Abstract
Postoperative delirium is a common and harrowing complication in older surgical patients. Those with cognitive impairment or dementia are at especially high risk for developing postoperative delirium; ominously, it is hypothesized that delirium can accelerate cognitive decline and the onset of dementia, or worsen the severity of dementia. Awareness of delirium has grown in recent years as various medical societies have launched initiatives to prevent postoperative delirium and alleviate its impact. Unfortunately, delirium pathophysiology is not well understood and this likely contributes to the current state of low-quality evidence that informs perioperative guidelines. Along these lines, recent prevention trials involving ketamine and dexmedetomidine have demonstrated inconsistent findings. Non-pharmacologic multicomponent initiatives, such as the Hospital Elder Life Program, have consistently reduced delirium incidence and burden across various hospital settings. However, a substantial portion of delirium occurrences are still not prevented, and effective prevention and management strategies are needed to complement such multicomponent non-pharmacologic therapies. In this narrative review, we examine the current understanding of delirium neurobiology and summarize the present state of prevention and management efforts.
Keywords: Anesthesia, Delirium, Cognitive Dysfunction, Cognitive Reserve, Neurocognitive, Neurophysiology, Postoperative, Surgery
Introduction
Delirium is an enigmatic clinical syndrome characterized by an acute and typically reversible failure of our brain’s basic cognitive and attentional functions. Delirium can be associated with alterations in level of consciousness and is characterized by a fluctuating course. Those with delirium are often either agitated (hyperactive type) or lethargic (hypoactive type) or alternate between these motor subtypes (mixed type). Delirium occurs commonly in older adults, especially when there is pre-existing neurocognitive impairment and also following an insult such as an infection or a trauma. With many vulnerable older adults requiring surgery, postoperative delirium specifically is a growing public health concern, occurring with an incidence of 20 to 50% in those older than 60 after major surgery 1, 2. When patients become delirious, this is often the most distressing element of the perioperative experience, both for them and for their family members. Furthermore, postoperative delirium is associated with increased mortality 3, cognitive and functional decline 4– 6, increased hospital length of stay 7, and substantial annual health-care costs 8. Despite the grave nature of delirium and its associated burdens, foundational problems have tempered the pace of clinical and scientific progress. Most fundamentally, the pathophysiology of delirium phenomenology 9 remains incompletely understood. Although it is appropriate that diagnosis of a clinical syndrome is informed by bedside observations, this ideally should be accompanied by a clear understanding of the underlying pathophysiology. Additionally, delirium screening tools (for example, the Confusion Assessment Method for the Intensive Care Unit and the Delirium Observation Screening scale) used in routine clinical practice demonstrate low sensitivity (about 30%) compared with expert-based delirium identification (that is, psychiatrist, geriatrician, or neurologist performing chart reviews and completing similar delirium screening assessments) 10. Furthermore, diagnostic disagreement may be common among such experts 10. In fact, diagnostic discrepancy even occurs with different Diagnostic and Statistical Manual of Mental Disorders (DSM) editions. In an examination of a pooled dataset of prospective studies investigating delirium, strict DSM-5 criteria identified only 30% of delirium cases diagnosed via DSM-IV criteria 11. Although guidelines have been published for the prevention of postoperative delirium 12– 14, they are often supported by low-quality evidence 12, 15, 16. Furthermore, implementation efforts may be limited by the required administrative support, resources, and health-care staff education 17, 18. Thus, with an incomplete pathophysiologic understanding, a deficient diagnostic toolbox, and limited guideline evidence and implementation capacity, prevention and management of delirium are inherently challenging.
Multiple steps can be taken to improve clinical understanding and management of delirium. First, elucidating the neurobiologic mechanisms of delirium will advance understanding of the syndrome. These efforts could help produce targeted therapeutic strategies that address and alleviate intrinsic pathophysiologic perturbations. Second, improvement in current guideline implementation and adherence may still reduce delirium incidence and improve related outcomes 19. In this narrative review, we examine the current understanding of delirium pathophysiology and summarize what is known regarding prevention and management efforts. Future directions are briefly discussed with a focus on improving diagnostic and management strategies.
Delirium pathogenesis: current understanding
Delirium classification and diagnosis currently rely on phenotypic descriptions of altered brain states (for example, inattention and disorganized thinking) rather than a neurobiologically informed framework. By comparison, perioperative cardiovascular perturbations (for example, wall motion abnormalities and tamponade) can be diagnosed at the bedside with currently available technology and diagnostic acumen. No analogous, standard neurophysiologic evaluation process exists for diagnosing or evaluating the severity of altered postoperative brain states. This deficit in pathophysiologic understanding likely contributes to the current state of ineffective prevention and management. This has been reinforced by recent large pharmacologic trials that have failed to demonstrate reductions in postoperative delirium risk despite promising preliminary data 2, 20. Additionally, a systematic review in 2016 demonstrated that then-current pharmacologic treatment strategies were ineffective for reducing delirium duration or severity 21. Our hope is that such informative trials will galvanize investigative efforts to better understand the pathophysiology of delirium and related brain states 22.
The pathophysiologic framework of delirium has evolved over recent years with advances in neurocognitive research. From a systems neuroscience perspective, neurotransmitter imbalances—particularly involving dopamine and acetylcholine—have been implicated in delirium pathogenesis 23. Sleep disruption and polypharmacy may contribute to such neurotransmitter alterations (reviewed in Watson et al. 24). Neuroinflammation may also play a role as specific neuroinflammatory protein signatures track with postoperative delirium 25, 26. The net effect of these perturbations may manifest as network-level alterations in information processing. In fact, using 21-channel electroencephalographic (EEG) data, van Dellen et al. demonstrated reduced functional connectivity, altered directionality of information flow, and network topology changes during delirious episodes in cardiac surgery patients 27. This group published subsequent data comparing EEG measures of hypoactive delirium with non-delirious controls and those recovering from anesthesia in the immediate postoperative setting 28. Hypoactive delirium was distinguished from these other states by network topology features, as measures of network integration in the alpha band were reduced. Network science may help shape our understanding of brain state transitions perioperatively (reviewed in Lee and Mashour 29) and this could apply to delirium as well as to other altered brain states. Shafi et al. have proposed a model by which transcranial magnetic stimulation could be used to assess connectivity and neuroplasticity in real time 22, hypothesizing that reduced baseline connectivity and plasticity contribute to delirium risk. This neurophysiologic line of investigation ultimately may produce bedside tools for objective risk stratification and diagnosis of altered brain states, based on the underlying neurobiology. Preliminary work, based on frontal-parietal oscillatory patterns, has already shown promise in identifying delirium with high reliability against reference DSM-IV–based criteria 30.
Recent prevention strategies
Despite the knowledge gaps in delirium pathogenesis, delirium may still be preventable with targeted, multicomponent interventions 31. Given the harmful nature of delirium and the apparent failure of currently used drugs (for example, haloperidol) for prophylaxis and treatment 21, prevention efforts have expanded through recent investigation of novel pharmacologic and non-pharmacologic strategies.
Pharmacologic
Ketamine has been found to reduce postoperative inflammation 32, improve perioperative pain outcomes 33, and reduce excitotoxicity in laboratory models 34. Results from a small trial also demonstrated decreased occurrence of delirium and decreased incidence of delayed neurocognitive recovery in cardiac surgery patients who received intraoperative ketamine compared with placebo 35, 36. With this background and rationale, an international team of investigators conducted the PODCAST (Prevention of Delirium and Complications Associated with Surgical Treatments) trial, hypothesizing that a sub-anesthetic, intraoperative dose of ketamine would reduce postoperative delirium 37. In this trial, ketamine had no statistically significant effect on delirium incidence (ketamine groups: 19.45%, placebo group: 19.82%, absolute difference 0.36%, 95% confidence interval [CI] −6.07 to 7.38; P = 0.92), delirium severity, or delirium recurrence 2. Instead, dose-dependent increases were reported for postoperative hallucinations (18% in the placebo group, 20% in the 0.5 mg/kg ketamine group, and 28% in the 1.0 mg/kg ketamine group; P = 0.01) and nightmares (8% in the placebo group, 12% in the 0.5 mg/kg ketamine group, and 15% in the 1.0 mg/kg ketamine group; P = 0.03). These findings align with previously known psychoactive side effects of ketamine 33, 38, 39. Thus, intraoperative ketamine probably does not prevent delirium; rather, ketamine may increase the risk of adverse perioperative psychoactive experiences.
Dexmedetomidine has also been tested in large randomized trials in relation to postoperative delirium. A 2014 meta-analysis examined dexmedetomidine use across 14 trials involving cardiac surgery and intensive care unit (ICU) patients 40. In these trials, dexmedetomidine was investigated as a sedation agent, primarily for mechanically ventilated patients, compared with gamma-aminobutyric acid–based sedative-hypnotics (for example, propofol and midazolam). In this context, dexmedetomidine use was associated with reduction in the composite outcome of delirium, agitation, and confusion (relative risk [RR] 0.68, 95% CI 0.49 to 0.96; P = 0.03). Similar findings were presented in a meta-analysis by Duan et al. 41. Delirium incidence was significantly lower in surgical populations receiving dexmedetomidine (odds ratio [OR] 0.35, 95% CI 0.24 to 0.51; I 2 = 53%). However, the two largest studies in the meta-analysis may provide a nuanced perspective. The largest trial was performed by Su et al., who conducted a 700-patient, double-blinded, randomized controlled trial in which dexmedetomidine, administered in the ICU until the morning after major non-cardiac surgery, was associated with an impressive 13.4% absolute reduction (95% CI 8.1% to 18.7%) in postoperative delirium incidence 42. However, there are some concerns regarding the methodology of this trial 43. The majority of participants (nearly 60%) were consented by proxy postoperatively, and participants were not tested for delirium at the time of enrollment. Thus, the primary outcome was likely present in some patients in both groups prior to the intervention. Furthermore, the biological plausibility remains in question, as a robust delirium reduction was achieved with a small, sub-sedative dose rate (0.1 μg/kg per hour). Nonetheless, preliminary data demonstrate that low-dose-rate dexmedetomidine may improve perioperative sleep 44– 46, which has been postulated to mitigate delirium risk 47. A trial by Deiner et al. demonstrated no significant difference in delirium incidence between dexmedetomidine (12.2%) and placebo (11.4%) groups (RR 1.06, 95% CI 0.79 to 1.41; P = 0.77) 20. The study design was different, as dexmedetomidine was started intraoperatively and continued for only 2 hours postoperatively. Nonetheless, the trial was stopped early for futility, as dexmedetomidine did not appear to influence delirium risk or cognitive function 3 to 6 months after surgery. Of note, however, relevant confounders (for example, anesthetic and analgesic consumption) were not comprehensively reported and this contributed to trial limitations. Overall, dexmedetomidine may mitigate delirium risk in mechanically ventilated, critically ill patients; however, its prophylactic use in the intraoperative and immediate postoperative setting, particularly for non-cardiac surgery patients, remains controversial 43, 48. Large multicenter trials, with preoperative delirium testing, rigorous delirium assessment, and multiple treatment arms (for dose comparisons), are warranted to refine the evidence regarding the role of dexmedetomidine in preventing or treating delirium.
Apart from ketamine and dexmedetomidine, other drugs have shown some promise as prophylactic agents in both cardiac and non-cardiac surgery. These include acetaminophen, ramelteon, gabapentin, statins, clonidine, and melatonin 49, 50. Recently, a small, industry-funded, single-center trial demonstrated that intravenous acetaminophen every 6 hours for 48 hours after cardiac surgery was associated with an impressive 18% (95% CI −32 to −5%; P = 0.01) absolute risk reduction in delirium incidence compared with placebo 51. However, this result, as noted by the investigators 51, should be viewed as hypothesis-generating only. The biological plausibility of acetaminophen decreasing delirium incidence, especially to such a large extent, is questionable. Therefore, even with this encouraging finding, the probability that acetaminophen is effective at preventing delirium should still be regarded as low 52. A common misunderstanding is that P values provide direct information regarding the probability of the truth or falsity of hypotheses 53. The P value, if inappropriately used for (null) hypothesis testing, substantially overstates the evidence against the null hypothesis 54. The fragility index (which suffers from the same limitations as P values) has been proposed to assess the robustness of positive results in clinical trials 55. The fragility index 55 calculation for this trial 51 indicates that if just two patients in the acetaminophen group were “converted” to having delirium, the results would lose statistical significance at an arbitrary P value of less than 0.05. Another major constraint of this trial was that the control group received placebo 56 rather than oral or rectal acetaminophen, which often is standard practice after cardiac surgery. In order to adopt a new expensive treatment, like intravenous acetaminophen, it would be necessary to show that it was superior to inexpensive alternatives, like the generic oral formulation of the same drug. Given these important limitations, the results of this trial should be tested for reproducibility in a large, multicenter trial, as the investigators themselves have recommended 51. Atypical antipsychotic agents, such as haloperidol and quetiapine, have not shown benefit in preventing or treating delirium 57. Similarly, steroids, which have non-specific anti-inflammatory properties, have not been effective at preventing postoperative delirium 58. Pending more compelling evidence, no pharmacologic agent currently can be recommended for prophylaxis of postoperative delirium 49.
Depth of anesthesia
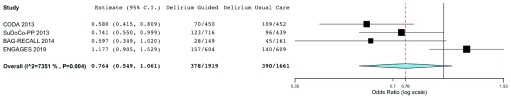
Within the last decade, a growing body of evidence has implicated anesthetic depth as a possible contributor to postoperative delirium. The Cognitive Dysfunction after Anesthesia (CODA) trial was published in 2013. Of 1000 patients who were planned for randomization, 921 older non-cardiac surgery patients (≥60 years of age) were randomly assigned to bispectral index (BIS)-guided anesthesia versus routine care 59. General anesthesia was achieved with ether-derived inhaled agents or with propofol intravenous anesthesia. Postoperative delirium, which was assessed in 902 patients, was reduced by 8.6% (95% CI 3.4 to 13.7) (relative reduction of 35%, 95% CI 16 to 51%) in the BIS-guided group, and cognitive dysfunction was also less common in the guided group 3 months after surgery. Of note, however, delirium was a secondary outcome of the trial, delirium was assessed only once daily, information on missing delirium data was not reported, delirium assessment training was not discussed, and protocol deviations were not reported. The same year, Radtke et al. reported findings from the Surgery Depth of Anaesthesia and Cognitive Outcome (SuDoCo) trial, which enrolled 1277 older non-cardiac surgery patients 60. Notably, the investigators specified a priori that 1600 patients would be enrolled (ISRCTN Register: 36437985), but the study was stopped early because of shortage of funds. General anesthesia was either with ether-derived inhaled agents or with propofol intravenous anesthesia. Interestingly, mean BIS values were almost identical (~39) in the BIS-guided and blinded groups. Delirium incidence was reported for 90.4% (1155 patients) and was significantly reduced in the BIS-guided group (16.5% versus 21.4%, absolute reduction 4.9%, 95% CI 0.3 to 9.4%; P = 0.036) 60. However, in the BIS-blinded group, clinicians deviated from the study protocol and unblinded themselves to BIS values for 141 patients at some point during surgery. By conducting the analysis with these patients in the BIS-guided group (that is, per-protocol approach), the association between BIS monitoring and delirium is not statistically significant (17.2% versus 21.9%, absolute reduction 4.7%, 95% CI −0.1 to 9.4%; P = 0.053). Observational data also demonstrate an association between intraoperative EEG suppression and postoperative delirium risk, even after adjustment for relevant confounders 61, 62. Collectively, these studies suggest that deep anesthesia—which is marked by EEG suppression—may causally contribute to postoperative delirium. Alternatively, the excessive presence of EEG suppression may reflect underlying neurologic vulnerability, indicating a higher inherent risk of delirium. The Electroencephalography Guidance of Anesthesia to Alleviate Geriatric Syndromes (ENGAGES) trial (ClinicalTrials.gov Identifier: NCT02241655) addressed this question by randomly assigning 1232 surgical patients to EEG-guided anesthesia—with a focus on avoiding EEG suppression—versus usual, EEG-blinded care 63, 64. Postoperative delirium occurred in 157 (26.0%) out of 604 patients in the EEG-guided group compared with the 140 (23.0%) out of 609 in the usual care group (absolute difference 3.0%, 95% CI −2.0 to 8.0; P = 0.22). The EEG-guided group had 46% (95% CI 16 to 76%) less EEG suppression time and 14% (95% CI 12 to 16%) less volatile anesthetic exposure. The findings suggest that EEG-guided anesthesia probably does not reduce postoperative delirium occurrence substantially in older surgical patients, even if EEG suppression time during surgery is decreased. The trial had specific methodological strengths, including structured delirium assessment training, fidelity checks for protocol compliance, and validated chart review methods to complement in-person delirium interviews. The ENGAGES trial also had several limitations, including the following: (i) single-center design, potentially limiting generalizability; (ii) lack of objective diagnostic criteria or biomarkers for delirium, which is a common consideration for all studies focusing on delirium; (iii) the potential for missed delirium occurrences given that delirium is a fluctuating disorder and could be missed with interval or insufficient assessments; and (iv) the potentially limited applicability to general anesthesia based on intravenous anesthetic agents. The results from these trials are illustrated meta-analytically in Figure 1.
Figure 1. Meta-analysis summarizing four trials in which the intervention group received electroencephalogram-guided anesthesia.
This analysis was conducted by using OpenMetaAnalyst 65 and was based on a binary, random effects, Hartung–Knapp–Sidik–Jonkman model 66, 67. The I 2 = 74%, tau 2 = 0.08, Q(df = 3) = 13.234, and heterogeneity P value = 0.004. The estimated odds ratio for delirium with intervention (electroencephalogram-guided [reduction in] anesthesia) = 0.764 (95% confidence interval 0.549 to 1.061, P = 0.108). BAG-RECALL, Bispectral Index or Anesthesia Gas to Reduce Explicit Recall; C.I., confidence interval; CODA, Cognitive Dysfunction after Anesthesia; ENGAGES, Electroencephalography Guidance of Anesthesia to Alleviate Geriatric Syndromes; SuDoCo-PP, Surgery Depth of Anaesthesia and Cognitive Outcome per-protocol.
Findings from the ENGAGES trial are consistent with those of systematic reviews of hip fracture surgery studies that have found no association between anesthetic technique (that is, general versus neuraxial anesthesia) and postoperative delirium risk 68– 70. Similar findings were demonstrated in the STRIDE (Strategy to Reduce the Incidence of Postoperative Delirium in Elderly Patients) trial 71, which randomly assigned patients undergoing surgery for hip fracture (n = 200) to light versus heavy sedation during spinal anesthesia. Overall, there was no significant difference in delirium incidence in the light sedation group (34/100, 34%) compared with the heavy sedation group (39/100, 39%; absolute reduction 5.0%, 95% CI −8.3 to 18.3%; P = 0.46). Thus, both the ENGAGES trial and data from the hip fracture surgery literature do not support current recommendations to use EEG-guided anesthesia for patients at risk in order to prevent postoperative delirium 12. This conclusion may be refined after the findings of the ENGAGES-Canada trial (ClinicalTrials.gov Identifier: NCT02692300) and Balanced Anesthesia Trial 72 are published.
Behavioral and multicomponent interventions
One of the most consistently effective delirium prevention strategies involves a multicomponent intervention that targets modifiable risk factors. The Hospital Elder Life Program (HELP), founded by Sharon K. Inouye et al., is a multidisciplinary program designed to prevent cognitive and functional decline in older hospitalized patients, and the focus is on delirium 73. HELP services include cognitive orientation, social support, sleep protocol implementation, assistance with nutrition and mobilization, and education for health-care staff. HELP has expanded to over 200 sites worldwide, and positive outcomes have been reproduced across several hospital settings and locations. A recent meta-analysis involving 14 studies demonstrated significant reductions in delirium incidence (OR 0.47, 95% CI 0.37 to 0.59; I 2 = 28%), risk of falls (OR 0.58, 95% CI 0.35 to 0.95; I 2 = 0%), and health-care costs ($16,000 USD per person-year) 31. Despite the paucity of effective delirium prevention strategies, HELP stands as a consistent, reproducible intervention for preventing delirium in high-risk patients.
Cognitive prehabilitation is also being studied as an approach for strengthening cognitive reserve in surgical patients 74. So-called “brain training” efforts have been hypothesized to curtail the risk of postoperative delirium and cognitive impairment. Computerized cognitive training exercises have demonstrated cognitive benefit in non-surgical patients across a wide variety of clinical settings 75. However, modest gains are generally observed in the short term, and training appears to require direct supervision, over several hours, and spaced out over multiple weeks to avoid cognitive fatigue 75, 76. In fact, preliminary data demonstrate that such training programs are unlikely to be feasible for many older patients 77. Time commitment and preoperative anxiety served as barriers to training adherence, and those randomly assigned to training were more likely to withdraw from the study. Although larger-scale trials are ongoing (ClinicalTrials.gov Identifier: NCT02230605 74), cognitive prehabilitation may not be feasible for many older patients prior to surgery.
Clinical management
Medical associations such as the UK’s National Institute for Health and Care Excellence, the European Society of Anaesthesiology, and the American Geriatrics Society offer evidence-based guidelines for postoperative delirium management 12, 13, 78. Initial steps focus on identifying and treating precipitating etiologies. In hospitalized patients, iatrogenic causes include infection, polypharmacy, fluid and electrolyte disturbances, and organ failure (with associated physiologic perturbations). Concurrent with treating the underlying medical condition, supportive efforts can be implemented to mitigate delirium severity. Non-pharmacologic interventions, such as delirium education programs for medical staff, have led to reductions in delirium duration, hospital length of stay, and mortality 79. Such programs can also improve delirium recognition and disposition and are associated with reductions in point prevalence 80. Pharmacologic interventions for treating active delirium have been studied for many years, although most studies have not found candidate drugs to be effective. Neufeld et al. recently published a systematic review to examine antipsychotic medication treatment for delirium 21. The authors reviewed 19 studies, which included various typical and atypical antipsychotics across diverse hospital settings, and found that antipsychotics demonstrated no significant effects on delirium incidence, duration, or severity, or on hospital length of stay. In fact, a subsequent clinical trial by Agar et al. demonstrated improved survival, reduced delirium severity scores, and fewer extrapyramidal effects in the placebo group compared with risperidone and haloperidol arms in palliative care patients 81. Thus, current guidelines recommend only pharmacologic treatment for select scenarios, such as severe agitation (that is, posing harm to self or others or both) and alcohol or benzodiazepine withdrawal 12, 13, 78.
Lastly, the lack of delirium guideline implementation may also impede delirium prevention and care, especially in the ICU. A recent prospective mixed-methods study by Balas et al. 18 examined barriers to guideline dissemination and implementation across various ICU settings. Participants reported that (1) knowledge deficits and (2) low confidence with using delirium screening tools, particularly as time elapsed after initial training and education, served as barriers for delirium guideline implementation. These findings align with similar studies involving medical wards, where a staff educational program reduced delirium incidence and related complications, including mortality and hospital length of stay 79. Thus, consistent educational and training efforts may help prevent delirium and associated deleterious outcomes.
Conclusions and future directions
Delirium is a distressing syndrome for older surgical patients and their families, and the societal consequences of delirium are likely to escalate with a growing older surgical population. Advancing our pathophysiologic understanding of delirium is likely to inform better screening and diagnostic strategies. Neurophysiologic investigation, shaped by a network science framework, may improve neurobiologic understanding of delirium mechanisms. Knowledge gaps in relation to pathophysiology may help explain why rigorous, large pharmacologic and non-pharmacologic trials for delirium prevention have generally been disappointing 2, 20, 64 and weigh against current guidelines 13. Non-pharmacologic, multicomponent interventions are not likely to increase the risk of harm and have repeatedly been shown to reduce the incidence and impact of delirium 31. With improving scientific and technological advances and the establishment of multidisciplinary neuroscience collaborations 82, 83, the time is ripe to improve delirium understanding and management.
Acknowledgments
The authors would like to acknowledge support from the Department of Anesthesiology at the University of Michigan Medical School and from the Dr. Seymour and Rose T. Brown Chair in Anesthesiology at Washington University’s Department of Anesthesiology.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Daqing Ma, Section of Anaesthetics, Pain Management and Intensive Care, Department of Surgery and Cancer, Faculty of Medicine, Imperial College London, Chelsea and Westminster Hospital, London, UK
Finn Radtke, Department of Anesthesiology, Naestved Hospital, Naestved, Denmark
Funding Statement
PEV is supported by the National Institutes of Health (NIH) under grant number K23GM126317. MSA is supported by the NIH under Network for Investigation of Delirium: Unifying Scientists (National Institute on Aging; principal investigator, Sharon K. Inouye; grant number R24AG054259) and under ENGAGES (National Institute on Aging, grant number UH3AG050312).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; peer review: 2 approved]
References
- 1. Dasgupta M, Dumbrell AC: Preoperative risk assessment for delirium after noncardiac surgery: a systematic review. J Am Geriatr Soc. 2006;54(10):1578–89. 10.1111/j.1532-5415.2006.00893.x [DOI] [PubMed] [Google Scholar]
- 2. Avidan MS, Maybrier HR, Abdallah AB, et al. : Intraoperative ketamine for prevention of postoperative delirium or pain after major surgery in older adults: an international, multicentre, double-blind, randomised clinical trial. Lancet. 2017;390(10091):267–75. 10.1016/S0140-6736(17)31467-8 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 3. Witlox J, Eurelings LS, de Jonghe JF, et al. : Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta-analysis. JAMA. 2010;304(4):443–51. 10.1001/jama.2010.1013 [DOI] [PubMed] [Google Scholar]
- 4. Hshieh TT, Saczynski J, Gou RY, et al. : Trajectory of Functional Recovery After Postoperative Delirium in Elective Surgery. Ann Surg. 2017;265(4):647–53. 10.1097/SLA.0000000000001952 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 5. Inouye SK, Marcantonio ER, Kosar CM, et al. : The short-term and long-term relationship between delirium and cognitive trajectory in older surgical patients. Alzheimers Dement. 2016;12(7):766–75. 10.1016/j.jalz.2016.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 6. Saczynski JS, Marcantonio ER, Quach L, et al. : Cognitive trajectories after postoperative delirium. N Engl J Med. 2012;367(1):30–9. 10.1056/NEJMoa1112923 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 7. Gleason LJ, Schmitt EM, Kosar CM, et al. : Effect of Delirium and Other Major Complications on Outcomes After Elective Surgery in Older Adults. JAMA Surg. 2015;150(12):1134–40. 10.1001/jamasurg.2015.2606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Leslie DL, Marcantonio ER, Zhang Y, et al. : One-year health care costs associated with delirium in the elderly population. Arch Intern Med. 2008;168(1):27–32. 10.1001/archinternmed.2007.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yang FM, Marcantonio ER, Inouye SK, et al. : Phenomenological subtypes of delirium in older persons: patterns, prevalence, and prognosis. Psychosomatics. 2009;50(3):248–54. 10.1176/appi.psy.50.3.248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Numan T, van den Boogaard M, Kamper AM, et al. : Recognition of Delirium in Postoperative Elderly Patients: A Multicenter Study. J Am Geriatr Soc. 2017;65(9):1932–8. 10.1111/jgs.14933 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 11. Meagher DJ, Morandi A, Inouye SK, et al. : Concordance between DSM-IV and DSM-5 criteria for delirium diagnosis in a pooled database of 768 prospectively evaluated patients using the delirium rating scale-revised-98. BMC Med. 2014;12:164. 10.1186/s12916-014-0164-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. American Geriatrics Society Expert Panel on Postoperative Delirium in Older Adults: American Geriatrics Society abstracted clinical practice guideline for postoperative delirium in older adults. J Am Geriatr Soc. 2015;63(1):142–50. 10.1111/jgs.13281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Aldecoa C, Bettelli G, Bilotta F, et al. : European Society of Anaesthesiology evidence-based and consensus-based guideline on postoperative delirium. Eur J Anaesthesiol. 2017;34(4):192–214. 10.1097/EJA.0000000000000594 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 14. Devlin JW, Skrobik Y, Gélinas C, et al. : Clinical Practice Guidelines for the Prevention and Management of Pain, Agitation/Sedation, Delirium, Immobility, and Sleep Disruption in Adult Patients in the ICU. Crit Care Med. 2018;46(9):e825–e873. 10.1097/CCM.0000000000003299 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 15. Atkins D, Best D, Briss PA, et al. : Grading quality of evidence and strength of recommendations. BMJ. 2004;328(7454):1490. 10.1136/bmj.328.7454.1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Qaseem A, Snow V, Owens DK, et al. : The development of clinical practice guidelines and guidance statements of the American College of Physicians: summary of methods. Ann Intern Med. 2010;153(3):194–9. 10.7326/0003-4819-153-3-201008030-00010 [DOI] [PubMed] [Google Scholar]
- 17. Balas MC, Weinhouse GL, Denehy L, et al. : Interpreting and Implementing the 2018 Pain, Agitation/Sedation, Delirium, Immobility, and Sleep Disruption Clinical Practice Guideline. Crit Care Med. 2018;46(9):1464–70. 10.1097/CCM.0000000000003307 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 18. Balas MC, Burke WJ, Gannon D, et al. : Implementing the awakening and breathing coordination, delirium monitoring/management, and early exercise/mobility bundle into everyday care: Opportunities, challenges, and lessons learned for implementing the ICU Pain, Agitation, and Delirium Guidelines. Crit Care Med. 2013;41(9 Suppl 1):S116–27. 10.1097/CCM.0b013e3182a17064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Barnes-Daly MA, Phillips G, Ely EW: Improving Hospital Survival and Reducing Brain Dysfunction at Seven California Community Hospitals: Implementing PAD Guidelines Via the ABCDEF Bundle in 6,064 Patients. Crit Care Med. 2017;45(2):171–8. 10.1097/CCM.0000000000002149 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 20. Deiner S, Luo X, Lin HM, et al. : Intraoperative Infusion of Dexmedetomidine for Prevention of Postoperative Delirium and Cognitive Dysfunction in Elderly Patients Undergoing Major Elective Noncardiac Surgery: A Randomized Clinical Trial. JAMA Surg. 2017;152(8):e171505. 10.1001/jamasurg.2017.1505 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 21. Neufeld KJ, Yue J, Robinson TN, et al. : Antipsychotic Medication for Prevention and Treatment of Delirium in Hospitalized Adults: A Systematic Review and Meta-Analysis. J Am Geriatr Soc. 2016;64(4):705–14. 10.1111/jgs.14076 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 22. Shafi MM, Santarnecchi E, Fong TG, et al. : Advancing the Neurophysiological Understanding of Delirium. J Am Geriatr Soc. 2017;65(6):1114–8. 10.1111/jgs.14748 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 23. Trzepacz PT: Is there a final common neural pathway in delirium? Focus on acetylcholine and dopamine. Semin Clin Neuropsychiatry. 2000;5(2):132–48. [DOI] [PubMed] [Google Scholar]
- 24. Watson PL, Ceriana P, Fanfulla F: Delirium: is sleep important? Best Pract Res Clin Anaesthesiol. 2012;26(3):355–66. 10.1016/j.bpa.2012.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vasunilashorn SM, Ngo LH, Chan NY, et al. : Development of a Dynamic Multi-Protein Signature of Postoperative Delirium. J Gerontol A Biol Sci Med Sci. 2019;74(2):261–8. 10.1093/gerona/gly036 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 26. Vasunilashorn SM, Ngo L, Inouye SK, et al. : Cytokines and Postoperative Delirium in Older Patients Undergoing Major Elective Surgery. J Gerontol A Biol Sci Med Sci. 2015;70(10):1289–95. 10.1093/gerona/glv083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. van Dellen E, van der Kooi AW, Numan T, et al. : Decreased functional connectivity and disturbed directionality of information flow in the electroencephalography of intensive care unit patients with delirium after cardiac surgery. Anesthesiology. 2014;121(2):328–35. 10.1097/ALN.0000000000000329 [DOI] [PubMed] [Google Scholar]
- 28. Numan T, Slooter AJC, van der Kooi AW, et al. : Functional connectivity and network analysis during hypoactive delirium and recovery from anesthesia. Clin Neurophysiol. 2017;128(6):914–24. 10.1016/j.clinph.2017.02.022 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 29. Lee U, Mashour GA: Role of Network Science in the Study of Anesthetic State Transitions. Anesthesiology. 2018;129(5):1029–44. 10.1097/ALN.0000000000002228 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 30. van der Kooi AW, Zaal IJ, Klijn FA, et al. : Delirium detection using EEG: what and how to measure. Chest. 2015;147(1):94–101. 10.1378/chest.13-3050 [DOI] [PubMed] [Google Scholar]
- 31. Hshieh TT, Yang T, Gartaganis SL, et al. : Hospital Elder Life Program: Systematic Review and Meta-analysis of Effectiveness. Am J Geriatr Psychiatry. 2018;26(10):1015–33. 10.1016/j.jagp.2018.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 32. Dale O, Somogyi AA, Li Y, et al. : Does intraoperative ketamine attenuate inflammatory reactivity following surgery? A systematic review and meta-analysis. Anesth Analg. 2012;115(4):934–43. 10.1213/ANE.0b013e3182662e30 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 33. Laskowski K, Stirling A, McKay WP, et al. : A systematic review of intravenous ketamine for postoperative analgesia. Can J Anaesth. 2011;58(10):911–23. 10.1007/s12630-011-9560-0 [DOI] [PubMed] [Google Scholar]
- 34. Wang L, Jing W, Hang YN: Glutamate-induced c-Jun expression in neuronal PC12 cells: the effects of ketamine and propofol. J Neurosurg Anesthesiol. 2008;20(2):124–30. 10.1097/ANA.0b013e3181667c27 [DOI] [PubMed] [Google Scholar]
- 35. Hudetz JA, Patterson KM, Iqbal Z, et al. : Ketamine attenuates delirium after cardiac surgery with cardiopulmonary bypass. J Cardiothorac Vasc Anesth. 2009;23(5):651–7. 10.1053/j.jvca.2008.12.021 [DOI] [PubMed] [Google Scholar]
- 36. Hudetz JA, Iqbal Z, Gandhi SD, et al. : Ketamine attenuates post-operative cognitive dysfunction after cardiac surgery. Acta Anaesthesiol Scand. 2009;53(7):864–72. 10.1111/j.1399-6576.2009.01978.x [DOI] [PubMed] [Google Scholar]
- 37. Avidan MS, Fritz BA, Maybrier HR, et al. : The Prevention of Delirium and Complications Associated with Surgical Treatments (PODCAST) study: protocol for an international multicentre randomised controlled trial. BMJ Open. 2014;4(9):e005651. 10.1136/bmjopen-2014-005651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fine J, Finestone SC: Sensory disturbances following ketamine anesthesia: recurrent hallucinations. Anesth Analg. 1973;52(3):428–30. [PubMed] [Google Scholar]
- 39. Lilburn JK, Dundee JW, Nair SG, et al. : Ketamine sequelae. Evaluation of the ability of various premedicants to attenuate its psychic actions. Anaesthesia. 1978;33(4):307–11. 10.1111/j.1365-2044.1978.tb12412.x [DOI] [PubMed] [Google Scholar]
- 40. Pasin L, Landoni G, Nardelli P, et al. : Dexmedetomidine reduces the risk of delirium, agitation and confusion in critically Ill patients: a meta-analysis of randomized controlled trials. J Cardiothorac Vasc Anesth. 2014;28(6):1459–66. 10.1053/j.jvca.2014.03.010 [DOI] [PubMed] [Google Scholar]
- 41. Duan X, Coburn M, Rossaint R, et al. : Efficacy of perioperative dexmedetomidine on postoperative delirium: systematic review and meta-analysis with trial sequential analysis of randomised controlled trials. Br J Anaesth. 2018;121(2):384–97. 10.1016/j.bja.2018.04.046 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 42. Su X, Meng ZT, Wu XH, et al. : Dexmedetomidine for prevention of delirium in elderly patients after non-cardiac surgery: a randomised, double-blind, placebo-controlled trial. Lancet. 2016;388(10054):1893–902. 10.1016/S0140-6736(16)30580-3 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 43. Kronzer VL, Avidan MS: Preventing postoperative delirium: all that glisters is not gold. Lancet. 2016;388(10054):1854–6. 10.1016/S0140-6736(16)31353-8 [DOI] [PubMed] [Google Scholar]
- 44. Wu XH, Cui F, Zhang C, et al. : Low-dose Dexmedetomidine Improves Sleep Quality Pattern in Elderly Patients after Noncardiac Surgery in the Intensive Care Unit: A Pilot Randomized Controlled Trial. Anesthesiology. 2016;125(5):979–91. 10.1097/ALN.0000000000001325 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 45. Chen Z, Tang R, Zhang R, et al. : Effects of dexmedetomidine administered for postoperative analgesia on sleep quality in patients undergoing abdominal hysterectomy. J Clin Anesth. 2017;36:118–22. 10.1016/j.jclinane.2016.10.022 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 46. Li HJ, Li CJ, Wei XN, et al. : Dexmedetomidine in combination with morphine improves postoperative analgesia and sleep quality in elderly patients after open abdominal surgery: A pilot randomized control trial. PLoS One. 2018;13(8):e0202008. 10.1371/journal.pone.0202008 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 47. Shelton KT, Qu J, Bilotta F, et al. : Minimizing ICU Neurological Dysfunction with Dexmedetomidine-induced Sleep (MINDDS): protocol for a randomised, double-blind, parallel-arm, placebo-controlled trial. BMJ Open. 2018;8(4):e020316. [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 48. Berian JR, Rosenthal RA, Robinson TN: Confusion Regarding Surgical Delirium-Is Dexmedetomidine the Answer? JAMA Surg. 2017;152(8):e171511. 10.1001/jamasurg.2017.1511 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 49. Tremblay P, Gold S: Prevention of Post-operative Delirium in the Elderly Using Pharmacological Agents. Can Geriatr J. 2016;19(3):113–26. 10.5770/cgj.19.226 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 50. Mu JL, Lee A, Joynt GM: Pharmacologic agents for the prevention and treatment of delirium in patients undergoing cardiac surgery: systematic review and metaanalysis. Crit Care Med. 2015;43(1):194–204. 10.1097/CCM.0000000000000673 [DOI] [PubMed] [Google Scholar]
- 51. Subramaniam B, Shankar P, Shaefi S, et al. : Effect of Intravenous Acetaminophen vs Placebo Combined With Propofol or Dexmedetomidine on Postoperative Delirium Among Older Patients Following Cardiac Surgery: The DEXACET Randomized Clinical Trial. JAMA. 2019;321(7):686–96. 10.1001/jama.2019.0234 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 52. Nuzzo R: Scientific method: statistical errors. Nature. 2014;506(7487):150–2. 10.1038/506150a [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 53. Goodman S: A dirty dozen: twelve p-value misconceptions. Semin Hematol. 2008;45(3):135–40. 10.1053/j.seminhematol.2008.04.003 [DOI] [PubMed] [Google Scholar]
- 54. Goodman SN: p values, hypothesis tests, and likelihood: implications for epidemiology of a neglected historical debate. Am J Epidemiol. 1993;137(5):485–96; discussion 497–501. 10.1093/oxfordjournals.aje.a116700 [DOI] [PubMed] [Google Scholar]
- 55. Walsh M, Srinathan SK, McAuley DF, et al. : The statistical significance of randomized controlled trial results is frequently fragile: a case for a Fragility Index. J Clin Epidemiol. 2014;67(6):622–8. 10.1016/j.jclinepi.2013.10.019 [DOI] [PubMed] [Google Scholar]
- 56. Millum J, Grady C: The ethics of placebo-controlled trials: methodological justifications. Contemp Clin Trials. 2013;36(2):510–4. 10.1016/j.cct.2013.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. van den Boogaard M, Slooter AJC, Brüggemann RJM, et al. : Effect of Haloperidol on Survival Among Critically Ill Adults With a High Risk of Delirium: The REDUCE Randomized Clinical Trial. JAMA. 2018;319(7):680–90. 10.1001/jama.2018.0160 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 58. Sauër AM, Slooter AJ, Veldhuijzen DS, et al. : Intraoperative dexamethasone and delirium after cardiac surgery: a randomized clinical trial. Anesth Analg. 2014;119(5):1046–52. 10.1213/ANE.0000000000000248 [DOI] [PubMed] [Google Scholar]
- 59. Chan MT, Cheng BC, Lee TM, et al. : BIS-guided anesthesia decreases postoperative delirium and cognitive decline. J Neurosurg Anesthesiol. 2013;25(1):33–42. 10.1097/ANA.0b013e3182712fba [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 60. Radtke FM, Franck M, Lendner J, et al. : Monitoring depth of anaesthesia in a randomized trial decreases the rate of postoperative delirium but not postoperative cognitive dysfunction. Br J Anaesth. 2013;110 Suppl 1:i98–105. 10.1093/bja/aet055 [DOI] [PubMed] [Google Scholar]
- 61. Fritz BA, Maybrier HR, Avidan MS: Intraoperative electroencephalogram suppression at lower volatile anaesthetic concentrations predicts postoperative delirium occurring in the intensive care unit. Br J Anaesth. 2018;121(1):241–8. 10.1016/j.bja.2017.10.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Fritz BA, Kalarickal PL, Maybrier HR, et al. : Intraoperative Electroencephalogram Suppression Predicts Postoperative Delirium. Anesth Analg. 2016;122(1):234–42. 10.1213/ANE.0000000000000989 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 63. Wildes TS, Winter AC, Maybrier HR, et al. : Protocol for the Electroencephalography Guidance of Anesthesia to Alleviate Geriatric Syndromes (ENGAGES) study: a pragmatic, randomised clinical trial. BMJ Open. 2016;6(6):e011505. 10.1136/bmjopen-2016-011505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wildes TS, Mickle AM, Ben Abdallah A, et al. : Effect of Electroencephalography-Guided Anesthetic Administration on Postoperative Delirium Among Older Adults Undergoing Major Surgery: The ENGAGES Randomized Clinical Trial. JAMA. 2019;321(5):473–83. 10.1001/jama.2018.22005 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 65. Wallace BC, Dahabreh IJ, Trikalinos TA, et al. : Closing the Gap between Methodologists and End-Users: R as a Computational Back-End. J Stat Soft. 2012;49(5). 10.18637/jss.v049.i05 [DOI] [Google Scholar]
- 66. IntHout J, Ioannidis JP, Borm GF: The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Med Res Methodol. 2014;14:25. 10.1186/1471-2288-14-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Serghiou S, Goodman SN: Random-Effects Meta-analysis: Summarizing Evidence With Caveats. JAMA. 2019;321(3):301–2. 10.1001/jama.2018.19684 [DOI] [PubMed] [Google Scholar]
- 68. Guay J, Parker MJ, Gajendragadkar PR, et al. : Anaesthesia for hip fracture surgery in adults. Cochrane Database Syst Rev. 2016;2:CD000521. 10.1002/14651858.CD000521.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 69. O'Donnell CM, McLoughlin L, Patterson CC, et al. : Perioperative outcomes in the context of mode of anaesthesia for patients undergoing hip fracture surgery: systematic review and meta-analysis. Br J Anaesth. 2018;120(1):37–50. 10.1016/j.bja.2017.09.002 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 70. Patel V, Champaneria R, Dretzke J, et al. : Effect of regional versus general anaesthesia on postoperative delirium in elderly patients undergoing surgery for hip fracture: a systematic review. BMJ Open. 2018;8(12):e020757. 10.1136/bmjopen-2017-020757 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 71. Sieber FE, Neufeld KJ, Gottschalk A, et al. : Effect of Depth of Sedation in Older Patients Undergoing Hip Fracture Repair on Postoperative Delirium: The STRIDE Randomized Clinical Trial. JAMA Surg. 2018;153(11):987–95. 10.1001/jamasurg.2018.2602 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 72. Short TG, Leslie K, Chan MT, et al. : Rationale and Design of the Balanced Anesthesia Study: A Prospective Randomized Clinical Trial of Two Levels of Anesthetic Depth on Patient Outcome After Major Surgery. Anesth Analg. 2015;121(2):357–65. 10.1213/ANE.0000000000000797 [DOI] [PubMed] [Google Scholar]
- 73. Inouye SK, Bogardus ST, Jr, Baker DI, et al. : The Hospital Elder Life Program: a model of care to prevent cognitive and functional decline in older hospitalized patients. Hospital Elder Life Program. J Am Geriatr Soc. 2000;48(12):1697–706. 10.1111/j.1532-5415.2000.tb03885.x [DOI] [PubMed] [Google Scholar]
- 74. Humeidan ML, Otey A, Zuleta-Alarcon A, et al. : Perioperative Cognitive Protection-Cognitive Exercise and Cognitive Reserve (The Neurobics Trial): A Single-blind Randomized Trial. Clin Ther. 2015;37(12):2641–50. 10.1016/j.clinthera.2015.10.013 [DOI] [PubMed] [Google Scholar]
- 75. Lampit A, Hallock H, Valenzuela M: Computerized cognitive training in cognitively healthy older adults: a systematic review and meta-analysis of effect modifiers. PLoS Med. 2014;11(11):e1001756. 10.1371/journal.pmed.1001756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Lampit A, Hallock H, Moss R, et al. : The Timecourse of Global Cognitive Gains from Supervised Computer-Assisted Cognitive Training: A Randomised, Active-Controlled Trial in Elderly with Multiple Dementia Risk Factors. J Prev Alzheimers Dis. 2014;1(1):33–9. 10.14283/jpad.2014.18 [DOI] [PubMed] [Google Scholar]
- 77. Vlisides PE, Das AR, Thompson AM, et al. : Home-based Cognitive Prehabilitation in Older Surgical Patients: A Feasibility Study. J Neurosurg Anesthesiol. 2019;31(2):212–217. 10.1097/ANA.0000000000000569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Young J, Murthy L, Westby M, et al. : Diagnosis, prevention, and management of delirium: summary of NICE guidance. BMJ. 2010;341:c3704. 10.1136/bmj.c3704 [DOI] [PubMed] [Google Scholar]
- 79. Lundström M, Edlund A, Karlsson S, et al. : A multifactorial intervention program reduces the duration of delirium, length of hospitalization, and mortality in delirious patients. J Am Geriatr Soc. 2005;53(4):622–8. 10.1111/j.1532-5415.2005.53210.x [DOI] [PubMed] [Google Scholar]
- 80. Tabet N, Hudson S, Sweeney V, et al. : An educational intervention can prevent delirium on acute medical wards. Age Ageing. 2005;34(2):152–6. 10.1093/ageing/afi031 [DOI] [PubMed] [Google Scholar]
- 81. Agar MR, Lawlor PG, Quinn S, et al. : Efficacy of Oral Risperidone, Haloperidol, or Placebo for Symptoms of Delirium Among Patients in Palliative Care: A Randomized Clinical Trial. JAMA Intern Med. 2017;177(1):34–42. 10.1001/jamainternmed.2016.7491 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 82. Fick DM, Auerbach AD, Avidan MS, et al. : Network for Investigation of Delirium across the U.S.: Advancing the Field of Delirium with a New Interdisciplinary Research Network. J Am Geriatr Soc. 2017;65(10):2158–60. 10.1111/jgs.14942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Baughman RW, Farkas R, Guzman M, et al. : The National Institutes of Health Blueprint for Neuroscience Research. J Neurosci. 2006;26(41):10329–31. 10.1523/JNEUROSCI.3979-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]