Abstract
Background
Opioid dependence is a significant public health problem in the United States and the number of opioid overdose deaths among women has increased dramatically in comparison to men in the last few years. In this context, understanding the biological mechanisms underlying gender differences in vulnerability to opioid dependence is essential.
Methods
The current pilot study examined gender differences in subjective stress, heart rate (HR), and cortisol/ dephydroepiandrosterone (DHEA) response to a laboratory stressor (Trier Social Stress Test; TSST) or a no-stress condition, and drug cue paradigm among men (n=21) and women (n=18) with opioid dependence.
Results
Significant group (TSST vs. no stress) differences emerged in self-reported stress [F(1,35)=41.77, p<.001], HR [F(1,31) =12.3; p=0.001] and cortisol (F1.34=5.0; p=0.032) response, such that the TSST group was more reactive than the no-stress group. Women reported greater subjective stress [F(1,35)=11.24, p<.01] in response to the TSST compared to men. However, men evidenced marginally greater cortisol and DHEA responses to the TSST compared to women [F(1,34) =2.7; p=0.113 and F(1,31)=3.4; p=0.073, respectively].
Conclusions
Although women with opioid dependence report greater subjective stress when exposed to a laboratory stress paradigm as compared to men, the neuroendocrine response was more robust in men. This pattern is similar to gender findings in men and women with cocaine and tobacco use disorders. The blunted cortisol combined with an increased subjective response among women may be a sign of HPA axis dysregulation which could increase vulnerability to relapse in women.
Keywords: opioid dependence, gender differences, stress, heart rate, cortisol, dephydroepiandrostrone
Introduction
Opioid dependence is a significant public health problem in the United States. According to the 2015 National Survey on Drug Use and Health, approximately 11.5 million adults reported misusing opioids in the past year, and nearly 2 million met criteria for an opioid use disorder (Han et al., 2017). While the number of opioid-dependent men in the United States remains larger than the number of opioid-dependent women, the rate of opioid overdose deaths and heroin use among women exceeds men (Marsh et al., 2018). Between 1999 and 2010, overdose deaths from opioids increased more than 400-fold in women compared to an increase of 237% in men (Prevention, 2013). Similarly, between 2002 and 2013, heroin use among women increased 100% compared to an increase of 50% among men (Jones et al., 2015; Prevention, 2013). Further, women are more likely than men to report opioids as their primary substance of abuse upon admission for substance abuse treatment (Jones, 2017).
There are biological and psychosocial differences between men and women that influence substance use patterns, disease course, treatment entry as well as risk for relapse (Greenfield et al., 2010). Women with substance use disorders are more likely than men to have comorbid depression and anxiety disorders (Brady et al., 1993; Brady and Randall, 1999) and are more likely to attribute use and relapse to negative emotional states and interpersonal conflict (Back et al., 2011; Connors et al., 1998). In one study comparing men and women with opioid dependence, psychological and emotional distress were identified as risk factors for hazardous opioid use among women, but not among men (Back et al., 2011).
Studies investigating the biological underpinning of sex differences in substance use suggest that dysregulation in the hypothalamic-pituitary-adrenal (HPA) axis and noradrenergic system in response to stress may increase women’s vulnerability to substance abuse and relapse (Sinha, 2001). In a human laboratory study following the administration of corticotrophin releasing factor (CRF), women with cocaine use disorder demonstrated greater evidence of HPA axis disruption and a more robust and prolonged HR response, suggesting a greater noradrenergic response to stress, as compared to men (Brady et al., 2009). Similarly, Back and colleagues (Back et al., 2008) demonstrated a blunted adrenocorticotropic hormone and cortisol response in women with tobacco use disorder, as compared to men, following exposure to stress and tobacco cues in women. These findings are significant because prior research has demonstrated women report a greater emotional intensity at lower levels of HPA arousal, thus HPA dysregulation may be one key to enhanced vulnerability to relapse in response to negative affect among women (Fox et al., 2008).
In opioid dependence, the clinical significance of stress system dysregulation is likely to be of major significance because of the complex circuitry by which stress-related neuropeptides and endogenous opioids co-regulate activity of the locus coeruleus-noradrenergic (LC-NE) system. The LC–NE system is reciprocally regulated by endogenous opioids and CRF (Curtis et al., 2001; Valentino et al., 2001). Preclinical studies have demonstrated that chronic morphine sensitizes the LC-NE system to CRF and this is expressed as increased sensitivity of the neurons to stress, providing a potential mechanism to link opioid use with stress-sensitive disorders (Xu et al., 2004). In addition to increasing vulnerability to stress-related disorders, opioid-induced sensitization of the LC-NE system may facilitate the maintenance of opioid use to counteract this sensitization. Withdrawal from opioids engages CRF and other stress-systems, including noradrenergic pathways, and is associated with anxiety and dysphoria that can increase susceptibility to relapse (Schluger et al., 2003).
Preclinical investigations have found significant sex differences in the LC-NE system including increased numbers of LC neurons and differences in dendritic structure allowing female rats to receive more CRF-containing afferents from limbic regions (Bangasser et al., 2016). In addition, estrogen can increase NE in LC target regions by enhancing the capacity for NE synthesis and reducing NE degradation, potentially increasing arousal in females (Bangasser et al., 2016). Finally, one study found that CRF was 10–30 times more potent in activating LC neurons in female vs. male rats (Curtis et al., 2006). These findings suggest that stress system dysregulation caused by chronic opioid dependence in humans may differ by gender, but this has not been systematically explored.
Current Study
Understanding the biological mechanisms that underlie gender differences in vulnerability to opioid dependence is greatly needed to adapt and enhance evidence-based treatments for opioid dependence to promote increased treatment initiation, maintenance, and time to relapse by addressing gender-specific differences. This pilot study prospectively investigated gender differences in subjective stress, heart rate and neuroendocrine response to a laboratory stressor and drug cue paradigm among women and men with opioid dependence. Further, gender-differences in predictors of relapse during the week following testing were examined.
Methods
Participants
The current study used the subsample of men (n=21) and women (n=18) with opioid dependence who were part of a larger study that involved testing associations between stress, HPA axis function, and drug use among non-treatment seeking individuals with opioid dependence or healthy controls (Back et al., 2015). Participants were recruited through advertisements in newspapers, Craigslist, and local clinics. Interested participants were screened for study eligibility over the phone and subsequently with a clinical assessment and physical examination in the clinic. Exclusion criteria included: pregnancy or nursing, major medical problems and psychiatric conditions that could impact the HPA axis, BMI greater or equal to 39, use of opioid replacement therapies in the past three months, use of treatment agents that may interfere with stress response such antihypertensive medications, and DSM-IV substance dependence other than opioid dependence (excluding caffeine and tobacco). Participants included adults who met criteria for current substance dependence as defined by the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (Association, 2000) on opioid analgesics. Laboratory procedures took place at the same time in the morning for all participants following a one-night hospital stay.
Measures
Demographic Characteristics.
Participants completed demographic questions including gender, age, race, marital status, and education level.
Substance Use.
The Time Line Follow-Back (Sobell & Sobell, 1992; TLFB) was used at baseline and the one-week follow-up visit to assess daily substance use during the past 30-days and past week respectively. To determine eligibility for the study and presence of opioid dependence, the Structured Clinical Interview for DSM-IV (First et al., 2002; SCID) and the Mini International Neuropsychiatric Interview (Sheehan et al., 1998; MINI) were used. On Track Test Cup® and breathalyzer tests were administered to verify abstinence from alcohol and other non-opioid substances prior to the hospital visit.
Lifetime Exposure to Traumatic Events.
The Life Stressor Checklist-Revised (Wolfe and Kimerling, 1997; LSC-R) was used to assess past exposure (yes or no) to 30 potentially traumatic life events, for example, serious accidents, interpersonal violence, and physical and sexual abuse. The LSC-R also includes women’s life experiences such as pregnancy abortion (Norris and Hamblen, 2004). The total number of lifetime events were summed.
Subjective Stress.
The Within Session Rating Scale (Childress et al., 1986) was used to create an analog scale for subjective stress, sadness, happiness, anger, craving, and amount of money participants were willing to pay for opioids. Participants rated the items from 0 (not at all) to 10 (extremely). The 20-item State-Trait Anxiety Inventory(Spielberger, 2010)(STAI) was used to assess current levels of anxiety using a 4-point Likert scale ranging from not at all to very much so. Measures were completed immediately prior to the TSST or no-stress condition, immediately after the TSST/prior to the drug cue paradigm, and at 15, 30, and 60 minutes after the drug-cue paradigm.
Heart rate.
Electrodes were placed on the ribcage and collar bone of participants and heart rate (HR) was continuously measured immediately prior to the TSST or no-stress condition, during the TSST, immediately after the TSST/prior to the drug-cue paradigm, during the drug-cue paradigm, and at 15, 30, and 60 minutes after the drug cue paradigm.
Neuroendocrine response.
Unstimulated salivary samples were collected to measure dephydroepiandrosterone (DHEA) and cortisol. DHEA and cortisol were assayed in duplicate using salivary DHEA (intra-assay precision of 5.6% with a sensitivity of 5pg/mL) and cortisol (intra-assay precision of 3.35% - 3.65% with a sensitivity of <0.003 ug/dL) enzyme immunoassay systems. PowerWave HT Microplate and Precision Series Automated Liquid Handling System (BioTek Instruments, Inc.) were used to analyze DHEA and cortisol. Samples were collected immediately prior to the TSST or no-stress condition, immediately after the TSST/prior to the drug-cue paradigm, and at 15, 30, and 60 minutes after the drug cue paradigm.
Laboratory Procedure
The local Institutional Review Board approved all study procedures for the larger study (Back et al., 2015). Upon arrival to the hospital, participants completed a breathalyzer and UDS to test for alcohol and other substances, excluding caffeine and tobacco. Participants who were abstinent from opioids, alcohol and other substances of abuse for three days and did not have significant withdrawal symptoms were admitted for the overnight stay at 2000h the evening prior to the testing. Twenty-four hour nicotine replacement therapy was provided to participants who smoked cigarettes for the duration of the hospital stay. The next morning, participants had a standard breakfast at 0730h followed by a 60-minute acclimation period from 0830-0930h, pre-testing assessments from 0930h and 0945h, and testing starting at 0950h.
Participants were randomly assigned to a standardized 15-minute Tier Social Stress Task (Kirschbaum et al., 1993; TSST) or a no-stress condition using urn randomization (Wei and Lachin, 1988). The TSST invokes stress by giving participants 5-minutes to prepare a speech followed by verbally providing the speech for 5-mintues and completing serial subtractions for 5-minutes to 3 confederates. Control participants relaxed during this 15-minute period. Next, all participants completed a 15 minute drug cue paradigm that involved three 5-minute components (Back et al., 2011) including an audio induction script, handling drug paraphernalia, and viewing a video of people using prescription opioids. Heart rate, neuroendocrine response, and subjective ratings were collected immediately prior to the TSST or no-stress condition, immediately after the TSST/prior to the drug cue paradigm and at 15, 30, and 60 minutes after the drug-cue paradigm. Participants were subsequently debriefed, compensated, and discharged.
Data Analysis
This secondary analysis focused on gender differences in the stress response of participants with opioid dependence who were randomized to a TSST stress task plus a drug cue paradigm (stress+drug cue group) to those randomized to the drug cue paradigm only (drug cue only group). Standard descriptive statistics were used to quantify demographic, clinical and substance use characteristics for the entire study cohort as well as across randomized study treatment assignments. Baseline continuous and count characteristics are presented as means and associated standard deviations while categorical characteristics are presented as a proportion of the group or total sample size. Continuous and count characteristics are compared across group assignment and gender using a Wilcoxon rank sum statistic while categorical characteristics are compared using a Pearson Chi-Square test statistic (or Fisher exact test when appropriate).
The primary analysis of longitudinal responses to the stress+drug cue or drug cue only was analyzed over all five post-TSST time points (matched timing accordingly for drug cue only group). Generalized linear mixed effects models assuming a Gaussian distribution (GLMMs) were used to assess the overall treatment assignment and gender differences over time. Initial models contain group assignment, gender, time, and baseline measures of the model outcome. Additionally, interaction terms of interest were added to the model; specifically, group assignment by gender and treatment assignment by time. Group level means were constructed using model based estimates and associated standard errors. Restricted maximum likelihood (REML) methods were used to estimate fixed effects and variance components in the presence of imbalanced data (Patterson and Thompson, 1971). Residual normality was assessed for each model using Q-Q plots and data transformations were performed when necessary; specifically, it was necessary that both cortisol and DHEA were log10 transformed prior to analysis. Although this pilot study was not powered to determine statistically significant gender by treatment interactions, a primary goal was to estimate gender differences in subjective stress response in a population with opioid dependence; thus group analyses are additionally stratified by gender. Secondarily, opioid use rates at a 1-week follow up visits were collected using the TLFB. Substance use rates (any use) are compared between groups and gender using Fisher’s exact test and presented as percentage of the available participants reporting use. Amount of substance use per using day is compared between groups and gender using a Wilcoxon rank sum test statistic and presented as the median daily use and associated interquartile range (IQR). All statistical analysis were conducted using SAS version 9.4 (SAS® 9.4) and significance for all comparisons was set at a 2 sided p-value of 0.05.
Results
Study Participant Characteristics
Baseline demographics and clinical characteristics were examined for the cohort as well as across groups (Table 1). At study baseline, the mean participant age was 34.9 (SD=12.6) years, 47% (n=18) were female and 82% (n=32) were Caucasian. There were no significant differences in age, race, gender or prior opioid use characteristics between the randomized groups (stress+drug cue vs. drug cue only). However, female study participants were significantly older [41.2 (SD=12.4) vs. 29.5 (10.2); p=0.008], began opioid use at a moderately older age [26.2 (13.1) vs. 18.3 (5.1); p=0.109], progressed to opioid dependence later [30.7 (12.3) vs. 23.5 (7.1); p=0.068], and reported more lifetime traumatic events [10.5 (4.7) vs. 5.7 (3.6); p = .001], than male participants. Additionally, female participants reported moderately greater subjective stress [4.2 (2.4) vs. 3.0 (2.2); p=0.066] and had higher HRs [73.3 (8.7) vs. 66.9 (10.6); p=0.019] prior to the study protocol initiation than male participants.
Table 1.
Sample demographics, history of substance use and baseline psychological and biological stress response variables.
| Overall Study Cohort (n=39) |
Treatment Assignment/Gender Group |
||||
|---|---|---|---|---|---|
| No Stress Group | Stress Group | ||||
| Male (n=10) |
Female (n=10) |
Male (n=11) |
Female (n=8) |
||
| Demographics | |||||
| Age | 34.9 (12.6) | 28.5 (10.5) | 38.3 (13.3) | 30.5 (10.3) | 44.8 (11.0)# |
| Female % (n) | 46.5 (18) | -- | -- | -- | -- |
| Race/Ethnicity % (n) | |||||
| Caucasian | 82.1 (32) | 80.0 (8) | 80.0 (8) | 90.9 (10) | 75.0 (6) |
| African American | 5.1 (2) | 0.0 (0) | 0.0 (0) | 0.0 (0) | 25.0 (2) |
| Hispanic | 5.1 (2) | 10.0 (1) | 0.0 (0) | 9.1 (1) | 0.0 (0) |
| Native American | 7.7 (3) | 10.0 (1) | 20.0 (2) | 0.0 (0) | 0.0 (0) |
| Lifetime number of traumatic experiences | 7.9 (4.8) | 6.0 (3.0) | 11.4 (5.2)# | 5.5 (4.2) | 9.4 (7.2) |
| Substance Use | |||||
| Age of first opiate use | 21.9 (10.3) | 20.3 (5.8) | 24.2 (14.3) | 16.5 (3.8) | 28.8 (11.7) # |
| Age OUD onset | 26.8 (10.4) | 24.8 (8.1) | 28.4 (14.3) | 22.4 (6.2) | 33.5 (9.4) # |
| Days using Opiates (past 30 days) | 18.2 (8.5) | 19.0 (8.4) | 18.3 (8.0) | 14.5 (8.1) | 22.1 (9.5) |
| Pills per using Day (past 30 days) | 4.3 (2.8) | 3.7 (1.8) | 5.8 (4.1) | 3.2 (1.9) | 4.7 (2.6) |
| Past month Substance Use % (n)* | |||||
| Heroin | 8.1 (3) | 0.0 (0) | 11.1 (1) | 10.0 (1) | 12.5 (1) |
| Alcohol | 59.5 (22) | 77.8 (7) | 33.3 (3) | 81.8 (9) | 37.5 (3) |
| Cannabis | 41.7 (15) | 44.4 (4) | 33.3 (3) | 60.0 (6) | 25.0 (2) |
| Cocaine | 30.6 (11) | 33.3 (3) | 55.6 (5) | 20.0 (2) | 12.5 (1) |
| Baseline Response Variables | |||||
| Subjective Stress | 3.5 (2.3) | 2.4 (2.1) | 4.4 (2.7) | 3.5 (2.3) | 4.0 (2.1) |
| Cortisol (ug/dL) | 0.27 (0.13) | 0.23 (0.14) | 0.30 (0.14) | 0.28 (0.11) | 0.25 (0.13) |
| DHEA (pg/mL) | 170.3 (108.9) | 226.9 (137.9) | 143.7 (64.9) | 149.3 (117.2) | 157.2 (90.6) |
| Heart Rate | 70.1 (10.1) | 66.6 (7.8) | 75.3 (9.1) # | 67.2 (13.3) | 70.8 (8.1) |
Continuous data are presented as means and associated standard deviations while categorical characteristics are presented as percentages and the number in each category.
2 participants in the stress group failed to report past 30 day heroin use status, 2 participants in the no stress group failed to report past 30 days alcohol use status, 2 participants in the no stress and 1 in the stress group failed to report past 30 days cannabis and cocaine use status.
p<0.05 as compared to male participants.
The majority of participants indicated that they were given opioids by a medical provider (52.1%). Other participants indicated that the opioids came from a friend or family member (43%) and a minority of participants indicated that the opioids were stolen from a friend or family member (4.9%). The majority of participants initially used the opioid medically to reduce pain (66.2%). However, only a minority indicated current use was to reduce pain (23.9%).
Although participants were excluded who had current substance dependence other than from opioids, many of the participants had a history of substance dependence. A total of 38.5% had a history of alcohol dependence, 35.9% had a history of cocaine dependence, 25.6% had a history of cannabis dependence, and 5.1% had a history of dependence on sedatives.
Response to Laboratory Tasks
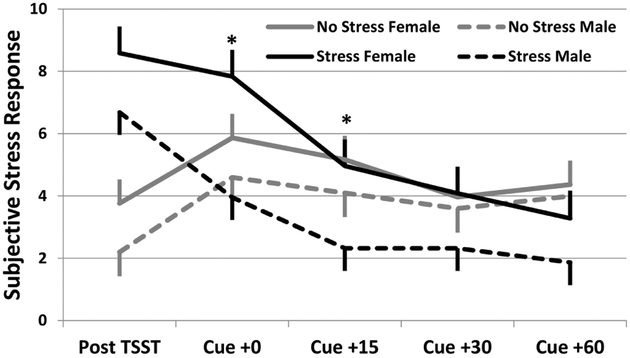
Subjective stress ratings, HR, and neuroendocrine responses were collected prior to and following the stress/cue paradigm. As anticipated, participants randomized to the stress+drug cue group reported a greater overall subjective stress response to the paradigm than those randomized to the drug cue only group (F1,34=23.8; p=0.001). Additionally, female participants reporter significantly greater subjective stress following the laboratory tasks than males (F1,34=6.5; p=0.015); specifically noted in the stress+drug cue task (Figure 1).
Figure 1.
Subjective stress response stratified by randomization group and gender. Data are shown as model based means and associated standard errors adjusted for baseline stress response. *p<0.05 Stress females as compared to Stress group male participants.
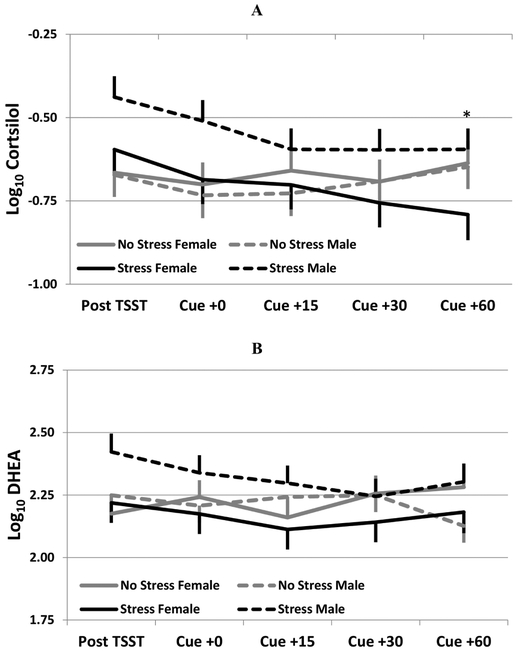
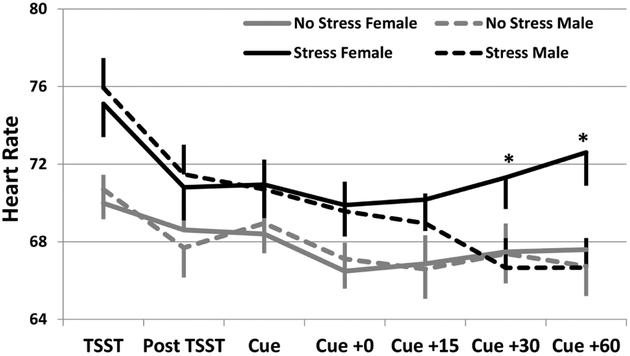
Cortisol and DHEA were collected at the same time points as subjective stress. Following the laboratory tasks, the stress+drug cue group had significantly greater cortisol levels as compared to the drug cue only group (F1,34=5.0; p=0.032). This difference is primarily driven by a numerically greater response in the male stress+drug cue group (Figure 2a; group x gender interaction F1,34=2.7; p=0.113). The DHEA response to the stress paradigm was similar in direction to the cortisol response, although not as strong (group effect, F1,31=0.8; p=0.377). However, the gender differential across group assignments is consistent with the cortisol pattern, with the men exhibiting a greater DHEA response as compared to women (Figure 2b; group x gender interaction F1,31=3.4; p=0.073). Heart rate data was collected continuously and means were collected during binned times according to the measurement timing as described above, additionally heart rate data was also collected during the paradigm. Participants randomized to the stress+drug cue group experienced higher mean heart rate values as compared to the drug cue only group (Figure 3; F1,31=12.3; p=0.001). Of interest, HR for women was significantly higher at the 30 and 60 minute post-laboratory task measurement as compared to men, whose HR had returned to baseline levels by the 30 minute post-laboratory task measurement.
Figure 2.
(A) Cortisol and (B) DHEA response stratified by randomization group and gender. Data are shown as log10 transformed model based means and associated standard errors adjusted for baseline cortisol/DHEA. *p<0.05 Stress females as compared to Stress group male participants.
Figure 3.
Heart Rate response stratified by randomization group and gender. Data are shown as model based means and associated standard errors adjusted for baseline heart rate. *p<0.05 Stress group females as compared to Stress group male participants.
Follow Up Use Rate
At the 1-week follow-up visit, 32 of the 39 randomized participants reported drug use data; 18 in the stress+drug cue group and 14 in the drug cue only group. Twenty-five of the 32 participants (78%) reported any use during follow-up. In the stress+drug cue group, 16 of the 18 participants (89%) reported any use while 9 of the 14 participants (64%) in the drug cue only group reported any use (Fisher exact p=0.195). Among participants that reported any use, those in the stress+drug cue group reported a median of 2.71 opioid pills (IQR 2.06-4.60 pills) per using day while those in the drug cue only group reported a median of 1.60 pills (IQR=1.10-3.50 pills) per using day [Wilcoxon Rank Sum test p=0.349]. Participant gender was not associated with significant differences in use rates [Females 71.4% vs. Males 83.3%; Fisher exact test p=0.419] or use per using day [Females Median=2.31 (1.50-2.71) vs. Males 3.00 (1.00-4.00) p=0.597].
Participants did report other drug use at follow-up. Specifically, 34.4% of participants reported using alcohol, 27.3% reported using cannabis, 12.1% reported using cocaine, and 6% reported using heroin.
Discussion
The current study examined gender differences in subjective stress, HR, and cortisol/DHEA in response to a laboratory stressor (TSST) and drug cue paradigm among men and women with opioid dependence to inform the biological mechanisms underlying gender differences in vulnerability to opioide dependence. While women reported greater subjective stress following the TSST compared to men, men had higher cortisol levels and DHEA response following the TSST compared to women. There was no difference in cortisol and DHEA response among women in the stress+drug cue group compared to the drug cue only group. While both men and women had an increase in HR in response to the TSST, for men the HR returned to baseline levels by 30 minutes post-stressor while for women the HR remained elevated at the 60-minute measurement time point. Although findings should be considered pilot data, they suggest important gender differences in stress response that are consistent with gender differences found in other SUDs and particular vulnerability among women.
While subjective distress was greater among those randomized to the stress+drug cue group compared to those in the drug cue only group, this finding was moderated by gender. Among participants randomized to the stress+drug cue group, women reported greater subjective stress than men. This finding is consistent with previous research indicating that women report greater emotional intensity at lower levels of HPA arousal (Fox et al., 2008). This finding is particularly concerning among individuals with opioid dependence, as women may be more likely than men to attribute use and relapse to negative emotional states (Back et al., 2011; Connors et al., 1998). Of interest, in a study of gender differences in response to CRF administration in cocaine dependence, high subjective stress ratings following CRF administration were positively associated with higher cocaine use in the month following testing (Back et al., 2010). It may be that women with opioid dependence experience stress more intensely than men with opioid dependence, and this stress may lead to increased use, thus increasing risk for overdose.
Despite the increased subjective stress, women did not demonstrate an increase in cortisol and DHEA response to the TSST consistent with a meta-analysis on gender differences post-TSST in cortisol reactivity (Liu et al., 2017). Men demonstrated increases in both cortisol and DHEA following the TSST. This finding suggests blunting of the normal HPA axis stress response in women with opioid dependence, consistent with the literature on cocaine and nicotine dependent individuals (Back et al., 2008; Brady et al., 2009). Back and colleagues (Back et al., 2010) reported that attenuated levels of ACTH and cortisol following the TSST in cocaine-dependent individuals was associated with significantly increased likelihood of use and a shorter time to relapse in the 30 days following testing. Similarly, several studies have demonstrated that blunted ACTH and cortisol responses to the laboratory paradigms in alcohol-dependent (Brady et al., 2006; Cooney et al., 1997; Junghanns et al., 2003) and nicotine-dependent individuals (al’Absi et al., 2005) is associated with relapse in the month following testing. Unfortunately, gender analysis was not conducted in any of these studies. The current study found that those in the stress+drug cue group used more in the week following the experiment compared to those in the drug cue only group. However, gender differences were not found. It is possible that the response to the TSST served as a mediator of the association between gender and post-experimental prescription opioid use, however, the sample size was too small to conduct this analysis. Nonetheless, this study combined with previous literature suggests that HPA axis dysregulation may be associated with vulnerability to relapse and the data from this and other studies suggest greater HPA axis dysregulation in women. Treatments targeting stress systems and HPA axis dysregulation may be particularly efficacious for women with substance use disorders.
DHEA is released from the adrenal gland with cortisol in response to stress, however DHEA exerts antiglucocorticoid and antiglutamatergic activity in the brain and may confer neuroprotection (Charney, 2004). When stress is chronic and cortisol levels remain high, this can have serious adverse effects on multiple physiologic systems (Ozbay et al., 2007). As such, DHEA may confer resilience to stress by helping to terminate HPA-activation and prevent harmful effects of prolonged exposure to glucocorticoids. Men in the stress+drug cue group had significantly higher levels of DHEA compared to men in the drug cue only group whereas women did not experience an increased DHEA response to the TSST. This may be further evidence for HPA dysregulation and vulnerability to adverse effects of stress in drug-dependent women as compared to men.
Finally, while the HR increased for both men and women following the TSST, the HR for women with opioid dependence remained elevated at 30 and 60 minutes post-stressor while men’s HR returned to baseline. HR elevations occur immediately after exposure to a stressor and are regulated the locus-coeruleus-noradrenergic (LC-NE) sympathetic nervous system, which is rapidly mobilized following stress. There is a robust literature suggesting increased sensitivity of the noradrenergic system in women with cocaine (McRae-Clark et al., 2017) and tobacco (McKee et al., 2015) use disorders as compared to men. For example, women with cocaine use disorder exhibit more physiologic response and anxiety when administered yohimbine, an agent that increases NE release (Moran-Santa Maria et al., 2014) and more diminution of craving and stress response following administration of an agent that decreases noradrenergic function (Fox et al., 2014). Similarly, women with tobacco use disorder show less craving and greater reduction in smoking following noradrenergic blocking agents as compared to men (Verplaetse et al., 2015). Although our sample size is small, these findings suggest increased noradrenergic sensitivity women as compared to men with opioid dependence. This warrants further investigation as this difference has clear implications for therapeutic development.
Women in the study did have significant differences from men that cannot be extrapolated from gender differences in stress response due to multicollinearity. Women were older, began opioid use at an older age, progressed to opioid dependence at a later age, reported more subjective stress prior to the experimental paradigms, and reported more traumatic experiences when compared to men. These sample differences may have contributed to differences in subjective stress and decreased neuroendocrine response after the stress paradigm. A review of the pre-clinical and human studies using non-drug dependent samples (Rasmusson et al., 2004) suggests the opposite, that individuals with traumatic event exposure have higher stress responsiveness due to long-term hyperactivity of CRF systems. This initial hyperactivity can result in a blunted stress response due to CRF hypersecretion (Heim and Nemeroff, 2001), and may explain the blunted cortisol response found among women in the current study despite higher subjective stress when compared to men. Previous research examining the association between traumatic event exposure and cortisol response to the TSST found similar results to the current study among non-drug-dependent women suggesting that individuals with prior traumatic event exposure (Carpenter et al., 2011) and those with posttraumatic stress disorder (Wichmann et al., 2017) experience a blunted cortisol response after the TSST. The blunted cortisol response related to traumatic event exposure mimicks that of potential HPA axis disruption among women with cocaine (Brady et al., 2009) and tobacco use (Back et al., 2008) disorders. Because HPA dysregulation is one possible mechanism explicating enhanced vulnerability to relapse in response to negative affect among women (Fox et al., 2008) and this blunted stress response may be related to both traumatic event exposure and opioid dependence among women, future research should be designed to differentiate the impact of trauma history from gender effects on stress responding in a larger sample of individuals with opioid dependence to best inform interventions.
Strengths and Limitations
This was the first study to examine gender differences in subjective stress and neuroendocrine response to a stress task among individuals with opioid dependence. These gender differences can potentially explicate the differences in vulnerability to opioid dependence and relapse based on gender. However, there are a number of limitations. First, the sample size is small and these findings should be interpreted as preliminary. In spite of the small sample size, significant gender differences with potential treatment implications were found. Future work is needed to examine subjective stress and neuroendocrine response to a stress task among a larger sample individuals with opioid dependence. Second, due to multicollinearity between gender and traumatic event exposure, it was not possible to tease out differences based on traumatic event exposure within the current participant pool. Future work should recruit an equal sample of men and women with and without traumatic event exposure to examine if the differences found in the current study are due to gender or traumatic event exposure. Finally, individuals were excluded if they were on opioid replacement therapy. Therefore, future research should examine differences between those on and not on opioid replacement therapy.
Conclusion
Although women with opioid dependence reported greater subjective stress to the TSST paradigm than men with opioid dependence, men had the expected post-stress increase in cortisol and DHEA, whereas for women this response was blunted. There was some evidence for increased noradrenergic sensitivity to stress in women as compared to men. These findings must be replicated in a larger sample, but they are consistent with findings from other studies in substance-using populations and have clear treatment implications.
Highlights.
Opioid dependence is a significant public health problem
We examined gender differences in vulnerability to opioid dependence
Women reported greater subjective stress to after a stress task than men
Women evidenced a blunted cortisol response to a stress task compared to men
Acknowledgments
Funding
Support for data collection and manuscript preparation came from the National Institute on Drug Abuse (K23DA021228; PI: Back; K23DA042935; PI: Gilmore; K23DA039318-01; PI: Guille; K23DA036566; PI: McCauley and U54DA01651 to support Mr. Baker). Manuscript preparation was also partially supported by the National Institute on Mental Health (T32MH018869 to support Dr. Hahn).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- al’Absi M, Hatsukami D, Davis GL, 2005. Attenuated adrenocorticotropic responses to psychological stress are associated with early smoking relapse. Psychopharmacology 181, 107–117. [DOI] [PubMed] [Google Scholar]
- Association, A.P., 2000. DSM-IV-TR: Diagnostic and statistical manual of mental disorders, text revision. American Psychiatric Association 75, 78–85. [Google Scholar]
- Back SE, Gros DF, Price M, LaRowe S, Flanagan J, Brady KT, Davis C, Jaconis M, McCauley JL, 2015. Laboratory-induced stress and craving among individuals with prescription opioid dependence. Drug & Alcohol Dependence 155, 60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back SE, Payne RL, Simpson AN, Brady KT, 2010. Gender and prescription opioids: findings from the national survey on drug use and health. Addictive Behaviors 35, 1001–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back SE, Payne RL, Wahlquist AH, Carter RE, Stroud Z, Haynes L, Hillhouse M, Brady KT, Ling W, 2011. Comparative profiles of men and women with opioid dependence: results from a national multisite effectiveness trial. The American Journal of Drug and Alcohol Abuse 37, 313–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back SE, Waldrop AE, Saladin ME, Yeatts SD, Simpson A, McRae AL, Upadhyaya HP, Sisson RC, Spratt EG, Allen J, 2008. Effects of gender and cigarette smoking on reactivity to psychological and pharmacological stress provocation. Psychoneuroendocrinology 33, 560–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, Wiersielis KR, Khantsis S, 2016. Sex differences in the locus coeruleus-norepinephrine system and its regulation by stress. Brain Research 1641, 177–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady KT, Back SE, Waldrop AE, McRae AL, Anton RF, Upadhyaya HP, Saladin ME, Randall PK, 2006. Cold pressor task reactivity: Predictors of alcohol use among alcohol-dependent individuals with and without comorbid posttraumatic stress disorder. Alcoholism: Clinical and Experimental Research 30, 938–946. [DOI] [PubMed] [Google Scholar]
- Brady KT, Grice DE, Dustan L, Randall C, 1993. Gender differences in substance use disorders. The American Journal of Psychiatry 150, 1707. [DOI] [PubMed] [Google Scholar]
- Brady KT, McRae AL, Moran-Santa Maria MM, DeSantis SM, Simpson AN, Waldrop AE, Back SE, Kreek MJ, 2009. Response to corticotropin-releasing hormone infusion in cocaine-dependent individuals. Archives of General Psychiatry 66, 422–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady KT, Randall CL, 1999. Gender differences in substance use disorders. Psychiatric Clinics 22, 241–252. [DOI] [PubMed] [Google Scholar]
- Carpenter LL, Shattuck TT, Tyrka AR, Geracioti TD, Price LH, 2011. Effect of childhood physical abuse on cortisol stress response. Psychopharmacology 214, 367–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charney DS, 2004. Psychobiological mechanisms of resilience and vulnerability. Focus. [DOI] [PubMed] [Google Scholar]
- Childress AR, McLellan AT, O'Brien CP, 1986. Conditioned responses in a methadone population: A comparison of laboratory, clinic, and natural settings. Journal of Substance Abuse Treatment 3, 173–179. [DOI] [PubMed] [Google Scholar]
- Connors GJ, Maisto SA, Zywiak WH, 1998. Male and female alcoholics' attributions regarding the onset and termination of relapses and the maintenance of abstinence. Journal of Substance Abuse 10, 27–42. [DOI] [PubMed] [Google Scholar]
- Cooney NL, Litt MD, Morse PA, Bauer LO, Gaupp L, 1997. Alcohol cue reactivity, negative-mood reactivity, and relapse in treated alcoholic men. Journal of Abnormal Psychology 106, 243. [DOI] [PubMed] [Google Scholar]
- Curtis AL, Bello NT, Valentino RJ, 2001. Evidence for functional release of endogenous opioids in the locus ceruleus during stress termination. Journal of Neuroscience 21, RC152–RC152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis AL, Bethea T, Valentino RJ, 2006. Sexually dimorphic responses of the brain norepinephrine system to stress and corticotropin-releasing factor. Neuropsychopharmacology 31, 544. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer RL, Gibbon M, Williams JB, 2002. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition Biometrics Research. New York State Psychiatric Institute, New York. [Google Scholar]
- Fox HC, Hong KA, Paliwal P, Morgan PT, Sinha R, 2008. Altered levels of sex and stress steroid hormones assessed daily over a 28-day cycle in early abstinent cocaine-dependent females. Psychopharmacology 195, 527–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox HC, Morgan PT, Sinha R, 2014. Sex differences in guanfacine effects on drug craving and stress arousal in cocaine-dependent individuals. Neuropsychopharmacology 39, 1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield SF, Back SE, Lawson K, Brady KT, 2010. Substance abuse in women. Psychiatric Clinics, 33, 339–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han B, Compton WM, Blanco C, Crane E, Lee J, Jones CM, 2017. Prescription opioid use, misuse, and use disorders in US adults: 2015 national survey on drug use and health. Annals of Internal Medicine 167, 293–301. [DOI] [PubMed] [Google Scholar]
- Heim C, Nemeroff CB, 2001. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biological Psychiatry 49, 1023–1039. [DOI] [PubMed] [Google Scholar]
- Jones CM, 2017. The paradox of decreasing nonmedical opioid analgesic use and increasing abuse or dependence - an assessment of demographic and substance use trends, United States, 2003–2014. Addictive Behaviors 65, 229–235. [DOI] [PubMed] [Google Scholar]
- Jones CM, Logan J, Gladden RM, Bohm MK, 2015. Vital signs: demographic and substance use trends among heroin users - United States, 2002-2013. Morbidity and Mortality Weekly Report 64, 719–725. [PMC free article] [PubMed] [Google Scholar]
- Junghanns K, Backhaus J, Tietz U, Lange W, Bernzen J, Wetterling T, Rink L, Driessen M, 2003. Impaired serum cortisol stress response is a predictor of early relapse. Alcohol and Alcoholism 38, 189–193. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke K-M, Hellhammer DH, 1993. The ‘Trier Social Stress Test’–a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology 28, 76–81. [DOI] [PubMed] [Google Scholar]
- Liu JJ, Ein N, Peck K, Huang V, Pruessner JC, Vickers K, 2017. Sex differences in salivary cortisol reactivity to the Trier Social Stress Test (TSST): A meta-analysis. Psychoneuroendocrinology 82, 26–37. [DOI] [PubMed] [Google Scholar]
- Marsh JC, Park K, Lin Y-A, Bersamira C, 2018. Gender differences in trends for heroin use and nonmedical prescription opioid use, 2007–2014. Journal of Substance Abuse Treatment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, Potenza MN, Kober H, Sofuoglu M, Arnsten AF, Picciotto MR, Weinberger AH, Ashare R, Sinha R, 2015. A translational investigation targeting stress-reactivity and prefrontal cognitive control with guanfacine for smoking cessation. Journal of psychopharmacology 29, 300–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae-Clark AL, Cason AM, Kohtz AS, Moran Santa-Maria M, Aston-Jones G, Brady KT, 2017. Impact of gender on corticotropin-releasing factor and noradrenergic sensitivity in cocaine use disorder. Journal of Neuroscience Research 95, 320–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran-Santa Maria MM, McRae-Clark A, Baker NL, Ramakrishnan V, Brady KT, 2014. Yohimbine administration and cue-reactivity in cocaine-dependent individuals. Psychopharmacology 231, 4157–4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris FH, Hamblen JL, 2004. Standardized self-report measures of civilian trauma and PTSD. Assessing Psychological Trauma and PTSD 2, 63–102. [Google Scholar]
- Ozbay F, Johnson DC, Dimoulas E, Morgan III C, Charney D, Southwick S, 2007. Social support and resilience to stress: from neurobiology to clinical practice. Psychiatry (Edgmont) 4, 35. [PMC free article] [PubMed] [Google Scholar]
- Patterson HD, Thompson R, 1971. Recovery of inter-block information when block sizes are unequal. Biometrika 58, 545–554. [Google Scholar]
- Prevention, C.f.D.C.a., 2013. Vital signs: overdoses of prescription opioid pain relievers and other drugs among women--United States, 1999-2010. Morbidity and Mortality Weekly Report 62, 537. [PMC free article] [PubMed] [Google Scholar]
- Rasmusson AM, Vasek J, Lipschitz DS, Vojvoda D, Mustone ME, Shi Q, Gudmundsen G, Morgan CA, Wolfe J, Charney DS, 2004. An increased capacity for adrenal DHEA release is associated with decreased avoidance and negative mood symptoms in women with PTSD. Neuropsychopharmacology 29, 1546. [DOI] [PubMed] [Google Scholar]
- Schluger JH, Bart G, Green M, Ho A, Kreek MJ, 2003. Corticotropin-releasing factor testing reveals a dose-dependent difference in methadone maintained vs control subjects. Neuropsychopharmacology 28, 985. [DOI] [PubMed] [Google Scholar]
- Sheehan D, Janavs J, Baker R, Harnett-Sheehan K, Knapp E, Sheehan M, Lecrubier Y, Weiller E, Hergueta T, Amorim P, 1998. MINI-mini international neuropsychiatric interview-english version 5.0. 0-DSM-IV. Journal of Clinical psychiatry 59, 34–57. [PubMed] [Google Scholar]
- Sinha R, 2001. How does stress increase risk of drug abuse and relapse? Psychopharmacology 158, 343–359. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB, 1992. Timeline follow-back, Measuring Alcohol Consumption. Springer, pp. 41–72. [Google Scholar]
- Spielberger CD, 2010. State-trait anxiety inventory. Wiley Online Library. [Google Scholar]
- Valentino R, Rudoy C, Saunders A, Liu X-B, Van Bockstaele E, 2001. Corticotropin-releasing factor is preferentially colocalized with excitatory rather than inhibitory amino acids in axon terminals in the peri-locus coeruleus region. Neuroscience 106, 375–384. [DOI] [PubMed] [Google Scholar]
- Verplaetse TL, Weinberger AH, Smith PH, Cosgrove KP, Mineur YS, Picciotto MR, Mazure CM, McKee SA, 2015. Targeting the noradrenergic system for gender-sensitive medication development for tobacco dependence. Nicotine & Tobacco Research 17, 486–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L, Lachin JM, 1988. Properties of the urn randomization in clinical trials. Controlled Clinical Trials 9, 345–364. [DOI] [PubMed] [Google Scholar]
- Wichmann S, Kirschbaum C, Bohme C, Petrowski K, 2017. Cortisol stress response in posttraumatic stress disorder, panic disorder, and major depressive disorder patients. Psychoneuroendocrinology 83, 135–141. [DOI] [PubMed] [Google Scholar]
- Wolfe J, Kimerling R, 1997. Life Stressor Checklist - Revised. National Center for Posttraumatic Stress Disorder. [Google Scholar]
- Xu M, Petraschka M, McLaughlin JP, Westenbroek RE, Caron MG, Lefkowitz RJ, Czyzyk TA, Pintar JE, Terman GW, Chavkin C, 2004. Neuropathic pain activates the endogenous κ opioid system in mouse spinal cord and induces opioid receptor tolerance. Journal of Neuroscience 24, 4576–4584. [DOI] [PMC free article] [PubMed] [Google Scholar]