Abstract
The present study was taken up to understand the phylogenetic relationship using ITS and TEF markers among 22 isolates of Fusarium oxysporum f. sp. lentis (Fol) causing lentil wilt belonging to eight races isolated from different geographic locations of India and to develop specific markers for its detection. The nucleotide sequences of ITS region varied from 490 to 560 bp whereas, 670–725 bp for TEF 1α. The phylogeny analysis revealed that the isolates were more than 98% similar based on the neighbour joining analysis and were grouped into two major clusters in both ITS and TEF. The first major cluster of ITS had twenty isolates whereas for TEF, there were 15 isolates. Two sets of SCAR markers MS1 (162 bp) and MS2 (125 bp) were designed and synthesised. These markers were used against race representative Fol isolates for amplification. While, MS 1 marker was able to detect the genomic DNA up to 0.1 ng, MS 2 could detect the Fol genomic DNA up to 0.05 ng. The specificity of these two markers to detect Fol and their inability to amplify most common lentil pathogens (Rhizoctonia solani, R. bataticola, Sclerotium rolfsii, Sclerotinia sclerotiarum, and Aschochyta rabiei) makes them a reliable tool for detection. The phylogenetic analysis is helpful in the understanding of variability in Fol populations and the SCAR markers help in rapid and reliable detection of an important pathogen of lentil.
Electronic supplementary material
The online version of this article (10.1007/s13205-019-1734-4) contains supplementary material, which is available to authorized users.
Keywords: Lentil, Fusarium oxysporum f. sp. lentis, Phylogeny, ITS, TEF, SCAR marker
Introduction
Lentil [Lens culinaris (L.) Medik.] is an important food legume crop grown in many regions like West Asia, the Indian subcontinent, Ethiopia and North Africa and to a lesser extent in southern Europe. In India, the crop is grown on an area of 1.48 mha with a production of 1.03 mt and productivity of 700 kg ha−1 (Anonymous 2014). Lentil wilt caused by Fusarium oxysporum Schlecht. Emend Snyder and Hansen f. sp. lentis Vasudeva and Srinivasan (Fol) is one of the most important biotic stresses of lentil. In India, the disease is more prevalent in Uttar Pradesh, Madhya Pradesh, Bihar, West Bengal and other areas where lentil is grown and reported to cause 25–95% infection in some fields (Khare 1980; Agrawal et al. 1991); while the losses are reported to be 5–10%, it may lead to total crop loss under conducive weather conditions (Chaudhary and Amarjit 2002). The isolates of F. oxysporum f. sp. lentis exhibit great variability in morphology and aggressiveness (Abbas 1995; Belabid et al. 2004). However, DNA-based techniques have increasingly become the tool of choice for understanding the genetic diversity (O’Donnell 2000). Molecular phylogenetic analyses have helped to clarify ambiguities in traditional classification systems of Fusarium spp. by ITS marker (LoBuglio et al. 1993). Regions of ribosomal DNA (rDNA) are highly conserved, have also been used in diversity and phylogeny analysis of Fusarium spp. (Alves-Santos et al. 2002). The genetic diversity among the Fol isolates has been reported to be high (Al-Husien et al. 2017; Mohammadi et al. 2011) as in a collection of Fol isolates from Iran, Syria, and Algeria found to have high molecular variation within the regions (Pouralibaba et al. 2018), whereas, it was found low in isolates belonging to Ilam provinces of western Iran (Nourollahi and Madahjalali 2017). Moreover, seven pathotypes originating from Iran, Syria and Algeria (Pouralibaba et al. 2016) and eight races from India (Hiremani and Dubey 2018) have recently been identified.
F. oxysporum f. sp. lentis being soil- and seed-borne, its early detection is very crucial to undertake management practices to a great extent. Earlier, a specific marker for the detection of F. oxysporum f. sp. ciceris was developed based on ITS-RFLP (Dubey et al. 2010). But, there are no Fol-specific diagnostic markers available to detect either from soil or seed. Thus, the present investigation was taken up to know the phylogenetic relationship among the identified races in the Indian population of Fol using the universal ITS and TEF markers and also to develop highly specific SCAR markers for detection of Fol.
Materials and methods
Culture of Fusarium oxysporum f. sp. lentis
A total 50 isolates of Fusarium oxysporum f. sp. lentis (Fol) representing 7 lentil growing states of India (Online Resource 1) were obtained from Pulse Pathology laboratory, Division of Plant Pathology, IARI-New Delhi, India; IIPR-Kanpur, Uttar Pradesh, India and RAK College-Sehore, Madhya Pradesh, India and also isolated from wilted lentil plants collected from infected fields of these areas. Single-spore cultures of the isolates were used for DNA extraction.
Genomic DNA extraction
The genomic DNA was extracted from the mycelium by modified CTAB method (Murray and Thompson 1980). Mycelial mat (1 g) was ground and was transferred into tubes containing 10 ml preheated (65 °C) 2% Cetyltrimethyl Ammonium Bromide (CTAB) extraction buffer (1 M Tris–HCl, pH 8.0; 5 M NaCl; 0.5 M EDTA, pH 8.0, and 2% CTAB). The contents were incubated at 65 °C for 1 h followed by addition of equal volume phenol: chloroform: isoamyl alcohol (25:24:1). After centrifugation at 11,000 rpm for 10 min. upper aqueous solution formed was transferred to another tube and precipitated with chilled 0.6 volume of isopropanol and 0.1 volume of sodium acetate and then centrifuged at 12,000 rpm for 10 min. The pellet obtained was washed twice with 70% ethanol and later dissolved in nuclease-free water. The extracted DNA was purified and stored at − 20 °C for further use (Dubey et al. 2014).
Internal transcribed spacer region analysis
The universal primers namely, ITS 1 (5′ TCCGTAGGTGAACCTGCGG 3′) and ITS 4 (5′ TCCTCCGCTTATTGATATGC 3′) described by White et al. (1990) were used to amplify internal transcribed spacer (ITS) region of genomic DNA of 50 Fol isolates. Although 50 isolates were used in ITS amplification, only 22 isolates representing different races and state were sequenced and used for phylogenetic analysis. The PCR amplification reaction was performed in a 25 µl mixture containing 1.5 mM MgCl2, 0.6 mM dNTP, 5 pmol of each primers, 2.5 mM Taq buffer, 1 unit of Taq polymerase and 25 ng of DNA template. The reaction cycle conditions were standardized as initial denaturation at 94 °C for 5 min followed by 35 cycles of denaturation at 94 °C for 1 min, annealing at 58 °C for 45 s and extension at 72 °C for 2 min and a final extension of 72 °C for 4 min. Amplified products were separated by gel electrophoresis in 1.2% agarose gel pre-stained with ethidium bromide (1 µg/mL) in 1 × TAE buffer.
Translation elongation factor 1-α gene analysis
The universal primers for Fusarium oxysporum complex namely, Ef 1 (5′ ATGGGTAAGGAAGGACAAG 3′) and Ef 2 (5′ GGAGAGTACCAGTGCATCAT 3′) given by O’Donnell et al. (1998) were used to amplify TEF region of genomic DNA of Fol isolates. The PCR amplification reaction was performed in a 25 µl mixture containing 1.5 mM MgCl2, 0.5 mM dNTP, 10 pmol of each primers, 2.5 mM Taq buffer, 1.5 unit of Taq polymerase and 50 ng DNA template. The reaction cycle conditions were standardized as initial denaturation at 94 °C for 2 min followed by 35 cycles of denaturation at 94 °C for 30 s, annealing at 62 °C for 30 s and extension at 72 °C for 30 s and a final extension of 74 °C for 4 min. Post PCR protocol is same as for ITS.
Phylogenetic relationship analysis
The bioinformatics algorithm basic local alignment search tool (BLAST) program (Altschul et al. 1990) was used for sequence analysis. Contigs were made in PRABI-Duoa:CAP3 program (Huang and Madan 1999) and the Fol sequences were subjected to BLAST analysis. All the ITS and TEF sequences of Fol isolates were submitted to NCBI GenBank (Table 1). The multiple sequence alignment and pair-wise alignment were made using BioEdit v 7.0.5 software (Hall 1999). Phylogenetic tree was constructed based on the maximum likelihood of nucleotide sequences using Clustal W2 sequence alignment selecting Bootstrap Neighbor-Joining by MEGA6 (v 6.06) program (Tamura et al. 2013).
Table 1.
Accession numbers of internal transcribed spacer (ITS) and translation elongation factor 1α gene (TEF) sequences of Fusarium oxysporum f. sp. lentis isolates submitted to NCBI GenBank
| State | Place | Isolate no. | Race | NCBI accession no. | |
|---|---|---|---|---|---|
| ITS | TEF | ||||
| Uttar Pradesh | Hamirpur | FLS 5 | 1 | KU671027 | MK303898 |
| Madhya Pradesh | Panna | FLS 32 | 1 | KU671028 | MK303899 |
| Uttar Pradesh | Budaun | FLS 4 | 2 | KU671029 | MK303900 |
| Rajasthan | Nagaur | FLS 67 | 2 | KU671030 | MK303901 |
| Chhattisgarh | Raigarh | FLS 61 | 2 | KU671031 | MK303902 |
| Uttar Pradesh | Lucknow | FLS 8 | 3 | KU671032 | MK303903 |
| Rajasthan | Jaipur | FLS 72 | 3 | KU671033 | MK303904 |
| Uttar Pradesh | Gazipur | FLS 10 | 4 | KU671034 | MK303905 |
| Bihar | Patna | FLS 27 | 4 | KU671035 | MK303906 |
| Jharkhand | Ranchi | FLS 58 | 4 | KU671036 | MK303907 |
| Madhya Pradesh | Damoh | FLS 31 | 4 | KU671037 | MK303908 |
| Uttar Pradesh | Karwi | FLS 2 | 5 | KU671038 | MK303909 |
| Bihar | Khagariya | FLS 23 | 5 | KU671039 | MK303910 |
| Madhya Pradesh | Betul | FLS 40 | 5 | KU671040 | MK303911 |
| Jharkhand | Chatra | FLS 52 | 5 | KU671041 | MK303912 |
| Chhattisgarh | Durg | FLS 65 | 5 | KU671042 | MK303913 |
| Madhya Pradesh | Sehore | FLS 29 | 6 | KU671043 | MK303914 |
| Jharkhand | Hazaribagh | FLS 51 | 6 | KU671044 | MK303915 |
| Bihar | Muzaffarpur | FLS 22 | 7 | KU671045 | MK303916 |
| Delhi | New Delhi | FLS 75 | 7 | KU671046 | MK303917 |
| Madhya Pradesh | Hoshangabad | FLS 30 | 8 | KU671047 | MK303918 |
| Chhattisgarh | Baloda-Bazar | FLS 62 | 8 | KU671048 | MK303919 |
Development of sequence-characterized amplified region (SCAR) markers for detection of the pathogen
A microsatellite marker MB 18 (Bogale et al. 2006) provided a monomorphic band of (~ 250 bp) in all the isolates of Fol, but absent in other species of Fusarium. This marker was used to amplify the DNA of FLS 75 isolate. Thus, the desirable amplified band (~ 250 bp) was cut from the gel and a QIAquick® gel extraction kit (QIAGEN, Hilden, Germany) was used for elution of purified DNA. The standard protocol given in the manufacturer’s manual was followed to elute the DNA. Finally, the purified DNA was given for sequencing (SciGenome Labs, Cochin, India) and both the forward and reverse sequences of the specific band obtained were used to make a contig from CAP3 software online.
Designing of specific SCAR primers
Primers for candidate SCAR markers were designed using Primer3 (v. 0.4.0) software (Untergasser et al. 2012) to test their specificity. From the contig sequence of the SSR fragment, markers MS1 (F and R) and MS2 (F and R) were designed and were synthesised from Eurofins Genomics India Pvt Ltd. Two sets of primers were made for each marker, to a common length of 18–22 bp. For both set of primers, care was taken to avoid secondary structures, primer dimer generation and cross hybridization.
Specificity and sensitivity of designed SCAR markers
The SCAR markers MS1 and MS2 were standardized for amplification of desired size of the band in eight race representative isolates of Fol viz., FLS 34, FLS 14, FLS 72, FLS 24, FLS 23, FLS 51, FLS 75, and FLS 62 along with six negative controls; Rhizoctonia solani, R. bataticola, Sclerotium rolfsii, Sclerotinia sclerotiarum, Fusarium oxysporum f. sp. ciceris, and Ascochyta rabiei. Polymerase chain reaction was carried out in 25 µl reaction volume containing 1 × Taq buffer, 1.5 mM MgCl2, 0.2 mM dNTP, 1.5 U Taq polymerase, 15 pmol of each primers and 25 ng DNA. Amplification was performed in a thermocycler, as initial denaturation at 94 °C for 5 min followed by 30 cycles of denaturation at 94 °C for 1 min, annealing temperature for each primer was standardized by gradient PCR and it was set at 65° C and 55° C for MS1 and MS2, respectively, for 45 s and extension at 72 °C for 45 s and a final elongation of 74 °C for 4 min. Reaction products were resolved by electrophoresis on 1.2% agarose gel in 1 × TAE buffer stained with ethidium bromide at 70 V for 60 min and observed under UV light in a gel doc system. All PCR reactions with SCAR primers were repeated at least two times.
Cloning and sequencing to validate SCAR markers
The purified DNA was cloned into the pGEM®-T Easy vector (Promega, Madison, USA) according to the manufacturer’s instructions. The ligation mixture included 3 µl gel-eluted DNA, 2 × rapid ligase buffer (5 µl), pGEM®-T Easy vector (1 µl) and DNA ligase (1 µl) with a total volume of 10 µl. The ligation mixture was incubated overnight at 4 °C. The competent cells were prepared in Luria broth (LB) by calcium chloride method (Mendel and Higa 1970). Luria broth (50 ml) was inoculated with overnight grown culture of DH 5α strain of Escherichia coli and incubated at 37 °C for 1 h and 15 min with constant shaking at 200 rpm in a shaking incubator. These competent cells were transferred as small aliquots into fresh, sterile tubes and used for transformation after incubating them on ice for 1 h. The transformed cells were selected by screening blue/white colonies (Ullmann et al. 1967). The white colonies were selected as recombinant cells, subsequently plated on IXA (IPTG, X-gal and ampicillin) plates and incubated at 37 °C. The plate having individual transformants served as master plate. The master plate was analyzed and the clones were selected and sub cultured on another IXA plate by streaking with a sterile tip and incubated at 37 °C. The single colonies from the sub cultured IXA plate were marked, then picked and streaked on fresh IXA plates, incubated at 37 °C and the tip was immediately dipped in the already prepared reaction mixture of colony PCR under aseptic conditions. The colony PCR was run as the earlier standardized thermocycler conditions of each SCAR marker.
Results
Internal transcribed spacer (ITS) region analysis and its sequencing
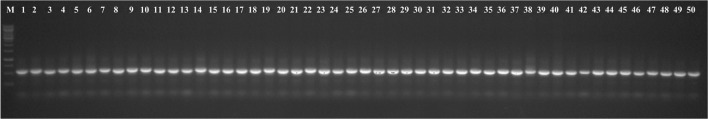
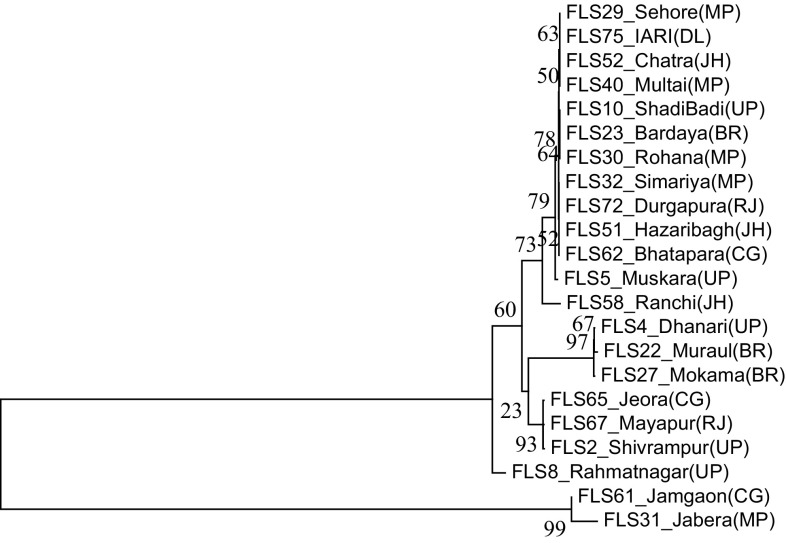
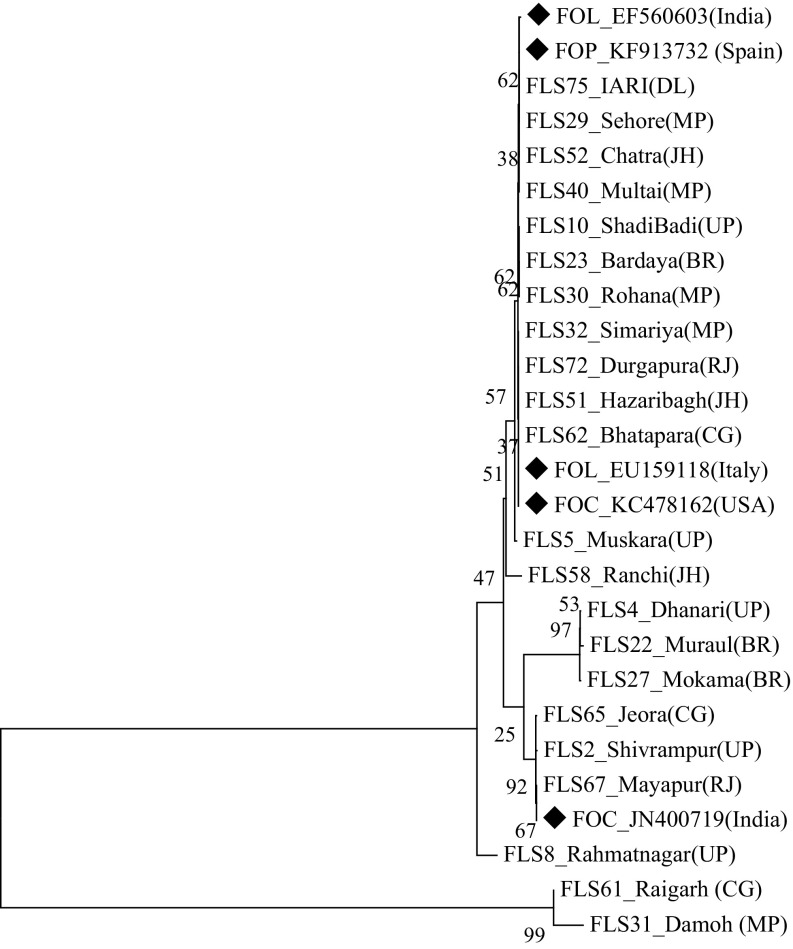
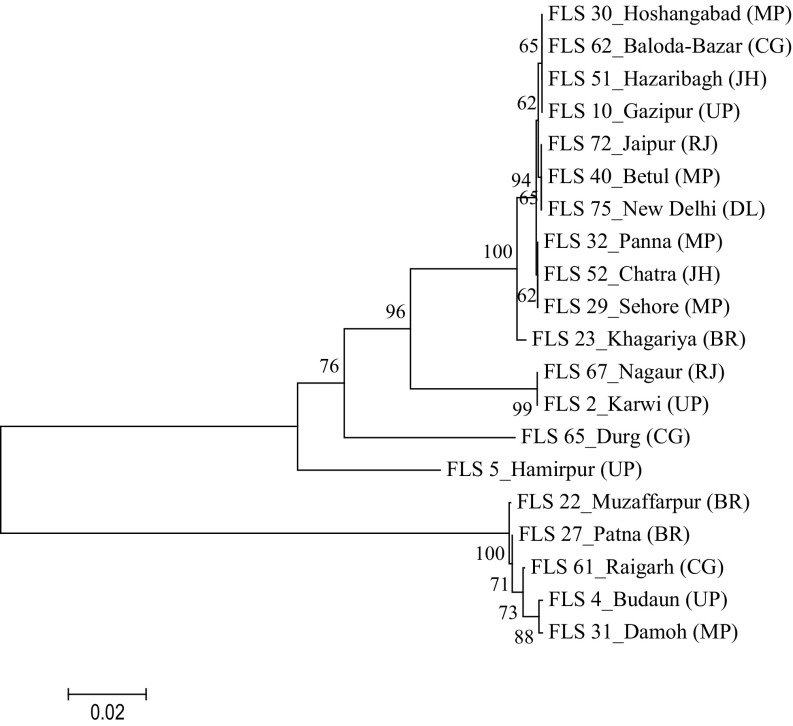
The genomic DNA of all the Fol isolates was amplified using the universal primers ITS 1 and ITS 4, which yielded the ITS products (ITS1 + 5.8S + ITS2) of approximately 550 bp (Fig. 1). Out of 50 isolates, 22 race-representative isolates belonging to different states within the racial groups were selected for sequencing. The nucleotide sequences of ITS 1, 5.8S rDNA and ITS 2 region of the 22 representative isolates varied from 490 to 560 bp. The sequences were deposited to NCBI GenBank database and accession numbers were obtained (Online Resource 2). The phylogeny tree constructed based on the nucleotide sequences using bootstrap neighbor-joining analysis produced two major clusters (Fig. 2). Twenty isolates belonging to the first major cluster were again divided into two subclusters with the first having 19 isolates and the second subcluster had only one isolate, FLS 8 from Uttar Pradesh, designated as race 3. The second major cluster consisted of two isolates, FLS 61 from Chhattisgarh and FLS 31 from Madhya Pradesh representing race 2 and 4, respectively. Further, NCBI GenBank sequences of two isolates of Fol (one each from India and Italy), two isolates of F. oxysporum f. sp. ciceris (one each from India and USA) and one isolate of F. oxysporum f. sp. pisi from Spain were compared in the phylogenetic analysis (Fig. 3) and they were also similar to Fol isolates used in the present study and were grouped in the first major cluster. But, from the major cluster one, two subclusters were formed where 24 isolates were present in first subcluster including all the reference sequences of NCBI GenBank and only one isolate FLS 8 from Uttar Pradesh was present in second subcluster. The subcluster one was further divided into two more sub subclusters and out of the two F. oxysporum f. sp. ciceris isolates, the isolate from India was present in sub subcluster two whereas, the isolate from USA and remaining other reference isolates were present in the first sub subcluster.
Fig. 1.
DNA profile generated using universal ITS 1 and ITS 4 primers for internal transcribed spacer (ITS) region analysis of 50 Fusarium oxysporum f. sp. lentis isolates; M = 1 kb marker; Lanes 1–13 (Uttar Pradesh), 14–19 (Bihar), 20–31 (Madhya Pradesh), 32–37 (Jharkhand), 38–43 (Chhattisgarh), 44–49 (Rajasthan) and 50 (Delhi) indicate isolates of Fusarium oxysporum f. sp. lentis
Fig. 2.
Neighbour-joining phylogenetic tree generated from the sequences of ITS region of Fusarium oxysporum f. sp. lentis isolates at bootstrap values of 1000 replicates. Abbreviations in bracket indicate states as UP—Uttar Pradesh, BR—Bihar, MP—Madhya Pradesh, JH—Jharkhand, CG—Chhattisgarh, RJ—Rajasthan and DL—Delhi. The bootstrap values < 95 are statistically non-significant
Fig. 3.
Neighbour-joining phylogenetic tree generated from the sequences of ITS region of Fusarium oxysporum f. sp. lentis isolates at bootstrap values of 1000 replicates along with NCBI GenBank reference sequences (represented by diamond). Abbreviations in bracket indicate states as UP—Uttar Pradesh, BR—Bihar, MP—Madhya Pradesh, JH—Jharkhand, CG—Chhattisgarh, RJ—Rajasthan and DL—Delhi whereas FOL—Fusarium oxysporum f. sp. lentis, FOC—Fusarium oxysporum f. sp. ciceris and FOP—Fusarium oxysporum f. sp. pisi
Translation elongation factor 1-α gene analysis
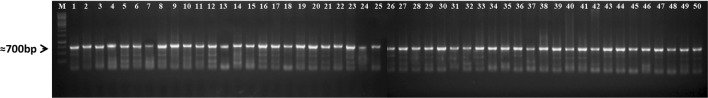
The genomic DNA of the 50 Fol isolates was amplified with universal primers Ef 1 and Ef 2 which yielded the products of approximately 700 bp (Fig. 4). Twenty race representative isolates were sequenced and the nucleotide sequences of the TEF 1 α gene varied from 670–725 bp. Based on these nucleotide sequences, a phylogeny tree was constructed using the bootstrap neighbor-joining analysis. The isolates were grouped into two major clusters with each having two subclusters. The first subcluster of the major cluster one had 14 Fol isolates originated from different states within the racial groups. Whereas, the second subcluster had only one isolate, FLS 5 (race 1) from Uttar Pradesh. The second major cluster had five isolates where only one isolate FLS 27 (race 4) from Bihar was present in the first subcluster and four isolates namely, FLS 22 (Bihar, race 7), FLS 61 (Chhattisgarh, race 2), FLS 4 (Uttar Pradesh, race 2) and FLS 31 (Madhya Pradesh, race 4) were present in the second subcluster (Fig. 5).
Fig. 4.
DNA profile generated using universal primers Ef 1 and Ef 2 for TEF 1 α gene analysis of 50 Fusarium oxysporum f. sp. lentis isolates; M = 1 kb marker; Lanes 1–13 (Uttar Pradesh), 14–19 (Bihar), 20–31 (Madhya Pradesh), 32–37 (Jharkhand), 38–43 (Chhattisgarh), 44–49 (Rajasthan) and 50 (Delhi) indicate isolates of Fusarium oxysporum f. sp. lentis
Fig. 5.
Neighbour-joining phylogenetic tree generated from the sequences of translation elongation factor 1α (TEF) gene of Fusarium oxysporum f. sp. lentis isolates at bootstrap value of 1000 replicates. Abbreviations in bracket indicate states as UP—Uttar Pradesh, BR—Bihar, MP—Madhya Pradesh, JH—Jharkhand, CG—Chhattisgarh, RJ—Rajasthan and DL—Delhi. The bootstrap values < 95 are statistically non-significant
Development of SCAR marker
A monomorphic band obtained in all isolates of Fol using SSR primer MB 18 (≈ 250 bp) was selected for development of sequence-characterized amplified region primers. The eluted fragment was purified and sequenced. The forward and reverse sequences obtained were used to make a contig (Table 2). Two sets of markers MS1 F and R and MS2 F and R were designed and synthesised. Both produced the expected monomorphic band in all the race-representative Fol isolates. These markers yielded the products with a size of 162 bp and 125 bp, respectively. The product sequences of each primer are given Table 2.
Table 2.
Sequences of the contig and specific markers
| Name of the contig/marker | Sequence of the contig/marker |
|---|---|
| Product sequence of monomorphic MB 18 band | ATGACGAAGCTGACAAGAAAGATAGTCGAGATAGTGCATCCCCTAAATTAGTTATCCGTGATTCCCTATACGTAGTACGTAATTCGGAGAGCAACTTCCTTCAAGAATTGAACTGCAACACAACACAACACACACAGCACAACAAAAAAGCTGGGGTGAGTCGACAGCCAACCACATGAGGTGGGGTTGACACCCGTTTGACCTAACAAGAGCCGCTTGAGTTACCGACCCCCGGGTTCTTGGGCGCCTCTTACAGAGGTTTGGAGTGCTAGAGTGCTCAA |
| Product sequence of MS1 primer | GAACTGCAACACAACACAACACAACACAGCACAACAAAAAAGCTGGGGTGAGTCGACAGCCAACCACATGAGGTGGGGTTGACACCCGTTTGACCTAACAAGAGCCGCTTGAGTTACCGACCCCCGGGTTCTTGGGCGCCTCTTACAGAGGTTTGGAGTGCT |
| Product sequence of MS2 primer | AAAAAGCTGGGGTGAGTCGACAGCCAACCACATGAGGTGGGGTTGACACCCGTTTGACCTAACAAGAGCCGCTTGAGTTACCGACCCCCGGGTTCTTGGGCGCCTCTTACAGAGGTTTGGAGTGC |
| MS1 forward | 5′-GAACTGCAACACAACACAAC-3′ |
| MS1 reverse | 5′-AGCACTCCAAACCTCTGTAA-3′ |
| MS2 forward | 5′-AAAAAGCTGGGGTGAGTC-3′ |
| MS2 reverse | 5′-GCACTCCAAACCTCTGTAAG-3′ |
Specificity and validation of SCAR markers
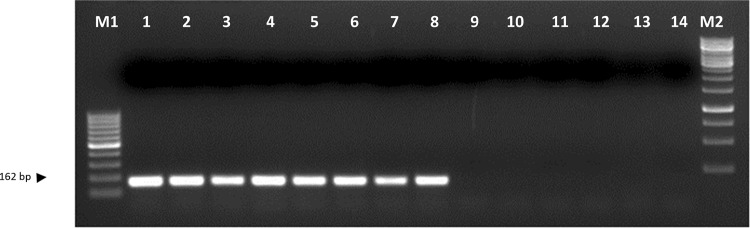
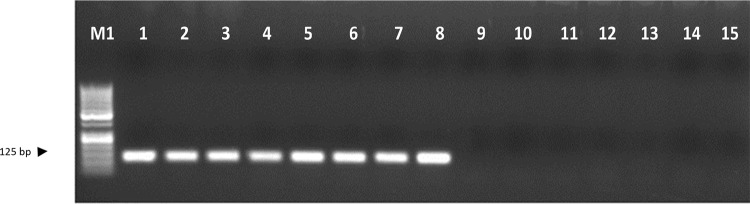
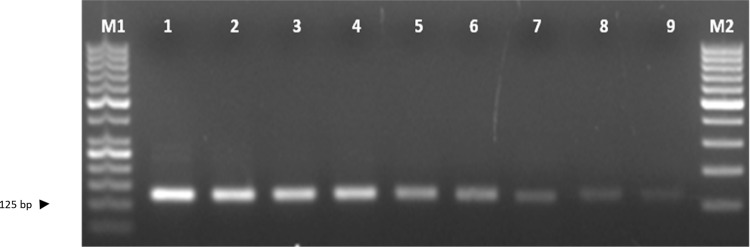
The primer pairs MS1F (5′-GAACTGCAACACAACACAAC-3′), MS1R (5′-AGCACTCCAAACCTCTGTAA-3′), MS2F (5′-AAAAAGCTGGGGTGAGTC-3′) and MS2R (5′-GCACTCCAAACCTCTGTAAG-3′) gave the single PCR product of the expected size in race-representative isolates of Fol. Validation against race-representative isolates confirmed the specificity of these markers wherein the expected amplification was obtained in Fol isolates but not in the other soil-borne fungi used as controls namely, Rhizoctonia solani, R. bataticola, Sclerotium rolfsii, Sclerotinia sclerotiarum, Fusarium oxysporum f. sp. ciceris, and Ascochyta rabiei in case of both MS1 F and R (Fig. 6) and MS2 F and R (Fig. 7) markers.
Fig. 6.
DNA profile generated by sequence-characterized amplified region (SCAR) marker MS1 F and R; Lane M1- 100 bp DNA ladder, lanes 1–8 indicate isolates of Fusarium oxysporum f. sp. lentis as 1—FLS 34, 2—FLS 14, 3—FLS 72, 4—FLS 24, 5—FLS 23, 6—FLS 51, 7—FLS 75, 8—FLS 62, 9—Rhizoctonia solani, 10—Rhizoctonia bataticola, 11—Sclerotium rolfsii, 12—Sclerotinia sclerotiarum, 13—Fusarium oxysporum f. sp. ciceris, 14—Ascochyta rabiei and lane M2—1 kb DNA ladder
Fig. 7.
DNA profile generated by sequence characterized amplified region (SCAR) marker MS2 F and R; Lane M1- 100 bp DNA ladder, lanes 1–8 indicate isolates of Fusarium oxysporum f. sp. lentis as 1—FLS 34, 2—FLS 14, 3—FLS 72, 4—FLS 24, 5—FLS 23, 6—FLS 51, 7—FLS 75, 8—FLS 62, 9—Rhizoctonia solani, 10—Rhizoctonia bataticola, 11—Sclerotium rolfsii, 12—Sclerotinia sclerotiarum, 13—Fusarium oxysporum f. sp. ciceris 14—Ascochyta rabiei and 1—non-template control
Sensitivity of the SCAR markers
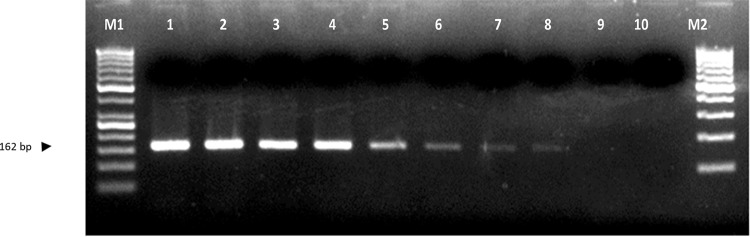
The detection limit of the SCAR markers MS1 F and R and MS2 F and R specific to the Fol isolates was also tested to assess their sensitivity through conventional PCR. Different DNA concentrations as 50 ng, 25 ng, 12 ng, 6 ng, 3 ng, 1 ng, 0.5 ng, 0.1 ng and 0.05 ng were tested with the respective reaction conditions for both the markers. While, MS 1 marker was able to detect the genomic DNA up to 0.1 ng (Fig. 8), the second marker MS 2 was able to detect the Fol genomic DNA up to 0.05 ng (Fig. 9).
Fig. 8.
Sensitivity of sequence-characterized amplified region (SCAR) marker MS1 F and R at different DNA concentrations. M1- 50 bp DNA ladder; Lane 1–50 ng; 2–25 ng; 3–12 ng; 4–6 ng; 5–3 ng; 6–1 ng; 7–0.5 ng; 8–0.1 ng; 9–0.05 ng; 10–non-template control and M2–100 bp DNA ladder
Fig. 9.
Sensitivity of sequence characterized amplified region (SCAR) marker MS 2 at different DNA concentrations. M1–50 bp DNA ladder; Lane 1–50 ng; 2–25 ng; 3–12 ng; 4–6 ng; 5–3 ng; 6–1 ng; 7–0.5 ng; 8–0.1 ng; 9–0.05 ng; and M2–100 bp DNA ladder
Cloning and sequencing to validate SCAR markers
The validation of both the SCAR markers MS1 and MS2 was done through cloning and sequencing. The target DNA of both the markers was ligated through pGEMT® Easy vector and then transformed into Escherichia coli DH 5 α competent cells which gave a turbid cell suspension. The mixture when plated on Luria agar amended with IXA gave blue/white colonies post-incubation overnight at 37 °C (Online resource 2). The clones were screened and transformed clone (single white colony) was picked and streaked on a LA-IXA plate and the bacterial colonies were seen after overnight incubation at 37 °C (Online resource 2), this plate was served as a master plate. From the master plate a single, isolated white colony was picked and again streaked on a sub-plate (Online resource 3) and kept for incubation overnight at 37 °C. Post incubation the transformed colonies (white) were observed and one of these clones (white colony) was picked up and colony PCR was performed which revealed the positive presence of insert DNA namely, FLS 23 and FLS 75 (Online resource 3). The sub-plate which gave positive result in colony PCR was given for sequencing and the results confirmed the sequence and exact size of the sequence as 162 bp and 125 bp for MS 1 and MS 2 markers, respectively. The sequence obtained was similar to the sequences used for designing the primers.
Discussion
Regions of ribosomal DNA (rDNA), which are highly conserved, are being used in diversity and phylogenetic studies of several Fusarium spp. (Alves-Santos et al. 2002). Out of various regions of rDNA, the internal transcribed spacer (ITS) of the nuclear rDNA repeat units has been reported to be evolved fast and may vary among species within a genus or among populations and hence can be used for phylogenetic studies at taxonomic levels (O’Donnell 2000). It has been proved that molecular phylogenetic analyses using ITS markers helped to elucidate ambiguities in traditional classification systems of Fusarium spp. (LoBuglio et al. 1993). In the present study, the ITS region (ITS1 + 5.8S + ITS2) of the 50 Fol isolates was amplified with universal ITS 1 and ITS 4 primers which produced approximately 550 bp amplicon. The nucleotide sequences obtained for 22 race representative isolates belonging to different states formed two major clusters in phylogenetic tree based on bootstrap neighbour-joining analysis. The isolates were more than 98% similar among them with 20 isolates belonging to first major cluster and two isolates belonging to second cluster. A slight difference in the nucleotide sequences of these two isolates may be the reason for grouping in second cluster. Similarly, nucleotide sequence homology of ITS region of 11 isolates of F. oxysporum f. sp. ciceris grouped them into 5 categories (Dubey et al. 2010). Similar observation has been made by Datta et al. (2011) who reported variability even in the isolates belonging to the same agro-climatic regions. Further, in the present study, it was found that the reference sequences of F. oxysporum f. sp. lentis (from India and Italy), F. oxysporum f. sp. ciceris (from India and USA) and F. oxysporum f. sp. pisi (from Spain) accessed from NCBI GenBank were similar with those of the Fol isolates used in the study and were present in the same major cluster. As the ITS region is highly conserved in the organisms it is probable that they are almost similar but for some slight variations over the period of time. However, in this study the sequence homology with other Fusarium oxysporum form species selected from NCBI Genbank is in agreement with the findings of Bogale et al. (2006) that pathogenicity of isolates does not necessarily correlate with phylogenetic grouping. The grouping of the reference isolates along with Fol isolates in the same cluster suggests that the form species of a fungus cannot be distinguished morphologically but a slight difference has made them pathogenic on different hosts. This has also been explained by Bogale et al. (2006) that sequence analysis lacked resolution among formae speciales and they are based on pathogenicity to specific plants which is influenced by several factors and not necessarily linked to phylogeny.
The difficulty in taxonomy of Fusarium can be properly addressed by translation elongation factor 1-alpha gene (EF1α) that encodes an essential part in the protein translation machinery and has high phylogenetic utility (Geiser et al. 2004). In the present study, the genomic DNA of 50 Fol isolates was amplified using Ef 1 and Ef 2 primers which yielded a product size of approximately 700 bp. Of these, 20 race-representative isolates originated from different states were sequenced and the nucleotide sequences varied from 670 to 725 bp. The phylogenetic tree based on the bootstrap neighbour joining analysis revealed that the isolates were more than 98% similar and grouped into two major clusters where first cluster had 15 isolates and second had 5 isolates. All the isolates belonging to the two clusters were identical among themselves regardless of their different geographical origins but slight differences in the nucleotides differentiated them into two groups, similar results have also been obtained by O’Donnell et al. (1998) for other taxa within the Fusarium oxysporum complex. Similarly, two groups were formed among the pathogenic and non-pathogenic isolates of F. oxysporum f. sp. ciceris using Ef1α gene sequences (Jimenez-Gasco et al. 2002).
In Fusarium, TEF gene appears to be consistently single copy and has become a marker of choice as a single-locus identification tool (Geiser et al. 2004). But, in the present study, despite the diversity in geographical origin, host-specific pathogenicity and symptoms, the isolates representing eight different races of lentil wilt pathogen had identical sequences except for five isolates which formed another group. Nevertheless, these five isolates were also identical among themselves. Thus, very narrow variability was observed among the isolates in respect of TEF gene sequences. Research findings from around the world using conserved gene regions have indicated that some of the form species of Fusarium oxysporum are polyphyletic (Koenig et al. 1997; Hill et al. 2011), while others have reported monophyletic groups (Jimenez-Gasco et al. 2002; Wunsch et al. 2009). The earlier work on genetic diversity analysis of Fol using different molecular markers was restricted to the isolates of a particular region or to a limited number of isolates (20–30). Besides, Fol being soil- and seed-borne, its early detection is very critical to minimise the losses using seed and soil treatment. Thus, the rapid detection of the pathogen will hasten the management approach to a great extent to apply fungicides as soil drenching at early stage of infections.
Apart from knowing the genetic relatedness among the isolates based on the EF1α sequence, these sequences can be used to develop specific primers or probes specific to a pathogen. Earlier, ITS-RFLP-based (Dubey et al. 2010) and TEF1α sequence-based (Dubey et al. 2014) detections have been developed for F. oxysporum f. sp. ciceris. But, there are no Fol-specific diagnostic markers available to detect either from soil or seed. Hence, in the present study two SCAR markers MS1 F and R and MS2 F and R were developed from a fragment of SSR marker MB 18 which amplified region of 162 bp and 125 bp, respectively, which were unique for Fol isolates. Further, the MS1 F and R and MS2 F and R markers were highly specific to Fol and did not detect or amplify the genomic DNA of other fungal pathogens used in the study namely F. oxysporum f. sp. ciceris, Rhizoctonia solani, R. bataticola, Sclerotium rolfsii, Sclerotinia sclerotiarum, and Ascochyta rabiei as control. These markers can be highly useful in the rapid detection of the wilt pathogen as they can eliminate the confusion regarding the other soil-borne pathogens encountering lentil crop. The sensitivity test of the MS1 F and R and MS2 F and R markers through conventional PCR revealed that the two markers can detect the genomic DNA of Fol up to 0.1 ng and 0.05 ng, respectively. Earlier, several workers have developed SCAR markers for the detection of Fusarium spp. in different crops like pigeon pea (Prasanthi et al. 2015), castor (Reddy et al. 2012), chickpea (Dubey et al. 2014; Farahani et al. 2015), tomato (Mutlu et al. 2015) and banana (Cunha et al. 2015). Whereas, Luongo et al. (2012) developed a SCAR marker based on RAPD which was highly specific to detect the race 2 of F. oxysporum f. sp. melonis unambiguously and even could not amplify any of the common melon pathogens.
The present study reports the phylogenetic relationship among the population of F. oxysporum f. sp. lentis originating from India and also between other subspecies within the F. oxysporum complex. The significant information generated will be helpful in understanding the variability in Fol which may be required in planning disease-resistance breeding against area-specific group of a population of the pathogen. Also, the two highly specific SCAR markers developed in this study, which were lacking earlier, can be effectively utilized in the rapid and reliable detection of Fol from diseased samples. Early and reliable detection even in asymptomatic plants/seeds is helpful in timely application of fungicides for disease management and also helpful in molecular epidemiology of the disease to correlate the weather variables with the frequency of infected plants at different growth stages determined by use of molecular markers.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Online Resource 1. Isolates of Fusarium oxysporum f. sp. lentis representing major lentil growing areas of India (PDF 197 kb)
Online Resource 2. The plates showing the blue/ white colonies after spreading (a) and only white colonies after streaking (b) a single white colony on the Luria agar plate amended with IPTG, X-gal and ampicillin after overnight incubation at 37 °C. The arrow indicates a white colony and plate B served as master plate (PDF 408 kb)
Online Resource 3. The plate showing the white colonies after streaking a single colony from master plate on the Luria agar plate amended with IPTG, X-gal and ampicillin (sub-plate) after overnight incubation at 37 °C (a), the arrows indicate single white transformed colonies. The gel picture (b) shows the amplification of inserted DNA through colony polymerase chain reaction for MS 2 marker. Lanes M- 100 bp ladder, 1- FLS 23 and 2- FLS 75 (PDF 397 kb)
Acknowledgements
The authors are thankful to Dr R. G. Chaudhary (IIPR, Kanpur) and Dr D. R. Saxena (RAK College, Sehore) for providing the Fol cultures. The first author is also thankful to Indian Agricultural Research Institute, New Delhi and UGC, New Delhi for Rajiv Gandhi National Fellowship. Thanks are also due to the advisory committee Drs. R. K. Jain, Pratibha Sharma, H. K. Dikshit and T. R. Sharma for their support and suggestions; also to Drs. B. K. Upadhyay and Birendra Singh for technical help and support. Further, the authors declare that they do not have conflict of interest of any kind.
Author contribution
SCD- Planning and supervision of the experiment, correction of the manuscript. NSH- Carried out the experiment, analysed the data and prepared the manuscript.
Compliance with ethical standards
Conflict of interest
All the help and financial assistance has been duly acknowledged. The authors declare that they have no conflict of interest.
References
- Abbas A (1995) Variation in some cultural and physiological characters and host pathogen interaction of Fusarium oxysporum f. sp. lentis and inheritance of resistance to lentil wilt in Syria. Syria: University of Aleppo, Dissertation
- Agrawal SC, Singh K, Lal SS (1991) Plant protection of lentil in India. In: Lentil in South Asia, New Delhi, India: ICAR-ICARDA seminar, 11–15 March, pp 147–167
- Al-Husien NH, Hamwieh A, Ahmed S, Bayaa B. Genetic diversity of Fusarium oxysporum f. sp. lentis population affecting lentil in Syria. J Phytopathol. 2017;165:306–312. doi: 10.1111/jph.12563. [DOI] [Google Scholar]
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Alves-Santos FM, Ramos B, Garcia-Sanchez MA, Eslava AP, Diaz-Minguez JM. A DNA based procedure for in planta detection of Fusarium oxysporum f. sp. phaseoli. Phytopathology. 2002;92:237–244. doi: 10.1094/PHYTO.2002.92.3.237. [DOI] [PubMed] [Google Scholar]
- Anonymous . Agricultural statistics at a glance. Government of India: Ministry of Agriculture and Farmer’s Welfare; 2014. [Google Scholar]
- Belabid L, Baum M, Fortas Z, Bouznad Z, Eujayl I. Pathogenic and genetic characterization of Algerian isolates of Fusarium oxysporum f. sp. lentis by RAPD and AFLP analysis. Afr J Biotechnol. 2004;3:25–31. doi: 10.5897/AJB2004.000-2005. [DOI] [Google Scholar]
- Bogale M, Wingfield BD, Wingfield MJ, Steenkamp ET. Characterization of Fusarium oxysporum isolates from Ethiopia using AFLP, SSR and DNA sequence analyses. Fungal Divers. 2006;23:51–66. [Google Scholar]
- Chaudhary RG, Amarjit K. Wilt disease as a cause of shift from lentil cultivation in Sangod tehsil of Kota, Rajasthan. Indian J Pulses Res. 2002;15:193–194. [Google Scholar]
- Cunha CMS, Hinz RH, Pereira A, Tcacenco FA, Paulino EC, Stadnik MJ. A SCAR marker for identifying susceptibility to Fusarium oxysporum f. sp. cubense in banana. Sci Hortic-Amsterdam. 2015;191:108–112. doi: 10.1016/j.scienta.2015.04.038. [DOI] [Google Scholar]
- Datta S, Choudhary RG, Shamim M, Singh RK, Dhar V. Molecular diversity in Indian isolates of Fusarium oxysporum f. sp. lentis inciting wilt disease in lentil (Lens culinaris Medik) Afr J Biotechnol. 2011;10:7314–7323. [Google Scholar]
- Dubey SC, Singh SR, Singh B. Morphological and pathogenic variability of Indian isolates of Fusarium oxysporum f. sp. ciceris causing chickpea wilt. Arch Phytopathology PFL. 2010;43:174–189. doi: 10.1080/03235400802021108. [DOI] [Google Scholar]
- Dubey SC, Priyanka K, Upadhyay BK. Development of molecular markers and probes based on TEF-1α, β-tubulin and ITS gene sequences for quantitative detection of Fusarium oxysporum f. sp. ciceris by using real-time PCR. Phytoparasitica. 2014;42:355–366. doi: 10.1007/s12600-013-0369-y. [DOI] [Google Scholar]
- Farahani S, Morid B, Maleki M, Saberi S. Using SCAR molecular marker to detect resistance genes to Fusarium oxysporum f. sp. ciceris in chickpea cultivars and lines. Biol Forum Int J. 2015;7:1369–1376. [Google Scholar]
- Geiser DM, Jimenez-Gasco MM, Kang S, Makalowska I, Veeraraghavan N, Ward TJ, Zhang N, Kuldau GA, O’Donnell K. Fusarium-ID v. 1.0: a DNA sequence database for identifying Fusarium. Eur J Plant Pathol. 2004;110:473–479. doi: 10.1023/B:EJPP.0000032386.75915.a0. [DOI] [Google Scholar]
- Hall TA. BIOEDIT; a user friendly biological sequences alignment, editor and analysis of program for windows 95/98/NT. Nucl Acid S. 1999;41:95–98. [Google Scholar]
- Hill AL, Reeves PA, Larson RL, Fenwick AL, Hanson LE, Panella L. Genetic variability among isolates of Fusarium oxysporum from sugar beet. Plant Pathol. 2011;60:496–505. doi: 10.1111/j.1365-3059.2010.02394.x. [DOI] [Google Scholar]
- Hiremani NS, Dubey SC. Race profiling of Fusarium oxysporum f. sp. lentis causing wilt in lentil. Crop Prot. 2018;108:23–30. doi: 10.1016/j.cropro.2018.02.010. [DOI] [Google Scholar]
- Huang X, Madan A. CAP3: a DNA sequence assembly program. Genome Res. 1999;9:868–877. doi: 10.1101/gr.9.9.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Gasco MM, Milgroom MG, Jimenez-Diaz RM. Gene genealogies support Fusarium oxysporum f. sp. ciceris as a monophyletic group. Plant Pathol. 2002;51:72–77. doi: 10.1046/j.0032-0862.2001.00610.x-i1. [DOI] [Google Scholar]
- Khare MN (1980) Wilt of lentil. First Technical Report: Project Pl-480. Jawaharlal Nehru Krishi Vishwa Vidyalaya, Jabalpur, India, pp 155
- Koenig RL, Ploetz RC, Kistler HC. Fusarium oxysporum f. sp. cubense consists of a small number of divergent and globally distributed clonal lineages. Phytopathology. 1997;87:915–923. doi: 10.1094/PHYTO.1997.87.9.915. [DOI] [PubMed] [Google Scholar]
- LoBuglio KF, Pitt JI, Taylor JW. Phylogenetic analysis of two ribosomal DNA regions indicates multiple independent losses of asexual alaromyces state among sexual alaromyces state among asexual Penicillium species in subgenus Biverticullm. Mycologia. 1993;85:592–604. doi: 10.1080/00275514.1993.12026313. [DOI] [Google Scholar]
- Luongo L, Vitale S, Haegi A, Belisario A. Development of SCAR markers and PCR assay for Fusarium oxysporum f. sp. melonis race 2-specific detection. J Plant Pathol. 2012;94:193–199. [Google Scholar]
- Mendel M, Higa A. Calcium dependent bacteriophages DNA. J Mol Bio. 1970;53:159–162. doi: 10.1016/0022-2836(70)90051-3. [DOI] [PubMed] [Google Scholar]
- Mohammadi N, Goltapeh EM, Dolatabadi HK, Ahari AB, Pouralibaba H. The genetic diversity of Iranian isolates causing Fusarium wilt of lentil. Int J Agric Technol. 2011;7:1809–1822. [Google Scholar]
- Murray MG, Thompson WF. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980;8:4321–4326. doi: 10.1093/nar/8.19.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutlu N, Demirelli A, Ilbi H, Ikten C. Development of codominant SCAR markers linked to resistant gene against the Fusarium oxysporum f. sp. radicis-lycopersici. Theor Appl Genet. 2015;128:1791–1798. doi: 10.1007/s00122-015-2547-4. [DOI] [PubMed] [Google Scholar]
- Nourollahi K, Madahjalali M. Analysis of population genetic structure of Iranian Fusarium oxysporum f. sp. lentis isolates using microsatellite markers. Australas Plant Path. 2017;46:35–42. doi: 10.1007/s13313-016-0458-8. [DOI] [Google Scholar]
- O’Donnell K. Molecular phylogeny of the Nectria haematococca- Fusarium solani species complex. Mycologia. 2000;92:919–938. doi: 10.2307/3761588. [DOI] [Google Scholar]
- O’Donnell K, Kistler HC, Cigelnik E, Ploetz RC. Multiple evolutionary origins of the fungus causing Panama disease of banana: concordant evidence from nuclear and mitochondrial gene genealogies. P Natl Acad Sci USA. 1998;95:2044–2049. doi: 10.1073/pnas.95.5.2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouralibaba HR, Rubiales D, Fondevilla S. Identification of pathotypes in Fusarium oxysporum f. sp. lentis. Eur J Plant Pathol. 2016;144:539–549. doi: 10.1007/s10658-015-0793-6. [DOI] [Google Scholar]
- Pouralibaba HR, Šatović Z, Cobos MJ, Rubiales D, Fondevilla S. Genetic diversity and structure of Fusarium oxysporum f.sp. lentis isolates from Iran, Syria and Algeria. Eur J Plant Pathol. 2018;15:10. doi: 10.1007/s10658-018-01617-7. [DOI] [Google Scholar]
- Prasanthi LR, Reddy BVB, Rani KR, Prasad YS, Rajeshwari T, Reddy KR. Development of sequence characterized amplified region (SCAR) marker for Fusarium wilt resistant gene in pigeonpea (Cajanus cajan L. Millsp.) Int J Plant Breed. 2015;3:134–138. [Google Scholar]
- Reddy JM, Raoof MA, Ulaganathan K. Development of specific markers for identification of Indian isolates of Fusarium oxysporum f. sp. ricini. Eur J Plant Pathol. 2012;134:713–719. doi: 10.1007/s10658-012-0047-9. [DOI] [Google Scholar]
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullmann A, Jacob F, Monod J. Characterization by in vitro complementation of a peptide corresponding to an operator-proximal segment of the beta-galactosidase structural gene of Escherichia coli. J Mol Biol. 1967;24:339–343. doi: 10.1016/0022-2836(67)90341-5. [DOI] [PubMed] [Google Scholar]
- Untergasser A, Cutcutache I, Koressaar T, Ye J, Faircloth BC, Remm M, Rozen SG. Primer3—new capabilities and interfaces. Nucleic Acids Res. 2012;40(15):e115. doi: 10.1093/nar/gks596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TJ, Bruns T, Lee S, Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for Phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR protocols: a guide to methods and applications. New York: Academic Press; 1990. pp. 315–322. [Google Scholar]
- Wunsch MJ, Baker AH, Kalb DW, Bergstrom GC. Characterization of Fusarium oxysporum f. sp. loti forma specialis nov., a monophyletic pathogen causing vascular wilt of birdsfoot trefoil. Plant Dis. 2009;93:58–66. doi: 10.1094/PDIS-93-1-0058. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Resource 1. Isolates of Fusarium oxysporum f. sp. lentis representing major lentil growing areas of India (PDF 197 kb)
Online Resource 2. The plates showing the blue/ white colonies after spreading (a) and only white colonies after streaking (b) a single white colony on the Luria agar plate amended with IPTG, X-gal and ampicillin after overnight incubation at 37 °C. The arrow indicates a white colony and plate B served as master plate (PDF 408 kb)
Online Resource 3. The plate showing the white colonies after streaking a single colony from master plate on the Luria agar plate amended with IPTG, X-gal and ampicillin (sub-plate) after overnight incubation at 37 °C (a), the arrows indicate single white transformed colonies. The gel picture (b) shows the amplification of inserted DNA through colony polymerase chain reaction for MS 2 marker. Lanes M- 100 bp ladder, 1- FLS 23 and 2- FLS 75 (PDF 397 kb)