Abstract
Phosphatidic acid produced by phospholipase D (PLD) as a result of signaling activity is thought to play a role in membrane vesicle trafficking, either as an intracellular messenger or as a cone-shaped lipid that promotes membrane fusion. We recently described that, in neuroendocrine cells, plasma membrane-associated PLD1 operates at a stage of Ca2+-dependent exocytosis subsequent to cytoskeletal-mediated recruitment of secretory granules to exocytotic sites. We show here that PLD1 also plays a crucial role in neurotransmitter release. Using purified rat brain synaptosomes subjected to hypotonic lysis and centrifugation, we found that PLD1 is associated with the particulate fraction containing the plasma membrane. Immunostaining of rat cerebellar granule cells confirmed localization of PLD1 at the neuronal plasma membrane in zones specialized for neurotransmitter release (axonal neurites, varicosities, and growth cone-like structures). To determine the potential involvement of PLD1 in neurotransmitter release, we microinjected catalytically inactive PLD1(K898R) into Aplysia neurons and analyzed its effects on evoked acetylcholine (ACh) release. PLD1(K898R) produced a fast and potent dose-dependent inhibition of ACh release. By analyzing paired-pulse facilitation and postsynaptic responses evoked by high-frequency stimulations, we found that the exocytotic inhibition caused by PLD1(K898R) was not the result of an alteration in stimulus-secretion coupling or in vesicular trafficking. Analysis of the fluctuations in amplitude of the postsynaptic responses revealed that the PLD1(K898R) blocked ACh release by reducing the number of active presynaptic-releasing sites. Our results provide evidence that PLD1 plays a major role in neurotransmission, most likely by controlling the fusogenic status of presynaptic release sites.
Membrane fusion has been intensively investigated in the context of Ca2+-dependent exocytosis at neurons and neuroendocrine cells (1, 2). Presently best defined are proteins such as the SNAREs that catalyze membrane bilayer mixing by assembling into highly stable helical bundles and bringing membranes into close proximity (3, 4). Less well understood is the role of membrane lipid composition at the fusion site; although it has been proposed that this should critically affect lipid bilayer intermediate structures formed during the fusion process (5, 6).
Phosphatidylcholine (PC)-specific phospholipase D (PLD) catalyzes the hydrolysis of PC to produce choline and phosphatidic acid (PA). PA produced by PLD as a result of signaling activity is thought to play a role in many cellular functions including membrane vesicle trafficking, either as an intracellular messenger or as a cone-shaped lipid that promotes negative membrane curvature and fusion (7–9). There are two mammalian PLD genes (10–12), and the encoded proteins, PLD1 and PLD2, are ≈50% identical. Within cells, the PLDs become activated in response to a wide variety of agonists, including hormones, neurotransmitters, growth factors, and cytokines, that signal through heterotrimeric G protein-coupled or tyrosine kinase receptors (8, 13).
We recently reported that in chromaffin cells and in their tumor cell derivatives, PC12, plasma membrane-associated PLD1 plays an important role in Ca2+-dependent exocytosis, operating a late stage subsequent to the cytoskeletal-mediated recruitment of secretory granules to exocytotic sites (14). We proposed that PLD1 constitutes a key factor for the late fusion event. Because Ca2+-dependent membrane fusion proceeds through a closely related mechanism in secretory cells and neurons, our results raised the question of whether PLD1 also may play a role in neurotransmitter release. We show here that PLD1 is present in rat nerve terminals at the presynaptic plasma membrane. By monitoring acetylcholine (ACh) release from identified cholinergic neurons in the Aplysia buccal ganglion, we found that PLD1 plays a major role in neurotransmission by controlling the number of functional release sites at nerve terminals.
Materials and Methods
Cerebellar Granule Cells.
Primary cultures of cerebellar granule cells were prepared according to Stewart et al. (15) from 5-day-old Wistar rats. Granule cells were placed in Petri dishes (35 mm) previously coated with polyornithine (0.5 mg/ml) and cultivated in DMEM containing horse serum (10%), insulin (5 × 10−7 M), and KCl (25 mM). Cells were used 7–14 days in culture.
Isolation and Fractionation of Synaptosomes from Rat Brain and Aplysia Nerve Tissue.
Synaptosomes prepared from rat brain according to Huttner et al. (16) were resuspended in buffered sucrose, diluted with 9 vol of ice-cold H2O (hypotonic lysis), immediately homogenized, and then centrifuged for 20 min at 25,500 × g. The pellet containing synaptosomal membranes (i.e., plasma membrane, mitochondria, and granules) was saved. The supernatant was centrifuged for 2 h at 48,000 rpm in a Ti60 rotor (Beckman Coulter). The resulting supernatant was centrifuged at 100,000 × g and the pellet (crude synaptic vesicles) was saved.
Nerve ganglia dissected from Aplysia californica (70–120 g body weight; Marinus, Long Beach, CA) were homogenized in Tris buffer containing: 10 mM Tris (pH 7.5), 2 mM MgCl2, 1 mM DTT, 10 mg/ml leupeptin, 1 μM pepstatin, and 0.1 mM PMSF. Complete lysis was achieved by three cycles of freeze-thawing. The preparations were then centrifuged (15,000 rpm for 15 min), and the pellet containing membrane fractions but not the cytosolic and crude vesicle fractions was saved. Proteins of the pellet were extracted in Tris buffer containing 1% Triton X-100 for 20 min at room temperature.
Samples, 10 μg (rat) or 20 μg (Aplysia) proteins were subjected to SDS/10% polyacrylamide gels, transferred to nitrocellulose for Western blotting, and incubated with 1:50 dilution rabbit Abs against PLD1 and PLD2 (QCB, BioSource International, Camarillo, CA) in PBS-BSA 1% at room temperature for 2 h. Immunoblots were analyzed by chemoluminescence with ECL plus kit (Amersham Pharmacia).
Immunofluorescence and Confocal Microscopy.
For double-labeling immunocytochemistry, cerebellar granule cells were fixed with 4% paraformaldehyde in PBS for 20 min, permeabilized for 10 min in PBS containing 0.1% Triton X-100, and further incubated for 1 h in 10% normal goat serum in PBS to block nonspecific staining. The cells were then incubated overnight at 4°C with primary Abs: rabbit anti-PLD1 (1:50), mouse monoclonal anti-SNAP25 (1:400; Sternberger–Meyer, Jarrettsville, MD), or mouse monoclonal anti-synaptophysin (1:400; Sigma), washed and incubated for 1 h with Alexa 488-labeled anti-mouse (1:1,000; Molecular Probes) and Cynanine 3-labeled anti-rabbit Abs (1:1,000; Jackson ImmunoResearch). Stained cells were visualized as described (17) by using a Zeiss confocal microscope LSM 510. For immunocytochemistry on Aplysia ganglion, a similar procedure was applied except that fixation buffer was corrected for osmolarity (1,260 mOsm, final). Transversal sections of the fixed buccal ganglion were done with a cryostat (10 μm) after cryo-protection overnight with 30% sucrose in PBS.
ACh Release and Electrical Recordings at Aplysia Synapses.
Experiments were performed at 22°C at identified cholinergic synapses in buccal ganglia of Aplysia according to previously published procedures (18–20). Ganglia were superfused continuously (50 ml/h) with physiological medium containing 460 mM NaCl, 10 mM KCl, 33 mM CaCl2, 50 mM MgCl2, 28 mM MgSO4, and 10 mM Tris (pH 7.5). When needed, the extracellular [Ca2+/Mg2+] ratio was modified as described (19, 20). In brief, two presynaptic cholinergic interneurons and one postsynaptic neuron in the buccal ganglion were impaled with two glass microelectrodes (3 M KCl, Ag/AgCl2, 2–10 Mohms). ACh release from a presynaptic neuron was stimulated by evoking an action potential every 40 sec. When needed, trains of stimuli (50 Hz, 1 sec) were generated (20). Postsynaptic Cl−-dependent responses caused by evoked ACh release were recorded as inhibitory postsynaptic current (IPSC) by using the conventional two-electrode voltage-clamp technique. The IPSC amplitude I subsequently was converted to an apparent membrane conductance change by taking into account the null potential for Cl− (i.e., the reversal potential of the postsynaptic response). The holding potential of the postsynaptic neuron was maintained 30 mV above ECl−.
Intraneuronal Application of PLD Proteins.
GluGlu-tagged catalytically inactive PLD1(K898R) or PLD2(K758R) proteins were prepared and purified on an affinity column as described (14, 21), mixed with a vital dye (fast green FCF, 10% vol/vol; Sigma), and air-pressure injected into presynaptic neurons under visual and electrophysiological monitoring as described (18–20). The injected volume was in the range of 1% of the cell body volume.
Determination IPSC Mean Amplitude and Variances.
When ACh release was stable, average IPSC amplitude Iav was calculated and corresponding variance, V, was determined from the fluctuation of the IPSCs around Iav. In nonstationary conditions, Iav values were obtained by local linear fitting of the I = f(t). The window width used for local fitting was between 9 and 15 data points. The ensemble of IPSC fluctuations around Iav was generated by subtracting the I data to the corresponding fit value. The variance of the fluctuations around fit (i.e., around Iav) was calculated in a window (width = 20) that was moving with a step of 1 along the whole data range to be analyzed. Then the V = f(Iav) plots were constructed.
Results
Subcellular Distribution of PLD1 in Rat Neurons.
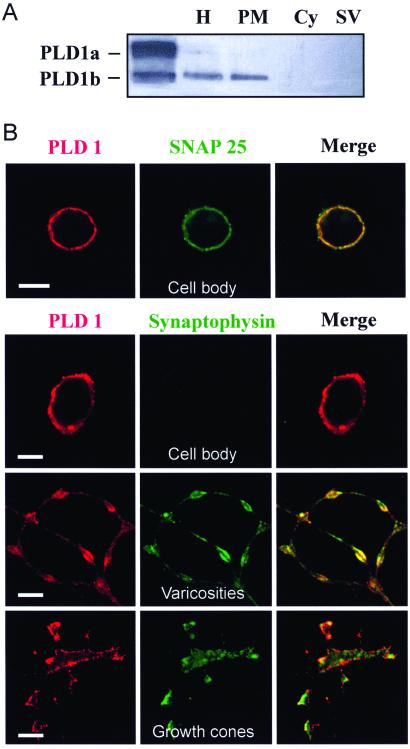
The intracellular distribution of PLD1 in presynaptic nerve terminals of rat brain neurons was first investigated (Fig. 1A). Immunoreactivity was detected in the particulate fraction containing the synaptosomal plasma membrane and comigrating with recombinant PLD1b, a splice variant lacking exon 17 (22). PLD1 immunoreactivity was not detected in the crude synaptic vesicle fraction. Immunostaining on cultured rat cerebellar granule cells with anti-PLD1 Abs revealed a peripheral fluorescent pattern very similar to that obtained with anti-SNAP-25 Abs (Fig. 1B), confirming the localization of PLD1 at the neuronal plasma membrane. To visualize the neuronal distribution of synaptic vesicles, granule cells were labeled with an anti-synaptophysin Ab (Fig. 1B). Synaptic vesicles were found in axonal neurites, varicosities, and growth cone-like structures but not in cell bodies. Although PLD1 did not precisely colocalize with the sites of punctuate synaptophysin staining, it was detected in membrane areas close to the regions enriched in synaptic vesicles, suggesting that it is present in zones specialized for transmitter release.
Figure 1.
PLD1 localization to the neuronal plasma membrane. (A) Brain synaptosomes lysed by hypotonic shock were homogenized (H) and processed to separate the cytosol (Cy), the membrane-bound compartment (PM), and the crude synaptic vesicle fraction (SV). Proteins (10 μg) were subjected to gel electrophoresis and immunodetection on nitrocellulose sheets by using anti-PLD1 Abs. Lysates from HEK293 cells transfected with pCGN-PLD1a and pCGN-PLD1b were used as positive controls. (B) Immunofluorescent confocal micrographs of cultured cerebellar granule cells double-labeled with anti-PLD1 Abs (red) and anti-SNAP-25 or anti-synaptophysin Abs (green). (Bar = 5 μm.)
Catalytically Inactive PLD1 Inhibits Evoked ACh Release.
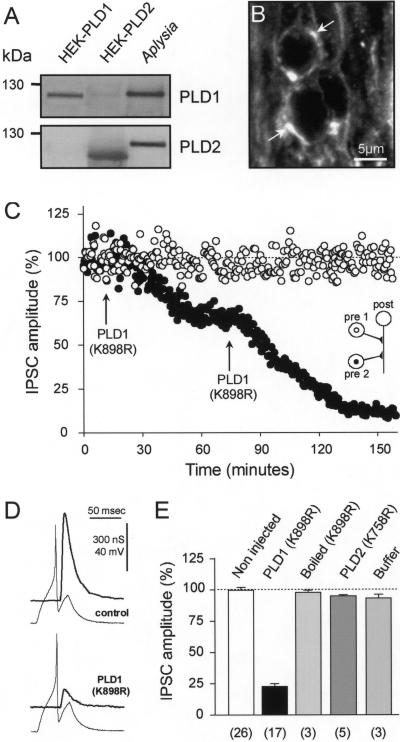
To functionally determine the synaptic role(s) of PLD1, we used identified cholinergic synapses in the buccal ganglion of Aplysia (18–20). As revealed by Western blotting, a protein corresponding by molecular weight to PLD was found in Aplysia nerve tissue (Fig. 2A). However, it was recognized by both anti-PLD1 and anti-PLD2 Abs and therefore cannot be referred as being PLD1 or PLD2 (Fig. 2A). PLD-like immunoreactivity was found in the ganglion neuropile where the periphery of thin axons processes was decorated (Fig. 2B). This finding suggests that PLD is associated with the membrane of axonal processes or nerve terminals making synapses with axons. To probe the role of PLD1 in ACh release, we examined the effect on IPSC amplitude of microinjecting the catalytically inactive PLD1(K898R) into one of the identified presynaptic cholinergic neurons in the buccal ganglion (see scheme in Fig. 2C). The second presynaptic neuron was kept noninjected to have an internal control for the ability of the postsynaptic neuron to respond to ACh during the course of the experiment. As illustrated in Fig. 2 C and D, PLD1(K898R) produced a fast and potent dose-dependent inhibition of ACh release. We estimated that after each intracellular injection, the intraneuronal concentration of PLD1(K898R) was increased by 20–40 nM. Upon repeating the injection, ACh release could be inhibited by >90% (Fig. 2C). Neither the action potential that triggers ACh release at nerve terminals nor the transmembrane-resting potential nor the membrane resistance of the injected neuron were significantly modified after injection of the recombinant protein (Fig. 2D). Thus, the inhibition of ACh release induced by the inactive PLD1(K898R) was not caused by a modification of membrane excitability.
Figure 2.
Injection of catalytically inactive PLD1 inhibits evoked-ACh release in Aplysia neurons. (A) Lyzed Aplysia ganglia membrane-bound proteins (20 μg) were subjected to gel electrophoresis and immunodetection by using anti-PLD1 or anti-PLD2 Abs. Lysates from HEK293 cells expressing PLD1 or PLD2 were used as controls. (B) Immunofluorescent confocal micrographs of Aplysia buccal ganglion neuropile labeled with anti-PLD1 Abs. Note that PLD1 outlined axon (arrows). (C) Amplitude of IPSCs evoked at identified synapses in the buccal ganglion of Aplysia was averaged for periods of 10 min (mean ± SD) and plotted against time. PLD1(K898R) was pressure injected (arrow) in one of the presynaptic neurons (●). The second presynaptic neuron was not injected (○). (D) Representative presynaptic action potentials and IPSCs recorded before (control) and 120 min after PLD1(K898R) injection. (E) ACh release measured 120 min after injection and expressed relative to the initial response obtained before injection. For control, neurons were injected with PLD2(K758R), buffer, or boiled PLD1(K898R). Values in brackets indicate the number of experiments.
The mean inhibition of ACh release induced by PLD1(K898R), calculated 120 min after injection, is shown in Fig. 2E. At the same concentration, PLD1(K898R) previously boiled at 100°C for 10 min did not significantly affect ACh release, nor did the unboiled catalytically inactive PLD2(K758R). Taken together, these observations strongly suggest that PLD1 regulates a rate-limiting step of exocytosis in neurons. The low concentration of inactive PLD1 required to efficiently block neurotransmission is compatible with PLD1 exerting its action on a very limited number of sites. Because PLD1 decorates the plasma membrane, these few sites may correspond to the presynaptic active zones.
PLD1 Does Not Control Ca2+ Entry or Ca2+ Sensitivity of the ACh Release Process in Aplysia Neurons.
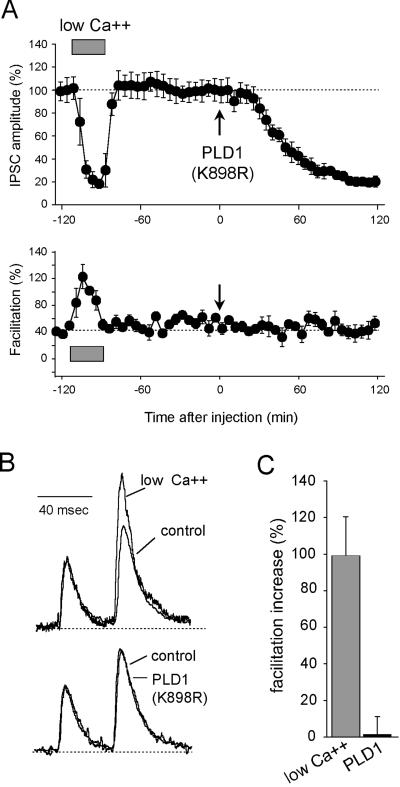
Next, we examined whether the catalytically inactive PLD1 inhibited ACh release by altering the coupling of the exocytotic machinery to Ca2+. Paired-pulse facilitation (PPF) denotes the increase in amplitude of a test response as compared to the conditioning response elicited by paired stimuli. PPF amplitude depends on two main parameters: (i) the residual intraterminal [Ca2+] that potentiates the release probability at the time when the test stimulus is applied and (ii) the number of synaptic vesicles that remain releasable after conditioning stimulation (i.e., vesicles brought by replenishment mechanisms and those that had not fused during conditioning stimulus). Both parameters depend on the release probability (i.e., the Ca2+ gradient) when the conditioning stimulus is applied. Hence, PPF may be used to detect eventual changes in the Ca2+-release coupling or/and Ca2+-buffering capacity in the nerve terminals. We compared the PPF observed when ACh release was inhibited either by application of low extracellular [Ca2+] or by injection of PLD1(K898R). Consistent with our previous observations (18, 19), PPF was strongly increased when extracellular [Ca2+/Mg2+] was reduced (Fig. 3; low Ca2+). In contrast, despite the strong reduction in ACh release, PPF was not significantly modified in PLD1(K898R)-injected neurons (Fig. 3). Thus, PLD1(K898R) is unlikely to inhibit ACh release by interfering with the influx and elevation of cytosolic calcium or by modifying in some way the Ca2+-sensitivity of the synaptic vesicle exocytotic machinery.
Figure 3.
PPF in PLD1(K898R)-injected neurons. Paired stimuli (40-msec interpulse interval) were applied in control and low extracellular [Ca2+/Mg2+] (0.42 and 0.14, respectively) and after injection of PLD1(K898R). (A) Amplitude of the first IPSCs averaged for periods of 10 min during the course of the experiment. The mean PPFs measured during the same periods of time are presented (Lower). External [Ca2+/Mg2+] = 0.14 (hatched bar). Pressure injection of PLD1(K898R) (Arrow). (B) Traces recorded at time −90 min (low [Ca2+/Mg2+]), −10 min (control), and +100 min [PLD1(K898R)]. For comparison, responses were scaled at the first IPSC in the pair. (C) At the same level of ACh release (see A), PPF was averaged and represented as the percentage increase in PPF relative to the control situation.
PLD1 Is Not Involved in the Recruitment/Trafficking of Synaptic Vesicles in Aplysia Neurons.
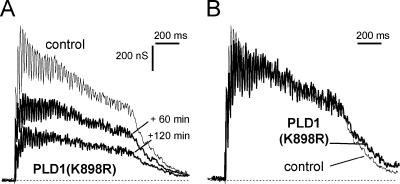
We have previously shown that alterations in vesicular trafficking modifies the time course of the complex postsynaptic response obtained by applying brief high-frequency train of stimuli to the presynaptic neuron (20). Trains of stimuli were elicited at 50 Hz for 1 sec before and after PLD1(K898R)-induced blockage of ACh release. Under control conditions, after a short-lasting facilitation, ACh release declined slightly during a 50-Hz train (Fig. 4A), probably because of an imbalance between the replenishment of the readily releasable pool of vesicles and the number of vesicles that undergo exocytosis. In PLD1(K898R)-injected neurons, ACh release evoked by a 50-Hz stimulation train was greatly inhibited (Fig. 4A) but, the overall time course of the train response, as seen when traces are normalized to first response of the train (Fig. 4B), was not modified. This finding suggests that injection of PLD1(K898R) did not affect the trafficking or recruitment of synaptic vesicles to the sites of exocytosis in the nerve terminals.
Figure 4.
Effect of repetitive stimulation on PLD1(K898R)-induced blockage of ACh release. (A) Trains of stimuli (50 Hz, 1 sec) were elicited before (control) and at the indicated times after presynaptic injection of PLD1(K898R), and postsynaptic responses were recorded. (B) For comparison, the responses to 50-Hz trains before (thin line) and 120 min after PLD1(K898R) injection (thick line) were scaled at the first response of the train.
PLD1 Affects the Number of Functional Release Sites in Aplysia Neurons.
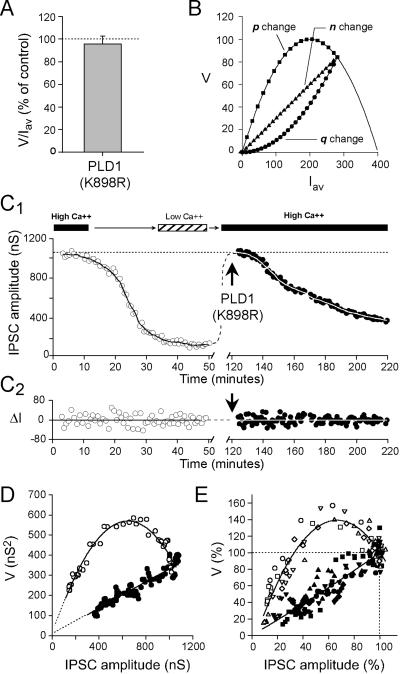
The analysis of the relationship between mean IPSC amplitude, Iav, and the variance of IPSC fluctuations, V, is a conventional way to get insights into the quantal parameters of the neurotransmitter release process (23–25). Indeed, Iav can be described by the combination of three parameters: n, the number of functional release sites, p, the average release probability at these sites, and q the average amplitude of the elementary response because of exocytosis of the vesicle content. Accordingly, Iav = npq and, if we assume that p and q are uniform between the release sites, and that quantal release follows binomial statistics, V = np(1 − p)q2. The ratio V/Iav = q(1 − p) can be used to determined whether q and/or p are changed after a treatment. We found that after near stabilization of PLD1(K898R)-induced inhibition of ACh release V/Iav was not distinct from that calculated before injection (Fig. 5A). Because unaffected PPF at PLD1(K898R)-injected neurons (Fig. 3) suggests that p is unmodified, unchanged V/Iav indicates that q remains unaltered at PLD1(K898R)-injected synapses. Therefore, a decrease in the amount ACh released upon fusion of individual synaptic vesicle is unlikely to account for the inhibition induced by PLD1(K898R). Hence, impairment of PLD1 activity would reduce n, the number of functional release sites. To confirm this deduction, we examined the V = f(Iav) relationship during the course of ACh release inhibition by PLD1(K898R). Indeed, V = f(Iav) plots allows to graphically determine which of n, p, or q is modified and responsible for the changes in IPSC amplitude. When only p is modified, V = f(Iav) follows a simple parabola V = Iavq − Iav2/n (Fig. 5B; refs. 24 and 25). When only n is changed, V is a linear function of Iav: V = q(1 − p)Iav. When only q is modified, V = f(Iav) is a quadratic function of positive curvature: V = [(1 − p)/(np)]Iav2 (Fig. 5B).
Figure 5.
Catalytically inactive PLD1 inhibits neurotransmission by decreasing the number of functional presynaptic release sites. (A) Variance to mean amplitude of IPSC ratio (V/Iav) determined 120 min after PLD1(K898R) injection and normalized to value before injection. (B) Using a binomial model for release, consequences on V = f(Iav) were simulated when IPSC amplitude was decreased after p, n, or q reduction. Note the typical graphical signature for each quantal parameter change. (C1) Representative experiment in which IPSC amplitude was reduced by decreasing the external [Ca2+/Mg2+] from 2.1 to low 0.14 (opens symbols). Then, after returning to high [Ca2+/Mg2+], PLD1(K898R) was injected (closed symbols). The solid lines corresponds to the ensemble Iav of the data. (C2) IPSC fluctuations (ΔI) around their mean are calculated by subtracting Iav to each IPSC amplitude value. (D) The corresponding V = f(Iav) plots. (E) Normalized cumulative plots of five similar experiments. Note that V data were illustrated by stepping five data.
Lowering the extracellular [Ca2+/Mg2+] ratio from 2.1 to 0.14 decreased ACh release (Fig. 5C) by affecting p and, accordingly, in the five experiments performed, V = f(Iav) followed a parabola (Fig. 5 D and E). The parabola started clearly beyond its peak. This indicated that by using [Ca2+/Mg2+] = 2.1 medium, clear distinction between n or p or q change could be made. In the same set of experiments, after returning the [Ca2+/Mg2+] ratio to 2.1, catalytically inactive PLD1 was injected to decrease ACh release. The corresponding V = f(Iav) plots were linear (Fig. 5 D and E). Consistent with above results, this indicates that the inactive PLD1 inhibited ACh release by progressively reducing n, the number of active release sites without altering p or q.
Discussion
PLD has been proposed previously to act in regulated exocytosis in various secretory cell types (9), such as neutrophils (26), platelets (27), and mast cells (28). By using a variety of direct means to probe the role of PLD, we recently demonstrated that a plasma membrane-associated PLD1 acts at a late stage of the secretory pathway in chromaffin and PC12 cells (14). We now extend this concept to the fastest known exocytotic process: the vesicular release of neurotransmitter. Our results show that PLD1 is associated with the neuronal plasma membrane. Moreover, PLD1 accumulates in neuronal regions known to exhibit high levels of exo-endocytosis activities such as varicosities that release neurotransmitter or the growth cones that sprout or retract by membrane incorporation or retrieval. Our observation that catalytically inactive PLD1(K898R) injected into neurons acts as a dominant negative and inhibits neurotransmitter release indicates that PLD1 activity is rate limiting for neurotransmitter release. This is fully consistent with our previous demonstration that plasma membrane-associated PLD1 acts at a late stage of the secretory pathway in chromaffin and PC12 cells. By analyzing PPF, sustained postsynapytic responses and the fluctuations in amplitude of postsynaptic responses, we found that the exocytotic block caused by catalytically inactive PLD1(K898R) was not the result of an alteration in the stimulus-secretion coupling. Moreover, it was not due to a change in the amount of transmitter released upon fusion of individual synaptic vesicles but resulted solely from a reduction in the number of active releasing sites. This important deduction is in line with our observation that a low concentration of inactive PLD1(K898R) was sufficient to cause a potent inhibition in ACh release. A direct action on synaptic vesicles (>1,000 vesicles/button in Aplysia) would have required many more inactive PLD molecules per synaptic button. Hence, our data are consistent with our findings in chromaffin and PC12 cells that PLD1 operates subsequent to the cytoskeletal-mediated recruitment of secretory granules to the exocytotic sites, possibly at the level of the fusion machinery or the fusion pore expansion (14).
Two possible mechanisms may explain how PLD1 regulates the transition of a release site from a nonfusogenic to a fusogenic state. First, PLD1 by generating PA-rich domains in the plasma membrane may recruit or activate a protein essential for fusion. Second, PA-rich domains in the plasma membrane may promote the mixing of vesicle and plasma membrane lipids during fusion. This latter possibility is consistent with a current proposal that the membrane curvature changes accompanying vesicle budding and fusion events should be facilitated by generating lipids that cause a local distortion of the lipid bilayer (29, 30). The propensity of the cone-shaped PA to promote membrane fusion by bending a lipid monolayer into a negative curvature as opposed to inverted-cones (lysophospholipids), which inhibit fusion when added between fusing membranes (6), brings additional support to this hypothesis.
The rapid blocking action of PLD1(K898R) on ACh release, which started after a delay of ≈10 min and became maximal in <1 h, suggests that the lifetime of PA-rich domains in the plasma membrane is limited and that there was a rapid exchange between the inactive PLD1 molecules and the endogenous PLD1 present at the plasma membrane. This raises the questions of the regulatory mechanisms that control PLD1 recruitment and activation at the release sites. PLD is synergistically stimulated by ADP-ribosylation factors (ARFs), members of the Rho family (including Rac1), Ral, and protein kinase C (22, 31). In chromaffin cells, ARF6 controls exocytosis via activation of downstream effectors including PLD1 (14, 32, 33). Overexpression of guanine nucleotide exchange factor for ARF (ARF-GEF) in Xenopus neuromuscular junction increases both spontaneous exocytosis frequency and the number of quanta released per stimulus (34), suggesting that the ARF pathway may also control neuronal neurotransmitter release. Moreover, our previous work on Aplysia synapses suggested as well a role for Rac1 (19). Because Rac1 and RalA associate with synaptic vesicles (19, 35), an attractive possibility would be that Rac1, RalA, and/or ARF stimulate the plasma membrane-associated PLD1 upon docking of a synaptic vesicle at the active zone. PLD1 activity also depends on the presence of polyphosphoinositides such as PI(4,5)P2 and PI(3,4,5)P3 (22), which are present at release sites (36–38). This finding suggests that the release site environment presents optimal conditions for PLD1 activation. The residency time during which synaptic vesicles stay docked on the plasma membrane before fusion has been evaluated to 2–5 sec (39, 40). One PLD1 molecule can produce ≈2 PA molecule⋅sec−1 according to Hammond et al. (22). This finding suggests that activation of one PLD1 molecule might be sufficient to create a local PA-rich microdomain that conditions the fusogenic status of the release site, in an all or none manner. This would explain why injection of catalytically inactive PLD1 leads to a reduction of the number of active release sites rather than modifying the release probability.
To conclude, this report demonstrates that PLD1 controls a late stage of Ca2+-dependent synaptic vesicle exocytosis at nerve terminals. This extends the idea that membrane lipid constituents are essential partners for proteins in the control of vesicular membrane trafficking events. Our report reinforces the concept that lipid cones such as PA, by locally remodeling membrane curvature and fluidity, mark sites of fusion (41).
Acknowledgments
We thank Drs. F. Doussau and H. Kojima for insightful discussions and T. Thahouly and G. Bombarde for technical assistance. This work was supported by Association pour la Recherche sur le Cancer Grant 5802 (to M.F.B.), Association Française Contre les Myopathies and Ministère de la Recherche Grant PRFMMIP-2000/2001 (to B.P.), and National Institutes of Health Grant GM54813 (to M.A.F). G.D. is a fellow of the American Heart Association. We acknowledge the confocal microscopy facility service of IFR37.
Abbreviations
- PLD1
phospholipase D1
- PA
phospatidic acid
- ACh
acetycholine
- IPSC
inhibitory postsynaptic current
- PPF
paired-pulse facilitation
- ARF
ADP-ribosylation factor
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Jahn R, Südhof T C. Annu Rev Biochem. 1999;68:863–911. doi: 10.1146/annurev.biochem.68.1.863. [DOI] [PubMed] [Google Scholar]
- 2.Neher E. Neuron. 1998;20:389–399. doi: 10.1016/s0896-6273(00)80983-6. [DOI] [PubMed] [Google Scholar]
- 3.Hanson P I, Roth R, Morisaki H, Jahn R, Heuser J E. Cell. 1997;90:523–535. doi: 10.1016/s0092-8674(00)80512-7. [DOI] [PubMed] [Google Scholar]
- 4.McNew J A, Weber T, Parlati F, Johnston R J, Melia T J, Sollner T H, Rothman J E. J Cell Biol. 2000;150:105–117. doi: 10.1083/jcb.150.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chernomordik L V, Chanturiya A, Green J, Zimmerberg J. Biophys J. 1995;69:922–929. doi: 10.1016/S0006-3495(95)79966-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Monck J R, Fernandez J M. Curr Opin Cell Biol. 1996;8:524–533. doi: 10.1016/s0955-0674(96)80031-7. [DOI] [PubMed] [Google Scholar]
- 7.Roth M G, Bi K, Ktistakis N T, Yu S. Chem Phys Lipids. 1999;98:141–152. doi: 10.1016/s0009-3084(99)00026-2. [DOI] [PubMed] [Google Scholar]
- 8.Liscovitch M, Czarny M, Fiucci G, Tang X. Biochem J. 2000;345:401–415. [PMC free article] [PubMed] [Google Scholar]
- 9.Jones D, Morgan C, Cockcroft S. Biochim Biophys Acta. 1999;1439:229–244. doi: 10.1016/s1388-1981(99)00097-9. [DOI] [PubMed] [Google Scholar]
- 10.Hammond S M, Altshuller Y M, Sung T, Rudge S A, Rose K, Engebrecht J, Morris A J, Frohman M A. J Biol Chem. 1995;270:29640–29643. doi: 10.1074/jbc.270.50.29640. [DOI] [PubMed] [Google Scholar]
- 11.Colley W C, Sung T, Roll R, Jenco J, Hammond S M, Altshuller Y, Bar-Sagi D, Morris A J, Frohman M A. Curr Biol. 1997;7:191–201. doi: 10.1016/s0960-9822(97)70090-3. [DOI] [PubMed] [Google Scholar]
- 12.Kodaki T, Yamashita S. J Biol Chem. 1997;272:11408–11413. doi: 10.1074/jbc.272.17.11408. [DOI] [PubMed] [Google Scholar]
- 13.Exton J. Biochim Biophys Acta. 1999;1439:121–133. doi: 10.1016/s1388-1981(99)00089-x. [DOI] [PubMed] [Google Scholar]
- 14.Vitale N, Caumont A S, Chasserot-Golaz S, Du G, Wu S, Sciorra V A, Morris A J, Frohman M A, Bader M F. EMBO J. 2001;20:2424–2434. doi: 10.1093/emboj/20.10.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stewart R R, Bossu J L, Muzet M, Dupont J L, Feltz A. J Neurobiol. 1995;28:419–432. doi: 10.1002/neu.480280403. [DOI] [PubMed] [Google Scholar]
- 16.Huttner W B, Schiebler W, Greengard P, De Camilli P. J Cell Biol. 1983;96:1374–1388. doi: 10.1083/jcb.96.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chasserot-Golaz S, Vitale N, Sagot I, Delouche B, Dirrig S, Pradel L A, Henry J P, Aunis D, Bader M F. J Cell Biol. 1996;133:1217–1236. doi: 10.1083/jcb.133.6.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doussau F, Clabecq A, Henry J P, Darchen F, Poulain B. J Neurosci. 1998;18:3147–3157. doi: 10.1523/JNEUROSCI.18-09-03147.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doussau F, Gasman S, Humeau Y, Vitiello F, Popoff M, Boquet P, Bader M F, Poulain B. J Biol Chem. 2000;275:7764–7770. doi: 10.1074/jbc.275.11.7764. [DOI] [PubMed] [Google Scholar]
- 20.Humeau Y, Doussau F, Vitiello F, Greengard P, Benfenati F, Poulain B. J Neurosci. 2001;21:4195–4206. doi: 10.1523/JNEUROSCI.21-12-04195.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Du G, Morris A J, Sciorra VA, Frohman M A. Methods Enzymol. 2002;345:265–274. doi: 10.1016/s0076-6879(02)45022-7. [DOI] [PubMed] [Google Scholar]
- 22.Hammond S M, Jenco J M, Nakashima S, Cadwallader K, Gu Q, Cook S, Nozawa Y, Prestwich G D, Frohman M A, Morris A J. J Biol Chem. 1997;272:3860–3868. doi: 10.1074/jbc.272.6.3860. [DOI] [PubMed] [Google Scholar]
- 23.Quastel D M J. Biophys J. 1997;72:728–753. doi: 10.1016/s0006-3495(97)78709-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clements J D, Silver R A. Trends Neurosci. 2000;23:105–113. doi: 10.1016/s0166-2236(99)01520-9. [DOI] [PubMed] [Google Scholar]
- 25.Schleuss V, Neher E. Biophys J. 2001;81:1970–1989. doi: 10.1016/S0006-3495(01)75848-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morgan C P, Sengelov H, Whatmore J, Borregaard N, Cockcroft S. Biochem J. 1997;325:581–585. doi: 10.1042/bj3250581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haslam R J, Coorssen J R. Adv Exp Med Biol. 1993;344:149–164. doi: 10.1007/978-1-4615-2994-1_11. [DOI] [PubMed] [Google Scholar]
- 28.Cohen J S, Brown H A. Biochemistry. 2001;40:6589–6597. doi: 10.1021/bi0103011. [DOI] [PubMed] [Google Scholar]
- 29.Schmidt A, Wolde M, Thiele C, Fest W, Kratzin H, Podtelejnikov A V, Witke W, Huttner W B, Söling H D. Nature (London) 1999;401:133–141. doi: 10.1038/43613. [DOI] [PubMed] [Google Scholar]
- 30.Weigert R, Silletta M G, Spano S, Turracchio G, Cercicola C, Colanzi A, Senatore S, Mancini R, Polishchuk E V, Salmona M, et al. Nature (London) 1999;402:429–433. doi: 10.1038/46587. [DOI] [PubMed] [Google Scholar]
- 31.Luo J Q, Liu X, Frankel P, Rotunda T, Ramos M, Flom J, Jiang H, Feig L A, Morris A J, Kahn R A, Foster D A. Proc Natl Acad Sci USA. 1998;95:3632–3637. doi: 10.1073/pnas.95.7.3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Caumont A S, Galas M C, Vitale N, Aunis D, Bader M F. J Biol Chem. 1998;273:1373–1379. doi: 10.1074/jbc.273.3.1373. [DOI] [PubMed] [Google Scholar]
- 33.Caumont A S, Vitale N, Gensse M, Galas M C, Casanova J E, Bader M F. J Biol Chem. 2000;275:15637–15644. doi: 10.1074/jbc.M908347199. [DOI] [PubMed] [Google Scholar]
- 34.Ashery U, Koch H, Scheuss V, Brose N, Rettig J. Proc Natl Acad Sci USA. 1999;96:1094–1099. doi: 10.1073/pnas.96.3.1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park J B, Lee J Y, Kim J W. Biochem Biophys Res Com. 1999;263:765–769. doi: 10.1006/bbrc.1999.1463. [DOI] [PubMed] [Google Scholar]
- 36.Hay J C, Fisette P L, Jenkins G H, Fukami K, Takenawa T, Anderson R E, Martin T F J. Nature (London) 1995;374:173–177. doi: 10.1038/374173a0. [DOI] [PubMed] [Google Scholar]
- 37.De Camilli P, Scott D E, McPherson P S, Novick P. Science. 1996;271:1533–1539. doi: 10.1126/science.271.5255.1533. [DOI] [PubMed] [Google Scholar]
- 38.Cockroft S, De Matteis M A. J Membr Biol. 2001;180:187–194. doi: 10.1007/s002320010069. [DOI] [PubMed] [Google Scholar]
- 39.Klingauf J, Kavalali E T, Tsien R W. Nature (London) 1998;394:581–585. doi: 10.1038/29079. [DOI] [PubMed] [Google Scholar]
- 40.Murthy V N, Stevens C F. Nat Neurosci. 1999;2:503–507. doi: 10.1038/9149. [DOI] [PubMed] [Google Scholar]
- 41.Barr F A, Shorter J. Curr Biol. 2001;10:141–144. [Google Scholar]