Abstract
Hidradenitis suppurativa (HS) is a debilitating chronic inflammatory skin disease resulting in non-healing wounds affecting body areas of high hair follicle and sweat gland density. The pathogenesis of HS is not well understood but appears to involve dysbiosis-driven aberrant activation of the innate immune system leading to excessive inflammation. Marked dysregulation of antimicrobial peptides and proteins (AMPs) in HS is observed, which may contribute to this sustained inflammation. Here, we analyzed HS skin transcriptomes from previously published studies and integrated these findings through a comparative analysis with a published wound healing data set and with immunofluorescence and qPCR analysis from new HS patient samples. Among the top differently expressed genes between lesional and non-lesional HS skin were members of the S100 family as well as dermcidin, the latter known as a sweat gland-associated AMP and one of the most downregulated genes in HS lesions. Interestingly, many genes associated with sweat gland function, such as secretoglobins and aquaporin 5, were decreased in HS lesional skin and we discovered that these genes demonstrated opposite expression profiles in healing skin. Conversely, HS lesional and wounded skin shared a common gene signature including genes encoding for S100 proteins, defensins, and genes encoding antiviral proteins. Overall, our results suggest that the pathogenesis of HS may be driven by changes in AMP expression and altered sweat gland function, and may share a similar pathology with chronic wounds.
Introduction
Hidradenitis suppurativa is a multifactorial disease characterized by chronic inflammatory non-healing sinus tracts leading to impaired quality of life
Hidradenitis suppurativa (HS) is characterized by recurrent painful nodules, abscesses and sinus tract formation leading to chronic non-healing wounds [1, 2]. Cutaneous nodules form, which over time may rupture, resulting in painful, deep dermal abscesses. With disease progression, draining sinus tracts, open wounds, fibrosis, and scarring can be observed, which together can often accompany significant disfigurement, in addition to pain, malodor, and drainage. HS is common; it is estimated that 1–4% of the general population is affected and this percentage varies by geographic region with disproportionately more females, young adults, and African Americans and biracial patients being affected [3, 4]. Overall, chronic wounds are a source of acute and chronic infections, chronic pain and have a profound negative impact on activities of daily living. This negative impact on quality of life has been found to have an impact similar to that seen in patients with renal and heart failure [5, 6].
Improved knowledge of immune response dysregulation in HS, including innate antimicrobial immunity, may unveil mechanisms of disease pathogenesis and may ultimately help develop therapies that lead to better disease outcome for patients. This understanding could lead to better healing, decreased risk of infection, and ultimately improved quality of life for those with HS. In this manuscript, we use a transcriptomics-centered approach to investigate the pathogenesis of HS, uncovering a wide range of gene expression changes that may cause disease.
The understanding of the inflammatory and antimicrobial processes in HS has begun but is incomplete [7–16]. Like chronic non-healing wounds such as chronic venous ulcers and diabetic ulcers, HS lesions are often associated with dysregulated immune responses including altered expression of various cytokines, chemokines and antimicrobial proteins (AMPs) [9, 17]. As effectors of innate immunity, AMPs act as endogenous microbicidals against invading microbes [18–23]. AMPs directly kill Gram-positive and Gram-negative bacteria, fungi, and certain viruses [24, 25]. Over- or under-expression of AMPs has been implicated in various cutaneous diseases, such as psoriasis, atopic dermatitis, and in chronic leg ulcers [26–28]. Although efforts have been made to begin characterizing AMP expression in HS, our understanding is very limited.
It is important to note that HS is thought to be primarily due to occlusion and subsequent inflammation of the hair follicle and not the sweat gland itself [1]. However, in about 50% of all HS patients, inflammation and/or secondary involvement of the sweat gland unit is observed, especially after rupture of dilated hair follicles and half of patients with HS report a change in their sweating behavior before an overt lesion occurs [2, 29, 30]. This raises the possibility that changes in sweat gland-associated AMPs may be functionally linked to HS. Consequently, a better understanding of the molecular and cellular mechanisms involved in HS antimicrobial immunity and the impact of the sweat gland and its antimicrobial products on inflammation and wound healing is needed to ultimately develop optimized therapies for HS.
Dysregulation of host innate antimicrobial immunity and sweat gland pathology could play a significant role in the inflammatory response in HS. However, this relationship has not yet been sufficiently characterized. In this manuscript, we employ first a biocomputational approach to examine the relationship between HS and innate antimicrobial defenses using a previously published data set (GSE72702) [14]. Additionally, we analyzed the gene expression signatures of a skin wound RNA-seq data set (GSE97615) [31]. Using differently expressed genes (DEGs) from both data sets, we show that HS lesional skin and skin wounds share distinct DEGs, demonstrating that HS and wounded skin have a common gene signature underlying possibly a common pathogenesis pathway. We further identified that expression of multiple AMPs and key sweat gland-associated genes were downregulated in HS lesional skin compared to non-lesional skin. Conversely, expression of many inflammation-associated AMPs was increased in HS lesional skin, confirming previous studies examining the roles of AMPs in HS and further adding to our understanding of this disease [32]. Increased expression of multiple S100 family members was confirmed by qPCR and immunofluorescence staining of donor-matched HS lesional and non-lesional samples. We also confirmed by qPCR and IF the decrease in expression of dermcidin (DCD), a key sweat gland-associated AMP and one of the most downregulated genes in HS lesions identified by the biocomputational approach. Further, analysis of microarray GSE72702 and IF on new HS samples verified that expression of multiple other genes associated with sweat gland function are decreased in HS lesional skin. Finally, we describe a common gene expression signature in HS lesions and wounded skin. A number of AMPs have increased expression in both HS lesions and wounded skin. Conversely, DCD, as well as many sweat gland-associated genes were greatly suppressed in lesional HS skin but highly upregulated in healing skin.
Overall, our results suggest that the pathogenesis of HS may be driven by changes in AMP expression, altered sweat gland function, and may also share a similar pathology with wounds.
Materials and methods
Analysis of gene expression data sets
Microarray data set
We used the publicly available microarray dataset from Blok et al. (GEO accession number: GSE72702) to evaluate changes in gene expression between lesional versus non-lesional skin in HS patients [14]. This dataset results from mRNA microarray experiments performed on skin biopsy samples from patients with HS. The samples were split between lesional skin (n = 17) and healthy non-lesional skin obtained from the upper arm or leg (n = 13). RNA was hybridized to the GeneChip HT HG-U133+PM Array (Affymetrix, Santa Clara, CA, U.S.A.). We used the normalized dataset submitted to the Gene Expression Omnibus [33]. The data had been previously normalized by Robust Multi-array Average (RMA) using ArrayStudio software version 8.0 (OmicSoft Corp., St Morrisville, NC, U.S.A.). To identify differentially expressed genes (DEGs) between the lesional and non-lesional samples we first removed the 4 lesional skin samples for which no matched healthy non-lesional skin sample had been obtained. Also, prior to analysis we filtered lowly-expressed and invariant microarray probe sets, i.e. those with an expression level < 4 in all but two samples or a standard deviation < 0.1 across all samples. After filtering, the dataset consisted of 26 (13 paired lesional and non-lesional) samples and 51,567 probe sets.
To identify DEGs between lesional and non-lesional samples, we used the R package nlme to implement a mixed-effects model including the patient ID as the random effect [34]. P-values were corrected for multiple testing using the Benjamini-Hochberg method [35]. Significantly changing genes were defined as probe sets with an adjusted p-value < 0.05.
To identify genes whose expression varies in similar fashion to the 1553946_PM_at probe (corresponding to the DCD gene), we calculated the Pearson correlation between the 1553946_PM_at probe and all other probes in the dataset across all samples using the R statistical programming environment.
RNA-seq data set
We used the publicly available RNA-seq dataset from Iglesias-Bartolome et al. (GEO accession number: GSE97615) to identify DEGs between wounded and non-wounded human skin samples [31]. This dataset contained human axillary skin wounds at baseline (Day 1, unwounded), two days after full-thickness 3-mm punch biopsy wounding (Day 3), and five days after wounding (Day 6). The raw data was reprocessed as initially described in Iglesias-Bartolome et al. using the Partek Genomics Suite Analysis Toolkit version 6.6 (www.partek.com) to generate read counts per gene. In brief, reads were aligned to the hg19 version of the human genome using the TopHat v2.1 alignment tool, and expression was quantified by the Partek E/M algorithm based on known RefSeq transcripts [31]. Genes that did not have at least 10 reads in any one skin sample were removed from subsequent analysis, resulting in a data set of 12 samples and 26,473 genes. To identify genes that change across time we calculated the moderated F-statistic in limma using voom to estimate the mean-variance relationship [36, 37]. P-values were corrected for multiple testing using the Benjamini-Hochberg method. Genes were considered differentially expressed if the adjusted p-value was < 0.05. The DEGs were then compared against those identified as differentially expressed (adjusted p-value < 0.05) in our analysis of the Blok et al. lesion/non-lesion microarray data of HS samples.
Heatmaps
Heatmaps were generated using the R package pheatmap [38]. For data visualization, probe sets were z-score transformed and capped when the absolute scaled values exceeded 2.5. Genes and samples were clustered using a correlation distance with complete linkage.
Preparation of skin samples
All qPCR analyses and immunofluorescence on HS samples as reported in this manuscript were performed using samples from skin punch biopsies (4-mm) of clinically affected, “lesional” skin obtained from patients visiting a dermatologist at Duke University Medical Center Dermatology Clinic. Clinically unaffected, but adjacent, “non-lesional” biopsies were also obtained. Written informed consent was obtained from all patients for participation in the study. This tissue was obtained in accordance with the Duke Health Institutional Review Board (IRB) protocol 0007979, "Immune Signaling in Psoriasis and other Immune-mediated Diseases". De-identified normal skin samples were obtained from surgical skin waste, in accordance with the Duke Health IRB protocol 00090566, "Access to de-identified skin samples". Biopsies for immunohistochemistry were immediately placed in Tissue-Tek O.C.T Compound (Sakura Finetek USA) and stored at -80°C. For future RT-qPCR, samples were homogenized by mincing into small pieces with surgical scissors, lysed in TRIzol Reagent (ThermoFisher, Waltham, MA) and stored at -80°C for RNA isolation.
Real-time polymerase chain reaction (qPCR)
RNA extraction was performed using the Direct-zol RNA Purification Kit (Zymo Research, Tustin, CA). cDNA was synthesized using iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA). qPCR was performed for determining gene expression using Fast SYBR Green Master Mix (ThermoFisher, Waltham, MA) and primers specific for DCD, S100A7, S100A8, and S100A7A (Integrated DNA Technologies, Skokie, IL) (see Table 1) on a StepOnePlus Real-Time PCR machine (Applied Biosystems, Foster City, CA). PCR was performed for 40 cycles with a melting temperature of 95°C for 3 seconds and an annealing/extension temperature of 60°C for 30 seconds. qPCR was performed on 6 (3 paired lesional and non-lesional) samples. All data was normalized to the average gene expression levels of HS non-lesional skin using the comparative ΔΔ CT method [39].
Table 1. Primer sequences and melting temperatures.
| Primer | Sequence | Tm (°C) |
|---|---|---|
| hGAPDH fwd | CAAGAGCACAAGAGGAAGAGAG | 55 |
| hGAPDH rev | CTACATGGCAACTGTGAGGAG | 55.3 |
| hDCD fwd | AAAGCCAAGGAAGCAGAGAT | 54.3 |
| hDCD rev | CTCCTTTACCCACGCTTTCT | 54.7 |
| hS100A7 fwd | CCTGCTGACGATGATGAA | 52 |
| hS100A7 rev | TGGCTCTGCTTGTGGTAG | 54.6 |
| hS100A8 fwd | AGTGTCCTCAGTATATCAG | 47.5 |
| hS100A8 rev | CTCTTTGTGGCTTTCTTC | 48.3 |
| hS100A7A fwd | GCTGACGATGATGAAGGAGAAC | 55.5 |
| hS100A7A rev | CAGTGGCGAGGTAATGTATGC | 55.9 |
Statistical analysis
To determine fold change (FC) in gene expression for genes examined via qPCR, statistical analysis was performed using the Student’s t-test with p-value < 0.05. Data are shown as mean +/- standard error of the mean. Analysis was performed in GraphPad Prism v8.0 (GraphPad Software, La Jolla, CA).
Immunofluorescence
Samples in Tissue-Tek O.C.T Compound (Sakura Finetek, Torrance, CA) were sectioned and fixed in 4% paraformaldehyde. Staining was performed using an established immunohistochemistry protocol [40]. Samples were permeabilized in 0.1% Triton X (Millipore-Sigma, St. Louis, MO) for 10 minutes. Samples were incubated with monoclonal mouse anti-human S100A7 antibody (Clone 47C1068, 1:200 dilution, ThermoFisher, Waltham, MA), monoclonal mouse anti-human S100A8 antibody (Clone CF-145, 1:500 dilution, ThermoFisher, Waltham, MA), monoclonal mouse anti-human DCD antibody (Clone G-81, 1:50 dilution, Santa Cruz Biotechnology, Dallas, TX), monoclonal mouse anti-human K77 antibody (Clone AE1/AE3, 1:250 dilution, Abcam, San Francisco, CA), or unlabeled IgG control (Southern Biotech, Birmingham, AL) overnight at 4°C. Samples were then washed with 0.01% Triton-X and incubated with Cy3-congugated anti-IgG secondary antibody (Invitrogen, Waltham, MA) for 45 minutes. Secondary antibody was used as control for all DCD and K77 co-stained samples. Nuclear counterstaining was performed with DAPI. Images were acquired using the IX73 inverted microscope (Olympus, Center Valley, PA). Staining of all directly-compared specimens was performed using the same antibody concentrations and exposure times were kept consistent throughout samples.
Results
Genes related to humoral immunity, AMPs, and response to bacterium are upregulated in HS lesions
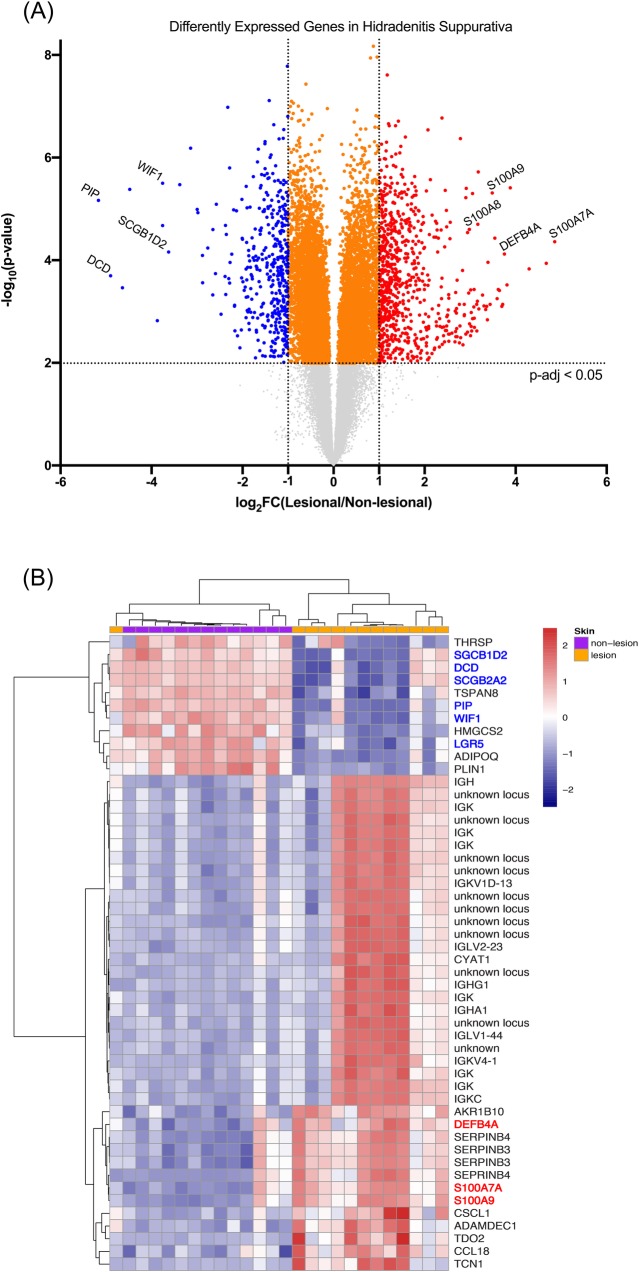
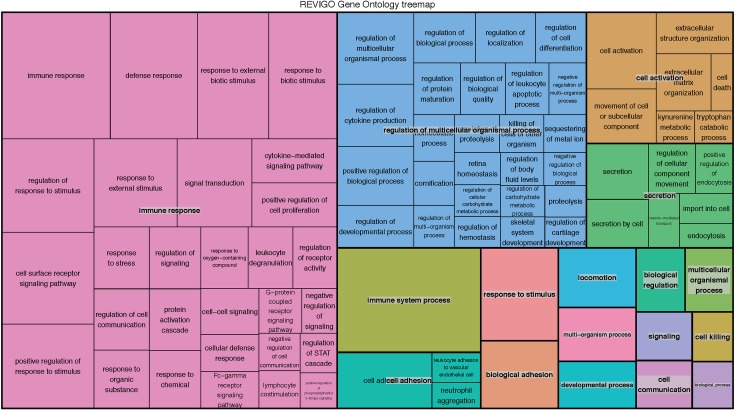
Analysis of microarray data published in Blok et al. comparing HS lesional skin to HS non-lesional skin identified a total of 6,352 DEGs (adjusted p-value < 0.05) (S1 Table). There were 804 unique significant DEGs with a FC > 2 [14]. Of the unique DEGs with a FC > 2, 524 genes were upregulated in the lesional skin relative to the non-lesional skin and an additional 280 genes were downregulated (Fig 1). Gene Ontology Enrichment Analysis (GOrilla) of microarray data revealed a number of Gene Ontology (GO) terms that were enriched [41]. Enriched GO terms included “chemokine production” (GO 0032602), “antimicrobial humoral response mediated by antimicrobial peptide” (GO 0051844), “complement activation” (GO 006956) and “keratinization” (GO 0031424) among others (Fig 2 & Table 2). Top upregulated genes, as previously reported, included AMPs, immunoglobulins, and some keratin types (Table 3) [14, 42]. Top downregulated genes included the AMP DCD, genes involved in keratinocytes development and proliferation, and a different subset of keratins (Table 4).
Fig 1. AMPs are increased in lesional HS, but DCD and other sweat-gland associated proteins are decreased.
(A) Volcano plot showing increased or decreased genes in HS. Graph shows log FC in gene expression of HS lesional skin over HS non-lesional skin samples plotted against negative log p-value of the difference in gene expression. Genes represented in red are upregulated by >2-fold in HS lesional skin (p-adj < 0.05). Genes represented in blue are downregulated by >2-fold in HS lesional skin (p-adj < 0.05). Genes represented in orange were unchanged (FC < 2, p-adj < 0.05) in HS lesional vs. HS non-lesional skin. Non-adjusted p-values were used for generation of the volcano plot to minimize points with tied y-values but significance level was set using the corresponding non-adjusted p-values. (B) Top 50 most differentially expressed probe sets between the HS lesional skin and the HS non-lesional skin. Highlighting shows DEGs. Genes highlighted in blue are downregulated genes of interest; genes highlighted in red are upregulated genes of interest. While DCD is downregulated in HS lesional skin, many other AMPs and interferon-associated molecules are enriched in lesional HS. The top 50 most differentially expressed probes were defined as genes with an adjusted p-value < 0.05 with the largest magnitude FC. Genes were z-score transformed and then the genes and samples were clustered using a correlation distance with complete linkage.
Fig 2. Enriched GO terms.
REVIGO treemap representing the most significantly enriched GO terms associated with DEGs [43]. Larger boxes indicate a smaller p-value and greater disease relevance. Colors indicate GO families in which HS DEGs fall.
Table 2. Enriched GO terms.
| GO Term | Description | Relevant genes | Enrichment | FDR p-value |
|---|---|---|---|---|
| GO 0032602 | Chemokine production | S100A8, S100A9 | 194.54 | 1.84*10−3 |
| GO 0002377 | Immunoglobulin production | IGLV1-44, IGKC, IL7R | 40.65 | 4.64*10−9 |
| GO 0019731 | Antibacterial humoral response | IGHM, IGKV3-20, LTF | 40.35 | 7.83*10−3 |
| GO 0050832 | Defense response to fungus | S100A12, S100A8, LTF, DCD, S100A9 | 28.73 | 1.67*10−4 |
| GO 0019730 | Antimicrobial humoral response | S100A12, PI3, LYZ, S100A8, S100A7, DCD, S100A9, DEFB4A | 27.81 | 6.81*10−18 |
| GO 0051844 | Antimicrobial humoral immune response mediated by antimicrobial peptide | S100A12, DEFB4A, S100A7, S100A9 | 27.45 | 9.56*10−9 |
| GO 0050829 | Defense response to Gram-negative bacterium | DEFB4A, LYZ, S100A7 | 27.15 | 3.38*10−4 |
| GO 0006956 | Complement activation | TRBC1, IGHM, C7 | 18.47 | 8.76*10−10 |
| GO 0031424 | Keratinization | KRT19, KRT6B, KRT16, KRT77 | 6 | 3.03*10−3 |
| GO 0006898 | Receptor-mediated endocytosis | ITGA4, SCARA5, IGKC | 5.83 | 2.38*10−4 |
Selected enriched GO terms from among top 100 GO terms with highest enrichment value, where enrichment is defined at (b/n)/(B/N) where N = number of genes total, B = number of genes associated with a given GO term, n = number of genes closer to the top of a ranked list of genes and b = the number of genes in the ranked list that are associated with a given GO term [41]. False discovery rate (FDR) p-values were calculated according to the minimum hypergeometric model corrected for multiple testing using the Benjamini and Hochberg method [35, 41].
Table 3. Significant upregulated genes in hidradenitis suppurativa.
| Gene | Protein | FC | Function | Reference |
|---|---|---|---|---|
| S100A7A (S100A15) | Koebnerisin | 29.02 | Member of S100 family of AMPs and the epidermal differentiation complex (EDC). Induced by E. coli via TLR4. Markedly increased in psoriatic skin. | [44, 45] |
| DEFB4A | Beta-defensin 2 | 13.48 | Antimicrobial activity against Gram-negative and Gram-positive bacteria. Has previously been shown to be upregulated in HS. | [32, 46] |
| S100A9 | Calprotecin L1H subunit | 11.22 | Members of S100 family of AMPs. Stress induced; increased following epidermal injury. Members of the EDC. | [47, 48] |
| S100A8 | Calprotecin L1L subunit | 7.69 | ||
| PI3 | Peptidase inhibitor 3 | 5.89 | AMP against Gram-positive and Gram-negative bacteria and fungi. | [49] |
| SPRR2B | Small proline rich protein 2B | 5.57 | Members of the SPRR family of genes in the EDC. Involved in cornified envelope formation. | [50, 51] |
| SPRR2C | Small proline rich protein 2C | 4.75 | ||
| KRT16 | Keratin 16 | 5.21 | Stress-induced keratin present in wounds. | [52] |
| S100A7 | Psoriasin | 4.89 | Member of S100 family of AMPs and the EDC. Strongly upregulated in psoriasis. | [53] |
| S100A12 | Calgranulin C | 4.18 | Member of S100 family of AMPs and the EDC. | [51] |
| OAS2 | Oligoadenylate synthetase 2 | 3.67 | Antiviral protein that degrades viral RNA through formation of 2’-5’ linked oligomers. | [54, 55] |
| OASL | Oligoadenylate synthetase-like protein | 3.40 | Antiviral protein that binds viral RNA but lacks classical 2’-5’OAS activity. | |
| KRT6A | Keratin 6A | 2.89 | Stress-induced keratin present in wounds. | [52] |
| LCE3D | Late cornified envelope protein 3D | 3.11 | Member of the LCE family of genes in the EDC. Expressed late in differentiation in upper granular layers of epidermis. Increased in psoriasis. | [56, 57] |
Select upregulated genes in HS lesional skin relative to HS non-lesional skin from among the 200 top upregulated genes. The top 200 upregulated genes are defined as the top 200 unique, significant DEGs (p-adj < 0.05) with the highest positive FC in expression.
Table 4. Significant downregulated genes in hidradenitis suppurativa.
| Gene | Protein Name | FC | Function | Reference |
|---|---|---|---|---|
| PIP | Prolactin-inducible protein | 0.03 | Expressed by sweat glands and also associated with breast cancer. | [58] |
| DCD | Dermcidin | 0.03 | AMP with activity against E. coli, S. aureus, and C. albicans. Optimal pH and salt conditions are those found in sweat. | [59] |
| SCGB2A2 | Mammaglobin-A | 0.04 | Produced in sweat glands. Members of the secretoglobin family are anti-inflammatory. Also likely involved in cell signaling, immune response, and chemotaxis. | [60] |
| SCGB1D2 | Lipophilin-B | 0.08 | ||
| SCGB2A1 | Mammaglobin-B | 0.27 | ||
| WIF1 | Wnt inhibitory factor 1 | 0.07 | Tumor suppressor gene. Inhibits Wnt protein signaling. Involved in sweat gland development. | [61] |
| LGR5 | Leucine rich repeat containing G protein-coupled receptor 5 | 0.13 | Wnt target. Marker of hair follicle stem cells. | [62] |
| ERBB4 | Erb-B2 Receptor Tyrosine Kinase 4 | 0.20 | Epidermal growth factor associated receptor that may play a role in keratinocyte proliferation. | [63] |
| KRT77 | Keratin 77 | 0.22 | Keratin expressed only in eccrine sweat glands. | [64, 65] |
| KRT19 | Keratin 19 | 0.27 | Keratin of simple epithelial cells. | [64] |
| KRT79 | Keratin 79 | 0.30 | Poorly characterized keratin found in skin. | [66] |
| KRT73 | Keratin 73 | 0.34 | Hair follicle-specific keratins. | [64] |
| KRT74 | Keratin 74 | 0.38 | ||
| KRT31 | Keratin 31 | 0.35 | Keratin of the hair fiber. | [64] |
| IL37 | Interleukin-37 | 0.29 | Suppressor of innate inflammatory responses. Inhibits dendritic cell activation. | [67] |
| AQP5 | Aquaporin-5 | 0.31 | Water cannel involved in generation of secretions. | [68] |
| NR1D1 | Rev-ErbA-Alpha | 0.34 | Negative regulator of BMAL1/CLOCK. Involved in regulation of hair follicle cycling. | [69] |
| PER1 | Periodic circadian regulator 1 | 0.39 | Negative regulator of BMAL1/CLOCK. | [70] |
| FOXA1 | Forkhead box A1 | 0.39 | Transcription factor involved in regulation of sweat secretion. | [71] |
| FOXQ1 | Forkhead box Q1 | 0.43 | Transcription factor with role in hair follicle differentiation. | [72] |
| IL17D | Interleukin 17D | 0.44 | Pro-inflammatory cytokine overexpressed in psoriasis. | [73] |
Select downregulated genes in HS from among the 200 top downregulated genes. The top 200 downregulated genes are defined as the top 200 unique, significant DEGs (p-adj < 0.05) with the most negative FC in expression.
A number of antibacterial and antiviral proteins are upregulated in HS lesions
The most significantly upregulated gene found in lesional HS skin was S100A7A (also known as S100A15, Koebnerisin) (Table 3). S100A7A is a member of the S100 family of AMPs, which display antimicrobial activity against gram-negative bacteria, such as E. coli [44]. Interestingly, S100A7A is highly increased in psoriatic skin and shares near-complete homology with S100A7 (Psoriasin), which has a well-established role in the pathogenesis of psoriasis and is also increased in atopic dermatitis [45, 53]. S100A7, which has been shown to be increased in HS lesions in one study, also showed significantly increased expression in our transcriptomic analysis (Table 3) [74]. S100A8 and S100A9, which combine to form Calprotectin, were also among the most increased genes in HS lesional skin [47]. These S100 proteins, which are similarly overexpressed in psoriasis, are also expressed in human wounds, ulcers, and by wound-infiltrating inflammatory cells [48, 75].
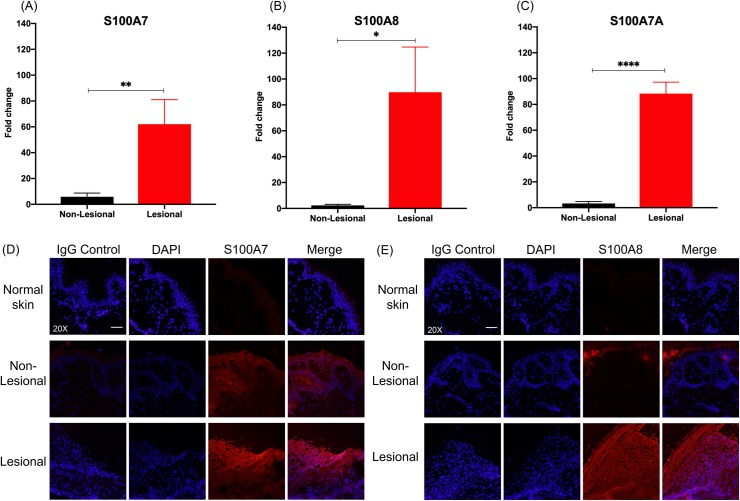
Expression patterns of S100 proteins were confirmed via qPCR and immunofluorescence on paired samples of HS lesional and non-lesional skin. Consistent with DEG analysis of microarray data, we found that expression of S100A7, S100A8, and S100A7A was significantly increased in HS lesional skin samples (Fig 3).
Fig 3. S100 proteins are strongly upregulated in HS lesional skin.
qPCR for (A) S100A7 (log2FC = 3.51, **p < 0.01), (B) S100A8 (log2FC = 3.41, *p < 0.05) and (C) S100A7A (log2FC = 6.47, **** p < 0.0001) in lesional and non-lesional HS skin. FC is expressed as average of skin samples from 3 patients. Measurements were collected in triplicate or duplicate, as allowed by RNA yield from samples. Data is shown as mean expression value compared to non-lesional skin +/- the standard error of the mean. Non-lesional and lesional samples were compared using paired t-test. (D) IF staining for S100A7 at 20X. Scale bar is 50μm. (E) IF staining for S100A8 at 20X. Scale bar is 50μm. Immunofluorescence intensity is highest in HS lesional skin, compared to non-lesional and normal skin.
As previously demonstrated, our analysis of DEGs showed that DEFB4, which encodes for human beta-defensin 2 (h-BD2), was strongly upregulated in HS lesional skin (Table 3) [32]. h-BD2 exhibits antimicrobial action against gram-negative bacteria, but not against gram-positive bacteria, such as S. aureus [46, 76]. Human beta-defensin 3 (hBD-3), which has broad-spectrum antimicrobial activity against gram-negative and gram-positive bacteria, such as S. aureus, was not significantly increased in HS lesions, which is consistent with prior reported data [77].
Antibacterial proteins are not the only group of AMPs that are increased in HS lesions. Interferon stimulated genes (ISGs), such as oligoadenylate synthetase 2 (OAS2) also had increased expression in HS lesions (Table 3). OAS2, a member of the OAS family of antiviral proteins targets viral RNA primarily through an RNase-L dependent mechanism [54]. OAS family members display potent antiviral activity against both RNA and DNA viruses [24].
S100 proteins and other members of the epidermal differentiation complex are upregulated in HS
Notably, S100 proteins are members of the epidermal differentiation complex (EDC), a cluster of genes located on human chromosome 1q21 that codes for proteins involved in keratinocyte terminal differentiation and cornified envelope formation [51]. S100A7, which was increased in HS lesions, increases in expression throughout the process of keratinocyte differentiation [78]. S100A8/9 are also upregulated in hyperproliferative epidermis, and levels of S100A8 increase as keratinocytes become more differentiated [51, 75, 78]. Other S100 proteins, such as S100A3, S100A6, and S100A13, which are decreased in differentiated keratinocytes, did not show changes in expression in HS lesions.
Additional members of the EDC had increased expression in HS lesional skin. Small proline-rich proteins (SPRR) 2B and 2C were increased in HS lesions (Table 3). SPRRs participate in cross-bridge formation during development of the cornified envelope of keratinocytes [50]. Late cornified envelope (LCE) proteins are also expressed late in differentiation in the upper granular layers of the epidermis [56]. LCE3D is increased in HS lesions; members of the LCE3 group are known to be increased in psoriasis [57].
Patterns of keratin expression are altered in HS lesional skin
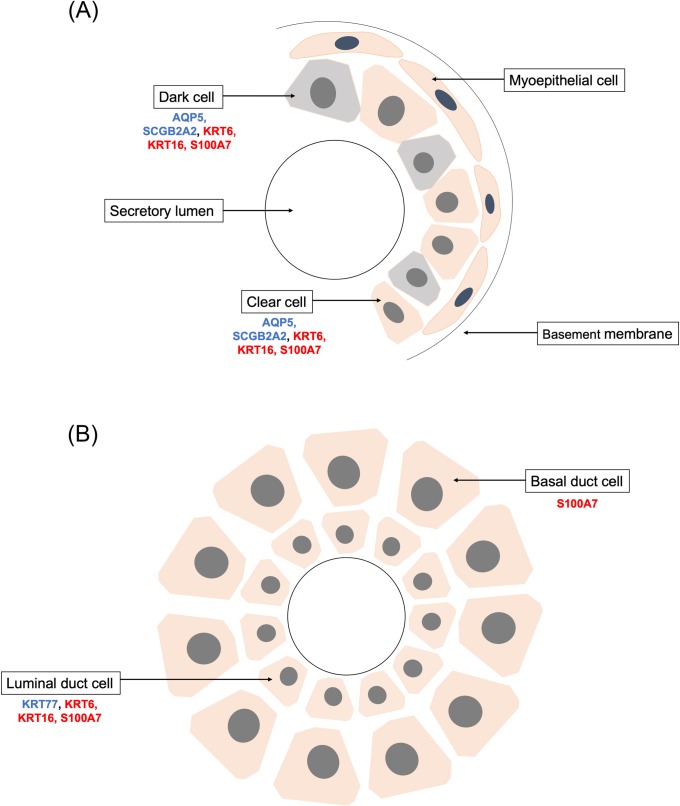
Keratins 6A and 16 are increased in HS lesional skin (Table 3). These keratins, constitutively expressed in the outer root sheath of hair follicles, are increased in multiple inflammatory skin conditions and are makers of a hyperproliferative epidermis [79, 80]. Wounding induces expression of keratins 6 and 16 in the inter-follicular epidermis even before re-epithelialization begins, and keratin 16 may play a role in reorganization of other keratin filaments during healing [52, 81]. However, mice overexpressing keratin 16 have delayed wound healing [82]. Notably, keratins 6A and 16 are also expressed in secretory and luminal cells of eccrine sweat glands (Fig 4) [83]. Increased expression of these keratins in HS lesions is not due to the number of eccrine sweat glands in the adnexal and inguinal regions, where HS is often found; keratin 77, which is exclusively expressed in eccrine sweat glands, is here shown to be decreased in HS lesions (Table 4) [65]. Other keratins are also decreased in HS lesions, including keratin 73 and keratin 74, which are specific to the hair follicle [64].
Fig 4. Schematic representation eccrine sweat gland and duct cells.
Diagram of gene expression of A) eccrine sweat gland and B) duct. Genes in blue are decreased in HS lesional skin. Genes in red are increased in HS lesional skin.
DCD, a sweat-gland associated AMP, is decreased in HS lesions
In drastic contrast to the large number of AMPs that were strongly upregulated in HS skin, we identified that DCD is one of the most significantly downregulated genes in HS lesions (Table 4). DCD is an AMP secreted by sweat glands to provide antimicrobial function against bacteria and a broad spectrum of microbes including fungi and viruses [84]. While most AMPs carry a strong positive charge, and prefer to bind to bacterial membranes, DCD has an overall negative charge, so it relies on positively-charged zinc ions, which are abundant in sweat, to assist with its specific interaction with bacterial lipids [59]. In human healthy skin, DCD is known to be expressed in the dark mucous cells of the secretory coil of eccrine sweat glands and is found in the Golgi complex and the secretory granules typical for a secreted protein [59].
DCD is a relatively newly discovered AMP, so its role in skin protection and/or disease is not yet fully understood. DCD is proteolytically processed into multiple peptides, including DCD-1L, which exhibits antimicrobial activity [85]. Y-P30, another peptide product of DCD, is considered a “survival-promoting peptide” for neurons [84]. The antimicrobial activity of DCD is broad and includes both gram-positive and gram-negative bacteria, fungi, and even viruses [84]. DCD is constitutively secreted, which suggests that it may play a role in maintaining a favorable skin microbiome under homeostatic conditions. This is in contrast to other AMPs, such as LL-37, which are induced by bacteria and wounding [26].
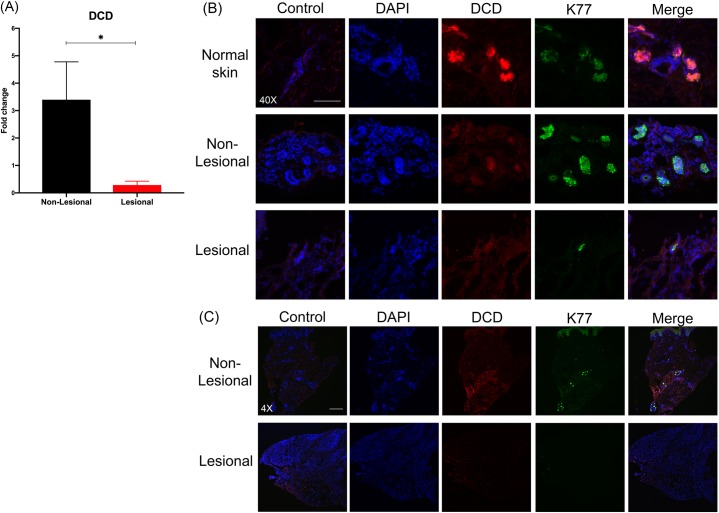
Given the strong downregulation of DCD in HS lesions, in contrast to the increase of most other AMPs, we sought to further elucidate the role of DCD in HS. Analysis via qPCR confirmed the significant decrease of nearly 12-fold in DCD in HS lesional samples (Fig 5A). Furthermore, we used immunofluorescence to examine expression of DCD in matched HS non-lesional and lesional skin from a single patient. Fluorescence intensity for DCD was stronger in non-lesional skin, compared to lesional skin (Fig 5B). In addition to the decreased fluorescence intensity of DCD exhibited by HS lesional skin, we also observed that fewer eccrine sweat glands (as marked by K77) were found in HS lesional skin than normal skin or HS non-lesional skin (Fig 5C).
Fig 5. DCD is strikingly decreased in HS lesions.
(A) qPCR for DCD (log2FC = -3.57, *p < 0.05). FC is expressed as average of skin samples from 3 patients. Measurements were collected in triplicate or duplicate, as allowed by RNA yield from samples. Data is shown as mean FC over non-lesional skin +/- the standard error of the mean. Non-lesional and lesional samples were compared using a paired t-test. (B) Immunofluorescence for DCD (red) at 40X in normal skin, HS non-lesional skin, and HS lesional skin. Co-staining was performed with K77 (green), which is a marker of eccrine sweat glands. Scale bar is 100μm. (C) Immunofluorescence for DCD (red) and K77 (green) at 4X showing decreased number of eccrine sweat glands in HS lesional skin. Scale bar is 500μm.
Correlation analysis was run to determine genes positively and negatively correlated with the DCD probe set (Table 5) in the Blok et al. dataset (S2 Table) [14]. Top correlated genes with DCD included members of the secretoglobin family (r = 0.97, 0.99; adj p-value = 3.25x10-18, 2.34x10-11), WIF1 (r = 0.87, adj p-value = 9.90x10-6), and AQP5 (r = 0.79, adj p-value = 1.84x10-4), which were some of the most downregulated genes overall in HS. On the other hand, DCD was negatively regulated with multiple molecules from the interferon and antiviral protein pathway, such as STAT1 (r = -0.88, adj p-value = 7.78x10-6), IRF1 (r = -0.84, adj p-value = 6.93x10-5), TLR8 (r = -0.79, adj p-value = 1.15x10-4), and IFNAR2 (r = -0.74, adj p-value = 8.41x10-4). IFNAR2 signals through a STAT-dependent mechanism to turn on production of ISGs, which have antiviral activity [86]. Findings from the correlation analysis were mirrored by expression analysis in the microarray data set, where both the interferon receptors 1 and 2 (IFNAR1 and 2) and OAS2, a downstream ISG, were upregulated in lesional HS skin relative to non-lesional skin.
Table 5. Selected genes most positively- or negatively-correlated with DCD.
| Gene | Protein Name | Correlation with DCD | Function | Reference |
|---|---|---|---|---|
| DCD | Dermcidin | 1 | AMP with activity against E. coli, S. aureus, and C. albicans. Optimal pH and salt conditions are those found in sweat. | [59] |
| SCGB2A2 | Secretoglobin 2A2 | 0.99 | Produced in sweat glands. Members of the secretoglobin family are anti-inflammatory. Also likely involved in cell signaling, immune response, and chemotaxis. | [60] |
| SCGB1D2 | Secretoglobin 1D2 | 0.97 | ||
| WIF1 | Wnt inhibitory factor 1 | 0.87 | Tumor suppressor gene. Inhibits Wnt protein signaling. Involved in sweat gland development. | [61] |
| AQP5 | Aquaporin 5 | 0.79 | Water cannel involved in generation of secretions. | [68] |
| STAT1 | Signal transducer and activator of transcription 1 | -0.88 | Transcription factor involved in upregulation of genes following type I, II, or III interferon signaling. | [87] |
| SAR1B | GTP- binding protein SAR1b | -0.84 | Sar1-ADP ribosylation factor family of small GTPases, which govern the intracellular trafficking of protein in coat proteins (COP)-coated vesicles. Plays a role in clathrin-mediated endocytosis signaling. | [88] |
| IRF1 | Interferon regulatory factor 1 | -0.83 | Regulates expression of target genes by binding to an interferon stimulated response element in their promoters. May contribute to multiple autoimmune diseases. | [89] |
| TLR8 | Toll-like receptor 8 | -0.81 | Recognizes single stranded viral RNA. Activation of TLR8 can initiate development of psoriatic lesions. | [90] |
| RAB31 | Ras-related protein Rab-31 | -0.79 | Member of the RAS oncogene family. Small GTP-binding protein. Has a role in targeting of vesicles and granules. | [91] |
| IFNAR2 | Interferon receptor | -0.74 | Receptor for type I IFN. Activation of the receptor stimulates Janus protein kinase (JAK), which in turn phosphorylate STAT1/2. May even have intrinsic antiviral activity. | [92] |
Sweat gland proteins were among the genes most positively correlated with DCD in HS skin samples (all statistically significant with an adjusted p-value < 0.05). Signaling molecules of the interferon and antiviral protein pathways were negatively correlated with DCD.
Proteins associated with sweat gland function are decreased in HS lesions
A number of genes related to sweat gland function had differential expression in HS lesions. Expression of genes coding for S100 proteins, which are present in multiple sweat glands cell types, was increased (Table 3) [93]. In contrast, expression of a number of other sweat-gland associated proteins was decreased. Secretoglobins B2A2, B1D2, and B2A1 were among the genes with the largest decrease in expression in HS lesions (Table 4 and Fig 4). Secretoglobins, for example, may play a role in sweat secretion [60]. Wnt inhibitory factor 1 (WIF1), an inhibitor of Wnt signaling, was decreased in HS lesional skin. Dynamic and tightly regulated expression of Wnt, Shh, and Eda has been previously linked to sweat gland development and function [94]. Aquaporin 5 (AQP5), which contributes to sweating by increasing permeability of sweat glands, was also decreased in HS lesions [68, 95]. Finally, we found that forkhead box A1 (FOXA1), a transcription factor that is required for sweat secretion in mice, is decreased in HS lesions [71]. In sum, we found that multiple genes involved in development, regulation, and homeostasis of sweat glands show decreased expression in HS lesions.
Interestingly, genes involved in hair follicle differentiation and proliferation were also decreased in HS lesions (Table 4). For example, the leucine-rich repeat containing G protein-coupled receptor 5 (LGR5) was decreased in lesional skin. LGR5 is a Wnt target gene that is a marker of proliferating stem cells in the hair follicle [62]. Additionally, forkhead box Q1 (FOXQ1), a regulatory target with a role in hair follicle differentiation, was also decreased [72]. Expression of hair follicle-associated genes, in addition to sweat gland-associated genes, is altered in HS.
HS lesions share a transcriptomic signature with wounds
Notably, the most severe HS lesions (Hurley stage III) are characterized by chronic non-healing sinuses, which form a wound-like environment [96]. Previous understanding of wound closure was that new skin cells originate from hair follicles and from intact skin at the edge of the wound [97]. More recent studies demonstrated that cells also arise from beneath the wound, and suggested that human eccrine sweat glands also store an important reservoir of adult stem cells that can quickly be recruited to aid wound healing [98]. Based on our findings that genes related to sweat gland development and re-epithelialization were downregulated in HS lesional skin, we compared the transcriptomes of HS skin with wounded skin.
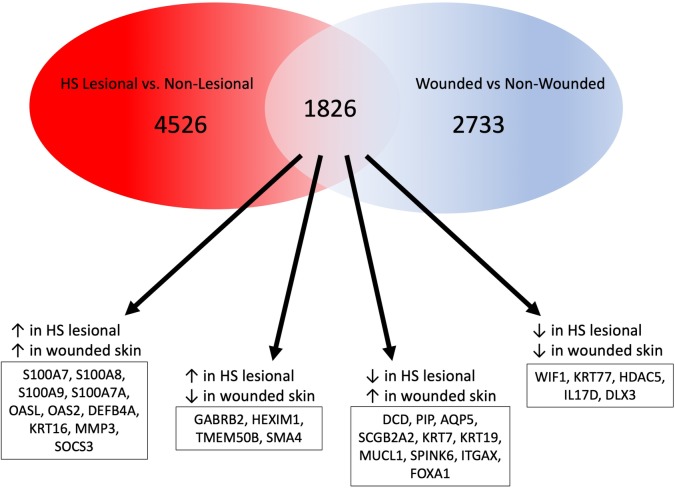
Re-analysis of wounded skin vs. non-wounded skin from Iglesias-Bartolome et al. revealed 4,599 DEGs (adjusted p-value < 0.05) (S3 Table) [31]. 1,826 of these genes were also differentially expressed in HS lesional skin compared to non-lesional skin (Fig 6). A number of the shared DEGs were increased in both HS lesional skin relative to non-lesional skin and in wounded skin relative to non-wounded skin. These include S100 family members (S100A7, S100A8, S100A9, and S100A7A), DEFB4A, the ISGs OASL and OAS2, and KRT16. The shared increased expression of antibacterial and antiviral proteins by HS lesions and wounded skin suggests that they are carrying out similar antimicrobial programs. Stress-induced keratins 6 and 16 were upregulated in both HS lesions and wounds [52]. Other shared DEGs were decreased in both HS lesional skin and wounded skin, including WIF1. Finally, a small number of genes showed opposite expression in HS lesions and wounded skin. Among these genes were DCD, AQP5, and SCGB2A2. Whereas these genes were upregulated in wounded skin, they were downregulated in HS skin, suggesting that they play a role in the pathology of HS, but are beneficial for wound healing.
Fig 6. Shared and dissimilar pathways in HS and healing skin wounds.
Venn diagram illustrating shared and dissimilar gene expression in HS lesions and wounded skin. AMP expression is increased in both HS lesional and wounded skin. DCD and other sweat-gland associated proteins show opposite expression; they are decreased in HS lesions but are increased in wounded skin.
Discussion
The striking increase in expression of many AMPs in HS lesions brings forth two hypotheses for pathogenesis of HS. Firstly, increased AMP expression could be the result of a general overactivation of the innate immune system in response to bacteria or other stimuli. Secondly, the increased AMP expression could be a reaction to an altered cutaneous microbiome of HS lesions. Whether changes in AMP expression are inherent to the disease or secondary to the altered microbiome of HS, dysregulation of AMPs may contribute to the initiating pathogenesis of HS or contribute to disease progression and aggravation. HS lesions are characterized by upregulation of defensins, a group of AMPs that target gram-negative bacteria, but not gram-positive bacteria, and this could provide a rationale for the increased prevalence of gram-positive microbes, particularly S. aureus, in HS lesions [99]. Although more research is needed to address these questions, our work shows that there is significant dysregulation of AMP expression in HS lesions.
A second theme that emerged from re-analysis of microarray data from Blok et al. is that many of the genes that were upregulated in HS are members of the EDC [14]. Interestingly, genes of the EDC were the focus of a recent study comparing the genes expression profiles of mice raised in the presence of commensal microbiota (specific pathogen free, SPF) with mice raised in a germ-free environment [100]. Meisel and colleagues found that S100A7 and many LCE proteins were increased in SPF mice. Based on the increased expression of these EDC genes, which are makers of terminal differentiation, it was hypothesized that SPF mice have decreased regenerative capacity compared to GF mice. Specifically, our findings suggest that there may be a greater number of terminally differentiated keratinocytes in HS lesions. Therefore, HS lesional skin may have less regenerative capacity than normal skin. Therefore, although significant research will be required for definitive conclusions to be drawn about the role of the EDC in HS, our analysis indicates that epidermal differentiation and regeneration pathways could be involved in the pathology of HS.
There is strong evidence emerging that human sweat glands contribute significantly to epidermal homeostasis and wound repair. It is well known that stem cells in the hair follicle bulge contribute to re-epithelialization [97]. More recently, it has been shown that eccrine sweat gland cells are able to reconstitute a stratified interfollicular epidermis with all features characteristic of a normal stratified squamous epithelium both in vitro and in rat models [101]. These findings have been replicated in humans using immunohistochemical staining of healing skin wounds [98]. In fact, there are distinct, multi-potent, stem progenitor cell populations residing within sweat glands [102]. In elderly skin, re-epithelialization by sweat gland cells is impaired and may account for poor wound healing in this population [103]. Severe HS lesions resemble chronic, non-healing wounds and are characterized by sinus tracts, scarring, abscesses, and bacterial superinfection [96]. Given that sweat glands and sweat gland function are integrally important for wound healing, it is possible that impaired sweat gland function and decreased sweat gland number contribute to the pathological non-healing wound-like environment of HS. Our analysis determined that multiple genes associated with sweat-gland function, such as WIF1, AQP5, FOXA1, and DCD were decreased in HS lesions. Wnt signaling is required for development of sweat glands, suggesting that sweat gland development may be impaired in HS [61]. WIF1 is also decreased in psoriatic skin [73]. AQP5 is a transmembrane protein that increases water permeability of cells, and therefore contributes to sweat formation [68, 95]. Knockout of AQP5 function impairs sweat secretion in mouse models, raising the possibility that the decreased expression of AQP5 in the eccrine glands of HS lesional skin impairs sweat generation [95]. Impaired sweat gland function via downregulation of these key sweat gland-associated genes could contribute to HS.
Impaired sweat secretion could be one reason for the reduced levels of DCD in HS lesions. However, a second possibility for decreased DCD level in HS lesional skin is a decreased overall number of eccrine sweat glands in HS lesions. We showed via immunofluorescence that fewer eccrine sweat glands are present in HS lesional skin than normal skin or HS non-lesional skin. Moreover, staining intensity of DCD in HS lesions within the HS lesional skin samples was also reduced compared to those found in donor-matched healthy non-lesional skin or healthy normal skin. This raises the possibility that the decreased gene expression levels of DCD seen in transcriptomic and qPCR data may be due to a combination of decreased DCD production by eccrine glands and a fewer number of total eccrine glands in HS lesional skin. Loss of normal skin architecture can be seen in HS, which may contribute to the decreased number of eccrine sweat glands observed in HS lesional skin samples [30].
Furthermore, we found that expression of many sweat gland-associated genes was different in HS and acute healing wounds. Wound samples in the RNA-seq data set from Iglesias-Bartolome et al. were acquired from the axillary region of subjects, therefore the density of eccrine glands in the wound samples is representative of wounded healthy skin from areas where HS often occurs [31]. It is important to note that the wound data that was used for overlap analysis with HS samples is from wounds that ultimately went on to heal, indicating that genes that were increased in this data set could be important for healing. Genes such as AQP5, FOXA1, and DCD were increased in acute wounds, but were decreased in HS. The finding that sweat gland-associated genes were decreased in HS lesional skin, which resembles a chronic non-healing wound, points to these genes as potential pathogenic explanations for the HS phenotype.
Conclusion
Hidradenitis suppurativa is a chronic and frequently debilitating cutaneous disorder that significantly impacts the quality of life of patients. Compared to other cutaneous disorders, such as psoriasis and atopic dermatitis, it is relatively poorly characterized. HS is significantly different from these classic inflammatory skin diseases through the clinical presentation of non-healing skin lesions and the formation of ducts and cysts that become highly inflamed. Our transcriptomic analysis of HS lesions suggests a significant role for innate antimicrobial immunity and altered sweat gland function in HS disease pathology and furthermore revealed a previously unknown set of DEG that overlap with healing wounds.
Recent studies have begun to illuminate the role of sweat gland cells, specifically sweat gland progenitors, in wound healing and re-epithelialization. Sweat glands contain multipotent progenitor cells that can migrate to epidermal layers of the skin and contribute to repair; in addition, eccrine ductal cells participate in re-epithelialization [98, 102]. Sweat glands may also contribute to cutaneous immunity beyond their role in wound repair through production of inflammatory cytokines and DCD, a sweat-gland associated AMP [59, 104]. Therefore, it is possible that sweat glands produce multiple host factors, including antimicrobial DCD that promote epithelial regeneration and that this pathway is dysregulated in HS prohibiting healing of severe HS lesions.
We also uncovered substantial transcriptional overlap between HS lesions and wounded skin, suggesting that HS may represent a wound-like environment. Our analysis could pave the way for development of new therapies for HS. For example, supplementation and activation of natural AMPs, such as DCD, may be promising therapeutic options for the treatment of HS.
Supporting information
(XLSX)
(XLSX)
(XLSX)
Acknowledgments
We would like to acknowledge the contributions of Kim Scoggins, Clinical Research Coordinator for the Department of Dermatology, Duke University.
Data Availability
Hidradenitis microarray data set (GSE72702) was previously published by Blok et al. 2016 and is publicly available at https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE72702. Wound healing RNA seq data set (GSE97615) was previously published by Iglesias-Bartolome et al. 2018 and is publicly available at https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE97615.
Funding Statement
ASM is supported by R01AI139207 01, receives funding from the Dermatology Foundation Research Grant and the Duke Physician-Scientist Strong Start Award. ASM also received funding from Silab Inc. to support her laboratory, but the sponsor did not have any control over the content nor results of the study. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Napolitano M, Megna M, Timoshchuk EA, Patruno C, Balato N, Fabbrocini G, et al. Hidradenitis suppurativa: from pathogenesis to diagnosis and treatment. Clinical, cosmetic and investigational dermatology. 2017;10:105–15. Epub 2017/05/02. 10.2147/CCID.S111019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoffman LK, Ghias MH, Lowes MA. Pathophysiology of hidradenitis suppurativa. Seminars in cutaneous medicine and surgery. 2017;36(2):47–54. Epub 2017/05/26. 10.12788/j.sder.2017.017 . [DOI] [PubMed] [Google Scholar]
- 3.Revuz JE, Canoui-Poitrine F, Wolkenstein P, Viallette C, Gabison G, Pouget F, et al. Prevalence and factors associated with hidradenitis suppurativa: results from two case-control studies. Journal of the American Academy of Dermatology. 2008;59(4):596–601. Epub 2008/08/05. 10.1016/j.jaad.2008.06.020 . [DOI] [PubMed] [Google Scholar]
- 4.Jemec GB, Heidenheim M, Nielsen NH. The prevalence of hidradenitis suppurativa and its potential precursor lesions. Journal of the American Academy of Dermatology. 1996;35(2 Pt 1):191–4. Epub 1996/08/01. . [DOI] [PubMed] [Google Scholar]
- 5.Miller IM, Ahlehoff O, Zarchi K, Rytgaard H, Mogensen UB, Ellervik C, et al. Hidradenitis suppurativa is associated with myocardial infarction, but not stroke or peripheral arterial disease of the lower extremities. The British journal of dermatology. 2018;178(3):790–1. Epub 2017/09/16. 10.1111/bjd.15998 . [DOI] [PubMed] [Google Scholar]
- 6.Esmann S, Jemec GB. Psychosocial impact of hidradenitis suppurativa: a qualitative study. Acta dermato-venereologica. 2011;91(3):328–32. Epub 2011/03/12. 10.2340/00015555-1082 . [DOI] [PubMed] [Google Scholar]
- 7.Ardon CB, Prens EP, Fuursted K, Ejaz RN, Shailes J, Jenssen H, et al. Biofilm production and antibiotic susceptibility of Staphylococcus epidermidis strains from Hidradenitis Suppurativa lesions. Journal of the European Academy of Dermatology and Venereology: JEADV. 2018. Epub 2018/07/20. 10.1111/jdv.15183 . [DOI] [PubMed] [Google Scholar]
- 8.Hoffman LK, Tomalin LE, Schultz G, Howell MD, Anandasabapathy N, Alavi A, et al. Integrating the skin and blood transcriptomes and serum proteome in hidradenitis suppurativa reveals complement dysregulation and a plasma cell signature. PloS one. 2018;13(9):e0203672 Epub 2018/09/29. 10.1371/journal.pone.0203672 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jemec GB. Hidradenitis suppurativa and immune dysregulation. The British journal of dermatology. 2012;166(2):237–8. Epub 2012/01/25. 10.1111/j.1365-2133.2012.10802.x . [DOI] [PubMed] [Google Scholar]
- 10.Riis PT, Ring HC, Themstrup L, Jemec GB. The Role of Androgens and Estrogens in Hidradenitis Suppurativa—A Systematic Review. Acta Dermatovenerol Croat. 2016;24(4):239–49. Epub 2017/01/28. . [PubMed] [Google Scholar]
- 11.Ring HC, Thorsen J, Saunte DM, Lilje B, Bay L, Riis PT, et al. The Follicular Skin Microbiome in Patients With Hidradenitis Suppurativa and Healthy Controls. JAMA dermatology. 2017;153(9):897–905. Epub 2017/05/26. 10.1001/jamadermatol.2017.0904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ring HC, Thorsen J, Saunte DM, Lilje B, Bay L, Theut Riis P, et al. Moderate to severe hidradenitis suppurativa patients do not have an altered bacterial composition in peripheral blood compared to healthy controls. Journal of the European Academy of Dermatology and Venereology: JEADV. 2018;32(1):125–8. Epub 2017/08/24. 10.1111/jdv.14538 . [DOI] [PubMed] [Google Scholar]
- 13.Blok JL, Li K, Brodmerkel C, Horvatovich P, Jonkman MF, Horvath B. Ustekinumab in hidradenitis suppurativa: clinical results and a search for potential biomarkers in serum. The British journal of dermatology. 2016;174(4):839–46. Epub 2015/12/08. 10.1111/bjd.14338 . [DOI] [PubMed] [Google Scholar]
- 14.Blok JL, Li K, Brodmerkel C, Jonkman MF, Horvath B. Gene expression profiling of skin and blood in hidradenitis suppurativa. The British journal of dermatology. 2016;174(6):1392–4. Epub 2015/12/29. 10.1111/bjd.14371 . [DOI] [PubMed] [Google Scholar]
- 15.Blok JL, van Hattem S, Jonkman MF, Horvath B. Systemic therapy with immunosuppressive agents and retinoids in hidradenitis suppurativa: a systematic review. The British journal of dermatology. 2013;168(2):243–52. Epub 2012/10/31. 10.1111/bjd.12104 . [DOI] [PubMed] [Google Scholar]
- 16.Buimer MG, Wobbes T, Klinkenbijl JH, Reijnen MM, Blokx WA. Immunohistochemical analysis of steroid hormone receptors in hidradenitis suppurativa. The American Journal of dermatopathology. 2015;37(2):129–32. Epub 2014/09/18. 10.1097/DAD.0000000000000206 . [DOI] [PubMed] [Google Scholar]
- 17.MacLeod AS, Mansbridge JN. The Innate Immune System in Acute and Chronic Wounds. Advances in wound care. 2016;5(2):65–78. Epub 2016/02/11. 10.1089/wound.2014.0608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Braff MH, Gallo RL. Antimicrobial peptides: an essential component of the skin defensive barrier. Current topics in microbiology and immunology. 2006;306:91–110. Epub 2006/08/17. . [DOI] [PubMed] [Google Scholar]
- 19.Lopez-Garcia B, Lee PH, Yamasaki K, Gallo RL. Anti-fungal activity of cathelicidins and their potential role in Candida albicans skin infection. J Invest Dermatol. 2005;125(1):108–15. 10.1111/j.0022-202X.2005.23713.x . [DOI] [PubMed] [Google Scholar]
- 20.Nizet V, Gallo RL. Cathelicidins and innate defense against invasive bacterial infection. Scand J Infect Dis. 2003;35(9):670–6. 10.1080/00365540310015629 . [DOI] [PubMed] [Google Scholar]
- 21.Nizet V, Ohtake T, Lauth X, Trowbridge J, Rudisill J, Dorschner RA, et al. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature. 2001;414(6862):454–7. Epub 2001/11/24. 10.1038/35106587 . [DOI] [PubMed] [Google Scholar]
- 22.Lai Y, Li D, Li C, Muehleisen B, Radek KA, Park HJ, et al. The antimicrobial protein REG3A regulates keratinocyte proliferation and differentiation after skin injury. Immunity. 2012;37(1):74–84. Epub 2012/06/26. 10.1016/j.immuni.2012.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.MacLeod AS, Hemmers S, Garijo O, Chabod M, Mowen K, Witherden DA, et al. Dendritic epidermal T cells regulate skin antimicrobial barrier function. The Journal of clinical investigation. 2013;123(10):4364–74. Epub 2013/09/21. 10.1172/JCI70064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Handfield C, Kwock J, MacLeod AS. Innate Antiviral Immunity in the Skin. Trends in immunology. 2018;39(4):328–40. Epub 2018/03/13. 10.1016/j.it.2018.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coates M, Blanchard S, MacLeod AS. Innate antimicrobial immunity in the skin: A protective barrier against bacteria, viruses, and fungi. PLoS pathogens. 2018;14(12):e1007353 Epub 2018/12/07. 10.1371/journal.ppat.1007353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frohm M, Agerberth B, Ahangari G, Stahle-Backdahl M, Liden S, Wigzell H, et al. The expression of the gene coding for the antibacterial peptide LL-37 is induced in human keratinocytes during inflammatory disorders. The Journal of biological chemistry. 1997;272(24):15258–63. Epub 1997/06/13. . [DOI] [PubMed] [Google Scholar]
- 27.Glaser R, Meyer-Hoffert U, Harder J, Cordes J, Wittersheim M, Kobliakova J, et al. The antimicrobial protein psoriasin (S100A7) is upregulated in atopic dermatitis and after experimental skin barrier disruption. The Journal of investigative dermatology. 2009;129(3):641–9. Epub 2008/08/30. 10.1038/jid.2008.268 . [DOI] [PubMed] [Google Scholar]
- 28.Heilborn JD, Nilsson MF, Kratz G, Weber G, Sorensen O, Borregaard N, et al. The cathelicidin anti-microbial peptide LL-37 is involved in re-epithelialization of human skin wounds and is lacking in chronic ulcer epithelium. The Journal of investigative dermatology. 2003;120(3):379–89. Epub 2003/02/27. 10.1046/j.1523-1747.2003.12069.x . [DOI] [PubMed] [Google Scholar]
- 29.Ring HC, Theut Riis P, Zarchi K, Miller IM, Saunte DM, Jemec GB. Prodromal symptoms in hidradenitis suppurativa. Clinical and experimental dermatology. 2017;42(3):261–5. Epub 2017/02/15. 10.1111/ced.13025 . [DOI] [PubMed] [Google Scholar]
- 30.Jemec GB, Hansen U. Histology of hidradenitis suppurativa. Journal of the American Academy of Dermatology. 1996;34(6):994–9. Epub 1996/06/01. . [DOI] [PubMed] [Google Scholar]
- 31.Iglesias-Bartolome R, Uchiyama A, Molinolo AA, Abusleme L, Brooks SR, Callejas-Valera JL, et al. Transcriptional signature primes human oral mucosa for rapid wound healing. Science translational medicine. 2018;10(451). Epub 2018/07/27. 10.1126/scitranslmed.aap8798 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bechara FG, Sand M, Skrygan M, Kreuter A, Altmeyer P, Gambichler T. Acne inversa: evaluating antimicrobial peptides and proteins. Annals of dermatology. 2012;24(4):393–7. Epub 2012/12/01. 10.5021/ad.2012.24.4.393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Edgar R, Domrachev M, Lash AE. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic acids research. 2002;30(1):207–10. Epub 2001/12/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pinheiro J, Bates D, DebRoy S, Sarkar D, Team RC. nlme: Linear and Nonlinear Mixed Effects Models. R package version 31–137, https://CRANR-projectorg/package=nlme. 2018. [Google Scholar]
- 35.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society Series B (Methodological). 1995;57(1):289–300. [Google Scholar]
- 36.Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic acids research. 2015;43(7):e47 Epub 2015/01/22. 10.1093/nar/gkv007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Law CW, Chen Y, Shi W, Smyth GK. voom: Precision weights unlock linear model analysis tools for RNA-seq read counts. Genome biology. 2014;15(2):R29 Epub 2014/02/04. 10.1186/gb-2014-15-2-r29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kolde R. pheatmap: Pretty Heatmaps. R package version 077 http://CRANR-projectorg/package=pheatmap. 2013. [Google Scholar]
- 39.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nature Protocols. 2008;3:1101 10.1038/nprot.2008.73 [DOI] [PubMed] [Google Scholar]
- 40.Suwanpradid J, Shih M, Pontius L, Yang B, Birukova A, Guttman-Yassky E, et al. Arginase1 Deficiency in Monocytes/Macrophages Upregulates Inducible Nitric Oxide Synthase To Promote Cutaneous Contact Hypersensitivity. Journal of immunology (Baltimore, Md: 1950). 2017;199(5):1827–34. Epub 2017/07/28. 10.4049/jimmunol.1700739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eden E, Navon R, Steinfeld I, Lipson D, Yakhini Z. GOrilla: a tool for discovery and visualization of enriched GO terms in ranked gene lists. BMC bioinformatics. 2009;10:48 Epub 2009/02/05. 10.1186/1471-2105-10-48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hofmann SC, Saborowski V, Lange S, Kern WV, Bruckner-Tuderman L, Rieg S. Expression of innate defense antimicrobial peptides in hidradenitis suppurativa. Journal of the American Academy of Dermatology. 2012;66(6):966–74. Epub 2011/10/11. 10.1016/j.jaad.2011.07.020 . [DOI] [PubMed] [Google Scholar]
- 43.Supek F, Bošnjak M, Škunca N, Šmuc T. REVIGO Summarizes and Visualizes Long Lists of Gene Ontology Terms. None. 2011;6(7). 10.1371/journal.pone.0021800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Buchau AS, Hassan M, Kukova G, Lewerenz V, Kellermann S, Wurthner JU, et al. S100A15, an antimicrobial protein of the skin: regulation by E. coli through Toll-like receptor 4. The Journal of investigative dermatology. 2007;127(11):2596–604. Epub 2007/07/13. 10.1038/sj.jid.5700946 . [DOI] [PubMed] [Google Scholar]
- 45.Wolf R, Mirmohammadsadegh A, Walz M, Lysa B, Tartler U, Remus R, et al. Molecular cloning and characterization of alternatively spliced mRNA isoforms from psoriatic skin encoding a novel member of the S100 family. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2003;17(13):1969–71. Epub 2003/08/19. 10.1096/fj.03-0148fje . [DOI] [PubMed] [Google Scholar]
- 46.Bals R, Wang X, Wu Z, Freeman T, Bafna V, Zasloff M, et al. Human beta-defensin 2 is a salt-sensitive peptide antibiotic expressed in human lung. The Journal of clinical investigation. 1998;102(5):874–80. Epub 1998/09/03. 10.1172/JCI2410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Teigelkamp S, Bhardwaj RS, Roth J, Meinardus-Hager G, Karas M, Sorg C. Calcium-dependent complex assembly of the myeloic differentiation proteins MRP-8 and MRP-14. The Journal of biological chemistry. 1991;266(20):13462–7. Epub 1991/07/15. . [PubMed] [Google Scholar]
- 48.Broome AM, Ryan D, Eckert RL. S100 protein subcellular localization during epidermal differentiation and psoriasis. The journal of histochemistry and cytochemistry: official journal of the Histochemistry Society. 2003;51(5):675–85. Epub 2003/04/22. 10.1177/002215540305100513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Simpson AJ, Maxwell AI, Govan JR, Haslett C, Sallenave JM. Elafin (elastase-specific inhibitor) has anti-microbial activity against gram-positive and gram-negative respiratory pathogens. FEBS letters. 1999;452(3):309–13. Epub 1999/07/01. . [DOI] [PubMed] [Google Scholar]
- 50.Greco MA, Lorand L, Lane WS, Baden HP, Parameswaran KN, Kvedar JC. The pancornulins: a group of small proline rich-related cornified envelope precursors with bifunctional capabilities in isopeptide bond formation. The Journal of investigative dermatology. 1995;104(2):204–10. Epub 1995/02/01. . [DOI] [PubMed] [Google Scholar]
- 51.Mischke D, Korge BP, Marenholz I, Volz A, Ziegler A. Genes encoding structural proteins of epidermal cornification and S100 calcium-binding proteins form a gene complex ("epidermal differentiation complex") on human chromosome 1q21. The Journal of investigative dermatology. 1996;106(5):989–92. Epub 1996/05/01. . [DOI] [PubMed] [Google Scholar]
- 52.Mazzalupo S, Wong P, Martin P, Coulombe PA. Role for keratins 6 and 17 during wound closure in embryonic mouse skin. Developmental dynamics: an official publication of the American Association of Anatomists. 2003;226(2):356–65. Epub 2003/01/31. 10.1002/dvdy.10245 . [DOI] [PubMed] [Google Scholar]
- 53.Jinquan T, Vorum H, Larsen CG, Madsen P, Rasmussen HH, Gesser B, et al. Psoriasin: a novel chemotactic protein. The Journal of investigative dermatology. 1996;107(1):5–10. Epub 1996/07/01. . [DOI] [PubMed] [Google Scholar]
- 54.Kristiansen H, Gad HH, Eskildsen-Larsen S, Despres P, Hartmann R. The oligoadenylate synthetase family: an ancient protein family with multiple antiviral activities. Journal of interferon & cytokine research: the official journal of the International Society for Interferon and Cytokine Research. 2011;31(1):41–7. Epub 2010/12/15. 10.1089/jir.2010.0107 . [DOI] [PubMed] [Google Scholar]
- 55.Rebouillat D, Marie I, Hovanessian AG. Molecular cloning and characterization of two related and interferon-induced 56-kDa and 30-kDa proteins highly similar to 2'-5' oligoadenylate synthetase. European journal of biochemistry. 1998;257(2):319–30. Epub 1998/11/24. . [DOI] [PubMed] [Google Scholar]
- 56.Marshall D, Hardman MJ, Nield KM, Byrne C. Differentially expressed late constituents of the epidermal cornified envelope. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(23):13031–6. Epub 2001/11/08. 10.1073/pnas.231489198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bergboer JG, Tjabringa GS, Kamsteeg M, van Vlijmen-Willems IM, Rodijk-Olthuis D, Jansen PA, et al. Psoriasis risk genes of the late cornified envelope-3 group are distinctly expressed compared with genes of other LCE groups. The American journal of pathology. 2011;178(4):1470–7. Epub 2011/03/26. 10.1016/j.ajpath.2010.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jadoon S, Karim S, Akram MR, Kalsoom Khan A, Zia MA, Siddiqi AR, et al. Recent developments in sweat analysis and its applications. International journal of analytical chemistry. 2015;2015:164974 Epub 2015/04/04. 10.1155/2015/164974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schittek B, Hipfel R, Sauer B, Bauer J, Kalbacher H, Stevanovic S, et al. Dermcidin: a novel human antibiotic peptide secreted by sweat glands. Nature immunology. 2001;2(12):1133–7. Epub 2001/11/06. 10.1038/ni732 . [DOI] [PubMed] [Google Scholar]
- 60.Sjodin A, Guo D, Hofer PA, Henriksson R, Hedman H. Mammaglobin in normal human sweat glands and human sweat gland tumors. The Journal of investigative dermatology. 2003;121(2):428–9. Epub 2003/07/26. 10.1046/j.1523-1747.2003.12374.x . [DOI] [PubMed] [Google Scholar]
- 61.Cui CY, Yin M, Sima J, Childress V, Michel M, Piao Y, et al. Involvement of Wnt, Eda and Shh at defined stages of sweat gland development. Development (Cambridge, England). 2014;141(19):3752–60. Epub 2014/09/25. 10.1242/dev.109231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jaks V, Barker N, Kasper M, van Es JH, Snippert HJ, Clevers H, et al. Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nature genetics. 2008;40(11):1291–9. Epub 2008/10/14. 10.1038/ng.239 . [DOI] [PubMed] [Google Scholar]
- 63.Hoesl C, Rohrl JM, Schneider MR, Dahlhoff M. The receptor tyrosine kinase ERBB4 is expressed in skin keratinocytes and influences epidermal proliferation. Biochimica et biophysica acta General subjects. 2018;1862(4):958–66. Epub 2018/02/08. 10.1016/j.bbagen.2018.01.017 . [DOI] [PubMed] [Google Scholar]
- 64.Moll R, Divo M, Langbein L. The human keratins: biology and pathology. Histochemistry and cell biology. 2008;129(6):705–33. Epub 2008/05/08. 10.1007/s00418-008-0435-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Langbein L, Rogers MA, Praetzel S, Cribier B, Peltre B, Gassler N, et al. Characterization of a novel human type II epithelial keratin K1b, specifically expressed in eccrine sweat glands. The Journal of investigative dermatology. 2005;125(3):428–44. Epub 2005/08/25. 10.1111/j.0022-202X.2005.23860.x . [DOI] [PubMed] [Google Scholar]
- 66.Rogers MA, Edler L, Winter H, Langbein L, Beckmann I, Schweizer J. Characterization of new members of the human type II keratin gene family and a general evaluation of the keratin gene domain on chromosome 12q13.13. The Journal of investigative dermatology. 2005;124(3):536–44. Epub 2005/03/02. 10.1111/j.0022-202X.2004.23530.x . [DOI] [PubMed] [Google Scholar]
- 67.Nold MF, Nold-Petry CA, Zepp JA, Palmer BE, Bufler P, Dinarello CA. IL-37 is a fundamental inhibitor of innate immunity. Nature immunology. 2010;11(11):1014–22. Epub 2010/10/12. 10.1038/ni.1944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Inoue R, Sohara E, Rai T, Satoh T, Yokozeki H, Sasaki S, et al. Immunolocalization and translocation of aquaporin-5 water channel in sweat glands. Journal of dermatological science. 2013;70(1):26–33. Epub 2013/03/12. 10.1016/j.jdermsci.2013.01.013 . [DOI] [PubMed] [Google Scholar]
- 69.Lin KK, Kumar V, Geyfman M, Chudova D, Ihler AT, Smyth P, et al. Circadian clock genes contribute to the regulation of hair follicle cycling. PLoS genetics. 2009;5(7):e1000573 Epub 2009/07/25. 10.1371/journal.pgen.1000573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sporl F, Schellenberg K, Blatt T, Wenck H, Wittern KP, Schrader A, et al. A circadian clock in HaCaT keratinocytes. The Journal of investigative dermatology. 2011;131(2):338–48. Epub 2010/10/22. 10.1038/jid.2010.315 . [DOI] [PubMed] [Google Scholar]
- 71.Cui CY, Childress V, Piao Y, Michel M, Johnson AA, Kunisada M, et al. Forkhead transcription factor FoxA1 regulates sweat secretion through Bestrophin 2 anion channel and Na-K-Cl cotransporter 1. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(4):1199–203. Epub 2012/01/10. 10.1073/pnas.1117213109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Potter CS, Peterson RL, Barth JL, Pruett ND, Jacobs DF, Kern MJ, et al. Evidence that the satin hair mutant gene Foxq1 is among multiple and functionally diverse regulatory targets for Hoxc13 during hair follicle differentiation. The Journal of biological chemistry. 2006;281(39):29245–55. Epub 2006/07/13. 10.1074/jbc.M603646200 . [DOI] [PubMed] [Google Scholar]
- 73.Gudjonsson JE, Johnston A, Stoll SW, Riblett MB, Xing X, Kochkodan JJ, et al. Evidence for altered Wnt signaling in psoriatic skin. The Journal of investigative dermatology. 2010;130(7):1849–59. Epub 2010/04/09. 10.1038/jid.2010.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schlapbach C, Yawalkar N, Hunger RE. Human beta-defensin-2 and psoriasin are overexpressed in lesions of acne inversa. Journal of the American Academy of Dermatology. 2009;61(1):58–65. Epub 2009/03/03. 10.1016/j.jaad.2008.12.033 . [DOI] [PubMed] [Google Scholar]
- 75.Thorey IS, Roth J, Regenbogen J, Halle JP, Bittner M, Vogl T, et al. The Ca2+-binding proteins S100A8 and S100A9 are encoded by novel injury-regulated genes. The Journal of biological chemistry. 2001;276(38):35818–25. Epub 2001/07/21. 10.1074/jbc.M104871200 . [DOI] [PubMed] [Google Scholar]
- 76.Schroder JM, Harder J. Human beta-defensin-2. The international journal of biochemistry & cell biology. 1999;31(6):645–51. Epub 1999/07/15. . [DOI] [PubMed] [Google Scholar]
- 77.Harder J, Bartels J, Christophers E, Schroder JM. Isolation and characterization of human beta -defensin-3, a novel human inducible peptide antibiotic. The Journal of biological chemistry. 2001;276(8):5707–13. Epub 2000/11/22. 10.1074/jbc.M008557200 . [DOI] [PubMed] [Google Scholar]
- 78.Sobiak B, Graczyk-Jarzynka A, Lesniak W. Comparison of DNA Methylation and Expression Pattern of S100 and Other Epidermal Differentiation Complex Genes in Differentiating Keratinocytes. Journal of cellular biochemistry. 2016;117(5):1092–8. Epub 2015/10/08. 10.1002/jcb.25392 . [DOI] [PubMed] [Google Scholar]
- 79.Weiss RA, Eichner R, Sun TT. Monoclonal antibody analysis of keratin expression in epidermal diseases: a 48- and 56-kdalton keratin as molecular markers for hyperproliferative keratinocytes. The Journal of cell biology. 1984;98(4):1397–406. Epub 1984/04/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Moll R, Franke WW, Volc-Platzer B, Krepler R. Different keratin polypeptides in epidermis and other epithelia of human skin: a specific cytokeratin of molecular weight 46,000 in epithelia of the pilosebaceous tract and basal cell epitheliomas. The Journal of cell biology. 1982;95(1):285–95. Epub 1982/10/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Paladini RD, Takahashi K, Bravo NS, Coulombe PA. Onset of re-epithelialization after skin injury correlates with a reorganization of keratin filaments in wound edge keratinocytes: defining a potential role for keratin 16. The Journal of cell biology. 1996;132(3):381–97. Epub 1996/02/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wawersik MJ, Mazzalupo S, Nguyen D, Coulombe PA. Increased levels of keratin 16 alter epithelialization potential of mouse skin keratinocytes in vivo and ex vivo. Molecular biology of the cell. 2001;12(11):3439–50. Epub 2001/11/06. 10.1091/mbc.12.11.3439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Demirkesen C, Hoede N, Moll R. Epithelial markers and differentiation in adnexal neoplasms of the skin: an immunohistochemical study including individual cytokeratins. Journal of cutaneous pathology. 1995;22(6):518–35. Epub 1995/12/01. . [DOI] [PubMed] [Google Scholar]
- 84.Schittek B. The multiple facets of dermcidin in cell survival and host defense. Journal of innate immunity. 2012;4(4):349–60. Epub 2012/03/30. 10.1159/000336844 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Baechle D, Flad T, Cansier A, Steffen H, Schittek B, Tolson J, et al. Cathepsin D is present in human eccrine sweat and involved in the postsecretory processing of the antimicrobial peptide DCD-1L. The Journal of biological chemistry. 2006;281(9):5406–15. Epub 2005/12/16. 10.1074/jbc.M504670200 . [DOI] [PubMed] [Google Scholar]
- 86.Schoggins JW, Rice CM. Interferon-stimulated genes and their antiviral effector functions. Current opinion in virology. 2011;1(6):519–25. Epub 2012/02/14. 10.1016/j.coviro.2011.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Luu K, Greenhill CJ, Majoros A, Decker T, Jenkins BJ, Mansell A. STAT1 plays a role in TLR signal transduction and inflammatory responses. Immunology and cell biology. 2014;92(9):761–9. Epub 2014/07/17. 10.1038/icb.2014.51 . [DOI] [PubMed] [Google Scholar]
- 88.Nie C, Wang H, Wang R, Ginsburg D, Chen XW. Dimeric sorting code for concentrative cargo selection by the COPII coat. Proceedings of the National Academy of Sciences of the United States of America. 2018;115(14):E3155–e62. Epub 2018/03/21. 10.1073/pnas.1704639115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Matta B, Song S, Li D, Barnes BJ. Interferon regulatory factor signaling in autoimmune disease. Cytokine. 2017;98:15–26. Epub 2017/03/12. 10.1016/j.cyto.2017.02.006 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lai CY, Su YW, Lin KI, Hsu LC, Chuang TH. Natural Modulators of Endosomal Toll-Like Receptor-Mediated Psoriatic Skin Inflammation. Journal of immunology research. 2017;2017:7807313 Epub 2017/09/13. 10.1155/2017/7807313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bao X, Faris AE, Jang EK, Haslam RJ. Molecular cloning, bacterial expression and properties of Rab31 and Rab32. European journal of biochemistry. 2002;269(1):259–71. Epub 2002/01/11. . [DOI] [PubMed] [Google Scholar]
- 92.Han CS, Chen Y, Ezashi T, Roberts RM. Antiviral activities of the soluble extracellular domains of type I interferon receptors. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(11):6138–43. Epub 2001/05/10. 10.1073/pnas.111139598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhu L, Okano S, Takahara M, Chiba T, Tu Y, Oda Y, et al. Expression of S100 protein family members in normal skin and sweat gland tumors. Journal of dermatological science. 2013;70(3):211–9. Epub 2013/04/30. 10.1016/j.jdermsci.2013.03.002 . [DOI] [PubMed] [Google Scholar]
- 94.Zafrakas M, Petschke B, Donner A, Fritzsche F, Kristiansen G, Knuchel R, et al. Expression analysis of mammaglobin A (SCGB2A2) and lipophilin B (SCGB1D2) in more than 300 human tumors and matching normal tissues reveals their co-expression in gynecologic malignancies. BMC cancer. 2006;6:88 Epub 2006/04/11. 10.1186/1471-2407-6-88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nejsum LN, Kwon TH, Jensen UB, Fumagalli O, Frokiaer J, Krane CM, et al. Functional requirement of aquaporin-5 in plasma membranes of sweat glands. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(1):511–6. Epub 2002/01/05. 10.1073/pnas.012588099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Horvath B, Janse IC, Blok JL, Driessen RJ, Boer J, Mekkes JR, et al. Hurley Staging Refined: A Proposal by the Dutch Hidradenitis Suppurativa Expert Group. Acta dermato-venereologica. 2017;97(3):412–3. Epub 2016/08/19. 10.2340/00015555-2513 . [DOI] [PubMed] [Google Scholar]
- 97.Cotsarelis G. Epithelial stem cells: a folliculocentric view. The Journal of investigative dermatology. 2006;126(7):1459–68. Epub 2006/06/17. 10.1038/sj.jid.5700376 . [DOI] [PubMed] [Google Scholar]
- 98.Rittie L, Sachs DL, Orringer JS, Voorhees JJ, Fisher GJ. Eccrine sweat glands are major contributors to reepithelialization of human wounds. The American journal of pathology. 2013;182(1):163–71. Epub 2012/11/20. 10.1016/j.ajpath.2012.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sartorius K, Killasli H, Oprica C, Sullivan A, Lapins J. Bacteriology of hidradenitis suppurativa exacerbations and deep tissue cultures obtained during carbon dioxide laser treatment. The British journal of dermatology. 2012;166(4):879–83. Epub 2011/11/22. 10.1111/j.1365-2133.2011.10747.x . [DOI] [PubMed] [Google Scholar]
- 100.Meisel JS, Sfyroera G, Bartow-McKenney C, Gimblet C, Bugayev J, Horwinski J, et al. Commensal microbiota modulate gene expression in the skin. Microbiome. 2018;6(1):20 Epub 2018/01/31. 10.1186/s40168-018-0404-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Biedermann T, Pontiggia L, Bottcher-Haberzeth S, Tharakan S, Braziulis E, Schiestl C, et al. Human eccrine sweat gland cells can reconstitute a stratified epidermis. The Journal of investigative dermatology. 2010;130(8):1996–2009. Epub 2010/04/09. 10.1038/jid.2010.83 . [DOI] [PubMed] [Google Scholar]
- 102.Lu CP, Polak L, Rocha AS, Pasolli HA, Chen SC, Sharma N, et al. Identification of stem cell populations in sweat glands and ducts reveals roles in homeostasis and wound repair. Cell. 2012;150(1):136–50. Epub 2012/07/10. 10.1016/j.cell.2012.04.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rittie L, Farr EA, Orringer JS, Voorhees JJ, Fisher GJ. Reduced cell cohesiveness of outgrowths from eccrine sweat glands delays wound closure in elderly skin. Aging cell. 2016;15(5):842–52. Epub 2016/05/18. 10.1111/acel.12493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dai X, Okazaki H, Hanakawa Y, Murakami M, Tohyama M, Shirakata Y, et al. Eccrine sweat contains IL-1alpha, IL-1beta and IL-31 and activates epidermal keratinocytes as a danger signal. PloS one. 2013;8(7):e67666 Epub 2013/07/23. 10.1371/journal.pone.0067666 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(XLSX)
(XLSX)
Data Availability Statement
Hidradenitis microarray data set (GSE72702) was previously published by Blok et al. 2016 and is publicly available at https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE72702. Wound healing RNA seq data set (GSE97615) was previously published by Iglesias-Bartolome et al. 2018 and is publicly available at https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE97615.