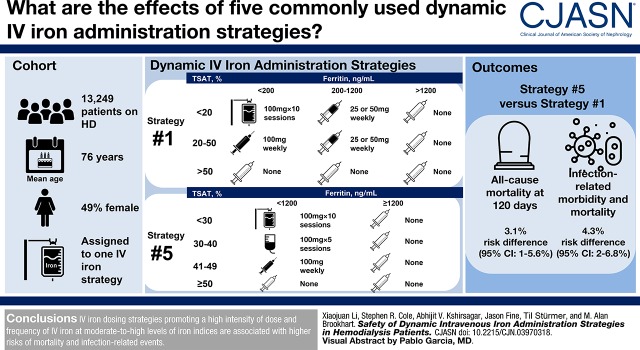
Visual Abstract
Keywords: intravenous iron administration strategies; comparative safety; infections; mortality; hemodialysis patients; anemia management; endstage kidney disease; dialysis; Iron; Confidence Intervals; Kidney Failure, Chronic; Proportional Hazards Models; Administration, Intravenous; anemia; Medicare
Abstract
Background and objectives
Intravenous iron therapy for chronic anemia management is largely driven by dosing protocols that differ in intensity with respect to dosing approach (i.e., dose, frequency, and duration). Little is known about the safety of these protocols.
Design, setting, participants, & measurements
Using clinical data from a large United States dialysis provider linked to health care utilization data from Medicare, we constructed a cohort of patients with ESKD aged ≥65 years who initiated and continued center-based hemodialysis for ≥90 days between 2009 and 2012, and initiated at least one of the five common intravenous iron administration strategies; ranked by intensity (the amount of iron given at moderate-to-high iron indices), the order of strategies was 3 (least intensive), 2 (less intensive), 1 (reference), 4 (more intensive), and 5 (most intensive). We estimated the effect of continuous exposure to these strategies on cumulative risks of mortality and infection-related events with dynamic Cox marginal structural models.
Results
Of 13,249 eligible patients, 1320 (10%) died and 1627 (12%) had one or more infection-related events during the 4-month follow-up. The most and least commonly initiated strategy was strategy 2 and 5, respectively. Compared with the reference strategy 1, more intensive strategies (4 and 5) demonstrated a higher risk of all-cause mortality (e.g., most intensive strategy 5: 60-day risk difference: 1.3%; 95% confidence interval [95% CI], 0.8% to 2.1%; 120-day risk difference: 3.1%; 95% CI, 1.0% to 5.6%). Similarly, higher risks were observed for infection-related morbidity and mortality among more intensive strategies (e.g., strategy 5: 60-day risk difference: 1.8%; 95% CI, 1.2% to 2.6%; 120-day risk difference: 4.3%; 95% CI, 2.2% to 6.8%). Less intensive strategies (2 and 3) demonstrated lower risks of all-cause mortality and infection-related events.
Conclusions
Among dialysis patients surviving 90 days, subsequent intravenous iron administration strategies promoting more intensive iron treatment at moderate-to-high iron indices levels are associated with higher risks of mortality and infection-related events.
Introduction
Intravenous (IV) iron is either provided via large doses over consecutive hemodialysis sessions (often termed “bolus dosing”) or via small doses provided every 1–2 weeks (often termed “maintenance dosing”) for anemia management in contemporary clinical practice. Decisions about the exact sequence of iron administration are typically guided by iron indices levels—serum ferritin and transferrin saturation (TSAT)—and hemoglobin (1,2). On the basis of these tests, providers make recommendations about the dosing approach (i.e., bolus dosing, maintenance dosing, or another variation) for the next treatment course.
Currently, dialysis clinics rely on dosing protocols that prescribe dosing approaches for IV iron administration. These protocols factor in a patient’s iron indices levels and evolving clinical characteristics to provide treatment recommendations with the primary goal of achieving a target hemoglobin level while not exceeding the upper limits of ferritin and TSAT. Consequently, the treatment dose, frequency, and duration (dosing approach) are repeatedly adjusted when updated iron indices and clinical characteristics become available. These dosing protocols are known as dynamic administration strategies (3–5).
Surprising variation exists in protocols used in clinical practice, perhaps reflective of the lack of consensus among expert guidelines (1,2,6–9). These protocols differ in intensity with respect to target levels of iron indices and dosing approach recommendations (10–12). For example, one protocol may specify 100 mg of iron administered over ten consecutive dialysis sessions for TSAT<30% and ferritin <1200 ng/ml, whereas another protocol would hold iron for any ferritin >500 ng/ml regardless of TSAT level.
Although previous studies have examined the safety of IV iron, none have formally assessed dosing protocols (13,14). Existing observational studies (15–17) have largely focused on the effect of cumulative iron exposure over a long period that may oversimplify patients’ heterogenous IV iron treatment trajectories (18). Cumulative iron exposures may also not align well with the treatment decisions that providers make regarding iron administration in clinical practice (19). Randomized, clinical trials assessing multiple dosing protocols are lacking. As such, the risks of infectious complications and mortality are unclear given conflicting conclusions of existing studies (12–17,20,21) and the insufficiency of the current literature on safety of dosing protocols.
Given the high prevalence of IV iron (22), we conducted a study to examine the effect of continues treatment with five commonly used dynamic IV iron administration strategies on risks of all-cause mortality and infection-related events in a contemporary cohort of patients on hemodialysis.
Materials and Methods
Data Sources
We constructed a large hemodialysis patient cohort using deidentified datasets derived from the electronic health records of a large dialysis organization in the United States, linked with the US Renal Data System (USRDS). We obtained detailed clinical information regarding patients’ dialysis treatments, vascular access, laboratory test data, IV medications, and anemia management using the clinical database from the dialysis organization. We obtained information regarding their demographics, comorbidities, health care system encounters, and outcomes from the USRDS. The study was approved by the Institutional Review Board at University of North Carolina at Chapel Hill (approval no. 15–1991).
Study Design and Study Population
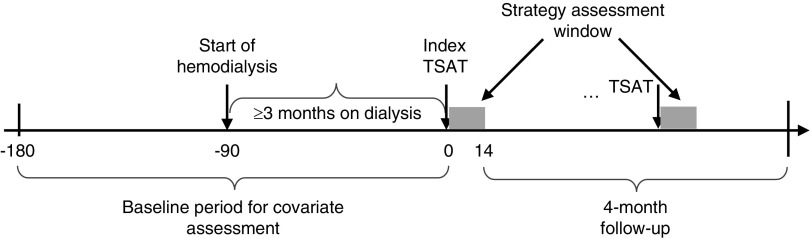
Detailed methods for cohort construction have been described elsewhere (23). Briefly, we used a retrospective cohort design with the index date for IV iron administration strategy defined as the day of the first TSAT test result within 90–136 days after dialysis initiation to ensure patients were receiving chronic anemia management. We anchored the index date on a TSAT measurement because in clinical practice: (1) decisions about subsequent IV iron dosing approach typically occur upon the availability of iron indices tests; (2) TSAT is updated slightly more frequently than ferritin (approximately monthly versus quarterly); and (3) when no current ferritin test is available, the last available ferritin is used with the updated TSAT to make treatment recommendations. The TSAT measurement on the index date was defined as the index TSAT. We used the 14-day window after the index TSAT to assess the index IV iron administration strategy in the index treatment interval anchored by the index TSAT and its subsequent TSAT. We defined the baseline period as the period starting 90 days before dialysis initiation and ending on the day before the index date. Eligible patients were followed for outcomes of interest in a 4-month follow-up period, starting on day 15 (the day after the index strategy assessment window) (Figure 1).
Figure 1.
Study design for assessing the effect of initiating and staying on a particular dynamic IV iron administration strategy. Patients are followed starting on the end of the first 14-day iron strategy assessment window (the gray block) for all-cause mortality or infection-related events. Those deviated from their index strategy are censored at the end of current assessment window.
Our study population comprised outpatients who initiated in-center hemodialysis between January 1, 2009 and September 16, 2012 and survived 90 days after initiation (Supplemental Figure 1). We excluded patients who (1) were aged <65 years at initiation (to ensure the collection of their comprehensive clinical history for confounding control), (2) did not have Medicare as primary insurer, (3) did not continue hemodialysis for ≥90 days, (4) had incomplete baseline covariates information (24), or (5) had fewer than nine dialysis sessions in the month preceding the index date to ensure they were receiving regular hemodialysis and anemia management. We also excluded patients with polycystic kidney disease because their iron administration strategies could differ because of their heterogeneous need for erythropoiesis stimulating agents.
IV Iron Administration Strategies
We considered five dynamic IV iron administration strategies that were adapted from existing protocols used by several dialysis organizations in contemporary routine practice. Each strategy consisted of a set of decision rules that specified a range of acceptable iron therapy values during a treatment course given a patient’s current iron indices (Tables 1 and 2). Ranked by intensity of iron treatment, the amount of iron given at moderate-to-high iron indices, the order of strategies was 3 (least intensive), 2 (less intensive), 1 (reference), 4 (more intensive), and 5 (most intensive).
Table 1.
Definitions of dynamic intravenous iron administration strategies
| Strategy Definitions | TSAT, % | Ferritin, ng/ml | ||
|---|---|---|---|---|
| Strategy 1 | ||||
| <200 | 200–1200 | >1200 | ||
| <20 | Bolus | Low maintenance | None | |
| 20–50 | Maintenance | Low maintenance | None | |
| >50 | None | None | None | |
| Strategy 2 | ||||
| <200 | 200–800 | >800 | ||
| <20 | Bolus | Low maintenance | None | |
| 20–50 | Maintenance | Low maintenance | None | |
| >50 | None | None | None | |
| Strategy 3 | ||||
| <200 | 200–500 | >500 | ||
| <20 | Bolus | Low maintenance | None | |
| 20–50 | Maintenance | Low maintenance | None | |
| >50 | None | None | None | |
| Strategy 4 | ||||
| <800 | 800–1200 | >1200 | ||
| <30 | Bolus | Half bolus | None | |
| 30–50 | Low maintenance | Low maintenance | None | |
| >50 | None | None | None | |
| Strategy 5 | ||||
| <1200 | ≥1200 | |||
| <30 | Bolus | None | ||
| 30–40 | Half bolus | None | ||
| 41–49 | Maintenance | None | ||
| ≥50 | None | None | ||
Table 2.
Dosing approach definition
| Dosing Approach | Iron Dosage Level | |
|---|---|---|
| 2-wk | Monthly Equivalencea | |
| Bolus | >500 | 100 mg×ten consecutive sessions |
| Half bolus | 201–500 | 100 mg×five consecutive sessions |
| Maintenance | 101–200 | 100 mg weekly |
| Low maintenance | 1–100 | 25 or 50 mg weekly |
| None | 0 | 0 mg |
The dosing approach presented was on the basis of iron sucrose. For other intravenous iron formulations, their monthly equivalence was used. For example, for the bolus dosing approach, the monthly iron dosage level was 1000 mg in one dialysis session with iron dextran, and 125 mg over eight consecutive dialysis sessions with ferric gluconate.
We identified the IV iron administration strategies initiated by eligible patients in the index treatment interval by matching a patient’s treatment pattern in the 14-day assessment window and current iron indices values with candidate strategies by concordance (23). The length of assessment window was chosen to maximize the representativeness of treatment experience in the assessment window for the treatment experience during the entire treatment course as well as minimizing the days required for assessment to maximize follow-up time for outcomes (Supplemental Figure 2). Patients were excluded from the main analyses if their treatment patterns in the assessment window were incompatible with all candidate strategies.
Effect Measure of Interest
We estimated the 120-day cumulative risks of all-cause mortality and infection-related events under continuous treatment with each IV iron administration strategy. We focused on this per-protocol effect of these administration strategies—the effect that would have been observed if patients had adhered to their assigned strategy throughout the 120-day follow-up—because the typical intention-to-treat effect may be suboptimal for assessment of comparative safety, particularly in the presence of nonadherence to the strategy (25,26).
Outcomes
Two safety outcomes examined were all-cause mortality and a composite outcome of infection-related hospitalization (sepsis, vascular access infection, or pneumonia) or death in the 4 months after initiation of IV iron administration strategy. These events were identified using claims-based definitions (Supplemental Table 1).
Patients were censored by death attributed to reasons other than infection (for the infection-related events outcome), receipt of kidney transplantation, time of switching modality, loss to follow-up, disenrollment from the dialysis provider, loss of Medicare coverage, or the administrative end of follow-up (December 31, 2012). For both analyses, patients were also censored by deviation from index strategy when they received treatment in a way inconsistent with their index strategy (Supplemental Material, Supplemental Figure 3).
Covariates
Covariates (defined in Supplemental Table 2) in the analyses included demographic characteristics (e.g., age, sex, race, year of strategy initiation), clinical characteristics (e.g., cause of ESKD, body mass index), baseline anemia treatment history (IV iron and epoetin dose), facility-related factors (geographical region of dialysis clinic, vascular access type), parameters reflective of anemia management (e.g., TSAT, ferritin, epoetin dose, hemoglobin, receipt of blood transfusion), parameters reflective of inflammation (albumin, creatinine, systolic BP, postdialysis body weight), health care system encounters (e.g., number of dialysis sessions, days of hospitalization), and a list of comorbidities.
Statistical Analyses
We compared IV iron administration strategies with respect to risks of all-cause mortality and infection-related events using inverse probability weighted estimation of Cox marginal structural models (27,28). We chose strategy 1 as the reference because of its frequent use and ranked intensity among the five strategies (Table 1). Standardized mortality ratio weighting (29) was used to adjust for potential baseline confounding. As a multivariable standardization method, this weighting method uses the treated study participants (i.e., the patients who initiated strategy 1 in this analysis) as the standard population and estimates the treatment effect in a population whose distribution of risk factors is equal to that of the treated study participants. Inverse probability of censoring weighting (30) was used to adjust for potential selection bias introduced by censoring patients who deviated from index strategies in the follow-up. The model for censoring weights included time-dependent factors for both outcomes and strategy deviation (including length of hospital stay, total epoetin doses, number of dialysis sessions, vascular access type, and current iron indices in the treatment course before deviation), and time-independent factors (including sex, cause of ESKD, and comorbidities). We estimated cumulative risk differences (RDs) for both mortality and infection-related outcomes comparing each strategy with the referent strategy 1 during follow-up, and their 95% confidence intervals (95% CIs) using a nonparametric bootstrap procedure with 200 repetitions (31).
We conducted sensitivity analyses using different covariates for censoring weights estimation and various definitions for strategy deviation. We also conducted additional analyses to estimate different effects of strategy exposure. These analyses were described more completely in Supplemental Material. All statistical analyses were performed using R 3.3.1 (R Foundation for Statistical Computing, Vienna, Austria) and SAS 9.4 (SAS Institute Inc., Cary, NC).
Results
Between 2009 and 2012, 18,697 patients met our study entry criteria (Supplemental Figure 1), and 15,518 (83%) patients were matched with at least one IV iron administration strategy under consideration. Among them, 13,249 (71%) patients initiated one of the five most commonly used strategies and were included in the analyses. At strategy initiation, the average age was 76 years. About half of the cohort were women, and 23% were black. The most and least commonly initiated strategy was strategy 2 and 5, respectively. More intensive strategies 4 and 5 recommended more aggressive iron therapy in broader ranges of TSAT and ferritin. For example, strategy 5 recommended bolus dosing if TSAT<30% and ferritin <1200 ng/ml, half bolus dosing if TSAT was between 30% and 40%, and maintenance dosing if TSAT was between 40% and 50%. Definitions of the strategies and dosing approaches are listed in Tables 1 and 2.
Table 3 presents patients’ baseline characteristics stratified by strategy. Baseline characteristics were similar among initiators of strategies 1, 2, and 3. Compared with these patients, initiators of more intensive strategies (4 and 5) were more likely to initiate in early years and used a catheter. Recent history of infections and comorbidities were more common among the most intensive strategy 5 initiators, who were also more likely to have had a gastrointestinal bleed or received blood transfusion. During the last baseline month, they received higher doses of epoetin and IV iron and spent more days in the hospital. Their index TSAT and ferritin levels were also higher.
Table 3.
Baseline characteristics of patients on hemodialysis by initiated IV iron administration strategy, 2009–2012
| Characteristics | Overall | Strategy 1 | Strategy 2 | Strategy 3 | Strategy 4 | Strategy 5 |
|---|---|---|---|---|---|---|
| N | 13,249 | 10,882 | 11,293 | 10,397 | 8089 | 6305 |
| Age, mean (SD) | 76 (7) | 76 (7) | 76 (7) | 76 (7) | 76 (7) | 76 (7) |
| Women, % | 49 | 49 | 50 | 49 | 49 | 49 |
| Race, % | ||||||
| Black | 23 | 22 | 23 | 22 | 22 | 22 |
| White | 72 | 73 | 72 | 73 | 73 | 73 |
| Other | 5 | 5 | 5 | 5 | 5 | 5 |
| Medicaid, % | 29 | 29 | 29 | 29 | 29 | 30 |
| Lower income subsidy, % | 34 | 34 | 34 | 34 | 35 | 35 |
| Region, % | ||||||
| Midwest | 23 | 23 | 23 | 23 | 23 | 23 |
| Northeast | 14 | 14 | 14 | 14 | 14 | 14 |
| Other | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| South | 42 | 41 | 42 | 42 | 43 | 43 |
| West | 21 | 21 | 21 | 21 | 20 | 20 |
| Cause of ESKD, % | ||||||
| Diabetes | 45 | 45 | 45 | 45 | 45 | 44 |
| GN | 5 | 5 | 5 | 5 | 5 | 5 |
| Hypertension | 36 | 36 | 36 | 36 | 36 | 36 |
| Other | 13 | 14 | 14 | 14 | 14 | 15 |
| Missing | 0.3 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 |
| Index year, % | ||||||
| 2009 | 25 | 23 | 24 | 25 | 27 | 29 |
| 2010 | 25 | 23 | 23 | 25 | 26 | 28 |
| 2011 | 25 | 27 | 27 | 26 | 24 | 22 |
| 2012 | 25 | 27 | 26 | 23 | 23 | 21 |
| Comorbidities, % | ||||||
| Vascular access infectiona | 1 | 1 | 1 | 1 | 2 | 2 |
| Pneumoniaa | 2 | 2 | 2 | 2 | 3 | 3 |
| Sepsisa | 2 | 3 | 2 | 3 | 3 | 4 |
| Infection (ADR definition)a | 4 | 4 | 4 | 4 | 5 | 6 |
| Antibiotic usea | 20 | 21 | 21 | 21 | 24 | 28 |
| IV antibiotics (dialysis center)a | 11 | 12 | 12 | 12 | 14 | 17 |
| Infection (broad definition)a | 24 | 26 | 26 | 26 | 29 | 34 |
| Diabetes | 69 | 70 | 69 | 70 | 69 | 70 |
| Hypertensive disease | 96 | 95 | 95 | 96 | 96 | 97 |
| Congestive heart failure | 62 | 62 | 63 | 63 | 64 | 66 |
| Myocardial infarction, acute | 10 | 10 | 10 | 10 | 10 | 10 |
| Angina | 7 | 7 | 7 | 7 | 7 | 7 |
| Coronary artery disease/atherosclerosis | 56 | 56 | 56 | 57 | 58 | 59 |
| Ischemic stroke | 8 | 8 | 8 | 8 | 8 | 9 |
| Intracerebral hemorrhage | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 |
| Subarachnoid hemorrhage | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Hemorrhagic stroke | 0.7 | 0.7 | 0.7 | 0.7 | 0.8 | 0.8 |
| Cerebrovascular disease | 24 | 24 | 24 | 24 | 24 | 25 |
| Chronic obstructive pulmonary disease and asthma | 33 | 33 | 33 | 34 | 34 | 36 |
| Hyperlipidemia | 61 | 60 | 60 | 61 | 60 | 60 |
| Cancer | 21 | 22 | 22 | 22 | 22 | 23 |
| Liver disease | 4 | 4 | 4 | 4 | 4 | 5 |
| Gastrointestinal bleeding and ulcer | 3 | 3 | 3 | 3 | 3 | 3 |
| Blood transfusion | 36 | 36 | 36 | 37 | 38 | 41 |
| Blood loss anemia | 5 | 5 | 5 | 5 | 6 | 6 |
| Peripheral vascular disease | 26 | 27 | 27 | 27 | 27 | 28 |
| Rheumatic heart disease | 6 | 6 | 6 | 6 | 6 | 7 |
| Psychiatric disorder | 6 | 6 | 6 | 6 | 6 | 7 |
| Substance abuse | 6 | 6 | 6 | 7 | 7 | 7 |
| Autoimmune disorder | 5 | 5 | 5 | 5 | 5 | 5 |
| Other neurologic disorders | 14 | 14 | 14 | 14 | 15 | 16 |
| Hyperparathyroidism | 4 | 4 | 4 | 4 | 4 | 4 |
| Chronic heart disease procedures | 6 | 5 | 6 | 6 | 5 | 6 |
| Rheumatoid arthritis | 3 | 3 | 3 | 3 | 3 | 3 |
| Neuropathy | 21 | 21 | 21 | 21 | 21 | 22 |
| Osteoarthritis | 20 | 20 | 20 | 20 | 20 | 21 |
| Osteoporosis | 5 | 5 | 5 | 5 | 5 | 5 |
| History of fall | 5 | 6 | 5 | 5 | 6 | 6 |
| Last month of baseline period, mean (SD)b | ||||||
| Total EPO dose, 1000 units/mo | 68 (73) | 68 (73) | 68 (73) | 71 (75) | 73 (79) | 83 (83) |
| Total iron dose, mg | 299 (311) | 293 (296) | 288 (299) | 289 (308) | 310 (322) | 334 (343) |
| TSAT, % | 25 (12) | 25 (12) | 25 (13) | 25 (13) | 25 (13) | 25 (14) |
| Ferritin, ng/ml | 490 (435) | 471 (437) | 487 (446) | 484 (463) | 488 (482) | 511 (525) |
| Hemoglobin, g/dl | 11.5 (1.4) | 11.4 (1.4) | 11.5 (1.4) | 11.5 (1.5) | 11.5 (1.5) | 11.4 (1.5) |
| Albumin, g/dl | 3.6 (0.5) | 3.6 (0.5) | 3.6 (0.5) | 3.6 (0.5) | 3.6 (0.5) | 3.5 (0.5) |
| Creatinine, mg/dl | 5.1 (1.9) | 5.1 (1.9) | 5.1 (1.9) | 5.1 (1.9) | 5.1 (1.9) | 5.0 (1.9) |
| Pretreatment systolic BP, mm Hg | 143 (22) | 143 (22) | 143 (22) | 143 (22) | 142 (22) | 142 (22) |
| Post-treatment weight, kg | 74 (18) | 75 (19) | 75 (19) | 75 (19) | 75 (19) | 74 (19) |
| Hospital days | 0.7 (2.0) | 0.8 (2.0) | 0.7 (2.0) | 0.8 (2.1) | 0.9 (2.2) | 1.0 (2.3) |
| No. of transfusions | 0.03 (0.20) | 0.03 (0.22) | 0.03 (0.21) | 0.03 (0.22) | 0.04 (0.25) | 0.05 (0.27) |
| Access, % | ||||||
| Catheter | 63 | 63 | 64 | 65 | 66 | 70 |
| Fistula | 27 | 26 | 26 | 25 | 24 | 21 |
| Graft | 11 | 10 | 10 | 10 | 10 | 9 |
| Index date, mean (SD)c | ||||||
| Index TSAT, % | 28 (14) | 29 (14) | 29 (14) | 29 (15) | 31 (16) | 29 (18) |
| Index ferritin, ng/ml | 546 (464) | 526 (488) | 543 (487) | 536 (506) | 560 (549) | 584 (608) |
| Index hemoglobin, g/dl | 11.6 (1.3) | 11.5 (1.3) | 11.5 (1.3) | 11.6 (1.3) | 11.6 (1.4) | 11.5 (1.4) |
| Index albumin, g/dl | 3.6 (0.5) | 3.6 (0.5) | 3.6 (0.5) | 3.6 (0.5) | 3.6 (0.5) | 3.5 (0.5) |
| Index creatine, mg/dl | 5.2 (2.0) | 5.2 (2.0) | 5.2 (2.0) | 5.2 (2.0) | 5.2 (2.0) | 5.2 (2.0) |
IV, intravenous; ADR, annual data report; EPO, epoetin; TSAT, transferrin saturation.
Prevalence during the last month of baseline period.
If a laboratory test in the last month of baseline was missing, the previous test value was used.
If a laboratory test was missing on the index date, the last nonmissing test value was used.
During follow-up, patients deviated from their index strategy quickly, especially among initiators of more intensive strategies 4 and 5 (Supplemental Figure 4). The median time to deviation was shortest among strategy 5 and longest in strategy 2 (49 versus 131 days). By the end of 4 months, 40%–80% of patients had deviated from their index strategies. Factors that increased the probability of deviation included vascular access-related infection in the last baseline month, use of a catheter, higher albumin level, fewer dialysis sessions, and higher epoetin doses in the previous treatment interval. In contrast, having blood transfusions and longer hospital stays in the previous treatment interval reduced the probability of deviation.
During the first 4 months of follow-up, 1320 (10%) patients died and 1627 (12%) patients had at least one infection-related events. The unadjusted RDs of all-cause mortality and infection-related risks comparing each strategy with referent strategy 1 during the 4-month follow-up are shown in Supplemental Figures 5 and 6.
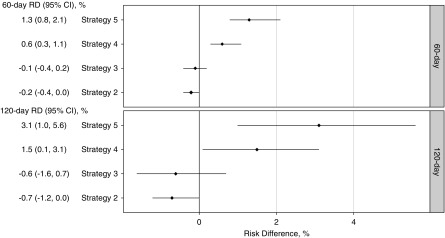
Figure 2 presents the estimated cumulative RDs of all-cause mortality among strategy groups in the first 4 months, adjusted for baseline confounding and strategy deviation in the follow-up. Compared with strategy 1, initiators of less intensive strategies (2 and 3) had lower but nonstatistically significant mortality risks; initiators of more intensive strategy 4 had higher risks, and the adjusted RDs and 95% CIs at 2 and 4 months were 0.6% (95% CI, 0.3% to 1.1%) and 1.5% (95% CI, 0.1% to 3.1%), respectively. The crude estimates were 1.0% and 1.7%, respectively. The highest risks were among initiators of the most intensive strategy 5, and RDs at 2 and 4 months were 1.3% (95% CI, 0.8% to 2.1%) and 3.1% (95% CI, 1.0% to 5.6%), respectively. Their respective crude estimates were 2.5% and 3.4%.
Figure 2.
After adjustment for baseline confounding and strategy deviation in the follow-up, higher cumulative risks of all-cause mortality were associated with more intense strategies compared with the reference strategy 1 in the 4-month follow-up. These RDs represent differences in risks of all-cause mortality if strategy 1 users, contrary to fact, initiated and stayed on another intravenous iron strategy instead of initiating and staying on strategy 1 during the 120-day follow-up.
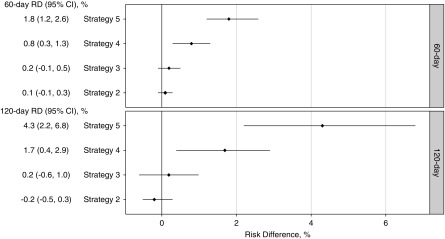
A similar trend was observed for the composite outcome of infection-related events in the 4 months (Figure 3). Compared with strategy 1, initiators of more intensive strategies (4 and 5) had higher risks, whereas those of less intensive strategies (2 and 3) had little difference. At 2 months, RDs for more intensive strategies (4 and 5) were 0.8% (95% CI, 0.3% to 1.3%) and 1.8% (95% CI, 1.2% to 2.6%), respectively. At 4 months, their respective RDs increased to 1.7% (95% CI, 0.4% to 2.9%) and 4.3 (95% CI, 2.2% to 6.8%).
Figure 3.
After adjustment for baseline confounding and strategy deviation in the follow-up, higher cumulative risks of infection-related events were associated with more intense strategies compared with the reference strategy 1 in the 4-month follow-up. These RDs represent differences in risks of infection-related events if strategy 1 users, contrary to fact, initiated and stayed on another intravenous iron strategy instead of initiating and staying on strategy 1 during the 120-day follow-up.
We conducted sensitivity analyses varying the definition for strategy deviation, and our results were robust to such changes. We also examined censoring models with different sets of covariates to adjust for strategy deviation, and the results were robust to the variations in the models. Little difference was seen among the estimates except for the intercept-only model with no covariate (Supplemental Figures 7 and 8).
Discussion
In a large cohort of older patients on hemodialysis, we assessed the effect of continuous use of five common IV iron administration strategies for chronic anemia management on risks of mortality and infection-related events. Higher risks were observed among users of strategies that adopted more intensive dosing approaches (4 and 5) at higher levels of iron indices. Compared with strategy 1, the most intensive strategy 5 may result in an additional 13 deaths (95% CI, 8 to 21) or 18 infection-related events (95% CI, 12 to 26) per 2 months per 1000 patients treated. Higher risks were also observed with more intensive strategy 4 but with a smaller magnitude.
Although previous studies, included in a recently published meta-analysis (13), have examined safety of cumulative doses of IV iron, to our knowledge, this study is the first to assess the safety profile of IV iron administration strategies reflective of clinical practice among patients on hemodialysis. To approximate the dynamic treatment decision process, we aligned exposure assessment with points of treatment decisions (19) and compared the multidimensional strategies adapted from complex protocols actually used by dialysis organizations in contemporary clinical practice.
Ideally, we could answer questions about the comparative safety and efficacy of different strategies with randomized, clinical trials. However, trials assessing multiple strategies are currently lacking and may be infeasible in many cases because of cost, time, and ethical constraints. The recent published Proactive IV irOn Therapy in haemodiALysis Patients (PIVOTAL) trial examined two strategies that were less intensive than all five strategies in this study: a proactive regimen administering 400 mg of iron sucrose monthly (equivalent of maintenance dosing) to ferritin ≤700 ng/ml or TSAT<40%, and a reactive regimen administering a monthly dose of 0–400 mg of iron sucrose to maintain ferritin at 200 ng/ml and TSAT at 20% (32). By examining five commonly used strategies in the United States at the same time, our study could augment the trial with information on safety of more intensive IV iron dosing strategies.
Our findings that intensive strategies had higher infection risks seemingly contrast with the PIVOTAL trial that found no difference in infection risks between the proactive and reactive regimens (32). The difference might be explained by the different intensity of iron therapy among the strategies examined in the trial and this study. Patients in the proactive regimen in the trial received a median monthly dose of 264 mg (and a maximum of 400 mg), which is lower than the median amount received by patients of all five strategies in this study and also the current practice patterns for bolus or “half-bolus” dosing. Additionally, the trial included incident patients on hemodialysis who may have a different safety profile from patients with longer dialysis vintage and more complex comorbidities included in this study.
Our findings confirm and complement findings from prior observational studies. One cohort study observed higher risk of infection-related events with bolus dosing approach compared with maintenance dosing (21). Yet the magnitude of current results was larger, which might be attributable to the difference in strategy definition and age of study population. Another cohort study also showed higher mortality risk associated with nonmaintenance over maintenance strategies (12). They defined maintenance strategy as having IV iron in a regular schedule and nonmaintenance strategy as having any other administration practices, which included patients that had sporadic or no use. Hence, no direct comparisons could be drawn because of the substantially different strategy definitions.
Among these strategies in our study, the main difference was the ferritin level at which iron treatment should be withheld. The findings suggest that aggressive iron treatment with moderate-to-high ferritin level could contribute to increased risks. Compared with strategy 1 that stopped iron at ferritin of 1200 ng/ml, less intensive strategies 2 and 3 that had same TSAT levels indicating a particular dosing approach but lower stopping ferritin levels (800 and 500 ng/ml, respectively) had modestly lower all-cause mortality risks, although not statistically significant. Compared with strategy 1, strategies 4 and 5 that made more intensive treatment recommendations at moderate-to-high levels of iron indices had higher risks of adverse events. The higher risks could potentially be explained by iron overload with persistent administration of IV iron in the setting of relatively high ferritin levels and were consistent with previous studies (22,33,34) and the anticipated adverse effects of excessive use of IV iron in this vulnerable population (35). These results suggest that the role and cutoff values of ferritin level should be thoroughly examined in determining IV iron dosing.
We focused on the per-protocol effect of these dynamic administration strategies using a “cloning and censoring” approach (27). With this approach, multiple strategies could be assigned to a single patient if the treatment patterns were consistent. This approach has been widely used for the estimation of per-protocol effect (36–39). However, with this approach, an intention-to-treat analysis, i.e., comparing the initial strategy, would not be informative, especially when considering dynamic treatment strategies that have overlapping regions and many patients may have been assigned to many or even all strategies (26).
The results of our study may not be generalizable to other patient populations different from those included in the analysis, who were aged 65 or older at dialysis initiation and have survived the first 90 days of dialysis. Compared with the general dialysis population, our study population was older and had higher proportion of male and white patients. Updated analyses are needed to evaluate more recent dosing protocols because anemia management may have changed, especially with the recent policy changes including the capitated reimbursement program. Also, exposure misclassification might occur in the classification of strategies using treatment experience in a 14-day assessment window. However, sensitivity analyses varying the length of assessment window showed the 14-day window was the most representative of the treatment experience in the entire treatment course while maximizing time for follow-up (Supplemental Figure 2). Additionally, we applied this identification approach in patients on hemodialysis in a longer time period and found the prevalence of match between strategies and treatment experience increased sharply, starting in 2010 to 91% in 2012 (23). The increasing trend of matches across time was consistent with the fact that the installation of administration strategies in dialysis clinics occurred in recent years. Moreover, we used ranges of iron dose to classify dosing approach, assuming identical treatment effect across the range for a single dosing approach. This assumption might be violated if treatment effect varies substantially across a range, which is unlikely as the ranges were narrow. Furthermore, we did not evaluate the safety profile by iron formulation.
Our analyses were subject to possible bias from residual confounding. Residual confounding by indication could occur if initiators of more aggressive strategies were inherently different and were treated more aggressively for some indication that we had no information on. If such unmeasured indication was also a risk factor for death or infection-related events, then the observed effects would be subject to bias. Similarly, residual selection bias would occur if there were unmeasured risk factors for both strategy deviation and outcomes of interest, adjustment of which would potentially attenuate the effect. However, we adjusted for an extensive list of clinical, laboratory, treatment, and demographic variables and achieved good balance in the distribution of these covariates after weighting. Sensitivity analyses examining different sets of covariates for the censoring model showed the results were robust to changes in the models, suggesting the possibility that the association between strategy deviation and the outcomes under study might not be strongly confounded by the measured covariates. We may have missed or misclassified comorbidities because they were ascertained only if coded claims are available in the USRDS. Furthermore, we only included limited facility-specific variables, which may not fully account for facility effect associated with iron use and outcomes of interest.
In conclusion, there remains variation in the guidelines for IV iron administration among patients on hemodialysis. We found that strategies recommending relatively aggressive IV iron supplementation, especially at the moderate-to-high levels of ferritin and TSAT, were associated with higher risks of all-cause mortality and infection-related events. Our findings suggest caution for intense IV iron administration among older patients with elevated ferritin levels.
Disclosures
Dr. Brookhart reports grants and personal fees from Amgen and AbbVie, grants from AstraZeneca, personal fees from Genetech, Fibrogen, and TargetPharma, and owns equity in NoviSci, LLC, outside the submitted work. Dr. Kshirsagar was on an Akebia Therapeutics advisory board in 2017 outside the submitted work. Dr. Stürmer reports grants from Amgen, grants from AstraZeneca, and grants from Novo Nordisk; he also receives salary support as Director of Comparative Effectiveness Research (CER), NC TraCS Institute, UNC Clinical and Translational Science Award (UL1TR002489), the Center for Pharmacoepidemiology (current members: GlaxoSmithKline, UCB BioSciences, Merck, Shire, and Takeda), and from pharmaceutical companies (GSK, Amgen, AstraZeneca, Novo Nordisk). Dr. Stürmer owns stock in Novartis, Roche, BASF, AstraZeneca, and Novo Nordisk. Dr. Cole, Dr. Fine, and Dr. Li have nothing to disclose.
Supplementary Material
Acknowledgments
The authors thank DaVita Clinical Research and the US Renal Data System (USRDS) for providing data for this study.
Dr. Li reports support from National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Disease Ruth L. Kirschstein National Research Service Award institutional research training grant (5T32DK007750; Principal Investigator R.J. Falk) during the conduct of the study. Dr. Cole has received investigator-initiated research funding from the US National Institute of Allergy and Infectious Diseases at the National Institutes of Health (R01 AI100654). Dr. Stürmer receives investigator-initiated research funding and support as Principal Investigator (R01 AG056479) from the National Institute on Aging, and as Co-Investigator (R01 HL118255, R01MD011680) from the National Institutes of Health. He received support from a generous contribution from Dr. Nancy A. Dreyer to the Department of Epidemiology, University of North Carolina at Chapel Hill.
Results of this study were presented as an abstract at the 2017 International Conference of Pharmacoepidemiology and Therapeutic Risk Management in Montreal, Canada, August 30, 2017.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the USRDS. DaVita Clinical Research had no role in the design or implementation of this study or the decision to publish.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi: 10.2215/CJN.03970318/-/DCSupplemental.
Detailed methods.
Supplemental Table 1. Claims-based definitions for study outcomes.
Supplemental Table 2. Claims-based definitions for study covariates.
Supplemental Figure 1. Flow chart showing how patients were selected into the cohort.
Supplemental Figure 2. Schematic illustration of the process to determine the length of assessment window.
Supplemental Figure 3. Schematic illustration of the process to assess patient adherence to their intravenous iron administration strategy during follow-up.
Supplemental Figure 4. Cumulative risk of deviation from their index strategy for initiators of the five dynamic intravenous iron strategies during the 120-day follow-up.
Supplemental Figure 5. Cumulative risk difference curves for all-cause mortality varying models for effect estimation.
Supplemental Figure 6. Cumulative risk difference curves for infection-related events varying models for effect estimation.
Supplemental Figure 7. Cumulative risk difference curves for all-cause mortality varying models for strategy deviation.
Supplemental Figure 8. Cumulative risk difference curves for infection-related events varying models for strategy deviation.
References
- 1.KDIGO : KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int Suppl 2: 279–335, 2012 [Google Scholar]
- 2.Kliger AS, Foley RN, Goldfarb DS, Goldstein SL, Johansen K, Singh A, Szczech L: KDOQI US commentary on the 2012 KDIGO clinical practice guideline for anemia in CKD. Am J Kidney Dis 62: 849–859, 2013 [DOI] [PubMed] [Google Scholar]
- 3.Robins JM: The analysis of randomized and nonrandomized AIDS treatment trials using a new approach to causal inference in longitudinal studies. In: Health Service Research Methodology: A Focus on AIDS, edited by Sechrest L, Freeman H, Mulley A, Washington, DC, NCHSR, US Public Health Service, 1989, pp 113–159 [Google Scholar]
- 4.Lavori PW, Dawson R: Introduction to dynamic treatment strategies and sequential multiple assignment randomization. Clin Trials 11: 393–399, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li X, Young JG, Toh S: Estimating effects of dynamic treatment strategies in pharmacoepidemiologic studies with time-varying confounding: A primer. Curr Epidemiol Rep 4: 288–297, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moist LM, Troyanov S, White CT, Wazny LD, Wilson JA, McFarlane P, Harwood L, Sood MM, Soroka SD, Bass A, Manns BJ: Canadian Society of Nephrology commentary on the 2012 KDIGO Clinical Practice Guideline for Anemia in CKD. Am J Kidney Dis 62: 860–873, 2013 [DOI] [PubMed] [Google Scholar]
- 7.Locatelli F, Bárány P, Covic A, De Francisco A, Del Vecchio L, Goldsmith D, Hörl W, London G, Vanholder R, Van Biesen W; ERA-EDTA ERBP Advisory Board : Kidney Disease: Improving Global Outcomes guidelines on anaemia management in chronic kidney disease: A European Renal Best Practice position statement. Nephrol Dial Transplant 28: 1346–1359, 2013 [DOI] [PubMed] [Google Scholar]
- 8.National Institute of Health and Care Excellence (NICE): Chronic Kidney Disease: Managing Anaemia, 2015. Available at: https://www.nice.org.uk/guidance/ng8. Accessed October 1, 2016
- 9.Macdougall IC, Bircher AJ, Eckardt KU, Obrador GT, Pollock CA, Stenvinkel P, Swinkels DW, Wanner C, Weiss G, Chertow GM; Conference Participants : Iron management in chronic kidney disease: Conclusions from a “Kidney Disease: Improving Global Outcomes” (KDIGO) Controversies Conference. Kidney Int 89: 28–39, 2016 [DOI] [PubMed] [Google Scholar]
- 10.Krishnan M, Weldon J, Wilson S, Goyhkman I, Van Wyck D: Effect of maintenance iron protocols on ESA dosing and anemia outcomes. Am J Kidney Dis 57: A55, 2011 [Google Scholar]
- 11.Schiller B: Implementing an IV Iron Administration Protocol within a Dialysis Organization, 2014. Available at: http://www.nephrologynews.com/implementing-an-iv-iron-administration-protocol-within-a-dialysis-organization/. Assessed March 22, 2016 [PubMed]
- 12.Michels WM, Jaar BG, Ephraim PL, Liu Y, Miskulin DC, Tangri N, Crews DC, Scialla JJ, Shafi T, Sozio SM, Bandeen-Roche K, Cook CJ, Meyer KB, Boulware LE; DEcIDE Network Patient Outcomes in End Stage Renal Disease Study Investigators : Intravenous iron administration strategies and anemia management in hemodialysis patients. Nephrol Dial Transplant 32: 173–181, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hougen I, Collister D, Bourrier M, Ferguson T, Hochheim L, Komenda P, Rigatto C, Tangri N: Safety of intravenous iron in dialysis: A systematic review and meta-analysis. Clin J Am Soc Nephrol 13: 457–467, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li X, Kshirsagar AV, Brookhart MA: Safety of intravenous iron in hemodialysis patients. Hemodial Int 21[Suppl 1]: S93–S103, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miskulin DC, Tangri N, Bandeen-Roche K, Zhou J, McDermott A, Meyer KB, Ephraim PL, Michels WM, Jaar BG, Crews DC, Scialla JJ, Sozio SM, Shafi T, Wu AW, Cook C, Boulware LE; Developing Evidence to Inform Decisions about Effectiveness (DEcIDE) network patient outcomes in end stage renal disease study investigators : Intravenous iron exposure and mortality in patients on hemodialysis. Clin J Am Soc Nephrol 9: 1930–1939, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tangri N, Miskulin DC, Zhou J, Bandeen-Roche K, Michels WM, Ephraim PL, McDermott A, Crews DC, Scialla JJ, Sozio SM, Shafi T, Jaar BG, Meyer K, Boulware LE; DEcIDE Network Patient Outcomes in End-Stage Renal Disease Study Investigators : Effect of intravenous iron use on hospitalizations in patients undergoing hemodialysis: A comparative effectiveness analysis from the DEcIDE-ESRD study. Nephrol Dial Transplant 30: 667–675, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bailie GR, Larkina M, Goodkin DA, Li Y, Pisoni RL, Bieber B, Mason N, Tong L, Locatelli F, Marshall MR, Inaba M, Robinson BM: Data from the Dialysis Outcomes and Practice Patterns Study validate an association between high intravenous iron doses and mortality. Kidney Int 87: 162–168, 2015 [DOI] [PubMed] [Google Scholar]
- 18.Li X, Kshirsagar AV: Rest easy with intravenous iron for dialysis patients? High dose IV iron safety. Clin J Am Soc Nephrol 13: 363–365, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brookhart MA: Counterpoint: The treatment decision design. Am J Epidemiol 182: 840–845, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freburger JK, Ellis AR, Kshirsagar AV, Wang L, Brookhart MA: Comparative short-term safety of bolus versus maintenance iron dosing in hemodialysis patients: A replication study. BMC Nephrol 15: 154, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brookhart MA, Freburger JK, Ellis AR, Wang L, Winkelmayer WC, Kshirsagar AV: Infection risk with bolus versus maintenance iron supplementation in hemodialysis patients. J Am Soc Nephrol 24: 1151–1158, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fishbane S, Mathew AT, Wanchoo R: Intravenous iron exposure and outcomes in patients on hemodialysis. Clin J Am Soc Nephrol 9: 1837–1839, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li X: Comparative effectiveness of intravenous iron treatment protocols in hemodialysis patients: Causal inference with dynamic treatment regimes [dissertation]. Chapel Hill, NC, University of North Carolina at Chapel Hill, 2017 [Google Scholar]
- 24.Krishnan M, Weinhandl ED, Jackson S, Gilbertson DT, Lacson E Jr: Comorbidity ascertainment from the ESRD Medical Evidence Report and Medicare claims around dialysis initiation: A comparison using US Renal Data System data. Am J Kidney Dis 66: 802–812, 2015 [DOI] [PubMed] [Google Scholar]
- 25.Hernán MA, Hernández-Díaz S: Beyond the intention-to-treat in comparative effectiveness research. Clin Trials 9: 48–55, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hernán MA, Robins JM: Using big data to emulate a target trial when a randomized trial is not available. Am J Epidemiol 183: 758–764, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cotton CA, Heagerty PJ: Evaluating epoetin dosing strategies using observational longitudinal data. Ann Appl Stat 8: 2356–2377, 2014 [Google Scholar]
- 28.Satten GA, Datta S: The Kaplan-Meier estimator as an inverse-probability-of-censoring weighted average. Am Stat 55: 207–210, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sato T, Matsuyama Y: Marginal structural models as a tool for standardization. Epidemiology 14: 680–686, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Robins JM: Correction for non-compliance in equivalence trials. Stat Med 17: 269–302; discussion 387–389, 1998 [DOI] [PubMed] [Google Scholar]
- 31.Efron B, Taibshirani R: Confidence intervals based on bootstrap percentiles. In: An Introduction to the Bootstrap, New York, Chapman & Hall, 1993, pp 168–177 [Google Scholar]
- 32.Macdougall IC, White C, Anker SD, Bhandari S, Farrington K, Kalra PA, McMurray JJV, Murray H, Tomson CRV, Wheeler DC, Winearls CG, Ford I; PIVOTAL Investigators and Committees : Intravenous iron in patients undergoing maintenance hemodialysis. N Engl J Med 380: 447–458, 2019 [DOI] [PubMed] [Google Scholar]
- 33.Rostoker G, Griuncelli M, Loridon C, Magna T, Machado G, Drahi G, Dahan H, Janklewicz P, Cohen Y: Reassessment of iron biomarkers for prediction of dialysis iron overload: An MRI Study. PLoS One 10: e0132006, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rostoker G, Vaziri ND, Fishbane S: Iatrogenic iron overload in dialysis patients at the beginning of the 21st century. Drugs 76: 741–757, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vaziri ND: Understanding iron: Promoting its safe use in patients with chronic kidney failure treated by hemodialysis. Am J Kidney Dis 61: 992–1000, 2013 [DOI] [PubMed] [Google Scholar]
- 36.Cain LE, Robins JM, Lanoy E, Logan R, Costagliola D, Hernán MA: When to start treatment? A systematic approach to the comparison of dynamic regimes using observational data. Int J Biostat 6: 18, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Orellana L, Rotnitzky A, Robins JM: Dynamic regime marginal structural mean models for estimation of optimal dynamic treatment regimes, Part II: Proofs of results. Int J Biostat 6: 9, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Orellana L, Rotnitzky A, Robins JM: Dynamic regime marginal structural mean models for estimation of optimal dynamic treatment regimes, Part I: Main content. Int J Biostat 6: 8, 2010 [PubMed] [Google Scholar]
- 39.van der Laan MJ, Petersen ML: Causal effect models for realistic individualized treatment and intention to treat rules. Int J Biostat 3: 3, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.