Significance
Functional connectivity within the default mode network in patients with major depressive disorder has been frequently reported to be abnormal but with contradicting directions in previous studies with small sample sizes. In creating the REST-meta-MDD consortium containing neuroimaging data of 1,300 depressed patients and 1,128 normal controls from 25 research groups in China, we found decreased default mode network functional connectivity in depressed patients, driven by patients with recurrent depression, and associated with current medication treatment but not with disease duration. These findings suggest that default mode network functional connectivity remains a prime target for understanding the pathophysiology of depression, with particular relevance to revealing mechanisms of effective treatments.
Keywords: default mode network, functional connectivity, major depressive disorder, resting-state fMRI, REST-meta-MDD
Abstract
Major depressive disorder (MDD) is common and disabling, but its neuropathophysiology remains unclear. Most studies of functional brain networks in MDD have had limited statistical power and data analysis approaches have varied widely. The REST-meta-MDD Project of resting-state fMRI (R-fMRI) addresses these issues. Twenty-five research groups in China established the REST-meta-MDD Consortium by contributing R-fMRI data from 1,300 patients with MDD and 1,128 normal controls (NCs). Data were preprocessed locally with a standardized protocol before aggregated group analyses. We focused on functional connectivity (FC) within the default mode network (DMN), frequently reported to be increased in MDD. Instead, we found decreased DMN FC when we compared 848 patients with MDD to 794 NCs from 17 sites after data exclusion. We found FC reduction only in recurrent MDD, not in first-episode drug-naïve MDD. Decreased DMN FC was associated with medication usage but not with MDD duration. DMN FC was also positively related to symptom severity but only in recurrent MDD. Exploratory analyses also revealed alterations in FC of visual, sensory-motor, and dorsal attention networks in MDD. We confirmed the key role of DMN in MDD but found reduced rather than increased FC within the DMN. Future studies should test whether decreased DMN FC mediates response to treatment. All R-fMRI indices of data contributed by the REST-meta-MDD consortium are being shared publicly via the R-fMRI Maps Project.
Major depressive disorder (MDD) is the second leading cause of disability worldwide, with point prevalence exceeding 4% (1). The pathophysiology of MDD remains unknown despite intensive efforts, including neuroimaging studies. However, the small sample size of most MDD neuroimaging studies entails low sensitivity and reliability (2, 3). An exception is the Enhancing NeuroImaging Genetics through Meta-Analysis (ENIGMA) consortium which meta- and mega-analyzed thousands of structural MRI scans from MDD patients and healthy controls (4, 5). The ENIGMA-MDD working group found a slight albeit robust reduction in hippocampal volume (4) and cortical thinning in medial orbitofrontal cortex (5). However, this approach does not consider communication among brain regions (i.e., functional brain networks).
Abnormal communication among functional brain networks has been reported in MDD using resting-state fMRI (R-fMRI) functional connectivity (FC), which detects synchronized spontaneous activity among anatomically distinct networks. MDD studies have focused on the default mode network (DMN), which has been linked to rumination (6). The first study focusing on the DMN in MDD reported increased DMN FC (7), although similar studies found both increased and decreased DMN FC in MDD (8, 9). Meta-analyses have reported increased DMN FC in MDD, albeit based on few studies (6, 10). As summarized in SI Appendix, Table S1, 38 studies have examined DMN FC alterations in MDD. Of these, 18 found increases, 8 decreases, 7 both increases and decreases, and 5 no significant changes. As shown in SI Appendix, Fig. S1, a voxelwise meta-analysis of 32 studies revealed increased orbitofrontal DMN FC and decreased FC between dorsomedial prefrontal cortex (dmPFC) and posterior DMN in MDD. Such complex results may have contributed to prior inconsistencies.
Inconsistencies may reflect limited statistical power (2) from small samples, but data analysis flexibility may also contribute, as a large number of preprocessing and analysis operations with many different parameter combinations have been used in fMRI analyses (11). MDD studies have used diverse multiple comparison correction methods, most likely inadequate (12). Data analysis flexibility also impedes large-scale meta-analysis (6, 10). Moreover, clinical characteristics such as number and type of episodes, medication status, and illness duration vary across studies, further contributing to heterogeneous results.
To address limited statistical power and analytic heterogeneity, we initiated the REST-meta-MDD Project. We implemented a standardized preprocessing protocol on Data Processing Assistant for Resting-State fMRI (DPARSF) (13) at local sites with only final indices provided to the consortium. We obtained R-fMRI indices (including FC matrices) corresponding to 1,300 patients with MDD and 1,128 normal controls (NCs) from 25 cohorts in China. To our knowledge, REST-meta-MDD is the largest MDD R-fMRI database (SI Appendix, Table S2). We used linear mixed models (LMMs) to identify abnormal FC patterns associated with DMN across cohorts and investigated whether episode type, medication status, illness severity, and illness duration contributed to abnormalities.
Results
Sample Composition.
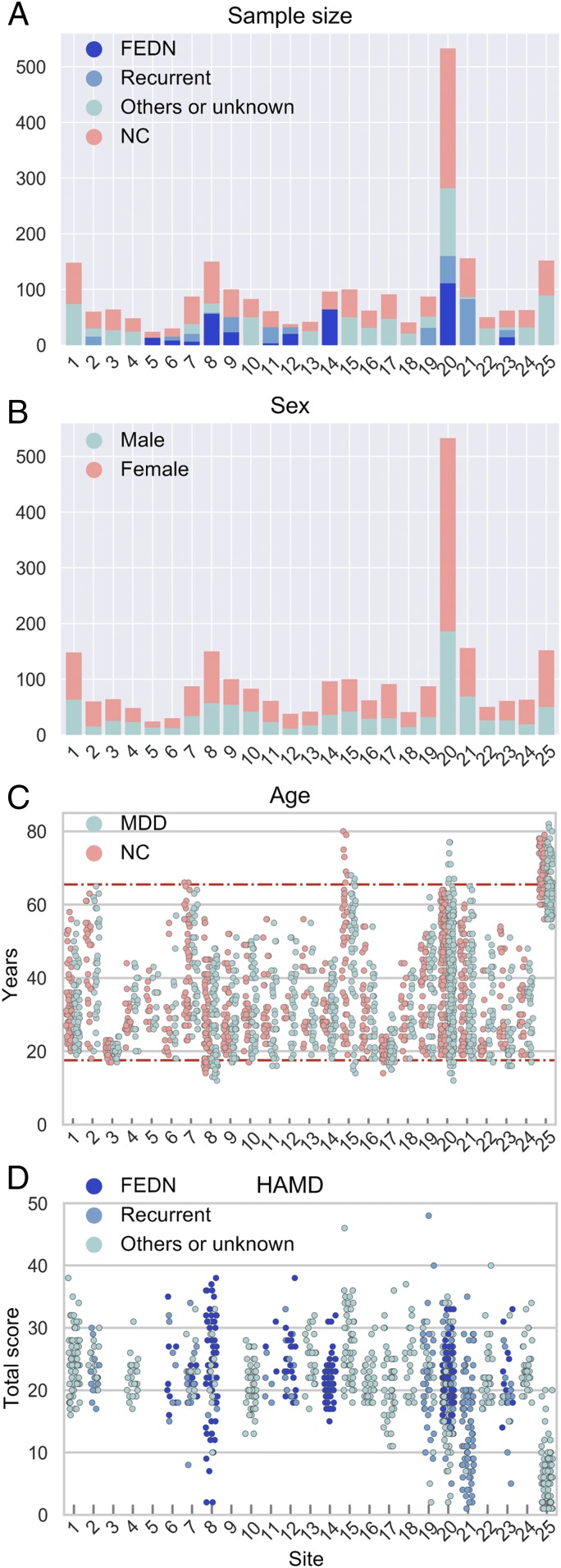
Contributions were requested from users of DPARSF, a MATLAB- and SPM-based R-fMRI preprocessing pipeline (13). Twenty-five research groups from 17 hospitals in China formed the REST-meta-MDD consortium and agreed to share final R-fMRI indices from patients with MDD and matched NCs (see SI Appendix, Table S3 for data composition; henceforth “site” refers to each cohort for convenience) from studies approved by local Institutional Review Boards. The consortium contributed 2,428 previously collected datasets (1,300 MDDs and 1,128 NCs) (Fig. 1 and SI Appendix, Tables S3–S5). On average, each site contributed 52.0 ± 52.4 patients with MDD (range 13 to 282) and 45.1 ± 46.9 NCs (range 6 to 251). Most MDD patients were female (826 vs. 474 males), as expected. The 562 patients with first-episode MDD included 318 first-episode drug-naïve (FEDN) MDD and 160 scanned while receiving antidepressants (medication status unavailable for 84). Of 282 with recurrent MDD, 121 were scanned while receiving antidepressants and 76 were not being treated with medication (medication status unavailable for 85). Episodicity (first or recurrent) and medication status were unavailable for 456 patients.
Fig. 1.
REST-meta-MDD sample characteristics. (A) Total number of participants per group for each contributing site. The MDD patients were subdivided into FEDN, recurrent, and others/unknown types. (B) Number of male subjects and female subjects for each site. (C) Age (in years) for all individuals per site for the MDD group and NC group. The two horizontal lines represent ages 18 and 65 y, the age limits for participants chosen for imaging analysis. (D) The score of HAMD for MDD patients, when available.
Decreased DMN FC in MDD Patients.
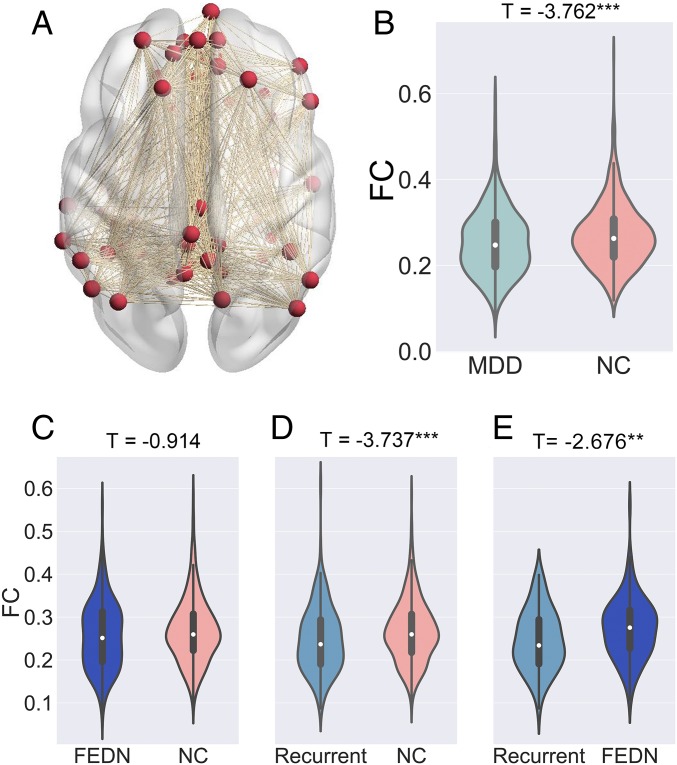
Individual-level imaging processing was performed at each site using standardized DPARSF processing parameters. After preprocessing, time series for the Dosenbach 160 functional regions of interest (ROIs) (14) were extracted. Individual-level imaging metrics (i.e., ROI time series and R-fMRI indices) and phenotypic data were then uploaded through the R-fMRI Maps Project (rfmri.org/maps) platform at the Institute of Psychology, Chinese Academy of Sciences for statistical analyses. We defined DMN ROIs as those overlapping with the DMN delineated by Yeo et al. (15). Average FC within the 33 DMN ROIs was taken to represent DMN within-network FC. We used the LMM (16) to compare MDDs with NCs while allowing the effect to vary across sites. Mean DMN within-network FC (averaged across 33*32/2 = 528 connections) was compared between 848 MDDs and 794 NCs (SI Appendix, Sample Selection) with the LMM. MDD patients demonstrated significantly lower DMN within-network FC than NCs (t = −3.762, P = 0.0002, d = −0.186; Fig. 2A). On subgroup analyses, FEDN MDDs did not differ significantly from NCs (t = −0.914, P = 0.361, d = −0.076; Fig. 2B), while DMN FC was significantly decreased in patients with recurrent MDD vs. NCs (t = −3.737, P = 0.0002, d = −0.326; Fig. 2C). Significantly reduced DMN FC in recurrent MDD patients directly compared with FEDN MDDs (t = −2.676, P = 0.008, d = −0.400; Fig. 2D), which suggests the recurrent MDDs were the major contributors to decreased DMN FC in MDD.
Fig. 2.
Decreased DMN FC in MDD patients. Mean DMN within-network FC was averaged across 33*32/2 = 528 connections as shown in A. The violin figures show the distribution of mean DMN within-network FC contrasting: MDD and NC groups (B); first episode drug naïve (FEDN) MDD and NC groups (C); recurrent MDD and NC groups (D); and FEDN MDD and recurrent MDD groups (E). Of note, for each comparison, only sites with sample size larger than 10 in each group were included. The t values were the statistics for these comparisons in LMM analyses. Please see SI Appendix, Fig. S3 for the forest plots of effect size per site generated by a metamodel in reproducibility analyses. **P < 0.01; ***P < 0.001.
Reduced DMN FC Was Not Associated with Illness Duration.
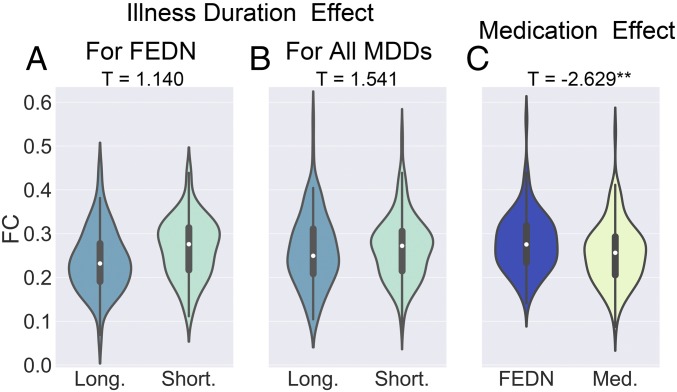
Reduced DMN FC in recurrent MDD but not in FEDN MDD could reflect illness duration or medication history. We first tested the effect of illness duration in FEDN MDDs to reduce medication confounds. The tercile with longest illness duration (≥12 mo, 70 MDDs from two sites) did not differ significantly from the tercile with shortest illness duration (≤3 mo, 48 MDDs from the same two sites) in DMN FC (t = 1.140, P = 0.257, d = 0.214; Fig. 3A). Similarly, when exploring in the entire sample, the tercile with longest illness duration (≥24 mo, 186 MDDs from four sites) did not differ significantly from the tercile with shortest illness duration (≤6 mo, 112 MDDs from the same four sites): t = 1.541, P = 0.124, d = 0.184 (Fig. 3B). Beyond chronicity, clinical subtypes could contribute to DMN FC. We examined subtypes characterized by core depression, anxiety, and neurovegetative symptoms of melancholia by mapping Hamilton Depression Rating Scale (HAMD) scale items to National Institute of Mental Health Research Domain Criteria constructs (17). However, subtype analyses did not reveal any significant effects (SI Appendix, Supplementary Results, Figs. S5 and S6, and Table S6).
Fig. 3.
The effects of illness duration and medication status on decreased DMN FC in MDD patients. The violin figures show the distribution of mean DMN within-network FC for FEDN MDD patients with long vs. short illness duration (A), for all MDD patients with long vs. short illness duration (B), and for first-episode MDD patients with vs. without medication usage (C). The t values are the statistics for these comparisons in LMM analyses. Please see SI Appendix, Fig. S4 for the forest plots of effect size per site generated by a metamodel in reproducibility analyses. **P < 0.01.
Medication Effect and Reduced DMN FC in MDD Patients.
To further examine medication treatment effects, we contrasted first-episode MDDs on medication (115 MDDs from site 20) with FEDN MDDs (97 MDDs from site 20) and found significantly reduced DMN FC (t = −2.629, P = 0.009, d = −0.362; Fig. 3C). When directly comparing 102 first-episode MDDs on medication with 266 NCs from two sites, we found a nonsignificant effect (t = −1.614, P = 0.108, d = −0.188). While FEDN MDDs showed higher DMN FC than recurrent MDDs, as shown in Decreased DMN FC in MDD Patients, 102 first-episode MDDs on medication and 57 recurrent MDDs from two sites did not differ significantly (t = 0.548, P = 0.585, d = −0.091). This suggests that medication treatment might account for our overall finding of reduced DMN FC in MDD. However, we could not address whether currently unmedicated recurrent MDDs had been previously treated with antidepressants. We were also unable to examine treatment duration, as medication status was binary.
Association of DMN FC with Symptom Severity.
The association between DMN FC and HAMD scores was tested on 734 MDD patients (excluding remitted patients with HAMD scores below 7) from 15 sites and was not significant (t = 1.591, P = 0.112, r = 0.059). The effect of symptom severity was not significant in FEDN MDDs (n = 197, three sites; t = −0.158, P = 0.874, r = −0.011) but significant in recurrent MDDs (n = 126, four sites; t = 2.167, P = 0.032, r = 0.194).
Reproducibility.
We assessed reproducibility through several strategies (SI Appendix, Table S7). (i) Using another functional clustering atlas generated by parcellating whole brain R-fMRI data into spatially coherent regions of homogeneous FC [i.e., Craddock’s 200 functional clustering atlas (18), with 48 DMN ROIs] confirmed our results, except that the effect of symptom severity in recurrent MDDs became insignificant (t = 1.424, P = 0.157, r = 0.129). (ii) Using a finer-grade parcellations [i.e., Zalesky’s random 980 parcellation (19), with 211 DMN ROIs] also confirmed our results, except that symptom severity in recurrent MDDs became insignificant (t = 1.264, P = 0.209, r = 0.115). (iii) Beyond LMM, we also performed meta-analyses: Within-site t values were converted into Hedge’s g and entered in a random effect metamodel (using R metansue, https://www.metansue.com/). Results were almost the same, although the difference between recurrent MDDs and FEDN MDDs became insignificant (Z = −1.732, P = 0.083, d = −0.251), and symptom severity in recurrent MDDs became insignificant (Z = 1.304, P = 0.192, r = 0.119). (iv) We also tested whether global signal regression (GSR) mattered. With GSR, we found similar results except for loss of significance for the difference between recurrent MDDs and FEDN MDDs (t = −0.974, P = 0.331, d = −0.145), the medication effect (t = −1.891, P = 0.060, d = −0.261), and symptom severity in recurrent MDD (t = 1.741, P = 0.084, r = 0.157). This overall confirmation is important since the global signal has been viewed as reflecting spurious noise (20), and its SD differed significantly between MDDs and NCs (t = −2.662, P = 0.008, d = −0.131). (v) For head motion control, despite already incorporating the Friston-24 model at the individual level and a motion covariate at the group level in primary analyses, we also used scrubbing [removing time points with framewise displacement >0.2 mm (21)] to verify results. All results remained the same using this aggressive head motion control strategy.
Exploratory Findings of Brain Networks Beyond DMN.
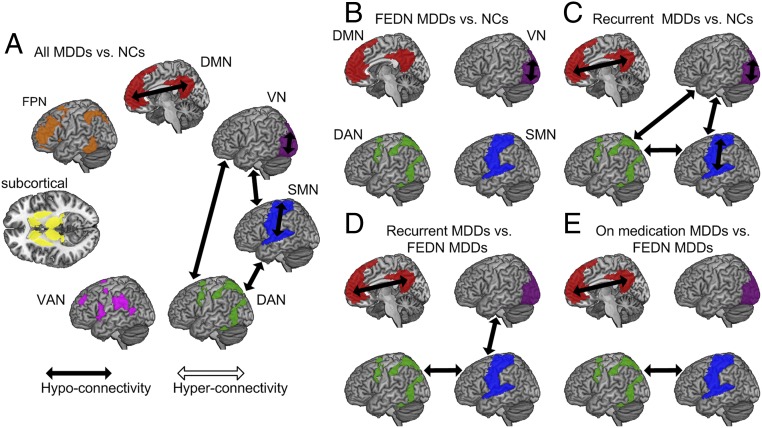
Although we focused on DMN FC in MDD, we also performed exploratory analyses comprising other brain networks beyond DMN using the seven-network atlas developed by Yeo et al. (15): visual network (VN), sensory-motor network (SMN), dorsal attention network (DAN), ventral attention network (VAN), subcortical network [instead of the limbic network defined by Yeo et al. (15), which is not covered by the 160 ROIs], frontoparietal network (FPN), and DMN. Comparing all 848 MDDs with 794 NCs, after false discovery rate (FDR) correction among 7 within-network and 21 between-network connections, we found VN, SMN, and DMN demonstrated decreased within-network connection in MDDs compared with NC. Furthermore, three between-network connections also demonstrated significant decreases in MDDs: VN-SMN, VN-DAN, and SMN-DAN (Fig. 4A and SI Appendix, Table S8). We further explored which subgroups contributed to these six abnormal within- and between-network connections by performing subgroup analyses. FEDN MDDs only demonstrated significant decrease in within-network connectivity of VN after FDR correction (Fig. 4B). Recurrent MDDs demonstrated the same abnormal pattern as the whole group, confirming again they were the major contributors (Fig. 4C). This was further supported by the direct comparisons between recurrent MDDs with FEDN MDDs, which showed lower within-network connectivity of DMN and between-network connectivity of VN-SMN and SMN-DAN in recurrent MDDs (Fig. 4D and SI Appendix, Table S9). Similar to the primary DMN analysis, we did not find any significant illness duration effect, whether within the whole group or within FEDN MDDS (SI Appendix, Table S9). When comparing MDDs on medication with FEDN MDDs, reduced within-network connectivity of DMN and between-network connectivity of SMN and DAN was found in MDDs with medication (Fig. 4E). Finally, none of the within- and between-network connectivities correlated significantly with illness severity (HAMD) after correction (SI Appendix, Table S10).
Fig. 4.
Exploratory analyses of FC within and between the seven brain networks delineated by Yeo et al. (15): (A) all MDDs vs. NCs; (B) FEDN MDDs vs. NCs; (C) recurrent MDDs vs. NCs; (D) recurrent MDDs vs. FEDN MDDs; and (E) MDDs on medication vs. FEDN MDDs. FDR correction was performed among 7 within-network and 21 between-network connections for the whole-group analysis (comparing all 848 MDDs with 794 NCs). For subgroup analyses, FDR corrected for the six abnormal connections found in the whole-group analysis. Subcortical, subcortical ROIs.
Discussion
Using an unprecedentedly large sample, we found decreased instead of increased FC within the DMN in MDD compared with NCs. However, this effect was only significant in recurrent MDD whether vs. controls or patients with FEDN MDD. Furthermore, decreased DMN FC in recurrent MDD was associated with being scanned on antidepressant medication rather than illness duration. DMN FC was also positively related to symptom severity but only in recurrent MDD. Exploratory analyses also revealed alterations in FC of visual, sensory-motor, and dorsal attention networks in MDD.
Our primary results contradict the prevailing notion that DMN FC is increased in MDD (6, 10). Several factors may account for this discrepancy. (i) Prior studies have also reported decreased DMN FC in MDD (SI Appendix, Table S1). Our voxelwise meta-analysis of 32 studies (SI Appendix, Fig. S1) revealed both increases (orbitofrontal DMN FC) and decreases (dmPFC/posterior DMN FC) in MDD. (ii) Prior inconsistent results may also reflect heterogeneous analysis strategies (11). We applied a standardized analysis protocol across sites, removing analytic variations. (iii) Average DMN FC might be insensitive to possible pairwise increases in MDD DMN FC. However, pairwise tests did not reveal even a single pair of significantly increased within-DMN connection in MDDs, even within the three DMN subsystems proposed by Andrews-Hanna et al. (22) (SI Appendix, Supplementary Results and Fig. S7). Finally, most studies reporting increased DMN FC in MDDs, albeit inconsistently, were conducted in Caucasian samples, while our sample was homogeneously Chinese. Ethnic differences may have contributed, as East Asians report lower lifetime prevalence of MDD (1), more somatic symptoms and fewer psychological symptoms (23), and differ in MDD risk genes (24). International studies will need to address this question.
In subgroup analyses, we only found decreased DMN FC in recurrent MDD patients, with nearly twice the effect size of the whole-group (d = −0.326 vs. −0.186). Similarly, ENIGMA-MDD found a robust reduction in hippocampal volume (a key DMN node) only in recurrent MDD and not in first-episode MDD (4). Illness duration in recurrent MDD was significantly longer than in FEDN MDD (Z = 6.419, P < 0.001), but it was unrelated to DMN FC on direct comparisons. An early MDD study (7) found that DMN FC was positively correlated with current episode duration but this was not confirmed subsequently (9, 25). We conclude that illness duration is likely unrelated to DMN FC. However, longitudinal studies are needed to determine whether DMN FC changes over the course of depressive episodes.
Decreased DMN FC in recurrent MDD was associated with antidepressant medication treatment. We confirmed that first-episode MDDs scanned while on medication had decreased DMN FC compared with FEDN MDDs. This result aligns with studies of antidepressants on DMN FC in MDD (26), dysthymia (27), and in healthy individuals (28). In MDD, antidepressant treatment for 12 wk reduced posterior DMN FC (26). In patients with dysthymia, 10 wk of duloxetine treatment reduced DMN FC (27). In healthy individuals, duloxetine for 2 wk reduced DMN FC and improved mood (28). Our finding of medication-associated reduction in DMN FC suggests antidepressant medications may alleviate depressive symptoms by reducing DMN FC. This medication effect (effect size d = −0.362) might also underlie the contradiction between our finding of reduced DMN FC in MDD and prior meta-analyses. However, this medication effect was observed in a retrospective cross-sectional sample that cannot be stratified by class, dosage, or length of use, and thus has it to be confirmed using longitudinal designs with medication follow-up.
We did not find significant associations between DMN FC and symptom severity in all MDDs or in FEDN MDDs. However, symptom severity was positively correlated with DMN FC in recurrent MDDs. Similarly, a prior report (29) found a positive correlation between DMN FC in a specific frontal subcircuit and illness severity in MDDs (half treated with medication). Our finding may reflect medication effects in recurrent MDD (the effect was stronger in recurrent MDDs on medication: n = 40, two sites; t = 3.268, P = 0.003, r = 0.489): The greater the medication benefit (indicated by lower HAMD score), the more DMN FC was reduced. However, this finding should be interpreted with caution, as these small sample size, secondary analyses might not reflect a true effect (2). Additionally, this result was not consistently confirmed with other parcellations (SI Appendix, Table S7). More importantly, testing this hypothesis requires longitudinal follow-up of medication effects.
To extend beyond the DMN, we explored other brain networks defined by Yeo et al. (15). We found decreased FC within VN, SMN, and DMN. Task-based fMRI studies have reported abnormal neural filtering of irrelevant visual information in visual cortex in MDD (30). R-fMRI studies have also found reduced VN FC in MDD patients (31), suggesting abnormal processing in the visual cortex in MDD. For SMN, a previous meta-analysis (32) reported reduced regional homogeneity in depressed patients, which could underlie psychomotor retardation, a core clinical manifestation of MDD (33). Besides changes in within-network FC, we also observed decreased between-network FC involving VN, SMN, and DAN. The reduced FC of the SMN with the VN and DMN may be interpreted as the neural underpinnings of the pervasive influence of psychomotor retardation on attentional processes, as revealed by previous studies (34). Similar to the primary analyses focused on the DMN, most of these other alterations in FC were contributed by recurrent MDD patients, which needs to be confirmed by future longitudinal designs.
Study limitations include an exclusively Chinese sample, with unknown generalization to other populations. As a next step, UK Biobank MDD data (35) should be analyzed. In addition, in conjunction with the ENIGMA-MDD consortium (36), we are inviting international MDD researchers to join the REST-meta-MDD Project to identify ethnicity/culture-general and ethnicity/culture-specific abnormal brain patterns in MDD. Second, we could not address longitudinal effects, such as response to treatment. We anticipate the REST-meta-MDD consortium will perform coordinated prospective longitudinal studies. Third, medication treatment was binary; future studies should quantify cumulative doses and include nonpharmacologic treatments. Finally, our findings require independent replication (11). To improve transparency and reproducibility, the analysis code has been openly shared at https://github.com/Chaogan-Yan/PaperScripts/tree/master/Yan_2019_PNAS. The R-fMRI indices of the 1,300 MDD patients and 1,128 NCs have been openly shared through the R-fMRI Maps Project (rfmri.org/REST-meta-MDD). These data derivatives will allow replication, secondary analyses and discovery efforts while protecting participant privacy and confidentiality. Future independent efforts could include generating neural biotypes of MDD (37), performing dynamic FC analysis, and data mining with machine learning algorithms.
In summary, based on the largest R-fMRI database of MDD, we confirmed the key role of the DMN in MDD, identifying a reduction of DMN FC in patients with recurrent MDD. This reduction appears to reflect medication usage rather than illness duration. These findings suggest that the DMN should remain a prime target for further MDD research, especially to determine whether reducing DMN FC mediates symptomatic improvement.
Materials and Methods
Phenotypic Data.
Consortium members (25 research groups from 17 Chinese hospitals) met on March 25, 2017, to establish the collaboration; all agreed to provide diagnosis, age at scan, sex, and education. When collected systematically, measures of first-episode or recurrent MDD (if a patient’s prior and current episode were diagnosed as MDD based on ICD10 or DSM-IV), medication status, illness duration, and 17-item HAMD were also provided. Deidentified and anonymized data were contributed from studies approved by local Institutional Review Boards. All study participants provided written informed consent at their local institution.
Individual-Level Image Processing.
Neuroimaging analysts from each site took a 2-d DPARSF training course on May 13 and 14, 2017, at the Institute of Psychology, Chinese Academy of Sciences to harmonize analyses of individual R-fMRI data and 3D T1-weighted images.
After preprocessing (SI Appendix, Supplementary Methods), time series for the Dosenbach 160 functional ROIs (14) were extracted. Dosenbach 160 functional ROIs were used for the primary analysis as these functionally defined regions were based on a series of five meta-analyses, focused on error processing, default mode (task-induced deactivations), memory, language, and sensorimotor functions. For each, we defined DMN ROIs as those overlapping with the DMN delineated by Yeo et al. (15). The average FC (Fisher’s r-to-z transformed Pearson’s correlation between time series of all ROI pairs) within DMN ROIs was defined as DMN within-network FC for patient–control contrasts.
Group-Level Image Processing.
Sample selection.
From 1,300 MDDs and 1,128 NCs, we selected 848 MDDs and 794 NCs from 17 sites for statistical analyses. Exclusion criteria (e.g., incomplete information, bad spatial normalization, bad coverage, excessive head motion, and sites with fewer than 10 subjects in either group) and final inclusions are provided in SI Appendix, Supplementary Methods and Fig. S2.
Statistical analyses.
We used the LMM to compare MDDs with NCs while allowing site-varying effects. LMM describes the relationship between a response variable (e.g., DMN FC) and independent variables (here, diagnosis and covariates of age, sex, education, and head motion), with coefficients that can vary with respect to grouping variables (here, site) (16). We utilized MATLAB’s command fitlme (https://www.mathworks.com/help/stats/fitlme.html) to test the model: y ∼1 + Diagnosis + Age + Sex + Education + Motion + (1 | Site) + (Diagnosis | Site), which yields t and P values for the fixed effect of Diagnosis. Cohen’s d effect size was computed as (38).
Subgroup analyses.
Several sites reported whether patients with MDD were in their first episode (and drug-naïve) or recurrent. We compared 232 FEDN MDD patients with 394 corresponding NCs from five sites. We also compared 189 recurrent MDD patients with 427 corresponding NCs from six sites. To compare 119 FEDN MDD patients with 72 recurrent MDD patients from two sites, we replaced Diagnosis with FEDN or recurrent status in the LMM model.
Analyses of effects of illness duration, medication, and symptom severity.
As the distribution of illness duration was skewed (most were brief), we contrasted the terciles with longest and shortest illness durations instead of Diagnosis in the LMM model. To test medication effects, we replaced Diagnosis with medication (on/off, assessed at time of scan) in the LMM model. Finally, to test symptom severity effects, we replaced Diagnosis with the 17-item HAMD total score regressor in the LMM model.
Supplementary Material
Acknowledgments
This work was supported by National Key R&D Program of China Grant 2017YFC1309902; National Natural Science Foundation of China Grants 81671774, 81630031, 81471740, 81621003, and 81371488; the Hundred Talents Program; 13th Five-Year Informatization Plan XXH13505 of the Chinese Academy of Sciences; Beijing Municipal Science & Technology Commission Grants Z161100000216152, Z171100000117016, Z161100002616023, and Z171100000117012; Department of Science and Technology, Zhejiang Province Grant 2015C03037; and National Basic Research (973) Program 2015CB351702.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The R-fMRI indices of the 1,300 patients with major depressive disorder and 1,128 normal controls are available through the R-fMRI Maps Project (rfmri.org/REST-meta-MDD). The analysis code has been deposited in GitHub (https://github.com/Chaogan-Yan/PaperScripts/tree/master/Yan_2019_PNAS).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1900390116/-/DCSupplemental.
References
- 1.Ferrari AJ, et al. Burden of depressive disorders by country, sex, age, and year: Findings from the global burden of disease study 2010. PLoS Med. 2013;10:e1001547. doi: 10.1371/journal.pmed.1001547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Button KS, et al. Power failure: Why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci. 2013;14:365–376. doi: 10.1038/nrn3475. [DOI] [PubMed] [Google Scholar]
- 3.Chen X, Lu B, Yan CG. Reproducibility of R-fMRI metrics on the impact of different strategies for multiple comparison correction and sample sizes. Hum Brain Mapp. 2018;39:300–318. doi: 10.1002/hbm.23843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schmaal L, et al. Subcortical brain alterations in major depressive disorder: Findings from the ENIGMA major depressive disorder working group. Mol Psychiatry. 2016;21:806–812. doi: 10.1038/mp.2015.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmaal L, et al. Cortical abnormalities in adults and adolescents with major depression based on brain scans from 20 cohorts worldwide in the ENIGMA Major Depressive Disorder Working Group. Mol Psychiatry. 2017;22:900–909. doi: 10.1038/mp.2016.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamilton JP, Farmer M, Fogelman P, Gotlib IH. Depressive rumination, the default-mode network, and the dark matter of clinical neuroscience. Biol Psychiatry. 2015;78:224–230. doi: 10.1016/j.biopsych.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greicius MD, et al. Resting-state functional connectivity in major depression: Abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry. 2007;62:429–437. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu X, et al. Evidence of a dissociation pattern in resting-state default mode network connectivity in first-episode, treatment-naive major depression patients. Biol Psychiatry. 2012;71:611–617. doi: 10.1016/j.biopsych.2011.10.035. [DOI] [PubMed] [Google Scholar]
- 9.Guo W, et al. Abnormal default-mode network homogeneity in first-episode, drug-naive major depressive disorder. PLoS One. 2014;9:e91102. doi: 10.1371/journal.pone.0091102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaiser RH, Andrews-Hanna JR, Wager TD, Pizzagalli DA. Large-scale network dysfunction in major depressive disorder: A meta-analysis of resting-state functional connectivity. JAMA Psychiatry. 2015;72:603–611. doi: 10.1001/jamapsychiatry.2015.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poldrack RA, et al. Scanning the horizon: Towards transparent and reproducible neuroimaging research. Nat Rev Neurosci. 2017;18:115–126. doi: 10.1038/nrn.2016.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eklund A, Nichols TE, Knutsson H. Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci USA. 2016;113:7900–7905. doi: 10.1073/pnas.1602413113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chao-Gan Y, Yu-Feng Z. DPARSF: A MATLAB toolbox for “pipeline” data analysis of resting-state fMRI. Front Syst Neurosci. 2010;4:13. doi: 10.3389/fnsys.2010.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dosenbach NU, et al. Prediction of individual brain maturity using fMRI. Science. 2010;329:1358–1361. doi: 10.1126/science.1194144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yeo BT, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.West BT, Welch KB, Galecki AT. Linear Mixed Models: A Practical Guide Using statistical software. CRC; Boca Raton, FL: 2014. [Google Scholar]
- 17.Ahmed AT, et al. Mood Disorders Precision Medicine Consortium (MDPMC) Mapping depression rating scale phenotypes onto research domain criteria (RDoC) to inform biological research in mood disorders. J Affect Disord. 2018;238:1–7. doi: 10.1016/j.jad.2018.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Craddock RC, James GA, Holtzheimer PE, 3rd, Hu XP, Mayberg HS. A whole brain fMRI atlas generated via spatially constrained spectral clustering. Hum Brain Mapp. 2012;33:1914–1928. doi: 10.1002/hbm.21333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zalesky A, et al. Whole-brain anatomical networks: Does the choice of nodes matter? Neuroimage. 2010;50:970–983. doi: 10.1016/j.neuroimage.2009.12.027. [DOI] [PubMed] [Google Scholar]
- 20.Power JD, Plitt M, Laumann TO, Martin A. Sources and implications of whole-brain fMRI signals in humans. Neuroimage. 2016;146:609–625. doi: 10.1016/j.neuroimage.2016.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- 22.Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional-anatomic fractionation of the brain’s default network. Neuron. 2010;65:550–562. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ryder AG, et al. The cultural shaping of depression: Somatic symptoms in China, psychological symptoms in North America? J Abnorm Psychol. 2008;117:300–313. doi: 10.1037/0021-843X.117.2.300. [DOI] [PubMed] [Google Scholar]
- 24.Long H, et al. The long rather than the short allele of 5-HTTLPR predisposes Han Chinese to anxiety and reduced connectivity between prefrontal cortex and amygdala. Neurosci Bull. 2013;29:4–15. doi: 10.1007/s12264-013-1299-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wise T, et al. Instability of default mode network connectivity in major depression: A two-sample confirmation study. Transl Psychiatry. 2017;7:e1105. doi: 10.1038/tp.2017.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li B, et al. A treatment-resistant default mode subnetwork in major depression. Biol Psychiatry. 2013;74:48–54. doi: 10.1016/j.biopsych.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 27.Posner J, et al. Antidepressants normalize the default mode network in patients with dysthymia. JAMA Psychiatry. 2013;70:373–382. doi: 10.1001/jamapsychiatry.2013.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Wingen GA, et al. Short-term antidepressant administration reduces default mode and task-positive network connectivity in healthy individuals during rest. Neuroimage. 2014;88:47–53. doi: 10.1016/j.neuroimage.2013.11.022. [DOI] [PubMed] [Google Scholar]
- 29.Davey CG, Harrison BJ, Yücel M, Allen NB. Regionally specific alterations in functional connectivity of the anterior cingulate cortex in major depressive disorder. Psychol Med. 2012;42:2071–2081. doi: 10.1017/S0033291712000323. [DOI] [PubMed] [Google Scholar]
- 30.Desseilles M, et al. Abnormal neural filtering of irrelevant visual information in depression. J Neurosci. 2009;29:1395–1403. doi: 10.1523/JNEUROSCI.3341-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Veer IM, et al. Whole brain resting-state analysis reveals decreased functional connectivity in major depression. Front Syst Neurosci. 2010;4:41. doi: 10.3389/fnsys.2010.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iwabuchi SJ, et al. Localized connectivity in depression: A meta-analysis of resting state functional imaging studies. Neurosci Biobehav Rev. 2015;51:77–86. doi: 10.1016/j.neubiorev.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 33.Buyukdura JS, McClintock SM, Croarkin PE. Psychomotor retardation in depression: Biological underpinnings, measurement, and treatment. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:395–409. doi: 10.1016/j.pnpbp.2010.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Snyder HR. Major depressive disorder is associated with broad impairments on neuropsychological measures of executive function: A meta-analysis and review. Psychol Bull. 2013;139:81–132. doi: 10.1037/a0028727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sudlow C, et al. UK biobank: An open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12:e1001779. doi: 10.1371/journal.pmed.1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thompson PM, et al. Alzheimer’s Disease Neuroimaging Initiative, EPIGEN Consortium, IMAGEN Consortium, Saguenay Youth Study (SYS) Group The ENIGMA consortium: Large-scale collaborative analyses of neuroimaging and genetic data. Brain Imaging Behav. 2014;8:153–182. doi: 10.1007/s11682-013-9269-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Drysdale AT, et al. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med. 2017;23:28–38. doi: 10.1038/nm.4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosenthal R, Rosnow RL. Essentials of Behavioral Research: Methods and Data Analysis. 3rd Ed McGraw-Hill; New York: 1991. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.