Abstract
Background
Migraine is characterized by a collection of neurological symptoms in the absence of injury or damage. However, several common preclinical migraine models require significant damage to the skull to stimulate the dura mater, the likely source of afferent signaling leading to head pain. The goal of this study was to determine whether dural stimulation can be performed in mice using an injection that does not cause injury or damage.
Methods
Using mice, injections of stimuli were administered to the dura mater through the soft tissue at the intersection between the lambdoidal and sagittal sutures. This technique did not require a permanent cannula nor did it cause damage to the skull or dura. Following injection of noxious stimuli, migraine-like behaviors were measured including cutaneous allodynia and facial grimace. The retrograde tracer fluorogold was applied onto the dura using the same injection technique to label trigeminal ganglion cell bodies, which were then testing in vitro using patch-clamp electrophysiology.
Results
Dural injection of allyl-isothiocyanate, low pH, interleukin-6, or inflammatory soup but not vehicles, led to cephalic/extracephalic allodynia. Facial grimace responses were also observed with allyl-isothiocyanate, pH 6.0, and interleukin-6. Stimulation with interleukin-6 causes priming to normally subthreshold pH 7.0 stimulation of the dura following resolution of the initial interleukin-6 behavior. Systemic injection of sumatriptan at the time of dural stimulation with inflammatory soup decreased the resulting cutaneous hypersensitivity. Trigeminal ganglion cell bodies retrogradely labeled from the dura had low pH-evoked currents similar to those generated by acid-sensing ion channels.
Conclusion
Non-invasive dural stimulation in mice can be used as a model of migraine in the absence of injury.
Keywords: Dura, mouse model, meninges, headache, migraine, interleukin, priming, trigeminal
Introduction
Migraines are recurrent episodes of aberrant neurological symptoms that cause disruption or impairment of normal sensory, social, and occupational functions (1–3). Over 36 million Americans suffer from migraine, and the World Health Organization ranks it as the third most prevalent disease in the world (4). Despite this prevalence, the etiology of migraine is enigmatic and the underlying pathology for progression of the disease remains unidentified (5). The most widely held hypothesis for the mechanism of migraine pain is that it is driven by the activation of nociceptors within the meninges. This is based on the observation that stimulation of the meninges in conscious humans causes headache (6,7) and activation of the trigeminovascular system that innervates the meninges can be seen in humans during migraine (2). Consistent with this human observation, experimental work in anesthetized animals demonstrates that stimulation of the meninges activates nociceptive afferent pathways (8–10). Further, behavioral models of migraine in rodents show that stimulation of the dura mater produces responses consistent with migraine headache, including cutaneous allodynia, decreased exploratory behavior (e.g. rearing), and conditioned-place preference (11–17). However, all of the currently available behavioral models assessing direct dural stimulation require a craniotomy to implant a delivery device (e.g. a cannula) that allows injection of stimuli onto the dura. This invasive procedure can potentially compromise the underlying tissue, it can lead to inflammation of the skull/meninges, and to activation/sensitization of meningeal nociceptors. Studies using this method allow a 7-day recovery period following surgery or administration of an anesthetic to the surrounding tissue during the procedure, but questions remain regarding whether the craniotomy influences the responses to subsequent dural stimulation in the behavioral experiments. Although preclinical migraine models that do not require this type of injury exist, for example using systemic administration of nitric oxide (NO) donors or calcitonin gene-related peptide (CGRP), or continued exposure to sumatriptan (18–23), these models are not capable of measuring the direct effects of the experimental manipulation on activation of meningeal nociceptors. Development of a novel method to directly stimulate meningeal afferents in the absence of tissue injury would allow for better evaluation of the role of this system in headache in its native state.
The purpose of these studies was to develop and characterize an approach for acute stimulation of the dura in mice that eliminates the need for a craniotomy surgery and an extended recovery period. This approach utilizes the natural separation between cranial plates prior to fusion as the injection point. At the intersection of the lambdoid and sagittal sutures, acute injections can be given through the fibrous tissue using an injector with a fixed-length projection to prevent damage to the underlying dura mater. Using this approach, the ability of stimuli to produce headache behavior without the confounds of a prior surgery can be assessed. The current studies used this non-invasive supradural injection method in mice to examine behavioral responses to stimuli previously found to induce headache behavior in cannulated rats, including allyl isothiocyanate (AITC, aka mustard oil (MO)), low pH, and interleukin-6 (IL-6), and tested whether priming to IL-6 occurs in the absence of a craniotomy. Additionally, these experiments determined whether the properties of dural afferents retrogradely labeled without a craniotomy are similar to those previously published in rats where surgical exposure of the dura was conducted to apply the tracer.
Methods
Animals
Male and female adult ICR (6–8 weeks; Envigo) mice were maintained in a temperature-controlled room on a 12-hour light/dark cycle with food and water ad libitum. All procedures were performed in accordance with the policies of the International Association for the Study of Pain, the ARRIVE guidelines, as well as the National Institutes of Health guidelines for use of laboratory animals. All procedures were conducted with prior approval of the Institutional Animal Care and Use Committee at the University of Texas at Dallas.
Injections
Mouse dural injectors were created by modifying a commercially-available cannula (Invivo1, part #C313I/SPC, Internal Cannula, Standard, 28 gauge, I.D. 0.18mm, O.D. 0.35mm) (Supplementary Figure 1). The projection depth of the internal cannula was determined by adjusting the location of the outer plastic pedestal manually down the length of the injector in order to shorten the projection. A digital caliper was used to measure the injector projection length (which can range from 0.5mm to 0.65mm), which is adjusted based on the weight of the mice. The projection length allows soft tissue to be displaced, bypassing the unfused suture plates, while limiting the length to maintain dural integrity. In 30–35g mice, the suture junction was measured post-mortem to be ~0.5mm thick, and a 0.15mm projection beyond the inner face of the skull (0.65mm total projection length) does not impact the dura at this location. For animals of less than 30 grams (which is commonly the case with C57BL6 mice; only ICR mice were used in these studies), the suture junction is thinner and there is less subcutaneous tissue (fascia and/or adipose tissue), requiring injectors that are shorter and may even approach 0.5mm total projection length. Suture junctions in these smaller mice can be as small as ~0.4mm thickness and require a 0.1mm projection beyond the inner face of the skull at the suture plate to avoid damage to the dura. Pilot experiments on mice not used in these studies were regularly performed to determine the thickness of the suture plate and the necessary injector projection length based on the weight of the mice. An additional age-related concern is the time point when the cranial plates fuse completely, a detail that is not completely clear and likely variable between laboratories, based on diet among other factors. This time point is also variable with strain of mouse. For example, in the outbred ICR mice used here, dural injections could be given up to about 7 weeks of age. In contrast, in C57BL6 mice (not used in the current study) injections can be given at least up to 12 weeks. Pilot experiments are generally necessary at the beginning of any study to determine the limitations of when dural injections can be given. Once the specific parameters were determined for these studies, the modified injectors were then attached to a 10ml glass syringe cemented needle (Hamilton Company, 700 series) via Tygon tubing (Cole-Palmer, Item # EW-96460–16).
Supradural injections are given ~4.8mm posterior to the bregma on the junction of the sagittal and lambdoid sutures. Differences in mouse age and body weight may require adjustments of the injection coordinates and/or injector length. Animals weighing more than 30g generally have slightly larger skulls, thus the structure junction may be >4.8mm posterior from bregma. Animals of differing weight/age are examined post mortem in order to identify the appropriate modifications to the injection site. Using the injector described above, the cranial bone sutures at bregma and lambda are identified via topographical features of the cranial plates. The junction of the sagittal and lambdoidal sutures is located by inserting the injector through the skin and gently probing the lambdoid suture line where it intersects the sagittal suture line using the tip of injector, and verified by re-positioning the injector along the skull. The injector is gently placed between the junction of the sagittal and lambdoidal sutures using light downward force to allow for injection. Injections (5μl) are performed under light isoflurane anesthesia administered via nose cone from a vaporizer. Mice are under isofluorane anesthesia for less than 2 minutes, and injections were administered onto the dura of the animals. The animals were then returned to the testing boxes. Fluorogold was injected onto mice dura using an identical technique 7–10 days prior to dissection of the trigeminal ganglia. At the end of experiments, mice are checked for integrity of the dura to ensure that no damage was caused during the injection procedure. See Supplementary Figure 2 for images of dural injections with dye showing spread of the injection within the dura but not onto the cortex.
Solution preparation
AITC (Sigma) 10% was prepared in mineral oil by diluting 1:10 from 100% MO. No stock solution of AITC was prepared. MO was prepared at a concentration known to induce dural nociceptor activation in our rat behavioral studies (13). The vehicle injection was 100% mineral oil (Min oil). Rat recombinant IL-6 (R&D Systems) stock solution (10 μg/ml) was prepared in sterile 0.1% bovine serum albumin (BSA) and diluted to final concentrations of 10 ng/ml in synthetic interstitial fluid (SIF) (pH 7.4, 310 osmolality). The SIF contained 10mM HEPES, 5mM KCl, 135mM NaCl, 1mM MgCl2, 2mM CaCl2, and 10mM glucose. SIF was used at pH 6.0, 6.8, 7.0, and 7.4. Inflammatory soup (IS) was SIF containing bradykinin, histamine, 5HT (all at 1 mM), PGE2 (100 μM), and pH 5.0. All components were from Sigma except PGE2, which was from Cayman Chemicals. Sumatriptan was prepared from a stock solution of 0.1mg/ml sumatriptan in sterile PBS. Fluorogold (Fluorochrome, LLC) was dissolved in SIF (pH 7.4, 310 osmolality) to 4%.
Grimace scale
Coding of facial expressions was conducted using a three-point scale (0 = not present, 1 = moderately present and 2 = obviously present) as previously described (24). All experimenters were blinded to experimental groups, which prevented experimenter bias when attempting to score facial expressions. Average grimace score was determined and analyzed as described below.
Behavioral testing: Allodynia and grimace
Animals were separated into experimental groups using a block randomization protocol with block size dependent on the number of experimental groups (e.g. the number of pH stimuli given). A statistical power analysis was performed for sample size estimation using GPower 3.1 for a repeated measures group comparison. The effect size of this study was estimated at f=0.4 based on data from previous similar studies. With an alpha of 0.05 and power at 0.80, the projected sample size needed with this effect size was approximately five per group. Male and female adult ICR mice were habituated to tapered single poly-coated paper cylindrical facial testing chambers (Choice 4oz. paper cups; 6.5cm top diameter, 4.5 cm bottom diameter, 72.5 cm length) while contained in clear acrylic compartments (5 cm length × 7.6 cm width × 23 cm height) for two hours each day for three days, prior to baseline (Supplementary Figure 3). The use of this larger compartment containing the facial testing chamber allows mice free access to explore the chamber without physical restraint, but they are unable to move beyond the acrylic compartment; that is, they are confined to the testing chamber. Animals were not deprived of food or water; they were given a food pellet and Hydrogel® (Clear H2O) during habituation. Once habituated, mice remain within the testing chamber without the need for the larger acrylic compartment. However, mice are kept in the larger compartment during testing time points, and only one mouse at a time is removed from the acrylic compartment for periorbital von Frey testing.
Prior to injection, baseline grimace, facial, and hindpaw thresholds were recorded for each animal. Once facial thresholds had been tested, animals were placed back in hindpaw testing chambers to habituate for approximately 5 mins before beginning hindpaw testing. Grimace was evaluated in the hindpaw testing chambers. For grimace, mice are observed over a 15min period starting at each time point (e.g. starting at 3 hours post injection). During this time window, the face of the mouse is not continually visible for the entire 15min. When a mouse face is in view, scoring takes place. Grimace is evaluated only once for each mouse at each time point during the 15min window when the scoring is conducted. Grimace was scored using the method and five expressions listed (24) that include a) orbital tightening; b) nose bulge; c) cheek bulge; d) ear position; e) whisker change. Following hindpaw testing and grimace measurement, mice are returned to the larger acrylic compartment in the facial testing chambers until the next time point. This sequence of testing was repeated at all data points.
For dural injection, animals were briefly anesthetized, an injection was made as described above, and the animals were then returned to the testing boxes. Grimace, facial and hindpaw thresholds were measured at 1, 3, 5, and 24 hours post-injection using the Von Frey up-down method of testing. The Von Frey filaments were applied to the peri-orbital region of the face or to the plantar surface of the hind paw perpendicularly, until the entire force was applied and held for approximately 3 seconds or until animals withdrew. Maximum filaments used were 0.6g for the peri-orbital region and 2g for the hindpaw. For biphasic behavioral experiments (where priming is induced), facial and hindpaw withdrawal thresholds were measured until mice returned to baseline, at which point the second dural stimulus was given using the same injection protocol described above. All experimenters were blinded to experimental groups, which prevented experimenter bias when attempting to measure withdrawal thresholds.
Trigeminal neuron culture
The left and right trigeminal ganglia were dissected 7–10 days post application of fluorogold to the dura mater, and tissue was enzymatically treated with Papain and Collagenase Type II. The tissue was triturated 4–5 times, plated onto Poly-D-Lysine and laminin coated 35mm Petri dishes, flooded with L-15 solution 2–3 hours post-plating, and kept at room temperature. Cells were used for patch-clamp experiments within 24 hours of plating. In separate animals, trigeminal ganglia were dissected 5 days post application of fluorogold to the dura mater from perfusion-fixed mice and sliced to visualize the location of labeling within the ganglia (Supplementary Figure 4).
Whole cell patch-clamp electrophysiology
A MultiClamp 700B patch-clamp amplifier with pClamp 10 acquisition software (Axon Instruments) was used on isolated rat trigeminal ganglia neurons. Pipettes (OD: 1.5mm, ID: 0.86mm, Sutter Instrument) were pulled using a P-97 puller (Sutter Instrument) and polished to 1.5–3 MΩ resistance using a microforge (MF-83, Narishige). During the recording, series resistance was less than 7 MΩ with 80% compensation. Solenoid valves attached to gravity-fed flow tubes were used to perfuse extracellular solution over the cells during recording. Recordings were sampled at 2kHz and filtered at 1kHz (Digidata 1322A, Axon Instruments). All recordings were performed at room temperature. A Nikon TE2000-S microscope equipped with a mercury arc lamp (X-Cite® 120) was used to identify FG-labeled dural afferents. Data were analyzed using Clampfit 10 (Molecular Devices) and Origin 8 (OriginLab). Cell sizes were not significantly different among groups. Labeled neurons fall in a range of capacitance values from 15–80 pF (average of 57.47±5.47). Pipette solution contained (in mM) 140 KCl, 11 EGTA, 2 MgCl2, 10 NaCl, 10 HEPES, 2 MgATP, 0.3 Na2GTP, 1CaCl2 pH 7.3 (adjusted with N-methyl glucamine), and was ~320 milli-Osmolar (mosM). External solution contained (in mM) 135 NaCl, 2 CaCl2, 1 MgCl2, 5 KCl, 10 glucose, 5 HEPES, 5 MES (4-morpholineethanesulfonic acid), pH 7.4 (adjusted with N-methyl glucamine), and was ~320 mosM. External solutions at pH 6.0 were prepared by adjusting the pH of the standard external solution. Solution exchange time was approximately 20ms.
Statistics
Behavioral data are graphed as means±SEM. Statistical evaluations of allodynia studies were conducted using GraphPad Prism Version 6.0 (GraphPad Software Inc., La Jolla, CA, USA). Data was analyzed among groups and across time by one- or two-way analysis of variance (ANOVA) for treatment and time, followed by Bonferroni post-test where appropriate. Significance was set at p<0.05 for all data analysis.
Results
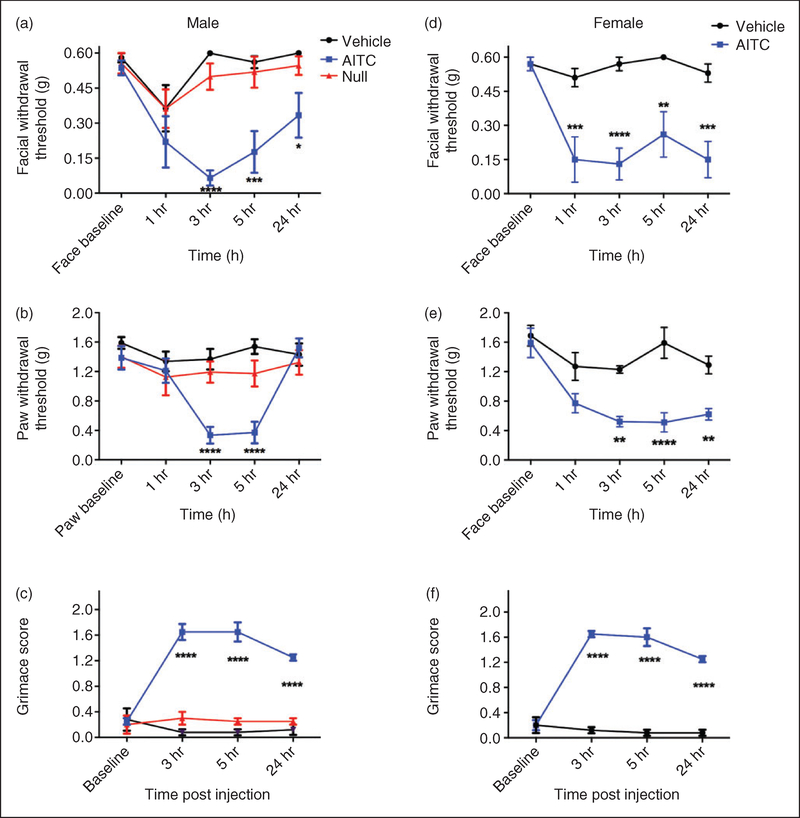
Behavioral responses following supradural injection in mice were evaluated for comparison to results published previously in cannulated rats. First, we Investigated the effects of dural application of 10% AITC, as we have shown previously that this stimulus causes facial and hindpaw allodynia as well as decreased rearing in rats (13). Dural application of AITC produced facial and hindpaw allodynia in both male (Figure 1(a) and (b)) and female mice (Figure 1(d) and (e)). Application of AITC to the dura also produced facial grimace in both male (Figure 1(c)) and female mice (Figure 1(e)). In order to determine whether the injection itself and not the AITC caused behavioral responses, vehicle (mineral oil) was also injected on the dura. No response was observed following dural injection of vehicle. There was significant hypersensitivity of both the facial and hindpaw regions from 3–5 hours post AITC injection compared to vehicles and animals had not returned to baseline by 24 hours (with the exception of male hindpaw responses). Grimace was still present in both males and females at 24 hours (Figure 1(c) and (f)). Similar responses were observed between male and female mice, although females had slightly more pronounced hypersensitivity at the 24-hour time point.
Figure 1.
Non-invasive dural application of 10% AITC produces headache-related behaviors in both male and female mice. Dural application of AITC produces cutaneous allodynia in both male ((a), (b)) and female mice ((d), (e)). Withdrawal thresholds to tactile stimuli applied to the face ((a), (d)) and hindpaws ((b), (e)) were measured in animals prior to and after dural application of AITC, vehicle (mineral oil) or a null injection (n = 6 for all groups, see methods for definition of null injections). In males, null injections failed to produce significant facial and hindpaw allodynia at all timepoints ((a), (b)). Administration of AITC produced significant allodynia facial (a) and hindpaw responses (b) in males. In females, administration of AITC produced significant facial allodynia (d) and hindpaw responses (e). Grimace behaviors for both males (n = 6 all groups, (c) and females (n = 6 all groups, (f) were significant at multiple time points. Two-factor analysis of variance (ANOVA) indicated a significant effect of both treatment and time of both the face and hindpaws. Significant differences among means for each group were determined by analysis of variance followed by Bonferroni post hoc test. Males (a) facial: Time F (4, 75) = 6.602, p= 0.0001, treatment F (2, 75) = 6.602, p < 0.0001; (b) hindpaw: Time F (4, 75) = 6.572, p < 0.0001, treatment F (2, 75) = 13.03, p < 0.0001; (c) grimace: Time F (3, 40) = 11.68, P < 0.0001, treatment F (2, 40) = 131.5, P < 0.0001. Females (d) facial: Time F (4, 50) = 5.473, p= 0.001, treatment F (1, 50) = 62.11, p < 0.0001; (e) hindpaw: Time F (4, 50) = 9.213, p < 0.0001, treatment F (1, 50) = 46.4, p < 0.0001; (f) grimace: Time F (3, 28) = 26.75, p < 0.0001, treatment F (1, 28) = 303.8, p < 0.0001.
It is possible that diffusion of AITC out of the tip of the injector while the open tip is in contact with surrounding tissue (even in the absence of pressure applied to the syringe plunger) could allow AITC actions within the fibrous tissue in the suture or on skin/fascia outside of the bone that is in addition to the AITC actions on the dura. These non-dural actions of AITC could contribute to the observed behavioral responses. To evaluate this possibility, null injections were given to male mice. A null injection is defined as an injection where the syringe and injector are loaded with 10% AITC, and the injector is advanced through the suture, but the contents of the syringe/tubing/injector are not expelled onto the dura. Similar to vehicle injections, null injections failed to produce significant changes in withdrawal thresholds. These data indicate that the observed allodynia is due to the exposure of the dura to AITC and not simply due to the injection procedure or diffusion of AITC out of the tubing before/during/after the injection.
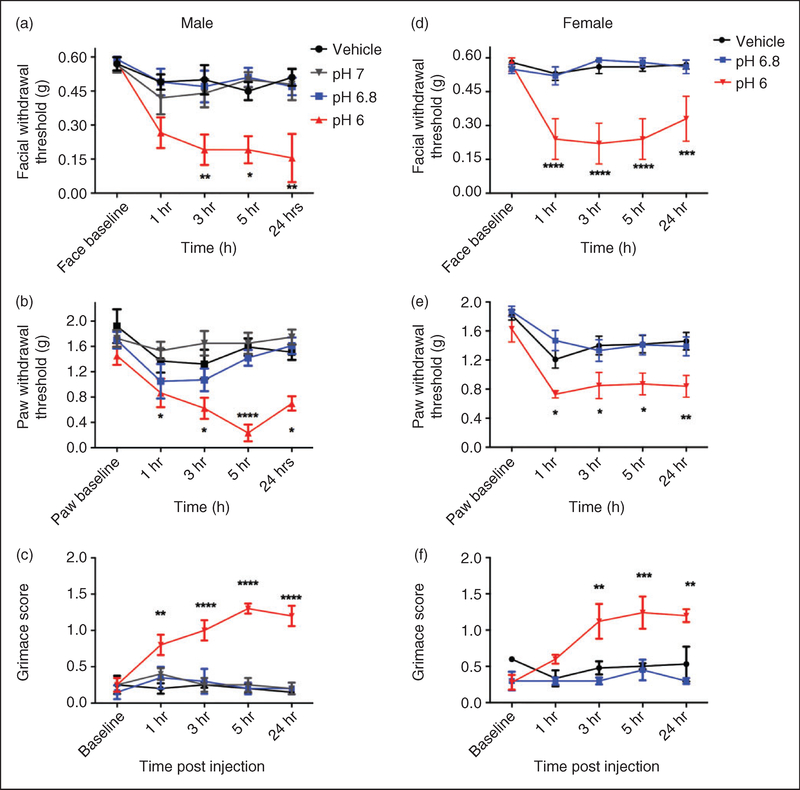
We have also shown that dural application of low pH solutions in cannulated rats causes facial and hindpaw allodynia (25,26). In the next series of experiments, pH 6.0, 6.8, and 7.0 SIF were compared with SIF at normal pH 7.4 (vehicle) to determine the pH sensitivity of male mouse dura. Dural application of pH 6, but not pH 6.8, 7.0, and vehicle, produce allodynia in male mice (Figure 2(a)) with significant facial responses between 1 hour and 24 hours, and significant paw responses at 5 hours and 24 hours. Similarly, dural application of pH 6, but not pH 6.8 or vehicle, produced allodynia in female mice from 1 hour to 24 hours post injection (Figure 2(b)). Similar to AITC in Figure 1, application of pH 6.0, but not 6.8 or vehicle, caused significant facial grimace responses in males from 1–24 hours (Figure 2(c)) and in females from 3–24 hours (Figure 2(f)).
Figure 2.
Non-invasive dural application of pH 6.0 produces headache-related behavior in male and female mice. Dural application of low pH (pH 6) produces cutaneous hypersensitivity in both male ((a), (b)) and female mice ((d), (e)). Withdrawal thresholds to tactile stimuli applied to the face (a) and hindpaws of male mice (b) were measured in animals prior to and following dural application of pH 6.0 (1–5 hr; n = 13, 24 hr; n = 7), pH 6.8 (n = 8) pH 7.0 (n = 7) or vehicle (pH 7.4; n = 8). In males, only administration of pH 6.0 produced significant facial (a) and hindpaw (b) responses. Similarly, female withdrawal thresholds to tactile stimuli applied to the face (d) and hindpaws (e) were measured in animals prior to and following dural application of pH 6.0 (n = 11), pH 6.8 (n = 8) or vehicle (pH 7.4, n = 16). In females, dural administration of pH 6 produced significant facial (d) and hindpaw (e) responses. Grimace behaviors were significant in males (n = 5, (c)) and in females (n = 5, (f)). Two-factor analysis of variance (ANOVA) indicated a significant effect of both treatment and time of both the face and hindpaws. Significant differences among means for each group were determined by analysis of variance followed by Bonferroni post hoc test. Males (a) facial: Time F (4, 154) = 6.631, p < 0.0001, treatment F (3, 154) = 18.12, p < 0.0001; (b) hindpaw: Time F (4, 154) = 5.7, p < 0.0001, treatment F (3, 154) = 22.75, p< 0.0001; (c) grimace: Time F (4,80) = 3.976, p= 0.00054, treatment F (3, 80) = 51.78, p < 0.0001. Females (d) facial: Time F (4, 160) = 4.64, p= 0.0014, treatment F (2, 160) = 54, p < 0.0001; (e) hindpaw: Time F (4, 160) = 11.26, p < 0.0001, treatment F (2, 160) = 22.64, p < 0.0001; (f) grimace: F (4, 60) = 4.332, p= 0.0038, treatment F (2, 60) = 24.41, p < 0.0001
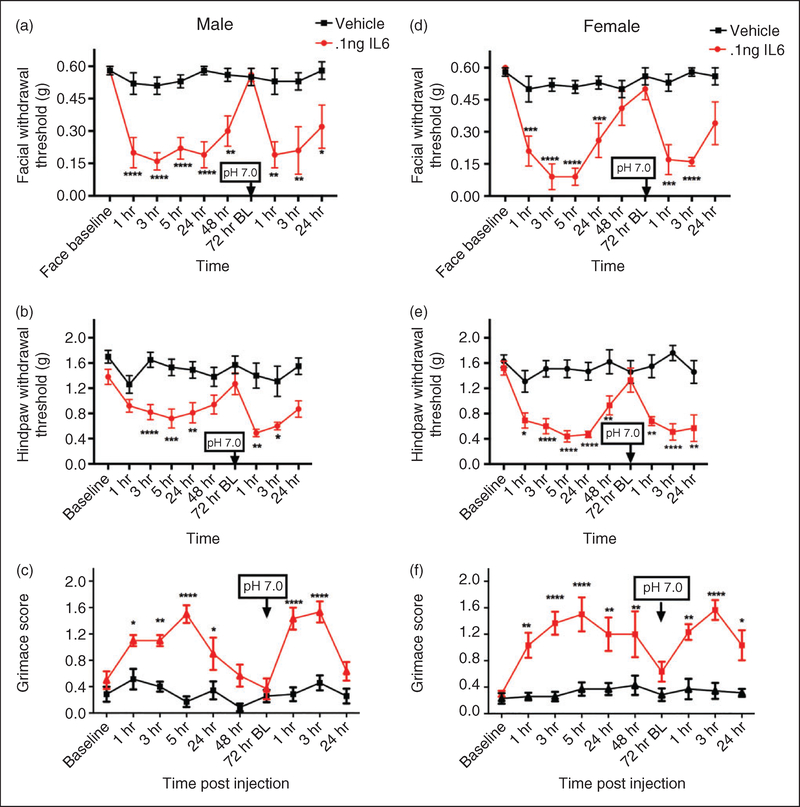
Next, we determined whether dural application of IL-6 to the mouse produces responses similar to those we have shown previously in cannulated rats. Prior studies in rats found that dural IL-6 produced cutaneous facial and hindpaw allodynia that developed with the typical time course but remained significantly different from baseline at 24 hours post injection (27). Subsequent studies found that headache behavior due to dural IL-6 resolved by 48 hours, but that at 72 hours rats were primed to normally subthreshold dural stimulation with a pH 7.0 solution (28). IL-6 was applied to the mouse dura in the current studies at the same concentration as the prior rat studies (0.1ng) to characterize both the acute hypersensitivity response as well as whether mice are also primed following resolution of the acute phase. Dural application of IL-6 in male mice produced both significant facial and hindpaw allodynia on day 1 and at 24 hours post injection but was returned to baseline at 72 hours (Figure 3(a) and (b)). To investigate if male mice were primed at the 72-hour time point, animals were given a subsequent application of dural pH 7.0. Only animals initially treated with IL-6 were primed to supradural application of pH 7.0, showing significant responses post injection; vehicle-treated animals showed no response to this pH value, consistent with a lack of response to pH 7.0 in naïve animals shown in Figure 2. Similar cutaneous hypersensitivity responses and time courses to males were observed in female mice treated with the same injections and doses (Figure 3(d) and (e)).
Figure 3.
Non-invasive dural application of IL-6 produces headache-related behavior in both male and female mice. Dural application of IL-6 (0.1 ng) produces cutaneous hypersensitivity in both male ((a), (b)) and female mice ((d), (e)). Males treated with IL-6 (1–48 hr; n = 13, 72 hr BL and later; n = 7), showed significant facial (a) and hindpaw (b) responses and also significant responses to subsequent dural pH 7.0. Similarly, females treated with IL-6/dural pH 7.0 (1–48 hr; n = 12, 72 hr BL and later; n = 7) had significant facial (d), and hindpaw (e) responses and also significant responses to subsequent dural pH 7.0. Grimace behaviors were significant in males (n = 6, all timepoints; (c) and in females (n = 7, (e)). Significant differences among means for each group were determined with one-way ANOVA followed by Bonferroni post hoc test. Males facial: Time F (9, 177) = 4.768, p < 0.0001, treatment F (1, 177) = 106.5, p < 0.0001; hindpaw: Time F (9, 166) = 3.135, p= 0.0016, treatment F (1, 166) = 82.65, p < 0.0001); grimace: Time F (9, 110) = 7.305, p < 0.0001, treatment F (1, 110) = 127.4, p < 0.0001. Females facial: Time F (9, 151) = 6.818, p < 0.0001, treatment F (1, 151) = 120.7, p < 0.0001; hindpaw: Time F (9, 151) = 3.758, p < 0.0001, treatment F (1, 151) = 130.4, p < 0.0001); grimace: Time F (9, 110) = 3.578, p= 0.0004, treatment F (1, 110) = 125.8, p < 0.0001.
As shown with AITC and pH 6.0, dural IL-6 also caused increased grimace responses in both males and females that was resolved by 72 hours post injection (Figure 3(c) and (f)). Application of a pH 7.0 solution to the dura at 72 hours caused reinstatement of grimace responses only in animals previously treated with IL-6, demonstrating that the IL-6 priming produces both cutaneous hypersensitivity and grimace following exposure to normally subthreshold stimuli. These data are consistent with those shown previously in rats, where priming to dural pH 7.0 solution is present following resolution of the acute IL-6 behavior (28).
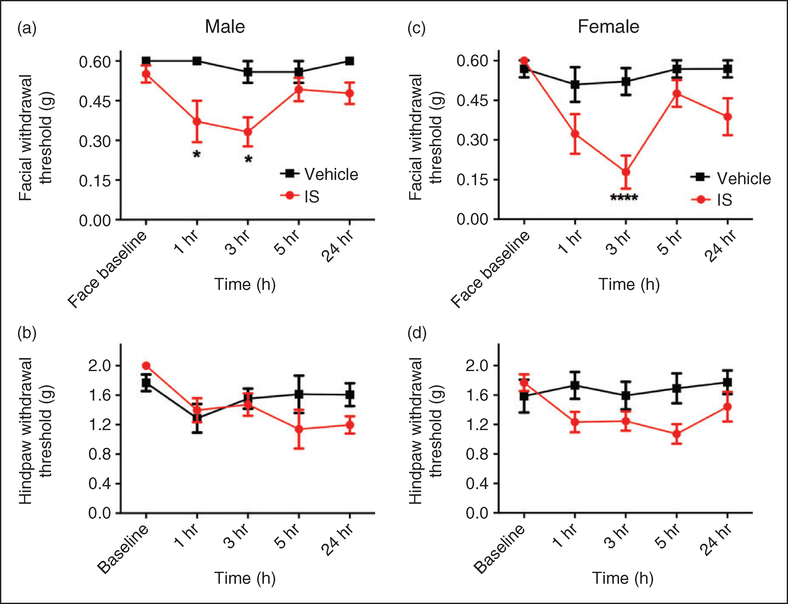
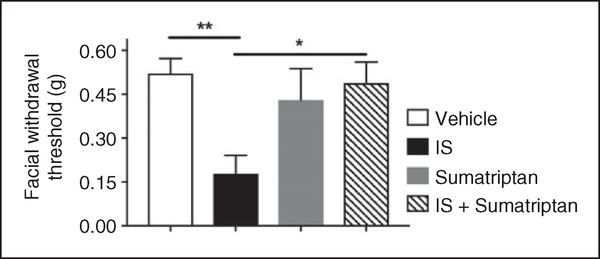
Inflammatory soup (IS) is a commonly used dural stimulus in preclinical migraine studies. This cocktail consists of a combination of bradykinin, histamine, 5HT, and PGE2, often at low pH. In order to determine whether this stimulus also causes behavioral responses using the mouse model described here, IS was applied to the mouse dura and behavioral responses were measured. Following IS injection, cutaneous facial allodynia was observed at 1 and 3 hours in males and at 3 hours in females (Figure 4(a) and (c)). In both sexes, allodynia had resolved by 5 hours and remained at baseline at 24 hours. Significant hindpaw hypersensitivity was not observed in either sex with this stimulus (Figure 4(b) and (d)), nor was facial grimace (data not shown). We also tested whether sumatriptan could attenuate IS-induced behavior in females, as efficacy of this drug would further support the model as producing migraine-related behavior. When sumatriptan (0.6mg/kg, i.p.) was injected at the same time as dural IS in female mice, there was significantly less facial allodynia at the 3-hour time point post injection compared to IS alone (Figure 5). There was no effect of sumatriptan treatment alone (i.e. in the absence of IS) on facial withdrawal thresholds. These data indicate that the behavioral responses following dural stimulation, at least with IS, are sensitive to this common acute anti-migraine agent.
Figure 4.
Non-invasive dural application of IS produces headache-related behavior in both male and female mice. Dural application of IS produces cutaneous facial hypersensitivity in both male (a) and female mice (d). Mice treated with IS (1–24 hr; n = 6–10), showed significant facial (a) and (c) responses, but no significant differences in hindpaw sensitivity were observed ((b) and (d)). Significant differences among means for each group were determined by two-way ANOVA followed by Bonferroni post hoc test. Males facial: Time F (4, 70) = 1.95, p= 0.1117, treatment F (1, 70) = 18.54, p < 0.0001; hindpaw: Time F (4, 55) = 2.903, p= 0.0299, treatment F (1, 55) = 1.128, p= 0.2928. Females facial: Time F (4, 75) = 5.908, p= 0.0003, treatment F (1, 75) = 21.28, p < 0.0001; hindpaw: Time F (4, 60) = 1.111, p= 0.3599, treatment F (1, 60) = 9.261, p= 0.0035.
Figure 5.
Cutaneous facial allodynia following non-invasive dural application of IS is blocked by sumatriptan. Dural application of IS produced cutaneous facial hypersensitivity in female mice. Withdrawal thresholds to tactile stimuli applied to the face were measured in mice at the 3-hour time point following dural application of vehicle, IS, sumatriptan, or IS + sumatriptan (n = 6–9). A significant decrease in withdrawal threshold was observed only in the IS group. Significant differences among means for each group were determined by analysis of variance followed by Bonferroni post hoc test. Females facial: Treatment F (3, 26) = 5.408, p= 0.005.
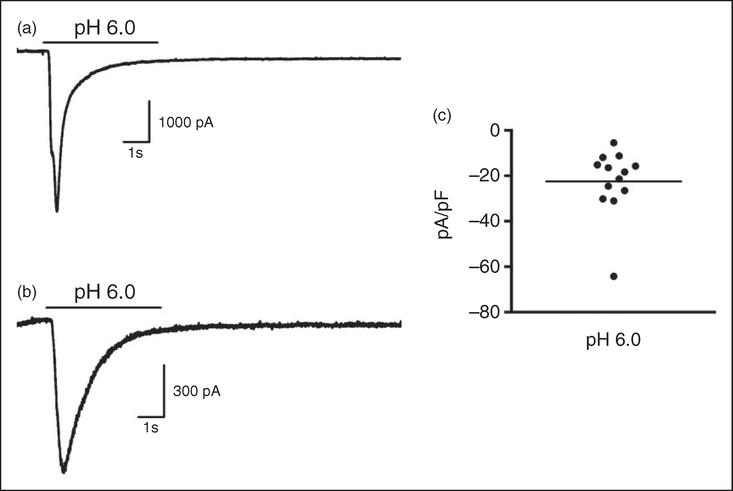
We have previously shown in rats that dural application of the retrograde tracer fluorogold leads to labeling of trigeminal ganglion cell bodies that can be identified in primary cell culture and recorded using in vitro electrophysiology (13,25,27,29). Using this technique, we found that approximately 80% of identified dural afferents respond to application of a pH 6.0 solution (25). However, fluorogold was applied to the dura in these studies following a procedure to thin the skull such that injections can easily be given through a thin layer of bone. To verify that supradural injections similarly access and label dural afferents in mice when applied via the suture injection described above, we applied fluorogold to mouse dura to label cell bodies in the trigeminal ganglion. As expected, fluorogold injections resulted in labeling of TG cell bodies when ganglia were removed 7–10 days later. Supplementary Figure 4 shows a section of mouse trigeminal ganglia with cell bodies labeled predominantly in the V1 region of the ganglia. Identified dural afferents from mice in vitro were tested using stimulation with an acidic pH of 6.0, identical to the experiments conducted previously in rats (25). The capacitances of retrogradelylabeled cells chosen for recording ranged from 22.7 to 62.02 pF. Approximately 65% (13 of 20 neurons) of mouse trigeminal ganglion neurons retrogradely labeled from the dura responded to pH 6.0 stimulation with currents consistent with those generated by acidsensing ion channels (ASICs) (Figure 6); this percentage is similar to the 80% response rate previously observed in rat dural afferents (25). Examples of typical currents generated from mouse dural afferents upon pH 6.0 application are shown in Figure 6(a) and (b) and were of both fast and slow desensitization kinetics, again consistent with different subtypes of ASICs. As seen in Figure 6(c), the current density (pA/pF) distribution for the 13 responding neurons ranged from ~5–30 (mean of 22.5), with one cell at 64pA/pF, also similar to our prior work (25).
Figure 6.
Mouse trigeminal ganglion neurons retrogradely labeled from the dura mater generate ASIC-like currents at pH 6.0. Recordings from retrogradely-labeled mouse dural afferents in response to a 5-sec pH change from 7.4 to 6.0 shown in (a) and (b) are characteristic of ASIC currents. (c) Current density (pA/pF) of the 13 neurons that responded to pH 6.0 (out of 20 total neurons recorded) range from 5–65 pA/pF with a mean of 22.5 pA/pF.
Discussion
Migraines occur in the absence of apparent tissue injury. Since the most likely mechanism for the pain of migraine is activation of meningeal afferents, preclinical models that study the activation, sensitization, and plasticity of these neurons in the absence of tissue injury are most applicable to the human state. Understanding the function and pathology of these neurons without injury may allow more accurate assessment of the underlying mechanisms contributing to migraine headaches.
The current studies report the development and characterization of such a mouse model of migraine, where dural afferents can be non-invasively activated. Similar to our previous work in rats, we demonstrate here that headache behavioral responses can be induced by stimulation of the mouse dura with AITC, pH 6.0, and IL-6 (13,25,27), and that IL-6 causes priming of the dural afferent system to subsequent challenge with pH 7.0 (28). We also show that IS produces cutaneous facial hypersensitivity and these responses are blocked by the commonly-used acute migraine therapeutic sumatriptan. Additionally, we show here that the responses to all of these stimuli are similar in males and females. The time courses of behavioral responses in mice to these stimuli are similar to those published previously in rats (although only male data exists in rats).
Questions have been raised with the prior data in rats regarding what role the craniotomy and cannula implantation surgery plays in the responses to the stimuli applied to the dura. The responses observed in the current study with mouse dural stimulation in the absence of a craniotomy are almost identical to those seen previously in rats. Thus, the likely conclusion is that there is little influence of the prior craniotomy on the responses to these specific stimuli, at least given the 7-day recovery period between surgery and dural stimulation. That said, the prior craniotomy may influence the responses to other stimuli not tested here. We report in a companion study that application of the mast cell degranulator compound 48/80 to the mouse dura produces cutaneous facial and hindpaw allodynia as well as increased grimace responses (30). It is highly likely that a craniotomy surgery in rats would cause mast cell degranulation, as this has been shown 4 days post dural cannulation using a similar model (15). Whether 7 days between the craniotomy and testing is enough time for mast cells to repopulate the rat dura and thus be available to degranulate by compound 48/80 is not known. However, this could be one example of how a prior craniotomy would influence responses to subsequent dural stimulation.
In addition to behavioral responses, we show here that retrograde labeling from the mouse dura allows identification of TG cell bodies that innervate the dura when ganglia are removed from mice and cultured. Similar techniques have been used previously in rats (25,27,30,31). Our prior studies showed that dural-projecting TG neurons respond to stimulation with pH 6.0 solutions with ASIC-like inward currents (25,26). Our current studies show similar ASIC-like currents from mouse dural afferents. Thus, mouse dural afferents in vitro show similar pH-evoked current properties to rats despite the less invasive retrograde labeling protocol in mice. These data demonstrate that dural afferents likely express ASICs natively and increased expression of these channels is not due to inflammation/sensitization caused by retrograde labeling.
Much of the data generated using the current approach suggests that mouse and rat preclinical models of migraine are similar, and that the craniotomy used in rats plays little role in the findings previously shown (given sufficient recovery between surgery and testing). This raises the question of whether mice or rats should be used in studies moving forward. Our data suggest that either species is suitable. However, conducting preclinical migraine studies in mice offers two critical advantages. First, dural stimulation experiments in mice do not require the time consuming and technically challenging cannula implantation surgery, or the waiting period between surgery and testing, and thus may be more widely utilized due to greater ease and lower costs. Second, and probably more importantly, conducting these studies in mice allows for the use of genetically-modified animals to better understand the underlying mechanisms of dural afferent activation/sensitization and ultimately migraine in the absence of tissue injury. For example, genetic tools such as ASIC knockout mice (32) can allow testing of a role for these channels in migraine behavioral assays, providing complementary support to the use of pharmacological agents. Our companion paper utilizes this approach to test the role of PAR2 in preclinical migraine models by conducting experiments using both selective PAR2 pharmacological tools and also conducting experiments on PAR2 knockout mice (Hassler et al., companion manuscript). Additionally, dural stimulation can be applied to familial-hemiplegic migraine (FHM) knock-in models (33–35) to address how these mutants differ in their responses to dural stimulation without the potential confounds of how these mice may be influenced by tissue damage. Finally, optogenetic/chemogenetic tools (36,37) can be used in genetically-modified mice to dissect the contribution of various cell types (e.g. neurons, fibroblasts) within the dura to nociceptive signaling. Taken together, development of a non-invasive dural stimulation model will substantially enhance the ability to test the role of the trigeminovascular system in migraine in a state more similar to human patients; that is, without cranial injury.
Supplementary Material
Article highlights.
The dura mater can be stimulated for behavioral experiments in mice without the need for a craniotomy or cannula implantation.
Non-invasive noxious dural stimulation causes behavioral responses consistent with migraine including cutaneous hypersensitivity and facial grimace.
Retrograde tracing of trigeminal neurons from the mouse dura mater can be performed without a craniotomy or cannula implantation.
This model can be used to test mouse behavioral responses following dural stimulation in the absence of tissue injury.
Acknowledgments
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: These studies were funded by the National Institutes of Health (NS072204), the Migraine Research Foundation, and The University of Texas System.
Footnotes
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Hougaard A, Amin FM and Ashina M. Migraine and structural abnormalities in the brain. Curr Opin Neurol 2014; 27: 309–314. [DOI] [PubMed] [Google Scholar]
- 2.Burstein R, Noseda R and Borsook D. Migraine: Multiple processes, complex pathophysiology. J Neurosci 2015; 35: 6619–6629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goadsby PJ, Holland PR, Martins-Oliveira M, et al. Pathophysiology of migraine: A disorder of sensory processing. Physiol Rev 2017; 97: 553–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380: 2163–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diener HC, Dodick DW, Goadsby PJ, et al. Chronic migraine – classification, characteristics and treatment. Nat Rev Neurol 2012; 8: 162–171. [DOI] [PubMed] [Google Scholar]
- 6.Ray BS and Wolff HG. Experimental studies on headache–pain-sensitive structures of the head and their significance in headache. Arch Surg-Chicago 1940; 41: 813–856. [Google Scholar]
- 7.Penfield W and McNaughton F. Dural headache and innervation of the dura mater. Arch Neuro Psychiatr 1940; 44: 43–75. [Google Scholar]
- 8.Noseda R and Burstein R. Migraine pathophysiology: Anatomy of the trigeminovascular pathway and associated neurological symptoms, CSD, sensitization and modulation of pain. Pain 2013; 154 DOI: 10.1016/j.pain.2013.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strassman AM, Raymond SA and Burstein R. Sensitization of meningeal sensory neurons and the origin of headaches. Nature 1996; 384: 560–564. [DOI] [PubMed] [Google Scholar]
- 10.Levy D Migraine pain and nociceptor activation – where do we stand? Headache 2010; 50: 909–916. [DOI] [PubMed] [Google Scholar]
- 11.Melo-Carrillo A and Lopez-Avila A. A chronic animal model of migraine, induced by repeated meningeal nociception, characterized by a behavioral and pharmacological approach. Cephalalgia 2013; 33: 1096–1105. [DOI] [PubMed] [Google Scholar]
- 12.Oshinsky ML and Gomonchareonsiri S. Episodic dural stimulation in awake rats: A model for recurrent headache. Headache 2007; 47: 1026–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edelmayer RM, Le LN, Yan J, et al. Activation ofTRPA1 on dural afferents: A potential mechanism of headache pain. Pain 2012; 153: 1949–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Felice M, Eyde N, Dodick D, et al. Capturing the aversive state of cephalic pain preclinically. Ann Neurol 2013; 74: 257–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wieseler J, Ellis A, Sprunger D, et al. A novel method for modeling facial allodynia associated with migraine in awake and freely moving rats. J Neurosci Methods 2009; 185: 236–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stucky NL, Gregory E, Winter MK, et al. Sex differences in behavior and expression of CGRP-related genes in a rodent model of chronic migraine. Headache 2011; 51: 674–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang D, Ren L, Qiu CS, et al. Characterization of amouse model of headache. Pain 2016; 157: 1744–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bates EA, Nikai T, Brennan KC, et al. Sumatriptan alleviates nitroglycerin-induced mechanical and thermal allodynia in mice. Cephalalgia 2010; 30: 170–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pradhan AA, Smith ML, McGuire B, et al. Characterization of a novel model of chronic migraine. Pain 2013; 155: 269–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tassorelli C, Greco R, Morocutti A, et al. Nitric oxideinduced neuronal activation in the central nervous system as an animal model of migraine: Mechanisms and mediators. Funct Neurol 2001; 16: 69–76. [PubMed] [Google Scholar]
- 21.Recober A, Kuburas A, Zhang Z, et al. Role of calcitonin gene-related peptide in light-aversive behavior: Implications for migraine. J Neurosci 2009; 29: 8798–8804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sufka KJ, Staszko SM, Johnson AP, et al. Clinically relevant behavioral endpoints in a recurrent nitroglycerin migraine model in rats. J Headache Pain 2016; 17: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Felice M, Ossipov MH, Wang R, et al. Triptan-induced latent sensitization: A possible basis for medication overuse headache. Ann Neurol 2010; 67: 325–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Langford DJ, Bailey AL, Chanda ML, et al. Coding of facial expressions of pain in the laboratory mouse. Nat Methods 2010; 7: 447–449. [DOI] [PubMed] [Google Scholar]
- 25.Yan J, Edelmayer RM, Wei X, et al. Dural afferents express acid-sensing ion channels: A role for decreased meningeal pH in migraine headache. Pain 2011; 152: 106–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yan J, Wei X, Bischoff C, et al. pH-evoked dural afferent signaling is mediated by ASIC3 and is sensitized by mast cell mediators. Headache 2013; 53: 1250–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yan J, Melemedjian OK, Price TJ, et al. Sensitization of dural afferents underlies migraine-related behavior following meningeal application of interleukin-6 (IL-6). Mol Pain 2012; 8: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burgos-Vega CC, Quigley LD, Avona A, et al. Dural stimulation in rats causes BDNF-dependent priming to subthreshold stimuli including a migraine trigger. Pain 2016; 157: 2722–2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wei X, Edelmayer RM, Yan J, et al. Activation ofTRPV4 on dural afferents produces headache-related behavior in a preclinical rat model. Cephalalgia 2011; 31: 1595–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hassler SN, Ahmad FB, Burgos-Vega CC, et al. Protease activated receptor 2 (PAR2) activation causes migraine-like pain behaviors in mice. Cephalalgia. Epub ahead of print 2018. DOI: 10.1177/0333102418779548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harriott AM and Gold MS. Electrophysiological properties of dural afferents in the absence and presence of inflammatory mediators. J Neurophysiol 2009; 101: 3126–3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deval E and Lingueglia E. Acid-sensing ion channels and nociception in the peripheral and central nervous systems. Neuropharmacology 2015; 94: 49–57. [DOI] [PubMed] [Google Scholar]
- 33.Tolner EA, Houben T, Terwindt GM, et al. From migraine genes to mechanisms. Pain 2015; 156: S64–S74. [DOI] [PubMed] [Google Scholar]
- 34.Chen SP, Tolner EA and Eikermann-Haerter K. Animal models of monogenic migraine. Cephalalgia 2016; 36: 704–721. [DOI] [PubMed] [Google Scholar]
- 35.Sutherland HG and Griffiths LR. Genetics of migraine: Insights into the molecular basis of migraine disorders. Headache 2017; 57: 537–569. [DOI] [PubMed] [Google Scholar]
- 36.Roth BL. DREADDs for Neuroscientists. Neuron 2016; 89: 683–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim CK, Adhikari A and Deisseroth K. Integration of optogenetics with complementary methodologies in systems neuroscience. Nat Rev Neurosci 2017; 18: 222–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.