Abstract
Unveiling past tipping points is a prerequisite for a better understanding of how individual species and entire ecosystems will respond to future climate change. Such knowledge is key for the implementation of biodiversity conservation. We identify the relationships between peatland vegetation and hydrological conditions over the past 2000 years using plant macrofossils, testate amoebae-based quantitative hydrological reconstructions and Sphagnum-moss functional traits from seven Polish peatland records. Using threshold indicator taxa analysis, we discovered that plant community composition strongly converged at a water level of ca 11.7 cm, indicating a community-level tipping point. We identified 45 plant taxa that showed either an increase or a decrease in their relative abundance between 8 and 17 cm of water-level depth. Our analysis of Sphagnum community traits further showed that Sphagnum functional diversity was remarkably stable over time despite Sphagnum species sensitivity to hydrological conditions. Our results suggest that past hydrological shifts did not influence major functions of the Sphagnum community, such as photosynthetic capacity, growth and productivity, owing to species replacement with a similar functional space. Although further studies including trait plasticity will be required, our findings suggest that the capacity of the Sphagnum community to gain carbon remained stable despite hydrological changes.
Keywords: critical transition, plant macrofossils, functional traits, resilience, trait variation, vegetation change
1. Introduction
Regime shifts of ecosystem functioning in the response of individual species to ongoing climate change are one of the most pressing issues facing ecologists [1]. Regime shifts are often marked by a tipping point—a threshold in environmental drivers and one or more biotic variables within the system that, when breached, causes a major change in ecosystem functioning [2,3]. Detecting tipping points is a key factor in both the prediction of such shifts and the provision of support for the management of natural ecosystems [4]. Most studies exploring regime shifts are based on observations over short timescales (up to 5 years) [2,5,6]. However, long timescales are necessary to understand the factors underlying resilience and possible existence of critical transitions of ecosystems [7–9]. In this context, past biodiversity in paleoecological archives can be explored to learn how communities have responded to environmental changes over the Holocene, thus offering a long-term view on the past dynamics of ecosystems [10].
Peatlands are important archives to study how environmental change affects biodiversity through time [11]. Secondary succession leads to abrupt changes in plant and microbial communities, divergent from their former pristine state [12]. Understanding whether long-term community changes either preserve species' functional diversity and then ecosystem functions owing to species' functional redundancy or shift ecosystem functions towards another state because of high species' functional turnover is key to the better prediction of the fate of ecosystem functions under ongoing global changes. In this paper, we address this gap in understanding by examining the understudied response of peatland vegetation to long-term hydrological changes in seven peat-records. Plant species composition is central to how peatlands respond to environmental change, given the strong links between plant community composition and ecosystem processes such as carbon cycling [13,14]. Specifically, Sphagnum is a key C-accreting genus, but Sphagnum species can diverge in their capacity to assimilate and store carbon [15]. While the composition of peatland plant communities can be remarkably stable through time [16,17], abrupt shifts in hydrological conditions may cause shifts in the relative abundance of Sphagnum species, and then negative or positive feedbacks [18–20] that may affect peatland carbon dynamics [2].
Our overarching goal is to identify a hydrological threshold in water table depth beyond which peatland vegetation undergoes compositional change using plant macrofossils and reconstructed depth to water table (DWT) from seven peat archives. More particularly, we aim to examine whether species' turnover above and below this hydrological threshold influences the functional diversity of peat-forming mosses, and thus potentially peatland C storage capacity. We test the often-stated hypothesis that functional redundancy among species should mitigate the effects of climate change on species' functional diversity.
2. Methods
(a). Summary of the approach
We selected seven peat profiles from Sphagnum-dominated peatlands in Poland, situated in a young glacial landscape (Stążki, Słowińskie Błoto, Linje, Gązwa, Mechacz Wielki and Bagno Kusowo) and in the Izery Mountains (electronic supplementary material, figure S1). Together, the sites represent a coherent group of Sphagnum-dominated peatlands that were affected by human activity in the past. We used high-resolution chronology, testate amoebae-based reconstructions of depth to the water table—as a proxy for hydrological changes (DWT, based on the Polish calibration dataset [21])—and plant macrofossil data. All the data from each site cover up to the past 2000 years.
As a first step in our data analysis, we used broken-line regression models [22] to identify whether plant community composition, assessed using a non-metric multidimensional scaling analysis (NMDS), and reconstructed DWT experienced different states over time [2,23]. Broken-points identified in plant communities and reconstructed DWT in each site were related using linear regression models to identify whether shifts in plant communities over time occurred either before or after a shift in DWT. We also analysed patterns in plant species along the DWT gradient (all sites were pooled) using a threshold indicator taxa analysis (TITAN; see [24] for a full description of the method). In short, TITAN reveals sharp, nonlinear transitions in an entire community dataset by detecting changes in species' distributions (species that increase or decrease in abundance) along a gradient. TITAN assesses synchrony among species' responses as evidence for community thresholds, but also identifies the main taxa changing.
The next step of our analyses aimed at understanding whether shifts in DWT through time influenced the functional traits (table 1) and functional space (i.e. the trait combination of each species that defines their functional niche) of Sphagnum mosses. To investigate change in community traits over time, we used a Bayesian hierarchical modelling approach, conducted in Stan through R (v. 3.5.1) [30,31], in which time varied at the site level. Change in community trait over DWT gradient was estimated by accounting for the hierarchical spatial and temporal structure of the data (DWT variations within sites and time).
Table 1.
| trait | unit | function | number of samples per species (n) | reference |
|---|---|---|---|---|
| bulk density | mg cm−3 | bulk density is strongly related to organic matter content, and then to C sequestered in Sphagnum tissues | 6–10 | Bengtsson et al. [15] |
| shoot density | cm−2 | species that grow in high shoot densities better retain water | 6–10 | Bengtsson et al. [15] |
| spore diameter | µm | large spore diameter favours dissemination of the species and then its capacity to cope with environmental changes | 2–17 | Sundberg et al. [25] |
| spore capsule diameter | mm | large spore capsule diameter favours dissemination of the species and then its capacity to cope with environmental changes | 2–17 | Sundberg et al. [25] |
| leaf C, N and P content | mg g−1 | determinants of Sphagnum litter quality; species with low C, N and P contents will be decomposed more slowly | 36 | V Jassey, C Signarbieux 2018, unpublished data |
| carbon exchange capacity (CEC) | µeq g−1 | CEC determines the capacity of Sphagnum species to acidify the environment and then slow down microbial activities | 5 | Bengtsson et al. [26] |
| height above water table (HWT) | mm | reflect the capacity of species to cope with drying conditions | 6–10 | Bengtsson et al. [15] |
| productivity | mgC cm−2 y−1 | growth in term of production; it reflects the capacity of species to fix and potentially accumulate carbon | 1–60 | Gunnarsson [28] |
| growth in biomass | g m−2 | growth in terms of production per surface; it reflects the capacity of species to fix and potentially accumulate carbon | 6–10 | Bengtsson et al. [15] |
| growth | mm | length increment; species with high growth will fix high rates of CO2 | Bengtsson et al. [15] | |
| photosynthetic capacity | mgC g−1 h−1 | net rate of CO2 fixation under standard conditions | 5–12 | Bengtsson et al. [15]; V Jassey, C Signarbieux 2018, unpublished data |
| decomposition rate (mass loss) | % | species that decompose slowly (low % mass loss) accumulate peat over time | 6–10 | Bengtsson et al. [15] |
| phenolics content | mg g−1 | recalcitrant compounds and/or anti-microbial compounds that favour accumulation of peat | 5–36 | Jassey et al. [29]; Bengtsson et al. [26] |
| sphagnan content | mg g−1 | recalcitrant compounds and/or anti-microbial compounds that favour accumulation of peat | 5 | Bengtsson et al. [26] |
| lignin-like phenolics content | mg g−1 | recalcitrant compounds that favour accumulation of peat | 5 | Bengtsson et al. [26] |
Detailed methods, including statements of data availability and any associated accession codes and references, are available in the electronic supplementary material. Data used in this study [32] are available in the Dryad Digital Repository using the following link: https://doi.org/10.5061/dryad.t50863s.
3. Results
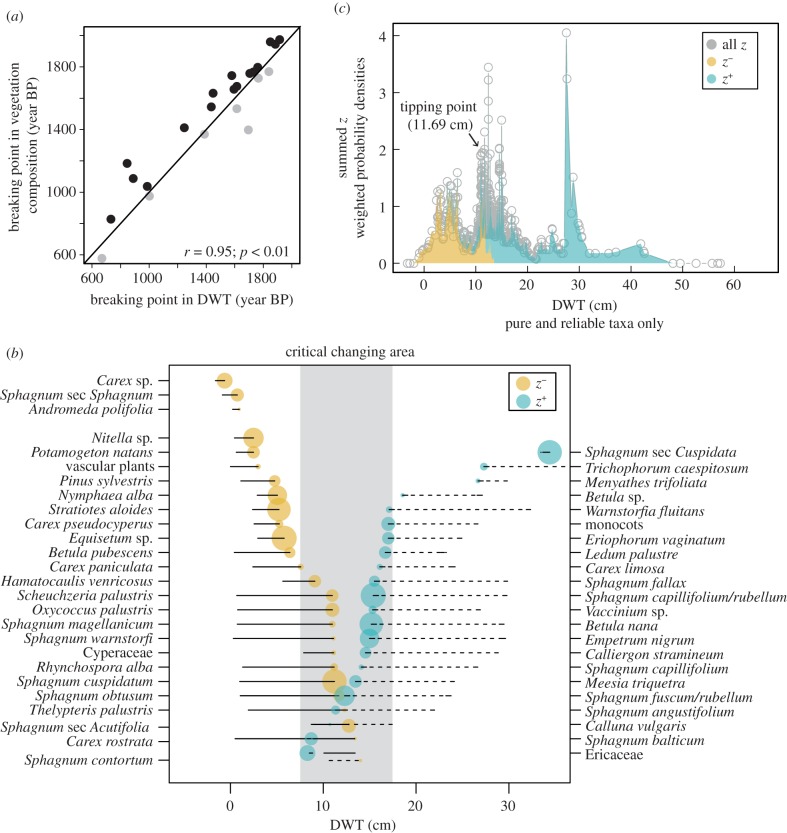
We identified several abrupt transitions in vegetation composition and DWT through time in all peat profiles, mostly between 500 and 2000 cal. CE (electronic supplementary material, figure S2). At almost every site, vegetation composition did not recover after transition and shifted towards unstable states in terms of species' composition (electronic supplementary material, figure S2). Only Gązwa showed that vegetation composition recovered following turnover event. At most of the vegetation turnover events, shifts in plant community composition occurred following hydrological changes (figure 1a; electronic supplementary material, figure S2), usually 5–100 years after DWT changes. All sites showed different hydrological phases, with DWT fluctuating between 5 and 20 cm. Only Słowińskie Bloto showed important hydrological variability, with DWT fluctuating between wet (DWT = 10 cm) and very dry conditions (DWT = 50 cm). Using TITAN, we identified 45 plant taxa (out of 51) that showed a significant changing point along the DWT gradient (figure 1b), at DWT between 5 and 18 cm. Among these, 29 taxa were closely related to wet conditions (figure 1b), while 22 species were related to drier conditions. As a result, vegetation community composition showed an important species' turnover at a DWT of c. 11.7 cm (figure 1c), which we interpret as indicating a community-level tipping point.
Figure 1.
Tipping point in vegetation community composition. (a) Biplot of tipping points in vegetation composition (NMDS axis 1) versus tipping points in DWT at each site. Black dots above the 1 : 1 line indicate vegetation tipping points that followed a DWT tipping point; grey dots below the 1 : 1 line indicate the opposite. (b) Plant species' change points along the water-level gradient (purity greater than 99%, p < 0.05 in greater than 99% bootstraps) showing 95% bootstrap percentiles; dot colours show the species that either increase (z+) or decrease (z−) in abundance along the DWT gradient. Critical changing area reflects the 5%–95% bootstrap percentile range (in grey) of community change point (see subset c). (c) Plant community change point along the DWT gradient showing community threshold (dotted line) at max([sum(z−)]) and 5–95% bootstrap percentile range. [sum(z−)] values represent the sum of responses for each possible change point along the gradient. (Online version in colour.)
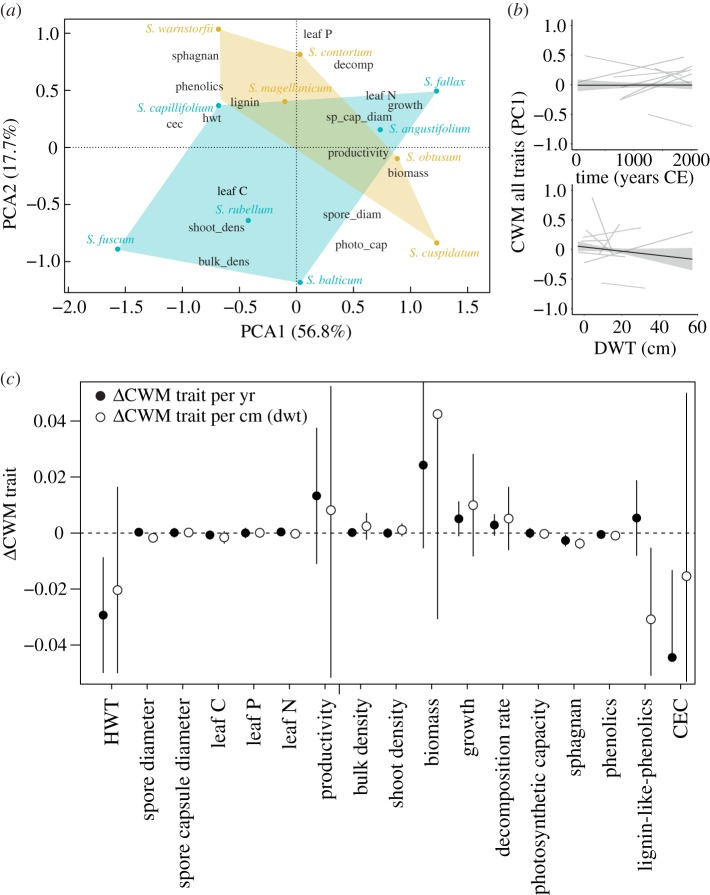
Based on TITAN outputs, we calculated the functional space for Sphagnum species related to wet (z− species) and dry (z+ species) conditions. Functional spaces for both groups of Sphagnum species were similar (figure 2a), although three Sphagnum species from dry conditions clearly differed in terms of trait combinations. Sphagnum fuscum, S. balticum and S. rubellum had higher shoot and bulk densities, and photosynthetic capacities, but lower decomposition rates, phenolic content and leaf P than other species (figure 2a). Community traits remained remarkably stable over time and across the DWT gradient (figure 2b), with slopes that did not differ from 0 (figure 2b). Sphagnum height above DWT (HWT) and carbon exchange capacity (CEC) were the only traits for which community-weighted mean (CWM) significantly changed over the 2000 years; both community traits decreased at nearly every site (electronic supplementary material, figure S3). Sphagnan and lignin-like phenolics were the only traits that changed over the DWT gradient; they slightly decreased along with drying conditions (electronic supplementary material, figures S3 and S4). However, detailed analyses of CWM of traits at each site showed that some variability in CWM can be observed within sites (electronic supplementary material, figures S3 and S4).
Figure 2.
Functional traits of Sphagnum from the macrofossil record. (a) Functional space of each Sphagnum species based on a PCA analysis computed on Sphagnum functional traits. Yellow colour: species below the plant community tipping point; blue colour: species above the plant community tipping point identified in TITAN. (b) Relationship between time, DWT and averaged Sphagnum community traits. Species scores from the PCA shown in (a) were used as a synthetic Sphagnum ‘trait’ to calculate Sphagnum averaged community traits. Lines are mixed-effect model fits for each site (thin grey line) or across sites (thick lines with bands indicating 95% confidence interval). (c) Moisture sensitivity of each community trait (CWM). Circles represent the mean water table sensitivity across six sites based on a Bayesian linear mixed model (see Methods); Słowińskie Błoto was removed from the analysis because it was the only site covering a large DWT gradient. Error bars are a 95% credible interval on the mean and indicate that the DWT–trait relationship differed from zero. Black circles indicate a DWT–trait relationship that differed from zero, while white circles indicate the opposite. (Online version in colour.)
4. Discussion
Peatland hydrological records often show complex temporal patterns including transitory perturbations (e.g. extreme drought) and rapid-onset (e.g. drainage) overlaid on a background of long-term climate changes. Identifying the level of hydrological changes beyond which peatland vegetation undergoes potentially irreversible degradation is crucial to better predict the controls over peatland functioning. To date, clear estimations of the critical water table that abruptly shifts peatland functioning remain scarce [2]. In our study, we show that peatland vegetation strongly diverges between a water table depth of 8 and 17 cm. This estimation corroborates recent findings from an experimental study that revealed a tipping point in peatland functioning around a water table depth of 24 cm [2]. However, the former tipping point value in DWT relates only to an extreme water table drop, while our long-term-based calculation takes into account both abrupt and gradual variations in DWT on a long temporal scale. Above-mentioned thresholds in water table depth represent tipping points in the functioning of peatland ecosystem when changes might be irreversible after crossing the critical DWT in a long time-scale exceeding decades.
Our findings suggest that hydrological changes mostly drove shifts in vegetation composition, which most of the time did not revert to its initial composition owing to species' turnover with different water table sensitivity. Peatland vegetation, through its effect on peat evapotranspiration, could also have affected water table position but evapotranspiration remains difficult to extrapolate using peat archives, and more importantly, is poorly related to DWT changes [33,34]. Despite the need for better determination of cause and effect in DWT–vegetation relationships, our study highlights the value of a long-term approach to identification of the biophysical tipping point in peatlands, and possibly abrupt functional shifts.
We showed that Sphagnum functional diversity was remarkably stable over time despite significant shifts in community composition (figure 2). This result corroborates recent findings in peatland vegetation, which demonstrated that plant taxonomic and functional turnover were decoupled [27]. Here, we further show that Sphagnum species sensitive to wet and dry conditions have similar functional spaces (figure 2a), which explains why peatlands can maintain ecosystem functioning over time despite species' turnover. More particularly, we found that major functions such as Sphagnum community photosynthetic capacity, growth, productivity and decomposition rates did not change over time, including along the DWT gradient (figure 2b). These results suggest that the capacity of the Sphagnum community to fix and store carbon remained stable despite hydrological changes. However, our findings may have been influenced by the use of averaged-trait values derived from a trait database that may not always accurately reflect species' intraspecific trait variability [35]. Although we caution that a decreasing water table through time might have influenced trait responses of certain species, as well as species' performance, our findings provide evidence that species' replacement with high functional redundancy confers high resistance and resilience [36] on a Sphagnum community over time (figure 2).
In conclusion, our study indicates that peatlands might be more resistant to future hydrological changes than previously thought. We showed that Sphagnum community functions were resistant to hydrological perturbations over the long term owing to species' functional redundancy. This result suggests that peatlands might represent a small negative feedback to climate change for the next century if it becomes drier [37]. However, the high resistance and resilience of the Sphagnum community to hydrological changes do not necessarily mean that overall vegetation function is not influenced by DWT variability. Our analysis also shows an increase in specific vascular plants along with decreasing water table position, such as Eriophorum vaginatum, Betula nana and Calluna vulgaris (figure 1b). These vascular plants have been shown to increase C loss in peatlands [2,13], and most likely negatively influence peatland C cycle. All of these changes are, however, regulated by the biophysical tipping point, and the strength of hydrological changes will determine whether or not vegetation shifts result in a smaller peatland sink [2]. Earth system models are increasingly moving to incorporate functional tipping points to estimate ecosystem changes [3,38]. The tipping point value we identified could further become a key target number for nature conservation.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We would like to thank Łukasz Lamentowicz and Miriam Marczewska for their contribution in the analysis of testate amoebae, as well as Barbara Fiałkiewicz-Kozieł and Pim van der Knaap for help during the fieldwork.
Ethics
All studies received the necessary permissions when conducted in nature reserves.
Data accessibility
Data available from the Dryad Digital Repository at: https://doi.org/10.5061/dryad.t50863s [32].
Authors' contributions
M.L. and V.E.J.J. conceived the research, V.E.J.J. and M.D.D. conducted statistical analyses, M.L. and V.E.J.J. interpreted results; M.L., M.G., K.M., K.K.-D. and M.S. conducted fieldwork; M.L. and V.E.J.J. prepared the initial manuscript and all authors contributed to subsequent revisions, gave final approval for publication and agreed to be held accountable for the work within the article. M.G. and M.S. made plant macrofossil analyses.
Competing interests
We declare no competing interests.
Funding
The research was supported by the National Science Centre (Poland) (grant nos. 2015/17/B/ST10/01656, 2014/13/B/ST10/02091, 2015/17/B/ST10/0343, 2011/01/D/ST10/02579) and the National Programme of Development of Humanities 2bH15015483. Scientific work was financed from the budgetary sources for scientific activity in 2016–2019, project no. 0342/IP1/2016/74. V.E.J.J. was supported by the French National Research Agency (MIXOPEAT project, grant no. ANR-17-CE01-0007). Authors acknowledge a grant [year 2018, no 254] of the Plenipotentiary of Poland at JINR. This research was supported by grant no. PSPB-013/2010 from Switzerland through the Swiss Contribution to the enlarged European Union. We acknowledge the support of the Scientific Exchange Programme from the Swiss Contribution to the New Member States of the European Union (Sciex-NMSch) - SCIEX Scholarship Found, project RE-FIRE 12.286.
References
- 1.Lenton TM, Held H, Kriegler E, Hall JW, Lucht W, Rahmstorf S, Schellnhuber HJ. 2008. Tipping elements in the Earth's climate system. Proc. Natl Acad. Sci. USA 105, 1786–1793. ( 10.1073/pnas.0705414105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jassey VEJ, et al. 2018. Tipping point in plant–fungal interactions under severe drought causes abrupt rise in peatland ecosystem respiration. Glob. Change Biol. 24, 972–986. ( 10.1111/gcb.13928) [DOI] [PubMed] [Google Scholar]
- 3.Moore JC. 2018. Predicting tipping points in complex environmental systems. Proc. Natl Acad. Sci. USA 115, 635–636. ( 10.1073/pnas.1721206115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Munson SM, Reed SC, Peñuelas J, McDowell NG, Sala OE. 2018. Ecosystem thresholds, tipping points, and critical transitions. New Phytologist 218, 1315–1317. ( 10.1111/nph.15145) [DOI] [PubMed] [Google Scholar]
- 5.Pascual M, Guichard F. 2005. Criticality and disturbance in spatial ecological systems. Trends Ecol. Evol. 20, 89–95. ( 10.1016/j.tree.2004.11.012) [DOI] [PubMed] [Google Scholar]
- 6.Scheffer M, et al. 2009. Early-warning signals for critical transitions. Nature 461, 53–59. ( 10.1038/nature08227) [DOI] [PubMed] [Google Scholar]
- 7.Willis KJ, Birks HJB. 2006. What is natural? The need for a long-term perspective in biodiversity conservation. Science 314, 1261–1265. ( 10.1126/science.1122667) [DOI] [PubMed] [Google Scholar]
- 8.Willis KJ, Araújo MB, Bennett KD, Figueroa-Rangel B, Froyd CA, Myers N. 2007. How can a knowledge of the past help to conserve the future? Biodiversity conservation and the relevance of long-term ecological studies. Phil. Trans. R. Soc. B 362, 175–186. ( 10.1098/rstb.2006.1977) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taranu ZE, Carpenter SR, Frossard V, Jenny J-P, Thomas Z, Vermaire JC, Perga M-E. 2018. Can we detect ecosystem critical transitions and signals of changing resilience from paleo-ecological records? Ecosphere 9, e02438 ( 10.1002/ecs2.2438) [DOI] [Google Scholar]
- 10.Barnosky AD, et al. 2017. Merging paleobiology with conservation biology to guide the future of terrestrial ecosystems. Science 355, eaah4787 ( 10.1126/science.aah4787) [DOI] [PubMed] [Google Scholar]
- 11.Charman DJ. 2002. Peatlands and environmental change. Chichester, UK: Wiley. [Google Scholar]
- 12.Marcisz K, Tinner W, Colombaroli D, Kołaczek P, Słowiński M, Fiałkiewicz-Kozieł B, Łokas E, Lamentowicz M. 2015. Long-term hydrological dynamics and fire history during the last 2000 years in CE Europe reconstructed from a high-resolution peat archive. Quat. Sci. Rev. 112, 138–152. ( 10.1016/j.quascirev.2015.01.019) [DOI] [Google Scholar]
- 13.Kuiper JJ, Mooij WM, Bragazza L, Robroek BJM. 2014. Plant functional types define magnitude of drought response in peatland CO2 exchange. Ecology 95, 123–131. ( 10.1890/13-0270.1) [DOI] [PubMed] [Google Scholar]
- 14.Robroek BJM, et al. 2015. Peatland vascular plant functional types affect methane dynamics by altering microbial community structure. J. Ecol. 103, 925–934. ( 10.1111/1365-2745.12413) [DOI] [Google Scholar]
- 15.Bengtsson F, Granath G, Rydin H. 2016. Photosynthesis, growth, and decay traits in Sphagnum – a multispecies comparison. Ecol. Evol. 6, 3325–3341. ( 10.1002/ece3.2119) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Backéus I. 1972. Bog vegetation re-mapped after sixty years. Studies on Skagershultamossen, central Sweden. Oikos 23, 348–393. ( 10.2307/3543178) [DOI] [Google Scholar]
- 17.Churchill AC, Turetsky MR, Mcguire AD, Hollingsworth TN. 2015. Response of plant community structure and primary productivity to experimental drought and flooding in an Alaskan fen. Can. J. For. Res. 45, 185–193. ( 10.1139/cjfr-2014-0100) [DOI] [Google Scholar]
- 18.Bridgham SD, Pastor J, Dewey B, Weltzin JF, Updegraff K. 2008. Rapid carbon response of peatlands to climate change. Ecology 89, 3041–3048. ( 10.1890/08-0279.1) [DOI] [PubMed] [Google Scholar]
- 19.Swindles GT, Morris PJ, Baird AJ, Blaauw M, Plunkett G. 2012. Ecohydrological feedbacks confound peat-based climate reconstructions. Geophys. Res. Lett. 39, L11401 ( 10.1029/2012GL051500) [DOI] [Google Scholar]
- 20.Morris PJ, Baird AJ, Young DM, Swindles G. 2015. Untangling climate signals from autogenic changes in long-term peatland development. Geophys. Res. Lett. 42, 10 788–10 797. ( 10.1002/2015GL066824) [DOI] [Google Scholar]
- 21.Lamentowicz M, Obremska M, Mitchell EAD. 2008. Autogenic succession, land-use change, and climatic influences on the Holocene development of a kettle-hole mire in Northern Poland. Rev. Palaeobot. Palynol. 151, 21–40. ( 10.1016/j.revpalbo.2008.01.009) [DOI] [Google Scholar]
- 22.Muggeo VMR. 2008. Segmented: an R package to fit regression models with broken-line relationships. R news 8, 20–25. [Google Scholar]
- 23.Vanacker M, Wezel A, Payet V, Robin J. 2015. Determining tipping points in aquatic ecosystems: the case of biodiversity and chlorophyll α relations in fish pond systems. Ecol. Indic. 52, 184–193. ( 10.1016/j.ecolind.2014.12.011) [DOI] [Google Scholar]
- 24.Baker M, King RS. 2010. A new method for detecting and interpreting biodiversity and ecological community thresholds. Methods Ecol. Evol. 1, 25–37. ( 10.1111/j.2041-210X.2009.00007.x) [DOI] [Google Scholar]
- 25.Sundberg S, Hansson J, Rydin H. 2006. Colonization of Sphagnum on land uplift islands in the Baltic Sea: time, area, distance and life history. J. Biogeogr. 33, 1479–1491. ( 10.1111/j.1365-2699.2006.01520.x) [DOI] [Google Scholar]
- 26.Bengtsson F, Rydin H, Hájek T. 2018. Biochemical determinants of litter quality in 15 species of Sphagnum. Plant Soil 425, 161–176. ( 10.1007/s11104-018-3579-8) [DOI] [Google Scholar]
- 27.Robroek BJM, et al. 2017. Taxonomic and functional turnover are decoupled in European peat bogs. Nat. Commun. 8, 1161 ( 10.1038/s41467-017-01350-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gunnarsson U. 2005. Global patterns of Sphagnum productivity. J. Bryol. 27, 269–279. ( 10.1179/174328205X70029) [DOI] [Google Scholar]
- 29.Jassey VEJ, Chiapusio G, Gilbert D, Buttler A, Toussaint M-L, Binet P. 2011. Experimental climate effect on seasonal variability of polyphenol/phenoloxidase interplay along a narrow fen-bog ecological gradient in Sphagnum fallax. Glob. Change Biol. 17, 2945–2957. ( 10.1111/j.1365-2486.2011.02437.x) [DOI] [Google Scholar]
- 30.Team RC. 2018. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 31.Team SD. 2016. RStan: the R interface to Stan. R package version 2.14.1.
- 32.Lamentowicz M, Gałka M, Marcisz K, Słowiński M, Kajukało K, Druguet Dayras M, Jassey V. 2019. Data from: Unveiling tipping points in long-term ecological records from Sphagnum-dominated peatlands Dryad Digital Repository. ( 10.5061/dryad.t50863s) [DOI]
- 33.Lafleur PM, Hember RA, Admiral SW, Roulet NT. 2005. Annual and seasonal variability in evapotranspiration and water table at a shrub-covered bog in southern Ontario, Canada. Hydrol. Processes 19, 3533–3550. ( 10.1002/hyp.5842) [DOI] [Google Scholar]
- 34.Parmentier FJW, Van Der Molen MK, De Jeu RAM, Hendriks DMD, Dolman AJ. 2009. CO2 fluxes and evaporation on a peatland in the Netherlands appear not affected by water table fluctuations. Agric. For. Meteorol. 149, 1201–1208. ( 10.1016/j.agrformet.2008.11.007) [DOI] [Google Scholar]
- 35.Cordlandwehr V, Meredith RL, Ozinga WA, Bekker RM, Van Groenendael JM, Bakker JP. 2013. Do plant traits retrieved from a database accurately predict on-site measurements? J. Ecol. 101, 662–670. ( 10.1111/1365-2745.12091) [DOI] [Google Scholar]
- 36.Holling CS. 1973. Resilience and stability of ecological systems. Annu. Rev. Ecol. Syst. 4, 1–23. ( 10.1146/annurev.es.04.110173.000245) [DOI] [Google Scholar]
- 37.Gallego-Sala AV, et al. 2018. Latitudinal limits to the predicted increase of the peatland carbon sink with warming. Nat. Clim. Change 8, 907–913. ( 10.1038/s41558-018-0271-1) [DOI] [Google Scholar]
- 38.Jiang J, Huang ZG, Seager TP, Lin W, Grebogi C, Hastings A, Lai YC. 2018. Predicting tipping points in mutualistic networks through dimension reduction. Proc. Natl Acad. Sci. USA 115, E639–E647. ( 10.1073/pnas.1714958115) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Lamentowicz M, Gałka M, Marcisz K, Słowiński M, Kajukało K, Druguet Dayras M, Jassey V. 2019. Data from: Unveiling tipping points in long-term ecological records from Sphagnum-dominated peatlands Dryad Digital Repository. ( 10.5061/dryad.t50863s) [DOI]
Supplementary Materials
Data Availability Statement
Data available from the Dryad Digital Repository at: https://doi.org/10.5061/dryad.t50863s [32].