Abstract
It was recently proposed that in Jurkat cells, after inhibition of respiration by NO, glycolytically generated ATP plays a critical role in preventing the collapse of mitochondrial membrane potential (Δψm) and thus apoptotic cell death. We have investigated this observation further in primary cultures of rat cortical neurons and astrocytes—cell types that differ greatly in their glycolytic capacity. Continuous and significant (≈85%) inhibition of respiration by NO (1.4 μM at 175 μM O2) generated by [(z)-1-[2-aminoethyl]-N-[2-ammonioethyl]amino]diazen-1-ium-1,2 diolate (DETA-NO) initially (10 min) depleted ATP concentrations by ≈25% in both cell types and increased the rate of glycolysis in astrocytes but not in neurons. Activation of glycolysis in astrocytes, as judged by lactate production, prevented further ATP depletion, whereas in neurons, which do not invoke this mechanism, there was a progressive decrease in ATP concentrations over the next 60 min. During this time, there was a persistent mitochondrial hyperpolarization and absence of apoptotic cell death in astrocytes, whereas in the neurons there was a progressive fall in Δψm and increased apoptosis. After glucose deprivation or treatment with inhibitors of the F1F0-ATPase and adenine nucleotide translocase, astrocytes responded to NO with a fall in Δψm and apoptotic cell death similar to the response in neurons. Finally, although treatment of astrocytes with NO partially prevented staurosporin-induced collapse in Δψm and cell death, NO and staurosporin synergized in decreasing Δψm and inducing apoptosis in neurons. These results demonstrate that although inhibition of cellular respiration by NO leads to neurotoxicity, it may also result in initial neuroprotection, depending on the glycolytic capacity of the particular cell.
Keywords: glycolysis‖mitochondrial membrane potential‖apoptosis‖ oxygen consumption‖neurodegeneration
Nitric oxide (NO) is a physiological messenger (1) that has been reported either to cause or prevent cellular apoptotic death (2, 3). The mitochondrion is now emerging as a key organelle playing a role in the NO-mediated apoptotic pathway. Disruption of the membrane potential across the mitochondrial inner membrane (Δψm) dissipates the electrochemical gradient necessary for ATP synthesis (4). Moreover, Δψm collapse is associated with mitochondrial swelling, disruption of the outer mitochondrial membrane, and the release of proapoptotic factors such as cytochrome c, apoptosis-inducing factor, and some procaspases (5, 6) from the intermembrane space. NO inhibits the activity of certain components of the mitochondrial respiratory chain, including cytochrome c oxidase (reviewed in ref. 7), and it has recently been shown that endogenous NO formation in primary neurons triggers a rapid and transient ATP depletion associated with Δψm collapse and apoptosis (8).
Beltrán et al. (9), however, have shown in Jurkat cells that inhibition of mitochondrial respiration by NO induces mitochondrial hyperpolarization. They suggested that this phenomenon may be associated with protection from apoptotic death. Furthermore, these authors suggested that glycolytically generated ATP is required to maintain the Δψm (9). This finding may explain why treatment of astrocytes with lipopolysaccharide and IFN-γ, which induces, among other things, the generation of NO, inhibited cytochrome c oxidase activity (10), and stimulated the rate of glycolysis, whereas no signs of cell death were detected (11). Thus, the different susceptibility of cells to NO-mediated apoptosis may be a function of the ability of the cells to increase their glycolytic activity after inhibition of mitochondrial respiration by NO.
We have now carried out further studies in neurons in which inhibition of mitochondrial respiration is known to be accompanied by mitochondrial depolarization (12–14) and have compared their responses to NO with those of astrocytes. We have found that NO-mediated inhibition of cellular respiration is followed by mitochondrial depolarization and cell death in neurons but is followed by hyperpolarization in astrocytes. Furthermore, we show that an increase in Δψm at the expense of glycolytically generated ATP prevents apoptotic death in astrocytes.
Materials and Methods
Reagents.
DMEM, poly(d-lysine), horse serum, cytosine arabinoside, carbonyl cyanide p[-trifluoromethoxy] phenyl hydrazone (FCCP), oligomycin, staurosporin, and propidium iodide (PI) were obtained from Sigma. DMEM was always supplemented with penicillin (100 units/ml), streptomycin (100 μg/ml), and amphotericin B (0.25 μg/ml) (Sigma). FCS was purchased from Roche Diagnostics (Heidelberg, Germany). DETA-NO ([(z)-1-[2-aminoethyl]-N-[2-ammonioethyl]amino]diazen-1-ium-1,2 diolate) was obtained from Alexis (San Diego). JC-1 (5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine iodide) was purchased from Molecular Probes. Bongkrekic acid was from Calbiochem. Other substrates, enzymes, and coenzymes were purchased from Sigma, Roche Diagnostics, or Merck. Plastic tissue culture flasks were purchased from Nunc.
Primary Culture of Astrocytes.
Astrocytes in primary culture were prepared from the forebrains of neonatal (24-h-old) Wistar rats. Cell suspensions were obtained as described (15) and were seeded at a density of 1.2 × 105 cells per cm2 in 175-cm2 flasks in culture medium [DMEM supplemented with 10% (vol/vol) FCS]. Cells were incubated at 37°C under a humidified 5% CO2-containing atmosphere for 12–14 days. Under these conditions, astrocytes represented ≈85% of the cells, whereas the remaining proportion of cells comprised microglia and progenitor cells, as determined immunocytochemically (16).
Primary Culture of Neurons.
Cerebral cortex neurons in primary culture were prepared from fetal rats at 16–17 days of gestation (16). Dissociated cell suspensions were plated at a density of 2.5 × 105 cells per cm2 in 175-cm2 flasks previously coated with poly(d-lysine) (15 μg/ml) in DMEM supplemented with 10% FCS. Cells were incubated at 37°C in a humidified atmosphere containing 5% CO2/95% air. Forty-eight hours after plating, the medium was replaced with DMEM supplemented with 5% horse serum and 20 mM d-glucose. On day 4 of culture, cytosine arabinoside (10 μM) was added to prevent nonneuronal proliferation, and neurons were used on day 9, when ≈99% of the cells showed neuronal neurofilament-positive staining (16).
Cell Incubations.
Cells were removed from the flasks by mild trypsinization and resuspended in prewarmed (37°C) buffered Hanks' solution (5.26 mM KCl/0.43 mM KH2PO4/132.4 mM NaCl/4.09 mM NaHCO3/0.33 mM Na2HPO4/20 mM glucose/2 mM CaCl2/20 mM Hepes, pH 7.4) at a density of 4 × 106 cells per ml. After a 30–45 min preincubation period at 37°C, the cell suspension was mixed with an equal volume of prewarmed (37°C; 15 min) buffered Hanks' solution containing DETA-NO at a final concentration of 0.5 mM, except for the dose–response experiments in which 0.03, 0.1, 0.5, or 1 mM DETA-NO was used. Preliminary experiments using a NO electrode (ISO-NO, World Precision Instruments, Sarasota, FL) revealed that the amount of NO released by 0.5 mM DETA-NO in buffered Hanks' solution at 37°C was 1.4 μM NO for at least 20 h. The resulting cell suspensions (2 × 106 cells per ml) were incubated at 37°C for the times indicated in the figures (10–60 min). When necessary, both the preincubation and incubation periods were carried out in glucose-free Hanks' solution. In some experiments, incubations of cells with DETA-NO were carried out in the presence of d-fructose (20 mM), bongkrekic acid (10 μM), oligomycin (8 μM), or staurosporin (100 nM).
Measurement of Oxygen Consumption.
A Clark-type electrode (Rank Brothers, Cambridge, U.K.) was used in experiments measuring oxygen consumption. Because NO-mediated inhibition of O2 consumption has been shown to depend strongly on the O2 concentration (17), we always assessed the rate of O2 consumption at an initial O2 concentration of ≈200 μM (saturation) for 15 min; after this period, the final O2 concentration was ≈175 μM. The rate of O2 consumption was calculated from the slopes and expressed as nmoles of O2 consumed per min per 2 × 106 cells.
Metabolite Determinations.
ATP and lactate concentrations were measured in the same experiments. Thus, aliquots of the cell suspensions were acidified with HClO4 and neutralized with KHCO3. Lactate concentrations were measured in the supernatants spectrophotometrically as described by Gutmann and Wahlefeld (18), and ATP concentrations by chemiluminescence using a commercially available kit (Sigma) following the manufacturer's instructions. Glycogen concentrations were measured in astrocytes as described (19).
Flow Cytometric Analysis of Mitochondrial Membrane Potential.
To measure the Δψm of both neurons and astrocytes, the fluorescent probe JC-1 was used (20). Thus, cell suspensions were incubated in buffered Hanks' solution containing JC-1 (3 μM) for a 45-min preincubation period, as described (9). After incubation in the presence of DETA-NO for the indicated times, data acquisition was performed in a FACScalibur flow cytometer (Becton Dickinson) equipped with a 15-mW argon ion laser tuned at 488 nm, by using CELLQUEST software (Becton Dickinson). The analyzer threshold was adjusted on the FSC channel to exclude noise and most of the subcellular debris. Photomultiplier settings were adjusted to detect JC-1 monomer fluorescence signals on the FL1 detector (green fluorescence, centered around 525 nm), and JC-1 aggregate fluorescence signals on the FL2 detector (red fluorescence, centered around 590 nm). Data analysis was performed with PAINT-A-GATE PRO software (Becton Dickinson). Mean fluorescence intensity values for FL1 and FL2, expressed as relative linear fluorescence channels (arbitrary units scaled from channel 0 to 10,000) were obtained for all experiments. The relative aggregate/monomer (red/green) fluorescence intensity values were used for data presentation. These values were expressed in a percentage scale, with the red/green fluorescence ratio values of control cells (2.98 ± 0.10 for neurons, 3.11 ± 0.20 for astrocytes) considered as 100% and the red/green fluorescence ratio values of FCCP (5 μM, 5 min)-treated cells (0.68 ± 0.04 for neurons, 0.84 ± 0.05 for astrocytes) considered as 0%.
Flow Cytometric Analysis of Apoptosis.
Early apoptotic cell death was determined after staining with FITC-conjugated annexin-V and PI, by using a commercially available kit (Bender MedSystems) following the manufacturer's instructions. Data acquisition was carried out in a FACScalibur flow cytometer. Annexin-V-stained cells that were PI-negative were considered to be apoptotic.
Statistical Analysis.
Measurements from individual cultures were always performed in triplicate. The results are expressed as mean ± SEM values for three different culture preparations. Statistical analysis of the results was performed by one-way ANOVA followed by the least significant difference multiple range test. In all cases, P < 0.05 was considered significant.
Results
Inhibition of Cellular Respiration by NO Stimulates Glycolysis in Astrocytes but Not in Neurons.
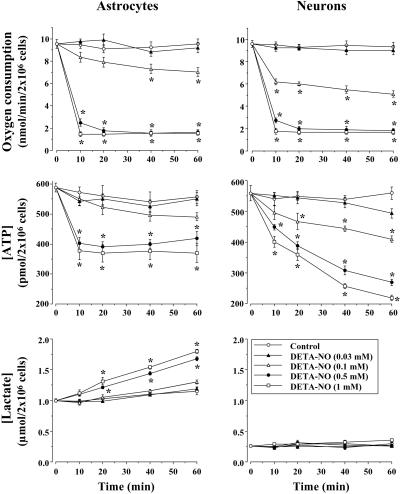
Untreated control astrocytes and neurons were found to consume O2 at a similar rate (Fig. 1). This finding is in agreement with previous results obtained in intact cells or isolated mitochondria (16, 21). Incubation of both of these cell types with the NO donor DETA-NO inhibited, in a dose- and time-dependent manner, the rate of O2 consumption at O2 concentrations ranging between 175 and 200 μM. In both cell types, the concentration of DETA-NO that inhibited respiration by ≈85% was 0.5 mM, which corresponded to a continuous release of NO to maintain a concentration of 1.4 μM NO.
Figure 1.
Inhibition of cellular respiration by NO stimulates glycolysis in astrocytes but not in neurons. Cell suspensions (2 × 106 cells per ml) were incubated at 37°C in buffered Hanks' solution either in the absence (control) or presence of DETA-NO for the indicated times. Oxygen consumption experiments were performed at an initial O2 concentration of ≈200 μM. For ATP and lactate concentrations, aliquots of the cell suspensions were lysed in HClO4, neutralized with KHCO3, and used for metabolite determinations in the supernatants as described in Materials and Methods. *, P < 0.05 versus appropriate control values.
Control ATP concentration (560–590 pmol per 2 × 106 cells) did not differ significantly between the cell types throughout the 60-min incubation period. However, although incubation with 0.1 mM DETA-NO resulted in a significant fall in ATP concentration in neurons, higher concentrations (0.5 and 1 mM) were required to have such an effect in astrocytes. Furthermore, the initial fall in ATP concentration (≈25%) observed in astrocytes 10 min after incubation with DETA-NO (0.5 and 1 mM) remained unchanged for the remainder of the incubation time, whereas neuronal ATP concentrations fell progressively throughout this period (Fig. 1).
Lactate concentrations were determined as an index of glycolytic activity. As shown in Fig. 1, control astrocytes generated more lactate than neurons. DETA-NO (0.5 and 1 mM) caused a significant activation of lactate production in the astrocytes but had no effect on lactate production by neurons at any time point (Fig. 1).
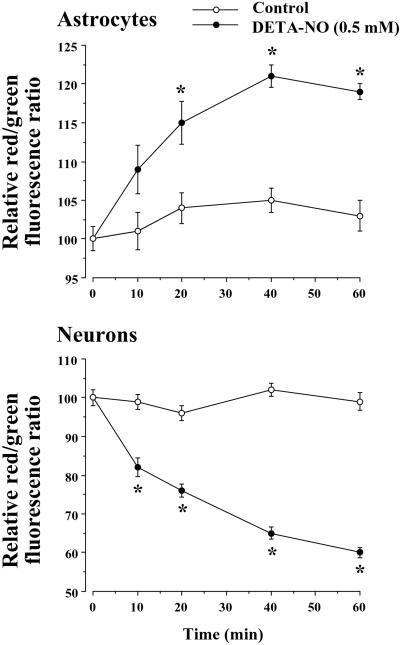
NO Hyperpolarizes Astrocytic Mitochondria, but Depolarizes Neuronal Mitochondria.
Astrocytes and neurons were incubated with a concentration of DETA-NO (0.5 mM) that caused a similar degree of inhibition of cellular respiration in both cell types and Δψm was measured by using flow cytometry with the fluorescent dye JC-1. DETA-NO caused a time-dependent increase (by 20% at 40–60 min) in the red/green fluorescence ratio in astrocytes, indicating hyperpolarization of the mitochondria. In contrast, the same concentration of DETA-NO caused a time-dependent decrease (by 35–40% at 40–60 min) in the red/green fluorescence ratio in neurons, indicating depolarization of the mitochondria (Fig. 2).
Figure 2.
NO hyperpolarizes astrocytic mitochondria, but depolarizes neuronal mitochondria. Cell suspensions (2 × 106 cells per ml) were incubated at 37°C in buffered Hanks' solution either in the absence (control) or presence of DETA-NO (0.5 mM) for the indicated times. In this buffer, 0.5 mM DETA-NO was found to generate a constant NO concentration of 1.4 μM. For Δψm measurements, cell suspensions were centrifuged, and the pellet was resuspended in buffered Hanks' solution containing JC-1 (3 μM) and incubated for a further 10-min at 37°C. Data acquisition was performed in a FACScalibur flow cytometer as detailed in Materials and Methods. Data are expressed as a percentage, with the red/green fluorescence ratio values of untreated cells (2.98 ± 0.10 for neurons, 3.11 ± 0.20 for astrocytes) considered as 100% and the red/green fluorescence ratio values of FCCP (5 μM)-treated cells (0.68 ± 0.04 for neurons, 0.84 ± 0.05 for astrocytes) considered as 0%. *, P < 0.05 versus appropriate control values.
NO-Dependent Glycolytic Activation Determines Mitochondrial Membrane Potential.
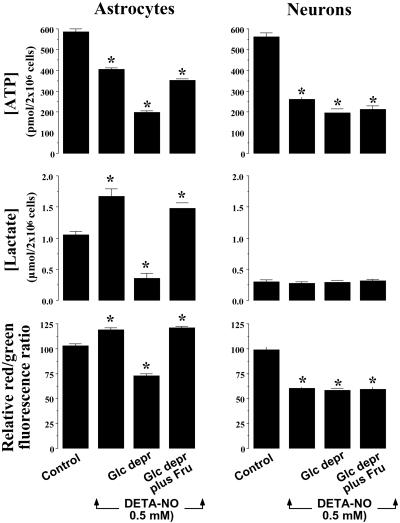
Astrocytes, but not neurons, contain a considerable amount of glycogen (19), the catabolism of which might provide sufficient glucose-1-phosphate for further glycolytic metabolism in these cells. Cells were therefore preincubated for 45 min in glucose-free buffered Hanks' solution, after which glycogen was measured and its content in astrocytes was found to be depleted (in nmols of glucosyl residues per 2 × 106 cells, 45.0 ± 1.0 at t = 0, and 2.0 ± 0.1 at t = 45 min). Glucose deprivation was found to enhance further the NO-mediated decrease in astrocytic ATP concentrations, reaching values similar to those found in DETA-NO-treated neurons (Fig. 3). Moreover, glucose deprivation prevented the NO-mediated increase in lactate concentrations in astrocytes; indeed, such treatment caused a reduction in lactate concentrations in astrocytes to values similar to those found in the neurons (Fig. 3). Glucose deprivation prevented NO-mediated hyperpolarization in astrocytes and instead caused depolarization in these cells (Fig. 3). In contrast, incubation in the absence of glucose had no effect on NO-dependent fall in ATP concentration, lactate production, or mitochondrial depolarization in the neurons (Fig. 3). Finally, glucose-depleted cells were incubated in the presence of fructose, a glycolytic intermediate that, though less efficient than glucose, is a substrate for this metabolic pathway. As shown in Fig. 3, addition of fructose to glucose-deprived astrocytes prevented the enhancement of NO-induced ATP depletion, the lactate depletion, and the mitochondrial depolarization, so that ATP, lactate, and Δψm values were restored to those found in glucose-fed astrocytes. The presence of fructose had no effect on any of these parameters in neurons (Fig. 3).
Figure 3.
NO-dependent glycolytic activation determines mitochondrial membrane potential. Cell suspensions (2 × 106 cells per ml) were incubated at 37°C in buffered Hanks' solution either in the absence (control) or presence of DETA-NO (0.5 mM) for 60 min. For glucose-deprivation experiments (Glc depr), both the preincubation (45 min) and incubation (60 min) periods were carried out in glucose-free Hanks' solution. Under these conditions, the astrocytic content of glycogen was shown to be depleted. Where indicated, cells were incubated in the presence of 20 mM fructose throughout. ATP and lactate concentrations, as well as Δψm, were determined as described in the legends to Figs. 1 and 2. *, P < 0.05 versus appropriate control values.
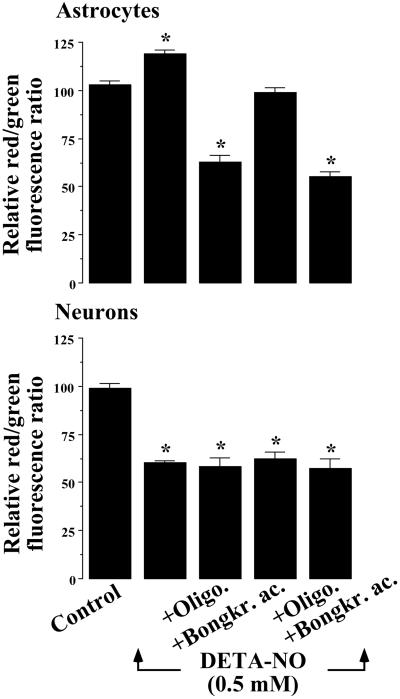
F1F0-ATPase Activity Accounts for NO-Mediated Mitochondrial Hyperpolarization and Prevention from Apoptotic Cell Death.
Glycolytically generated ATP may be hydrolyzed by the F1F0–ATPase-catalyzed reaction, a mechanism that has previously been demonstrated to support membrane potential in isolated mitochondria (22, 23). Cells were therefore incubated with DETA-NO in either the absence or presence of oligomycin, which blocks this enzyme. In astrocytes, treatment with oligomycin prevented DETA-NO mediated mitochondrial hyperpolarization and led instead to depolarization (Fig. 4). Blockade of adenine nucleotide translocase with bongkrekic acid prevented DETA-NO-induced hyperpolarization, whereas the use of both inhibitors together prevented DETA-NO hyperpolarization and resulted in a depolarization comparable to that induced by oligomycin alone (Fig. 4). The presence of these inhibitors did not modify DETA-NO-mediated depolarization in neurons (Fig. 4).
Figure 4.
F1F0-ATPase activity accounts for NO-mediated mitochondrial hyperpolarization. Cell suspensions (2 × 106 cells per ml) were incubated at 37°C in buffered Hanks' solution either in the absence (control) or presence of DETA-NO (0.5 mM) for 60 min. Where indicated, the 60-min incubation period of the cells was carried out in the presence of oligomycin (8 μM), bongkrekic acid (10 μM), or both inhibitors together. Mitochondrial membrane potential measurements were made and expressed as described in the legend to Fig. 2. *, P < 0.05 versus appropriate control values.
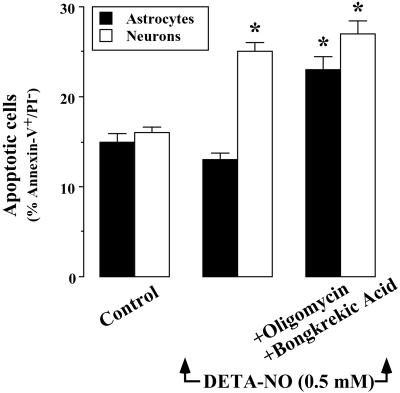
Under control conditions ≈15% of both astrocytes and neurons were annexin V+/PI− (Fig. 5). This considerable degree of apoptosis may be caused by serum withdrawal (9). Incubation of the cells with DETA-NO (0.5 mM; 60 min) did not modify the number of apoptotic astrocytes; however, it significantly increased the number of apoptotic neurons. In the presence of oligomycin plus bongkrekic acid, however, DETA-NO increased the number of apoptotic astrocytes compared to the number observed in neurons. The presence of these inhibitors had no effect on DETA-NO-triggered neuronal apoptosis (Fig. 5).
Figure 5.
F1F0-ATPase activity accounts for NO-mediated prevention from apoptotic cell death. Cell suspensions (2 × 106 cells per ml) were incubated at 37°C in buffered Hanks' solution either in the absence (control) or presence of DETA-NO (0.5 mM) for 60 min. Where indicated, the 60-min incubation period of the cells was carried out in the presence of oligomycin (8 μM) plus bongkrekic acid (10 μM). Apoptotic cell death was determined after staining with FITC-conjugated annexin-V and PI. Data acquisition was carried out in a FACScalibur flow cytometer, as described in Materials and Methods. Annexin V+/PI− cells were considered to be apoptotic. *, P < 0.05 versus appropriate control values.
NO Partially Protects Astrocytes, but Not Neurons, from Staurosporin-Triggered Δψm Collapse and Apoptosis.
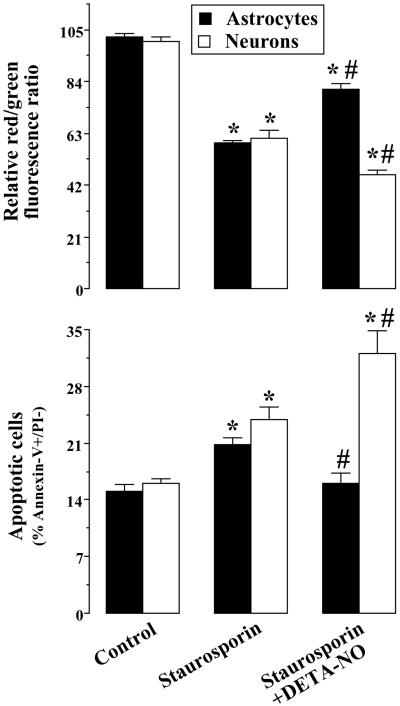
The apoptotic stimulus staurosporin triggered cellular apoptosis in both astrocytes and neurons, an effect that was accompanied by mitochondrial depolarization. Incubation of cells with DETA-NO (0.5 mM) partially, but significantly, prevented both staurosporin-mediated mitochondrial depolarization and apoptotic death in astrocytes. In contrast, under these conditions, neurons underwent further mitochondrial depolarization and apoptotic death (Fig. 6).
Figure 6.
NO partially protects astrocytes, but not neurons, from staurosporin-triggered Δψm collapse and apoptosis. Cell suspensions (2 × 106 cells per ml) were incubated at 37°C in buffered Hanks' solution either in the absence (control) or presence of DETA-NO (0.5 mM) for 60 min. Where indicated, the 60-min incubation period of the cells were carried out in the presence of staurosporin (100 nM). Mitochondrial membrane potential measurements were carried out and expressed as described in the legend to Fig. 2. Apoptotic cell death was determined and expressed as described in the legend to Fig. 5. *, P < 0.05 versus appropriate control values. #, P < 0.05 versus appropriate staurosporin values.
Discussion
Previous work carried out in a variety of biological systems has yielded conflicting results on cellular susceptibility to NO-mediated apoptosis (2, 3). We have now shown that the same degree of inhibition of mitochondrial respiration by NO is proapoptotic in neurons but antiapoptotic in astrocytes. Astrocytes are considered to be “glycolytic” cells because mitochondrial toxins, such as antimycin, strongly stimulate glucose metabolism through the glycolytic pathway, whereas neurons do not invoke glycolysis to maintain ATP production (24, 25).
Our experiments have shown that NO in a concentration- and time-dependent manner inhibited cellular respiration, in good agreement with previous data reported in a range of biological systems (reviewed in refs. 7 and 26). Cellular respiration was rapidly blocked (≈85% of inhibition) in both cell types after incubation with 0.5 mM DETA-NO (1.4 μM NO). This resulted in stimulation of glycolysis in astrocytes, but not in neurons. Furthermore, the observed increased glycolysis in astrocytes was associated with maintenance of ATP concentrations, strongly suggesting that NO-dependent inhibition of cellular respiration up-regulates glycolysis and thus prevents depletion of ATP. We did not investigate the mechanism by which inhibition of mitochondrial respiration leads to glycolytic stimulation; however, it is possible that the low ATP/ADP ratio activates phosphofructokinase 1 (27).
The decrease in ATP concentrations in neurons was accompanied by a collapse in Δψm, in agreement with previous results observed in hippocampal neurons (28). Those authors also showed that high concentrations of NO inhibit glycolysis. However, the lower concentrations that we used in our experiments did not inhibit glycolysis, as determined by production of lactate. Therefore, our results suggest that mitochondrial depolarization alone accounts for the neuronal ATP depletion that we observed. In contrast to neurons, astrocytes maintained ATP concentrations and showed hyperpolarization of mitochondria. Prevention of the up-regulation of glycolysis by glucose deprivation, and the restoration of this by fructose confirms the key role for glycolysis in maintaining the concentration of ATP and the subsequent hyperpolarization of mitochondria.
We further studied the mechanism whereby glycolytically generated ATP supports Δψm. For this purpose, we explored the possible association of this phenomenon with the F1F0-ATPase-catalyzed reaction, which hydrolyzes ATP on inhibition of the mitochondrial respiratory chain (22, 23). Inhibition of the adenine nucleotide translocase activity with bongkrekic acid alone prevented NO-mediated astrocytic mitochondrial hyperpolarization, but did not cause depolarization. This result suggests that an efficient transport of ATP into the mitochondrion is required for the hyperpolarization that follows inhibition of respiration by NO. However, when F1F0-ATPase activity was abolished in these cells with oligomycin, the Δψm collapsed, suggesting that F1F0-ATPase activity is essential for maintenance of Δψm. In neurons, blockade of these mechanisms had no effect on Δψm, suggesting that glycolytically generated ATP is required to enter the mitochondrion where it is hydrolyzed and thus maintains Δψm.
The correlation observed between Δψm and apoptotic death suggests that NO-mediated modulation of Δψm may be a factor controlling the cellular death/survival outcome in neurons and astrocytes. Collapse of the Δψm was associated with apoptosis, whereas no apoptotic death was observed as long as mitochondria were hyperpolarized. Moreover, our data show that, in the presence of NO, the effects of staurosporin on Δψm and apoptosis were partly prevented in astrocytes but not in neurons. This finding indicates that NO-mediated inhibition of respiration may influence the susceptibility of the cell to a variety of proapoptotic stimuli by eliciting a glycolysis-dependent defense mechanism.
An increase in Δψm has been observed before in Jurkat cells exposed to proapoptotic stimuli (29, 30) and in a variety of cells during activation with lipopolysaccharide and IFN-γ or high concentrations of NO (31). This increase has been interpreted as either part of the apoptotic process or as independent from the proapoptotic cytochrome c mitochondrial signaling cascade. It is likely that, in all those circumstances, the increase in Δψm represents the initiation of a cellular defense mechanism that may or may not result in cell death.
In conclusion, in this work we demonstrate that, when NO is present at concentrations that inhibit mitochondrial respiration, it is proapoptotic, unless the ability of the cell to switch to glycolytic metabolism is sufficient to provide ATP to maintain Δψm. Thus, NO appears to have both beneficial and detrimental effects on the cellular death/survival outcome, depending on the glycolytic capacity of the cell.
Acknowledgments
This work was funded by grants from the Ministerio de Ciencia y Tecnología, Junta de Castilla y León, and Fundación Ramón Areces (to J.P.B.), and Fondo de Investigaciones Sanitarias de la Seguridad Social (Spain) (to A.A.). S.M. is the recipient of a grant from the Medical Research Council.
Abbreviations
- DETA-NO
[(z)-1-[2-aminoethyl]-N-[2-ammonioethyl]amino] diazen-1-ium-1,2 diolate
- FCCP
carbonyl cyanide p-[trifluoromethoxy]phenyl hydrazone
- JC-1
5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine iodide
- PI
propidium iodide
References
- 1.Moncada S, Palmer R M J, Higgs E A. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- 2.Melino G, Bernassola F, Catani M V, Rossi A, Corazzari M, Sabatini S, Vilbois F, Green D R. Cancer Res. 2000;60:2377–2383. [PubMed] [Google Scholar]
- 3.Sastry P S, Rao K S. J Neurochem. 2000;74:1–20. doi: 10.1046/j.1471-4159.2000.0740001.x. [DOI] [PubMed] [Google Scholar]
- 4.Mitchell P. Nature (London) 1961;191:144–148. doi: 10.1038/191144a0. [DOI] [PubMed] [Google Scholar]
- 5.Liu X, Kim C N, Yang J, Jemmerson R, Wang X. Cell. 1996;86:147–157. doi: 10.1016/s0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- 6.Susin S, Lorenzo H, Zamzami N, Marzo I, Brenner C, Larochette N, Prevost M, Alzari P, Kroemer G. J Exp Med. 1999;189:381–393. doi: 10.1084/jem.189.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bolaños J P, Almeida A, Stewart V, Peuchen S, Land J M, Clark J B, Heales S J R. J Neurochem. 1997;68:2227–2240. doi: 10.1046/j.1471-4159.1997.68062227.x. [DOI] [PubMed] [Google Scholar]
- 8.Almeida A, Bolaños J P. J Neurochem. 2001;77:676–690. doi: 10.1046/j.1471-4159.2001.00276.x. [DOI] [PubMed] [Google Scholar]
- 9.Beltrán B, Mathur A, Duchen M R, Erusalimsky J D, Moncada S. Proc Natl Acad Sci USA. 2000;97:14602–14607. doi: 10.1073/pnas.97.26.14602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown G C, Bolaños J P, Heales S J R, Clark J B. Neurosci Lett. 1995;193:201–204. doi: 10.1016/0304-3940(95)11703-y. [DOI] [PubMed] [Google Scholar]
- 11.Bolaños J P, Peuchen S, Heales S J R, Land J M, Clark J B. J Neurochem. 1994;63:910–916. doi: 10.1046/j.1471-4159.1994.63030910.x. [DOI] [PubMed] [Google Scholar]
- 12.Khodorov B, Pinelis V, Vergun O, Storozhevykh T, Vinskaya N. FEBS Lett. 1996;397:230–234. doi: 10.1016/s0014-5793(96)01139-8. [DOI] [PubMed] [Google Scholar]
- 13.White R J, Reynolds I J. J Neurosci. 1996;16:5688–5697. doi: 10.1523/JNEUROSCI.16-18-05688.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duchen M R. J Physiol (London) 2000;529:57–68. doi: 10.1111/j.1469-7793.2000.00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bolaños J P, Peuchen S, Land J M, Clark J B, Heales S J R. Brain Res Prot. 1997;1:258–262. doi: 10.1016/s1385-299x(96)00039-6. [DOI] [PubMed] [Google Scholar]
- 16.Almeida A, Medina J M. Brain Res Prot. 1998;2:209–214. doi: 10.1016/s1385-299x(97)00044-5. [DOI] [PubMed] [Google Scholar]
- 17.Brown G C, Cooper C E. FEBS Lett. 1994;356:295–298. doi: 10.1016/0014-5793(94)01290-3. [DOI] [PubMed] [Google Scholar]
- 18.Gutmann I, Wahlefeld A W. In: Methods of Enzymatic Analysis. Bergmeyer H U, editor. Vol. 2. Weinheim, Germany: Verlag Chemie; 1974. pp. 1464–1468. [Google Scholar]
- 19.Dringen R, Hamprecht B. J Neurochem. 1992;58:511–517. doi: 10.1111/j.1471-4159.1992.tb09750.x. [DOI] [PubMed] [Google Scholar]
- 20.Cossarizza A, Baccarini-Contri M, Kalashnikova G, Franceschi C. Biochem Biophys Res Commun. 1993;197:40–45. doi: 10.1006/bbrc.1993.2438. [DOI] [PubMed] [Google Scholar]
- 21.Hertz L, Drejer J, Schousboe A. Neurochem Res. 1988;13:605–610. doi: 10.1007/BF00973275. [DOI] [PubMed] [Google Scholar]
- 22.Akerman K E O, Jarvisalo J O. Biochem J. 1980;192:183–190. doi: 10.1042/bj1920183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buchet K, Godinot C. J Biol Chem. 1998;273:22983–22989. doi: 10.1074/jbc.273.36.22983. [DOI] [PubMed] [Google Scholar]
- 24.Pauwels P J, Opperdoes F R, Trouet A. J Neurochem. 1985;44:143–148. doi: 10.1111/j.1471-4159.1985.tb07123.x. [DOI] [PubMed] [Google Scholar]
- 25.Walz W, Mukerji S. Glia. 1988;1:366–370. doi: 10.1002/glia.440010603. [DOI] [PubMed] [Google Scholar]
- 26.Brown G C. FEBS Lett. 1995;369:136–139. doi: 10.1016/0014-5793(95)00763-y. [DOI] [PubMed] [Google Scholar]
- 27.Lehninger A L, Nelson D L, Cox M M. Principles of Biochemistry. New York: Worth; 1995. [Google Scholar]
- 28.Brorson J R, Schumacker P T, Zhang H. J Neurosci. 1999;19:147–158. doi: 10.1523/JNEUROSCI.19-01-00147.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Banki K, Hutler E, Gonchoroff N J, Perl A. J Immunol. 1999;162:1466–1479. [PMC free article] [PubMed] [Google Scholar]
- 30.Sanchez-Alcazar J A, Ault J G, Khodjakov A, Schneider E. Cell Death Differ. 2000;7:1090–1100. doi: 10.1038/sj.cdd.4400740. [DOI] [PubMed] [Google Scholar]
- 31.Hortelano S, Alvarez A M, Bosca L. FASEB J. 1999;13:2311–2317. doi: 10.1096/fasebj.13.15.2311. [DOI] [PubMed] [Google Scholar]