Abstract
Thioredoxin (Trx), a redox enzyme with a conserved active site (Cys-32–Gly–Pro–Cys-35), is induced and secreted into circulation in response to inflammation. Studies here demonstrate that elevating Trx levels in circulation either by i.v. injection of recombinant Trx or stimulating Trx release in Trx-transgenic mice dramatically blocks lipopolysaccharide (LPS)-stimulated neutrophil migration in the murine air pouch chemotaxis model. Furthermore, we show that leukocyte recruitment induced by the murine chemokines KC/GROα, RANTES (regulated upon activation, normal T cell expressed and secreted), and monocyte chemoattractant protein-1 (MCP-1) is suppressed also in Trx-transgenic mice. Addressing the mechanism responsible for this suppression, we show that circulating Trx blocks (i) the LPS-stimulated in vitro activation of neutrophil p38 mitogen-activated protein kinase, (ii) the normal down-regulation of CD62L on neutrophils migrating into the LPS-stimulated air pouch, and (iii) the in vitro adhesion of LPS-activated neutrophils on endothelial cells. However, as we also show, Trx does not alter the expression of endothelial cell adhesion molecules (intercellular adhesion molecule-1, vascular cell adhesion molecule-1, CD62P, and CD62E) within 3 h. Collectively, these findings indicate that elevated levels of circulating Trx interfere with chemotaxis by acting directly on neutrophils. We discuss these findings in the context of recent studies reporting beneficial effects of acutely elevated Trx in ischemic injury and negative effects associated with chronically elevated Trx in HIV disease.
Thioredoxin (Trx), a small (12-kDa), well characterized protein with a highly conserved active site (Cys-32–Gly–Pro–Cys-35), plays a variety of redox-related roles in organisms ranging from Escherichia coli to man (1). Intracellular Trx, together partly with peroxiredoxin (2), plays crucial roles in the scavenging of reactive oxygen species and the regulation of redox-sensitive transcription factors including activator protein-1 and nuclear factor-κB (3, 4). In addition, it plays key roles in the regulation of glucocorticoid receptor-mediated signal transduction and thus in the host defense against reactive oxygen species-mediated inflammation (5–7).
In addition to its intracellular functions, Trx is released by cells and has been shown to have several cytokine and chemokine-like activities. In fact, human Trx was cloned originally as an immunologically active “factor” released in vivo and in vitro by human T cell leukemia virus type I-transformed cells (8) and as an autocrine growth factor produced by Epstein–Barr virus (EBV)-transformed B cells (9). A series of studies demonstrate the cytokine-like activities of the intact Trx protein (10, 11) and, most recently, of the truncated form of Trx (Trx-80), which has been shown to activate monocytes and to be selectively mitogenic for lymphocytes (12, 13).
Trx is released from cells in response to oxidative stress (14, 15). In HIV disease, plasma Trx levels are chronically elevated in a subset of subjects (16) and, when elevated, are associated with poor prognosis in subjects with CD4 T cell counts below 200/μl of blood (15). Chronically elevated plasma Trx levels also have been detected in hepatitis C virus infection, where the elevated levels are associated with decreased responsiveness to IFN-α therapy (17). In contrast, beneficial effects of acute plasma Trx elevation caused by injection of recombinant human Trx have been demonstrated in ischemic reperfusion injury (18, 19) and other situations in which tissue damage results from neutrophil invasion and oxidant production.
Studies reported here, in which we document the role of circulating Trx in regulating the neutrophil extravasation into inflammatory sites, grow from the recent demonstration that locally administered Trx is a potent chemoattractant for neutrophils, monocytes, and lymphocytes in the mouse air pouch chemotaxis model (20). This chemokine-like activity of Trx suggests that circulating Trx might inhibit chemotaxis, because previous studies have demonstrated that pretreatment with chemokines blocks chemotaxis in in vitro assays (21, 22) and that, importantly, injection of IL-8 in rabbits (23, 24) also inhibits local chemotaxis induction. We confirm this hypothesis in a recent report that focuses primarily on Trx elevation in HIV disease but also includes our initial studies showing that i.v. injection of recombinant human Trx blocks lipopolysaccharide (LPS)-stimulated neutrophil chemotaxis in the mouse air pouch model (15).
Here we continue the characterization of this suppression and the mechanisms that mediate it. Thus, we show that (i) elevated plasma Trx levels raised either by i.v. injection of human recombinant Trx into wild-type mice or by stimulation of Trx release in mice that express a human Trx transgene inhibits neutrophil extravasation into the LPS-stimulated air pouch, (ii) Trx directly blocks the in vitro adhesion of LPS-stimulated neutrophils on endothelial cells, and (iii) circulating Trx suppresses the activation of p38 mitogen-activated protein kinase (MAPK) in LPS-stimulated neutrophils and prevents the normal down-regulation of CD62L on neutrophils that migrate into the LPS-stimulated air pouch.
We discuss these studies in the context of the beneficial effects mediated by acutely elevated plasma Trx in ischemia reperfusion injury and interstitial pneumonia and the potentially negative effects mediated by chronic elevation of plasma Trx in HIV and other diseases. In essence, we propose that in both cases the underlying mechanism traces to a physiologically significant inhibition of neutrophil chemotaxis by the circulating Trx.
Methods
Reagents.
LPS from E. coli was purchased from Sigma. Recombinant murine KC, monocyte chemoattractant protein-1 (JE), and RANTES (regulated upon activation, normal T cell expressed and secreted) were obtained from PeproTech Ltd. (London). Human recombinant Trx and C32S/C35S mutant Trx, in which two cysteines at positions 32 and 35 in the active site were replaced with serines, were prepared as described previously (25) and provided by Ajinomoto (Kawasaki, Japan). Anti-human Trx antibody (mouse monoclonal IgG1) was provided by Fuji Rebio. Anti-mouse neutrophil 7/4 antibody recognizing a polymorphic 40-kDa antigen expressed by polymorphonuclear cells was from Serotec. Anti-CD3 and anti-CD45R antibodies were from Immunotech (Luminy, France). FITC-mouse IgG1, anti-mouse CD62L antibody, and anti-human intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), CD62P, and CD62E antibodies were from PharMingen.
Chemotaxis in Vivo (Air Pouch Model).
Animal experiments adhered to the Declaration of Helsinki. A dorsal air pouch was created as described previously by injecting male 7–9-week-old BALB/c or C57BL/6 mice with 4 ml of air s.c. at days −7 and −3. On day 0, 1 μg of LPS in 1 ml of sterile pyrogen-free saline was injected into the air pouch. Four hours later, the air pouch was flushed quantitatively with PBS, the recovered volume was measured, and the number of recovered cells was determined by hemocytometer count. The frequency of neutrophils, lymphocytes, and other leukocyte subsets was determined by flow cytometry.
Flow Cytometry.
Cells were incubated for 30 min at 4°C with saturating concentrations of the indicated mouse monoclonal antibody labeled either with FITC or phycoerythrin. Cells then were washed and resuspended in PBS, and the cell-associated light scatter and fluorescence were determined with a FACScaliber instrument (Becton Dickinson).
ELISA for Trx.
Blood levels of human Trx in mice were measured by sandwich ELISA as described previously (16). The monoclonal antibodies used to detect human Trx in this ELISA do not crossreact with mouse Trx. Therefore, endogenously produced murine Trx or murine Trx introduced by hemolysis does not influence measurement of human Trx levels in mouse blood.
Detection of p38 MAPK.
Human neutrophils were prepared from whole venous blood by Mono-Poly resolving medium (Dainippon Pharmaceutical Co. Ltd., Osaka, Japan). Neutrophils were preincubated with control PBS, 10 μg/ml recombinant wild-type Trx, or 10 μg/ml C32S/C35S mutant for 30 min at 37°C and then incubated in the absence or presence of 1 μg/ml LPS for 15 min at 37°C. The cells were lysed in lysis buffer containing 2% SDS/10% glycerol/50 mM DTT/0.1% bromophenol blue in 62.5 mM Tris⋅HCl, pH 6.8, and separated by SDS/PAGE. p38 MAPK and phosphorylated p38 MAPK were detected by Western blotting using anti-p38 MAPK (Cell Signaling Technology, Beverly, MA) and anti-phosphorylated p38 MAPK (New England Biolabs) antibodies. After electrophoresis, the proteins were transferred to a polyvinylidene difluoride membrane (Millipore). The membrane was incubated in blocking buffer containing 10% skim milk/0.05% Tween 20 in PBS followed by incubation with a primary antibody against either p38 MAPK or phosphorylated p38 MAPK. The membrane then was incubated with the respective secondary antibody conjugated with horseradish peroxidase, and the protein of interest was visualized by enhanced chemiluminescence (ECL Western blot detection kit, Amersham Pharmacia Biotech).
Neutrophil Adherence to Endothelial Cells.
Human umbilical vein endothelial cells (HUVECs, BioWhittaker) were maintained with endothelial cell basal medium (EBM-2, BioWhittaker) supplemented with EGM-2 (Clonetics Human Cell Systems, BioWhittaker) in a humidified atmosphere of 5% CO2/95% air at 37°C. For the adherence assay, an endothelial cell monolayer was established by culturing 1 × 105 HUVECs per ml for 4 h. Neutrophils (5 × 106 per ml) then were introduced and allowed to adhere for 4 h at 37°C, after which the floating cells were decanted, the plate was washed with PBS, and the monolayer and adherent cells were photographed with an Olympus IX70 photomicrography system. To test for inhibition of neutrophil adherence, recombinant Trx or mutant Trx was introduced into the culture before addition of the neutrophils.
Adhesion Assay.
A cell adhesion assay was performed as described previously (26) with some modification. Human neutrophils were labeled with 5 μM 2′,7′-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein acetoxymethyl ester (Molecular Probes), which was prepared as a 1 mM stock in DMSO, for 1 h at 37°C. After washing with PBS twice, 1 × 106 labeled neutrophils per ml were incubated with or without 1 μg/ml of LPS for 3 h and then cocultured with a HUVEC monolayer pretreated with or without 1 μg/ml of LPS in a 96-microwell plate for 1 h in a humidified atmosphere of 5% CO2/95% air at 37°C. Recombinant Trx or mutant recombinant Trx (C32S/C35S) was added when the coculture was started. After the coculture, the plate was washed with PBS three times by using a multipipette with wide, open tips. The fluorescence intensity was measured by using a fluorescence microplate reader (Spectra Fluora, Wako, Japan) at the excitation and emission wavelengths of 485 and 530 nm, respectively. Background fluorescence was measured for each plate and subtracted, and 1 × 106 labeled neutrophils per ml was totally lysed with 1% Nonidet P-40, with PBS used for sequential standard.
Results
LPS Induces Leukocyte Recruitment in the Air Pouch Chemotaxis Model.
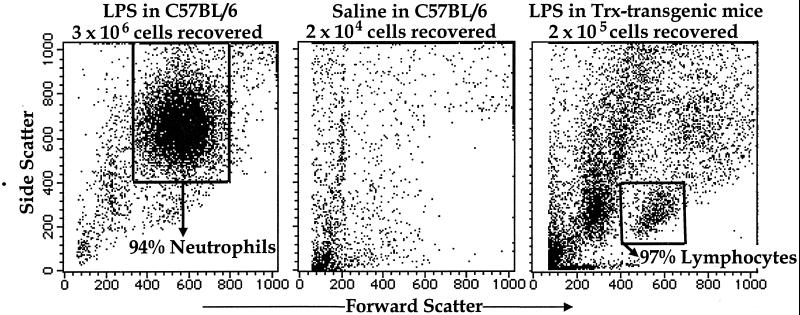
In wild-type C57BL/6 mice, the introduction of 1 μg of LPS suspended in 1 ml of pyrogen-free saline into a previously created air pouch on the animal's back induces rapid recruitment of leukocytes into the pouch. Roughly 3 × 106 leukocytes are recovered from the pouch 4 h after LPS is introduced (Fig. 1 Left), whereas only 2 × 104 cells are recovered from control pouches injected with only the saline carrier (Fig. 1 Middle). Most of the LPS-recruited leukocytes are neutrophils (accompanied by a small proportion of lymphocytes), whereas virtually no neutrophils are recovered from the control pouches (Fig. 1 Left and Middle).
Figure 1.
Four hours after LPS injection in the air pouch, neutrophils predominate among cells recovered from C57BL/6 (wild type, Left), whereas very few cells (2 × 104 cells) were collected after saline injection (Middle). Cells (2 × 105) were recovered from the Trx-transgenic air pouch where lymphocytes predominate among viable cells recovered (Right). FACS analyses are shown for 10,000 cells in each case; forward scatter units are shown for the x axis, and side scatter units are shown for the y axis.
LPS-Induced Leukocyte Recruitment Is Suppressed in Trx-Transgenic Mice.
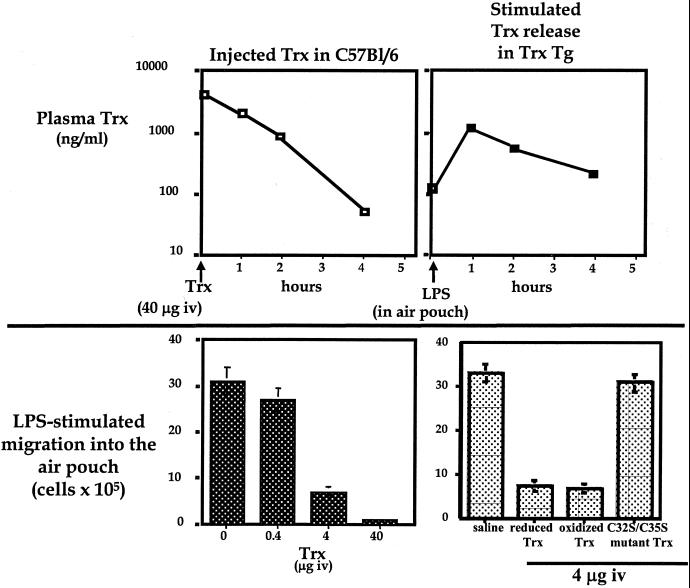
Trx-transgenic mice carry a human Trx transgene introduced into C57BL/6 animals under control of the β-actin promoter. The human Trx protein encoded by this transgene is functionally equivalent to murine Trx (27). Intracellular levels of the human Trx are substantially higher than endogenous (murine) intracellular Trx levels in the transgenic mice. However, despite this overexpression, human Trx is only detectable marginally in blood (<120 ng/ml) in untreated mice (Fig. 2 Upper Right). Injection of LPS into air pouches on the Trx-transgenic mice induces rapid release of human Trx into circulation and hence a rapid rise in Trx blood levels. One hour after LPS injection, blood levels of Trx in the Trx-transgenic mice reach ≈1 μg/ml. Even 4 h after LPS injection Trx blood levels are still 320 ng/ml (Fig. 2 Upper Right). This rapid and relatively prolonged increase in Trx blood levels is accompanied by severe inhibition of the recruitment of leukocytes into the pouch. Less than 7% of the cells recovered in LPS-injected pouches on wild-type animals are recovered from the pouches on the Trx-transgenic mice (Fig. 1 Right). Most of this decrease is caused by inhibition of neutrophil recruitment, which reaches only 5% of the recruitment level in wild-type C57BL/6. Lymphocyte recruitment, in contrast, is much less inhibited (Fig. 1 Left and Right).
Figure 2.
When C57BL/6 mouse was i.v. injected with 40 μg of human recombinant Trx, blood levels of human Trx were measured by ELISA at the indicated times. Similar results were obtained in three mice (Upper Left). Trx blood levels measured by ELISA after i.v. injection of 1 μg of LPS in the air pouch in Trx-transgenic mice reveal rapid release of Trx into circulation (Upper Right). Air pouches were injected with LPS immediately after animals were i.v. injected with the indicated amount of human recombinant Trx. The bars show the numbers of leukocytes recovered from the LPS-injected air pouches 4 h later (Lower Left). Numbers of infiltrated cells induced by LPS were decreased by both reduced and oxidized recombinant wild-type Trx but not C32S/C35S mutant (Lower Right). Reduced recombinant Trx was prepared by incubation with DTT at 37°C for 30 min, and excess DTT was removed by a Sephadex G-25 column (NAP-5 column, Amersham Pharmacia Biotech). Oxidized recombinant Trx was prepared by air-bubbling on ice for 30 min. The redox status of Trx was determined by 5,5′-dithiobis(2-nitrobenzoic acid) assay. The sulfhydryl residues per molecule measured by the DTNB assay were 4.9 and 2.9 in reduced and oxidized Trx, respectively. Oxidized or reduced Trx was i.v. injected just before LPS injection in the air pouch. Four hours later infiltrated cells were collected. Infiltrated cell numbers are shown as the mean ± SD (Lower Right).
Injection of Trx Blocks Leukocyte Recruitment.
Injection of 40 μg of Trx i.v. raises circulating Trx levels to levels comparable to those in LPS-injected Trx-transgenic mice (Fig. 2 Upper Left). The half-life of Trx in the blood calculated from the observed decay in Trx levels is ≈1 h.
When the Trx is injected just before introduction of LPS into the air pouch, the total numbers of LPS-recruited leukocytes decrease in proportion to the amount of Trx injected: injection of 0.4 μg of Trx minimally decreases recruitment; injection of 4 μg decreases recruitment by nearly 80%; and injection of 40 μg virtually completely inhibits recruitment (Fig. 2 Lower Left). Thus, exogenously introduced Trx in wild-type mice behaves identically to the internally produced human Trx that is released into blood in the transgenic mice. In both cases, the elevated levels of circulating Trx strongly inhibit LPS-induced chemotaxis.
Both oxidized Trx (Trx-S2, actually the oxidized form of the Trx active site) and reduced Trx (Trx-(sulfhydryl)2) inhibit chemotaxis equally when injected into animals. In contrast, redox-inactive recombinant C32S/C35S mutant Trx, in which two cysteines in the active site were replaced with serines, did not inhibit LPS-induced chemotaxis (Fig. 2 Lower Right). Because reduced Trx tends to be oxidized rapidly in circulation, it is likely that oxidized Trx is the active mediator of the Trx chemotaxis inhibition.
Trx Suppresses p38 MAPK Activation in Neutrophils.
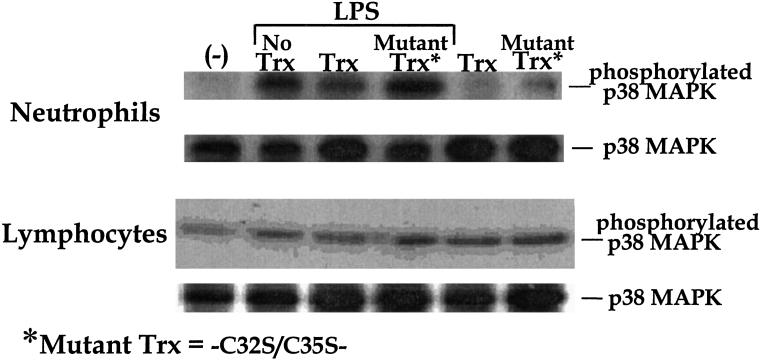
Incubating human neutrophils with 1 μg/ml LPS for 15 min increases the levels of phosphorylated p38 MAPK detectable in cell lysates. Preincubation of the neutrophils with recombinant wild-type Trx suppresses this phosphorylation, whereas preincubation with C32S/C35S mutant Trx results in a slight enhancement (Fig. 3). In contrast, incubating lymphocytes with LPS does not dramatically increase p38 MAPK phosphorylation, and there is no significant effect of Trx preincubation (Fig. 3).
Figure 3.
Human neutrophils and lymphocytes were preincubated with 10 μg/ml recombinant wild type Trx or C32S/C35S mutant at 37°C for 30 min and then treated with 1 μg/ml LPS at 37°C for 1 h. Recombinant Trx but not C32S/C35S mutant suppresses the phosphorylation of p38 MAPK in LPS-stimulated neutrophils.
Trx Selectively Suppresses the LPS-Induced Down-Regulation of CD62L on Neutrophils.
In LPS-stimulated animals, CD62L (L-selectin) is down-regulated and shed from neutrophils just before they start rolling on the vessel wall preparative to adhering to the endothelial cells, although it is not down-regulated markedly on circulating neutrophils (28–30). In the air pouch model, CD62L is markedly lower on the neutrophils that enter the pouch by LPS stimulation (Fig. 4 Top). However, when circulating Trx is present, this LPS-induced CD62L down-regulation is inhibited, as is migration into the pouch (Fig. 4). Thus, the few neutrophils that succeed in entering the pouch when Trx is elevated in circulation show substantially more CD62L than the uninhibited migrants that enter the pouch in the absence of circulating Trx.
Figure 4.
CD62L expression on neutrophils found in the air pouch when sterile saline was injected (Bottom) or recruited when LPS was injected into the pouch in C57/BL6 mice (Top). The middle panels show CD62L expression on neutrophils recovered from LPS-injected air pouches in Trx-transgenic mice (Lower Middle) or in mice pretreated by i.v. injection of 40 μg of Trx (Upper Middle). Leukocytes (10,000) gated in a neutrophil region similar to that in Fig. 1 (Left) were analyzed.
In contrast, the expression of CD11b/CD18 on infiltrated neutrophils in the pouch is up-regulated in LPS-stimulated animals but is not influenced by elevated Trx either in Trx-injected or Trx-transgenic mice stimulated to release Trx into circulation (data not shown).
Trx Inhibits in Vitro Adhesion of Neutrophils on Endothelial Cells.
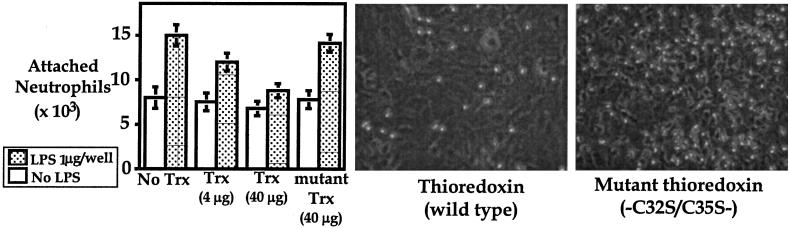
When human leukocytes are activated in vitro by LPS, they readily adhere to monolayers of HUVECs. This adhesion is inhibited by the addition of 40 μg/ml recombinant Trx but not mutant recombinant Trx (C32S/C35S) to the culture just before the addition of the leukocytes (Fig. 5). Thus, the site that mediates the classical Trx redox functions is necessary for the inhibition of leukocyte adhesion. This inhibition is not caused by an alteration of the expression of adhesion molecules such as ICAM-1, VCAM-1, E-selectin, and P-selectin, which are expressed on the endothelial cells and known to play important roles in the adherence of neutrophils (Table 1).
Figure 5.
Recombinant Trx, but not C32S/C35S mutant Trx, suppresses the adhesion of neutrophils on HUVEC by adhesion assay using 2′,7′-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein acetoxymethyl ester (Left). Shown are photographs of neutrophils adhering on endothelial cells. The adhesion of LPS-activated human neutrophils on HUVECs is inhibited by the administration of Trx (Middle) but not by C32S/C35S mutant Trx (Right).
Table 1.
Expressions of adhesion molecules on endothelial cells
| ICAM-1 | VCAM-1 | CD62P | CD62E | |
|---|---|---|---|---|
| No treatment | 72 | 3.8 | 4.1 | 7.8 |
| LPS | 93 | 16.8 | 8.8 | 16.8 |
| Trx | 75 | 9.4 | 6.5 | 10.3 |
| LPS + Trx | 91 | 15.4 | 10.3 | 21.6 |
HUVECs were precultured in the absence or presence of LPS and Trx for 3 h. Expressions of ICAM-1, VCAM-1, CD62P (P-selectin), and CD62E (E-selectin) were analyzed by flow cytometry. The mean fluorescence intensity of each is shown. Similar results were obtained in three different experiments.
Leukocyte Recruitment Induced by KC, Monocyte Chemoattractant Protein-1 (MCP-1), and RANTES Is Suppressed in Trx-Transgenic Mice.
Similar to LPS, murine chemokines (KC, MCP-1, or RANTES) stimulate chemotaxis in the air pouch model in C57BL/6 mice. Similarly, as with LPS stimulation, chemotaxis by the murine chemokines is inhibited dramatically in the Trx-transgenic mice (Table 2). Of the three chemokines, KC is the most chemotactic for neutrophils and is the most inhibited in the transgenic mice. This inhibition of classical chemokine activity is consistent with the known ability of LPS to stimulate production of multiple chemokines. Thus, these findings validate the Trx inhibition of chemotaxis in the LPS model used for most of our studies and show that the inhibition reflects a broad regulation of chemokine activity by elevated levels of circulating Trx.
Table 2.
Numbers of infiltrated cells in the air pouch by murine chemokines KC, MCP-1, or RANTES in C57BL/6 (wild-type) and Trx-transgenic mice
| KC, 100 ng/ml | MCP-1, 100 ng/ml | RANTES, 1 ng/ml | |
|---|---|---|---|
| C57BL/6 (wild type) | 8.8 ± 1.8 | 0.9 ± 0.2 | 5.0 ± 0.5 |
| Trx-transgenic | 1.0 ± 0.1 | 0.4 ± 0.2 | 1.0 ± 0.5 |
Infiltrated cell numbers (× 105) were shown as the mean ± SD.
Discussion
The studies presented here demonstrate that elevated levels of Trx in circulation, caused by either stimulated release of Trx in Trx-transgenic mice or to i.v. injection of Trx, block chemotaxis induced by LPS or chemokines in a standard chemotaxis (air pouch) model. This finding is consistent with the recent demonstration that Trx is itself chemotactic for neutrophils, macrophages, and lymphocytes (20), because previous studies have shown that elevated levels of a classical chemokine such as IL-8 blocks the extravasation of leukocytes into inflammatory sites (31). Thus, Trx takes its place next to classical chemokines, both with respect to the ability to recruit leukocytes when injected locally and the ability, as a circulating molecule, to inhibit leukocyte recruitment by other chemotactic agents.
We have shown also that circulating Trx inhibits the LPS-induced neutrophil recruitment more dramatically than lymphocyte recruitment. Furthermore, we have shown that Trx inhibits chemotaxis induced by murine chemokine KC, which primarily recruits neutrophils into the air pouch, more dramatically than chemotaxis induced by MCP-1 or RANTES, which tend to recruit more lymphocytes than neutrophils. Thus, we have demonstrated clearly that circulating Trx is more effective in controlling neutrophil recruitment to inflammatory sites than it is for controlling lymphocyte recruitment.
The mechanism(s) responsible for this differential recruitment and its selective inhibition have yet to be elucidated fully. We have presented in vitro data showing that Trx inhibits the LPS-induced activation of p38 MAPK in neutrophils but not in lymphocytes. Activation of p38 MAPK is one of the initial events in the intracellular signal transduction in LPS-activated neutrophils. Although previous studies have shown that intracellular overexpression of Trx suppresses the activation of p38 MAPK (32), the studies presented here show that exogenous Trx suppresses intracellular activation of p38 MAPK. The demonstration of this activation in neutrophils and its suppression by Trx introduces a key mechanism through which Trx can selectively inhibit LPS-induced neutrophil extravasation.
The demonstration that circulating Trx inhibits down-regulation of the CD62L, which occurs in the absence of Trx as neutrophils migrate into the LPS-stimulated air pouch, is consistent with this idea. Importantly, CD62 down-regulation may be mediated wholly or in part by the suppression of p38 MAPK activation (33). Furthermore, the Trx-mediated down-regulation of neutrophil CD62L in the absence of Trx-mediated alteration in the expression of endothelial cell adhesion molecules known to be involved in neutrophil extravasation, i.e., ICAM-1, VCAM-1, CD62P (P-selectin), and CD62E (E-selectin; ref. 34), indicates a selective Trx effect on neutrophil physiology. Thus, the available evidence collectively supports the idea that circulating Trx selectively interferes with neutrophil extravasation at sites of inflammation.
The recognition that circulating Trx can block neutrophil invasion in inflammation has sharp implications in the medical arena. Administration (i.v.) of human recombinant Trx has been shown to attenuate ischemia-reperfusion injury in rats, rabbits, and dogs (18, 19). Similarly, focal ischemic brain damage is decreased in the Trx-transgenic mice studied here (27), and resistance to autoimmune-induced and streptozotocin diabetes is increased in Trx-transgenic mice that specifically express Trx under control of the insulin promoter in pancreatic β cells (35). Moreover, in lung fibrosis induced by bleomycin and interstitial pneumonia induced by daily cytokine injection, leukocyte infiltration into the interstitial space is attenuated dramatically in Trx-transgenic mice or in mice injected with recombinant Trx (T. Hoshino, M. Nakamura, M. Okamoto, S. Araya, O. Shimazato, H. Young, K. Oizumi, and J. Yodoi, unpublished observation). A similar observation was reported in transgenic mice expressing an acute phase protein, C-reactive protein, where elevated plasma C-reactive protein suppresses infiltration of neutrophil into bronchoalveolar lavage fluid and alveolitis (36).
The beneficial effects of Trx in these models were ascribed initially to the ability of Trx to relieve oxidative stress under the assumption that the reduced form of Trx was the active agent. However, more recent data (37) indicates that Trx, whether injected or released from cells, is oxidized rapidly in circulation and therefore must be acting in a capacity other than as a reductant. Thus, it is more likely that the protective effects of Trx in inflammatory situations reflect the ability of Trx to inhibit leukocyte extravasation.
Leukocyte extravasation, however, is not always harmful. It is a key component of innate immunity and provides early protection when certain pathogens invade the host. Under these conditions, Trx inhibition of leukocyte extravasation may be harmful rather than helpful. For example, we have shown previously that chronic elevation of circulating Trx is associated with decreased survival in HIV disease, particularly among subjects in which the adaptive immune system is compromised severely (15). The decreased survival in these subjects, we suggested, is caused by the Trx-mediated impairment in innate immunity in individuals who lack functional adaptive immunity. In other words, although administration of recombinant Trx offers a new tool for the prevention and treatment of pathogen-free disorders initiated by leukocyte infiltration, it may worsen the inflammation caused by some pathogens. Therefore, therapeutic use of Trx, similar to the therapeutic use of glucocorticoid, requires caution.
Acknowledgments
This work was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan and by a Grant-in-Aid for Research for the Future from the Japan Society for the Promotion of Science. In addition, it was supported by grants from the National Cancer Institute (CA-42509 and CA-81543) and National Institutes of Health (Bethesda, MD).
Abbreviations
- Trx
thioredoxin
- LPS
lipopolysaccharide
- MAPK
mitogen-activated protein kinase
- RANTES
regulated upon activation, normal T cell expressed and secreted
- ICAM-1
intercellular adhesion molecule-1
- VCAM-1
vascular cell adhesion molecule-1
- MCP-1
monocyte chemoattractant protein-1
- HUVEC
human umbilical vein endothelial cell
References
- 1.Holmgren A. Annu Rev Biochem. 1985;54:237–271. doi: 10.1146/annurev.bi.54.070185.001321. [DOI] [PubMed] [Google Scholar]
- 2.Chae H Z, Robison K, Poole L B, Church G, Storz G, Rhee S G. Proc Natl Acad Sci USA. 1994;91:7017–7021. doi: 10.1073/pnas.91.15.7017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hirota K, Matsui M, Iwata S, Nishiyama A, Mori K, Yodoi J. Proc Natl Acad Sci USA. 1997;94:3633–3638. doi: 10.1073/pnas.94.8.3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hirota K, Murata M, Sachi Y, Nakamura H, Takeuchi J, Mori K, Yodoi J. J Biol Chem. 1999;274:27891–27897. doi: 10.1074/jbc.274.39.27891. [DOI] [PubMed] [Google Scholar]
- 5.Makino Y, Okamoto K, Yoshikawa N, Aoshima M, Hirota K, Yodoi J, Umesono K, Makino I, Tanaka H. J Clin Invest. 1996;98:2469–2477. doi: 10.1172/JCI119065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Makino Y, Yoshikawa N, Okamoto K, Hirota K, Yodoi J, Makino I, Tanaka H. J Biol Chem. 1999;274:3182–3188. doi: 10.1074/jbc.274.5.3182. [DOI] [PubMed] [Google Scholar]
- 7.Wang H C, Zentner M D, Deng H T, Kim K J, Wu R, Yang P C, Ann D K. J Biol Chem. 2000;275:8600–8609. doi: 10.1074/jbc.275.12.8600. [DOI] [PubMed] [Google Scholar]
- 8.Tagaya Y, Maeda Y, Mitsui A, Kondo N, Matsui H, Hamuro J, Brown N, Arai K, Yokota T, Wakasugi H, Yodoi J. EMBO J. 1989;8:757–764. doi: 10.1002/j.1460-2075.1989.tb03436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wollman E E, d'Auriol L, Rimsky L, Shaw A, Jacquot J P, Wingfield P, Graber P, Dessarps F, Robin P, Galibert F, et al. J Biol Chem. 1988;263:15506–15512. [PubMed] [Google Scholar]
- 10.Wakasugi N, Tagaya Y, Wakasugi H, Mitsui A, Maeda M, Yodoi J, Tursz T. Proc Natl Acad Sci USA. 1990;87:8282–8286. doi: 10.1073/pnas.87.21.8282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosen A, Lundman P, Carlsson M, Bhavani K, Srinivasa B R, Kjellstrom G, Nilsson K, Holmgren A. Int Immunol. 1995;7:625–633. doi: 10.1093/intimm/7.4.625. [DOI] [PubMed] [Google Scholar]
- 12.Pekkari K, Gurunath R, Arner E S, Holmgren A. J Biol Chem. 2000;275:37474–37480. doi: 10.1074/jbc.M001012200. [DOI] [PubMed] [Google Scholar]
- 13.Pekkari K, Avila-Carino J, Bengtsson A, Gurunath R, Scheynius A, Holmgren A. Blood. 2001;97:3184–3190. doi: 10.1182/blood.v97.10.3184. [DOI] [PubMed] [Google Scholar]
- 14.Nakamura H, Nakamura K, Yodoi J. Annu Rev Immunol. 1997;15:351–369. doi: 10.1146/annurev.immunol.15.1.351. [DOI] [PubMed] [Google Scholar]
- 15.Nakamura H, De Rosa S C, Yodoi J, Holmgren A, Ghezzi P, Herzenberg L A. Proc Natl Acad Sci USA. 2001;98:2688–2693. doi: 10.1073/pnas.041624998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakamura H, De Rosa S, Roederer M, Anderson M T, Dubs J G, Yodoi J, Holmgren A, Herzenberg L A. Int Immunol. 1996;8:603–611. doi: 10.1093/intimm/8.4.603. [DOI] [PubMed] [Google Scholar]
- 17.Sumida Y, Nakashima T, Yoh T, Nakajima Y, Ishikawa H, Mitsuyoshi H, Sakamoto Y, Okanoue T, Kashima K, Nakamura H, Yodoi J. J Hepatol. 2000;33:616–622. doi: 10.1034/j.1600-0641.2000.033004616.x. [DOI] [PubMed] [Google Scholar]
- 18.Yokomise H, Fukuse T, Hirata T, Ohkubo K, Go T, Muro K, Yagi K, Inui K, Hitomi S, Mitsui A, et al. Respiration. 1994;61:99–104. doi: 10.1159/000196315. [DOI] [PubMed] [Google Scholar]
- 19.Okubo K, Kosaka S, Isowa N, Hirata T, Hitomi S, Yodoi J, Nakano M, Wada H. J Thorac Cardiovasc Surg. 1997;113:1–9. doi: 10.1016/S0022-5223(97)70393-3. [DOI] [PubMed] [Google Scholar]
- 20.Bertini R, Howard O M, Dong H F, Oppenheim J J, Bizzarri C, Sergi R, Caselli G, Pagliei S, Romines B, Wilshire J A, et al. J Exp Med. 1999;189:1783–1789. doi: 10.1084/jem.189.11.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith W B, Gamble J R, Clark-Lewis I, Vadas M A. Immunology. 1993;78:491–497. [PMC free article] [PubMed] [Google Scholar]
- 22.Wang J M, McVicar D W, Oppenheim J J, Kelvin D J. J Exp Med. 1993;177:699–705. doi: 10.1084/jem.177.3.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hechtman D H, Cybulsky M I, Fuchs H J, Baker J B, Gimbrone M A., Jr J Immunol. 1991;147:883–892. [PubMed] [Google Scholar]
- 24.Ley K, Baker J B, Cybulsky M I, Gimbrone M A, Jr, Luscinskas F W. J Immunol. 1993;151:6347–6357. [PubMed] [Google Scholar]
- 25.Mitsui A, Hirakawa T, Yodoi J. Biochem Biophys Res Commun. 1992;186:1220–1226. doi: 10.1016/s0006-291x(05)81536-0. [DOI] [PubMed] [Google Scholar]
- 26.Roy S, Sen C K, Packer L, Strausbaugh H J, Green P G, Lo E, Tangemann K, Reichling D B, Rosen S D, Levine J D. Methods Enzymol. 1999;300:395–401. doi: 10.1016/s0076-6879(99)00144-5. [DOI] [PubMed] [Google Scholar]
- 27.Takagi Y, Mitsui A, Nishiyama A, Nozaki K, Sono H, Gon Y, Hashimoto N, Yodoi J. Proc Natl Acad Sci USA. 1999;96:4131–4136. doi: 10.1073/pnas.96.7.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Finn A, Strobel S, Levin M, Klein N. Ann NY Acad Sci. 1994;725:173–182. doi: 10.1111/j.1749-6632.1994.tb39799.x. [DOI] [PubMed] [Google Scholar]
- 29.Tedder T F, Steeber D A, Pizcueta P. J Exp Med. 1995;181:2259–2264. doi: 10.1084/jem.181.6.2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Malhotra R, Priest R, Bird M I. Biochem J. 1996;320:589–593. doi: 10.1042/bj3200589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simonet W S, Hughes T M, Nguyen H Q, Trebasky L D, Danilenko D M, Medlock E S, Westerlund-Wikstrom B. J Clin Invest. 1994;94:1310–1319. doi: 10.1172/JCI117450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hashimoto S, Matsumoto K, Gon Y, Furuichi S, Maruoka S, Takeshita I, Hirota K, Yodoi J, Horie T. Biochem Biophys Res Commun. 1999;258:443–447. doi: 10.1006/bbrc.1999.0658. [DOI] [PubMed] [Google Scholar]
- 33.Rizoli S B, Rotstein O D, Kapus A. J Biol Chem. 1999;274:22072–22080. doi: 10.1074/jbc.274.31.22072. [DOI] [PubMed] [Google Scholar]
- 34.Vestweber D, Blanks J E. Physiol Rev. 1999;79:181–213. doi: 10.1152/physrev.1999.79.1.181. [DOI] [PubMed] [Google Scholar]
- 35.Hotta M, Tashiro F, Ikegami H, Niwa H, Ogihara T, Yodoi J, Miyazaki J. J Exp Med. 1998;188:1445–1451. doi: 10.1084/jem.188.8.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ahmed N, Thorley R, Xia D, Samols D, Webster R O. Am J Respir Crit Care Med. 1996;153:1141–1147. doi: 10.1164/ajrccm.153.3.8630558. [DOI] [PubMed] [Google Scholar]
- 37.Nakamura H, Vaage J, Valen G, Padilla C A, Bjornstedt M, Holmgren A. Free Radical Biol Med. 1998;24:1176–1186. doi: 10.1016/s0891-5849(97)00429-2. [DOI] [PubMed] [Google Scholar]