Abstract
We conducted a 93-day experiment investigating the independent and combined effects of acidification (280−3300 µatm pCO2) and warming (28°C and 31°C) on calcification and linear extension rates of four key Caribbean coral species (Siderastrea siderea, Pseudodiploria strigosa, Porites astreoides, Undaria tenuifolia) from inshore and offshore reefs on the Belize Mesoamerican Barrier Reef System. All species exhibited nonlinear declines in calcification rate with increasing pCO2. Warming only reduced calcification in Ps. strigosa. Of the species tested, only S. siderea maintained positive calcification in the aragonite-undersaturated treatment. Temperature and pCO2 had no effect on the linear extension of S. siderea and Po. astreoides, and natal reef environment did not impact any parameter examined. Results suggest that S. siderea is the most resilient of these corals to warming and acidification owing to its ability to maintain positive calcification in all treatments, Ps. strigosa and U. tenuifolia are the least resilient, and Po. astreoides falls in the middle. These results highlight the diversity of calcification responses of Caribbean corals to projected global change.
Keywords: ocean acidification, ocean warming, Caribbean corals, calcification, linear extension
1. Introduction
Increasing carbon dioxide (CO2) from anthropogenic sources is of growing concern as global average atmospheric pCO2 has now increased from a pre-industrial level of 280 µatm to 410 µatm [1]. This rapid change has resulted in negative and often irreversible impacts on both terrestrial and marine ecosystems [2,3]. In terrestrial ecosystems, rising surface temperatures pose serious threats to animals and plants that are unable to cope with hotter, longer and more frequent thermal stress events [4,5]. Marine ecosystems are under similarly intense pressure from ocean warming and acidification [6], affecting everything from biogeochemical cycling to habitat and population structure [7].
Ocean warming is a major concern for marine organisms, especially at lower latitudes where sea surface temperature is predicted by the Intergovernmental Panel on Climate Change (IPCC) to rise between 0.6°C and 3.0°C by the end of the twenty-first century [8]. Reef-building corals in these low-latitude regions, including the Caribbean, are already living within a degree of their thermal maxima [9] and are therefore considered to be at particular risk [10]. Abnormally high seawater temperatures disrupt the symbiosis between the coral animal and its algal endosymbiont (Symbiodiniaceae) [11] through a process known as ‘coral bleaching’ [12], resulting in deterioration of corals' physiological processes [9,13,14]. Mortality rates increase because of the strong dependence of corals on their endosymbionts, which contribute up to 100% of their daily metabolic requirements [15], impacting the corals’ ability to withstand additional environmental stress.
Rising atmospheric pCO2 is not only warming surface seawater, but also causing more CO2 to dissolve into oceans, reducing carbonate ion concentration [CO3−2], pH and aragonite saturation state (ΩA) of seawater—a process known as ocean acidification [16]. The IPCC projects that atmospheric pCO2 will surpass 600 µatm by 2100, which would cause surface ocean pH to decrease by 0.1–0.3 [8]. Scleractinian corals rely heavily on elevated pH and ΩA at their site of calcification to form calcium carbonate skeletons [17–20], making it harder for some species to maintain conditions within these sites that are supportive of skeletal formation under acidification [21]. However, previous research has revealed inconsistencies in scleractinian corals' response to acidification [22,23]. Simulations of past [24] and future [25] pCO2 conditions in a natural reef system on the Great Barrier Reef revealed a decrease in net community calcification with increasing pCO2, while ex situ experiments demonstrated negative [23,26,27], threshold [28], parabolic [14] and no significant [26,27] response of corals to increased pCO2. Numerous explanations for the wide array of responses include differences in experimental design [29], evolutionary divergence among corals with respect to mechanisms of calcification and/or resilience to acidification [30], and differences among coral species’ physiological control of calcifying fluid chemistry [19,31–33]. Moreover, although studies have investigated the effects of increasing pCO2 on coral calcification and health, few have investigated the combined effects of temperature and pCO2.
In isolation, warming has been shown to more negatively impact coral calcification than pCO2 [14,34–37]. However, numerous studies have observed that the combination of pCO2 and temperature causes a more severe negative response in corals than either stressor alone [31,37–40], although few studies report a truly synergistic interaction between warming and acidification. This highlights the importance of studying the response of multiple coral species to global change scenarios under a common suite of conditions. Using multiple species in the same experiment minimizes differential outcomes that arise from differences in experimental design, allowing for direct comparison among species. The few studies that have investigated multiple coral species have yielded important insights into reef-community-level responses to acidification and warming, including projecting rates of whole-reef accretion under future IPCC scenarios [37].
Here, we investigate the independent and combined effects of ocean acidification and warming on four abundant and widespread Caribbean scleractinian coral species―Siderastrea siderea, Pseudodiploria strigosa, Porites astreoides and Undaria tenuifolia―in a 93-day laboratory experiment. These four species were selected because they span a range of skeletal morphologies (foliate—domical), possess similar life-history strategies [41] and occupy similar depth and geographical ranges [42]. Corals collected from the Belize Mesoamerican Barrier Reef System (MBRS) were reared under projected temperature and pCO2 stress with the aim of characterizing the effects of future global change on a suite of genetically and morphologically diverse Caribbean coral species.
2. Material and methods
(a). Experimental design
Six colonies of S. siderea, Ps. strigosa, Po. astreoides and U. tenuifolia were collected from inshore and offshore reef environments along the southern portion of the Belize MBRS (see the electronic supplementary material for details of the coral collection and figure S1). Forty-eight coral colonies were transported to Northeastern University's Marine Science Centre in Nahant, Massachusetts and sectioned into eight comparably sized fragments and placed into aquaria for a recovery period of 23 days. After recovery, temperature and pCO2 were adjusted gradually over a 20-day interval until target experimental conditions were approximately achieved for each treatment (temperature: 28°C and 31°C; pCO2: 280, 400, 700, 2800 µatm). Coral fragments were acclimated to treatment conditions for 30 days and then maintained in each experimental treatment for 93 days. Four pCO2 treatments corresponding to pre-industrial (311/288 µatm), present-day (pCO2 control; 405/447 µatm), end-of-century (701/673 µatm) and an extreme (3309/3285 µatm) pCO2 were maintained at two temperatures corresponding to the corals' approximate present-day mean annual temperature (28°C; determined by over 10 years of in situ records [43–45]) and projected end-of-century annual mean temperature (31°C) [8]. The extreme pCO2 treatment was formulated at a value approaching that predicted for the year 2500 [8] and was selected to push the corals closer to their physiological limits. Experimental tanks were illuminated on a 10 L : 14 D cycle with photosynthetically active radiation of approximately 300 µmol photons m–2 s–1 (see the electronic supplementary material for detailed experimental conditions and maintenance and figures S2 and S3).
(b). Measured and calculated parameters
Temperature, salinity and pH were measured every other day throughout the experiment (table 1). Water samples were obtained every 10 days for measurement of total alkalinity (TA) and dissolved inorganic carbon (DIC) and analysed with a VINDTA 3C (Marianda Corporation, Kiel, Germany). Temperature, salinity, TA and DIC were used to calculate carbonate parameters using CO2SYS [46] with Roy et al. carbonic acid constants K1 and K2 [47], Mucci's value for the stoichiometric aragonite solubility product [48] and an atmospheric pressure of 1.015 atm (table 1; electronic supplementary material, figure S4 and tables S2 and S3). The two temperatures at a given pCO2 level exhibited slight differences in carbonate chemistry because the solubility of CO2 in seawater varies with temperature.
Table 1.
Treatment conditions measured either every other day (T, pH, salinity) or every 10 days (pCO2, TA, DIC, ΩA).
| treatment | T (°C) | pCO2 (µatm) | pH | TA (µM) | DIC (µM) | ΩA | salinity |
|---|---|---|---|---|---|---|---|
| 1 | 27.9 ± 0.04 | 311 ± 18 | 8.30 ± 0.01 | 2052 ± 8 | 1708 ± 15 | 4.0 ± 0.1 | 31.7 ± 0.02 |
| 2 | 28.0 ± 0.04 | 405 ± 17 | 8.20 ± 0.01 | 2081 ± 3 | 1788 ± 10 | 3.4 ± 0.1 | 31.8 ± 0.02 |
| 3 | 28.1 ± 0.05 | 701 ± 17 | 8.01 ± 0.03 | 2092 ± 7 | 1901 ± 8 | 2.4 ± 0.1 | 31.7 ± 0.02 |
| 4 | 28.1 ± 0.02 | 3309 ± 76 | 7.31 ± 0.01 | 2131 ± 5 | 2156 ± 6 | 0.7 ± 0.1 | 31.8 ± 0.02 |
| 5 | 31.0 ± 0.04 | 288 ± 12 | 8.34 ± 0.01 | 2101 ± 6 | 1710 ± 11 | 4.6 ± 0.1 | 31.7 ± 0.02 |
| 6 | 31.1 ± 0.05 | 447 ± 28 | 8.21 ± 0.01 | 2077 ± 6 | 1773 ± 15 | 3.6 ± 0.1 | 31.7 ± 0.02 |
| 7 | 30.9 ± 0.03 | 673 ± 19 | 8.00 ± 0.01 | 2082 ± 6 | 1865 ± 8 | 2.7 ± 0.1 | 31.7 ± 0.02 |
| 8 | 31.0 ± 0.05 | 3285 ± 99 | 7.29 ± 0.01 | 2123 ± 4 | 2135 ± 5 | 0.8 ± 0.1 | 31.7 ± 0.02 |
(c). Quantification of calcification and linear extension
Net calcification rates were estimated from surviving coral fragments using a buoyant weight method [49] performed at the beginning of the pre-acclimation period and every 30 days throughout the experiment (see the electronic supplementary material for empirical derivation of buoyant weight–dry weight relationships for all four coral species and for survivorship, figures S5 and S6).
Extension was quantified from vertical cross sections of the corals as the total area of skeleton above the calcein dye line incorporated into coral skeletons at the beginning of the experiment, divided by the length of the region of active growth (see the electronic supplementary material for detailed methodology and figure S7). Linear extension was not quantified for U. tenuifolia or Ps. strigosa because their irregular skeletal morphologies rendered the method too inaccurate.
(d). Colony-level effects of basal calcification rate on calcification response to stress
Recent work has shown that coral species which calcify faster are generally more vulnerable to the effects of ocean acidification than slower calcifying species [50]—raising the possibility that similar trends exist within species among colonies with differing calcification rates. Colony-specific relationships between basal calcification rate and response to pCO2 and thermal stress were investigated by assessing correlation between the random effect of colony on each colony's calcification rate within the control treatment (pre-industrial pCO2 at 28°C) versus each colony's calcification response to pCO2 or thermal stress (i.e. change in calcification rate between the control treatment and the stress treatments). Small sample size prevented fitting a frequentist model to estimate these colony-level effects, so a Bayesian hierarchical regression model was fitted to calculate credible intervals of the corresponding extracted correlation coefficient using R package brms (version 2.7.0) with default priors [51]. Random effects relating colony-specific relationships between basal calcification rate and response to pCO2 and thermal stress were calculated for all species together, as the study lacked the statistical power to assess this correlation within individual species.
(e). Statistical analyses
Three-way mixed-model analyses of variance selected using Akaike information criterion (electronic supplementary material, table S4) were used to assess impacts of pCO2 and temperature on calcification and linear extension (lme4 (1.1–12)) [52]. Parametric bootstraps were performed to model 95% confidence intervals with 1500 iterations [53]. Significant differences between treatments were defined as non-overlapping 95% confidence intervals. Because reef environment was not a significant predictor of any parameter, colonies were pooled across reef environments and these effects were not further addressed (see the electronic supplementary material for detailed analyses; tables S12 and S13). To further evaluate the effects of acidification and warming on U. tenuifolia, survival rates were assessed using a Kaplan–Meier estimate of survival (survfit, survival, 2.39–5) [54]. Cox proportional hazard models, with colony nested within the tank as a random effect, were performed using coxme (2.2–5) [55].
3. Results
(a). Calcification rates
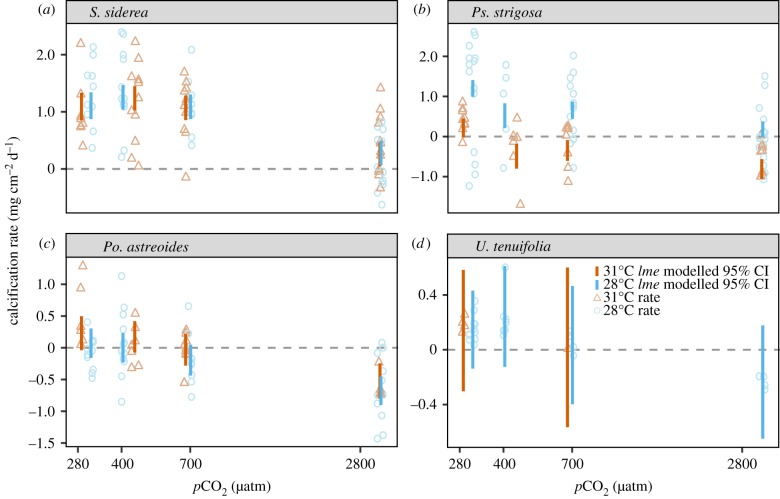
All four coral species exhibited nonlinear declines in calcification rate with increasing pCO2 (figure 1). Notably, S. siderea maintained positive net calcification across all temperature and pCO2 treatments (figure 1a), while the other species exhibited net dissolution in at least one treatment. Pseudodiploria. strigosa maintained net calcification at 28°C but exhibited net dissolution in all but pre-industrial pCO2 at 31°C (figure 1b). Porites astreoides yielded negligible net calcification or net dissolution in all treatments except under pre-industrial pCO2 at 31°C (figure 1c), and U. tenuifolia exhibited net calcification in all treatments except under the extreme pCO2 treatment (figure 1d). Temperature had no significant effect on S. siderea or Po. astreoides calcification rates; however, elevated temperature significantly reduced calcification rate in Ps. strigosa under all pCO2 conditions (figure 1; electronic supplementary material, tables S5 and S6). The effect of temperature on calcification rates of U. tenuifolia could not be quantified owing to low survival in the elevated-temperature treatments.
Figure 1.
Net calcification rates (mg cm−2 day−1) for S. siderea (a), Ps. strigosa (b), Po. astreoides (c) and U. tenuifolia (d) cultured over a range of pCO2 and temperature conditions. Blue circles represent net calcification rates for fragments in the 28°C treatments and orange triangles represent net calcification rates for fragments in the 31°C treatments. Blue and orange vertical bars represent modelled 95% confidence intervals (CI) for each pCO2 treatment at 28°C and 31°C, respectively.
(b). Colony-level calcification response to stress
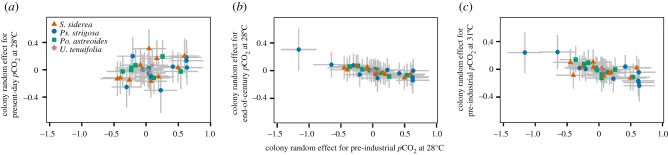
A negative slope of the correlation between random effects of colony on calcification rate in the control treatment (pre-industrial pCO2 at 28°C) versus those in the stress treatments (figure 2) would support the hypothesis that faster calcifying colonies (relative to the treatment mean) under control conditions calcify slower (relative to the treatment mean) under pCO2 and thermal stress (figure 2). While the best estimates of these correlations were negative, only the 75% credible intervals, and not the 95% credible intervals, did not always overlap zero (electronic supplementary material, figure S8)—suggesting that the results of the current experiment provide weak evidence for the inverse correlations between basal calcification rate and calcification response to pCO2 and thermal stress. However, the current study possibly lacked the statistical power to confirm the statistical significance of this correlation owing to a combination of low within-colony replication and high mortality rate.
Figure 2.
Estimated random effects and 95% credible intervals of colony on calcification rate of all four species under the control treatment (pre-industrial pCO2 at 28°C) versus random effects of colony on calcification rate under stress treatments of present-day pCO2 at 28°C (a), end-of-century pCO2 at 28°C (b) and pre-industrial pCO2 at 31°C (c).
(c). Linear extension
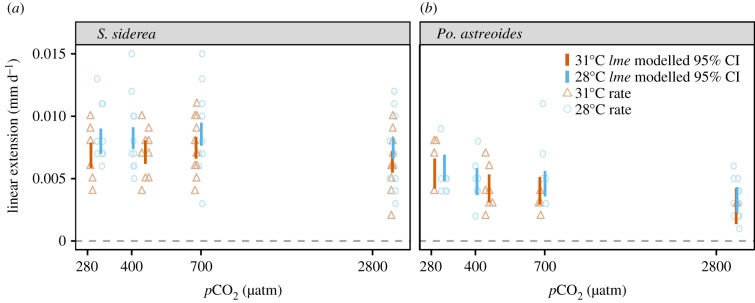
Siderastrea siderea and Po. astreoides exhibited positive linear extension rates in all treatments. Neither temperature, nor pCO2, nor their interaction had a significant impact on linear extension rates of S. siderea or Po. astreoides (figure 3; electronic supplementary material, tables S7 and S8).
Figure 3.
Linear extension rates (mm day−1) for S. siderea (a) and Po. astreoides (b) cultured over a range of pCO2 and temperature conditions. Blue circles represent extension rates for fragments in the 28°C treatments and orange triangles represent extension rates for fragments in the 31°C treatments. Blue and orange vertical bars represent modelled 95% confidence intervals (CI) for each pCO2 treatment at 28°C and 31°C, respectively.
4. Discussion
(a). Caribbean corals exhibit nonlinear calcification responses to pCO2 and temperature
All four coral species exhibited nonlinear calcification responses to pCO2 driven primarily by stability in calcification rates across the three lowest pCO2 treatments and major declines under extreme pCO2 (figure 1). One exception to this trend was Ps. strigosa, which exhibited an abrupt decline in calcification rate at present-day pCO2. Similar nonlinear calcification responses have been reported in previous studies for several temperate [28,56] and tropical corals [14,27,37], indicating that such pCO2 thresholds exist for a diverse range of coral species. Interspecific differences in corals' calcification responses to pCO2 may be influenced by differences in a coral's ability to control ΩA at their calcification site [18,19]. It has been proposed that corals transport Ca2+ into the calcifying fluid from the surrounding seawater in exchange for two protons using the enzyme Ca2+-ATPase [18], increasing the ΩA by elevating [Ca2+] and by converting HCO3− to CO32− [18,19,57]. However, this process requires energy (1 mole ATP consumed per mole of Ca2+-ATPase [17]), which should increase under more acidic conditions as more protons must be removed to deprotonate HCO3−. This suggests that the threshold pCO2 for maintaining stable rates of calcification is determined, at least in part, by the energetic costs of regulating ionic concentrations at the coral's site of calcification [19,36,57].
Increased temperature had no significant effect on calcification rates of either S. siderea or Po. astreoides (figure 1a,c). Similarly, in a prior study, S. siderea from the Florida Keys demonstrated stability in calcification rates with a temperature increase from 27°C to 30.3°C [37]. However, two studies on S. siderea from the Belize MBRS reported reduced calcification rates with a temperature increase from 28°C to 32°C [14,38]. Other studies have also reported reduced calcification for Po. astreoides under thermal stress [37,58], although the present study found that an increase in temperature from 28°C to 31°C did not significantly impact calcification rate of this species. These apparent discrepancies in coral species' calcification responses to warming may arise from evaluating temperature effects across different portions of these species’ thermal performance curves. Rates of biological processes, including calcification, are known to increase with increasing temperature to a maximum before declining with continued temperature increases, resulting in a thermal performance curve [59], which is typically parabolic in shape. It is possible that the two temperatures investigated in the present experiment are symmetrically distributed about this species' optimal temperature, resulting in equivalent calcifications rates at both temperatures.
Notably, only Ps. strigosa exhibited reduced calcification rates under thermal stress (figure 1b), contrasting previous work on this species showing no calcification response to thermal stress [37]. Again, this discrepancy between studies may result from assessing temperature effects across different portions of this species’ thermal performance curve (28–31°C versus 27.0–30.3°C in prior study). Differences in populations may also contribute to these discrepancies among studies with respect to a species' calcification responses to temperature [60] and pCO2 [61,62].
The effect of temperature on U. tenuifolia calcification rate could not be fully evaluated owing to low survival at 31°C, although these results highlight the thermal sensitivity of this species—as previously observed on the Belize MBRS after thermal bleaching events [63,64] (electronic supplementary material, figure S6d and tables S9–S11). Previous studies suggest that the susceptibility of U. tenuifolia to thermal stress arises from lack of compensatory stress responses [65–68], including insufficient production of heat shock proteins to protect against thermal events [66] and reduced endosymbiont photosynthesis owing to oxidative stress induced by warming [67]. Owing to its reliance on endosymbiont photosynthesis over heterotrophy for energy [65], oxidative bleaching may effectively starve this species of nutrition.
The interaction between pCO2 and temperature did not significantly impact calcification rates for any of the coral species. Absence of an interactive effect of pCO2 and temperature on coral calcification rate is relatively common and has been observed for multiple species [37,40,69]. A previous study that exposed S. siderea to elevated temperature (32°C), elevated pCO2 (approx. 900 µatm) and the combination of these two stressors found calcification rates were most negatively affected by the combined high-pCO2/high-temperature treatment, resulting in additive, but not synergistic, effects on calcification rates [38]. Thus, the evidence to date suggests that scleractinian corals exposed to both pCO2 and thermal stress rarely experience effects that are truly synergistic. Finally, calcification rates in the present study were generally comparable to those reported for corals from the Florida Keys [37] and Belize [38].
(b). Faster-growing colonies may be more vulnerable to pCO2 and thermal stress
Colonies that exhibited faster calcification in the control treatment (pre-industrial pCO2 at 28°C) tended to exhibit slower calcification in the elevated pCO2 and elevated-temperature treatments, suggesting a trade-off in which faster calcifying colonies may be more vulnerable to the negative impacts of pCO2 and thermal stress on calcification. Unsurprisingly, this correlation was weakest when comparing pre-industrial to present-day pCO2 treatments—the two most similar treatments. This variation in calcification rates was evident across the four coral species, which is consistent with previous literature suggesting that divergent calcification strategies exist across populations [70–74]. Our analysis provides preliminary support for two end-member strategies of calcification: (i) fast calcifying colonies that divert more energy towards flourishing during favourable environmental regimes but flounder during periods of environmental stress (potentially owing to lack of energetic reserves); and (ii) slower calcifying colonies that store more energy during environmentally favourable conditions, yet are able to continue calcifying under environmentally stressful conditions (potentially owing to their ability to tap energy stored during environmentally favourable times).
These divergent calcification strategies within coral populations may confer stability to populations faced with environmental stress over both short and long timescales. Over short timescales, these strategies increase the probability that at least some colonies (faster calcifiers) flourish when conditions are favourable, while ensuring that there are also survivors (slower calcifiers) during unfavourable times that allow populations to persist [75]. Over longer timescales, these divergent strategies may provide a high degree of genotypic variability upon which natural selection can act, thereby facilitating the evolution of the population towards optimal weightings of these calcification strategies [74], depending on the magnitude and duration of the environmental perturbation (e.g. short-term anthropogenic cycles [76] versus medium-term glacial cycles [77] versus longer-term secular trends in pCO2 associated with tectonics [78]). Although populations of coral species that exhibit these divergent calcification strategies could become more tolerant of anthropogenic stressors in the future, they would also become slower growing through time. Although our current study was not designed to specifically address colony-level calcification responses, our analysis demonstrates a potential trade-off within species that may allow populations to persist under projected global change. This apparent relationship between a colony's basal calcification rate and its response to pCO2 and thermal stress merits further investigation given its potentially far-reaching implications for corals' response to global change.
(c). All coral species, except Siderastrea siderea, exhibited net skeletal dissolution under the highest pCO2
Specimens of S. siderea maintained positive net calcification under all treatments (figure 1a), suggesting greater resilience to pCO2 and thermal stress compared to the other species examined [28,31,57]. Indeed, correcting net calcification rates with empirically derived gross dissolution rates [79] yields high rates of gross calcification for S. siderea even in undersaturated seawater conditions (electronic supplementary material, figure S9a), providing support for the assertion that S. siderea is able to maintain conditions supportive of aragonite precipitation at its site of calcification, despite external seawater supporting dissolution of its aragonite skeleton [14,37,38]. The combination of resilient calcification responses to thermal and pCO2 stress with the high survival exhibited by S. siderea in the present study (electronic supplementary material, figure S6a and tables S9–S11), as well as in prior studies [14,37,38], suggests that S. siderea possesses unique physiological mechanisms for maintaining basic life processes under pCO2 and thermal stress, and may contribute to its abundant distribution on reefs throughout the Caribbean [80].
Specimens of Ps. strigosa, Po. astreoides and U. tenuifolia exhibited net skeletal dissolution in at least one pCO2–temperature treatment, with the greatest net dissolution observed under the highest pCO2 treatment (figure 1; electronic supplementary material, figure S9b–d). Pseudodiploria strigosa exhibited the highest rates of net dissolution at the elevated temperature, probably owing, at least in part, to the loss of algal symbionts (i.e. partial bleaching; electronic supplementary material, figure S10) from which corals obtain a significant portion of their energy [15]. Thus, under thermal stress, reduced symbiont densities may lead to diminished photosynthate, reducing the energy available for calcification and eventually leading to thermally induced mortality as observed in the present study (electronic supplementary material, figure S6b and tables S9–S11) and previous experiments on juvenile corals [81]. Under these conditions, corals may be unable to produce enough new skeleton to counter the effects of skeletal dissolution in undersaturated conditions [79].
(d). Siderastrea siderea and Porites astreoides maintain constant rates of linear extension under pCO2 and thermal stress
Increasing pCO2 had no significant effect on linear extension rates of either S. siderea or Po. astreoides (figure 3), providing support for prior assertions that symbiotic corals exert strong control over the chemical milieu at their site of calcification [18–21]. This constant rate of extension (i.e. volume addition) combined with the threshold decrease in net calcification (i.e. mass addition) with increasing pCO2 suggests that both species produce less-dense skeletons and/or that the gain in skeletal mass associated with the new linear extension is offset by the loss of previously formed skeletal mass via dissolution under extreme pCO2 (figure 3; electronic supplementary material, figure S9a). Additionally, the observation that Po. astreoides exhibited net dissolution at both temperatures under several pCO2 treatments, yet maintained constant rates of linear extension, suggests that dissolution, rather than decreasing skeletal density, is driving the decline in calcification rate of this species under increasing pCO2—as the addition of new, less-dense skeleton alone could not cause a net decrease in skeletal mass (i.e. net dissolution).
Linear extension of S. siderea and Po. astreoides did not differ significantly across temperatures (figure 3). This contrasts previous reports linking historical ocean warming to reductions in the extension of wild specimens of S. siderea, although this decrease was observed only for forereef colonies along the southern MBRS [44]. Extension rates of S. siderea observed in the present study were generally comparable to those reported for wild specimens in Belize [44]. Conversely, the lack of temperature effect on the extension of Po. astreoides is consistent with the measured calcification response, supporting prior observations that rates of net calcification within this species is driven by the rate of linear extension, rather than by changes in skeletal density [34,82].
(e). Experiments reveal corals’ differential resilience to future oceanic change
Diverse responses to pCO2 and warming exhibited by the corals investigated here reveal a spectrum of resilience to future global oceanic change. We confirm the relatively high resilience of S. siderea to thermal and pCO2 stress [37], the moderate sensitivity of Po. astreoides and the relatively high sensitivity of Ps. strigosa [80,83] and U. tenuifolia [64,66,67]. The results also highlight the relative resilience of the investigated species (excluding Ps. strigosa) to moderate pCO2 stress, while revealing their high sensitivity to extreme pCO2. Faster-growing colonies tended to exhibit increased vulnerability to pCO2 and thermal stress, suggesting variability in tolerance of pCO2 and thermal stress within populations of these corals—a potential pathway for evolutionary resilience. Collectively, these results reveal the wide spectrum of responses exhibited by four common Caribbean corals in response to changes in ocean pH and temperature, a necessary step in understanding and forecasting the response of coral reef systems to future global change.
Supplementary Material
Acknowledgements
We thank Belize Fisheries Department for all associated permits, the Toledo Institute for Development and Environment (TIDE) and the Southern Environmental Association (SEA) for their continued support. We thank Garbutt's Marine for assistance in the field, A. Dwyer, S. Williams, L. Cameron and H. Aichelman for assisting with experimental maintenance and I. DeCorte for reading drafts of this manuscript and providing helpful feedback.
Ethics
All work undertaken in this study complied with current laws of Belize and United States of America for collecting and importing/exporting coral specimens.
Data accessibility
The data reported in this paper can be accessed at https://www.bco-dmo.org/person/735588 and all R code can be accessed at https://github.com/seabove7.
Authors' contributions
C.B.B., J.B.R., S.W.D. and K.D.C. conceived and designed the study. C.B.B., S.W.D. and K.D.C. collected specimens. C.B.B. executed the experiment, data analyses and statistical analyses with input from S.W.D., J.B.R. and K.D.C. I.T.W. and J.B.R. assisted with experimental maintenance, C.B.B. and I.T.W. analysed seawater carbonate chemistry and J.U. assisted with statistical analyses. C.B.B. drafted the manuscript with contributions from co-authors. All authors gave final approval of this manuscript for publication.
Competing interests
Authors declare that research was conducted in the absence of any commercial or financial relationships that could be construed as the potential conflict of interest.
Funding
Experiments were supported by NOAA award NA13OAR4310186 (to J.B.R. and K.D.C.) and NSF award OCE-1437371 (to J.B.R.). Salary and travel for C.B.B. and S.W.D. were supported by KC's start-up, NSF OCE-1459706 (to J.B.R.) and NSF OCE-1459522 (to K.D.C.). C.B.B. was supported through a UNC Summer Research Fellowship and S.W.D. was a Simons Foundation Fellow of the Life Sciences Research Foundation while preparing this work.
References
- 1.Tans P, Keeling R. 2017. Recent monthly average Mauna Loa CO2. San Diego, CA: NOAA/ESRL and Scripps Institution of Oceanography. [Google Scholar]
- 2.Solomon S, Plattner GK, Knutti R, Friedlingstein P. 2009. Irreversible climate change due to carbon dioxide emissions. Proc. Natl Acad. Sci. USA 106, 1704–1709. ( 10.1073/pnas.0812721106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pecl GT, et al. 2017. Biodiversity redistribution under climate change: impacts on ecosystems and human well-being. Science 355, 1389 ( 10.1126/science.aai9214) [DOI] [PubMed] [Google Scholar]
- 4.Caruso NM, Sears MW, Adams DC, Lips KR. 2014. Widespread rapid reductions in body size of adult salamanders in response to climate change. Glob. Change Biol. 20, 1751–1759. ( 10.1111/gcb.12550) [DOI] [PubMed] [Google Scholar]
- 5.Fitter AH, Fitter RSR. 2002. Rapid changes in flowering time in British plants. Science 296, 1689–1691. ( 10.1126/science.1071617) [DOI] [PubMed] [Google Scholar]
- 6.Hoegh-Guldberg O, et al. 2007. Coral reefs under rapid climate change and ocean acidification. Science 318, 1737–1742. ( 10.1126/science.1152509) [DOI] [PubMed] [Google Scholar]
- 7.Hoegh-Guldberg O, Bruno JF. 2010. The impact of climate change on the world's marine ecosystems. Science 328, 1523–1528. ( 10.1126/science.1189930) [DOI] [PubMed] [Google Scholar]
- 8.Stocker TF, et al. 2013. Climate change 2013: the physical science basis. In Contribution of working group I to the fifth assessment report of the intergovernmental panel on climate change (ed. IPCC), p. 1535, 5th edn Cambridge, UK: Cambridge University Press. [Google Scholar]
- 9.Jokiel PL, Coles SL. 1977. Effects of temperature on mortality and growth of Hawaiian reef corals. Mar. Biol. 43, 201–208. ( 10.1007/bf00402312) [DOI] [Google Scholar]
- 10.Somero GN. 2010. The physiology of climate change: how potentials for acclimatization and genetic adaptation will determine ‘winners’ and ‘losers’. J. Exp. Biol. 213, 912–920. ( 10.1242/jeb.037473) [DOI] [PubMed] [Google Scholar]
- 11.LaJeunesse TC, Parkinson JE, Gabrielson PW, Jeong HJ, Reimer JD, Voolstra CR, Santos SR. 2018. Systematic revision of symbiodiniaceae highlights the antiquity and diversity of coral endosymbionts. Curr. Biol. 28, 2570–2580. ( 10.1016/j.cub.2018.07.008) [DOI] [PubMed] [Google Scholar]
- 12.Glynn PW. 1991. Coral-reef bleaching in the 1980s and possible connections with global warming. Trends Ecol. Evol. 6, 175–179. ( 10.1016/0169-5347(91)90208-f) [DOI] [PubMed] [Google Scholar]
- 13.Grottoli AG, Rodrigues LJ, Palardy JE. 2006. Heterotrophic plasticity and resilience in bleached corals. Nature 440, 1186–1189. ( 10.1038/nature04565) [DOI] [PubMed] [Google Scholar]
- 14.Castillo KD, Ries JB, Bruno JF, Westfield IT. 2014. The reef-building coral Siderastrea siderea exhibits parabolic responses to ocean acidification and warming. Proc. R. Soc. B 281, 20141856 ( 10.1098/rspb.2014.1856) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muscatine L, McCloskey LR, Marian RE. 1981. Estimating the daily contribution of carbon from zooxanthellae to coral animal respiration. Limnol. Oceanogr. 26, 601–611. ( 10.4319/lo.1981.26.4.0601) [DOI] [Google Scholar]
- 16.Orr JC, et al. 2005. Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature 437, 681–686. ( 10.1038/nature04095) [DOI] [PubMed] [Google Scholar]
- 17.Al-Horani FA, Al-Moghrabi SM, de Beer D.. 2002. The mechanism of calcification and its relation to photosynthesis and respiration in the scleractinian coral Galaxea fascicularis. Mar. Biol. 142, 419–426. ( 10.1007/s00227-002-0981-8) [DOI] [Google Scholar]
- 18.Cohen AL, McConnaughey TA. 2003. Geochemical perspectives on coral mineralization. Biomineralization 54, 151–187. ( 10.2113/0540151) [DOI] [Google Scholar]
- 19.Ries JB. 2011. A physicochemical framework for interpreting the biological calcification response to CO2-induced ocean acidification. Geochim. Cosmochim. Acta 75, 4053–4064. ( 10.1016/j.gca.2011.04.025) [DOI] [Google Scholar]
- 20.Venn AA, Tambutte E, Holcomb M, Laurent J, Allemand D, Tambutte S. 2013. Impact of seawater acidification on pH at the tissue-skeleton interface and calcification in reef corals. Proc. Natl Acad. Sci. USA 110, 1634–1639. ( 10.1073/pnas.1216153110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCulloch M, Falter J, Trotter J, Montagna P. 2012. Coral resilience to ocean acidification and global warming through pH up-regulation. Nat. Clim. Change 2, 623–633. ( 10.1038/nclimate1473) [DOI] [Google Scholar]
- 22.Ries JB, Cohen AL, McCorkle DC. 2009. Marine calcifiers exhibit mixed responses to CO2-induced ocean acidification. Geology 37, 1131–1134. ( 10.1130/g30210a.1) [DOI] [Google Scholar]
- 23.Comeau S, Carpenter RC, Edmunds PJ. 2013. Coral reef calcifiers buffer their response to ocean acidification using both bicarbonate and carbonate. Proc. R. Soc. B 280, 20122374 ( 10.1098/rspb.2012.2374) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Albright R, et al. 2016. Reversal of ocean acidification enhances net coral reef calcification. Nature 531, 362–365. ( 10.1038/nature17155) [DOI] [PubMed] [Google Scholar]
- 25.Albright R, Takeshita Y, Koweek DA, Ninokawa A, Wolfe K, Rivlin T, Nebuchina Y, Young J, Caldeira K. 2018. Carbon dioxide addition to coral reef waters suppresses net community calcification. Nature 555, 516 ( 10.1038/nature25968) [DOI] [PubMed] [Google Scholar]
- 26.Reynaud S, Leclercq N, Romaine-Lioud S, Ferrier-Pages C, Jaubert J, Gattuso JP. 2003. Interacting effects of CO2 partial pressure and temperature on photosynthesis and calcification in a scleractinian coral. Glob. Change Biol. 9, 1660–1668. ( 10.1046/j.1529-8817.2003.00678.x) [DOI] [Google Scholar]
- 27.Jury CP, Whitehead RF, Szmant AM. 2010. Effects of variations in carbonate chemistry on the calcification rates of Madracis auretenra (= Madracis mirabilis sensu Wells, 1973): bicarbonate concentrations best predict calcification rates. Glob. Change Biol. 16, 1632–1644. ( 10.1111/j.1365-2486.2009.02057.x) [DOI] [Google Scholar]
- 28.Ries JB, Cohen AL, McCorkle DC. 2010. A nonlinear calcification response to CO2-induced ocean acidification by the coral Oculina arbuscula. Coral Reefs 29, 661–674. ( 10.1007/s00338-010-0632-3) [DOI] [Google Scholar]
- 29.Cornwall CE, Hurd CL. 2016. Experimental design in ocean acidification research: problems and solutions. ICES J. Mar. Sci. 73, 572–581. ( 10.1093/icesjms/fsv118) [DOI] [Google Scholar]
- 30.Brown D, Edmunds PJ. 2016. Differences in the responses of three scleractinians and the hydrocoral Millepora platyphylla to ocean acidification. Mar. Biol. 163, 1–10. ( 10.1007/s00227-016-2837-7) [DOI] [Google Scholar]
- 31.Agostini S, Fujimura H, Higuchi T, Yuyama I, Casareto BE, Suzuki Y, Nakano Y. 2013. The effects of thermal and high-CO2 stresses on the metabolism and surrounding microenvironment of the coral Galaxea fascicularis. C. R. Biol. 336, 384–391. ( 10.1016/j.crvi.2013.07.003) [DOI] [PubMed] [Google Scholar]
- 32.Holcomb M, Venn AA, Tambutte E, Tambutte S, Allemand D, Trotter J, McCulloch M. 2014. Coral calcifying fluid pH dictates response to ocean acidification. Sci. Rep. 4, e5207 ( 10.1038/srep05207) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barott KL, Venn AA, Perez SO, Tambutte S, Tresguerres M. 2015. Coral host cells acidify symbiotic algal microenvironment to promote photosynthesis. Proc. Natl Acad. Sci. USA 112, 607–612. ( 10.1073/pnas.1413483112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carricart-Ganivet JP, Cabanillas-Teran N, Cruz-Ortega I, Blanchon P. 2012. Sensitivity of calcification to thermal stress varies among genera of massive reef-building corals. PLoS ONE 7, e0032859 ( 10.1371/journal.pone.0032859) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Venti A, Andersson A, Langdon C. 2014. Multiple driving factors explain spatial and temporal variability in coral calcification rates on the Bermuda platform. Coral Reefs 33, 979–997. ( 10.1007/s00338-014-1191-9) [DOI] [Google Scholar]
- 36.Davies SW, Marchetti A, Ries JB, Castillo KD. 2016. Thermal and pCO2 stress elicit divergent transcriptomic responses in a resilient coral. Front. Mar. Sci. 3, 1–12. ( 10.3389/fmars.2016.00112) [DOI] [Google Scholar]
- 37.Okazaki RR, et al. 2017. Species-specific responses to climate change and community composition determine future calcification rates of Florida Keys reefs. Glob. Change Biol. 23, 1023–1035. ( 10.1111/gcb.13481) [DOI] [PubMed] [Google Scholar]
- 38.Horvath KM, Castillo KD, Armstrong P, Westfield IT, Courtney T, Ries JB. 2016. Next-century ocean acidification and warming both reduce calcification rate, but only acidification alters skeletal morphology of reef-building coral Siderastrea siderea. Sci. Rep. 6, 29613 ( 10.1038/srep29613) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Edmunds PJ, Brown D, Moriarty V. 2012. Interactive effects of ocean acidification and temperature on two scleractinian corals from Moorea, French Polynesia. Glob. Change Biol. 18, 2173–2183. ( 10.1111/j.1365-2486.2012.02695.x) [DOI] [Google Scholar]
- 40.Schoepf V, et al. 2013. Coral energy reserves and calcification in a high-CO2 world at two temperatures. PLoS ONE 8, e75049 ( 10.1371/journal.pone.0075049) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Szmant AM. 1986. Reproductive ecology of Caribbean reef corals. Coral Reefs 5, 43–53. ( 10.1007/bf00302170) [DOI] [Google Scholar]
- 42.Veron JEN. 2000. Corals of the world. Townsville, Australia: Australian Institute of Marine Science. [Google Scholar]
- 43.Castillo KD, Lima FP. 2010. Comparison of in situ and satellite-derived (MODIS-Aqua/Terra) methods for assessing temperatures on coral reefs. Limnol. Oceanogr. 8, 107–117. ( 10.4319/lom.2010.8.0107) [DOI] [Google Scholar]
- 44.Castillo KD, Ries JB, Weiss JM, Lima FP. 2012. Decline of forereef corals in response to recent warming linked to history of thermal exposure. Nat. Clim. Change 2, 756–760. ( 10.1038/nclimate1577) [DOI] [Google Scholar]
- 45.Baumann JH, Townsend JE, Courtney TA, Aichelman HE, Davies SW, Lima FP, Castillo KD. 2016. Temperature regimes impact coral assemblages along environmental gradients on lagoonal reefs in Belize. PLoS ONE 11, e0162098 ( 10.1371/journal.pone.0162098) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pierrot D, Lewis E, Wallace D. 2006. MS Excel program developed for CO2 system calculations. ORNL/CDIAC-105a Oak Ridge, TN: Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory. U.S Department of Energy. [Google Scholar]
- 47.Roy RN, Roy LN, Vogel KM, Portermoore C, Pearson T, Good CE, Millero FJ, Campbell DM. 1993. The dissociation-constants of carbonic-acid in seawater at salinities 5 to 45 and temperatures 0-degrees-C to 45-degrees-C. Mar. Chem. 44, 249–267. ( 10.1016/0304-4203(93)90207-5) [DOI] [Google Scholar]
- 48.Mucci A. 1983. The solubility of calcite and aragonite in seawater at various salinities, temperatures, and one atmosphere total pressure. Am. J. Sci. 283, 780–799. ( 10.2475/ajs.283.7.780) [DOI] [Google Scholar]
- 49.Davies PS. 1989. Short-term growth measurements of corals using an accurate buoyant weighing technique. Mar. Biol. 101, 389–395. ( 10.1007/BF00428135) [DOI] [Google Scholar]
- 50.Comeau S, Edmunds PJ, Spindel NB, Carpenter RC. 2014. Fast coral reef calcifiers are more sensitive to ocean acidification in short-term laboratory incubations. Limnol. Oceanogr. 59, 1081–1091. ( 10.4319/lo.2014.59.3.1081) [DOI] [Google Scholar]
- 51.Bürkner P.-C. 2017. brms: an R package for Bayesian multilevel models using Stan. J. Stat. Softw. 80, 1–28. ( 10.18637/jss.v080.i01) [DOI] [Google Scholar]
- 52.Bates D, Maechler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. ( 10.18637/jss.v067.i01) [DOI] [Google Scholar]
- 53.Wilcox RR. 2010. Fundamental of modern statistical methods: substantially improving power and accuracy, 2nd edn New York, NY: Springer. [Google Scholar]
- 54.Therneau TM. 2015. A package for survival analysis in S. See https://CRAN.R-project.org/package=survival.
- 55.Therneau TM. 2015. coxme: mixed effects cox models. See https://CRAN.R-project.org/package=coxme.
- 56.Rodolfo-Metalpa R, Martin S, Ferrier-Pages C, Gattuso JP. 2010. Response of the temperate coral Cladocora caespitosa to mid- and long-term exposure to pCO(2) and temperature levels projected for the year 2100 AD. Biogeosciences 7, 289–300. ( 10.5194/bg-7-289-2010) [DOI] [Google Scholar]
- 57.Von Euw S, et al. 2017. Biological control of aragonite formation in stony corals. Science 356, 933 ( 10.1126/science.aam6371) [DOI] [PubMed] [Google Scholar]
- 58.Kenkel CD, Goodbody-Gringley G, Caillaud D, Davies SW, Bartels E, Matz MV. 2013. Evidence for a host role in thermotolerance divergence between populations of the mustard hill coral (Porites astreoides) from different reef environments. Mol. Ecol. 22, 4335–4348. ( 10.1111/mec.12391) [DOI] [PubMed] [Google Scholar]
- 59.Portner HO, Bennett AF, Bozinovic F, Clarke A, Lardies MA, Lucassen M, Pelster B, Schiemer F, Stillman JH. 2006. Trade-offs in thermal adaptation: the need for a molecular to ecological integration. Physiol. Biochem. Zool. 79, 295–313. ( 10.1086/499986) [DOI] [PubMed] [Google Scholar]
- 60.Marshall PA, Baird AH. 2000. Bleaching of corals on the Great Barrier Reef: differential susceptibilities among taxa. Coral Reefs 19, 155–163. ( 10.1007/s003380000086) [DOI] [Google Scholar]
- 61.Manzello DP, Enochs IC, Melo N, Gledhill DK, Johns EM. 2012. Ocean acidification refugia of the Florida reef tract. PLoS ONE 7, e0041715 ( 10.1371/journal.pone.0041715) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Melendez M, Salisbury J. 2017. Impacts of ocean acidification in the coastal and marine environments of Caribbean small island developing states (SIDS). Caribbean Mar. Climate Change Rep. Card Sci. Rev. 2017, 31–30. [Google Scholar]
- 63.Aronson RB, Macintyre IG, Precht WF, Murdoch TJT, Wapnick CM. 2002. The expanding scale of species turnover events on coral reefs in Belize. Ecol. Monogr. 72, 233–249. ( 10.1890/0012-9615(2002)072[0233:tesost]2.0.co;2) [DOI] [Google Scholar]
- 64.Aronson RB, Precht WF, Macintyre IG, Murdoch TJ.T. 2000. Coral bleach-out in Belize. Nature 405, 36 ( 10.1038/35011132) [DOI] [PubMed] [Google Scholar]
- 65.Seemann J, Carballo-Bolaños R, Berry KL, González CT, Richter C, Leinfelder RR. 2012. Importance of heterotrophic adaptations of corals to maintain energy reserves. In Proceedings of the 12th International Coral Reef Symposium, Cairns, Australia, (19A), pp. 9–13. Fort Lauderdale, FL: National Coral Reef Institute. [Google Scholar]
- 66.Robbart ML, Peckol P, Scordilis SP, Curran HA, Brown-Saracino J. 2004. Population recovery and differential heat shock protein expression for the corals Agaricia agaricites and A-tenuifolia in Belize. Mar. Ecol. Progr. Ser. 283, 151–160. ( 10.3354/meps283151) [DOI] [Google Scholar]
- 67.Lesser MP. 1997. Oxidative stress causes coral bleaching during exposure to elevated temperatures. Coral Reefs 16, 187–192. ( 10.1007/s003380050073) [DOI] [Google Scholar]
- 68.Feder ME, Hofmann GE. 1999. Heat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Annu. Rev. Physiol. 61, 243–282. ( 10.1146/annurev.physiol.61.1.243) [DOI] [PubMed] [Google Scholar]
- 69.Muehllehner N, Edmunds P. 2008. Effects of ocean acidification and increased temperature on skeletal growth of two scleractinian corals, Pocillopora meandrina and Porites rus. In Proceedings of the 11th International Coral Reef Symposium, Cairns, Australia, pp. 57–61. Fort Lauderdale, FL: National Coral Reef Institute. [Google Scholar]
- 70.Leong W, Pawlik JR. 2010. Evidence of a resource trade-off between growth and chemical defenses among Caribbean coral reef sponges. Mar. Ecol. Progr. Ser. 406, 71–78. ( 10.3354/meps08541) [DOI] [Google Scholar]
- 71.Metcalfe NB, Monaghan P. 2001. Compensation for a bad start: grow now, pay later? Trends Ecol. Evol. 16, 254–260. ( 10.1016/s0169-5347(01)02124-3) [DOI] [PubMed] [Google Scholar]
- 72.Rinkevich B. 1996. Do reproduction and regeneration in damaged corals compete for energy allocation? Mar. Ecol. Progr. Ser. 143, 297–302. ( 10.3354/meps143297) [DOI] [Google Scholar]
- 73.Szmant AM, Gassman NJ. 1990. The effects of prolonged bleaching on the tissue biomass and reproduction of the reef coral Montastrea annularis. Coral Reefs 8, 217–224. ( 10.1007/bf00265014) [DOI] [Google Scholar]
- 74.Arnott SA, Chiba S, Conover DO. 2006. Evolution of intrinsic growth rate: metabolic costs drive trade-offs between growth and swimming performance in Menidia menidia. Evolution 60, 1269–1278. ( 10.1111/j.0014-3820.2006.tb01204.x) [DOI] [PubMed] [Google Scholar]
- 75.Conover DO, Schultz ET. 1995. Phenotypic similarity and the evolutionary significance of countergradient variation. Trends Ecol. Evol. 10, 248–252. ( 10.1016/s0169-5347(00)89081-3) [DOI] [PubMed] [Google Scholar]
- 76.Hughes TP, et al. 2003. Climate change, human impacts, and the resilience of coral reefs. Science 301, 929–933. ( 10.1126/science.1085046) [DOI] [PubMed] [Google Scholar]
- 77.Daly R. 1915. The glacial-control theory of coral reefs. Proc. Am. Acad. Arts Sci. 51, 155–251. ( 10.2307/20025572) [DOI] [Google Scholar]
- 78.Honisch B, et al. 2012. The geological record of ocean acidification. Science 335, 1058–1063. ( 10.1126/science.1208277) [DOI] [PubMed] [Google Scholar]
- 79.Ries JB, Ghazaleh MN, Connolly B, Westfield I, Castillo KD. 2016. Impacts of seawater saturation state (ΩA = 0.4–4.6) and temperature (10, 25°C) on the dissolution kinetics of whole-shell biogenic carbonates. Geochim. Cosmochim. Acta 192, 318–337. ( 10.1016/j.gca.2016.07.001) [DOI] [Google Scholar]
- 80.Alemu JI, Clement Y. 2014. Mass coral bleaching in 2010 in the southern Caribbean. PLoS ONE 9, e83829 ( 10.1371/journal.pone.0083829) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bassim KM, Sammarco PW. 2003. Effects of temperature and ammonium on larval development and survivorship in a scleractinian coral (Diploria strigosa). Mar. Biol. 142, 241–252. ( 10.1007/S00227-002-0953-z) [DOI] [Google Scholar]
- 82.Elizalde-Rendon EM, Horta-Puga G, Gonzalez-Diaz P, Carricart-Ganivet JP. 2010. Growth characteristics of the reef-building coral Porites astreoides under different environmental conditions in the western Atlantic. Coral Reefs 29, 607–614. ( 10.1007/s00338-010-0604-7) [DOI] [Google Scholar]
- 83.Pratte ZA, Richardson LL. 2014. Impacts of temperature increase and acidification on thickness of the surface mucopolysaccharide layer of the Caribbean coral Diploria spp. Coral Reefs 33, 487–496. ( 10.1007/s00338-013-1115-0) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data reported in this paper can be accessed at https://www.bco-dmo.org/person/735588 and all R code can be accessed at https://github.com/seabove7.