Abstract
Identification of defined epithelial cell populations with progenitor properties is critical for understanding prostatic development and disease. Here, we demonstrate that Sox2 expression is enriched in the epithelial cells of the proximal prostate adjacent to the urethra. We use lineage tracing of Sox2-positive cells during prostatic development, homeostasis, and regeneration to show that the Sox2 lineage is capable of self-renewal and contributes to prostatic regeneration. Persisting luminal cells express Sox2 after castration, highlighting a potential role for Sox2 in cell survival and castration-resistance. In addition to revealing a novel progenitor population in the prostate, these data implicate Sox2 as a regulatory factor of adult prostate epithelial stem cells.
Keywords: Sox2, Prostate, Stem cell, Regeneration
INTRODUCTION
Prostate disease, including cancer and benign prostatic hyperplasia, continues to be a significant health challenge. Insight into regulatory mechanisms of epithelial stem cell function in the prostate is essential to our understanding of normal epithelial formation and homeostasis as well as disease initiation. Notably, the intrinsic properties of these progenitor cells may dictate clinical behaviors of prostatic disease. The gene expression networks that regulate their behavior, therefore, may represent potential therapeutic targets for disease prevention and intervention.
The pseudostratified prostate epithelium is comprised of three cell types. Terminally differentiated, androgen-dependent luminal cells are marked by cytokeratin (CK) 8, 18, and androgen receptor (AR). Luminal cells comprise the vast majority of epithelial cells and produce the secretory components of prostatic fluid. Basal cells lie adjacent to the basement membrane and express CK 5, 14, and ΔNp63 [1]. Rare neuroendocrine (NE) cells, thought to influence proliferation of the prostatic epithelium through paracrine signaling, express synaptophysin and/or chromogranin A [2, 3]. Morphogenesis and maintenance of the prostate relies upon AR-mediated paracrine signaling from the stroma [4]. In mice, androgen deprivation via castration causes preferential apoptosis of luminal cells, whereas basal cells appear largely unaffected [5]. This prostatic atrophy can be reversed upon androgen replacement, suggesting the existence of castration-resistant progenitor cells that can give rise to differentiated daughters and regenerate the gland [6, 7]. Additionally, androgens can be cycled repeatedly with little difference in morphology or function of each resultant prostate [8]. Therefore, the murine prostate is a tractable model in which to investigate characteristics and behavior of epithelial progenitor cells, particularly in tissue regeneration.
Previous studies have suggested that there is a population of stem-like cells located in the adult murine proximal prostate, immediately adjacent to the urethra [7, 9]. They are reported to be slow-cycling, possess a high proliferative potential in vitro, and recapitulate the proximal–distal axis in tissue grafting assays [7, 9]. Importantly, this population of cells does not rely on androgen signaling for survival, in contrast to more distal cells in the adult prostate [9, 10].
Recently, molecular markers of prostatic epithelial progenitors have begun to be examined. Multiple lineage tracing studies have revealed the existence of castration-resistant luminal cell populations that contribute to prostate regeneration. The prototypical study focused on luminal cells expressing Nkx3.1, a downstream target gene of AR and well-known regulator of prostate epithelial differentiation [11, 12]. Castration-resistant Nkx3.1-expressing cells (CARNs) were shown to be bipotent, able to give rise to both luminal and basal cells in prostate regeneration [13]. Other reported castration-resistant populations are marked by Bmi1, a member of the polycomb-repressing complex, and Lgr5, a G-protein coupled receptor and member of the canonical Wnt family [14, 15]. Notably, castration-resistant Bmi1-expressing cells (CARBs), were shown to be a distinct, nonoverlapping population from CARNs, although both populations can serve as cells of origin in a murine model of prostate cancer [15]. Molecular characterization of these populations is ongoing.
In the mature adult prostate, epithelial lineages are considered to be self-sustained by lineage-specific progenitors [16]. During regeneration new luminal cells are largely derived from castration-resistant luminal cells that survive androgen deprivation [17, 18]. Although bipotent basal cells have been reported, they appear to make a small contribution to homeostasis and regeneration and require AR expression to do so [16, 18–20]. This is in contrast to the development of the postnatal prostate, during which basal cells serve as multipotent progenitors that can derive basal, luminal, and NE cells [21, 22]. Controlled by mitotic spindle orientation, basal cells can divide symmetrically (parallel to the basement membrane), generating two basal daughter cells, or asymmetrically (perpendicular to the basement membrane), generating a basal and a luminal daughter cell [23, 24].
The transcription factor SOX2 (sex determining region Y-box 2) is a putative stem cell factor that maintains embryonic pluripotency and contributes to fetal epithelium formation [25–27]. SOX2 has also been reported as both a driver of stem-ness in normal and malignant adult tissues, as well as a marker of progenitor populations in multiple tissues [28–40]. Notably, Sox2 was recently reported to promote lineage plasticity and resistance to antiandrogen therapy, a frontline strategy to treat prostate cancer [38, 41]. We and others have shown that a portion of ΔNp63-positive human basal epithelial cells express SOX2 [41, 42]. However, whether Sox2 marks a progenitor compartment competent for prostate homeostasis and regeneration in vivo has not been examined. In this study, we use lineage tracing to demonstrate that Sox2+ cells are castration-resistant and contribute to prostate regeneration.
MATERIALS AND METHODS
Animals
Sox2-CreER; ROSA26-lox-stop-lox-EYFP mice were recreated from commercially available strains (Sox2-CreER: 017593; R26-lsl-EYFP: 006148) sold by the Jackson Laboratory (Bar Harbor, ME) [27]. To induce Cre-mediated activity, mice were administered 2 mg tamoxifen (TAM; Sigma, St. Louis, MO) suspended in corn oil by intraperitoneal injection daily for 4 consecutive days. For in utero lineage tracing, a single pulse of 2 mg TAM with 1 mg progesterone (Sigma) was given to pregnant females at E11.5. All animal care and use was approved and monitored by the University of Chicago Institutional Animal Care and Use Committee.
Animal Procedures
Males were castrated as previously described [41]. After castration, silastic hormone pellets containing 12.5 mg testosterone (Steraloids, Newport, RI) were surgically implanted to induce prostatic regeneration. A 1 cm implant maintains host testoster-one levels at 5.3 T 0.5 ng/ml (18.2 nM) which is similar to eugonadal adult human males [43]. Animals were age-matched across conditions. All procedures were done in accordance with Institutional Animal Care and Use Committee (IACUC) guidelines, all efforts were made to minimize suffering. Prostatic regression and regeneration each took place over 3 weeks.
Histology and Immunofluorescence Staining
Prostates were fixed with freshly made 4% paraformaldehyde, infiltrated with sucrose and embedded in Optimal Cutting Temperature (OCT). Cryosections (5 μM) were blocked with 10% normal donkey serum (Sigma) in phosphate-buffered saline with Mouse-On-Mouse Blocking Reagent (catalog no. MKB-2213, Vector Labs, Burlinggame, CA) and incubated with primary antibodies (Supporting Information Table S1) diluted in block buffer. Sections then were incubated with secondary antibodies (Jackson ImmunoResearch, Westgrove, PA; Supporting Information Table S1). Sections were counterstained with Hoechst 33342 (catalog no. H3570, ThermoFisher Scientific, Hampton, NH) and mounted with ProLong Gold Antifade (Invitrogen/Molecular Probes, Eugene, OR).
Microscopy and Image Analysis
Immunofluorescence images were visualized using a Marianas Yokogawa type spinning disk inverted confocal fluorescent microscope (SlideBook, version 6). Maximal projections were composed in ImageJ, each image is scaled to its normalization time point for each lobe. Image analysis was performed using Fiji [44]. Automated cell counts were generated from 16-bit tiffs by subtracting background, and using threshold, water-shed, analyze particles to count cells. In cases where cells were unable to be accurately separated, cells were counted manually with the assistance of the Cell Counter Plugin (Kurt De Vos, release 2.2.2, http://imagej.net/Cell_Counter). Manual counting determined the number of YFP+/CK8+ or YFP+/p63+ cells with the aid of the Process → Math → AND command to identify costained cells.
Statistical Analysis
Statistics for all mouse experiments were analyzed as indicated in the figure legends. Data are displayed as mean ± SEM. n is the number of biological replicates unless otherwise specified. For image analysis, statistical analysis between groups was performed using one-way analysis of variance and post hoc Tukey Honest Significant Difference unless noted otherwise.
RESULTS
Embryonic Sox2+ Cells Can Serve as Precursors to Adult Basal and Luminal Cells
Sox2 has been shown to play an important role in the fetal development of multiple tissues, including the nervous system, anterior foregut endoderm and derivatives, retina, lens epithelium, taste bud, inner ear, stomach epithelium, lung, and testes [27, 32, 36, 45–50]. Therefore, we sought to determine whether Sox2 is expressed during embryonic formation of the urogenital sinus (UGS), the embryonic anlagen of the prostate. At embryonic day E12.5, during early UGS epithelial formation, we noted prominent and specific expression of Sox2 in the UGS by Immunohistochemistry (IHC) (Fig. 1A) that persisted through postnatal day 5 (Supporting Information Fig. S1, panel J) [51–53]. As the UGS epithelium begins to differentiate and p63 expression begins to stratify to the basal cell compartment, we observed Sox2 expression in both p63+ basal and luminal cells of the UGS at E18.5 (Supporting Information Fig. S1, panels N and I, respectively) [22]. In contrast, the mesoderm-derived Wolffian duct—the anlagen tissue of the seminal vesicle—did not contain cells with detectable Sox2 expression (Supporting Information Fig. S1, panel H).
Figure 1.
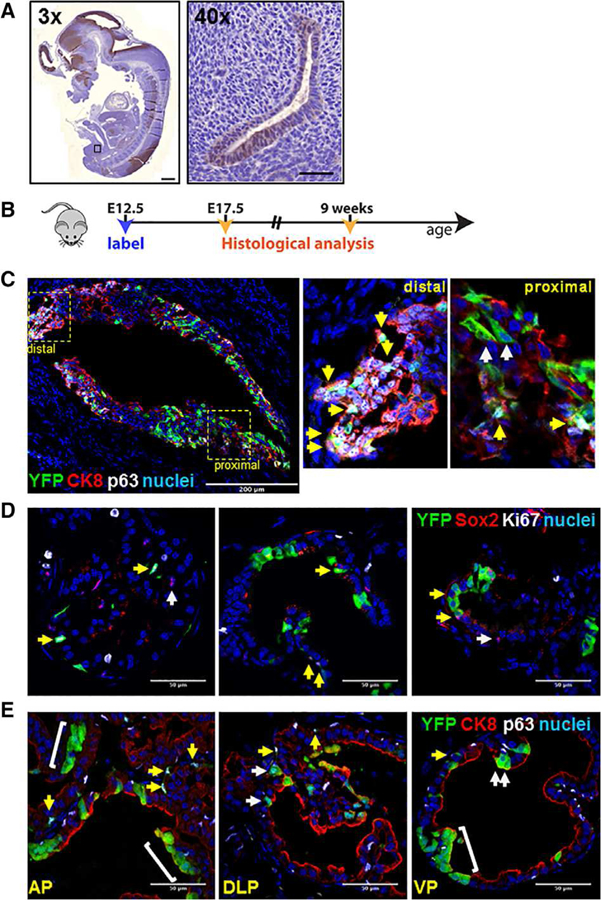
Embryonic Sox2+ cells can give rise to adult Sox2+ cells as well as basal and luminal cells. (A): Representative immunohistochemistry of Sox2 in the urogenital sinus epithelium (UGSE) of E12.5 embryos. Left panel: ×3 whole-mount magnification, scale bar is 500 μm. Right panel: ×40 magnification, scale bar is 50 μm. (B): Scheme for labeling embryos in utero. (C): Representative immunofluorescent (IF) staining shows colocalization of YFP with basal cell marker p63 and luminal cell marker CK8 in the UGSE of E17.5 embryo (montage images of sagittal sections). Scale bar: 200 μm. Insets are of proximal and distal regions of the UGSE. White arrows: YFP+ cells positive for CK8+ (luminal). Yellow arrows: YFP+ cells positive for p63+ (basal). (D): IF staining shows colocalization of YFP with Sox2 and Ki67 in postpubescent murine prostate. Yellow arrows: Sox2+ cells positive for YFP. White arrows: Sox2+ cells negative for YFP. (E): IF staining shows colocalization of YFP with p63 and CK8 in postpubescent murine prostate basal and luminal epithelial cells. Yellow arrows: YFP+ cells positive for basal cell markers. White arrows: YFP+ cells positive for luminal cell markers. Scale bar: 50 μm. Abbreviations: AP, anterior prostate; VP, ventral prostate; DLP, dorsolateral prostate.
Given that Sox2 is robustly expressed during early stages of prostate development, we investigated whether these Sox2+ cells contributed to embryonic prostate development. We adapted a previously published genetic lineage tracing approach to fate-map the Sox2 lineage in the prostate (Fig. 1B) [27]. Briefly, a TAM-inducible Cre construct (CreERT2) was knocked into the endogenous Sox2 locus; these mice were then crossed with a homozygous ROSA26-lox-Stop-lox-YFP reporter mouse [54]. TAM administration drives YFP expression permanently in Sox2+ cells and daughter progeny. To fate-map fetal Sox2+ cells, we injected pregnant females carrying Sox2-CreERT2; ROSA26-lsl-EYFP (hereafter Sox2-LT) embryos with TAM and progesterone at E12.5, coincident with UGS formation. We examined the distribution of YFP at E17.5, when the UGS epithelium begins to bud into the surrounding UGS mesenchyme [51–53]. We observed YFP expression in cells of both the distal and proximal regions of the urogenital sinus epithelium (UGSE), in double-positive CK8+/p63+ cells as well as single-positive CK8+ luminal and p63+ basal cells (Fig. 1C). There was no detectable YFP-expression in control mice not administered TAM (Supporting Information Fig. S2A), and throughout our studies we did not observe stromal Sox2-positivity. These data indicate that embryonic Sox2+ cells in the UGS are capable of deriving both basal and luminal lineages in the developing mouse prostate.
Given the robust expression of Sox2 in the embryonic and postnatal prostate, we next investigated whether Sox2+ cells emerging in the UGS are embryonic precursors for Sox2+ cells in the adult prostate. Pregnant dams were pulsed at E12.5, and their postpubescent Sox2-LT male progeny were sacrificed at 9 weeks of age to evaluate YFP expression. We noted strong coexpression of YFP and Sox2, particularly in the anterior and ventral lobes (Fig. 1D; of 1,878 total cells counted, 9.9% of total YFP+ cells coexpress Sox2, 25% of all Sox2+ cells coexpress YFP). We cannot exclude the possibility of incomplete YFP staining due to a partial Cre efficiency. A costain with the proliferation marker Ki67 demonstrates that Sox2+ cells are mostly negative for Ki67, indicating that these cells do not actively divide in the adult prostate (0.8% of Sox2+ cells costained for Ki67). We next sought to explicitly test whether these adult YFP+ cells constituted both the luminal and basal lineages of the adult murine prostate. Indeed, we observed YFP positivity in both the CK8+ luminal and p63+ basal lineages (Fig. 1E). Taken together, these data suggest that embryonic Sox2+ cells in the UGSE can derive both epithelial lineages of the prostate and may be the embryonic precursors to adult Sox2+ cells.
Sox2 Is Predominantly Expressed in the Proximal Adult Murine Prostate
Considering previous reports of stem-like populations in the prostate, we next sought to investigate the location, distribution, and cell-type expression of adult Sox2+ cells in the adult murine prostate [7]. Cre activity was induced by TAM treatment within hormonally intact adult male Sox2-LT mice for 4 days, and 24 hours later prostates were harvested and examined for YFP (Fig. 2A). Low doses of TAM were chosen to induce infrequent labeling of a small minority of cells, in order to more efficiently analyze clonal dynamics [55]. Indeed, we observed an average labeling efficiency of approximately 26% across all three lobes of the adult prostate (Supporting Information Fig. S2B, S2C), in accordance with previous reports [27]. Labeling efficiency was uniform across both proximal and distal prostatic regions demonstrating uniform TAM penetration. We next quantified the percentage of YFP+ cells in proximal, intermediate, and distal regions [10]. YFP marked 2.5% of cells in the proximal region of the prostate as compared with 0.2% of cells in the more distal regions (Fig. 2B, 2C; p = .01).
Figure 2.
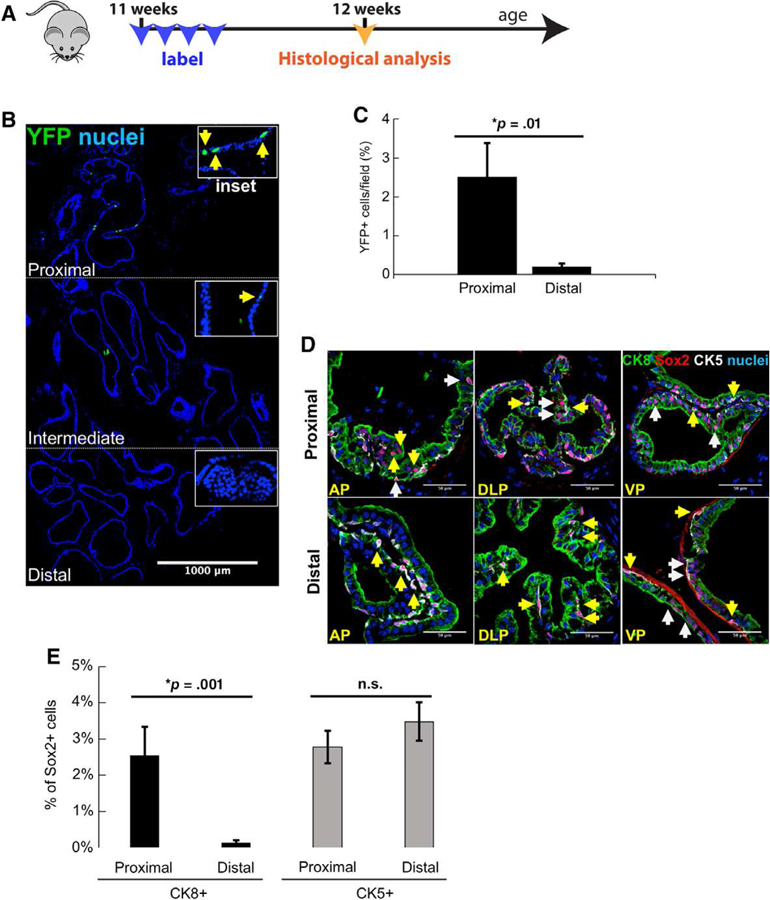
Sox2 expression is enriched in the proximal adult murine prostate. (A): Scheme for labeling Sox2+ cells in the adult mouse prostate. (B): Immunofluorescent (IF) staining of YFP expression (green) in adult mouse prostate 24 hours after last tamoxifen pulse. Scale bar: 1,000 μm. (C): Quantitation of YFP+ cells in proximal and distal regions of anterior prostate (n = 3 mice). Data represent the mean T SEM, two-tailed Student’s t test; *, p = .01. (D): IF staining shows colocalization of Sox2 with CK8 and CK5. Yellow arrows: Sox2+ cells positive for basal cell markers. White arrows: Sox2+ cells positive for luminal cell markers. Scale bar: 50 μm. Abbreviations: AP, anterior prostate; VP, ventral prostate; DLP, dorsolateral prostate. (E): Quantitation of Sox2+ cells in proximal and distal regions of prostates from adult, intact mice (n = 3–6 mice). Data represent the mean T SEM, one-tailed homoscedastic Student’s t test; *, p < .05.
Considering previous reports of heterogeneity within prostate epithelial lineages, we next determined lineage-specific expression of Sox2 [16, 56–62]. We observed endogenous Sox2 expression in CK5+ basal cells of both proximal and distal regions. Notably, Sox2 appeared to mark significantly more CK8+ luminal cells in the proximal region, as compared with more distal regions (Fig. 2D). Indeed, quantification of Sox2+ positivity reveals significantly more luminal cells labeled in the proximal region, as compared with distal (Fig. 2E 2.54% versus 0.13%; p = .001). The percentage of Sox2+/CK5+ basal cells was not significantly different between the proximal and distal regions (2.78% versus 3.48%; p = .15). Taken together, these data suggest that Sox2 expression delineates proximal and distal luminal cells and may contribute to their differential plasticity.
Sox2+ Cells Contribute to Homeostatic Turnover of Prostate Epithelium
Two crucial criteria of stem cells, sometimes collectively referred to as stem-ness, are the abilities for self-renewal and deriving all the cell types specific to the tissue they are found in reference [63]. To determine whether Sox2-expressing cells in the adult prostate epithelium fulfill these criteria of stem cells in homeostasis, we used genetic lineage tracing [64]. Sox2-LT mice were pulsed with TAM and then chased for extended periods of time. We noted individual, dispersed YFP+ p63+ basal as well as YFP+ CK8+ luminal cells immediately after TAM administration in Sox2-LT mice (Fig. 3, left column). YFP+ cells appear to remain dispersed throughout the 2-and 6-month chase periods, probably due to the low proliferation rate of the adult murine prostate [65]. Notably, 12 months after the pulse, we observed emergence of YFP+ CK8+ ribbons, defined as three or more cells (Fig. 3, right column, brackets) [14]. These data suggest that Sox2-expressing cells are able to generate progeny during epithelial homeostasis.
Figure 3.
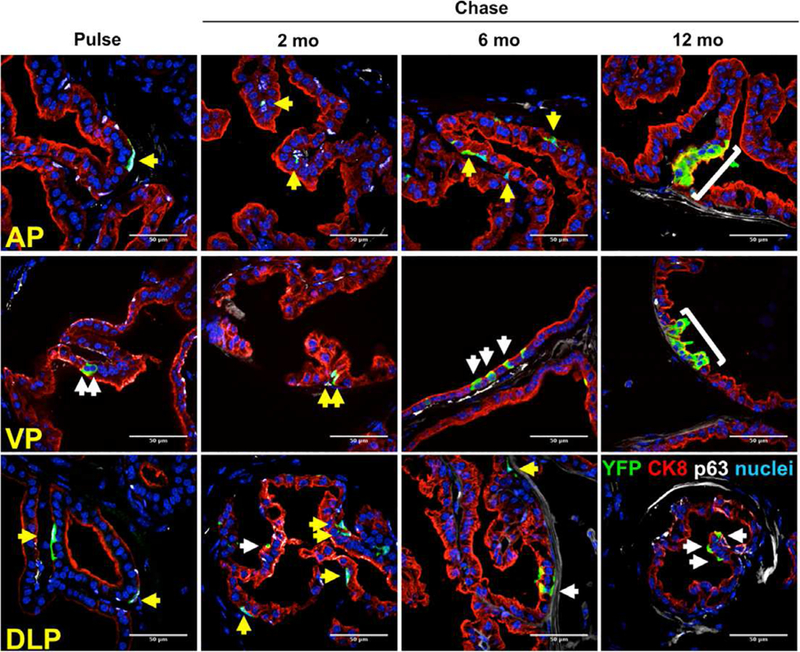
Sox2+ cells contribute to homeostatic turnover in the adult murine prostate. (A): Immunofluorescent staining shows colocalization of YFP (green) with either CK8 or p63 (n = 3 mice per time point). Yellow arrows: YFP+ cells positive for basal cell markers. White arrows: YFP+ cells positive for luminal cell markers. Scale bar: 50 μm. Abbreviations: AP, anterior prostate; VP, ventral prostate; DLP, dorsolateral prostate.
Sox2+ Cells Are Castration-Resistant and Contribute to Prostatic Regeneration
A hallmark of bona fide prostate progenitor cells is the ability to persist during castration and contribute to epithelial regeneration after androgen supplementation. To determine the castration-resistance of Sox2+ cells, we TAM-pulsed hormonally intact Sox2-LT mice to follow YFP+ cells over two cycles of castration and regeneration (Fig. 4A). At the pulse time point, 1.82% of cells were YFP+, with a majority coexpressing the basal cell marker p63 as compared with the luminal cell marker CK8+ (87% versus 13%; Fig. 4B, 4C). After host castration, we observed 2.75% YFP+ cells in the regressed prostate, a significant enrichment of YFP+ cells (p < .05), most coexpressing CK8+ (40% in regressed versus 13% in intact prostates). As expected, the percentage of YFP+/p63+ basal cells increased during castration due to diminished luminal cell populations and the established castration-resistance of basal cells. Furthermore, costaining with Nkx3.1 demonstrated no detectable Sox2+/Nkx3.1+ cells within the hormonally intact condition (0% of 2,807 nuclei analyzed), nor the castrate condition (0% of 4,041 cells analyzed; Supporting Information Fig. S3A, S3B). These data suggest that Sox2-lineage luminal cells are protected from castration, but that Sox2 is not expressed is castration-resistant cells expressing Nkx3.1 (i.e., CARN cells) [13].
Figure 4.
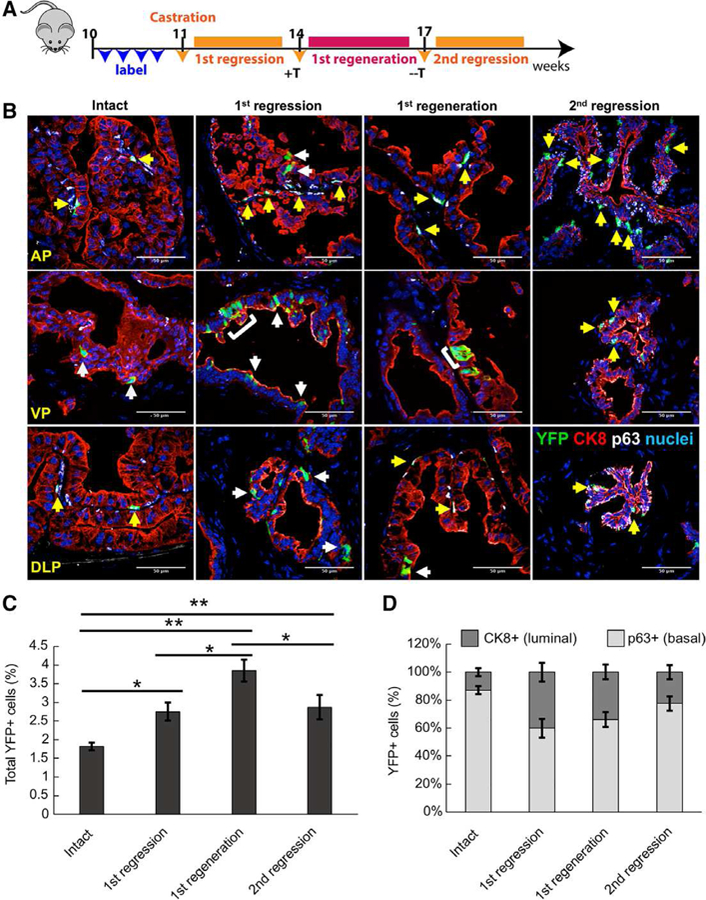
Sox2+ cells are castration-resistant and contribute to prostatic regeneration. (A): Scheme for Sox2 lineage marking during serial prostate regression and regeneration. Sox2+ cells were labeled for YFP-expression prior to host castration. (B): Immunofluorescent staining to assess YFP+/CK8+ luminal cells (white arrows) or YFP+/p63+ basal cells (yellow arrows) after serial prostate regeneration (n = 3 mice per time point). Abbreviations: AP, anterior prostate; VP, ventral prostate; DLP, dorsolateral prostate. (C): Graph showing percentage of total YFP+ cells in intact, castrated and regenerated prostates examined over all lobes. Data represent the mean T SEM. **, p < .01; *, p < .05; Tukey’s Honest Significant Difference test. (D): Graph showing percentage of YFP+ cells coexpressing luminal or basal markers in intact, castrated and regenerated prostates.
Upon testosterone supplementation, the percentage of YFP+ cells grew significantly from 2.75% in the regressed prostate to 3.85% in the regenerated prostates (p < .05). The significant enrichment of YFP+ cells in regenerated versus pulsed prostates (p < .01) indicate that Sox2-lineage cells that survive castration can then contribute to glandular regeneration. Notably, the YFP+ CK8+ population markedly increased from pulsed prostates to regenerated prostates (13% versus 34%), underscoring an expansion in the luminal cell compartment.
Removal of the testosterone pellet reduced YFP+ cells from 3.85% in regenerated prostates to 2.87% (p = .02). The second regression was not statistically different as compared with the first regression (p = .85). These data suggest that, while castration-resistant Sox2-expressing cells contribute to prostatic regeneration, the daughters are castration-sensitive. Altogether our results demonstrate that Sox2+ cells in the intact murine prostate are castration-resistant, and substantially contribute to prostatic regeneration upon testosterone supplementation.
Persisting Luminal Cells Express Sox2 after Host Castration
We and others have previously demonstrated that Sox2 expression is sufficient to promote in vivo castration resistance of tumor xenografts, and that androgen-mediated signaling influences Sox2 expression [38, 41]. Here, we sought to determine whether Sox2 is endogenously expressed in persisting castration-resistant cells. We used immunofluorescent microscopy to costain for Sox2 and basal-specific CK5 and luminal-specific CK8 in castrated males (3-weeks postcastration; Fig. 5A). Image quantification revealed that significantly more Sox2+ CK8 + luminal cells were located proximally, closer to the urethra, as compared with more distally (Fig. 5B 4.28% versus 0.20%; *, p = .04). The percentage of Sox2+/CK5+ basal cells was not significantly different between the proximal and distal regions (9.45% versus 10.51%; p = .26). Interestingly, the percentage of Sox2+ cells that are colabeled with CK8 is not significantly different between the proximal regions of hormonally intact and castrated mice (2.54% versus 4.28%; p = .19). Taken together, these data indicate that Sox2 expression marks CK8+ luminal cells in the proximal region that are nonsusceptible to castration-induced death. These results highlight a potential role for Sox2 in promoting cellular survival and castration-resistance within prostate luminal cells.
Figure 5.
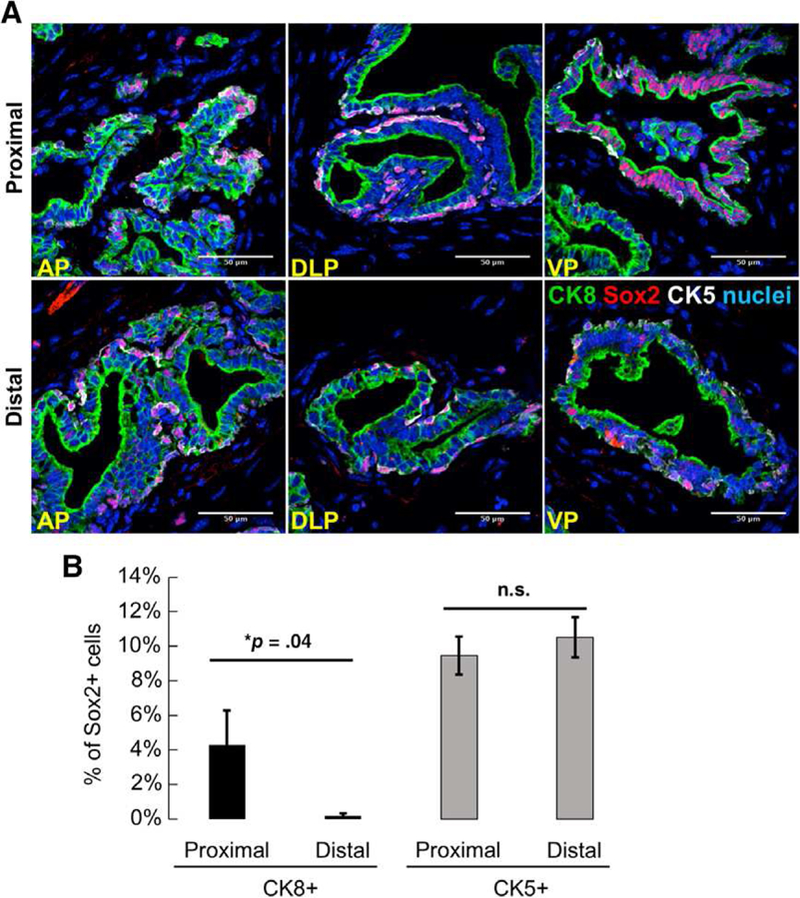
Persisting luminal cells express Sox2 after host castration. (A): Immunofluorescent staining shows colocalization of Sox2 with CK5 (white) or CK8 (green) in postpubescent castrated murine prostates (n = 3 mice, 3-weeks postcastration). Yellow arrows: Sox2+ cells positive for basal cell markers. White arrows: Sox2+ cells positive for luminal cell markers. Scale bar: 50 μm. Abbreviations: AP, anterior prostate; DLP, dorsolateral prostate, VP, ventral prostate. (B): Quantitation of Sox2+ cells in proximal and distal regions of prostates from adult, castrated mice. Data represent the mean T SEM, one-tailed homoscedastic Student’s t test; *, p = .04.
Castration-Resistant Sox2+ Cells Have Regenerative Capacity In Vivo
Our observation that Sox2 expression persists in castration-resistant luminal epithelial cells led us to investigate the fate of such cells during prostatic regeneration. To do this, we castrated Sox2-LT mice and allowed maximal involution prior to TAM-induced YFP labeling. We then induced two cycles of castration and regeneration (Fig. 6A). It should be noted that, in contrast to data presented in Figure 4, Sox2+ cells were labeled for YFP expression after host castration and prostatic involution. YFP expression labeled 2.7% of cells in the castrated prostate (44% CK8+ luminal, 56% p63+ basal). Administration of testosterone significantly increased YFP+ cells to 4.7% (58% CK8+, 42% p63+; p < .05), and resulted in the emergence of patches of CK8+ cells. The overall fraction of YFP+ cells did not significantly increase between regeneration cycles (5.06% after second regeneration versus 4.36% in the first; p = .66), supporting a model whereby castration-resistant Sox2+ cells can regenerate epithelial cells following castration, but cells derived from the Sox2+ lineage can be castration-sensitive (Fig. 6B, 6C).
Figure 6.
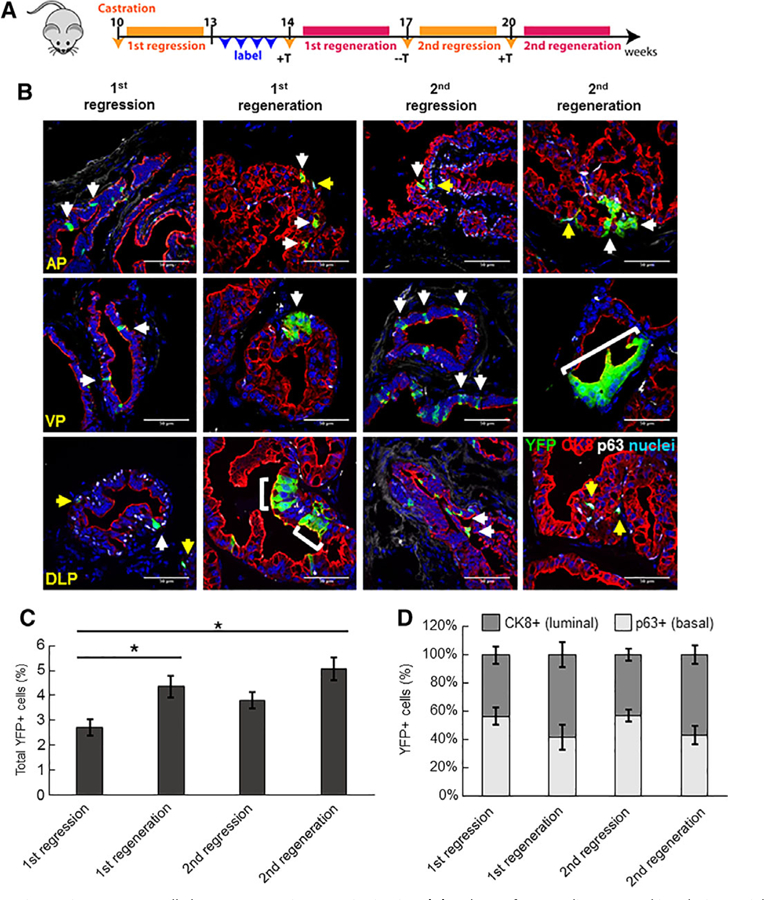
Castration-resistant Sox2+ cells have regenerative capacity in vivo. (A): Scheme for Sox2 lineage marking during serial prostate regression and regeneration. In this approach, Sox2+ cells were labeled for YFP-expression after host castration and prostatic involution. (B): Immunofluorescent staining to assess YFP+/CK8+ luminal cells (white arrows) or YFP+/p63+ basal cells (yellow arrows) after serial prostate regeneration (n = 3 mice per time point). Scale bar: 50 μm. Abbreviations: AP, anterior prostate; VP, ventral prostate; DLP, dorsolateral prostate. (C): Graph showing percentage of total YFP+ cells in intact, castrated or regenerated prostates examined over all lobes. Data represent the mean T SEM; **, p < .01; *, p < .05; Tukey’s Honest Significant Difference test. (D): Graph showing percentage of YFP+ cells coexpressing luminal or basal markers in intact, castrated and regenerated prostates.
These numbers mirror previously published reports on castration-resistant Lgr5-expressing and Bmi1-expressing cells [14, 15]. In the case of castration-resistant Lgr5-expressing cells, lineage tracing revealed an increase from 0.068% in fully regressed prostates to 1.942% RFP+ cells after the first round of regeneration [14]. In the case of CARBs, the percentage of YFP+ cells increased from 0.6% to 2.8% after the first round of regeneration [15].
DISCUSSION
Our study has identified a population of castration-resistant Sox2-expressing epithelial cells that contribute to epithelial development, homeostasis, and regeneration in the prostate. In intact animals, Sox2 labels significantly more luminal cells located in the proximal region as compared with distal. Sox2 expression is also observed in persisting luminal cells that survive castration, indicating that Sox2+ luminal cells are protected from castration-induced death. Therefore, additional characterization of these castration-resistant Sox2-expressing cells is warranted. Specifically, it is unknown whether Sox2 expression itself is required for self-renewal and potency. Furthermore, it remains unclear whether Sox2 expression and/or the Sox2-expressing population is required for prostatic regeneration. Whether these castration-resistant Sox2-expressing cells exist and behave similarly in humans is also unknown. Furthermore, underpinning mechanisms of specification of castration-resistant populations remain incompletely characterized.
The lineage marking efficiency of the Sox2CreER driver in the prostate labeled ~25% of all Sox2+ cells. Thus, while clonal lineage tracing experiments can draw definitive conclusions about the cells labeled in each experiment, they may omit information about the behavior of unlabeled cells, particularly if the labeled population is not representative of the entire lineage of interest [55]. In our system, Sox2+ and YFP+ populations were similar in percentages and distribution across lobes and distal/proximal axes (Fig. 2C versus Fig. 2E). We observed a gradient of both Sox2 and YFP positivity along the proximal–distal axis, and both populations were present through castration and regeneration, indicating that both the YFP+ and Sox2+ cells observed in regressed prostates are castration-resistant. Taken together, these data demonstrate that the lineage marking of the Sox2C-reER driver is likely representative of the Sox2+ population.
Our data is consistent with previous reports suggesting that adult prostate basal and luminal lineages are largely self-sustained [18]. However, we cannot completely exclude the possibility that Sox2 expression marks multipotent luminal or basal cell populations, also described in previous publications [13, 23]. Moreover, it has been documented that p63-null embryonic tissue grafts do not derive Sox2+ luminal cells, suggesting that Sox2+ luminal cells are the progeny of p63+ basal cells [23]. Lineage-tracing of p63-positive cells, however, demonstrate their ability to form luminal cells [22]. These results may reflect the different progenitor relationships in the embryonic and adult prostate. More experiments are required to definitively determine whether this is the case.
The percentage of YFP+ cells in multiple rounds of castration-regeneration hints at the existence of multiple progenitor pools. This is not entirely surprising, as recent reports have revealed castration-resistant populations marked by Nkx3.1, Lgr5, and Bmi1 [13–15]. Castration-resistant cells expressing either Nkx3.1 (CARNs) or Bmi1 (CARBs), were shown to be distinct, nonover-lapping populations [15]. Our analyses of Nkx3.1 and Sox2 coexpression in castrated hosts demonstrated no detectable overlap between Sox2 and CARNs. Molecular characterization of each population is warranted, and mechanistic approaches to understand the function of Sox2, Nkx3.1, and Bmi1 have the potential to more clearly define functional overlap [13].
CONCLUSION
Although it is possible for both basal and luminal cells to serve as the cells of origin for prostate cancer, luminal cells appear to be more sensitive to malignant transformation [66]. These mechanisms of cell survival in the context of androgen ablation may shed light on how prostate cancer cells escape frontline, luminal cell-specific therapeutics that target AR [37, 38, 41, 67, 68]. As expression of SOX2 in human prostate cancer might indicate a phenotypic switch to a more therapy-resistant cancer, it is crucial to understand resultant changes in gene expression throughout tumor initiation and progression as a result of SOX2 signaling. Future studies are required to understand how SOX2 expression in tumor cells may alter disease natural history and patient outcomes. Therefore, investigation of SOX2-expressing lineages within the normal and malignant human prostate epithelium should also be a high priority.
Supplementary Material
SIGNIFICANCE STATEMENT.
The murine prostate is a tractable model for understanding the contribution of adult stem cells to tissue regeneration, as host castration induces prostatic regression which can be regenerated by androgen replacement. Using lineage tracing, it has been demonstrated that Sox2-expressing luminal cells are protected from castration-induced death and contribute to androgen-mediated prostatic regeneration. These results support a key role for Sox2-positive cells in adult prostate biology and lay the groundwork for future studies. Indeed, an ongoing critical area of investigation is whether cellular resistance to hormone-targeted therapies is preprogrammed or acquired, and whether such mechanisms can be targeted for increased therapeutic efficacy.
ACKNOWLEDGMENTS
We acknowledge Vander Griend and Szmulewitz lab members for their input. We also acknowledge the support of the University of Chicago Section of Urology led by Dr. Arieh Shalhav, and the Ben May Institute for Cancer Research led by Dr. Geoffrey Greene. We would also like to acknowledge the support of the University of Chicago Comprehensive Cancer Center (UCCCC) led by Dr. Michelle Le Beau. We thank Dr. Yi Cai for his expertise and contribution to the work. We are thankful for the expert technical assistance of the Human Tissue Resource Center core facility led by Dr. Mark Lingen and Terri Li. Finally, we acknowledge the support of Dr. Fred Behm, Alan Diamond, and Gail Prins at the University of Illinois at Chicago, Departments of Pathology and Urology. This work was supported by R01CA178431 (D.J.V.G.); the University of Chicago Comprehensive Cancer Center (UCCCC), especially the Cancer Center Support Grant (P30CA014599), the Alvin Baum Family Fund, and the Pierce Family Foundation. E.M. was supported by the UChicago Molecular and Cellular Biology Training Grant (NIH NIGMS T32GM007183) and currently by an F31 (NIH NIDDK DK111131). C.V.O. was supported by the Cancer Biology Training Grant (T32 CA 009594) and an F31 (CA232651). D.M. is supported by the UChicago Molecular and Cellular Biology Training Grant (NIH NIGMS T32GM007183).
Footnotes
DATA AVAILABILITY
The data that support the findings of this study are available from the corresponding author upon reasonable request.
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
The authors indicated no potential conflicts of interest.
See www.StemCells.com for supporting information available online.
REFERENCES
- 1.Signoretti S, Waltregny D, Dilks J et al. p63 is a prostate basal cell marker and is required for prostate development. Am J Pathol 2000;157:1769–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abate-Shen C, Shen MM. Molecular genetics of prostate cancer. Genes Dev 2000;14: 2410–2434. [DOI] [PubMed] [Google Scholar]
- 3.Kasper S Exploring the origins of the normal prostate and prostate cancer stem cell. Stem Cell Rev 2008;4:193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cunha GR, Ricke W, Thomson A et al. Hormonal, cellular, and molecular regulation of normal and neoplastic prostatic development. J Steroid Biochem Mol Biol 2004;92: 221–236. [DOI] [PubMed] [Google Scholar]
- 5.English HF, Santen RJ, Isaacs JT. Response of glandular versus basal rat ventral prostatic epithelial cells to androgen withdrawal and replacement. Prostate 1987;11:229–242. [DOI] [PubMed] [Google Scholar]
- 6.Evans GS, Chandler JA. Cell proliferation studies in rat prostate. I. The proliferative role of basal and secretory epithelial cells during normal growth. Prostate 1987;10:163–178. [DOI] [PubMed] [Google Scholar]
- 7.Tsujimura A, Koikawa Y, Salm S et al. Proximal location of mouse prostate epithelial stem cells: A model of prostatic homeostasis. J Cell Biol 2002;157:1257–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Isaacs JT. Control of Cell Proliferation and Cell Death in the Normal and Neoplastic Prostate: A Stem Cell Model U.S. Dept. of Health and Human Services, 1987:85–94, Bethesda, MD: NIH Publication #87–2881. [Google Scholar]
- 9.Goto K, Salm SN, Coetzee S et al. Proximal prostatic stem cells are programmed to regenerate a proximal-distal ductal axis. STEM CELLS 2006;24:1859–1868. [DOI] [PubMed] [Google Scholar]
- 10.Salm SN, Burger PE, Coetzee S et al. TGF-{beta} maintains dormancy of prostatic stem cells in the proximal region of ducts. J Cell Biol 2005;170:81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sciavolino PJ, Abrams EW, Yang L et al. Tissue-specific expression of murine Nkx3.1 in the male urogenital system. Dev Dyn 1997; 209:127–138. [DOI] [PubMed] [Google Scholar]
- 12.Bhatia-Gaur R, Donjacour AA, Sciavolino PJ et al. Roles for Nkx3.1 in prostate development and cancer. Genes Dev 1999;13:966–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang X, Kruithof-de Julio M, Economides KD et al. A luminal epithelial stem cell that is a cell of origin for prostate cancer. Nature 2009; 461:495–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang BE, Wang X, Long JE et al. Castration-resistant Lgr5(+) cells are long-lived stem cells required for prostatic regeneration. Stem Cell Rep 2015;4:768–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoo YA, Roh M, Naseem AF et al. Bmi1 marks distinct castration-resistant luminal progenitor cells competent for prostate regeneration and tumour initiation. Nat Commun 2016; 7:12943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang ZA, Mitrofanova A, Bergren SK et al. Lineage analysis of basal epithelial cells reveals their unexpected plasticity and supports a cell-of-origin model for prostate cancer heterogeneity. Nat Cell Biol 2013;15: 274–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu J, Pascal LE, Isharwal S et al. Regenerated luminal epithelial cells are derived from preexisting luminal epithelial cells in adult mouse prostate. Mol Endocrinol 2011;25: 1849–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi N, Zhang B, Zhang L et al. Adult murine prostate basal and luminal cells are self-sustained lineages that can both serve as targets for prostate cancer initiation. Cancer Cell 2012;21:253–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xie Q, Wang ZA. Transcriptional regulation of the Nkx3.1 gene in prostate luminal stem cell specification and cancer initiation via its 3′ genomic region. J Biol Chem 2017; 292:13521–13530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xie Q, Liu Y, Cai T et al. Dissecting cell-type-specific roles of androgen receptor in prostate homeostasis and regeneration through lineage tracing. Nat Commun 2017;8:14284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ousset M, Van Keymeulen A, Bouvencourt G et al. Multipotent and unipotent progenitors contribute to prostate postnatal development. Nat Cell Biol 2012;14: 1131–1138. [DOI] [PubMed] [Google Scholar]
- 22.Pignon JC, Grisanzio C, Geng Y et al. p63-expressing cells are the stem cells of developing prostate, bladder, and colorectal epithelia. Proc Natl Acad Sci USA 2013;110: 8105–8110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang J, Zhu HH, Chu M et al. Symmetrical and asymmetrical division analysis provides evidence for a hierarchy of prostate epithelial cell lineages. Nat Commun 2014;5:4758. [DOI] [PubMed] [Google Scholar]
- 24.Shafer MER, Nguyen AHT, Tremblay M et al. Lineage specification from prostate progenitor cells requires Gata3-dependent mitotic spindle orientation. Stem Cell Rep 2017;8:1018–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boyer LA, Lee TI, Cole MF et al. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell 2005;122:947–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006;126:663–676. [DOI] [PubMed] [Google Scholar]
- 27.Arnold K, Sarkar A, Yram MA et al. Sox2 (+) adult stem and progenitor cells are important for tissue regeneration and survival of mice. Cell Stem Cell 2011;9:317–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boumahdi S, Driessens G, Lapouge G et al. SOX2 controls tumour initiation and cancer stem-cell functions in squamous-cell carcinoma. Nature 2014;511:246–250. [DOI] [PubMed] [Google Scholar]
- 29.Juuri E, Saito K, Ahtiainen L et al. Sox2+ stem cells contribute to all epithelial lineages of the tooth via Sfrp5+ progenitors. Dev Cell 2012;23:317–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brazel CY, Limke TL, Osborne JK et al. Sox2 expression defines a heterogeneous population of neurosphere-forming cells in the adult murine brain. Aging Cell 2005;4:197–207. [DOI] [PubMed] [Google Scholar]
- 31.Driskell RR, Giangreco A, Jensen KB et al. Sox2-positive dermal papilla cells specify hair follicle type in mammalian epidermis. Development 2009;136:2815–2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ellis P, Fagan BM, Magness ST et al. SOX2, a persistent marker for multipotential neural stem cells derived from embryonic stem cells, the embryo or the adult. Dev Neurosci 2004;26:148–165. [DOI] [PubMed] [Google Scholar]
- 33.Fauquier T, Rizzoti K, Dattani M et al. SOX2-expressing progenitor cells generate all of the major cell types in the adult mouse pituitary gland. Proc Natl Acad Sci USA 2008; 105:2907–2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gu R, Brown RM 2nd, Hsu CW et al. Lineage tracing of Sox2-expressing progenitor cells in the mouse inner ear reveals a broad contribution to non-sensory tissues and insights into the origin of the organ of Corti. Dev Biol 2016;414:72–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kempfle JS, Turban JL, Edge AS. Sox2 in the differentiation of cochlear progenitor cells. Sci Rep 2016;6:23293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kiernan AE, Pelling AL, Leung KK et al. Sox2 is required for sensory organ development in the mammalian inner ear. Nature 2005;434:1031–1035. [DOI] [PubMed] [Google Scholar]
- 37.Ku SY, Rosario S, Wang Y et al. Rb1 and Trp53 cooperate to suppress prostate cancer lineage plasticity, metastasis, and antiandrogen resistance. Science 2017;355:78–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mu P, Zhang Z, Benelli M et al. SOX2 promotes lineage plasticity and antiandrogen resistance in TP53-and RB1-deficient prostate cancer. Science 2017;355:84–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Que J, Luo X, Schwartz RJ et al. Multiple roles for Sox2 in the developing and adult mouse trachea. Development 2009;136:1899–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steevens AR, Sookiasian DL, Glatzer JC et al. SOX2 is required for inner ear neurogenesis. Sci Rep 2017;7:4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kregel S, Kiriluk KJ, Rosen AM et al. Sox2 is an androgen receptor-repressed gene that promotes castration-resistant prostate cancer. PLoS One 2013;8:e53701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu X, Cates JM, Morrissey C et al. SOX2 expression in the developing, adult, as well as, diseased prostate. Prostate Cancer Prostatic Dis 2014;17:301–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Michiel Sedelaar JP, Dalrymple SS, Isaacs JT. Of mice and men—Warning: Intact versus castrated adult male mice as xenograft hosts are equivalent to hypogonadal versus abiraterone treated aging human males, respectively. Prostate 2013;73:1316–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schindelin J, Arganda-Carreras I, Frise E et al. Fiji: An open-source platform for biological-image analysis. Nat Methods 2012;9:676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bylund M, Andersson E, Novitch BG et al. Vertebrate neurogenesis is counteracted by Sox1–3 activity. Nat Neurosci 2003;6:1162–1168. [DOI] [PubMed] [Google Scholar]
- 46.Graham V, Khudyakov J, Ellis P et al. SOX2 functions to maintain neural progenitor identity. Neuron 2003;39:749–765. [DOI] [PubMed] [Google Scholar]
- 47.Que J, Okubo T, Goldenring JR et al. Multiple dose-dependent roles for Sox2 in the patterning and differentiation of anterior foregut endoderm. Development 2007;134: 2521–2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Taranova OV, Magness ST, Fagan BM et al. SOX2 is a dose-dependent regulator of retinal neural progenitor competence. Genes Dev 2006;20:1187–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kamachi Y, Uchikawa M, Collignon J et al. Involvement of Sox1, 2 and 3 in the early and subsequent molecular events of lens induction. Development 1998;125:2521–2532. [DOI] [PubMed] [Google Scholar]
- 50.Okubo T, Clark C, Hogan BL. Cell lineage mapping of taste bud cells and keratinocytes in the mouse tongue and soft palate. STEM CELLS 2009;27:442–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Staack A, Donjacour AA, Brody J et al. Mouse urogenital development: A practical approach. Differentiation 2003;71:402–413. [DOI] [PubMed] [Google Scholar]
- 52.Georgas KM, Armstrong J, Keast JR et al. An illustrated anatomical ontology of the developing mouse lower urogenital tract. Development 2015;142:1893–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Toivanen R, Shen MM. Prostate organo-genesis: Tissue induction, hormonal regulation and cell type specification. Development 2017; 144:1382–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Srinivas S, Watanabe T, Lin CS et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol 2001;1:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wuidart A, Ousset M, Rulands S et al. Quantitative lineage tracing strategies to resolve multipotency in tissue-specific stem cells. Genes Dev 2016;30:1261–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ceder JA, Aalders TW, Schalken JA. Label retention and stem cell marker expression in the developing and adult prostate identifies basal and luminal epithelial stem cell subpopulations. Stem Cell Res Ther 2017;8:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goldstein AS, Lawson DA, Cheng D et al. Trop2 identifies a subpopulation of murine and human prostate basal cells with stem cell characteristics. Proc Natl Acad Sci USA 2008; 105:20882–20887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kwon OJ, Valdez JM, Zhang L et al. Increased Notch signalling inhibits anoikis and stimulates proliferation of prostate luminal epithelial cells. Nat Commun 2014;5:4416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chua CW, Shibata M, Lei M et al. Single luminal epithelial progenitors can generate prostate organoids in culture. Nat Cell Biol 2014;16:951–961. 951–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kwon OJ, Zhang L, Xin L. Stem cell antigen-1 identifies a distinct androgen-independent murine prostatic luminal cell lineage with bipotent potential. STEM CELLS 2016;34:191–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang D, Jeter C, Gong S et al. Histone 2B-GFP label-retaining prostate luminal cells possess progenitor cell properties and are intrinsically resistant to castration. Stem Cell Rep 2018;10:228–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chua CW, Epsi NJ, Leung EY et al. Differential requirements of androgen receptor in luminal progenitors during prostate regeneration and tumor initiation. Elife 2018;7:e28768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Potten CS, Loeffler M. Stem cells: Attributes, cycles, spirals, pitfalls and uncertainties. Lessons for and from the crypt. Development 1990;110:1001–1020. [DOI] [PubMed] [Google Scholar]
- 64.Snippert HJ, Clevers H. Tracking adult stem cells. EMBO Rep 2011;12:113–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Di Cristofano A, Pesce B, Cordon-Cardo C et al. Pten is essential for embryonic development and tumour suppression. Nat Genet 1998;19:348–355. [DOI] [PubMed] [Google Scholar]
- 66.Wang ZA, Toivanen R, Bergren SK et al. Luminal cells are favored as the cell of origin for prostate cancer. Cell Rep 2014;8:1339–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kar S, Sengupta D, Deb M et al. SOX2 function and Hedgehog signaling pathway are co-conspirators in promoting androgen independent prostate cancer. Biochim Biophys Acta 2017;1863:253–265. [DOI] [PubMed] [Google Scholar]
- 68.Jiang N, Ke B, Hjort-Jensen K et al. YAP1 regulates prostate cancer stem cell-like characteristics to promote castration resistant growth. Oncotarget 2017;8:115054–115067 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


