Two repressors of light signaling, BBX30 and BBX31, are transcriptionally and negatively regulated by the transcription factor HY5 through direct binding to the G-box cis-element present in their promoters.
Abstract
Light-mediated seedling development is coordinately controlled by a variety of key regulators. Here, we identified two B-box (BBX)-containing proteins, BBX30 and BBX31, as repressors of photomorphogenesis. ELONGATED HYPOCOTYL5, a central regulator of light signaling, directly binds to the G-box cis-element present in the promoters of BBX30 and BBX31 and negatively controls their transcription levels in the light. Seedlings with mutations in BBX30 or BBX31 are hypersensitive to light, whereas the overexpression of BBX30 or BBX31 leads to hypo-photomorphogenic growth in the light. Furthermore, transgenic and phenotypic analysis revealed that the B-box domain of BBX30 or BBX31 is essential for their respective functioning in the regulation of photomorphogenic development in plants. In conclusion, BBX30 and BBX31 act as key negative regulators of light signaling, and their transcription is repressed by ELONGATED HYPOCOTYL5 through directly associating with their promoters.
As sessile organisms, plants respond dynamically to changes in light conditions throughout their life cycle. To optimize their growth and development, plants have evolved the ability to continuously monitor the quality, quantity, duration, and direction of light (Jiao et al., 2007). Plants undergo two distinct developmental processes depending on the absence or presence of light at the seedling stage. In the absence of light, germinated seeds develop elongated hypocotyls, closed cotyledons, and apical hooks, which is termed skotomorphogenesis. In the presence of light, seedlings display short hypocotyls and expanded cotyledons, which is known as photomorphogenesis (Sullivan and Deng, 2003). These two light-controlled developmental processes enable a seedling to emerge from a buried seed and penetrate through the soil.
At least four classes of photoreceptors are responsible for perceiving various wavelength spectra of light, which in turn initiate and control transcriptional reprogramming during the transition between skotomorphogenic and photomorphogenic development (Huang et al., 2014; Podolec and Ulm, 2018). Up to one-third of the genes in the Arabidopsis (Arabidopsis thaliana) genome are significantly altered during dark-to-light transition, indicating that these light-activated photoreceptors mediate the massive transcriptional reprogramming of molecular events to promote photomorphogenesis (Ma et al., 2001; Tepperman et al., 2004; Lau and Deng, 2012; Huang et al., 2014; Podolec and Ulm, 2018). ELONGATED HYPOCOTYL5 (HY5) acts downstream of all photoreceptors and is a central regulator of the light signal transduction pathway. Mutants impaired in HY5 exhibit dramatically elongated hypocotyls in white, blue, red, and far-red light (Oyama et al., 1997; Ang et al., 1998). The abundance of HY5 is tightly controlled by CONSTITUTIVELY PHOTOMORPHOGENIC/DE-ETIOLATED/FUSCA (COP/DET/FUS) proteins, the biochemical activities of which are inhibited by photoreceptors upon light irradiation (Osterlund et al., 2000; Podolec and Ulm, 2018). As a b-ZIP-type transcription factor, HY5 preferentially binds to the ACGT-containing (ACE) cis-elements present in the promoters of approximately one-third of Arabidopsis genes and controls their transcription, which in turn ultimately promotes photomorphogenic development in the light (Lee et al., 2007; Zhang et al., 2011).
There are 32 B-box (BBX)-containing proteins in Arabidopsis, multiple members of which have been documented to play critical roles in the regulation of photomorphogenesis. BBX4, BBX19, BBX20, BBX21, BBX22, and BBX23 are positive regulators of light signaling (Datta et al., 2006, 2007, 2008; Fan et al., 2012; Wang et al., 2015a; Xu et al., 2016, 2018; Zhang et al., 2017), while BBX24, BBX25, BBX28, and BBX32 repress photomorphogenetic development (Indorf et al., 2007; Holtan et al., 2011; Gangappa et al., 2013; Lin et al., 2018). Extensive genetic and biochemical studies have revealed that all of these BBX proteins converge on HY5 at transcriptional and/or protein levels through distinct regulatory mechanisms that mediate photomorphogenesis, suggesting that these BBX proteins together with HY5 constitute a complex but delicate signaling network in light-mediated seedling development that acts downstream of photoreceptors and the COP/DET/FUS system (Holm et al., 2001, 2002; Datta et al., 2006, 2007, 2008; Indorf et al., 2007; Chang et al., 2011; Holtan et al., 2011; Fan et al., 2012; Wang et al., 2015a; Xu et al., 2016, 2018; Zhang et al., 2017; Lin et al., 2018). BBX21 promotes photomorphogenesis by directly binding to the T/G-box cis-element present in the HY5 promoter to activate its expression (Xu et al., 2016, 2018). HY5 interacts with the promoter of BBX22 and up-regulates its gene expression (Chang et al., 2008). Each of BBX21, BBX22, BBX23, BBX24, BBX25, and BBX28 can form heterodimers with HY5, which serve to modulate the biochemical activity of HY5. Interestingly, BBX21, BBX22, and BBX23 may enhance the activity of HY5 (Datta et al., 2007, 2008; Zhang et al., 2017), whereas BBX24, BBX25, and BBX28 repress the transcriptional activity of HY5 (Gangappa et al., 2013; Lin et al., 2018). Specifically, BBX24 and BBX25 repress HY5 transcriptional activity toward BBX22 (Gangappa et al., 2013). In addition, BBX32 forms a potentially inactive heterodimer with BBX21 that interferes with the biochemical activity of the BBX21-HY5 complex (Holtan et al., 2011). Thus, the BBXs-HY5 module appears to act as a central regulatory hub in the light signal transduction pathway.
In this study, we report that BBX30 and BBX31, two B-box-containing proteins, constitute two previously uncharacterized negative regulators of light signaling that act directly downstream of the transcription factor HY5. HY5 directly binds to the G-box DNA cis-element within the BBX30 and BBX31 promoters and represses their transcription. bbx30 and bbx31 mutant seedlings displayed shortened hypocotyls, while transgenic seedlings overexpressing BBX30 or BBX31 showed elongated hypocotyls. In addition, the destruction of the B-box domain in BBX30 and BBX31 results in the full impairment of their biochemical activity in plants. Collectively, we have identified BBX30 and BBX31 as two repressors of photomorphogenesis that are negatively regulated by HY5 at the transcriptional level.
RESULTS
HY5 Represses the Expression of BBX30 and BBX31
As a previous genome-wide expression study indicated that BBX31 might be a negative target of HY5 (Lee et al., 2007), we thus tested if HY5 represses the transcript levels of BBX30 (a close homolog of BBX31; Khanna et al., 2009) and BBX31 using real-time quantitative PCR (RT-qPCR). As expected, the expression of both BBX30 and BBX31 dramatically increased in two independent hy5 (hy5-215 and hy5-51) mutants but decreased in HA-HY5 hy5-215 transgenic seedlings compared to the wild type (Fig. 1, A and B), demonstrating that HY5 indeed negatively controls the transcript levels of BBX30 and BBX31 in plants.
Figure 1.
The transcript levels of BBX30 and BBX31 are repressed by HY5 but induced by light. A and B, The expression levels of BBX30 (A) and BBX31 (B) in 4-d-old Col, hy5-215, hy5-51, and HA-HY5 hy5-215 seedlings grown in white light. C, The expression levels of BBX30 and BBX31 in 4-d-old Col grown in dark, white, blue (B), red (R), and far-red (FR) light conditions. D, The expression levels of BBX30 and BBX31 in 4-d-old dark-grown Col upon being transferred to white light at indicated time points. E to G, The expression levels of BBX30 and BBX31 in 4-d-old Col, phyA-211, phyB-9, cry1, and cry2 under different light (FR, R, and B) conditions as indicated. Data are means ± se; n = 3. PCR was performed in triplicate for each sample, and the expression levels were normalized to that of a PP2A gene. The experiments were performed three times with similar results. The graphs depict the results of one of three experiments.
Next, we examined whether BBX30 and BBX31 are regulated by light signals. The expression of both BBX30 and BBX31 in wild-type seedlings grown under various light conditions (white [W], blue [B], red [R], and far-red [FR]) was significantly increased when compared to that in etiolated seedlings (Fig. 1C). Moreover, the transcript levels of both BBX30 and BBX31 were markedly elevated when the dark-grown wild-type seedlings were transferred to W light for the various indicated times (1, 3, 6, 12, and 24 h; Fig. 1D). As distinct wavelength-specific light is perceived by various photoreceptors in plants, we thus investigated whether photoreceptors, including phyA (FR light photoreceptor), phyB (R light photoreceptor), CRYPTOCHROME 1 (CRY1), and CRY2 (B light photoreceptors), affect the expression levels of BBX30 and BBX31. The transcripts of both BBX30 and BBX31 were obviously decreased in phyA-211 grown in FR, in phyB-9 grown in R, as well as in cry1 and cry2 grown in B light compared to those in the wild type grown in the corresponding wavelength-specific light conditions (Fig. 1, E–G). Together, these data suggest that light induces the expression of both BBX30 and BBX31 at the transcriptional level, and photoreceptors play positive roles in the light-induced transcription of BBX30 and BBX31.
To examine whether light affects the stability of BBX30 and BBX31, we generated yellow fluorescent protein (YFP)-tagged BBX30 or BBX31 transgenic lines in the Col background, in which BBX30 or BBX31 was overexpressed and their respective gene products were detectable (Supplemental Fig. S1, A and B). Either YFP-BBX30 Col #3 or YFP-BBX31 Col #5 transgenic seedlings grown under various light conditions (dark [D], W, B, R, and FR) accumulated comparable YFP-BBX30 or YFP-BBX31 protein levels, respectively (Supplemental Fig. S2, A and B). When these two dark-grown transgenic lines were transferred to W light for different time periods (1, 3, 6, 12, and 24 h), they also accumulated similar abundances of YFP-BBX30 or YFP-BBX31 (Supplemental Fig. S2, C and D). These data suggest that light may have little effect on BBX30 and BBX31 accumulation in plants.
HY5 Directly Binds to the Promoters of BBX30 and BBX31
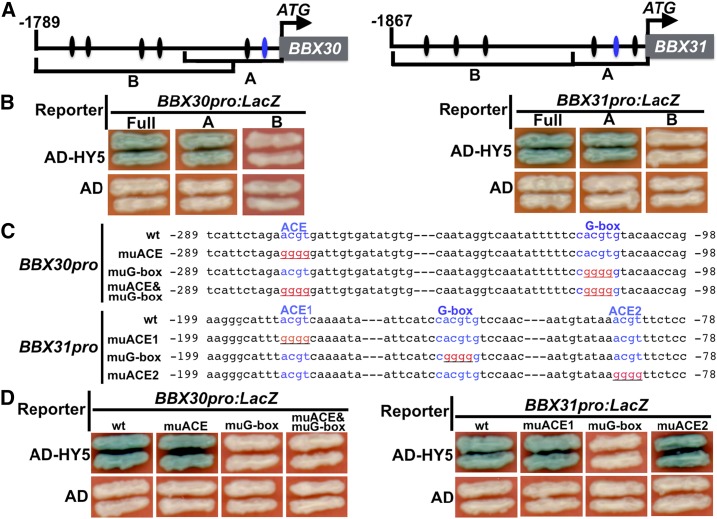
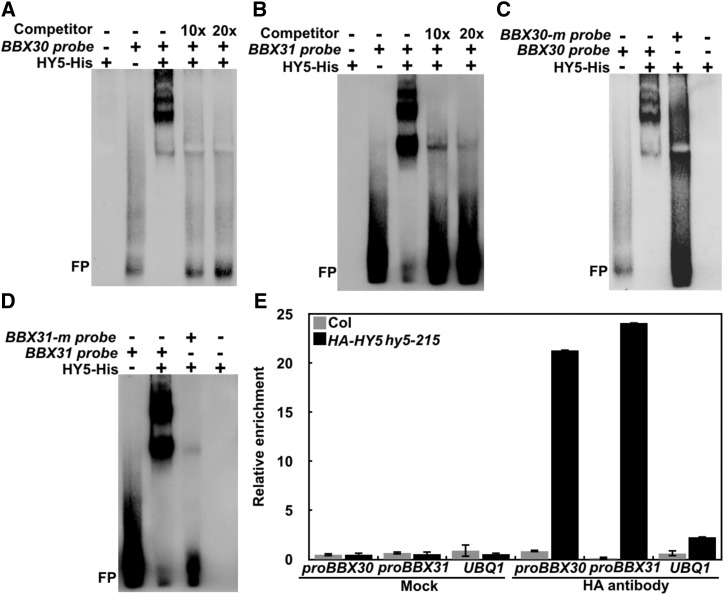
Being a b-ZIP-type transcription factor, HY5 can directly bind to the ACE cis-element within the promoters of its downstream target genes (Lee et al., 2007; Zhang et al., 2011). We therefore analyzed the 1789-bp BBX30 promoter and 1869-bp BBX31 promoter upstream of the start codon (ATG). This indicated five ACE cis-elements and one typical G-box in either the BBX30 or BBX31 promoter (Fig. 2A). We thus performed yeast-one hybrid assays, and the results showed that HY5 could activate both the BBX30pro:LacZ and BBX31pro:LacZ reporters (Fig. 2B). Next, we divided the BBX30 or BBX31 promoter into two portions (A and B; Fig. 2A). Furthermore, yeast-one hybrid assays revealed that HY5 was able to activate BBX30proA:LacZ and BBX31proA:LacZ, but not BBX30proB:LacZ and BBX31proB:LacZ (Fig. 2B), suggesting that HY5 binding sites may reside within the BBX30proA and BBX31proA portions. BBX30proA contains one ACE and one G-box, and BBX31proA possesses two ACE and one G-box (Fig. 2C). The activation of HY5 on the reporters was completely abolished when G-box was mutated, but not ACE (Fig. 2, C and D), in the yeast cells, suggesting that the G-box sites in BBX30 and BBX31 promoters are necessary and required for HY5 binding. To substantiate these results, we further performed an in vitro electrophoretic mobility shift (EMSA) assay. Recombinant HY5-His indeed was able to bind to the BBX30 or BBX31 promoter DNA subfragments containing an intact G-box motif (Fig. 3, A and B). As the amount of non-biotin-labeled BBX30 or BBX31 promoter DNA probes (competitor) increased, the HY5 binding affinity to biotin-labeled probes was clearly decreased (Fig. 3, A and B). Moreover, HY5 binding of these DNA subfragments was completely abolished when the G-box cis-elements were mutated (cacgtg was mutated to cggggg; Fig. 3, C and D). Next, chromatin immunoprecipitation (ChIP) experiments assays using HA-HY5 hy5-215 transgenic plants and an anti-HA antibody were employed to confirm the binding of HY5 to the BBX30 and BBX31 promoters in vivo. The HA-HY5 protein could immunoprecipitate the BBX30 and BBX31 promoter region containing an intact G-box (Fig. 3E), suggesting that HY5 associates with the BBX30 and BBX31 promoters in vivo. Together, these data suggest that HY5 can directly bind to the promoters of BBX30 and BBX31 and the G-box cis-elements present in the BBX30 and BBX31 promoters are the binding sites for HY5.
Figure 2.
HY5 binds to the G-box present in the promoters of BBX30 and BBX31 in yeast cells. A, Diagram of the promoter fragments of the BBX30 and BBX31. The adenine residue of the translational start codon (ATG) was assigned position +1, and the numbers flanking the sequences of the subfragment were counted based on this number. A and B indicate the corresponding promoter subfragments used in yeast one-hybrid assays shown in B. The ovals in black indicate ACE motifs, and the ovals in blue represent G-box motifs. B, Yeast one-hybrid assays showing that HY5 binds to the A fragments of BBX30 and BBX31 promoters. Empty vector expressing AD domain alone was used as negative control. C, Diagram of the wild type (wt) and mutant (mu) BBX30 and BBX31 subfragments used to drive LacZ reporter gene expression in yeast one-hybrid assays. Wild-type G-box elements are shown in blue, and nucleotide substitutions in the mutant subfragments are shown in red and underlined. D, Yeast one-hybrid assays showing that G-box motifs mediate HY5 binding to BBX30 and BBX31 promoters. The subfragments of the BBX30 and BBX31 promoters were mutated to abolish ACE and G-box alone or both ACE and G-box used to drive LacZ reporter gene expression.
Figure 3.
Binding of HY5 to BBX30 and BBX31 promoters both in vitro and in vivo. A and B, EMSA showing that the HY5 binds to the BBX30 and BBX31 promoter subfragments in vitro. “−” indicates the absence of corresponding of probes or proteins. For HY5-His, “+” indicates that 5.4 pmol is present; for BBX30 and BBX31 probes, “+” indicate that 2.1 pmol are present. Competitor indicates non-biotin-labeled BBX30 or BBX31 probes. FP, Free probe. C and D, EMSA showing that HY5 does not bind to BBX30 or BBX31 promoter subfragments carrying mutated G-box motifs in vitro. E, ChIP-qPCR showing binding of HY5 to BBX30 and BBX31 promoters in vivo. Col and UBQ1 were used as negative controls.
bbx30 and bbx31 Mutants Are Hypersensitive to Light
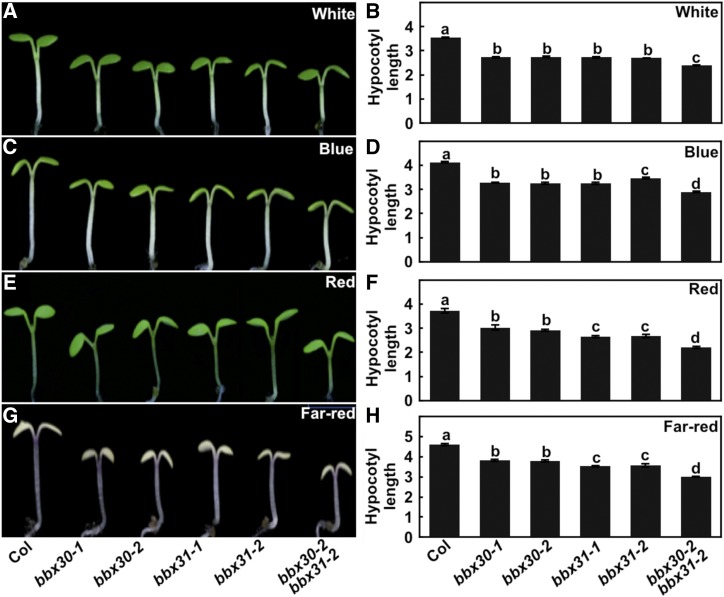
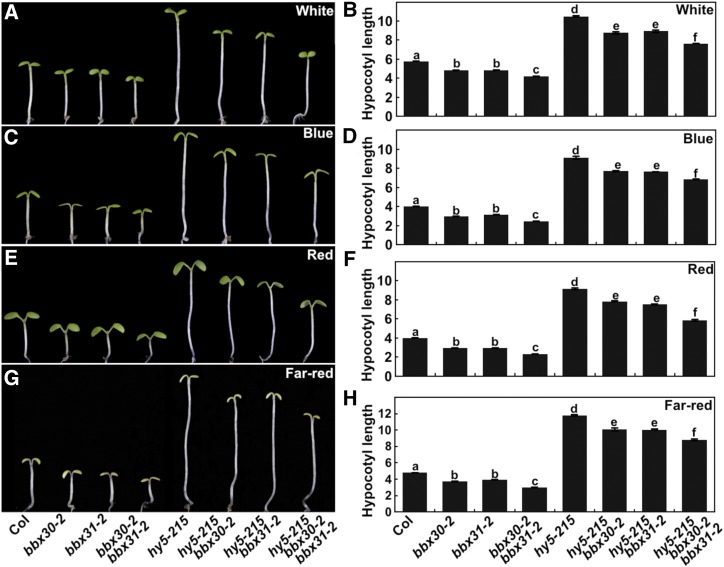
To examine the function of BBX30 and BBX31 in light signaling, we generated two bbx30 (bbx30-1 and bbx30-2) and two bbx31 (bbx31-1 and bbx31-2) mutants using the clustered regulatory interspaced short palindromic repeats (CRISPR)/Cas9 technique (Wang et al., 2015b; Supplemental Fig. S3). Each of two independent bbx30 and bbx31 mutant lines were grown in darkness for 4 d, and they displayed indistinguishable hypocotyl phenotypes compared to wild type (Supplemental Fig. S4). However, all the bbx30 and bbx31 single-mutant seedlings developed shortened hypocotyls in the various constant light conditions tested (W, B, R, and FR; Fig. 4). In addition, the hypocotyl length of the bbx30-2 bbx31-2 double mutant was significantly shorter than the wild type, bbx30-2, or bbx31-2 single mutant in the various light conditions tested (W, B, R, and FR; Fig. 4). These genetic results suggest that both BBX30 and BBX31 act as negative regulators of light signaling, and these two factors may function additively in the promotion of hypocotyl elongation in the light.
Figure 4.
bbx30 and bbx31 are hypersensitive to light. Hypocotyl length and phenotype of 4-d-old Col, bbx30, bbx31, and bbx30 bbx31 mutant seedlings W (17.5 μmol/m2/s; A and B), B (3.88 μmol/m2/s; C and D), R (75.1 μmol/m2/s; E and F), and FR (2.05 μmol/m2/s; G and H) light conditions. Hypocotyl length is expressed in mm. Data are means ± se; n ≥ 20. Letters above the bars indicate significant differences (P < 0.05), as determined by one-way ANOVA with Tukey’s post-hoc analysis. The experiments were performed three times with similar results. The graphs depict the results of one of three experiments.
Transgenic Seedlings Overexpressing BBX30 or BBX31 Are Hyposensitive to Light
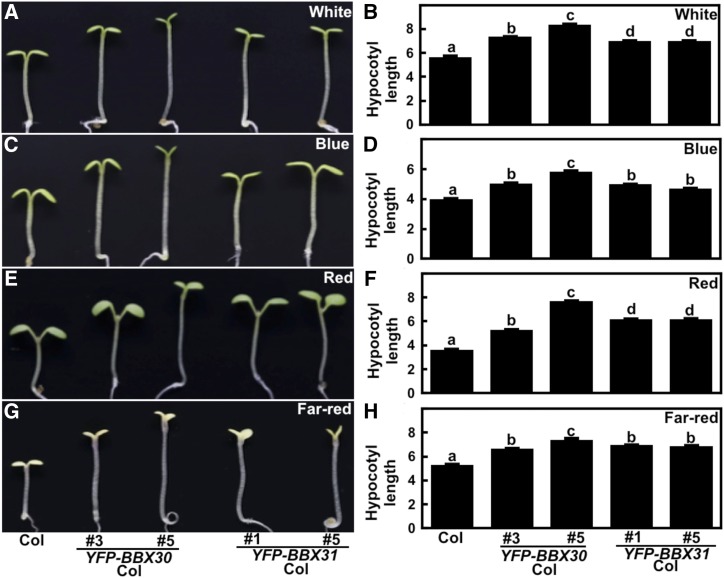
To verify the negative roles of BBX30 and BBX31 in controlling photomorphogenesis, we analyzed the hypocotyl phenotypes of YFP-tagged BBX30 or BBX31 transgenic lines (Supplemental Fig. S1, A and B). Each of the BBX30 or BBX31 overexpressors showed similar hypocotyl lengths compared with wild type when grown in the dark (Supplemental Fig. S1, C and D) but exhibited significantly elongated hypocotyls compared to wild type when grown in the various light conditions tested (W, B, R, and FR; Fig. 5). These genetic results are consistent with the notion that both BBX30 and BBX31 negatively regulate photomorphogenic development.
Figure 5.
BBX30 and BBX31 transgenic seedlings are hyposensitive to light. Hypocotyl length and phenotype of 4-d-old Col and each of two independent BBX30 and BBX31 transgenic lines grown in W (33.3 μmol/m2/s; A and B), B (3.28 μmol/m2/s; C and D), R (75.1 μmol/m2/s; E and F), and FR (2.05 μmol/m2/s; G and H) conditions. Hypocotyl length is expressed in mm. Data are means ± se; n ≥ 20. Letters above the bars indicate significant differences (P < 0.05) as determined by one-way ANOVA with Tukey’s post-hoc analysis. The experiments were performed three times with similar results. The graphs depict one of three experiments.
Global Analysis of Genes Targeted by BBX30 and BBX31
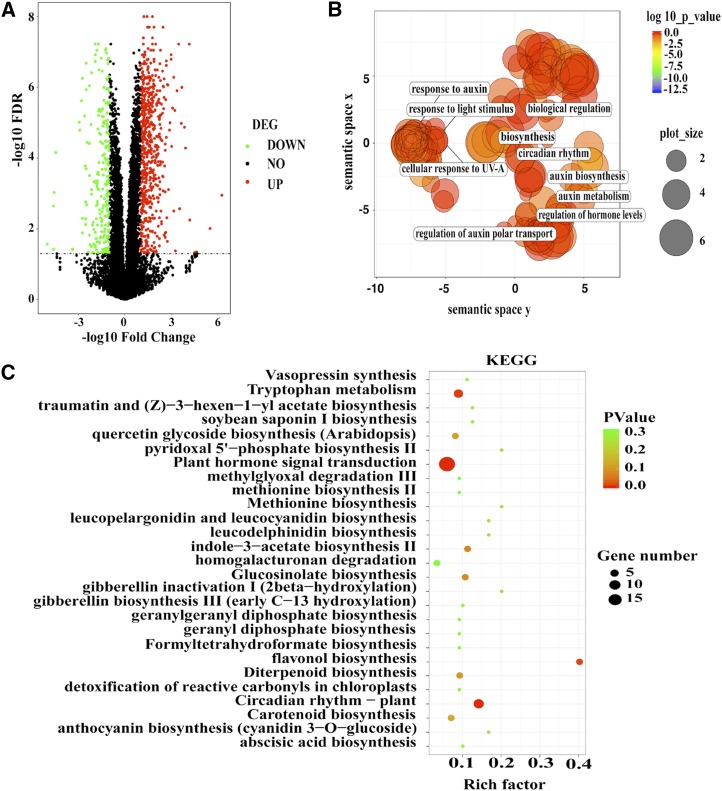
To identify the genes regulated by BBX30 and BBX31 at the genome-wide scale, we performed a paired-end RNA sequencing (RNA-Seq) assay and compared the gene expression profile between the Col (wild type) and bbx30-2 bbx31-2 double mutant. Our RNA-Seq data showed that the expression of 861 genes was significantly changed in bbx30-2 bbx31-2 compared with that in Col (Fig. 6A; Supplemental Table S1). Of these genes, 627 (72.8%) were up-regulated in bbx30-2 bbx31-2, while 234 (27.2%) were down-regulated in bbx30-2 bbx31-2 when compared to Col. To validate the RNA-Seq data, we conducted RT-qPCR analyses and validated selected differential genes (Supplemental Fig. S5). Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis revealed that BBX30- and BBX31-regulated genes are involved in the response to multiple stimuli, particularly light and auxin (Fig. 6, B and C; Supplemental Tables S2 and S3), suggesting that BBX30 and BBX31 promote hypocotyl elongation, at least in part, by controlling the expression of a set of light- and auxin-regulated genes.
Figure 6.
Globe analysis of target genes regulated by BBX30 and BBX31. A, Volcano plot shows the differentially expressed genes in the bbx30-2 bbx31-2 double mutant. Green spot indicates significant down-regulated genes in the mutant; red spot indicates significantly up-regulated genes; black spot indicates no-change genes. Genes with ratios above 2 or below 0.5. Corrected P value (FDR) ≤ 0.05 was considered as the significant differentially expressed genes (DEG). B, The scatterplot shows the cluster representatives in a two-dimensional space derived by applying multidimensional scaling to a matrix of the enriched GO terms’ semantic similarities (down-regulated genes in the bbx30-2 bbx31-2 double mutant). Bubble color indicates enriched GO term’s P value; size indicates the frequency of the GO term in the GO database (bubbles of more general terms are larger). C, The scatterplot shows the significantly enriched KEGG pathways in the bbx30-2 bbx31-2 double mutant. Bubble color indicates the enriched KEGG pathway’s P value. Significant enriched pathways are marked as red color. Bubble size indicates the gene number in the specific KEGG pathway. Rich factor defines the ratio of input gene number (differentially expressed genes detected in this pathway/background genes of this pathway).
The Short Hypocotyl Phenotype of bbx30 bbx31 Is Dependent on a Functional HY5
HY5 directly bound to the G-box present in the promoters of BBX30 and BBX31 and repressed their transcription (Figs. 1–3). We therefore generated hy5-215 bbx30-2, hy5-215 bbx31-1, and hy5-215 bbx30-2 bbx31-2 double or triple mutants to examine the genetic interactions among BBX30, BBX31, and HY5. All of the mutants we tested showed similar hypocotyl phenotypes in darkness (Supplemental Fig. S6). Consistent with previous studies and our data (Fig. 7; Oyama et al., 1997), bbx30-2, bbx31-2, and bbx30-2 bbx31-2 displayed shortened hypocotyls, while hy5-215 exhibited dramatically elongated hypocotyls when grown under various light conditions (W, B, R, and FR). The hypocotyl length of hy5-215 bbx30-2, hy5-215 bbx31-2, and hy5-215 bbx30-2 bbx31-2 was obviously longer than that of Col, bbx30-2, bbx31-2, and bbx30-2 bbx31-2. The double mutant hy5-215 bbx30-2 or hy5-215 bbx31-2 showed intermediate hypocotyl length compared with hy5-215 and hy5-215 bbx30-2 bbx31-2 (Fig. 7). These genetic data suggest that BBX30 and BBX31 may function downstream or independently of HY5 in the regulation of light-inhibited hypocotyl elongation.
Figure 7.
Genetic interaction between BBX30, BBX31, and HY5. Hypocotyl length and phenotype of 4-d-old Col, bbx30-2, bbx31-2, bbx30-2 bbx31-2, hy5-215, hy5-215 bbx30-2, hy5-215 bbx31-2, and hy5-215 bbx30-2 bbx31-2 seedlings grown in W (33.3 μmol/m2/s; A and B), B (3.28 μmol/m2/s; C and D), R (75.1 μmol/m2/s; E and F), and FR (2.05 μmol/m2/s; G and H) conditions. Hypocotyl length is expressed in mm. Data are means ± se; n ≥ 20. Letters above the bars indicate significant differences (P < 0.05) as determined by one-way ANOVA with Tukey’s post-hoc analysis. The experiments were performed three times with similar results. The graphs depict one of three experiments.
BBX30 and BBX31 Repress Flowering
The ectopic overexpression of either BBX30 or BBX31 (also known as miP1a or miP1b) cause delayed flowering under inductive long-day conditions (16 h light/8 h dark; Graeff et al., 2016). We thus verified the function of BBX30 and BBX31 in the regulation of flowering using bbx30-2 bbx31-2 and transgenic plants overexpressing BBX30 or BBX31. The bbx30-2 bbx31-2 double mutant consistently flowered earlier compared with wild type (Col), while the overexpression of BBX30 or BBX31 led to a significantly delayed flowering phenotype under long-day conditions (Supplemental Fig. S7). These data are consistent with a previous study showing that BBX30 and BBX31 play negative and essential roles in controlling flowering under inductive long-day conditions (Graeff et al., 2016).
The B-Box Domain in BBX30 or BBX31 Is Essential for Repressing Photomorphogenesis in Plants
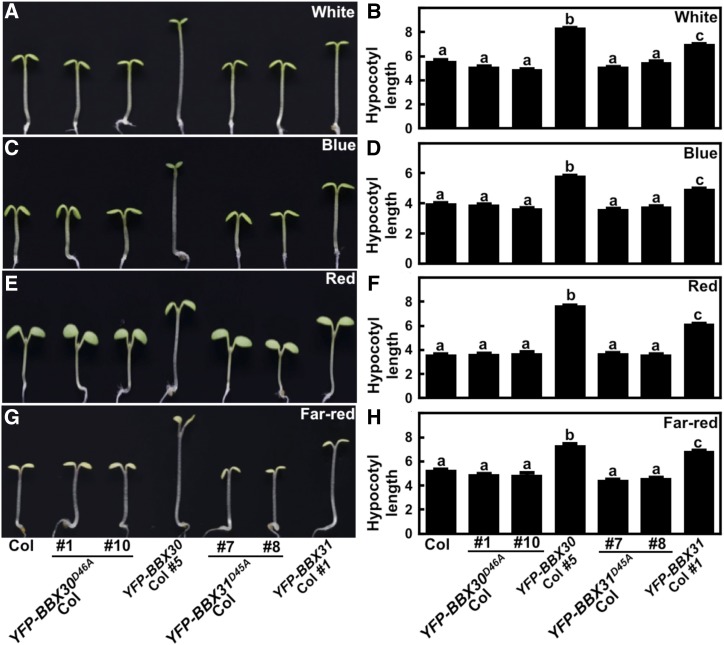
Either BBX30 or BBX31 possesses one B-box domain in the N-terminal region (Khanna et al., 2009), and the B-box domain plays critical roles in developmental processes in plants (Wang et al., 2015a; Xu et al., 2018). We thus investigated whether the B-box domain in BBX30 or BBX31 is essential for their functioning in plants. We substituted a conserved Asp residue (D46) in BBX30 or D45 in BBX31. Each of them is the fourth zinc ion-ligating residue in the B-box domain of BBX30 or BBX31 (Supplemental Fig. S8, A and B). Both YFP-tagged BBX30D46A carrying a D46A substitution (YFP-BBX30D46A) and YFP-tagged BBX31D45A carrying a D45A substitution (YFP-BBX31D45A) driven by the 35S promoter were transformed into wild type (Col). As shown in Supplemental Fig. S8, C to F, a mutated BBX30 or BBX31 gene and protein was obviously overexpressed and detectable in each of two independent YFP-BBX30D46A or YFP-BBX31D45A transgenic lines, respectively. However, unlike the YFP-BBX30 or YFP-BBX31 overexpressors, these independent transgenic plants exhibited indistinguishable hypocotyl phenotypes from wild type grown under various light conditions (D, W, B, R, and FR; Supplemental Fig. S9; Fig. 8). These results indicate that neither BBX30D46A nor BBX31D45A could confer light hyposensitive responsiveness in plants, and the intact structure of the B-box domain of BBX30 or BBX31 is indispensable for their biological function in plants.
Figure 8.
BBX30D46A and BBX31D45A transgenic seedlings show similar hypocotyl phenotypes with wild type. Hypocotyl length and phenotype of 4-d-old Col, BBX30D46A, BBX30, BBX31D45A, and BBX31 transgenic seedlings grown in W (33.3 μmol/m2/s; A and B), B (0.62 μmol/m2/s; C and D), R (6.78 μmol/m2/s; E and F), and FR (1.46 μmol/m2/s; G and H) conditions. Hypocotyl length is expressed in mm. Data are means ± se; n ≥ 20. Letters above the bars indicate significant differences (P < 0.05) as determined by one-way ANOVA with Tukey’s post-hoc analysis. The experiments were performed three times with similar results. The graphs depict one of three experiments.
DISCUSSION
BBX proteins play critical roles in a variety of cellular and developmental processes, including photomorphogenesis, thermomorphogenesis, flowering, and the responsiveness of various phytohormones (Datta et al., 2006; Gangappa and Botto, 2014; Xu et al., 2014, 2016, 2018; Zhang et al., 2017; Ding et al., 2018). A previous study indicated that BBX30 and BBX31 could interact with CONSTANSE and TOPLESS, thereby likely forming a CO-BBX30/31-TOPLESS complex. This leads to a severe delay in flowering due to the inhibition of CO transcriptional activity toward FT under long-day conditions (Graeff et al., 2016). Here, we not only verified that BBX30 and BBX31 indeed repress flowering but also provided evidence showing that they negatively regulate photomorphogenesis downstream of HY5. HY5 directly binds to the G-box cis-element present in the promoters of BBX30 and BBX31 to repress their transcription. An HY5-BBX30/BBX31-mediated regulatory module may play a critical role in the regulation of seedling development.
A group of BBX proteins mediates photomorphogenetic development, either positively or negatively (Datta et al., 2007; Gangappa and Botto, 2014; Xu et al., 2016; Zhang et al., 2017; Lin et al., 2018). BBX30 or BBX31 loss-of-function mutants showed shortened hypocotyls, while BBX30 or BBX31 gain-of-function transgenic seedlings exhibited elongated hypocotyls in the light (Figs. 4 and 5). These phenotypic and genetic observations firmly demonstrated that both BBX30 and BBX31 promote hypocotyl growth and act as negative regulators of light signaling. A set of 32 BBX proteins is divided into five groups according to the domain structure in Arabidopsis (Khanna et al., 2009). Either BBX30 or BBX31 only contain one conserved B-box domain in the N terminus and belong to subfamily V, which consists of seven members (BBX26–BBX32; Khanna et al., 2009). Of these, BBX28 and BBX32 have been shown to repress photomorphogenesis. bbx28 mutant seedlings, but not bbx32, show a shortened hypocotyl phenotype, while both BBX28 and BBX32 overexpressors display elongated hypocotyl phenotypes (Holtan et al., 2011; Lin et al., 2018). Thus, at least four members in subfamily V are involved in light signaling, and all of them play negative roles. Whether the other three members (BBX26, BBX27, and BBX29) function in the regulation of seedling photomorphogenesis requires further detailed genetic and biochemical evaluation. It has been documented that the disruption of the B-box domain structure in BBXs led to the impairment of their biological function in regulating developmental processes such as hypocotyl growth and flowering (Wang et al., 2014; Xu et al., 2018). Similarly, neither BBX30D46A nor BBX31D45A with a disrupted B-box structure could regulate hypocotyl elongation (Fig. 8). All of this evidence suggests that the intact B-box domain structure in BBX proteins is essential for their proper functioning in plants.
Extensive genetic and biochemical studies have revealed a very tight link between BBX proteins and the HY5-mediated transduction pathway in the regulation of photomorphogenesis (Gangappa and Botto, 2014). BBX21 directly binds to the T/G-box present in the HY5 promoter through its second B-box domain, and its C-terminal half likely activates HY5 transcription (Xu et al., 2016, 2017, 2018). BBX32 forms heterodimers with BBX21, which in turn serves to inhibit HY5 activity (Holtan et al., 2011). In addition, BBX21, BBX22, BBX24, BBX25, and BBX28 directly interact with HY5 to enhance or repress HY5 biochemical activity (Datta et al., 2007, 2008; Gangappa et al., 2013; Lin et al., 2018). Therefore, BBX proteins regulate HY5 at least at two distinct layers, both at the transcriptional and protein levels. BBX30 and BBX31 function directly downstream of HY5, and they are transcriptionally and negatively modulated by HY5 (Figs. 1–3). Upon light exposure, both HY5 transcript and protein levels increase in plants predominantly due to the light-mediated inactivation of the COP/DET/FUS system (Osterlund et al., 2000; Hoecker, 2017; Podolec and Ulm, 2018). However, BBX30 and BBX31 transcript levels are up-regulated by light signals but down-regulated by HY5 (Fig. 1). These facts imply that light-induced BBX30 and BBX31 expression is possibly independent of HY5 and/or additional yet unidentified factor(s) are likely responsible for the induction of BBX30 and BBX31 in response to light.
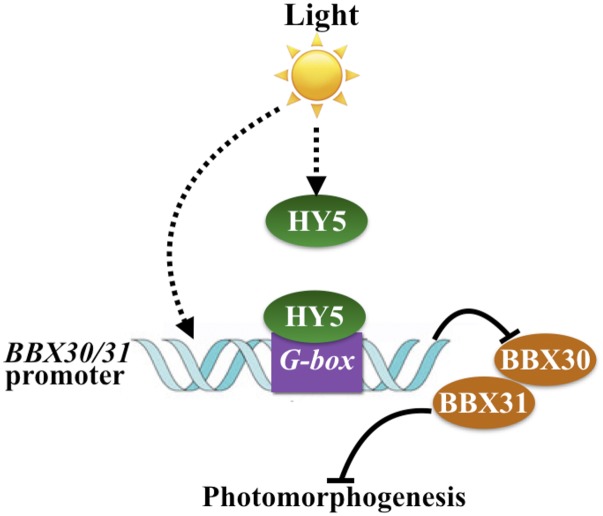
HY5, acting downstream of photoreceptors and COP/DET/FUS in the light signal transduction pathway, directly or indirectly controls the expression of over 3000 genes in Arabidopsis (Lee et al., 2007; Zhang et al., 2011). BBX30 and BBX31 are two direct and negative targets of HY5. HY5 promotes photomorphogenesis, whereas BBX30 and BBX31 function as negative regulators of light-mediated seedling development, suggesting that HY5 positively influences photomorphogenic development not only by controlling numerous positive regulators but also by regulating a portion of negative regulators of light signaling, including BBX30 and BBX31. In summary, HY5 directly binds to the G-box present in the BBX30 and BBX31 promoters, thereby repressing their transcription. BBX30 and BBX31 negatively regulate photomorphogenic development in the light (Fig. 9).
Figure 9.
A working model depicting BBX30 and BBX31 in the regulation of photomorphogenesis. Light induces the accumulation of transcription factor HY5 and expression of BBX30 and BBX31. Accumulated HY5 directly binds to the G-boxes present in the promoters of BBX30 and BBX31, thereby inhibiting their expression. Both BBX30 and BBX31 negatively regulate photomorphogenic development.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
The hy5-215 (Ang et al., 1998) and hy5-51 (Salk_096651; Ruckle et al., 2007, 2012) are in the Columbia-0 (Col-0) ecotype. Seeds were surface sterilized with 30% (v/v) commercial Clorox bleach and 0.02% (v/v) Triton X-100 for 5 min, washed three times with sterile water, and sown on 1× Murashige and Skoog (MS) medium supplemented with 0.8% (v/w) Agar (Difco) and 1% (w/v) Suc. The seeds were stratified in darkness for 3 d at 4°C and then transferred to light chambers maintained at 22°C.
Generation of bbx30 and bbx31 Mutant Using CRISPR/Cas9 Technique
Gene editing using CRISPR/Cas9 technique was performed as previously described (Wang et al., 2015b). Twenty-three-base-pair target sites (5′-N20NGG-3′) within exons of genomic DNA sequences of BBX30 or BBX31 were manually searched and identified, and then each of these sites was evaluated target specificities on the website of potential off-target finder (http://www.rgenome.net/cas-offinder/). Two independent sgRNA target sites of BBX30 or BBX31 were subcloned into pHEE401E vector. These vectors were transformed into Agrobacterium tumefaciens GV3101 by the freeze-thaw method, respectively, and then introduced into Col plants via the floral-dip method (Clough and Bent, 1998). T1 transgenic plants were selected on MS medium containing 25 mg/L hygromycin. The specific mutations in BBX30 or BBX31 were analyzed using gene-specific primers by PCR amplification and sequencing. The pHEE401 transfer-DNA insertion region including CRISPER/Cas9 in bbx30 or bbx31 single mutant at T1 generation was removed by genetic crossing with Col.
Construction of Plasmids
The full-length BBX30, BBX30D46A, BBX31, or BBX31D45A open reading frame were cloned into the pDONR-223 vector (Invitrogen) and introduced into the plant binary vector pEarley Gateway 104 (Earley et al., 2006) under the 35S promoter using Gateway LR Clonase enzyme mix (Invitrogen). pEarley Gateway-YFP-BBX30, pEarley Gateway-YFP-BBX30D46A, pEarley Gateway-YFP-BBX31, and Gateway-YFP- BBX31D45A constructs were generated.
One-thousand-seven-hundred-eighty-nine-base-pair BBX30 and 1869-bp BBX31 promoters upstream of ATG were amplified by PCR with the respective pairs of primers and then cloned into the KpnI/XhoI sites of the pLacZ-2u vector (Lin et al., 2007). pET28a-HY5-His (Xu et al., 2016) and pB42AD-HY5 (Lin et al., 2018) constructs were described previously. The primers used for plasmids construction were listed in Supplemental Table S1.
Transgenic Plants
The pEarley Gateway-YFP-BBX30, pEarley Gateway-YFP- BBX30D46A, pEarley Gateway-YFP-BBX31, and pEarley Gateway-YFP-BBX31D45A constructs were transformed into Agrobacterium tumefaciens GV3101 by the freeze-thaw method and then introduced into Col plants via the floral-dip method (Clough and Bent, 1998). Transgenic plants were selected on MS medium containing 20 mg/L Basta.
Measurement of Hypocotyl Length
To measure the hypocotyl length of seedlings, seeds were sown on plates and stratified at 4°C in darkness for 3 d and then kept in continuous white light for 8 h in order to induce uniform germination. The seeds were then transferred to D, W, B, R, and FR light conditions and incubated at 22°C for 4 d (Xu et al., 2016). The hypocotyl length of seedlings was measured using ImageJ software.
Immunoblot Analysis
For immunoblots, Arabidopsis (Arabidopsis thaliana) wild-type or transgenic seedlings were homogenized in a protein extraction buffer containing 100 mm NaH2PO4, 10 mm Tris·HCl, 200 mm NaCl, 8 m Urea, pH 8.0, 1 mm phenylmethylsulfonyl fluoride, and 1× complete protease inhibitor cocktail (Roche). Primary antibodies used in this study were anti-GFP (Abmart) and anti-Actin (Sigma-Aldrich).
EMSA Assays
EMSA assays were performed using biotin-labeled probes and the Light Shift Chemiluminescent EMSA kit (Thermo Scientific) as described previously (Xu et al., 2014). The promoter subfragment of BBX30 (32 bp, −94 to −125 bp) and BBX31 (32bp, −143 to −174 bp) upstream of ATG were PCR amplified and then mixed with biotin and kept in UV light for 30 min for biotin labeling. Next, purified HY5-His protein as indicated were incubated together with 40 fmol biotin-labeled probes in a 20-μL reaction mixture containing 10 mm Tris-HCl, pH 7.5, 0.05% NP40, 10 mm MgCl2, 5% (v/v) glycerol, and 0.1 μg/mL poly (dI∙dC). Reactions were incubated at 25°C for 20 min and separated on 6% native polyacrylamide gels in 0.5× Tris-borate/EDTA buffer. Then, gels were electroblotted to Hybond N+ (Milipore) nylon membranes in 0.5× Tris-borate/EDTA for 40 min, and the labeled probes were detected according to the manufacturer’s protocols provided with the EMSA kit. The probes used in this study were listed in Supplemental Table S1.
Yeast One-Hybrid Assays
For the yeast one-hybrid assay, the respective combinations of activation domain-fusion effectors and LacZ reporters were cotransformed into yeast strain EGY48, and transformants were selected and grown on SD/-Trp-Ura dropout media. Yeast transformation and liquid assay were conducted as described in the Yeast Protocols Handbook (BD Clontech).
ChIP
The ChIP assays were performed as described by Xu et al. (2016). Chromatin isolation was performed using Col and HA-HY5 hy5-215 transgenic seedlings grown under constant white light for 4 d. The resuspended chromatin was sonicated at 4°C to 250- to 500-bp fragments. The sheared chromatin was immunoprecipitated, washed, reverse cross linked, and finally amplified. About 10% of sonicated but nonimmunoprecipitated chromatin was reverse cross linked and used as an input DNA control. Both immunoprecipitated DNA and input DNA were analyzed by RT-PCR (Applied Biosystems). Monoclonal anti-HA antibody (Sigma-Aldrich) was used for the assays. All primers used for this assay are listed in Supplemental Table S1.
RNA-Seq Analysis
Total RNA was extracted from the 4-d-old Col and bbx30-2 bbx31-2 seedlings grown in constant W light (17.5 μmol/m2/s) conditions. Then, mRNA sequencing libraries were constructed, and sequencing was performed using the Illumina HiSeq 2500 platform according to the manufacturer’s instruction (HiSequation 2500 user guide) by Shanghai Hanyu-Bio. Three independent biological replicates were performed. RNA-Seq reads were mapped to Arabidopsis TAIR 10 using Hisat2 (version 2.05; Kim et al., 2015) software using default parameters. Then, raw reads for each gene were calculated by HTseq (Anders et al., 2015) before calculating differential gene expression. Then, differentially expressed genes among two conditions were identified using the general liner models method in the edgeR package (version 3.12.0; Robinson et al., 2010) withfalse positive rate of 0.05 and fold-change of 2. GO analysis was performed by topGO (Bioconductor) software (http://www.bioconductor.org/packages/2.11/bioc/HTML/topGO.html), and the result was plotted using REVIGO with R (Supek et al., 2011). KEGG enrichment analysis was conducted by KOBAS 3.0 (Xie et al., 2011) and the enriched pathways were plotted by R software.
RT-qPCR
Total RNA was extracted from 4-d-old Arabidopsis seedlings grown under white light using the RNeasy plant mini kit (QIAGEN). Complementary DNAs (cDNA) were synthesized from 2 μg of total RNA using the 5× All-In-One RT Master Mix cDNA synthesis system (Applied Biological Materials) according to the manufacturer’s instructions. Then, cDNA were subjected to RT-qPCR assays. RT-qPCR was performed using the Step One Plus RT-PCR detection system (Applied Biosystems) and SYBR Green PCR Master Mix (Takara). PCR was performed in triplicate for each sample, and the expression levels were normalized to that of a PP2A gene. The primers used in this study were listed in Supplemental Table S1.
Accession Numbers
Sequence data from this article can be found in the Genome Initiative or GenBank/EMBL data libraries under the following accession numbers: BBX30 (At4g15248), BBX31 (At3g21890), and HY5 (At5g11260).
Supplemental Data
The following supplemental materials are available:
Supplemental Figure S1. Hypocotyl phenotype and length in various BBX30 and BBX31 transgenic seedlings grown in darkness.
Supplemental Figure S2. Light does not affect the accumulation of YFP-BBX30 or YFP-BBX31.
Supplemental Figure S3. Mutations in each of the two independent bbx30 and bbx31 alleles created by the CRISPER/Cas9 method.
Supplemental Figure S4. Hypocotyl phenotype and length in bbx30, bbx31, and bbx30 bbx31 seedlings grown in darkness.
Supplemental Figure S5. Validation of 18 target genes of BBX30 and BBX31 identified by RNA-Seq using RT-qPCR.
Supplemental Figure S6. Hypocotyl phenotype and length in hy5-215 bbx30-2, hy5-215 bbx31-2, and hy5-215 bbx30-2 bbx31-2 grown in darkness.
Supplemental Figure S7. bbx30-2 bbx31-2 display earlier flowering phenotype, but BBX30 and BBX31 transgenic plants show delayed flowering phenotype under long-day conditions.
Supplemental Figure S8. BBX30D46A and BBX31D45A gene and protein expression levels in each of two independent transgenic lines.
Supplemental Figure S9. Hypocotyl phenotype and length in BBX30D46A and BBX31D45A transgenic seedlings grown in darkness.
Supplemental Table S1. List of up- and down-regulated gene in the bbx30-2 bbx31-2 double mutant compared with the wild type.
Supplemental Table S2. Enriched GO terms (biological process) and genes of the down-regulated genes in the bbx30-2 bbx31-2 double mutant.
Supplemental Table S3. Enriched KEGG pathways and genes of the down-regulated genes in the bbx30-2 bbx31-2 double mutant.
Supplemental Table S4. List of primers used in this study.
Acknowledgments
The authors thank all the lab members at SUSTech for their discussion on the project.
Footnotes
This work was supported by grants from National Key R&D Program of China (2017YFA0503800), National Natural Science Foundation of China (31330048, 31621001), Peking-Tsinghua Center for Life Sciences (to X.W.D.), US NIH grant (GM-47850), and by Nanjing Agricultural University and Southern University of Science and Technology (to D.X.).
References
- Anders S, Pyl PT, Huber W (2015) HTSeq—a Python framework to work with high-throughput sequencing data. Bioinformatics 31: 166–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang LH, Chattopadhyay S, Wei N, Oyama T, Okada K, Batschauer A, Deng XW (1998) Molecular interaction between COP1 and HY5 defines a regulatory switch for light control of Arabidopsis development. Mol Cell 1: 213–222 [DOI] [PubMed] [Google Scholar]
- Chang CS, Li YH, Chen LT, Chen WC, Hsieh WP, Shin J, Jane WN, Chou SJ, Choi G, Hu JM, et al. (2008) LZF1, a HY5-regulated transcriptional factor, functions in Arabidopsis de-etiolation. Plant J 54: 205–219 [DOI] [PubMed] [Google Scholar]
- Chang CS, Maloof JN, Wu SH (2011) COP1-mediated degradation of BBX22/LZF1 optimizes seedling development in Arabidopsis. Plant Physiol 156: 228–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Datta S, Hettiarachchi GH, Deng XW, Holm M (2006) Arabidopsis CONSTANS-LIKE3 is a positive regulator of red light signaling and root growth. Plant Cell 18: 70–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S, Hettiarachchi C, Johansson H, Holm M (2007) SALT TOLERANCE HOMOLOG2, a B-box protein in Arabidopsis that activates transcription and positively regulates light-mediated development. Plant Cell 19: 3242–3255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S, Johansson H, Hettiarachchi C, Irigoyen ML, Desai M, Rubio V, Holm M (2008) LZF1/SALT TOLERANCE HOMOLOG3, an Arabidopsis B-box protein involved in light-dependent development and gene expression, undergoes COP1-mediated ubiquitination. Plant Cell 20: 2324–2338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L, Wang S, Song ZT, Jiang Y, Han JJ, Lu SJ, Li L, Liu JX (2018) Two B-box domain proteins, BBX18 and BBX23, interact with ELF3 and regulate thermomorphogenesis in Arabidopsis. Cell Reports 25: 1718–1728.e4 [DOI] [PubMed] [Google Scholar]
- Earley KW, Haag JR, Pontes O, Opper K, Juehne T, Song K, Pikaard CS (2006) Gateway-compatible vectors for plant functional genomics and proteomics. Plant J 45: 616–629 [DOI] [PubMed] [Google Scholar]
- Fan XY, Sun Y, Cao DM, Bai MY, Luo XM, Yang HJ, Wei CQ, Zhu SW, Sun Y, Chong K, et al. (2012) BZS1, a B-box protein, promotes photomorphogenesis downstream of both brassinosteroid and light signaling pathways. Mol Plant 5: 591–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangappa SN, Botto JF (2014) The BBX family of plant transcription factors. Trends Plant Sci 19: 460–470 [DOI] [PubMed] [Google Scholar]
- Gangappa SN, Crocco CD, Johansson H, Datta S, Hettiarachchi C, Holm M, Botto JF (2013) The Arabidopsis B-BOX protein BBX25 interacts with HY5, negatively regulating BBX22 expression to suppress seedling photomorphogenesis. Plant Cell 25: 1243–1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graeff M, Straub D, Eguen T, Dolde U, Rodrigues V, Brandt R, Wenkel S (2016) MicroProtein-mediated recruitment of CONSTANS into a TOPLESS trimeric complex represses flowering in Arabidopsis. PLoS Genet 12: e1005959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoecker U. (2017) The activities of the E3 ubiquitin ligase COP1/SPA, a key repressor in light signaling. Curr Opin Plant Biol 37: 63–69 [DOI] [PubMed] [Google Scholar]
- Holm M, Hardtke CS, Gaudet R, Deng XW (2001) Identification of a structural motif that confers specific interaction with the WD40 repeat domain of Arabidopsis COP1. EMBO J 20: 118–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm M, Ma LG, Qu LJ, Deng XW (2002) Two interacting bZIP proteins are direct targets of COP1-mediated control of light-dependent gene expression in Arabidopsis. Genes Dev 16: 1247–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtan HE, Bandong S, Marion CM, Adam L, Tiwari S, Shen Y, Maloof JN, Maszle DR, Ohto MA, Preuss S, et al. (2011) BBX32, an Arabidopsis B-box protein, functions in light signaling by suppressing HY5-regulated gene expression and interacting with STH2/BBX21. Plant Physiol 156: 2109–2123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Ouyang X, Deng XW (2014) Beyond repression of photomorphogenesis: Role switching of COP/DET/FUS in light signaling. Curr Opin Plant Biol 21: 96–103 [DOI] [PubMed] [Google Scholar]
- Indorf M, Cordero J, Neuhaus G, Rodríguez-Franco M (2007) Salt tolerance (STO), a stress-related protein, has a major role in light signalling. Plant J 51: 563–574 [DOI] [PubMed] [Google Scholar]
- Jiao Y, Lau OS, Deng XW (2007) Light-regulated transcriptional networks in higher plants. Nat Rev Genet 8: 217–230 [DOI] [PubMed] [Google Scholar]
- Khanna R, Kronmiller B, Maszle DR, Coupland G, Holm M, Mizuno T, Wu SH (2009) The Arabidopsis B-box zinc finger family. Plant Cell 21: 3416–3420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Langmead B, Salzberg SL (2015) HISAT: A fast spliced aligner with low memory requirements. Nat Methods 12: 357–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau OS, Deng XW (2012) The photomorphogenic repressors COP1 and DET1: 20 years later. Trends Plant Sci 17: 584–593 [DOI] [PubMed] [Google Scholar]
- Lee J, He K, Stolc V, Lee H, Figueroa P, Gao Y, Tongprasit W, Zhao H, Lee I, Deng XW (2007) Analysis of transcription factor HY5 genomic binding sites revealed its hierarchical role in light regulation of development. Plant Cell 19: 731–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F, Jiang Y, Li J, Yan T, Fan L, Liang J, Chen ZJ, Xu D, Deng XW (2018) B-BOX DOMAIN PROTEIN28 negatively regulates photomorphogenesis by repressing the activity of transcription factor HY5 and undergoes COP1-mediated degradation. Plant Cell 30: 2006–2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin R, Ding L, Casola C, Ripoll DR, Feschotte C, Wang H (2007) Transposase-derived transcription factors regulate light signaling in Arabidopsis. Science 318: 1302–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Li J, Qu L, Hager J, Chen Z, Zhao H, Deng XW (2001) Light control of Arabidopsis development entails coordinated regulation of genome expression and cellular pathways. Plant Cell 13: 2589–2607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterlund MT, Hardtke CS, Wei N, Deng XW (2000) Targeted destabilization of HY5 during light-regulated development of Arabidopsis. Nature 405: 462–466 [DOI] [PubMed] [Google Scholar]
- Oyama T, Shimura Y, Okada K (1997) The Arabidopsis HY5 gene encodes a bZIP protein that regulates stimulus-induced development of root and hypocotyl. Genes Dev 11: 2983–2995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podolec R, Ulm R (2018) Photoreceptor-mediated regulation of the COP1/SPA E3 ubiquitin ligase. Curr Opin Plant Biol 45(Pt A): 18–25 [DOI] [PubMed] [Google Scholar]
- Robinson MD, McCarthy DJ, Smyth GK (2010) edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26: 139–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruckle ME, DeMarco SM, Larkin RM (2007) Plastid signals remodel light signaling networks and are essential for efficient chloroplast biogenesis in Arabidopsis. Plant Cell 19: 3944–3960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruckle ME, Burgoon LD, Lawrence LA, Sinkler CA, Larkin RM (2012) Plastids are major regulators of light signaling in Arabidopsis. Plant Physiol 159: 366–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan JA, Deng XW (2003) From seed to seed: the role of photoreceptors in Arabidopsis development. Dev Biol 260: 289–297 [DOI] [PubMed] [Google Scholar]
- Supek F, Bošnjak M, Škunca N, Šmuc T (2011) REVIGO summarizes and visualizes long lists of gene ontology terms. PLoS One 6: e21800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepperman JM, Hudson ME, Khanna R, Zhu T, Chang SH, Wang X, Quail PH (2004) Expression profiling of phyB mutant demonstrates substantial contribution of other phytochromes to red-light-regulated gene expression during seedling de-etiolation. Plant J 38: 725–739 [DOI] [PubMed] [Google Scholar]
- Wang CQ, Guthrie C, Sarmast MK, Dehesh K (2014) BBX19 interacts with CONSTANS to repress FLOWERING LOCUS T transcription, defining a flowering time checkpoint in Arabidopsis. Plant Cell 26: 3589–3602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CQ, Sarmast MK, Jiang J, Dehesh K (2015a) The transcriptional regulator BBX19 promotes hypocotyl growth by facilitating COP1-mediated EARLY FLOWERING3 degradation in Arabidbopsis. Plant Cell 27: 1128–1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZP, Xing HL, Dong L, Zhang HY, Han CY, Wang XC, Chen QJ (2015b) Egg cell-specific promoter-controlled CRISPR/Cas9 efficiently generates homozygous mutants for multiple target genes in Arabidopsis in a single generation. Genome Biol 16: 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie C, Mao X, Huang J, Ding Y, Wu J, Dong S, Kong L, Gao G, Li CY, Wei L (2011) KOBAS 2.0: A web server for annotation and identification of enriched pathways and diseases. Nucleic Acids Res 39(suppl_2): W316-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D, Li J, Gangappa SN, Hettiarachchi C, Lin F, Andersson MX, Jiang Y, Deng XW, Holm M (2014) Convergence of Light and ABA signaling on the ABI5 promoter. PLoS Genet 10: e1004197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D, Jiang Y, Li J, Lin F, Holm M, Deng XW (2016) BBX21, an Arabidopsis B-box protein, directly activates HY5 and is targeted by COP1 for 26S proteasome-mediated degradation. Proc Natl Acad Sci USA 113: 7655–7660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu DB, Gao SQ, Ma YN, Wang XT, Feng L, Li LC, Xu ZS, Chen YF, Chen M, Ma YZ (2017) The G-protein β subunit AGB1 promotes hypocotyl elongation through inhibiting transcription activation function of BBX21 in Arabidopsis. Mol Plant 10: 1206–1223 [DOI] [PubMed] [Google Scholar]
- Xu D, Jiang Y, Li J, Holm M, Deng XW (2018) The B-box domain protein BBX21 promotes photomorphogenesis. Plant Physiol 176: 2365–2375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, He H, Wang X, Wang X, Yang X, Li L, Deng XW (2011) Genome-wide mapping of the HY5-mediated gene networks in Arabidopsis that involve both transcriptional and post-transcriptional regulation. Plant J 65: 346–358 [DOI] [PubMed] [Google Scholar]
- Zhang X, Huai J, Shang F, Xu G, Tang W, Jing Y, Lin R (2017) A PIF1/PIF3-HY5-BBX23 transcription factor cascade affects photomorphogenesis. Plant Physiol 174: 2487–2500 [DOI] [PMC free article] [PubMed] [Google Scholar]