Abstract
The abuse potential of opioid analgesics in humans appears to increase rapidly during initial regimens of opioid exposure. Previous work using intracranial self-stimulation (ICSS), a preclinical procedure useful for studying rewarding drug effects in drug-naïve animals, has similarly shown that rewarding effects of mu opioid receptor (MOR) agonists increase rapidly in rats during initial regimens of opioid administration. The goal of the present study was to evaluate the role of MOR agonist efficacy as a determinant in eliciting this trajectory of increased rewarding effects during initial opioid exposure in opioid-naïve rats. Separate groups of adult, male Sprague-Dawley rats responded for electrical brain stimulation using a frequency-rate ICSS procedure and received repeated daily treatment with vehicle or one of five MOR agonists that ranged from low to high efficacy (NAQ, nalbuphine, buprenorphine, fentanyl, methadone). Two additional groups were used to evaluate effects of repeated treatment with non-opioids (the cannabinoid CP55940 or the monoamine releaser amphetamine). Morphine was tested after each repeated treatment. In opioid-naïve rats tested before repeated dosing, MOR agonists produced primarily dose- and efficacy-dependent decreases in ICSS. Following repeated treatment, all MOR agonists except NAQ produced tolerance to opioid-induced rate-decreasing effects and enhanced expression of ICSS facilitation (indicative of opioid reward) by both the repeatedly administered drug and morphine. Repeated treatment with CP55940 and amphetamine produced different effects. Collectively, these results provide evidence to suggest that enhanced expression of opioid reward after initial regimens of opioid exposure has a low requirement for MOR agonist efficacy and is pharmacologically selective.
Keywords: intracranial self-stimulation, morphine, mu opioid receptor, efficacy, repeated dosing
1. Introduction
Morphine and other mu opioid receptor (MOR) agonists are a mainstay for the clinical treatment of pain; however, a variety of factors have contributed to a recent increase in illicit opioid use and overdose deaths in the United States (Volkow and Collins, 2017). This public health crisis has stimulated renewed efforts to investigate determinants of opioid abuse with the ultimate goal of developing improved strategies for prevention and treatment. One distinguishing characteristic of opioids as drugs of abuse is that they often produce a constellation of dysphoric subjective effects in opioid-naïve users (Zacny et al., 1997), but repeated exposure for as few as five days (such as might occur during post-operative pain management, for example) can increase the risk of long-term opioid use (Shah et al., 2017). This apparent increase in opioid abuse potential during initial regimens of opioid exposure has prompted revised prescription guidelines that seek to reduce the number of doses patients can receive in a single prescription and thereby limit the extent of opioid exposure during pain-management episodes (Dowell et al., 2016).
Intracranial self-stimulation (ICSS) is a preclinical behavioral procedure that can be used to investigate the expression and mechanisms of changes in opioid reward that occur during initial regimens of opioid exposure. In ICSS procedures, experimental subjects with chronically implanted microelectrodes targeting a brain-reward area are trained to press an operant-response lever to receive pulses of electrical brain stimulation. Many drugs of abuse increase (or “facilitate”) low rates of ICSS responding maintained by low frequencies or intensities of brain stimulation, and as a result, ICSS facilitation is often interpreted as an abuse-related drug effect (Negus and Miller, 2014). Morphine and other MOR agonists often fail to produce ICSS facilitation in opioid-naïve subjects, and instead produce primarily dose-dependent ICSS depression; however, repeated daily treatment over the course of several days can produce tolerance to opioid-induced ICSS depression and heightened expression of abuse-related ICSS facilitation (Altarifi et al., 2013; Legakis and Negus, 2018; Miller et al., 2015; Negus and Moerke, 2019; Reid, 1987). This trajectory of increasing opioid-induced ICSS facilitation in rats resembles the increase in opioid abuse potential that can occur during initial regimens of opioid exposure in humans.
MOR agonists differ in their efficacy to activate G-protein signaling pathways coupled to MORs (Emmerson et al., 1996; Selley et al., 1998; Traynor and Nahorski, 1995), and in vitro measures of agonist efficacy correlate with in vivo agonist effectiveness to produce some behavioral and physiological effects, such as thermal antinociception (Morgan and Picker, 1996; Negus and Mello, 1999; Walker et al., 1998). The abuse-related effects of MOR agonists in ICSS and other preclinical procedures (e.g. place conditioning and drug self-administration) also appear to be mediated by MOR-coupled G-protein signaling (Altarifi et al., 2017; Bohn et al., 2003; Siuda et al., 2017), but the role of MOR agonist efficacy as a determinant of increasing ICSS facilitation during repeated opioid treatment has not been systematically examined. Accordingly, the goal of this study was to compare effects on ICSS produced by repeated daily treatment with MOR agonists that ranged from low to high efficacy in stimulating MOR-coupled G-protein signaling (from lowest to highest efficacy: NAQ, nalbuphine, buprenorphine, fentanyl, methadone) (Emmerson et al., 1996; Li et al., 2009; Selley et al., 1998). All drugs were tested using an experimental design that has been used previously to show a transition from initial ICSS depression to subsequent ICSS facilitation after repeated treatment with the prototype MOR agonist morphine (Legakis and Negus, 2018; Miller et al., 2015), which has MOR agonist efficacy slightly lower than that of fentanyl (Emmerson et al., 1996; Selley et al., 1998). Morphine was also tested after repeated dosing with each test drug. We hypothesized that repeated treatment with higher efficacy MOR agonists would increase abuse-related ICSS facilitation by both the repeatedly administered drug and by morphine, whereas repeated treatment with lower efficacy MOR agonists would not. Effects of repeated treatment with cannabinoid receptor agonist CP55940 (Grim et al., 2015; Kwilasz and Negus, 2012) and the monoamine releaser amphetamine (Bauer et al., 2013) were also examined for comparison with opioid effects, and morphine was also tested after repeated treatment with each of these drugs. Studies with these non-opioids were included to assess the degree to which dynamic changes in opioid effects on ICSS might be shared by non-opioid drugs of abuse, and whether prior exposure to these non-opioids would also increase morphine-induced ICSS facilitation.
2. Methods
2.1. Subjects
Adult male Sprague-Dawley rats were obtained from Envigo (Indianapolis, IN), weighed 310–350 g at the time of surgery, and were individually housed and maintained on a 12-h light/dark cycle with lights on from 6:00 a.m. to 6:00 p.m. All rats had free access to food and water except during testing. Animal maintenance and research were in compliance with National Institutes of Health guidelines, and all animal-use protocols were approved by the Institutional Animal Care and Use Committee.
2.2. Surgery
Subjects were anesthetized with isoflurane gas (3–4% in oxygen; Webster Veterinary, Phoenix, AZ) until unresponsive to toe-pinch and placed in a stereotaxic apparatus (Kopf Instruments, Tujunga, CA). The cathode of a stainless-steel electrode (0.25mm diameter and insulated except at the flattened tip; MS303/1-AIU/SPC, Plastics One, Roanoke, VA) was implanted in the medial forebrain bundle at the level of the lateral hypothalamus (2.8mm posterior to bregma, 1.7mm lateral to the midsagittal suture, 8.8mm ventral to the skull). Electrodes were secured to the skull using screws (Plastics One, Inc., Roanoke, VA) and orthodontic resin (Butler Schein, Dublin, OH), and the anode of the electrode (0.125mm diameter, uninsulated) was wrapped around one of the screws to act as a ground. Animals were allowed at least seven recovery days prior to initiation of ICSS training.
2.3. Apparatus
Experiments were conducted in sound-attenuating chambers that contained modular acrylic test chambers (29.2 × 30.5 × 24.1) equipped with a response lever (4.5 cm wide, extended 2.0 cm through the center of one wall, 3 cm off the floor), stimulus lights (three lights colored red, yellow and green positioned 7.6 cm directly above the lever), a 2-W white house light, and an ICSS stimulator (Med Associates, St. Albans, VT). Electrodes were connected to the stimulator via bipolar cables and a commutator (Model SL2C, Plastics One, Roanoke, VA). A computer and software program (Med Associates, St. Albans, VT) controlled the stimulator, programming parameters, and data collection.
2.4. Training
Rats were trained under a fixed-ratio 1 (FR 1) schedule of brain stimulation using procedures similar to those described previously (Altarifi et al., 2017; Altarifi et al., 2013; Altarifi et al., 2015; Legakis and Negus, 2018; Miller et al., 2015). Each lever press resulted in the delivery of a 0.5-s train of square wave cathodal pulses (0.1-ms pulse duration), and stimulation was accompanied by illumination of the stimulus lights above the lever. Responses during the 0.5-s stimulation period did not result in additional stimulation. During the initial phase of training, sessions lasted 30 to 60 min, the frequency of stimulation was held constant at 2.2 log Hz, and the stimulation intensity was adjusted to the lowest value that would sustain responding for at least 30 stimulations per minute. Frequency manipulations were then introduced during sessions that consisted of sequential 10-min components. During each component, a descending series of 10 current frequencies (2.2–1.75 log Hz in 0.05 log increments) was presented, with a 60-s trial at each frequency. A frequency trial began with a 5-s time out followed by a 5-s “priming” phase, during which five non-contingent stimulations were delivered at a rate of one per second. This non-contingent stimulation was followed by a 50-s “response” phase, during which responding produced electrical stimulation under a FR 1 schedule. Training continued with 3 to 6 sequential components per day, and the current intensity was adjusted until rats reliably responded during the first three to four frequency trials of all components for at least three consecutive days. This intensity (range: 80–250 μA) was held constant for the remainder of the study. Before testing began, rats were habituated to saline injections until there was no significant effect of injection on the frequency-rate function as determined by two-way ANOVA (see 2.6. Data Analysis).
2.5. Testing
Once training was completed, ICSS testing began. Eight different groups (n=6 per group) were used to test the effects of repeated treatment with saline or one of seven different drugs (NAQ, nalbuphine, buprenorphine, fentanyl, methadone, CP55940, amphetamine). Each treatment was tested using a nine-day protocol, and all rats were drug naïve at the start of this protocol. On Day 1, a range of doses was tested for the designated treatment. The test session consisted of three sequential baseline components followed by four 50-min test periods, each of which consisted of a 30-min time out followed by a pair of test components. Saline was administered s.c. at the beginning of the first time out, and three increasing doses of the test drug were administered s.c. at the beginning of each subsequent time out. Test doses for each drug are shown in Table 1. When saline was the test treatment, then saline was administered at the beginning of each time out. On Days 2–7, test sessions consisted of three sequential baseline components followed by a single 50-min test period, and a selected dose of the treatment drug was administered at the beginning of the time out on each day. On Day 8, effects of multiple test-drug doses were re-determined using procedures identical to those on Day 1. On Day 9, a range of morphine doses (0.32, 1.0, 3.2 mg/kg, s.c.) was evaluated in all groups using procedures identical to those on Days 1 and 8, with the exception that morphine was administered instead of the designated treatment. The dose range for each test drug was based on previous studies and was intended to span a range from ineffective doses to doses that either significantly altered ICSS or that produced significant behavioral effects in rats under other conditions (Altarifi et al., 2012; Altarifi et al., 2013; Altarifi et al., 2015). Half-log dose increments were used for most drugs, but one-log increments were used for the low-efficacy mu agonists NAQ, nalbuphine, and buprenorphine to assure evaluation of an adequately large dose range. The dose of each drug for repeated administration on Days 2–7 was selected based on Day 1 test data, and the rationale for each dose is provided in Results.
Table 1.
Experimental design: timeline of experiment, doses of drugs administered repeatedly in each group of rats, and sequence of doses administered in each test component on days with multiple test components. Sal is 1.0 ml/kg saline.
| Treatment Drug Dose Effect (Before) | Daily Repeated Treatment Drug | Treatment Drug Dose Effect (After) | Morphine Dose Effect (After) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | Day 8 | Day 9 | |
| Treatment | Sequence Treatment Drug Doses (mg/kg) | Repeated Treatment Drug Dose (mg/kg) | Sequence Treatment Drug Doses (mg/kg) | Sequence Morphine Doses (mg/kg) | |||||
| Saline | Sal, Sal, Sal, Sal | Sal | Sal | Sal | Sal | Sal | Sal | Sal, Sal, Sal, Sal | Sal, 0.32, 1.0, 3.2 |
| NAQ | Sal, 0.1, 1.0, 10 | 10 | 10 | 10 | 10 | 10 | 10 | Sal, 0.1, 1.0, 10 | Sal, 0.32, 1.0, 3.2 |
| Nalbuphine | Sal, 0.1, 1.0, 10 | 10 | 10 | 10 | 10 | 10 | 10 | Sal, 0.1, 1.0, 10 | Sal, 0.32, 1.0, 3.2 |
| Buprenorphine | Sal, 0.001, 0.01, 0.1 | 0.032 | 0.032 | 0.032 | 0.032 | 0.032 | 0.032 | Sal, 0.001, 0.01, 0.1 | Sal, 0.32, 1.0, 3.2 |
| Fentanyl | Sal, 0.0032, 0.01, 0.032 | 0.032 | 0.032 | 0.032 | 0.032 | 0.032 | 0.032 | Sal, 0.0032, 0.01, 0.032 | Sal, 0.32, 1.0, 3.2 |
| Methadone | Sal, 0.32, 1.0, 3.2 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | Sal, 0.32, 1.0, 3.2 | Sal, 0.32, 1.0, 3.2 |
| CP55940 | Sal, 0.32, 1.0, 3.2 | 3.2 | 3.2 | 3.2 | 3.2 | 3.2 | 3.2 | Sal, 0.32, 1.0, 3.2 | Sal, 0.32, 1.0, 3.2 |
| Amphetamine | Sal, 0.032, 0.1, 0.32 | 0.32 | 0.32 | 0.32 | 0.32 | 0.32 | 0.32 | Sal, 0.032, 0.1, 0.32 | Sal, 0.32, 1.0, 3.2 |
2.6. Data analysis
The first baseline component of each session was considered an acclimation component, and data were discarded. The primary dependent variable for all remaining components was the reinforcement rate in stimulations per trial during each frequency trial. To normalize these raw data, reinforcement rates from each trial in each rat were converted to percent Maximum Control Rate (% MCR), which was defined as the mean of the maximal rates observed during the second and third “baseline” components for that rat on that day. Thus, % MCR = [(rate during a frequency trial) / (MCR)] × 100. Normalized ICSS rates at each frequency were averaged across test components within each rat and then across rats to yield a “frequency-rate” curve for each experimental manipulation. Two-way ANOVA was used to compare frequency-rate curves, with ICSS frequency as one variable and dose as the second variable. ANOVA results are reported below only for the main effect of dose and the frequency × dose interaction, because the main effect of frequency was always significant. A significant ANOVA was followed by the Holm Sidak post hoc test, and the criterion for significance was set at p<0.05.
To provide an additional summary measure of ICSS performance in these studies, the EF50 for each ICSS curve was defined as the “effective frequency” that maintained 50% MCR (Johnson et al., 2018). EF50 values and 95% confidence limits were interpolated by linear regression from the linear portion of each ICSS curve, and EF50 values were considered to be different if 95% confidence limits did not overlap. In some cases, all points were below 50% MCR; in these cases, interpolation of EF50 values was not possible, and EF50 is shown as ND (not determined).
2.7. Drugs
Morphine sulfate, methadone HCl, fentanyl HCl, buprenorphine HCl, CP55940 and amphetamine hemisulfate were provided by the National Institute on Drug Abuse Drug Supply Program (Bethesda, MA). Nalbuphine HCl was provided by Dr. Kenner Rice (Chemical Biology Branch, National Institute on Drug Abuse, Bethesda, MA) and NAQ (17-cyclopropylmethyl-3,14ß-dihydroxy-4,5α-epoxy-6α-[(3′-isoquinolyl)acetamido] morphinan) was provided by Dr. Yan Zhang (Department of Medicinal Chemistry, Virginia Commonwealth University) (Li et al., 2009). All drugs were prepared in sterile saline, with the exceptions of NAQ, which was prepared in sterile water and CP55940, which was prepared in a vehicle of ethanol, emulphor and saline in a ratio of 1:1:18, respectively. All drugs were injected subcutaneously in a volume of 1 ml/kg.
3. Results
3.1. ICSS baseline
Under baseline conditions, electrical brain stimulation maintained a frequency-dependent increase on ICSS rates of responding. For the 48 rats used in these studies, the mean ± S.E.M. baseline maximum control rate was 245.68 ± 10.67 stimulations per trial, and the mean ± S.E.M. number of total baseline stimulations was 57.51 ± 1.32 stimulations per component.
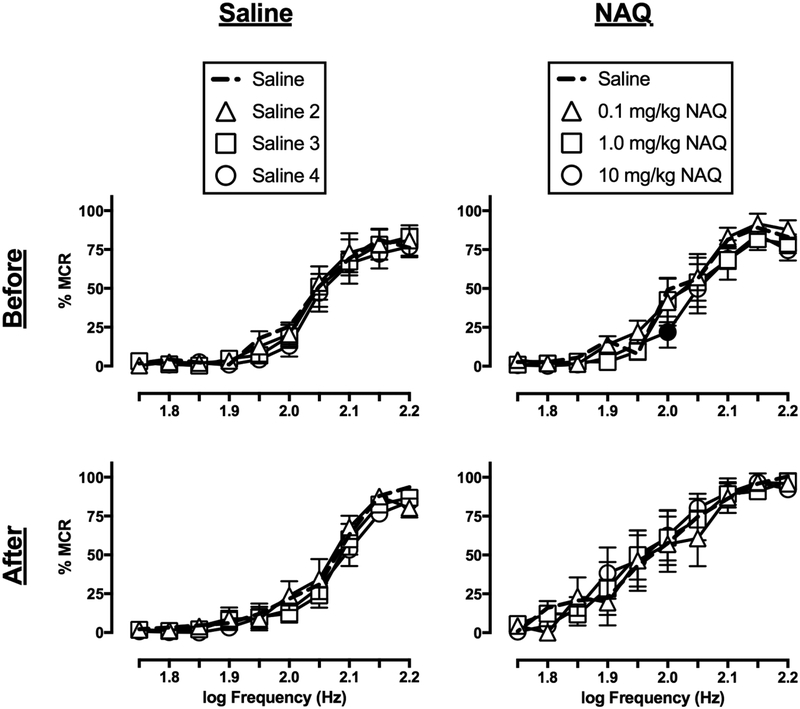
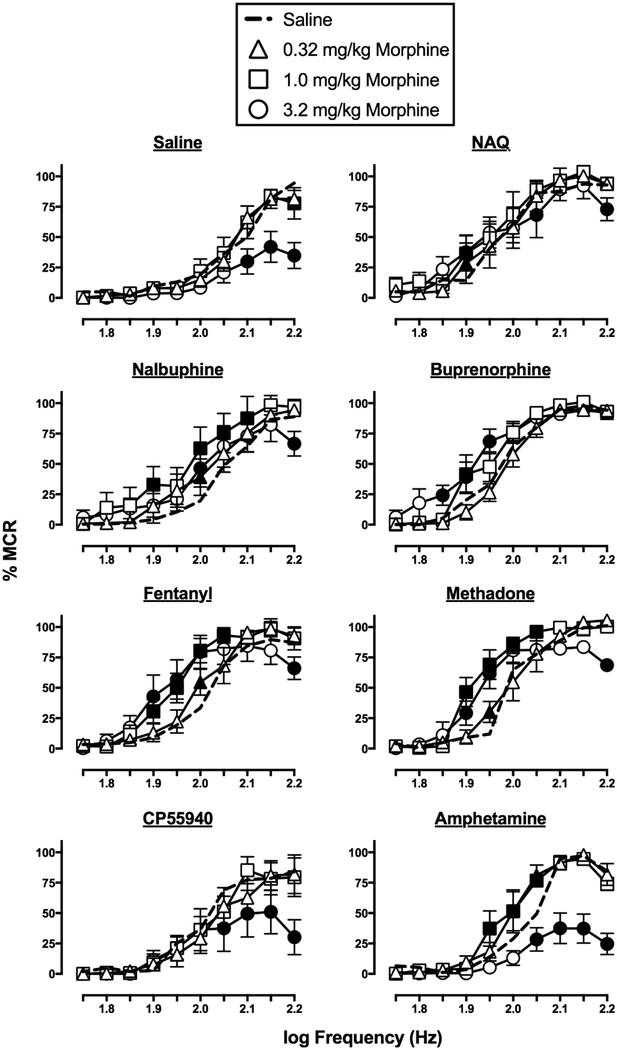
3.2. Effects of saline and NAQ
Figure 1 shows the effects of saline and NAQ on ICSS before and after repeated daily treatment, and EF50 values for this and all subsequent studies are reported in Table 2. Saline on Day 1, before repeated saline treatment, did not significantly alter ICSS frequency-rate curves (test period: F(3,15)=1.32, ns; frequency × cycle: F(27,135)=0.6438, ns), and there were no differences in EF50 values across test periods. On Day 8, after repeated daily saline treatment, there was a significant effect of test period but no interaction between frequency and test period (test period: F(3,15)=4.93, p=0.0141; frequency × cycle: F(27,135)=0.5807, ns), and post hoc analysis revealed no points on the frequency-rate curves that were different from saline in the first cycle. Additionally, there were no differences in EF50 values across test periods.
Figure 1.
Effects of saline (left panels) and NAQ (right panels) on ICSS frequency-rate curves in rats before (top panels) and after (bottom panels) seven-day treatment with saline or 10 mg/kg/day NAQ, respectively. Abscissae: electrical brain stimulation frequency in Hertz (Hz, log scale). Ordinates: ICSS reinforcement rate expressed as a percentage of the maximum control rate (% MCR). Filled symbols indicate statistical significance (p<0.05) as compared to saline. All points show mean ± S.E.M. for n=6 rats.
Table 2.
EF50 values with 95% confidence intervals before and after seven-day treatment for each group. * Indicates confidence limits do not overlap with saline (Sal) within a given treatment period (before or after repeated treatment). # Indicates confidence limits do not overlap across treatment periods within a given drug dose.
| Before Repeated Treatment | After Repeated Treatment | ||||||
|---|---|---|---|---|---|---|---|
| EF50 | Lower Limit | Upper Limit | EF50 | Lower Limit | Upper Limit | ||
| Saline | Sal | 2.053 | 2.032 | 2.074 | 2.068 | 2.033 | 2.113 |
| Sal 2 | 2.065 | 2.019 | 2.106 | 2.064 | 2.039 | 2.092 | |
| Sal 3 | 2.069 | 1.988 | 2.112 | 2.086 | 2.052 | 2.130 | |
| Sal 4 | 2.083 | 1.978 | 2.147 | 2.096 | 2.075 | 2.116 | |
| NAQ | Sal | 2.028 | 1.944 | 2.127 | 1.967 | 1.942 | 1.994 |
| 0.1 | 2.019 | 1.997 | 2.045 | 1.986 | 1.940 | 2.023 | |
| 1 | 2.046 | 2.013 | 2.078 | 1.968# | 1.955 | 1.980 | |
| 10 | 2.055 | 2.036 | 2.076 | 1.954# | 1.938 | 1.972 | |
| Nalbuphine | Sal | 2.028 | 2.008 | 2.045 | 2.024 | 2.005 | 2.043 |
| 0.1 | 2.017 | 2.010 | 2.023 | 2.009 | 1.973 | 2.052 | |
| 1 | 1.995 | 1.982 | 2.008 | 1.941*# | 1.895 | 1.974 | |
| 10 | 2.013 | 1.963 | 2.049 | 1.971* | 1.954 | 1.987 | |
| Buprenorphine | Sal | 1.964 | 1.951 | 1.977 | 2.015 | 1.977 | 2.044 |
| 0.001 | 1.978 | 1.958 | 1.999 | 2.016 | 1.990 | 2.048 | |
| 0.01 | 1.989 | 1.975 | 2.001 | 2.029 | 1.981 | 2.084 | |
| 0.1 | ND | ND | ND | 1.869*# | 1.835 | 1.897 | |
| Fentanyl | Sal | 1.994 | 1.982 | 2.006 | 2.002 | 1.985 | 2.020 |
| 0.0032 | 1.994 | 1.978 | 2.010 | 1.990 | 1.958 | 2.032 | |
| 0.01 | 1.956* | 1.938 | 1.975 | 1.920*# | 1.913 | 1.926 | |
| 0.032 | 2.056* | 2.023 | 2.109 | 1.885*# | 1.854 | 1.918 | |
| Methadone | Sal | 1.991 | 1.967 | 2.013 | 1.992 | 1.980 | 2.004 |
| 0.32 | 1.984 | 1.949 | 2.027 | 1.941* | 1.913 | 1.966 | |
| 1 | 1.996 | 1.969 | 2.026 | 1.911*# | 1.906 | 1.916 | |
| 3.2 | ND | ND | ND | ND | ND | ND | |
| CP55940 | Sal | 1.998 | 1.961 | 2.033 | 2.059 | 2.049 | 2.07 |
| 0.032 | 2.034 | 1.905 | 2.107 | 2.051 | −inf | +inf | |
| 0.1 | 2.118* | 2.074 | 2.182 | 2.048 | 2.019 | 2.077 | |
| 0.32 | ND | ND | ND | 2.069# | 2.026 | 2.105 | |
| Amphetamine | Sal | 2.003 | 1.991 | 2.014 | 2.026 | 1.976 | 2.077 |
| 0.032 | 2.009 | 1.988 | 2.026 | 2.01 | −inf | +inf | |
| 0.1 | 1.946 | 1.815 | 2.023 | 1.957 | 1.935 | 1.986 | |
| 0.32 | 1.839* | 1.791 | 1.917 | 1.876* | 1.834 | 1.948 | |
NAQ (0.1–10 mg/kg) on Day 1, before repeated NAQ treatment, significantly altered ICSS frequency-rate curves (dose: F(3,15)=5.866, p=0.0074; frequency × dose: F(27,135)=1.246, ns). However, depression was only observed at a single frequency (2.0 log Hz) at the high NAQ dose (10 mg/kg), and NAQ did not alter EF50 values. A dose of 10 mg/kg/day NAQ was selected for repeated treatment given evidence that this dose can facilitate ICSS after morphine exposure (Altarifi et al., 2015). However, on Day 8, after repeated treatment, NAQ administration did not alter ICSS frequency-rate curves (dose: F(3,15)=0.5425, ns; frequency × dose: F(27,135)=1.009, ns) or EF50 values. EF50 values were significantly lower for 1 and 10 mg/kg NAQ after repeated treatment relative to before treatment, suggesting a weak trend for NAQ effects to change after repeated treatment.
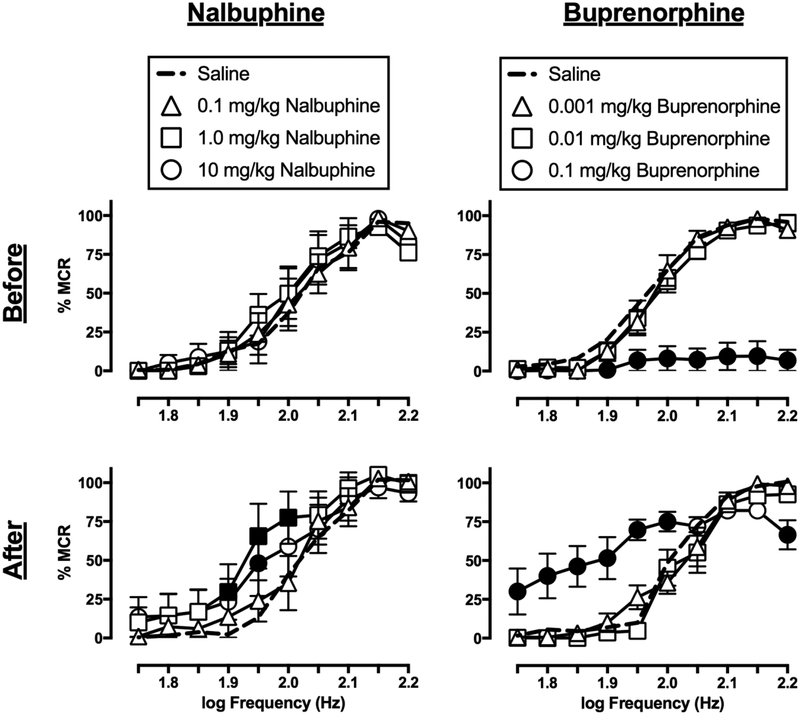
3.3. Effects of nalbuphine and buprenorphine
Figure 2 shows the effects of nalbuphine and buprenorphine on ICSS before and after repeated daily treatment. Nalbuphine (0.1–10 mg/kg) on Day 1, before repeated nalbuphine, did not significantly alter ICSS frequency-rate curves (dose: F(3,15)=0.5242, ns; frequency × dose: F(27,135)=1.539, ns) or EF50 values. A dose of 10 mg/kg nalbuphine was selected for repeated treatment given evidence that this dose can facilitate ICSS in rats with prior morphine exposure (Altarifi et al., 2013). On Day 8, after repeated nalbuphine, there was a significant effect of nalbuphine dose (dose: F(3,15)=4.625, p=0.0175; frequency × dose: F(27,135)=1.493, ns) resulting in facilitation across a range of frequencies (1.9–2.0 log Hz) for the intermediate nalbuphine dose (1.0 mg/kg) and at one frequency (1.95 log Hz) for the high dose (10 mg/kg). The EF50 values for these two doses were significantly lower than the saline EF50 value, indicating leftward shifts of the frequency-rate curve. Additionally, the EF50 value for 1.0 mg/kg nalbuphine was lower after repeated treatment than before treatment.
Figure 2.
Effects of nalbuphine (left panels) and buprenorphine (right panels) on ICSS frequency-rate curves in rats before (top panels) and after (bottom panels) seven-day treatment with 10 mg/kg/day nalbuphine or 0.032 mg/kg/day buprenorphine, respectively. Abscissae: electrical brain stimulation frequency in Hertz (Hz, log scale). Ordinates: ICSS reinforcement rate expressed as a percentage of the maximum control rate (% MCR). Filled symbols indicate statistical significance (p<0.05) as compared to saline. All points show mean ± S.E.M. for n=6 rats.
Buprenorphine on Day 1, before repeated buprenorphine, had no effect on ICSS at the low (0.001 mg/kg) and intermediate (0.01 mg/kg) doses; however, the high (0.1 mg/kg) dose resulted in nearly complete suppression of behavior (dose: F(3,15)=72.44, p<0.0001; frequency × dose: F(27,135)=18.93, p<0.0001). There were no differences in EF50 values for saline and the lower buprenorphine doses, and an EF50 value could not be determined for the high dose. A dose of 0.032 mg/kg/day buprenorphine was selected for repeated treatment because this dose was the half-log increment between a behaviorally inactive dose (0.01 mg/kg) and a dose that essentially eliminated responding (0.1 mg/kg). On Day 8, after repeated buprenorphine, the low (0.001 mg/kg) and intermediate (0.01 mg/kg) buprenorphine doses remained ineffective; however, biphasic effects of the high (0.1 mg/kg) buprenorphine dose emerged (dose: F(3,15)=9.326, p=0.001; frequency × dose: F(27,135)=6.533, p<0.0001), with facilitation across a range of lower frequencies (1.75–2.0 log Hz), and depression at the highest frequency (2.2 log Hz). Additionally, the EF50 value for 0.1 mg/kg buprenorphine was significantly less than that for saline, indicating a leftward shift in the frequency-rate curve. The EF50 value for 0.1 mg/kg buprenorphine was also lower after repeated treatment than before treatment.
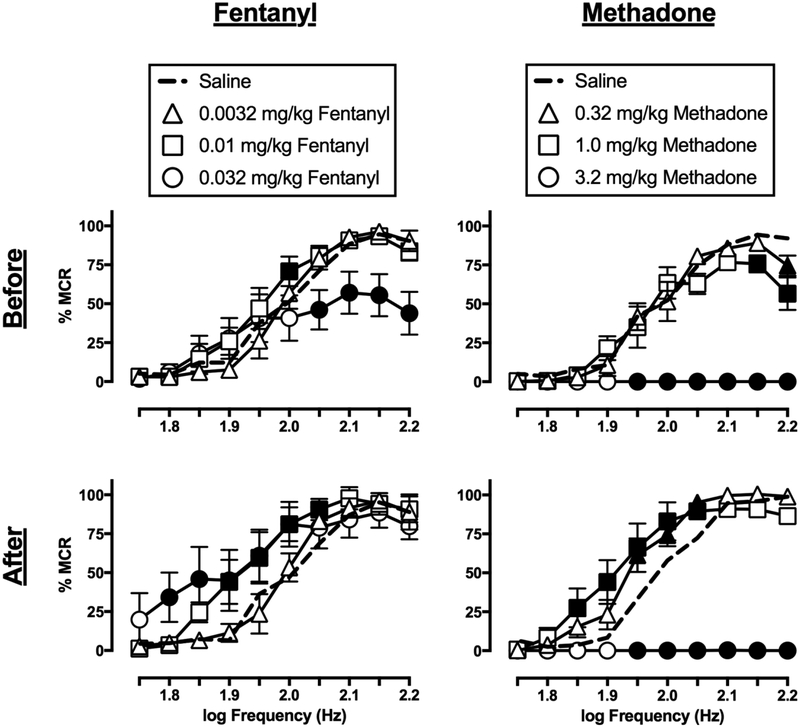
3.4. Effects of fentanyl and methadone
Figure 3 shows the effects of fentanyl and methadone on ICSS before and after repeated daily treatment. Fentanyl (0.0032–0.032 mg/kg) on Day 1, before repeated fentanyl, facilitated ICSS at a single frequency (2.0 log Hz) with the intermediate (0.01 mg/kg) dose, but produced rate depression across a range of frequencies (2.05–2.2 log Hz) at the high (0.032 mg/kg) dose (dose: F(3,15)=2.846, ns; frequency × dose: F(27,135)=5.871, p<0.0001). The EF50 values for 0.01 and 0.032 mg/kg fentanyl were significantly less than that for saline, indicating a leftward shift of the frequency-rate function. A dose of 0.032 mg/kg/day fentanyl was selected for repeated treatment because it depressed, but did not eliminate, responding in ICSS. On Day 8, after repeated fentanyl, only facilitation across a range of frequencies for both the intermediate (0.01 mg/kg; 1.9–2.05 log Hz) and high (0.032 mg/kg; 1.8–2.05 log Hz) doses was observed (dose: F(3,15)=2.054, ns; frequency × dose: F(27,135)=3.077, p<0.0001). Both 0.01 and 0.032 mg/kg fentanyl also significantly decreased EF50 values, indicating a dose-dependent leftward shift in the frequency-rate function. The EF50 values for these two fentanyl doses were also lower after repeated treatment than before treatment.
Figure 3.
Effects of fentanyl (left panels) and methadone (right panels) on ICSS frequency-rate curves in rats before (top panels) and after (bottom panels) seven-day treatment with 0.032 mg/kg/day fentanyl or 1.0 mg/kg/day methadone, respectively. Abscissae: electrical brain stimulation frequency in Hertz (Hz, log scale). Ordinates: ICSS reinforcement rate expressed as a percentage of the maximum control rate (% MCR). Filled symbols indicate statistical significance (p<0.05) as compared to saline. All points show mean ± S.E.M. for n=6 rats.
Methadone (0.32–3.2 mg/kg) on Day 1, before repeated methadone, dose-dependently decreased ICSS (dose: F(3,15)=81.69, p<0.0001; frequency × dose: F(27,135)=14.46, p<0.0001). The low (0.32 mg/kg) dose decreased responding at the highest frequency (2.2 log Hz), while the intermediate (1.0 mg/kg) dose depressed responding at the two highest frequencies (2.15–2.2 log Hz), and the high (3.2 mg/kg) methadone dose eliminated responding. EF50 values for the lower methadone doses were not significantly different from saline, and an EF50 value after the high dose could not be determined. A dose of 1.0 mg/kg/day methadone was selected for repeated treatment because it was the highest dose that did not eliminate responding. On Day 8, after repeated methadone, significant facilitation was observed across a range of frequencies for both the low (0.32 mg/kg; 1.95–2.05 log Hz) and intermediate (1.0 mg/kg; 1.85–2.05 log Hz) methadone doses (dose: F(3,15)=72.23, p<0.0001; frequency × dose: F(27,135)=19.16, p<0.0001), and EF50 values indicated dose-dependent leftward shifts in the frequency-rate curve. The EF50 value for 1.0 mg/kg methadone was also lower after repeated treatment than before treatment. However, the high (3.2 mg/kg) methadone dose still eliminated responding, and an EF50 value could not be determined.
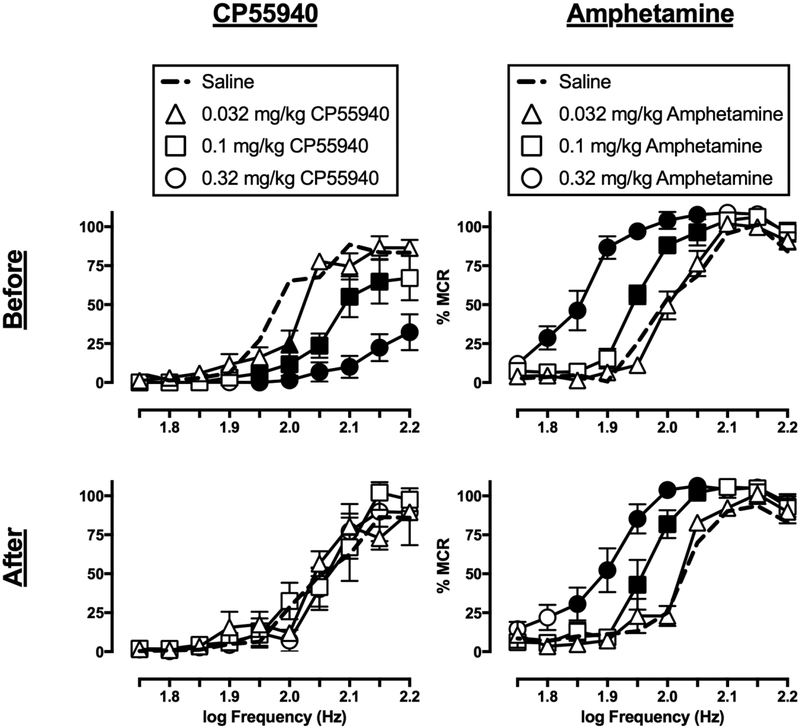
3.5. Effects of CP55940 and amphetamine
Figure 4 shows the effects of CP55940 and amphetamine on ICSS before and after repeated daily treatment. CP55940 (0.032–0.32 mg/kg) on Day 1, before repeated CP55940, dose-dependently decreased ICSS (dose: F(3,15)=44.98, p<0.0001; frequency × dose: F(27,135)=7.998, p<0.0001). The EF50 value for 0.1 mg/kg CP55940 was significantly greater than that for saline, indicating a rightward shift of the frequency-rate function, and an EF50 value could not be determined for 0.32 mg/kg CP55940. A dose of 0.32 mg/kg/day CP55940 was selected for repeated treatment because it reduced but did not eliminate responding. On Day 8, after repeated treatment, CP55940 failed to alter ICSS frequency-rate curves (dose: F(3,15)=0.2567, ns; frequency × dose: F(27,135)=0.8621, ns) or EF50 values.
Figure 4.
Effects of CP 55940 (left panels) and amphetamine (right panels) on ICSS frequency-rate curves in rats before (top panels) and after (bottom panels) seven-day treatment with 0.32 mg/kg/day CP 55940 or 0.32 mg/kg/day amphetamine, respectively. Abscissae: electrical brain stimulation frequency in Hertz (Hz, log scale). Ordinates: ICSS reinforcement rate expressed as a percentage of the maximum control rate (% MCR). Filled symbols indicate statistical significance (p<0.05) as compared to saline. All points show mean ± S.E.M. for n=6 rats.
Amphetamine (0.032–0.32 mg/kg) on Day 1, before repeated amphetamine, dose-dependently facilitated ICSS (dose: F(3,15)= 88.86, p<0.0001; frequency × dose: F(27,135)= 8.184, p<0.0001). Only the EF50 value determined for the high amphetamine dose was significantly less than the EF50 value for saline, indicating a left shift in the frequency-rate function. A dose of 0.32 mg/kg/day amphetamine was selected for repeated treatment because it significantly increased ICSS by analysis of both frequency-rate curves and EF50 values. On Day 8, after repeated treatment, amphetamine again produced dose-dependent ICSS facilitation (dose: F(3,15)= 48.75, p<0.0001; frequency × dose: F(27,135)= 6.183, p<0.0001), and again only the EF50 value for the high amphetamine dose was significantly less than the EF50 value for saline. There was no difference in EF50 values determined before vs. after repeated amphetamine for any amphetamine dose.
3.6. Effects of morphine after repeated treatment with other drugs
Figure 5 shows the effects of morphine (0.32–3.2 mg/kg) determined on Day 9, after repeated treatment with saline or each of the other drugs in each group. Following repeated saline treatment, morphine dose-dependently decreased ICSS (dose: F(3,15)=11.17, p=0.0004; frequency × dose: F(27,135)=3.444, p<0.0001). Following repeated NAQ, the low (0.32 mg/kg) and intermediate (1.0 mg/kg) morphine doses facilitated ICSS at a single frequency (1.9 log Hz), and weak but significant depression was evident at higher frequencies (2.05, 2.2 log Hz) for the high (3.2 mg/kg) morphine dose (dose: F(3,15)=1.887, ns; frequency × dose: F(27,135)=1.761, p=0.019).
Figure 5.
Effects of morphine on ICSS frequency-rate curves in rats following the second dose-effect determination for a given drug after seven-day treatments as indicated in Table 1. Abscissae: electrical brain stimulation frequency in Hertz (Hz, log scale). Ordinates: ICSS reinforcement rate expressed as a percentage of the maximum control rate (% MCR). Filled symbols indicate statistical significance (p<0.05) as compared to saline. All points show mean ± S.E.M. for n=6 rats.
Following repeated nalbuphine, the low (0.32 mg/kg) and intermediate (1.0 mg/kg) morphine doses facilitated ICSS at a single frequency (2.0 log Hz), whereas the high (3.2 mg/kg) morphine dose facilitated ICSS across a range of frequencies (1.9, 2.0–2.1 log Hz) and depressed ICSS at the highest frequency (2.2 log Hz) (dose: F(3,15)=4.091, p=0.0262; frequency × dose: F(27,135)=1.275, ns). Following repeated buprenorphine, morphine facilitated ICSS at a single frequency (1.9 log Hz) at the intermediate (1.0 mg/kg) dose and across a range of frequencies (1.85–1.95 log Hz) at the high (3.2 mg/kg) dose (dose: F(3,15)=9.265, p=0.001; frequency × dose: F(27,135)=2.067, p=0.0036).
Following repeated fentanyl, the low (0.032 mg/kg) morphine dose facilitated ICSS at a single frequency (2.0 log Hz), and the higher morphine doses facilitated ICSS across a broader range of frequencies (1.0 mg/kg, 1.9–2.05 log Hz; 3.2 mg/kg, 1.9–2.0 log Hz); 3.2 mg/kg morphine also depressed ICSS at the highest frequency (2.2 log Hz) (dose: F(3,15)=1.834, ns; frequency × dose: F(27,135)=3.623, p<0.0001). Similarly, following repeated methadone, the low (0.032 mg/kg) morphine dose facilitated ICSS at a single frequency (1.95 log Hz), and the higher morphine doses facilitated ICSS across a broader range of frequencies (1.0 mg/kg, 1.9–2.05 log Hz; 3.2 mg/kg, 1.9–1.95 log Hz); 3.2 mg/kg morphine also depressed ICSS at the highest frequency (2.2 log Hz) (dose: F(3,15)=7.316, p=0.003; frequency × dose: F(27,135)=5.316, p<0.0001).
Following repeated CP55940, no morphine dose produced facilitation at any brain-stimulation frequency, and the high (3.2 mg/kg) dose of morphine significantly depressed ICSS across a range of frequencies (2.05–2.2 log Hz) (dose: F(3,15)=3.962, p=0.0290; frequency × dose: F(27,135)=2.482, p=0.0003). Following repeated amphetamine, morphine facilitated ICSS at both the low and intermediate (0.32 mg/kg, 2.0–2.05 log Hz; 1.0 mg/kg, 1.95–2.05 log Hz) doses, however, the high (3.2 mg/kg) morphine dose significantly only depressed ICSS across a range of frequencies (2.05–2.2 log Hz) (dose: F(3,15)= 33.91, p<0.0001; frequency × dose: F(27,135)= 5.172, p<0.0001).
4. Discussion
The abuse potential of opioids can change rapidly during periods of initial exposure, and determinants of this phenomenon can be studied using ICSS procedures. The goal of the present study was to evaluate the role of MOR agonist efficacy as a determinant of increasing reward during a regimen of repeated opioid exposure in rats that were initially opioid naïve. There were three main findings. First, in opioid-naïve subjects tested before repeated dosing, MOR agonists rarely produced ICSS facilitation suggestive of abuse potential, and instead produced primarily efficacy-dependent decreases in ICSS. Second, repeated opioid dosing produced tolerance to opioid-induced rate-decreasing effects and an efficacy-dependent increase in abuse-related ICSS facilitation. Notably, repeated treatment with even the low-efficacy MOR agonists nalbuphine and buprenorphine was sufficient to reveal ICSS facilitation both by the repeatedly administered opioid and by morphine. Third, repeated treatment with the non-opioids CP55940 and amphetamine produced constellations of effects distinct from those of opioids. Taken together, these results provide evidence to suggest that enhanced expression of opioid reward after initial opioid exposure has a low requirement for MOR agonist efficacy and is pharmacologically selective.
4.1. Effects of opioids in drug-naïve rats
Results of the present study agree with previous findings that MOR agonists produce little or no ICSS facilitation in opioid-naïve male Sprague-Dawley rats, and instead produce primarily an efficacy-dependent ICSS depression (Altarifi et al., 2017; Altarifi et al., 2012; Altarifi et al., 2013; Altarifi et al., 2015; Miller et al., 2015; Negus and Moerke, 2019; Reid, 1987; Wiebelhaus et al., 2016). Thus, morphine administration in rats treated with repeated saline produced only a dose-dependent depression of ICSS. Similarly, initial treatment with the other intermediate-to-high efficacy MOR agonists buprenorphine, fentanyl, and methadone also produced primarily only dose-dependent ICSS depression. The only exception was that 0.01 mg/kg fentanyl facilitated ICSS at a single brain-stimulation frequency and significantly decreased the EF50 value, but even here, the degree of ICSS facilitation was small. The production of ICSS depression with little or no ICSS facilitation by these compounds in opioid-naïve subjects is consistent with the largely dysphoric subjective effects of MOR agonists in opioid-inexperienced humans (Zacny et al., 1997) and contrasts with the robust ICSS facilitation in drug-naïve subjects produced by psychomotor stimulant drugs of abuse like amphetamine or cocaine (Bauer et al., 2013; Bonano et al., 2014; Johnson et al., 2018) (also present study with amphetamine).
The lower efficacy MOR agonists NAQ and nalbuphine produced little or no ICSS facilitation or depression in opioid-naïve subjects. This agrees with previous reports that NAQ and nalbuphine produce little change in ICSS in opioid-naive rats (Altarifi et al., 2012; Altarifi et al., 2015). Moreover, NAQ and nalbuphine can antagonize the ICSS rate-decreasing effects of higher efficacy MOR agonists (Altarifi et al., 2012; Altarifi et al., 2015), indicating that both drugs were tested at doses sufficient to occupy MORs. The weak ICSS-depressing effects of NAQ and nalbuphine suggest that an intermediate degree of MOR efficacy is required to produce ICSS depression, just as intermediate degrees of MOR efficacy are required to produce some other effects, such as thermal antinociception against high thermal stimulus intensities (Li et al., 2009; Morgan and Picker, 1996; Negus and Mello, 1999; Walker et al., 1998).
4.2. Effects of repeated opioid treatment
We and others have shown previously that repeated daily treatment with morphine produces tolerance to its ICSS rate-decreasing effects and increased expression of abuse-related ICSS facilitation (Legakis and Negus, 2018; Miller et al., 2015; Reid, 1987). Similar results have been reported for the other intermediate-efficacy MOR agonists oxycodone (Wiebelhaus et al., 2016) and oliceridine (Altarifi et al., 2017). The present study extended these previous results by evaluating effects of repeated treatment with agonists varying from low to high efficacy at the MOR. Although initial treatment with nalbuphine, buprenorphine, fentanyl, and methadone produced little or no ICSS facilitation, all of these MOR agonists produced ICSS facilitation after repeated treatment, and repeated treatment with all of these MOR agonists also enabled ICSS facilitation by morphine. Two other nuances of these data warrant mention. First, for buprenorphine, fentanyl, and methadone, doses that produced ICSS depression in opioid-naïve rats produced ICSS facilitation after repeated treatment. This finding indicates that, as with morphine, repeated treatment produced both tolerance to ICSS depression as well as emergence of ICSS facilitation. Second, nalbuphine produced neither ICSS facilitation nor depression in opioid-naïve rats, but repeated treatment nonetheless enabled ICSS facilitation by nalbuphine itself and by morphine. This suggests that opioid-induced ICSS facilitation after repeated opioid treatment (a) has a lower efficacy requirement than ICSS depression in naïve subjects, (b) does not require initial ICSS depression, and (c) may involve an agonist-induced sensitization to opioid reward in addition to an unmasking of reward initially obscured by rate-decreasing effects (Negus and Moerke, 2019).
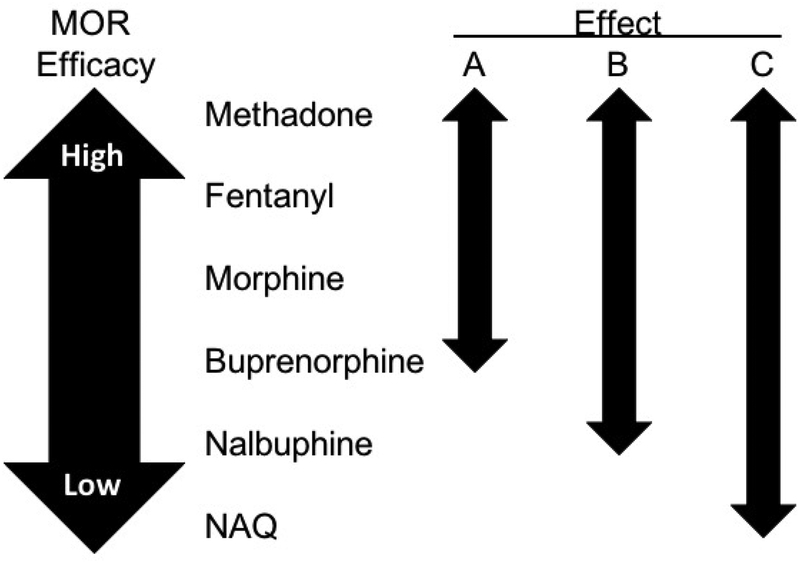
In comparison to effects of repeated treatment with the other MOR agonists, repeated treatment with the low-efficacy MOR agonist NAQ was less effective to reveal ICSS facilitation either by NAQ itself or by morphine. Intriguingly, though, we found previously that NAQ did produce dose-dependent ICSS facilitation in rats treated with repeated morphine (Altarifi et al., 2015). These results suggest that NAQ lacks sufficient efficacy itself to trigger adaptions leading to opioid-induced ICSS facilitation; however, NAQ does have sufficient efficacy to produce ICSS facilitation in subjects already exposed to higher efficacy MOR agonists. Figure 6 summarizes results of the present study, which suggest the following three conclusions. First, MOR agonists produce little or no ICSS facilitation in opioid-naïve subjects, but do produce efficacy-dependent ICSS depression. Second, repeated opioid treatment can produce tolerance to ICSS depression and promote ICSS facilitation both by the repeatedly administered drug and by other MOR agonists. Lastly, different opioid effects on ICSS require different levels of MOR efficacy. ICSS depression in opioid-naïve rats requires intermediate to high efficacy, sensitization to opioid-induced ICSS facilitation after repeated opioid treatment requires lower efficacy, and ICSS facilitation in sensitized rats requires the lowest efficacy.
Figure 6.
Relationship between MOR agonist efficacy and effects on ICSS. MOR agonists tested in this study range in efficacy from methadone at the high end to NAQ at the low end of the efficacy continuum. A-Efficacy range to produce ICSS depression in opioid-naïve rats. B-Efficacy range to trigger adaptations that promote opioid-induced ICSS facilitation after repeated treatment. C-Efficacy range to facilitate ICSS in rats already sensitized to opioid-induced ICSS facilitation.
4.3. Effects of CP55940 and amphetamine
Like morphine and consistent with the literature, CP55940 in the current study depressed ICSS in drug-naïve subjects, and repeated treatment produced tolerance to these rate-decreasing effects (Grim et al., 2015; Kwilasz and Negus, 2012). Unlike morphine and also consistent with previous literature examining the effects of repeated cannabinoid treatments, there was no evidence of facilitation with CP55940 despite the development of tolerance to rate-decreasing effects following repeated treatment (Grim et al., 2015; Kwilasz and Negus, 2012; Mavrikaki et al., 2010). Furthermore, when morphine was administered to rats following repeated CP55940 treatment, it produced only rate-decreasing effects similar to those observed in rats that received repeated saline treatment. Thus, tolerance to the rate-decreasing effects of CPP55940 was not sufficient to produce cross tolerance to the rate-decreasing effects of morphine or to enhance expression of ICSS facilitation by morphine. As a result, these findings do not provide evidence to suggest that prior exposure to a cannabinoid receptor agonist is sufficient to increase abuse-related effects of an opioid. Although prior self-exposure to cannabis has been correlated to increased risk of opioid abuse in some clinical studies (Butelman et al., 2018), the present results suggest that this heightened risk may reflect factors other than a cannabinoid-induced increase in mechanisms of opioid reward (e.g. a common liability to opioid and cannabinoid abuse (Vanyukov et al., 2012)).
Amphetamine was the only drug examined in the current study that produced robust facilitation of ICSS in drug-naïve rats, and this facilitation remained after repeated amphetamine administration. These results are consistent with previous studies demonstrating that amphetamine and other dopamine releasers produce robust ICSS facilitation following both initial acute administration and after repeated treatments (Bauer et al., 2013; Bauer et al., 2014; Johnson et al., 2018). As with CP55940, repeated amphetamine treatment failed to produce opioid-like tolerance to the rate-decreasing effects of 3.2 mg/kg morphine, and this agrees with previous findings that repeated amphetamine failed to produce tolerance to morphine-induced rate-decreasing effects in rats responding for food presentation (Brocco and McMillan, 1983). However, unlike CP55940, repeated amphetamine did produce an opioid-like increase in ICSS facilitation by lower morphine doses of 0.32 and 1.0 mg/kg. This agrees with an earlier study reporting that amphetamine pre-exposure increased rewarding effects of morphine in a place-conditioning procedure (Lett, 1989). Taken together, these results raise the possibility that prior exposure to amphetamine in humans may be sufficient to increase subsequent risk of opioid abuse.
Additionally, these results agree with other evidence for a dissociation between the mechanisms of tolerance to opioid-induced rate-decreasing effects vs. sensitization of opioid-induced rate-increasing effects in ICSS. For example, the results of previous studies have indicated that the rate-increasing and rate-decreasing effects of morphine are mediated by different populations of MORs in the brain (Broekkamp et al., 1976) and that, following chronic morphine treatment, MOR desensitization occurs selectively in brain regions associated with rate-decreasing and not rate-increasing effects of morphine (Sim et al., 1996). Furthermore, repeated administration of morphine also appears to produce sensitization of MORs in brain regions associated with rate-increasing effects via promotion of signaling cascades leading to increased expression of ΔFosB (Zachariou et al., 2006). Amphetamine may sensitize the MORs that mediate opioid-induced ICSS facilitation without altering signaling by MORs that mediate ICSS depression.
MOR agonists produce little/no abuse-related ICSS facilitation in opioid-naïve rats Repeated daily MOR agonist treatment increases MOR agonist-induced ICSS facilitation Emergence of ICSS facilitation depends on efficacy of repeatedly administered opioid The efficacy requirement for the switch to facilitation is relatively low
Acknowledgements
This work was supported by the National Institutes of Health [grant numbers R01NS07015, T32DA007027]. The authors thank Dr. Kenner Rice (Chemical Biology Research Branch, National Institute on Drug Abuse and National Institute on Alcohol Abuse and Alcoholism) for providing nalbuphine and Dr. Yan Zhang (Department of Medicinal Chemistry, Virginia Commonwealth University) for providing NAQ.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altarifi AA, David B, Muchhala KH, Blough BE, Akbarali H, Negus SS, 2017. Effects of acute and repeated treatment with the biased mu opioid receptor agonist TRV130 (oliceridine) on measures of antinociception, gastrointestinal function, and abuse liability in rodents. J Psychopharmacol 31, 730–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altarifi AA, Miller LL, Negus SS, 2012. Role of micro-opioid receptor reserve and micro-agonist efficacy as determinants of the effects of micro-agonists on intracranial self-stimulation in rats. Behav Pharmacol 23, 678–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altarifi AA, Rice KC, Negus SS, 2013. Abuse-related effects of micro-opioid analgesics in an assay of intracranial self-stimulation in rats: modulation by chronic morphine exposure. Behav Pharmacol 24, 459–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altarifi AA, Yuan Y, Zhang Y, Selley DE, Negus SS, 2015. Effects of the novel, selective and low-efficacy mu opioid receptor ligand NAQ on intracranial self-stimulation in rats. Psychopharmacology (Berl) 232, 815–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer CT, Banks ML, Blough BE, Negus SS, 2013. Use of intracranial self-stimulation to evaluate abuse-related and abuse-limiting effects of monoamine releasers in rats. Br J Pharmacol 168, 850–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer CT, Banks ML, Negus SS, 2014. The effect of chronic amphetamine treatment on cocaine-induced facilitation of intracranial self-stimulation in rats. Psychopharmacology (Berl) 231, 2461–2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn LM, Gainetdinov RR, Sotnikova TD, Medvedev IO, Lefkowitz RJ, Dykstra LA, Caron MG, 2003. Enhanced rewarding properties of morphine, but not cocaine, in beta(arrestin)-2 knock-out mice. J Neurosci 23, 10265–10273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonano JS, Glennon RA, De Felice LJ, Banks ML, Negus SS, 2014. Abuse-related and abuse-limiting effects of methcathinone and the synthetic “bath salts” cathinone analogs methylenedioxypyrovalerone (MDPV), methylone and mephedrone on intracranial self-stimulation in rats. Psychopharmacology (Berl) 231, 199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocco MJ, McMillan DE, 1983. Tolerance to d-amphetamine and lack of cross-tolerance to other drugs in rats under a multiple schedule of food presentation. J Pharmacol Exp Ther 224, 34–39. [PubMed] [Google Scholar]
- Broekkamp CL, Van den Bogaard JH, Heijnen HJ, Rops RH, Cools AR, Van Rossum JM, 1976. Separation of inhibiting and stimulating effects of morphine on self-stimulation behaviour by intracerebral microinjections. Eur J Pharmacol 36, 443–446. [DOI] [PubMed] [Google Scholar]
- Butelman ER, Maremmani AGI, Bacciardi S, Chen CY, Correa da Rosa J, Kreek MJ, 2018. Non-medical Cannabis Self-Exposure as a Dimensional Predictor of Opioid Dependence Diagnosis: A Propensity Score Matched Analysis. Front Psychiatry 9, 283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowell D, Haegerich TM, Chou R, 2016. CDC Guideline for Prescribing Opioids for Chronic Pain - United States, 2016. MMWR Recomm Rep 65, 1–49. [DOI] [PubMed] [Google Scholar]
- Emmerson PJ, Clark MJ, Mansour A, Akil H, Woods JH, Medzihradsky F, 1996. Characterization of opioid agonist efficacy in a C6 glioma cell line expressing the mu opioid receptor. J Pharmacol Exp Ther 278, 1121–1127. [PubMed] [Google Scholar]
- Grim TW, Wiebelhaus JM, Morales AJ, Negus SS, Lichtman AH, 2015. Effects of acute and repeated dosing of the synthetic cannabinoid CP55,940 on intracranial self-stimulation in mice. Drug Alcohol Depend 150, 31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AR, Banks ML, Selley DE, Negus SS, 2018. Amphetamine maintenance differentially modulates effects of cocaine, methylenedioxypyrovalerone (MDPV), and methamphetamine on intracranial self-stimulation and nucleus accumbens dopamine in rats. Neuropsychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwilasz AJ, Negus SS, 2012. Dissociable effects of the cannabinoid receptor agonists Delta9-tetrahydrocannabinol and CP55940 on pain-stimulated versus pain-depressed behavior in rats. J Pharmacol Exp Ther 343, 389–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legakis LP, Negus SS, 2018. Repeated Morphine Produces Sensitization to Reward and Tolerance to Antiallodynia in Male and Female Rats with Chemotherapy-Induced Neuropathy. J Pharmacol Exp Ther 365, 9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lett BT, 1989. Repeated exposures intensify rather than diminish the rewarding effects of amphetamine, morphine, and cocaine. Psychopharmacology (Berl) 98, 357–362. [DOI] [PubMed] [Google Scholar]
- Li G, Aschenbach LC, Chen J, Cassidy MP, Stevens DL, Gabra BH, Selley DE, Dewey WL, Westkaemper RB, Zhang Y, 2009. Design, synthesis, and biological evaluation of 6alpha- and 6beta-N-heterocyclic substituted naltrexamine derivatives as mu opioid receptor selective antagonists. J Med Chem 52, 1416–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavrikaki M, Markaki E, Nomikos GG, Panagis G, 2010. Chronic WIN55,212–2 elicits sustained and conditioned increases in intracranial self-stimulation thresholds in the rat. Behav Brain Res 209, 114–118. [DOI] [PubMed] [Google Scholar]
- Miller LL, Altarifi AA, Negus SS, 2015. Effects of repeated morphine on intracranial self-stimulation in male rats in the absence or presence of a noxious pain stimulus. Exp Clin Psychopharmacol 23, 405–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D, Picker MJ, 1996. Contribution of individual differences to discriminative stimulus, antinociceptive and rate-decreasing effects of opioids: importance of the drug’s relative intrinsic efficacy at the mu receptor. Behav Pharmacol 7, 261–284. [PubMed] [Google Scholar]
- Negus SS, Mello NK, 1999. Opioid antinociception in ovariectomized monkeys: comparison with antinociception in males and effects of estradiol replacement. J Pharmacol Exp Ther 290, 1132–1140. [PubMed] [Google Scholar]
- Negus SS, Miller LL, 2014. Intracranial self-stimulation to evaluate abuse potential of drugs. Pharmacol Rev 66, 869–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Moerke MJ, 2019. Determinants of opioid abuse potential: insights using intracranial self-stimulation. Peptides 112, 23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid LD, 1987. Tests involving pressing for intracranial stimulation as an early procedure for screening the likelihood of addiction of opioids and other drugs In: Bozarth MJ, (Ed), Methods of assessing the reinforcing properties of abused drugs. Springer-Verlag, Berlin, pp. 391–420. [Google Scholar]
- Selley DE, Liu Q, Childers SR, 1998. Signal transduction correlates of mu opioid agonist intrinsic efficacy: receptor-stimulated [35S]GTP gamma S binding in mMOR-CHO cells and rat thalamus. J Pharmacol Exp Ther 285, 496–505. [PubMed] [Google Scholar]
- Shah A, Hayes CJ, Martin BC, 2017. Characteristics of Initial Prescription Episodes and Likelihood of Long-Term Opioid Use - United States, 2006–2015. MMWR Morb Mortal Wkly Rep 66, 265–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim LJ, Selley DE, Dworkin SI, Childers SR, 1996. Effects of chronic morphine administration on mu opioid receptor-stimulated [35S]GTPgammaS autoradiography in rat brain. J Neurosci 16, 2684–2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siuda ER, Carr R 3rd, Rominger DH, Violin JD, 2017. Biased mu-opioid receptor ligands: a promising new generation of pain therapeutics. Curr Opin Pharmacol 32, 77–84. [DOI] [PubMed] [Google Scholar]
- Traynor JR, Nahorski SR, 1995. Modulation by mu-opioid agonists of guanosine-5’-O-(3-[35S]thio)triphosphate binding to membranes from human neuroblastoma SH-SY5Y cells. Mol Pharmacol 47, 848–854. [PubMed] [Google Scholar]
- Vanyukov MM, Tarter RE, Kirillova GP, Kirisci L, Reynolds MD, Kreek MJ, Conway KP, Maher BS, Iacono WG, Bierut L, Neale MC, Clark DB, Ridenour TA, 2012. Common liability to addiction and “gateway hypothesis”: theoretical, empirical and evolutionary perspective. Drug Alcohol Depend 123 Suppl 1, S3–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Collins FS, 2017. The Role of Science in Addressing the Opioid Crisis. N Engl J Med 377, 391–394. [DOI] [PubMed] [Google Scholar]
- Walker EA, Zernig G, Young AM, 1998. In vivo apparent affinity and efficacy estimates for mu opiates in a rat tail-withdrawal assay. Psychopharmacology (Berl) 136, 15–23. [DOI] [PubMed] [Google Scholar]
- Wiebelhaus JM, Walentiny DM, Beardsley PM, 2016. Effects of Acute and Repeated Administration of Oxycodone and Naloxone-Precipitated Withdrawal on Intracranial Self-Stimulation in Rats. J Pharmacol Exp Ther 356, 43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachariou V, Bolanos CA, Selley DE, Theobald D, Cassidy MP, Kelz MB, Shaw-Lutchman T, Berton O, Sim-Selley LJ, Dileone RJ, Kumar A, Nestler EJ, 2006. An essential role for DeltaFosB in the nucleus accumbens in morphine action. Nat Neurosci 9, 205–211. [DOI] [PubMed] [Google Scholar]
- Zacny JP, Conley K, Marks S, 1997. Comparing the subjective, psychomotor and physiological effects of intravenous nalbuphine and morphine in healthy volunteers. J Pharmacol Exp Ther 280, 1159–1169. [PubMed] [Google Scholar]