Arabidopsis, which does not normally emit isoprene, was engineered to emit isoprene, and growth and development as well as gene expression were analyzed to determine how isoprene affects plants.
Abstract
Isoprene synthase converts dimethylallyl diphosphate to isoprene and appears to be necessary and sufficient to allow plants to emit isoprene at significant rates. Isoprene can protect plants from abiotic stress but is not produced naturally by all plants; for example, Arabidopsis (Arabidopsis thaliana) and tobacco (Nicotiana tabacum) do not produce isoprene. It is typically present at very low concentrations, suggesting a role as a signaling molecule; however, its exact physiological role and mechanism of action are not fully understood. We transformed Arabidopsis with a Eucalyptus globulus isoprene synthase. The regulatory mechanisms of photosynthesis and isoprene emission were similar to those of native emitters, indicating that regulation of isoprene emission is not specific to isoprene-emitting species. Leaf chlorophyll and carotenoid contents were enhanced by isoprene, which also had a marked positive effect on hypocotyl, cotyledon, leaf, and inflorescence growth in Arabidopsis. By contrast, leaf and stem growth was reduced in tobacco engineered to emit isoprene. Expression of genes belonging to signaling networks or associated with specific growth regulators (e.g. gibberellic acid that promotes growth and jasmonic acid that promotes defense) and genes that lead to stress tolerance was altered by isoprene emission. Isoprene likely executes its effects on growth and stress tolerance through direct regulation of gene expression. Enhancement of jasmonic acid-mediated defense signaling by isoprene may trigger a growth-defense tradeoff leading to variations in the growth response. Our data support a role for isoprene as a signaling molecule.
Isoprene (2-methyl-1,3-butadiene), the lowest molecular weight hydrocarbon in the terpene family, is released by some but not all plants (Sharkey et al., 2008). Isoprene is synthesized by the methylerythritol 4-phosphate (MEP) pathway in the chloroplast stroma, and the conversion of dimethylallyl diphosphate (DMADP) to isoprene is catalyzed by isoprene synthase (ISPS; Silver and Fall, 1995; Miller et al., 2001; Sharkey et al., 2013). Production of DMADP by the MEP pathway requires two substrates derived from the Calvin-Benson cycle, pyruvate and glyceraldehyde 3-phosphate. Therefore, the rate of isoprene synthesis is dependent on photosynthesis and is driven by environmental factors such as light, temperature, and [CO2] (Monson and Fall, 1989; Sharkey and Yeh, 2001; Sanadze, 2004; Sun et al., 2013a, 2013b). Abiotic stress conditions such as drought and salt stress increase isoprene synthesis, while ozone decreases isoprene synthesis in plants expressing ISPS (Loreto and Delfine, 2000; Genard-Zielinski et al., 2014; Yuan et al., 2016).
The carbon (C) and energy cost of isoprene production has been found to vary depending on the plant species and environmental conditions. For example, trees that normally produce isoprene, such as eastern cottonwood (Populus deltoides), consume about 2% (Schnitzler et al., 2010; Way et al., 2011), while kudzu (Pueraria montana) can consume up to 64% (Sharkey and Loreto, 1993) of photosynthetic C for isoprene production. Under high temperature, C consumption for isoprene synthesis increases: gray poplar (Populus × canescens) at 42°C (Way et al., 2011) and oak (Quercus robur) at 40°C (Way et al., 2013) release about 25% and 14% of photosynthesized C as isoprene, respectively. In addition, the energy expenditure required for isoprene production is quite high. One isoprene molecule made via the MEP pathway requires 20 ATP and 14 NADPH (Sharkey and Yeh, 2001). The fact that the ISPS gene and the ability to synthesize isoprene were evolutionarily retained in some plants despite the comparatively high C and energy demand associated with isoprene production has been interpreted to indicate that isoprene is likely beneficial, although perhaps only in some species and under specific environmental conditions (Monson et al., 2013; Sharkey, 2013). Phylogenetic analyses show that, while present in ancestral lines, including Glycine soja, ISPS was lost from Glycine max probably fairly recently during the domestication process (Sharkey et al., 2013). Understanding how isoprene affects plant growth and physiology will allow researchers to determine whether ISPS will be a beneficial trait to be reintroduced to plants, especially for the purpose of crop improvement.
In general, isoprene emission has been shown to protect plants from abiotic stress, such as high-temperature stress, exposure to ozone and other reactive oxygen species (ROS), and herbivory (Loreto et al., 2001; Loreto and Velikova, 2001; Affek and Yakir, 2002; Sasaki et al., 2007; Laothawornkitkul et al., 2008; Loivamäki et al., 2008; Vickers et al., 2009a; Loreto and Schnitzler, 2010; Centritto et al., 2014). Inhibition of the MEP pathway by fosmidomycin blocks isoprene synthesis in plants, enhancing sensitivity to high temperature and ozone and leading to more severe oxidative damage (Loreto and Velikova, 2001; Sharkey et al., 2001; Velikova and Loreto, 2005; Velikova et al., 2006). In contrast, when plants are fumigated with isoprene, they suffer less damage when exposed to ozone and oxidative stress and recover more rapidly from thermal stress than untreated controls (Singsaas et al., 1997; Loreto et al., 2001; Sharkey et al., 2001; Velikova et al., 2006).
However, while isoprene synthesis, regulation, and its role in plant stress tolerance have been studied for several decades, its exact physiological role and the mechanism of action are still not fully understood. For many years, the underlying mechanisms of the above protective roles of isoprene were hypothesized to be mainly through stabilizing thylakoid membranes (Sharkey and Singsaas, 1995; Singsaas and Sharkey, 1998, 2000; Owen and Peñuelas, 2005; Behnke et al., 2007; Vickers et al., 2009a; Velikova et al., 2011), dissipating excess energy absorbed by photosynthetic pigments (Sanadze, 2010; Pollastri et al., 2014), antioxidant properties and ozone quenching (Loreto et al., 2001; Affek and Yakir, 2002; Sharkey et al., 2008; Vickers et al., 2009b), and indirect effects on ROS signaling (Vickers et al., 2009a). However, recently, Harvey et al. (2015) showed that isoprene is normally present at very low concentration (about 60 isoprene molecules per million lipid molecules), and even at very high concentration it is unable to cause changes to membrane fluidity. Thus, at normal concentrations, isoprene is very unlikely to affect membrane stability, and it also is not an effective method for quenching ROS compared with molecules such as β-carotene that are present at higher levels and act at much faster rates than isoprene (Harvey et al., 2015; Harvey and Sharkey, 2016).
New evidence based on RNA interference (RNAi)-mediated suppression of isoprene emission in gray poplar (Behnke et al., 2010) and Arabidopsis (Arabidopsis thaliana) fumigated with isoprene (Harvey and Sharkey, 2016) shows that isoprene leads to changes in the expression of many gene networks important for stress responses and also plant growth. Isoprene has also been shown to cause changes in the proteome (Vanzo et al., 2016), metabolome (Way et al., 2013), and metabolic fluxes (Behnke et al., 2010; Ghirardo et al., 2014) in plants. Evidence that shows isoprene can affect ATP-synthase activity in thylakoid membranes in Arabidopsis expressing ISPS (Velikova et al., 2011) allowed the hypothesis that isoprene may interact with membrane-bound proteins to induce signal transduction networks (Harvey and Sharkey, 2016). When Arabidopsis plants were fumigated for 24 h with a physiologically relevant concentration (20 μL L−1) of isoprene, microarray analysis found altered expression of genes that code for proteins functionally associated with many pathways, such as photosynthetic light reaction machinery, cell wall synthesis, stress responses, flavonoid and phenylpropanoid biosynthesis, etc. (Harvey and Sharkey, 2016). Based on these data, Harvey and Sharkey (2016) proposed that isoprene can act as a signaling molecule to alter gene expression and thereby bring about its effects on abiotic stress tolerance. The specific growth-related and stress-related signaling pathways likely affected by isoprene and specific genes of these pathways that are responsive to isoprene are not known.
The aim of this study was to investigate in detail the effects of isoprene on plant growth under normal environmental conditions in Arabidopsis transformed with a Eucalyptus globulus ISPS. Arabidopsis does not naturally produce isoprene. Our goal was to investigate the effects on the MEP pathway metabolome and photosynthesis and how isoprene emission is regulated under different environmental conditions when Arabidopsis is engineered to emit isoprene. We tested whether the effects of isoprene seen in Arabidopsis were common to other species by comparing our Arabidopsis model system with an isoprene-emitting tobacco (Nicotiana tabacum) model system developed by Vickers et al. (2009b). Like Arabidopsis, tobacco does not naturally produce isoprene. We also carried out gene expression analyses and a comprehensive comparison of isoprene-responsive genes from three different systems: (1) Arabidopsis and (2) tobacco expressing ISPS as well as (3) nontransformed wild-type Arabidopsis fumigated with isoprene. In doing so, we hoped to gain insight into the mechanisms through which isoprene can affect stress responses in plants.
RESULTS
Characterization of Arabidopsis Transgenic Lines Expressing ISPS
Expression of the ISPS Transgene in Arabidopsis
Arabidopsis wild-type Columbia-0 (Col-0) plants were transformed with an ISPS gene from E. globulus with an Arabidopsis Rubisco small subunit promoter (rbcS-1A; At1g67090), because this ISPS has a high affinity for DMADP. Results from PCR analyses confirmed the presence of vector DNA without ISPS in EV-A1, EV-B3, and EV-F1 and the presence of the ISPS gene in A1, B2, B4, C1, C3, C4, and F2 lines (Supplemental Fig. S1).
Photosynthesis and Isoprene Emission
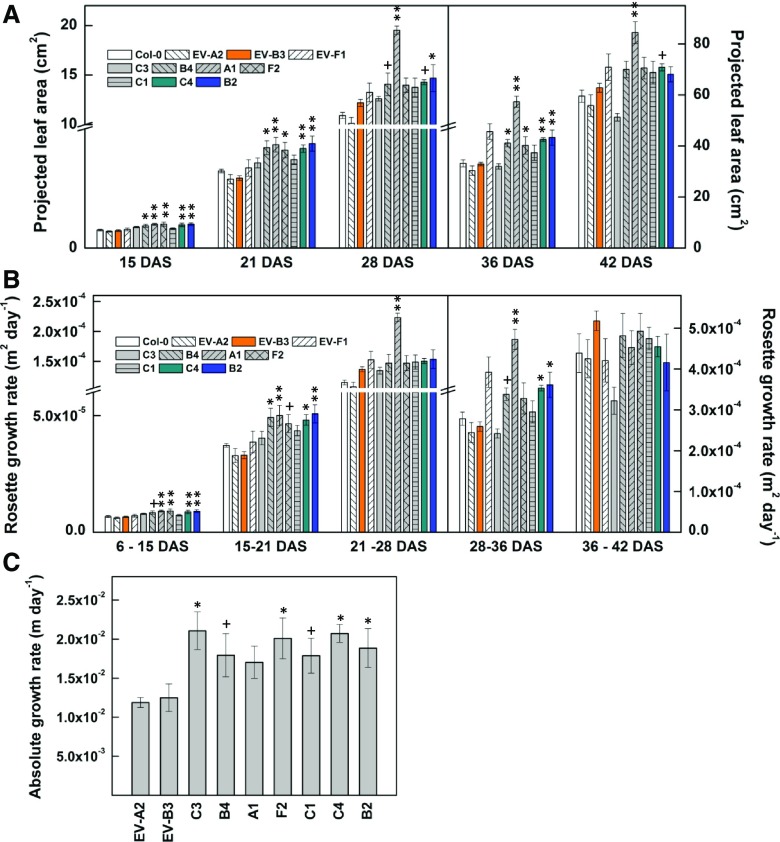
Photosynthesis rates were similar between lines carrying the ISPS gene and control lines (Fig. 1A). Significant rates of isoprene emission were detected in Arabidopsis lines carrying the ISPS gene (Fig. 1B). The Col-0 line and empty vector lines had trivial isoprene emission rates, although EV-F1 had a higher rate than the other empty vector lines. We believe this to be nonenzymatic conversion of DMADP to isoprene. Within isoprene-emitting lines, C3 and B4 exhibited lower rates of emission, A1 and F2 emitted moderate amounts of isoprene, and C1, C4, and B2 exhibited the highest rates of isoprene emission compared with the control lines.
Figure 1.
Carbon assimilation and isoprene emission in Arabidopsis expressing ISPS. Photosynthesis (A) and isoprene emission (B) were measured simultaneously in Arabidopsis wild-type Col-0, empty vector controls (EV-A2, EV-B3, and EV-F1), and lines expressing ISPS (C3, B4, A1, F2, C1, C4, and B2). Measurements were made on plants 45 to 47 d old. All transgenic lines belonged to the T3 generation. Values in graphs represent means ± se (n = 5–7 plants per line). Arabidopsis lines expressing ISPS that are significantly different from Col-0 and empty vector lines EV-A2 and EV-B3 are marked with asterisks: **, P < 0.01 using one-way ANOVA followed by Fisher’s lsd test.
Plant Growth
Hypocotyls were taller (Fig. 2, A and B) and cotyledons were larger (Fig. 2, C and D) in seedlings of isoprene-emitting lines than the controls. Except C1, cotyledon area seemed to be largest in plants that produced the highest amounts of isoprene at maturity. This relationship was not seen in terms of hypocotyl elongation.
Figure 2.
Early seedling growth in Arabidopsis expressing ISPS. Comparison of hypocotyl length (A and B) and cotyledon size (C and D) in 7-d-old Arabidopsis wild-type Col-0, empty vector controls (EV-A2, EV-B3, and EV-F1), and lines expressing ISPS (C3, B4, A1, F2, C1, C4, and B2). All transgenic lines belonged to the T3 generation. Values in graphs represent means ± se. In B, n = 22 to 41 plants per line; in D, n = 12 to 26 plants per line. Arabidopsis lines expressing ISPS that are significantly different from Col-0 and empty vector lines EV-A2 and EV-B3 are marked with symbols: +, P < 0.1; *, P < 0.05; and **, P < 0.01 using one-way ANOVA followed by Fisher’s lsd test. In D, C1 was only statistically different from Col-0, EV-B3, and EV-F1.
During the initial phenotypic characterization of the Arabidopsis mutant lines expressing ISPS, we focused mainly on rosette growth as a whole and rosette and stem growth rates. Projected leaf area (rosette area as seen from above with no correction for overlapping or curling leaves) was consistently larger in isoprene-emitting lines throughout the life cycle compared with the control lines (Figs. 3, A and B, and 4A). At maturity (50 d after seeding [DAS]), rosette dry weight was greater in F2, C4, and B2 compared with all control lines (Fig 3C). All isoprene-emitting lines except C3 exhibited greater total shoot dry weight than Col-0, EV-A2, and EV-B3 (Fig. 3D). In contrast to the wild type and other empty vector lines, EV-F1 showed a similarly increased shoot dry weight to the isoprene-producing lines (Fig. 3D). Most of the isoprene-emitting lines maintained higher rosette growth rates throughout their life span (Fig. 4B). In addition, stem growth rates were higher in isoprene-emitting lines than in EV-A2 and EV-B3 (Fig. 4C). Moreover, all isoprene-emitting lines flowered earlier than EV-A2 and EV-B3 (Fig. 3E) and produced taller inflorescences (Fig. 3F).
Figure 3.
Rosette and inflorescence growth in Arabidopsis expressing ISPS. Photographs comparing rosette size and projected leaf area of 21-d-old (A) and 50-d-old (B) T3 plants, and leaf dry weight (C), shoot (leaf + inflorescence) dry weight (D), number of days required for inflorescence initiation (E), and height of the primary (1°) inflorescence axis (F) in 50-d-old Arabidopsis wild-type Col-0, empty vector controls (EV-A2, EV-B3, and EV-F1), and lines expressing ISPS (C3, B4, A1, F2, C1, C4, and B2), are shown. All transgenic lines belonged to the T3 generation. Values in graphs represent means ± se. In C and D, n = 4 to 8 plants per line; in E and F, n = 3 to 6 plants per line. Arabidopsis lines expressing ISPS that are significantly different from Col-0 and empty vector lines EV-A2 and EV-B3 are marked with symbols: +, P < 0.1; *, P < 0.05; and **, P < 0.01 using one-way ANOVA followed by Fisher’s lsd test; in E and F, symbols denote statistical comparisons relative to only EV-A2 and EV-B3.
Figure 4.
Rosette and inflorescence stem growth rates in Arabidopsis expressing ISPS. Projected leaf area over time (A), absolute growth rates calculated as the increase in projected leaf area per day (B), and increase in the height of the primary inflorescence axis (stem) per day (C) are shown. All transgenic lines belonged to the T3 generation. Values in graphs represent means ± se. In A and B, n = 4 to 6 plants per line. In C, n = 3 to 5 plants per line. Arabidopsis lines expressing ISPS (C3, B4, A1, F2, C1, C4, and B2) significantly different from Col-0 and empty vector lines EV-A2 and EV-B3 are marked with symbols. +, P < 0.1; *, P < 0.05; and **, P < 0.01 using one-way ANOVA followed by Fisher’s lsd test. In C, symbols denote statistical comparisons relative to only EV-A2 and EV-B3.
In summary, while the magnitude of growth enhancement was variable between the seven isoprene-emitting lines, isoprene had a consistent positive effect on early seedling growth and both vegetative and reproductive growth in Arabidopsis.
Comparison of the Isoprene-Emitting Arabidopsis and the Isoprene-Emitting Tobacco Model Systems
After characterization of the isoprene-emitting Arabidopsis lines, one of the empty vector lines (EV-B3) and the two lines that exhibited the highest rates of isoprene emission (B2 and C4) were selected for more in-depth growth analysis and comparison with tobacco azygous plants not emitting isoprene (NE) and transformed, isoprene-emitting (IE) lines to study in more detail the regulation of isoprene emission and photosynthesis under varying temperature, CO2, light and darkness, postillumination isoprene emission, MEP pathway metabolites, and growth. Data were compared among three systems: fumigated Arabidopsis, ISPS-transformed Arabidopsis, and ISPS-transformed tobacco. To find out if the observed effects were affected by growth light levels, Arabidopsis was grown under two light intensities: 100 and 200 µmol m−2 s−1.
Arabidopsis
Photosynthesis and Isoprene Emission
Whether Arabidopsis was grown under 200 or 100 μmol m−2 s−1 light, there was no significant difference in photosynthesis among EV-B3, B2, and C4 lines at either of the two time points analyzed (Supplemental Fig. S2). B2 had the highest isoprene emission rate, while no isoprene emission was detected from EV-B3 (Supplemental Fig. S2). Behnke et al. (2007) also reported that rates of photosynthesis were not different between isoprene-emitting gray poplar and gray poplar lacking isoprene emission due to RNAi-mediated suppression of ISPS.
Response of Photosynthesis and Isoprene Emission to Temperature, CO2, Light, Darkness, and N2 Break
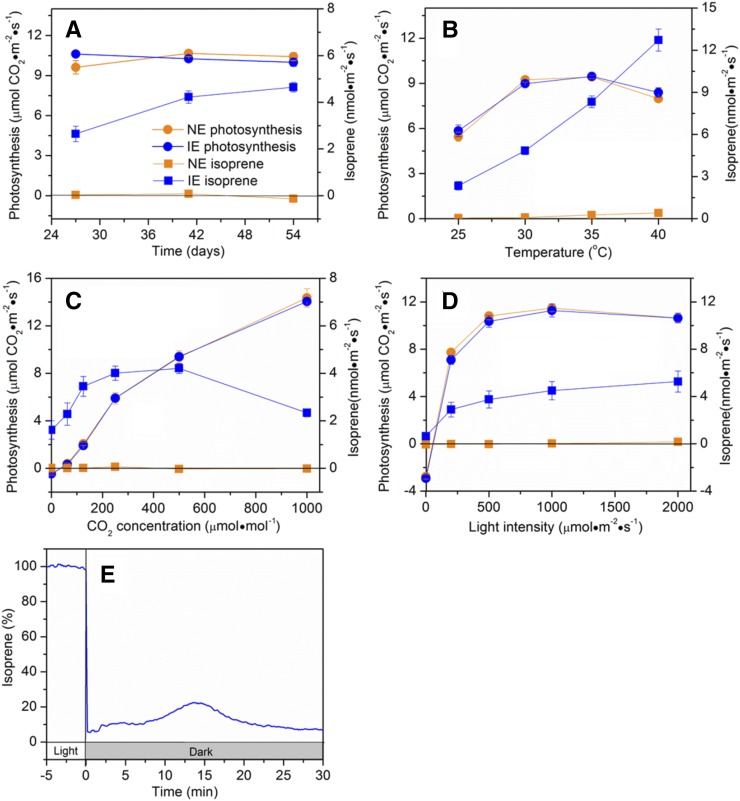
Increasing temperature from 23°C to 30°C did not have a significant effect on photosynthesis in wild-type Arabidopsis or B2 (Fig. 5A). However, at temperatures above 30°C, photosynthesis declined gradually with increasing temperature. High temperature promoted isoprene emission in transgenic line B2. These data are consistent with data previously published (Possell and Hewitt, 2009, 2011; Vickers et al., 2009b; Niinemets and Sun, 2015). To our surprise, wild-type plants released isoprene at 35°C, at an emission rate of 0.1 nmol m−2 s−1, and the emission rate increased by 1.5- and 3.8-fold, respectively, at 40°C and 45°C (Fig. 5A). This emission was also observed in NE tobacco and may result from nonenzymatic conversion of DMADP to isoprene (Brüggemann and Schnitzler, 2002; Ghirardo et al., 2010).
Figure 5.
Photosynthesis and isoprene emission under varying environmental conditions in Arabidopsis expressing ISPS. The responses of photosynthesis (Photo) and isoprene emission to temperature (A), CO2 (B), light (C), darkness (D), and N2 break (E) in 39- to 42-d-old Arabidopsis empty vector control (EV-B3) and lines expressing ISPS (B2 and C4) are shown. In A, the wild type (WT) was used as the control. Data from B2 are shown in D and E. All plants were grown in growth chambers under a light intensity of 200 μmol m−2 s−1. Measurements were taken 30°C (B–E) and 500 μmol m−2 s-1 light intensity (A, B, and E). In D, plants were exposed to 500 μmol m−2 s−1 light before being exposed to sudden darkness. Values in A to C represent means ± se; values in D represent means (n = 5). In E, representative data from a single leaf are presented.
Control line EV-B3 and transgenic line B2 showed similar photosynthesis responses to CO2 whether they were grown at a light intensity of 200 μmol m−2 s−1 (Fig. 5B) or 100 μmol m−2 s−1 (Supplemental Fig. S3A). Photosynthesis in EV-B3 and B2 increased with rising CO2 concentration, and there was no obvious difference between the two lines (Fig. 5B). Isoprene emission from B2 increased gradually as CO2 concentration was increased from 0 to 250 μmol mol−1, and then remained stable up to 500 μmol mol−1 CO2. Above 500 μmol mol−1, the rate of isoprene emission declined. There was no isoprene emission from EV-B3.
When EV-B3 and B2 plants grown under a light intensity of 200 μmol m−2 s−1 were subjected to varying light intensities, photosynthetic rates were highest at 500 μmol m−2 s−1 and remained nearly stable at intensities above 500 μmol m−2 s−1 (Fig. 5C). The response of isoprene emission from B2 to light was similar to that of photosynthesis when the light intensity was below 1,000 μmol m−2 s−1; above that a slight decline occurred. No isoprene was released from EV-B3. Similar responses were also found in EV-B3 and B2 grown at 100 μmol m−2 s−1 (Supplemental Fig. S3B).
B2 grown at 200 μmol m−2 s−1 (Fig. 5D) and 100 μmol m−2 s−1 (Supplemental Fig. S3C) showed a rapid decline in isoprene in response to sudden darkness. This has been observed in previous studies in oak and eastern cottonwood (Sharkey and Loreto, 1993; Li et al., 2011). However, unlike in oak and poplar, no postillumination peak was detected in Arabidopsis plants (Fig. 5D; Supplemental Fig. S3C).
Under illumination, when B2 was exposed to pure N2, isoprene emission declined to low levels (Fig. 5E). When it was exposed to air again after 17 min in pure N2, isoprene emission rapidly increased and reached a maximum about 10 min after restoring oxygen and CO2, with an increase of 91% compared with the isoprene emission rate before exposure to N2. This behavior has also been reported for aspen (Populus tremula × Populus alba) by Li and Sharkey (2013a), who hypothesized that it is caused by accumulation of methylerythritol cyclodiphosphate (MEcDP) during the N2 exposure.
MEP Pathway Metabolomics
There were no significant differences between Arabidopsis EV-B3, B2, and C4 in the levels of MEP pathway metabolites in plants grown under a light intensity of 200 μmol m−2 s−1 (Fig. 6A). MEcDP levels were lower and MEP levels were higher in Arabidopsis than in tobacco (Fig. 6). Because of compounds interfering with the mass spectrometer measurement of DMADP/isopentenyl diphosphate (IDP), we assessed DMADP/IDP by integrating the postillumination isoprene emission (Rasulov et al., 2009; Weise et al., 2013). This technique can only be used with emitting plants, so there are no data available for DMADP in nonemitting lines. The concentration of DMADP/IDP was low (note the extremely fast decline in isoprene emission upon darkening in Fig. 5D) relative to the values found in aspen, which were around 800 nmol m−2 s−1 (Li and Sharkey, 2013a). The low MEcDP content of Arabidopsis leaves is consistent with the lack of the postillumination emission described above (Fig. 5D). For a comparison of metabolite levels in Arabidopsis and tobacco, the data in Figure 6 are plotted together in Supplemental Figure S4.
Figure 6.
MEP pathway metabolites in Arabidopsis and tobacco expressing ISPS. Metabolite levels in leaves harvested from 42-d-old Arabidopsis empty vector control (EV-B3) and lines expressing ISPS (B2 and C4; A), and 42-d-old NE and IE tobacco (B), are shown. Arabidopsis plants were grown in a growth chamber under a light intensity of 200 μmol m−2 s−1. Tobacco was grown in the greenhouse. CDP-ME, Diphosphocytidylyl methylerythritol; DXP, deoxyxylulose 5-phosphate; HMBDP, hydroxymethylbutenyl diphosphate. Lowercase letters indicate significant differences among the three Arabidopsis lines or between NE and IE at P < 0.05 using one-way ANOVA followed by Fisher’s lsd test (i.e. there were no differences). Values represent means ± se. For Arabidopsis, n ≥ 5 plants per line; for tobacco, n = 7. Because DMADP + IDP were measured by postillumination isoprene emission, there are no data for Arabidopsis EV-B3 and tobacco NE lines.
Alterations in Plant Growth in Isoprene-Emitting Arabidopsis Lines
Arabidopsis lines B2 and C4 expressing ISPS produced larger cotyledons and longer hypocotyls than the empty vector control line EV-B3 (Supplemental Fig. S5), as had been seen in the preliminary screen (Fig. 2). This experiment showed that the effect was not dependent on light intensity.
While whole rosette growth was the main focus during characterization of the seven Arabidopsis lines expressing ISPS, here we explored in more detail leaf characteristics and growth of the two highest emitting lines. Compared with EV-B3, projected leaf area was consistently larger in B2 and C4 (Fig. 7, A–C). In addition, B2 and C4 also produced longer leaves and a greater number of leaves than EV-B3 (Table 1; Fig. 7, D–F; Supplemental Table S1), regardless of the light level the plants were grown in. Overall, the total leaf area was larger in B2 and C4 than in the control at both 28 and 35 DAS in plants grown under 200 μmol m−2 s−1 (Table 1; Fig. 7, D–F). Similar results were obtained from plants grown under 100 μmol m−2 s−1 (Supplemental Table S1). In fact, at maturity (50 DAS), the total leaf area was greater in most of the isoprene-emitting lines than in the four control lines (Fig. 7G).
Figure 7.
Leaf growth in Arabidopsis expressing ISPS. A comparison of rosette size reflecting projected leaf area (A–C) and leaves after being separated from the rosettes reflecting apparent total leaf area (D–F) in 35-d-old Arabidopsis empty vector control (EV-B3) and lines expressing ISPS (B2 and C4) are shown. For A to F, all plants were grown in growth chambers under a light intensity of 200 μmol m−2 s−1. G shows a comparison of total leaf area of all lines used in this study. Plants used in G were 50 d old and grown at 120 μmol m−2 s−1. In G, Arabidopsis lines expressing ISPS that are significantly different from Col-0 and empty vector lines EV-A2 and EV-B3 are marked with asterisks: *, P < 0.05 and **, P < 0.01 using one-way ANOVA followed by Fisher’s lsd test.
Table 1. Plant growth in Arabidopsis expressing ISPS.
Leaf area, leaf curling, dry weights of leaf, stem, and aboveground plant organs, LMA, length of leaf, number of leaves, and inflorescence stem height were measured in 28- and 35-d-old Arabidopsis empty vector control (EV-B3) and lines expressing ISPS (B2 and C4). All plants were grown in growth chambers under a light intensity of 200 μmol m−2 s−1. Nd, Not determined. Different lowercase letters indicate significant differences between the three lines at P < 0.05 using one-way ANOVA followed by Fisher’s lsd test. Values represent means ± se (n = 5 plants per line).
| Age | Plant Line | Leaf Area (cm2) | Curling | Weight (mg) | LMA (g/m2) | Leaf Length (cm) | Leaf No. | Inflorescence Stem Height | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Leaf | Stem | Aboveground | ||||||||
| Day 28 | EV-B3 | 46.0 ± 4.2 b | Nd | 89 ± 4 b | Nd | Nd | 19.7 ± 1.0 a | 4.88 ± 0.16 b | 27.4 ± 1.3 a | Nd |
| B2 | 57.1 ± 4.3 a,b | Nd | 129 ± 5 a | Nd | Nd | 23.1 ± 1.7 a | 5.41 ± 0.21 a,b | 28.2 ± 0.9 a | Nd | |
| C4 | 60.1 ± 2.6 a | Nd | 129 ± 4 a | Nd | Nd | 21.7 ± 1.3 a | 5.48 ± 0.11 a | 29.6 ± 1.1 a | Nd | |
| Day 35 | EV-B3 | 104 ± 5 b | 14.7 ± 1.5 a | 262 ± 1.6 b | 7 ± 0.0 b | 269 ± 16 b | 25.2 ± 0.5 a | 6.05 ± 0.13 b | 31.4 ± 1.7 b | 0.86 ± 0.07 b |
| B2 | 126 ± 3 a | 12.3 ± 1.3 a | 335 ± 6 a | 10 ± 1 a,b | 345 ± 7 a | 26.5 ± 0.5 a | 6.61 ± 0.07 a | 37.2 ± 1.6 a | 1.54 ± 0.21 a | |
| C4 | 118 ± 4 a | 10.1 ± 1.8 a | 319 ± 6 a | 15 ± 2 a | 334 ± 13 a | 27.0 ± 0.5 a | 6.46 ± 0.09 a | 38.0 ± 1.9 a | 1.68 ± 0.15 a | |
Regardless of the growth light intensity, leaf, inflorescence stem, and total aboveground dry weight in B2 and C4 was greater than in EV-B3 (Table 1; Supplemental Table S1). However, there was no significant difference in the leaf mass per unit leaf area (LMA; Table 1; Supplemental Table S1).
In 35-d-old B2 and C4 lines grown under a light intensity of 200 μmol m−2 s−1, leaves curled less compared with those of EV-B3, although the difference was not statistically significant (Table 1; Fig. 7). However, a significant difference in the degree of leaf curling was found between emitting and nonemitting lines when these plants were grown under 100 μmol m−2 s−1 light (Supplemental Table S1).
Isoprene-emitting lines B2 and C4 also showed early inflorescence initiation when grown under 100 μmol m−2 s−1 (Supplemental Table S1). The height of the primary inflorescence axis or inflorescence stem was also significantly longer in B2 and C4 than in EV-B3 (Table 1).
Plant Pigment Content in Arabidopsis Expressing ISPS
While lines B2 and C4 with the highest isoprene emission rates always produced the highest leaf pigment concentrations, other isoprene-emitting lines also produced higher pigment concentrations relative to the controls (Fig. 8). The chlorophyll (Chl) a/b ratio was unchanged in isoprene-emitting lines. Enhanced Chl and carotenoid levels in B2 and C4 were also seen despite growth light conditions.
Figure 8.
Comparison of photosynthetic pigment concentrations in Arabidopsis expressing ISPS. Concentrations of Chl a (A), Chl b (B), and total Chl (C), Chl a/b ratio, and carotenoid concentration (E) in leaves harvested from 49-d-old Arabidopsis wild-type Col-0, empty vector controls (EV-A2, EV-B3, and EV-F1), and lines expressing ISPS (A1, F2, C1, C4, and B2) are shown. All transgenic lines belonged to the T2 generation. Values represent means ± se (n = 4 or 5 plants per line). Arabidopsis lines expressing ISPS that are significantly different from Col-0 and empty vector lines EV-A2 and EV-B3 are marked with symbols: +, P < 0.1 and *, P < 0.05 using one-way ANOVA followed by Fisher’s lsd test.
Tobacco
Plants grown from seeds of a nonemitting, azygous control (NE) and an emitting line (IE) of tobacco corresponding to line 32 originally described by Vickers et al. (2009b) were studied for comparison with the Arabidopsis model system generated by us.
Photosynthesis and Isoprene Emission
The isoprene emission status of the tobacco lines was confirmed. The photosynthetic rate in NE and IE tobacco was stable from day 27 to day 54, and no significant difference was found between the two lines (Fig. 9A). Isoprene was not released from NE lines, while its emission rate increased during growth of the transformed line, with increases of 60% and 75% on day 41 and day 54, respectively, compared with day 27 (Fig. 9A). A modest but significant improvement in thermotolerance was observed (Supplemental Fig. S6). To compare with that seen in Arabidopsis, we also reconfirmed the responses of isoprene to temperature and light studied in tobacco by Vickers et al. (2009b) and examined isoprene responses to CO2 and exposure to sudden darkness.
Figure 9.
Comparison of photosynthesis and isoprene emission under varying environmental conditions in NE and IE tobacco. Photosynthesis and isoprene emission measured simultaneously in 27-, 41-, and 54-d-old NE and IE tobacco (A), and responses of photosynthesis and isoprene emission to temperature (B), CO2 (C), light (D), and darkness (E) in 43- to 47-d-old NE and IE tobacco, are shown. Measurements were taken at 30°C (A and C–E) and 800 μmol m−2 s-1 light intensity (A–C). In E, plants were under 800 μmol m−2 s-1 light before being exposed to sudden darkness. Values in A to D represent means ± se; values in E represent means (n = 5).
Response of Photosynthesis and Isoprene Emission to Temperature, CO2, Light, and Darkness
Photosynthesis in NE and IE lines increased with increasing temperature up to 35°C and then declined above that temperature (Fig. 9B), but there was no substantial difference between the two lines. Isoprene emission rate from IE plants increased with increasing temperature, and NE plants began to release isoprene at high temperature at very low but easily measured rates. At 35°C, NE plants had an emission rate of 0.27 nmol m−2 s−1, which was further elevated with increasing temperature.
Photosynthesis rates increased with rising CO2 concentration both in NE and IE lines and did not differ between the two lines (Fig. 9C). Isoprene emission from IE plants increased with CO2 concentration from 0 to 250 μmol mol−1, remained stable between 250 and 500 μmol mol−1 CO2, then declined above 500 μmol mol−1. Isoprene emission was not detected in NE plants at any CO2 level.
Photosynthesis increased with light intensity in NE and IE plants (Fig. 9D). A weak decrease was found above 1,000 μmol m−2 s−1. However, there was no significant difference in photosynthesis rate between the two lines. Isoprene emission increased with increasing light intensity in IE, while no isoprene emission was seen in NE lines.
When IE leaves kept under a light intensity of 800 μmol m−2 s−1 were abruptly exposed to darkness, isoprene emission quickly declined by 94% (Fig. 9E). However, unlike Arabidopsis, an emission peak appeared after 13.7 min in darkness in tobacco (Figs. 5D and 9E), similar to that observed in oak and poplar (Li et al., 2011).
MEP Pathway Metabolomics
Tobacco leaves had lower levels of MEP and higher levels of MEcDP and HMBDP than Arabidopsis regardless of whether they were making isoprene or not (Fig. 6B; Supplemental Fig. S4). In tobacco, both MEcDP and HMBDP were higher than deoxyxylulose phosphate or MEP. The high level of MEcDP is consistent with the postillumination burst of isoprene described above (Fig. 9E). The high level of HMBDP was not seen in metabolomics of aspen (Li and Sharkey, 2013a).
Plant Growth
Vickers et al. (2009b) did not see a statistically significant difference in growth between mature NE and IE plants (see plant line 32 in supplementary data in Vickers et al., 2009b). We examined the growth of these lines under normal (unstressed) growth conditions in both young seedlings and mature plants.
There was a significant difference in cotyledon area between NE and IE tobacco grown in soil, whether they were grown in the greenhouse or in growth chambers. In contrast to that seen in Arabidopsis, the cotyledon area of IE lines grown in the greenhouse and in growth chambers was reduced by 34% and 24%, respectively, compared with that in NE (Fig. 10A). This difference was also found in tobacco grown in rock wool in the growth chamber (Fig. 10B). However, similar to Arabidopsis, IE seedling hypocotyls were taller than those of NE plants (Fig. 10C).
Figure 10.
Comparison of cotyledon and hypocotyl growth in NE and IE young tobacco seedlings. Cotyledon areas of NE and IE young tobacco seedlings grown in Suremix either in the greenhouse or in the growth chamber (under a light intensity of 200 μmol m−2 s−1) at 12 DAS (A), and representative photographs of cotyledons (B) and hypocotyls (C) of tobacco grown hydroponically in rock wool in 2-mL tubes in a chamber with a light intensity of 200 μmol m−2 s−1 at 10 DAS, are shown. Different lowercase letters indicate significant differences between NE and IE at P < 0.05 using one-way ANOVA followed by Fisher’s lsd test. Values in A represent means ± se (n ≥ 12 plants per line).
Leaf area in 27-, 41-, and 54-d-old IE lines was smaller than that in NE lines (Table 2; Fig. 11, A–E). Stem height of the IE lines was also significantly shorter than NE lines (Table 2; Fig. 11, A, C, and F). Consistently, the weight of leaf, stem, and aboveground parts in IE plants was also less (Table 2). Opposite trends were seen in Arabidopsis (Table 1; Figs. 3, 4, and 7). The decrease in leaf area and leaf mass in IE agrees with data obtained by Vickers et al. (2009b). In general, LMA was greater in IE leaves than in NE leaves (Table 2).
Table 2. Comparison of plant growth in NE and IE tobacco.
Leaf area, dry weights of leaf, stem, and aboveground plant organs, LMA, and plant stem height were measured in 27-, 41-, and 54-d-old tobacco plants. Different lowercase letters indicate significant differences between NE and IE at P < 0.05 using one-way ANOVA followed by Fisher’s lsd test. Values represent means ± se (n = 5 plants per line).
| Age | Plant Line | Leaf Area (cm2) | Weight (g) | LMA (g⋅m−2) | Stem Height (cm) | ||
|---|---|---|---|---|---|---|---|
| Leaf | Stem | Aboveground | |||||
| Day 27 | NE | 56.4 ± 1.9 a | 0.069 ± 0.004 a | 0.009 ± 0.001 a | 0.078 ± 0.004 a | 12.2 ± 0.3 b | 3.3 ± 0.2 a |
| IE | 43.4 ± 2.2 b | 0.058 ± 0.003 b | 0.006 ± 0.001 b | 0.064 ± 0.004 b | 13.5 ± 0.4 a | 2.3 ± 0.1 b | |
| Day 41 | NE | 532 ± 16 a | 0.80 ± 0.05 a | 0.32 ± 0.02 a | 1.13 ± 0.07 a | 15.1 ± 0.5 a | 24.9 ± 0.8 a |
| IE | 314 ± 18 b | 0.51 ± 0.02 b | 0.21 ± 0.01 b | 0.71 ± 0.03 b | 16.2 ± 0.4 a | 19.8 ± 0.6 b | |
| Day 54 | NE | 2,320 ± 61 a | 3.75 ± 0.06 a | 2.39 ± 0.06 a | 6.16 ± 0.11 a | 16.2 ± 0.3 a | 61.0 ± 2.0 a |
| IE | 1,531 ± 51 b | 2.42 ± 0.09 b | 1.49 ± 0.22 b | 3.91 ± 0.30 b | 15.8 ± 0.3 a | 46.7 ± 0.7 b | |
Figure 11.
Comparison of leaf and plant growth over time in NE and IE tobacco. Photographs depict the front and top views of tobacco plants taken at day 27 (A and B), day 41 (C and D), and day 54 (E and F) of plant growth. In G, the shape of leaf tips of the sixth mature leaf is compared.
Interestingly, the shape of the leaf tip in leaves appearing after the sixth leaf differed between IE and NE lines. The sixth leaf produced by NE lines still had a sharp, pointed leaf tip, while in IE it was notched (Fig. 11G).
Differences in Pigment Content
Tobacco IE leaves had more Chl a, Chl b, and carotenoids than NE leaves (Table 3). However, the Chl a/b ratio was not different between the two lines. Vickers et al. (2009b) did not see a difference in pigment levels measured in µg g−1 fresh weight between NE and IE. For comparison, we also estimated pigment content in mg g−1 dry weight, taking into account differences in LMA ( Table 3). The increase in pigment concentration in IE lines was still evident even when expressed in mg g−1 leaf dry weight, which shows that observed differences in pigment content between NE and IE when measured based on leaf area are not a result of variations in LMA.
Table 3. Pigment content in NE and IE tobacco.
The Chl a, Chl b, and carotenoid (Car) pigment contents obtained from mature leaves harvested from 27-, 41-, and 54-d-old tobacco plants are shown. Raw data for LMA (g m−2 leaf area) and pigment content (μg m−2 leaf area) were used to estimate pigment content (mg g−1 leaf dry weight). Different lowercase letters indicate significant differences between NE and IE at P < 0.05 using one-way ANOVA followed by Fisher’s lsd test. Values represent means ± se (n = 5 plants per line).
| Age | Plant Line | Pigment Content (μg⋅m−2) | Pigment Content (mg⋅g−1 DW) | ||||
|---|---|---|---|---|---|---|---|
| Chl a | Chl b | Car | Chl a | Chl b | Car | ||
| Day 27 | NE | 12.3 ± 0.2 b | 4.1 ± 0.1 a | 2.6 ± 0.1 b | 10.06 ± 0.19 a | 3.36 ± 0.07 a | 2.16 ± 0.04 a |
| IE | 13.4 ± 0.3 a | 4.4 ± 0.1 a | 2.9 ± 0.1 a | 9.97 ± 0.22 a | 3.26 ± 0.06 a | 2.19 ± 0.04 a | |
| Day 41 | NE | 17.7 ± 0.4 b | 5.6 ± 0.1 b | 3.7 ± 0.1 a | 11.75 ± 0.28 b | 3.70 ± 0.07 b | 2.46 ± 0.07 a |
| IE | 19.8 ± 0.5 a | 6.5 ± 0.1 a | 4.0 ± 0.1 a | 12.31 ± 0.32 a | 4.06 ± 0.08 a | 2.49 ± 0.07 a | |
| Day 54 | NE | 19.5 ± 0.4 b | 6.8 ± 0.1 b | 3.8 ± 0.1 a | 12.04 ± 0.25 b | 4.18 ± 0.07 b | 2.37 ± 0.06 b |
| IE | 21.7 ± 0.7 a | 7.4 ± 0.2 a | 4.1 ± 0.2 a | 13.79 ± 0.45 a | 4.72 ± 0.14 a | 2.59 ± 0.10 a | |
Effects of Isoprene on Gene Expression in Arabidopsis and Tobacco
In Arabidopsis expressing ISPS (line B2), 551 genes were differentially expressed when compared with expression in the empty vector line EVB3. Of these, 292 had higher expression and 259 had lower expression at α = 0.1 (at α = 0.05, 108 genes were up-regulated and 87 were down-regulated; Supplemental File S1). In tobacco expressing ISPS (IE), 3,779 genes were differentially expressed, out of which 1,732 were up-regulated and 2,067 were down-regulated at α = 0.1 (at α = 0.05, 1,321 genes were up-regulated and 1,345 were down-regulated; Supplemental File S2). We compared differentially expressed genes in the above two systems and those found in Arabidopsis fumigated with isoprene (Harvey and Sharkey, 2016) to identify isoprene-responsive genes common to at least two or all three systems. These data are summarized in Supplemental Tables S2 to S5. Although only one isoprene-emitting line was used for Arabidopsis (B2) and tobacco (IE), we believe that selecting differentially expressed genes common to more than one system facilitated the selection of genes reflective of the isoprene treatment regardless of both interspecies variation and position-of-integration effects. Although altered in expression in all three systems, uncharacterized genes were not included in Supplemental Tables S2 to S5. A list of descriptive gene names of all the genes mentioned in the following text is provided in the Supplemental Materials under the title "List of gene/domain names used in the paper".
Isoprene Alters Expression of Genes of Specific Growth Regulator Signaling Pathways
Transcriptomic analyses revealed that expression of key genes important for the synthesis of specific growth regulators, as well as key components of these growth regulator-signaling pathways, were altered in plants exposed to isoprene either internally (Arabidopsis and tobacco expressing ISPS) or externally (Arabidopsis fumigated with isoprene; Supplemental Table S2). Isoprene altered gene expression to support the enhancement of jasmonic acid (JA), cytokinin (CK), gibberellins (GA), and ethylene signaling. The altered genes affecting JA include BBD1, BBD2, LOX1, LOX3, LOX4, LOX5, OPR3, and MYB59. Genes affecting cytokinins include KFB20 and WIND1. Those affecting gibberellins include TZF5, MARD5, TEM1, and DFL2. Genes related to ethylene include EBF1, EBF2, and ACO4. Changes in transcription of key genes in brassinosteroid (BR; BRI1), auxin (MYB73, BPC, and KFB20), and salicylic acid, (SA; CBP60A, ATAF1, EBF1, and EBF2) signaling provided evidence for the down-regulation of these pathways in the presence of isoprene. Interestingly, expression of genes belonging to the abscisic acid (ABA) signaling branch required to achieve drought tolerance (NCED3, NCED5, and ATAF1) was enhanced in response to isoprene, while expression of genes involved in ABA signaling associated with seed dormancy (TZF5, MARD1, NCED4, and MYBS2), pathogen resistance (BBD1, BBD2, and MPK7), and heat (NCED4) and salt (CIPK20) stress appeared to be down-regulated (Supplemental Table S2).
Among the isoprene-responsive genes associated with the biosynthesis of specific growth regulators and their signaling were a number encoding MYB family transcription factors (MYB59, MYB73, and MYBS2), an AP2/ERF transcription factor (WIND1), a RAV1-like ethylene-responsive transcription factor (TEM1), a NAC transcription factor (ATAF1), a basic penta-Cys transcription factor (BPC4), and a calmodulin-binding transcription factor (CBP60A; Supplemental Table S2). Genes responsive to isoprene also encoded different types of proteins, such as F-box proteins (KFB20, EBF1, and EBF2), zinc finger proteins (TZF5 and MARD1), GH3-related proteins (DFL2), and nucleases (BBD1 and BBD2), oxygenases (LOX1, LOX3, LOX4, LOX5, NCED3, NCED4, and NCED5), oxidases (ACO4), reductases (OPR3), and different types of kinases, including Ser/Thr (CIPK20), mitogen-activated (MPK7), and Leu-rich repeat receptor (BRI1) kinases (Supplemental Table S2).
Isoprene Alters Expression of Genes That Lead to Abiotic Stress Tolerance
Even though we used unstressed plants for gene expression analyses, the expression of a number of genes in abiotic stress signaling pathways was altered in response to isoprene (Supplemental Table S3). Isoprene seemed to affect gene expression that leads to detoxification of heavy metals (MRP2, MRP4, MRP5, MRP8, MRP11, HIPP32, and MYB59), protection against xenobiotics and excess secondary metabolites (MRPs), and enhanced tolerance to soil acidity (NRT1.1), high/fluctuating/low light (MPH1, MPH2, NDHL, NDHN, and SAUL1), heat (NCED4, MRP5, NRT1.1, ADC1, ADC2, CuAO3, NDHL, NDHN, PTI1, COP1, PAL, and other genes of the phenylpropanoid pathway), salinity (MYB73, CIPK20, NCED3, ADC1, ADC2, and PIP5K2), drought (NCED3, NCED5, ATAF1, MRP4, CuAO3, and WIND1), and oxidative stress (NADK1, AXS2, CATHB3, ADC1, ADC2, and PTI1). Tolerance against heat and oxidative stress are well-known protective functions of isoprene in plants. In contrast, gene expression associated with freezing tolerance appeared to be reduced in the presence of isoprene (CRPK1 and LTP3).
Interestingly, isoprene-responsive genes involved in abiotic stress tolerance included many proteins involved in transport mechanisms: ABC transporters (MRP2, MRP4, MRP5, MRP8, and MRP11), metallochaperones (HIPP32), NO3− transporters (NRT1.1), and proteins associated with lipid transport (LPT3) and those that induce bulk-flow endocytosis (PIP5K2; Supplemental Table S3). Isoprene-responsive genes that promoted abiotic stress coded for proteins important for cation (Ca2+ and NO3−) signaling and homeostasis (MYB59, MRPs, and NRT1.1), membrane proteins (MRPs, MPH1, NRT1.1, CRPK1, NDHL, and NDHN), different types of kinases, including Ser/Thr (CIPK20 and PTI1), cold-responsive (CRPK1), phosphatidylinositol monophosphate 5-kinase2 (PIP5K2), and NAD(H) kinase (NADK1), transcription factors (WIND1, MYB59, ATAF1, and MYB73), and secondary metabolite synthesis and sequestration, such as glucosinolate sequestration (MRPs), polyamine biosynthesis (ADC1, ADC2, and CuAO3), phenylpropanoid biosynthesis and regulation (PAL1, PAL2, PAL4, CPK1, 4CLI1, 4CLI2, 4CLI3, CCOAMT, DHQ-SDH, and KFB20; Supplemental Tables S3 and S4), phosphatidylinositol-4,5-bisphosphate synthesis (PIP5K2), and apiose and apiin biosynthesis (AXS2; Supplemental Table S3). There were also E3 ubiquitin ligases (SAUL1), oxidases (CuAO3), decarboxylases (ADC1 and ADC2), proteases (CATHB3), synthases (AXS2), and oxygenases (NCED3, NCED4, and NCED5) associated with abiotic stress responses whose expression was altered by isoprene (Supplemental Table S3).
Functions of proteins encoded by ADC1, ADC2, CuAO3, NADK1, MPH1, MPH2, PAL1, PAL2, PAL4, CPK1, 4CLI1, 4CLI2, 4CLI3, CCOAMT, DHQ-SDH, and KFB20 include stabilization of thylakoid membranes under heat, salt, and oxidative stress, remedying of oxidative damage under a variety of stresses, including hydrogen peroxide (H2O2) and ozone, prevention of photoinhibition, repair of PSII under high light, etc.
Isoprene Alters Expression of Genes That Enhance Resistance to Biotic Stress
Transcriptomic data also revealed that isoprene affects the expression of key genes that are components of signaling pathways related to biotic stress, such as herbivory and wounding, and infection by necrotrophic and biotrophic pathogens (Supplemental Table S4). Isoprene-responsive gene expression supported resistance against herbivory and wounding (LOX1, LOX3, LOX4, LOX5, OPR3, MRP2, MRP4, MRP5, MRP8, MRP12, ADC1, ADC2, MYB59, WIND1, PAL1, PAL2, PAL4, CPK1, 4CLI, CCOAMT, DHQ-SDH, KFB20, AXS2, and CYP81D8). Most of these genes belong to JA-mediated defense signaling, phenylpropanoid biosynthesis and regulation, CK-mediated wound repair, as well as signaling mediated by oxylipin, glutathione, and apiose (Supplemental Table S4). Isoprene-mediated resistance against herbivory has been reported (Laothawornkitkul et al., 2008). Almost all enzymes of the phenylpropanoid pathway were up-regulated in Arabidopsis fumigated with isoprene (Harvey and Sharkey, 2016), and expression of PAL, 4CLI, CCOAMT, and DHQ-SDH was also up-regulated in tobacco expressing ISPS (Supplemental Table S4). In addition, expression of CPK1, which encodes a calcium-dependent protein kinase that phosphorylates and activates PAL (Cheng et al., 2001; Boudsocq and Sheen, 2013), was up-regulated in all three model systems tested; expression of KFB20, which encodes a protein that physically interacts with and targets PAL isozymes for degradation through ubiquitination (Zhang et al., 2013), was suppressed in both Arabidopsis expressing ISPS and Arabidopsis fumigated with isoprene. Expression of PAL, 4CLI, CCOAMT, and DHQ-SDH was significantly down-regulated in poplar subjected to RNAi-mediated suppression of ISPS under moderate heat stress (Behnke et al., 2010), supporting the hypothesis that the changes in gene expression observed in our study were mediated by isoprene. There were no statistically significant changes in the expression of phenylpropanoid pathway enzymes in Arabidopsis expressing ISPS.
Isoprene altered gene expression that can enhance resistance against biotrophic pathogens (MORC7, BRI1, PCRK1, PTI1, GH9C2, and BRI1; Supplemental Table S4). However, some isoprene-responsive genes appeared to reduce resistance against the biotrophic pathogens Pseudomonas syringae pv maculicola strain ES4326 (CBP60A) and Pseudomonas syringae pv tomato strain DC3000 (ATAF1, EBF1, EBF2, and MPK7; Supplemental Table S4). Data indicated that isoprene-responsive genes that supported enhanced resistance to biotrophs belonged to signaling pathways independent of SA and ABA and that isoprene-mediated gene expression that supports the suppression of SA and ABA pathways increases the susceptibility of isoprene-emitting plants to biotrophs.
Isoprene suppressed the expression of the Leu-rich repeat receptor kinase BRI1 (Supplemental Table S4), the main BR receptor localized in the plasma membrane, which launches a signaling cascade with subsequent induction of BR-induced changes in gene expression (Lozano-Durán and Zipfel, 2015). Suppression of this BRI1 has been shown to enhance resistance against many necrotrophic pathogens, like Fusarium pseudo-graminearum, Fusarium culmorum, Pyrenophora teres, Gaeumannomyces graminis, Oculimacula spp., and Barley stripe mosaic virus (Lozano-Durán and Zipfel, 2015). However, alteration of the expression of some genes may lead to enhanced susceptibility to the necrotrophic pathogens Botrytis cinerea (BBD1, BBD2, and ATAF1) and Alternaria brassicicola (ATAF1; Supplemental Table S4). Thus, suppression of BRI1 and WAKL6 by isoprene supported resistance against many necrotrophic pathogens.
Isoprene-responsive genes that favored resistance to herbivory and wounding, as well as biotrophic and necrotrophic pathogens, also included additional proteins such as calcium-dependent protein kinase (CPK1), MAPK (MPK7), cytochrome P450 SA-independent systemic acquired resistance gene (CYP81D8), and membrane proteins such as GHKL ATPase (MORC7), receptor-like cytoplasmic kinase (PCRK1), Leu-rich repeat receptor kinase (BRI1), and transmembrane protein with a cytoplasmic Ser/Thr kinase domain (WAKL6; Supplemental Table S4). Among the isoprene-responsive genes that enhance response to biotic stress were three genes that enhance the synthesis of structural carbohydrates: apiose (also a secondary metabolite; AX2), callose (WIND1, BBD1, BBD2, and PCRK1), and cellulose (G69C2).
Isoprene Alters Expression of Genes That Affect Photosynthesis, Seed Germination, and Seedling and Plant Growth
Expression of genes important for photosynthesis and plant growth was also significantly affected by isoprene (Supplemental Table S5). For example, expression of PRPS20 (Wu et al., 2007; Romani et al., 2012; Gong et al., 2013; Tiller and Bock, 2014) and RPL24 (Tiller et al., 2012; Liu et al., 2014; Tiller and Bock, 2014), which lead to enhancement of chloroplast development and Chl production, was up-regulated in the presence of isoprene. CK can also promote Chl production and delay senescence (Supplemental Table S2). These gene expression data are in agreement with enhanced pigment concentrations in leaves of both Arabidopsis and tobacco emitting isoprene.
Genes encoding subunits of the NADH dehydrogenase-like complex (NDHL and NDHN) associated with PSI, which is involved in cyclic electron flow that protects PSII under abiotic stress, as well as thylakoid and stromal proteins important for protecting and stabilizing PSII under high light stress (MPH1 and MPH2), were also up-regulated by isoprene (Supplemental Table S5). Isoprene also altered gene expression in a manner that can lead to reduced chloroplast movement under blue light (WEB1) and increase polyamine biosynthesis and protection of photosynthetic components against a variety of stresses (ADC1, ADC2, and CuAO3), including heat, salt, and oxidative stress.
A number of genes that are crucial for early seedling establishment were altered by isoprene, which included genes essential for seed dormancy inhibition and enhanced seed germination (MYBS2, EBS, TZF5, NCED4, MARD1, ADC1, and ADC2), proper embryo development (PRPS20, RPL24, EMB2184, and MYBS2), seedling growth (PRPS20, RPL24, EMB2184, MYBS2, DFL2, and COP1), and elongation of hypocotyls (DFL2, TEM1, and COP1; Supplemental Table S5). Isoprene also altered gene expression in favor of enhanced leaf expansion and growth (TEM1, TZF5, AXS2, and PIP5K2), root growth (PIP5K2, MYB59, NRT1.1), and early flowering (TZF5, TEM1, COP1, ZFP8, EBS, WIND1, and AGP3). Growth-promoting genes included chloroplast ribosomal subunit genes (PRPS20, RPL24, and EMB2184), GA-signaling pathway genes (TZF5 TEM1, DFL2, and MARD1), interactors of phytochrome interacting factors or PIF (COP1), CK-signaling pathway genes (WIND1), cell cycle regulators (MYB59), transcription factors (ZFP8, MYBS2, MYB59, and EBS), apiose (AXS2) and polyamine synthesis genes (ADC1 and ADC2), and an auxin transport and phosphoinositide synthesis gene (PIP5K2). These data correspond well with the observed growth enhancement in seedlings and rosettes as well as the early-flowering phenotype seen in Arabidopsis isoprene-emitting lines. Interestingly, the growth-promoting genes were associated with GA and PIF signaling and were mostly differentially expressed in the two Arabidopsis model systems (Supplemental Table S5). Growth-promoting genes expressed in tobacco were PRPS20, RPL24, EMB218, COP1, ZFP8, MYBS2, EBS, AXS2, and PIP5K2. The reasons why tobacco showed reduced growth will be discussed later. A few genes were altered in a manner that can have a negative impact on growth (LTP3, PATL1, ZIM, BRI1, and BPC4). Isoprene also altered gene expression that can prevent (TEM1 and SAUL1) or enhance (ATAF1 and ADC7) senescence.
DISCUSSION
Genetically engineering nonemitting wild-type plant species (e.g. Arabidopsis and tobacco) to express ISPS (Sharkey et al., 2005; Loivamäki et al., 2007; Sasaki et al., 2007; Vickers et al., 2009b) and to suppress ISPS in plants known to be strong emitters (Behnke et al., 2007) has been a useful method to investigate the effects of isoprene on plant stress tolerance. In this study, we used Arabidopsis lines transformed to express ISPS to determine growth, photosynthesis, and isoprene emission in unstressed plants and to study how the regulation of isoprene emission takes place when ISPS is introduced to plants such as tobacco and Arabidopsis that do not normally have ISPS. We also investigated the role of isoprene as a signaling molecule and the key signaling pathways affected by isoprene. We saw that although Arabidopsis and tobacco do not normally produce isoprene, when engineered to do so, the same responses to temperature, CO2, and light can be observed as in native emitters. Hypocotyl elongation was promoted in isoprene-emitting Arabidopsis and tobacco. Isoprene promoted cotyledon and leaf expansion, length and number of leaves, growth rates, and leaf and whole-plant dry weights and resulted in early flowering and taller inflorescences in Arabidopsis; cotyledon and leaf growth was reduced in tobacco IE lines. Transformation with ISPS brought about significant increases in Chl and carotenoid contents in both Arabidopsis and tobacco. These phenotypes observed in the isoprene-emitting transgenic lines were a result of isoprene and not an effect of growth light or as a result of changes in the MEP pathway metabolites. The mechanisms through which isoprene may exert changes in plant growth, the role of isoprene as a signaling molecule, and the significance of our findings are discussed in the following sections.
The DMADP Pools in Nonemitting Plants Is Sufficient to Sustain Isoprene Synthesis under Different Environmental Conditions
Examination of the patterns of isoprene emission under normal conditions as well as under different levels of temperature, CO2, and light revealed that the chloroplastic DMADP pools in both tobacco and Arabidopsis are sufficient to maintain isoprene production under different environmental conditions. Tobacco had a higher level of MEcDP, similar to levels seen in aspen (Li and Sharkey, 2013a). Arabidopsis had relatively more MEP. DMADP synthesis from MEcDP is highly dependent on reducing power (Banerjee and Sharkey, 2014). Conversion of MEP to MEcDP is dependent on ATP (Banerjee and Sharkey, 2014). Higher levels of MEP in Arabidopsis compared with tobacco, and higher levels of MEcDP in tobacco compared with Arabidopsis, indicate that Arabidopsis may be more limited by ATP while tobacco may be more limited by reducing power. The fact that MEP pathway metabolites were not altered suggests that the alteration in phenotypes observed in the isoprene-emitting transgenic lines is in fact a result of isoprene and not a result of changes in the MEP pathway metabolites, such as MEcDP, which can act as a retrograde signal (Xiao et al., 2012). As only one representative tobacco line was used for the biochemical analysis above, the use of more transgenic lines is necessary to further confirm the above comparisons.
Although the DMADP pools in Arabidopsis were lower than those in tobacco (this study) and aspen (Li and Sharkey, 2013a), the emission rates were slightly higher than those reported previously in transformed Arabidopsis (Sharkey et al., 2005; Loivamäki et al., 2007). For example, isoprene emission rates in Arabidopsis transformed with an ISPS from gray poplar showed uppers limit of about 2.5 nmol m−2 s−1 at 30°C and 3 nmol m−2 s−1 at 40°C (see Figure 7I in Loivamäki et al., 2007). In Arabidopsis transformed with an ISPS from kudzu, an isoprene emission rate of 1.32 nmol m−2 s−1 was detected at 30°C (see Figure 10 in Sharkey et al., 2005). In this study, the emission rate was greater than 6 nmol m−2 s−1 at 40°C in B2 (Fig. 5A).
While DMADP limitation may play a role in restricting isoprene emission in Arabidopsis compared with that in tobacco (this study) and other species such as aspen, poplar, etc., the difference in isoprene emission rates between our study and Loivamäki et al. (2007) and Sharkey et al. (2005) may be due to the different promoters used to express ISPS and/or differences between kinetic properties of the ISPS. A constitutive Cauliflower mosaic virus 35S promoter with gray poplar ISPS was used by Loivamäki et al. (2007) and a promoter from kudzu with a kudzu ISPS coding sequence was used by Sharkey et al. (2005) to transform Arabidopsis. In contrast, we used an rbcS-1A promoter to express an ISPS gene from E. globulus. It is known that rbcS-1A drives high rates of gene expression (Izumi et al., 2012). The Km values of ISPS for DMADP reported for gray poplar and kudzu are 2.4 mm (Schnitzler et al., 2005) and 7.7 mm (Sharkey et al., 2005), respectively. In contrast, the Km of E. globulus ISPS is much lower, around 0.03 mm (Sharkey et al., 2013). Thus, we hypothesize that the comparatively higher rates of isoprene emission observed in Arabidopsis during this study may be the result of the chloroplast-specific rbcS-1A promoter and the higher affinity E. globulus ISPS for DMADP.
The Regulation of Isoprene Emission Is Similar in Genetically Engineered and Natural Isoprene-Emitting Species
The regulatory mechanisms of photosynthesis and isoprene emission in Arabidopsis and tobacco lines genetically engineered to express ISPS functioned in a manner similar to that in native emitters, as evident by the responses to temperature, CO2, light, darkness, and N2. In addition, isoprene synthesis in these plants did not have a significant effect on photosynthesis under nonstressed conditions or under varying temperature, CO2, or light levels. While photosynthesis is the principal source of C and energetic cofactors (ATP, NADPH, and ferredoxin) for the MEP pathway (Sharkey et al., 2008; Banerjee and Sharkey, 2014), the rate of isoprene emission responds to higher temperature, CO2, and light differently than does the rate of photosynthesis (Li et al., 2011; Li and Sharkey, 2013b; Sun et al., 2013a; Way et al., 2013; Potosnak et al., 2014). Similarly, during our study isoprene and photosynthesis showed very different temperature responses and even some differences in light response.
It has been found that the effects of temperature on isoprene emission are complex because it affects both ISPS activity and substrate DMADP levels (Wiberley et al., 2008; Rasulov et al., 2010; Li et al., 2011; Vickers et al., 2011). As temperature gradually increases to about 35°C, isoprene emission increases because both the activities of ISPS and DMADP pools increase. Above this temperature, the DMADP pool begins to decline but ISPS activity still increases even at 40°C to 45°C, resulting in an increase in isoprene emission (Rasulov et al., 2010; Li et al., 2011; Li and Sharkey, 2013b; Sharkey and Monson, 2014). The optimum temperature of isoprene emission is usually between 40°C and 48°C (Singsaas and Sharkey, 1998; Niinemets et al., 1999; Singsaas et al., 1999; Rasulov et al., 2010).
Interestingly, a weak isoprene emission was found in Arabidopsis empty vector lines and tobacco NE at 35°C. This was also seen in nonemitting poplar (Behnke et al., 2013) and is assumed to be due to nonenzymatic conversion of DMADP to isoprene in the leaf (Brüggemann and Schnitzler, 2002; Ghirardo et al., 2010). The trivial amounts of isoprene measured in EV-F1 (30°C) and EV-B3 and NE at temperatures above 35°C are likely not due to bacteria in the soil, because nonenzymatic isoprene production in bacteria occurs only when grown on specific growth media (Kuzma et al., 1995; Wagner et al., 2000), and in the case of NE (and poplar), only the leaf was in the leaf chamber during isoprene measurements. In addition, even though the presence of isoprene-degrading bacteria on leaf surfaces has been reported (Crombie et al., 2018), the presence of isoprene-emitting bacteria on the leaf phyllosphere and their contribution to nonenzymatic isoprene production are not known.
Elevated [CO2] has different effects on isoprene emission: no or moderate effects in P. alba (Loreto and Velikova, 2001; Loreto et al., 2007), Populus tremuloides (Calfapietra et al., 2008), and Populus × euramericana (Centritto et al., 2004) as well as promoting effects in Gingko biloba (Li et al., 2009) and P. tremula × P. tremuloides (Sun et al., 2012). Elevated [CO2] inhibited isoprene emission in Phragmites australis (Scholefield et al., 2004), Liquidambar styraciflua (Monson et al., 2007; Wilkinson et al., 2009), Platanus orientalis (Velikova et al., 2009), Acacia nigrescens (Possell and Hewitt, 2011), and E. globulus, P. tremuloides, and P. deltoides (Wilkinson et al., 2009). Both Arabidopsis and tobacco exhibited a high CO2 suppression of isoprene emission in this study. This decrease has been shown to result from the reduction of DMADP pools under elevated [CO2] (Possell and Hewitt, 2011; Sun et al., 2012).
While we only used one Arabidopsis (B2) and one tobacco isoprene-emitting line to test how isoprene emission varies under different environmental conditions, the temperature and light response curves were consistent with those published before for tobacco (Vickers et al., 2009b). In addition, although not performed in this study, measurements of ISPS activity and DMADP pool sizes under varying environmental conditions would further greatly clarify how isoprene formation is regulated in Arabidopsis and tobacco transgenic lines.
The Postillumination Burst May Be Species Specific Owing to Differences in MEcDP Pool Sizes
When a plant exposed to light is transferred to darkness, a discrete peak of emission of isoprene is detected after about 10 min in darkness. This is called the postillumination burst (Monson et al., 1991; Rasulov et al., 2010, 2011; Li et al., 2011) and is representative of the pool size of plastidic MEcDP (Li and Sharkey, 2013b). The reasons for the postillumination burst have been characterized as follows. Initially, isoprene emission following the imposition of darkness results from the consumption of DMADP plus IDP pools (Rasulov et al., 2009; Li et al., 2011; Li and Sharkey, 2013a, 2013b). In the MEP pathway, the conversion of MEcDP to HMBDP by HMBDP synthase is dependent on photosynthetic light reactions for reducing power (Seemann et al., 2002, 2006; Okada and Hase, 2005; Seemann and Rohmer, 2007; Li and Sharkey, 2013a, 2013b). However, with time the conversion of MEcDP to HMBDP and then to DMADP and IDP resumes, either because reducing power is supplied by an alternative source, possibly the pentose phosphate pathway, or because HMBDP synthase changes from requiring ferredoxin to accepting NADPH (Seemann et al., 2006), leading to isoprene emission in the dark (Li and Sharkey, 2013a, 2013b).
Arabidopsis had a small pool of MEcDP and no postillumination burst, while tobacco had a high level of MEcDP and showed a significant postillumination burst. Holding Arabidopsis leaves in N2 did allow for an overshoot in isoprene emission, likely the result of the accumulation of MEcDP in the N2 atmosphere. Thus, although we only used one representative isoprene-emitting Arabidopsis (B2) and tobacco line for the above experiments, the responses were similar to those reported previously.
In summary, our data show that regulation of isoprene emission in tobacco and Arabidopsis transformed with ISPS is similar to that in native isoprene emitters. Therefore, these responses are likely related to inherent regulatory mechanisms of the MEP pathway rather than reflecting regulation specific to isoprene.
Isoprene Has a Positive Effect on Chlorophyll and Carotenoid Production
Besides isoprene, the chloroplastic DMADP pool is used to synthesize myriad other volatile terpenoids, secondary products, and growth regulators. Geranyl diphosphate (GDP) synthesized from DMADP and IDP is the precursor for monoterpenes (Rohmer et al., 1993). Geranyl geranyl diphosphate synthesized from GDP is used to synthesize the phytol tail of Chl, phytoalexins, phylloquinones, plastoquinones, carotenoids, and tocopherols (Laule et al., 2003; Hwang and Sakakibara, 2006; Lichtenthaler, 2007, 2009; Kim et al., 2013). Carotenoids act as precursors for ABA and strigolactone biosynthesis (Beltran and Stange, 2016). Geranylgeranyl diphosphate also acts as a precursor for GA biosynthesis (Sun, 2008). Cytokinins can be made from HMBDP of the MEP pathway, although they are also made in the cytosol from DMADP from the mevalonic acid pathway (Kakimoto, 2003), which should not interact with isoprene synthesis.
The intuitive assumption would be that the production of Chl and carotenoids would decrease when chloroplastic DMADP is diverted to isoprene production when ISPS is introduced to nonemitting plants. But this was not the case both in Arabidopsis and tobacco. We saw a positive relationship between isoprene emission and Chl a, Chl b, and carotenoid content in Arabidopsis, which indicates that isoprene production favors Chl and carotenoid production in a dosage-dependent manner. In contrast to Vickers et al. (2009b), where a change was not observed, leaf pigment levels were also enhanced in tobacco IE lines during this study. Vickers et al. (2009b) expressed pigment concentration in µg g−1 fresh weight, and it is possible that variations in leaf fresh weight obscured the small differences in pigment concentrations between IE and NE lines evident in other studies. For example, a substantial positive correlation between isoprene emission and Chl, carotenoids, and xanthophyll cycle pigment synthesis (measured in µmol m−2) was found in basket willow (Salix viminalis; Harris et al., 2016). Behnke et al. (2007) saw reduced Chl and carotenoid contents (measured in nmol mg−1 dry weight) when isoprene emission was reduced in gray poplar by RNAi-mediated suppression of ISPS. However, in another study, when measured in µmol g−1 fresh weight, a difference in pigment concentration was not observed between isoprene-emitting gray poplar and RNAi lines (Behnke et al., 2010). Similarly, there was no difference (but no decline) in photosynthetic pigment concentrations (measured in mg g−1 fresh weight) in controls and Arabidopsis expressing ISPS from gray poplar (Loivamäki et al., 2007). The above examples show that the differences in pigment concentrations between isoprene-emitting and nonemitting lines are usually not seen when expressed on a fresh weight basis.
The fact that isoprene production does not negatively affect pigment production in transgenic lines may be because the MEP pathway is controlled primarily by feedback (Banerjee and Sharkey, 2014) and because GDP synthase that forms GDP from DMADP and IDP has a much higher affinity for DMADP than ISPS (Tholl et al., 2001; Laule et al., 2003; Orlova et al., 2009; Wang and Dixon, 2009; Rasulov et al., 2014).
The increase in Chl and carotenoid biosynthesis may be as a result of increased expression of genes associated with pigment biosynthesis by isoprene. For example, expression of the plastid ribosomal proteins PRPS20 and RPL24 was increased in both Arabidopsis and tobacco model systems during this study. These have been shown to enhance the expression of important Chl biosynthesis genes, including HEMA1, CAO1, YGL1, and PORAl, to increase rRNA processing in chloroplasts, and to promote chloroplast development (Wu et al., 2007; Romani et al., 2012; Tiller et al., 2012; Gong et al., 2013; Liu et al., 2014; Tiller and Bock, 2014). In addition, isoprene-responsive gene expression favored enhanced CK signaling (KFB20), reduction of premature senescing (SAUL1 and TEM1), and polyamine biosynthesis genes in favor of spermidine and spermine synthesis (ADC1, ADC2, and CuAO3), all of which can promote Chl biosynthesis and retention (Capell et al., 2004; Mason et al., 2005; Raab et al., 2009; Woo et al., 2010; Liu et al., 2015; Groß et al., 2017).
In summary, our study along with data from previous studies provide strong evidence that the enhancement in photosynthetic pigment content in Arabidopsis and tobacco expressing ISPS is indeed due to isoprene and its effects on gene expression that promotes Chl and carotenoid biosynthesis.
Isoprene Affects Plant Growth and Development and Growth-Defense Tradeoffs
Isoprene caused significant effects on growth in both Arabidopsis and tobacco grown under unstressed conditions. The differences in growth between the isoprene-emitting plants and nonemitting plants in Arabidopsis and tobacco could be detected soon after germination. Both Arabidopsis and tobacco lines expressing ISPS produced seedlings with taller hypocotyls than the control lines, and cotyledons were larger in Arabidopsis and smaller in tobacco. We showed that these changes were not due to differences in growth conditions such as light level and growth media. This study found that isoprene affected the expression of a number of genes that promote embryo growth, seed germination, and seedling establishment, such as PRPS20, RPL24, EMB2184, MYBS2, EBS, NCED4, and COP1 (Gómez-Mena et al., 2001; Wu et al., 2007; Bryant et al., 2011; Muralla et al., 2011; Romani et al., 2012; Tiller et al., 2012; Bogamuwa and Jang, 2013, 2016; Gong et al., 2013; Huo et al., 2013; Liu et al., 2014; Tiller and Bock, 2014; Chen et al., 2017; Li et al., 2018), in both Arabidopsis and tobacco, which may have contributed to hypocotyl elongation.
Two genes that specifically reduce hypocotyl elongation, DFL2 and TEM1 (Takase et al., 2003; Woo et al., 2010; Matías-Hernández et al., 2014), were down-regulated by isoprene in the two Arabidopsis systems (Fig. 12). These genes are located downstream of PIF in the Phytochrome B (PHYB)-mediated red light signaling pathway (Takase et al., 2003; Woo et al., 2010; Matías-Hernández et al., 2014). Interestingly, TZF5, which is down-regulated by isoprene, is located upstream of PHYB and PIF and is a negative regulator of expression of GA synthesis genes (GA3OX2; Bogamuwa and Jang, 2013, 2016; Fig. 12). The fact that the expression of TZF5, MARD1, DFL2, TEM1, and COP1 is altered by isoprene in a manner that enhances hypocotyl growth indicates that isoprene can activate PIF activity without PHYB and that the positive effects of isoprene on hypocotyl elongation in Arabidopsis may occur through this pathway. In tobacco, hypocotyl elongation may have been induced by isoprene-mediated enhancement of COP1 expression or a different isoprene-responsive genetic pathway, likely AXS2 (Eckey-Kaltenbach et al., 1993; Ahn et al., 2006; Pičmanová and Møller, 2016) and/or PIP5K2 (Ischebeck et al., 2013).
Figure 12.
Proposed model for how isoprene signaling can affect GA-mediated growth regulation and JA-mediated defense responses. Transcriptomic data revealed that isoprene can alter genes required for both GA accumulation and JA synthesis (genes shown in green font), which can promote both GA-mediated growth and JA-mediated defense simultaneously. We speculate that the observed growth enhancement in Arabidopsis engineered to emit isoprene is likely a result of the up-regulation of PIF. However, interactions between GA and JA pathways occur through DELLA and JAZ proteins (Campos et al., 2016). JA synthesis leads to the degradation of JAZ proteins that release the inhibition of transcription factors to enhance defense-related processes (Campos et al., 2016). Antagonistic interactions between JAZ and DELLA proteins play a part in regulating the growth-defense tradeoff mediated by GA and JA (Campos et al., 2016). Therefore, one possible explanation for the observed variations in isoprene-mediated growth effects in different species is the likely effect of isoprene on the growth-defense tradeoff. Genes belonging to other signaling pathways whose expression was altered by isoprene were omitted from this diagram for the sake of simplicity. Dotted lines and genes written in green font denote isoprene-responsive gene expression revealed during this study. Asterisks denote genes differentially expressed in both Arabidopsis expressing ISPS and Arabidopsis fumigated by isoprene but not differentially expressed in tobacco. Up-regulation and down-regulation of gene expression are denoted by pointed and blunt-ended arrows, respectively (DFL2 and TEM1 expression was down-regulated in the presence of isoprene in Arabidopsis). Solid lines denote signaling pathways that are well established. BBD, Bifunctional nuclease in basal defense response; CBF, CRT/DRE-binding factor; COP1, Constitutively photomorphogenic1, a ubiquitin ligase; CRPK1, Cold-responsive protein kinase1; DELLA, PIF transcription factor repressors; DFL2, Dwarf in light2, a GH3-related (auxin response-related) protein; FT, Flowering locus T; HY5, Elongated hypocotyl5; JAZ, Jasmonate ZIM-domain repressors; JMT, Jasmonic acid carboxyl methyltransferase; LOX, Lipoxygenase; MARD1, Mediator of ABA-regulated dormancy1, a novel zinc finger protein; MYB59, MYB domain protein59; MYC2, a basic helix-loop-helix transcription factor and a master regulator of JA signaling; OPR3, Oxophytodienoate-reductase3; TEM1, Tempranillo1, a RAV transcription factor; TZF5,Tandem CCCH zinc finger protein5; 14-3-3, highly conserved acidic proteins of the 14-3-3 protein family.
In addition to hypocotyl elongation, isoprene also had a positive effect on the growth of cotyledons, leaf area, leaf length, number of leaves, growth rates, leaf and inflorescence dry weight, early inflorescence initiation, and inflorescence stem height in Arabidopsis. Interestingly, mutant lines of phyB also exhibit elongated hypocotyls, larger cotyledons and larger leaf area and petiole length, reduced leaf curling, early flowering, and longer stems (Campos et al., 2016; Weraduwage et al., 2018). We speculate that these growth phenotypes in isoprene-emitting Arabidopsis lines may be in part a result of isoprene-mediated up-regulation of GA accumulation through TZF5 (and TEM1), with subsequent effects on GA-responsive genes through PIF (Fig. 12). In addition, alterations in AXS2 and PIPK5 may also have contributed to enhanced leaf growth in Arabidopsis. Loivamäki et al. (2007) also reported higher growth rates under both normal growth conditions and moderate heat stress in Arabidopsis lines transformed with an ISPS gene from gray poplar. Another lab that studied interactions between CK production and isoprene emission in the same Arabidopsis lines used in our study also saw faster growth rates and early inflorescence initiation in isoprene-emitting lines B2 and C4 (K.G.S. Dani, S. Pollastri, M. Reichelt, T.D. Sharkey, and F. Loreto, unpublished data). Evidence for higher levels of active CK and significant accumulation of its immediate precursors was also found in B2 and C4. It is proposed that isoprene-mediated higher CK levels facilitate better and faster growth and may be especially beneficial under short-term stress conditions. Overall, it is clear that isoprene-mediated changes to the transcriptome associated with CK signaling does in fact translate to changes in CK production, and this may, in part, explain the enhanced growth in Arabidopsis.
In contrast, cotyledon area, leaf area, leaf and plant stem dry weight, and plant stem height were reduced in the tobacco IE line (Fig. 12). There was no statistically significant effect on growth in the four tobacco IE lines studied by Vickers et al. (2009b) except line 22, where there was a significant enhancement in growth. However, tobacco IE line 32 that was also used in this study exhibited a trend of reduced leaf area and leaf fresh weight (Vickers et al., 2009b). It is possible that variations in isoprene-mediated growth effects are due to slight variations in growth conditions. For example, Arabidopsis lines transformed with ISPS from gray poplar showed faster growth rates on a leaf area basis than the wild type, especially under moderate heat stress (Loivamäki et al., 2007), and also grew better under severe heat stress (Sasaki et al., 2007). Thus, isoprene emission may offer a growth benefit only under a narrow range of environmental conditions. However, we can also provide several explanations for the opposite growth effects seen in Arabidopsis and tobacco, based on how isoprene affects the expression of growth- and defense-signaling genes. First, in contrast to Arabidopsis, the expression of genes of the GA signaling pathway was not altered in tobacco (Fig. 12; Supplemental Tables S2 and S5). Thus, GA-mediated growth effects may not be induced in tobacco. Second, it is possible that the reduction in growth in tobacco is JA mediated. A number of genes involved in JA signaling and JA-associated defense responses were enhanced in both Arabidopsis and tobacco (Fig. 12; Supplemental Table S2 and S3). While GA promotes growth, JA promotes defense responses and inhibits growth (Campos et al., 2016). This tradeoff between growth and defense in plants is mediated through interactions between DELLA and JAZ proteins, in the GA and JA signaling pathways, respectively (Fig. 12).
As our transcription data clearly show, by expressing ISPS or by exposing plants to isoprene, we have enhanced both GA-mediated growth and JA-mediated defense signaling simultaneously (Fig. 12; Supplemental Tables S2 and S3). It appears that isoprene-induced JA effects are more prominent in the tobacco IE line, resulting in changes in gene expression that inhibit growth (Fig. 12). This reciprocal interaction between GA and JA that determines tradeoffs between growth and defense may also be one reason why previous studies have seen varying effects of isoprene on growth.
In summary, the variations in isoprene-mediated growth effects observed in different species and even between plant lines emitting different levels of isoprene within the same species (e.g. as seen between ISPS-expressing Arabidopsis and tobacco and between IE lines in tobacco) is likely to occur because of differences in the degree of enhancement of GA-mediated growth and JA-mediated defense pathways by isoprene; the net effects of the growth-defense tradeoff will determine the net growth phenotype (Fig. 12). Moreover, in Arabidopsis, it is possible that we have uncoupled the tradeoff between growth and defense and created a scenario where enhanced growth is accompanied by enhanced defense; whether this is true will be tested during future experiments.
Our data clearly show that isoprene leads to early inflorescence initiation and also promotes the growth of larger inflorescences, which agrees with previous findings where exposure to isoprene led to similar inflorescence phenotypes in unstressed barley (Hordeum vulgare), rapeseed (Brassica napus), and Arabidopsis (Terry et al., 1995). Gene expression data suggest that isoprene likely promotes early inflorescence initiation, inflorescence growth, and reproductive growth by stimulating the expression of genes associated with GA and CK signaling as well as other genes important for reproductive development.
Isoprene Executes Its Protective Functions by Altering Expression of Genes Involved in Defense against Abiotic and Biotic Stress
Isoprene has been shown to protect plants against heat stress, ozone and other ROS, and herbivory (Loreto et al., 2001; Loreto and Velikova, 2001; Affek and Yakir, 2002; Sasaki et al., 2007). Harvey et al. (2015) showed that, contrary to the general opinion, isoprene in its normally low concentration is very unlikely to affect membrane stability and that it is not an effective method for quenching ROS. Harvey and Sharkey (2016) proposed that isoprene is likely acting as a signaling molecule to alter gene expression and thereby bring about its effects on abiotic stress tolerance.
In this study, although Arabidopsis and tobacco expressing ISPS and Arabidopsis fumigated with isoprene were unstressed, exposure to isoprene caused differential expression of many stress-responsive genes. Isoprene altered the expression of genes that enhance tolerance to heavy metals, xenobiotics, secondary metabolites, soil acidity and proton toxicity, high light, heat, salt, drought, and oxidative stress. For example, phenylpropanoids are known to enhance tolerance to heat stress as well as biotic stress like herbivory/wounding (Commisso et al., 2016; Lv et al., 2016). Expression of phenylpropanoid pathway enzymes was higher in gray poplar control lines than in those with RNAi-mediated suppression of isoprene under moderate heat stress (Behnke et al., 2010). Interestingly, in this study, under unstressed conditions, phenylpropanoid pathway enzyme gene expression was significantly altered in all three model systems in favor of the pathway, indicating that isoprene is important for stimulating gene expression under abiotic stress conditions, such as heat stress. Behnke et al. (2010) also showed that, corresponding with the reduced expression of phenylpropanoid pathway enzymes, total phenolic and condensed tannin content was reduced and H2O2 accumulation was enhanced in poplar subjected to RNAi-mediated suppression of ISPS under moderate heat stress. This provides clear evidence that alterations in the abiotic and biotic stress-related transcriptome by isoprene also translates to changes in the proteome, ultimately resulting in responses to abiotic (and biotic) stresses.
Tolerance of heat and oxidative stress are two of the well-known protective functions of isoprene in plants. Tobacco fumigated with isoprene maintained higher photochemical efficiency and showed less ultrastructural damage to chloroplasts and less chlorosis when exposed to ozone (Loreto et al., 2001). Using Arabidopsis transformed with an ISPS from kudzu and fosmidomycin-fed sycamore (Platanus orientalis) leaves, it was shown that isoprene can enhance the integrity and operation of the thylakoid membranes under heat stress (Velikova et al., 2011). Behnke et al. (2007) showed that in poplar lacking isoprene due to RNAi-mediated suppression, photosynthesis rates were reduced under heat stress but not in the absence of stress. It was hypothesized that isoprene or its internal presence can have a protective role in photosynthetic electron transport to maintain photosynthesis under stressful conditions (Behnke et al., 2007). When the same poplar lines were subjected to heat and drought treatments, the isoprene-emitting poplar genotypes maintained higher photosynthetic electron transport during stress and during recovery from stress (Vanzo et al., 2015). In fact, when tobacco IE lines, including IE 32 used in our study, were tested under drought stress, isoprene-emitting plants showed both higher operating and maximum efficiencies of PSII (Ryan et al., 2014).
Data from this study showed that isoprene up-regulates the expression of key genes (ADC1 and ADC2) required to synthesize the polyamine putrescine as well as down-regulates the expression of CuAO3 that favors spermidine and spermine synthesis from putrescine. Polyamines can alter partitioning of proton motive force between its electrical and osmotic components under stress, alter the unsaturated fatty acid content, change gene expression in thylakoid membranes, enhance thylakoid membrane protein content, and increase thylakoid membrane stability under salt stress (Urano et al., 2004; Ioannidis et al., 2012; Liu et al., 2015; Saha et al., 2015). Alterations in putrescine and spermidine content enhance thermotolerance, alleviate heat-induced damage, increase antioxidant enzyme activity, and reduce the accumulation of H2O2 (Sagor et al., 2013; Kumar et al., 2014; Liu et al., 2015). Enhanced spermine and spermidine helps retain Chl and protects plants against salt and drought stress (Capell et al., 2004; Zarei et al., 2016; Groß et al., 2017; Montilla-Bascón et al., 2017). NAD(H) kinase1 remedies oxidative damage under a variety of stresses, including H2O2 and ozone (Berrin et al., 2005; Chai et al., 2006; Hashida et al., 2009). Phenylpropanoids have been shown to protect the actin cytoskeleton in cells against heat damage (Commisso et al., 2016), and the expression of several PAL genes was up-regulated by isoprene. Thus, we see a clear connection between isoprene-mediated alterations in the transcriptome and physiological responses to stress by isoprene-emitting lines.
Isoprene also up-regulated MPH1 and MPH2 gene expression in both Arabidopsis and tobacco. MPH1 is a thylakoid membrane protein that is required for the accumulation of PSII core proteins (Liu and Last, 2015b); MPH2 is a stromal protein essential for PSII repair and regeneration of dimeric functional PSII supercomplexes (Liu and Last, 2017). Both proteins have been shown to be essential to stabilize PSII, especially under high and fluctuating light stress (Liu and Last, 2015a, 2015b, 2017). In this study, isoprene also affected the gene expression of subunits of the NADH dehydrogenase-like complex in the chloroplast, which is associated with PSI and mediated the cyclic electron transport important for protecting PSI against photoinhibition under normal and high-temperature stress (Peng et al., 2009, 2012; Shikanai, 2016; Essemine et al., 2017). As mentioned above, isoprene also altered a number of genes coding for ribosomal proteins in chloroplasts that promote chloroplast development and Chl synthesis. Sharkey et al. (1996) showed that in white oak (Quercus alba), isoprene emission is highest in leaves located at the top of the canopy. These leaves are exposed to higher light intensities, hence higher temperature stress as well as water shortage. However, owing to higher isoprene emission rates, these leaves are the ones that are most protected from these abiotic stresses (Sharkey et al., 1996). Overall, our data provide evidence that isoprene can alter the expression of specific genes in a manner that can increase membrane stability and photochemical efficiency and minimize damage to chloroplasts.
Another point of debate has been the role of isoprene as an antioxidant (Loreto et al., 2001; Loreto and Velikova, 2001; Sharkey and Yeh, 2001; Affek and Yakir, 2002; Sharkey et al., 2008; Vickers et al., 2009a, 2009b). It was proposed that isoprene must either directly react with oxidizing species or indirectly alter ROS signaling (Vickers et al., 2009a). However, we show that isoprene can directly alter gene expression to promote polyamine synthesis, especially putrescine, spermine, and spermidine synthesis (ADC1, ADC2, and CuOA3), and genes that synthesize apiose/apiin (AXS2); products of these genes are known to have antioxidant activity, promote ROS homeostasis, and protect against oxidative stress induced by ozone, UV, heat, and salt stress (Eckey-Kaltenbach et al., 1993; Perez-Amador et al., 2002; Urano et al., 2004, 2005; Ahn et al., 2006; Ioannidis et al., 2012; Sagor et al., 2013; Kumar et al., 2014; Liu et al., 2015; Saha et al., 2015; Pičmanová and Møller, 2016). Isoprene also down-regulated genes that lead to ROS accumulation and programmed cell death in response to oxidative stress (CATHB; Ge et al., 2016; Cai et al., 2018) and up-regulated cytoplasmic NAD(H) kinase1 (NADK1). It has been reported that ROS-damaged proteins can be repaired by glutathione, which can only be regenerated by NADPH. Presumably, the expression of NADK1 would enhance the concentration of NADPH (Berrin et al., 2005; Chai et al., 2006; Hashida et al., 2009).
Isoprene is known to exert protection against herbivory (Laothawornkitkul et al., 2008). Isoprene altered the expression of genes belonging to a number of different signaling pathways required for resistance against wounding, such as JA biosynthesis, glutathione transport, phenylpropanoid synthesis, apiose, callose, and cellulose synthesis, and CK signaling. A recent report of isoprene-mediated enhancement of CK (K.G.S. Dani, S. Pollastri, M. Reichelt, T.D. Sharkey, and F. Loreto, unpublished data) is important because more evidence shows that CK is not only important for growth regulation but can also act as a priming agent and is required to protect plants against both abiotic and biotic stress (Mason et al., 2005; Lin et al., 2008; Zhang et al., 2013; Iwase et al., 2017; Cortleven et al., 2018;). Often, stress causes plants to accelerate generation time and thereby reproduce before damage occurs to the plant. As seen in Arabidopsis, it is possible that isoprene triggers such a response through the induction of CK-mediated signaling, which can prime plants under normal unstressed conditions and protect plants during stress.
Considering that isoprene alters gene expression such that JA signaling is up-regulated and SA signaling is down-regulated with isoprene, we expected the transcriptome of isoprene-emitting lines to be richer in genes supporting resistance against necrotrophic pathogens and susceptibility toward biotrophs. In fact, while we could not detect an enhancement in the number of genes supporting defense against necrotrophic pathogens, the data indicated that genes altered by isoprene in isoprene-emitting Arabidopsis and tobacco are involved in mediating resistance against a larger number of necrotrophs. Genes associated with resistance against biotrophs belonged mostly to SA and ABA signaling pathways, and we saw that isoprene altered genes to suppress these pathways. This may result in isoprene-emitting Arabidopsis and tobacco becoming more susceptible to various strains of biotrophs. This is a hypothesis that will be tested in the future.
Isoprene Acts as a Signaling Molecule by Acting Upstream of Many Growth Regulator Signaling Pathways
The ability of isoprene to alter gene expression has been reported previously (Behnke et al., 2010), and the possibility of isoprene acting directly as a signaling molecule was proposed by Harvey and Sharkey (2016). The ability of isoprene to indirectly affect ROS signaling was proposed by Vickers et al. (2009a). In this study, we provide evidence from extensive transcriptomic analyses to show that isoprene is able to act directly as a signaling molecule to alter the expression of many genes located upstream of key signaling pathways.
Key genes associated with growth regulator signaling pathways, whose expression was altered in at least two if not all three model systems studied here (Arabidopsis or tobacco expressing ISPS and Arabidopsis fumigated with isoprene), provide evidence that these growth regulatory networks are responsive to isoprene (Fig. 12; Supplemental Table S2). Isoprene affected the expression of key genes necessary for the synthesis and accumulation of JA, GA, ethylene, ABA (only during water stress), and SA, suggesting that isoprene acts upstream of these growth regulators and their signaling pathways to exert its own signaling. In addition, isoprene appeared to selectively alter genes to enhance JA, GA, CK, ethylene, and the signaling branch of ABA under the regulation of ATAF1 and to down-regulate the remaining branch of ABA that is antagonistic to GA and also BR, SA, and auxin signaling.
While it can be argued that the isoprene-responsive transcriptome may not represent the proteome accurately, the facts that isoprene-emitting Arabidopsis showed phenotypes similar to those seen in phyB mutants and isoprene-emitting tobacco showed phenotypes similar to JA signaling indicate that the gene expression patterns seen in the transcriptome is also represented in the phenome of isoprene-emitting lines. Isoprene-mediated enhancement of CK biosynthesis and growth in Arabidopsis (K.G.S. Dani, S. Pollastri, M. Reichelt, T.D. Sharkey, and F. Loreto, unpublished data) shows that isoprene-mediated effects on gene expression do in fact translate to isoprene-mediated changes in the proteome. As mentioned before, GA, CK, and ABA are all synthesized by prenyl phosphates from the MEP pathway, and it is possible that isoprene, through its effects on gene expression, is altering flux through the MEP pathway in favor of hormone biosynthesis. Additional evidence showing that alterations of the isoprene-responsive transcriptome leads to representative changes in the metabolome was provided by Behnke et al. (2010) and was discussed in detail in the previous sections. In addition, the up-regulated and down-regulated genes of a certain growth regulator pathway corresponded to a similar trend, either toward an overall enhancement or overall suppression of that particular pathway. These data provide clear evidence that isoprene can act as a signaling molecule. In addition, the ability of isoprene to target genes encoding for membrane proteins and cell surface receptors, transporters, proteins that can trigger signaling cascades, key proteins in secondary metabolite and growth regulator biosynthesis, and a variety of transcription factors solidifies the potential role of isoprene as a signaling molecule.
Given the facts that ISPS has a higher temperature optimum and isoprene synthesis increases with increasing temperature (and light), isoprene as a signaling molecule may be especially effective under high-temperature and high-light stress. If isoprene is a signaling molecule, why evolution favored a volatile hydrocarbon as a signaling molecule and why it is produced in such large quantities can only be speculated. For example, the intercellular ethylene concentration is about 0.5 µL L−1 (Aharoni and Lieberman, 1979); for isoprene it is about 20 µL L−1 (Singsaas et al., 1997). It is possible that isoprene, while protecting plants under stress, also acts as a plant-to-plant long-distance signal to prime neighboring plants for impending stress or acts as an attractant to pollinators, or predators, or a repellant of other herbivores or pathogens. Perhaps this is why isoprene is made in such large amounts. However, this hypothesis can be argued against because once airborne, isoprene is short lived and gets diluted to minute amounts (Baker et al., 2005). While it is possible that insects can detect minute amounts of volatile chemicals in the air, and that isoprene concentrations within a microenvironment of a plant population may increase to high enough levels to be sufficient to induce signaling in neighboring plants, these hypotheses remain to be tested.
CONCLUSION
This study was designed to understand how isoprene affects growth and physiology in unstressed plants, whether isoprene can act as a signaling molecule, and to shed light on the potential mechanisms through which isoprene influences stress responses in plants. Although Arabidopsis (and tobacco) do not normally produce isoprene, when engineered to do so, the same responses to temperature, CO2, and light can be observed as in native emitters. Regardless of the species, isoprene had a positive effect on leaf pigment production. Isoprene also had a significant positive effect on seedling growth and on both vegetative and reproductive growth in Arabidopsis under unstressed conditions. Transcriptomic data revealed that isoprene probably exerts its effects on growth and stress tolerance by affecting the gene expression of many growth regulators and molecular networks involved in abiotic and biotic stress tolerance. Up-regulation of growth- and defense-related signaling pathways simultaneously may lead to plants that grow better and are also protected against stress. In some cases, growth-defense tradeoffs may be one reason why isoprene-mediated growth phenotypes are variable between species. Our data show that isoprene is beneficial for plants not only under stress conditions but also under unstressed conditions and introduce a novel role of isoprene as a signaling molecule. Some isoprene-responsive signaling networks revealed during this study were affiliated with abiotic stresses (e.g. soil acidity, salt stress, and heavy metal toxicity) and biotic stresses (e.g. necrotrophic pathogens) that were not known to be protected against by isoprene in plants. The ability of isoprene to protect plants against these stresses should be further explored.
MATERIALS AND METHODS
Plant Materials
Arabidopsis
For the transgenic Arabidopsis (Arabidopsis thaliana), the complete complementary DNA sequence for ISPS from Eucalyptus globulus including the sequence encoding the native transit peptide was placed behind the Arabidopsis Rubisco small subunit promoter rbcS-1A (At1g67090). The first 1,000 bp of the promoter sequence starting from the adenine immediately preceding the ATG start codon and counting backward was used. It has been shown by deletion analysis that this region promotes a high transcript amount (Donald and Cashmore, 1990). An octopine synthase terminator was used following the ISPS gene. DNA for the E. globulus ISPS and the rbcS-1A promoter and octopine synthase terminator were synthesized by Bio Basic and placed in the pUC57 plasmid vector. The construct was then transferred to the pART27 destination vector (Gleave, 1992). The construct was also transferred to the pART27 vector without ISPS to generate the empty vector control. The pART27 vector constructs were transformed into Agrobacterium tumefaciens strain GV3101 by electroporation. Arabidopsis Col-0 was transformed using the floral dip method (Clough and Bent, 1998). Transformation of Arabidopsis was carried out at the Arabidopsis Transformation Facility for the Plant Research Laboratory, Michigan State University.
Seeds from primary transformants were selected on plates containing 0.8% (w/w) Phytoblend (Caisson Laboratories), 4.3 g L−1 Murashige and Skoog salts (MSP C0130; Caisson Laboratories), 1% Suc, 2.5 mm MES, and 50 μg mL−1 kanamycin using a rapid selection method to generate T1 plants (Harrison et al., 2006). T1 plants were grown in Redi-Earth growing medium (Michigan Grower Products) and allowed to self-pollinate. Approximately 100 seeds from T1 plants were plated on kanamycin selection plates, and those with a 3:1 ratio of resistant plants to susceptible plants were selected and grown to maturity on Redi-Earth (T2 generation). Approximately 100 seeds from each plant selected in the T2 generation were planted, and those plants with 100% resistance were selected and used for all further experiments (T3 generation). Transgene insertion was confirmed by PCR.
Three empty vector lines (EV-A2, EV-B3, and EV-F1) and seven ISPS-containing lines (A1, B2, B4, C1, C3, C4, and F2) of Arabidopsis representing independent transformation events were characterized during this study in terms of isoprene emission, photosynthesis, and growth. For this purpose, T3 plants along with the wild type (Col-0) were grown on Suremix growing medium (Michigan Grower Products) under a 12-h photoperiod, 120 μmol m−2 s−1 light intensity, 23°C daytime and 20°C nighttime temperatures, and 60% relative humidity. Plants were fertilized every 3 d using one-half-strength Hoagland nutrient solution.
After characterization of the seven isoprene-emitting lines, one of the empty vector lines (EV-B3) and the two lines that exhibited the highest rates of isoprene emission (B2 and C4) were selected and grown simultaneously with the tobacco lines to compare the effects of ISPS expression on isoprene emission and photosynthesis, the response of isoprene emission and photosynthesis to temperature, CO2, light, and darkness, the postillumination isoprene emission, MEP pathway metabolites, and growth, between the two species. To test whether the observed effects of isoprene on the above parameters in Arabidopsis were not due to a light effect, EV-B3, B2, and C4 were grown on Redi-Earth growing medium and kept in either a low-light growth chamber (100 μmol m−2 s−1) or a high-light growth chamber (200 μmol m−2 s−1).
Tobacco
Seeds of tobacco (Nicotiana tabacum ‘Samsun’) transformed with ISPS from Populus alba to produce isoprene-emitting homozygous plants (IE) and the corresponding nonemitting azygous (null segregants) control (NE) were obtained from Claudia Vickers, University of Queensland. The NE and IE lines used in this study correspond to line 32 described by Vickers et al. (2009b), where IE was reported to produce the highest amount of isoprene compared with the other isoprene-emitting tobacco lines. More information regarding plasmid construction, transformation of tobacco, and line selection is described by Vickers et al. (2009b). These seeds were used to grow plants in a growth chamber (Big-Foot, BioChambers; under a 16-h photoperiod at a light intensity of 400 μmol m−2 s−1, day/night temperatures of 25°C/22°C, and humidity of 60%) to collect seeds. For experiments reported here, plants were grown from these seeds in Suremix growing medium (Michigan Grower Products) and kept in Michigan State University greenhouses. Plants were fertilized every 2 to 3 d using one-half-strength Hoagland nutrient solution (Hoagland and Arnon, 1938).
Transgene Detection in Arabidopsis Transformed with ISPS
DNA Extraction
DNA was extracted from 7-week-old rosette leaves using a DNeasy Plant Mini Kit (Qiagen) based on the manufacturer’s instructions. The DNA concentration and purity were determined with the aid of a DS-11 spectrophotometer (DeNovix).
PCR
PCR was carried out using 1 μL of the extracted DNA as the template in a total volume of 50 μL that contained 1× PCR buffer, 2.5 mm MgCl2, 150 µm deoxyribonucleoside triphosphate mix, 1 unit of Taq DNA polymerase (all reagents supplied by Invitrogen), and 1 µm each of the forward and reverse primers. Primer sequences were 5′-TGTGGAATTGTGAGCGGATA-3′ (forward) and 5′-GTGTCTCTAAGGGTGCGGTC-3′ (reverse). PCR was carried out based on the manufacturer’s instructions in a Mastercycler RealPlex2 PCR machine (Eppendorf). A PCR mix without the template DNA was used as a negative control. A PCR mix with empty vector plasmid was used as a positive control. After PCR, 10 μL of each resulting amplicon was mixed with 2.5 μL of EZ-vision loading buffer/DNA dye (Amresco) and run on a 1% agarose gel (0.5 g of agarose and 50 mL of 1× Tris-acetate-EDTA buffer) at 85 V for 30 min. A 1-kb Plus DNA ladder (Invitrogen) was run alongside the amplicons. The separated amplicons were visualized under UV light.
Gas-Exchange Measurements
The experimental setup for gas exchange was as described by Li et al. (2011). A custom-built rosette gas-exchange cuvette connected to an LI-6400 was used for Arabidopsis. Tobacco leaves were enclosed in the light-emitting diode leaf chamber (6 cm2) of the LI-6400 Portable Photosynthesis System (LI-COR Biosciences). The exhaust gas of the LI-6400 was connected to a Fast Isoprene Sensor (Hills Scientific), where isoprene emission was averaged over 5-s intervals. Experiments were carried out under standard conditions (30°C, 400 μmol mol−1 CO2 concentration, and 800 μmol m−2 s−1 photosynthetic photon flux density at leaf level for tobacco and 500 μmol m−2 s−1 for Arabidopsis). The temperature of the leaf chamber was controlled by a water bath. Light for Arabidopsis was provided with an SL3500 white light-emitting diode array (Photon Systems Instruments).
To determine the temperature response of photosynthesis and isoprene production, the temperature of the leaf chamber was controlled by two water baths linked by a six-way valve that allowed ultrafast switching between different temperatures, and simultaneous measurements of photosynthesis and isoprene emissions were recorded at each temperature point: at 23°C, 30°C, 35°C, 40°C, and 45°C for Arabidopsis and at 25°C, 30°C, 35°C, and 40°C for tobacco.
For the CO2 response, the reference CO2 concentration in the leaf chamber was set at 0, 62.5, 125, 250, 500, and 1,000 μmol mol−1. When the set CO2 concentration was achieved, a 4-min stabilization period was allowed prior to recording photosynthesis and isoprene emission.
For the light response, photosynthesis and isoprene emissions were recorded after a 4-min stabilization period under each light level. For Arabidopsis, it was set at 0, 100, 200, 500, 1,000, and 1,500 μmol m−2 s−1. For tobacco, the light intensity was set at 0, 200, 500, 1,000, and 2,000 μmol m−2 s−1.
Measurement of Postillumination Isoprene Emission
Postillumination isoprene emission was measured using the procedures described by Li et al. (2011). Light in the leaf chamber was turned off by either covering the Arabidopsis rosette gas-exchange cuvette with black cloth or by instantly switching off the LI-6400 light source in the case of tobacco. The postillumination isoprene emission was measured for 30 min. The chamber clearing time was determined by injecting isoprene directly into the chamber and then abruptly stopping the injection to follow how quickly isoprene cleared from the chamber and tubing.
Determination of MEP Pathway Metabolites
Whole rosettes of Arabidopsis and whole leaves from tobacco were harvested into liquid N2. The tissue was then ground in liquid N2, and cell contents were extracted with a solvent containing acetonitrile:water (4:1). The extract was centrifuged at 14,000g for 10 min, and the supernatant was stored at −80°C for later analysis. The MEP pathway metabolites were separated and quantified by an Acquity TQD Tandem Quadrupole Mass Spectrometer with an Acquity BEH Amide column (Acquity Group). Standards of MEP pathway metabolites were separated using a binary gradient consisting of 50 mm ammonium acetate in acetonitrile adjusted to pH 10 with ammonium hydroxide. The measurement procedure was described in detail by Li and Sharkey (2013a). All metabolites were measured using mass spectrometry as described above except DMADP, which was calculated using the postillumination isoprene response (Rasulov et al., 2009; Weise et al., 2013).
Plant Growth Analyses
Comparison of Young Seedling Growth Characteristics
Cotyledons of Arabidopsis and tobacco seedlings were photographed and heights of the hypocotyls were measured at different time points up to 12 d after seed sowing. Photographs were used to measure cotyledon area using ImageJ software (National Institutes of Health).
Determination of Leaf Area, Curling, Dry Weight, Growth Rate, and Inflorescence Growth
Every week, intact Arabidopsis rosettes were photographed from the top to obtain projected leaf area. After gas-exchange measurements, leaves were harvested from each rosette, placed on a benchtop, and photographed to obtain apparent total leaf area. Next, the same leaves were flattened by covering with transparent Plexiglas and photographed again to obtain actual total leaf area. The ImageJ software was used to measure leaf area from the photographs. Leaf curling in Arabidopsis was calculated as (actual total leaf area – apparent total leaf area)/actual total leaf area × 100%. Tobacco leaves were also photographed to determine leaf area. The leaf number was noted in Arabidopsis rosettes. After photographing, leaves and stems from Arabidopsis and tobacco were freeze dried (Labconco freeze-dryer; Labconco) followed by dry weight measurements. LMA was calculated as the ratio between leaf dry weight and total leaf area. The number of days required for inflorescence initiation and height of the inflorescence stem over time was measured in Arabidopsis. Absolute growth rates, leaf growth (m2 d−1) or stem growth (m d−1), were calculated using projected leaf area and stem heights obtained at different time points of the life cycle in Arabidopsis.
Measurement of Photosynthetic Pigment Content
Two leaf discs were harvested with the aid of a cork borer (diameter of 8.75 mm) from two mature leaves. Leaf discs were ground in liquid N2 using a mortar and pestle, and the photosynthetic pigments were extracted with 2 mL of 96% ethanol. The content of Chl a, Chl b, and carotenoids were calculated using the equations described by Lichtenthaler and Wellburn (1983), and the pigment concentration was calculated using the leaf disc area. As shading can affect pigment content, tobacco plants were maintained under uniform light and kept sufficiently apart to avoid shading.
Analysis of Gene Expression
RNA Extraction
Out of the seven isoprene-emitting Arabidopsis lines, the empty vector line EV-B3 and B2, the line that exhibited the highest isoprene emission rate, were selected for gene expression profiling; for tobacco, NE and IE (line 32) lines were used. Leaves were harvested from 5½-week-old Arabidopsis and 7-week-old tobacco plants grown under normal growth conditions (unstressed) as described above. Arabidopsis plants grown under 200 μmol m−2 s−1 were used. Leaves from three plants were harvested from each line (three independent RNA samples per line). Total RNA was extracted using the RNeasy Mini Kit (Qiagen). The RNA concentration, integrity, and quality were determined with the aid of a Qubit RNA Broad-Range Assay Kit (Invitrogen) and a Qubit 4 benchtop fluorometer (Invitrogen). RNA integrity was further assessed with a 2100 Bioanalyzer (Agilent Technologies). All samples used for sequencing had an RNA integrity number of at least 7.0 (1 being the most degraded RNA profile and 10 being the most intact).
mRNA Sequencing
Single-end 50-bp mRNA sequencing was performed at the Michigan State University Research Technology Support Facility Genomics Core (https://rtsf.natsci.msu.edu/genomics/). Libraries were prepared using the TruSeq Stranded mRNA Library Preparation Kit (Illumina) following the manufacturer’s recommendations. Completed libraries were assessed for quality and quantified using a combination of Qubit dsDNA High Sensitivity Assay Kit (Qubit), Caliper LabChipGX System and a DNA High Sensitivity Assay Kit (Caliper Life Sciences), and Kapa Illumina Library Quantification qPCR assays (Illumina). Each set of libraries was pooled in equimolar quantities and loaded on one lane of an Illumina HiSeq 2500 High Output flow cell (v4). Sequencing was carried out in a 1- × 50-bp (SE50) format using Illumina HiSeq SBS reagents (v4). Base calling was done by Illumina Real Time Analysis v1.18.64, and the output of Real Time Analysis was demultiplexed and converted to FastQ format with Illumina Bcl2fastq v1.8.4. The average number of sequencing reads was 44.7 ± 4.3 million per sample for Arabidopsis and 46.7 ± 2.8 million per sample for tobacco.
Data Analysis
Next-generation RNA sequencing data were subjected to gene differential expression analysis. Briefly, sequencing adapters and low-quality bases were trimmed from sequencing reads using Trimmomatic version 0.32 (Bolger et al., 2014), and cleaned reads were examined with FASTQC (https://www.bioinformatics.babraham.ac.uk/projects/fastqc/) for quality evaluation. Reads were aligned to the reference genomes, Arabidopsis TAIR10 (Berardini et al., 2015) and tobacco TN90 (Sierro et al., 2014). To calculate read counts for each gene, the Rsubread version 3 (Liao et al., 2013) tool (featureCounts function) was used to build a read count matrix. The read count matrix was analyzed using the linear model-based Limma::Voom package (Law et al., 2014) to calculate the gene expression values (reads per kilobase of transcript per million mapped reads) and perform gene differential expression statistical analyses. Data presented in Supplemental Files S1 and S2 represent the gene expression values (reads per kilobase of transcript per million mapped reads) per gene. Statistical data analyses were adjusted for multiple comparisons, and adjusted P values are provided at a false discovery rate threshold of 0.05. Differentially expressed genes identified in Arabidopsis and tobacco expressing ISPS from this study and Arabidopsis fumigated with isoprene (Harvey and Sharkey, 2016) were compared to identify differentially expressed genes common to at least two or all three systems; these genes were considered as isoprene-responsive genes.
Calculations and Statistical Analyses
Growth analyses and measurements of photosynthesis, isoprene emission, and leaf pigment concentrations were repeated to confirm the repeatability of results. Unless stated otherwise, data were collected from five or more biological replicates from each Arabidopsis and tobacco line. Statistical analyses were performed using SPSS 25 (IBM). One-way ANOVA was carried out followed by a Fisher’s Least Significant Difference test to compare the differences between means. Graphs were drawn with Origin 8.0.
Accession Numbers
The raw and processed data were deposited in the Gene Expression Omnibus (Edgar et al., 2002) and are accessible through GEO Series accession number GSE121676 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE121676). Other accession numbers are as follows: E. globulus ISPS used to transform Arabidopsis, AB266390; and P. alba ISPS used to transform tobacco, EF638224 (Vickers et al., 2009b).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Confirmation of the presence of the transgene in Arabidopsis transformed with ISPS.
Supplemental Figure S2. C assimilation and isoprene emission over time in Arabidopsis expressing ISPS grown under two light regimes.
Supplemental Figure S3. Photosynthesis and isoprene emission under varying environmental conditions in Arabidopsis expressing ISPS.
Supplemental Figure S4. MEP pathway metabolites in Arabidopsis and tobacco expressing ISPS.
Supplemental Figure S5. Cotyledon and hypocotyl growth in Arabidopsis expressing ISPS grown under two light regimes.
Supplemental Figure S6. Effects of heat treatment on photosynthesis in tobacco expressing ISPS.
Supplemental Table S1. Plant growth in Arabidopsis expressing ISPS.
Supplemental Table S2. Isoprene-responsive genes in signaling pathways of specific growth regulators.
Supplemental Table S3. Isoprene-responsive genes that mediate abiotic stress tolerance.
Supplemental Table S4. Isoprene-responsive genes that mediate biotic stress tolerance.
Supplemental Table S5. Isoprene-responsive genes that affect photosynthesis and plant growth.
Supplemental File S1. Gene expression induced in Arabidopsis expressing ISPS.
Supplemental File S2. Gene expression induced in tobacco expressing ISPS.
Acknowledgments
We thank Drs. D.M. Kramer (Plant Research Laboratory), Alicia Withrow and Melinda Frame (Center for Advanced Microscopy), Kevin Carr, Colleen Curry, and Shari Tjugum-Holland (Research Technologies Service Facility Genomics Core), and Jim Klug and Cody Keilen (Growth Chamber Facility), along with Dan Ter-Avest (Kramer lab), of Michigan State University for their expertise and assistance. We are also grateful to Linda Danhoff (Arabidopsis Transformation Facility for the Plant Research Laboratory, Michigan State University), who carried out Arabidopsis transformations. We thank Laura Cheaney (Department of Biochemistry and Molecular Biology, Michigan State University) and Amesha Wijesinghe (Honor Research Program of Okemos High School, Okemos, MI) for assisting with the selection of transgenic lines. We also thank all members of the Sharkey lab for their support.
Footnotes
This work was supported by the National Natural Science Foundation of China (31300364), the Natural Science Foundation of Zhejiang Province (LY17C160004), and the Personnel Startup Project of the Scientific Research and Development Foundation of Zhejiang Agriculture and Forestry University (2013FR069) to Z.Z.; by the Chemical Sciences, Geosciences, and Biosciences Division, Office of Basic Energy Sciences, Office of Science, U.S. Department of Energy (DE-FG02-91ER 20021) to S.M.W.; by Michigan AgBioResearch to T.D.S.; by the U.S. National Science Foundation (IOS-0950574); and by the Plant Genomics at Michigan State University REU Program NSF DBI-1358474 in 2016 to L.M.S.
Articles can be viewed without a subscription.
References
- Affek HP, Yakir D (2002) Protection by isoprene against singlet oxygen in leaves. Plant Physiol 129: 269–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aharoni N, Lieberman M (1979) Patterns of ethylene production in senescing leaves. Plant Physiol 64: 796–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn JW, Verma R, Kim M, Lee JY, Kim YK, Bang JW, Reiter WD, Pai HS (2006) Depletion of UDP-D-apiose/UDP-D-xylose synthases results in rhamnogalacturonan-II deficiency, cell wall thickening, and cell death in higher plants. J Biol Chem 281: 13708–13716 [DOI] [PubMed] [Google Scholar]
- Baker B, Bai JH, Johnson C, Cai ZT, Li QJ, Wang YF, Guenther A, Greenberg J, Klinger L, Geron C, et al. (2005) Wet and dry season ecosystem level fluxes of isoprene and monoterpenes from a southeast Asian secondary forest and rubber tree plantation. Atmos Environ 39: 381–390 [Google Scholar]
- Banerjee A, Sharkey TD (2014) Methylerythritol 4-phosphate (MEP) pathway metabolic regulation. Nat Prod Rep 31: 1043–1055 [DOI] [PubMed] [Google Scholar]
- Behnke K, Ehlting B, Teuber M, Bauerfeind M, Louis S, Hänsch R, Polle A, Bohlmann J, Schnitzler JP (2007) Transgenic, non-isoprene emitting poplars don’t like it hot. Plant J 51: 485–499 [DOI] [PubMed] [Google Scholar]
- Behnke K, Kaiser A, Zimmer I, Brüggemann N, Janz D, Polle A, Hampp R, Hänsch R, Popko J, Schmitt-Kopplin P, et al. (2010) RNAi-mediated suppression of isoprene emission in poplar transiently impacts phenolic metabolism under high temperature and high light intensities: A transcriptomic and metabolomic analysis. Plant Mol Biol 74: 61–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behnke K, Ghirardo A, Janz D, Kanawati B, Esperschütz J, Zimmer I, Schmitt-Kopplin P, Niinemets Ü, Polle A, Schnitzler JP, et al. (2013) Isoprene function in two contrasting poplars under salt and sunflecks. Tree Physiol 33: 562–578 [DOI] [PubMed] [Google Scholar]
- Beltran JCM, Stange C (2016) Apocarotenoids: A new carotenoid-derived pathway. In Stange C, ed, Carotenoids in Nature: Subcellular Biochemistry. Springer, Cham, Switzerland, pp 239–272 [DOI] [PubMed] [Google Scholar]
- Berardini TZ, Reiser L, Li D, Mezheritsky Y, Muller R, Strait E, Huala E (2015) The Arabidopsis Information Resource: Making and mining the “gold standard” annotated reference plant genome. Genesis 53: 474–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrin JG, Pierrugues O, Brutesco C, Alonso B, Montillet JL, Roby D, Kazmaier M (2005) Stress induces the expression of AtNADK-1, a gene encoding a NAD(H) kinase in Arabidopsis thaliana. Mol Genet Genomics 273: 10–19 [DOI] [PubMed] [Google Scholar]
- Bogamuwa S, Jang JC (2013) The Arabidopsis tandem CCCH zinc finger proteins AtTZF4, 5 and 6 are involved in light-, abscisic acid- and gibberellic acid-mediated regulation of seed germination. Plant Cell Environ 36: 1507–1519 [DOI] [PubMed] [Google Scholar]
- Bogamuwa S, Jang JC (2016) Plant tandem CCCH zinc finger proteins interact with ABA, drought, and stress response regulators in processing-bodies and stress granules. PLoS ONE 11: e0151574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger AM, Lohse M, Usadel B (2014) Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 30: 2114–2120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudsocq M, Sheen J (2013) CDPKs in immune and stress signaling. Trends Plant Sci 18: 30–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brüggemann N, Schnitzler JP (2002) Diurnal variation of dimethylallyl diphosphate concentrations in oak (Quercus robur) leaves. Physiol Plant 115: 190–196 [DOI] [PubMed] [Google Scholar]
- Bryant N, Lloyd J, Sweeney C, Myouga F, Meinke D (2011) Identification of nuclear genes encoding chloroplast-localized proteins required for embryo development in Arabidopsis. Plant Physiol 155: 1678–1689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai YM, Yu J, Ge Y, Mironov A, Gallois P (2018) Two proteases with caspase-3-like activity, cathepsin B and proteasome, antagonistically control ER-stress-induced programmed cell death in Arabidopsis. New Phytol 218: 1143–1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calfapietra C, Mugnozza GS, Karnosky DF, Loreto F, Sharkey TD (2008) Isoprene emission rates under elevated CO2 and O3 in two field-grown aspen clones differing in their sensitivity to O3. New Phytol 179: 55–61 [DOI] [PubMed] [Google Scholar]
- Campos ML, Yoshida Y, Major IT, de Oliveira Ferreira D, Weraduwage SM, Froehlich JE, Johnson BF, Kramer DM, Jander G, Sharkey TD, et al. (2016) Rewiring of jasmonate and phytochrome B signalling uncouples plant growth-defense tradeoffs. Nat Commun 7: 12570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capell T, Bassie L, Christou P (2004) Modulation of the polyamine biosynthetic pathway in transgenic rice confers tolerance to drought stress. Proc Natl Acad Sci USA 101: 9909–9914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centritto M, Nascetti P, Petrilli L, Raschi A, Loreto F (2004) Profiles of isoprene emission and photosynthetic parameters in hybrid poplars exposed to free-air CO2 enrichment. Plant Cell Environ 27: 403–412 [Google Scholar]
- Centritto M, Haworth M, Marino G, Pallozzi E, Tsonev T, Velikova V, Nogues I, Loreto F (2014) Isoprene emission aids recovery of photosynthetic performance in transgenic Nicotiana tabacum following high intensity acute UV-B exposure. Plant Sci 226: 82–91 [DOI] [PubMed] [Google Scholar]
- Chai MF, Wei PC, Chen QJ, An R, Chen J, Yang S, Wang XC (2006) NADK3, a novel cytoplasmic source of NADPH, is required under conditions of oxidative stress and modulates abscisic acid responses in Arabidopsis. Plant J 47: 665–674 [DOI] [PubMed] [Google Scholar]
- Chen YS, Chao YC, Tseng TW, Huang CK, Lo PC, Lu CA (2017) Two MYB-related transcription factors play opposite roles in sugar signaling in Arabidopsis. Plant Mol Biol 93: 299–311 [DOI] [PubMed] [Google Scholar]
- Cheng SH, Sheen J, Gerrish C, Bolwell GP (2001) Molecular identification of phenylalanine ammonia-lyase as a substrate of a specific constitutively active Arabidopsis CDPK expressed in maize protoplasts. FEBS Lett 503: 185–188 [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Commisso M, Toffali K, Strazzer P, Stocchero M, Ceoldo S, Baldan B, Levi M, Guzzo F (2016) Impact of phenylpropanoid compounds on heat stress tolerance in carrot cell cultures. Front Plant Sci 7: 1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortleven A, Leuendorf JE, Frank M, Pezzetta D, Bolt S, Schmülling T (2018) Cytokinin action in response to abiotic and biotic stress in plants. Plant Cell Environ 42: 998–1018 [DOI] [PubMed] [Google Scholar]
- Crombie AT, Larke-Mejia NL, Emery H, Dawson R, Pratscher J, Murphy GP, McGenity TJ, Murrell JC (2018) Poplar phyllosphere harbors disparate isoprene-degrading bacteria. Proc Natl Acad Sci USA 115: 13081–13086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donald RGK, Cashmore AR (1990) Mutation of either G box or I box sequences profoundly affects expression from the Arabidopsis rbcS-1A promoter. EMBO J 9: 1717–1726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckey-Kaltenbach H, Heller W, Sonnenbichler J, Zetl I, Schäfer W, Ernst D, Sandermann H (1993) Oxidative stress and plant secondary metabolism: 6″-O-malonyl apiin in parsley. Phytochemistry 34: 687–691 [Google Scholar]
- Edgar R, Domrachev M, Lash AE (2002) Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res 30: 207–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essemine J, Xiao Y, Qu M, Mi H, Zhu XG (2017) Cyclic electron flow may provide some protection against PSII photoinhibition in rice (Oryza sativa L.) leaves under heat stress. J Plant Physiol 211: 138–146 [DOI] [PubMed] [Google Scholar]
- Ge Y, Cai Y-M, Bonneau L, Rotari V, Danon A, McKenzie EA, McLellan H, Mach L, Gallois P (2016) Inhibition of cathepsin B by caspase-3 inhibitors blocks programmed cell death in Arabidopsis. Cell Death Differ 23: 1493–1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genard-Zielinski AC, Ormeño E, Boissard C, Fernandez C (2014) Isoprene emissions from downy oak under water limitation during an entire growing season: What cost for growth? PLoS ONE 9: e112418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghirardo A, Koch K, Taipale R, Zimmer I, Schnitzler JP, Rinne J (2010) Determination of de novo and pool emissions of terpenes from four common boreal/alpine trees by 13CO2 labelling and PTR-MS analysis. Plant Cell Environ 33: 781–792 [DOI] [PubMed] [Google Scholar]
- Ghirardo A, Wright LP, Bi Z, Rosenkranz M, Pulido P, Rodríguez-Concepción M, Niinemets Ü, Brüggemann N, Gershenzon J, Schnitzler JP (2014) Metabolic flux analysis of plastidic isoprenoid biosynthesis in poplar leaves emitting and nonemitting isoprene. Plant Physiol 165: 37–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleave AP. (1992) A versatile binary vector system with a T-DNA organisational structure conducive to efficient integration of cloned DNA into the plant genome. Plant Mol Biol 20: 1203–1207 [DOI] [PubMed] [Google Scholar]
- Gómez-Mena C, Piñeiro M, Franco-Zorrilla JM, Salinas J, Coupland G, Martínez-Zapater JM (2001) early bolting in short days: An Arabidopsis mutation that causes early flowering and partially suppresses the floral phenotype of leafy. Plant Cell 13: 1011–1024 [PMC free article] [PubMed] [Google Scholar]
- Gong X, Jiang Q, Xu J, Zhang J, Teng S, Lin D, Dong Y (2013) Disruption of the rice plastid ribosomal protein s20 leads to chloroplast developmental defects and seedling lethality. G3 (Bethesda) 3: 1769–1777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groß F, Rudolf EE, Thiele B, Durner J, Astier J (2017) Copper amine oxidase 8 regulates arginine-dependent nitric oxide production in Arabidopsis thaliana. J Exp Bot 68: 2149–2162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris A, Owen SM, Sleep D, Pereira MDGDS (2016) Constitutive changes in pigment concentrations: Implications for estimating isoprene emissions using the photochemical reflectance index. Physiol Plant 156: 190–200 [DOI] [PubMed] [Google Scholar]
- Harrison SJ, Mott EK, Parsley K, Aspinall S, Gray JC, Cottage A (2006) A rapid and robust method of identifying transformed Arabidopsis thaliana seedlings following floral dip transformation. Plant Methods 2: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey CM, Sharkey TD (2016) Exogenous isoprene modulates gene expression in unstressed Arabidopsis thaliana plants. Plant Cell Environ 39: 1251–1263 [DOI] [PubMed] [Google Scholar]
- Harvey CM, Li Z, Tjellström H, Blanchard GJ, Sharkey TD (2015) Concentration of isoprene in artificial and thylakoid membranes. J Bioenerg Biomembr 47: 419–429 [DOI] [PubMed] [Google Scholar]
- Hashida SN, Takahashi H, Uchimiya H (2009) The role of NAD biosynthesis in plant development and stress responses. Ann Bot 103: 819–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoagland DR, Arnon DI (1938) The Water-Culture Method for Growing Plants without Soil, Vol 347 College of Agriculture, University of California, Berkeley [Google Scholar]
- Huo H, Dahal P, Kunusoth K, McCallum CM, Bradford KJ (2013) Expression of 9-cis-EPOXYCAROTENOID DIOXYGENASE4 is essential for thermoinhibition of lettuce seed germination but not for seed development or stress tolerance. Plant Cell 25: 884–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang I, Sakakibara H (2006) Cytokinin biosynthesis and perception. Physiol Plant 126: 528–538 [Google Scholar]
- Ioannidis NE, Cruz JA, Kotzabasis K, Kramer DM (2012) Evidence that putrescine modulates the higher plant photosynthetic proton circuit. PLoS ONE 7: e29864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ischebeck T, Werner S, Krishnamoorthy P, Lerche J, Meijón M, Stenzel I, Löfke C, Wiessner T, Im YJ, Perera IY, et al. (2013) Phosphatidylinositol 4,5-bisphosphate influences PIN polarization by controlling clathrin-mediated membrane trafficking in Arabidopsis. Plant Cell 25: 4894–4911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwase A, Harashima H, Ikeuchi M, Rymen B, Ohnuma M, Komaki S, Morohashi K, Kurata T, Nakata M, Ohme-Takagi M, et al. (2017) WIND1 promotes shoot regeneration through transcriptional activation of ENHANCER OF SHOOT REGENERATION1 in Arabidopsis. Plant Cell 29: 54–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi M, Tsunoda H, Suzuki Y, Makino A, Ishida H (2012) RBCS1A and RBCS3B, two major members within the Arabidopsis RBCS multigene family, function to yield sufficient Rubisco content for leaf photosynthetic capacity. J Exp Bot 63: 2159–2170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakimoto T. (2003) Biosynthesis of cytokinins. J Plant Res 116: 233–239 [DOI] [PubMed] [Google Scholar]
- Kim S, Schlicke H, Van Ree K, Karvonen K, Subramaniam A, Richter A, Grimm B, Braam J (2013) Arabidopsis chlorophyll biosynthesis: An essential balance between the methylerythritol phosphate and tetrapyrrole pathways. Plant Cell 25: 4984–4993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar RR, Sharma SK, Rai GK, Singh K, Choudhury M, Dhawan Gaurav, Singh GP, Goswami S, Pathak H, Rai RD (2014) Exogenous application of putrescine at pre-anthesis enhances the thermotolerance of wheat (Triticum aestivum L.). Indian J Biochem Biophys 51: 396–406 [PubMed] [Google Scholar]
- Kuzma J, Nemecek-Marshall M, Pollock WH, Fall R (1995) Bacteria produce the volatile hydrocarbon isoprene. Curr Microbiol 30: 97–103 [DOI] [PubMed] [Google Scholar]
- Laothawornkitkul J, Paul ND, Vickers CE, Possell M, Taylor JE, Mullineaux PM, Hewitt CN (2008) Isoprene emissions influence herbivore feeding decisions. Plant Cell Environ 31: 1410–1415 [DOI] [PubMed] [Google Scholar]
- Laule O, Fürholz A, Chang HS, Zhu T, Wang X, Heifetz PB, Gruissem W, Lange M (2003) Crosstalk between cytosolic and plastidial pathways of isoprenoid biosynthesis in Arabidopsis thaliana. Proc Natl Acad Sci USA 100: 6866–6871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law CW, Chen Y, Shi W, Smyth GK (2014) voom: Precision weights unlock linear model analysis tools for RNA-seq read counts. Genome Biol 15: R29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Sharkey TD (2013a) Metabolic profiling of the methylerythritol phosphate pathway reveals the source of post-illumination isoprene burst from leaves. Plant Cell Environ 36: 429–437 [DOI] [PubMed] [Google Scholar]
- Li Z, Sharkey TD (2013b) Molecular and pathway controls on biogenic volatile organic compound emissions. In Niinemets Ü, Monson RK, eds, Biology, Controls and Models of Tree Volatile Organic Compound Emissions. Springer, Berlin, pp 119–151 [Google Scholar]
- Li B, Gao K, Ren H, Tang W (2018) Molecular mechanisms governing plant responses to high temperatures. J Integr Plant Biol 60: 757–779 [DOI] [PubMed] [Google Scholar]
- Li D, Chen Y, Shi Y, He X, Chen X (2009) Impact of elevated CO2 and O3 concentrations on biogenic volatile organic compounds emissions from Ginkgo biloba. Bull Environ Contam Toxicol 82: 473–477 [DOI] [PubMed] [Google Scholar]
- Li Z, Ratliff EA, Sharkey TD (2011) Effect of temperature on postillumination isoprene emission in oak and poplar. Plant Physiol 155: 1037–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y, Smyth GK, Shi W (2013) The Subread aligner: Fast, accurate and scalable read mapping by seed-and-vote. Nucleic Acids Res 41: e108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenthaler HK. (2007) Biosynthesis, accumulation and emission of carotenoids, α-tocopherol, plastoquinone, and isoprene in leaves under high photosynthetic irradiance. Photosynth Res 92: 163–179 [DOI] [PubMed] [Google Scholar]
- Lichtenthaler HK. (2009) Biosynthesis and accumulation of isoprenoid carotenoids and chlorophylls and emission of isoprene by leaf chloroplasts. Bull Georgian Natl Acad Sci 3: 81–94 [Google Scholar]
- Lichtenthaler HK, Wellburn AR (1983) Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem Soc Trans 11: 591–592 [Google Scholar]
- Lin RC, Park HJ, Wang HY (2008) Role of Arabidopsis RAP2.4 in regulating light- and ethylene-mediated developmental processes and drought stress tolerance. Mol Plant 1: 42–57 [DOI] [PubMed] [Google Scholar]
- Liu J, Last RL (2015a) A land plant-specific thylakoid membrane protein contributes to photosystem II maintenance in Arabidopsis thaliana. Plant J 82: 731–743 [DOI] [PubMed] [Google Scholar]
- Liu J, Last RL (2015b) MPH1 is a thylakoid membrane protein involved in protecting photosystem II from photodamage in land plants. Plant Signal Behav 10: e1076602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Last RL (2017) A chloroplast thylakoid lumen protein is required for proper photosynthetic acclimation of plants under fluctuating light environments. Proc Natl Acad Sci USA 114: E8110–E8117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JH, Wang W, Wu H, Gong X, Moriguchi T (2015) Polyamines function in stress tolerance: From synthesis to regulation. Front Plant Sci 6: 827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XD, Xie L, Wei Y, Zhou X, Jia B, Liu J, Zhang S (2014) Abiotic stress resistance, a novel moonlighting function of ribosomal protein RPL44 in the halophilic fungus Aspergillus glaucus. Appl Environ Microbiol 80: 4294–4300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loivamäki M, Gilmer F, Fischbach RJ, Sörgel C, Bachl A, Walter A, Schnitzler JP (2007) Arabidopsis, a model to study biological functions of isoprene emission? Plant Physiol 144: 1066–1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loivamäki M, Mumm R, Dicke M, Schnitzler JP (2008) Isoprene interferes with the attraction of bodyguards by herbaceous plants. Proc Natl Acad Sci USA 105: 17430–17435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loreto F, Delfine S (2000) Emission of isoprene from salt-stressed Eucalyptus globulus leaves. Plant Physiol 123: 1605–1610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loreto F, Schnitzler JP (2010) Abiotic stresses and induced BVOCs. Trends Plant Sci 15: 154–166 [DOI] [PubMed] [Google Scholar]
- Loreto F, Velikova V (2001) Isoprene produced by leaves protects the photosynthetic apparatus against ozone damage, quenches ozone products, and reduces lipid peroxidation of cellular membranes. Plant Physiol 127: 1781–1787 [PMC free article] [PubMed] [Google Scholar]
- Loreto F, Mannozzi M, Maris C, Nascetti P, Ferranti F, Pasqualini S (2001) Ozone quenching properties of isoprene and its antioxidant role in leaves. Plant Physiol 126: 993–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loreto F, Centritto M, Barta C, Calfapietra C, Fares S, Monson RK (2007) The relationship between isoprene emission rate and dark respiration rate in white poplar (Populus alba L.) leaves. Plant Cell Environ 30: 662–669 [DOI] [PubMed] [Google Scholar]
- Lozano-Durán R, Zipfel C (2015) Trade-off between growth and immunity: Role of brassinosteroids. Trends Plant Sci 20: 12–19 [DOI] [PubMed] [Google Scholar]
- Lv M, Kong H, Liu H, Lu Y, Zhang C, Liu J, Ji C, Zhu J, Su J, Gao X (2016) Induction of phenylalanine ammonia-lyase (PAL) in insect damaged and neighboring undamaged cotton and maize seedlings. Int J Pest Manage 63: 166–171 [Google Scholar]
- Mason MG, Mathews DE, Argyros DA, Maxwell BB, Kieber JJ, Alonso JM, Ecker JR, Schaller GE (2005) Multiple type-B response regulators mediate cytokinin signal transduction in Arabidopsis. Plant Cell 17: 3007–3018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matías-Hernández L, Aguilar-Jaramillo AE, Marín-González E, Suárez-López P, Pelaz S (2014) RAV genes: Regulation of floral induction and beyond. Ann Bot 114: 1459–1470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller B, Oschinski C, Zimmer W (2001) First isolation of an isoprene synthase gene from poplar and successful expression of the gene in Escherichia coli. Planta 213: 483–487 [DOI] [PubMed] [Google Scholar]
- Monson RK, Fall R (1989) Isoprene emission from aspen leaves: Influence of environment and relation to photosynthesis and photorespiration. Plant Physiol 90: 267–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monson RK, Hills AJ, Zimmerman PR, Fall RR (1991) Studies of the relationship between isoprene emission rate and CO2 or photon-flux density using a real-time isoprene analyser. Plant Cell Environ 14: 517–523 [Google Scholar]
- Monson RK, Trahan N, Rosenstiel TN, Veres P, Moore D, Wilkinson M, Norby RJ, Volder A, Tjoelker MG, Briske DD, et al. (2007) Isoprene emission from terrestrial ecosystems in response to global change: Minding the gap between models and observations. Philos Trans A Math Phys Eng Sci 365: 1677–1695 [DOI] [PubMed] [Google Scholar]
- Monson RK, Jones RT, Rosenstiel TN, Schnitzler JP (2013) Why only some plants emit isoprene. Plant Cell Environ 36: 503–516 [DOI] [PubMed] [Google Scholar]
- Montilla-Bascón G, Rubiales D, Hebelstrup KH, Mandon J, Harren FJM, Cristescu SM, Mur LAJ, Prats E (2017) Reduced nitric oxide levels during drought stress promote drought tolerance in barley and is associated with elevated polyamine biosynthesis. Sci Rep 7: 13311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muralla R, Lloyd J, Meinke D (2011) Molecular foundations of reproductive lethality in Arabidopsis thaliana. PLoS ONE 6: e28398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niinemets Ü, Sun Z (2015) How light, temperature, and measurement and growth [CO2] interactively control isoprene emission in hybrid aspen. J Exp Bot 66: 841–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niinemets Ü, Tenhunen JD, Harley PC, Steinbrecher R (1999) A model of isoprene emission based on energetic requirements for isoprene synthesis and leaf photosynthetic properties for Liquidambar and Quercus. Plant Cell Environ 22: 1319–1336 [Google Scholar]
- Okada K, Hase T (2005) Cyanobacterial non-mevalonate pathway: (E)-4-Hydroxy-3-methylbut-2-enyl diphosphate synthase interacts with ferredoxin in Thermosynechococcus elongatus BP-1. J Biol Chem 280: 20672–20679 [DOI] [PubMed] [Google Scholar]
- Orlova I, Nagegowda DA, Kish CM, Gutensohn M, Maeda H, Varbanova M, Fridman E, Yamaguchi S, Hanada A, Kamiya Y, et al. (2009) The small subunit of snapdragon geranyl diphosphate synthase modifies the chain length specificity of tobacco geranylgeranyl diphosphate synthase in planta. Plant Cell 21: 4002–4017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen SM, Peñuelas J (2005) Opportunistic emissions of volatile isoprenoids. Trends Plant Sci 10: 420–426 [DOI] [PubMed] [Google Scholar]
- Peng L, Fukao Y, Fujiwara M, Takami T, Shikanai T (2009) Efficient operation of NAD(P)H dehydrogenase requires supercomplex formation with photosystem I via minor LHCI in Arabidopsis. Plant Cell 21: 3623–3640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng L, Fukao Y, Fujiwara M, Shikanai T (2012) Multistep assembly of chloroplast NADH dehydrogenase-like subcomplex A requires several nucleus-encoded proteins, including CRR41 and CRR42, in Arabidopsis. Plant Cell 24: 202–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Amador MA, Leon J, Green PJ, Carbonell J (2002) Induction of the arginine decarboxylase ADC2 gene provides evidence for the involvement of polyamines in the wound response in Arabidopsis. Plant Physiol 130: 1454–1463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pičmanová M, Møller BL (2016) Apiose: One of nature’s witty games. Glycobiology 26: 430–442 [DOI] [PubMed] [Google Scholar]
- Pollastri S, Tsonev T, Loreto F (2014) Isoprene improves photochemical efficiency and enhances heat dissipation in plants at physiological temperatures. J Exp Bot 65: 1565–1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Possell M, Hewitt CN (2009) Gas exchange and photosynthetic performance of the tropical tree Acacia nigrescens when grown in different CO2 concentrations. Planta 229: 837–846 [DOI] [PubMed] [Google Scholar]
- Possell M, Hewitt CN (2011) Isoprene emissions from plants are mediated by atmospheric CO2 concentrations. Glob Change Biol 17: 1595–1610 [Google Scholar]
- Potosnak MJ, Lestourgeon L, Nunez O (2014) Increasing leaf temperature reduces the suppression of isoprene emission by elevated CO2 concentration. Sci Total Environ 481: 352–359 [DOI] [PubMed] [Google Scholar]
- Raab S, Drechsel G, Zarepour M, Hartung W, Koshiba T, Bittner F, Hoth S (2009) Identification of a novel E3 ubiquitin ligase that is required for suppression of premature senescence in Arabidopsis. Plant J 59: 39–51 [DOI] [PubMed] [Google Scholar]
- Rasulov B, Copolovici L, Laisk A, Niinemets U (2009) Postillumination isoprene emission: In vivo measurements of dimethylallyldiphosphate pool size and isoprene synthase kinetics in aspen leaves. Plant Physiol 149: 1609–1618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasulov B, Hüve K, Bichele I, Laisk A, Niinemets U (2010) Temperature response of isoprene emission in vivo reflects a combined effect of substrate limitations and isoprene synthase activity: A kinetic analysis. Plant Physiol 154: 1558–1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasulov B, Hüve K, Laisk A, Niinemets Ü (2011) Induction of a longer term component of isoprene release in darkened aspen leaves: Origin and regulation under different environmental conditions. Plant Physiol 156: 816–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasulov B, Bichele I, Laisk A, Niinemets Ü (2014) Competition between isoprene emission and pigment synthesis during leaf development in aspen. Plant Cell Environ 37: 724–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohmer M, Knani M, Simonin P, Sutter B, Sahm H (1993) Isoprenoid biosynthesis in bacteria: A novel pathway for the early steps leading to isopentenyl diphosphate. Biochem J 295: 517–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romani I, Tadini L, Rossi F, Masiero S, Pribil M, Jahns P, Kater M, Leister D, Pesaresi P (2012) Versatile roles of Arabidopsis plastid ribosomal proteins in plant growth and development. Plant J 72: 922–934 [DOI] [PubMed] [Google Scholar]
- Ryan AC, Hewitt CN, Possell M, Vickers CE, Purnell A, Mullineaux PM, Davies WJ, Dodd IC (2014) Isoprene emission protects photosynthesis but reduces plant productivity during drought in transgenic tobacco (Nicotiana tabacum) plants. New Phytol 201: 205–216 [DOI] [PubMed] [Google Scholar]
- Sagor GH, Berberich T, Takahashi Y, Niitsu M, Kusano T (2013) The polyamine spermine protects Arabidopsis from heat stress-induced damage by increasing expression of heat shock-related genes. Transgenic Res 22: 595–605 [DOI] [PubMed] [Google Scholar]
- Saha J, Brauer EK, Sengupta A, Popescu SC, Gupta K, Gupta B (2015) Polyamines as redox homeostasis regulators during salt stress in plants. Front Environ Sci 3: 21 [Google Scholar]
- Sanadze GA. (2004) Biogenic isoprene (a review). Russ J Plant Physiol 51: 729–741 [Google Scholar]
- Sanadze G. (2010) Photobiosynthesis of isoprene as an example of leaf excretory function in the light of contemporary thermodynamics. Russ J Plant Physiol 57: 1–6 [Google Scholar]
- Sasaki K, Saito T, Lämsä M, Oksman-Caldentey KM, Suzuki M, Ohyama K, Muranaka T, Ohara K, Yazaki K (2007) Plants utilize isoprene emission as a thermotolerance mechanism. Plant Cell Physiol 48: 1254–1262 [DOI] [PubMed] [Google Scholar]
- Schnitzler JP, Zimmer I, Bachl A, Arend M, Fromm J, Fischbach RJ (2005) Biochemical properties of isoprene synthase in poplar (Populus × canescens). Planta 222: 777–786 [DOI] [PubMed] [Google Scholar]
- Schnitzler JP, Louis S, Behnke K, Loivamäki M (2010) Poplar volatiles: Biosynthesis, regulation and (eco)physiology of isoprene and stress-induced isoprenoids. Plant Biol (Stuttg) 12: 302–316 [DOI] [PubMed] [Google Scholar]
- Scholefield PA, Doick KJ, Herbert BMJ, Hewitt CNS, Schnitzler JP, Pinelli P, Loreto F (2004) Impact of rising CO2 on emissions of volatile organic compounds: Isoprene emission from Phragmites australis growing at elevated CO2 in a natural carbon dioxide spring. Plant Cell Environ 27: 393–401 [Google Scholar]
- Seemann M, Rohmer M (2007) Isoprenoid biosynthesis via the methylerythritol phosphate pathway: GcpE and LytB, two novel iron-sulphur proteins. C R Chim 10: 748–755 [Google Scholar]
- Seemann M, Bui BTS, Wolff M, Tritsch D, Campos N, Boronat A, Marquet A, Rohmer M (2002) Isoprenoid biosynthesis through the methylerythritol phosphate pathway: The (E)-4-hydroxy-3-methylbut-2-enyl diphosphate synthase (GcpE) is a [4Fe-4S] protein. Angew Chem Int Ed Engl 41: 4337–4339 [DOI] [PubMed] [Google Scholar]
- Seemann M, Tse Sum Bui B, Wolff M, Miginiac-Maslow M, Rohmer M (2006) Isoprenoid biosynthesis in plant chloroplasts via the MEP pathway: Direct thylakoid/ferredoxin-dependent photoreduction of GcpE/IspG. FEBS Lett 580: 1547–1552 [DOI] [PubMed] [Google Scholar]
- Sharkey TD. (2013) Is it useful to ask why plants emit isoprene? Plant Cell Environ 36: 517–520 [DOI] [PubMed] [Google Scholar]
- Sharkey TD, Loreto F (1993) Water stress, temperature, and light effects on the capacity for isoprene emission and photosynthesis of kudzu leaves. Oecologia 95: 328–333 [DOI] [PubMed] [Google Scholar]
- Sharkey TD, Loreto F (1993) Water stress, temperature, and light effects on the capacity for isoprene emission and photosynthesis of kudzu leaves. Oecologia 95: 328–333 [DOI] [PubMed] [Google Scholar]
- Sharkey TD, Monson RK (2014) The future of isoprene emission from leaves, canopies and landscapes. Plant Cell Environ 37: 1727–1740 [DOI] [PubMed] [Google Scholar]
- Sharkey TD, Singsaas EL (1995) Why plants emit isoprene. Nature 374: 769 [Google Scholar]
- Sharkey TD, Yeh S (2001) Isoprene emission from plants. Annu Rev Plant Physiol Plant Mol Biol 52: 407–436 [DOI] [PubMed] [Google Scholar]
- Sharkey TD, Singsaas EL, Vanderveer PJ, Geron C (1996) Field measurements of isoprene emission from trees in response to temperature and light. Tree Physiol 16: 649–654 [DOI] [PubMed] [Google Scholar]
- Sharkey TD, Chen X, Yeh S (2001) Isoprene increases thermotolerance of fosmidomycin-fed leaves. Plant Physiol 125: 2001–2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey TD, Yeh S, Wiberley AE, Falbel TG, Gong D, Fernandez DE (2005) Evolution of the isoprene biosynthetic pathway in kudzu. Plant Physiol 137: 700–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey TD, Wiberley AE, Donohue AR (2008) Isoprene emission from plants: Why and how. Ann Bot 101: 5–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey TD, Gray DW, Pell HK, Breneman SR, Topper L (2013) Isoprene synthase genes form a monophyletic clade of acyclic terpene synthases in the TPS-B terpene synthase family. Evolution 67: 1026–1040 [DOI] [PubMed] [Google Scholar]
- Shikanai T. (2016) Chloroplast NDH: A different enzyme with a structure similar to that of respiratory NADH dehydrogenase. Biochim Biophys Acta 1857: 1015–1022 [DOI] [PubMed] [Google Scholar]
- Sierro N, Battey JN, Ouadi S, Bakaher N, Bovet L, Willig A, Goepfert S, Peitsch MC, Ivanov NV (2014) The tobacco genome sequence and its comparison with those of tomato and potato. Nat Commun 5: 3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver GM, Fall R (1995) Characterization of aspen isoprene synthase, an enzyme responsible for leaf isoprene emission to the atmosphere. J Biol Chem 270: 13010–13016 [DOI] [PubMed] [Google Scholar]
- Singsaas EL, Sharkey TD (1998) The regulation of isoprene emission responses to rapid leaf temperature fluctuations. Plant Cell Environ 21: 1181–1188 [Google Scholar]
- Singsaas EL, Sharkey TD (2000) The effects of high temperature on isoprene synthesis in oak leaves. Plant Cell Environ 23: 751–757 [Google Scholar]
- Singsaas EL, Lerdau M, Winter K, Sharkey TD (1997) Isoprene increases thermotolerance of isoprene-emitting species. Plant Physiol 115: 1413–1420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singsaas EL, Laporte MM, Shi JZ, Monson RK, Bowling DR, Johnson K, Lerdau M, Jasentuliytana A, Sharkey TD (1999) Kinetics of leaf temperature fluctuation affect isoprene emission from red oak (Quercus rubra) leaves. Tree Physiol 19: 917–924 [DOI] [PubMed] [Google Scholar]
- Sun TP. (2008) Gibberellin metabolism, perception and signaling pathways in Arabidopsis. The Arabidopsis Book 6: e0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z, Niinemets Ü, Hüve K, Noe SM, Rasulov B, Copolovici L, Vislap V (2012) Enhanced isoprene emission capacity and altered light responsiveness in aspen grown under elevated atmospheric CO2 concentration. Glob Change Biol 18: 3423–3440 [Google Scholar]
- Sun Z, Hüve K, Vislap V, Niinemets Ü (2013a) Elevated [CO2] magnifies isoprene emissions under heat and improves thermal resistance in hybrid aspen. J Exp Bot 64: 5509–5523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z, Niinemets Ü, Hüve K, Rasulov B, Noe SM (2013b) Elevated atmospheric CO2 concentration leads to increased whole-plant isoprene emission in hybrid aspen (Populus tremula × Populus tremuloides). New Phytol 198: 788–800 [DOI] [PubMed] [Google Scholar]
- Takase T, Nakazawa M, Ishikawa A, Manabe K, Matsui M (2003) DFL2, a new member of the Arabidopsis GH3 gene family, is involved in red light-specific hypocotyl elongation. Plant Cell Physiol 44: 1071–1080 [DOI] [PubMed] [Google Scholar]
- Terry GM, Stokes NJ, Hewitt CN, Mansfield TA (1995) Exposure to isoprene promotes flowering in plants. J Exp Bot 46: 1629–1631 [Google Scholar]
- Tholl D, Croteau R, Gershenzon J (2001) Partial purification and characterization of the short-chain prenyltransferases, gernayl diphospate synthase and farnesyl diphosphate synthase, from Abies grandis (grand fir). Arch Biochem Biophys 386: 233–242 [DOI] [PubMed] [Google Scholar]
- Tiller N, Bock R (2014) The translational apparatus of plastids and its role in plant development. Mol Plant 7: 1105–1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiller N, Weingartner M, Thiele W, Maximova E, Schöttler MA, Bock R (2012) The plastid-specific ribosomal proteins of Arabidopsis thaliana can be divided into non-essential proteins and genuine ribosomal proteins. Plant J 69: 302–316 [DOI] [PubMed] [Google Scholar]
- Urano K, Yoshiba Y, Nanjo T, Ito T, Yamaguchi-Shinozaki K, Shinozaki K (2004) Arabidopsis stress-inducible gene for arginine decarboxylase AtADC2 is required for accumulation of putrescine in salt tolerance. Biochem Biophys Res Commun 313: 369–375 [DOI] [PubMed] [Google Scholar]
- Urano K, Hobo T, Shinozaki K (2005) Arabidopsis ADC genes involved in polyamine biosynthesis are essential for seed development. FEBS Lett 579: 1557–1564 [DOI] [PubMed] [Google Scholar]
- Vanzo E, Jud W, Li Z, Albert A, Domagalska MA, Ghirardo A, Niederbacher B, Frenzel J, Beemster GTS, Asard H, et al. (2015) Facing the future: Effects of short-term climate extremes on isoprene-emitting and nonemitting poplar. Plant Physiol 169: 560–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanzo E, Merl-Pham J, Velikova V, Ghirardo A, Lindermayr C, Hauck SM, Bernhardt J, Riedel K, Durner J, Schnitzler JP (2016) Modulation of protein S-nitrosylation by isoprene emission in poplar. Plant Physiol 170: 1945–1961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velikova V, Loreto F (2005) On the relationship between isoprene emission and thermotolerance in Phragmites australis leaves exposed to high temperatures and during the recovery from a heat stress. Plant Cell Environ 28: 318–327 [Google Scholar]
- Velikova V, Loreto F, Tsonev T, Brilli F, Edreva A (2006) Isoprene prevents the negative consequences of high temperature stress in Platanus orientalis leaves. Funct Plant Biol 33: 931–940 [DOI] [PubMed] [Google Scholar]
- Velikova V, Tsonev T, Barta C, Centritto M, Koleva D, Stefanova M, Busheva M, Loreto F (2009) BVOC emissions, photosynthetic characteristics and changes in chloroplast ultrastructure of Platanus orientalis L. exposed to elevated CO2 and high temperature. Environ Pollut 157: 2629–2637 [DOI] [PubMed] [Google Scholar]
- Velikova V, Várkonyi Z, Szabó M, Maslenkova L, Nogues I, Kovács L, Peeva V, Busheva M, Garab G, Sharkey TD, et al. (2011) Increased thermostability of thylakoid membranes in isoprene-emitting leaves probed with three biophysical techniques. Plant Physiol 157: 905–916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickers CE, Gershenzon J, Lerdau MT, Loreto F (2009a) A unified mechanism of action for volatile isoprenoids in plant abiotic stress. Nat Chem Biol 5: 283–291 [DOI] [PubMed] [Google Scholar]
- Vickers CE, Possell M, Cojocariu CI, Velikova VB, Laothawornkitkul J, Ryan A, Mullineaux PM, Nicholas Hewitt C (2009b) Isoprene synthesis protects transgenic tobacco plants from oxidative stress. Plant Cell Environ 32: 520–531 [DOI] [PubMed] [Google Scholar]
- Vickers CE, Possell M, Laothawornkitkul J, Ryan AC, Hewitt CN, Mullineaux PM (2011) Isoprene synthesis in plants: Lessons from a transgenic tobacco model. Plant Cell Environ 34: 1043–1053 [DOI] [PubMed] [Google Scholar]
- Wagner WP, Helmig D, Fall R (2000) Isoprene biosynthesis in Bacillus subtilis via the methylerythritol phosphate pathway. J Nat Prod 63: 37–40 [DOI] [PubMed] [Google Scholar]
- Wang G, Dixon RA (2009) Heterodimeric geranyl(geranyl)diphosphate synthase from hop (Humulus lupulus) and the evolution of monoterpene biosynthesis. Proc Natl Acad Sci USA 106: 9914–9919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Way DA, Schnitzler JP, Monson RK, Jackson RB (2011) Enhanced isoprene-related tolerance of heat- and light-stressed photosynthesis at low, but not high, CO2 concentrations. Oecologia 166: 273–282 [DOI] [PubMed] [Google Scholar]
- Way DA, Ghirardo A, Kanawati B, Esperschütz J, Monson RK, Jackson RB, Schmitt-Kopplin P, Schnitzler JP (2013) Increasing atmospheric CO2 reduces metabolic and physiological differences between isoprene- and non-isoprene-emitting poplars. New Phytol 200: 534–546 [DOI] [PubMed] [Google Scholar]
- Weise SE, Li Z, Sutter AE, Corrion A, Banerjee A, Sharkey TD (2013) Measuring dimethylallyl diphosphate available for isoprene synthesis. Anal Biochem 435: 27–34 [DOI] [PubMed] [Google Scholar]
- Weraduwage SM, Campos ML, Yoshida Y, Major IT, Kim YS, Kim SJ, Renna L, Anozie FC, Brandizzi F, Thomashow MF, et al. (2018) The leaf: A platform for performing photosynthesis. In Sharkey TD, Eaton-Rye J, eds, Advances in Photosynthesis and Respiration Including Bioenergy and Related Processes. Springer, Berlin, pp 209–254 [Google Scholar]
- Wiberley AE, Donohue AR, Meier ME, Westphal MM, Sharkey TD (2008) Regulation of isoprene emission in Populus trichocarpa leaves subjected to changing growth temperature. Plant Cell Environ 31: 258–267 [DOI] [PubMed] [Google Scholar]
- Wilkinson MJ, Monson RK, Trahan N, Lee S, Brown E, Jackson RB, Polley HW, Fay PA, Fall RAY (2009) Leaf isoprene emission rate as a function of atmospheric CO2 concentration. Glob Change Biol 15: 1189–1200 [Google Scholar]
- Woo HR, Kim JH, Kim J, Kim J, Lee U, Song IJ, Kim JH, Lee HY, Nam HG, Lim PO (2010) The RAV1 transcription factor positively regulates leaf senescence in Arabidopsis. J Exp Bot 61: 3947–3957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Zhang X, He B, Diao L, Sheng S, Wang J, Guo X, Su N, Wang L, Jiang L, et al. (2007) A chlorophyll-deficient rice mutant with impaired chlorophyllide esterification in chlorophyll biosynthesis. Plant Physiol 145: 29–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y, Savchenko T, Baidoo EE, Chehab WE, Hayden DM, Tolstikov V, Corwin JA, Kliebenstein DJ, Keasling JD, Dehesh K (2012) Retrograde signaling by the plastidial metabolite MEcPP regulates expression of nuclear stress-response genes. Cell 149: 1525–1535 [DOI] [PubMed] [Google Scholar]
- Yuan X, Calatayud V, Gao F, Fares S, Paoletti E, Tian Y, Feng Z (2016) Interaction of drought and ozone exposure on isoprene emission from extensively cultivated poplar. Plant Cell Environ 39: 2276–2287 [DOI] [PubMed] [Google Scholar]
- Zarei A, Trobacher CP, Shelp BJ (2016) Arabidopsis aldehyde dehydrogenase 10 family members confer salt tolerance through putrescine-derived 4-aminobutyrate (GABA) production. Sci Rep 6: 35115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Gou M, Liu CJ (2013) Arabidopsis Kelch repeat F-box proteins regulate phenylpropanoid biosynthesis via controlling the turnover of phenylalanine ammonia-lyase. Plant Cell 25: 4994–5010 [DOI] [PMC free article] [PubMed] [Google Scholar]