the exposed Au nanotubes ( = 600 nm ± 90 nm, = 400 nm ± 50 nm, needle density n = 4 × 107) using SEM (Fig. 2e). We summarize the geometry of Au nanotubes on TEPC membranes with different pore sizes (400, 600, and 1000 nm) in Table S1. From these data, a 100 ± 30 nm Au layer was formed on the TEPC membrane upon immersion in gold plating solution for 24 h. Additional etching of exposed TEPC with O2 plasma produces hollow Au nanoneedles, which we refer to as nanostraws. The height (H) of Au nanostraws was controlled by adjusting the O2 plasma exposure time: as 0 min (Fig. 2e, H: 0 µm), 10 min (Fig. 2f, H: 1.3 µm), 20 min (Fig. 2g, H: 2.4 µm), or 30 min (Fig. 2h, H: 5.0 µm). Since the exposed TEPC membrane was damaged by activated O2 molecules in plasma, we could not perform O2 exposure for longer than 40 min to make free-standing Au nanostraws/TEPC membranes.
Molecular injection into cells
To calibrate the molecular flow through the Au nanostraw/TEPC membrane, we first investigated the injection flux of calcein molecules from a calcein-containing source chamber to the target chamber of the stirred phosphate buffer solution (PBS) (Fig. 3a). We made a needle-type source chamber consisting of an 8-mm-diameter membrane including Au nanostraws (outer diameter: 400 ± 50 nm, inner diameter r: 200 ± 30 nm), a glass tube (outer diameter: 8 mm, inner diameter R: 6 mm), and 10 mM PBS (pH 7) including target calcein molecules at different concentrations. When we contacted the needle source chamber with the stirring PBS solution in the collection target chamber, the calcein diffused from a high concentration in the source chamber to the target chamber via the Au nanostraw duct. We measured the amount of transported calcein in the target chamber using a microplate reader. This calcein flux J through the nanostraw membrane is defined as follows29:
| 1 |
where D is the diffusion coefficient, C is the calcein concentration, πr2 is the inner area of Au nanostraw, n is number of Au nanostraws on membrane area (πR2), πR2 is the membrane area, and l is the thickness of the membrane.
Figure 3.
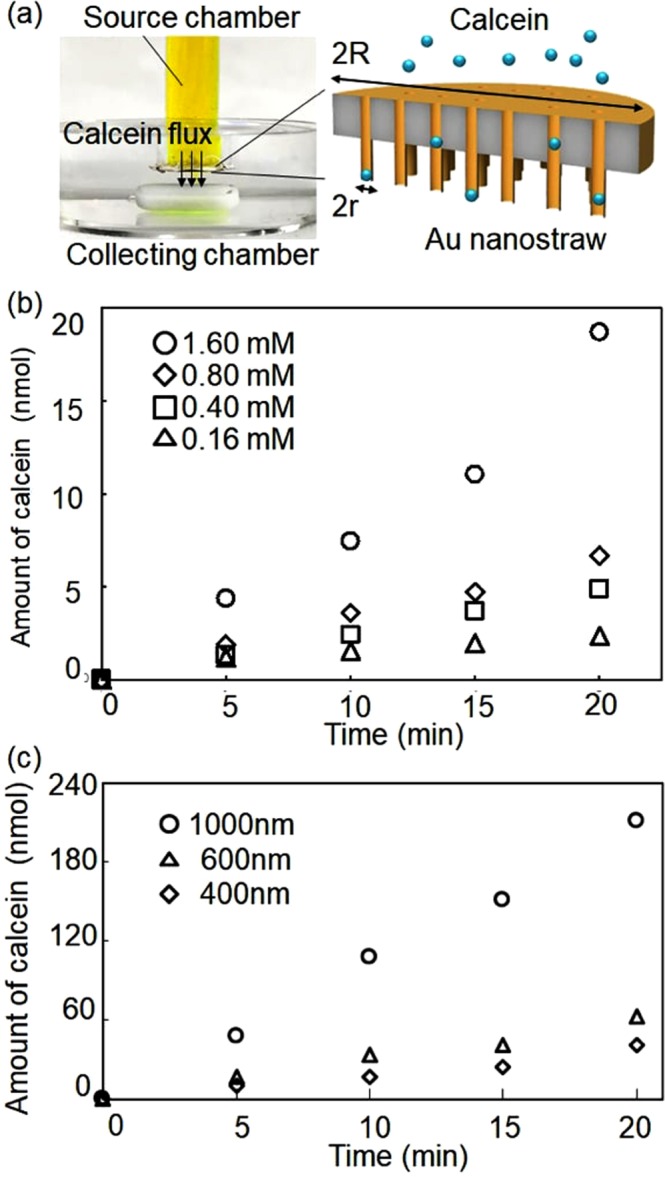
Calcein flow through Au nanostraw/TEPC membrane. (a) Photograph and schematic of calcein flow from the source chamber to the target chamber via Au nanostraws/TEPC membrane. (b) Amount of transported calcein at the different concentrations of calcein in the source chamber. (c) Amount of transported calcein with the different diameter Au nanostraws/TEPC at 1.6 mM calcein concentration.
According to equation (1), the calcein flows through the Au nanostraw membrane as a function of C and πr2. When we used the Au nanostraws (400-nm diameter)/TEPC membrane with 0.16 mM calcein solution, the amount of transported calcein in the target chamber increased linearly up to 2.3 nmol for 20 min (Fig. 3b), resulting in calcein flux J of 0.3 nmol s−1 cm−2. J was enhanced to 0.7 nmol s−1 cm−2 by increasing the calcein concentration to 0.4 mM, 1.1 nmol s−1 cm−2 when increasing it to 0.8 mM, and 2.3 nmol s−1 cm−2 when increasing it to 1.6 mM. Further improvement was achieved by modifying the inner area of nanostraws (Fig. 3c). J was 1.4 times greater (3.0 nmol s−1 cm−2) with Au nanostraws (600-nm diameter)/TEPC membrane, and 2.3 times greater (5.1 nmol s−1 cm−2) with Au nanostraws (1000-nm diameter)/TEPC membrane than the J obtained with the Au nanostraws (400-nm diameter)/TEPC membrane. These results indicate that the amount of delivered calcein target molecules can be controlled by adjusting C and πr2.
Similar to micro/nanoneedle intracellular delivery systems, our Au nanostraw membrane injection system requires precise x-y-z manipulation. To this end, we combined an optical microscope, including an x-y stage with an Au nanostraw membrane stamp moving manually in the z-axis with 1-µm step accuracy (Fig. 4a). The Au nanostraw membrane stamp consists of an 8-mm-diameter membrane including Au nanostraws with a 600-nm outer diameter and different heights (0, 1.3, 2.4, and 5.0 µm), a glass tube (outer diameter: 8 mm, inner diameter: 6 mm), and 1.6 mM calcein dye in 10 mM PBS at pH 7. As an adhesive cell, we used NIH-3T3 fibroblast cells cultured on a plastic Petri dish filled with Dulbecco’s modified Eagle medium at 37 °C in 5% CO2 for 2–3 days. Before calcein injection, we confirmed 80–100% confluency of the NIH-3T3 cells with a confocal microscope and then stamped the Au nanostraw membrane including calcein dye onto adhesive cells (Fig. 4b). After the stamping, we observed optical (Fig. 4c) and fluorescence (Fig. 4d) images focused on the region where stamped and unstamped areas met. From these images, we clearly confirmed the presence of stained cells only in the stamped area. In control experiments of the cultured cells exposed to 1.6 mM calcein medium for 10 min (Fig. S2), we did not observe any stained cells. These results indicate that calcein molecules can be delivered through Au nanostraws.
Figure 4.
Calcein delivery into NIH-3T3 cells with Au nanostraw stamping. (a) Photograph of Au nanostraw stamping system for precise x-y-z manipulation. (b) Schematic illustration of stamping area using Au nanostraw membrane on the cell-cultured dish. (c,d) Optical (c) and fluorescence (d) images at the edge of stamped and unstamped areas.
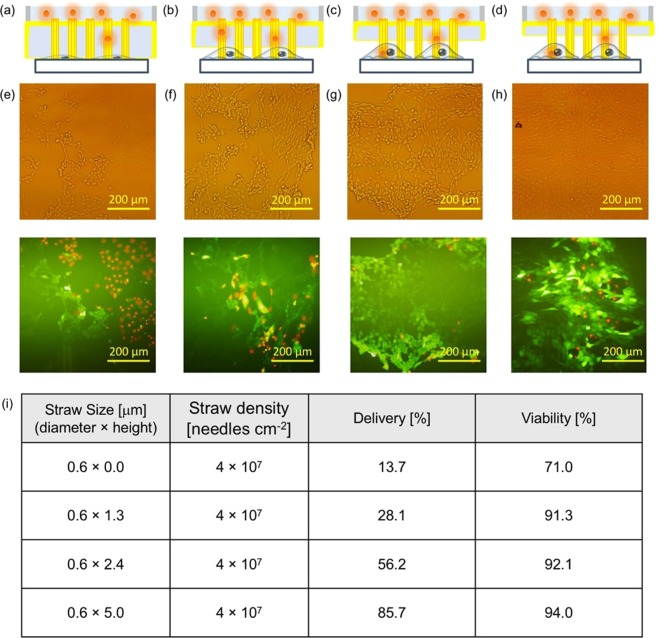
To confirm the feasibility of intracellular molecular delivery using Au nanostraw stamping, we investigated the viability (%) and delivery (%) after stamping with Au nanostraw membranes of different heights (Fig. 5). The viability and the delivery are defined as follows: Viability = live cells/live and dead cells and Delivery = live cells/total cells, where live cells are the number of calcein-stained cells, dead cells are the number of propidium iodide (PI)-stained cells, and total cells are the number of optically observed cells. Since calcein is a membrane-impermeable dye, the cells were not stained in calcein-containing medium (Fig. S2). Instead, we could inject a calcein dye into the cells through the Au nanostraw duct. In general, such Au nanostraw stamping leads to the generation of insertion holes in the cellular membrane that are repaired rapidly in healthy cells, resulting in the injected calcein being trapped inside the cells, which we refer to as live cells. In contrast, when the cells are damaged, the membrane holes remain until cell death, resulting in the binding of PI to intracellular DNA, which we refer to as dead cells. After stamping with Au nanostraws, the stamped cells retained high viability of over 90% with Au nanostraws of different heights of 1.3, 2.4, and 5.0 µm (Fig. 5i). However, the delivery rate varied from low at 13.7% with 0-µm-high nanostraws to high at 85.7% with 5.0-µm-high nanostraws. This is because higher Au nanostraws can penetrate easily into the adsorbed NIH-3T3 fibroblast cells, the height of which is less than 4 µm at the center30 and less than 0.8 µm at the edge31. Furthermore, we demonstrated the delivery of molecules into NIH-3T3 cells with different nanostraw diameters of 0.4 and 1.0 µm, at a height of 5.0 µm (Fig. S3). We confirmed similar results of 90.1% viability/75.7% delivery using 400-nm-diameter Au nanostraw and 92.6% viability/87.2% delivery using 1000-nm-diameter Au nanostraw membrane, when compared with the data using 600-nm-diameter nanostraws. Our nanostraw stamping technique allows the delivery of calcein and large molecule (FAM-labeled oligo DNA) into the different types of the cell (HeLa cancer cell) (Fig. S4). We confirmed the results of 94.6% viability/87.2% delivery with small calcein and 90.3% viability/83.0% delivery with oligo DNA, so our nanostraw stamping system provide a versatile method for molecular delivery into the different types of adhesive cells.
Figure 5.
Direct delivery of molecules into NIH-3T3 cells. (a–d) Schematics of Au nanostraw membrane stamping into cells with different nanostraw heights of 0 (a), 1.3 (b), 2.4 (c), and 5.0 µm (d). (e–h) Optical and fluorescence images after the calcein and PI delivery using 0- (e), 1.3- (f), 2.4- (g), and 5.0-µm-high nanostraws (h). Total cell number N = 224 in (e), 476 in (f), 535 in (g) and 494 in (h). (i) Summarized data of nanostraw size and density, molecular delivery, and viability.
Discussion and Conclusions
We have demonstrated the direct delivery of molecules into adhesive NIH-3T3 cells with Au nanostraw membrane stamping, resulting in high cell viability of over 90% and highly efficient delivery of 85%. Such high viability and efficient delivery were achieved by the modification of Au nanostraw insertion geometry. The flow rate of calcein molecules at the Au nanostraws was controlled by adjusting the calcein concentration in the source chamber and the inner diameter of the Au nanostraws. These improvements reflect two critical advantages of using Au nanostraw membrane for intracellular molecular delivery. First, Au nanostraws provide sufficient mechanical properties to penetrate the cell membrane and to minimize the damage to cells during the molecular delivery. Second, with the performance of stamping a single time, we can inject the target molecules into multiple adhesive cells. This is the first demonstration of the use of Au nanostraw membranes for intracellular molecular delivery. In future applications, our nanostraw stamping system could be applied to deliver functional molecules of mRNA to edit and manipulate cellular functions24, as well as peptides and proteins32 to modulate and characterize cellular signaling pathways, and also could be used to intracellular extraction33 and medicine delivery34.
Materials and Methods
Electroless Au thin-film deposition
The TEPC membranes (it4ip S.A.) were treated in 1.25 M NaOH solution for 20 min at 40 °C. After washing the membranes with water, they were immersed in 1.25 M SnCl2 solution for 10 min at 25 °C and then washed with water. Sn2+-coated TEPC membrane was immersed in 1.25 M PdCl2 solution for 10 min at 25 °C to form the metallic catalysts of Sn4+-Pd on the membrane surface. We repeated this metallic formation cycle one to four times. The catalyst-coated TEPC membrane was immersed in an electroless gold planting solution including 200 ml/L NC gold PDII (NC Gold II; Kojima Chemicals) and 20 ml/L gold(1) trisodium disulphite, for 24 h at 40 °C. After washing the Au/TEPC membrane with water, we dried it in a vacuum chamber.
Au nanostraw fabrication with wet and dry etching
An Au/TEPC membrane was floated on aqua regia (ITO-02; Kanto Chemical Co., Inc.) for 4 min to etch the top surface of the Au nanolayer on the Au/TEPC membrane. After washing the etched Au/TEPC membrane with distilled water, it was dried in a vacuum chamber. To make Au nanostraws, the TEPC surface on the Au/TEPC membrane was etched with oxygen-based reactive ion etching. By changing the etching time, the height of the Au nanostraws could be controlled. After etching, scanning electron microscopy (SEM) images of Au nanostraws were obtained using a HITACHI SEM S-3400N, and use HITACHI SEM software to measure the outer and inner diameters of more than 100 Au nanostraws.
Cell culture
We used NIH 3T3 cells (JCRB0615; Health Science Research Resources Bank, Japan Health Science Foundation, Japan) and HeLa cells (JCRB9004; Health Science Research Resources Bank, Japan Health Science Foundation, Japan) as adhesive cells. The cells were cultured in Dulbecco’s minimal essential medium (Invitrogen Corp., USA) supplemented with 10% fetal bovine serum, 100 U mL−1 penicillin, and 100 µg mL−1 streptomycin. Each cell suspension was obtained by treating the confluent monolayer formed on the tissue culture dish with 0.25% trypsin (Invitrogen). The cells were cultured under a humidified atmosphere of 5% CO2 and 95% air at 37 °C.
Intracellular molecular delivery
We made a needle-type source chamber consisting of Au nanostraws/TEPC membrane (8-mm diameter), a glass tube (outer diameter: 8 mm, inner diameter R: 6 mm), and 10 mM PBS (pH 7), including target molecules at different concentrations. Typically we used 1.6 mM calcein (MP Biomedicals, Inc., MW: 622.5) and 0.1 mM oligo DNA (FAM-labeled TTTTATTTTGTTTTCTTTTG, BED.CO., MW: 6603.51) solution. A needle-type source chamber was set in a stand that moved along the z-axis at 1-µm steps and then combined it with an optical microscope (Olympus IX71), including an x-y stage, to establish a precise x-y-z manipulation system. By using this manipulator, we inserted Au nanostraws into adhesive NIH-3T3 cells and then delivered the calcein molecules into the cells through Au nanostraw ducts for 1 min. After exchanging the medium solution, we observed the calcein-stained cells with a fluorescent microscope (Olympus IX71). To confirm the dead cells, we injected 0.05 ml of PI at 4 μM into a 10-ml cell-culture dish and then performed culturing for 30 min. After exchanging the medium solution, we counted the PI-stained cells in the fluorescent images.
Supplementary information
Acknowledgements
The research presented in this article was supported by a Waseda University Grant for special research projects. Part of this work was conducted at Nanotechnology Platform Kitakyushu User Facility.
Author Contributions
T.M. conceived the research. T.M. and K.N. designed the experiments. T.M., B.Z., Y.S. and D.M. performed the experiments. T.M., B.Z., Y.S. and K.N. analyzed the data. B.Z. and Y.S. fabricated the devices. D.M. and K.N. cultured the cells. B.Z. and T.M. wrote the manuscript with input from all authors. All authors revised the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-43340-1.
References
- 1.Cao Y, et al. Nondestructive nanostraw intracellular sampling for longitudinal cell monitoring. Proceedings of the National Academy of Sciences of the United States of America. 2017;114:1866–1874. doi: 10.1073/pnas.1615375114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim D, et al. Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell stem cell. 2009;4:472–476. doi: 10.1016/j.stem.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hannon GJ. RNA interference. Nature. 2002;418:244–251. doi: 10.1038/418244a. [DOI] [PubMed] [Google Scholar]
- 4.Stewart MP, et al. In vitro and ex vivo strategies for intracellular delivery. Nature. 2016;538:183–192. doi: 10.1038/nature19764. [DOI] [PubMed] [Google Scholar]
- 5.Kay MA, Glorioso JC, Naldini L. Viral vectors for gene therapy: the art of turning infectious agents into vehicles of therapeutics. Nature medicine. 2001;7:33. doi: 10.1038/83324. [DOI] [PubMed] [Google Scholar]
- 6.Andaloussi SE, Mäger I, Breakefield XO, Wood MJ. Extracellular vesicles: biology and emerging therapeutic opportunities. Nature reviews Drug discovery. 2013;12:347. doi: 10.1038/nrd3978. [DOI] [PubMed] [Google Scholar]
- 7.Sahay G, et al. Efficiency of siRNA delivery by lipid nanoparticles is limited by endocytic recycling. Nature biotechnology. 2013;31:653. doi: 10.1038/nbt.2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fox CB, et al. Fabrication of Sealed Nanostraw Microdevices for Oral Drug Delivery. ACS Nano. 2016;10:5873–5881. doi: 10.1021/acsnano.6b00809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park K, et al. Suppressing mosaicism by Au nanowire injector-driven direct delivery of plasmids into mouse embryos. Biomaterials. 2017;138:169–178. doi: 10.1016/j.biomaterials.2017.05.044. [DOI] [PubMed] [Google Scholar]
- 10.Guillaume-Gentil O, et al. Force-Controlled Fluidic Injection into Single Cell Nuclei. Small. 2013;9:1904–1907. doi: 10.1002/smll.201202276. [DOI] [PubMed] [Google Scholar]
- 11.Caprettini V, et al. Soft electroporation for delivering molecules into tightly adherent mammalian cells through 3D hollow nanoelectrodes. Scientific Reports. 2017;7:8524. doi: 10.1038/s41598-017-08886-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Messina GC, et al. Spatially, Temporally, and Quantitatively Controlled Delivery of Broad Range of Molecules into Selected Cells through Plasmonic Nanotubes. Advanced Materials. 2015;27:7145–7149. doi: 10.1002/adma.201503252. [DOI] [PubMed] [Google Scholar]
- 13.Tsukakoshi M, Kurata S, Nomiya Y, Ikawa Y, Kasuya T. A novel method of DNA transfection by laser microbeam cell surgery. Applied Physics B. 1984;35:135–140. doi: 10.1007/BF00697702. [DOI] [Google Scholar]
- 14.VanDersarl JJ, Xu AM, Melosh NA. Nanostraws for direct fluidic intracellular access. Nano Letters. 2012;12:3881–3886. doi: 10.1021/nl204051v. [DOI] [PubMed] [Google Scholar]
- 15.Singhal R, et al. Multifunctional carbon-nanotube cellular endoscopes. Nature Nanotechnology. 2011;6:57–64. doi: 10.1038/nnano.2010.241. [DOI] [PubMed] [Google Scholar]
- 16.Actis P, Mak AC, Pourmand N. Functionalized nanopipettes: toward label-free, single cell biosensors. Bioanalytical reviews. 2010;1:177–185. doi: 10.1007/s12566-010-0013-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moe AM, Golding AE, Bement WM. Biology. 2015. Cell healing: Calcium, repair and regeneration. Seminars in Cell and Development; pp. 18–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Warren L, et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell stem cell. 2010;7:618–630. doi: 10.1016/j.stem.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shalek AK, et al. Vertical silicon nanowires as a universal platform for delivering biomolecules into living cells. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:1870–1875. doi: 10.1073/pnas.0909350107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He Y, Che X, Que L. A Top-Down Fabrication Process for Vertical Hollow Silicon Nanopillars. Journal of Microelectromechanical Systems. 2016;25:662–667. doi: 10.1109/JMEMS.2016.2582341. [DOI] [Google Scholar]
- 21.De Angelis F, et al. 3D hollow nanostructures as building blocks for multifunctional plasmonics. Nano Letters. 2013;13:3553–3558. doi: 10.1021/nl401100x. [DOI] [PubMed] [Google Scholar]
- 22.Han S-W, et al. High-efficiency DNA injection into a single human mesenchymal stem cell using a nanoneedle and atomic force microscopy. Nanomedicine: Nanotechnology, Biology and Medicine. 2008;4:215–225. doi: 10.1016/j.nano.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 23.Peer E, Artzy-Schnirman A, Gepstein L, Sivan U. Hollow nanoneedle array and its utilization for repeated administration of biomolecules to the same cells. ACS nano. 2012;6:4940–4946. doi: 10.1021/nn300443h. [DOI] [PubMed] [Google Scholar]
- 24.Xie X, et al. Nanostraw–electroporation system for highly efficient intracellular delivery and transfection. ACS nano. 2013;7:4351–4358. doi: 10.1021/nn400874a. [DOI] [PubMed] [Google Scholar]
- 25.Wu Y-C, et al. Massively parallel delivery of large cargo into mammalian cells with light pulses. Nature methods. 2015;12:439. doi: 10.1038/nmeth.3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.VanDersarl JJ, Xu AM, Melosh NA. Nanostraws for direct fluidic intracellular access. Nano Lett. 2012;12:3881–3886. doi: 10.1021/nl204051v. [DOI] [PubMed] [Google Scholar]
- 27.Kavaldzhiev MN, et al. Inductively actuated micro needles for on-demand intracellular delivery. Sci Rep. 2018;8:9918. doi: 10.1038/s41598-018-28194-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singhal R, et al. Multifunctional carbon-nanotube cellular endoscopes. Nat Nanotechnol. 2011;6:57–64. doi: 10.1038/nnano.2010.241. [DOI] [PubMed] [Google Scholar]
- 29.Yamada K, Gasparac R, Martin CR. Electrochemical and transport properties of templated gold/polypyrrole-composite microtube membranes. Journal of the Electrochemical Society. 2004;151:E14–E19. doi: 10.1149/1.1629097. [DOI] [Google Scholar]
- 30.Folkman J, Moscona A. Role of cell shape in growth control. Nature. 1978;273:345–349. doi: 10.1038/273345a0. [DOI] [PubMed] [Google Scholar]
- 31.Rotsch C, Jacobson K, Radmacher M. Dimensional and mechanical dynamics of active and stable edges in motile fibroblasts investigated by using atomic force microscopy. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:921–926. doi: 10.1073/pnas.96.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu AM, Wang DS, Shieh P, Cao Y, Melosh NA. Direct Intracellular Delivery of Cell‐Impermeable Probes of Protein Glycosylation by Using Nanostraws. ChemBioChem. 2017;18:623–628. doi: 10.1002/cbic.201600689. [DOI] [PubMed] [Google Scholar]
- 33.Cao Y, et al. Nondestructive nanostraw intracellular sampling for longitudinal cell monitoring. Proceedings of the National Academy of Sciences. 2017;114:E1866–E1874. doi: 10.1073/pnas.1615375114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fox CB, et al. Fabrication of sealed nanostraw microdevices for oral drug delivery. ACS nano. 2016;10:5873–5881. doi: 10.1021/acsnano.6b00809. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.