Significance
The combination of two newly emerging methods for chemical synthesis enables access to molecular space that was previously challenging or impossible to access. Thus, a C–H activation of ubiquitous carboxylic acids followed by their decarboxylative functionalization provides modular access to difunctionalized carbon frameworks with distinctly controlled stereochemistry. Application of this strategy to simplify the synthesis of medicinally important entities and to discover potent antimalarial compounds is described.
Keywords: C–H activation, decarboxylative cross-coupling, modular, stereocontrolled, carboxylic acids
Abstract
The union of two powerful transformations, directed C–H activation and decarboxylative cross-coupling, for the enantioselective synthesis of vicinally functionalized alkyl, carbocyclic, and heterocyclic compounds is described. Starting from simple carboxylic acid building blocks, this modular sequence exploits the residual directing group to access more than 50 scaffolds that would be otherwise extremely difficult to prepare. The tactical use of these two transformations accomplishes a formal vicinal difunctionalization of carbon centers in a way that is modular and thus, amenable to rapid diversity incorporation. A simplification of routes to known preclinical drug candidates is presented along with the rapid diversification of an antimalarial compound series.
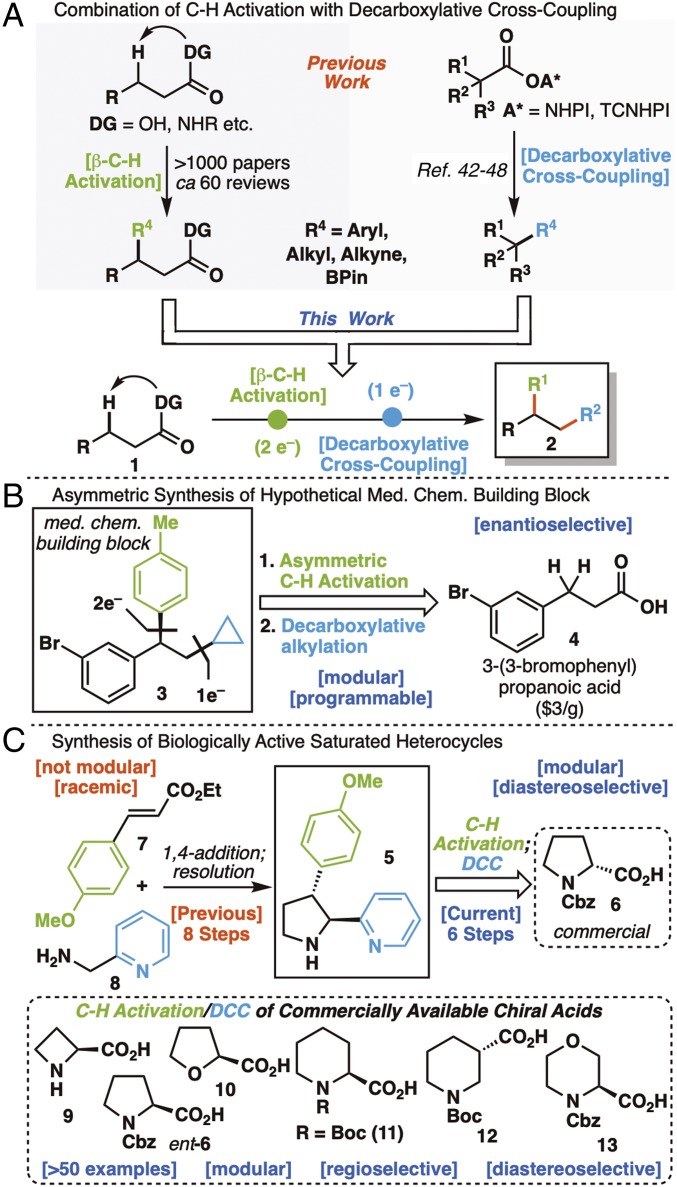
It is becoming increasingly clear that practitioners are no longer bound by the notion that the pervasive C–H bond is unresponsive to manipulation. In fact, the past two decades have seen a dramatic increase in the use of C–H functionalization logic (1–3) to assemble molecules (4–16). At this juncture, it can be considered part of the mainstream in terms of the way that students learn retrosynthetic analysis (17, 18). One of today’s workhorse C–H activation strategies involves the use of native functional groups to direct and guide the site of functionalization (19–24). As the most ubiquitous functional group in organic chemistry, carboxylic acids and their derivatives have naturally risen to the top in terms of directed C–H functionalization reactions available to the practitioner (Fig. 1A) (25–29). With over 1,000 reports now present for the use of such guided C–H activations (1–3) in synthesis, it is fair to say that this is a staple reaction manifold for modern organic synthesis. In this context, an exploration of serial reactivity in which the lingering carboxylate group is used in successive reactions has been limited in scope. Of the few notable examples, nearly all are restricted to restoration of the parent carboxylic acid followed by classic reactions, such as amidation and esterification (Fig. 1A) (30, 31). The recent development of robust methods to decarboxylate such systems and programmably replace them with new C–C and C–B bonds in a stereochemically predictable way, a formal type of C–C activation, opens opportunities to leverage the power of carboxylate-directed C–H activation chemistry. This combination of one- (32) and two-electron disconnections would enable pathways to potentially valuable chiral acyclic building blocks, such as 3, that could be considered “retrosynthetically opaque,” as it is not immediately apparent how a simple building block, like 3-(3-bromophenyl) propanoic acid (4), could be used as its precursor (Fig. 1B) (33). Within the privileged realm of saturated cyclic heterocycles, such logic could be used to rapidly access libraries of enantiopure scaffolds that would be rather difficult to otherwise prepare (Fig. 1C) (34–37). For example, chiral pyrrolidines, such as 5, have previously been prepared through labor-intensive routes that require chiral resolution and are not amenable to late-stage diversity incorporation (38, 39). In stark contrast, a combination of C–H activation and radical cross-coupling strategies (33) could access the same architectures in fewer steps with exquisite control of stereochemistry and allow for diverse arenes to be installed at the end of the route starting from simple commercial carboxylic acids. The difficulty in preparing such seemingly simple molecules is directly related to the challenge of “escaping the flatland” as articulated by many in the field (40, 41). Herein, we present a strategy for the net vicinal difunctionalization of cyclic and acyclic systems via sequential functionalization initiated by stereoselective C–H activation followed by decarboxylative cross-coupling (dCC) to form a variety of C–C and C–X bonds, including aryl (42, 43), alkenyl (44), alkynyl (45), alkyl (26, 46), and boryl (47, 48). The inherent modularity of this strategic advance allows access to a wealth of acyclic and cyclic systems, some of which have been prepared before in more laborious ways. Application to a promising series of heretofore inaccessible azetidine-based antimalarial agents is also disclosed.
Fig. 1.
Introduction to the modular, stereocontrolled Cβ–H/Cα–C activation of alkyl carboxylic acids. (A) Combination of C–H activation with decarboxylative cross-coupling. (B) Asymmetric synthesis of hypothetical medicinal chemistry (Med. Chem.) building block. (C) Synthesis of biologically active saturated heterocycles.
Results and Discussion
Proof of Concept.
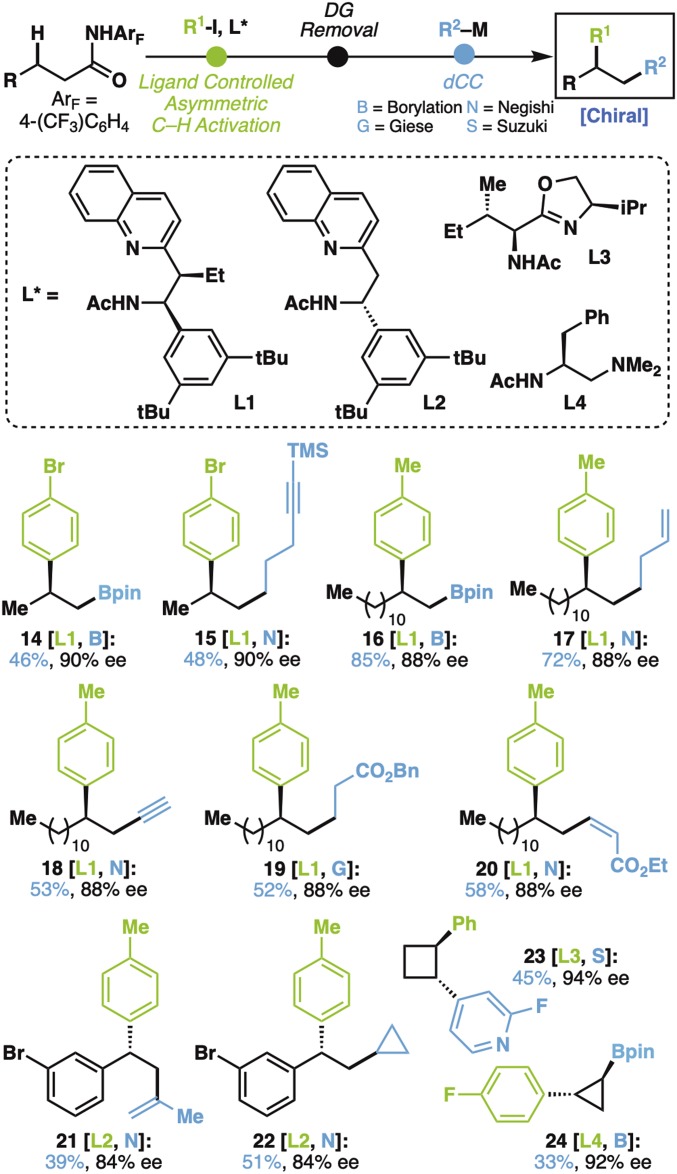
To obtain a first proof of concept for the underlying strategy, a set of enantiopure carboxylic acids, prepared using Pd-catalyzed ligand-enabled asymmetric sp3 C–H activation, was used (Fig. 2) (49–51). Recalling the suite of dCC reactions developed over the last several years (52), the succeeding reaction was found to be tolerant of a variety of pendant functional groups, including esters (19 and 20) and boronic esters (14 and 16), as well as both free (18) and silyl-capped alkynes (15), all of which can be used in yet another reaction sequence on liberation. Importantly, pyridine and boron can be incorporated in the challenging context of strained carbocycles to access trans-disubstituted cyclobutane (23) and cyclopropane (24) rings with high enantiomeric purity, the latter of which could be conducted without a directing group. The ligand-controlled nature of the C–H activation step allows for easily tunable access to either desired enantiomer (i.e., 14 vs. 21) (49–51).
Fig. 2.
Proof of concept for the Cβ–H/Cα–C activation strategy; enantiomeric excess (ee) were measured after the C–H activation step. General conditions for C–H activation reaction (SI Appendix has details): amide (1 eq), Aryl-I (2 eq), Pd(OAc)2 (10 mol %), ligand L* (12 mol %), Ag2CO3 (2 eq), hexafluoroisopropanol (HFIP) (0.1 M), 80 °C, 36 h. General conditions for directing group removal (SI Appendix has details): arylated amide (1 eq), Et2O•BF3 (35 eq), MeOH (0.025 M), 100 °C, 12 h; then, LiOH•H2O (2 eq), THF:H2O = 1:1, 0 °C, 1 h. General conditions for dCC reaction (SI Appendix has details): [N] TCNHPI ester (0.1 mmol, 1 eq), zinc reagent (0.2 mmol, 2 eq), NiCl2•glyme (30 mol %), ditBuBipy (60 mol %), THF:N,N-dimethyformamide (DMF) = 3:2, room temperature (rt), 12 h. [S] TCNHPI ester (0.1 mmol, 1 eq), boronic acid (0.3 mmol, 3 eq), NiCl2•6H2O (30 mol %), Bathophenantroline (30 mol %), Et3N (1 mmol, 10 eq), 1,4-dioxane:DMF = 10:1, 75 °C, 12 h. [G] NHPI ester (0.1 mmol, 1 eq), Michael acceptor (0.2 mmol, 2 eq), Ni(ClO4)2•6H2O (20 mol %), Zn powder (0.2 mmol, 2.0 eq), LiCl (0.3 mmol, 3 eq), MeCN, rt, 24 h. [B] TCNHPI ester (0.1 mmol, 1 eq), [B2Pin2Me]Li (0.33 mmol, 3.3 eq), NiCl2•6H2O (20 mol %), diOMeBipy (26 mol %), MgBr2•OEt2 (0.15 mmol, 1.5 eq), THF, rt, 2 h. DG, directing group.
Scope of Saturated Heterocycles.
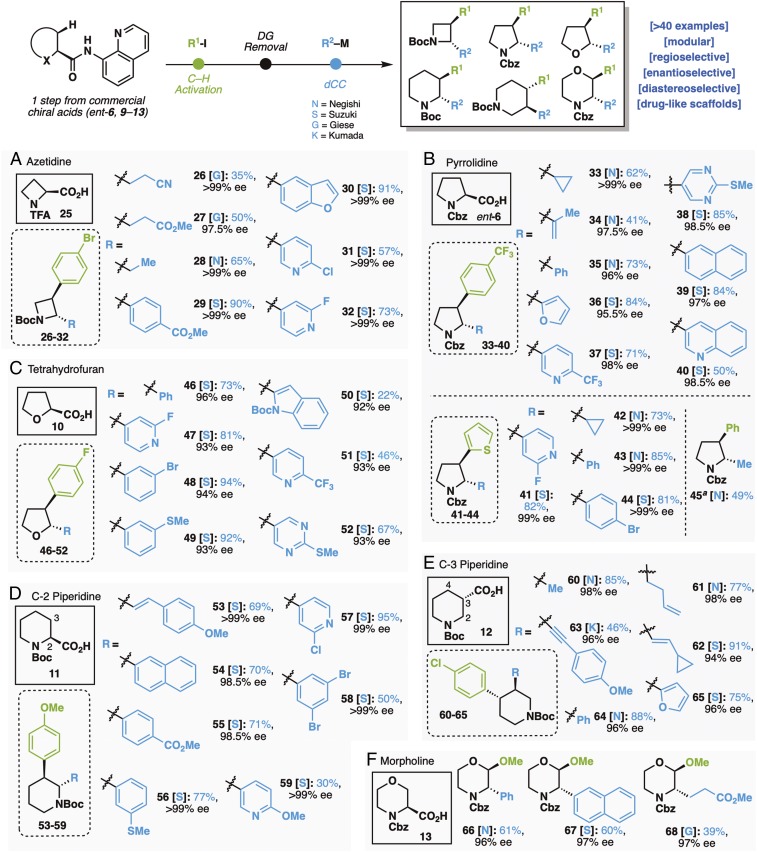
In acknowledgment of the increasing demand for saturated heterocycles in drug development (53, 54), the efficacy of this reaction sequence was demonstrated using an array of commercial heterocyclic acids (both enantiomers of each are available) as shown in Fig. 3. Beyond their importance as a framework for the celebrated β-lactam therapeutic class (55–58), azetidines have garnered recent interest as scaffolds in diversity-oriented synthesis, through which a wide variety of constrained (i.e., bridged or fused) or densely substituted nitrogenous ring systems can be accessed (59). To this point, arylated intermediates derived from N-protected azetidine-2-carboxylic acid (Fig. 3A) can be rapidly diversified under the Suzuki (29–32), Negishi (28), and Giese (26 and 27) protocols. In a similar vein, elaboration of Cbz-protected proline via directed C–H arylation at C3 followed by dCC furnished a range of enantiopure trans-1,2-difunctionalized pyrrolidines bearing diverse substituents, such as aryl (35 and 39), heteroaryl (36–38 and 40), cycloalkyl (33), and alkenyl (34) functionalities (Fig. 3B). Substrate 45 is of particular note, as prior routes to access such scaffolds involved early-stage incorporation of methyl and phenyl substituents and a tedious separation of diastereomers (60). Fig. 3C features the same titular sequence as applied to a THF core to furnish a variety of enantiopure THF-based building blocks (46–52). Among the privileged N-heterocyclic fragments, piperidine remains prevalent, with disubstitution identified as the most common decorative pattern (53, 54). Here, commercially available N-Boc-l-pipecolic acid (Fig. 3D) first furnishes the 3-(4-methoxyphenyl) analog, after which the hydrolysis/dCC sequence provides a variety of 2,3-trans-substitutions, including fused aryl (54), heteroaryl (57 and 59), and alkenyl (53) moieties at C2. All of these structures are new chemical entities despite their simplicity—even distant relatives are rare. It is difficult to conceive of a more direct and modular approach to such scaffolds with either enantiomeric form available simply by choosing l or d forms of pipecolic acid. When 3,4-piperidine substitution is desired, N-Boc-piperidine-3-carboxylic acid (Fig. 3E) may be used (60–65), as the initial C–H activation takes place selectively at C–4 vs. C–2. Given the structural similarity to the blockbuster drug Paxil (paroxetine), rapid access to such systems is noteworthy. Finally, the logic outlined above can be applied to substituted morpholines (53, 54)—one of the rare saturated, bis-heteroatom–containing systems to top frequency lists in Food and Drug Administration approvals—as detailed in Fig. 3F. Although the C–H activation step is limited to methoxylation at this juncture (SI Appendix discusses attempted C–H arylation and methylation), it does represent an example of C–H activation of such heterocycle.
Fig. 3.
Scope of saturated heterocycles. (A) Azetidine core; (B) pyrrolidine core; (C) tetrahydrofuran core; (D) C-2 piperidine core; (E) C-3 piperidine core; (F) morpholine. General conditions for C–H activation reaction (SI Appendix has details): amide (1 eq), Aryl-I (3 eq), Pd(OAc)2 (10 mol %), and AgOAc (2 eq), 110 °C, 38 h. General conditions for directing group removal (SI Appendix has details): arylated amide (1 eq), Boc2O (20 eq), 4-dimethylaminopyridine (DMAP) (3 eq), MeCN (1 M), 70 °C, 12 h. Then, LiOH•H2O (2 eq), 30% H2O2 (5.0 eq), THF:H2O = 3:1, 0 °C to room temperature (rt), 18 h. General conditions for dCC reaction (SI Appendix has details): [N] TCNHPI ester (0.1 mmol, 1 eq), zinc reagent (0.2 mmol, 2 eq), NiCl2•glyme (10–50 mol %), ditBuBipy (20–60 mol %), THF:N,N-dimethylformamide (DMF) = 3:2, rt, 12 h. [S] TCNHPI ester (0.1 mmol, 1 eq), boronic acid (0.3 mmol, 3 eq), NiCl2•6H2O (20–50 mol %), Bathophenantroline (22–60 mol %), Et3N (1 mmol, 10 eq), 1,4-dioxane:DMF = 10:1, 75 °C, 12 h. [G] TCNHPI ester (0.1 mmol, 1 eq), Michael acceptor (0.2 mmol, 2 eq), Ni(ClO4)2•6H2O (20 mol %), Zn powder (0.2 mmol, 2.0 eq), LiCl (0.3 mmol, 3 eq), MeCN, rt, 24 h. [K] TCNHPI ester (0.1 mmol, 1 eq), Grignard reagent (0.15 mmol, 1.5 eq), FeBr2•H2O (20 mol %), NMP, −15 °C, 15 min. aNo ee reported for this example, as racemic compound was not prepared. DG, directing group.
Synthesis of Hit-to-Lead Candidates and Late-Stage Intermediates.
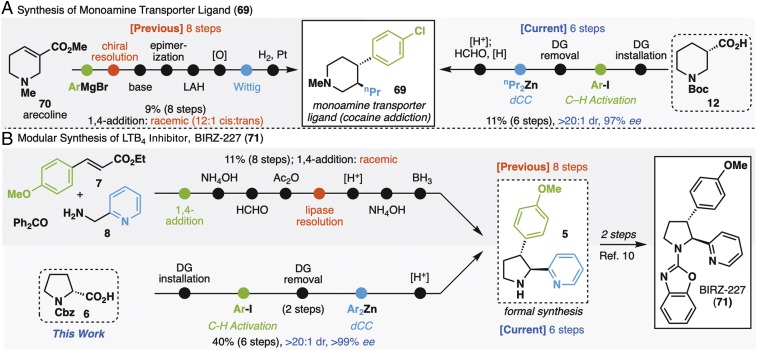
In addition to the diverse scope outlined above, the described reaction series was next evaluated for its capacity to simplify the synthesis of active hit-to-lead series and late-stage intermediates (Fig. 4). Of particular note in these case studies is how the logic presented herein can be used as both a means to effectively access a specific target or a library of similar structures simply by changing coupling partners. Monoamine transporter ligand 69 is a vivid demonstration of this (61, 62). The first synthesis of this molecule utilized conjugate addition to arecoline 70 followed by a series of functional group manipulations to arrive at 69 in 8.6% overall yield after chiral resolution (63). Use of the current vicinal difunctionalization strategy deleted many of those concession steps and could be used to access 69 (11.2% overall yield; >20:1 diastereomeric ratio; 97% enantiomeric excess) and in principle, a whole library of enantiopure analogs in only six steps. The leukotriene B4 inhibitor BIRZ-227 (Fig. 4B) (71), a trans-diarylpyrrolidine, is another prime example of how customary logic falters if a diverse, modularly assembled library is targeted. Indeed, the sole reported preparative-scale protocol to its precursor 5 opts for a pyrrolidinone construction in the initial step, placing severe limitations for rapid diversification (37, 38). To access enantiopure intermediates, an enzyme-assisted resolution is required, which itself requires multiple extraneous steps. Instead, the sequential C–H arylation/dCC approach begins from an inexpensive enantiopure amino acid building block (Pro), which is subjected to standard directing group installation, followed by C–H arylation/hydrolysis and the desired dCC to arrive at diarylated compound 5. Should further diversification be of interest, any number of analogs may be forged in short order, with no resolution necessary.
Fig. 4.
Synthesis of hit-to-lead candidates and late-stage intermediates. (A) Synthesis of monoamine transporter ligand (69); (B) modular synthesis of LBT4 inhibitor, BIRZ-227 (71). DG, directing group.
Structural Diversification of Azetidines with in Vitro Antimalarial Activity.
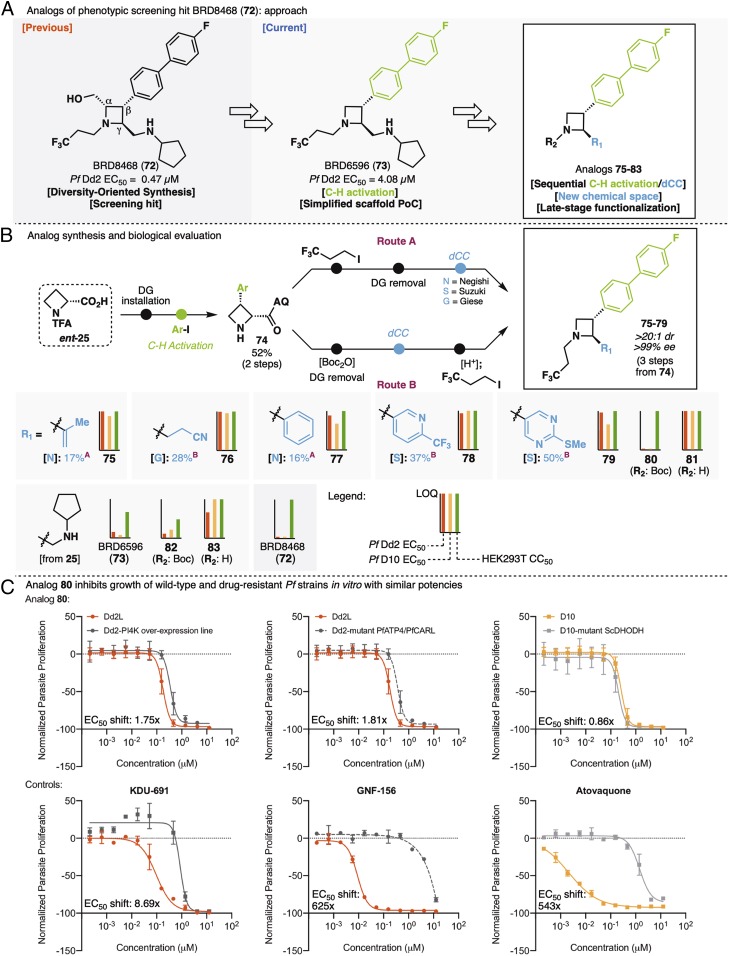
We finally applied the sequential functionalization tactic to the design and synthesis of azetidine-containing small molecules that potently inhibit the asexual blood stages of Plasmodium parasites. Plasmodium infections continue to cause over 200 million clinical cases of malaria each year, leading to an estimated 435,000 deaths in 2017 (64). Emerging resistance to frontline drugs, an issue currently curbed by means of combination therapies (64, 65), underscores the need for antimalarials that act via novel mechanisms of action (nMoA). Recently, Kato et al. (66) reported the discovery of potent nMoA antimalarials enabled by phenotypic high-throughput screening (see Malaria Therapeutics Response Portal, https://portals.broadinstitute.org/mtrp/). An unpursued promising hit from the same screening campaign is the trisubstituted azetidine BRD8468 (72; Malaria Therapeutics Response Portal) (Fig. 5A) (Malaria Therapeutics Response Portal). Importantly, BRD8468 may inhibit parasite growth via nMoA, because it remains equipotent against a panel of drug-resistant lines that have been used to identify compounds acting through known mechanisms of action, such as inhibitors of Plasmodium falciparum (Pf) ATP4, PfPI4K, and various targets in the mitochondrial electron transport chain, like PfDHODH (SI Appendix) (Pf is the deadliest species of Plasmodium that causes malaria in humans). Also of note, Cγ-epimer BRD5530 showed significantly lower potency than BRD8468 (up to 25-fold) (SI Appendix), indicating in turn, that the trans-relationship between the biaryl substituent and the vicinal alkyl group is critical for activity. In this context, we envisioned a structural simplification of BRD8468 consisting of the removal of the hydroxymethyl group at Cα. If successful (i.e., conducive to potent analogs), this strategy would enable both (i) significant abbreviation of synthetic routes, rendering the chemical series more attractive in terms of developability [guidelines on antimalarial development, including recommended maximal cost of goods, are in Burrows et al. (67)] and (ii) late-stage exploration of chemical space via the sequential C–H activation/dCC tactic described above. The Diversity-Oriented Synthesis compound collection that originally included BRD8468 72 was constructed via functionalization of a Cγ-nitrile (21) (SI Appendix)—the ability to instead perform dCC at Cγ would, therefore, allow the preparation of a host of previously inaccessible analogs. To establish proof of concept, we first synthesized disubstituted azetidine 73 (Fig. 5A). As expected, this could be achieved in four steps from C–H arylation product 74 (Fig. 5B). Encouragingly, in vitro evaluation of 73 showed that the structural simplification only led to a modest loss in potency (fourfold in PfD10) relative to parent compound 72, and no significant shift in potency was observed on treatment of drug-resistant strains compared with their wild-type counterparts. Given this positive preliminary result, two synthetic routes were designed to rapidly access simplified, structurally novel Cγ analogs of 72 (Routes A and B in Fig. 5B). The former would enable late-stage diversification at Cγ; the latter would be optimal for diversification at nitrogen, although outside the scope of this study. In practice, Route A led to vinyl and aryl analogs (75 and 77, respectively) (Fig. 5B) via Negishi-type dCC, albeit in low yield. Route B, in which the dCC step is performed on a Boc-protected azetidine intermediate, allowed higher-yielding dCC. Suzuki-dCC readily led to pyridine 78 and pyrimidine 79; Giese-dCC, in turn, gave rise to nitrile 76. Evaluation in vitro of these N-trifluoroalkyl analogs revealed inhibition of Pf growth with only micromolar potencies (Fig. 5B; details are in SI Appendix, Table S11), with pyrimidine 79 being one of the more active congeners (PfD10 EC50 = 10.3 µM; PfDd2 EC50 = 12.3 µM). Generally, no effect was observed on human cell line HEK293T at concentrations below 20 µM, suggesting that these compounds lack overt toxicity in human cells. Given that the parent scaffold (BRD8468; 72) had previously been shown to tolerate modifications at the azetidine nitrogen (Malaria Therapeutics Response Portal), we also evaluated 80 and 81, tert-butyl-carbamate and N-unsubstituted analogs (and synthetic intermediates), respectively, of 79. For reference, we introduced similar modifications on BRD6596 73, leading, in turn, to carbamate 82 and N-unsubstituted azetidine 83. Potencies varied following the same trend in both series of N-substitutions, with EC50 values decreasing in the order H > alkyl > carbamate (Fig. 5B). We were, however, delighted to find that C2-pyrimidine analog 80 [PfD10 EC50 = 0.27 µM; PfDd2 EC50 = 0.17 µM; HEK293T cytotoxic concentration 50 (CC50) > 20 µM], accessible in short order via sequential C–H arylation and dCC, exhibited superior potency and selectivity relative to both BRD6596 (73; PfD10 EC50 = 1.62 µM; PfDd2 EC50 = 4.08 µM; HEK293T CC50 = 16.7 µM) and the original screening hit BRD8468 (72; PfD10 EC50 = 0.38 µM; PfDd2 EC50 = 0.47 µM; HEK293T CC50 > 20 µM). Finally, we demonstrated that 80 retains activity against drug-resistant Pf strains (Fig. 5C and SI Appendix, Table S12). Specifically, 80 remained equipotent against a PfPI4K overexpression line, a PfCARL/PfATP4 mutant, and transgenic Pf-expressing ScDHODH (less than two times EC50 shifts relative to wild-type parasites). In good agreement with previously reported data, these parasites were resistant to KDU-691 (8.7× EC50 shift) (68), GNF-156 (625× EC50 shift) (69), and atovaquone (543× EC50 shift) (70), respectively. As mentioned previously, these observations suggest that 80 may inhibit parasite growth via nMoA and therefore, warrant additional study. To this end, additional structural modifications at Cγ and nitrogen and their impact on antimalarial activity are now being investigated. Although certainly preliminary, the encouraging results reported here demonstrate how the C–H activation/dCC tactic may easily and productively augment the scope of medicinal chemistry campaigns.
Fig. 5.
Structural diversification of azetidines with in vitro antimalarial activity. (A) Analogs of phenotypic screening hit BRD8488 (72): approach; (B) analog synthesis and biological evaluation; (C) analog 80 inhibits growth of wild-type and drug-resistant Pf strains in vitro with similar potencies. DG, directing group.
Limitations.
The strategy presented herein is not without limitations. Foremost among these is the unfortunate requirement for directing group installation and removal, as the free carboxylates can only be utilized for cyclopropane substrates (e.g., 24). For example, despite extensive efforts, piperazine heterocycles are completely inert to C–H activation attempts (SI Appendix has details). In addition, when the C–H alkynylation, alkoxylation, and fluorination of nitrogen-based saturated heterocycles were successfully achieved, the subsequent removal of the directing group has proven challenging. A trans-relationship between vicinal substituents is also an implicit limitation of the sequence. Finally, it is worth noting that, although an asymmetric conjugate addition strategy could be envisaged to access acyclic (Fig. 2) and C–3 piperidine systems (Fig. 3E), the remaining scaffolds would be difficult to obtain, as asymmetric conjugate addition to heterocyclic enamides and enol ethers is poorly developed.
Conclusion.
The tactical combination of two powerful, recently invented transformations (carboxylate-guided C–H activation and dCC) can thus be brought to bear to simplify the preparation of useful enantiopure building blocks (71). This formal vicinal difunctionalization permits the modular installation of an almost limitless variety of trans-1,2-disubstituted frameworks bearing aryl, alkyl, ether, and boron functional groups. Two distinct applications of these combined polar/radical retrosynthetic disconnections illustrate the power of this approach. Finally, the logic outlined herein shows promise in accessing a generation of antimalarial leads based on a diverse library of azetidine scaffolds.
Materials and Methods
All reagents were commercially available and used as supplied without additional purification. Solvents were obtained by passing the previously degassed solvents through an activated alumina column. The details of the materials, methods (including synthesis and characterization of compounds), and reaction optimizations are described in SI Appendix.
Note.
During the preparation of this manuscript, an elegant combination of C–H activation and dCC was instituted by Reisman and coworkers (71) for the synthesis of cyclobutene-based natural products and related molecules.
Supplementary Material
Acknowledgments
We thank Dr. Dee-Hua Huang and Dr. Laura Pasternack (Scripps Research) for assistance with NMR spectroscopy; Dr. Jason Chen and Brittany Sanchez (Scripps Automated Synthesis Facility) for assistance with HPLC, high-resolution mass spectrometry, and liquid chromatography mass spectrometry; and Dr. Arnold Rheingold, Dr. Curtis Moore, and Dr. Milan Gembicky (University of California, San Diego) for assistance with X-ray crystallographic analysis. We also thank Cindy Hon (Broad Institute) for logistical support, Dr. Florence Wagner and Ryan Babcock (Broad Institute) for assistance obtaining analytical samples, Dr. Eamon Comer (Broad Institute) for helpful discussions regarding compound synthesis, and Dr. Guoqin Xia (Scripps Research) for sharing valuable reagents. Financial support for this work was provided by NIH Grant GM-118176, Bill & Melinda Gates Foundation Grant OPP11823830, Vividion, Zhejiang Yuanhong Medicine Technology Co. Ltd. (M.S.), São Paulo Research Foundation Grant 2017/08128-7 (to K.S.F.), NSF Graduate Research Fellowship Program (L.M.B.), the China Scholarship Council (L.H. and S.N.), and Spanish Ministerio de Economía, Industria y Competitividad Formacion de Personal Investigador Predoctoral Fellowships BES-2014-069879 (to P.F.-C.) and EEBB-I-2018-12962 (to P.F.-C.).
Footnotes
Conflict of interest statement: S.L.S. is a member of the Board of Directors of the Genomics Institute of the Novartis Research Foundation (GNF); a shareholder and member of the Board of Directors of Jnana Therapeutics; a shareholder of Forma Therapeutics; a shareholder of and adviser to Decibel Therapeutics; an adviser to Eisai, Inc., the Ono Pharma Foundation, and F-Prime Capital Partners; and a Novartis Faculty Scholar. J.-Q.Y. and P.S.B. are cofounders of Vividion.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1903048116/-/DCSupplemental.
References
- 1.Gutekunst WR, Baran PS. C-H functionalization logic in total synthesis. Chem Soc Rev. 2011;40:1976–1991. doi: 10.1039/c0cs00182a. [DOI] [PubMed] [Google Scholar]
- 2.Brückl T, Baxter RD, Ishihara Y, Baran PS. Innate and guided C-H functionalization logic. Acc Chem Res. 2012;45:826–839. doi: 10.1021/ar200194b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamaguchi J, Yamaguchi AD, Itami K. C-H bond functionalization: Emerging synthetic tools for natural products and pharmaceuticals. Angew Chem Int Ed Engl. 2012;51:8960–9009. doi: 10.1002/anie.201201666. [DOI] [PubMed] [Google Scholar]
- 4.Arndtsen BA, et al. Selective intermolecular carbon-hydrogen bond activation by synthetic metal complexes in homogeneous solution. Acc Chem Res. 1995;28:154–162. [Google Scholar]
- 5.Shilov AE, Shul’pin GB. Activation of Cminus signH bonds by metal complexes. Chem Rev. 1997;97:2879–2932. doi: 10.1021/cr9411886. [DOI] [PubMed] [Google Scholar]
- 6.Labinger JÁ, Bercaw JE. Understanding and exploiting C-H bond activation. Nature. 2002;417:507–514. doi: 10.1038/417507a. [DOI] [PubMed] [Google Scholar]
- 7.Alberico D, Scott ME, Lautens M. Aryl-aryl bond formation by transition-metal-catalyzed direct arylation. Chem Rev. 2007;107:174–238. doi: 10.1021/cr0509760. [DOI] [PubMed] [Google Scholar]
- 8.Chen X, Engle KM, Wang DH, Yu JQ. Palladium(II)-catalyzed C-H activation/C-C cross-coupling reactions: Versatility and practicality. Angew Chem Int Ed Engl. 2009;48:5094–5115. doi: 10.1002/anie.200806273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daugulis O, Do H-Q, Shabashov D. Palladium- and copper-catalyzed arylation of carbon-hydrogen bonds. Acc Chem Res. 2009;42:1074–1086. doi: 10.1021/ar9000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jazzar R, Hitce J, Renaudat A, Sofack-Kreutzer J, Baudoin O. Functionalization of organic molecules by transition-metal-catalyzed C(sp3)-H activation. Chemistry. 2010;16:2654–2672. doi: 10.1002/chem.200902374. [DOI] [PubMed] [Google Scholar]
- 11.He J, Wasa M, Chan KSL, Shao Q, Yu JQ. Palladium-catalyzed transformations of alkyl C-H bonds. Chem Rev. 2017;117:8754–8786. doi: 10.1021/acs.chemrev.6b00622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karimov RR, Hartwig JF. Transition-metal-catalyzed selective functionalization of C(sp3 )-H bonds in natural products. Angew Chem Int Ed Engl. 2018;57:4234–4241. doi: 10.1002/anie.201710330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ackermann L. Carboxylate-assisted ruthenium-catalyzed alkyne annulations by C-H/Het-H bond functionalizations. Acc Chem Res. 2014;47:281–295. doi: 10.1021/ar3002798. [DOI] [PubMed] [Google Scholar]
- 14.Huang Z, Lim HN, Mo F, Young MC, Dong G. Transition metal-catalyzed ketone-directed or mediated C-H functionalization. Chem Soc Rev. 2015;44:7764–7786. doi: 10.1039/c5cs00272a. [DOI] [PubMed] [Google Scholar]
- 15.Lyons TW, Sanford MS. Palladium-catalyzed ligand-directed C-H functionalization reactions. Chem Rev. 2010;110:1147–1169. doi: 10.1021/cr900184e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Z, et al. Transition metal-catalyzed C–H bond functionalizations by the use of diverse directing groups. Org Chem Front. 2015;2:1107–1295. [Google Scholar]
- 17.Corey EJ, Cheng X-M. The Logic of Chemical Synthesis. Wiley; New York: 1995. [Google Scholar]
- 18.Reddy BVS, Reddy LR, Corey EJ. Novel acetoxylation and C-C coupling reactions at unactivated positions in α-amino acid derivatives. Org Lett. 2006;8:3391–3394. doi: 10.1021/ol061389j. [DOI] [PubMed] [Google Scholar]
- 19.Jain P, Verma P, Xia G, Yu JQ. Enantioselective amine α-functionalization via palladium-catalysed C-H arylation of thioamides. Nat Chem. 2017;9:140–144. doi: 10.1038/nchem.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schinkel M, Wang L, Bielefeld K, Ackermann L. Ruthenium(II)-catalyzed C(sp3)-H α-alkylation of pyrrolidines. Org Lett. 2014;16:1876–1879. doi: 10.1021/ol500300w. [DOI] [PubMed] [Google Scholar]
- 21.Prokopcová H, et al. C-2 arylation of piperidines through directed transition-metal-catalyzed sp3 C-H activation. Chemistry. 2010;16:13063–13067. doi: 10.1002/chem.201001887. [DOI] [PubMed] [Google Scholar]
- 22.Pastine SJ, Gribkov DV, Sames D. sp3 C-H bond arylation directed by amidine protecting group: α-arylation of pyrrolidines and piperidines. J Am Chem Soc. 2006;128:14220–14221. doi: 10.1021/ja064481j. [DOI] [PubMed] [Google Scholar]
- 23.Chatani N, et al. Carbonylation at sp3 C−H bonds adjacent to a nitrogen atom in alkylamines catalyzed by rhodium complexes. J Am Chem Soc. 2000;122:12882–12883. [Google Scholar]
- 24.Wang B, et al. Palladium-catalyzed trifluoroacetate-promoted mono-arylation of the β-methyl group of alanine at room temperature: Synthesis of β-arylated α-amino acids through sequential C–H functionalization. Chem Sci (Camb) 2014;5:3952–3957. [Google Scholar]
- 25.Goossen LJ, Rodríguez N, Goossen K. Carboxylic acids as substrates in homogeneous catalysis. Angew Chem Int Ed Engl. 2008;47:3100–3120. doi: 10.1002/anie.200704782. [DOI] [PubMed] [Google Scholar]
- 26.Qin T, et al. A general alkyl-alkyl cross-coupling enabled by redox-active esters and alkylzinc reagents. Science. 2016;352:801–805. doi: 10.1126/science.aaf6123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Engle KM, Mei TS, Wasa M, Yu JQ. Weak coordination as a powerful means for developing broadly useful C-H functionalization reactions. Acc Chem Res. 2012;45:788–802. doi: 10.1021/ar200185g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Novák P, Correa A, Gallardo-Donaire J, Martin R. Synergistic palladium-catalyzed C(sp3)-H activation/C(sp3)-O bond formation: A direct, step-economical route to benzolactones. Angew Chem Int Ed Engl. 2011;50:12236–12239. doi: 10.1002/anie.201105894. [DOI] [PubMed] [Google Scholar]
- 29.Giri R, et al. Palladium-catalyzed methylation and arylation of sp2 and sp3 C-H bonds in simple carboxylic acids. J Am Chem Soc. 2007;129:3510–3511. doi: 10.1021/ja0701614. [DOI] [PubMed] [Google Scholar]
- 30.Affron DP, Davis OA, Bull JA. Regio- and stereospecific synthesis of C-3 functionalized proline derivatives by palladium catalyzed directed C(sp3)-H arylation. Org Lett. 2014;16:4956–4959. doi: 10.1021/ol502511g. [DOI] [PubMed] [Google Scholar]
- 31.Antermite D, Affron DP, Bull JÁ. Regio- and stereoselective palladium-catalyzed C(sp3)-H arylation of pyrrolidines and piperidines with C(3) directing groups. Org Lett. 2018;20:3948–3952. doi: 10.1021/acs.orglett.8b01521. [DOI] [PubMed] [Google Scholar]
- 32.Smith JM, Harwood SJ, Baran PS. Radical retrosynthesis. Acc Chem Res. 2018;51:1807–1817. doi: 10.1021/acs.accounts.8b00209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Myers AG, Movassaghi M. Highly efficient methodology for the reductive coupling of aldehyde tosylhydrazones with alkyllithium reagents. J Am Chem Soc. 1998;120:8891–8892. [Google Scholar]
- 34.Vo CVT, Bode JW. Synthesis of saturated N-heterocycles. J Org Chem. 2014;79:2809–2815. doi: 10.1021/jo5001252. [DOI] [PubMed] [Google Scholar]
- 35.Wolfe JP. Palladium-catalyzed carboetherification and carboamination reactions of γ-hydroxy- and γ-aminoalkenes for the synthesis of tetrahydrofurans and pyrrolidines. Eur J Org Chem. 2007;2007:571–582. [PMC free article] [PubMed] [Google Scholar]
- 36.Schultz DM, Wolfe JP. Recent developments in Pd-catalyzed alkene aminoarylation reactions for the synthesis of nitrogen heterocycles. Synthesis (Stuttg) 2012;44:351–361. doi: 10.1055/s-0031-1289668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blakemore DC, et al. Organic synthesis provides opportunities to transform drug discovery. Nat Chem. 2018;10:383–394. doi: 10.1038/s41557-018-0021-z. [DOI] [PubMed] [Google Scholar]
- 38.Yee NKA. A Practical one-pot synthesis of trans-4,5-disubstituted 2-pyrrolidinones and the related pyrrolidines. Tetrahedron Lett. 1997;38:5091–5094. [Google Scholar]
- 39.Yee NK, Nummy LJ, Byrne DP, Smith LL, Roth GP. Practical synthesis of an enantiomerically pure trans-4,5-disubstituted 2-pyrrolidinone via enzymatic resolution. Preparation of the LTB4 inhibitor BIRZ-227. J Org Chem. 1998;63:326–330. [Google Scholar]
- 40.Lovering F, Bikker J, Humblet C. Escape from flatland: Increasing saturation as an approach to improving clinical success. J Med Chem. 2009;52:6752–6756. doi: 10.1021/jm901241e. [DOI] [PubMed] [Google Scholar]
- 41.Nielsen TE, Schreiber SL. Towards the optimal screening collection: A synthesis strategy. Angew Chem Int Ed Engl. 2008;47:48–56. doi: 10.1002/anie.200703073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cornella J, et al. Practical Ni-catalyzed aryl-alkyl cross-coupling of secondary redox-active esters. J Am Chem Soc. 2016;138:2174–2177. doi: 10.1021/jacs.6b00250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang J, et al. Nickel-catalyzed cross-coupling of redox-active esters with boronic acids. Angew Chem Int Ed Engl. 2016;55:9676–9679. doi: 10.1002/anie.201605463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Edwards JT, et al. Decarboxylative alkenylation. Nature. 2017;545:213–218. doi: 10.1038/nature22307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith JM, et al. Decarboxylative alkynylation. Angew Chem Int Ed Engl. 2017;56:11906–11910. doi: 10.1002/anie.201705107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qin T, et al. Nickel-catalyzed barton decarboxylation and giese reactions: A practical take on classic transforms. Angew Chem Int Ed Engl. 2017;56:260–265. doi: 10.1002/anie.201609662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li C, et al. Decarboxylative borylation. Science. 2017;356:eaam7355. doi: 10.1126/science.aam7355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang J, et al. Cu-catalyzed decarboxylative borylation. ACS Catal. 2018;8:9537–9542. doi: 10.1021/acscatal.8b02928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen G, et al. Ligand-accelerated enantioselective methylene C(sp3)-H bond activation. Science. 2016;353:1023–1027. doi: 10.1126/science.aaf4434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu QF, Wang XB, Shen PX, Yu JQ. Enantioselective C-H arylation and vinylation of cyclobutyl carboxylic amides. ACS Catal. 2018;8:2577–2584. doi: 10.1021/acscatal.8b00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shen PX, Hu L, Shao Q, Hong K, Yu JQ. Pd(II)-catalyzed enantioselective C(sp3)-H arylation of free carboxylic acids. J Am Chem Soc. 2018;140:6545–6549. doi: 10.1021/jacs.8b03509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen TG, et al. Building C(sp3)-rich complexity by combining cycloaddition and C-C cross-coupling reactions. Nature. 2018;560:350–354. doi: 10.1038/s41586-018-0391-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vitaku E, Smith DT, Njardarson JT. Analysis of the structural diversity, substitution patterns, and frequency of nitrogen heterocycles among U.S. FDA approved pharmaceuticals. J Med Chem. 2014;57:10257–10274. doi: 10.1021/jm501100b. [DOI] [PubMed] [Google Scholar]
- 54.Das P, et al. A Survey of the structures of US FDA approved combinational drugs. J Med Chem. November 30, 2018 doi: 10.1021/acs.jmedchem.8b01610. [DOI] [PubMed] [Google Scholar]
- 55.Sheehan JC. The Enchanted Ring: The Untold Story of Penicillin. MIT; Cambridge, MA: 1982. [Google Scholar]
- 56.Drawz SM, Bonomo RA. Three decades of β-lactamase inhibitors. Clin Microbiol Rev. 2010;23:160–201. doi: 10.1128/CMR.00037-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Leonard DA, Bonomo RA, Powers RA. Class D β-lactamases: A reappraisal after five decades. Acc Chem Res. 2013;46:2407–2415. doi: 10.1021/ar300327a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fisher JF, Meroueh SO, Mobashery S. Bacterial resistance to β-lactam antibiotics: Compelling opportunism, compelling opportunity. Chem Rev. 2005;105:395–424. doi: 10.1021/cr030102i. [DOI] [PubMed] [Google Scholar]
- 59.Lowe JT, et al. Synthesis and profiling of a diverse collection of azetidine-based scaffolds for the development of CNS-focused lead-like libraries. J Org Chem. 2012;77:7187–7211. doi: 10.1021/jo300974j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Veinberg G, et al. Synthesis and biological evaluation of 2-(5-methyl-4-phenyl-2-oxopyrrolidin-1-yl)-acetamide stereoisomers as novel positive allosteric modulators of sigma-1 receptor. Bioorg Med Chem. 2013;21:2764–2771. doi: 10.1016/j.bmc.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 61.Kozikowski AP, et al. 2000. US Patent WO2000/020390.
- 62.Petukhov PA, et al. Synthesis, molecular modeling, and biological studies of novel piperidine-based analogues of cocaine: Evidence of unfavorable interactions proximal to the 3alpha-position of the piperidine ring. J Med Chem. 2004;47:3009–3018. doi: 10.1021/jm0303296. [DOI] [PubMed] [Google Scholar]
- 63.Mobele BI, et al. Process development toward the pilot scale synthesis of the piperidine-based cocaine analogue and potent dopamine and norepinephrine reuptake inhibitor CTDP 31,446. Org Process Res Dev. 2006;10:914–920. [Google Scholar]
- 64.WHO 2018 World malaria report. Available at https://www.who.int/malaria/publications/world-malaria-report-2018/report/en/. Accessed December 3, 2018.
- 65.Flannery EL, Chatterjee AK, Winzeler EA. Antimalarial drug discovery–Approaches and progress towards new medicines. Nat Rev Microbiol. 2013;11:849–862. doi: 10.1038/nrmicro3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kato N, et al. Diversity-oriented synthesis yields novel multistage antimalarial inhibitors. Nature. 2016;538:344–349. doi: 10.1038/nature19804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Burrows JN, et al. New developments in anti-malarial target candidate and product profiles. Malar J. 2017;16:26, and erratum (2017) 16:151. doi: 10.1186/s12936-017-1809-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McNamara CW, et al. Targeting Plasmodium PI(4)K to eliminate malaria. Nature. 2013;504:248–253. doi: 10.1038/nature12782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lim MYX, et al. UDP-galactose and acetyl-CoA transporters as Plasmodium multidrug resistance genes. Nat Microbiol. 2016;1:16166. doi: 10.1038/nmicrobiol.2016.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Painter HJ, Morrisey JM, Mather MW, Vaidya AB. Specific role of mitochondrial electron transport in blood-stage Plasmodium falciparum. Nature. 2007;446:88–91. doi: 10.1038/nature05572. [DOI] [PubMed] [Google Scholar]
- 71.Beck JC, Lacker CR, Chapman LM, Reisman SE. A modular approach to prepare enantioenriched cyclobutanes: Synthesis of (+)-rumphellaone A. Chem Sci. 2018;10:2315–2319. doi: 10.1039/c8sc05444d. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.