Abstract
Opioid drugs are important tools to alleviate pain of different origins, but they have strong addictive potential and their abuse at higher doses often results in serious health complications. Respiratory depression that leads to brain hypoxia is perhaps the most dangerous symptom of acute intoxication with opioids, and it could result in lethality. The development of substrate-specific sensors coupled with amperometry made it possible to directly evaluate physiological and drug-induced fluctuations in brain oxygen levels in awake, freely-moving rats. The goal of this review paper is to consider changes in brain oxygen levels induced by several opioid drugs (heroin, fentanyl, oxycodone, morphine). While some of these drugs are widely used in clinical practice, they all are abused, often at doses exceeding the clinical range and often resulting in serious health complications. First, we consider some basic knowledge regarding brain oxygen, its physiological fluctuations, and mechanisms involved in regulating its entry into brain tissue. Then, we present and discuss data on brain oxygen changes induced by each opioid drug within a wide range of doses, from low, behaviorally relevant, to high, likely to be self-administered by drug users. These data allowed us to compare the effects of these drugs on brain oxygen in terms of their potency, time-course, and their potential danger when used at high doses via rapid-onset administration routes. While most data discussed in this work were obtained in rats, we believe that these data have clear human relevance in addressing the alarming rise in lethality associated with the opioid abuse.
Keywords: opiates, health complications, brain hypoxia, metabolic brain activation, nucleus accumbens, rats
1. Introduction
Opioids are widely used as therapeutic drugs to alleviate pain of different origins. In addition to their pain-relieving effects, opioid drugs have strong addictive potential, predisposing individuals for their repeated, non-medical use. The abuse of opioid drugs typically results in progressive increases in drug doses, well above the usual analgesic or exploratory range. While the short-term therapeutic use of opioids usually does not result in any serious health complications, opioid drugs used at higher doses have a number of side effects, including sedation, inhibition of gastro-intestinal activity, and respiratory depression (Baud, 2009; Jaffe et al., 1997; Simon, 1997). The latter effect is especially dangerous, being primarily responsible for the development of acute brain hypoxia, coma and lethality following overdose of these drugs.
While respiratory depression is a minor problem during therapeutic use of opioid drugs such as morphine and oxycodone and it could be well controlled in clinical settings, respiratory depression appears to be a quite dangerous symptom following non-medical use of highly efficacious opioid drugs such as heroin and fentanyl. Heroin has exceptionally rapid and strong psychoactive and physiological effects and it is usually self-administered via rapid-onset, intravenous (iv) route and often at high doses. Despite the extensive use of fentanyl for general anesthesia and analgesia (Peng and Sandler, 1999; Dahan et al, 2005; Jaffe et al, 1997; Pattinson, 2008; Yeadon and Kitchen, 1989), this drug emerged as a recreational drug only recently due to its availability in clandestine drug markets and relatively low prices. This drug is often used by habitual heroin users in combination with heroin (Compton et al., 2016; McLaughlin, 2017). Since fentanyl is much more potent than heroin (Wade et al., 2015), illicit fentanyl use can result in dramatic health complications, including death during overdose (Compton et al, 2016; Suzuki and El-Haddad, 2017). Street heroin is often contaminated with unknown amounts of fentanyl, which makes a drug’s impact especially unpredictable if an individual believes that he or she is consuming a known, standard dose of heroin but is actually consuming the much more potent drug combination. Opioid drugs are also often combined with alcohol and benzodiazepines, the drugs that have strong sedative potential. Such multi-drug combinations, often tried by young adults, could also result in unpredicted health complications.
Considering the role of respiratory depression as the most dangerous effect of opioid drugs, we recently conducted a serious of studies, in which we employed oxygen sensors coupled with high-speed amperometry to examine how different opioid drugs affect brain oxygen levels in awake, freely-moving rats (Solis et al., 2017a, b, c; Solis et al., 2018a, b, c). While the executive manifestations of breathing, i.e., changes in rate, tidal volume, and regularity, are usually measured in animal studies using whole body plethysmography to assess drug-induced respiratory depression (Kuo et al., 2015; Emery et al., 2016), this approach employed in rats requires expensive instrumentation and it is not very accurate, failing to detect very shallow breathing and its rapid changes (Kabir et al., 2010). Pulse oximetry, a noninvasive method to monitor blood oxygen saturation in skin, is another approach to detect changes in blood oxygen levels and this approach is widely used in humans (Bowes et al., 1989). However, it is also quite difficult to adopt this technology in freely-moving rats. In contrast, our approach—direct, high-speed monitoring of oxygen levels in the extracellular space within a discrete brain area—allowed us to examine a functionally significant outcome of respiration. Along with monitoring brain oxygen changes, we also conducted oxygen measurements in the subcutaneous space, a densely-vascularized area with no metabolic activity of its own. Measurements from this location provide a proxy for changes in systemic blood oxygen levels – a parameter directly related to respiratory activity.
Four different opioid drugs were examined in our studies. We began with heroin, which is perhaps the most common opioid drug of abuse. While heroin is currently not used for therapeutic purposes, it has exceptionally rapid and strong psychoactive and physiological effects due its rapid entry into brain tissue of heroin itself and 6-MAM, its highly potent metabolite with very high affinity to μ-opioid receptors (Boix et al, 2013; Gottas et al, 2013). In contrast to heroin, which is purely a drug of abuse, fentanyl is an important therapeutic drug widely used in surgical practice. However, due to its rapid entry into brain tissue and very high affinity to μ-opioid receptors (Maguire et al., 1992), fentanyl as a drug of abuse is highly potent, being responsible for many deaths following overdose. Morphine and oxycodone, the last two drugs of our interest, are widely used in clinical settings for pain relief. They have about the same analgesic potency, and similar pharmacokinetics and pharmacodynamics, which are much slower than those for heroin and fentanyl. Both of these drugs are also abused, often at doses that greatly exceed the therapeutic range. While new knowledge on the effects of each of these four drugs by themselves is important, our current findings allowed us to compare these drugs in terms of their potency and potential danger.
2. Oxygen sensors and high-speed amperometry as a unique tool to examine physiological and drug-induced oxygen fluctuations in brain tissue
Commercially produced oxygen sensors (Pinnacle Technology Inc., Lawrence, Kansas, USA) were coupled in our experiments with fixed-potential amperometry to examine physiological and drug-induced fluctuations in oxygen levels. These sensors consist of an epoxy-sheathed disc electrode that is grounded to a fine surface using a diamond-lapping disc. These sensors are prepared from Pt-Ir wire 180 μm in diameter, with a relatively small sensing area of 0.025 mm2 at the sensor’s tip. In contrast to other sensor designs, an active electrode in the Pinnacle oxygen sensors is incorporated with an integrated Ag/AgCl reference electrode. Dissolved oxygen is reduced on the active surface of these sensors, which is held at a stable potential of −0.6 V vs. the reference electrode, producing an amperometric current. The current from the sensor is relayed to a computer via a potentiostat and recorded at 1-s intervals. In our work, we utilized two types of recording: an acute, in which the sensor was inserted in a previously implanted cannula and removed after the recording session, and chronic, where the sensor was surgically implanted into a target brain structure, thus allowing electrochemical recordings to be continued during several daily sessions. In most of our studies, brain oxygen recordings were conducted in the nucleus accumbens (NAc), a deep brain structure critically involved in sensorimotor integration and which is a part of the brain’s motivation-reinforcement circuit (Wise and Bozarth, 1987; Di Chiara, 2002). To clarify the mechanisms responsible for drug-induced brain oxygen responses, electrochemical measurements were also conducted in the subcutaneous space (mediofrontal area of the rat’s head), a densely vascularized area with no or minimal metabolism of its own. Thus, oxygen measurements from the subcutaneous space served as a valuable proxy of systemic blood oxygen levels. During data analyses, current values were transferred into concentration values, which were averaged for different time intervals. All our experiments were conducted in freely-moving rats, which had a previously implanted intravenous (iv) catheter and which were extensively habituated to the recording environment (Figure 1). Moreover, the use of catheter extensions and an electrical swivel allowed us to administer drugs when the rat was in quiet resting conditions without any sensory and arousing influences typical to standard subcutaneous or intraperitoneal drug administration.
Figure 1. All electrochemical evaluations of drug-induced changes in oxygen were conducted in freely-moving rats habituated to the recording environment.
Inset shows oxygen sensor (Pinnacle Technology) used in our experiments. This sensor was either inserted into a previously implanted cannula or chronically implanted in brain tissue or subcutaneous space. The testing drugs were injected via chronically implanted catheter, catheter extension, and electrical swivel from outside of the cage, thus eliminating any stress associated with injection procedure.
Measurement selectivity has been a long-standing troublesome issue for voltammetric techniques, which are used to assess fluctuations in oxidizable brain substances such as monoamines (Bucher and Wightman, 2015). While measurement selectivity is greatly improved by using enzyme-based biosensors, which allow the assessment of fluctuations in brain levels of non-oxidizable substances such as glutamate and glucose, multiple selectivity controls are still necessary due to possible contamination of specific currents by other chemical and physical contributions (Kiyatkin et al., 2013; Kiyatkin and Wakabayashi, 2015). However, oxygen is reduced by applied potentials, thus excluding possible contamination by multiple oxidizable substances, and the brain’s extracellular concentrations of oxygen are relatively large (10–30 μM), which generate relatively large-magnitude currents during its reduction by the applied potential. Therefore, changes in currents detected by oxygen sensors can accurately represent oxygen fluctuations in brain’s extracellular space.
3. Brain oxygen as an important homeostatic parameter
All oxygen used for brain metabolic activity arrives into brain tissue from arterial blood, where its levels depend on respiratory activity and are higher than in the brain’s extracellular space. Therefore, entry of oxygen into brain tissue is gradient-dependent, increasing or decreasing depending upon changes in blood oxygen levels that are determined by efficiency of respiratory activity. This parameter can be changed within wide limits during physiological and behavioral activation and following exposure to certain drugs that affect respiration. Oxygen entry into brain tissue also depends on the state of cerebral vessels, which can be dilated or constricted due to neurovascular coupling, thus enhancing or decreasing intra-brain entry of oxygen independently of the brain-blood concentration gradient (Attwell et al., 2010). In addition to these two basic factors affecting oxygen entry into brain tissue, oxygen is continuously consumed for metabolic activity and oxygen consumption is enhanced during neural activation. Therefore, these three variables are primary determinants affecting both basal levels of brain oxygen and their physiological and drug-induced fluctuations. While the respiration-dependent changes in blood oxygen content will definitely affect oxygen levels in brain tissue, it is quite difficult to distinguish the contribution of this factor due to simultaneous changes in neuronal activity, brain metabolism, and the tone of cerebral vessels, which all are affected by physiological and drug stimuli. While the slowly-acting influences related to oxygen content in the external medium and the efficiency of respiration affect oxygen levels in brain tissue, more complex phasic changes in brain oxygen levels occur after exposure to sensory and arousing stimuli, which elicit changes in neuronal activity, brain metabolism, and cardio-vascular functions, including perfusion pressure and the tone of peripheral and cerebral vessels.
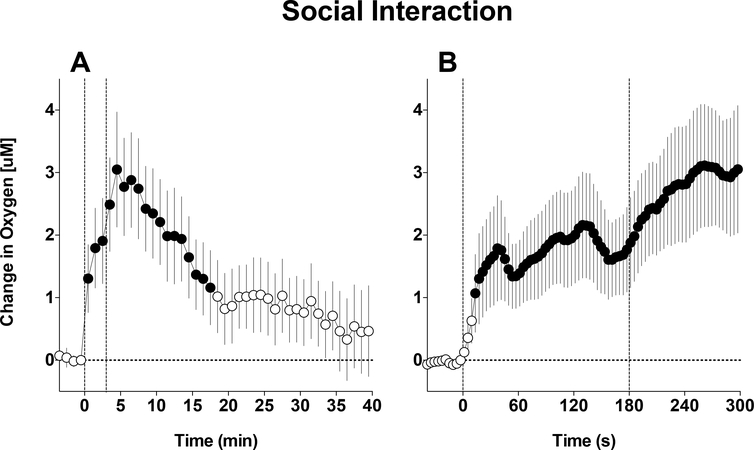
Before we initiated our oxygen studies with drugs, we examined how brain oxygen levels fluctuate in brain tissue under physiological conditions. Most of our brain recordings were conducted in the NAc, an important brain structure involved in sensori-motor integration and mechanisms of natural and drug reinforcement (Wise and Bozarth, 1987; Mogenson et al., 1980; Di Chiara, 2002). Male rats were extensively habituated to the recording environment and exposed to a number of arousing stimuli from a simple auditory tone to a tail-pinch and social interaction with male conspecific. In these experiments, we found that NAc oxygen levels rapidly increase following exposure to all of the arousing stimuli (Solis et al., 2017a). Figure 2 shows an example of NAc oxygen response induced by a 3-min social interaction of the recorded male rat with a male conspecific, a biologically relevant (salient) stimulation with no clear valence. When analyzed at slow (1-min) time-resolution, we found that NAc oxygen increased very rapidly at the start of stimulation, further grew thereafter, and peaked at the end of stimulation, when the rat actively investigated the cage after guest removal. By using, rapid (4-s) time resolution, we showed that changes in NAc oxygen are very rapid, showing maximal acceleration immediately after the start and after the end of stimulation. Other arousing stimuli also increased NAc oxygen levels, but there were distinct differences in responses depending on the stimulus duration and its presumed biological significance.
Figure 2. Mean (±SEM) changes in NAc oxygen levels induced by 3-min social interaction.
A shows data averaged with slow, 1-min time-resolution and B shows the same data analyzed with rapid, 4-s time resolution. Filled symbols show values significantly different from pre-injection baseline. Original data for this figure were published in Solis et al., 2017a.
While the rapidity of physiological fluctuations in NAc oxygen levels suggests a rapid neural mechanism, this mechanism was also confirmed by demonstrating rapid oxygen increases induced by intra-NAc microinjections of glutamate nearby the oxygen sensing area (Solis et al., 2017a). It is well known that accumbal neurons receive dense glutamate inputs (Rebec, 2005) and they are universally sensitive to and excited by both locally applied or iontophoretically delivered glutamate (Kiyatkin and Rebec, 1999). Therefore, the rapid oxygen increases induced by natural arousing stimuli are triggered by proximal neural activation, which induces local cerebral vasodilation, increased cerebral blood flow, and enhanced oxygen entry from arterial blood into the brain tissue. As shown in our previous thermorecording studies, natural arousing stimuli induce skin vasoconstriction that decreases blood flow in peripheral tissues and increases global cerebral blood flow to the brain (Kiyatkin, 2010).
4. NAc oxygen responses induced by heroin
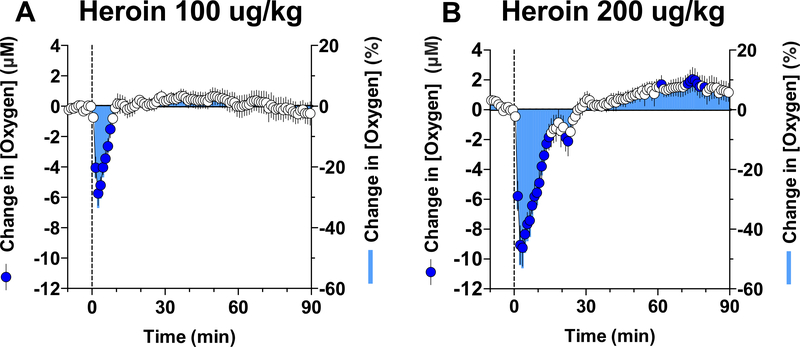
In contrast to oxygen increases elicited by natural arousing stimuli, iv heroin induced a rapid and strong decrease in NAc oxygen levels (Figure 3). At a low dose (0.1 mg/kg) optimal for self-administration in rats (Wise and Bozarth, 1987), the hypoxic effect was rapid, appearing within the first minute of drug administration, strong with a 30% drop from baseline, but relatively transient in its duration (A). At a higher heroin dose (0.2 mg/kg) within the range injected by experienced drug users, this effect greatly increased in magnitude (~50% drop from baseline) and duration (~20 min), suggesting robust brain hypoxia (B). Importantly, heroin-induced drop in oxygen was exceptionally rapid, displaying maximal change within 3–4 minutes after the start of iv injection.
Figure 3. Mean (±SEM) changes in NAc oxygen levels induced by iv injections of heroin at 100 (A) and 200 (B) μg/kg doses.
The changes in oxygen concentration are shown in both graphs in two ways: as an absolute change, in μM (left Y-axis) and relative change in percent (tight Y axes). Filled symbols show values significantly different from pre-injection baseline. Original data for this figure were published in Solis et al., 2017b.
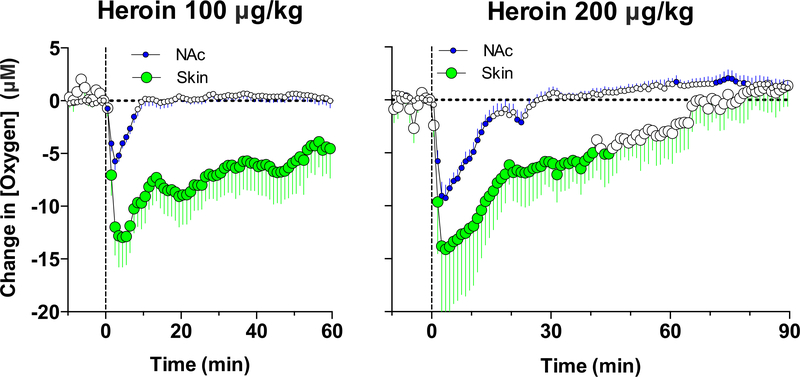
While we observed that heroin induces shallow breathing, suggesting respiratory depression as a cause of NAc oxygen decreases, this mechanism was confirmed by oxygen measurements in the subcutaneous space. As shown in Figure 4, heroin significantly decreased oxygen levels in the subcutaneous space; this effect was larger than that in the NAc in terms of μM change, due to higher basal oxygen levels in this location, but it was similar in term of percent change. In contrast to the NAc, where the oxygen decrease was transient, in the subcutaneous space it was much longer in duration. Therefore, respiratory depression and a decrease in blood oxygen levels is the primary cause of the oxygen drop in brain tissue induced by iv heroin. Importantly, the hypoxic effect of heroin is exceptionally rapid and strong even at behaviorally relevant doses. These rapid dynamics could reflect the unusual properties of heroin, which by itself has relatively low binding affinity (Inturrisi et al, 1983) and efficacy (Selley et al, 2001) at μ-opioid receptors but is quickly metabolized into two pharmacologically active metabolites, 6-monoacetylmorphine (6-MAM) and morphine, that have greater affinity to μ-opioid receptors (Gottas et al., 2014). While the traditional point of view postulates that the rapid effects of heroin results from its rapid entry into brain tissue (Oldendorf et al., 1972) and metabolic transformation into morphine, more recent data from direct measurements of heroin and its metabolites in the blood and brain’s extracellular space revealed the importance of 6-MAM, which appears in brain tissue at a high concentration (~200% of the injected dose) and prior to morphine, within minutes after heroin injection, and which has high affinity for μ-opioid receptors (Boix et al, 2013; Gottas et al, 2013).
Figure 4. Mean (±SEM) changes in oxygen levels in the NAc and subcutaneous space (Skin) induced by iv injections of heroin at 100 (A) and 200 (B) μg/kg doses.
Filled symbols show values significantly different from pre-injection baseline. Original data for this figure were published in Solis et al., 2017b.
5. NAc oxygen responses induced by fentanyl and heroin-fentanyl mixtures
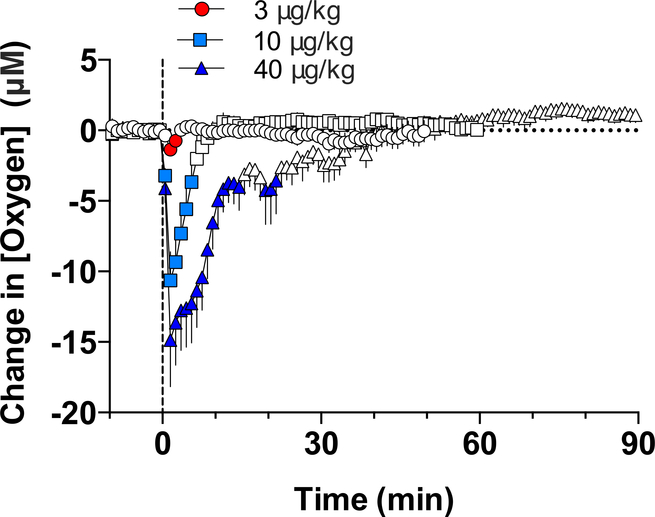
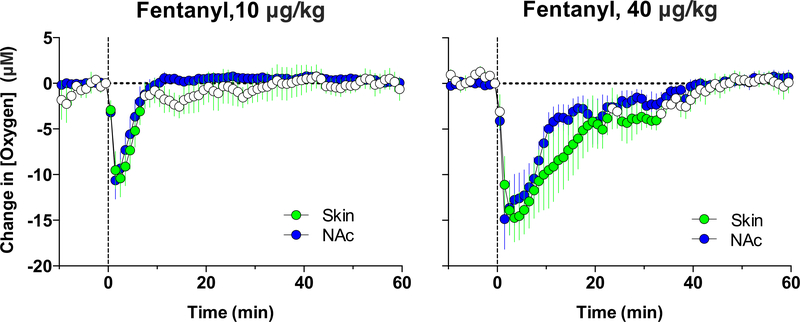
Fentanyl also decreased NAc oxygen levels; this effect appeared at very low doses and became stronger with dose increases (Figure 5). Similar to heroin, the effect was very rapid, appearing within the first minute and peaking at the second minute after the onset of iv injection. Based on the amplitude of oxygen decrease, fentanyl in terms of dose is 10–20-fold more potent than heroin. To confirm that fentanyl-induced decreases in brain oxygen result from respiratory depression, we examined the effects of this drug in the subcutaneous space, a proxy of arterial blood. As shown in Figure 6, similar to the NAc, fentanyl decreased oxygen levels in the subcutaneous space. Therefore, respiratory depression and a decrease in blood oxygen levels is the primary cause of the oxygen drop in brain tissue induced by iv fentanyl.
Figure 5. Mean (±SEM) changes in NAc oxygen levels induced by iv injections of fentanyl at different doses.
Filled symbols show values significantly different from pre-injection baseline. Original data for this figure were published in Solis et al., 2018a.
Figure 6. Mean (±SEM) changes in oxygen levels in the NAc and subcutaneous space (Skin) induced by iv injections of fentanyl at two doses.
Filled symbols show values significantly different from pre-injection baseline. Original data for this figure were published in Solis et al., 2018a.
Street heroin currently available to drug users is often contaminated by fentanyl. Due to fentanyl’s higher potency in terms of inducing respiratory depression (Dahan et al., 2005; Pattinson, 2008; Yeadon et al., 1989), intake of heroin-fentanyl mixtures may result in serious health complications, including comatose state and lethality (Compton et al., 2016; Suzuki and El-Haddad, 2017). To mirror such a real-life scenario, in which an individual believing that he or she is consuming a standard dose of heroin is actually consuming a heroin sample laced with a smaller amount of fentanyl, we examined changes in NAc oxygen induced by heroin (0.4 mg/kg) contaminated with 10% fentanyl (0.04 mg/kg). These doses of both drugs are larger than those typically used by humans, but they are only a fraction of the LD50s assessed in rats (heroin: 15–20 mg/kg; Strandberg et al., 2006; Gable 2004; fentanyl: 1–3 mg/kg; von Gunten et al., 2010) and are within the range of consumption by experienced drug users (see erowid.org).
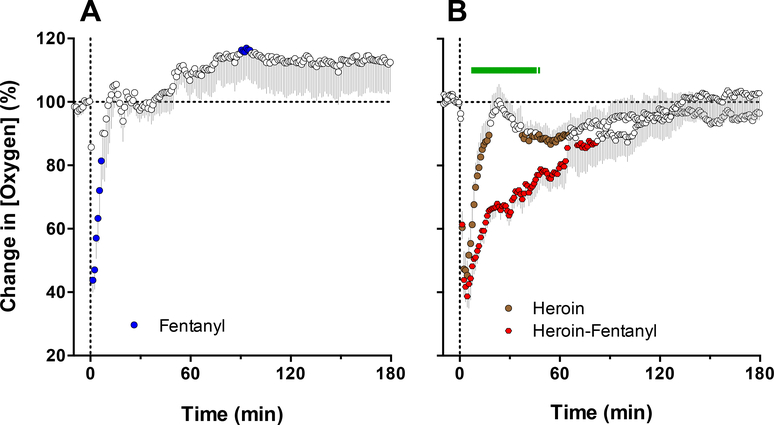
Consistent with our previous studies (Solis et al., 2017b, 2018a), both heroin and fentanyl injected individually induced strong but transient decreases in NAc oxygen levels which were rapid and relatively similar in magnitude (Figure 7; Solis et al., 2017c). A rapid and strong drop in NAc oxygen also occurred after administration of the heroin-fentanyl combination, but the response was much more prolonged than with each drug alone. As determined by the area under the curve for oxygen decrease, the effect of the heroin-fentanyl mixture was about 10-fold larger than fentanyl alone and ~5-fold larger than heroin alone. It is interesting that a clear potentiation of the NAc oxygen decrease following fentanyl-heroin mixture was not coupled with changes the response magnitude, which was only slightly larger for the mixture than with heroin alone. This prolongation of brain hypoxia is functionally important because brain cells may tolerate transient decreases in oxygen inflow, but they are damaged to a greater extent when hypoxia is more prolonged (Hossmann, 1999).
Figure 7. Mean (±SEM) changes in NAc oxygen levels induced by iv injections of fentanyl (40 μg/kg), heroin (400 μg/kg) and fentanyl-heroin mixture (40 + 360 μg/kg).
Filled symbols show values significantly different from pre-injection baseline. Green line in B shows time interval, where two curves significantly differed. Original data for this figure were published in Solis et al., 2017c.
6. NAc oxygen responses induced by morphine and oxycodone
Morphine is the prototypical opioid analgesic drug, which is still widely used to alleviate pain of different origins. As an analgesic drug, morphine is used in humans at low doses, varying from 5 to 20 mg for a 70 kg individual (or 0.07–0.3 mg/70 kg) (Paris and Yealy, 2006; Zimmer, 2004). Within this dose range (0.1–0.3 mg/kg), morphine is also effective in rats in sensitive nociceptive tests reflecting “affective components of pain” and during experimental noxious states (Kayser and Guilbaud, 1983, 1990; Kiyatkin, 1989). In contrast to heroin, morphine enters brain tissue much more slowly but remains in the brain for a longer time. Recent high-speed microdialysis measurements revealed that morphine levels in brain tissue peaked at only ~10–15% of the iv injected dose, with a subsequent slow and prolonged decay toward baseline (Gottas et al. 2014). Oxycodone, a semi-synthetic opioid analgesic, has slightly lower affinity to μ-receptors compared to morphine but it also interacts with a subtype of k-opioid receptors (Nielsen et al., 2007). Oxycodone also has similar liposolubility as morphine, slightly stronger analgesic properties and a slightly longer duration of action (Chan et al., 2008; Kalso, 2005). Both of these drugs are usually viewed as less dangerous in their acute effects than heroin or fentanyl.
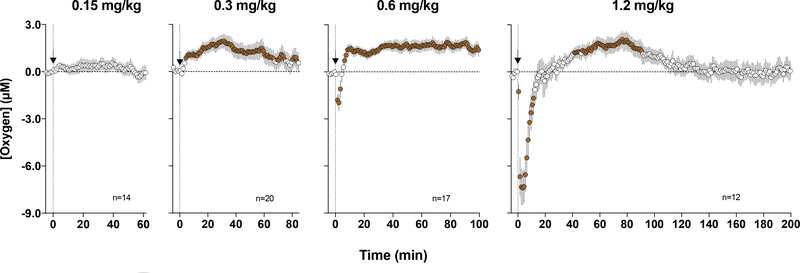
To examine how oxycodone affects NAc oxygen levels, we injected this drug to freely-moving rats at a wide range of doses (Solis et al., 2018b). In contrast to heroin, oxycodone at low and moderate doses (0.3–0.6 mg/kg) increased NAc oxygen levels (Figure 8). While significant, this effect was relatively weak, within 15% above the pre-injection baseline, but more tonic. However, at the highest dose (1.2 mg/kg), the effect became biphasic, with a transient decrease followed by an increase. A weak, transient drop in oxygen was also seen at the moderate oxycodone dose (0.6 mg/kg). When compared based on its ability to induce NAc oxygen decrease following iv injection, oxycodone was about 6 times less potent than heroin and about 60–120-fold weaker than fentanyl. In other words, fentanyl and heroin are 6- and 60–120-fold more potent in inducing brain hypoxia than oxycodone.
Figure 8. Mean (±SEM) changes in NAc oxygen levels induced by iv injections of oxycodone at different doses.
Filled symbols show values significantly different from pre-injection baseline. Original data for this figure were published in Solis et al., 2018b.
While the decrease in NAc oxygen induced by oxycodone at two high doses is likely a consequence of respiratory depression and a subsequent fall in blood oxygen levels, oxygen increases observed at low and moderate doses suggest that this drug has another effect that could be related to cerebral vasodilation and increased cerebral blood flow. This effect is relatively independent of respiratory depression and could occur due to neural activation via a neuro-vascular coupling mechanism (Attwell et al., 2010) as well as due to strong peripheral vasoconstriction (which results in redistribution of blood from the periphery to the brain and heart), which was found through our parallel thermorecording experiments (Solis et al., 2018b). Our data with parallel measurements of NAc glucose, which was dose-dependently increased by oxycodone, strongly support the role of this latter mechanism. Since glucose levels in arterial blood are maintained at very stable levels, the only possibility for their rapid increase in the brain is cerebral vasodilation that enhances glucose entry into brain tissue independently of concentration gradient (Kiyatkin and Lenoir, 2013).
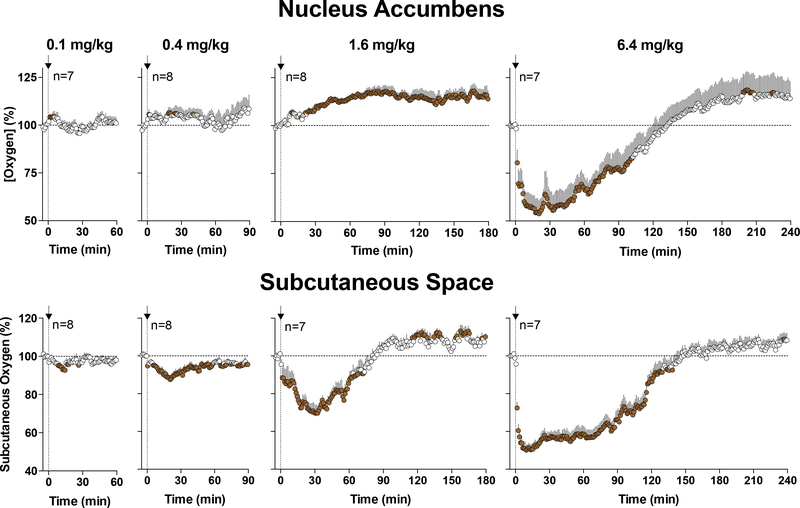
Our recent experiments with morphine allowed us to further clarify and confirm the role of this gradient-independent mechanism (Solis et al., 2018c). Morphine in these experiments was delivered at wide range of doses from low, within the traditional analgesic range (0.1 mg/kg), to large (6.4 mg/kg), within the range of possible overdose. As shown in Figure 9, morphine at a 0.1 mg/kg dose induces a very small and transient NAc oxygen increase. This increase becomes larger at the 0.4 and 1.6 mg/kg doses, but oxygen strongly decreased at the 6.4 mg/kg dose. While the morphine-induced oxygen decrease reflects respiratory depression, it is unclear why morphine at lower doses increased brain oxygen levels. To clarify this issue, we conducted oxygen measurements in the subcutaneous space (Figure 9). In this case, we found that subcutaneous oxygen levels were decreased at all morphine doses, displaying a clear dose-dependency. Since the subcutaneous space is metabolically inactive, we surmise that oxygen decreases in this location reflect decreases in blood oxygen levels due to a modest decrease in respiratory activity. Therefore, it appears that morphine at a wide range of doses depresses respiratory activity which results in decreases in oxygen content in blood and peripheral tissue. However, in the brain this gradient-dependent mechanism that tends to decrease brain oxygen content is opposed by another, neurovascular coupling mechanism that tends to increase its levels. Due to the prevalence of this mechanism, brain oxygen levels are increased by morphine at low doses to counteract weak decreases in blood oxygen caused by modest respiratory depression. At higher morphine doses, this adaptive mechanism is unable to compensate for the enhanced depression of respiratory activity, resulting in brain hypoxia. Hence, morphine appears to be safe at clinically relevant doses, but poses great risks at high doses likely to be abused by drug users.
Figure 9. Mean (±SEM) changes in oxygen levels in the NAc and subcutaneous space induced by iv injections of morphine at different doses.
Filled symbols show values significantly different from pre-injection baseline. Original data for this figure were published in Solis et al., 2018c.
7. Between-drug differences in NAc oxygen decreases
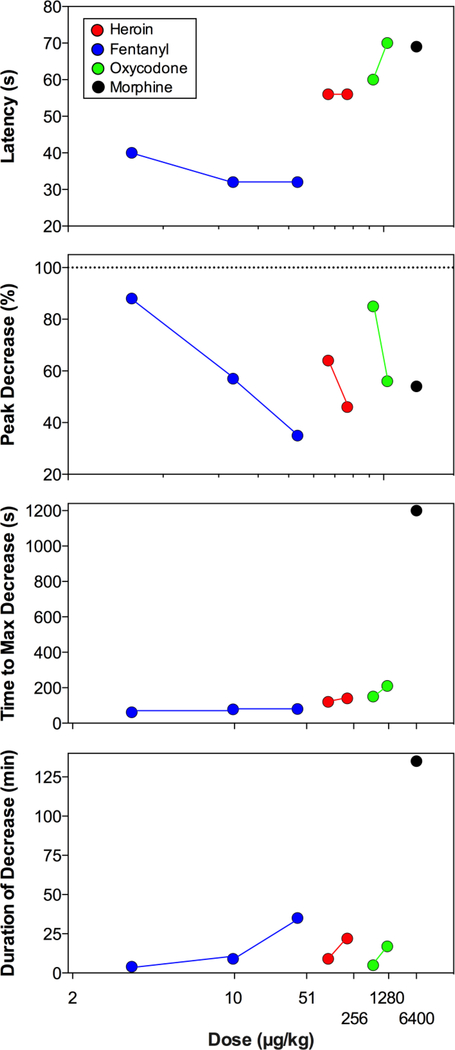
During the final stage of our analyses we compared four primary parameters of NAc oxygen decrease (latency, peak, time to peak, and duration) induced by heroin, fentanyl, oxycodone, and morphine at different doses (Figure 10). As can be seen, NAc oxygen decreases occurred with relatively similar onset latencies for each drug and each dose (40–70 s), with minimal values for fentanyl (32 s) and maximal for oxycodone (65 s) and morphine (70 s). These onset latencies also remained relatively stable with increases in drug doses. These latencies obviously reflect a definite time that is necessary for the drug delivered intravenously to reach centrally and peripherally located opioid receptors and induce centrally-mediated depression of respiratory activity. Each of the four drugs tested also decreased oxygen levels with different potency, and this effect was clearly dose-dependent for each drug. The most robust between-drug differences in NAc oxygen response were found with respect to the time to maximal effect, which was shortest for fentanyl (62, 78, and 80 s for 3, 10 and 40 μg/kg, respectively), slightly larger for heroin (120 and 140 s for 0.1 and 0.2 mg/kg doses) and oxycodone (150 and 210 s for 0.6 and 1.2 mg/kg doses) but much larger for morphine (20 min). Similar between-drug differences were found in duration of NAc oxygen decrease, which was short for fentanyl, heroin, and oxycodone, but clearly longer for morphine. While morphine was found to be ~10-fold less potent than fentanyl in respiratory-depressive effects as assessed by plethysmography following intracerebral injections, the effects of morphine were much more prolonged (Kuo et al., 2014). As shown in latter study, centrally administered oxycodone was 2–3-fold less potent than morphine and its effects were shorter than those for morphine.
Figure 10. Mean values of different parameters of NAc oxygen decreases induced by heroin, fentanyl, oxycodone and morphine, shown in log-scale.
Latency to decrease is the time from the onset of the injection to the first significant point of decrease. Peak decrease is the lowest point of oxygen decrease vs. baseline (=100%). Time to maximum decrease is the time from the onset of the injection to the point of maximum decrease. Duration of decrease is the time from onset of the injection to the last significant value in drug-induced brain oxygen decrease.
8. Conclusions and Clinical Implications
Respiratory depression appears to be a basic effect of all μ-opioid receptor agonists (Baud, 2009; Jaffe et al., 1997; Simon, 1997). As shown above, each of four opioids considered in this review decreased NAc oxygen levels, indicating brain hypoxia. However, these drugs had significant differences in their potency to decrease brain oxygen levels. In terms of dose, fentanyl was the most potent of four tested drugs and it induced a tiny, but significant transient drop in NAc oxygen at a very small, 3 μg/kg dose that maintains self-administration behavior in rats (Wade et al., 2015). This hypoxic response greatly progressed with 10 and 40 μg/kg doses, which are still much smaller than the LD50 assessed in rats with this route of drug delivery (1–3 mg/kg; Gunten et al., 2010). While about 10–20-fold less potent, heroin also induced a strong drop in oxygen at 100 μg/kg dose that is optimal for self-administration behavior (Gerber and Wise, 1979) but is also much smaller than the LD50 for iv drug administration in rats (20–23 mg/kg; Strandberg et al., 2006; Gable, 2004). Despite differences in potency, oxygen responses induced by both fentanyl and heroin developed with equally short latencies and rapidly peaked, but they were relatively transient. Therefore, rats could tolerate even strong decreases in oxygen levels (up to 40–20% of baseline) if these decreases are transient. It is also known that brain cells tolerate robust but transient hypoxia, but cellular damage is greatly progressing if hypoxia becomes prolonged (Hossmann, 1999).
Morphine and oxycodone have important differences from heroin and fentanyl in their effects on brain oxygen levels and these two drugs shared important common features. Unexpectedly, we found that both oxycodone and morphine at low and moderate doses, which are effective in sensitive analgesic tests and can maintain self-administration behavior, slightly increase brain oxygen levels, but clearly decrease them at high doses. As tested for morphine using subcutaneous oxygen measurements, these differential responses appear to result from the co-existence of two independent mechanisms that oppose each other in regulating oxygen entry into brain tissue. While at low doses, oxygen levels are increased due to neuro-vascular coupling mechanism, at higher doses this adaptive mechanism could not compete with robust drop in blood oxygen levels occurring due to respiratory depression.
Both morphine and oxycodone were clearly less potent to induce brain hypoxia than heroin and fentanyl. Based on the amplitude of brain oxygen decreases, oxycodone, heroin, and fentanyl are ~4-, 40-, and 400-fold stronger than morphine. Furthermore, the effects of heroin and fentanyl are more rapid than the effects of morphine, placing significant timing restraints for the use of opiate antagonists to treat an acute overdose. While morphine was the least potent of the tested drugs, its effects on brain oxygen were much slower but much longer than for other drugs. This lower potency and slower time-course of the oxygen decrease is consistent with pharmacokinetics of this drug, which has a limited and slow entry into brain tissue.
Most data discussed in this review were obtained in rats that place natural limitations in their translation to humans. For example, rats appear to be much more tolerant to transient hypoxia than humans tolerating apnea up to 6 min (Willette et al., 1987), which would likely result in lethality if apnea of such duration were to occur in humans (Dahan et al., 2005). Therefore, potentially dangerous doses of opioids in humans are likely lower than those in rats. Rats also have important differences in metabolism (Schmidt-Nielsen, 1997), thus affecting dynamic aspects of most neural processes, as well as metabolic transformation of drugs. While, our initial focus was on pure effects of the drug in drug-naive animals, humans expose themselves to multiple drugs and repeated drug administrations that also change the effects of these drugs. Nevertheless, we believe that these data have clear human relevance to explain the alarming rise in lethality associated with opioid abuse.
Highlights:
Brain oxygen is an important homeostatic parameter
Electrochemical sensors are tools to examine fluctuations in brain oxygen levels
Brain oxygen levels fluctuate under physiological conditions
All opioid drugs at high doses decrease brain oxygen due to respiratory depression
Opioid drugs differ in their ability to inhibit respiration and induce brain hypoxia
Acknowledgements
The original research reviewed in this manuscript was supported by the Intramural Research Program of the NIH, NIDA. The Author wants to thank Ernesto Solis Jr, Aaron Bola, Keaton Cameron-Burr, and Anum Afzal for assisting in experiments described in this work, analyses of data, and fruitful discussions on their functional implications. I also want to specially thank Anum Afzal for careful editing of the present text and helpful comments on its matters.
Footnotes
Conflict of Interest: The Author report no conflict of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, and Newman EA (2010). Glial and neuronal control of brain blood flow. Nature 468, 232–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baud FJ (2009). Mechanisms of opioid-induced overdose: experimental approach to clinical concerns. Ann. Pharm. Fr 67, 353–359. [DOI] [PubMed] [Google Scholar]
- Battisti-Charbonney A, Fisher J, and Duffin J (2011). The cerebrovascular response to carbon dioxide in humans. J. Physiol 589, 3039–3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazzu G, Puggioni GGM, Dedola S, Calla G, Rocchitta G, Mgheli R, Desole MS, Lowry JP, O’Neill RD, and Serra PA (2009). Real-time monitoring of brain tissue oxygen using a miniaturized biotelemetric device implanted in freely moving rats. Anal. Chem 81, 2235–2241. [DOI] [PubMed] [Google Scholar]
- Bedford TG, Tipton CM, Wilson NC, Oppliger RA, and Gisolfi NC (1979). Maximum oxygen consumption of rats and its changes with various experimental procedures. J. App.l Physiol.: Respirat. Environ. Exercise Phys 47, 1278–1283. [DOI] [PubMed] [Google Scholar]
- Bolger FB, Bennett R, and Lowry JP (2011). An in vitro characterization comparing carbon paste and Pt microelectrodes for real-time detection of brain tissue oxygen.” Analyst 136, 4028–4035. [DOI] [PubMed] [Google Scholar]
- Cannon W (1929). Bodily Changes in Pain, Hunger, Fear and Rage. New York: Littleton. [Google Scholar]
- Carelli RM, and West MO (1991). Representation of the body by single neurons in the dorsolateral striatum of the awake, unrestrained rats. J. Comp. Neurol 309, 231–249. [DOI] [PubMed] [Google Scholar]
- Clark LC, Misrahy G, and Fox RP (1958). Chronically implanted polarographic electrodes. J. Appl. Physiol 13, 85–91. [DOI] [PubMed] [Google Scholar]
- Compton WM, Jones CM, and Baldwin GT (2016). Relationship between Nonmedical PrescriptionOpioid Use and Heroin Use. N. Engl. J. Med 374, 154–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahan A, Yassen A, Bijl H, Romberg R, Sarton E, Teppema L, et al. (2005). Comparison of the respiratory effects of intravenous buprenorphine and fentanyl in humans and rats. Br. J. Anaesth 94, 825–834. [DOI] [PubMed] [Google Scholar]
- Davies PW, and Brink FJ (1942). Microelectrodes for measuring local oxygen tension in animal tissues. Rev. Sci. Instrum 13, 524–533. [Google Scholar]
- Davies PW, and Bronk DW (1957). Oxygen tension in mammalian brain. Fed. Proc 16, 689–692. [PubMed] [Google Scholar]
- Di Chiara G (2002). Nucleus accumbens shell and core dopamine: differential role in behavior and addiction. Behav. Brain Res 137, 75–114. [DOI] [PubMed] [Google Scholar]
- Duelli R, and Kuschinsky W (2001). Brain glucose transporters: Relationship to local energy demand. News Physiol. Sci 16, 71–76. [DOI] [PubMed] [Google Scholar]
- Emery MJ, Groves CC, Kruse TN, Shi C, and Terman GW (2016). Ventilation and the response to hypercapnia after morphine in opioid-naive and opioid-tolerant rats. Anesthesiology 124, 945957. [DOI] [PubMed] [Google Scholar]
- Fellows LK, Boutelle MG, and Fillenz M (1992). Extracellular brain glucose levels reflect local neuronal activity: a microdialysis study in awake, freely moving rats. J. Neurochem 59, 2141–2147. [DOI] [PubMed] [Google Scholar]
- Fellows LK, and Boutelle MG (1993). Rapid changes in extracellular glucose levels and blood flow in the striatum of the freely moving rat. Brain Res 604, 225–231. [DOI] [PubMed] [Google Scholar]
- Fox PT, and Raichle ME (1986). Focal physiological uncoupling of cerebral blood flow and oxidative metabolism during somatosensory stimulation in human subjects. Proc. Natl. Acad. Sci. USA 83, 1140–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin AL (1967). The Conduction of the Nervous Impulse. Liverpool: Liverpool University Press. [Google Scholar]
- Hossmann KA (1999). The hypoxic brain. Insights from ischemic research. Adv. Exp. Med. Biol 474, 155–169. [PubMed] [Google Scholar]
- Jaffe JH, Knapp CM, and Ciraulo DA (1997). Opiates: Clinical Aspects In: Substance Abuse. A comprehensive Textbook (3rd Ed) edited by Lowinson JH, Ruis P, Millman RB Langrod JG. (pp. 158–165). Baltimore: et al. : Williams and Wilkins. [Google Scholar]
- Kabir MM, Beig MI, Baumert M, Trombini M, Mastorci F, Sgoifo A, Walker FR, Day TA, and Nalivaiko E (2010). Respiratory pattern in awake rats: Effects of motor activity and alerting stimuli. Physiol. Behav 101, 22–31. [DOI] [PubMed] [Google Scholar]
- Kayser V, and Guilbaud G. (1983). The analgesic effects of morphine, but not those of the enkephalinase inhibitor thiorphan, are enhanced in arthritic rats. Brain Res 267, 131–138. [DOI] [PubMed] [Google Scholar]
- Kealy J, Bennett R, and Lowry JP (2013). Simultaneous recording of hippocampal oxygen and glucose in real time using constant potential amperometry in freely-moving rat. J. Neurosci. Meth 215, 110–120. [DOI] [PubMed] [Google Scholar]
- Kealy J, Bennett R, and Lowry JP (2015). Real-time effects of insulin-induced hypoglycaemia on hippocampal glucose and oxygen. Brain Res. 1598, 76–87. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA (1989). Morphine: some puzzles of well-known substance. Int. J. Neurosci 45, 231–246. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA (2010). Brain temperature homeostasis: physiological fluctuations and pathological shifts. Front. Biosci 15, 73–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyatkin EA, and Lenoir M (2012). Rapid fluctuations in extracellular brain glucose levels induced by natural arousing stimuli and intravenous cocaine: fueling the brain during neural activation. J. Neurophysiol 108, 1669–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyatkin EA, and Rebec GV (1996). Dopaminrgic modulation of glutamate-induced excitations of neurons in the neostriatum and nucleus accumbens of awake, unrestrained rats. J. Neurophysiol 75, 142–53. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA, and Rebec GV (1999). Modulation of striatal neuronal activity by glutamate and GABA: iontophoresis in awake, unrestrained rats. Brain Res. 822, 88–106. [DOI] [PubMed] [Google Scholar]
- Kontos HA (1981). Regulation of the cerebral circulation. Annu. Rev. Physiol 43, 397–407. [DOI] [PubMed] [Google Scholar]
- Kuo A, Wyse BD, Meutermans W, and Smith MT (2014). In vivo profiling of seven common opioids for antinociception, constipation, and respiratory depression: no two opioids have the same profile. Br. J. Pharmacol 172, 532–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecrux C, and Hamel E (2011) The neurovascular unit in brain function and disease. Acta Physiol (Oxf) 203: 47–59. [DOI] [PubMed] [Google Scholar]
- Li J, Bravo DS, Gilmor G, Tricklebank MD, Fillnz M, Martin C, Lowry JP, Bannerman DM, and McHugh SD (2011). Close temporal coupling of neuronal activity and tissue oxygen responses in rodent whisker barrel cortex. Eur. J. Neurosci 34, 1983–1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry JP, O’Neill RD, Boutille MG, and Fillenz M (1998). Continuous monitoring of extracellular glucose concentrations in the striatum of freely moving rats with an implanted glucose biosensor. J. Neurochem 70, 391–396. [DOI] [PubMed] [Google Scholar]
- Maguire P, Tsai N, Kamal J, Cometta-Morrini C, Upton C, and Loew G (1992). Pharmacological profile of fentanyl analogs at mu, delta, and kappa receptors. Eur. J. Pharmacol 213, 219–225. [DOI] [PubMed] [Google Scholar]
- Martin C., Martindale J., Berwick J., Mayhew J. (2006). Investigating neural-hemodynamic coupling and the hemodynamic response function in the awake rat. Neuroimage 32, 33–48. [DOI] [PubMed] [Google Scholar]
- McNay EC, McCarty RC, and Gold PE (2001). Fluctuations in brain glucose concentrations during behavioral testing: dissociation between brain areas and between brain and blood. Neurobiol. Learn. Mem 75, 325–337. [DOI] [PubMed] [Google Scholar]
- McLaughlin K (2017). Deadly chemistry. Science 355, 1364–1366. [DOI] [PubMed] [Google Scholar]
- Mergenthaler P, Lindauer U, Dienel G, and Meisel A (2013). Sugar for the brain: The role of glucose in physiological and pathological brain function. Trends Neurosci.. 36, 587–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogenson GJ, Jones DL, and Yim CY (1980). From motivation to action: functional interface between the limbic system and the motor system. Prog. Neurobiol 14, 69–97. [DOI] [PubMed] [Google Scholar]
- Muoio V, Persson PB, and Sendeski MM (2014). The neurovascular unit – concept review. Acta Physiol. (Oxf) 210, 790–798. [DOI] [PubMed] [Google Scholar]
- Pattinson KT (2008). Opioids and the control of respiration. Br. J. Anaesth 100, 747–758. [DOI] [PubMed] [Google Scholar]
- Paulson OB, Hasselbalch SG, Rostrup E, Knudsen GM, Pellegrino D (2010). Cerebral blood flow response to functional activation. J. Cereb. Blood Flow Metab 30, 2–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng PW, and Sandler AN (1999). A review of the use of fentanyl analgesia in the management of acute pain in adults. Anesthesiology 90, 576–599. [DOI] [PubMed] [Google Scholar]
- Rebec GV (2006). Behavioral electrophysiology of psychostimulants. Neuropsychopharmacology 31, 2341–2348. [DOI] [PubMed] [Google Scholar]
- Ritchie JM (1973). Energetic aspects of nerve conductance: The relationships between heat production, electrical activity and metabolism. Progr. Biophys. Molec. Biol 26, 147–187. [DOI] [PubMed] [Google Scholar]
- Rolfe DF, and Brown GC (1997). Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol. Rev 77, 731–758. [DOI] [PubMed] [Google Scholar]
- Schmidt CF, and Kety SS (1947). Recent studies of cerebral blood flow and cerebral metabolism in man. Trans. Assoc. Am. Physicians 60, 52–58. [PubMed] [Google Scholar]
- Schmidt-Nielsen K (1997). Animal Physiology. Adaptation and Environment. Cambridge, Cambridge University Press. [Google Scholar]
- Silver IA, and Erecinska M (1994). Extracellular glucose concentration in mammalian brain: continuous monitoring of changes during increased neuronal activity and upon limitation in oxygen supply in normo-, hypo-, and hyperglycemic animals. J. Neurosci 14, 5068–5076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverthorn DU (2004). Human Physiology: An Integrative Approach. San Francisco, CA: Benjamin Cummings. [Google Scholar]
- Simon EJ (1997). Opiates: Neurobiology In: Substance Abuse, Third Edition (Lowinson JH, Ruiz P, Millman RB, Langrod JG, eds), pp 148–158. Baltimore: Williams & Wilkins. [Google Scholar]
- Siesjo B (1978). Brain Energy Metabolism. New York: Wiley. [Google Scholar]
- Sokoloff L (1999). Energetics of functional activation in neural tissues. Neurochem. Res 24, 321–329. [DOI] [PubMed] [Google Scholar]
- Solis E Jr., Cameron-Burr KT, and Kiyatkin EA (2017a). Rapid physiological fluctuations in nucleus accumbens oxygen levels induced by arousing stimuli: Relationships with changes in brain glucose and metabolic brain activation. Front. Integr. Neurosci April 24; 11:9. doi: 10.3389/fnint.2017.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solis E Jr., Cameron-Burr KT, Shaham Y, and Kiyatkin EA (2017b). Intravenous heroin induces rapid brain hypoxia and hyperglycemia that precede brain metabolic response. eNeuro. 2017 June 7;4(3). pii: ENEURO.0151–17.2017. doi: 10.1523/ENEURO.0151-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solis E Jr., Cameron-Burr KT, and Kiyatkin EA (2017c). Heroin contaminated with fentanyl dramatically enhances brain hypoxia and induces brain hypothermia. eNeuro 4(5) e0323–17, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solis E Jr., Cameron-Burr KT, Shaham Y, and Kiyatkin EA (2018a). Fentanyl-induced brain hypoxia triggers brain hyperglycemia and biphasic changes in brain temperature. Neuropsychopharmacology 43, 810–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solis E Jr., Afzal A, and Kiyatkin EA (2018b). Relationships between central and peripheral effects of oxycodone: brain oxygen, glucose, and temperature. Neuropharmacology 133, 481–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solis E Jr., Afzal A, and Kiyatkin EA (2018c). Opposing mechanisms underlying differential changes in brain oxygen and temperature induced by intravenous morphine. J. Neurophysiol 120, 2513–2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki J, and El-Haddad S (2017). A review: Fentanyl and non-pharmaceutical fentanyls. Drug Alcohol Depend. 171, 107–116. [DOI] [PubMed] [Google Scholar]
- Travis RP, and Clark LC (1965). Changes in evoked brain oxygen during sensory stimulation and conditioning. Electroencephalog. Clin Neurophysiol. 19, 484–491. [DOI] [PubMed] [Google Scholar]
- Wade CL, Vendruscolo LF, Schlosburg JE, Hernandez DO, and Koob GF (2015). Compulsive-like responding for opioid analgesics in rats with extended access. Neuropsychopharmacology 40, 421–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi KT, and Kiyatkin EA.(2015). Behavior-associated and post-consumption glucose entry into the nucleus accumbens extracellular space during glucose free-drinking in trained rats. Front. Behav. Neurosci 9, 173; doi: 10.3389/fnbeh.2015.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, and Bozarth MA (1987). A psychomotor stimulant theory of addiction. Psychol. Rev 94, 469–492. [PubMed] [Google Scholar]
- Yeadon M, and Kitchen I (1990). Multiple opioid receptors mediate the respiratory depressant effects of fentanyl-like drugs in the rat. Gen. Pharmacol 21, 655–664. [DOI] [PubMed] [Google Scholar]