Abstract
Acetylcholine serves an important modulatory role in the central nervous system. Pharmacological evidence has suggested that cholinergic activity can modulate central dopaminergic transmission; however, the nature of this interaction and the receptors involved remain undefined. In this study we have generated mice lacking the M1 muscarinic acetylcholine receptor and examined the effects of M1 deletion on dopaminergic transmission and locomotor behavior. We report that M1 deficiency leads to elevated dopaminergic transmission in the striatum and significantly increased locomotor activity. M1-deficient mice also have an increased response to the stimulatory effects of amphetamine. Our results provide direct evidence for regulation of dopaminergic transmission by the M1 receptor and are consistent with the idea that M1 dysfunction could be a contributing factor in psychiatric disorders in which altered dopaminergic transmission has been implicated.
Muscarinic acetylcholine receptors are members of the family of G protein-coupled receptors and are widely expressed in the central nervous system and periphery (1, 2). Molecular cloning studies identified genes for five distinct muscarinic receptors, M1–M5, which are expressed in overlapping patterns in the brain. Of these, M1 is predominant in the cortex and hippocampus (3) and, together with M4, is the major muscarinic receptor in the striatum (4), where it is expressed on the majority of cell types (5, 6). M1 receptors couple with a Gq type of G protein, and activation of this pathway leads to stimulation of phospholipase C-β, mobilization of intracellular calcium, and increased neuronal excitability (2). Activation of pharmacologically defined M1 receptors has been shown to enhance the excitability of cortical (7) and striatal (8) neurons. Muscarinic receptors mediate many of the cholinergic effects described in the central nervous system and have been implicated in a variety of central processes, including locomotion, sleep, thermoregulation, generation of seizures, antinociception, and learning and memory (1, 2, 9).
A large body of evidence suggests the existence of a complex balance between the cholinergic and dopaminergic systems in the basal ganglia and that disruption of this balance could contribute to movement disorders such as parkinsonism (10, 11). Dopamine can exert both positive and negative regulatory influences on striatal cholinergic transmission, with D1 and D2 receptors exerting facilitative and inhibitory actions, respectively (11). Cholinergic regulation of striatal dopaminergic transmission is less well defined and can occur at multiple levels of the nigrostriatal system. Although a number of pharmacological studies have addressed the effects of muscarinic function on striatal dopaminergic transmission, such studies are limited by the lack of highly subtype-specific muscarinic antagonists (2) and the expression of multiple muscarinic receptors affecting nigrostriatal function. Thus, evidence for both facilitative (12–15) and inhibitory (16–19) muscarinic effects has been reported, and the role of specific muscarinic receptor subtypes in the regulation of striatal dopaminergic transmission has remained unclear.
We report here the generation and characterization of mice deficient for the M1 muscarinic receptor. These mice exhibit increased locomotor activity and have elevated levels of extracellular dopamine in the striatum, which likely underlies their locomotor behavioral abnormalities. In addition, these mice display an increased response to the stimulatory effects of amphetamine. Our results demonstrate that disruption of M1 receptor function leads to increased striatal dopaminergic transmission with consequent behavioral abnormalities. These findings support the notion that M1 receptor dysfunction could contribute to psychiatric disorders such as schizophrenia, in which altered subcortical dopaminergic transmission has been implicated.
Materials and Methods
Generation of M1−/− Mice.
Genomic clones spanning the M1 locus were isolated from a C57BL/6 genomic phage library, mapped by restriction analysis, and used to create the targeting construct. Fifty micrograms of the targeting vector was linearized with SacII and introduced into C57BL/6 embryonic stem cells (a gift from Colin Stewart, National Cancer Institute, Frederick, MD) by electroporation (Bio-Rad Gene Pulser set at 800 V and 3 μF). G418 selection was applied 24 h after transfection, and G418-resistant colonies were isolated on days 5–8 of selection. Isolated colonies were screened for homologous recombination by Southern hybridization, and clones that were correctly recombined on both sides were injected into BALB/c blastocysts to generate chimeras. Resulting chimeras were bred to C57BL/6 mice to obtain pure C57BL/6 M1+/− mice. These mice were used to generate a breeding colony from which all M1+/+, +/−, and −/− mice were derived. Behavioral, pharmacological, and dopamine measurement analyses were performed on 3- to 6-month-old male mice. All procedures relating to animal care and treatment conformed to institutional and National Institutes of Health guidelines.
Probes and Genotyping.
The cDNA probe used for library screening and Northern hybridization was generated by PCR and consists of nucleotides 24–656 of the M1 coding sequence. For in situ hybridization this PCR product was cloned into the pSP72 vector (Promega), so that a cRNA probe could be generated. The 5′ external probe is a 900-bp HindIII–PstI fragment located just upstream of the 5′ arm. To generate the 3′ external probe, a 2.5-kb BamHI–KpnI fragment adjacent to the 3′ arm was digested with RsaI, yielding four fragments, of which the 1-kb fragment was used for the probe. Initially, mice were genotyped by Southern hybridization of tail DNA. Tail DNA was digested with HindIII and hybridized with the 5′ probe, which distinguishes the 6-kb targeted allele and 11-kb endogenous allele. Subsequently, mice were typed by PCR with the use of primers for the M1 coding sequence, m1pr1: 5′-TGTCAGTCCCAACATCACCG-3′ and m1pr2: 5′-GCTCGGTTTTCTGTCTCCCG-3′ and for the neo gene, neo1: 5′-GCTTGGGTGGAGAGGCTATTC-3′ and neo2: 5′-CAAGGTGAGATGACAGGAGATC-3′.
Northern Analysis.
Total brain RNA was isolated with the use of TriReagent (Sigma). Fifteen micrograms of RNA per lane was electrophoresed on a 1% agarose gel containing 2.2 M formaldehyde, transferred in 20× SSC (1× SSC = 0.15 M sodium chloride/0.015 M sodium citrate, pH 7) to zetaprobe nylon membrane (Bio-Rad), and hybridized with the M1 cDNA probe. After washing (final wash, 0.1× SSC, 0.1% SDS, 65°C), the membrane was exposed to Biomax MS film (Kodak) at −80°C. The membrane was stripped and reprobed with a glyceraldehyde-3-phosphate dehydrogenase (G3PDH) probe (CLONTECH) as a positive control.
In Situ Hybridization.
In situ hybridization was performed according to a described protocol (20) with some modifications.
Analysis of Locomotor Behavior.
Mice were tested for motor behaviors with the use of an automated Digiscan apparatus (Accuscan Instruments, Columbus, OH) in which activity is measured by IR beam interruption. Horizontal activity, measured as the total distance traveled by each mouse, and vertical movements, measured as total independent interruptions of an elevated array of beams, were recorded in 5-min intervals over a 90-min or 2-h period. Raw numbers were averaged to give values for each 5-min interval. To evaluate the effects of amphetamine or saline on locomotor behavior, mice were habituated to the activity monitoring chamber for 1 h, and then drugs were administered i.p. Amphetamine was dissolved in saline and administered at 0.1 ml/10 g body weight. Clozapine and haloperidol (Sigma) were dissolved in a drop of glacial acetic acid and quickly diluted in saline. Clozapine and haloperidol were freshly prepared before each experiment and delivered by i.p. injection in a volume of 0.1 ml/25 g body weight, 15 min before activity was monitored.
HPLC Assessment of Brain Content of Monoamines and Metabolites.
Striatum or frontal cortex of mice was homogenized in 0.1 M HClO4 containing 100 ng/ml 3,4-dihydroxybenzylamine as an internal standard. Homogenates were centrifuged for 10 min at 10,000 × g. Supernatants were filtered through a 0.22-μm filter and analyzed for levels of dopamine, serotonin, 3,4-dihydroxyphenylacetic acid (DOPAC), homovanillic acid (HVA), and 5-hydroxyindoleacetic acid with the use of HPLC with electrochemical detection. Monoamines and metabolites were separated on a microbore reverse-phase column (C-18, 5 μm, 1 × 150 mm, Unijet; BAS, West Lafayette, IN) with a mobile phase consisting of 0.03 M citrate-phosphate buffer with 2.1 mM octyl sodium sulfate, 0.1 mM EDTA, 10 mM NaCl, and 17% methanol (pH 3.6) at a flow rate of 90 μl/min and detected by a 3-mm glass carbon electrode (Unijet; BAS) set at +0.8 V. The volume of injection was 5 μl.
Determination of Monoamine Synthesis Rates in Vivo.
To measure rates of dopamine synthesis in the striatum, mice were injected i.p. with the l-aromatic acid decarboxylase inhibitor 3-hydroxybenzylhydrazine (NSD-1015) at 100 mg/kg. Forty minutes later, the concentration of l-DOPA in the striatum was determined with the use of HPLC with electrochemical detection for in vivo measurement of tyrosine hydroxylase activity. Determinations were performed with the use of the same column and apparatus as described above with a mobile phase consisting of 50 mM monobasic sodium phosphate, 0.2 mM octyl sodium sulfate, 0.1 mM EDTA, 10 mM NaCl, and 10% methanol (pH 2.6). The potential applied was +0.65 V.
In Vivo Microdialysis.
Mice were anesthetized with chloral hydrate (400 mg/kg, i.p.) and placed in a stereotaxic frame. Dialysis probes (2-mm membrane length, 0.24-mm o.d., Cuprophane, 6-kDa cut-off, CMA-11; CMA/Microdialysis, Solna, Sweden) with CMA-11 guide cannulae were implanted in the right striatum. The stereotaxic coordinates for implantation of microdialysis probes were 0.0 mm AP, −4.4 DV mm, L2.5 relative to bregma. Placement of the probe was verified by histological examination subsequent to the experiments.
After surgery, animals were returned to their home cages with free access to food and water. Twenty-four hours after surgery, the dialysis probe was connected to a syringe pump and perfused at 1 μl/min with artificial cerebrospinal fluid (147 mM NaCl/2.7 mM KCl/1.2 mM CaCl2/0.85 mM MgCl2) (CMA/Microdialysis). After an equilibration period of at least 1 h, the perfusates were collected every 20 min. At least four control samples were taken before amphetamine, cocaine, or saline was administered i.p. Data are presented as a percentage of the basal levels.
To measure the “absolute” extracellular concentration of dopamine, quantitative “low perfusion rate” microdialysis was performed (21). Ringer's solution was perfused at a flow rate of 80 nl/min for 6–7 h and collected into a tube containing 2 μl of 0.5 M HClO4 each 90 min. Perfusate samples were assayed for dopamine, DOPAC, and HVA with the use of HPLC with electrochemical detection under the chromatographic conditions described above.
Statistical Analysis.
All data are presented as means ± SEM. Data were statistically analyzed by a two-tailed Student's t test with the use of the Microsoft excel program, or by two-way or repeated measure ANOVA with STATVIEW 5.0.1.
Results
Generation of M1-Deficient Mice.
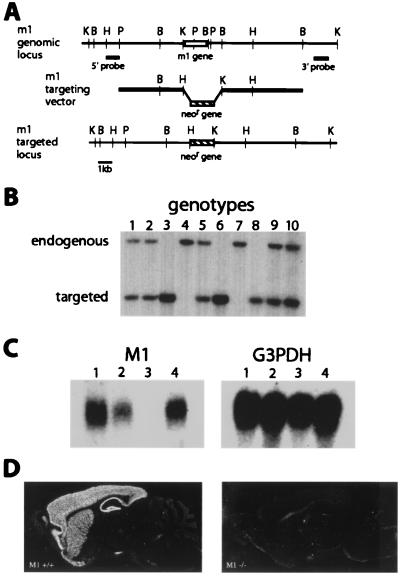
To generate inbred C57BL/6 mice lacking the M1 receptor gene, we first isolated DNA clones flanking the M1 gene from a B6 genomic library. These clones were used to create a targeting construct designed to replace a 3-kb genomic fragment containing the entire M1 coding sequence with the neomycin resistance gene upon homologous integration (Fig. 1A). The construct was transfected by electroporation into C57BL/6 (B6) embryonic stem cells, and desired recombinant clones were identified by Southern hybridization with 5′ and 3′ external probes. Injection of two clones, 126 and 348, into BALB/c blastocysts yielded chimeras that transmitted the targeted allele when crossed with B6 breeders (Fig. 1B). Experiments described in this article were performed with mice derived from the 348 clone.
Figure 1.
Targeted disruption of the mouse M1 gene. (A) Homologous recombination between the targeting construct and the M1 genomic locus replaces a 3-kb KpnI–BamHI genomic fragment containing the entire M1 coding sequence with the neomycin resistance gene. B, BamHI; H, HindIII; K, KpnI; P, PstI. Not all sites are shown. (B) Germ-line transmission of the targeted allele. HindIII digests of tail DNA from progeny of a heterozygous cross were screened with the 5′ probe. The endogenous allele is 11 kb, and the targeted allele is 6 kb. Lanes 4 and 7, +/+; lanes 1, 2, 5, 9, and 10, +/−; lanes 3, 6, and 8, −/−. (C) Absence of M1 message in the M1 −/− mice. Total forebrain RNA was prepared from M1 +/+, +/−, and −/− mice and analyzed by Northern hybridization with a M1 cDNA probe (Left). Lanes 1 and 4, +/+; lane 2, +/−; lane 3, −/−. The filter was stripped and rehybridized with a glyceraldehyde-3-phosphate dehydrogenase (G3DPH) control probe (Right). (D) In situ hybridization of sagittal brain sections from M1 +/+ (Left) and M1 −/− (Right) mice. M1 mRNA is strongly expressed in the cortex, hippocampus, and striatum of +/+ mice and absent in −/− mice.
Heterozygous M1 mutant mice (M1+/−) were interbred to produce homozygous mutant mice (M1−/−). As described (22), M1−/− mice are viable and fertile, and heterozygous crosses yielded the expected Mendelian ratio of genotypes, 45/180 (+/+) = 25%, 46/180 (−/−) = 25.6%, and 89/180 (+/−) = 49.4%. To confirm that M1 function is disrupted in the targeted mice, Northern analysis of total forebrain RNA from M1−/−, M1+/−, and M1+/+ mice was performed with the use of a M1 cDNA probe. As shown in Fig. 1C, M1 mRNA is completely absent in the forebrain of M1−/− mice and reduced in M1+/− mice relative to wild-type controls. Glyceraldehyde-3-phosphate dehydrogenase (G3PDH) hybridization was used to control for RNA quality and loading (Fig. 1C Right). In situ hybridization of sagittal brain sections with an M1 cRNA probe revealed strong expression of M1 RNA in the cortex, hippocampus, and striatum of M1+/+ mice and the absence of M1 RNA in the M1−/− brain (Fig. 1D). Thus, as expected, the targeted M1 allele is a null allele.
Abnormal Motor Behaviors in M1 Mutant Mice.
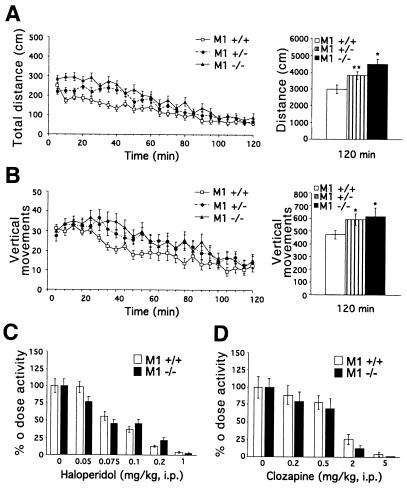
Casual inspection of M1 mutant mice suggested that they are more active than their wild-type littermates. To confirm that M1−/− mice are hyperactive, locomotor activity was analyzed over a 2-h period in a digital activity monitor that comprised a novel environment. As shown in Fig. 2A Left, M1−/− mice displayed an increase in locomotion throughout the monitoring period relative to M1+/+ mice. The average total distance traveled by M1−/− mice in 2 h was ≈1.5-fold higher (P < 0.0006) than that of M1+/+ controls (Fig. 2A Right). An intermediate increase (1.3-fold, P < 0.022) in locomotor activity was observed in M1+/− mice, suggesting that decreased M1 expression is sufficient to cause abnormal motor activity (Fig. 2A). Habituation to the novel environment was similar in M1−/− and M1+/+ mice (Fig. 2A). Total vertical rearing activity was also significantly increased (P < 0.05) in M1−/− and M1+/− mice relative to M1+/+ controls (Fig. 2B). The increased locomotion of M1−/− mice could be attenuated by the administration of haloperidol (Fig. 2C), which has dopamine D2 receptor antagonistic activity, or clozapine (Fig. 2D), which has dopamine D1, D2, and serotonin 5HT2A receptor antagonistic properties, in a dose-dependent manner. The dose–response to these compounds was similar in M1−/− and M1+/+ mice (Fig. 2 C and D).
Figure 2.
Increased locomotion of M1 mutant mice. (A) Locomotor activity of M1+/+ (n = 18), M1+/− (n = 19), and M1−/− (n = 18) mice. The distance traveled during a 2-h period was monitored in 5-min intervals (Left). The total distance traveled by M1−/− and M1+/− mice in 2 h was 1.5-fold higher (**, P < 0.00052) and 1.3-fold higher (*, P < 0.022), respectively, than the distance traveled by M1+/+ mice (Right). (B) Vertical activity of M1+/+ (n = 18), M1+/− (n = 19), and M1−/− (n = 18) mice. The number of vertical movements made during a 2-h period was monitored in 5-min intervals (Left). The total number of vertical movements made by M1−/− mice was 1.3-fold greater (*, P < 0.05) and that by M1 +/− mice was 1.25-fold greater (*, P < 0.05) than that by M1+/+ mice (Right). (C) The dose response to haloperidol is similar in M1+/+ and M1−/− mice. The total distance traveled in 90 min after drug or saline administration was measured. A significant difference between M1+/+ and M1−/− mice was not observed at any dose tested (P > 0.05). M1+/+ and M1−/− mice: for 0 and 0.05 doses, n = 16–18; for all other doses, n = 8–9). (D) The dose–response to clozapine is similar in M1+/+ and M1−/− mice. The total distance traveled in 90 min after drug or saline administration was measured. A significant difference between M1+/+ and M1−/− mice was not observed at any dose tested (P > 0.05). M1+/+ and M1−/− mice: n = 7–8 for all doses tested.
Elevated Striatal Extracellular Dopamine in M1−/− Mice.
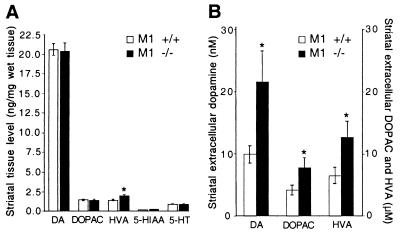
Increased locomotor activity in rodents is often associated with elevated dopaminergic tone (23). To investigate whether deletion of M1 muscarinic receptors could have an effect on dopaminergic function, HPLC analysis of striatal tissue contents of dopamine and two metabolites, DOPAC and HVA, was performed. Whereas total tissue contents of dopamine and DOPAC were unchanged in the striatum of M1−/− mice, the level of striatal HVA was significantly elevated by about 40% (P < 0.004) (Fig. 3A). Inasmuch as HVA accumulation is primarily a reflection of extracellular metabolism of dopamine, this observation indirectly suggests that M1−/− mice may have an elevation in the concentration of extracellular striatal dopamine.
Figure 3.
Altered dopaminergic transmission in M1−/− mice. (A) HPLC analysis of striatal tissue content of dopamine, serotonin, and metabolites in M1−/− (n = 6) and M1+/+ (n = 8) mice. HVA content is increased 1.4-fold (*, P < 0.004) in M1−/− mice. (B) Extracellular levels of dopamine and metabolites in M1−/− (n = 6) and M1+/+ (n = 7) mice. The extracellular levels of dopamine, DOPAC, and HVA are increased by 2-fold (*, P < 0.036), 1.9-fold (*, P < 0.04), and 1.9-fold (*, P < 0.05), respectively, in M1−/− mice. DA, dopamine; 5-HT, 5-hydroxytryptamine; 5-HIAA, 5-hydroxyindoleacetic acid.
To directly determine whether the level of extracellular striatal dopamine is altered in M1−/− mice, quantitative in vivo microdialysis [low perfusion rate technique (21)] was performed on freely moving animals. This analysis revealed a 2-fold elevation in striatal extracellular dopamine (P < 0.036), as well as DOPAC and HVA in M1−/− mice relative to M1+/+ controls (Fig. 3B). Assessment of the rate of dopamine biosynthesis by analysis of l-DOPA accumulation after inhibition of l-aromatic acid decarboxylase with NSD-1015 (21) did not reveal a significant difference between M1−/− and M1+/+ mice, suggesting that dopamine synthesis is not significantly altered in M1−/− mice (data not shown). No alterations in the striatal tissue content of serotonin or its metabolite, 5-hydroxyindoleacetic acid, were detected in M1−/− mice, suggesting that striatal serotonergic function is normal in these mice (Fig. 3A). HPLC analysis of frontal cortical tissue content of dopamine and serotonin and their metabolites detected no difference between M1−/− mice and M1+/+ controls (data not shown).
Response of M1−/− Mice to Amphetamine.
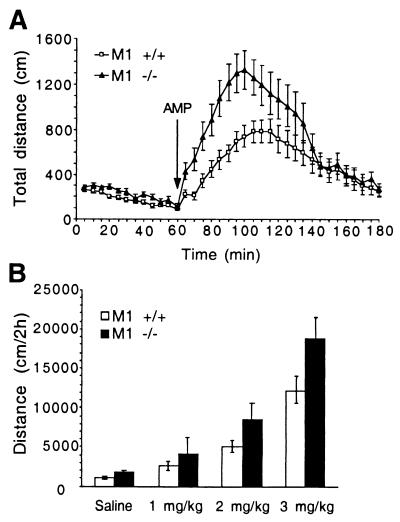
The elevated dopaminergic tone in M1−/− mice suggested that these mice might be prone to enhanced potentiation of dopaminergic transmission induced by indirect dopaminergic agonists, such as amphetamine. Administration of amphetamine (3 mg/kg i.p.), which elevates extracellular dopamine by reversing the plasma membrane dopamine transporter (24), resulted in significantly stronger behavioral effects in M1−/− mice relative to M1+/+ mice (P < 0.02, repeated measure ANOVA), as indicated by increased locomotion (Fig. 4A). Analysis of the dose–response of M1+/+ and M1−/− mice to amphetamine (Fig. 4B) revealed significant genotype (P < 0.02) and dose (P < 0.0001) effects (two-way ANOVA). The total distance traveled by M1−/− mice was 1.5-fold higher (P < 0.05) than that of M1+/+ mice after administration of amphetamine (3 mg/kg i.p.) (Fig. 4B). A similar trend was observed for doses of 1 and 2 mg/kg amphetamine (Fig. 4B). Thus the increased locomotion of M1−/− mice in response to amphetamine appears to be proportional to that observed in untreated M1−/− mice relative to M1+/+ controls (Fig. 2A).
Figure 4.
M1−/− mice exhibit increased locomotor activity in response to amphetamine. (A) Locomotor activity of M1+/+ (n = 15) and M1−/− (n = 13) mice in response to d-amphetamine (AMP) administration (3 mg/kg i.p.). Locomotor activity was monitored in 5-min intervals 1 h before and 2 h after AMP administration. Activity of M1−/− mice was significantly increased during an 80-min period after amphetamine administration (P < 0.02, repeated measure ANOVA). (B) Dose–response to amphetamine (0, 1, 2, and 3 mg/kg) in M1−/− and M1+/+ mice. Significant genotype (P < 0.02) and dose effects (P < 0.0001) were observed (two-way ANOVA). M1−/−: saline (n = 10), 1 mg/kg (n = 13), 2 mg/kg (n = 17), 3 mg/kg (n = 13); M1 +/+: saline (n = 10), 1 mg/kg (n = 15), 2 mg/kg (n = 18), 3 mg/kg (n = 15).
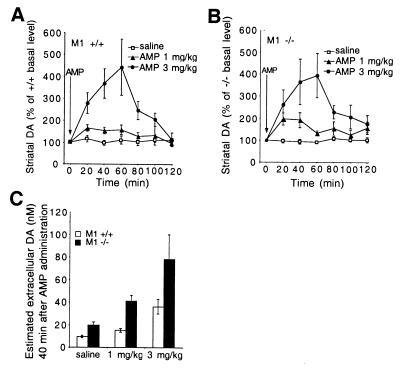
Consistent with the observed behavioral response of M1−/− mice to amphetamine, assessment of the dynamics of striatal extracellular dopamine accumulation by in vivo microdialysis revealed a similar percentage elevation in striatal extracellular dopamine concentration in M1+/+ and M1−/− mice in response to amphetamine administration (1 and 3 mg/kg i.p.) (Fig. 5 A and B). However, because the basal level of striatal dopamine in M1−/− mice is on average 2-fold higher than that of M1+/+ mice (Fig. 3B), this would result in substantially enhanced dopaminergic tone in the M1−/− mice (Fig. 5C), which likely underlies their heightened locomotor activity in response to amphetamine. Similar effects on locomotor behavior and striatal dopamine outflow were observed after administration of cocaine (data not shown).
Figure 5.
Dynamics of striatal extracellular dopamine levels in response to amphetamine. (A) Extracellular dopamine dynamics after administration of saline or amphetamine (AMP) (1 mg/kg or 3 mg/kg i.p.) to M1+/+ mice. Saline (n = 9): 1 mg/kg, n = 7; 3 mg/kg, n = 6. (B) Extracellular dopamine dynamics after the administration of saline or amphetamine (AMP) (1 mg/kg or 3 mg/kg i.p.) to M1 −/− mice. Saline (n = 9): 1 mg/kg, n = 6; 3 mg/kg, n = 5. Administration of amphetamine (AMP) (1 mg/kg or 3 mg/kg i.p.) causes a similar percentage increase in striatal extracellular dopamine levels in M1+/+ and M1−/− mice. In vivo microdialysis was performed in freely moving mice as described in Materials and Methods. (C) Estimated extracellular dopamine (DA) levels 40 min after amphetamine administration. The basal extracellular levels determined in Fig. 3B (M1+/+, 9.9 nM; M1−/−, 21.4 nM) were used to estimate the extracellular levels at 40 min after saline or amphetamine (1, 3 mg/kg) administration.
Discussion
We have generated mice deficient for the M1 muscarinic acetylcholine receptor and characterized the effects of M1 deletion on locomotor behavior and striatal dopaminergic transmission. M1 mutant mice were found to have a significantly elevated level of extracellular dopamine in the striatum with correspondingly increased locomotion and vertical rearing activity. The increased locomotion of M1 mutant mice could be attenuated by administration of haloperidol or clozapine, and the dose–response to these compounds was similar in M1 mutant mice and wild-type controls. Administration of amphetamine yielded higher levels of locomotor activity and striatal extracellular dopamine accumulation in M1−/− mice relative to M1+/+ mice. Moreover, these increases were proportional to those observed in M1 mutants relative to controls under basal conditions. These findings demonstrate that M1 receptor function is required for regulation of subcortical dopaminergic transmission and for maintaining normal control of locomotor behavior.
Several additional studies have described analysis of mice deficient for the M1 muscarinic receptor. One study reported deficits in muscarinic regulation of the M current and absence of pilocarpine-induced seizures in M1 mutant mice (22). Another recent report also describes hyperactivity in M1-deficient mice, and those mice were shown to have intact hippocampal learning (25). The mechanism of the observed hyperactivity was not addressed in that study. Both reports demonstrated that M1 deletion does not lead to compensatory changes in the expression of other muscarinic receptors (22, 25). Previous studies of M1 mutant mice did not assess the consequences of M1 deficiency on dopaminergic transmission. Our finding of elevated striatal dopaminergic transmission in M1 mutant mice elucidates the mechanism by which M1 deficiency leads to abnormal motor behavior and provides primary genetic evidence for a role of the M1 receptor in regulating dopaminergic transmission. In addition to the increased dopaminergic tone, direct effects of M1 deficiency on cortical, hippocampal, or striatopallidal function could contribute to the abnormal locomotor behavior observed in the M1 mutant mice.
Elevated extracellular dopamine could be a consequence of decreased uptake or increased release. The higher dopamine level observed in the M1 mutant mice is likely a consequence of increased release, based on two lines of reasoning. First, in situ (4) and immunohistochemical (3) analyses have failed to detect M1 expression in neurons of the substantia nigra. Therefore, it is unlikely that M1 receptors are present on the nerve terminals of dopaminergic cells, which express the dopamine transporter and comprise the major site of dopamine uptake (26, 27). Thus, modulation of dopamine uptake by the M1 receptor appears unlikely. Second, administration of amphetamine or cocaine, which affect dopamine uptake (28, 29), to the M1−/− mice caused an increase in extracellular dopamine proportional to that observed in wild-type mice. This proportional increase should not be observed if dopamine uptake is already attenuated by M1 deficiency. Indeed, elevated extracellular dopamine in response to cocaine and amphetamine is not observed in dopamine transporter-deficient mice, which have increased extracellular dopamine as a consequence of decreased uptake (30).
A number of pharmacological studies have addressed the role of muscarinic receptors in striatal dopaminergic transmission; however, specific effects of muscarinic receptor function on the regulation of striatal extracelluar dopamine levels have been difficult to define. Inasmuch as expression of M1 receptors has not been observed in the substantia nigra (3, 4), it is unlikely that M1 function could exert direct or presynaptic effects on dopaminergic neurons. Cholinergic modulation has been suggested to indirectly affect striatal dopaminergic transmission by enhancing an inhibitory striatonigral feedback pathway (31). Consistent with this idea, in vivo studies have shown that systemic administration of general muscarinic antagonists can lead to increased dopamine outflow in the striatum (19).
Several previous in vivo studies of the effects of pirenzepine on striatal dopamine release have suggested that M1 receptors may play a facilitative role in striatal dopaminergic transmission (32, 33). Conclusions from such studies are complicated by the lack of a high degree of subtype specificity among the muscarinic receptor antagonists (2). Although it is difficult to compare the consequences of acute pharmacological intervention with those of chronic, specific genetic ablation, our results do not support these previous interpretations and suggest rather that M1 function serves to restrict striatal dopaminergic transmission.
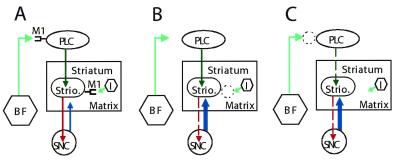
How could M1 deficiency lead to increased dopamine release in the striatum? We suggest two related explanatory models for indirect regulation of striatal dopaminergic transmission by M1 receptors based on the prominent localization of M1 receptors to the cortex and striatum. The striatum is divided into two anatomically and functionally distinct compartments, the striosome and matrix (34). Inhibitory projection neurons of the striosome receive excitatory glutamatergic inputs from the prelimbic cortex, an area of frontal cortex that processes inputs from the limbic system (35). A subset of inhibitory striosomal neurons projects to the substantia nigra pars compacta and regulates the resident dopaminergic neurons (35, 36). We propose that activation of M1 receptors expressed on these striosomal cells by locally released acetylcholine facilitates this inhibitory pathway (Fig. 6A). In the absence of M1, inhibition of the dopaminergic cells is dampened, leading to elevation of dopamine release (Fig. 6B). A similar disinhibition model has been proposed to explain increased striatal dopamine levels caused by general muscarinic blockade (19). Alternatively, it is possible that optimal excitation of the prelimbic cortical cells that project to the striosomal cells requires facilitative cholinergic input via cortical M1 receptors. In the absence of M1 expression, activity of cortical cells is decreased, leading to decreased excitation of the striosomal projection neurons and disinhibition of nigral dopaminergic cells (Fig. 6C). This idea is reminiscent of a neuro-anatomical model of schizophrenia that proposes that dysfunction of the frontal cortex could lead to increased subcortical dopaminergic transmission underlying the positive symptoms of that disease (37). In addition to a lack of striatal or cortical M1 receptors, a possible effect of hippocampal M1 deficiency on interactions between the hippocampus and the cortex or the nucleus accumbens that influences striatal dopaminergic transmission cannot be formally excluded.
Figure 6.
Disinhibition models for increased dopamine release in M1 mutant mice. (A) Under normal conditions, striosomal inhibitory projection neurons receive excitatory glutamatergic input from prelimbic cortical cells. A subset of the striosomal neurons projects to the substantia nigra pars compacta and inhibits the resident dopaminergic neurons, thereby regulating dopaminergic transmission. Cortical and striosomal cells receive facilitative cholinergic modulation by means of M1 receptors. (B) When M1 expression is absent or reduced on striosomal cells, facilitative cholinergic modulation of these cells is reduced, causing a decrease in their firing, leading to disinhibition of dopaminergic neurons and consequent increased dopamine release. (C) When M1 expression is absent or reduced on cortical cells, facilitative cholinergic modulation of these cells is lowered, causing decreased firing of the cortical cells and consequent decreased excitation of their striosomal target neurons, leading to disinhibition of nigral dopaminergic neurons and higher dopamine release. Dark green, excitatory input; light green, cholinergic facilitative input; red, inhibitory input; blue, dopaminergic input. Dashed lines indicate attenuated pathways. BF, basal forebrain; I, cholinergic interneuron; PLC, prelimbic cortex; SNC, substantia nigra pars compacta; Strio., striosome.
A number of studies have suggested that muscarinic dysfunction could be a contributing factor in schizophrenia (38–40). Moreover, several recent pharmacological studies have raised the possibility that compounds with muscarinic agonistic properties may have useful therapeutic effects in the treatment of psychiatric diseases such as schizophrenia (41, 42), although a potential mechanism of action has not been determined. Elevated subcortical dopaminergic transmission has been a long-standing element of one of the major explanatory hypotheses for the molecular basis of schizophrenia (23, 37, 43), and recent studies have provided direct evidence supporting this concept (44–46). The observation that M1 deficiency leads to increased striatal dopaminergic transmission with consequent behavioral abnormalities is consistent with the possibility that M1 dysfunction could contribute to alterations in dopaminergic transmission that have been implicated in certain manifestations of schizophrenia. These findings also provide direct genetic evidence supporting the idea that primary deficits in neurotransmitter systems other than the dopaminergic system could lead to secondary alterations in dopaminergic transmission associated with this disease (47). It will be of considerable interest to determine whether additional genetic or pharmacological manipulations in combination with M1 deficiency can evoke further behavioral manifestations associated with schizophrenia or related psychiatric disorders (48).
Acknowledgments
We thank Akira Kato for his very helpful suggestions and advice, Colin Stewart for kindly providing the B6 embryonic stem cells, Wenjiang Yu and Chanel Lovett for excellent technical assistance, and members of the Tonegawa laboratory for helpful support and encouragement. This work was supported in part by National Institutes of Health Grants NS19576 (M.G.C.) and NS32925 and AG05134 (S.T.). D.J.G. is a Howard Hughes Medical Institute postdoctoral fellow. M.G.C. and S.T. are Howard Hughes Medical Institute Investigators.
Abbreviations
- DOPAC
3,4-dihydroxyphenylacetic acid
- HVA
homovanillic acid
References
- 1.Nathanson N M. Annu Rev Neurosci. 1987;10:195–236. doi: 10.1146/annurev.ne.10.030187.001211. [DOI] [PubMed] [Google Scholar]
- 2.Wess J. Crit Rev Neurobiol. 1996;10:69–99. doi: 10.1615/critrevneurobiol.v10.i1.40. [DOI] [PubMed] [Google Scholar]
- 3.Levey A I, Kitt C A, Simonds W F, Price D L, Brann M R. J Neurosci. 1991;11:3218–3226. doi: 10.1523/JNEUROSCI.11-10-03218.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weiner D M, Levey A I, Brann M R. Proc Natl Acad Sci USA. 1990;87:7050–7054. doi: 10.1073/pnas.87.18.7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernard V, Normand E, Bloch B. J Neurosci. 1992;12:3591–3600. doi: 10.1523/JNEUROSCI.12-09-03591.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hersch S M, Gutekunst C-A, Rees H D, Heilman C J, Levey A I. J Neurosci. 1994;14:3351–3363. doi: 10.1523/JNEUROSCI.14-05-03351.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aramakis V B, Bandrowski A E, Ashe J H. Synapse. 1999;32:262–275. doi: 10.1002/(SICI)1098-2396(19990615)32:4<262::AID-SYN3>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 8.Calabresi P, Centonze D, Gubellini P, Pisani A, Bernardi G. Trends Neurosci. 2000;23:120–126. doi: 10.1016/s0166-2236(99)01501-5. [DOI] [PubMed] [Google Scholar]
- 9.Brown J H, Taylor P. In: The Pharmacological Basis of Therapeutics. Hardman J G, Limberd L E, editors. New York: McGraw–Hill; 1996. pp. 141–160. [Google Scholar]
- 10.Graybiel A M. Trends Neurosci. 1990;13:244–253. doi: 10.1016/0166-2236(90)90104-i. [DOI] [PubMed] [Google Scholar]
- 11.Di Chiara G, Morelli M, Consolo S. Trends Neurosci. 1994;17:228–233. doi: 10.1016/0166-2236(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 12.Giorguieff M F, Le Floc'h M L, Glowinski J, Besson M J. J Pharmacol Exp Ther. 1977;200:535–544. [PubMed] [Google Scholar]
- 13.Raiteri M, Marchi M, Maura G. Eur J Pharmacol. 1982;83:127–129. doi: 10.1016/0014-2999(82)90296-5. [DOI] [PubMed] [Google Scholar]
- 14.Lehmann J, Langer S Z. Brain Res. 1982;248:61–69. doi: 10.1016/0006-8993(82)91147-7. [DOI] [PubMed] [Google Scholar]
- 15.Hernandez-Lopez S, Gongora-Alfaro J L, Martinez-Fong D, Aceves J. Brain Res. 1992;598:114–120. doi: 10.1016/0006-8993(92)90174-8. [DOI] [PubMed] [Google Scholar]
- 16.Westfall T C. Life Sci. 1974;14:1641–1652. doi: 10.1016/0024-3205(74)90266-5. [DOI] [PubMed] [Google Scholar]
- 17.De Belleroche J, Bradford H F. Brain Res. 1978;142:53–68. doi: 10.1016/0006-8993(78)90176-2. [DOI] [PubMed] [Google Scholar]
- 18.Kudernatsch M, Sutor B. Neurosci Lett. 1994;181:107–112. doi: 10.1016/0304-3940(94)90571-1. [DOI] [PubMed] [Google Scholar]
- 19.Dewey S L, Smith G S, Logan J, Brodie J D, Simkowitz P, MacGregor R R, Fowler J S, Volkow N D, Wolf A P. Proc Natl Acad Sci USA. 1993;90:11816–11820. doi: 10.1073/pnas.90.24.11816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simmons D M, Arriza J L, Swanson L W. J Histotechnol. 1989;12:169–181. [Google Scholar]
- 21.Wang Y M, Gainetdinov R R, Fumagalli F, Xu F, Jones S R, Bock C B, Miller G W, Wightman R M, Caron M G. Neuron. 1997;19:1285–1296. doi: 10.1016/s0896-6273(00)80419-5. [DOI] [PubMed] [Google Scholar]
- 22.Hamilton S E, Loose M D, Qi M, Levey A I, Hille B, McKnight G S, Idzerda R L, Nathanson N M. Proc Natl Acad Sci USA. 1997;94:13311–13316. doi: 10.1073/pnas.94.24.13311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carlsson A, Waters N, Holm-Waters S, Tedroff J, Nilsson M, Carlsson M L. Annu Rev Pharmacol Toxicol. 2001;41:237–260. doi: 10.1146/annurev.pharmtox.41.1.237. [DOI] [PubMed] [Google Scholar]
- 24.Jones S R, Gainetdinov R R, Wightman R M, Caron M G. J Neurosci. 1998;18:1979–1986. doi: 10.1523/JNEUROSCI.18-06-01979.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miyakawa T, Yamada M, Duttaroy A, Wess J. J Neurosci. 2001;14:5239–5250. doi: 10.1523/JNEUROSCI.21-14-05239.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giros B, el Mestikawy S, Bertrand L, Caron M G. FEBS Lett. 1991;295:149–154. doi: 10.1016/0014-5793(91)81406-x. [DOI] [PubMed] [Google Scholar]
- 27.Cerruti C, Walther D M, Kuhar M J, Uhl G R. Brain Res Mol Brain Res. 1993;18:181–186. doi: 10.1016/0169-328x(93)90187-t. [DOI] [PubMed] [Google Scholar]
- 28.Ritz M C, Lamb R J, Goldberg S R, Kuhar M J. Science. 1987;237:1219–1223. doi: 10.1126/science.2820058. [DOI] [PubMed] [Google Scholar]
- 29.Jones S R, Gainetdinov R R, Jaber M, Giros B, Wightman R M, Caron M G. Proc Natl Acad Sci USA. 1998;95:4029–4034. doi: 10.1073/pnas.95.7.4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giros B, Jaber M, Jones S R, Wightman R M, Caron M G. Nature (London) 1996;379:606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- 31.Davis K L, Rosenberg G S. Life Sci. 1981;28:1953–1959. doi: 10.1016/0024-3205(81)90304-0. [DOI] [PubMed] [Google Scholar]
- 32.Xu M, Mizobe F, Yamamoto T, Kato T. Brain Res. 1989;495:232–242. doi: 10.1016/0006-8993(89)90217-5. [DOI] [PubMed] [Google Scholar]
- 33.De Klippel N, Sarre S, Ebinger G, Michotte Y. Brain Res. 1993;630:57–64. doi: 10.1016/0006-8993(93)90642-z. [DOI] [PubMed] [Google Scholar]
- 34.Graybiel A M, Ragsdale C W., Jr Proc Natl Acad Sci USA. 1978;75:5723–5726. doi: 10.1073/pnas.75.11.5723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gerfen C R. Nature (London) 1984;311:461–464. doi: 10.1038/311461a0. [DOI] [PubMed] [Google Scholar]
- 36.Jimenez-Castellanos J, Graybiel A M. Neuroscience. 1989;32:297–321. doi: 10.1016/0306-4522(89)90080-8. [DOI] [PubMed] [Google Scholar]
- 37.Weinberger D R. Arch Gen Psychiatry. 1987;44:660–669. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]
- 38.Dean B, Crook J M, Opeskin K, Hill C, Keks N, Copolov D L. Mol Psychiatry. 1996;1:54–58. [PubMed] [Google Scholar]
- 39.Crook J M, Tomaskovic-Crook E, Copolov D L, Dean B. Am J Psychiatry. 2001;158:918–925. doi: 10.1176/appi.ajp.158.6.918. [DOI] [PubMed] [Google Scholar]
- 40.Yeomans J S. Neuropsychopharmacology. 1995;12:3–16. doi: 10.1038/sj.npp.1380235. [DOI] [PubMed] [Google Scholar]
- 41.Bymaster F P, Shannon H E, Rasmussen K, DeLapp N W, Ward J S, Calligaro D O, Mitch C H, Whitesitt C, Ludvigsen T S, Sheardown M, et al. Life Sci. 1999;64:527–534. doi: 10.1016/s0024-3205(98)00597-9. [DOI] [PubMed] [Google Scholar]
- 42.Felder C C, Porter A C, Skillman T L, Zhang L, Bymaster F P, Nathanson N M, Hamilton S E, Gomeza J, Wess J, McKinzie D L. Life Sci. 2001;68:2605–2613. doi: 10.1016/s0024-3205(01)01059-1. [DOI] [PubMed] [Google Scholar]
- 43.Seeman P. Synapse. 1987;1:133–152. doi: 10.1002/syn.890010203. [DOI] [PubMed] [Google Scholar]
- 44.Abi-Dargham A, Rodenhiser J, Printz D, Zea-Ponce Y, Gil R, Kegeles L S, Weiss R, Cooper T B, Mann J J, Van Heertum R L, et al. Proc Natl Acad Sci USA. 2000;97:8104–8109. doi: 10.1073/pnas.97.14.8104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Laruelle M, Abi-Dargham A, van Dyck C H, Gil R, D'Souza C D, Erdos J, McCance E, Rosenblatt W, Fingado C, Zoghbi S S, et al. Proc Natl Acad Sci USA. 1996;93:9235–9240. doi: 10.1073/pnas.93.17.9235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Breier A, Su T P, Saunders R, Carson R E, Kolachan B S, de Bartolomeis A, Weinberger D R, Weisenfeld N, Malhotra A K, Eckelman W C, et al. Proc Natl Acad Sci USA. 1997;94:2569–2574. doi: 10.1073/pnas.94.6.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reynolds G P. Br J Psychiatry. 1989;155:305–316. doi: 10.1192/bjp.155.3.305. [DOI] [PubMed] [Google Scholar]
- 48.Gainetdinov R R, Mohn A R, Caron M G. Trends Neurosci. 2001;9:527–533. doi: 10.1016/s0166-2236(00)01886-5. [DOI] [PubMed] [Google Scholar]