Abstract
Within the past 25 years, tissue engineering (TE) has grown enormously as a science and as an industry. Although classically concerned with the recapitulation of tissue and organ formation in our body for regenerative medicine, the evolution of TE research is intertwined with progress in other fields through the examination of cell function and behaviour in isolated biomimetic microenvironments. As such, TE applications now extend beyond the field of tissue regeneration research, operating as a platform for modifiable, physiologically-representative in vitro models with the potential to improve the translation of novel therapeutics into the clinic through a more informed understanding of the relevant molecular biology, structural biology, anatomy, and physiology. By virtue of their biomimicry, TE constructs incorporate features of extracellular macrostructure, molecular adhesive moieties, and biomechanical properties, converging with computational and structural biotechnology advances. Accordingly, this mini-review serves to contextualise TE for the computational and structural biotechnology reader and provides an outlook on how the disciplines overlap with respect to relevant advanced analytical applications.
Keywords: Tissue engineering, Biomaterials, Polymer, Mechanobiology, Mechanotransduction
Graphical Abstract
1. Introduction
Since the seminal paper of Langer and Vacanti over 25 years ago [1], tissue engineering (TE) has grown enormously as a science and as an industry. In 1993, it was introduced to the wider scientific community as “an interdisciplinary field that applies the principles of engineering and the life sciences toward the development of biological substitutes that restore, maintain, or improve tissue function”. As an industry, TE has stabilised and become profitable since the turn of this decade [2]; within Europe, for example, more TE products are regularly emerging in registered clinical trials as advanced therapeutic medicinal products (ATMPs) [3]. Classically, TE recapitulates tissue and organ formation in our body to varying degrees, bringing together cells in a three-dimensional (3D) fabricated environment where appropriate signals are provided for tissue formation. In parallel, the evolution of TE research is intertwined with progress in other fields through the examination of cell function and behaviour in isolated biomimetic microenvironments. Expanding our knowledge of stem cell biology [4,5], disease [6,7], and improved maintenance of cells in culture without dedifferentiation [8,9]. As such, TE applications now extend beyond regeneration strategies alone, operating as a platform for modifiable, physiologically-representative in vitro models [10]. Indeed, studies with TE in vitro models can answer questions that potentially improve the translation of novel therapeutics and ATMPs from the laboratory bench into the clinic, through a more informed understanding of the relevant molecular biology, structural biology, anatomy, and physiology.
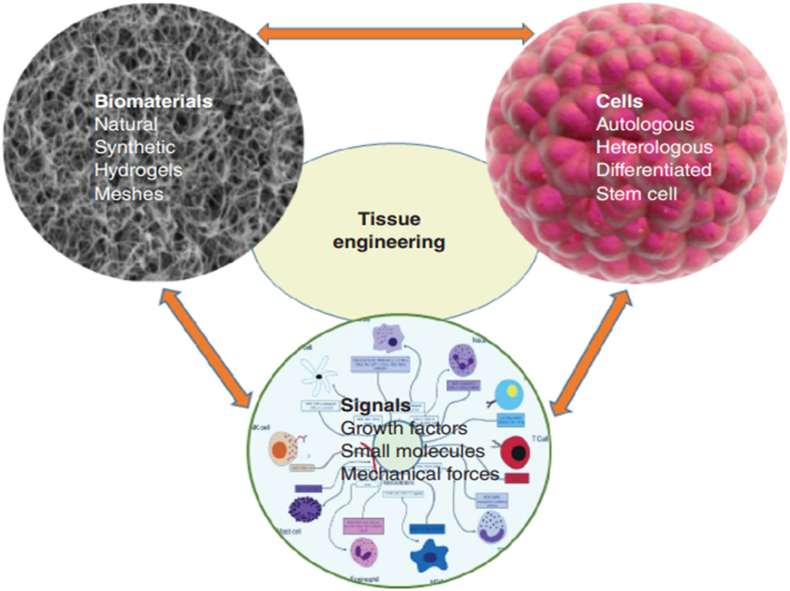
The manufacture of a successful TE construct is underpinned by three crucial components, referred to as the tissue engineering triad (Fig. 1): a relevant selection of cells, a biomaterial scaffold for 3D culture, and the presence of appropriate signals such as biophysical cues and chemical mediators that coordinate to ultimately recreate tissue [11]. Scaffolds produced should be biocompatible to preclude an immune response in the host following implantation, maintain a biodegradability rate that facilitates the replacement of scaffold with physiological tissue without collapse of the construct, have mechanical properties that mimic that of its surrounding in vivo environment, an architecture that facilitates cellular processes like diffusion, vascularisation and waste removal, and finally, be feasible in efficient and economic manufacture [12]. Signals to encourage extracellular matrix (ECM) production by cells can be provided by biophysical cues such as those applied by a bioreactor in which the construct is cultured [13], by delivery of bioactive molecules or genes [14,15], or even by the cell substrate's biophysical properties [16]. Cell sources include stem cells and host-derived cells that are typically cultured ex vivo on the biomaterial before implantation [17].
Fig. 1.
The tissue engineering triad. A combination of cells cultured on a biomaterial scaffold with appropriate biophysical and chemical signals coordinate to recapitulate the desired tissue. Image adapted from [11].
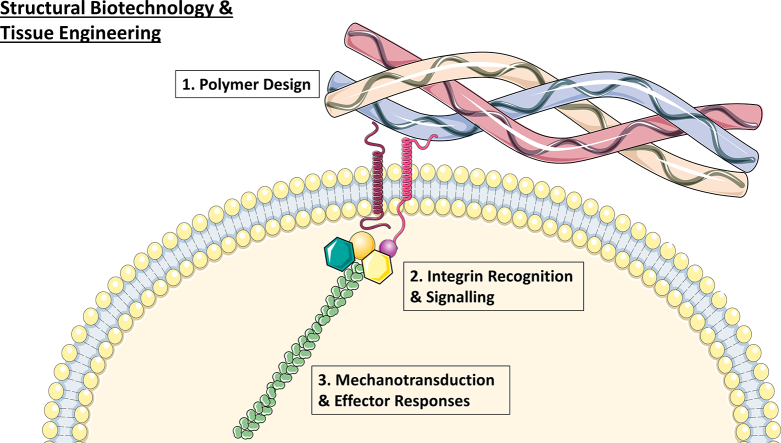
As the field of TE has evolved, an increased convergence with computational and structural biotechnology research has occurred as a function of the former's inherent biomimicry. Conventionally, structural biotechnology is primarily concerned with nanoscale molecules and how they interact within a biological system, such as inside a cell or tissue [18]. The ability to investigate the interaction between complex macromolecules like proteins and nucleic acids has been enabled on an unprecedented scale by advances in imaging, informatics, and big data omics, permitting analytical outputs of biological function and disease that were hitherto impossible or unfeasible [19,20]. On the other hand, the discipline of TE conventionally explores the “macro” level, creating constructs on the scale of the tissue or organ with anatomical architecture and robust bulk physical properties. However, as more knowledge has been accrued from the life sciences and as manufacture technologies have advanced to the high-resolution fabrication techniques of electrospinning and 3D printing additive manufacture [[21], [22], [23]], TE constructs can now accurately recapitulate elements of an in vivo microenvironment such as extracellular macrostructure, molecular adhesive moieties, and biomechanical properties [24]. Taken together, structural biotechnology and TE thus converge in a reciprocal fashion, whereby TE model and construct design provide the inputs to elicit certain cell and tissue functions, while the outputs that are stimulated can be identified, quantified, and interpreted by structural biotechnology approaches. Indeed, techniques such as high resolution microscopy and next generation sequencing, coupled with bioinformatics, can provide a deeper analysis of TE platforms and are being increasingly recognised within the field as powerful methods for extensive analysis of in vitro 3D models [25].However, the respective core concepts, strengths, and opportunity for synergy are not yet widely recognised or understood across both fields, prompting the need to create some context that can act as a springboard for further interest and collaboration among disciplines.
Accordingly, this mini-review serves to contextualise TE for the computational and structural biotechnology reader and provides an outlook on how the disciplines overlap with respect to relevant advanced analytical applications. While cell sources are a critical consideration within TE platforms, this review considers the structural biologist to have extensive experience in cellular biology. Therefore, we focus on the role of extracellular polymeric structures and the fundamentals of how their properties can transduce intracellular signals as integral facets to the biomaterial and signal pillars of the TE triad.
2. The Use of Natural Polymers in Tissue Engineering as Biomaterials
In fundamental terms, biomaterials are natural, synthetic, or composite polymer constructs that have been manufactured to a defined set of parameters in order to interact with a biological system (Fig. 2). The critical role of the biomaterial is to mimic the extracellular matrix (ECM) to which cells anchor and orientate themselves to form tissue, which itself is primarily composed of elastic fibres, collagens, and integrated glycosaminoglycans [26]. Thus, in addition to being non-toxic for the cells, biomaterials need to be structurally designed to reflect the elastic and tensile properties of these ECM components, in addition to exhibiting the capability for further crosslinking reactions that can occur in vivo [27]. Also, biomaterials must display cell-binding moieties for attachment, either by resembling the interactions of native ligands or by direct incorporation of peptide sequences, such as the triple helical GFOGER sequence of fibrillar collagens [28], the RGD epitope within fibronectin [29], or the GRKRK motif of tropoelastin [30]. Finally, and particularly in the case of synthetic materials, biodegradability and bioresorption are important issues to consider in order to ensure that cells have the capability to break down polymeric units and replace them with their own secreted ECM, if desired.
Fig. 2.
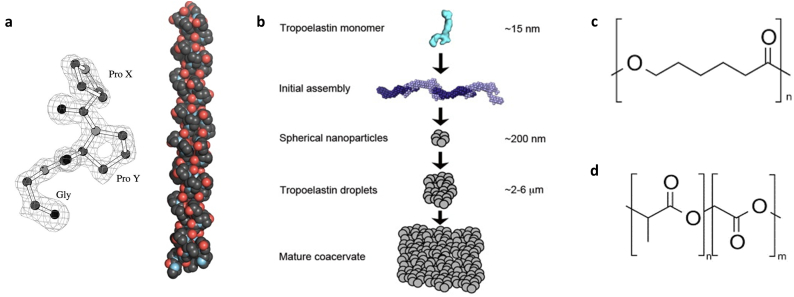
(a) Collagen structure, composed of repeating Gly-X-Y units that assemble into a heterotrimeric structure. Adapted from [31,32]. (b) Formation of tropoelastin coacervates in elastin synthesis. Adapted from [33]. (c) Poly-Ɛ-caprolactone (PCL). (d) Poly(lactic-co-glycolic) acid (PLGA) structure.
Of course, one of the simplest methods to mimic the ECM is to isolate and use the natural polymers that constitute it. As the most abundant structural protein in vivo, collagen is one of the most popular biomaterial choices in the TE field [34]. Collagens form a large protein family containing more than 40 genes encoding various alpha chains which can form at least 29 members, within which type I collagen forms the bedrock of the ECM of many tissues [35]. Collagen I is fibrillar in structure, composed of a distinctive heterotrimeric left-handed helix (Fig. 2a). The repeating Gly-X-Y amino acid sequence in its primary structure is critical to the formation of the polypeptide and resultant fibrils, reducing steric hindrance to permit helical formation and outward projection of other amino acid side chains to maximise adhesion and crosslinking. For almost every TE application, type I collagen has been explored as a biomaterial substrate, in many forms: as polymerised hydrogels [[36], [37], [38], [39]], porous polymeric scaffolds [[40], [41], [42]] and within decellularised tissue [[43], [44], [45]].
Other natural polymers can offer different mechanical or functional properties to those of collagen in biomaterials, which can be useful for particular regenerative medicine applications or TE in vitro disease models. For instance, while collagen confers stability and tensile strength to the ECM, other components such as elastin endow tissue with improved elastic properties [33]. Composed of monomeric units of tropoelastin that form a mature coacervate for subsequent deposition on a fibrillar network and crosslinking (Fig. 2b), the presence of hydrophobic regions within elastin's protein structure is integral for recoil following distension, whereby the exposure of non-polar residues in stretched form provoke a thermodynamically-favourable structural retraction to shield them from aqueous surroundings [46]. Accordingly, this natural polymer has been predominantly explored in TE for vascular and dermal applications, where such mechanical properties are inherently critical [[47], [48], [49], [50], [51], [52]]. As highlighted in these studies, elastin biomaterials have generally been fabricated as co-polymers with collagen, as well as silk fibroin, a versatile natural polymer [53]. Other natural polymers of notable interest in TE are hyaluronic acid and alginate. Neural ECM in the body contains higher concentrations of the glycosaminoglycan hyaluronic acid and its inclusion in biomaterials has been found to enhance tissue regeneration in intervertebral disc injury and reduce degeneration in retinal nervous tissue [54,55]. Other tissue where hyaluronic acid is concentrated, such as hyaline cartilage, has also benefited from its presence in TE constructs [56]. Interestingly, hyaluronic acid is also an intriguing polymer for TE in vitro disease models, where an upregulation of its presence can exacerbate metastasis and mortality in cancer [57]; indeed, different structural features of hyaluronic acid appear to mediate different inflammatory and healing responses in vivo [58], and in effect, different cell behaviours in healthy and diseased states.
Alginate, on the other hand, is derived from sea weed and is not present in the human body [59]. However, it is an interesting polymer for biomimetic TE in vitro models due to the ability to modulate its tensile and viscoelastic properties without resorting to crosslinking methods that can adversely affect cell-binding epitopes. Moreover, epitopes can be attached to alginate, providing further control of cell ligand density. In this way, alginate biomaterials have great utility in platforms where examination of the effects of mechanical properties on cell behaviour is sought that is independent of cell-ligand effects [60,61]. This is in stark contrast to collagen, where commonly used crosslinkers can hinder cell recognition of peptide sequences that are altered in the formation of ester and amide covalent bonds (discussed in [34]). Albeit a strength of alginate, this ligand-material decoupling highlights a major limitation of the use of other natural polymers: reduced capacity for customisation of physical and chemical structural properties, which translates into reduced capability for finely tuned control of TE systems for different applications. To address this issue, the TE field has investigated the use of synthetic biomaterials to provide a more controllable means of ECM biomimicry.
3. Structural Biomimicry With Synthetic and Composite Polymer Biomaterials
In general, the principal advantage of synthetic polymers in TE applications is their versatility. The control afforded over factors such as the structure of the monomeric units, the ratio of co-polymer structures, polymer sequence, chain length, and inter-chain facilitates a finer degree of control of biomaterial characteristics like biodegradability and mechanical properties [62] than that which can be achieved with natural polymers that have in effect, been pre-synthesised by nature, and exhibit a greater degree of structural heterogeneity [24]. Moreover, synthetic polymers can be designed to be more stable than protein-based polymers, permitting the use of manufacturing methods that use extremes of some conditions like charge and temperature; typical examples are electrospinning and melt electrospinning, respectively, for which the use of collagen solutions to prepare nanofibrous structures is doubtful [23,63]. Finally, synthetic materials can be manufactured more easily in bulk and are not a cost prohibitive as the time-consuming and onerous isolation and purification of natural polymers. However, a synthetic polymer's biocompatibility and facility for cell attachment is not necessarily as guaranteed as it can be for natural polymers. While the latter issue can be resolved through the incorporation of functional groups to conjugate cell ligands to in stoichiometric amounts [64], more extensive testing of novel synthetic polymers and their breakdown by-products could be required before their routine use becomes widespread in TE applications. Although many different types have been explored in the TE field (recently outlined in [62]), two archetypical biomaterials that illustrate these strengths and limitations of synthetic polymers are the polyesters poly-Ɛ-caprolactone (PCL; Fig. 2c) and poly(lactic-co-glycolic) acid (PLGA, Fig. 2d) [65,66].
PCL, a hydrophobic and semi-crystalline material, is renowned for its long in vivo residence time without chemical breakdown and for its viscoelastic behaviour that is similar to native tissue [[67], [68], [69], [70]]. Unlike natural polymers such as collagen or proteoglycans, the human body lacks enzymes that can cleave PCL chains, with the net result that its breakdown proceeds slowly via hydrolytic polymeric surface erosion over an average of three years [71,72]. When this information is taken together with its biomimetic material properties, PCL can be a very useful choice of biomaterial where prolonged scaffold support is warranted before robust tissue regeneration has occurred in situ that can bear significant mechanical loading. In this regard, PCL holds great promise for bone TE [[73], [74], [75]], tracheal TE [[76], [77], [78]], and intervertebral disc regeneration [79,80]. However, although PCL's structure confers resistance to rapid degradation, the polymer backbone also lacks an abundance of functional groups that can be modified for ligand attachment; as such, PCL has a reduced capacity for cell attachment. Thus, PCL is often employed in a composite biomaterial that compensates for cell binding, discussed below.
Of course, depending on the application, more flexible or rapid degradation rates might be desired for a biomaterial. In this regard, PLGA is another versatile, biocompatible, aliphatic polymer that has been widely used for drug delivery and TE applications with such properties [81]. PLGA also degrades by hydrolysis on hydrated surfaces, but unlike PCL, it degrades more rapidly because the glycolic acid monomers are more hydrophilic in nature and therefore interact more readily with water in the degradation process. Conversely, lactic acid does not degrade as rapidly as a result of its hydrophobic character. Thus, through fabricating polymers with different ratios of monomeric units present, a faster or slower degradation rate can be built into the polymer, purely as a result of its primary structure. Additionally, different molecular weights can also be utilised to control biodegradation, with slower degradation as molecular weight increases. Following hydrolysis, however, the build-up of acidic breakdown products can be damaging for surrounding local tissue, with is particularly undesired in cases of tissue regeneration [82]. For TE applications where these products can be readily removed, such as in 3D in vitro models that have regular media changes, cellular damage and toxicity might not be as significant. In summary, taking the advantages and disadvantages of both synthetic polymers together, synthetic polymers are indeed useful biomaterials with customisable and versatile features for TE technologies, but their absence of the inherent biomimicry that is a feature of natural polymers can oppose their universal application.
In order to address the respective shortcomings of both natural and synthetic polymers in TE, composite biomaterials entailing combinations of both have been the subject of many studies, particularly in tissue regeneration applications. Typically, natural and synthetic composites have been designed with the aim of bolstering the stability and robust mechanical properties of synthetic materials with the cell adhesive and cell instructive cues of natural polymers. For example, PCL has been combined with a wide range of natural biomaterials including collagen [77], gelatin [83,84], hyaluronic acid [85], and cellulose [86]. The myriad of all combinations of synthetic-natural composite combinations in the TE field are vast; a comprehensive list is beyond the scope of this mini-review. However, from a structural perspective, the common theme of improved mechanical properties prevails within the literature. As recently illustrated by Jakus and colleagues [87], however, improving structural strength can also yield resultant effects on cell behaviour and the improved development of organotypic tissue. This material, a mix of PCL or PLGA with hydroxyapatite, exhibited high material stiffness, osteogenic differentiation of seeded stem cells, and formed vascularised bone tissue in vivo. It well-known in TE that a stiffer material substrate can stimulate stem cell differentiation into bone cells [4], and the presence of the bone mineral hydroxyapatite also contributed to osteogenesis, as with other studies of doping PCL constructs with mineral [83,88]. Once again, the core structural features of the polymers provide a resultant effect on tissue formation as a function of biomaterial composition, mechanical properties, and biodegradation.
4. Biophysical Signalling in Tissue Engineering: Cell-Substrate Mechanotransduction
Regardless of whether the biomaterial substrate is natural or synthetic, once cells can attach to a polymer, a combination of receptor-mediated and mechanical-mediated signals will regulate their phenotype and function. This process is defined as mechanotransduction, in which cells sense and respond to mechanical stimuli by converting them to biochemical signals, commencing with cell recognition of specific extracellular motifs to bind, subsequent probing of the physical nature of its surrounding environment, and resultant effector responses [26]. Effector responses to cell ligand density and matrix elasticity include differentiation of stem cells [4,5,89], migration [90,91], and disease progression [[92], [93], [94], [95]].
Mechanotransduction initiates with cell recognition of ligands at the cell-substrate interface, followed by tension generation in the cell and kinase activity, before culminating in downstream signalling responses (Fig. 3a). A fully comprehensive review of all protein-material interactions, including nanotopographical characteristics of the biomaterial and ligand spacing (reviewed in [96]), is beyond the scope of this mini-review but the critical concept of cell responses to adjacent polymeric structures is illustrated through canonical integrin receptor signalling, a family of transmembrane proteins that are the major cell adhesion receptor for the ECM [97,98]. As heterodimeric proteins, distinct α- and β-subunits of integrins can propagate different downstream effects, even upon recognition of the same extracellular ligand. Thus, while integrins are typically classified by their recognition of collagen, fibronectin or laminin motifs, resultant cell responses can range from homeostatic to pathogenic [99].
Fig. 3.
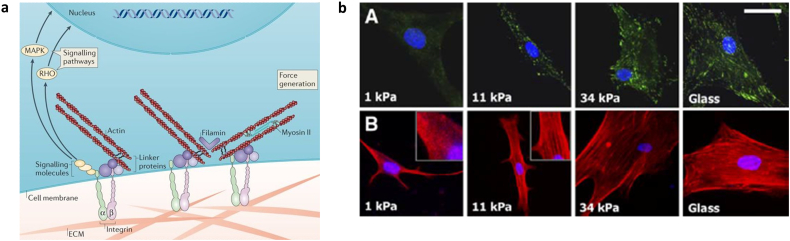
(a) Overview of mechanotransduction. Integrin receptors recognise and bind to cell-adhesive ligands in the extracellular matrix (ECM), initiating the formation of intracellular focal adhesion complexes (left panel). Signalling molecules directly stimulate downstream transcription and linker proteins in the complex bind to actin filaments, which can generate a tensile force in conjunction with myosin II activity. Adapted from [26]. (b) Increased substrate stiffness induces the formation of focal adhesion complexes (green) and actin polymerisation and alignment (red) in human mesenchymal stem cells. Adapted from [4]. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
In either case, extracellular integrin-ligand binding induces conformational changes in the receptor's intracellular domain, stimulating the association of linker proteins including talin [100], vinculin [101], and paxillin [102] to form a focal adhesion complex. The linker proteins in turn facilitate the association of the cytoskeletal actin framework with the focal adhesion complex, generating a tensional force across the cell in proportion to the substrate stiffness sensed in resistance to the net inward flow of actin towards the nucleus. As the stiffness increases, more focal adhesions cluster and actin filaments align to generate a greater tensional force (Fig. 3b). Consequently, the polymerisation of actin filaments and tensional force is felt along the entire cytoskeleton, regulating differential gene expression in response.
In addition to acting as key transducers of mechanical signalling at the cell-substrate interface, focal adhesion subunits, including focal adhesion kinase (FAK), can phosphorylate and activate a range of other pathways with a multitude of effects [103]. In one of its roles, FAK mediates linker protein recruitment as filament tension increases [104], but its kinase activity affects MAPK signalling and Rho kinase activity, among others [[105], [106], [107], [108], [109]]. Herein, the variety of pathways that FAK is linked to reveal its potential for both normal and aberrant signalling in different cells and tissue. For example, FAK knockout in keratinocytes abrogates adequate cell coverage and tissue formation in wound healing [110], while in breast cancer, FAK can operate as a central regulator of invadopodia formation and consequently, matrix proteolysis, cell migration, and metastasis [111]. Thus, via FAK activity, integrin coupling to different biomaterial structures can instigate a complex but coordinated overlap of cellular processes, with a net influence on both essential and unwanted outcomes in our bodies [112]. From a TE perspective, careful biomaterial design can ultimately harness biophysical signalling pathways to elicit the required cell responses in different applications.
Interestingly, biomaterials can also trigger biophysical cell responses in a dynamic fashion through stimuli-responsive polymers. Piezoelectric polymers, for example, develop a voltage in response to a mechanical stress with resultant changes in electroconductive properties and surface charge [113]. Many native tissues are piezoelectric in nature and accordingly, it is little surprise that stem cells respond by differentiating towards myogenic [114], osteogenic [115,116], or chondrogenic [117] lineages. Moreover, electroactive biomaterials have the potential to be combined with external magnetic fields to induce differential cell responses as a function of applied electrical fields [118], potentially enabling “real-time” control of signal transduction. As well electroactive biomaterials, other stimuli-responsive polymers have been explored using pH, temperature, and photo-catalysed reactions [119]. Indeed, in the case of the latter, Ondeck and colleagues have recently shown in an elegant study how stimuli-responsive biomaterials can be used to explore in situ cell transformation. Using a methacrylated hyaluronic acid substrate that stiffens under UV light, a mechanosensitive epithelial-mesenchymal transition in precancerous breast epithelial cells was dynamically stimulated, observing an increase in cell invasion as the substrate adopted the mechanical properties of tumour tissue [120]. Ultimately, from a TE perspective, such careful biomaterial design can ultimately harness biophysical signalling pathways, whether regenerative, aberrant, or otherwise to elicit the required cell responses in different applications.
5. Summary & Outlook: Convergence of Tissue Engineering With Computational and Structural Biotechnology Applications
The central objective of all TE research is to emulate the anatomical and physiological or pathophysiological traits of a tissue or organ: multicellular systems with accurate spatial distribution of cells and ECM with the appropriate architecture, and resultant coordinated biological responses within this system. Through our knowledge of the structural influence of the 3D scaffold structures on cellular function, our capacity to engineer platforms that repair injury, model disease, and test novel therapeutics has increased significantly. Thus, as the complexity of these platforms increase, so too does the need for advanced methods of analysis that can be found within the realm of computational and structural biotechnology. This outlook serves to highlight recent examples of TE engaging with several methods in this domain to elevate the field to a deeper level of understanding of cellular function in a 3D environment (Table 1).
Table 1.
Advanced analytical methods from computational and structural biotechnology and their use in analysis of biomaterials. FLIM = Fluorescence-lifetime imaging microscopy; PEG = Polyethylene glycol; PVA = polyvinyl alcohol; RNA-seq = RNA sequencing.
| Method | Biomaterial system | Comments | Ref |
|---|---|---|---|
| FLIM | Cellulose-based scaffold | FLIM permitted the real-time acidification of 3D environments by colon cancer cells and stem cell organoids | [121] |
| Bovine pericardium tissue | Collagenase-mediated degradation was observable using FLIM | [122] | |
| Live imaging of recellularisation and vascularisation detectable using FLIM | [123] | ||
| Collagen-based hydrogel | Longitudinal monitoring of collagen crosslinking in real time detectable using LFIM | [124] | |
| RNA-seq | PVA hydrogel | RNA-seq identified enrichment of differentially expressed genes in metabolic activity and cytoskeletal proteins in response to different PVA substrate stiffness | [125] |
| Collagen-Matrigel hydrogel | RNA-seq validated the ability of a customised miniature ventricular heart chamber to induce expression of cardiac-specific cellular markers derived from human pluripotent stem cells | [126] | |
| Silk film | RNA-seq revealed that alignment of silk fibres in films induced differential gene expression in cell adhesion and cytoskeletal dynamics | [127] | |
| Alginate hydrogel | RNA-seq identified enrichment of differentially expressed genes in cell differentiation and immunomodulatory function as a response to different alginate substrate stiffness | [60] | |
| PEG hydrogel | RNA-seq validated the ability of the hydrogel biomaterial to induce expression of vascularisation genes in endothelial cells derived from induced pluripotent stem cells | [128] |
As a science that develops complex 3D biomimetic structures, advanced imaging capabilities that can combine spatial orientation with functional analysis show great promise for TE platforms. Moreover, the possibility of non-destructive imaging of cells in TE constructs would be clearly of benefit to monitor their in situ behaviour in real time. Fluorescence-lifetime imaging microscopy (FLIM) is one such technique [129]. FLIM utilises contrast in live images by spatial variations in fluorescence lifetime of a probe that is largely concentration- and intensity-independent but is sensitive to the environmental surroundings of the fluorophore. In this manner, cell responses including intracellular protein-protein interactions [130] and metabolism [131,132] can be evaluated, with the potential to identify spatially-dependent or other microenvironmental responses. For the TE field, FLIM has only recently began to be recognised as a powerful technique [[121], [122], [123], [124],133]; it remains as an exciting technique for further exploitation, with notable possibilities for detailed analysis of integrin agonism [134]. Moreover, interferometric photoactivated localisation microscopy (iPALM) has the capability to examine integrin structures on a nanoscale, offering new insights into differential receptor conformations and related interactions with other biomolecules and signals [[135], [136], [137]]. Finally, as next generation sequencing becomes more commonplace in TE [60], recent work from the Church lab has developed a protocol to combine single cell transcriptomics with spatial orientation [138]; although there are still several technical and logistical hurdles to overcome for widespread use of this technology, it emphasises yet again the potential advanced imaging technologies available to embrace for enhanced analysis of structural interactions in TE systems.
Coupled with advanced imaging techniques for merging structural biotechnology investigations with TE, detailed quantification of the composition of TE platforms can be performed through large-scale analysis with bioinformatics. This is of particular interest as 3D in vitro models evolve in conjunction with a deeper understanding of ECM and cell-substrate interactions in disease. Quantitative proteomic analysis of ECM changes in disease, for example, can reveal matrix signatures that could be recapitulated for biomimetic disease models; such studies of the matrisome in cancer and fibrosis are of great interest in this regard [[139], [140], [141]]. RNA sequencing has also begun to feature more in analyses of the biophysical effects of different microenvironments [60,[125], [126], [127], [128]]. Naturally, bioinformatics will have a role to play in the processing of large data related to spatially-relevant transcriptomics [138], and additionally, computational technology will enable intelligent combinatorial analyses of spectroscopic and microscopic data, as has been recently reported in studies that blended histology with Raman spectra and atomic force microscopy to evaluate the mechanical, compositional, and structural characteristics of diseased tissue [142,143].
In summary, the design and development of TE constructs, whether for therapeutic or scientific applications, hinges upon its biomimetic structural features that affect mechanical properties, stability, degradation, cell adhesion, and cell functionality. As TE studies integrate techniques that have been traditionally restricted to computational and structural biologists, greater opportunities for complementary investigations within both disciplines will present themselves. Ultimately, future collaboration provides greater success for both fields; and in order for further advances in a discipline that continues to evolve and address new challenges in the treatment of injury and disease, we propose increased engagement between the interrelated disciplines of structural biotechnology and tissue engineering.
Conflict of Interest Disclosure
The authors declare no competing financial interest.
Acknowledgements
The authors acknowledge the funding received for this research by the Government of the State of Kuwait. The graphical abstract was prepared using images available from Servier Medical Art by Servier, licensed under a Creative Commons Attribution 3.0 Unported License (CC BY 3.0).
Contributor Information
Nour Almouemen, Email: nouralmouemen@rcsi.ie.
Helena M. Kelly, Email: helenakelly@rcsi.ie.
Cian O'Leary, Email: cianoleary@rcsi.ie.
References
- 1.Langer R., Vacanti J.P. Tissue engineering. Science. 1993;260(5110):920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 2.Jaklenec A., Stamp A., Deweerd E., Sherwin A., Langer R. Progress in the tissue engineering and stem cell industry "are we there yet?". Tissue Eng Part B Rev. 2012;18(3):155–166. doi: 10.1089/ten.TEB.2011.0553. [DOI] [PubMed] [Google Scholar]
- 3.Koci Z., Boran T., Krupa P., Kubinova S. The current state of advanced therapy medicinal products in the Czech Republic. Hum Gene Ther Clin Dev. 2018;29(3):132–147. doi: 10.1089/humc.2018.035. [DOI] [PubMed] [Google Scholar]
- 4.Engler A.J., Sen S., Sweeney H.L., Discher D.E. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 5.Cameron A.R., Frith J.E., Cooper-White J.J. The influence of substrate creep on mesenchymal stem cell behaviour and phenotype. Biomaterials. 2011;32(26):5979–5993. doi: 10.1016/j.biomaterials.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 6.Benam K.H., Dauth S., Hassell B., Herland A., Jain A., Jang K.J. Engineered in vitro disease models. Annu Rev Pathol. 2015;10:195–262. doi: 10.1146/annurev-pathol-012414-040418. [DOI] [PubMed] [Google Scholar]
- 7.Fitzgerald K.A., Malhotra M., Curtin C.M., O’Brien F.J., O’Driscoll C.M. Life in 3D is never flat: 3D models to optimise drug delivery. J Control Release. 2015;215:39–54. doi: 10.1016/j.jconrel.2015.07.020. [DOI] [PubMed] [Google Scholar]
- 8.Malda J., van Blitterswijk C.A., Grojec M., Martens D.E., Tramper J., Riesle J. Expansion of bovine chondrocytes on microcarriers enhances redifferentiation. Tissue Eng. 2003;9(5):939–948. doi: 10.1089/107632703322495583. [DOI] [PubMed] [Google Scholar]
- 9.Brodkin K.R., Garcia A.J., Levenston M.E. Chondrocyte phenotypes on different extracellular matrix monolayers. Biomaterials. 2004;25(28):5929–5938. doi: 10.1016/j.biomaterials.2004.01.044. [DOI] [PubMed] [Google Scholar]
- 10.O'Leary C., Gilbert J.L., O'Dea S., O'Brien F.J., Cryan S.A. Respiratory tissue engineering: current status and opportunities for the future. Tissue Eng Part B Rev. 2015;21(4):323–344. doi: 10.1089/ten.TEB.2014.0525. [DOI] [PubMed] [Google Scholar]
- 11.Mhanna R., Hasan A. Introduction to tissue engineering. In: Hasan A., editor. Tissue engineering for artifical organs: regenerative medicine, smart diagnostics and personalized medicine. Wiley-VCH; NJ, USA: 2017. [Google Scholar]
- 12.Hutmacher D.W. Scaffolds in tissue engineering bone and cartilage. Biomaterials. 2000;21(24):2529–2543. doi: 10.1016/s0142-9612(00)00121-6. [DOI] [PubMed] [Google Scholar]
- 13.Partap S., Plunkett N.A., O'Brien F.J. Bioreactors in tissue engineering. In: Eberli D., editor. Tissue Engineering. InTech; 2010. [Google Scholar]
- 14.Vo T.N., Kasper F.K., Mikos A.G. Strategies for controlled delivery of growth factors and cells for bone regeneration. Adv Drug Deliv Rev. 2012;64(12):1292–1309. doi: 10.1016/j.addr.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Leary C., O'Brien F.J., Cryan S.A. Retinoic acid-loaded collagen-hyaluronate scaffolds: a bioactive material for respiratory tissue regeneration. ACS Biomater Sci Eng. 2017;3(7):1381–1393. doi: 10.1021/acsbiomaterials.6b00561. [DOI] [PubMed] [Google Scholar]
- 16.Murphy W.L., McDevitt T.C., Engler A.J. Materials as stem cell regulators. Nat Mater. 2014;13(6):547–557. doi: 10.1038/nmat3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weiss D.J., Kolls J.K., Ortiz L.A., Panoskaltsis-Mortari A., Prockop D.J. Stem cells and cell therapies in lung biology and lung diseases. Proc Am Thorac Soc. 2008;5(5):637–667. doi: 10.1513/pats.200804-037DW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Campbell I.D. The march of structural biology. Nat Rev Mol Cell Biol. 2002;3:377. doi: 10.1038/nrm800. [DOI] [PubMed] [Google Scholar]
- 19.Hasin Y., Seldin M., Lusis A. Multi-omics approaches to disease. Genome Biol. 2017;18(1):83. doi: 10.1186/s13059-017-1215-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karczewski K.J., Snyder M.P. Integrative omics for health and disease. Nat Rev Genet. 2018;19:299. doi: 10.1038/nrg.2018.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barnes C.P., Sell S.A., Boland E.D., Simpson D.G., Bowlin G.L. Nanofiber technology: designing the next generation of tissue engineering scaffolds. Adv Drug Deliv Rev. 2007;59(14):1413–1433. doi: 10.1016/j.addr.2007.04.022. [DOI] [PubMed] [Google Scholar]
- 22.Murphy S.V., Atala A. 3D bioprinting of tissues and organs. Nat Biotechnol. 2014;32(8):773–785. doi: 10.1038/nbt.2958. [DOI] [PubMed] [Google Scholar]
- 23.Zhang L.-H., Duan X.-P., Yan X., Yu M., Ning X., Zhao Y. Recent advances in melt electrospinning. RSC Adv. 2016;6(58):53400–53414. [Google Scholar]
- 24.von der Mark K., Park J. Engineering biocompatible implant surfaces: part II: cellular recognition of biomaterial surfaces: lessons from cell–matrix interactions. Prog Mater Sci. 2013;58(3):327–381. [Google Scholar]
- 25.Horvath P., Aulner N., Bickle M., Davies A.M., Nery E.D., Ebner D. Screening out irrelevant cell-based models of disease. Nat Rev Drug Discov. 2016;15(11):751–769. doi: 10.1038/nrd.2016.175. [DOI] [PubMed] [Google Scholar]
- 26.Humphrey J.D., Dufresne E.R., Schwartz M.A. Mechanotransduction and extracellular matrix homeostasis. Nat Rev Mol Cell Biol. 2014;15(12):802–812. doi: 10.1038/nrm3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grau-Bové X., Ruiz-Trillo I., Rodriguez-Pascual F. Origin and evolution of lysyl oxidases. Sci Rep. 2015;5:10568. doi: 10.1038/srep10568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Knight C.G., Morton L.F., Peachey A.R., Tuckwell D.S., Farndale R.W., Barnes M.J. The collagen-binding A-domains of integrins alpha(1)beta(1) and alpha(2)beta(1) recognize the same specific amino acid sequence, GFOGER, in native (triple-helical) collagens. J Biol Chem. 2000;275(1):35–40. doi: 10.1074/jbc.275.1.35. [DOI] [PubMed] [Google Scholar]
- 29.Pytela R., Pierschbacher M.D., Argraves S., Suzuki S., Ruoslahti E. Arginine-glycine-aspartic acid adhesion receptors. Methods Enzymol. 1987;144:475–489. doi: 10.1016/0076-6879(87)44196-7. [DOI] [PubMed] [Google Scholar]
- 30.Bax D.V., Rodgers U.R., Bilek M.M., Weiss A.S. Cell adhesion to tropoelastin is mediated via the C-terminal GRKRK motif and integrin alphaVbeta3. J Biol Chem. 2009;284(42):28616–28623. doi: 10.1074/jbc.M109.017525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berisio R., Vitagliano L., Mazzarella L., Zagari A. Crystal structure of the collagen triple helix model [(Pro-Pro-Gly)10]3. Protein Sci. 2002;11(2):262–270. doi: 10.1110/ps.32602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shoulders M.D., Raines R.T. Collagen structure and stability. Annu Rev Biochem. 2009;78:929–958. doi: 10.1146/annurev.biochem.77.032207.120833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wise S.G., Yeo G.C., Hiob M.A., Rnjak-Kovacina J., Kaplan D.L., Ng M.K.C. Tropoelastin: a versatile, bioactive assembly module. Acta Biomater. 2014;10(4):1532–1541. doi: 10.1016/j.actbio.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pawelec K.M., Best S.M., Cameron R.E. Collagen: a network for regenerative medicine. J Mater Chem B Mater Biol Med. 2016;4(40):6484–6496. doi: 10.1039/c6tb00807k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stamov D.R., Pompe T. Structure and function of ECM-inspired composite collagen type I scaffolds. Soft Matter. 2012;8:10200–10212. [Google Scholar]
- 36.Xu T., Molnar P., Gregory C., Das M., Boland T., Hickman J.J. Electrophysiological characterization of embryonic hippocampal neurons cultured in a 3D collagen hydrogel. Biomaterials. 2009;30(26):4377–4383. doi: 10.1016/j.biomaterials.2009.04.047. [DOI] [PubMed] [Google Scholar]
- 37.Pageau S.C., Sazonova O.V., Wong J.Y., Soto A.M., Sonnenschein C. The effect of stromal components on the modulation of the phenotype of human bronchial epithelial cells in 3D culture. Biomaterials. 2011;32(29):7169–7180. doi: 10.1016/j.biomaterials.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 38.Antoine E.E., Vlachos P.P., Rylander M.N. Tunable collagen I hydrogels for engineered physiological tissue micro-environments. PLoS One. 2015;10(3) doi: 10.1371/journal.pone.0122500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuo K.C., Lin R.Z., Tien H.W., Wu P.Y., Li Y.C., Melero-Martin J.M. Bioengineering vascularized tissue constructs using an injectable cell-laden enzymatically crosslinked collagen hydrogel derived from dermal extracellular matrix. Acta Biomater. 2015;27:151–166. doi: 10.1016/j.actbio.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O'Leary C., Cavanagh B., Unger R.E., Kirkpatrick C.J., O'Dea S., O'Brien F.J. The development of a tissue-engineered tracheobronchial epithelial model using a bilayered collagen-hyaluronate scaffold. Biomaterials. 2016;85:111–127. doi: 10.1016/j.biomaterials.2016.01.065. [DOI] [PubMed] [Google Scholar]
- 41.Roche P., Alekseeva T., Widaa A., Ryan A., Matsiko A., Walsh M. Olfactory derived stem cells delivered in a biphasic conduit promote peripheral nerve repair in vivo. Stem Cells Transl Med. 2017;6(10):1894–1904. doi: 10.1002/sctm.16-0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matsiko A., Thompson E.M., Lloyd-Griffith C., Cunniffe G.M., Vinardell T., Gleeson J.P. An endochondral ossification approach to early stage bone repair: use of tissue-engineered hypertrophic cartilage constructs as primordial templates for weight-bearing bone repair. J Tissue Eng Regen Med. 2018;12(4):e2147–e2150. doi: 10.1002/term.2638. [DOI] [PubMed] [Google Scholar]
- 43.Mertsching H., Walles T., Hofmann M., Schanz J., Knapp W.H. Engineering of a vascularized scaffold for artificial tissue and organ generation. Biomaterials. 2005;26(33):6610–6617. doi: 10.1016/j.biomaterials.2005.04.048. [DOI] [PubMed] [Google Scholar]
- 44.Dziki J.L., Giglio R.M., Sicari B.M., Wang D.S., Gandhi R.M., Londono R. The effect of mechanical loading upon extracellular matrix bioscaffold-mediated skeletal muscle remodeling. Tissue Eng Part A. 2018;24(1–2):34–46. doi: 10.1089/ten.tea.2017.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reing J.E., Brown B.N., Daly K.A., Freund J.M., Gilbert T.W., Hsiong S.X. The effects of processing methods upon mechanical and biologic properties of porcine dermal extracellular matrix scaffolds. Biomaterials. 2010;31(33):8626–8633. doi: 10.1016/j.biomaterials.2010.07.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rauscher S., Pomes R. Structural disorder and protein elasticity. Adv Exp Med Biol. 2012;725:159–183. doi: 10.1007/978-1-4614-0659-4_10. [DOI] [PubMed] [Google Scholar]
- 47.Ryan A.J., O'Brien F.J. Insoluble elastin reduces collagen scaffold stiffness, improves viscoelastic properties, and induces a contractile phenotype in smooth muscle cells. Biomaterials. 2015;73:296–307. doi: 10.1016/j.biomaterials.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 48.Singh R., Sarker B., Silva R., Detsch R., Dietel B., Alexiou C. Evaluation of hydrogel matrices for vessel bioplotting: vascular cell growth and viability. J Biomed Mater Res A. 2016;104(3):577–585. doi: 10.1002/jbm.a.35590. [DOI] [PubMed] [Google Scholar]
- 49.Chouhan D., Chakraborty B., Nandi S.K., Mandal B.B. Role of non-mulberry silk fibroin in deposition and regulation of extracellular matrix towards accelerated wound healing. Acta Biomater. 2017;48:157–174. doi: 10.1016/j.actbio.2016.10.019. [DOI] [PubMed] [Google Scholar]
- 50.Schmidt V.J., Wietbrock J.O., Leibig N., Gloe T., Henn D., Hernekamp J.F. Collagen-elastin and collagen-glycosaminoglycan scaffolds promote distinct patterns of matrix maturation and axial vascularization in arteriovenous loop-based soft tissue flaps. Ann Plast Surg. 2017;79(1):92–100. doi: 10.1097/SAP.0000000000001096. [DOI] [PubMed] [Google Scholar]
- 51.Silva R., Singh R., Sarker B., Papageorgiou D.G., Juhasz-Bortuzzo J.A., Roether J.A. Hydrogel matrices based on elastin and alginate for tissue engineering applications. Int J Biol Macromol. 2018;114:614–625. doi: 10.1016/j.ijbiomac.2018.03.091. [DOI] [PubMed] [Google Scholar]
- 52.Goins A., Ramaswamy V., Lichlyter D., Webb A., Allen J.B. Fabrication of a bilayer scaffold for smalldiameter vascular applications. J Biomed Mater Res Part A. 2018:2850–2862. doi: 10.1002/jbm.a.36473. (106A) [DOI] [PubMed] [Google Scholar]
- 53.Qi Y., Wang H., Wei K., Yang Y., Zheng R.Y., Kim I.S. A review of structure construction of silk fibroin biomaterials from single structures to multi-level structures. Int J Mol Sci. 2017;18(3) doi: 10.3390/ijms18030237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gansau J., Buckley C.T. Incorporation of collagen and hyaluronic acid to enhance the bioactivity of fibrin-based hydrogels for nucleus pulposus regeneration. J Funct Biomater. 2018;9(3) doi: 10.3390/jfb9030043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hunt N.C., Hallam D., Chichagova V., Steel D.H., Lako M. The application of biomaterials to tissue engineering neural retina and retinal pigment epithelium. Adv Healthc Mater. 2018;7:1800226. doi: 10.1002/adhm.201800226. [DOI] [PubMed] [Google Scholar]
- 56.Matsiko A., Levingstone T.J., O'Brien F.J., Gleeson J.P. Addition of hyaluronic acid improves cellular infiltration and promotes early-stage chondrogenesis in a collagen-based scaffold for cartilage tissue engineering. J Mech Behav Biomed Mater. 2012;11:41–52. doi: 10.1016/j.jmbbm.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 57.Oskarsson T. Extracellular matrix components in breast cancer progression and metastasis. Breast (Edinburgh, Scotland) 2013;22(Suppl. 2) doi: 10.1016/j.breast.2013.07.012. (S66–72) [DOI] [PubMed] [Google Scholar]
- 58.Papakonstantinou E., Karakiulakis G. The 'sweet' and 'bitter' involvement of glycosaminoglycans in lung diseases: pharmacotherapeutic relevance. Br J Pharmacol. 2009;157(7):1111–1127. doi: 10.1111/j.1476-5381.2009.00279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee K.Y., Mooney D.J. Alginate: properties and biomedical applications. Prog Polym Sci. 2012;37(1):106–126. doi: 10.1016/j.progpolymsci.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Darnell M., Gu L., Mooney D. RNA-seq reveals diverse effects of substrate stiffness on mesenchymal stem cells. Biomaterials. 2018;181:182–188. doi: 10.1016/j.biomaterials.2018.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Darnell M., O'Neil A., Mao A., Gu L., Rubin L.L., Mooney D.J. Material microenvironmental properties couple to induce distinct transcriptional programs in mammalian stem cells. Proc Natl Acad Sci U S A. 2018;115(36):E8368–e8377. doi: 10.1073/pnas.1802568115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.BaoLin G.U.O., Ma P.X. Synthetic biodegradable functional polymers for tissue engineering: a brief review. Sci China Chem. 2014;57(4):490–500. doi: 10.1007/s11426-014-5086-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zeugolis D.I., Khew S.T., Yew E.S., Ekaputra A.K., Tong Y.W., Yung L.Y. Electro-spinning of pure collagen nano-fibres - just an expensive way to make gelatin? Biomaterials. 2008;29(15):2293–2305. doi: 10.1016/j.biomaterials.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 64.Hersel U., Dahmen C., Kessler H. RGD modified polymers: biomaterials for stimulated cell adhesion and beyond. Biomaterials. 2003;24(24):4385–4415. doi: 10.1016/s0142-9612(03)00343-0. [DOI] [PubMed] [Google Scholar]
- 65.Puppi D., Piras A.M., Detta N., Dinucci D., Chiellini F. Poly(lactic-co-glycolic acid) electrospun fibrous meshes for the controlled release of retinoic acid. Acta Biomater. 2010;6(4):1258–1268. doi: 10.1016/j.actbio.2009.08.015. [DOI] [PubMed] [Google Scholar]
- 66.Mondal D., Griffith M., Venkatraman S.S. Polycaprolactone-based biomaterials for tissue engineering and drug delivery: current scenario and challenges. Int J Polym Mater Polym Biomater. 2016;65(5):255–265. [Google Scholar]
- 67.Johnson G.A., Tramaglini D.M., Levine R.E., Ohno K., Choi N.Y., Woo S.L. Tensile and viscoelastic properties of human patellar tendon. J Orthop Res. 1994;12(6):796–803. doi: 10.1002/jor.1100120607. [DOI] [PubMed] [Google Scholar]
- 68.Woodruff M.A., Hutmacher D.W. The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog Polym Sci. 2010;35(10):1217–1256. [Google Scholar]
- 69.Kurniawan D., Nor F.M., Lee H.Y., Lim J.Y. Elastic properties of polycaprolactone at small strains are significantly affected by strain rate and temperature, proceedings of the institution of mechanical engineers. Part H. J Eng Med. 2011;225(10):1015–1020. doi: 10.1177/0954411911413059. [DOI] [PubMed] [Google Scholar]
- 70.Safshekan F., Tafazzoli-Shadpour M., Abdouss M., Shadmehr M.B. Viscoelastic properties of human tracheal tissues. J Biomech Eng. 2017;139(1) doi: 10.1115/1.4034651. [DOI] [PubMed] [Google Scholar]
- 71.Vert M. Degradable and bioresorbable polymers in surgery and in pharmacology: beliefs and facts. J Mater Sci Mater Med. 2009;20(2):437–446. doi: 10.1007/s10856-008-3581-4. [DOI] [PubMed] [Google Scholar]
- 72.Zopf D.A., Hollister S.J., Nelson M.E., Ohye R.G., Green G.E. Bioresorbable airway splint created with a three-dimensional printer. N Engl J Med. 2013;368(21):2043–2045. doi: 10.1056/NEJMc1206319. [DOI] [PubMed] [Google Scholar]
- 73.Yilgor P., Sousa R.A., Reis R.L., Hasirci N., Hasirci V. 3D plotted PCL scaffolds for stem cell based bone tissue engineering. Macromol Symp. 2008;269(1):92–99. [Google Scholar]
- 74.Cheng G., Ma X., Li J., Cheng Y., Cao Y., Wang Z. Incorporating platelet-rich plasma into coaxial electrospun nanofibers for bone tissue engineering. Int J Pharm. 2018;547(1–2):656–666. doi: 10.1016/j.ijpharm.2018.06.020. [DOI] [PubMed] [Google Scholar]
- 75.Abdal-Hay A., Hamlet S., Ivanovski S. Fabrication of a thick three-dimensional scaffold with an open cellular-like structure using airbrushing and thermal cross-linking of molded short nanofibers. Biofabrication. 2019;11:015006. doi: 10.1088/1758-5090/aae421. [DOI] [PubMed] [Google Scholar]
- 76.Lin C.H., Su J.M., Hsu S.H. Evaluation of type II collagen scaffolds reinforced by poly(epsilon-caprolactone) as tissue-engineered trachea. Tissue Eng Part C Methods. 2008;14(1):69–77. doi: 10.1089/tec.2007.0336. [DOI] [PubMed] [Google Scholar]
- 77.Lin C.H., Hsu S.H., Huang C.E., Cheng W.T., Su J.M. A scaffold-bioreactor system for a tissue-engineered trachea. Biomaterials. 2009;30(25):4117–4126. doi: 10.1016/j.biomaterials.2009.04.028. [DOI] [PubMed] [Google Scholar]
- 78.Tsao C.K., Ko C.Y., Yang S.R., Yang C.Y., Brey E.M., Huang S. An ectopic approach for engineering a vascularized tracheal substitute. Biomaterials. 2014;35(4):1163–1175. doi: 10.1016/j.biomaterials.2013.10.055. [DOI] [PubMed] [Google Scholar]
- 79.Wismer N., Grad S., Fortunato G., Ferguson S.J., Alini M., Eglin D. Biodegradable electrospun scaffolds for annulus fibrosus tissue engineering: effect of scaffold structure and composition on annulus fibrosus cells in vitro. Tissue Eng Part A. 2014;20(3–4):672–682. doi: 10.1089/ten.TEA.2012.0679. [DOI] [PubMed] [Google Scholar]
- 80.van Uden S., Silva-Correia J., Correlo V.M., Oliveira J.M., Reis R.L. Custom-tailored tissue engineered polycaprolactone scaffolds for total disc replacement. Biofabrication. 2015;7(1) doi: 10.1088/1758-5090/7/1/015008. [DOI] [PubMed] [Google Scholar]
- 81.Makadia H.K., Siegel S.J. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers (Basel) 2011;3(3):1377–1397. doi: 10.3390/polym3031377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sung H.J., Meredith C., Johnson C., Galis Z.S. The effect of scaffold degradation rate on three-dimensional cell growth and angiogenesis. Biomaterials. 2004;25(26):5735–5742. doi: 10.1016/j.biomaterials.2004.01.066. [DOI] [PubMed] [Google Scholar]
- 83.Ezati M., Safavipour H., Houshmand B., Faghihi S. Development of a PCL/gelatin/chitosan/beta-TCP electrospun composite for guided bone regeneration. Prog Biomater. 2018;7(3):225–237. doi: 10.1007/s40204-018-0098-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Blackstone B.N., Hahn J.M., McFarland K.L., DeBruler D.M., Supp D.M., Powell H.M. Inflammatory response and biomechanical properties of coaxial scaffolds for engineered skin in vitro and post-grafting. Acta Biomater. 2018;80:247–257. doi: 10.1016/j.actbio.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 85.Sudheesh Kumar P.T., Hashimi S., Saifzadeh S., Ivanovski S., Vaquette C. Additively manufactured biphasic construct loaded with BMP-2 for vertical bone regeneration: a pilot study in rabbit, materials science & engineering. C Mater Biol Appl. 2018;92:554–564. doi: 10.1016/j.msec.2018.06.071. [DOI] [PubMed] [Google Scholar]
- 86.Rashad Ahmad, Mohamed-Ahmed Samih, Ojansivu Miina, Berstad Kaia, Yassin Mohammed A., Kivijarvi Tove, Bævre Heggset Ellinor, Syverud Kristin, Mustafa Kamal. Coating 3D Printed Polycaprolactone Scaffolds with Nanocellulose Promotes Growth and Differentiation of Mesenchymal Stem Cell. Biomacromolecules. 2018;19(11):4307–4319. doi: 10.1021/acs.biomac.8b01194. [DOI] [PubMed] [Google Scholar]
- 87.Jakus A.E., Rutz A.L., Jordan S.W., Kannan A., Mitchell S.M., Yun C. Hyperelastic "bone": a highly versatile, growth factor-free, osteoregenerative, scalable, and surgically friendly biomaterial. Sci Transl Med. 2016;8(358) doi: 10.1126/scitranslmed.aaf7704. [DOI] [PubMed] [Google Scholar]
- 88.Pae H.C., Kang J.H., Cha J.K., Lee J.S., Paik J.W., Jung U.W. 3D-printed polycaprolactone scaffold mixed with β‐tricalcium phosphate as a bone regenerative material in rabbit calvarial defects. J Biomed Mater Res B Part B. 2019:1254–1263. doi: 10.1002/jbm.b.34218. (107B) [DOI] [PubMed] [Google Scholar]
- 89.Wen J.H., Vincent L.G., Fuhrmann A., Choi Y.S., Hribar K.C., Taylor-Weiner H. Interplay of matrix stiffness and protein tethering in stem cell differentiation. Nat Mater. 2014;13(10):979–987. doi: 10.1038/nmat4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Harland B., Walcott S., Sun S.X. Adhesion dynamics and durotaxis in migrating cells. Phys Biol. 2011;8(1):015011. doi: 10.1088/1478-3975/8/1/015011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pieuchot L., Marteau J., Guignandon A., Dos Santos T., Brigaud I., Chauvy P.-F. Curvotaxis directs cell migration through cell-scale curvature landscapes. Nat Commun. 2018;9(1):3995. doi: 10.1038/s41467-018-06494-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ulrich T.A., de Juan Pardo E.M., Kumar S. The mechanical rigidity of the extracellular matrix regulates the structure, motility, and proliferation of glioma cells. Cancer Res. 2009;69(10):4167–4174. doi: 10.1158/0008-5472.CAN-08-4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liu F., Lagares D., Choi K.M., Stopfer L., Marinkovic A., Vrbanac V. Mechanosignaling through YAP and TAZ drives fibroblast activation and fibrosis. Am J Physiol Lung Cell Mol Physiol. 2015;308(4):L344–L357. doi: 10.1152/ajplung.00300.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ko P., Kim D., You E., Jung J., Oh S., Kim J. Extracellular matrix rigidity-dependent sphingosine-1-phosphate secretion regulates metastatic cancer cell invasion and adhesion. Sci Rep. 2016;6:21564. doi: 10.1038/srep21564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Miroshnikova Y.A., Rozenberg G.I., Cassereau L., Pickup M., Mouw J.K., Ou G. alpha5beta1-Integrin promotes tension-dependent mammary epithelial cell invasion by engaging the fibronectin synergy site. Mol Biol Cell. 2017;28(22):2958–2977. doi: 10.1091/mbc.E17-02-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cipitria A., Salmeron-Sanchez M. Mechanotransduction and growth factor signalling to engineer cellular microenvironments. Adv Healthc Mater. 2017;6(15):1700052. doi: 10.1002/adhm.201700052. [DOI] [PubMed] [Google Scholar]
- 97.Puklin-Faucher E., Sheetz M.P. The mechanical integrin cycle. J Cell Sci. 2009;122(Pt 2):179–186. doi: 10.1242/jcs.042127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Humphries J.D., Byron A., Humphries M.J. Integrin ligands at a glance. J Cell Sci. 2006;119(Pt 19):3901–3903. doi: 10.1242/jcs.03098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hamidi H., Ivaska J. Every step of the way: integrins in cancer progression and metastasis. Nat Rev Cancer. 2018;18(9):533–548. doi: 10.1038/s41568-018-0038-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Goult B.T., Yan J., Schwartz M.A. Talin as a mechanosensitive signaling hub. J Cell Biol. 2018;217(11):3776–3784. doi: 10.1083/jcb.201808061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Atherton P., Stutchbury B., Jethwa D., Ballestrem C. Mechanosensitive components of integrin adhesions: role of vinculin. Exp Cell Res. 2016;343(1):21–27. doi: 10.1016/j.yexcr.2015.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.López-Colomé A.M., Lee-Rivera I., Benavides-Hidalgo R., López E. Paxillin: a crossroad in pathological cell migration. J Hematol Oncol. 2017;10(1):50. doi: 10.1186/s13045-017-0418-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhao X., Guan J.L. Focal adhesion kinase and its signaling pathways in cell migration and angiogenesis. Adv Drug Deliv Rev. 2011;63(8):610–615. doi: 10.1016/j.addr.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pasapera A.M., Schneider I.C., Rericha E., Schlaepfer D.D., Waterman C.M. Myosin II activity regulates vinculin recruitment to focal adhesions through FAK-mediated paxillin phosphorylation. J Cell Biol. 2010;188(6):877. doi: 10.1083/jcb.200906012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Schlaepfer D.D., Hanks S.K., Hunter T., van der Geer P. Integrin-mediated signal transduction linked to Ras pathway by GRB2 binding to focal adhesion kinase. Nature. 1994;372(6508):786–791. doi: 10.1038/372786a0. [DOI] [PubMed] [Google Scholar]
- 106.Ren X.D., Kiosses W.B., Sieg D.J., Otey C.A., Schlaepfer D.D., Schwartz M.A. Focal adhesion kinase suppresses rho activity to promote focal adhesion turnover. J Cell Sci. 2000;113(Pt 20):3673–3678. doi: 10.1242/jcs.113.20.3673. [DOI] [PubMed] [Google Scholar]
- 107.Li W., Duzgun A., Sumpio B.E., Basson M.D. Integrin and FAK-mediated MAPK activation is required for cyclic strain mitogenic effects in Caco-2 cells. Am J Physiol Gastrointest Liver Physiol. 2001;280(1):G75–G87. doi: 10.1152/ajpgi.2001.280.1.G75. [DOI] [PubMed] [Google Scholar]
- 108.Chen B.H., Tzen J.T., Bresnick A.R., Chen H.C. Roles of Rho-associated kinase and myosin light chain kinase in morphological and migratory defects of focal adhesion kinase-null cells. J Biol Chem. 2002;277(37):33857–33863. doi: 10.1074/jbc.M204429200. [DOI] [PubMed] [Google Scholar]
- 109.Song J., Ye B., Liu H., Bi R., Zhang N., Hu J. Fak-Mapk, hippo and Wnt signalling pathway expression and regulation in distraction osteogenesis. Cell Prolif. 2018;51(4) doi: 10.1111/cpr.12453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wong V.W., Garg R.K., Sorkin M., Rustad K.C., Akaishi S., Levi K. Loss of keratinocyte focal adhesion kinase stimulates dermal proteolysis through upregulation of MMP9 in wound healing. Ann Surg. 2014;260(6):1138–1146. doi: 10.1097/SLA.0000000000000219. [DOI] [PubMed] [Google Scholar]
- 111.Genna A., Gil-Henn H. FAK family kinases: the yin and Yang of cancer cell invasiveness. Mol Cell Oncol. 2018;5(4) doi: 10.1080/23723556.2018.1449584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Huveneers S., Danen E.H.J. Adhesion signaling – crosstalk between integrins, Src and rho. J Cell Sci. 2009;122(8):1059. doi: 10.1242/jcs.039446. [DOI] [PubMed] [Google Scholar]
- 113.Ribeiro C., Sencadas V., Correia D.M., Lanceros-Méndez S. Piezoelectric polymers as biomaterials for tissue engineering applications. Colloids Surf B Biointerfaces. 2015;136:46–55. doi: 10.1016/j.colsurfb.2015.08.043. [DOI] [PubMed] [Google Scholar]
- 114.Ribeiro S., Gomes A.C., Etxebarria I., Lanceros-Méndez S., Ribeiro C. Electroactive biomaterial surface engineering effects on muscle cells differentiation. Mater Sci Eng C. 2018;92:868–874. doi: 10.1016/j.msec.2018.07.044. [DOI] [PubMed] [Google Scholar]
- 115.Ribeiro C., Parssinen J., Sencadas V., Correia V., Miettinen S., Hytonen V.P. Dynamic piezoelectric stimulation enhances osteogenic differentiation of human adipose stem cells. J Biomed Mater Res A. 2015;103(6):2172–2175. doi: 10.1002/jbm.a.35368. [DOI] [PubMed] [Google Scholar]
- 116.Parssinen J., Hammaren H., Rahikainen R., Sencadas V., Ribeiro C., Vanhatupa S. Enhancement of adhesion and promotion of osteogenic differentiation of human adipose stem cells by poled electroactive poly(vinylidene fluoride) J Biomed Mater Res A. 2015;103(3):919–928. doi: 10.1002/jbm.a.35234. [DOI] [PubMed] [Google Scholar]
- 117.Damaraju S.M., Shen Y., Elele E., Khusid B., Eshghinejad A., Li J. Three-dimensional piezoelectric fibrous scaffolds selectively promote mesenchymal stem cell differentiation. Biomaterials. 2017;149:51–62. doi: 10.1016/j.biomaterials.2017.09.024. [DOI] [PubMed] [Google Scholar]
- 118.Ribeiro C., Correia V., Martins P., Gama F.M., Lanceros-Mendez S. Proving the suitability of magnetoelectric stimuli for tissue engineering applications. Colloids Surf B Biointerfaces. 2016;140:430–436. doi: 10.1016/j.colsurfb.2015.12.055. [DOI] [PubMed] [Google Scholar]
- 119.Hoffman A.S. Stimuli-responsive polymers: biomedical applications and challenges for clinical translation. Adv Drug Deliv Rev. 2013;65(1):10–16. doi: 10.1016/j.addr.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 120.Ondeck M.G., Kumar A., Placone J.K., Plunkett C.M., Matte B.F., Wong K.C. Dynamically stiffened matrix promotes malignant transformation of mammary epithelial cells via collective mechanical signaling. Proc Natl Acad Sci. 2019;116(9):3502–3507. doi: 10.1073/pnas.1814204116. (201814204) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.O'Donnell N., Okkelman I.A., Timashev P., Gromovykh T.I., Papkovsky D.B., Dmitriev R.I. Cellulose-based scaffolds for fluorescence lifetime imaging-assisted tissue engineering. Acta Biomater. 2018;80:85–96. doi: 10.1016/j.actbio.2018.09.034. [DOI] [PubMed] [Google Scholar]
- 122.Li C., Shklover J., Parvizi M., Sherlock B.E., Alfonso Garcia A., Haudenschild A.K. Label-free assessment of collagenase digestion on bovine pericardium properties by fluorescence lifetime imaging. Ann Biomed Eng. 2018;46(11):1870–1881. doi: 10.1007/s10439-018-2087-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Alfonso-Garcia A., Shklover J., Sherlock B.E., Panitch A., Griffiths L.G., Marcu L. Fiber-based fluorescence lifetime imaging of recellularization processes on vascular tissue constructs. J Biophotonics. 2018;11(9) doi: 10.1002/jbio.201700391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sherlock B.E., Harvestine J.N., Mitra D., Haudenschild A., Hu J., Athanasiou K.A. Nondestructive assessment of collagen hydrogel cross-linking using time-resolved autofluorescence imaging. J Biomed Opt. 2018;23(3):1–9. doi: 10.1117/1.JBO.23.3.036004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Xia T., Zhao R., Feng F., Song Y., Zhang Y., Dong L. Gene expression profiling of human hepatocytes grown on differing substrate stiffness. Biotechnol Lett. 2018;40(5):809–818. doi: 10.1007/s10529-018-2536-1. [DOI] [PubMed] [Google Scholar]
- 126.Li R.A., Keung W., Cashman T.J., Backeris P.C., Johnson B.V., Bardot E.S. Bioengineering an electro-mechanically functional miniature ventricular heart chamber from human pluripotent stem cells. Biomaterials. 2018;163:116–127. doi: 10.1016/j.biomaterials.2018.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kang K.B., Lawrence B.D., Gao X.R., Luo Y., Zhou Q., Liu A. Micro- and nanoscale topographies on silk regulate gene expression of human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2017;58(14):6388–6398. doi: 10.1167/iovs.17-22213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zanotelli M.R., Ardalani H., Zhang J., Hou Z., Nguyen E.H., Swanson S. Stable engineered vascular networks from human induced pluripotent stem cell-derived endothelial cells cultured in synthetic hydrogels. Acta Biomater. 2016;35:32–41. doi: 10.1016/j.actbio.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Levitt J.A., Matthews D.R., Ameer-Beg S.M., Suhling K. Fluorescence lifetime and polarization-resolved imaging in cell biology. Curr Opin Biotechnol. 2009;20(1):28–36. doi: 10.1016/j.copbio.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 130.Sun Y., Day R.N., Periasamy A. Investigating protein-protein interactions in living cells using fluorescence lifetime imaging microscopy. Nat Protoc. 2011;6:1324. doi: 10.1038/nprot.2011.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Stringari C. Metabolic imaging of living tissues by fluorescence lifetime microscopy (FLIM) and endogenous biomarkers. Biophys J. 2015;108(2) (8a) [Google Scholar]
- 132.Meleshina A.V., Dudenkova V.V., Shirmanova M.V., Shcheslavskiy V.I., Becker W., Bystrova A.S. Probing metabolic states of differentiating stem cells using two-photon FLIM. Sci Rep. 2016;6:21853. doi: 10.1038/srep21853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Okkelman I.A., Dmitriev R.I., Foley T., Papkovsky D.B. Use of fluorescence lifetime imaging microscopy (FLIM) as a timer of cell cycle S phase. PLoS One. 2016;11(12) doi: 10.1371/journal.pone.0167385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Parsons M., Messent A.J., Humphries J.D., Deakin N.O., Humphries M.J. Quantitation of integrin receptor agonism by fluorescence lifetime imaging. J Cell Sci. 2008;121(Pt 3):265–271. doi: 10.1242/jcs.018440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Shtengel G., Galbraith J.A., Galbraith C.G., Lippincott-Schwartz J., Gillette J.M., Manley S. Interferometric fluorescent super-resolution microscopy resolves 3D cellular ultrastructure. Proc Natl Acad Sci U S A. 2009;106(9):3125–3130. doi: 10.1073/pnas.0813131106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Legg K. Getting to the core. Nat Rev Mol Cell Biol. 2010;12:4. doi: 10.1038/nrm3030. [DOI] [PubMed] [Google Scholar]
- 137.Moore T.I., Aaron J., Chew T.L., Springer T.A. Measuring integrin conformational change on the cell surface with super-resolution microscopy. Cell Rep. 2018;22(7):1903–1912. doi: 10.1016/j.celrep.2018.01.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lee J.H., Daugharthy E.R., Scheiman J., Kalhor R., Ferrante T.C., Terry R. Fluorescent in situ sequencing (FISSEQ) of RNA for gene expression profiling in intact cells and tissues. Nat Protoc. 2015;10(3):442–458. doi: 10.1038/nprot.2014.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gocheva V., Naba A., Bhutkar A., Guardia T., Miller K.M., Li C.M. Quantitative proteomics identify Tenascin-C as a promoter of lung cancer progression and contributor to a signature prognostic of patient survival. Proc Natl Acad Sci U S A. 2017;114(28):E5625–e5634. doi: 10.1073/pnas.1707054114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Naba A., Pearce O.M.T., Del Rosario A., Ma D., Ding H., Rajeeve V. Characterization of the extracellular matrix of Normal and diseased tissues using proteomics. J Proteome Res. 2017;16(8):3083–3091. doi: 10.1021/acs.jproteome.7b00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Socovich A.M., Naba A. The cancer matrisome: from comprehensive characterization to biomarker discovery. Semin Cell Dev Biol. 2019;89:157–166. doi: 10.1016/j.semcdb.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 142.Brauchle E., Bauer H., Fernes P., Zuk A., Schenke-Layland K., Sengle G. Raman microspectroscopy as a diagnostic tool for the non-invasive analysis of fibrillin-1 deficiency in the skin and in the in vitro skin models. Acta Biomater. 2017;52:41–48. doi: 10.1016/j.actbio.2016.12.019. [DOI] [PubMed] [Google Scholar]
- 143.Brauchle E., Kasper J., Daum R., Schierbaum N., Falch C., Kirschniak A. Biomechanical and biomolecular characterization of extracellular matrix structures in human colon carcinomas. Matrix Biol. 2018;68-69:180–193. doi: 10.1016/j.matbio.2018.03.016. [DOI] [PubMed] [Google Scholar]