Significance
Addiction is characterized by compulsive drug use despite negative consequences. In a rat model of methamphetamine self-administration, we found that compulsive-like drug taking was correlated with alterations in the balance between an increased orbitofrontal cortex-dorsomedial striatal “go” circuit and a decreased prelimbic cortex-ventrolateral striatal “stop” circuit only in an addictive-like subgroup resistant to foot shock punishment paired with drug taking. In nonaddictive-like rats, the go-stop balance, which was similarly changed after repeated self-administration without punishment, normalized toward baseline levels after punishment. These results indicate that restoring the go-stop physiological balance, perhaps with noninvasive brain stimulation, may provide a promising avenue for addiction treatment when coupled with neuroimaging to assess clinical responses to determine the adequacy of a course of treatment.
Keywords: methamphetamine self-administration, compulsive behavior, frontostriatal functional circuits, functional connectivity, foot shock punishment
Abstract
Substance use disorders (SUDs) impose severe negative impacts upon individuals, their families, and society. Clinical studies demonstrate that some chronic stimulant users are able to curtail their drug use when faced with adverse consequences while others continue to compulsively use drugs. The mechanisms underlying this dichotomy are poorly understood, which hampers the development of effective individualized treatments of a disorder that currently has no Food and Drug Administration-approved pharmacological treatments. In the present study, using a rat model of methamphetamine self-administration (SA) in the presence of concomitant foot shocks, thought to parallel compulsive drug taking by humans, we found that SA behavior correlated with alterations in the balance between an increased orbitofrontal cortex-dorsomedial striatal “go” circuit and a decreased prelimbic cortex-ventrolateral striatal “stop” circuit. Critically, this correlation was seen only in rats who continued to self-administer at a relatively high rate despite receiving foot shocks of increasing intensity. While the stop circuit functional connectivity became negative after repeated SA in all rats, “shock-resistant” rats showed strengthening of this negative connectivity after shock exposure. In contrast, “shock-sensitive” rats showed a return toward their baseline levels after shock exposure. These results may help guide novel noninvasive brain stimulation therapies aimed at restoring the physiological balance between stop and go circuits in SUDs.
Substance use disorder (SUD) is a chronic brain disease characterized by compulsive drug seeking and taking despite harmful consequences (1, 2). Numerous findings suggest that chronic drug administration leads to maladaptive neural plasticity in widespread brain systems modulated by complex interactions between drug, genetics, and environment (1, 3–5). While dysfunctions in striatal circuits may underlie distinct features of the addiction phenotype (3–7), both preclinical (8–10) and clinical models (11–13) point toward dysregulated frontostriatal circuits contributing to the compulsive behaviors defining the addiction phenotype. More specifically, exaggerated motivation/drive for drug use underlain by an enhanced orbitofrontal cortex (OFC)-striatal circuit and compromised executive/inhibitory control underpinned by an impaired prefrontal cortex (PFC)-striatal circuit, along with alterations in limbic- and motor-striatal pathways, are thought to differentiate the brains of those addicted to drugs from those who are not (6). Using resting state functional connectivity (rsFC), which characterizes the interregional relationship between two or more brain regions, circuits, or networks (14, 15), we previously demonstrated that the balance in the strength between an OFC-superior ventral striatum (hypothesized “go”) circuit and a dorsal anterior cingulate cortex (dACC)-inferior ventral striatum (hypothesized “stop”) circuit correlates positively with those DSM-IV (16) diagnostic symptoms concerning loss-of-control over drug use (12). Taken together with other findings (8, 9, 11), these data suggest that pathological dysregulation of OFC- and dACC-striatal circuits plays a critical role in the development of compulsive addiction behavior.
However, a complete picture of the dynamic circuit plasticity that occurs across the full trajectory of drug use—initiation, maintenance and recovery from the disease—has not yet been delineated. Due to logistical and ethical limitations encountered when studying human SUD individuals, most of our knowledge comes from cross-sectional studies between drug-dependent and healthy control individuals examined at a single time point, generally when the disease is fully manifest and clinically diagnosed. Therefore, the trajectory of neural circuit plasticity preceding the disease and following prolonged abstinence have rarely been investigated (13, 17).
Importantly, both clinical (18, 19) and preclinical (8–10, 19–21) studies have clearly demonstrated that only a relatively small portion of recreational drug users progress to compulsive drug use. Longitudinal preclinical studies have further demonstrated that there are no discernable differences in drug self-administration (SA) behavior between addictive-like and nonaddictive-like subgroups before the introduction of a negative consequence (8–10, 19–21), raising the question of when and how punishment-resistant individuals develop differential neural processes that support compulsive drug use despite negative consequences.
To address these questions, we performed a longitudinal fMRI study (Fig. 1A) using a rat model of stimulant SUD (20, 21). Our goal was to (i) identify and then assess putative go and stop circuit alterations across the different stages of the addiction trajectory, and (ii) examine the relationship between these circuits and the selective development of compulsive SA behavior for methamphetamine (METH), a widely abused stimulant drug (22). Based upon previous human and preclinical studies (6, 8–10, 12), we hypothesized (i) up-regulation of an OFC-striatal circuit and down-regulation of a prelimbic (PrL) cortex (homolog of human dACC; ref. 23)-striatal circuit, after the development of METH SA and subsequent introduction of concomitant punishment, and (ii) that the balance between the OFC-striatal and PrL-striatal connectivity will be associated with compulsive-like SA behaviors.
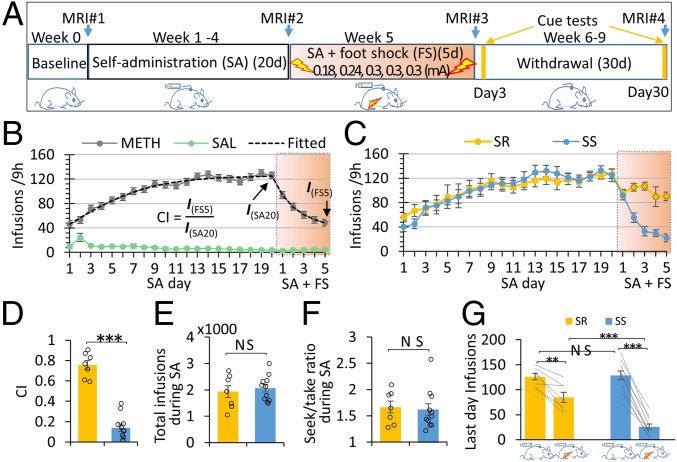
Fig. 1.
Experimental design and behavioral results. (A) Two groups of rats METH (n = 18) and SAL (n = 11) experienced four distinct conditions designed to model the addiction cycle: baseline, SA training (9 h/d for 20 d, FR-1, 0.1 mg/kg per infusion of METH/SAL), SA plus foot shock, and withdrawal (cue-reactivity tests performed on withdrawal days 3 and 30). MRI data were collected at the end of each phase. (B) The METH SA rats increased lever pressing during the SA development phase (one-way ANOVA, P < 0.001 for both groups), and SA behavior decreased when foot shocks were introduced (one-way ANOVA, P < 0.001 for both groups). Drug intake was computationally modeled, and the estimated infusion on the last punishment day was normalized to that on the last SA development day to define a CI. (C) K-mean clustering on the CI differentiated METH rats into SR (n = 7) and SS (n = 11) subgroups. While both subgroups reduced drug taking after shock imposition, the SR but not the SS subgroup “recovered” and took more drug after the second shock day. There was no difference in SA behavior between the two subgroups before shock imposition. (D) The SR group showed significantly higher CI than the SS subgroup. (E) The total number of drug infusions during the SA development phase did not predict the subsequent categorization of SR and SS rats. (F) The total number of lever presses normalized by drug infusions (seek/take ratio) during SA phase did not differ between subgroups. (G) While both SS and SR rats significantly reduced drug intake at the end of SA+FS phase, the SR group took more drug than did the SS group, although there was no difference between them at the end of SA phase. #P < 0.1; *P < 0.05; **P < 0.01; ***P < 0.001; NS, not significant; error bar stands for SEM.
Results
Behavioral Results.
Groups of rats were trained to press a lever to obtain saline (SAL, n = 11) or METH (n = 18; 0.1 mg/kg per infusion) for 20 d (Fig. 1A). A 5-s tone-light compound cue was paired with the active (METH or SAL) lever but not with an inactive (control) lever. The METH group quickly escalated their drug intake during the SA phase [repeated measure one-way ANOVA of infusions from SA day 1–20, F(4.91,73.67) = 25.34, P < 0.00001], whereas the SAL group maintained a very low level of responding on both active and inactive levers (Fig. 1B and SI Appendix, Fig. S3 A and B). When drug SA was paired with foot shock (FS) during the subsequent 5-d punishment phase, the METH rats as a group reduced their SA behavior and drug intake [repeated measure one-way ANOVA of infusions from the last SA day to the last FS day, F(2.63, 44.64) = 22.81, P < 0.00001, Fig. 1B, see SI Appendix, Fig. S3B for lever presses].
The escalation during the SA phase and the decreased SA during the punishment phase were computationally modeled (SI Appendix, Fig. S2, also see Methods). Model estimate of SA on the last FS day was normalized to that on the last SA day to create a “compulsivity index (CI)” (Fig. 1B). To account for the heterogeneity in individual SA behavior, the METH group was parsed into shock-resistant (SR) and shock-sensitive (SS) subgroups using k-mean clustered CI results, yielding 7 SR and 11 SS rats (Fig. 1 C and D and SI Appendix, Fig. S3C). The SR group showed a higher CI than the SS group [SR vs. SS, T(16) = 10.24, P < 0.00001, Fig. 1D]. Critically, the total number of drug infusions during the 20-d SA phase did not differ significantly between the two subgroups [SR vs. SS, T(16) = 0.48, P = 0.64, Fig. 1E], suggesting that the total pharmacological dosage of METH received during SA acquisition cannot by itself predict future compulsive drug taking behavior. The two subgroups also did not differ significantly on the total number of active lever presses normalized to METH infusions (seek/take ratio) during SA [SS vs. SR, T(16) = 0.28, P = 0.79, Fig. 1F], once again suggesting that future compulsive-like drug seeking cannot be predicted by drug-seeking behavior and drug intake in the absence of punishment. However, the subgroups did differ after the aversive FS was introduced [repeated measure two-way ANOVA of infusions from the last SA day to the last FS day, GROUP × DAY interaction, F(5, 80) = 10.08, P < 0.0001, Fig. 1C]. While both SS and SR rats continued to SA under punishment and both subgroups significantly reduced drug intake from their preshock levels [paired t tests SR: T(6) = −4.61, P = 0.004; SS: T(10) = −14.67, P < 0.0001, Fig. 1G], SR rats responded significantly more on the active lever than the SS group [average infusions of the last two shock days, SR vs. SS, T(16) = 5.32, P = 0.00007, Fig. 1G]. The clear gap between the lowest CI of the SR subgroup and the highest CI of the SS subgroup supports the idea that clustering dichotomy reflects two truly different subgroups.
For the withdrawal phase, a (GROUP: SS, SR) × (LEVER: active, inactive) × (TIME: day 3, day 30) repeated-measures ANOVA revealed a significant main effect of LEVER (P < 0.001) and TIME (P < 0.03), which is consistent with clinical observations (24, 25) and the well-known incubation of craving phenomenon (26). There were no significant effects for GROUP or any two-way or three-way interactions (SI Appendix, Fig. S4).
Imaging Results.
Voxel-wise analyses of rsFC.
Hypothesis-driven, whole-brain voxel-wise rsFC using seeds from the OFC (Fig. 2A) and PrL (Fig. 3A) was used to assess the strength of and behavioral relationship with frontostriatal circuits across the drug-dependence cycle (i.e., predrug exposure, stable SA, after pairing FS with SA, and after protracted withdrawal). RsFC was calculated individually for each seed and each animal and was submitted to a 3 × 4 linear mixed-effects model ANOVA (27), with GROUP (SR, SS, SAL) and SESSION (baseline, SA, SA+FS, withdrawal) as factors. GROUP × SESSION interactions were of primary interest to determine longitudinal between-group differences across the four phases of the study. Both main ANOVAs and corresponding follow up analyses were corrected for whole-brain, voxel-wise multiple comparisons (Pcorr < 0.05, see Methods for details).
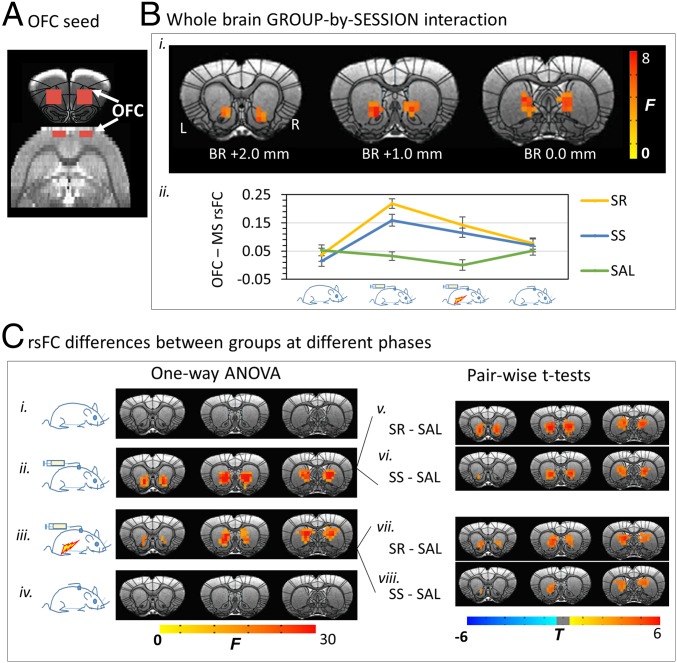
Fig. 2.
Changes in the OFC circuit in the SR, SS, and SAL groups, along the cycle of addiction. (A) OFC seed definition. (B) ANOVAs [(GROUP: SR, SS, SAL) × (SESSION: baseline, SA, SA+FS, withdrawal)] revealed a significant GROUP-by-SESSION interaction in rsFC between the OFC and MS (i); the average rsFC from the MS mask was plotted as a function of addiction cycle in each group for illustrative purpose (ii). (C) Post hoc one-way ANOVAs across groups indicated that rsFC in the three groups did not differ at baseline (i) or following 30-d withdrawal (iv). However, a significant difference was shown in OFC-MS rsFC after SA phase (ii), which was maintained after foot shock (iii). Paired t tests demonstrated an increase in OFC-MS rsFC after SA phase in both SR (v) and SS (vi) rats compared with the SAL control group. The punishment did not differentiate the two SA subgroups, such that both the SR and SS rats continued to demonstrate increased OFC-MS rsFC compared with the SAL group (Lower Right). Results were corrected for whole-brain multiple comparisons at P < 0.05. Error bar stands for SEM.
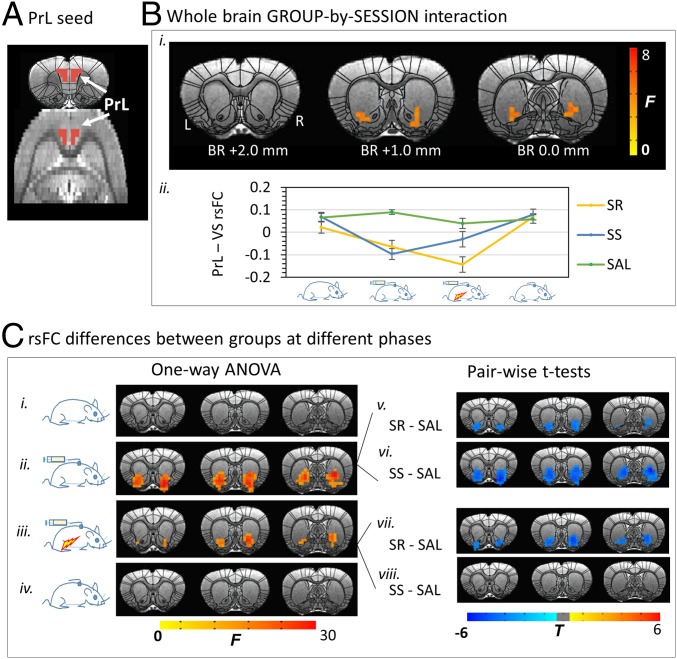
Fig. 3.
Changes in the PrL rsFC circuit in the SR, SS, and SAL groups, along the cycle of addiction. (A) PrL seed definition. (B) ANOVA [(GROUP: SR, SS, SAL) × (SESSION: baseline, SA, SA+FS, withdrawal)] revealed a significant GROUP-by-SESSION interaction in rsFC between the PrL and VS. (C) Post hoc one-way ANOVAs across groups indicated that rsFC in the three groups did not differ at baseline or following 30-d withdrawal. However, a significant difference was shown in PrL-VS rsFC after SA development phase, which was maintained after foot shock (Left). Paired t tests demonstrated a lower negative PrL-VS rsFC after SA development in both SR and SS rats compared with SAL control group (Upper Right). The punishment differentiated the two METH subgroups, such that the SR but not the SS rats continued to demonstrate lower negative PrL-VS rsFC, significantly different from the SAL group (Lower Right). Results were corrected for whole-brain multiple comparisons at P < 0.05. Error bar stands for SEM.
There was a significant GROUP × SESSION interaction in rsFC between the OFC seed and a medial striatum (MS) cluster, which encompassed the nucleus accumbens (NAc) core extending caudally and superiorly along the medial wall into the dorsal striatum (Fig. 2 B, i). The orbitofrontal-medial striatum (OFC-MS) rsFC strength for each group is shown in Fig. 2 B, ii. Follow-up one-way ANOVAs across the three groups at each time point revealed (i) no group difference in the OFC-seeded rsFC at baseline (Fig. 2 C, i); (ii) emergence of group differences in OFC-MS rsFC after SA (Fig. 2 C, ii); follow-up t tests indicated greater rsFC strength in both METH SA subgroups (SS and SR) vs. the SAL SA group (Fig. 2 C, v and vi); (iii) maintenance of group differences after 5 d of FS plus SA (Fig. 2 C, iii); follow-up t tests again indicated that both METH SA subgroups differed from the SAL group (Fig. 2 C, vii and viii); and (iv) no group differences after 30-d withdrawal, at which time both SS and SR subgroups were again similar to the SAL group (Fig. 2 C, iv). Qualitatively, the difference of OFC rsFC in the caudal MS seemed to be driven by a SA-induced increase in both SR and SS subgroups compared with the SAL group, whereas the difference in the NAc core appeared to be primarily driven by higher rsFC in SR rats compared with the SAL group (Fig. 2 C, v–viii).
There was a significant GROUP × SESSION interaction in rsFC strength between the PrL seed and the ventral striatum (VS), which included the lateral NAc shell and the ventral tip of the caudate-putamen (Fig. 3 B, i). The prelimbic-ventral striatum (PrL-VS) rsFC circuit strength trajectory for each group is illustrated in Fig. 3 B, ii. Follow-up one-way ANOVAs across groups at each time point indicated (i) no difference in PrL rsFC strength between groups at baseline (Fig. 3 C, i); (ii) emergence of rsFC differences after SA between PrL and NAc core, shell and ventral caudate-putamen (Fig. 3 C, ii); follow-up t tests indicated lower rsFC strength in both SS and SR subgroups vs. the SAL group (Fig. 3 C, v and vi); (iii) maintenance of group differences after 5 d of FS plus SA (Fig. 3 C, iii); follow-up t tests indicated that the PrL-VS circuit strength remained lower in the SR but not SS subgroup (Fig. 3 C, vii) vs. SAL group (Fig. 3 C, viii); and (iv) no group difference after 30-d withdrawal, at which time both SS and SR subgroups were again similar to the SAL group (Fig. 3 C, iv).
Critically, using the same ANOVA model and correction criteria for multiple comparisons, there was no significant GROUP × SESSION interaction in rsFC strength in the S1HL negative control circuits (SI Appendix, Fig. S1 B–E), suggesting region-specific, drug SA-induced circuit alterations. The low cortical-striatal connectivity strength (around 0.1) observed is consistent with previous neuroimaging studies (12, 28–30) and in line with the dense striatal afferent inputs from multiple cortical and midbrain regions (23, 31).
Changes in the [go (-) stop] circuitry balance and circuit-behavioral relationships.
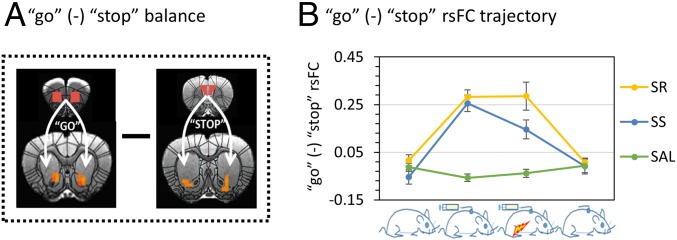
Based upon previous studies (6, 12, 23), we defined the OFC-MS (the entire cluster showing the GROUP × SESSION interaction) as the go circuit and the PrL-VS (the entire cluster showing the GROUP × SESSION interaction) as the stop circuit. The rsFC strength difference between the OFC-MS and PrL-VS were defined as the [go (-) stop] circuit balance (Fig. 4A). After the SA phase, we found that the [go (-) stop] rsFC balance was biased toward the go direction in both the SR [paired t test, SA vs. baseline, T(10) = 7.25, P = 0.0003] and SS [paired t test, SA vs. baseline, T(10) = 6.74, P = 0.00005] subgroups. This deviation in circuit balance was reduced following 5 d of SA+FS only in the SS (paired t test, FS + SA vs. SA T(10) = 2.97, P = 0.014) but not the SR subgroup (paired t test, FS + SA vs. SA T(6) = −0.056, P = 0.96). After long-term (30-d) withdrawal and two cue-SA extinction sessions, the [go (-) stop] circuit balance was restored back to baseline levels in both SR and SS subgroups (paired t test, baseline vs. withdrawal, P values ≥0.30; Fig. 4B). Importantly, no difference in the [go (-) stop] circuit balance was seen in the SAL group over time (repeated one-way ANOVA F(3,9) = 1.28, P = 0.30).
Fig. 4.
The [go (-) stop] circuitry balance. (A) Illustration of the OFC-MS go and PrL-VS stop circuits and the concept of [go (-) stop] balance. (B) The [go (-) stop] balance changed similarly in SS and SR rats except that the balance was reduced toward baseline level after shock in the SS rats (paired t test, shock vs. SA, P = 0.014), but not in the SR rats (paired T, shock vs. SA, P = 0.96). Error bar stands for SEM.
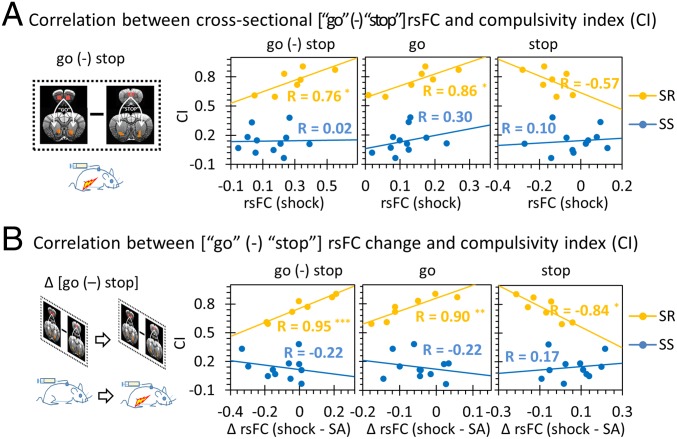
Next, the relationship between the CI and the [go (-) stop] balance after FS punishment was examined. We found that CI was positively correlated with the [go (-) stop] rsFC balance in the SR subgroup (R = 0.76, P = 0.047; Fig. 5A), driven by a positive correlation in go circuit strength with CI (R = 0.86, P = 0.014; Fig. 5A) whereas the stop circuit strength only showed a weak, trend-level negative correlation (R = −0.57, P = 0.19; Fig. 5A). In addition, we leveraged the longitudinal design to examine if CI was correlated with circuit strength changes induced by punishment. We found that FS induced changes in the [go (-) stop] circuit balance correlated significantly with CI (R = 0.96, P = 0.00078; Fig. 5B) in SR but not SS individuals, with both go and stop circuits individually contributing to the relationship, but in opposite directions (go: R = 0.90, P = 0.006; stop: R = −0.84, P = 0.018; Fig. 5B). Further analyses showed that both go and stop circuits independently explained the variance in CI (SI Appendix, Tables S3–S5). Notably, there was no significant relationship between CI and the [go (-) stop] balance in the SS subgroup, either when circuit measures were taken alone or with changes in these circuits between the SA and punishment phases (Fig. 5).
Fig. 5.
The relationship between the [go (-) stop] rsFC balance and the CI (Fig. 1B). (A) The cross-sectional [go (-) stop] balance significantly correlated with CI in SR rats (P = 0.047), with the go circuit significantly potentiating compulsive-like behavior (P = 0.014). (B) Longitudinal change in the [go (-) stop] balance after punishment positively correlated with the CI but only in the SR individuals (P = 0.0008), with a positive correlation with the go circuit change (P = 0.006) and negative correlation with the stop circuit change (P = 0.018). The CI was not correlated with rsFC change of the [go (-) stop] balance, nor any single circuit in the SS rats. *P < 0.05; **P < 0.01; ***P < 0.001.
Discussion
Using a preclinical model that closely captures the principal human addiction phenotype of compulsive drug use despite negative consequences, we identified two functional frontostriatal circuits that are differentially regulated as a function of the drug-dependence trajectory. The circuit strength of an OFC-dorsal medial striatal increased, while a PrL-ventral striatal circuit connectivity became negative following 20 d of METH SA (Figs. 2 and 3). The balance between the OFC-striatal (go) and PrL-striatal (stop) circuits, [go (-) stop] deviated toward the go direction in both SS and SR subgroups by the end of the SA phase; this deviation was significantly reduced following 5 d of FS punishment only in the SS but not SR subgroup (Fig. 4B). Importantly, the severity of dependence, as reflected by a SA Compulsivity Index, was related most closely to the balance of the two circuits, with the brain-behavior relationship unique to the subset of rats (SR) who exhibit continued drug SA despite the aversive FS consequence (Fig. 5).
Together, these results suggest that frontostriatal circuits dynamically change over the course of addiction, with both similarities and important differences between SS and SR rats. At the time of disease “diagnoses” (i.e., the punishment phase), we found higher OFC-striatal rsFC (Fig. 2) and lower PrL-striatal rsFC (Fig. 3) in those “addictive-like” rats (i.e., SR), with the difference in the strength of these two circuits significantly correlated with compulsive drug taking (Fig. 5A). These findings are highly consistent with existing cross-sectional human studies contrasting addicts with healthy controls (6, 11, 12). In addition, findings regarding the balance of frontostriatal circuits and their relationship to behavior were discovered. First, only the SR subgroup of rats demonstrated significant correlation between an index of compulsive behavior (CI) and changes in the [go (-) stop] circuit balance (Fig. 5B), despite both groups having a similar CI dynamic range. The absence of a brain circuit-behavior correlation with CI in the SS subgroup suggests that plasticity in the “vulnerable” SR subpopulation supports a neurobiological distinction between a compulsive vs. recreational drug use phenotype. Second, the [go (-) stop] circuit strength returned toward baseline only in the SS subgroup after FS (Fig. 4), suggesting a selective ability to reverse SA-evoked neural plasticity in response to punishment only in rats sensitive to negative consequences. Third, these brain circuits–behavioral correlations emerged only after FS exposure, suggesting punishment is critical, even necessary, for the emergence of compulsive behavior and related neural substrates to manifest in those individuals vulnerable to addiction.
These longitudinal results provided a unique insight into the role of frontostriatal circuits in the addiction cycle. While the stop (PrL-NAc) functional circuit strength was changed in both SS and SR animals after METH SA, it was partially reversed only in the SS but not SR rats after the introduction of punishment (Fig. 3B). This systems-level finding is highly consistent with a previous study demonstrating that, while the SA-induced abnormal synaptic plasticity progressively recovers in animals that maintain controlled drug intake, it remains abnormal in those rats that displayed addiction-like behavior during an extended punishment phase (32). In addition, our data may link the dACC hypoactivity seen in drug-dependent humans (33–35) with the reduced circuit strength in the putative homologous rodent region, the PrL (23). Hypoactivation during inhibitory control tasks (33–37) as well as reduced dACC-striatal connectivity (12, 38) have been reported across several human SUDs, although frontal hyperactivation in response to acute drug administration or presentation of drug-related cues has also been documented (13). The dACC has long been thought to be a key region exerting top-down control following error monitoring to promote appropriate behavioral adaptation (39–41). That punishment induced opposite changes in the putative stop circuit (Fig. 3 B, ii) herein may suggest difficulty adequately engaging the dACC/PrL region or even an active suppression of PrL by ventral striatum, resulting in a failure to adapt behavior properly when faced with negative consequences in vulnerable subjects. Unfortunately, rsFC by itself cannot provide information on the direction of influence between regions. In contrast to PrL hypoactivity, OFC hyperactivity has been reported after cocaine SA (9, 42–44). Notably, repeated optogenetic stimulation of OFC as well as its downstream striatal projection terminals can generate compulsive-like repetitive behavior in transgenic mice (45). Moreover, hyperconnectivity between the VS and OFC has been observed in cocaine-dependent (12) and OCD (28) individuals, both characterized by compulsive behaviors. Here, we showed that while OFC-striatal rsFC can be significantly enhanced by METH SA, only circuit changes that occurred after the introduction of FS in the susceptible animals (SR) were related to compulsive drug use.
Combined with previous findings, our results also suggest that restoring the physiological balance between stop and go circuits via noninvasive brain stimulation therapies is an emerging and promising treatment strategy for SUDs. A previous study demonstrated that optogenetically stimulating or inhibiting PrL neurons can, respectively, down- or up-regulate the manifestation of compulsive drug-seeking behaviors (8). Interestingly, both our previous human data (12) and current preclinical results demonstrate that circuit strength balance, i.e., [go (-) stop], is a better predictor of compulsive drug taking than either circuit alone (SI Appendix, Tables S1–S4), pointing to the benefit of differentiating individuals based on their circuit endophenotypes such that some individuals may benefit more from targeting the OFC-striatal circuit rather than, or in addition to, the dACC/PrL-striatal circuit. Indeed, inhibition of the OFC has also been shown to reduce compulsive drug taking in a rat model of addiction (9). Inhibitory transcranial magnetic stimulation directed at OFC/medial PFC may be beneficial in reducing drug cue responsivity in cocaine use disorder (46). Here, we propose that assessing the individual strength of both circuits, and especially their relative strengths, may assist in individualizing therapeutic options for those who may require either strengthening of the stop circuit, weakening of the go circuit, or both. Finally, such pretreatment neuroimaging measures may allow assessment of clinical responsivity and help determine the adequacy of a specific course of treatment.
Limitations
Since drug addiction is a complex neuropsychiatric disease involving multiple cognitive and affective constructs, any given animal model may only capture a limited aspect of the disease. In this study, we sought to investigate the compulsive nature of drug self-administration with FS serving as the homolog of negative consequence associated with human drug use, although the physical pain of FS might not fully reflect the range of negative consequences experienced by dependent drug abusers. In addition, while rsFC of the go and stop circuits returned to baseline levels after 30-d withdrawal of drug, other addictive behaviors (i.e., cue reactivity) continued even after a long period of withdrawal and was not related to the circuits identified in this study. How the go and stop circuits contribute to or interact with changes in other neural pathways underlying different aspects of addiction warrants further study. Finally, we did not include female rats and, therefore, cannot rule out gender differences in these findings.
Materials and Methods
Subjects.
All animal procedures were approved by the Animal Care and Use Committee of the National Institute of Drug Abuse Intramural Research Program and followed the Guide for the Care and Use of Laboratory Animals (ISBN 0-309-05377-3). Male Sprague–Dawley rats (Charles River Labs), receiving at 14–16 wk old and weighing 350–400 g before surgery, were used in the experiments.
Self-Administration Rat Model.
Surgery.
Under ketamine and xylazine (50 and 5 mg/kg, i.p., respectively) anesthesia, silastic catheters were inserted into the jugular vein, as described (20, 21). The catheters were attached to a modified 22-gauge cannula mounted underneath the skin of the back between the shoulder blades; catheters were flushed every 24–48 h throughout the experiment with gentamicin (Butler Schein; 5 mg/mL) and sterile saline. Buprenorphine (0.1 mg/kg, s.c.) was administered after surgery to relieve pain; rats were allowed 7 d recovery before METH SA training.
Self-administration training and foot shock punishment.
Rats were randomly assigned to either a METH (n = 18) or SAL (n = 11) SA control group (Fig. 1A). SA chambers were located inside sound-attenuating cabinets controlled by a Med Associates system (Med Associates). Each chamber was equipped with two levers (active and inactive). Presses on the retractable active lever activated an infusion pump that delivered either METH or saline. Presses on the inactive lever had no reinforced consequences.
The SA training procedure has also been described (20, 21). Briefly, rats were trained to self-administer DL-methamphetamine HCl received from the pharmacy of National Institute on Drug Abuse (0.1 mg/kg per infusion over 3.5 s) or saline during three, 3-h sessions per day (each separated by 30 min) for 20 d under a fixed-ratio (FR)-1 reinforcement schedule with a 20-s timeout. A 5-s compound tone-light cue was paired with each infusion. Rats were trained in four cycles of 5 d on and 2 d off to minimize weight loss. Following 20 d of SA training, a 5-d punishment phase was initiated with pseudorandom 0.5-s FS now accompanying METH SA on half of the reinforced lever-presses. Foot shock intensity varied over the 5 d in a predetermined fixed order (0.18, 0.24, 0.3, 0.3, and 0.3 mA) (20, 21).
Withdrawal and cue-reactive tests.
After the 5-d punishment phase, rats underwent forced withdrawal for 30 d. Cue-presentation (extinction) testing occurred for 30 min on days 3 and 30 of withdrawal (Fig. 1A). On these occasions, rats were returned to the SA chambers with presses on the formally active lever resulting only in the presentation of the tone-light cue previously paired with METH/saline infusions.
MRI Experiments.
Animal preparation.
For magnetic resonance imaging (MRI) scans, animals were anesthetized with a combination of isoflurane (Piramal Critical Care Inc.) and dexmedetomidine hydrochloride (Zoetis Services LLC) using a previously described protocol (47). For more details, please see SI Appendix.
Image acquisition and preprocessing.
MRI data were acquired on a Bruker Biospin 9.4T scanner (Bruker Medizintechnik). The fMRI data acquisition was initiated 90 min after anesthesia induction (48) using a T2*-weighted EPI sequence (echo time = 13 ms, repetition time = 1,000 ms, field of view = 35 × 35 mm2, matrix size = 64 × 64, slice thickness = 1 mm, slice number = 15). FSL (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/) and AFNI (49) were used in fMRI data preprocessing, which included slice timing correction, motion correction, spatial smoothing, and normalization to a rat stereotaxic atlas (47). Independent component analysis was applied to eliminate nonneural components in the fMRI data (50). For data acquisition parameters and more preprocessing details, please see SI Appendix.
Statistical Analyses.
Behavioral data analyses.
The escalation of drug intake during the SA phase and the reduction of drug intake during the punishment phase were analyzed. Mixed ANOVAs, independent two-sample t tests, or paired t tests were employed to examine the behavioral differences between or within groups.
Voxel-wise analyses of rsFC.
Based upon our previous human study (12) and the homology between the primate and rodent brain (23), the bilateral OFC (Fig. 2A) and bilateral PrL (Fig. 3A) were chosen as “seed” regions for rsFC analyses. Additionally, the primary somatosensory cortex of hind limb (S1HL, SI Appendix, Fig. S1A) was chosen as a control seed region.
Whole-brain voxel-wise rsFC was calculated individually for each seed and each animal, and then submitted to a 3 × 4 linear mixed-effects model ANOVA (27), with GROUP (SR, SS, SAL) and SESSION (baseline, SA, SA+FS, withdrawal) as factors. GROUP × SESSION interactions were of primary interest to examine between-group rsFC differences across sessions. Post hoc one-way (SR, SS, SAL) ANOVAs were used to demonstrate group differences at each session, while post hoc t tests compared pairs of groups to examine the source of interaction, both in whole brain voxel-wise (51). All voxel-wise statistics were corrected for whole-brain multiple comparisons (corrected P < 0.05, determined with the criteria of voxel-level P < 0.001 and cluster size >8 voxels based on Monte Carlo simulation in AFNI; ref. 49).
Changes of the [go (-) stop] circuitry balance and the circuit-behavior relationships.
We defined the OFC-MS (the whole cluster showing the GROUP x SESSION interaction) as the go circuit and the PrL-VS (the whole cluster showing the GROUP x SESSION interaction) as the stop circuit. The rsFC strength difference between the OFC-MS and PrL-VS were defined as the [go (-) stop] circuit balance. Changes in the [go (-) stop] circuit balance during the course of four study phases were examined. Next, relationships between the CI and the [go (-) stop] balance were also examined cross-sectionally. In addition, we leveraged our longitudinal design to examine if CI was correlated with the circuit strength changes induced by punishment.
Supplementary Material
Acknowledgments
We thank Bruce Ladenheim, Michael McCoy, and Ndeah Terry for their assistance in animal behavioral training and Alice Morgunova and Yang Qiao for their assistance in neuroimaging data collection. This study was supported by the Intramural Research Program of the National Institute on Drug Abuse.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1819978116/-/DCSupplemental.
References
- 1.Leshner AI. Addiction is a brain disease, and it matters. Science. 1997;278:45–47. doi: 10.1126/science.278.5335.45. [DOI] [PubMed] [Google Scholar]
- 2.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders: DSM-5. 5th Ed American Psychiatric Publishing; Arlington, VA: 2013. [Google Scholar]
- 3.Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: From actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- 4.Lüscher C. The emergence of a circuit model for addiction. Annu Rev Neurosci. 2016;39:257–276. doi: 10.1146/annurev-neuro-070815-013920. [DOI] [PubMed] [Google Scholar]
- 5.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Volkow ND, Wang GJ, Fowler JS, Tomasi D, Telang F. Addiction: Beyond dopamine reward circuitry. Proc Natl Acad Sci USA. 2011;108:15037–15042. doi: 10.1073/pnas.1010654108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koob GF, Volkow ND. Neurobiology of addiction: A neurocircuitry analysis. Lancet Psychiatry. 2016;3:760–773. doi: 10.1016/S2215-0366(16)00104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen BT, et al. Rescuing cocaine-induced prefrontal cortex hypoactivity prevents compulsive cocaine seeking. Nature. 2013;496:359–362. doi: 10.1038/nature12024. [DOI] [PubMed] [Google Scholar]
- 9.Pascoli V, Terrier J, Hiver A, Lüscher C. Sufficiency of mesolimbic dopamine neuron stimulation for the progression to addiction. Neuron. 2015;88:1054–1066. doi: 10.1016/j.neuron.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 10.Kasanetz F, et al. Prefrontal synaptic markers of cocaine addiction-like behavior in rats. Mol Psychiatry. 2013;18:729–737. doi: 10.1038/mp.2012.59. [DOI] [PubMed] [Google Scholar]
- 11.Ersche KD, et al. Abnormal structure of frontostriatal brain systems is associated with aspects of impulsivity and compulsivity in cocaine dependence. Brain. 2011;134:2013–2024. doi: 10.1093/brain/awr138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu Y, Salmeron BJ, Gu H, Stein EA, Yang Y. Impaired functional connectivity within and between frontostriatal circuits and its association with compulsive drug use and trait impulsivity in cocaine addiction. JAMA Psychiatry. 2015;72:584–592. doi: 10.1001/jamapsychiatry.2015.1. [DOI] [PubMed] [Google Scholar]
- 13.Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: Neuroimaging findings and clinical implications. Nat Rev Neurosci. 2011;12:652–669. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van den Heuvel MP, Hulshoff Pol HE. Exploring the brain network: A review on resting-state fMRI functional connectivity. Eur Neuropsychopharmacol. 2010;20:519–534. doi: 10.1016/j.euroneuro.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 15.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 16.American Psychiatric Association 2000. Diagnostic and Statistical Manual of Mental Disorders (American Psychiatric Association, Washington, DC), 4th Ed, Text Revision (DSM-IV-TR)
- 17.Volkow ND, et al. The conception of the ABCD study: From substance use to a broad NIH collaboration. Dev Cogn Neurosci. 2018;32:4–7. doi: 10.1016/j.dcn.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wagner FA, Anthony JC. From first drug use to drug dependence; developmental periods of risk for dependence upon marijuana, cocaine, and alcohol. Neuropsychopharmacology. 2002;26:479–488. doi: 10.1016/S0893-133X(01)00367-0. [DOI] [PubMed] [Google Scholar]
- 19.Deroche-Gamonet V, Belin D, Piazza PV. Evidence for addiction-like behavior in the rat. Science. 2004;305:1014–1017. doi: 10.1126/science.1099020. [DOI] [PubMed] [Google Scholar]
- 20.Krasnova IN, et al. Incubation of methamphetamine and palatable food craving after punishment-induced abstinence. Neuropsychopharmacology. 2014;39:2008–2016. doi: 10.1038/npp.2014.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cadet JL, et al. Genome-wide DNA hydroxymethylation identifies potassium channels in the nucleus accumbens as discriminators of methamphetamine addiction and abstinence. Mol Psychiatry. 2017;22:1196–1204. doi: 10.1038/mp.2016.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Radfar SR, Rawson RA. Current research on methamphetamine: Epidemiology, medical and psychiatric effects, treatment, and harm reduction efforts. Addict Health. 2014;6:146–154. [PMC free article] [PubMed] [Google Scholar]
- 23.Heilbronner SR, Rodriguez-Romaguera J, Quirk GJ, Groenewegen HJ, Haber SN. Circuit-based corticostriatal homologies between rat and primate. Biol Psychiatry. 2016;80:509–521. doi: 10.1016/j.biopsych.2016.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dutra L, et al. A meta-analytic review of psychosocial interventions for substance use disorders. Am J Psychiatry. 2008;165:179–187. doi: 10.1176/appi.ajp.2007.06111851. [DOI] [PubMed] [Google Scholar]
- 25.Gawin FH, Kleber HD. Abstinence symptomatology and psychiatric diagnosis in cocaine abusers. Clinical observations. Arch Gen Psychiatry. 1986;43:107–113. doi: 10.1001/archpsyc.1986.01800020013003. [DOI] [PubMed] [Google Scholar]
- 26.Grimm JW, Hope BT, Wise RA, Shaham Y. Neuroadaptation. Incubation of cocaine craving after withdrawal. Nature. 2001;412:141–142. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen G, Saad ZS, Britton JC, Pine DS, Cox RW. Linear mixed-effects modeling approach to FMRI group analysis. Neuroimage. 2013;73:176–190. doi: 10.1016/j.neuroimage.2013.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harrison BJ, et al. Altered corticostriatal functional connectivity in obsessive-compulsive disorder. Arch Gen Psychiatry. 2009;66:1189–1200. doi: 10.1001/archgenpsychiatry.2009.152. [DOI] [PubMed] [Google Scholar]
- 29.Choi EY, Yeo BT, Buckner RL. The organization of the human striatum estimated by intrinsic functional connectivity. J Neurophysiol. 2012;108:2242–2263. doi: 10.1152/jn.00270.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu H, et al. Abstinence from cocaine and sucrose self-administration reveals altered mesocorticolimbic circuit connectivity by resting state MRI. Brain Connect. 2014;4:499–510. doi: 10.1089/brain.2014.0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haber SN, Knutson B. The reward circuit: Linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kasanetz F, et al. Transition to addiction is associated with a persistent impairment in synaptic plasticity. Science. 2010;328:1709–1712. doi: 10.1126/science.1187801. [DOI] [PubMed] [Google Scholar]
- 33.Kaufman JN, Ross TJ, Stein EA, Garavan H. Cingulate hypoactivity in cocaine users during a GO-NOGO task as revealed by event-related functional magnetic resonance imaging. J Neurosci. 2003;23:7839–7843. doi: 10.1523/JNEUROSCI.23-21-07839.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hester R, Nestor L, Garavan H. Impaired error awareness and anterior cingulate cortex hypoactivity in chronic cannabis users. Neuropsychopharmacology. 2009;34:2450–2458. doi: 10.1038/npp.2009.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luo X, et al. Error processing and gender-shared and -specific neural predictors of relapse in cocaine dependence. Brain. 2013;136:1231–1244. doi: 10.1093/brain/awt040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zubieta JK, et al. Regional cerebral blood flow responses to smoking in tobacco smokers after overnight abstinence. Am J Psychiatry. 2005;162:567–577. doi: 10.1176/appi.ajp.162.3.567. [DOI] [PubMed] [Google Scholar]
- 37.Li S, Yang Y, Hoffmann E, Tyndale RF, Stein EA. CYP2A6 genetic variation alters striatal-cingulate circuits, network hubs, and executive processing in smokers. Biol Psychiatry. 2017;81:554–563. doi: 10.1016/j.biopsych.2016.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hong LE, et al. A genetically modulated, intrinsic cingulate circuit supports human nicotine addiction. Proc Natl Acad Sci USA. 2010;107:13509–13514. doi: 10.1073/pnas.1004745107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hampton AN, O’doherty JP. Decoding the neural substrates of reward-related decision making with functional MRI. Proc Natl Acad Sci USA. 2007;104:1377–1382. doi: 10.1073/pnas.0606297104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Williams ZM, Bush G, Rauch SL, Cosgrove GR, Eskandar EN. Human anterior cingulate neurons and the integration of monetary reward with motor responses. Nat Neurosci. 2004;7:1370–1375. doi: 10.1038/nn1354. [DOI] [PubMed] [Google Scholar]
- 41.Botvinick M, Nystrom LE, Fissell K, Carter CS, Cohen JD. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature. 1999;402:179–181. doi: 10.1038/46035. [DOI] [PubMed] [Google Scholar]
- 42.Lu H, et al. Cocaine-induced brain activation detected by dynamic manganese-enhanced magnetic resonance imaging (MEMRI) Proc Natl Acad Sci USA. 2007;104:2489–2494. doi: 10.1073/pnas.0606983104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kufahl PR, et al. Neural responses to acute cocaine administration in the human brain detected by fMRI. Neuroimage. 2005;28:904–914. doi: 10.1016/j.neuroimage.2005.06.039. [DOI] [PubMed] [Google Scholar]
- 44.Risinger RC, et al. Neural correlates of high and craving during cocaine self-administration using BOLD fMRI. Neuroimage. 2005;26:1097–1108. doi: 10.1016/j.neuroimage.2005.03.030. [DOI] [PubMed] [Google Scholar]
- 45.Ahmari SE, et al. Repeated cortico-striatal stimulation generates persistent OCD-like behavior. Science. 2013;340:1234–1239. doi: 10.1126/science.1234733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kearney-Ramos TE, et al. Transdiagnostic effects of ventromedial prefrontal cortex transcranial magnetic stimulation on cue reactivity. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018;3:599–609. doi: 10.1016/j.bpsc.2018.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lu H, et al. Rat brains also have a default mode network. Proc Natl Acad Sci USA. 2012;109:3979–3984. doi: 10.1073/pnas.1200506109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brynildsen JK, et al. Physiological characterization of a robust survival rodent fMRI method. Magn Reson Imaging. 2017;35:54–60. doi: 10.1016/j.mri.2016.08.010. [DOI] [PubMed] [Google Scholar]
- 49.Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 50.Hsu LM, et al. Constituents and functional implications of the rat default mode network. Proc Natl Acad Sci USA. 2016;113:E4541–E4547. doi: 10.1073/pnas.1601485113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kriegeskorte N, Simmons WK, Bellgowan PS, Baker CI. Circular analysis in systems neuroscience: The dangers of double dipping. Nat Neurosci. 2009;12:535–540. doi: 10.1038/nn.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.