Abstract
Caenorhabditis elegans lives in a complex habitat in which they routinely experience large fluctuations in temperature, and encounter physical obstacles that vary in size and composition. Their habitat is shared by other nematodes, by beneficial and harmful bacteria, and nematode-trapping fungi. Not surprisingly, these nematodes can detect and discriminate among diverse environmental cues, and exhibit sensory-evoked behaviors that are readily quantifiable in the laboratory at high resolution. Their ability to perform these behaviors depends on <100 sensory neurons, and this compact sensory nervous system together with powerful molecular genetic tools has allowed individual neuron types to be linked to specific sensory responses. Here, we describe the sensory neurons and molecules that enable C. elegans to sense and respond to physical stimuli. We focus primarily on the pathways that allow sensation of mechanical and thermal stimuli, and briefly consider this animal’s ability to sense magnetic and electrical fields, light, and relative humidity. As the study of sensory transduction is critically dependent upon the techniques for stimulus delivery, we also include a section on appropriate laboratory methods for such studies. This chapter summarizes current knowledge about the sensitivity and response dynamics of individual classes of C. elegans mechano- and thermosensory neurons from in vivo calcium imaging and whole-cell patch-clamp electrophysiology studies. We also describe the roles of conserved molecules and signaling pathways in mediating the remarkably sensitive responses of these nematodes to mechanical and thermal cues. These studies have shown that the protein partners that form mechanotransduction channels are drawn from multiple superfamilies of ion channel proteins, and that signal transduction pathways responsible for temperature sensing in C. elegans share many features with those responsible for phototransduction in vertebrates.
Keywords: C. elegans, mechanosensation, thermosensation, WormBook
THE ability of animals to detect mechanical, thermal, and other physical stimuli is conserved across phyla and plays a key role in their navigation of variable and harsh environmental conditions. These senses enable animals to mate, find food, and avoid danger, and depend on the functions of neurons specialized to detect these stimuli. Transduction of these stimuli in sensory neurons is mediated via signaling pathways that converge on ion channels, thereby converting physical stimuli into electrical signals that propagate through the nervous system to trigger appropriate behavioral responses. Animals use diverse receptors and signaling pathways to reliably sense and respond to physical cues. An intriguing feature of sensory transduction is the convergent and divergent evolution of sensory molecules and mechanisms allowing optimization of animal survival in specialized ecological niches. For instance, a signaling pathway used in a sensory modality in one species may evolve to detect a different cue in another. Similarly, the same cue may be sensed via distinct mechanisms in different species. Thus, a complete understanding of mechano- and thermosensation requires identification and comparison of transduction mechanisms across animals.
This chapter describes the current state of knowledge regarding the transduction of mechanical and thermal cues in Caenorhabditis elegans nematodes. Research on this topic is enabled by the ability of researchers to combine genetic discovery with quantitative analyses of behaviors and physiological measurements of sensory neuron responses in living animals. Neuroanatomical studies presciently predicted the identities of mechanosensory and thermosensory neurons, which were confirmed by cell ablation followed by behavioral analyses (Chalfie et al. 1985; Mori and Ohshima 1995). Genetic analyses that identified mutants with sensory defects have now provided detailed molecular insights into sensory signal transduction mechanisms employed by individual sensory neuron types in this organism. The development of genetically encoded sensors for calcium and other second messengers, and of techniques for electrophysiology, have also now allowed studies of sensory neuron physiology in living animals. Findings in C. elegans have contributed several pivotal concepts to the broader field of sensory transduction. Analyses in C. elegans revealed that even simple animals rely on a collection of specialized sensory neurons for mechanosensory transduction, and that multiple classes of ion channels have been harnessed to subserve this function. Paradigms for linking mechanosensory transduction to the action of specific ion channels via mutations that alter ion selectivity were established in C. elegans and have been adopted in other systems, including the hair cells responsible for hearing and balance in mammals. Analyses of thermosensation in C. elegans have provided insights into the molecular mechanisms that enable an organism to respond sensitively and robustly to a cue over a wide dynamic range in an experience-dependent manner, and have described principles that are broadly applicable to diverse sensory systems.
Here, we focus primarily on the neurons, molecules, and signaling pathways used by C. elegans to detect mechanical and thermal stimuli and briefly describes the neurons and molecules known to mediate responses to magnetic and electrical fields, light, and humidity. Laboratory methods for delivering physical stimuli to C. elegans nematodes are presented as an Appendix. The molecular events that give rise to chemosensation of soluble and volatile attractants and repellents, of gases, and of pheromones, are discussed in an upcoming Wormbook chapter.
Sensory Anatomy of C. elegans Nematodes
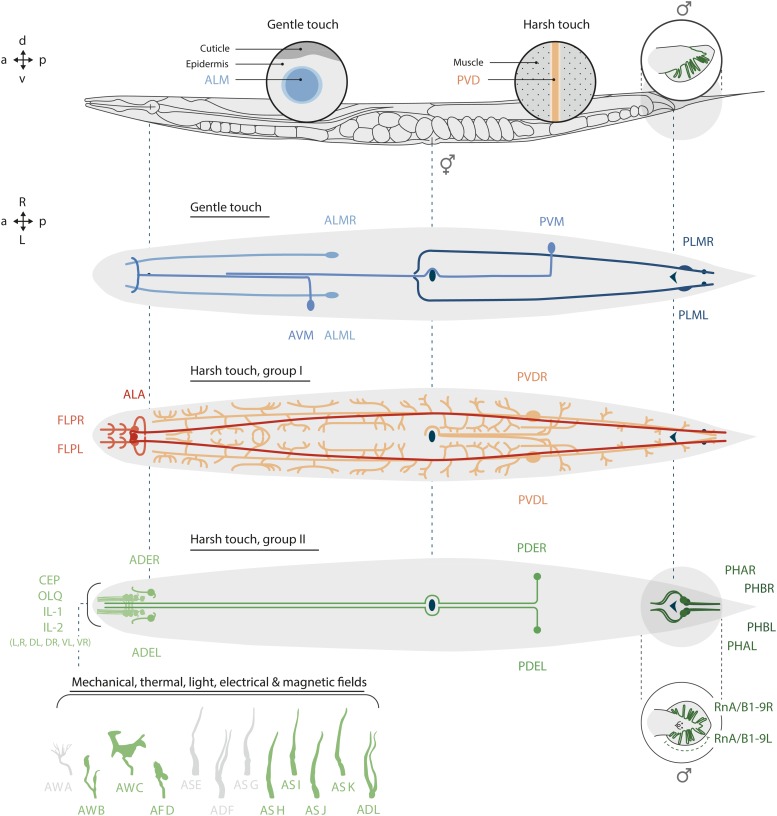
Despite their compact nervous system, C. elegans nematodes have at least 70 neurons predicted to function as sensory neurons (Ward et al. 1975; Perkins et al. 1986; White et al. 1986; Hall et al. 2006). These neurons (Figure 1 and Table 1) detect soluble and volatile chemicals, gases, mechanical stress, temperature, light, and additional sensory cues. Sixty of the sensory neurons in the adult hermaphrodite, and an additional 52 neurons in the adult male, contain primary cilia at their sensory endings (Ward et al. 1975; Perkins et al. 1986; Doroquez et al. 2014). These cilia are microtubule-based structures that are structurally specialized for the unique functions of each sensory neuron type, and house all primary sensory signal transduction molecules. A subset of nonciliated sensory neurons including PVD and FLP exhibits highly complex dendritic morphologies that also shape the functions of these neurons (Halevi et al. 2002; Oren-Suissa et al. 2010; Smith et al. 2010; Albeg et al. 2011) (Figure 1). Readers are referred to the chapter by Leroux and Buelow (Wormbook) for details regarding the formation, maintenance, and function of sensory dendrites and cilia.
Figure 1.
Positions of sensory neurons in adult C. elegans. (Top) Lateral view of an adult hermaphrodite and male tail. Insets illustrate the association of gentle touch receptor neurons (TRNs; only ALM is shown) and the multidendritic nociceptive neurons (PVD) with the epidermis and muscle, respectively. (Middle two panels) Inside view of the six TRNs (ALML/R, AVM, PVM, PLML/R), the dendritic arbors of the multidendritic FLP and PVD neurons and the ALA harsh touch receptor. (Bottom) Positions of ciliated sensory neurons in hermaphrodites and in the male tail. Inset shows the shapes of the ciliated endings that terminate in the amphid sensilla. Ciliated neurons indicated in green are discussed in this chapter; those indicated in gray will be discussed in a forthcoming Wormbook chapter on chemical sensing.
Table 1. Primary sensory neurons mediating responses to physical stimuli in C. elegans hermaphrodites.
Chemosensory properties of a subset of neurons in this Table are discussed in a forthcoming WormBook chapter.
Mechanosensation and Sensory Mechanotransduction
All animals are endowed with sensory neurons specialized to detect mechanical energy in the form of touch, potential injury (nociception), or body movement (proprioception). The proper function of internal organs also depends on feedback from mechanoreceptor neurons (interoreception). Mechanoreceptor neurons differ in their anatomy and intimate association with skin, muscle, and internal organs, but share the vital function of performing mechanotransduction. Studies in C. elegans were the first to use unbiased, forward genetic screens to identify proteins specifically required for mechanosensation (Chalfie and Sulston 1981; Chalfie and Au 1989) and the first to combine genetic dissection with optical imaging (Suzuki et al. 2003) and electrophysiology (O’Hagan et al. 2005) of identified mechanoreceptor neurons in living animals. Readers interested in how mechanosensory transduction in C. elegans relates to parallel processes in other animals are referred to other sources (Arnadóttir and Chalfie 2010; Katta et al. 2015; Wu et al. 2017).
C. elegans hermaphrodites have 45, and males have an additional 42, putative mechanoreceptor neurons (Figure 1 and Table 1). Responses to mechanical stimulation have been observed using calcium imaging in ca. one-third of these sensory neurons (see Figure 2 and text below). Each neuron is embedded in a specific place in the body and specialized to detect mechanical stresses that originate in that location. They differ in their sensitivity to mechanical loads: some detect low-intensity, gentle touch, while others detect high-intensity, harsh stimuli. As in other animals, C. elegans mechanoreceptors are believed to rely on ion channels gated by mechanical cues. Techniques for patch-clamp recordings from identified C. elegans neurons (Goodman et al. 1998) have been adapted to measure mechanoreceptor currents (MRCs) in several mechanoreceptor neurons (Figure 3).
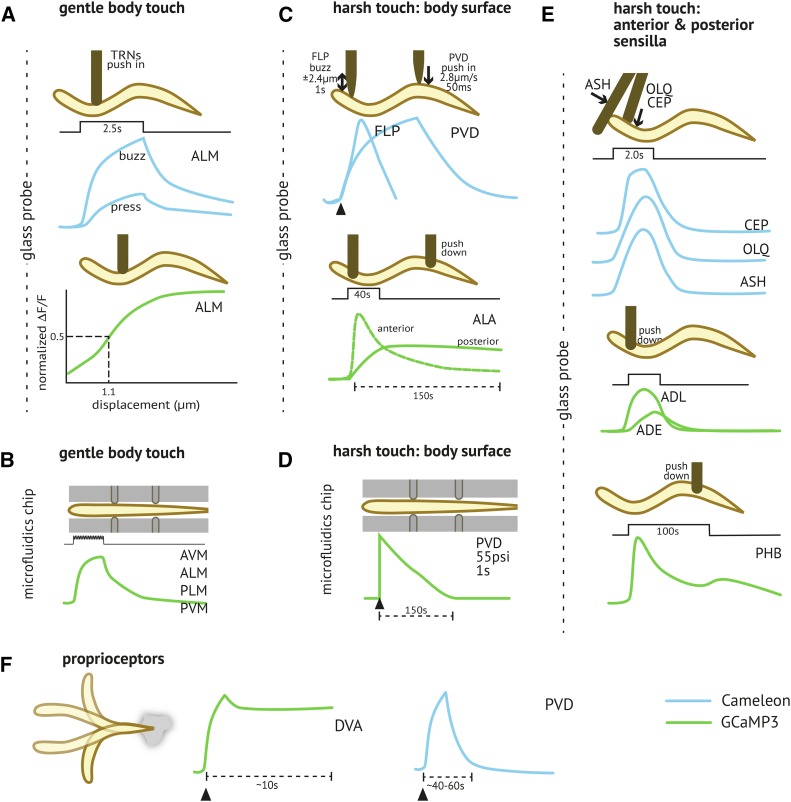
Figure 2.
Mechanical stimuli activate calcium transients in mechanoreceptor neurons. Schematics depict the stimulation position, intensity, and dynamics as well as calcium transients monitored using the ratiometric indicator, Cameleon (blue), or the single-wavelength indicator GCaMP (green). (A) Touch receptor neurons respond to a simple stimulus (press) and more strongly to a complex one (buzz) delivered via glass probe. Based on calcium imaging, the ALM neurons can detect submicrometer displacements (bottom). Data source(s): (Suzuki et al. 2003; Chatzigeorgiou et al. 2010; Chen and Chalfie 2014). (B) Touch receptor neurons activated in a microfluidic chip also demonstrate stronger response to buzz stimuli. Data source(s): (Cho et al. 2017; Nekimken et al. 2017a). (C) High intensity or harsh touch stimuli activate multidendritic and simple nociceptors. Each panel shows the time course of calcium transients evoked by mechanical stimuli delivered by pushing a stiff glass probe into the dorsal or ventral side of an immobilized animal (PVD, FLP) or pushing a probe down onto the side of an animal (ALA). Data source(s): (Chatzigeorgiou et al. 2010; Sanders et al. 2013; Cho et al. 2017). (D) Harsh touch stimuli delivered in a microfluidic chamber (PVD). Data source(s): (Cho et al. 2017), (E) High intensity or harsh touch stimuli activate nociceptors innervating anterior and posterior sensilla. Each panel shows the time course of calcium transients evoked by mechanical stimuli delivered by pushing a stiff glass probe into the dorsal or ventral side of an immobilized animal (ASH, OLQ, CEP) or by pushing down in an anterior (ADE, ADL) or posterior position (PHB) Data source(s): (Kindt et al. 2007a,b; Chatzigeorgiou et al. 2010; Chatzigeorgiou and Schafer 2011; Sanders et al. 2013; Zou et al. 2017). (F) Proprioceptors activated during body bending. Data source(s): (Li et al. 2006; Albeg et al. 2011).
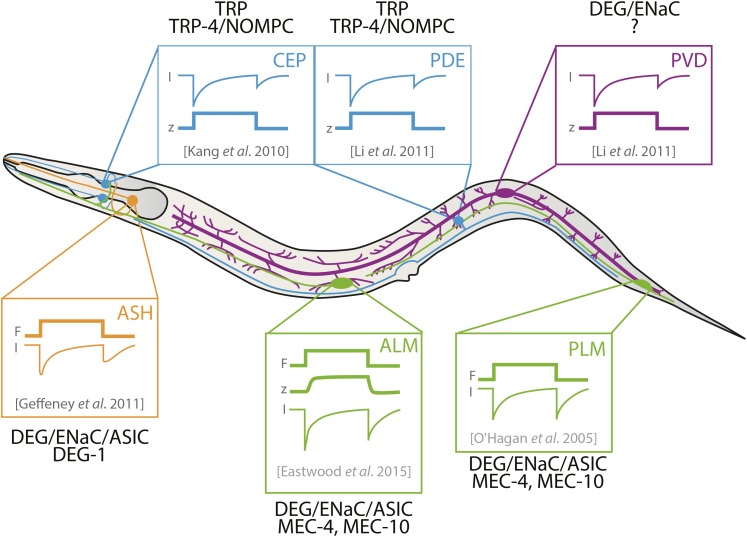
Figure 3.
Dynamics of mechanoreceptor currents (MRCs) recorded from C. elegans neurons in vivo. Shown (schematically) are the first reported measurements of MRCs in PDE (Li et al. 2011), ASH (Geffeney et al. 2011), ALM (Eastwood et al. 2015), CEP (Kang et al. 2010), PLM (O’Hagan et al. 2005), and PVD (Li et al. 2011). MRCs in ADE and CEP depend on expression of the trp-4 gene, which encodes the C. elegans homolog of the Drosophila NOMPC mechanosensitive ion channel. MRCs in ASH, ALM, PVD, and PLM are all carried by sodium ions and blocked by the diuretic drug, amiloride, and depend on DEG-1 in ASH and on MEC-4 in ALM and PLM. [The mec-10 gene is dispensable for MRC generation in ALM and PLM, but contributes to the pore; the pore forming subunits of MRCs in PVD remain to be discovered].
The integration of mechanosensory neurons into neural circuits, and how behavioral responses are linked to external and self-generated mechanical cues are described elsewhere (e.g., Goodman 2006; Schafer 2015). In reviewing current knowledge, we also seek to alert readers to gaps in knowledge and to unifying concepts that are emerging from recent research. For instance, current evidence suggests that ion channels are the principal receptors for light touch, painful or harsh touch, and proprioception, and that the proximal effect leading to their activation is indentation-induced changes in local mechanical strain (aka stretch).
Gentle or light touch
The gentle touch receptor neurons (TRNs) innervate the body surface and are required for touch-evoked avoidance behaviors. Optogenetic activation of these neurons is sufficient to evoke avoidance behaviors (Leifer et al. 2011; Stirman et al. 2011). Activation of the anterior TRNs suppresses rapid head swings during backward movement, an effect thought to enable nematodes to escape from traps set by predatory fungi (Maguire et al. 2011). The TRNs also contribute to habituation (reviewed in Bozorgmehr et al. 2013), a form of behavioral plasticity in which animals become less sensitive to repeated sensory stimulation. The simplicity of TRN-dependent behaviors enabled Chalfie and colleagues to perform comprehensive, forward genetic screens that identified hundreds of mutant alleles that impair or eliminate touch sensitivity in C. elegans hermaphrodites (Chalfie and Sulston 1981; Chalfie and Au 1989). In turn, these so-called mec (mechanosensory abnormal) mutants have been critical tools for advancing our understanding of mechanical senses more generally.
Freely moving wild-type animals can respond to forces as small as 100 nN and the response probability saturates near 0.8 (or 8 of 10 trials) for forces exceeding 2 µN (Petzold et al. 2013). The force required for half-activation (F1/2), depends on body stiffness, indicating that a complex relationship exists between applied force and touch-evoked behavior. Indeed, touch sensitivity is more directly related to body indentation than it is to applied force. An indentation of only 100 nm is sufficient to evoke an avoidance response and ∼450 nm is the indentation required to evoke a half-maximal response (Petzold et al. 2013). Thus, C. elegans hermaphrodites are extraordinarily sensitive to mechanical stimuli and touch sensation depends on skin indentation, rather than the applied force.
TRN cytoskeleton and extracellular matrix:
Wild-type TRN neurites contain a cross-linked bundle of ca. 40–50 microtubules; individual microtubules are long (10–15 µm) and staggered to fill the length of the neurite (Chalfie and Thomson 1979). TRN microtubules are composed of 15 protofilaments and are highly acetylated (Chalfie and Thomson 1982; Fukushige et al. 1999). The C. elegans genome contains two genes encoding α-tubulin acetyltransferases, mec-17 and atat-2, and both paralogs are coexpressed in the TRNs (Akella et al. 2010; Shida et al. 2010; Topalidou et al. 2012). The TRNs express multiple α- and β-tubulin isoforms, including four α-tubulins, tba-1, tba-2, tba-7, mec-12, and three β-tubulins, tbb-1, tbb-2, mec-7 (Savage et al. 1989; Fukushige et al. 1995, 1999; Lockhead et al. 2016; Zheng et al. 2017). The mec-12 α-tubulin and mec-7 β-tubulin genes are expressed at levels more than fivefold higher than other tubulin genes in TRNs (Lockhead et al. 2016), underscoring their critical importance in TRN development and function. Loss of mec-12 or mec-7 function is sufficient to disrupt 15-protofilament microtubules in the TRNs (Chalfie and Thomson 1982; Chalfie et al. 1986; Cueva et al. 2012; Zheng et al. 2017). Null mutants of both genes are touch-insensitive or Mec (Chalfie and Au 1989) and have mechanoreceptor currents that are dramatically reduced in amplitude (O’Hagan et al. 2005; Bounoutas et al. 2009). These tubulins also play critical roles in vesicle transport, as demonstrated by transport defects in animals carrying missense mutations in both mec-7 and mec-12 (Krieg et al. 2017; Zheng et al. 2017). The contribution of other tubulins to TRN development and function may be partially redundant or specialized. tba-1;mec-12 double mutants exhibit stronger defects in axon outgrowth than either single mutant (Lockhead et al. 2016), and tba-7 mutants exhibit ectopic neurites (Zheng et al. 2017).
In newly hatched larvae, the lateral TRNs (ALM, PLM) lie next to muscle cells. As animals mature and epidermal cells expand, TRN neurites separate from the muscle. This process is disrupted in him-4 hemicentin mutants (Vogel and Hedgecock 2001) and in mec-1 mutants with defects in the N-terminal portion of the encoded MEC-1 protein (Emtage et al. 2004). The mec-1 gene produces several transcripts and the longer ones encode a large polypeptide of >2000 amino acids. Consistent with its influence on neuronal attachment and the presence of a predicted N-terminal signal sequence, MEC-1 is thought to reside in the ECM. Indeed, mec-1 mutants lack the electron-dense mantle (Chalfie and Sulston 1981). Two additional proteins are thought to contribute to the specialized ECM: MEC-5 and MEC-9. The MEC-5 protein is an atypical collagen that is not expressed by the TRNs, but is required for the proper distribution of MEC-4 channels (see below) along the TRNs (Du et al. 1996; Emtage et al. 2004) and for touch-evoked mechanoreceptor currents (O’Hagan 2005). A long isoform of the MEC-9 protein harbors multiple Kunitz and EGF domains and is expressed selectively in the TRNs (Du et al. 1996). As found for MEC-5, loss of MEC-9 also disrupts the distribution of MEC-4 channels and the production of touch-evoked mechanoreceptor currents (Emtage et al. 2004; O’Hagan 2005).
TRN physiology—touch-evoked calcium signals:
One of the earliest applications of genetically encoded calcium indicators in living animals involved expressing Cameleon, a FRET-based calcium indicator (Miyawaki et al. 1997) in C. elegans muscles (Kerr et al. 2000) and in the TRNs (Suzuki et al. 2003). By imaging touch-evoked changes in Cameleon signals, Suzuki et al. (2003) showed that mechanical stimulation activated the TRNs (Figure 2A, top). Touch-evoked calcium signals were abolished by null mutations in the mec-4, mec-2, and mec-6 genes previously known to abrogate touch sensation, and impaired by reduction-of-function mutations in the egl-19 voltage-gated calcium channel (Suzuki et al. 2003). This study was the first to establish that any of the putative mechanosensory neurons in C. elegans function as bona fide mechanoreceptor neurons, and to link genes encoding the ion channel proteins, mec-4, mec-2, and mec-6 to touch-evoked activation of the TRNs. As illustrated schematically in Figure 2A (top), this study also showed that a brief, sinusoidal stimulus (buzz) was more effective than a single pulse (press).
The amplitude of touch-evoked calcium transients decreases during repeated stimulation; this effect is stronger in ALM than it is in PLM (Kindt et al. 2007a). Subsequent studies using more sensitive genetically encoded calcium indicators (e.g., GCaMP3, GCaMP6s) showed that calcium transients increase with probe displacement (Figure 2A, bottom) and that response amplitude, but not displacement sensitivity, is decreased by mutations in the EGL-19 L-type voltage-gated calcium channel (Chen and Chalfie 2014). The latter finding is consistent with the idea that voltage-gated calcium channels amplify touch-evoked calcium transients, but do not determine mechanosensitivity.
TRN physiology:
Touch depolarizes the ALM and PLM neurons and activates an inward mechanoreceptor current (MRC) at both the onset and withdrawal of mechanical loads (O’Hagan et al. 2005; Bounoutas et al. 2009; Arnadottir et al. 2011; Chen and Chalfie 2015; Eastwood et al. 2015; Chen et al. 2016a; Han et al. 2017) (Figure 3). These response dynamics are not unique to the TRNs; rather they are shared by other mechanoreceptor neurons (Figure 3). Given that a buzz or brief sinusoidal stimulus activates MRCs about twice during each cycle (Eastwood et al. 2015), these response dynamics are likely to account for the empirical observation that a buzz generates larger calcium signals than a press (Suzuki et al. 2003; Nekimken et al. 2017a). Studies using a feedback-control device to control applied force and measure indentation (or vice versa) show that MRC activation depends on the indentation produced, rather than the force applied (Petzold et al. 2013; Eastwood et al. 2015). Like the responses of mammalian Pacinian corpuscles (Sato 1961; Loewenstein and Mendelson 1965), MRC activation is also velocity- and frequency-dependent: neither pushing slowly against the animal’s body nor a brief, sinusoidal stimulus of <3 Hz could activate any current. Thus, C. elegans TRNs appear to be tuned to fast stimulation and are insensitive to slow stimuli such as those generated during movement, and, therefore, unlikely to function as proprioceptors. Consistent with this prediction, animals that lack TRNs have no obvious defects in movement.
In the TRNs, mechanoreceptor currents depend on extracellular sodium ions and are inhibited by the diuretic drug, amiloride. These properties are shared by channels formed by members of the superfamily of DEG/ENaC/ASIC channel proteins (Eastwood and Goodman 2012; Kellenberger and Schild 2015; Boscardin et al. 2016), including MEC-4 (Goodman et al. 2002). Loss of MEC-4 eliminates MRCs (O’Hagan et al. 2005), while loss of MEC-10 has only minor effects on these touch-evoked currents (Arnadottir et al. 2011). Mutations affecting conserved glycines in the second transmembrane domain of MEC-4 and MEC-10 alter the ionic permeability of MRCs recorded in the TRNs (O’Hagan 2005; Arnadottir et al. 2011), demonstrating that these proteins are pore-forming subunits of the native mechano-electrical transduction (MeT) channel responsible for touch sensation in the TRNs. This study was the first to link specific ion channel subunits to a native MeT channel in any mechanoreceptor neuron.
Harsh touch (mechanical nociception)
Harsh touch is detected primarily by sensory neurons innervating the nose and by multidendritic neurons that tile the body surface (Figure 1 and Table 1), and is linked most often to avoidance behaviors. For instance, head-on collisions evoke a robust evasive behavior consisting of a quick reversal followed by an omega turn, a behavior commonly described as a nose-touch response. Laser-mediated ablation studies reveal that this response depends on the ASH, CEP, and FLP neurons (Kaplan and Horvitz 1993). Optogenetic activation of the ASH neurons alone (Guo et al. 2009) or the FLP neurons alone (Li et al. 2011) is sufficient to evoke a reversal response. Mechanical stimuli delivered along the ventral side of the animal’s nose evoke head withdrawal and depend on the OLQ, FLP, and IL1 neurons (Hart et al. 1995). The OLQ, FLP, and CEP neurons are interconnected through gap junctions with RIH, a so-called hub interneuron, and this network appears to operate in parallel with the ASH neurons to govern sensorimotor integration in a complex manner (Chatzigeorgiou and Schafer 2011; Rabinowitch et al. 2013).
FLP and PVD are bilaterally symmetric, multidentric sensory neurons that extend primary dendrites bearing menorah-shaped dendritic structures that extend laterally across the body surface and are embedded between body wall muscles and the epidermal layer (Figure 1) (Halevi et al. 2002; Albeg et al. 2011). In contrast with FLP, optogenetic activation of PVD triggers forward movement (Li et al. 2011). The PVD and FLP neurons mediate responses to high-intensity mechanical cues (harsh touch) as well as to extreme cold (PVD) and heat (FLP) (Way and Chalfie 1989; Chatzigeorgiou et al. 2010; Albeg et al. 2011; Chatzigeorgiou and Schafer 2011; Schild et al. 2014).
Based on laser killing experiments (Li et al. 2011), these other sensory neurons have been implicated in harsh touch avoidance behaviors: ADE, AQR, BDU, SDQ, PHA, PHB, PQR, and PDE. It is not yet clear how mechanical loads affect signaling by these neurons or whether they function as primary mechanoreceptors.
Cellular responses to harsh touch—calcium signals:
Compressing the worm’s nose or body evokes transient increases in intracellular calcium in certain neurons and some glia linked to harsh touch sensation. The ASH neurons are activated by rapid compression delivered perpendicular to the anterior-posterior body axis (Kindt et al. 2002; Hilliard et al. 2005; Walker et al. 2009), which is illustrated schematically in Figure 2E. Their response is independent of unc-13-dependent synaptic transmission (Hilliard et al. 2005) and decreased in itr-1 IP3 receptor mutants (Walker et al. 2009). These findings imply that ASH is a primary mechanoreceptor neuron and that release of calcium from ITR-1-dependent intracellular stores contributes to compression-evoked calcium transients. Mechanical stimuli similar to those that activate the ASH neurons also evoke large inward currents and calcium transients in the amphid sheath cells (Ding et al. 2015). These observations raise the possibility that ASH signals downstream of glia or that ASH mechanoresponses involve signals from both non-neuronal cells (glia) and sensory neurons. A scenario analogous to the second possibility occurs in mammals in which both specialized epidermal cells and the neurons that innervate them function as primary mechanoreceptors (Reviewed by Vásquez et al. 2014; Woo et al. 2015).
The OLQ (Kindt et al. 2002; Chatzigeorgiou and Schafer 2011) and CEP neurons (Kindt et al. 2007b) also behave like primary mechanoreceptor neurons (Figure 2E and Table 1). Mechanoresponses in OLQ and CEP depend on the osm-9 TRPV channel and the trp-4 TRPN channel, respectively (Chatzigeorgiou and Schafer 2011). Consistent with the idea that these channels are needed for mechanosensitivity, OLQ mechanoresponses are restored by expression of osm-9 in the OLQ neurons and CEP mechanoresponses are restored by expression of trp-4 in the CEP neurons (Chatzigeorgiou and Schafer 2011). Compressing the nose in both a harsh and gentle manner activates calcium transients in FLP (Chatzigeorgiou and Schafer 2011) (Figure 2C). Sensitivity to modest or gentle indentation is facilitated by two other mechanoreceptor neurons, OLQ and CEP, via the common hub neuron, RIH (Chatzigeorgiou and Schafer 2011). By contrast, sensitivity to harsh stimuli is thought to be cell autonomous based on a requirement for the expression of an intact mec-10 DEG/ENaC/ASIC channel gene in FLP itself (Chatzigeorgiou and Schafer 2011).
Body indentation evokes calcium transients in PVD (Chatzigeorgiou and Schafer 2011) whose amplitude increase with stimulus intensity and stimulus duration (Cho et al. 2017) (Figure 2C). However, PVD neurons are also activated by body bends (Albeg et al. 2011) (Figure 2F)—a result that suggests that these neurons may also function as proprioceptors. Consistent with this idea, genetic ablation of the PVD neurons alters body posture (Albeg et al. 2011) and affects locomotion speed (Cohen et al. 2012). It will be interesting to discover if both classes of multidendritic neurons, PVD and FLP, have dual roles as nociceptors and proprioceptors, and how the behaviors linked to activation of these mechanoreceptor neurons depend on neural circuitry. The shape of the ALA neuron is much simpler than that of PVD and FLP (Figure 1), but it has one of the longest neurites in the C. elegans nervous system. Calcium imaging (Figure 2C) demonstrates that ALA neurites are sensitive to high-intensity (harsh touch) mechanical stimulation along their lengths, and that distal stimuli generate slower responses than proximal stimuli (Sanders et al. 2013). As shown schematically in Figure 2E, mechanical stimuli evoke calcium transients in two other classes of sensory neurons not previously considered mechanoreceptors: PHA/PHB, ADL (Sanders et al. 2013).
Cellular responses to harsh touch—mechanoreceptor currents:
The finding that compressing the worm’s nose evokes transient increases in intracellular calcium implies that mechanical stimuli depolarize mechanoreceptor neurons by activating inward currents. Indeed, the onset and withdrawal of such mechanical loads activates an inward mechanoreceptor current (MRC) in the CEP neurons (Kang et al. 2010; Han et al. 2017) (Figure 3). The channels carrying these currents are permeable to both Na+ and K+ ions and depend on expression of the trp-4 gene encoding the C. elegans ortholog of the mechanosensitive NOMPC protein (Kang et al. 2010). MRC activation is displacement-dependent and adapts to a conditioning stimulus by shifting its activation curve to larger displacements (Kang et al. 2010). Polymorphisms in the trp-4 gene may govern the relationship between MRC activation and displacement (Han et al. 2017).
As shown schematically in Figure 3, electrophysiological recordings from ASH reveal robust mechanoreceptor potentials and sodium-dependent, amiloride-sensitive mechanoreceptor currents (Geffeney et al. 2011; Ding et al. 2015). As found in the touch receptor neurons (O’Hagan et al. 2005) and CEP neurons (Kang et al. 2010), MRCs activate with a millisecond latency and occur in response to both the onset and removal of mechanical deformations of the nose (Geffeney et al. 2011). They are dramatically reduced in mutants carrying a deletion in the deg-1 DEG/ENaC/ASIC gene. Unexpectedly, MRCs are unaffected by loss of the osm-9 or the ocr-2 TRPV channel genes alone or in combination (Geffeney et al. 2011). This counterintuitive result implies that these two TRPV channels contribute to ASH signaling downstream of the initial transduction event. Similar to the TRNs and ASH, electrical recordings from PVD and PDE demonstrate that mechanical stimulation activates an inward current at both the onset and removal of the stimulus. Mechanoreceptor currents in these cells are blocked by amiloride and likely to be carried primarily by sodium ions (Li et al. 2011).
Although electrophysiological recordings have not been reported for any other sensory neuron linked to harsh touch sensation in C. elegans, recordings from the amphid sheath cells reveal large, mechanically activated inward currents. These currents reverse polarity near 0 mV and are not sensitive to amiloride (Ding et al. 2015)—properties that make it unlikely that they are carried by a DEG/ENaC/ASIC channel. The protein partners that make up this channel are not currently known, nor is it known how mechanosensitivity in these cells contributes to harsh touch sensation in particular or to other functions more broadly.
Texture sensing
Wild-type animals can discriminate between different surface textures in a manner that depends on CEP and ADE in the nose and PDE in the tail (Sawin et al. 2000; Han et al. 2017). As noted above, CEP and PDE generate currents in response to both the onset and withdrawal of an indentation (Kang et al. 2010; Li et al. 2011). This response pattern is consistent with mechanoreceptor neurons being sensitive to the temporal dynamics of mechanical stimulation. In this context, it is tempting to speculate it is these response dynamics that enable CEP, ADE and PDE to detect the vibration-like mechanical stimuli produced as the worm crawls over a textured surface.
Proprioception
The role of proprioception in regulating gait in C. elegans has been investigated using both experimental and computational approaches (reviewed by Schafer 2015; Zhen and Samuel 2015). One model emerging from this effort is that the B class of motor neurons helps to propagate signals related to body curvature (Wen et al. 2012), but it is not known if their activity is directly affected by the mechanical stresses generated during movement. The DVA (Kang et al. 2010) and PVD (Albeg et al. 2011; Cohen et al. 2012) neurons have also been linked to proprioception (Figure 2F). In addition to these neurons innervating the central and posterior domains of the worm’s body, the fourfold symmetric SMD neurons innervating the head were also proposed to function as proprioceptors based upon their position in the body and neural network (White et al. 1986). Consistent with such a function, the SMDD and SMDV neurons are activated by dorsal and ventral bending, respectively, and are activated in an antiphase manner during forward locomotion (Yeon et al. 2018). The sensitivity of the SMDD neurons to body bending, and the coordination of the SMDV and SMDD neurons, depend upon expression of TRP-1 and TRP-2 TRPC channels in the SMD neurons. Expression of TRP-1 confers sensitivity to dorsal-ventral bending on the AWC chemosensory neurons. Together, these findings suggest that a TRPC ion channel may function as a proprioceptive receptor in C. elegans neurons (Yeon et al. 2018).
Mating and mechanoreceptor neurons
C. elegans males have 42 sex-specific, ciliated mechanoreceptor neurons that innervate the tail, hook, post-cloacal sensilla and spicules (Figure 1). Based on laser ablation studies (Liu and Sternberg 1995), all of these sensory neurons are thought to play essential roles in mating behavior. The sensilla that innervate the ventral surface of the male tail are required for responses to ventral contact with hermaphrodites, while dorsally directed rays mediate responses to dorsal contact with hermaphrodites (Liu and Sternberg 1995). The hook sensilla function in vulva location, while the sensory neurons that innervate the spicule are likely to provide feedback for spicule insertion into the vulva and subsequent sperm release. It is likely that many or perhaps all of these sensory neurons contribute to mate-sensing, while also providing proprioceptive feedback for ensuring the precise execution of mating behaviors, including response, turning, vulva detection, spicule insertion, and sperm transfer. For additional details regarding male mating behavior, please see Barr et al. (2018).
Like other mechanoreceptor neurons, the ray sensory neurons in the male tail respond to applied mechanical loads with a transient increase in intracellular calcium, as reported by GCaMP5 fluorescence in cell bodies (Zhang et al. 2018). Such responses are independent of the expression of unc-13 and unc-31 genes essential for release of clear- and dense-core synaptic vesicles, respectively (Zhang et al. 2018), indicating that touch-evoked calcium responses originate in the sensory neurons themselves. Calcium responses in the ray neurons are much slower than those of other C. elegans mechanoreceptors, reaching their peak amplitude over tens of seconds. Loss of the PKD-2 TRPP ion channel, which is required for male mating behavior (Barr et al. 2001), had no detectable effect on touch-evoked calcium signals. Loss of the OSM-9 TRPV channel decreased and slowed the mechanoreceptor calcium signals, but did not eliminate them. These signals were blocked by application of the DEG/ENaC/ASIC channel blocker, amiloride (Zhang et al. 2018). The picture emerging from these results is that, similar to ASH neurons in hermaphrodites, ray neurons in the male tail rely upon a DEG/ENaC/ASIC channel to detect mechanical stimuli and the OSM-9 TRPV channel to amplify such responses.
Concluding remarks
These studies demonstrate that C. elegans relies on both TRP and DEG/ENaC channels to convert mechanical stimuli into electrical signals and that these channels activate in response to stimulus application and removal (summarized in Figure 3). Thus, divergent ion channels can function as mechanoelectrical transduction (MeT) channels (Katta et al. 2015). A corollary of this idea is that it is unlikely that all TRP or DEG/ENaC channels function as MeT channels. In support of this inference, the osm-9 gene encoding a TRPV channel was initially thought to have this function (Colbert et al. 1997), but subsequent studies indicate that this TRPV channel is dispensable for mechanotransduction (Geffeney et al. 2011; Zhang et al. 2018). Future work will be needed to determine how this TRPV channel and others expressed in mechanosensory neurons contribute to mechanosensation.
Whether the in vivo activation of MeT channels depends on the force-from-lipid (Anishkin and Kung 2013; Anishkin et al. 2014), the force-from-filament (Katta et al. 2015), or a combination of both principles, remains to be determined. To date, none of the channels identified as sensory MeT channels in C. elegans have been shown to retain mechanosensitivity when purified and reconstituted. This is also true of most channels thought to function as MeT channels in mammals, with the exception of Piezo1 (Syeda et al. 2016). Even Piezo1 depends on an intimate relationship with the plasma membrane for mechanosensitivity (Guo and MacKinnon 2017; Haselwandter and MacKinnon 2018), suggesting that the mechanosensitivity of each MeT channel is a combined function of its biophysical properties and the cellular machinery. Owing to its diversity of mechanoreceptor neurons and the availability of techniques in single-neuron physiology and genetic dissection, studies in C. elegans are ideally suited to investigate whether a givent MeT channel is activated following the force-from-lipid or the force-from-filament principle or a combination of these biophysical mechanisms.
Thermosensation and Molecular Mechanisms of Thermotransduction
Temperature is a ubiquitous stimulus that regulates the rate of every biochemical reaction. It is thus imperative for animals to sense temperature changes in order to appropriately alter their physiology and behavior. Thermosensation is particularly critical for ectotherms whose body temperature is dictated by the environment. Faced with large diurnal and seasonal temperature fluctuations, these animals must seek temperatures conducive to survival, and avoid noxious temperature ranges. Mechanisms underlying thermosensation are not fully understood. Members of the TRP family of cation channels have been implicated in thermosensation in both the physiological and noxious temperature ranges in multiple species, but species-specific thermosensory molecules and mechanisms have also been described (Sengupta and Garrity 2013; Li and Gong 2017; Hoffstaetter et al. 2018). The molecular basis of thermoreception in animals remains unknown.
C. elegans provides a particularly attractive system in which to study the molecular and neuronal basis of thermosensation. C. elegans is able to survive and reproduce across a relatively broad range of temperatures ranging from 12 to 26°, and can thus be considered a temperature generalist (Angilletta 2009; Anderson et al. 2011; Schulenburg and Felix 2017). These nematodes are remarkably thermosensitive and are able to detect, and behaviorally respond to, temperature changes of as little as 0.01° in the laboratory (Luo et al. 2006; Ramot et al. 2008a). In addition to regulating behavior, thermal cues regulate multiple aspects of nematode physiology. Studies in this organism have provided insights into the transduction pathways that allow animals to respond with high sensitivity and fidelity to temperature changes over a broad temperature range. Intriguingly, thermosensory pathways in C. elegans appear to share remarkable parallels with vertebrate phototransduction mechanisms, revealing a surprising conservation of signaling pathways that mediate responses to distinct sensory cues. This section reviews current knowledge about the neurons and signaling pathways that detect and translate environmental temperature changes into changes in locomotion, navigation, and physiology in C. elegans.
Behavioral responses to thermal stimuli
In the laboratory, the Bristol N2 strain of C. elegans does not exhibit a fixed preferred temperature. Instead, the behavior of worms on thermal spatial gradients is dictated by the animal’s previous temperature experience. When animals grown for several hours at a given temperature (cultivation temperature or Tc) are placed at a temperature T > Tc, worms migrate toward cooler temperatures on the gradient (negative thermotaxis) (Hedgecock and Russell 1975; Mori and Ohshima 1995). Conversely, when placed at a T that is a few degrees cooler than Tc on shallow thermal gradients, they migrate toward warmer temperatures (positive thermotaxis) (Mori and Ohshima 1995; Ramot et al. 2008b; Jurado et al. 2010). However, if placed at T << Tc, they are atactic (Ryu and Samuel 2002; Ramot et al. 2008b). In a narrow temperature band within 2° of Tc, worms track isotherms (Hedgecock and Russell 1975; Mori and Ohshima 1995; Luo et al. 2006) (summarized in Figure 4). The exact behavior exhibited depends not only on the difference between T and Tc, but also the gradient steepness and the animal’s internal state. Thus, on steep thermal gradients of >1.5°/cm, worms perform negative thermotaxis regardless of their temperature experience (Yamada and Ohshima 2003; Ramot et al. 2008b). In contrast, if animals are starved, while they continue to track isotherms, they no longer perform negative thermotaxis (Hedgecock and Russell 1975; Mohri et al. 2005; Biron et al. 2006; Chi et al. 2007). Tc behavioral “memory” can be reset upon shifting adult animals to a new Tc for a few hours (Hedgecock and Russell 1975; Mori and Ohshima 1995; Biron et al. 2006; Ramot et al. 2008b), indicating that this is a highly plastic process.
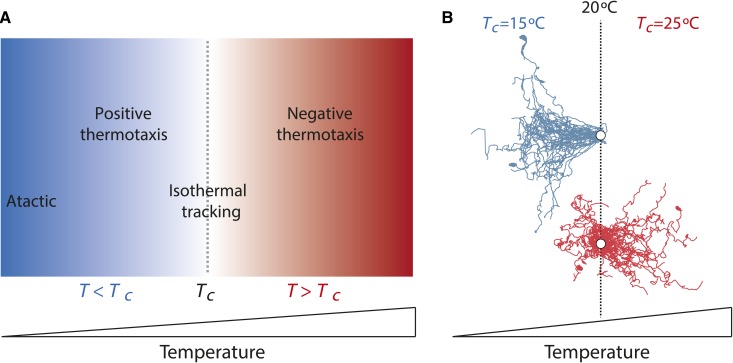
Figure 4.
C. elegans exhibits Tc-dependent navigation behaviors on spatial thermal gradients. (A) Schematic of navigation behaviors exhibited at temperatures relative to Tc (Tc is defined here as the temperature experienced 3–5 hr prior to the assay). T: ambient temperature. (B) Example trajectories of individual animals grown at either 15° (blue) or 25° (red) and placed at 20° on a shallow spatial thermal gradient. Note movement of animals toward cooler temperatures when T > Tc, and movement toward warmer temperatures when T < Tc. Trajectories are adjusted to the same starting point (white circles). Adapted from Luo et al. (2014a).
Detailed analyses of worm locomotion have described the navigation strategies underlying thermotaxis. Negative thermotaxis is mediated primarily via a biased random walk strategy (klinokinesis) (Ryu and Samuel 2002; Clark et al. 2007b; Ramot et al. 2008b; Luo et al. 2014a). In this strategy, worms extend the duration of their forward movement when moving toward cooler temperatures, and increase the frequency of reorientations when moving toward warmer temperatures (Ryu and Samuel 2002; Clark et al. 2007b). In addition, animals preferentially move toward cooler temperatures following a reorientation (Luo et al. 2014a). Movement toward warmer temperatures is mediated primarily via preferential movement toward warmer temperatures following reorientation; klinokinesis does not appear to contribute to this behavior (Luo et al. 2014a). Isothermal tracking is mediated via a distinct motor program. Worms do not seek isotherms but if they are serendipitously on an isotherm within T±∼2°, the temperature changes detected by the sinusoidal oscillation of their heads is translated via as yet uncharacterized mechanisms to suppress turns and maintain extended periods of forward movement (Ryu and Samuel 2002; Luo et al. 2006). Modeling experiments suggest that both negative thermotaxis and isothermal tracking behaviors are likely to contribute to the ability of worms to tolerate the daily temperature fluctuations they experience in their terrestrial habitats (Ramot et al. 2008b).
Temperature responses in sensory neurons driving thermosensory navigation behaviors
Three pairs of sensory neurons (AFD, AWC, ASI) in the bilateral amphid sense organs of the head have been implicated in regulating thermosensory navigation behaviors (Table 1). Of these, the AFD neurons are the primary thermoreceptors regulating thermotaxis, with AWC and ASI playing minor modulatory roles.
Temperature responses in AFD:
Bilateral ablation of AFD fully abolishes isothermal tracking behavior and disrupts negative and positive thermotaxis, supporting a major role of this sensory neuron type in mediating thermotaxis (Mori and Ohshima 1995). Responses of AFD to temperature changes can be robustly measured using genetically encoded calcium indicators in vivo. Calcium levels in AFD rise as temperatures warm and fall when temperatures drop (Kimura et al. 2004; Clark et al. 2006) (Figure 5A). Temperature responses in AFD are strongly correlated with temperature fluctuations and can be observed in animals freely navigating a thermal gradient, as well as in immobilized animals subjected to temperature oscillations (Clark et al. 2006, 2007a; Tsukada et al. 2016; Venkatachalam et al. 2016). Moreover, these responses are observed in dissociated embryonic AFD neurons in culture (Kobayashi et al. 2016), indicating that these temperature responses are an intrinsic property of AFD.
Figure 5.
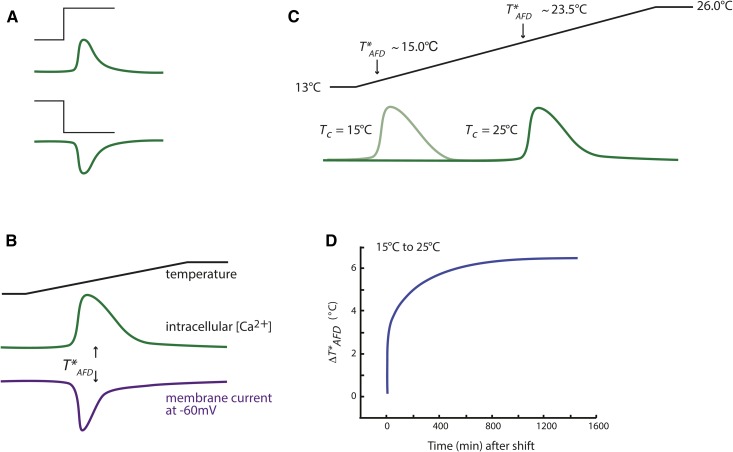
The AFD neurons exhibit responses to warming and cooling in a Tc experience-dependent manner. (A) Schematic of changes in intracellular calcium in AFD (green lines) in response to a warming (top) or cooling (bottom) step (black lines) (Kimura et al. 2004; Clark et al. 2006). (B) Schematic of calcium transients (green) and thermoreceptor current (purple) (Ramot et al. 2008a) in response to a rising temperature ramp (black line). Responses are observed at temperatures above a Tc-regulated threshold T*AFD. (C) T*AFD shifts upon acclimation to a new Tc. Schematic of calcium transients (green lines) in response to a rising temperature ramp (black line) in animals acclimated to different temperatures (Kimura et al. 2004; Biron et al. 2006; Clark et al. 2006). Adapted with permission from Goodman and Sengupta (2018). (D) Schematic showing dynamics of T*AFD adaptation as measured by calcium transients upon temperature shift from 15 to 25°. Data source(s): (Yu et al. 2014).
Consistent with the observed calcium flux in AFD upon temperature fluctuations, warming and cooling increase and decrease a nonselective cation current, respectively (Figure 5B), resulting in depolarization of AFD upon warming, and hyperpolarization upon cooling (Ramot et al. 2008b). Quantification of the temperature dependence of the thermoreceptor current in AFD has shown that this neuron type is extraordinary in its thermosensitivity. The unitless value Q10 (defined as the change in the rate of a reaction for every 10° change in temperature) provides a measure of the temperature-dependence of a reaction (Garrity et al. 2010). The Q10 of warming-evoked thermoreceptor currents in AFD is >1020 (Ramot et al. 2008a). This value is reminiscent of those estimated for thermosensory neurons in pit viper snakes (Bullock and Diecke 1956) and is many orders of magnitude larger than that reported for mammalian thermosensory neurons (Vyklický et al. 1999) and individual thermosensitive TRP channels. This extraordinary thermal dependence implies the existence of a nonlinear amplification process that culminates in ion channel activation, similar to the signal cascade responsible for single-photon sensing in vertebrates.
Interestingly, similar to the Tc-dependent modulation of the operating range of thermotaxis, the response threshold of AFD (T*AFD) is also Tc-correlated (Kimura et al. 2004; Biron et al. 2006; Clark et al. 2006; Kobayashi et al. 2016). Thus, in animals grown at 15°, AFD responds to temperature changes when the temperature rises above ∼15°, whereas upon growth at 25°, T*AFD shifts to ∼23° (Figure 5C). These response ranges of AFD correspond to the temperature ranges at which animals perform positive (e.g., grown at 25°, and placed at ∼22°) and negative (e.g., grown at 15°, and placed at T > Tc) thermotaxis behaviors (Luo et al. 2014b), raising the question of how similar thermosensory responses in AFD drive two distinct motor programs in different temperature ranges (see below). Neither T*AFD nor temperature responses in AFD are altered upon starvation (Ramot et al. 2008a; Tsukada et al. 2016), indicating that feeding state disrupts negative thermotaxis behaviors downstream or in parallel to AFD thermosensory responses.
While resetting of behavioral Tc memory to a new value requires growth at a new T for a few hours, T*AFD can shift on two distinct timescales upon a T upshift (Figure 5D). Both calcium imaging and electrophysiology experiments have indicated that shifting animals to a higher T results in a very rapid (timescale of minutes) corresponding shift in T*AFD (Ramot et al. 2008a; Yu et al. 2014; Hawk et al. 2018) (Figure 5D). If T >> Tc, adaptation of T*AFD to the final value additionally occurs on an hours-long timescale (Yu et al. 2014) (Figure 5D). In contrast to the biphasic T*AFD adaptation upon T upshift, T*AFD adapts slowly (timescale of hours) upon T downshift (Biron et al. 2006; Yu et al. 2014; Hawk et al. 2018). Rapid adaptation of T*AFD upon T upshift may allow animals to retain the ability to respond to small temperature changes across a broad temperature range, whereas the slower adaptation rate may allow precise adaptation to the warmer Tc.
While the motor program driving isothermal tracking behavior is unknown, temperature responses in AFD are also likely to be important for this behavior (Mori and Ohshima 1995). To track isotherms, animals must be able to detect and respond to rapid and small amplitude T changes around a constant T that is within ±2° of the Tc (Luo et al. 2006). Indeed, AFD is able to reliably respond to sinusoidal temperature variations only near the Tc (Wasserman et al. 2011). Moreover, the operating range for these responses is reset upon T*AFD adaptation to a new T (Wasserman et al. 2011). These observations suggest that the ability of AFD to track small T changes around T*AFD may drive isothermal tracking behavior.
Temperature responses in additional sensory neurons:
In addition to AFD, the AWC and ASI sensory neurons have been implicated in regulating thermosensory navigation behaviors (Biron et al. 2008; Kuhara et al. 2008; Beverly et al. 2011). Both these neuron types have been extensively studied in the context of mediating attraction to food-related odors (Bargmann and Horvitz 1991; Bargmann et al. 1993). Increased activity in AWC and ASI promotes and suppresses reorientations, respectively (Tsalik and Hobert 2003; Gray et al. 2005; Albrecht and Bargmann 2011; Larsch et al. 2013; Gordus et al. 2015). Consequently, physical or genetic ablation of AWC or ASI, or manipulation of their activity modulates, but does not fully disrupt, thermotaxis navigation primarily via alteration of reorientation frequency (Biron et al. 2008; Kuhara et al. 2008; Beverly et al. 2011). Consistently, AWC-ablated animals, or animals in which AWC is hyperactive, exhibit prolonged or abbreviated sojourns on isotherms, respectively (Biron et al. 2008). Both AWC and ASI respond to temperature changes, with the operating range of the responses dependent on Tc (Biron et al. 2008; Kuhara et al. 2008; Beverly et al. 2011). However, unlike temperature responses in AFD, calcium dynamics in AWC or ASI are not phase-locked to non-nociceptive time-varying thermal stimuli (Biron et al. 2008; Kuhara et al. 2008; Beverly et al. 2011). Together, these results indicate that under defined conditions, AWC and ASI contribute to the modulation of thermotaxis behaviors.
Molecular mechanisms of thermotransduction
Thermotransduction mechanisms have been studied more extensively in AFD than in AWC and ASI. It has long been established that cGMP signaling plays a critical role in thermotransduction based on the characterization of animals mutant for the tax-2 and tax-4 cGMP-gated channel subunit genes (Dusenbery et al. 1975; Hedgecock and Russell 1975). tax-2 and tax-4 mutants are atactic (Dusenbergy et al. 1975; Hedgecock and Russell 1975; Mori and Ohshima 1995), and the AFD neurons fail to exhibit changes in calcium dynamics (Kimura et al. 2004) or thermoreceptor current (Ramot et al. 2008a) in response to temperature changes in these mutants. Similarly, TAX-2 and TAX-4 are also essential for temperature responses in both AWC and ASI (Kuhara et al. 2008; Beverly et al. 2011).
TAX-2 and TAX-4 are unlikely to form temperature-gated channels. While these proteins are necessary for thermotransduction, they are expressed in multiple additional nonthermosensory neurons (Coburn et al. 1998; Kimura et al. 2004) indicating that they are not sufficient to mediate temperature responses. Moreover, a lag of ∼130 ms was observed between changes in temperature steps and opening of these channels in AFD (Ramot et al. 2008a), suggesting that these channels are gated by a soluble second messenger (such as cGMP) whose concentration is regulated in a temperature-dependent manner.
Soluble and transmembrane or receptor guanylyl cyclase (rGCs) enzymes catalyze the production of cGMP from GTP (Potter 2011; Kuhn 2016). Of the 27 rGCs encoded by the C. elegans genome, three (gcy-8, gcy-18, gcy-23) are expressed exclusively in AFD and are localized to their sensory endings (Yu et al. 1997; Inada et al. 2006; Ortiz et al. 2006). Similar to tax-2 and tax-4 mutants, animals mutant for all AFD-rGCs are atactic, and temperature changes fail to elicit changes in intracellular calcium or thermoreceptor current in the AFD neurons of these mutants (Inada et al. 2006; Ramot et al. 2008a; Wasserman et al. 2011; Wang et al. 2013; Takeishi et al. 2016). However, animals singly or doubly mutant for the AFD-rGCs exhibit subtle but nevertheless significant behavioral and imaging phenotypes (Inada et al. 2006; Wasserman et al. 2011; Wang et al. 2013; Takeishi et al. 2016). In particular, single and double mutant combinations of AFD-rGCs result in defects in thermotaxis navigation behaviors (Inada et al. 2006; Wasserman et al. 2011), and a lower T*AFD (Wasserman et al. 2011; Takeishi et al. 2016). These observations suggest that AFD-rGCs act partly redundantly to mediate thermotransduction in AFD.
Are these AFD-rGCs themselves the thermosensors in AFD? Misexpression of either GCY-23 or GCY-18, but not GCY-8, was found to be sufficient to confer highly thermosensitive responses in chemosensory neurons and vulval muscles (Takeishi et al. 2016). Interestingly, the thresholds of response of GCY-23 and GCY-18 were distinct from each other upon misexpression in a chemosensory neuron type (Takeishi et al. 2016). Moreover, the response threshold of GCY-23 was cell type-specific and, unlike in AFD, largely uncorrelated with Tc (Takeishi et al. 2016). Together, these results suggest that temperature may directly modulate AFD-rGC activity, but that both protein- and cell type-specific mechanisms define the temperature at which these proteins are activated. Whether other members of the rGC family similarly mediate thermosensation in AWC and ASI is not yet clear.
In addition to rGCs, several phosphodiesterase-encoding genes have been implicated in regulating thermotaxis behaviors. Of these pde phosphodiesterase genes, pde-1, pde-2 and pde-5 are expressed in AFD (Wang et al. 2013; Singhvi et al. 2016). In contrast to the lower T*AFD of rGC mutants (Wasserman et al. 2011; Takeishi et al. 2016), T*AFD in pde-2 mutants is higher (Wang et al. 2013), similar to T*AFD of animals grown on a nonhydrolyzable cGMP analog (Wasserman et al. 2011). Moreover, thermoreceptor currents in AFD are prolonged in pde-2 mutants (Wang et al. 2013). These observations indicate that correct regulation of both rGC and PDE enzyme functions is essential for fine-tuning thermosensory properties of AFD. Whether similar to AFD-rGCs, temperature also modulates AFD-expressed PDE activity is not yet known.
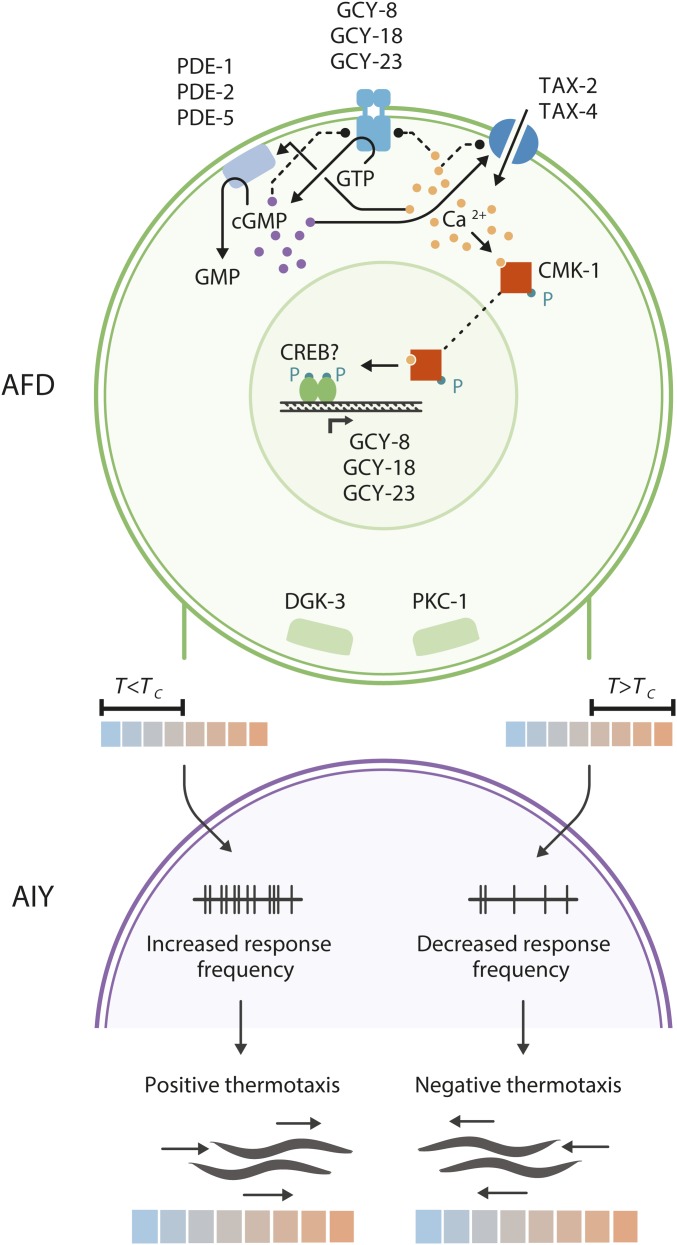
To summarize the current working model for thermotransduction in AFD, at T > T*AFD, thermosensory rGCs are activated to generate cGMP. Increased cGMP opens TAX-2/TAX-4 channels to permit cation influx and depolarization. Temperature changes and/or increased cGMP and/or calcium concentrations may also activate PDEs to hydrolyze cGMP and terminate signaling (summarized in Figure 6). The thermosensitivity of AFD-rGCs, amplification of a small temperature change via cGMP production, and rapid adaptation in part account for the extraordinary thermosensitivity of AFD. The molecular and cellular mechanisms of thermosensation in AFD are remarkably similar to those mediating light responses in vertebrate photoreceptors. Similar to AFD, photoreceptors are also highly sensitive; they can detect a single photon over a wide range of light intensities. It is tempting to speculate that these signaling pathways are an example of convergent evolution in cell types that are optimized for highly sensitive detection of environmental cues.
Figure 6.
Schematic of thermosensory signal transduction and synaptic output of AFD. Upon warming, GCY-8, GCY-18, and GCY-23 are activated to increase intracellular cGMP levels. The TAX-2/TAX-4 cGMP-gated channels open and enable calcium ion influx and depolarization. cGMP is degraded by PDEs whose functions may also be temperature-dependent. Rapid adaptation is hypothesized to be mediated by cGMP and/or calcium-mediated feedback; targets of this feedback may be the rGCs, PDEs or the cGMP-gated channels. Long-term adaptation of the sensory response threshold is mediated in part via CMK-1-regulated changes in rGC and other gene expression. AFD synaptic output is also regulated upon long-term temperature acclimation via DGK-3 and PKC-1. The probability and amplitude of temperature-regulated responses in AIY, the major postsynaptic partner of AFD, is decreased at T > Tc and promotes negative thermotaxis, whereas increased response probability in AIY at T < Tc promotes positive thermotaxis. See text for additional details and references.
Molecular mechanisms of thermosensory adaptation
As described above, T*AFD is reset on both a short (minutes) and long (hours) timescale upon T upshift allowing these neurons to both retain responsiveness to small temperature changes across a broad temperature range, as well as to precisely shift their operating range in a Tc-correlated manner. However, behavioral adaptation to Tc is only observed upon a hours-long exposure to a higher T, suggesting that additional mechanisms downstream of T*AFD adaptation—likely acting to modulate AFD synaptic output—must account for the altered behavioral operating range.
Mechanisms of adaptation of AFD thermosensory responses:
How does T*AFD adapt on both a fast and slow timescale? The fast timescale of minutes precludes transcription-dependent mechanisms. Calcium buffering slows the timescale of this fast adaptation suggesting that calcium modulates this process (Ramot et al. 2008a). Consistent with this hypothesis, animals mutant for the calcium-regulated NCS-1 frequenin-like protein are defective in fast adaptation (Wang et al. 2013). Since frequenin modulates phosphodiesterase activity (Schaad et al. 1996), calcium-dependent modulation of AFD-expressed PDEs may reset T*AFD. In addition, manipulation of intracellular cGMP levels in AFD alters T*AFD (Wasserman et al. 2011; Wang et al. 2013; Takeishi et al. 2016). Thus, a current notion is that upon temperature upshift, calcium and/or cGMP provide feedback signals to terminate signaling and rapidly reset T*AFD. Recent work has also implicated the SLO-1 BK+channels and the CNG-3 cGMP-gated channels in AFD adaptation (Aoki et al. 2018). The targets of this adaptation process are hypothesized to be the AFD-rGCs, AFD-expressed PDEs, cGMP-gated or K+ channels, or multiple combinations thereof (Figure 6).
Precise adaptation of T*AFD upon a large T shift requires several hours and is mediated via changes in gene expression (Yu et al. 2014). Expression of AFD-rGCs is higher in animals grown at a warmer temperature, and this temperature-dependent change in gene expression requires the CMK-1 calcium/calmodulin-dependent protein kinase I gene (Yu et al. 2014) (Figure 6). In cmk-1 mutants, expression of these (and likely additional genes) is not temperature-regulated, resulting in a decreased magnitude of T*AFD adaptation and lower final T*AFD (Yu et al. 2014). CMK-1-mediated changes in gene expression might require the Raf kinase pathway and possibly CREB (Nishida et al. 2011; Kobayashi et al. 2016) (Figure 6). Although T*AFD adaptation has also been shown to occur in dissociated AFD neurons grown in culture (Kobayashi et al. 2016), cell nonautonomous mechanisms may also play a role in fine-tuning T*AFD (see below).
Mechanisms of adaptation in AFD synaptic output:
The AIY interneurons are the primary postsynaptic partners of AFD (White et al. 1986). Thus, analyses of temperature responses in AIY can serve as a reasonable proxy for measurements of AFD synaptic output. The threshold of AIY temperature responses adapts on a behavioral hours-long timescale (Biron et al. 2006; Hawk et al. 2018) suggesting that adaptation of T*AFD and AFD synaptic output can be partially decoupled molecularly. Indeed, mutations in the dgk-3 diacylglycerol kinase and pkc-1 nPKCε genes alter the rate of behavioral adaptation and AFD synaptic output without affecting T*AFD adaptation (Okochi et al. 2005; Biron et al. 2006; Luo et al. 2014b; Hawk et al. 2018).
AFD to AIY synaptic transmission is modulated by the animal’s thermal experience in a complex manner. The AFD neurons are glutamatergic and are likely to signal to AIY via both glutamate-mediated inhibitory and peptidergic excitatory transmission (Clark et al. 2007b; Kuhara et al. 2011; Narayan et al. 2011; Ohnishi et al. 2011). Interestingly, in the operating range of negative thermotaxis at T > Tc, both the probability and amplitude of AFD-driven AIY temperature responses are decreased, whereas conversely, in the operating range for positive thermotaxis, probability and amplitude of AIY responses are increased (Kuhara et al. 2011; Hawk et al. 2018) (Figure 6). The ranges in which these responses are exhibited in AIY adapt on a timescale corresponding to behavioral adaptation to Tc (Hawk et al. 2018). How AFD-driven AIY responses are modulated bidirectionally as a function of Tc experience is not yet clear.
Nonautonomous regulation of AFD thermosensory properties
The complex sensory endings of AFD are comprised of multiple actin-based microvilli and a microtubule-based cilium; these endings are fully embedded in the processes of the amphid sheath cell at the worm nose (Perkins et al. 1986; Doroquez et al. 2014; Nguyen et al. 2014). The amphid sheath cells express the KCC-3 K+/Cl− transporter which localizes around the AFD microvilli and regulates the concentration of extracellular Cl− ions (Singhvi et al. 2016; Yoshida et al. 2016). Chloride ions in turn inhibit the basal activity of the GCY-8 AFD-rGC by directly binding its extracellular domain (Singhvi et al. 2016). Decreased GCY-8 activity and reduction of intracellular cGMP levels modulate actin cytoskeleton remodeling and elongate AFD microvilli (Singhvi et al. 2016). Conversely, in kcc-3 mutants and upon constitutive activation of GCY-8, microvilli are shortened (Singhvi et al. 2016). While T*AFD is unaffected in kcc-3 mutants, AFD temperature response dynamics are markedly altered (Yoshida et al. 2016), and thermotaxis behaviors are disrupted (Singhvi et al. 2016; Yoshida et al. 2016). The amphid sheath cells have previously been shown to exhibit temperature-dependent, but AFD-independent, changes in gene expression (Procko et al. 2011). Thus, in one possible model, sheath cells could shape AFD temperature responses via temperature-dependent regulation of KCC-3 and extracellular Cl− concentration. In addition to glia, AFD thermosensory properties may also be fine-tuned by temperature-regulated systemic signaling from the intestine (Nishida et al. 2011; Land and Rubin 2017).
Temperature-dependent regulation of physiological processes
Environmental thermal stimuli regulate not only behavior but also physiological responses. These responses are mediated by AFD as well as additional thermosensory neurons. AFD has been implicated in coordinating systemic heat shock responses via serotonergic signaling (Prahlad et al. 2008; Tatum et al. 2015), and loss of AFD thermotransduction decreases thermotolerance (Prahlad et al. 2008). AFD also regulates temperature-dependent modulation of longevity. Warmer temperatures decrease the lifespan of ectotherms such as C. elegans (Lee and Kenyon 2009; Zhang et al. 2015). AFD antagonizes this heat-mediated shortening of lifespan via CMK-1-mediated upregulation of FLP-6 neuropeptidergic signaling in AFD at warmer temperatures (Lee and Kenyon 2009; Chen et al. 2016b). FLP-6 in turn increases lifespan via insulin and sterol hormone signaling (Chen et al. 2016b). Cold-dependent increase in lifespan is modulated in an AFD-independent manner and requires temperature sensation via the TRPA1 cation channel in the gut as well as in other as yet unidentified neurons (Xiao et al. 2013).
Avoidance of noxious thermal stimuli
Similar to other animals, C. elegans avoid noxious cold or warm temperatures. Interestingly, avoidance is triggered not only by the absolute temperature but also by the rate of temperature change (Wittenburg and Baumeister 1999; Chatzigeorgiou et al. 2010; Glauser et al. 2011; Ghosh et al. 2012; Mohammadi et al. 2013; Schild and Glauser 2013). Thus, a rapid increase or decrease in temperature within or beyond the physiological temperature range can elicit avoidance (Chatzigeorgiou et al. 2010; Mohammadi et al. 2013). Detailed analyses of locomotion parameters have suggested that worms employ both avoidance and escape behaviors to decrease exposure to noxious heat (Schild and Glauser 2013).
Noxious heat avoidance has been analyzed using spatial or temporal thermal gradients or via the use of infrared lasers (Wittenburg and Baumeister 1999; Glauser et al. 2011; Mohammadi et al. 2013; Kotera et al. 2016). Multiple sensory neurons have been implicated in mediating noxious thermal avoidance (Table 1), possibly due to the use of diverse assays. AFD has been implicated in heat avoidance, and these neurons respond in a deterministic manner to stimuli that elicit avoidance (Liu et al. 2012; Kotera et al. 2016). In addition, the AWC neurons respond to noxious heat stimuli and are required for heat avoidance. Previous work has shown that the two AWC neurons are functionally asymmetric (referred to as AWCON and AWCOFF), and that this asymmetry is determined in a stochastic manner (Troemel et al. 1999; Wes and Bargmann 2001; Chalasani et al. 2007, 2016). Interestingly, these neurons also respond asymmetrically to oscillating thermal stimuli that elicit avoidance, such that only the AWCOFF neuron is required for avoidance (Kotera et al. 2016). Additional neurons implicated in nociceptive thermal avoidance include the PVD (cold) (Chatzigeorgiou et al. 2010), FLP (heat) (Liu et al. 2012; Schild et al. 2014) and PHC (heat) (Liu et al. 2012) sensory neurons (Table 1). Of note, while the majority of these sensory neurons mediate avoidance of nociceptive thermal stimuli presented to the head of C. elegans, PVD and PHC have been suggested to mediate avoidance of thermal stimuli presented to the midbody and tail, respectively (Liu et al. 2012; Mohammadi et al. 2013). Unlike thermotaxis navigation behaviors, avoidance of nociceptive thermal stimuli requires TRP channels. TRPA1 (Chatzigeorgiou et al. 2010), as well as the TRPV channels OCR-2 and OSM-9 (Glauser et al. 2011; Liu et al. 2012), are required for avoidance of noxious temperatures, although it is not yet clear whether these channels are directly gated by temperature.
In parallel with the contribution of temperature experience in modulating thermosensory navigation behaviors, prior thermal history can also alter the range of noxious temperature responses. Acclimation at 28° for >1 hr shifts the threshold for warm avoidance to warmer values as compared to animals grown at 20° (Schild et al. 2014). Analogous to the role of CMK-1 CaMKI in mediating temperature experience-dependent alteration of the operating range of AFD (Yu et al. 2014), CMK-1 also acts in the FLP neurons to regulate the operating range of noxious heat avoidance (Schild et al. 2014). Similar to heat acclimation, acclimation to cooler temperatures promotes survival of C. elegans at low temperatures. Thus, while extended exposure to cold temperatures of 5° or lower kills C. elegans, growing animals at 15° for as little as 2–3 hr promotes survival (Ohta et al. 2014). Cold tolerance is negatively regulated by the bilateral ASJ sensory neuron pair in the head amphid organs (Ohta et al. 2014). ASJ responds tonically to a temperature change, with the amplitude of the response correlated with the acclimation temperature (Ohta et al. 2014). Temperature responses in ASJ are mediated by G proteins and the cGMP second messenger modulate insulin signaling to the intestine and other tissues to regulate cold tolerance (Ohta et al. 2014; Ujisawa et al. 2016).
Concluding remarks
Analyses of thermosensation in C. elegans have identified rGCs as a new family of putative thermosensors. Intriguingly, the GC-G rGC has recently been implicated in sensing cooling in the mouse Grueneberg ganglion (Chao et al. 2015), suggesting that the functions of these molecules in sensing temperature may be conserved. It is currently unclear how the enzymatic activity of these molecules is regulated by temperature changes. How rapid adaptation of AFD temperature responses is mediated is also unknown. Given the conservation with vertebrate phototransduction mechanisms, it is possible that adaptation mechanisms similar to those operating in photoreceptors may also mediate temperature adaptation in AFD. Additional open questions include the molecular mechanisms driving long-term temperature adaptation of both the thermosensory and presynaptic thresholds of AFD, as well as the molecular components of thermotransduction pathways in neurons other than AFD. For additional details regarding AFD-mediated thermotransduction, we refer to the reader to other sources (Aoki and Mori 2015; Goodman and Sengupta 2018). Further studies of thermosensation in C. elegans are likely to reveal insights into both divergent mechanisms of thermotransduction, as well as the conservation between C. elegans thermosensory and vertebrate visual transduction pathways.
Molecules and Neurons Mediating Responses to Magnetic and Electrical Fields, Light, and Humidity
Sensing magnetic cues
Although many animals use the earth’s magnetic field to orient themselves and to travel long distances, molecular mechanisms of magnetoreception are remarkably poorly understood (Wiltschko and Wiltschko 2012; Mouritsen et al. 2016; Mouritsen 2018). Chains of magnetosomes containing magnetite have been shown to act as a compass and mediate magnetotaxis in magnetotactic bacteria (Blakemore 1975; Greene and Komeili 2012; Lefevre and Bazylinski 2013), but the identity and location of the magnetoreceptor in animals have been elusive and are a matter of ongoing debate. In C. elegans, the pair of AFD sensory neurons has been suggested to be magnetosensory (Vidal-Gadea et al. 2015) (Table 1), in addition to their well-described role in thermosensation. The AFD neurons respond weakly to earth-strength magnetic fields, and this response appears to be mediated via cGMP signaling (Vidal-Gadea et al. 2015). The three thermosensory rGCs GCY-8, GCY-18, and GCY-23, as well as the TAX-2 and TAX-4 cGMP-gated channels are required for the response of AFD to magnetic fields (Vidal-Gadea et al. 2015), suggesting that the signal is amplified and transduced via the cGMP second messenger. The identity of the magnetoreceptor in AFD is unknown, but both the cilia and microvilli located at the sensory endings of AFD are required for C. elegans to orient correctly in a magnetic field (Bainbridge et al. 2016). It has been posited that iron particles localized to the microvilli and/or in the surrounding glia may be directly associated with the membrane or transmembrane mechanosensory channels (Clites and Pierce 2017). Physical deformation of the membrane or channels induced by a magnetic field acting on the iron beads could then transduce an electrical signal (Clites and Pierce 2017). Although the ability of C. elegans to sense magnetic fields has been debated (Landler et al. 2018; Vidal-Gadea et al. 2018), it will be interesting to further corroborate this behavior and advance our knowledge of this poorly understood sensory modality in this genetically tractable organism.
Sensing electrical cues
Electroreception has been extensively described in aquatic animals, but is less prevalent in terrestrial animals (Baker et al. 2013; Czech-Damal et al. 2013). C. elegans exhibits robust responses to imposed electrical fields (Sukul and Croll 1978; Gabel et al. 2007; Chrisman et al. 2016). These responses appear to be mediated by a distributed set of sensory neurons, with the specific neurons required dictated by the parameters of the imposed electrical field. Physical ablation of ASJ results in strong behavioral defects in electrosensory steering on a rotating electrical field (Gabel et al. 2007) (Table 1). Consistently, ASJ exhibits robust calcium responses in response to a rotating electrical stimuli (Gabel et al. 2007). Weak responses were also observed in ASH as well as in AWB, AWC, or ASK, although ablation of these neurons led to weak (ASH) or no (AWB, AWC, ASK) defects in electrosensory steering (Gabel et al. 2007). In contrast, electrotaxis on a fixed uniform electrical field appears to be mediated primarily by the functionally asymmetric AWCON member of the AWC sensory neuron pair, with a minor role for ASH (Chrisman et al. 2016). Electrosensory transduction pathways and their ethological function have yet to be uncovered.
Sensing light
Although C. elegans lacks eyes, an obvious eyespot, or ocellus, it can detect and respond to light. For instance, ultraviolet light evokes a robust avoidance behavior that is similar to that observed following activation of mechanoreceptor neurons such as the TRNs, ASH, and PVD. UV light stimulation has also been proposed to modulate pharyngeal function by catalyzing the generation of reactive oxygen species (Bhatla and Horvitz 2015). Additionally, C. elegans may be sensitive to visible light—a recent study reported that C. elegans uses blue light to detect and avoid pathogenic bacteria based on their synthesis of colored compounds such as pyocyanin (Ghosh et al. 2017).
Remarkably, all of these light-dependent behaviors depend upon expression of the lite-1 gene (Edwards et al. 2008; Liu et al. 2010; Ghosh et al. 2017). lite-1 encodes a transmembrane protein homologous to insect gustatory receptors (Edwards et al. 2008; Liu et al. 2010). In the context of UV light mediated avoidance behavior, lite-1 acts in the ASJ and ASH neurons and appears to depend on a G protein-mediated signal transduction pathway to exert its influence of these sensory neurons (Liu et al. 2010) (Table 1). The lite-1 gene is also expressed at very high levels in the TRNs and linked to blue light-evoked increases in cytoplasmic calcium (Nekimken et al. 2017a). The LITE-1 protein is sufficient to confer UV sensitivity upon muscle cells (Edwards et al. 2008; Liu et al. 2010), suggesting that it functions as an autonomous photoreceptor. Consistent with this idea, purified LITE-1 protein efficiently absorbs light at 280 and 320 nm and missense mutations in the lite-1 gene selectively affect absorption at 280 and 320 nm (Gong et al. 2016). Collectively, these findings indicate C. elegans and possibly other nematodes lacking eyes rely on sensory neurons to detect optical stimuli and use a membrane protein more closely related to insect gustatory receptors than to rhodopsin to detect photons.
Humidity
Animals migrate toward the dry side on humidity gradients, even on gradients as shallow as 0.03% relative humidity per millimeter (Russell et al. 2014). This preference is somewhat plastic and is modulated by the relative humidity of their growth conditions as well as their satiety state (Russell et al. 2014). Although hygrosensation may be mediated via integration of mechanical cues due to changes in skin hydration, and temperature changes from evaporative cooling, ablation of either the mechanosensory FLP or thermosensory AFD neurons significantly impairs hygrotaxis in C. elegans (Russell et al. 2014) (Table 1). Consistently, hygrotaxis is impaired in animals mutant for molecules implicated in mechanosensation by FLP including MEC-10, ASIC-1, and MEC-6, as well as the TAX-4 cGMP-gated channel required for thermotransduction in AFD (Russell et al. 2014).
Conclusions
Genetic dissection of sensory-guided behaviors and the responses of sensory neurons in C. elegans have provided detailed insights into the sensory signaling pathways that allow these nematodes to detect physical stimuli. C. elegans is highly sensitive to thermal and mechanical stimuli, while also being able to detect and respond to potential harmful (noxious) levels of these same stimuli. As in other animals, ion channels play key roles in sensory transduction; mechanical cues activate ion channels directly, while thermal fluctuations act indirectly through regulating the synthesis and degradation of soluble molecules (e.g., cGMP). One theme that emerges from this work is that molecular pathways and signaling principles are remarkably conserved, although certain pathways can be deployed in different sensory contexts, and signaling principles may be preserved in the absence of conserved signal transduction pathways. For instance, the thermotransduction pathway used by AFD to detect thermal fluctuations of as little as <1° is remarkably similar to the one that enables vertebrate photoreceptors to detect single photons. Similarly, the response dynamics and frequency selectivity of the TRNs are reminiscent of those that govern the function of Pacinian corpuscles in mammals. The ASH, CEP, and PVD neurons share these properties, but rely on distinct genes to form ion channels activated by mechanical stimuli. Another theme that arises is that many of the sensory neurons in C. elegans are polymodal, responding to multiple types of physical and chemical stimuli (Table 1). The polymodal nature of these sensory neurons is perhaps unsurprising given that the animal accomplishes complex sensorimotor tasks with only 302 neurons. For instance, AFD responds to temperature, magnetic fields, and gases such as carbon dioxide (Bretscher et al. 2011), and is proposed to play a key role in humidity sensing (Russell et al. 2014) (Table 1). The ASH neurons are activated by noxious chemical, osmotic and mechanical stimuli (Hilliard et al. 2005) and contribute to the avoidance of UV light (Liu et al. 2010) (Table 1). The PVD mechanoreceptors also contribute to proprioception (Cohen et al. 2012), and are activated by noxious cold (Chatzigeorgiou et al. 2010) (Table 1).
Future work will address several important challenges. An important unsolved issue is to obtain a biophysical understanding of sensory transduction. We do not yet understand how small temperature changes are sensed by the rGCs expressed in AFD to alter their enzymatic properties, nor is it clear how mechanical stress is transmitted across the skin to activate MeT channels in mechanoreceptor neurons. Another important issue is to decode whether the signaling pathways mediating responses to different sensory stimuli in a single sensory neuron type are compartmentalized according to the nature of the stimulus or whether they are integrated, enabling the neuron to deliver information regarding the coexistence of multiple sensory stimuli. Together with ongoing work on circuit computations in the C. elegans nervous system, a more detailed understanding of sensory transduction principles in this organism will allow us to derive general principles by which an animal navigates and thrives in its complex sensory environment.
Acknowledgments
We thank our past and current laboratory members who contributed to work from our laboratories described here, and the current members of the Goodman and Sengupta laboratories for comments on the manuscript. We thank Inna Nechipurenko for assistance with Figure 5 and Gil Costa for designing Figure 1 and Figure 6. Related work in our laboratories is supported by the National Institutes of Health (R35 GM122463 and P01 GM103770 to P.S.; R35 NS105092 to M.B.G.).
Appendix
Laboratory Methods for Delivering Physical Stimuli to C. elegans Nematodes
The study of sensory transduction depends critically upon techniques for stimulus delivery. Below, we briefly review existing techniques for the delivery of physical stimuli (summarized in Table 2 and Table 3). We note that experimental designs often balance ease-of-use and quantitative precision.
Table 2. Mechanical stimulus delivery methods.
| Tool or devicea | Stimulus amplitude | References | |
|---|---|---|---|
| Classical or qualitative | Eyebrow hair | Variable, 20–800 µN | Sulston et al. (1975), Chalfie and Sulston (1981), Nekimken et al. (2017b) |
| Wire pick | Unknown | Way and Chalfie (1989) | |
| Plate tap | Unknown | Rankin and Broster (1992) | |
| Quantitative approaches | Flexible glass probe (open loop) | Force, nN - µN | O’Hagan et al. (2005) |
| Stiff glass probe (open loop) | Displacement, µm | Suzuki et al. (2003) | |
| Microcantilever (feedback control, closed-loop) | Displacement (µm) or force (nN-µN) | Park et al. (2007), Petzold et al. (2013), Eastwood et al. (2015) | |
| Pneumatic, microfluidic devices | Pressure-induced displacement (µm) | Cho et al. (2017), Nekimken et al. (2017a) |
See also Chalfie et al. (2014).
Table 3. Thermal stimulus delivery methods.
| Tool or devicea | Thermal range, gradient type | References | |
|---|---|---|---|
| Spatial gradient | Frozen acetone | 15° - ambient, Gaussian | Hedgecock and Russell (1975) |
| Water bath plus metal substrate | Arbitrary, linear | Hedgecock and Russell (1975) | |
| Thermoelectric devices plus metal substrate | Arbitrary, linear | Ryu and Samuel (2002), Ramot et al. (2008b), Luo et al. (2014a) | |
| Temporal variation | Substrate control | ||
| Superfusion | ±10° from ambient, depending on thermal control device, slow (seconds) | Ramot et al. (2008a) | |
| Microfluidic device | ±10° from ambient, depending on thermal control device, fast (sub-second) | Zariwala et al. (2003) | |
| Liquid droplet | ±5° from ambient, slow (seconds) | Ryu and Samuel (2002), Clark et al. (2007b) |
See also Goodman et al. (2014).
Mechanical stress:
Mechanical stimuli are thought to generate stress within mechanoreceptor neurons and are generally reported as a function of the force applied, stimulator displacement, or the indentation produced in the tissue. The choice among these measures is primarily a matter of technical convenience and most tools operate in an open-loop control mode in which the output does not feed back onto the input. Classical tools for delivering mechanical stimuli to C. elegans are simple and hand operated (Chalfie et al. 2014)—an eyebrow hair glued to a toothpick or a platinum wire that doubles as a device for transferring worms between growth plates (Table 2). These tools are simple to use, but the amplitude, duration, timing, and location of stimulation are not under experimental control and are difficult to quantify. Moreover, the forces delivered by an eyebrow hair vary by an order of magnitude within and across human experimenters (Nekimken et al. 2017b). Fortunately, experimenters using an eyebrow hair tool consistently deliver forces that exceed the force required to saturate gentle touch responses (1–2 µN) by a factor of 5 (Petzold et al. 2013; Nekimken et al. 2017b; Mazzochette et al. 2018) (Table 2). Thus, the classical touch assay remains a valuable method for examining responses to touch in C. elegans nematodes.
The apparatus used for a plate tap withdrawal assay enables precise experimental control of stimulus timing and limited control over amplitude, but no control over stimulus location or duration (Table 2). An apparatus for plate tap accelerates a plunger into the side of a Petri dish to generate substrate displacements on the order of 10–80 µm with displacement amplitude depending on the strength of the plate tap (Timbers et al. 2013). Substrate displacement sufficient to evoke avoidance responses can also be achieved by using a piezoelectric sheet speaker to generate displacements up to 5 µm at frequencies between 0.5 and 3 kHz (Sugi et al. 2016).
The most common tool for controlling the amplitude, duration, timing, and location of mechanical stimulation is a piezoelectric motor pushing a glass probe into the animal’s body (Table 2). This general set-up is also used to study mechanosensitive ion channels in cells and typically operates without feedback control (Delmas et al. 2011). Although such open-loop systems offer good control over stimulus timing, duration, and position, their users must grapple with ambiguity regarding stimulus amplitude because it is not obvious how to determine the position of zero displacement or the indentation produced in response to a measured force or vice versa. Force-sensing devices provide this information and can be operated under feedback control to deliver a user-specified force or indentation. By contrast with open-loop systems, these closed-loop systems automatically regulate the applied force or indentation and two have been reported for use with C. elegans nematodes: one is based on custom-fabricated microforce-sensing probes (Park et al. 2007; Petzold et al. 2011, 2013; Eastwood et al. 2015) (Table 2) or other commercially available microforce sensing probes (Shaw et al. 2016; Elmi et al. 2017). Intracellular calcium responses generated in response to mechanical stimuli delivered by open-loop mechanical stimulators pushed into the ventral or dorsal side (TRNs, OLQ, CEP, FLP, PVD), down on the lateral aspect (ADL, ADE, PHB, ALA), or the tip of the nose (ASH) of animals immobilized by superglue are illustrated in Figure 2, A, B, and E, respectively. Closed-loop mechanical stimulators have yet to be applied in combination with calcium imaging.
Microfluidics-based devices for delivering mechanical stimuli to freely moving or immobilized C. elegans nematodes are emerging as an additional approach (reviewed by Kim et al. 2018, and summarized in Table 2). These include so-called artificial dirt chips that can test if C. elegans can distinguish between different textures (Han et al. 2017), as well as devices that deliver mechanical stimuli to immobilized animals (Cho et al. 2017; Nekimken et al. 2017a), or to animals allowed to move within defined channels (McClanahan et al. 2017). Because both the worms and the microfluidics chips are transparent, it is straightforward to combine these devices with calcium imaging, as illustrated in Figure 2, B and D.
Temperature:
Linear, spatial thermal gradients can be generated using several approaches, including thermoelectric devices or a temperature-controlled water bath coupled to a thermally conductive substrate such as an aluminum plate (Hedgecock and Russell 1975; Ryu and Samuel 2002; Yamada and Ohshima 2003; Ramot et al. 2008b) (Table 3). These gradients allow effective and efficient establishment of temperature gradients of the desired steepness and range. A glass substrate coated with indium tin oxide can be used to conduct thermal stimuli, and such a tool has been used in studies evaluating the response of thermosensory neurons to thermal gradients in restrained (Clark et al. 2006) and freely moving animals (Clark et al. 2007a). Strategies for controlled thermal variations in temperature include superfusion with temperature controlled saline (Ramot et al. 2008a; Wang et al. 2013), heating with an infrared laser (Wittenburg and Baumeister 1999; Clark et al. 2007b; Mohammadi et al. 2013), and rapid heating and cooling of droplets containing a single worm (Ryu and Samuel 2002) (Table 3). Microfluidic chips (Zariwala et al. 2003; McCormick et al. 2011) have also been developed for delivering acute thermal stimuli to restrained animals (Table 3). Additional details of these devices have been described recently (Goodman et al. 2014).
Others: magnetic fields, electric fields, light, humidity:
The study of responses to other physical stimuli including light, magnetic, and electrical fields, and relative humidity are in their infancy. Consequently, the tools for applying these stimuli have not yet been extensively refined. Current published methods for assessing responses to magnetic fields, electric fields, light, and humidity are described in brief below.
Magnetic and electrical fields:
Sensitivity to magnetic fields has been tested by exposing crawling animals to fields generated by a current-carrying coil surrounding a behavioral testing arena (Vidal-Gadea et al. 2015). Wild-type animals have been reported to be able to sense and respond to magnetic fields (Vidal-Gadea et al. 2015), although the inference that C. elegans can sense earth’s magnetic field has been disputed (Landler et al. 2018; Vidal-Gadea et al. 2018). To assess the response of C. elegans nematodes to electrical fields, animals are allowed to crawl on agar surfaces upon which a linear or rotating electrical field has been imposed (Sukul and Croll 1978; Gabel et al. 2007; Chrisman et al. 2016). Animals travel along remarkably straight paths toward the anode of a constant linear electrical field (Sukul and Croll 1978; Chrisman et al. 2016), and crawl in circles in response to a slowly rotating electrical field (Gabel et al. 2007). This behavior has been exploited in microfluidics devices to sort animals (Han et al. 2012; Wang et al. 2015).
Light:
To assess behavioral responses to light, pulses are delivered by coupling a shutter and a standard mercury bulb illuminator via an objective on a dissection microscope. To deliver light of specific wavelengths, the light source is filtered via standard microscope filters (Edwards et al. 2008; Liu et al. 2010; Bhatla and Horvitz 2015). Color-specific light-emitting diodes (LEDs) can also be used to deliver optical stimuli.
Humidity:
To establish a humidity gradient, distilled water or desiccant (such as DRIERITE(TM)) is placed into two troughs on either side of an elevated central stage in a plastic box. Worms are placed in the middle of a thin agarose pad on a glass plate placed on the central area, and the box sealed to establish the gradient (Russell and Pierce-Shimomura 2014). The gradient can be visualized using the colorimetric cobalt (II) chloride indicator.
Footnotes
Communicating editor: Y. Iino
Literature Cited
- Akella J. S., Wloga D., Kim J., Starostina N. G., Lyons-Abbott S., et al. , 2010. MEC-17 is an alpha-tubulin acetyltransferase. Nature 467: 218–222. 10.1038/nature09324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albeg A., Smith C. J., Chatzigeorgiou M., Feitelson D. G., Hall D. H., et al. , 2011. C. elegans multi-dendritic sensory neurons: morphology and function. Mol. Cell. Neurosci. 46: 308–317. 10.1016/j.mcn.2010.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht D. R., Bargmann C. I., 2011. High-content behavioral analysis of Caenorhabditis elegans in precise spatiotemporal chemical environments. Nat. Methods 8: 599–605. 10.1038/nmeth.1630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J. L., Albergotti L., Ellebracht B., Huey R. B., Phillips P. C., 2011. Does thermoregulatory behavior maximize reproductive fitness of natural isolates of Caenorhabditis elegans? BMC Evol. Biol. 11: 157 10.1186/1471-2148-11-157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angilletta M. J., 2009. Thermal Adaptation: A Theoretical and Empirical Synthesis. Oxford University Press, Oxford: 10.1093/acprof:oso/9780198570875.001.1 [DOI] [Google Scholar]
- Anishkin A., Kung C., 2013. Stiffened lipid platforms at molecular force foci. Proc. Natl. Acad. Sci. USA 110: 4886–4892. 10.1073/pnas.1302018110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anishkin A., Loukin S. H., Teng J., Kung C., 2014. Feeling the hidden mechanical forces in lipid bilayer is an original sense. Proc. Natl. Acad. Sci. USA 111: 7898–7905. 10.1073/pnas.1313364111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki I., Mori I., 2015. Molecular biology of thermosensory transduction in C. elegans. Curr. Opin. Neurobiol. 34: 117–124. 10.1016/j.conb.2015.03.011 [DOI] [PubMed] [Google Scholar]
- Aoki I., Tateyama M., Shimomura T., Ihara K., Kubo Y., et al. , 2018. SLO potassium channels antagonize premature decision making in C. elegans. Commun Biol 1: 123 10.1038/s42003-018-0124-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnadóttir J., Chalfie M., 2010. Eukaryotic mechanosensitive channels. Annu. Rev. Biophys. 39: 111–137. 10.1146/annurev.biophys.37.032807.125836 [DOI] [PubMed] [Google Scholar]
- Arnadottir J., O’Hagan R., Chen Y., Goodman M. B., Chalfie M., 2011. The DEG/ENaC protein MEC-10 regulates the transduction channel complex in Caenorhabditis elegans touch receptor neurons. J. Neurosci. 31: 12695–12704. 10.1523/JNEUROSCI.4580-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainbridge C., Rodriguez A., Schuler A., Cisneros M., Vidal-Gadea A. G., 2016. Magnetic orientation in C. elegans relies on the integrity of the villi of the AFD magnetosensory neurons. J. Physiol. Paris 110: 76–82. 10.1016/j.jphysparis.2016.12.002 [DOI] [PubMed] [Google Scholar]
- Baker C. V., Modrell M. S., Gillis J. A., 2013. The evolution and development of vertebrate lateral line electroreceptors. J. Exp. Biol. 216: 2515–2522. 10.1242/jeb.082362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargmann C. I., Horvitz H. R., 1991. Chemosensory neurons with overlapping functions direct chemotaxis to multiple chemicals in C. elegans. Neuron 7: 729–742. 10.1016/0896-6273(91)90276-6 [DOI] [PubMed] [Google Scholar]
- Bargmann C. I., Hartwieg E., Horvitz H. R., 1993. Odorant-selective genes and neurons mediate olfaction in C. elegans. Cell 74: 515–527. 10.1016/0092-8674(93)80053-H [DOI] [PubMed] [Google Scholar]
- Barr M. M., DeModena J., Braun D., Nguyen C. Q., Hall D. H., et al. , 2001. The Caenorhabditis elegans autosomal dominant polycystic kidney disease gene homologs lov-1 and pkd-2 act in the same pathway. Curr. Biol. 11: 1341–1346. 10.1016/S0960-9822(01)00423-7 [DOI] [PubMed] [Google Scholar]
- Barr M. M., Garcia L. R., Portman D. S., 2018. Sexual dimorphism and sex differences in Caenorhabditis elegans neuronal development and behavior. Genetics 208: 909–935. 10.1534/genetics.117.300294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beverly M., Anbil S., Sengupta P., 2011. Degeneracy and neuromodulation among thermosensory neurons contribute to robust thermosensory behaviors in Caenorhabditis elegans. J. Neurosci. 31: 11718–11727. 10.1523/JNEUROSCI.1098-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatla N., Horvitz H. R., 2015. Light and hydrogen peroxide inhibit C. elegans feeding through gustatory receptor orthologs and pharyngeal neurons. Neuron 85: 804–818. 10.1016/j.neuron.2014.12.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biron D., Shibuya M., Gabel C., Wasserman S. M., Clark D. A., et al. , 2006. A diacylglycerol kinase modulates long-term thermotactic behavioral plasticity in C. elegans. Nat. Neurosci. 9: 1499–1505. 10.1038/nn1796 [DOI] [PubMed] [Google Scholar]
- Biron D., Wasserman S., Thomas J. H., Samuel A. D., Sengupta P., 2008. An olfactory neuron responds stochastically to temperature and modulates Caenorhabditis elegans thermotactic behavior. Proc. Natl. Acad. Sci. USA 105: 11002–11007. 10.1073/pnas.0805004105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore R., 1975. Magnetotactic bacteria. Science 190: 377–379. 10.1126/science.170679 [DOI] [PubMed] [Google Scholar]
- Boscardin E., Alijevic O., Hummler E., Frateschi S., Kellenberger S., 2016. The function and regulation of acid-sensing ion channels (ASICs) and the epithelial Na(+) channel (ENaC): IUPHAR Review 19. Br. J. Pharmacol. 173: 2671–2701. 10.1111/bph.13533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bounoutas A., O’Hagan R., Chalfie M., 2009. The multipurpose 15-protofilament microtubules in C. elegans have specific roles in mechanosensation. Curr. Biol. 19: 1362–1367. 10.1016/j.cub.2009.06.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozorgmehr T., Ardiel E. L., McEwan A. H., Rankin C. H., 2013. Mechanisms of plasticity in a Caenorhabditis elegans mechanosensory circuit. Front. Physiol. 4: 88 10.3389/fphys.2013.00088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher A. J., Kodama-Namba E., Busch K. E., Murphy R. J., Soltesz Z., et al. , 2011. Temperature, oxygen, and salt-sensing neurons in C. elegans are carbon dioxide sensors that control avoidance behavior. Neuron 69: 1099–1113. 10.1016/j.neuron.2011.02.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock T. H., Diecke F. P., 1956. Properties of an infra-red receptor. J. Physiol. 134: 47–87. 10.1113/jphysiol.1956.sp005624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalasani S. H., Chronis N., Tsunozaki M., Gray J. M., Ramot D., et al. , 2007. Dissecting a circuit for olfactory behaviour in Caenorhabditis elegans. Nature 450: 63–70 [corrigenda: Nature 533: 130 (2016)] 10.1038/nature06292 [DOI] [PubMed] [Google Scholar]
- Chalasani S. H., Chronis N., Tsunozaki M., Gray J. M., Ramot D., et al. , 2016. Corrigendum: dissecting a circuit for olfactory behaviour in Caenorhabditis elegans. Nature 533: 130 10.1038/nature16515 [DOI] [PubMed] [Google Scholar]
- Chalfie M., Au M., 1989. Genetic control of differentiation of the Caenorhabditis elegans touch receptor neurons. Science 243: 1027–1033. 10.1126/science.2646709 [DOI] [PubMed] [Google Scholar]
- Chalfie M., Sulston J., 1981. Developmental genetics of the mechanosensory neurons of Caenorhabditis elegans. Dev. Biol. 82: 358–370. 10.1016/0012-1606(81)90459-0 [DOI] [PubMed] [Google Scholar]
- Chalfie M., Thomson J. N., 1979. Organization of neuronal microtubules in the nematode Caenorhabditis elegans. J. Cell Biol. 82: 278–289. 10.1083/jcb.82.1.278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalfie M., Thomson J. N., 1982. Structural and functional diversity in the neuronal microtubules of Caenorhabditis elegans. J. Cell Biol. 93: 15–23. 10.1083/jcb.93.1.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalfie M., Sulston J. E., White J. G., Southgate E., Thomson J. N., et al. , 1985. The neural circuit for touch sensitivity in Caenorhabditis elegans. J. Neurosci. 5: 956–964. 10.1523/JNEUROSCI.05-04-00956.1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalfie M., Dean E., Reilly E., Buck K., Thomson J. N., 1986. Mutations affecting microtubule structure in Caenorhabditis elegans. J. Cell Sci. Suppl. 5: 257–271. 10.1242/jcs.1986.Supplement_5.17 [DOI] [PubMed] [Google Scholar]
- Chalfie M., Hart A. C., Rankin C. H., Goodman M. B., 2014. Assaying mechanosensation. (July 31, 2014), WormBook, ed. The C. elegans Research Community, WormBook, /10.1895/wormbook.1.172.1. http://www.wormbook.org 10.1895/wormbook.1.172.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao Y. C., Chen C. C., Lin Y. C., Breer H., Fleischer J., et al. , 2015. Receptor guanylyl cyclase-G is a novel thermosensory protein activated by cool temperatures. EMBO J. 34: 294–306. 10.15252/embj.201489652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatzigeorgiou M., Schafer W. R., 2011. Lateral facilitation between primary mechanosensory neurons controls nose touch perception in C. elegans. Neuron 70: 299–309. 10.1016/j.neuron.2011.02.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatzigeorgiou M., Yoo S., Watson J. D., Lee W. H., Spencer W. C., et al. , 2010. Specific roles for DEG/ENaC and TRP channels in touch and thermosensation in C. elegans nociceptors. Nat. Neurosci. 13: 861–868. 10.1038/nn.2581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Chalfie M., 2014. Modulation of C. elegans touch sensitivity is integrated at multiple levels. J. Neurosci. 34: 6522–6536. 10.1523/JNEUROSCI.0022-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Chalfie M., 2015. Regulation of mechanosensation in C. elegans through ubiquitination of the MEC-4 mechanotransduction channel. J. Neurosci. 35: 2200–2212. 10.1523/JNEUROSCI.4082-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Bharill S., O’Hagan R., Isacoff E. Y., Chalfie M., 2016a. MEC-10 and MEC-19 reduce the neurotoxicity of the MEC-4(d) DEG/ENaC channel in Caenorhabditis elegans. G3 (Bethesda) 6: 1121–1130. 10.1534/g3.115.023507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. C., Chen H. J., Tseng W. C., Hsu J. M., Huang T. T., et al. , 2016b. A C. elegans thermosensory circuit regulates longevity through crh-1/CREB-dependent flp-6 neuropeptide signaling. Dev. Cell 39: 209–223. 10.1016/j.devcel.2016.08.021 [DOI] [PubMed] [Google Scholar]
- Chi C. A., Clark D. A., Lee S., Biron D., Luo L., et al. , 2007. Temperature and food mediate long-term thermotactic behavioral plasticity by association-independent mechanisms in C. elegans. J. Exp. Biol. 210: 4043–4052. 10.1242/jeb.006551 [DOI] [PubMed] [Google Scholar]
- Cho Y., Porto D. A., Hwang H., Grundy L. J., Schafer W. R., et al. , 2017. Automated and controlled mechanical stimulation and functional imaging in vivo in C. elegans. Lab Chip 17: 2609–2618 (erratum: Lab Chip 17: 3935) 10.1039/C7LC00465F [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrisman S. D., Waite C. B., Scoville A. G., Carnell L., 2016. C. elegans demonstrates distinct behaviors within a fixed and uniform electric field. PLoS One 11: e0151320 10.1371/journal.pone.0151320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark D. A., Biron D., Sengupta P., Samuel A. D., 2006. The AFD sensory neurons encode multiple functions underlying thermotactic behavior in Caenorhabditis elegans. J. Neurosci. 26: 7444–7451. 10.1523/JNEUROSCI.1137-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark D. A., Gabel C. V., Gabel H., Samuel A. D., 2007a. Temporal activity patterns in thermosensory neurons of freely moving Caenorhabditis elegans encode spatial thermal gradients. J. Neurosci. 27: 6083–6090. 10.1523/JNEUROSCI.1032-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark D. A., Gabel C. V., Lee T. M., Samuel A. D., 2007b. Short-term adaptation and temporal processing in the cryophilic response of Caenorhabditis elegans. J. Neurophysiol. 97: 1903–1910. 10.1152/jn.00892.2006 [DOI] [PubMed] [Google Scholar]
- Clites B. L., Pierce J. T., 2017. Identifying cellular and molecular mechanisms for magnetosensation. Annu. Rev. Neurosci. 40: 231–250. 10.1146/annurev-neuro-072116-031312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coburn C. M., Mori I., Ohshima Y., Bargmann C. I., 1998. A cyclic nucleotide-gated channel inhibits sensory axon outgrowth in larval and adult Caenorhabditis elegans: a distinct pathway for maintenance of sensory axon structure. Development 125: 249–258. [DOI] [PubMed] [Google Scholar]
- Cohen E., Yemini E., Schafer W., Feitelson D. G., Treinin M., 2012. Locomotion analysis identifies roles of mechanosensory neurons in governing locomotion dynamics of C. elegans. J. Exp. Biol. 215: 3639–3648. 10.1242/jeb.075416 [DOI] [PubMed] [Google Scholar]
- Colbert H. A., Smith T. L., Bargmann C. I., 1997. OSM-9, a novel protein with structural similarity to channels, is required for olfaction, mechanosensation, and olfactory adaptation in Caenorhabditis elegans. J. Neurosci. 17: 8259–8269. 10.1523/JNEUROSCI.17-21-08259.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cueva J. G., Hsin J., Huang K. C., Goodman M. B., 2012. Posttranslational acetylation of alpha-tubulin constrains protofilament number in native microtubules. Curr. Biol. 22: 1066–1074. 10.1016/j.cub.2012.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czech-Damal N. U., Dehnhardt G., Manger P., Hanke W., 2013. Passive electroreception in aquatic mammals. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 199: 555–563. 10.1007/s00359-012-0780-8 [DOI] [PubMed] [Google Scholar]
- Delmas P., Hao J., Rodat-Despoix L., 2011. Molecular mechanisms of mechanotransduction in mammalian sensory neurons. Nat. Rev. Neurosci. 12: 139–153. 10.1038/nrn2993 [DOI] [PubMed] [Google Scholar]
- Ding G., Zou W., Zhang H., Xue Y., Cai Y., et al. , 2015. In vivo tactile stimulation-evoked responses in Caenorhabditis elegans amphid sheath glia. PLoS One 10: e0117114 10.1371/journal.pone.0117114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doroquez D. B., Berciu C., Anderson J. R., Sengupta P., Nicastro D., 2014. A high-resolution morphological and ultrastructural map of anterior sensory cilia and glia in Caenorhabditis elegans. eLife 3: e01948 10.7554/eLife.01948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du H., Gu G., William C. M., Chalfie M., 1996. Extracellular proteins needed for C. elegans mechanosensation. Neuron 16: 183–194. 10.1016/S0896-6273(00)80035-5 [DOI] [PubMed] [Google Scholar]
- Dusenbery D. B., Sheridan R. E., Russell R. L., 1975. Chemotaxis-defective mutants of the nematode Caenorhabditis elegans. Genetics 80: 297–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastwood A. L., Goodman M. B., 2012. Insight into DEG/ENaC channel gating from genetics and structure. Physiology (Bethesda) 27: 282–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastwood A. L., Sanzeni A., Petzold B. C., Park S. J., Vergassola M., et al. , 2015. Tissue mechanics govern the rapidly adapting and symmetrical response to touch. Proc. Natl. Acad. Sci. USA 112: E6955–E6963 [corrigenda: Proc. Natl. Acad. Sci. USA 113: E2471 (2016)] 10.1073/pnas.1514138112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S. L., Charlie N. K., Milfort M. C., Brown B. S., Gravlin C. N., et al. , 2008. A novel molecular solution for ultraviolet light detection in Caenorhabditis elegans. PLoS Biol. 6: e198 10.1371/journal.pbio.0060198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmi M., Pawar V. M., Shaw M., Wong D., Zhan H., et al. , 2017. Determining the biomechanics of touch sensation in C. elegans. Sci. Rep. 7: 12329 10.1038/s41598-017-12190-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emtage L., Gu G., Hartwieg E., Chalfie M., 2004. Extracellular proteins organize the mechanosensory channel complex in C. elegans touch receptor neurons. Neuron 44: 795–807. 10.1016/j.neuron.2004.11.010 [DOI] [PubMed] [Google Scholar]
- Fukushige T., Yasuda H., Siddiqui S. S., 1995. Selective expression of the tba-1 alpha tubulin gene in a set of mechanosensory and motor neurons during the development of Caenorhabditis elegans. Biochim. Biophys. Acta 1261: 401–416. 10.1016/0167-4781(95)00028-F [DOI] [PubMed] [Google Scholar]
- Fukushige T., Siddiqui Z. K., Chou M., Culotti J. G., Gogonea C. B., et al. , 1999. MEC-12, an alpha-tubulin required for touch sensitivity in C. elegans. J. Cell Sci. 112: 395–403. [DOI] [PubMed] [Google Scholar]
- Gabel C. V., Gabel H., Pavlichin D., Kao A., Clark D. A., et al. , 2007. Neural circuits mediate electrosensory behavior in Caenorhabditis elegans. J. Neurosci. 27: 7586–7596. 10.1523/JNEUROSCI.0775-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrity P. A., Goodman M. B., Samuel A. D., Sengupta P., 2010. Running hot and cold: behavioral strategies, neural circuits, and the molecular machinery for thermotaxis in C. elegans and Drosophila. Genes Dev. 24: 2365–2382. 10.1101/gad.1953710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geffeney S. L., Cueva J. G., Glauser D. A., Doll J. C., Lee T. H., et al. , 2011. DEG/ENaC but not TRP channels are the major mechanoelectrical transduction channels in a C. elegans nociceptor. Neuron 71: 845–857. 10.1016/j.neuron.2011.06.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh, D. D., X. Jin, and M. N. Nitabach, 2017 C. elegans discriminate colors without eyes or opsins. 10.1101/092072 [DOI]
- Ghosh R., Mohammadi A., Kruglyak L., Ryu W. S., 2012. Multiparameter behavioral profiling reveals distinct thermal response regimes in Caenorhabditis elegans. BMC Biol. 10: 85 10.1186/1741-7007-10-85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glauser D. A., Chen W. C., Agin R., Macinnis B. L., Hellman A. B., et al. , 2011. Heat avoidance is regulated by transient receptor potential (TRP) channels and a neuropeptide signaling pathway in Caenorhabditis elegans. Genetics 188: 91–103. 10.1534/genetics.111.127100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong, J., Y. Yuan, A. Ward, L. Kang, B. Zhang et al., 2016 The C. elegans taste receptor homolog LITE-1 is a photoreceptor. Cell 167: 1252–1263.e10 [corrigenda: Cell 168: 325 (2017)] DOI: 10.1016/j.cell.2016.10.053 [DOI] [PMC free article] [PubMed]
- Goodman, M. B., 2006 Mechanosensation (January 06, 2006), WormBook, ed. The C. elegans Research Community, WormBook, /10.1895/wormbook.1.62.1. http://www.wormbook.org
- Goodman M. B., Sengupta P., 2018. The extraordinary AFD thermosensor of C. elegans. Pflugers Arch. 470: 839–849. 10.1007/s00424-017-2089-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman M. B., Hall D. H., Avery L., Lockery S. R., 1998. Active currents regulate sensitivity and dynamic range in C. elegans neurons. Neuron 20: 763–772. 10.1016/S0896-6273(00)81014-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman M. B., Ernstrom G. G., Chelur D. S., O’Hagan R., Yao C. A., et al. , 2002. MEC-2 regulates C. elegans DEG/ENaC channels needed for mechanosensation. Nature 415: 1039–1042. 10.1038/4151039a [DOI] [PubMed] [Google Scholar]
- Goodman M. B., Klein M., Lasse S., Luo L., Mori I., et al. , 2014. Thermotaxis navigation behavior (February 20, 2014), WormBook, ed. The C. elegans Research Community, WormBook, /10.1895/wormbook.1.168.1. http://www.wormbook.org 10.1895/wormbook.1.168.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordus A., Pokala N., Levy S., Flavell S. W., Bargmann C. I., 2015. Feedback from network states generates variability in a probabilistic olfactory circuit. Cell 161: 215–227. 10.1016/j.cell.2015.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray J. M., Hill J. J., Bargmann C. I., 2005. A circuit for navigation in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 102: 3184–3191. 10.1073/pnas.0409009101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene S. E., Komeili A., 2012. Biogenesis and subcellular organization of the magnetosome organelles of magnetotactic bacteria. Curr. Opin. Cell Biol. 24: 490–495. 10.1016/j.ceb.2012.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y. R., MacKinnon R., 2017. Structure-based membrane dome mechanism for Piezo mechanosensitivity. eLife 6: e33660 10.7554/eLife.33660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z. V., Hart A. C., Ramanathan S., 2009. Optical interrogation of neural circuits in Caenorhabditis elegans. Nat. Methods 6: 891–896. 10.1038/nmeth.1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halevi S., McKay J., Palfreyman M., Yassin L., Eshel M., et al. , 2002. The C. elegans ric-3 gene is required for maturation of nicotinic acetylcholine receptors. EMBO J. 21: 1012–1020. 10.1093/emboj/21.5.1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall D. H., Lints R., Altun Z., 2006. Nematode neurons: anatomy and anatomical methods in Caenorhabditis elegans. Int. Rev. Neurobiol. 69: 1–35. [DOI] [PubMed] [Google Scholar]
- Han B., Kim D., Ko U. H., Shin J. H., 2012. A sorting strategy for C. elegans based on size-dependent motility and electrotaxis in a micro-structured channel. Lab Chip 12: 4128–4134. 10.1039/c2lc40209b [DOI] [PubMed] [Google Scholar]
- Han B., Dong Y., Zhang L., Liu Y., Rabinowitch I., et al. , 2017. Dopamine signaling tunes spatial pattern selectivity in C. elegans. eLife 6: e22896 10.7554/eLife.22896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart A. C., Sims S., Kaplan J. M., 1995. Synaptic code for sensory modalities revealed by C. elegans GLR-1 glutamate receptor. Nature 378: 82–85. 10.1038/378082a0 [DOI] [PubMed] [Google Scholar]
- Haselwandter C. A., MacKinnon R., 2018. Piezo’s membrane footprint and its contribution to mechanosensitivity. eLife 7: e41968 10.7554/eLife.41968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawk J. D., Calvo A. C., Liu P., Almoril-Porras A., Aljobeh A., et al. , 2018. Integration of plasticity mechanisms within a single sensory neuron of C. elegans actuates a memory. Neuron 97: 356–367.e4. 10.1016/j.neuron.2017.12.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedgecock E. M., Russell R. L., 1975. Normal and mutant thermotaxis in the nematode Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 72: 4061–4065. 10.1073/pnas.72.10.4061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilliard M. A., Apicella A. J., Kerr R., Suzuki H., Bazzicalupo P., et al. , 2005. In vivo imaging of C. elegans ASH neurons: cellular response and adaptation to chemical repellents. EMBO J. 24: 63–72. 10.1038/sj.emboj.7600493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffstaetter L. J., Bagriantsev S. N., Gracheva E. O., 2018. TRPs et al.: a molecular toolkit for thermosensory adaptations. Pflugers Arch. 470: 745–759. 10.1007/s00424-018-2120-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inada H., Ito H., Satterlee J., Sengupta P., Matsumoto K., et al. , 2006. Identification of guanylyl cyclases that function in thermosensory neurons of Caenorhabditis elegans. Genetics 172: 2239–2252. 10.1534/genetics.105.050013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurado P., Kodama E., Tanizawa Y., Mori I., 2010. Distinct thermal migration behaviors in response to different thermal gradients in Caenorhabditis elegans. Genes Brain Behav. 9: 120–127. 10.1111/j.1601-183X.2009.00549.x [DOI] [PubMed] [Google Scholar]
- Kang L., Gao J., Schafer W. R., Xie Z., Xu X. Z., 2010. C. elegans TRP family protein TRP-4 is a pore-forming subunit of a native mechanotransduction channel. Neuron 67: 381–391. 10.1016/j.neuron.2010.06.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan J. M., Horvitz H. R., 1993. A dual mechanosensory and chemosensory neuron in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 90: 2227–2231. 10.1073/pnas.90.6.2227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katta S., Krieg M., Goodman M. B., 2015. Feeling force: physical and physiological principles enabling sensory mechanotransduction. Annu. Rev. Cell Dev. Biol. 31: 347–371. 10.1146/annurev-cellbio-100913-013426 [DOI] [PubMed] [Google Scholar]
- Kellenberger S., Schild L., 2015. International Union of basic and clinical pharmacology. XCI. structure, function, and pharmacology of acid-sensing ion channels and the epithelial Na+ channel. Pharmacol. Rev. 67: 1–35. 10.1124/pr.114.009225 [DOI] [PubMed] [Google Scholar]
- Kerr R., Lev-Ram V., Baird G., Vincent P., Tsien R. Y., et al. , 2000. Optical imaging of calcium transients in neurons and pharyngeal muscle of C. elegans. Neuron 26: 583–594. 10.1016/S0896-6273(00)81196-4 [DOI] [PubMed] [Google Scholar]
- Kim A. A., Nekimken A. L., Fechner S., O’Brien L. E., Pruitt B. L., 2018. Microfluidics for mechanobiology of model organisms. Methods Cell Biol. 146: 217–259. 10.1016/bs.mcb.2018.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura K. D., Miyawaki A., Matsumoto K., Mori I., 2004. The C. elegans thermosensory neuron AFD responds to warming. Curr. Biol. 14: 1291–1295. 10.1016/j.cub.2004.06.060 [DOI] [PubMed] [Google Scholar]
- Kindt K. S., Tam T., Whiteman S., Schafer W. R., 2002. Serotonin promotes G(o)-dependent neuronal migration in Caenorhabditis elegans. Curr. Biol. 12: 1738–1747. 10.1016/S0960-9822(02)01199-5 [DOI] [PubMed] [Google Scholar]
- Kindt K. S., Quast K. B., Giles A. C., De S., Hendrey D., et al. , 2007a. Dopamine mediates context-dependent modulation of sensory plasticity in C. elegans. Neuron 55: 662–676. 10.1016/j.neuron.2007.07.023 [DOI] [PubMed] [Google Scholar]
- Kindt K. S., Viswanath V., Macpherson L., Quast K., Hu H., et al. , 2007b. Caenorhabditis elegans TRPA-1 functions in mechanosensation. Nat. Neurosci. 10: 568–577. 10.1038/nn1886 [DOI] [PubMed] [Google Scholar]
- Kobayashi K., Nakano S., Amano M., Tsuboi D., Nishioka T., et al. , 2016. Single-cell memory regulates a neural circuit for sensory behavior. Cell Rep. 14: 11–21. 10.1016/j.celrep.2015.11.064 [DOI] [PubMed] [Google Scholar]
- Kotera I., Tran N. A., Fu D., Kim J. H., Byrne Rodgers J., et al. , 2016. Pan-neuronal screening in Caenorhabditis elegans reveals asymmetric dynamics of AWC neurons is critical for thermal avoidance behavior. eLife 5: e19021 10.7554/eLife.19021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg M., Stuhmer J., Cueva J. G., Fetter R., Spilker K., et al. , 2017. Genetic defects in β-spectrin and tau sensitize C. elegans axons to movement-induced damage via torque-tension coupling. eLife 6: e20172 10.7554/eLife.20172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhara A., Okumura M., Kimata T., Tanizawa Y., Takano R., et al. , 2008. Temperature sensing by an olfactory neuron in a circuit controlling behavior of C. elegans. Science 320: 803–807. 10.1126/science.1148922 [DOI] [PubMed] [Google Scholar]
- Kuhara A., Ohnishi N., Shimowada T., Mori I., 2011. Neural coding in a single sensory neuron controlling opposite seeking behaviours in Caenorhabditis elegans. Nat. Commun. 2: 355 10.1038/ncomms1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn M., 2016. Molecular physiology of membrane guanylyl cyclase receptors. Physiol. Rev. 96: 751–804. 10.1152/physrev.00022.2015 [DOI] [PubMed] [Google Scholar]
- Land M., Rubin C. S., 2017. A calcium- and diacylglycerol-stimulated protein kinase C (PKC), Caenorhabditis elegans PKC-2, links thermal signals to learned behavior by acting in sensory neurons and intestinal cells. Mol. Cell. Biol. 37: e00192-17 10.1128/MCB.00192-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landler L., Nimpf S., Hochstoeger T., Nordmann G. C., Papadaki-Anastasopoulou A., et al. , 2018. Comment on “Magnetosensitive neurons mediate geomagnetic orientation in Caenorhabditis elegans”. eLife 7: e30187 10.7554/eLife.30187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsch J., Ventimiglia D., Bargmann C. I., Albrecht D. R., 2013. High-throughput imaging of neuronal activity in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 110: E4266–E4273. 10.1073/pnas.1318325110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. J., Kenyon C., 2009. Regulation of the longevity response to temperature by thermosensory neurons in Caenorhabditis elegans. Curr. Biol. 19: 715–722. 10.1016/j.cub.2009.03.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefevre C. T., Bazylinski D. A., 2013. Ecology, diversity, and evolution of magnetotactic bacteria. Microbiol. Mol. Biol. Rev. 77: 497–526. 10.1128/MMBR.00021-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leifer A. M., Fang-Yen C., Gershow M., Alkema M. J., Samuel A. D., 2011. Optogenetic manipulation of neural activity in freely moving Caenorhabditis elegans. Nat. Methods 8: 147–152. 10.1038/nmeth.1554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K., Gong Z., 2017. Feeling hot and cold: thermal sensation in Drosophila. Neurosci. Bull. 33: 317–322. 10.1007/s12264-016-0087-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Feng Z., Sternberg P. W., Xu X. Z., 2006. A C. elegans stretch receptor neuron revealed by a mechanosensitive TRP channel homologue. Nature 440: 684–687. 10.1038/nature04538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Kang L., Piggott B. J., Feng Z., Xu X. Z., 2011. The neural circuits and sensory channels mediating harsh touch sensation in Caenorhabditis elegans. Nat. Commun. 2: 315 10.1038/ncomms1308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K. S., Sternberg P. W., 1995. Sensory regulation of male mating behavior in Caenorhabditis elegans. Neuron 14: 79–89. 10.1016/0896-6273(95)90242-2 [DOI] [PubMed] [Google Scholar]
- Liu J., Ward A., Gao J., Dong Y., Nishio N., et al. , 2010. C. elegans phototransduction requires a G protein-dependent cGMP pathway and a taste receptor homolog. Nat. Neurosci. 13: 715–722. 10.1038/nn.2540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Schulze E., Baumeister R., 2012. Temperature- and touch-sensitive neurons couple CNG and TRPV channel activities to control heat avoidance in Caenorhabditis elegans. PLoS One 7: e32360 10.1371/journal.pone.0032360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockhead D., Schwarz E. M., O’Hagan R., Bellotti S., Krieg M., et al. , 2016. The tubulin repertoire of C. elegans sensory neurons and its context-dependent role in process outgrowth. Mol. Biol. Cell. 27: 3717–3728. 10.1091/mbc.e16-06-0473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewenstein W. R., Mendelson M., 1965. Components of receptor adaptation in a Pacinian corpuscle. J. Physiol. 177: 377–397. 10.1113/jphysiol.1965.sp007598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L., Clark D. A., Biron D., Mahadevan L., Samuel A. D., 2006. Sensorimotor control during isothermal tracking in Caenorhabditis elegans. J. Exp. Biol. 209: 4652–4662. 10.1242/jeb.02590 [DOI] [PubMed] [Google Scholar]
- Luo L., Cook N., Venkatachalam V., Martinez-Velazquez L. A., Zhang X., et al. , 2014a. Bidirectional thermotaxis in Caenorhabditis elegans is mediated by distinct sensorimotor strategies driven by the AFD thermosensory neurons. Proc. Natl. Acad. Sci. USA 111: 2776–2781. 10.1073/pnas.1315205111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L., Wen Q., Ren J., Hendricks M., Gershow M., et al. , 2014b. Dynamic encoding of perception, memory, and movement in a C. elegans chemotaxis circuit. Neuron 82: 1115–1128. 10.1016/j.neuron.2014.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire S. M., Clark C. M., Nunnari J., Pirri J. K., Alkema M. J., 2011. The C. elegans touch response facilitates escape from predacious fungi. Curr. Biol. 21: 1326–1330. 10.1016/j.cub.2011.06.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzochette E. A., Nekimken A. L., Loizeau F., Whitworth J., Huynh B., et al. , 2018. The tactile receptive fields of freely moving Caenorhabditis elegans nematodes. Integr. Biol. 10: 450–463. 10.1039/c8ib00045j [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClanahan P. D., Xu J. H., Fang-Yen C., 2017. Comparing Caenorhabditis elegans gentle and harsh touch response behavior using a multiplexed hydraulic microfluidic device. Integr. Biol. (Camb). 9: 800–809. 10.1039/c7ib00120g [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick K. E., Gaertner B. E., Sottile M., Phillips P. C., Lockery S. R., 2011. Microfluidic devices for analysis of spatial orientation behaviors in semi-restrained Caenorhabditis elegans. PLoS One 6: e25710 10.1371/journal.pone.0025710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyawaki A., Llopis J., Heim R., McCaffery J. M., Adams J. A., et al. , 1997. Fluorescent indicators for Ca2+ based on green fluorescent proteins and calmodulin. Nature 388: 882–887. 10.1038/42264 [DOI] [PubMed] [Google Scholar]
- Mohammadi A., Byrne Rodgers J., Kotera I., Ryu W. S., 2013. Behavioral response of Caenorhabditis elegans to localized thermal stimuli. BMC Neurosci. 14: 66 10.1186/1471-2202-14-66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohri A., Kodama E., Kimura K. D., Koike M., Mizuno T., et al. , 2005. Genetic control of temperature preference in the nematode Caenorhabditis elegans. Genetics 169: 1437–1450. 10.1534/genetics.104.036111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori I., Ohshima Y., 1995. Neural regulation of thermotaxis in Caenorhabditis elegans. Nature 376: 344–348. 10.1038/376344a0 [DOI] [PubMed] [Google Scholar]
- Mouritsen H., 2018. Long-distance navigation and magnetoreception in migratory animals. Nature 558: 50–59. 10.1038/s41586-018-0176-1 [DOI] [PubMed] [Google Scholar]
- Mouritsen H., Heyers D., Gunturkun O., 2016. The neural basis of long-distance navigation in birds. Annu. Rev. Physiol. 78: 133–154. 10.1146/annurev-physiol-021115-105054 [DOI] [PubMed] [Google Scholar]
- Narayan A., Laurent G., Sternberg P. W., 2011. Transfer characteristics of a thermosensory synapse in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 108: 9667–9672. 10.1073/pnas.1106617108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nekimken A. L., Fehlauer H., Kim A. A., Manosalvas-Kjono S. N., Ladpli P., et al. , 2017a. Pneumatic stimulation of C. elegans mechanoreceptor neurons in a microfluidic trap. Lab Chip 17: 1116–1127. 10.1039/C6LC01165A [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nekimken A. L., Mazzochette E. A., Goodman M. B., Pruitt B. L., 2017b. Forces applied during classical touch assays for Caenorhabditis elegans. PLoS One 12: e0178080 10.1371/journal.pone.0178080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen P. A., Liou W., Hall D. H., Leroux M. R., 2014. Ciliopathy proteins establish a bipartite signaling compartment in a C. elegans thermosensory neuron. J. Cell Sci. 127: 5317–5330. 10.1242/jcs.157610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida Y., Sugi T., Nonomura M., Mori I., 2011. Identification of the AFD neuron as the site of action of the CREB protein in Caenorhabditis elegans thermotaxis. EMBO Rep. 12: 855–862. 10.1038/embor.2011.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Hagan R., 2005. Components of a mechanotransduction complex in C. elegans touch receptor neuorns: an in vivo electrophysiological study, pp. 135 in Biological Sciences. Columbia University, New York. [Google Scholar]
- O’Hagan R., Chalfie M., Goodman M. B., 2005. The MEC-4 DEG/ENaC channel of Caenorhabditis elegans touch receptor neurons transduces mechanical signals. Nat. Neurosci. 8: 43–50. 10.1038/nn1362 [DOI] [PubMed] [Google Scholar]
- Ohnishi N., Kuhara A., Nakamura F., Okochi Y., Mori I., 2011. Bidirectional regulation of thermotaxis by glutamate transmissions in Caenorhabditis elegans. EMBO J. 30: 1376–1388. 10.1038/emboj.2011.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta A., Ujisawa T., Sonoda S., Kuhara A., 2014. Light and pheromone-sensing neurons regulates cold habituation through insulin signalling in Caenorhabditis elegans. Nat. Commun. 5: 4412 10.1038/ncomms5412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okochi Y., Kimura K. D., Ohta A., Mori I., 2005. Diverse regulation of sensory signaling by C. elegans nPKC-epsilon/eta TTX-4. EMBO J. 24: 2127–2137. 10.1038/sj.emboj.7600697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oren-Suissa M., Hall D. H., Treinin M., Shemer G., Podbilewicz B., 2010. The fusogen EFF-1 controls sculpting of mechanosensory dendrites. Science 328: 1285–1288. 10.1126/science.1189095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz C. O., Etchberger J. F., Posy S. L., Frokjaer-Jensen C., Lockery S., et al. , 2006. Searching for neuronal left/right asymmetry: genomewide analysis of nematode receptor-type guanylyl cyclases. Genetics 173: 131–149. 10.1534/genetics.106.055749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S. J., Goodman M. B., Pruitt B. L., 2007. Analysis of nematode mechanics by piezoresistive displacement clamp. Proc. Natl. Acad. Sci. USA 104: 17376–17381. 10.1073/pnas.0702138104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins L. A., Hedgecock E. M., Thomson J. N., Culotti J. G., 1986. Mutant sensory cilia in the nematode Caenorhabditis elegans. Dev. Biol. 117: 456–487. 10.1016/0012-1606(86)90314-3 [DOI] [PubMed] [Google Scholar]
- Petzold B. C., Park S. J., Ponce P., Roozeboom C., Powell C., et al. , 2011. Caenorhabditis elegans body mechanics are regulated by body wall muscle tone. Biophys. J. 100: 1977–1985. 10.1016/j.bpj.2011.02.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petzold B. C., Park S. J., Mazzochette E. A., Goodman M. B., Pruitt B. L., 2013. MEMS-based force-clamp analysis of the role of body stiffness in C. elegans touch sensation. Integr. Biol. 5: 853–864. 10.1039/c3ib20293c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter L. R., 2011. Guanylyl cyclase structure, function and regulation. Cell. Signal. 23: 1921–1926. 10.1016/j.cellsig.2011.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prahlad V., Cornelius T., Morimoto R. I., 2008. Regulation of the cellular heat shock response in Caenorhabditis elegans by thermosensory neurons. Science 320: 811–814. 10.1126/science.1156093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Procko C., Lu Y., Shaham S., 2011. Glia delimit shape changes of sensory neuron receptive endings in C. elegans. Development 138: 1371–1381. 10.1242/dev.058305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinowitch I., Chatzigeorgiou M., Schafer W. R., 2013. A gap junction circuit enhances processing of coincident mechanosensory inputs. Curr. Biol. 23: 963–967. 10.1016/j.cub.2013.04.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramot D., MacInnis B. L., Goodman M. B., 2008a. Bidirectional temperature-sensing by a single thermosensory neuron in C. elegans. Nat. Neurosci. 11: 908–915. 10.1038/nn.2157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramot D., MacInnis B. L., Lee H. C., Goodman M. B., 2008b. Thermotaxis is a robust mechanism for thermoregulation in Caenorhabditis elegans nematodes. J. Neurosci. 28: 12546–12557. 10.1523/JNEUROSCI.2857-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin C. H., Broster B. S., 1992. Factors affecting habituation and recovery from habituation in the nematode Caenorhabditis elegans. Behav. Neurosci. 106: 239–249. 10.1037/0735-7044.106.2.239 [DOI] [PubMed] [Google Scholar]
- Russell J., Pierce-Shimomura J. T., 2014. Apparatus for investigating the reactions of soft-bodied invertebrates to controlled humidity gradients. J. Neurosci. Methods 237: 54–59. 10.1016/j.jneumeth.2014.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J., Vidal-Gadea A. G., Makay A., Lanam C., Pierce-Shimomura J. T., 2014. Humidity sensation requires both mechanosensory and thermosensory pathways in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 111: 8269–8274. 10.1073/pnas.1322512111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu W. S., Samuel A. D., 2002. Thermotaxis in Caenorhabditis elegans analyzed by measuring responses to defined Thermal stimuli. J. Neurosci. 22: 5727–5733. 10.1523/JNEUROSCI.22-13-05727.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders J., Nagy S., Fetterman G., Wright C., Treinin M., et al. , 2013. The Caenorhabditis elegans interneuron ALA is (also) a high-threshold mechanosensor. BMC Neurosci. 14: 156 10.1186/1471-2202-14-156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M., 1961. Response of Pacinian corpuscles to sinusoidal vibration. J. Physiol. 159: 391–409. 10.1113/jphysiol.1961.sp006817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage C., Hamelin M., Culotti J. G., Coulson A., Albertson D. G., et al. , 1989. mec-7 is a beta-tubulin gene required for the production of 15-protofilament microtubules in Caenorhabditis elegans. Genes Dev. 3: 870–881. 10.1101/gad.3.6.870 [DOI] [PubMed] [Google Scholar]
- Sawin E. R., Ranganathan R., Horvitz H. R., 2000. C. elegans locomotory rate is modulated by the environment through a dopaminergic pathway and by experience through a serotonergic pathway. Neuron 26: 619–631. 10.1016/S0896-6273(00)81199-X [DOI] [PubMed] [Google Scholar]
- Schaad N. C., De Castro E., Nef S., Hegi S., Hinrichsen R., et al. , 1996. Direct modulation of calmodulin targets by the neuronal calcium sensor NCS-1. Proc. Natl. Acad. Sci. USA 93: 9253–9258. 10.1073/pnas.93.17.9253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer W. R., 2015. Mechanosensory molecules and circuits in C. elegans. Pflugers Arch. 467: 39–48. 10.1007/s00424-014-1574-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schild L. C., Glauser D. A., 2013. Dynamic switching between escape and avoidance regimes reduces Caenorhabditis elegans exposure to noxious heat. Nat. Commun. 4: 2198 10.1038/ncomms3198 [DOI] [PubMed] [Google Scholar]
- Schild L. C., Zbinden L., Bell H. W., Yu Y. V., Sengupta P., et al. , 2014. The balance between cytoplasmic and nuclear CaM kinase-1 signaling controls the operating range of noxious heat avoidance. Neuron 84: 983–996. 10.1016/j.neuron.2014.10.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulenburg H., Felix M. A., 2017. The natural biotic environment of Caenorhabditis elegans. Genetics 206: 55–86. 10.1534/genetics.116.195511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta P., Garrity P., 2013. Sensing temperature. Curr. Biol. 23: R304–R307. 10.1016/j.cub.2013.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw M., Elmi M., Pawar V., Srinivasan M. A., 2016. Investigation of mechanosensation in C. elegans using light field calcium imaging. Biomed. Opt. Express 7: 2877–2887. 10.1364/BOE.7.002877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shida T., Cueva J. G., Xu Z., Goodman M. B., Nachury M. V., 2010. The major alpha-tubulin K40 acetyltransferase alphaTAT1 promotes rapid ciliogenesis and efficient mechanosensation. Proc. Natl. Acad. Sci. USA 107: 21517–21522. 10.1073/pnas.1013728107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhvi A., Liu B., Friedman C. J., Fong J., Lu Y., et al. , 2016. A glial K/Cl transporter controls neuronal receptive ending shape by chloride inhibition of an rGC. Cell 165: 936–948. 10.1016/j.cell.2016.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. J., Watson J. D., Spencer W. C., O’Brien T., Cha B., et al. , 2010. Time-lapse imaging and cell-specific expression profiling reveal dynamic branching and molecular determinants of a multi-dendritic nociceptor in C. elegans. Dev. Biol. 345: 18–33. 10.1016/j.ydbio.2010.05.502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirman J. N., Crane M. M., Husson S. J., Wabnig S., Schultheis C., et al. , 2011. Real-time multimodal optical control of neurons and muscles in freely behaving Caenorhabditis elegans. Nat. Methods 8: 153–158. 10.1038/nmeth.1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugi T., Okumura E., Kiso K., Igarashi R., 2016. Nanoscale mechanical stimulation method for quantifying C. elegans mechanosensory behavior and memory. Anal. Sci. 32: 1159–1164. 10.2116/analsci.32.1159 [DOI] [PubMed] [Google Scholar]
- Sukul N. C., Croll N. A., 1978. Influence of potential difference and current on the electrotaxis of Caenorhaditis elegans. J. Nematol. 10: 314–317. [PMC free article] [PubMed] [Google Scholar]
- Sulston J., Dew M., Brenner S., 1975. Dopaminergic neurons in the nematode Caenorhabditis elegans. J. Comp. Neurol. 163: 215–226. 10.1002/cne.901630207 [DOI] [PubMed] [Google Scholar]
- Suzuki H., Kerr R., Bianchi L., Frokjaer-Jensen C., Slone D., et al. , 2003. In vivo imaging of C. elegans mechanosensory neurons demonstrates a specific role for the MEC-4 channel in the process of gentle touch sensation. Neuron 39: 1005–1017. 10.1016/j.neuron.2003.08.015 [DOI] [PubMed] [Google Scholar]
- Syeda R., Florendo M. N., Cox C. D., Kefauver J. M., Santos J. S., et al. , 2016. Piezo1 channels are inherently mechanosensitive. Cell Rep. 17: 1739–1746. 10.1016/j.celrep.2016.10.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeishi A., Yu Y. V., Hapiak V. M., Bell H. W., O’Leary T., et al. , 2016. Receptor-type guanylyl cyclases confer thermosensory responses in C. elegans. Neuron 90: 235–244. 10.1016/j.neuron.2016.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatum M. C., Ooi F. K., Chikka M. R., Chauve L., Martinez-Velazquez L. A., et al. , 2015. Neuronal serotonin release triggers the heat shock response in C. elegans in the absence of temperature increase. Curr. Biol. 25: 163–174. 10.1016/j.cub.2014.11.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timbers T. A., Giles A. C., Ardiel E. L., Kerr R. A., Rankin C. H., 2013. Intensity discrimination deficits cause habituation changes in middle-aged Caenorhabditis elegans. Neurobiol. Aging 34: 621–631. 10.1016/j.neurobiolaging.2012.03.016 [DOI] [PubMed] [Google Scholar]
- Topalidou I., Keller C., Kalebic N., Nguyen K. C., Somhegyi H., et al. , 2012. Genetically separable functions of the MEC-17 tubulin acetyltransferase affect microtubule organization. Curr. Biol. 22: 1057–1065. 10.1016/j.cub.2012.03.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troemel E. R., Sagasti A., Bargmann C. I., 1999. Lateral signaling mediated by axon contact and calcium entry regulates asymmetric odorant receptor expression in C. elegans. Cell 99: 387–398. 10.1016/S0092-8674(00)81525-1 [DOI] [PubMed] [Google Scholar]
- Tsalik E. L., Hobert O., 2003. Functional mapping of neurons that control locomotory behavior in Caenorhabditis elegans. J. Neurobiol. 56: 178–197. 10.1002/neu.10245 [DOI] [PubMed] [Google Scholar]
- Tsukada Y., Yamao M., Naoki H., Shimowada T., Ohnishi N., et al. , 2016. Reconstruction of spatial thermal gradient encoded in thermosensory neuron AFD in Caenorhabditis elegans. J. Neurosci. 36: 2571–2581. 10.1523/JNEUROSCI.2837-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ujisawa T., Ohta A., Uda-Yagi M., Kuhara A., 2016. Diverse regulation of temperature sensation by trimeric G-protein signaling in Caenorhabditis elegans. PLoS One 11: e0165518 10.1371/journal.pone.0165518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vásquez V., Scherrer G., Goodman M. B., 2014. Sensory biology: it takes Piezo2 to tango. Curr. Biol. 24: R566–R569. 10.1016/j.cub.2014.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatachalam V., Ji N., Wang X., Clark C., Mitchell J. K., et al. , 2016. Pan-neuronal imaging in roaming Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 113: E1082–E1088. 10.1073/pnas.1507109113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal-Gadea A., Ward K., Beron C., Ghorashian N., Gokce S., et al. , 2015. Magnetosensitive neurons mediate geomagnetic orientation in Caenorhabditis elegans. eLife 4 10.7554/eLife.07493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal-Gadea A., Bainbridge C., Clites B., Palacios B. E., Bakhtiari L., et al. , 2018. Response to comment on “Magnetosensitive neurons mediate geomagnetic orientation in Caenorhabditis elegans”. eLife 7: e31414 10.7554/eLife.31414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel B. E., Hedgecock E. M., 2001. Hemicentin, a conserved extracellular member of the immunoglobulin superfamily, organizes epithelial and other cell attachments into oriented line-shaped junctions. Development 128: 883–894. [DOI] [PubMed] [Google Scholar]
- Vyklický L., Vlachova V., Vitaskova Z., Dittert I., Kabat M., et al. , 1999. Temperature coefficient of membrane currents induced by noxious heat in sensory neurones in the rat. J. Physiol. 517: 181–192. 10.1111/j.1469-7793.1999.0181z.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker D. S., Vazquez-Manrique R. P., Gower N. J., Gregory E., Schafer W. R., et al. , 2009. Inositol 1,4,5-trisphosphate signalling regulates the avoidance response to nose touch in Caenorhabditis elegans. PLoS Genet. 5: e1000636 10.1371/journal.pgen.1000636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., O’Halloran D., Goodman M. B., 2013. GCY-8, PDE-2, and NCS-1 are critical elements of the cGMP-dependent thermotransduction cascade in the AFD neurons responsible for C. elegans thermotaxis. J. Gen. Physiol. 142: 437–449. 10.1085/jgp.201310959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Hu R., Ge A., Hu L., Wang S., et al. , 2015. Highly efficient microfluidic sorting device for synchronizing developmental stages of C. elegans based on deflecting electrotaxis. Lab Chip 15: 2513–2521. 10.1039/C5LC00354G [DOI] [PubMed] [Google Scholar]
- Ward S., Thomson N., White J. G., Brenner S., 1975. Electron microscopical reconstruction of the anterior sensory anatomy of the nematode Caenorhabditis elegans.?2UU. J. Comp. Neurol. 160: 313–337. 10.1002/cne.901600305 [DOI] [PubMed] [Google Scholar]
- Wasserman S. M., Beverly M., Bell H. W., Sengupta P., 2011. Regulation of response properties and operating range of the AFD thermosensory neurons by cGMP signaling. Curr. Biol. 21: 353–362. 10.1016/j.cub.2011.01.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Way J. C., Chalfie M., 1989. The mec-3 gene of Caenorhabditis elegans requires its own product for maintained expression and is expressed in three neuronal cell types. Genes Dev. 3: 1823–1833. 10.1101/gad.3.12a.1823 [DOI] [PubMed] [Google Scholar]
- Wen Q., Po M. D., Hulme E., Chen S., Liu X., et al. , 2012. Proprioceptive coupling within motor neurons drives C. elegans forward locomotion. Neuron 76: 750–761. 10.1016/j.neuron.2012.08.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wes P. D., Bargmann C. I., 2001. C. elegans odour discrimination requires asymmetric diversity in olfactory neurons. Nature 410: 698–701. 10.1038/35070581 [DOI] [PubMed] [Google Scholar]
- White J. G., Southgate E., Thomson J. N., Brenner S., 1986. The structure of the nervous system of the nematode Caenorhabditis elegans. Philos. Trans. R. Soc. Lond. B Biol. Sci. 314: 1–340. 10.1098/rstb.1986.0056 [DOI] [PubMed] [Google Scholar]
- Wiltschko R., Wiltschko W., 2012. Magnetoreception. Adv. Exp. Med. Biol. 739: 126–141. 10.1007/978-1-4614-1704-0_8 [DOI] [PubMed] [Google Scholar]
- Wittenburg N., Baumeister R., 1999. Thermal avoidance in Caenorhabditis elegans: an approach to the study of nociception. Proc. Natl. Acad. Sci. USA 96: 10477–10482. 10.1073/pnas.96.18.10477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo S. H., Lumpkin E. A., Patapoutian A., 2015. Merkel cells and neurons keep in touch. Trends Cell Biol. 25: 74–81. 10.1016/j.tcb.2014.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Lewis A. H., Grandl J., 2017. Touch, tension, and transduction - the function and regulation of Piezo ion channels. Trends Biochem. Sci. 42: 57–71. 10.1016/j.tibs.2016.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao R., Zhang B., Dong Y., Gong J., Xu T., et al. , 2013. A genetic program promotes C. elegans longevity at cold temperatures via a thermosensitive TRP channel. Cell 152: 806–817. 10.1016/j.cell.2013.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada Y., Ohshima Y., 2003. Distribution and movement of Caenorhabditis elegans on a thermal gradient. J. Exp. Biol. 206: 2581–2593. 10.1242/jeb.00477 [DOI] [PubMed] [Google Scholar]
- Yeon J., Kim J., Kim D. Y., Kim H., Kim J., et al. , 2018. A sensory-motor neuron type mediates proprioceptive coordination of steering in C. elegans via two TRPC channels. PLoS Biol. 16: e2004929 10.1371/journal.pbio.2004929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida A., Nakano S., Suzuki T., Ihara K., Higashiyama T., et al. , 2016. A glial K(+)/Cl(-) cotransporter modifies temperature-evoked dynamics in Caenorhabditis elegans sensory neurons. Genes Brain Behav. 15: 429–440. 10.1111/gbb.12260 [DOI] [PubMed] [Google Scholar]
- Yu S., Avery L., Baude E., Garbers D. L., 1997. Guanylyl cyclase expression in specific sensory neurons: a new family of chemosensory receptors. Proc. Natl. Acad. Sci. USA 94: 3384–3387. 10.1073/pnas.94.7.3384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y. V., Bell H. W., Glauser D., Van Hooser S. D., Goodman M. B., et al. , 2014. CaMKI-dependent regulation of sensory gene expression mediates experience-dependent plasticity in the operating range of a thermosensory neuron. Neuron 84: 919–926. 10.1016/j.neuron.2014.10.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zariwala H. A., Miller A. C., Faumont S., Lockery S. R., 2003. Step response analysis of thermotaxis in Caenorhabditis elegans. J. Neurosci. 23: 4369–4377. 10.1523/JNEUROSCI.23-10-04369.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., Xiao R., Ronan E. A., He Y., Hsu A. L., et al. , 2015. Environmental temperature differentially modulates C. elegans longevity through a thermosensitive TRP channel. Cell Rep. 11: 1414–1424. 10.1016/j.celrep.2015.04.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Yue X., Cheng H., Zhang X., Cai Y., et al. , 2018. OSM-9 and an amiloride-sensitive channel, but not PKD-2, are involved in mechanosensation in C. elegans male ray neurons. Sci. Rep. 8: 7192 10.1038/s41598-018-25542-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen M., Samuel A. D., 2015. C. elegans locomotion: small circuits, complex functions. Curr. Opin. Neurobiol. 33: 117–126. 10.1016/j.conb.2015.03.009 [DOI] [PubMed] [Google Scholar]
- Zheng C., Diaz-Cuadros M., Nguyen K. C. Q., Hall D. H., Chalfie M., 2017. Distinct effects of tubulin isotype mutations on neurite growth in Caenorhabditis elegans. Mol. Biol. Cell 28: 2786–2801. 10.1091/mbc.e17-06-0424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou W., Cheng H., Li S., Yue X., Xue Y., et al. , 2017. Polymodal responses in C. elegans phasmid neurons rely on multiple intracellular and intercellular signaling pathways. Sci. Rep. 7: 42295 10.1038/srep42295 [DOI] [PMC free article] [PubMed] [Google Scholar]