Introduction
Adeno-associated virus (AAV) is a small single-stranded DNA-containing non- pathogenic human parvovirus, which has gained attention as an efficient and safe vector for gene transfer [1-6]. Recombinant AAV vectors have been, or are currently being, used in 165 Phase I/II/III clinical trials, and thus far, no serious adverse events have ever been observed or reported. AAV serotype 2 (AAV2) vectors have shown clinical efficacy in 3 human diseases: Leber’s congenital amaurosis (LCA) [7-10], aromatic L-amino acid decarboxylase deficiency (AADC) [11], and choroideremia [12]. In the past decade, at least 12 additional AAV serotype vectors, some derived from non-human primates, have also become available [13-21]. AAV1 vectors have successfully been used in the gene therapy of lipoprotein lipase deficiency [22], and AAV8 vectors have shown clinical efficacy in the potential gene therapy of hemophilia B [23-25]. More recently, AAV5 vectors have been to be effective in hemophilia A [26,27]. AAV9 vectors have successfully been used in gene therapy for Pompe disease [28] and in spinal muscular atrophy [29]. The AAV1-LPL vector was approved as a drug, designated as Alipogene tiparvovec, and marketed under the trade name Glybera®, in Europe in 2012. In 2017, an AAV2 vector expressing retinal pigment epithelium-specific 65 kDa protein (RPE65) was approved by the Food and Drug Administration as a drug, Voretigene Neparvovec (Luxturna), in the USA. A number of additional Phase I/II clinical trials have been, or are currently being pursued with AAV1, AAV2, AAV3, AAV5, AAV6, AAV8, AAV9, and AAV10 vectors for the potential gene therapy of a wide variety of human diseases [30].
Despite these remarkable achievements, it has become increasingly clear that the full potential of the first generation of AAV vectors will only be realized after these vectors have been modified to evade the host immune response [31]. A brief account of the use of AAV vectors in targeting the liver in general, and gene therapy of hemophilia in particular, follows.
Liver-tropic AAV vectors
Liver has long been considered among the ideal targets for the potential gene therapy of a wide variety of human diseases. In 1997, Ponnazhagan et al. [32] first reported the liver-tropism of rAAV2 vectors, following intra-venous administration of rAAV2-lacZ vectors, in a murine model in vivo. Koeberl et al. [33] and Snyder et al. [34] documented persistent expression of human clotting factor IX (F.IX) following intravenous injection in mice as well. Subsequently, persistent expression of canine factor IX in hemophilia B canines was also reported by Chao et al. [35]. Based on those pre-clinical studies, a Phase I clinical trial for hemophilia B was carried out with AAV2 vectors expressing h.FIX [35]. Even though in pre-clinical studies with both hemophilic murine and canine models, rAAV2-F.IX vectors provided complete phenotypic correction of the disease for the entire life-spans of these animals, the predicted dose of these vectors in humans did not express therapeutic levels of F.IX in humans. The results of the first Phase I clinical trial for the potential gene therapy of hemophilia B with the first generation of rAAV2 vectors were reported in 2006 [36]. At low (8×1010 vgs/kg), and medium (4×10n vgs/kg) vector doses, rAAV2 vectors failed to express F.IX in two patients. At the high dose (2×1012 vgs/kg), rAAV2 vectors did lead to expression of therapeutic levels of F.IX in one patient, but it was short-lived due to the host immune response to AAV2 capsid proteins. Uptake of AAV2 vectors by dendritic cells, followed by proteasomal degradation of capsid proteins, led to activation of AAV2 capsid-specific CD8+ memory T cells, which in turn, led to the destruction of transduced hepatocytes, and consequently, the loss of FIX levels in this patient [37]. The lesson learned from this first liver-directed gene therapy trial was that AAV2 serotype vectors, although extremely effective in mice and dogs, were not optimal for humans.
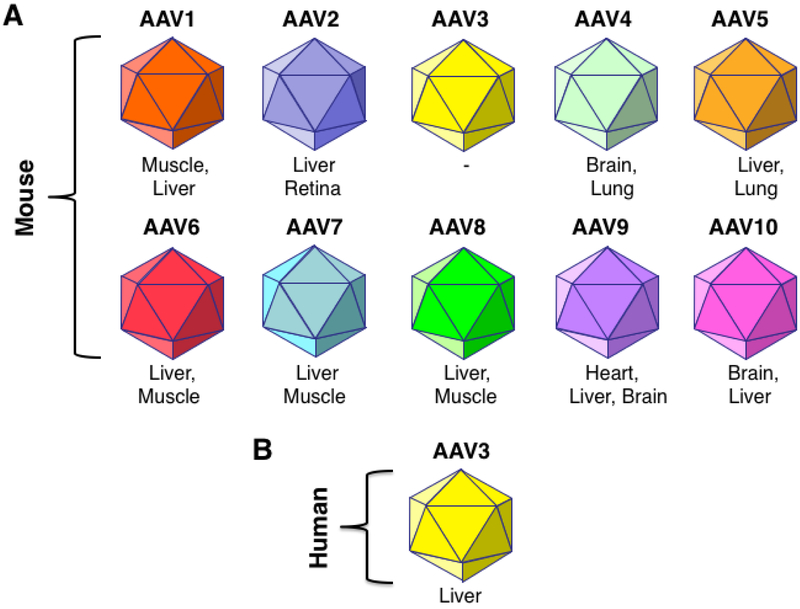
In the ensuing years a number of additional AAV serotype vectors became available, and their tissue/organ tropisms were determined [13-21,38]. The 10 most commonly used AAV serotype vectors are depicted schematically in Figure 1A. Several investigators reported remarkably high efficiency of transduction of murine liver with AAV8 serotype vectors [18,39,40]. A subsequent clinical trial with AAV8 vectors, performed by Nathwani et al. [23] proved to be a significant step forward, but a similar immune response was also observed in the high-dose cohort of patients [24]. Although the choice of AAV8 vector was undoubtedly based on its superior performance over AAV2 vectors in transducing murine hepatocytes, it was not readily apparent whether AAV8 vectors would be ideal for transducing human hepatocytes. This question proved to be particularly difficult to address since AAV8 failed to transduce any cell type, including hepatocytes in vitro.
FIGURE 1: Schematic representation of the most commonly used recombinant AAV serotype vectors and their tissue-tropism.
Various murine tissues and organs that have been reported to be transduced efficiently with various AAV serotype vectors are indicated. AAV3 serotype vectors in particular, have been shown to transduce human hepatocytes well. Data from Glushakova LG, Lisankie MJ, Eruslanov EB, et al.. AAV3-mediated transfer and expression of the pyruvate dehydrogenase E1 alpha subunit gene causes metabolic remodeling and apoptosis of human liver cancer cells. Mol Genet Metab 2009;98:289-99, Cheng B, Ling C, Dai Y, et al.. Development of optimized AAV3 serotype vectors: Mechanism of high-efficiency transduction of human liver cancer cells. Gene Ther 2012; 19:375-84, Ling C, Wang Y, Zhang Y, Ejjigani A, Yin Z, Lu Y, Wang L, Wang M, Li J, Hu Z, Aslanidi GV et al.: Selective in vivo targeting of human liver tumors by optimized AAV3 vectors in a murine xenograft model. Hum Gene Ther (2014) 25(12):1023-1034, Ling C, Lu Y, Kalsi JK, Jayandharan GR, Li B, Ma W, Cheng B, Gee SW, McGoogan KE, Govindasamy L, Zhong L et al.: Human hepatocyte growth factor receptor is a cellular coreceptor for adeno-associated virus serotype 3. Hum Gene Ther (2010) 21 (12):1741- 1747, Li S, Ling C, Zhong L, Li M, Su Q, He R, Tang Q, Greiner DL, Shultz LD, Brehm MA, Flotte TR et al.: Efficient and targeted transduction of nonhuman primate liver with systemically delivered optimized AAV3b vectors. Mol Ther (2015) 23(12):1867-1876, Wang L, Bell P, Somanathan S, Wang Q, He Z, Yu H, McMenamin D, Goode T, Calcedo R, Wilson JM: Comparative study of liver gene transfer with AAV vectors based on natural and engineered aav capsids. Mol Ther (2015) 23(12):1877-1887, and Vercauteren K, Hoffman BE, Zolotukhin I, et al.. Superior in vivo transduction of human hepatocytes using engineered AAV3 capsid. Mol Ther 2016;24:1042-49 with permission.
Selective human liver-tropism of AAV3 vectors
In the quest to identify an alternative AAV serotype for efficient transduction of human hepatocytes, Glushakova et al. [41] made an unexpected observation nearly a decade ago that AAV3 vectors, shown schematically in Figure 1B, could efficiently transduce human liver cancer cell lines as well as primary human hepatocytes efficiently in vitro. Cheng et al. [42] and Ling et al. [43] documented that human liver tumors could also be transduced remarkably well in xenograft mouse model in vivo. Ling et al. [44] provided the underlying basis of the remarkable tropism of AAV3 vectors for human hepatic cells because AAV3 uses human hepatocyte growth factor receptor (huHGFR) as a cellular co-receptor. Although murine HGFR (muHGFR) shares 88% identity with huHGFR,38 AAV3 fails to bind to muHGFR because of the location of amino acid differences along the interaction interface, including amino residues that make contact with hepatocyte growth factor (HGF). Lisowski et al. [45] reported that a shuffled rAAV vector, designated LK-03, which shares 97.7 and 98.9% homology with rAAV3B at the DNA and amino acids level, respectively, transduced human primary hepatocytes ~20-fold more efficiently than AAV8.
AAV3 and non-human primate studies
Although murine xenograft mouse models, used by Cheng et al. [42] and Ling et al. [43] provided a useful system to evaluate the efficacy of AAV3 vectors in human hepatocyte transduction, the safety of these vectors could not be assessed in such models since AAV3 vectors do not transduce any tissues or organs in mice [46]. Because of the close phylogenetic and physiologic similarities between non-human primates (NHPs) and humans]. The safety and efficacy of rAAV3 vectors was evaluated by Li et al. in a NHP model in vivo [47]. Interestingly, the human and the non-human primate HGFRs share 99% identity. High-efficiency transduction of NHP livers, both short-term [7-days] and long-term [91-days], following intravenous delivery of rAAV3 vectors, was indeed documented, with no apparent toxicity at a relatively high dose of 1×1013 vgs/kg. These findings revealed the efficient liver-targeted gene transfer by rAAV3 vectors following systematic administration. Remarkably, the vector-mediated EGFP expression and vector genome accumulation were largely restricted to the NHP liver. Although the use of the EGFP reporter gene allowed us to address both vector tropism and gene transfer efficiency simultaneously, it was less quantitative and likely immunogenic leading to transient expression. The use of a secreted self-antigen, rhCG, allowed us to study not only the efficiency, but also the onset and kinetics of transgene expression and long-term stability. In addition, since the rhCG used in this study can limit the potential host humoral and cellular immune responses to the transgene product, we were also able to evaluate the potential host immune responses against the vector capsid proteins for up to 3 months. The vector genome biodistribution study clearly revealed that the persisting vector genomes of rAAV3 vectors were predominantly harbored in the monkey liver. Furthermore, the rhCG mRNA expression pattern in six selected organs displayed that the transgene expression was largely restricted to the liver, which also did not lead to any overt cytotoxicity. In addition, no obvious vector-related pathological changes in the liver, spleen and other organs were observed in any of the treated animals. These studies documented the remarkable specificity, efficacy, and safety of rAAV3 vectors in NHP livers following systemic administration. These studies were further corroborated by Wang et al. [48], which further established the remarkable specificity, efficacy, and safety of AAV3 vectors [49].
Next generation of AAV vectors
Hansen et al. [50] made the original observation that following infection of cells, only ~20% of the input AAV2 vectors gain entry into the nucleus, whereas ~80% fail to escape the endosome in the cytoplasm. It was subsequently reported by Duan et al. [51] that AAV2 capsids become ubiquitinated in the cytoplasm, where they are targeted for degradation by the host cell proteasomal machinery, thereby negatively impacting the transduction efficiency of the first generation of AAV vectors. One of the major obstacles that limit the transduction efficiency of AAV vectors in general is ubiquitination, followed by proteasomal-mediated degradation. Previously, Mah et al. [52] had observed that inhibition of the host cell epidermal growth factor receptor protein tyrosine kinase (EGFR-PTK) resulted in a significant increase in the transduction efficiency of AAV2 vectors. Thus, it was hypothesized that following infection, the AAV2 capsid protein becomes phosphorylated at surface-exposed tyrosine residues by EGFR-PTK, and that tyrosine phosphorylation leads to ubiquitination, followed by proteasomal degradation of AAV2 vectors in the cytoplasm [51,53]. Indeed, Zhong et al. [54] provided experimental evidence in 2007 to support this hypothesis. These studies provided the impetus to mutagenize the surface- exposed tyrosine residues in the AAV2 capsid to circumvent this barrier.
There are 7 tyrosine (Y) residues in the AAV2 capsid that are surface-exposed (Y252, Y272, Y444, Y500, Y700, Y704, Y730). Each of these Y residues was mutagenized by Zhong et al. [55] to phenylalanine (F) residues to generate 7 single-mutants (Y252F, Y272F, Y444F, Y500F, Y700F, Y704F, Y730F), the transduction efficiency of 3 of which (Y444F, Y500F, Y730F) was significantly higher than their WT counterpart [56]. The Y730F single-mutant AAV2 vector was the most efficient, the use of which resulted in the expression of therapeutic levels of h.FIX in 3 different strains of mice following intravenous or portal vein administration at 10-fold reduced vector doses [55]. When the 3 most efficient mutations were combined into one capsid, the resulting triple-mutant (Y444+500+730F) vectors showed a further 3-fold increase in F.IX expression in hemophilia B mice [56]. Furthermore, this triple-mutant AAV2 vector was shown to minimizes in vivo targeting of transduced hepatocytes by capsid-specific CD8+ T cells [57].
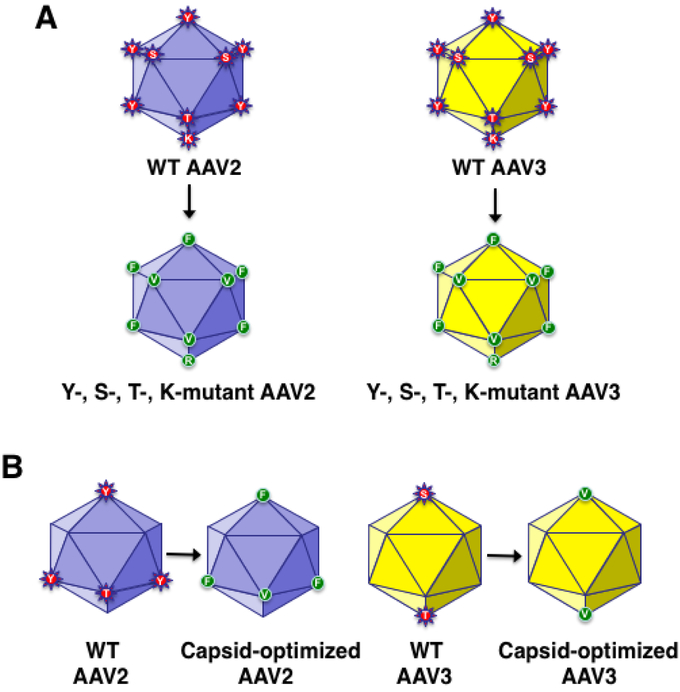
In the quest to develop more efficient and potentially less immunogenic AAV vectors, these studies were extended to include two additional amino acid residues in the AAV capsid that are surface-exposed, and can also be phosphorylated by cellular serine/threonine protein kinases. For example, in addition to 7 tyrosine (Y) residues, the AAV2 capsid also contains 17 surface- exposed serine (S), and 15 surface-exposed threonine (T) residues, each of which were mutagenized, and AAV2 vectors containing various permutations and combinations thereof, depicted schematically in Figure 2A, were generated [58,59]. In addition, since ubiquitination occurs on lysine (K) residues, all 7 surface-exposed residues in the AAV2 capsid were mutagenized, and a limited numbers of Y+S+T+K-mutant AAV2 vectors were generated [60].
FIGURE 2: Schematic representation of capsid-modified next generation of recombinant AAV vectors.
Surface-exposed, specific tyrosine (Y), serine (S), and threonine (T) residues on AAV capsids can be phosphorylated, which is a signal for ubiquitination. Surface-exposed, specific lysine (K) residues on AAV capsids can be ubiquitinated, and subsequently degraded by the host cell proteasome machinery. Site-directed mutagenesis of these residues leads to the generation of AAV vectors that are more efficient at reduced vector doses, and consequently, less immunogenic. Specific examples of the most efficient AAV2 [55-57,59,61], and AAV3 [42,43,47,61,63], serotype vectors generated thus far, are also depicted. AAV2 data from Zhong L, Li B, Mah CS, et al.. Next generation of adeno-associated virus 2 vectors: Point mutations in tyrosines lead to high-efficiency transduction at lower doses. Proc Natl Acad Sci USA 2008;105:7827-32, Markusic DM, Herzog RW, Aslanidi GV, et al.. High-efficiency transduction and correction of murine hemophilia B using AAV2 vectors devoid of multiple surface-exposed tyrosines. Mol Ther 2010;18:2048-56, Martino AT, Basner-Tschakarjan E, Markusic DM, et al.. Engineered AAV vector minimizes in vivo targeting of transduced hepatocytes by capsid-specific CD8+ T cells. Blood 2013;121:2224-33, Aslanidi GV, Rivers AE, Ortiz L, et al.. Optimization of the capsid of recombinant adeno-associated virus 2 (AAV2) vectors: The final threshold? PLoS One 2013;8:e59142, and Ling C, Li B, Ma W, et al.. Development of optimized AAV serotype vectors for high-efficiency transduction at further reduced doses. Hum Gene Ther Meth 2016;27:143-9, with permission. AAV3 data from Cheng B, Ling C, Dai Y, et al.. Development of optimized AAV3 serotype vectors: Mechanism of high-efficiency transduction of human liver cancer cells. Gene Ther 2012; 19:375-84, Ling C, Wang Y, Zhang Y, Ejjigani A, Yin Z, Lu Y, Wang L, Wang M, Li J, Hu Z, Aslanidi GV et al.: Selective in vivo targeting of human liver tumors by optimized AAV3 vectors in a murine xenograft model. Hum Gene Ther (2014) 25(12):1023-1034, Li S, Ling C, Zhong L, Li M, Su Q, He R, Tang Q, Greiner DL, Shultz LD, Brehm MA, Flotte TR et al.: Efficient and targeted transduction of nonhuman primate liver with systemically delivered optimized AAV3b vectors. Mol Ther (2015) 23(12):1867-1876, Ling C, Li B, Ma W, et al.. Development of optimized AAV serotype vectors for high-efficiency transduction at further reduced doses. Hum Gene Ther Meth 2016;27:143-9, and Vercauteren K, Hoffman BE, Zolotukhin I, et al.. Superior in vivo transduction of human hepatocytes using engineered AAV3 capsid. Mol Ther 2016;24:1042-49 with permission.
Interestingly, however, most, if not all, of the surface-exposed Y, S, T, and K residues are highly conserved among all 10 commonly used AAV serotype vectors, and most of these residues have also been mutagenized in each of 10 AAV serotype vectors. The corresponding Y-, S-, T-, and K-mutants of AAV3 vectors are also shown in Figure 2A. A quadruple-mutant (Y444+500+730F+T491V) for AAV2, and a double-mutant (S663V+T492V) for AAV3, shown schematically in Figure 2B, have been identified to be the most efficient in transducing the normal and murine xenograft models, respectively [61].
In addition to the WT AAV3 vectors, Li et al. [47] also evaluated the safety and efficacy of the S663V+T492V double-mutant AAV3 (AAV3.ST) vectors in NHPs. The results revealed that the transduction efficiency of this capsid-modified vector was ~5-fold higher compared with its WT counterpart, with no apparent vector-related toxicity. Thus, the use of capsid-modified next generation of AAV vectors is likely to overcome some of the limitations associated with the first generation of AAV vectors.
AAV3 vectors and “humanized” mouse models
In 2014, Lisowski et al. [45] reported that a shuffled rAAV vector, designated LK-03, which shares 97.7 and 98.9% homology with rAAV3 at the DNA and amino acids level, respectively, transduced human primary hepatocytes in a humanized mouse model in vivo, and it was ~20-fold more efficient than AAV8. In studies performed by Li et al. [47] with a different humanized liver xenograft mouse model [62], it was observed that AAV3 was ~12- fold more efficient than AAV8 in transducing human hepatocytes in vivo [14]. In a more recent study, Vercauteren et al. [63] compared the transduction efficiencies of AAV3-ST vectors with AAV8 and AAV5 vectors, that are currently being used in gene therapy of hemophilia [Table 1]. In this humanized mouse model, it was reported that rAAV3-ST vectors were ~8-times more efficient than rAAV8 vectors, and ~82-times more efficient than rAAV5 vectors in transducing primary human hepatocytes [63]. Although the ultimate efficacy of AAV3 vectors will only be revealed by clinical trials in humans, it appears likely that these vectors will prove to be safer and more efficient than AAV8 and AAV5 vectors in targeting human liver diseases in general, and gene therapy of hemophilia in particular.
Table 1:
First generation of recombinant AAV serotype vectors used/being used for the potential gene therapy of hemophilia.
| Investigators/ Sponsors |
Vector | Dose | Expression level |
Total dose* | Ref. | |
|---|---|---|---|---|---|---|
| Hemophilia B | High/Kay | ssAAV2 | 8×1010 vgs/kg 4×1011 vgs/kg 2×1012 vgs/kg |
0% 0% 11%➔0% |
5.6 Trillion 28 Trillion 140 Trillion |
[36] |
| Nathwani/ Davidoff |
scAAV8 | 2×1011 vgs/kg 6×1011 vgs/kg 2×1012 vgs/kg |
2% 2-4% 8-12%➔5% |
14 Trillion 42 Trillion 140 Trillion |
[24,25] | |
| Baxalta** | scAAV8 | 2×1011 vgs/kg 1×1012 vgs/kg 3×1012 vgs/kg |
2-5% 20-25% 50%➔?% |
14 Trillion 70 Trillion 210 Trillion |
[65,69] | |
| Spark Therapeutics |
Modified ssAAV8 |
5×1011 vgs/kg | 28-41 %➔26-33% | 35 Trillion | [25] | |
| Dimension Therapeutics*** |
ssAAVrh10 | 1.6×1012 vgs/kg 3×1012 vgs/kg |
<2% <2% |
112 Trillion 210 Trillion |
[66 ] | |
| uniQure | scAAV5 | 5×1012 vgs/kg 2×1013 vgs/kg |
3-6.8% 3-12.7% |
350 Trillion 1.4 Quadrillion |
[26] | |
| Hemophilia A | BioMarin | ssAAV5 | 2×1013 vgs/kg 6×1013 vgs/kg |
2-5% 50-200%➔32-59% |
1.4 Quadrillion 4.2 Quadrillion |
[27,67] |
| Spark Therapeutics |
ssLK-03 | 5×1011 vgs/kg 1×1012 vgs/kg 2×1012 vgs/kg |
6-37% 7-24% 16-49%**** |
35 Trillion 70 Trillion 140 Trillion |
[68] |
Based on an average patient’s weight of 70 kg (Estimated number of cells in a 70 kg “reference man” = 3 x 1013, or 30 Trillion) Data from Sender R, Fuchs S, Milo R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol 2016;14:e1002533 with permission.
Acquired by Shire.
Acquired by Ultragenyx.
Serious adverse events in 2 patients.
Gene therapy of hemophilia with AAV vectors
Table 1 lists all clinical trials in which various AAV serotypes and their variants that have been used, or are currently being used, in the potential gene therapy of both hemophilia B and hemophilia A. As stated above, following the short-lived expression h.FIX mediated by the first generation AAV2 vectors [37], the use of AAV8 vectors proved to be successful [23]. However, the h.FIX levels declined from ~8-12% to ~5% over a 3-year follow-up period [24]. Although the superior performance of AAV8 vectors in humans appeared to parallel that observed in murine models, the vector genome used was double-stranded self-complementary (sc), in contrast to that used in the first clinical trial with AAV2 vectors, which was single-stranded (ss), and ssAAV DNA is known to transcriptionally-inactive, and viral second strand-DNA synthesis is known to be a rate-limiting step during AAV vector-mediated transgene expression. [64]. Thus, it appears that the use of scAAV genome, rather than the AAV8 serotype, contributed to the successful outcome since AAV8 vectors transduce human hepatocytes less efficiently than mouse hepatocytes [47,48,63]. The next trial, sponsored by Baxalta, also with scAAV8 vectors, yielded inconsistent results, and as a consequence, this trial was halted [65]. Interestingly, however, the trial sponsored by Spark Therapeutics, using a capsid-modified ssAAV vector, has led to therapeutic levels of h.FIX at relatively low doses [25]. The trial sponsored by Dimension Therapeutics using ssAAVrh10 vectors failed to lead to expression therapeutic levels of h.FIX, even at significantly high doses and a consequence, this trial was also halted [66]. Using scAAV5 vectors, uniQure recently reported modest levels of expression of h.FIX, however, enormously high vector doses were required [26]. (Table 1)
The results of two Phase I clinical trials for gene therapy of hemophilia A have also been reported. In the Biomarin-sponsored trial with ssAAV5 vectors, h.FVTII levels ranging from ~50- 200% were achieved [27]. However, there has been a steady decline over time, ranging between ~32-59% [67]. More recently, Spark Therapeutics reported that with the use of ssLK-03, a shuffled ssAAV vector with significant homology to AAV3, h.FVIII levels ranging from ~16- 49% were achieved, but at the highest vector dose, two patients experienced severe adverse events [68].
Taken together, the following conclusions can be drawn: (i) The tropism, safety, and efficacy of AAV vectors in animal models do not necessarily translate well in humans; (ii) The use of AAV vectors composed of naturally occurring capsids are likely to induce immune responses, especially at high doses since the host immune system cannot distinguish between AAV as a virus versus AAV as a vector; (iii) The host immune response is directly correlated with the AAV vector dose; and (iv) Since the wild-type AAV did not evolve for the purposes of delivery of therapeutic genes, recombinant AAV vectors composed of naturally occurring capsid are unlikely to be optimal in human clinical trials [31]. Overall, our prediction is that, in contrast to the high vector doses that are currently being used with AAV8 and AAV5 vectors for hemophilia B and hemophilia A, respectively, the optimized AAV3 serotype vectors, in addition to being far more efficacious, will also offer the potential advantages of being less immunogenic, and more cost- effective, for their use in human liver diseases in general, and hemophilia in particular [4].
KEY POINTS.
The first generation of recombinant AAV vectors have shown efficacy in a number of Phase I/II/III clinical trials targeting various human diseases, but the host immune response at high vector doses remains a challenge.
The tissue-tropism of AAV vectors observed in animal models does not always correlate well with that in humans.
A specific AAV serotype, AAV3, has been identified with which targeted delivery as well as high-efficiency transduction of human hepatocytes can be achieved.
Next generation of AAV3 vectors have also been developed that are likely to circumvent most, if not all, of the problems associated with the first generation of AAV vectors in general.
Further optimized AAV3 vectors should prove safe and effective for the potential gene therapy of human liver diseases in general, and hemophilia in particular.
SYNOPSIS.
Recombinant vectors based on a non-pathogenic parvovirus, the adeno-associated virus (AAV) have taken center stage in the past decade. The safety of AAV vectors in 165 Phase I/II/III clinical trials in humans to date, and clinical efficacy in several human diseases, are now well documented. Despite these remarkable achievements, it is becoming increasingly clear that the full potential of AAV vectors composed of the naturally occurring capsids is unlikely to be realized. In this article, we describe the advances that have been made, and the challenges that remain, in the optimal use of AAV vectors in human gene therapy applications. More specifically, we provide an account of the progress that has been made in the development of AAV vectors to ensure both safety and efficacy of these vectors in targeting human liver diseases in general, and hemophilia in particular.
Acknowledgements
The authors gratefully acknowledge their colleagues and collaborators, both past and present, for helpful scientific discussions. This work was supported in part by Public Health Service grants R01 HL-097088, R41 AI-122735, and R21 EB-015684 from the National Institutes of Health; a grant from the Children’s Miracle Network; and support from the Kitzman Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE STATEMENT
Dr. Berns is a board member of Lacerta Therapeutics, a recently launched AAV gene therapy company. Dr. Srivastava is a co-founder of, and holds equity in, Lacerta Therapeutics, aaVective, and Nirvana Therapeutics, and is an inventor on several issued patents on recombinant AAV vectors that have been licensed to various gene therapy companies.
References
- 1.Samulski RJ, Muzyczka N. AAV-mediated gene therapy for research and therapeutic purposes. Annu Rev Virol 2014;1:427–51. [DOI] [PubMed] [Google Scholar]
- 2.Muzyczka N, Berns KI. AAV’s golden jubilee. Mol Ther 2015;23:807–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grimm D, Zolotukhin S. E Pluribus Unum: 50 years of research, millions of viruses, and one goal - Tailored acceleration of AAV evolution. Mol Ther 2015;23:1819–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Srivastava A Advances and challenges in the use of recombinant adeno-associated virus vectors for human gene therapy. Cell and Gene Ther Insights 2016;2:553–75. [Google Scholar]
- 5.Berns KI, Muzyczka N. AAV: An overview of unanswered questions. Hum Gene Ther 2017;28:308–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weinmann J, Grimm D. Next-generation AAV vectors for clinical use: An ever- accelerating race. Virus Genes 2017;53:707–13. [DOI] [PubMed] [Google Scholar]
- 7.Bainbridge JW, Smith AJ, Barker SS, et al. Effect of gene therapy on visual function in Leber’s congenital amaurosis. N Engl J Med 2008;358:2231–39. [DOI] [PubMed] [Google Scholar]
- 8.Maguire AM, Simonelli F, Pierce EA, et al. Safety and efficacy of gene transfer for Leber’s congenital amaurosis. N Engl J Med 2008;358:2240–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cideciyan AV, Aleman TS, Boye SL, et al. Human gene therapy for RPE65 isomerase deficiency activates the retinoid cycle of vision but with slow rod kinetics. Proc Natl Acad Sci USA 2008;105:15112–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hauswirth WW, Aleman TS, Kaushal S, et al. Treatment of Leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: Short-term results of a phase I trial. Hum Gene Ther 2008;19:979–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hwu WL, Muramatsu S, Tseng SH, et al. Gene therapy for aromatic L-amino acid decarboxylase deficiency. Sci Transl Med 2012;4:134ra61. [DOI] [PubMed] [Google Scholar]
- 12.MacLaren RE, Groppe M, Barnard AR, et al. Retinal gene therapy in patients with choroideremia: Initial findings from a phase 1/2 clinical trial. Lancet 2014;383:1129–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xiao W, Chirmule N, Berta SC, et al. Gene therapy vectors based on adeno-associated virus type 1. J Virol 1999;73:3994–03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muramatsu S, Mizukami H, Young NS, et al. Nucleotide sequencing and generation of an infectious clone of adeno-associated virus 3. Virol 1996;221:208–17. [DOI] [PubMed] [Google Scholar]
- 15.Chiorini JA, Yang L, Liu Y, et al. Cloning of adeno-associated virus type 4 (AAV4) and generation of recombinant AAV4 particles. J Virol 1997;71:6823–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bantel-Schaal U, Delius H, Schmidt R, et al. Human adeno-associated virus type 5 is only distantly related to other known primate helper-dependent parvoviruses. J Virol 1999; 73:939–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rutledge EA, Halbert CL, Russell DW. Infectious clones and vectors derived from adeno- associated virus (AAV) serotypes other than AAV type 2. J Virol 1998;72:309–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao GP, Alvira MR, Wang L, et al. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc Natl Acad Sci USA 2002;99:11854–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mori S, Wang L, Takeuchi T, et al. Two novel adeno-associated viruses from cynomolgus monkey: Pseudotyping characterization of capsid protein. Virol 2004;330:375–83. [DOI] [PubMed] [Google Scholar]
- 20.Schmidt M, Voutetakis A, Afione S, et al. Adeno-associated virus type 12 (AAV12): A novel AAV serotype with sialic acid- and heparan sulfate proteoglycan-independent transduction activity. J Virol 2008;82:1399–06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmidt M, Govindasamy L, Afione S, et al. Molecular characterization of the heparin- dependent transduction domain on the capsid of a novel adeno-associated virus isolate, AAV(VR-942). J Virol 2008;82:8911–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gaudet D, Methot J, Dery S, et al. Efficacy and long-term safety of alipogene tiparvovec (AAV1-LPLs447x) gene therapy for lipoprotein lipase deficiency: An open-label trial. Gene Ther 2013;20:361–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nathwani AC, Tuddenham EG, Rangarajan S, et al. Adenovirus-associated virus vector- mediated gene transfer in hemophilia B. N Engl J Med 2011;365:2357–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nathwani AC, Reiss UM, Tuddenham EG, et al. Long-term safety and efficacy of factor IX gene therapy in hemophilia B. N Engl J Med 2014;371:1994–04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.George LA, Sullivan SK, Giermasz JEJ, et al. Hemophilia B gene therapy with a high- specific-activity factor IX variant. New Engl J Med 2017;377:2215–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miesbach W, Meijer K, Coppens M, et al. Gene therapy with adeno-associated virus vector 5-human factor IX in adults with hemophilia B. Blood 2018;131:1022–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rangarajan S, Walsh L, Lester W, et al. AAV5-Factor VIII gene transfer in severe hemophilia A. New Engl J Med 2017;377:2519–30. [DOI] [PubMed] [Google Scholar]
- 28.Byrne PI, Collins S, Mah CS, et al. Phase I/II trial of diaphragm delivery of recombinant adeno-associated virus acid alpha-glucosidase (rAAV1-CMV-GAA) gene vector in patients with Pompe disease. Hum Gene Ther Clin Dev 2014;25:134–63. [DOI] [PubMed] [Google Scholar]
- 29.Mendell JR, Al-Zaidy S, Shell R, WD, et al. , Single-dose gene replacement therapy for spinal muscular atrophy, N Engl J Med 2017;377:1713–22. [DOI] [PubMed] [Google Scholar]
- 30.Ling C, Zhong L, Srivastava A. Adeno-associated viral vectors in gene therapy. In: eLS John Wiley & Sons, Ltd: Chichester; 2018. DOI: 10.1002/9780470015902.a0005738. [DOI] [Google Scholar]
- 31.Srivastava A Adeno-associated virus: The naturally occurring virus versus the recombinant vector. Hum Gene Ther (2016) 27(1): 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ponnazhagan S, Mukherjee P, Yoder MC, et al. Adeno-associated virus 2-mediated gene transfer in vivo: Organ-tropism and expression of transduced sequences in mice. Gene 1997;190:203–10. [DOI] [PubMed] [Google Scholar]
- 33.Koeberl DD, Alexander IE, Halbert CL, et al. Persistent expression of human clotting factor IX from mouse liver after intravenous injection of adeno-associated virus vectors. Proc Natl Acad Sci USA 1997;94:1426–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Snyder RO, Miao CH, Patijn GA, et al. Persistent and therapeutic concentrations of human factor IX in mice after hepatic gene transfer of recombinant AAV vectors. Nat Genet 1997; 16:270–6. [DOI] [PubMed] [Google Scholar]
- 35.Chao H, Samulski R, Bellinger D, et al. Persistent expression of canine factor IX in hemophilia B canines. Gene Ther 1990;6:1695–704. [DOI] [PubMed] [Google Scholar]
- 36.Manno CS, Pierce GF, Arruda VR, et al. Successful transduction of liver in hemophilia by AAV-factor IX and limitations imposed by the host immune response. Nat Med 2006; 12:342–7. [DOI] [PubMed] [Google Scholar]
- 37.Mingozzi F, Maus MV, Hui DJ, et al. CD8(+) T-cell responses to adeno-associated virus capsid in humans. Nat Med 2007;13:419–22. [DOI] [PubMed] [Google Scholar]
- 38.Srivastava A In vivo tissue-tropism of adeno-associated viral vectors. Curr Opin Virol 2016;21:75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sarkar R, Tetreault R, Gao G, et al. Total correction of hemophilia A mice with canine FVIII using an AAV8 serotype. Blood 2004;103:1253–60. [DOI] [PubMed] [Google Scholar]
- 40.Thomas CE, Storm TA, Huang Z, et al. Rapid uncoating of vector genomes is the key to efficient liver transduction with pseudotyped adeno-associated virus vectors. J Virol 2004; 78:3110–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Glushakova LG, Lisankie MJ, Eruslanov EB, et al. AAV3-mediated transfer and expression of the pyruvate dehydrogenase E1 alpha subunit gene causes metabolic remodeling and apoptosis of human liver cancer cells. Mol Genet Metab 2009;98:289–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheng B, Ling C, Dai Y, et al. Development of optimized AAV3 serotype vectors: Mechanism of high-efficiency transduction of human liver cancer cells. Gene Ther 2012; 19:375–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ling C, Wang Y, Zhang Y, Ejjigani A, Yin Z, Lu Y, Wang L, Wang M, Li J, Hu Z, Aslanidi GV et al. : Selective in vivo targeting of human liver tumors by optimized AAV3 vectors in a murine xenograft model. Hum Gene Ther (2014) 25(12):1023–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ling C, Lu Y, Kalsi JK, Jayandharan GR, Li B, Ma W, Cheng B, Gee SW, McGoogan KE, Govindasamy L, Zhong L et al. : Human hepatocyte growth factor receptor is a cellular coreceptor for adeno-associated virus serotype 3. Hum Gene Ther (2010) 21(12): 1741–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lisowski L, Dane AP, Chu K, Zhang Y, Cunningham SC, Wilson EM, Nygaard S, Grompe M, Alexander IE, Kay MA: Selection and evaluation of clinically relevant AAV variants in a xenograft liver model. Nature (2014) 506(7488):382–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zincarelli C, Soltys S, Rengo G, Rabinowitz JE. Analysis of AAV serotypes 1-9 mediated gene expression and tropism in mice after systemic injection. Mol Ther. 2008. June;16(6):1073–80 Human hepatocyte growth factor receptor is a cellular coreceptor for adeno-associated virus serotype 3. [DOI] [PubMed] [Google Scholar]
- 47.Li S, Ling C, Zhong L, Li M, Su Q, He R, Tang Q, Greiner DL, Shultz LD, Brehm MA, Flotte TR et al. : Efficient and targeted transduction of nonhuman primate liver with systemically delivered optimized AAV3b vectors. Mol Ther (2015) 23(12):1867–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang L, Bell P, Somanathan S, Wang Q, He Z, Yu H, McMenamin D, Goode T, Calcedo R, Wilson JM: Comparative study of liver gene transfer with AAV vectors based on natural and engineered aav capsids. Mol Ther (2015) 23(12):1877–1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Srivastava A Advances and challenges in the use of recombinant adeno-associated virus vectors for human gene therapy. Cell & Gene Therapy Insights (2016) 2: 553–575. [Google Scholar]
- 50.Hansen J, Qing K, Kwon HJ, Mah C, Srivastava A: Impaired intracellular trafficking of adeno-associated virus type 2 vectors limits efficient transduction of murine fibroblasts. J Virol (2000) 74(2):992–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Duan D, Yue Y, Yan Z, Yang J, Engelhardt JF: Endosomal processing limits gene transfer to polarized airway epithelia by adeno-associated virus. J Clin Invest (2000) 105(11): 1573–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mah C, Qing K, Khuntirat B, Ponnazhagan S, Wang XS, Kube DM, Yoder MC, Srivastava A: Adeno-associated virus type 2-mediated gene transfer: Role of epidermal growth factor receptor protein tyrosine kinase in transgene expression. J Virol (1998) 72(12):9835–9843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhong L, Li B, Jayandharan G, Mah CS, Govindasamy L, Agbandje-McKenna M, Herzog RW, Weigel-Van Aken KA, Hobbs JA, Zolotukhin S, Muzyczka N et al. : Tyrosine- phosphorylation of AAV2 vectors and its consequences on viral intracellular trafficking and transgene expression. Virology (2008) 381(2):194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhong L, Zhao W, Wu J, et al. A dual role of EGFR protein tyrosine kinase signaling in ubiquitination of AAV2 capsids and viral second-strand DNA synthesis. Mol Ther 2007; 15:1323–30. [DOI] [PubMed] [Google Scholar]
- 55.Zhong L, Li B, Mah CS, et al. Next generation of adeno-associated virus 2 vectors: Point mutations in tyrosines lead to high-efficiency transduction at lower doses. Proc Natl Acad Sci USA 2008;105:7827–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Markusic DM, Herzog RW, Aslanidi GV, et al. High-efficiency transduction and correction of murine hemophilia B using AAV2 vectors devoid of multiple surface-exposed tyrosines. Mol Ther 2010;18:2048–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Martino AT, Basner-Tschakarjan E, Markusic DM, et al. Engineered AAV vector minimizes in vivo targeting of transduced hepatocytes by capsid-specific CD8+ T cells. Blood 2013;121:2224–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aslanidi GV, Rivers AE, Ortiz L, et al. High-efficiency transduction of human monocyte- derived dendritic cells by capsid-modified recombinant AAV2 vectors. Vaccine 2012; 30:3908–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Aslanidi GV, Rivers AE, Ortiz L, et al. Optimization of the capsid of recombinant adeno-associated virus 2 (AAV2) vectors: The final threshold? PLoS One 2013;8:e59142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li B, Ma W, Ling C, et al. Site-directed mutagenesis of surface-exposed lysine residues leads to improved transduction by AAV2, but Not AAV8, vectors in murine hepatocytes in vivo. Hum Gene Ther Meth 2015;26:211–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ling C, Li B, Ma W, et al. Development of optimized AAV serotype vectors for high- efficiency transduction at further reduced doses. Hum Gene Ther Meth 2016;27:143–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Borel F, Tang Q, Gernoux G, et al. Survival advantage of both human hepatocyte xenografts and genome-edited hepatocytes for treatment of α-1 antitrypsin deficiency. Mol Ther 2017;25:2477–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vercauteren K, Hoffman BE, Zolotukhin I, et al. Superior in vivo transduction of human hepatocytes using engineered AAV3 capsid. Mol Ther 2016;24:1042–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McCarty DM, Monahan PE, Samulski RJ. Self-complementary recombinant adeno- associated virus (scAAV) vectors promote efficient transduction independently of DNA synthesis. Gene Ther 2001;8:1248–54. [DOI] [PubMed] [Google Scholar]
- 65.Terry M Shire kills Baxalta’s hemophilia B program; clears path for BioMarin, Spark Therapeutics and uniQure. Avaialble at: https://www.biospace.com/article/shire-kills-baxalta-s-hemophilia-b-program-clears-path-for-biomarin-spark-therapeutics-and-uniqure-/.
- 66.Semedo D Dimension to End Development of DTX101 as Gene Therapy for Hemophilia B. Available at: https://hemophilianewstoday.com/2017/05/15/dimension-therapeutics-to-end-hemophilia-b-gene-therapy-dtx101-development/.
- 67.Gatlin A Why Analysts Remain Bullish On This Hemophilia Gene-Therapy Play. Available at: https://www.investors.com/news/technology/biomarin-pharmaceutical-hemophilia-a-gene-therapy/.
- 68.Pagliarulo N Spark sheds $1B in value on hemophilia gene therapy data. Available at: https://www.biopharmadive.com/news/spark-gene-therapy-hemophilia-data-stock/529526/.
- 69.Herzog RW. A cure for hemophilia: The promise becomes a reality. Mol Ther 2016. 24:1503–04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sender R, Fuchs S, Milo R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol 2016;14:e1002533. [DOI] [PMC free article] [PubMed] [Google Scholar]