Abstract
Self-inflicted injury (SII) in adolescence is a serious public health concern that portends prospective vulnerability to internalizing and externalizing psychopathology, borderline personality development, suicide attempts, and suicide. To date, however, our understanding of neurobiological vulnerabilities to SII is limited. Behaviorally, affect dysregulation is common among those who self-injure. This suggests ineffective cortical modulation of emotion, as observed among adults with borderline personality disorder (BPD). In borderline samples, structural and functional abnormalities are observed in several frontal regions that subserve emotion regulation (e.g., anterior cingulate, insula, dorsolateral prefrontal cortex). However, no volumetric analyses of cortical brain regions have been conducted among self-injuring adolescents. We used voxel-based morphometry to compare cortical gray matter volumes between self-injuring adolescent girls, ages 13–19 years (n=20), and controls (n=20). Whole brain analyses revealed reduced gray matter volumes among self-injurers in the insular cortex bilaterally, and in the right inferior frontal gyrus (rIFG), an adjacent neural structure also implicated in emotion- and self-regulation. Insular and IFG gray matter volumes correlated inversely with self-reported emotion dysregulation—over-and-above effects of psychopathology. Findings are consistent with an emotion dysregulation construal of SII, and indicate structural abnormalities in some but not all cortical brain regions implicated in BPD among adults.
Keywords: self-injury, adolescence, MRI, insula, borderline personality disorder
Self-inflicted injury (SII) in adolescence is an increasingly prevalent phenomenon that marks prospective vulnerability to a wide range of adverse outcomes, including academic underachievement, internalizing and externalizing psychopathology, suicide attempts, and eventual death by suicide (see Beauchaine, Hinshaw, & Bridge, 2018; Jacobson & Gould, 2007; Klonsky, Victor & Saffer, 2014). SII affects between 15% and 20% of adolescents in North America, with some studies reporting even higher percentages (Brent, 2011; Heath, Baxter, Toste, & McLouth, 2010; Hilt, Cha, & Nolen-Hoeksema, 2008; Somer et al., 2015). Troublingly, SII age of onset, which is associated with lethality of episodes and hospitalizations, is decreasing (Ammerman, Jacobucci, Kleiman, Uyeji, & McCloskey, 2018). Moreover, lifetime prevalence rates of suicidal ideation and attempts, which are often preceded by SII, are now 12.1% and 4.1%, respectively, and increasing among teens (Nock et al., 2013). These findings are especially troubling given that SII is a strong predictor of eventual death by suicide (Joiner, Brown, & Wingate, 2005; Swannell, Martin, Page, Hasking, & St John, 2014). Yet despite the urgency of this public health concern, our understanding of etiology and associated neurobiology remains limited (e.g., Beauchaine, Crowell, & Hsiao, 2015).
SII is typically associated with extreme psychological distress (see Crowell, Beauchaine, & Linehan, 2009; Derbidge & Beauchaine, 2014). Adolescents who self-injure are more anxious, more depressed, and more hostile than clinically-referred peers who do not self-injure (Andover, Pepper, Ryabchenko, Orrico & Gibb 2005; Klonsky, Oltmanns, & Turkheimer, 2003). Furthermore, nearly all adolescents who self-injure are affected by at least one psychiatric disorder, and nearly half have two or more comorbid conditions (Bodfish & Lewis, 2002; Haw, Hawton, Houston & Townsend, 2001). Diagnoses of depressive disorders are most common, but externalizing behaviors—often accompanied by clinically significant impairment—are also observed (e.g., Crowell et al., 2005; Nock, Joiner, Gordon, Lloyd-Richardson, & Prinstein 2006).
Notably, however, self-injury is not, in and of itself, a psychiatric disorder, and SII is often not associated with attempts or desire to kill oneself (Wilkinson, 2013). Nevertheless, self-injury is classified as a condition for further study in the Diagnostic and Statistical Manual of Mental Disorders (DSM-5; American Psychiatric Association, 2013), and is a symptom of both major depressive disorder (MDD) and borderline personality disorder (BPD). Given longstanding proscriptions against diagnosing personality disorders among youth, BPD remains a controversial diagnosis for children and adolescents (see Beauchaine, Klein, Crowell, Derbidge, & Gatzke-Kopp, 2009). These and other nosologic concerns have led some to suggest that the DSM-5 does not effectively integrate self-injury into the existing psychiatric nomenclature (see Kaufman, Crowell, & Lenzenweger, 2017).
Despite unresolved diagnostic issues, there is growing consensus that etiological precursors to BPD, including SII, often appear by adolescence (see Beauchaine et al., 2009; Derbidge & Beauchaine, 2014; Hinshaw et al., 2012). In fact, a plurality of adolescents who self-injure already meet full diagnostic criteria for BPD (e.g., Crowell et al., 2005). Following from these and other findings, our research group has proposed a developmental model whereby SII and BPD emerge from a common etiology. According to this perspective, heritable trait impulsivity confers vulnerability to both SII and BPD, but only when coupled with deficiencies in emotion regulation (Beauchaine et al., 2009, 2018; Crowell et al., 2009). As described immediately below, evidence for emotion dysregulation in SII is extensive. This, coupled with (1) the role that self-injury serves in marking prospective vulnerability to BPD and other psychiatric disorders, and (2) over-lapping neural correlates of emotion dysregulation and BPD, provides a basis for hypothesizing about cortical volumetric abnormalities among adolescents who self-injure, even though no such analyses have been reported in the literature to date.
Self-inflicted Injury, Emotion Dysregulation, and Borderline Personality Development
Over the past 25 years, research on SII and borderline personality development has been influenced heavily by Linehan’s (1993) biosocial theory. Linehan proposed that self-injury functions to downregulate overwhelmingly negative affect, and usually precedes development of BPD (see also Bentley, Nock, & Barlow, 2014; Klonsky, 2007, 2009; Korfine & Hooley, 2000). Support for an emotion regulation function of SII is substantial, and spans neural, physiological, self-report, and behavioral levels of analysis (for reviews see Klonsky, 2007, 2009). Similar to adults with BPD, adolescents who engage in SII report high levels of emotion dysregulation and affective distress, which are dampened by self-injurious behaviors (Lloyd-Richardson, Perrine, Dierker, & Kelley, 2007; Turner, Chapman, & Layden, 2012).
At the central nervous system level, an extensive literature implicates functional subdivisions of the prefrontal, anterior cingulate, and insular cortices in volitional downregulation of negative affect (e.g., Tone, Garn, & Pine, 2016; Zilverstand, Parvaz, & Goldstein, 2017). For example, in their foundational paper, Goldin, McRae, Ramel, and Gross (2008) demonstrated that effortful reappraisal of negative emotion elicits increased BOLD responding across a wide range of frontal structures, including the dorsolateral, medial, and ventrolateral prefrontal cortices (PFC), the lateral orbitofrontal cortex (OFC), the inferior frontal gyrus (IFG), and the insular cortex (see also Giuliani, Drabant, & Gross, 2011; Grecucci, Giorgetta, Bonini, & Sanfey, 2013). Furthermore, effective PFC recruitment when regulating negative emotions dampens amygdala reactivity—a subcortical neural substrate of strong affective responses (see Ochsner et al., 2004). Notably, both adolescents who engage in SII and adults with BPD show bilateral amygdala reactivity abnormalities to a host of eliciting events (e.g., Sauder, Derbidge, & Beauchaine, 2016; Hazlett et al., 2012).
Although our focus here is on brain structure rather than function, it should be noted that adults with BPD exhibit functional abnormalities in many of the cortical structures listed above when regulating or attempting to regulate negative affect. These regions include the anterior cingulate cortex (ACC), the dlPFC, the mPFC, the vlPFC, the OFC, and the insular cortex (e.g. Domsalla et al., 2014; King-Cases et al., 2008; Krause-Utz, Winter, Niedtfeld, & Schmahl, 2014; Lis, Greenfield, Henry, Guilé, & Dougherty, 2007; Malhi et al., 2013; Niedtfeld et al., 2012; Ruocco, Amirthavasagam, Choi-Kain, & McMain, 2013). Thus, BPD—a disorder characterized by emotion dysregulation—is associated with functional deficiencies in frontal structures that are ordinarily recruited to regulate emotion (see also Beauchaine, 2015).
Strutural Neuroimaging Findings
Structural analyses of cortical volumes are consistent with functional data. Adults with BPD show reduced gray matter volumes across a wide range of cortical regions including the ACC, the vlPFC, the OFC, and the insular cortex (e.g., Morandotti et al., 2013; Rossi et al., 2013; Soloff, Nutche, Goradia, & Diwadkar, 2008; Soloff et al., 2012). Moreover, adolescents who are already diagnosed with BPD show reduced gray matter volumes in the dlPFC and OFC compared with controls (Brunner et al., 2010). We are aware of no studies conducted with samples recruited specifically for self-injury. Important for purposes of the present study, however—where our objective is to evaluate premorbid neural vulnerability among self-injuring youth—are findings of decreased gray matter volumes in the insular cortex in adolescents at first presentation with BPD (Takahashi et al., 2009). This suggests that at least some reductions in cortical volume may precede development of more serious psychiatric impairment among vulnerable adolescents. Accordingly, the primary objective of this study was to evaluate cortical volumes among adolescent girls, ages 13–19 years, who were recruited based solely on histories of SII, compared with age-matched peers. As reviewed above, these girls suffer from considerable impairments in emotion regulation (Crowell et al., 2005, 2009), and are vulnerable to developing BPD, yet it is currently unknown whether neural deficiencies observed in adolescents and adults with BPD are observed among self-injuring samples. Consistent with the literature outlined above, we hypothesized reduced frontal gray matter volumes among participants who engage in SII. If confirmed, this would be the first such finding among girls who are at significant prospective vulnerability to BPD and suicide attempts. Given the wide range of frontal cortex regions implicated in emotion dysregulation and BPD, we conducted a whole-brain analysis—not a region-of-interest analysis.
Method
Participants
Self-injuring (n=22) and control (n=22) adolescent females, ages 13–19 years, were enrolled. One control participant was taking an SSRI and therefore excluded. Of the remaining participants, two self-injuring and one control were excluded due to excessive motion or scanner artifact. Twenty-eight participants identified as Caucasian, 4 as Hispanic Caucasian, and 8 as biracial. We did not include males given lower prevalence rates of self-injury and an inadequate sample size for evaluating sex effects. Participants were recruited from previous studies, direct mailings to families, and advertisements/letters sent to mental health providers, community centers, and pediatricians’ offices. Study procedures were approved by the local institutional review board. Informed consent was obtained from both adolescents and their parents.
To be included in the self-injury group, adolescents were required to report at least 3 self-injury episodes in the last year or 5 or more in their lifetime. At least one of these episodes had to have a lethality rating of 2 or higher on the Lifetime Suicide Attempt and Self-Injury Interview (L-SASI Count; Linehan & Comtois, 1996)1. Potential control group participants were screened out if they endorsed DSM-IV (APA, 1994) criteria for a depressive disorder on the Diagnostic Interview Schedule for Children (DISC-C; Shaffer, Fisher, Lucas, Mina, & Schwab-Stone, 2000), or if they reported any lifetime self-injury event. Those who reported a history of bipolar disorder or schizophrenia, possible mental retardation, IV drug use, left-handedness, and/or MRI contraindications (e.g., metal implants, braces) were excluded from both groups.
Evaluation
Potential participants completed a preliminary phone screen, which included questions that assessed lifetime and current self-injurious behaviors, previous psychiatric diagnoses, current major depressive disorder (MDD), possible mental retardation, IV drug use, current medications, handedness, and MRI safety screening. Respondents who met preliminary phone screen criteria were evaluated more stringently during a subsequent lab visit.
The DISC was used to evaluate current MDD and substance use disorders. Other current disorders, including attention-deficit/hyperactivity disorder (ADHD), conduct disorder (CD) generalized anxiety disorder, panic disorder, obsessive-compulsive disorder, posttraumatic stress disorder, social phobia, schizophrenia, dysthymia, bipolar disorder, anorexia, and bulimia were assessed using the Youth’s Inventory (YI; Gadow et al., 2002), an adolescent self-report measure. The YI produces both symptom counts and diagnosis cutoffs. In addition, adolescents completed the Youth Self Report (YSR; Achenbach, 1991), which provides T-scores for internalizing and externalizing problems. The Structured Clinical Interview for DSM-IV, Axis II (SCID-II; First, Gibbon, Spitzer, Williams, & Benjamin, 1997) was used to assess BPD symptoms.
Participants also completed measures of self-injury, suicide attempts, and emotion dysregulation. The L-SASI (formerly the Lifetime Parasuicide Count; Linehan & Comtois, 1996) is a structured interview that assesses lethality, suicidal intent, level of medical treatment received for, and specific circumstances surrounding adolescents’ first, most recent, and most severe SII episode. It includes ratings of both the number and lethality of both non-suicidal and suicidal behaviors. The Suicidal Ideation Questionnaire (SIQ; Reynolds, 1987, 1988) was used to assess suicidal ideation at each study visit. The Difficulties in Emotion Regulation Scale (DERS; Gratz & Roemer, 2004) was also administered. Although validated initially among adults, the DERS is effective in assessing individual differences in emotion dysregulation among adolescents (Neumann, van Lier, Gratz, & Koot, 2010). Finally, participants completed the Kaufman Brief Intelligence Test, 2nd ed. (KBIT-2; Kaufman & Kaufman, 2004).
MRI Scanning
All participants completed a mock scanning session to familiarize them with MRI procedures. Structural scans were acquired as part of an hour-long session that included two functional tasks that are not presented herein (see Sauder et al., 2016). Scans were performed on a 3 Telsa Philips Achieva MR System (version 2.63, Philips Medical Systems, Best, the Netherlands) with dual Quasar gradients (80 mT/m at a slew rate of 110 mT/m/s; or 40 mT/m at a slew rate of 220 mT/m/s) and an 8-channel SENSE head coil. High resolution 3D FFE T1-weighted magnetization prepared-rapid gradient echo (MPRAGE) fast imaging sequences generated 200 contiguous axial slices spanning the entire brain (TR = 7.7 ms; TE = 3.6 ms; flip angle = 8°; FOV = 220×220×200; matrix size = 220 × 205; voxel dimension = 1 × 1.07 × 1 mm; SENSE factor = 1).
MRI Analysis
Data were analyzed using statistical parametric mapping software (SPM12; http://www.fil.ion.ucl.ac.uk/spm/software/spm12/). Anatomical scans were segmented, spatially normalized, and bias corrected in the same model, using default parameters. The process corrects for image intensity non-uniformity and provides better results than serial application of these steps (Ashburner & Friston, 2005). Segmented gray matter volumes were aligned to a common group space prior to normalization to MNI space using Diffeomorphic Anatomical Registration Through Expotentiated Lie Algebra (DARTEL; Ashburner, 2007), a high dimensional warping process. Data were smoothed using an 8mm Gaussian kernel. To examine regional differences in gray matter volumes between self-injuring adolescents and controls, data were compared in a mixed-effects second level analysis. A between-groups t-test was computed including total intracranial volumes and age as covariates, masked to remove areas of non-interest (e.g., ventricles, skull, large white-matter bundles [corpus callosum]). Results were evaluated with a voxel threshold of <.001, uncorrected with a minimum cluster size of 500 voxels (~1700mm3 p), and subsequently corrected for multiple comparisons and non-stationarity using a family-wise error cluster extent threshold of p<.05.
Results
As indicated in Table 1, the SII group engaged in significant levels of self-harm, with large effect sizes separating them from the control group on all measures. The average number of lifetime nonsuicidal self-injurious behaviors among those in the SII group, as defined as self-injury in the absence of intent to die, was 180 but widely variable (SD = 256). Self-injuring adolescents also reported a substantial number of self-injurious behaviors with ambivalent intent to die (M=19.60), and an average of 1.25 suicide attempts with high intent to die. On average, those in the SII group endorsed 3 symptoms of BPD on the SCID (5 are required for a diagnosis). The SII group also scored higher on both the YSR internalizing and externalizing scales, and on emotion dysregulation as assessed by the DERS. These effects were significant and large, all ts (39) ≥ 4.02, all ds ≥ 1.36. As reported elsewhere, similar effect sizes were observed for DSM-IV symptoms of ADHD, CD, MDD, and dysthymia on the YI (Sauder et al., 2016).
Table 1.
Demographic variables, levels of self-injury and suicidal ideation, and self-reported internalizing and externalizing scores, by group
| Variable | Control Mean (SD) | Self-injury Mean (SD) | t-statistic/z-statistica | effect size |
|---|---|---|---|---|
| Descriptive statistics | ||||
| Age | 15.93 (2.03) | 15.70 (1.77) | t = 0.36 | d = 0.11 |
| KBIT IQ | 113.68 (9.73) | 108.42 (10.17) | t = 1.63 | d = 0.52 |
| Self-reported symptoms | ||||
| SIQ-Jr | 1.63 (2.00) (n=8) | 14.88 (15.72) (n=8) | t = 2.36* | d = 1.26 |
| SIQ | 2.54 (5.28) (n=11) | 49.63 (32.57) (n=11) | t = 4.73*** | d = 2.12 |
| L-SASI | ||||
| SII | 0.00 (0.00) | 184.16 (262.05) | z = 5.63*** | r = .91 |
| Ambivalent attempts | 0.00 (0.00) | 1.32 (2.40) | z = 2.62** | r = .42 |
| High intent attempts | 0.00 (0.00) | 20.42 (36.13) | z = 3.81*** | r = .62 |
| Total suicide attempts | 0.00 (0.00) | 21.74 (38.27) | z = 4.04*** | r = .66 |
| SCID-II BPD symptoms | 0.00 (0.00) | 3.00 (1.70) | z = 5.66*** | r = .92 |
| DERS total scores | 51.58 (10.23) | 92.06 (26.57) | t = 6.41*** | d = 2.01 |
| Youth Self Report | ||||
| Externalizing (T) | 46.63 (8.50) | 58.42 (9.84) | t = 4.02*** | d = 1.36 |
| Internalizing (T) | 38.68 (6.13) | 61.26 (12.70) | t = 6.98*** | d = 2.36 |
Notes. KBIT = Kaufman Brief Intelligence Test, 2nd ed. (KBIT-2; Kaufman & Kaufman, 2004); SIQ=Suicide Ideation Questionnaire (Reynolds, 1987, 1988), standardized by grade level. SIQ-Jr (grades 7–9) raw scores ≥ 31 and SIQ (grades 10–12) raw scores ≥ 41 indicate significant clinical concern regarding suicide risk; L-SASI= Lifetime Suicide Attempt Self-Injury Count (Linehan and Comtois, 1996); SCID-II = Structured Clinical Interview for DSM-IV Axis II (First et al., 1997); DERS = Difficulties in Emotion Regulation Scale (Gratz & Roemer, 2004); SII = self-inflicted injury. SA = suicide attempt.
t-tests were conducted on normally-distributed data; Mann-Whitney U tests (z) were conducted on skewed data.
p ≤ .05.
p .01.
p ≤ .001.
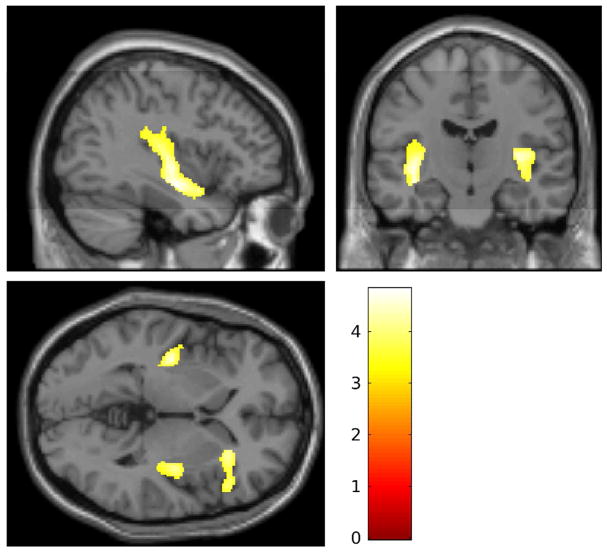
To evaluate our structural hypothesis, we first conducted a whole brain analysis with a liberal voxel-wise correction of p<.001 uncorrected, and a strict FWE-corrected cluster extent threshold of p<.05. This error rate was chosen to balance probabilities of type I and type II error given the modest sample size (n=40). No group difference was observed in total brain volumes, t(39) = 0.33, p > .25. However, the cluster extent threshold was exceeded bilaterally for the posterior insula and the right anterior insula/inferior frontal gyrus (rIFG; see Table 2 and Figure 1). The group difference spanned multiple anatomical structures; the two larger clusters are almost entirely insula, whereas the anterior portion spans the insula and IFG. Notably, the IFG is adjacent to the insula, and is implicated in both emotion and self-regulation more broadly (e.g., Depue, Burgess, Bidwell, Willcutt, & Banich, 2010; Grecucci et al., 2013). In each case, regional reductions in gray matter were observed for SII vs. control participants. There were no regions of greater gray matter volume among self-injuring adolescents than controls, and no significant differences between groups in regional white-matter volumes. Inspection for outliers revealed three z-scores that exceeded 2.07 (98th percentile)—one in each brain region (rIFG, insula bilaterally). Each outlier came from a different participant in the self-injury group. However, since all outliers were positive, these data worked against our hypotheses and were retained.
Table 2.
Gray matter volume differences between self-injuring and control participants for regions identified by whole brain analysis
| Vol. (RESEL) | Vol. (cm3) | pFWE-NS | Coordinate (MNI) | z-score | |
|---|---|---|---|---|---|
| Left hemisphere | |||||
| Insula | 3.496 | 7.840 | .000 | −38, −10, −14 | 4.20 |
| Right hemisphere | |||||
| IFG/Insula | 1.287 | 2.801 | .018 | 33, 33, 6 | 3.82 |
| Insula | 2.428 | 5.281 | .019 | 34, −18, 7 | 3.99 |
Notes. Self-injuring adolescents had reduced gray matter densities in each region, indicating reduced gray matter volumes. Volumes given in RESEL, reflecting volume corrected for smoothness (Worsley, Evans, Marrett, & Neelin, 1992), and cm3. P-values reflect cluster-level corrections for family wise error (FWE) rate, corrected for non-stationarity (Hayasaka, Phan, Liberzon, Worsley, & Nichols, 2004). Coordinates are given in Montreal Neurological Institute (MNI) standard brain space, and reflect locations of maximal gray matter differences, with corresponding z-scores at each location. Hemisphere labels are derived from the automated anatomical labelling (AAL) atlas (Tzourio-Mazoyer et al., 2002), and reflect anatomical regions most associated with the regional difference in gray matter density. IFG = inferior frontal gyrus.
Figure 1.
Regional reductions in gray matter volume for self-injuring adolescents relative to controls [−37, −14, 0]. Shaded regions depict bilateral reductions in posterior insula volumes, and reductions in volumes spanning and portions of the anterior insula and the right inferior frontal gyrus.
Findings of reduced insular and IFG gray matter volumes among SII participants could be attributable to overall levels of psychopathology rather than emotion dysregulation per se. Since we had no psychiatric controls without SII for comparison, we addressed this possibility by conducting regression analyses in which (1) associations between regional gray matter volumes and both ADHD and MDD were evaluated (see Table 3), and (2) regional gray matter volumes were correlated with emotion dysregulation scores from the DERS, controlling for all symptoms of psychopathology from the YI (see Table 4). As reported in Table 3, when entered alone, both ADHD and MDD symptoms accounted for significant variance in bilateral insula volumes and rIFG volumes, over-and-above effects of total intracranial volume. However, when entered into regression models together, neither ADHD nor MDD symptoms made independent contributions. They therefore contributed overlapping variance.
Table 3.
Linear regression of total intracranial volumes, attention-deficit/hyperactivity disorder (ADHD) symptoms, and major depressive disorder (MDD) symptoms on gray matter volumes in areas of group difference centered within the left insula and right interior frontal gyrus
| Variable | Insula (mL) | Inferior Frontal Gyrus (mL) | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| R2 | B | SE | β | R2 | B | SE | β | |
| TIV | 0.60 | 2.118 | 0.314 | 0.737*** | 0.40 | 0.654 | 0.145 | 0.602*** |
| ADHD symptoms | −0.012 | 0.005 | −0.299** | −0.004 | 0.002 | −0.238Ŧ | ||
|
| ||||||||
| TIV | 0.61 | 2.054 | 0.308 | 0.715*** | 0.44 | 0.636 | 0.139 | 0.585*** |
| MDD symptoms | −0.010 | 0.003 | −0.318** | −0.004 | 0.002 | −0.318* | ||
|
| ||||||||
| TIV | 0.62 | 2.085 | 0.311 | 0.726*** | 0.44 | 0.637 | 0.142 | 0.586*** |
| ADHD symptoms | −0.006 | 0.007 | −0.142 | 0.000 | 0.003 | −0.015 | ||
| MDD symptoms | −0.007 | 0.005 | −0.215 | −0.004 | 0.002 | −0.307 | ||
Notes. TIV = total volume in liters; ADHD and MDD symptom scores defined as the sum score of DSM-IV symptoms. Gray matter volumes are reported for the left insula and right interior frontal gyrus. Similar results were found for the right insula, and are not reported.
p<.05;
p<.05;
p<.001;
p = 0.084.
Table 4.
Linear regression of total intracranial volume, symptoms of psychopathology, and emotion dysregulation on gray matter volumes in areas of group difference centered within the left insula and right inferior frontal gyrus.
| Variable | Insula (mL) | Inferior Frontal Gyrus (mL) | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| R2 | B | SE | β | R2 | B | SE | β | |
| TIV | 0.57 | 1.973 | 0.322 | 0.664*** | 0.46 | 0.600 | 0.201 | 0.527*** |
| DERS-Total | −0.120 | 0.040 | −0.334** | −0.057 | 0.017 | −0.414** | ||
|
| ||||||||
| TIV | 0.58 | 1.961 | 0.322 | 0.660*** | 0.46 | 0.600 | 0.139 | 0.527*** |
| YI total symptoms | −0.020 | 0.018 | −0.182 | 0.000 | 0.008 | −0.002 | ||
| DERS-Total | −0.070 | 0.061 | −0.194 | −0.057 | 0.026 | −0.413* | ||
Notes. TIV = total volume in liters; DERS = Difficulties with Emotion Regulation Scale (Gratz & Roemer, 2004); YI = Youth Inventory (Gadow et al., 2002). Gray matter volumes are reported for the left insula and the right interior frontal gyrus. Similar results were found for the right insula, and are not reported.
p<.05;
p<.05;
p<.001.
As reported in Table 4, DERS scores also accounted for significant variance in bilateral insula volumes and rIFG volumes, over-and-above effects of total intracranial volume. Furthermore, when entered into a regression with all YI symptoms, DERS scores accounted for independent variance in rIFG volumes. Thus, emotion dysregulation accounted for variance in rIFG volumes—over and above effects of general psychopathology.
Discussion
Self-inflicted injury is a significant public health concern that confers prospective vulnerability to depression, borderline personality development, and eventual suicide (see e.g., Beauchaine et al., 2018; Crowell et al., 2009; Jacobson & Gould, 2007; Klonsky et al., 2014). To date, however, relatively little is known about neurobiological vulnerabilities to SII. Characterizing this neurobiology may improve our understanding of traits that predispose to significant distress and impairment, with direct implications for improved treatments (Beauchaine, Neuhaus, Brenner, & Gatzke-Kopp, 2008). We hypothesized that self-injuring adolescents would exhibit reduced cortical volumes compared with controls, consistent with findings from adult samples with emotion regulation difficulties, including but not limited to BPD. Volumetric abnormalities in the bilateral insula and rIFG are consistent with etiological models that emphasize emotion dysregulation as a predisposing vulnerability to both SII and later BPD (Crowell et al., 2009; Derbidge & Beauchaine, 2014; Linehan, 1993; Klonsky, 2007). Insular cortex function is implicated consistently in emotion regulation (e.g., Goldin et al., 2008; Grecucci et al., 2013; Zilverstand et al., 2017). In this study, regional reductions in gray matter volume were localized to the insular cortex and rIFG, perhaps reflecting abnormalities in neural development of emotional processing. To date, there is limited evidence to suggest that reduced insular volumes are associated directly with suicidality (e.g., Hwang et al., 2010). Indeed, any such link would almost certainly be indirect. Insular and IFG dysfunction predispose to emotion dysregulation, which likely interacts with environmental adversities to potentiate vulnerability to more debilitating psychiatric impairment (see e.g., Beauchaine et al., 2009).
Volumetric abnormalities in the insula are observed in a wide range of psychiatric syndromes characterized by emotion dysregulation. For example, internalizing and externalizing boys and girls exhibit reduced insular volumes (Fairchild et al., 2011; Fairchild et al., 2013; Snyder et al., 2017; Sterzer, Stadler, Poustka, & Kleinschmidt, 2007), as do adults who incur cumulative life stress and more acute forms of trauma (e.g., Herringa, Phillips, Almeida, Insana, & Germain, 2012). These latter findings are of particular interest given the increasingly well documented role of trauma and correlated environmental adversities in development of both (1) SII and suicidal behaviors (e.g., Guendelman, Owens, Galan, Gard, & Hinshaw, 2016; Hinshaw et al., 2012), and (2) cortical brain growth (e.g., Ansell, Rando, Tuit, Guarnaccia, & Sinha, 2012; Hair, Hanson, Wolfe, & Pollak, 2015). Taken together, accumulating research suggests that the insula and other cortical structures are sensitive to neuroplastic degenerative effects of stress and trauma, with harmful consequences for emerging emotion regulation skills (see above; Kohn et al., 2014; Zilverstand et al., 2017). Moreover, reduced insula and other cortical volumes across various disorders are consistent with the transdiagnostic nature of emotion dysregulation in psychopathology (Beauchaine & Zisner, 2017; Kring & Sloan, 2009).
Gray matter reductions extended to the rIFG, an adjacent neural structure implicated in both emotion- and self-regulation—particularly in social contexts (Grecucci et al., 2013; Kohn et al., 2014; Urgesi, Mattiassi, Buiatti, & Marini, 2016). Lesions to this region result in impaired behavioral inhibition and impulsive decision making (Aron, Robbins, & Poldrack, 2004; Chamberlain & Sahakian, 2007). Individuals with ADHD show reduced rIFG gray matter volumes, and smaller rIFG volumes are associated with slower processing speed and reduced response inhibition (Depue et al., 2010). Thus, reduced rIFG gray matter volumes may help to account for high rates of impulsivity and other externalizing behaviors observed among adolescents with SII.
It is important to acknowledge that abnormalities in neural structure are not necessarily manifested in neural or behavioral function. In fact, it is relatively common to see atypical morphology in subsets of individuals who undergo MRI, despite no identifiable abnormalities in neural function or behavior. As mentioned above, however, reductions in rIFG volumes are associated with impairments in inhibition, whereas healthy emotion awareness and regulation are associated with larger insular volumes (Giuliani, Drabant, Bhatnagar, & Gross, 2011). Given the large body of evidence indicating bilateral volumetric abnormalities in the insula among emotionally dysregulated samples, it is reasonable to conclude that reduced gray matter in this brain region is a vulnerability to emotional lability and its psychiatric correlates.
In contrast to observed insular cortex volumes in several forms of psychopathology, as reviewed immediately above, insular volumes correlate positively with self-control (Rosso et al., 2010; Uchida et al., 2008). Such findings lend more support to the apparent emotion regulation/emotion suppression function of the insula, and may help to explain consistent associations between emotion dysregulation and vagally-mediated autonomic dysfunction observed across these differing manifestations of psychopathology—including SII (e.g., Crowell et al., 2005, 2017)—given insular involvement in regulating the peripheral nervous system (see e.g., Beauchaine & Thayer, 2015).
Of note, reduced cortical volumes were not observed in many other cortical structures that are consistently compromised in BPD samples (e.g., ACC, dlPFC, mPFC, vlPFC, OFC). There are at least three explanations for this finding. First, only a subset of self-injuring adolescences will go on to develop BPD. Although that fraction is likely to be high in the present sample given (1) the number of BPD symptoms and degree of emotion dysregulation endorsed (see Table 1) and (2) the severity of self-injury that participants were recruited for, it is nevertheless possible that heterogeneity in vulnerability to BPD diluted cortical effects.
A second possibility is that broader PFC structural deficiencies were not yet evident because neuromaturational trajectories for the SII and control groups had not yet diverged enough. It is possible that girls in the SII group will be “left behind” their typically developing peers in frontal neuromaturation. Effects of emerging cortical structural deficiencies across early adolescence have been observed for other disorders (e.g., de Brito et al., 2009; Shaw et al., 2013). Addressing this possibility will require future longitudinal studies with vulnerable samples.
Finally, although many studies conducted with adults show volumetric abnormalities in BPD using whole brain analyses of similarly sized samples, statistical power in a sample of n=40 is limited. It is therefore possible that structural abnormalities in cortical regions other than the insular cortex and IFG were missed. Addressing this question will also require additional research.
There are a number of caveats to consider in interpreting our findings. As is usually the case with cross-sectional data, we cannot know whether insular and IFG gray matter reductions observed preceded or followed SII onset, or whether they mark prospective vulnerability to BPD. Our article represents one small step toward disentangling such questions by demonstrating—for the first time—that reduced insular cortex volumes are present among emotionally dysregulated adolescents who self-injure. Nevertheless, more longitudinal studies of gray matter development are needed to identify prospective vulnerabilities, especially regarding development of self-injury. As alluded to above, gray matter densities follow typical neurodevelopmental time courses across adolescence and young adulthood (see Gogtay & Thompson, 2010). Insular volumes increase throughout early and middle adolescence, with later reductions in late adolescence and early adulthood (Shaw et al., 2008).
It is important to restate that this is not a study of existing BPD. Rather, it study of likely prospective vulnerability. Such vulnerability is almost certainly more general than specific. Girls who participated are vulnerable to a range of adverse multifinal outcomes, including depression, externalizing behaviors, and substance use disorders—in addition to BPD. They are also vulnerable to developing other personality disorders. Nevertheless, given our recruitment strategy, the severity of SII, and the fact that many girls recruited using very similar inclusion criteria have been diagnosed with BPD in our previous work (e.g., Beauchaine et al., 2015; Crowell et al., 2017), we are confident our findings are relevant to evaluating premorbid vulnerability to BPD. That said, very few neural vulnerabilities are specific to single disorders, and the expectation that a specific neural signature could predict single disorders may me misplaced. Rather, most psychiatric disorders share neural vulnerabilities that transcend traditional diagnostic categories. Accordingly, specifying neural substrates of transdiagnostic vulnerability traits, such as emotion dysregulation, may be more fruitful in the upcoming years than persistently searching for pathognomonic sins (see Beauchaine & Constantino, 2017).
Finally, it is important to note that cortical thickness measures and the VBM methods used here are not necessarily interchangeable. Although the measures yield similar results, cortical thickness may be more sensitive to normative developmental changes (Hutton, Draganski, Ashburner, & Weiskopf, 2009). Future studies are therefore needed to determine how our findings fit within contexts of typical and atypical neural development.
Caveats aside, this is the first study to demonstrate volumetric abnormalities in the insula and IFG among adolescent girls who are vulnerable to depression, suicidal behavior, and BPD. Our findings indicate that brain abnormalities that are characteristic of adults with BPD are already present among adolescents who self-injure, which should motivate the field to intervene earlier (see Beauchaine et al., 2008, 2018). Cortical structures in particular follow protracted neuromaturational time courses (Brain Development Cooperative Group, 2012), and are responsive to environmental input through mechanisms of neural plasticity (e.g., Hair et al., 2015). Prevention and early intervention programs may hold great promise in redressing abnormal patterns of neural structure and function during adolescence—a critical period for cortical neuromaturation (e.g., Casey & Caudle, 2013).
Acknowledgments
Research reported in this article was supported by Grants MH86198 and DE025980 from the National Institute of Mental Health, and by the National Institutes of Health Science of Behavior Change (SoBC) Common Fund.
Footnotes
Historically, authors have parsed self-injurious behaviors in a number of ways, sometimes without empirical justification (for extended discussion see Kaufman, Crowell, & Stepp, 2017). These parsings focus on different but overlapping features, including psychological functions of self-harm, lethality of events, level of suicidal intent, and physical outcomes. More recent conceptualizations often view self-harm along a continuum spanning nonsuicidal self-injury (NSSI) to attempted/completed suicide. This approach is consistent with dimensional models of psychopathology, and well characterized progressions of NSSI to more lethal forms of self-injury across development. A comprehensive account of these issues would require a full-length review, and is therefore beyond the scope of this article. Readers are referred elsewhere for such discussions (e.g., Andover & Morris, 2014; Joiner et al., 2009; Klonsky, 2007, 2009; Klonsky, May, & Glenn, 2013).
Contributor Information
Theodore P. Beauchaine, The Ohio State University
Colin L. Sauder, University of Texas San Antonio
Christina M. Derbidge, University of Utah
Lauren L. Uyeji, Temple University
References
- Achenbach TM. Manual for the Youth Self Report and 1991 profile. Burlington, VT: University of Vermont Department of Psychiatry; 1991. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: Author; 1994. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5. Arlington, VA: Author; 2013. [Google Scholar]
- Ammerman BA, Jacobucci R, Kleiman EM, Uyeji L, McCloskey MS. Suicide and Life-Threatening Behavior. 2018;48:31–37. doi: 10.1111/sltb.12330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andover MS, Morris BW. Expanding and clarifying the role of emotion regulation in nonsuicidal self-injury. Canadian Journal of Psychiatry. 2014;59:569–575. doi: 10.1177/070674371405901102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andover MS, Pepper CM, Ryabchenko KA, Orrico EG, Gibb BE. Self-mutilation and symptoms of depression, anxiety, and borderline personality disorder. Suicide and Life-Threatening Behavior. 2005;35:581–591. doi: 10.1521/suli.2005.35.5.581. [DOI] [PubMed] [Google Scholar]
- Ansell EB, Rando K, Tuit K, Guarnaccia J, Sinha R. Cumulative adversity and smaller gray matter volume in medial prefrontal, anterior cingulate, and insula regions. Biological Psychiatry. 2012;72:57–64. doi: 10.1016/j.biopsych.2011.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends in Cognitive Sciences. 2004;8:170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Beauchaine TP. Future directions in emotion dysregulation and youth psychopathology. Journal of Clinical Child and Adolescent Psychology. 2015;44:875–896. doi: 10.1080/15374416.2015.1038827. [DOI] [PubMed] [Google Scholar]
- Beauchaine TP, Constantino JN. Redefining the endophenotype concept to accommodate transdiagnostic vulnerabilities and etiological complexity. Biomarkers in Medicine. 2017;11:769–780. doi: 10.2217/bmm-2017-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchaine TP, Crowell SE, Hsiao R. Post-dexamethasone cortisol, self-inflicted injury, and suicidal ideation among depressed adolescent girls. Journal of Abnormal Child Psychology. 2015;43:619–632. doi: 10.1007/s10802-014-9933-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchaine TP, Hinshaw SP, Bridge JA. Nonsuicidal self-injury and suicidal behaviors in maltreated girls with ADHD: The case for targeted prevention in preadolescence. 2018 doi: 10.1177/2167702618818474. Manuscript submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchaine TP, Klein DN, Crowell SE, Derbidge C, Gatzke-Kopp L. Multifinality in the development of personality disorders: A Biology × Sex × Environment interaction model of antisocial and borderline traits. Development and Psychopathology. 2009;21:735–770. doi: 10.1017/S0954579409000418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchaine TP, Neuhaus E, Brenner SL, Gatzke-Kopp L. Ten good reasons to consider biological processes in prevention and intervention research. Development and Psychopathology. 2008;20:745–774. doi: 10.1017/S0954579408000369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchaine TP, Thayer JF. Heart rate variability as a transdiagnostic biomarker of psychopathology. International Journal of Psychophysiology. 2015;98:338–350. doi: 10.1016/j.ijpsycho.2015.08.004. [DOI] [PubMed] [Google Scholar]
- Beauchaine TP, Zisner A. Motivation, emotion regulation, and the latent structure of psychopathology: An integrative and convergent historical perspective. International Journal of Psychophysiology. 2017;119:108–118. doi: 10.1016/j.ijpsycho.2016. [DOI] [PubMed] [Google Scholar]
- Bentley KH, Nock MK, Barlow DH. The four-function model of nonsuicidal self-injury: Key directions for future research. Clinical Psychological Science. 2014;2:638–656. doi: 10.1177/2167702613514563. [DOI] [Google Scholar]
- Bodfish JW, Lewis MH. Self-injury and comorbid behaviors in developmental, neurological, psychiatric, and genetic disorders. In: Schroeder SR, Oster-Granite ML, Thompson T, editors. Self-injurious behavior: Gene-brain-behavior relationships. Washington, DC: American Psychological Association; 2002. pp. 23–39. [DOI] [Google Scholar]
- Brain Development Cooperative Group. Total and regional brain volumes in a population-based normative sample from 4 to 18 years: The NIH MRI Study of Normal Brain Development. Cerebral Cortex. 2012;22:1–12. doi: 10.1093/cercor/bhr018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brent D. Nonsuicidal self-injury as a predictor of suicidal behavior in depressed adolescents. American Journal of Psychiatry. 2011;168:452–454. doi: 10.1176/appi.ajp.2011.11020215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner R, Henze R, Parzer P, Kramer J, Feigl N, Lutz K, Stieltjes B. Reduced prefrontal and orbitofrontal gray matter in female adolescents with borderline personality disorder: Is it disorder specific? NeuroImage. 2010;49:114–120. doi: 10.1016/j.neuroimage.2009.07.070. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Caudle K. The teenage brain: Self-control. Current Directions in Psychological Science. 2013;22:82–87. doi: 10.1177/0963721413480170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain SR, Sahakian BJ. The neuropsychiatry of impulsivity. Current Opinion in Psychiatry. 2007;20:255–261. doi: 10.1097/YCO.0b013e3280ba4989. [DOI] [PubMed] [Google Scholar]
- Crowell SE, Beauchaine TP, Linehan MM. A biosocial developmental model of borderline personality: Elaborating and extending Linehan’s theory. Psychological Bulletin. 2009;135:495–510. doi: 10.1037/a0015616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowell SE, Butner J, Wiltshire TJ, Munion AK, Yaptangco M, Beauchaine TP. Evaluating emotional and biological sensitivity to maternal behavior among depressed and self-injuring adolescent girls using nonlinear dynamics. Clinical Psychological Science. 2017;5:272–285. doi: 10.1177/2167702617692861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowell SE, Beauchaine TP, McCauley E, Smith C, Stevens AL, Sylvers P. Psychological, autonomic, and serotonergic correlates of parasuicidal behavior in adolescent girls. Development and Psychopathology. 2005;17:1105–1127. doi: 10.1017/s0954579405050522. doi:10.10170S0954579405050522. [DOI] [PubMed] [Google Scholar]
- De Brito SA, Mechelli A, Wilke M, Laurens KR, Jones AP, Barker GJ, … Viding E. Size matters: Increased grey matter in boys with conduct problems and callous-unemotional traits. Brain. 2009;132:843–852. doi: 10.1093/brain/awp011. [DOI] [PubMed] [Google Scholar]
- Depue BE, Burgess GC, Bidwell LC, Willcutt EG, Banich MT. Behavioral performance predicts grey matter reductions in the right inferior frontal gyrus in young adults with combined type ADHD. Psychiatry Research: Neuroimaging. 2010;182:231–237. doi: 10.1016/j.pscychresns.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derbidge C, Beauchaine TP. A developmental model of self-inflicted injury, borderline personality, and suicide risk. In: Lewis M, Rudolph K, editors. Handbook of developmental psychopathology. 3. New York: Springer; 2014. pp. 521–542. [DOI] [Google Scholar]
- Domsalla M, Koppe G, Niedtfeld I, Vollstädt-Klein S, Schmahl C, Bohus M, Lis S. Cerebral processing of social rejection in patients with borderline personality disorder. Social Cognitive and Affective Neuroscience. 2014;9:1789–1797. doi: 10.1093/scan/nst176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairchild G, Hagan CC, Walsh ND, Passamonti L, Calder AJ, Goodyer IM. Brain structure abnormalities in adolescent girls with conduct disorder. Journal of Child Psychology and Psychiatry. 2013;54:86–95. doi: 10.1111/j.1469-7610.2012.02617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairchild G, Passamonti L, Hurford G, Hagan C, von dem Hagen E, van Goozen S, … Calder A. Brain structure abnormalities in early-onset and adolescent-onset conduct disorder. American Journal of Psychiatry. 2011;168:624–633. doi: 10.1176/appi.ajp.2010.10081184. [DOI] [PubMed] [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JB, Benjamin LS. User’s guide for the Structured Clinical Interview for DSM-IV Axis II personality disorders. Washington DC: American Psychiatric Press; 1997. [Google Scholar]
- Gadow KD, Sprafkin J, Carlson G, Schneider J, Nolan EE, Mattison RE, Rundberg-Rivera A DSM-IV-referenced adolescent self-report rating scale. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41:671–679. doi: 10.1097/00004583-200206000-00006. [DOI] [PubMed] [Google Scholar]
- Giuliani NR, Drabant EM, Bhatnagar R, Gross JJ. Emotion regulation and brain plasticity: Expressive suppression use predicts anterior insula volume. Neuroimage. 2011;58:10–15. doi: 10.1016/j.neuroimage.2011.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliani NR, Drabant EM, Gross JJ. Anterior cingulate cortex volume and emotion regulation: Is bigger better? Biological Psychology. 2011;86:379–382. doi: 10.1016/j.biopsycho.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Thompson PM. Mapping gray matter development: implications for typical development and vulnerability to psychopathology. Brain and Cognition. 2010;72:6–15. doi: 10.1016/j.bandc.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin PR, McRae K, Ramel W, Gross JJ. The neural bases of emotion regulation: Reappraisal and suppression of negative emotion. Biological Psychiatry. 2008;63:577–586. doi: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratz KL, Roemer L. Multidimensional assessment of emotion regulation and dysregulation: Development, factor structure, and initial validation of the Difficulties in Emotion Regulation Scale. Journal of Psychopathology and Behavioral Assessment. 2004;26:41–54. doi: 10.1023/B:JOBA.0000007455.08539.94. [DOI] [Google Scholar]
- Grecucci A, Giorgetta C, Bonini N, Sanfey AG. Reappraising social emotions: The role of inferior frontal gyrus, temporo-parietal junction and insula in interpersonal emotion regulation. Frontiers in Human Neuroscience. 2013;7:523. doi: 10.3389/fnhum.2013.00523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guendelman M, Owens EB, Galan C, Gard A, Hinshaw SP. Early adult correlates of maltreatment in girls with ADHD: Increased risk for internalizing problems and suicidality. Development and Psychopathology. 2016;28:1–14. doi: 10.1017/S0954579414001485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hair NL, Hanson JL, Wolfe BL, Pollak SD. Association of child poverty, brain development, and academic achievement. JAMA Pediatrics. 2015;169:822–829. doi: 10.1001/jamapediatrics.2015.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haw C, Hawton K, Houston K, Townsend E. Psychiatric and personality disorders in deliberate self-harm patients. British Journal of Psychiatry. 2001;178:48–54. doi: 10.1192/bjp.178.1.48. [DOI] [PubMed] [Google Scholar]
- Hayasaka S, Phan KL, Liberzon I, Worsley KJ, Nichols TE. Nonstationary cluster-size inference with random field and permutation methods. Neuroimage. 2004;22:676–687. doi: 10.1016/j.neuroimage.2004.01.041. doi:0.1016/j.neuroimage.2004.01.041. [DOI] [PubMed] [Google Scholar]
- Hazlett EA, Zhang J, New AS, Zelmanova Y, Goldstein KE, Haznedar MM, … Chu K-W. Potentiated amygdala response to repeated emotional pictures in borderline personality disorder. Biological Psychiatry. 2012;72:448–456. doi: 10.1016/j.biopsych.2012.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath NL, Baxter AL, Toste JR, McLouth R. Adolescents’ willingness to access school-based support for nonsuicidal self-injury. Canadian Journal of School Psychology. 2010;25:260–276. doi: 10.1177/0829573510377979. [DOI] [Google Scholar]
- Herringa R, Phillips M, Almeida J, Insana S, Germain A. Post-traumatic stress symptoms correlate with smaller subgenual cingulate, caudate, and insula volumes in unmedicated combat veterans. Psychiatry Research Neuroimaging. 2012;203:139–145. doi: 10.1016/j.pscychresns.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilt LM, Cha CB, Nolen-Hoeksema S. Nonsuicidal self-injury in young adolescent girls: Moderators of the distress-function relationship. Journal of Consulting and Clinical Psychology. 2008;76:63–71. doi: 10.1037/0022-006X.76.1.63. [DOI] [PubMed] [Google Scholar]
- Hinshaw SP, Owens EB, Zalecki C, Huggins SP, Montenegro-Nevado AJ, Schrodek E, Swanson EN. Prospective follow-up of girls with ADHD into early adulthood: Continuing impairment includes elevated risk for suicide attempts and self-injury. Journal of Consulting and Clinical Psychology. 2012;80:1041–1051. doi: 10.1037/a0029451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton C, Draganski B, Ashburner J, Weiskopf N. A comparison between voxel-based cortical thickness and voxel-based morphometry in normal aging. Neuroimage. 2009;48:371–380. doi: 10.1016/j.neuroimage.2009.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang JP, Lee TW, Tsai SJ, Chen TJ, Yang CH, Lirng JF, Tsai CF. Cortical and subcortical abnormalities in late-onset depression with history of suicide attempts investigated with MRI and voxel-based morphometry. Journal of Geriatric Psychiatry and Neurology. 2010;23:171–184. doi: 10.1177/0891988710363713. [DOI] [PubMed] [Google Scholar]
- Jacobson CM, Gould M. The epidemiology and phenomenology of non-suicidal self-injurious behavior among adolescents: A critical review of the literature. Archives of Suicide Research. 2007;11:129–147. doi: 10.1080/13811110701247602. [DOI] [PubMed] [Google Scholar]
- Joiner TE, Brown JS, Wingate LR. The psychology and neurobiology of suicidal behavior. Annual Review of Psychology. 2005;56:287–314. doi: 10.1146/annurev.psych.56.091103.070320. [DOI] [PubMed] [Google Scholar]
- Joiner TE, Jr, Van Orden KA, Witte TK, Selby EA, Ribeiro JD, Lewis R, Rudd MD. Main predictions of the interpersonal–psychological theory of suicidal behavior: Empirical tests in two samples of young adults. Journal of Abnormal Psychology. 2009;118:634–646. doi: 10.1037/a0016500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman AS, Kaufman NL. Kaufman Brief Intelligence Test. 2. Circle Pines, MN: AGS Publishing; 2004. [Google Scholar]
- Kaufman EA, Crowell SE, Lenzenweger MF. The development of borderline personality and self-inflicted injury. In: Beauchaine TP, Hinshaw SP, editors. Child and adolescent psychopathology. 3. Hoboken, NJ: Wiley; 2017. pp. 642–679. [Google Scholar]
- Kaufman EA, Crowell SE, Stepp SD. Self-injury, borderline personality development, and the externalizing spectrum. In: Beauchaine TP, Hinshaw SP, editors. The Oxford handbook of externalizing spectrum disorders. New York, NY: Oxford University Press; 2017. pp. 61–78. [DOI] [Google Scholar]
- King-Casas B, Sharp C, Lomax-Bream L, Lohrenz T, Fonagy P, Montague R. The rupture and repair of cooperation in borderline personality disorder. Science. 2008;321:806–810. doi: 10.1126/science.1156902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klonsky ED. The functions of deliberate self-injury: A review of the evidence. Clinical Psychology Review. 2007;27:226–239. doi: 10.1016/j.cpr.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Klonsky ED. The functions of self-injury in young adults who cut themselves: Clarifying the evidence for affect-regulation. Psychiatry Research. 2009;166:260–268. doi: 10.1016/j.psychres.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klonsky ED, May AM, Glenn CR. The relationship between nonsuicidal self-injury and attempted suicide: Converging evidence from four samples. Journal of Abnormal Psychology. 2013;122:231–237. doi: 10.1037/a0030278. [DOI] [PubMed] [Google Scholar]
- Klonsky ED, Oltmanns TF, Turkheimer E. Deliberate self-harm in a nonclinical population: prevalence and psychological correlates. American Journal of Psychiatry. 2003;160:1501–1508. doi: 10.1176/appi.ajp.160.8.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klonsky ED, Victor SE, Saffer BY. Nonsuicidal self-injury: What we know, and what we need to know. Canadian Journal of Psychiatry. 2014;59:565–568. doi: 10.1177/070674371405901101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn N, Eickhoff SB, Scheller M, Laird AR, Fox PT, Habel U. Neural network of cognitive emotion regulation—An ALE meta-analysis and MACM analysis. NeuroImage. 2014;87:345–355. doi: 10.1016/j.neuroimage.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korfine L, Hooley JM. Directed forgetting of emotional stimuli in borderline personality disorder. Journal of Abnormal Psychology. 2000;109:214–221. doi: 10.1037/0021-843X.109.2.214. [DOI] [PubMed] [Google Scholar]
- Krause-Utz A, Winter D, Niedtfeld I, Schmahl C. The latest neuroimaging findings in borderline personality disorder. Current Psychiatry Reports. 2014;16:438. doi: 10.1007/s11920-014-0438-z. [DOI] [PubMed] [Google Scholar]
- Kring AM, Sloan DM. Emotion regulation and psychopathology: A transdiagnostic approach to etiology and treatment. New York, NY: Guilford; 2009. [Google Scholar]
- Linehan M. Cognitive-behavioral treatment of borderline personality disorder. New York: Guilford Press; 1993. [Google Scholar]
- Linehan MM, Comtois K. Unpublished manuscript. University of Washington; Seattle: 1996. Lifetime parasuicide history. [Google Scholar]
- Lis E, Greenfield B, Henry M, Guilé JM, Dougherty G. Neuroimaging and genetics of borderline personality disorder: A review. Journal of Psychiatry and Neuroscience. 2007;32:162–173. [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Richardson EE, Perrine N, Dierker L, Kelley ML. Characteristics and functions of non-suicidal self-injury in a community sample of adolescents. Psychological Medicine. 2007;37:1183–1192. doi: 10.1017/S003329170700027X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhi GS, Tanious M, Fritz K, Coulston CM, Bargh DM, Phan KL, … Das P. Differential engagement of the fronto-limbic network during emotion processing distinguishes bipolar and borderline personality disorder. Molecular Psychiatry. 2013;18:1247–1248. doi: 10.1038/mp.2013.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morandotti N, Dima D, Jogia J, Frangou S, Sala M, De Vidovich GZ, … Brambilla P. Childhood abuse is associated with structural impairment in the ventrolateral prefrontal cortex and aggressiveness in patients with borderline personality disorder. Psychiatry Research Neuroimaging. 2013;213:18–23. doi: 10.1016/j.pscychresns.2013.02.002. [DOI] [PubMed] [Google Scholar]
- Niedtfeld I, Kirsch P, Schulze L, Herpertz SC, Bohus M, Schmahl C. Functional connectivity of pain-mediated affect regulation in borderline personality disorder. PlosOne. 2012;7:e33293. doi: 10.1371/journal.pone.0033293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nock MK, Green JG, Hwang I, McLaughlin KA, Sampson NA, Zaslavsky AM, Kessler RC. Prevalence, correlates, and treatment of lifetime suicidal behavior among adolescents: Results from the National Comorbidity Survey Replication Adolescent Supplement. JAMA Psychiatry. 2013;70:300–310. doi: 10.1001/2013.jamapsychiatry.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nock MK, Joiner TE, Gordon KH, Lloyd-Richardson E, Prinstein MJ. Non-suicidal self-injury among adolescents: Diagnostic correlates and relation to suicide attempts. Psychiatry Research. 2006;144:65–72. doi: 10.1016/j.psychres.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Neumann A, van Lier PC, Gratz KL, Koot HM. Multidimensional assessment of emotion regulation difficulties in adolescents using the Difficulties in Emotion Regulation Scale. Assessment. 2010;17:138–149. doi: 10.1177/1073191109349579. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JDE, Gross JJ. For better or for worse: Neural systems supporting the cognitive down- and up-regulation of negative emotion. NeuroImage. 2004;23:483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Reynolds WM. Suicidal Ideation Questionnaire—Junior. Odessa, FL: Psychological Assessment Resources; 1987. [Google Scholar]
- Reynolds WM. Suicidal Ideation Questionnaire: Professional Manual. Odessa, FL: Psychological Assessment Resources; 1988. [Google Scholar]
- Rossi R, Pievani M, Lorenzi M, Boccardi M, Beneduce M, Bignotti S, … Frisoni G. Structural brain features of borderline personality and bipolar disorders. Psychiatry Research Neuroimaging. 2013;213:83–91. doi: 10.1016/j.pscychresns.2012.07.002. [DOI] [PubMed] [Google Scholar]
- Rosso IM, Makris N, Britton JC, Price LM, Gold AL, Zai D, … Rauch SL. Anxiety sensitivity correlates with two indices of right anterior insula structure in specific animal phobia. Depression and Anxiety. 2010;27:1104–1110. doi: 10.1002/da.20765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruocco AC, Amirthavasagam S, Choi-Kain LW, McMain SF. Neural correlates of negative emotionality in borderline personality disorder: An activation-likelihood-estimation meta-analysis. Biological Psychiatry. 2013;73:153–160. doi: 10.1016/j.biopsych.2012.07.014. [DOI] [PubMed] [Google Scholar]
- Sauder CL, Derbidge CM, Beauchaine TP. Neural responses to monetary incentives among self-injuring adolescent girls. Development and Psychopathology. 2016;28:277–291. doi: 10.1017/S0954579415000449. [DOI] [PubMed] [Google Scholar]
- Shaffer D, Fisher P, Lucas CP, Mina K, Schwab-Stone ME. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): Description, differences from previous versions, and reliability of some common diagnoses. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39:28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- Shaw P, Kabani NJ, Lerch JP, Eckstrand K, Lenroot R, Gogtay N, … Giedd JN. Neurodevelopmental trajectories of the human cerebral cortex. Journal of Neuroscience. 2008;28:3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Malek M, Watson B, Greenstein D, de Rossi P, Sharp W. Biological Psychiatry. 2013;74:599–606. doi: 10.1016/j.biopsych.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder HR, Hankin BL, Sandman CA, Head K, Davis EP. Distinct patterns of reduced prefrontal and limbic gray matter volume in childhood general and internalizing psychopathology. Clinical Psychological Science. 2017 doi: 10.1177/2167702617714563. epublished ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soloff P, Nutche J, Goradia D, Diwadkar V. Structural brain abnormalities in borderline personality disorder: a voxel-based morphometry study. Psychiatry Research: Neuroimaging. 2008;164:223–236. doi: 10.1016/j.pscychresns.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soloff PH, Pruitt P, Sharma M, Radwan J, White R, Diwadkar VA. Structural brain abnormalities and suicidal behavior in borderline personality disorder. Journal of Psychiatry Research. 2012;46:516–525. doi: 10.1016/j.jpsychires.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somer O, Bildik T, Kabukçu-Başay B, Güngör D, Başay Ö, Farmer RF. Prevalence of non-suicidal self-injury and distinct groups of self-injurers in a community sample of adolescents. Social Psychiatry and Psychiatric Epidemiology. 2015;50:1163–1171. doi: 10.1007/s00127-015-1060-z. [DOI] [PubMed] [Google Scholar]
- Sterzer P, Stadler C, Poustka F, Kleinschmidt A. A structural neural deficit in adolescents with conduct disorder and its association with lack of empathy. Neuroimage. 2007;37:335–342. doi: 10.1016/j.neuroimage.2007.04.043. [DOI] [PubMed] [Google Scholar]
- Swannell SV, Martin GE, Page A, Hasking P, St John NJ. Prevalence of nonsuicidal self-injury in nonclinical samples: Systematic review, meta-analysis and meta-regression. Suicide and Life-Threatening Behavior. 2014;44:273–303. doi: 10.1111/sltb.12070. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Chanen AM, Wood SJ, Yücel M, Tanino R, Suzuki M, … McGorry PD. Insular cortex volume and impulsivity in teenagers with first-presentation borderline personality disorder. Progress in Neuropsychopharmacology and Biological Psychiatry. 2009;33:1395–1400. doi: 10.1016/j.pnpbp.2009.07.017. [DOI] [PubMed] [Google Scholar]
- Tone EB, Garn CL, Pine DS. Anxiety regulation: A developmental psychopathology perspective. In: Cicchetti D, editor. Developmental psychopathology, Vol. 2: Developmental neuroscience. 3. Hoboken, NJ: Wiley; 2016. pp. 523–556. [DOI] [Google Scholar]
- Turner BJ, Chapman AL, Layden BK. Intrapersonal and interpersonal functions of non-suicidal self-injury: Associations with emotional and social functioning. Suicide and Life Threatening Behavior. 2012;42:36–55. doi: 10.1111/j.1943-278X.2011.00069.x. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, … Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Uchida RR, Del-Ben CM, Busatto GF, Duran FL, Guimarães FS, Crippa JA, … Graeff FG. Regional gray matter abnormalities in panic disorder: a voxel-based morphometry study. Psychiatry Research: Neuroimaging. 2008;163:21–29. doi: 10.1016/j.pscychresns.2007.04.015. [DOI] [PubMed] [Google Scholar]
- Urgesi C, Mattiassi ADA, Buiatti T, Marini A. Tell it to a child! A brain stimulation study of the role of left inferior frontal gyrus in emotion regulation during storytelling. NeuroImage. 2016;136:26–36. doi: 10.1016/j.neuroimage.2016.05.039. [DOI] [PubMed] [Google Scholar]
- Wilkinson P. Non-suicidal self-injury. European Child and Adolescent Psychiatry. 2013;22:75–79. doi: 10.1007/s00787-012-0365-7. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Evans AC, Marrett S, Neelin P. A three-dimensional statistical analysis for CBF activation studies in human brain. Journal of Cerebral Blood Flow and Metabolism. 1992;12:900–918. doi: 10.1038/jcbfm.1992.127. [DOI] [PubMed] [Google Scholar]
- Zilverstand A, Parvaz MA, Goldstein RZ. Neuroimaging cognitive reappraisal in clinical populations to define neural targets for enhancing emotion regulation. A systematic review. NeuroImage. 2017;151:105–116. doi: 10.1016/j.neuroimage.2016.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]