Abstract
Background
We evaluated five key proteins involved in various cancer-related pathways and assessed their relation to breast cancer recurrence.
Methods
We used the Kentucky Cancer Registry to retrospectively identify primary invasive breast cancer cases (n=475) that were diagnosed and treated at University of Kentucky Medical Center between 2000 and 2007. Breast cancer recurrence was observed in 62 cases during the five-year followup after diagnosis. Protein expression or activity level was analyzed from surgery tissue using immunohistochemical assays.
Results
Compared to ER+/PR+/HER2- patients without recurrence, those with recurrence had higher TWIST expression (p=0.049) but lower ABL1/ABL2 activity (p=0.003) in primary tumors. We also found that triple-negative breast cancer patients with recurrence had higher SNAI1 expression compared to those without recurrence (p=0.03). After adjusting for potential confounders, the higher ABL1/ABL2 activity in primary tumors was associated with a decreased risk of recurrence (OR=0.72, 95%CI = 0.85-0.90) among ER+/PR+/HER2- patients. In addition, among patients with recurrence we observed that the activity level of ABL1/ABL2 was significantly increased in recurrent tumors compared to the matched primary tumors regardless of the subtype (p=0.013).
Conclusions
These findings provide evidence that the expression/activity level of various proteins may be differentially associated with risk of recurrence of breast tumor subtypes.
Keywords: breast cancer, recurrence, biomarkers, protein expression, protein activity, tumor subtypes
Introduction
Despite advances in breast cancer therapy, over 40,000 women die from the disease each year in the United States [1]. Recurrent breast tumors tend to be highly resistant to chemotherapy and targeted therapies. The average survival after recurrence of breast cancer is less than 12 months [2]. Breast tumors may recur at both local and distant sites [3], and distant metastasis accounts for virtually all breast cancer related deaths [4]. Approximately 20-30% of early stage breast cancer cases eventually develop distance recurrence several months or up to 20 years after the initial diagnosis [5]. One major challenge is to identify biomarkers that can distinguish patients who will develop recurrence from those who will not at diagnosis or before clinical manifestation of recurrence, so that more effective and precise targeted therapies can be selected and unnecessary treatments and toxicities can be avoided. For example, small molecule ABL kinase inhibitors have been used to effectively treat chronic myeloid leukemia [6].
In past decades, molecular prognostic profiles have been developed and used in clinical practice to predict recurrence and guide treatment selection, including the 21-gene Recurrence Score (Oncotype DX), the Amsterdam 70-gene profile (Mammaprint), and the Risk of Recurrence score (PAM50 assay) [7-10]. These molecular profiles are based on mRNA expression levels in primary tumor specimens. However, expression levels of mRNA and protein are not necessarily well correlated [11, 12] due to post-transcriptional regulation between transcript and protein product [13]. Thus, tumor protein expression or activity levels as measured with tissue microarrays (TMAs) may provide additional information and lead to the identification of new markers that can predict cancer recurrence.
Several proteins involved in various cancer biological pathways have been implicated in breast cancer recurrence. PAWR (aka PAR-4) is a pro-apoptotic tumor suppressor protein that is downregulated in breast cancer recurrence at local and distant sites [14-17]. In contrast, SNAI1 (aka SNAIL), TWIST1 (aka TWIST), and CTNNB1 (aka β-catenin, as a readout of Wnt signaling) are transcriptional factors that promote cell survival, epithelial-mesenchymal transition (EMT), and metastasis; and their expression levels are elevated in metastatic and recurrent tumors [18-20]. Because ABL1 (aka c-Abl) and its homolog ABL2 (aka Arg) are oncoproteins that promote cell survival, proliferation and metastasis activities in solid tumors including breast cancer [21, 22], ABL1/ABL2 activation may be associated with recurrence. However, the relationship between these proteins and breast cancer recurrence has not been previously evaluated in an epidemiological cohort study. In this study, we used the Kentucky Cancer Registry (KCR) to retrospectively identify a cohort of women who were diagnosed with primary invasive breast cancer and conducted a molecular epidemiological study to explore the association between the expression/activity level of the five proteins (TWIST1, SNAI1, PAWR, CTNNB1, and ABL1/ABL2) and breast cancer recurrence.
Materials and Methods
Study Population
Study population was identified through the KCR, which is both a National Cancer Institute Surveillance Epidemiology and End Results cancer registry and a participant in the Centers for Disease Control National Program of Cancer Registries. The study population includes women who had their first primary invasive breast cancer diagnosed and treated at the University of Kentucky (UK) Chandler Medical Center between January 1, 2000 and December 31, 2007, and were determined to be disease-free following surgery (no clinical evidence of disease). Only patients who had first course treatment at UK and TNM stage group I through III were included. After applying these criteria, the KCR identified 503 eligible breast cancer patients. Among the identified patients, there were no deaths observed over the five years after the initial breast cancer diagnosis. Tissue blocks with adequate tumor to construct TMAs were available for 479 cases. This cohort was traced forward in time for five years after diagnosis using the KCR to identify the patients who had a recurrence and those who did not. Medical record reviews were conducted on all 479 cases to ensure that no recurrent cases were missed. Both primary breast cancer diagnosis and recurrence were pathologically confirmed. Information concerning demographics and clinical data, including age, race, residence (Appalachian/non-Appalachia regions/Out of State), tumor histology, hormone receptors and HER2 status, size, grade, stage, lymph node involvement, and treatment modality, were obtained using the KCR registry data and the KCR electronic pathology records. Of note, Appalachian regions are located in Eastern Kentucky with much lower socioeconomic status compared to other regions of Kentucky [23]. The study was approved by the UK Institutional Review Board.
Breast Tumor Block Collection
The 503 eligible primary breast cancer cases identified by the KCR were screened for tumor block collection eligibility (confirmed cases with a pathology report and surgery) by the UK Markey Cancer Center (MCC) Cancer Research Informatics Shared Resource Facility using the KCR electronic pathology records. Of these, 497 cases were eligible for tumor collection. Paraffin-embedded tissue blocks prior to any treatment were received for 483 cases and appropriate tissues for TMAs were obtained for 479 cases. After excluding the cores with poor staining quality and uninterpretable results, the final analytic samples included 475 primary breast cancer cases with tissue blocks from the original primary breast cancer surgery, of which 62 subsequently developed a recurrence and 413 did not during the five-year follow-up. Because a number of the patients who had a recurrence had moved or were not treated for their recurrence at UK, the tissue blocks from the surgery or biopsy at the time of recurrence were only available for 22 out of the 62 recurrent cases. Breast cancer cases from which tumor blocks were obtained were generally similar to cases from which tumor blocks were not obtained with respect to patient demographics and tumor characteristics.
TMA Construction and Immunohistochemistry
For each case, original hematoxylin and eosin (H&E) stained slides were reviewed by a pathologist to confirm the histologic diagnosis and to select optimal tumor blocks for inclusion in the study. After block selection, fresh H&E sections were cut and reviewed by the team pathologist, who then designated tumor areas for inclusion in the TMAs. TMAs were then prepared by the UK MCC Biospecimen and Tissue Procurement Shared Resource Facility from formalin-fixed, paraffin embedded (FFPE) tumor blocks by aligning the circled H&E slide with the associated block. Two representative 2mm-diameter tissue cores were taken from one FFPE block for each case, and these were then transferred to the recipient paraffin-embedded block using a TMArrayer (Pathology Devices, Westminster, MD).
Immunohistochemistry was performed on 4 μm thick TMA sections using the following antibodies: 1) rabbit polyclonal anti-phosphorylated Crk/CrkL (Cell Signaling #3181), which is a reliable read-out of ABL1/ABL2 activity [24-27]; 2) mouse monoclonal anti-μ-catenin (BD Biosceince #610153) as a surrogate for the activation of Wnt pathway as its nuclear localization indicates activation of Wnt signaling [28, 29]; 3) rabbit polyclonal anti-snail (Novus NBP2-27293) measured SNAI1 expression; 4) mouse monoclonal anti-twist (Abcam ab 175430) measured TWIST1 expression; 5) rabbit polyclonal anti-par4 (sc-1807, SantaCruz Biotech. Inc.) measured PAWR expression; and 6) IgG control antibodies (species-matched). Details of the immunohistochemistry process are described in supplementary materials. Scoring was performed by a pathologist who was blinded to clinical variables. Each tissue core was assigned an intensity score using the following semi-quantitative scale: negative (0), weak (1), moderate (2), and strong (3). In addition, the percentage of invasive carcinoma cells with positive staining was recorded. A proportion score was assigned based on the estimated proportion of positivestaining cells (0, none; 1, <1/100; 2, 1/100 to 1/10; 3, 1/10 to 1/3; 4, 1/3 to 2/3; and 5, >2/3). Since the cytoplasmic scaffold proteins Crk and CrkL were also observed in the nucleus, subcellular localization was further assessed to measure the ABL1/ABL2 activity levels in cytoplasm and nucleus separately. The combined ABL1/ABL2 activity level took into account both cytoplasm and nucleus levels.
Statistical Analysis
The average score of the two cores for each case was used in the analysis using equal weighting [30]. Protein expression/activity level in each case was estimated using intensity score as well as two other scoring methods that took into consideration of the percentage of cells with positive staining and staining intensity: Allred score sums percentage score (a scale of 0-5) and intensity score (on a scale of 0-3); and conventional total score multiplies percentage and intensity [30, 31].
Statistical analyses were first performed considering all breast cancer cases as one group, and then by tumor subtypes defined by estrogen receptor (ER), progesterone receptor (PR), and HER2 status. Sample size for each tumor subtype is: ER+ (n=354); ER- (n=105); PR+ (n=316); PR- (n=145); HER2+ (n=35); HER2- (n=403); ER+/PR+/HER2- (n=280); ER+/PR-/HER2- (n=32); and ER-/PR-/HER2- (n=75). The Wilcoxon rank-sum test was used to compare the expression/activity level of each protein in primary tumors from breast cancer patients with and without recurrence. Logistic regression was used to examine the association between the expression/activity level of each protein in primary tumors and breast cancer recurrence, where the protein expression/activity level was dichotomized into a high or low category based on the median score. A backward model selection procedure based on the Akaike information criterion was used to select and control for potential confounders including age, race, residence, and tumor stage. Tissue block age was considered in the model but it did not materially change the results, and therefore was not included in the final model. Odds ratios (OR) and 95% confidence intervals (CIs) of recurrence were estimated for high compared to low expression/activity level. Among the subset of breast cancer patients who developed recurrence and had available recurrent tumor tissue, the Wilcoxon signed-rank test was used to compare the expression/activity level of each protein between primary and recurrent tumors. All p-values were based on two-sided tests and were considered statistically significant if p<0.05. Statistical analyses were performed using R package version 3.3.1 (https://cran.r-project.org).
Results
Of the 475 patients included in the final data set, 62 (13%) developed recurrence during the five-year follow-up period after the initial primary breast cancer diagnosis. Patients with recurrence were younger than patients without recurrence (Table 1). Although no significant differences were observed regarding race and residence between patients with and without recurrence, patients with recurrence were more likely to have advanced disease, and to be hormone receptor (ER or PR) negative and triple negative (TNBC) (Table 1).
Table 1.
Demographic and clinical characteristics of the breast cancer patients in the study.
| Variable | All Patients | Patients with Recurrence |
Patients without Recurrence |
p-valueb |
|---|---|---|---|---|
| N (%) | 475 (100%) | 62 (13%) | 413 (87%) | |
| Age, mean±SDa | 51.7±11.8 | 55.7±12.6 | 0.015 | |
| Race, N (%) | 0.087 | |||
| White | 445 (93.7%) | 55 (88.7%) | 390 (94.4%) | |
| Black | 25 (5.3%) | 7 (11.3%) | 18 (4.4%) | |
| Other | 5 (1.0%) | 0 (0%) | 5 (1.2%) | |
| Residence at Diagnosis, N (%) | 0.12 | |||
| Appalachian | 233 (49.1%) | 25 (40.3%) | 208 (50.4%) | |
| Non-Appalachian | 231 (48.6%) | 34 (54.8%) | 197 (47.7%) | |
| Out of State | 11 (2.3%) | 3 (4.8%) | 8 (1.9%) | |
| Tumor Stage, N (%) | <0.001 | |||
| Stage I | 176 (37.1%) | 8 (12.9%) | 168 (40.7%) | |
| Stage II | 229 (48.2%) | 25 (40.3%) | 204 (49.4%) | |
| Stage III | 70 (14.7%) | 29 (46.8%) | 41 (9.9%) | |
| HER2 Status, N (%) | 0.34 | |||
| HER2+ | 35 (7.4%) | 7 (11.3%) | 28 (6.8%) | |
| HER2− | 403 (84.8%) | 50 (80.6%) | 353 (85.5%) | |
| Equivocal | 20 (4.2%) | 3 (4.8%) | 17 (4.1%) | |
| Unknown | 17 (3.6%) | 2 (3.2%) | 15 (3.6%) | |
| ER Status, N (%) | 0.003 | |||
| ER+ | 354 (74.5%) | 35 (56.5%) | 319 (77.2%) | |
| ER− | 105 (22.1%) | 24 (38.7%) | 81 (19.6%) | |
| Borderline | 2 (0.4%) | 0 (0%) | 2 (0.5%) | |
| Unknown | 14 (3.0%) | 3 (4.8%) | 11 (2.7%) | |
| PR status, N (%) | <0.001 | |||
| PR+ | 316 (66.5%) | 26 (41.9%) | 290 (70.2%) | |
| PR− | 145 (30.5%) | 33 (53.2%) | 112 (27.1%) | |
| Unknown | 14 (3.0%) | 3 (4.8%) | 11 (2.7%) | |
| ER/PR/HER2 Status, N (%) | 0.007 | |||
| HER2+ | 35 (7.4%) | 7 (11.3%) | 28 (6.8%) | |
| ER+/PR+/HER2− | 280 (58.9%) | 25 (40.3%) | 255 (61.7%) | |
| ER+/PR−/HER2− | 32 (6.7%) | 6 (9.7%) | 26 (6.3%) | |
| ER−/PR−/HER2− | 75 (15.8%) | 16 (25.8%) | 59 (14.3%) | |
| Other and Unknown | 53 (11.2%) | 8 (12.9%) | 45 (10.9%) |
SD, standard deviation;
p-value was from student’s t-test for continuous variables and chi-square test for categorical variables, comparing patients with and without recurrence.
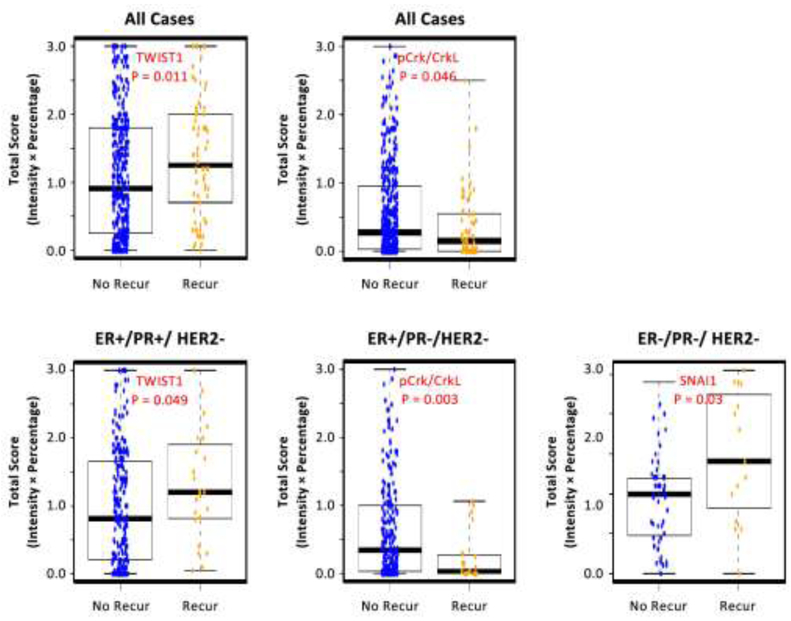
The expression level of each protein was compared in primary tumors from patients with and without recurrence considering all breast cancer cases in one group as well as by tumor subtypes defined by ER, PR and HER2 status (Supplemental Figure 1). Results are generally consistent across the three scoring methods (Intensity Score, Allred, and Conventional Total Score) (Supplemental Table 1). We chose to report the total score for its straightforward biological interpretation. When considering all breast cancer cases in one group, we found that TWIST1 and pCrk/CrkL (a readout of ABL1/ABL2 activation) were differentially expressed in primary tumors from patients with and without recurrence (Figure 1, upper panel). Compared to patients without recurrence, patients with recurrence had higher expression level of TWIST1 but lower expression level of pCrk/CrkL (reflecting lower ABL1/ABL2 activity) (p-values, 0.011 and 0.046, respectively). This is likely driven by ER+/PR+/HER2- patients, as a similar pattern was observed among this largest tumor subtype (Figure 1, lower panel). Compared to the ER+/PR+/HER2- subtype, the expression pattern of TWIST1 appeared to be similar in other subtypes except for ER+/PR-/HER2- subtype, and the expression pattern of pCrk/CrkL appeared to be different in ER+/PR-/HER2- and ER-/PR-/HER2- subtypes. However, none of these other subtypes had significant p-values likely due to the smaller sample size (Supplemental Figure 1a and 1e). Moreover, we found that TNBC patients with recurrence had higher SNAI1 expression in primary tumors compared to those without (p=0.03) (Figure 1, lower panel). Interestingly, the expression pattern of SNAI1 appeared to be different in other subtypes, with a borderline significance in ER+/PR-/HER2- subtype (p-value, 0.053; Supplemental Figure 1b). We also found that tumor grade was associated with protein expression levels in our data and it represented a potential confounder in the association between the protein expression level and breast cancer recurrence. We thus examined the association between the expression level of each protein and breast cancer recurrence, adjusting potential confounders including age, race, residence, tumor grade and stage in logistic regression models (Supplemental Table 2). We found that high expression of pCrk/CrkL, reflecting high activity level of ABL1/ABL2, was significantly associated with a decreased risk of recurrence (OR=0.72, 95% CI= 0.58-0.90) among ER+/PR+/HER2- patients (Table 2).
Figure 1.
Box-and-whisker plots of three proteins showing different expression levels in primary tumors from breast cancer patients with and without recurrence in all breast cancer cases (upper panel) and in different tumor subtypes (lower panel). No Recur, no recurrence; Recur, recurrence. Blue dots: cases without recurrence; Yellow dots: cases with recurrence. The expression level of each proteins was estimated using the total score (intensity x percentage) method. The pCrk/CrkL expression (a readout of ABL1/ABL2 activity level) is the combined levels from cytoplasm and nucleus. Results using the other two score methods (Intensity and Allred) are similar and not shown. P-value was obtained from Wilcoxon signed rank test.
Table 2.
Association between protein expression level and breast cancer recurrence.
| ALL | HER2+ | ER+/PR+/HER2− | ER+/PR−/HER2− | ER−/PR−/HER2− | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR (95%CI) | p- value |
OR (95%CI) | p- value |
OR (95%CI) | p- value |
OR (95%CI) | p- value |
OR (95%CI) | p- value |
|
| TWIST1 | 1.16 (1.00, 1.35) | 0.05 | 0.84(0.50, 1.41) | 0.51 | 1.23 (0.97, 1.57) | 0.093 | 0.87 (0.58, 1.32) | 0.52 | 1.41 (0.94, 2.13) | 0.10 |
| SNAI1 | 0.96 (0.78, 1.17) | 0.67 | 1.14(0.56, 2.33) | 0.72 | 0.75 (0.53, 1.06) | 0.11 | 0.81 (0.51, 1.29) | 0.38 | 1.43 (0.95, 2.15) | 0.08 |
| PAWR | 1.09 (0.95, 1.25) | 0.24 | 0.91 (0.61, 1.35) | 0.63 | 1.00 (0.82, 1.22) | 1.00 | 2.03 (0.94, 4.41) | 0.07 | 1.40 (0.99, 2.00) | 0.06 |
| β-catenin | 0.97 (0.85, 1.10) | 0.63 | 2.79 (0.61, 12.7) | 0.19 | 0.90 (0.75, 1.07) | 0.22 | 1.39 (0.66, 2.90) | 0.38 | 0.80 (0.51, 1.26) | 0.34 |
| pCrk/CrkL_Cytoplasm | 0.92 (0.82, 1.03) | 0.15 | 0.93 (0.63, 1.38) | 0.73 | 0.69 (0.55, 0.87) | 0.002 | 0.97 (0.70, 1.35) | 0.86 | 1.21 (0.98, 1.48) | 0.08 |
| pCrk/CrkL_Nucleus | 0.98(0.87, 1.10) | 0.69 | 0.78(0.53, 1.14) | 0.20 | 0.82 (0.69, 0.98) | 0.03 | 1.57 (0.84, 2.91) | 0.16 | 1.14(0.88, 1.48) | 0.32 |
| pCrk/CrkL_Combined | 0.94(0.83, 1.06) | 0.31 | 0.79 (0.50, 1.24) | 0.31 | 0.72 (0.58, 0.90) | 0.003 | 1.05 (0.71, 1.54) | 0.81 | 1.23 (0.96, 1.58) | 0.11 |
Odds ratio of recurrence for higher expression level compared to lower level; b p-value was obtained from logistic regression model controlling for age, race, residence, tumor grade and stage. The expression level of each protein was estimated using the total score (intensity x percentage) method. The expression level of each protein was dichotomized based on median value. Results using the other two score methods (Intensity and Allred) are similar and not shown. The pCrk/CrkL expression is a readout of ABL1/ABL2 activity level, and the β-catenin expression is a surrogate for the Wnt signaling activation.
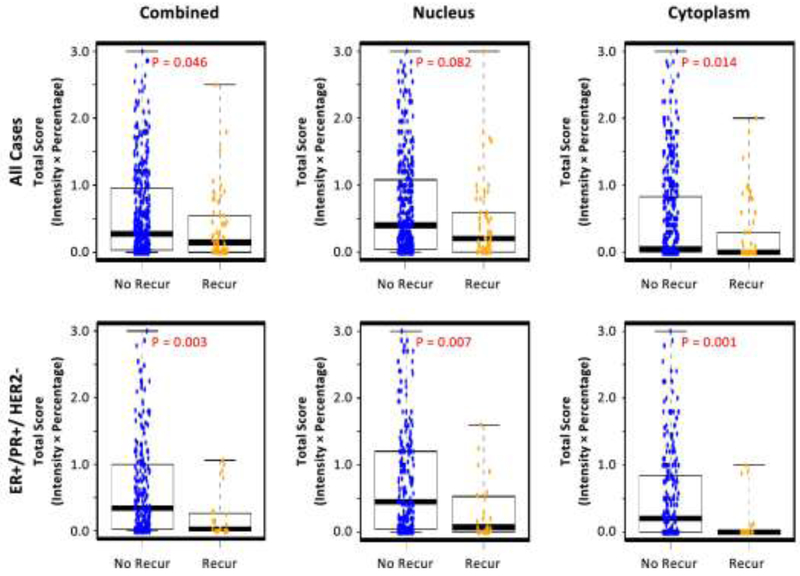
Interestingly, pCrk/CrkL was observed in the nucleus and cytoplasm in patient samples. Although Crk and CrkL are thought to be cytoplasmic proteins there are reports indicating the proteins can reside in the nucleus [32, 33]. Thus, we further investigated the subcellular localization of the pCrk/CrkL proteins in the primary tumor from the initial surgeries (Figure 2). Both nuclear and cytoplasmic pCrk/CrkL proteins were significantly lower in the primary tumors from patients that eventually recurred as compared to those that did not recur, and the pattern was similar in all cases and in ER+/PR+/HER2- subtype (Figure 2). Although, in general, there was less cytoplasmic pCrk/CrkL than nuclear pCrk/CrkL expression, the extremely low levels of pCrk/CrkL proteins in cytoplasm (close to the detection limit) in the primary tumors from the majority of patients with recurrence was particularly interesting and notable. Future research is needed to better understand the role of this protein in breast cancer recurrence.
Figure 2.
Box-and-whisker plots showing subcellular localization of pCrk/CrkL expression levels in primary tumors from breast cancer patients with and without recurrence in all breast cancer cases (upper panel) and in ER+/PR+/HER2- subtype (lower panels). Blue dots: cases without recurrence; Yellow dots: cases with recurrence. Combined (Left), Nucleus (Middle), and Cytoplasm (Right). The pCrk/CrkL expression level (a readout of ABL1/ABL2 activity level) was estimated using the total score (intensity x percentage) method. Results using the other two score methods (Intensity and Allred) are similar and not shown. P-value was obtained from Wilcoxon signed rank test.
Out of the 62 patients who developed recurrence, 22 had recurrent tumor tissue available. Information on the sites of primary and recurrent tumors for the 22 patients is present in Supplementary Table 2. We compared the expression level of each protein between matched primary and recurrent tumors for each patient (Supplemental Figure 2). We found pCrk/CrkL expression level (corresponding to ABL1/ABL2 activation) was significantly increased in the recurrent tumors compared to the matched primary tumors (p=0.004) regardless of the tumor subtype, and the increase in cytoplasm appeared to be more pronounced compared to that in nucleus (Figure 3).
Figure 3.
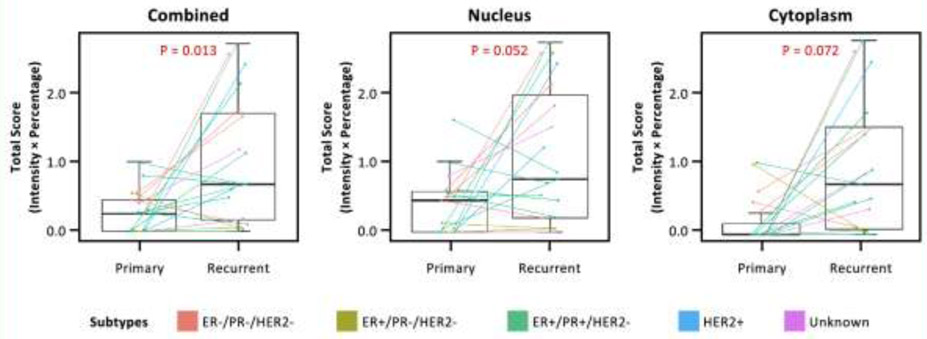
Box-and-whisker plots showing pCrk/CrkL expression levels between primary and recurrent tumors from the 22 patients who developed recurrence. Combined (Left), Nucleus (Middle), and Cytoplasm (Right). Colors show different tumor subtypes of the primary tumor. The pCrk/CrkL expression level (a readout of ABL1/ABL2 activity level) was estimated using total score (intensity x percentage) method. Results using the other two score methods (Intensity and Allred) are similar and are not shown. P-value was obtained from Wilcoxon signed rank test.
Discussion
Breast cancer recurrence is the major cause of breast cancer-related deaths [4] and is largely attributed to metastases and resistance to therapies [34]. A differential pattern of recurrence has been observed among different breast cancer subtypes defined by ER, PR and HER2 status, showing higher risk of recurrence in ER-negative compared to ER-positive breast cancer [35-37]. In particular, TNBC, characterized by ER-/PR-/HER2-, is associated with high risk of recurrence (especially of distant recurrence) compared to hormone receptor (ER/PR) positive cases [38]. Consistent with this line of evidence, in our study we observed that breast cancer patients with recurrence during the initial 5 years after diagnosis were more likely to be ER- negative and triple-negative cases compared to patients without recurrence. This result is also consistent with the observation of late recurrence in ER-positive breast cancer cases [39].
The mechanisms underlying breast cancer recurrence are not completely understood. However, multiple cancer-related biological pathways have been implicated in breast cancer recurrence, including those involved in proliferation, apoptosis, metastasis, cell invasion and cell movement [34, 40]. In this study, we investigated the five candidate proteins in these pathways, including TWIST1, SNAI1, PAR4, CTNNB1 (β-catenin, as a readout of Wnt signaling), and pCrk/CrkL (a readout of ABL1/ABL2 activation), and assessed their protein expression levels in association with breast cancer recurrence. Among all cases and ER+/PR+/HER2- cases, we found that patients with recurrence had a higher expression level of TWIST1 but lower expression of pCrk/CrkL (a readout for low ABL1/ABL2 activation) in their primary tumors compared to patients without recurrence. Intriguingly, we observed that the expression level of SNAI1 was significantly higher in primary tumors from TNBC patients with recurrence than those without. Some of these observed differences in protein expression levels were partially explained by tumor grade. However, higher level of pCrk/CrkL expression (corresponding to higher ABL1/ABL2 activation) was associated with a 28% reduced risk of recurrence in ER+/PR+/HER2- cases even after controlling for potential confounders including tumor grade. We did not observe any statistically significant difference in protein expression levels for PAWR and CTNNB1 (β-catenin) in primary tumors from patients with and without recurrence.
EMT, a complex process in which epithelial cells acquire mesenchymal cell-like properties and enhanced migration capacity, is an important event in breast cancer progression that promotes metastasis, drug resistance and tumor recurrence [41-43]. Both TWIST1 and SNAI1 play a fundamental role in EMT as master regulators and have been linked to breast cancer recurrence [18, 19]. Aberrantly high expression levels of TWIST1 and SNAI1 have been previously observed in primary and metastatic breast cancer [44]. Consistent with these observations, our study found higher protein expression levels of TWIST1 and SNAI1 in the primary tumors from the initial diagnosis in patients who developed recurrence compared to those who did not. In addition, this pattern appeared to be breast cancer subtype-specific, as a significant difference of TWIST1 and SNAI1 expression was observed in ER+/PR+/HER2- and TNBC cases, respectively. These results are in line with previous research indicating that TWIST1 expression in ER-positive cells was associated with drug resistance and shorter survival [45-47] and that high SNAI1 levels were correlated with low differentiation, invasiveness, and ER-negative status [19, 48]. Our study suggests that TWIST1 and SNAI1 may be involved in breast cancer recurrence in a subtype-specific fashion.
Increasing evidence supports a role of the ABL family of non-receptor tyrosine kinases, ABL1 and ABL2, in progression of breast cancer [21, 22]. ABL1 shuttles between the nucleus and the cytoplasm while ABL2 primarily localizes to the cytoplasm [49]. ABL1 and ABL2 phosphorylate a large body of nuclear and cytoplasmic proteins and are involved in diverse biological processes including proliferation, differentiation, cell death and survival, morphogenesis, EMT, migration, and invasion [50, 51]. Although ABL kinases were first identified as oncogenes, they can function as suppressors or promoters in tumorigenesis in a tissue- and context-specific manner [21, 50]. Activation as well as elevated expression of ABL proteins have been observed in advanced high-grade breast tumors [22, 52]. In ER+/PR+/HER2- subtype we found lower activity level of ABL1/ABL2 in the primary tumors from patients who eventually developed recurrence compared to those who did not, suggesting a protective effect of early ABL1/ABL2 activation on recurrence in this subtype. In contrast, we observed an opposite trend in TNBC where patients with recurrence had higher ABL1/ABL2 activity level than patients without (Supplementary Figure 1E). Although the difference was not statistically significant in TNBC patients (P=0.11), likely due to the smaller numbers of patients in this subtype, these results are consistent with other data in the literature, suggesting that ABL1/ABL2 may play opposing roles in ER+/PR+ versus TNBC breast cancer subtypes [53, 54]. Interestingly, we found that the activity level of ABL1/ABL2 was significantly increased in recurrent tumors compared to the matched primary tumors regardless of the subtype, indicating that ABL1/ABL2 likely plays a critical role during breast cancer metastasis/recurrence. These results are consistent with previous studies demonstrating that ABL kinases promote invasion and metastasis as well as drug resistance in breast cancer cells [25, 53-55]. We speculate that ABL1/ABL2 may interact with treatment and the hyper-activation or overexpression of ABL1/ABL2 after initial diagnosis is critical for cancer progression, and such dynamic changes may be associated with drug resistance and metastasis that contribute to cancer recurrence. In this regard, ABL1/ABL2 may serve as potential therapeutic targets, in addition to their potential as markers for breast cancer progression and recurrence. Nonetheless, further research is needed to better understand the differential role of ABL1/ABL2 in various subtypes as well as in different stages of cancer progression.
It has been suggested that the Wnt/ β-catenin signaling pathway plays a role in breast cancer recurrence [20]. Wnt signaling is upregulated at tumor recurrence, especially in TNBC [56-58]. A recent study suggested that co-activation of hedgehog and Wnt signaling pathway was associated with risk of tumor recurrence for TNBC patients [59]. However, in our study, we did not find statistically significant difference in Wnt signaling activation in primary tumors from patients with and without recurrence nor between recurrent and primary tumors. A Wnt target gene and stem cell marker, LGR5, has been found to be associated with recurrence and poor outcomes, suggesting that mechanisms in addition to the β-catenin levels and localization could contribute to Wnt signaling activation in breast cancer stem cells [60]. Similarly, we did not observe statistically significant differences in PAWR protein expression. PAWR is a pro-apoptotic, tumor suppressor protein; downregulation of PAWR is necessary and sufficient for breast cancer recurrence [14-17]. In our study, we observed a non-significant decrease in PAWR expression in primary tumors from ER+/PR+/HER2- patients with recurrence compared to those without recurrence (P=0.19).
Our study has several strengths. First, this is a relatively large molecular epidemiological study that included 475 primary tumors at the initial diagnosis. We used the KCR to retrospectively identify a cohort of patients and retrieve relevant tissue samples to study the association between expression/activity levels of five candidate proteins and breast cancer recurrence. Our study has 80% power to detect a 0.4 standard deviation difference in protein expression level in primary tumors between recurrent and non-recurrent patients based on a two-sided significance level of 5%. Second, we used three scoring methods to measure protein expression level and the results were generally consistent across the three methods. Total score (intensity x percentage) was reported for its straightforward biological interpretation, although the Allred score showed better separation of patients compared to the total score method [61]. Third, the availability of matched primary and recurrent tumor tissue from patients with recurrence allowed us to examine the dynamic change in the protein expression during cancer progression. To our knowledge, this is the first epidemiological study showing a dramatic increase of ABL1/ABL2 activity level in recurrent tumors regardless of the subtype, providing rationale for targeting ABL1/ABL2 in cancer treatment. Importantly, many FDA-approved ABL inhibitors are available and have been used for decades to treat leukemia with relatively few side-effects [55]. Finally, our study assessed the subcellular localization of pCrk/CrkL protein in light of the diverse functions of ABL1/ABL2 in nucleus and cytoplasm. The extremely low level of pCrk/CrkL in cytoplasm in primary tumor and subsequent dramatic increase in recurrent tumor is worth noting. Further research into the potential mechanisms on how the subcellular localization and activation of ABL1/ABL2 impacts tumor recurrence is warranted.
Limitations of our study include a modest sample size for subtype analyses, especially for rare subtypes in our study including HER2+ and ER+/PR+/HER2-. This could be one possible explanation of why we did not observe any significant result for these two rare subtypes. These subtype analyses are considered exploratory. Larger studies are needed to further examine and validate these associations. Despite the small sample size in comparison between primary and recurrent tumor, we were able to observe a significant increase in ABL1/ABL2 activity level. This is likely due to the dramatic activation of these proteins during cancer progression. This result also needs to be further validated in future larger studies. Another potential limitation of the study is to examine the protein expression levels using two representative cores of TMA instead of whole section, which may not fully take into account the possible heterogeneous expression of these proteins in breast tumor cells [62-66]. Our study examined breast cancer recurrence in the initial five years after the diagnosis, which represents a relatively short time window to evaluate the recurrence of ER-positive breast cancer cases, as ER-positive cases tend to have later recurrence. In addition, our study lacks detailed information on treatment, such as specific chemotherapy agents used as well as radiation therapy doses and fractions, which is important for understanding molecular changes during tumor progression, as there is evidence that specific types of treatment can trigger activation of certain signaling molecules that are believed to be associated with the recurrence of breast cancer [67]. Furthermore, the interactions between these five candidate proteins are biologically plausible [68, 69] in breast cancer recurrence. However, the limited sample size does not allow us to assess statistical interactions between these proteins. Larger studies are needed to examine the interaction of these proteins in association with breast cancer recurrence.
In conclusion, our results underscore the relevance of different biological pathways to breast cancer recurrence in different tumor subtypes. We identified potential subtype-specific markers for breast cancer recurrence that are differentially expressed or activated in primary tumors from patients with and without recurrence. In addition, one of these markers (ABL1/ABL2) had dynamic changes between primary and recurrent tumors regardless of the subtype and can potentially be used to actively monitor cancer progression or be targeted therapeutically in cancer treatment. Further research is needed to understand the underlying mechanisms, consider interaction between these proteins, and build a prediction model of recurrence by incorporating these protein markers.
Supplementary Material
Acknowledgements
Special thanks to Dana Napier for her expertise in TMA construction and talent with immunohistochemistry. Drs. He, Plattner, Rangnekar, Zhou, and Tucker are supported by the University of Kentucky Markey Cancer Center (P30CA177558). Dr. Stewart is supported by NIH fellowship grant T32 CA160003. The Markey Cancer Center’s Research Communications Office assisted with preparation of this manuscript.
Funding: This research project was supported by Markey Cancer Center pilot funding IRB# 14-0172-P3H, the Biospecimen Procurement and Translational Pathology, the Biostatistics and Bioinformatics, and the Cancer Research Informatics Shared Resource Facilities of the University of Kentucky Markey Cancer Center (P30CA177558).
Footnotes
Disclosure: The authors declare no conflicts of interest.
References:
- 1.Siegel R, Naishadham D, Jemal A (2013) Cancer statistics, 2013. CA Cancer J Clin 63(1):11–30 [DOI] [PubMed] [Google Scholar]
- 2.Guarneri V, Dieci MV, Conte P (2013) Relapsed triple-negative breast cancer: challenges and treatment strategies. Drugs 73(12):1257–1265 [DOI] [PubMed] [Google Scholar]
- 3.Hockel M, Dornhofer N (2005) The hydra phenomenon of cancer: why tumors recur locally after microscopically complete resection. Cancer Res 65(8):2997–3002 [DOI] [PubMed] [Google Scholar]
- 4.Redig AJ, McAllister SS (2013) Breast cancer as a systemic disease: a view of metastasis. J Intern Med 274(2):113–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tevaarwerk AJ, Gray RJ, Schneider BP, Smith ML, Wagner LI, Fetting JH, Davidson N, Goldstein LJ, Miller KD, Sparano JA (2013) Survival in patients with metastatic recurrent breast cancer after adjuvant chemotherapy: little evidence of improvement over the past 30 years. Cancer 119(6):1140–1148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Druker BJ, Talpaz M, Resta DJ, Peng B, Buchdunger E, Ford JM, Lydon NB, Kantarjian H, Capdeville R, Ohno-Jones S et al. (2001) Efficacy and safety of a specific inhibitor of the BCRABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med 344(14):1031–1037 [DOI] [PubMed] [Google Scholar]
- 7.Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, Baehner FL, Walker MG, Watson D, Park T et al. (2004) A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med 351(27):2817–2826 [DOI] [PubMed] [Google Scholar]
- 8.van de Vijver MJ, He YD, van't Veer LJ, Dai H, Hart AA, Voskuil DW, Schreiber GJ, Peterse JL, Roberts C, Marton MJ et al. (2002) A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med 347(25):1999–2009 [DOI] [PubMed] [Google Scholar]
- 9.Buyse M, Loi S, van't Veer L, Viale G, Delorenzi M, Glas AM, d'Assignies MS, Bergh J, Lidereau R, Ellis P et al. (2006) Validation and clinical utility of a 70-gene prognostic signature for women with node-negative breast cancer. J Natl Cancer Inst 98(17):1183–1192 [DOI] [PubMed] [Google Scholar]
- 10.Parker JS, Mullins M, Cheang MC, Leung S, Voduc D, Vickery T, Davies S, Fauron C, He X, Hu Z et al. (2009) Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol 27(8):1160–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Sousa Abreu R, Penalva LO, Marcotte EM, Vogel C (2009) Global signatures of protein and mRNA expression levels. Mol Biosyst 5(12):1512–1526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vogel C, Marcotte EM (2012) Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat Rev Genet 13(4):227–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maier T, Guell M, Serrano L (2009) Correlation of mRNA and protein in complex biological samples. FEBS Lett 583(24):3966–3973 [DOI] [PubMed] [Google Scholar]
- 14.Alvarez JV, Pan TC, Ruth J, Feng Y, Zhou A, Pant D, Grimley JS, Wandless TJ, Demichele A, Investigators IST et al. (2013) Par-4 downregulation promotes breast cancer recurrence by preventing multinucleation following targeted therapy. Cancer Cell 24(1):30–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hebbar N, Wang C, Rangnekar VM (2012) Mechanisms of apoptosis by the tumor suppressor Par-4. J Cell Physiol 227(12):3715–3721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mendez-Lopez LF, Zapata-Benavides P, Zavala-Pompa A, Aguado-Barrera ME, Pacheco-Calleros J, Rodriguez-Padilla C, Cerda-Flores RM, Cortes-Gutierrez EI, Davila-Rodriguez MI (2010) Immunohistochemical analysis of prostate apoptosis response-4 (Par-4) in Mexican women with breast cancer: a preliminary study. Arch Med Res 41(4):261–268 [DOI] [PubMed] [Google Scholar]
- 17.Nagai MA, Gerhard R, Salaorni S, Fregnani JH, Nonogaki S, Netto MM, Soares FA (2010) Down-regulation of the candidate tumor suppressor gene PAR-4 is associated with poor prognosis in breast cancer. Int J Oncol 37(1):41–49 [DOI] [PubMed] [Google Scholar]
- 18.Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A, Weinberg RA (2004) Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell 117(7):927–939 [DOI] [PubMed] [Google Scholar]
- 19.Moody SE, Perez D, Pan TC, Sarkisian CJ, Portocarrero CP, Sterner CJ, Notorfrancesco KL, Cardiff RD, Chodosh LA (2005) The transcriptional repressor Snail promotes mammary tumor recurrence. Cancer Cell 8(3):197–209 [DOI] [PubMed] [Google Scholar]
- 20.Karamboulas C, Ailles L (2013) Developmental signaling pathways in cancer stem cells of solid tumors. Biochim Biophys Acta 1830(2):2481–2495 [DOI] [PubMed] [Google Scholar]
- 21.Wang J, Pendergast AM (2015) The Emerging Role of ABL Kinases in Solid Tumors. Trends Cancer 1(2):110–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Srinivasan D, Sims JT, Plattner R (2008) Aggressive breast cancer cells are dependent on activated Abl kinases for proliferation, anchorage-independent growth and survival. Oncogene 27(8):1095–1105 [DOI] [PubMed] [Google Scholar]
- 23.[https://www.arc.gov/appalachian_region/TheAppalachianRegion.asp]
- 24.Ganguly SS, Fiore LS, Sims JT, Friend JW, Srinivasan D, Thacker MA, Cibull ML, Wang C, Novak M, Kaetzel DM et al. (2012) c-Abl and Arg are activated in human primary melanomas, promote melanoma cell invasion via distinct pathways, and drive metastatic progression. Oncogene 31(14):1804–1816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ganguly SS, Plattner R (2012) Activation of Abl family kinases in solid tumors. Genes Cancer 3(5-6):414–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith-Pearson PS, Greuber EK, Yogalingam G, Pendergast AM (2010) Abl kinases are required for invadopodia formation and chemokine-induced invasion. J Biol Chem 285(51):40201–40211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fiore LS, Ganguly SS, Sledziona J, Cibull ML, Wang C, Richards DL, Neltner JM, Beach C, McCorkle JR, Kaetzel DM et al. (2014) c-Abl and Arg induce cathepsin-mediated lysosomal degradation of the NM23-H1 metastasis suppressor in invasive cancer. Oncogene 33(36):4508–4520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu T, Chen X, Zhang W, Colon D, Shi J, Napier D, Rychahou P, Lu W, Lee EY, Weiss HL et al. (2012) Regulation of the Potential Marker for Intestinal Cells, Bmi1, by beta-Catenin and the Zinc Finger Protein KLF4: Implications for Colon Cancer. J Biol Chem 287:3760–3768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu C, Li Y, Semenov M, Han C, Baeg GH, Tan Y, Zhang Z, Lin X, He X (2002) Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell 108(6):837–847 [DOI] [PubMed] [Google Scholar]
- 30.Harvey JM, Clark GM, Osborne CK, Allred DC (1999) Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol 17(5):1474–1481 [DOI] [PubMed] [Google Scholar]
- 31.Collins LC, Botero ML, Schnitt SJ (2005) Bimodal frequency distribution of estrogen receptor immunohistochemical staining results in breast cancer: an analysis of 825 cases. Am J Clin Pathol 123(1):16–20 [DOI] [PubMed] [Google Scholar]
- 32.Rhodes J, York RD, Tara D, Tajinda K, Druker BJ (2000) CrkL functions as a nuclear adaptor and transcriptional activator in Bcr-Abl-expressing cells. Exp Hematol 28(3):305–310 [DOI] [PubMed] [Google Scholar]
- 33.Kar B, Reichman CT, Singh S, O'Connor JP, Birge RB (2007) Proapoptotic function of the nuclear Crk II adaptor protein. Biochemistry 46(38):10828–10840 [DOI] [PubMed] [Google Scholar]
- 34.Ahmad A (2013) Pathways to breast cancer recurrence. ISRN Oncol 2013:290568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goss PE, Chambers AF (2010) Does tumour dormancy offer a therapeutic target? Nat Rev Cancer 10(12):871–877 [DOI] [PubMed] [Google Scholar]
- 36.Early Breast Cancer Trialists' Collaborative G (2005) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 365(9472):1687–1717 [DOI] [PubMed] [Google Scholar]
- 37.Saphner T, Tormey DC, Gray R (1996) Annual hazard rates of recurrence for breast cancer after primary therapy. J Clin Oncol 14(10):2738–2746 [DOI] [PubMed] [Google Scholar]
- 38.Chacon RD, Costanzo MV (2010) Triple-negative breast cancer. Breast Cancer Res 12 Suppl 2:S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wangchinda P, Ithimakin S (2016) Factors that predict recurrence later than 5 years after initial treatment in operable breast cancer. World J Surg Oncol 14(1):223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hanahan D, Weinberg RA (2000) The hallmarks of cancer. Cell 100(1):57–70 [DOI] [PubMed] [Google Scholar]
- 41.Thiery JP (2002) Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer 2(6):442–454 [DOI] [PubMed] [Google Scholar]
- 42.Blick T, Widodo E, Hugo H, Waltham M, Lenburg ME, Neve RM, Thompson EW (2008) Epithelial mesenchymal transition traits in human breast cancer cell lines. Clin Exp Metastasis 25(6):629–642 [DOI] [PubMed] [Google Scholar]
- 43.Trimboli AJ, Fukino K, de Bruin A, Wei G, Shen L, Tanner SM, Creasap N, Rosol TJ, Robinson ML, Eng C et al. (2008) Direct evidence for epithelial-mesenchymal transitions in breast cancer. Cancer Res 68(3):937–945 [DOI] [PubMed] [Google Scholar]
- 44.Martin TA, Goyal A, Watkins G, Jiang WG (2005) Expression of the transcription factors snail, slug, and twist and their clinical significance in human breast cancer. Ann Surg Oncol 12(6):488–496 [DOI] [PubMed] [Google Scholar]
- 45.Vesuna F, Lisok A, Kimble B, Domek J, Kato Y, van der Groep P, Artemov D, Kowalski J, Carraway H, van Diest P et al. (2012) Twist contributes to hormone resistance in breast cancer by downregulating estrogen receptor-alpha. Oncogene 31(27):3223–3234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Riaz M, Sieuwerts AM, Look MP, Timmermans MA, Smid M, Foekens JA, Martens JW (2012) High TWIST1 mRNA expression is associated with poor prognosis in lymph node-negative and estrogen receptor-positive human breast cancer and is co-expressed with stromal as well as ECM related genes. Breast Cancer Res 14(5):R123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van Nes JG, de Kruijf EM, Putter H, Faratian D, Munro A, Campbell F, Smit VT, Liefers GJ, Kuppen PJ, van de Velde CJ et al. (2012) Co-expression of SNAIL and TWIST determines prognosis in estrogen receptor-positive early breast cancer patients. Breast Cancer Res Treat 133(1):49–59 [DOI] [PubMed] [Google Scholar]
- 48.Blanco MJ, Moreno-Bueno G, Sarrio D, Locascio A, Cano A, Palacios J, Nieto MA (2002) Correlation of Snail expression with histological grade and lymph node status in breast carcinomas. Oncogene 21(20):3241–3246 [DOI] [PubMed] [Google Scholar]
- 49.Bradley WD, Koleske AJ (2009) Regulation of cell migration and morphogenesis by Abl-family kinases: emerging mechanisms and physiological contexts. J Cell Sci 122(Pt 19):3441–3454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang JY (2014) The capable ABL: what is its biological function? Mol Cell Biol 34(7):1188–1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Colicelli J (2010) ABL tyrosine kinases: evolution of function, regulation, and specificity. Sci Signal 3(139):re6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cancer Genome Atlas N (2012) Comprehensive molecular portraits of human breast tumours. Nature 490(7418):61–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhao H, Ou-Yang F, Chen IF, Hou MF, Yuan SS, Chang HL, Lee YC, Plattner R, Waltz SE, Ho SM et al. (2010) Enhanced resistance to tamoxifen by the c-ABL proto-oncogene in breast cancer. Neoplasia 12(3):214–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weigel MT, Banerjee S, Arnedos M, Salter J, A'Hern R, Dowsett M, Martin LA (2013) Enhanced expression of the PDGFR/Abl signaling pathway in aromatase inhibitor-resistant breast cancer. Ann Oncol 24(1):126–133 [DOI] [PubMed] [Google Scholar]
- 55.Greuber EK, Smith-Pearson P, Wang J, Pendergast AM (2013) Role of ABL family kinases in cancer: from leukaemia to solid tumours. Nat Rev Cancer 13(8):559–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Paulson KE, Rieger-Christ K, McDevitt MA, Kuperwasser C, Kim J, Unanue VE, Zhang X, Hu M, Ruthazer R, Berasi SP et al. (2007) Alterations of the HBP1 transcriptional repressor are associated with invasive breast cancer. Cancer Res 67(13):6136–6145 [DOI] [PubMed] [Google Scholar]
- 57.Debies MT, Gestl SA, Mathers JL, Mikse OR, Leonard TL, Moody SE, Chodosh LA, Cardiff RD, Gunther EJ (2008) Tumor escape in a Wnt1-dependent mouse breast cancer model is enabled by p19Arf/p53 pathway lesions but not p16 Ink4a loss. J Clin Invest 118(1):51–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Smid M, Wang Y, Zhang Y, Sieuwerts AM, Yu J, Klijn JG, Foekens JA, Martens JW (2008) Subtypes of breast cancer show preferential site of relapse. Cancer Res 68(9):3108–3114 [DOI] [PubMed] [Google Scholar]
- 59.Arnold KM, Pohlig RT, Sims-Mourtada J (2017) Co-activation of Hedgehog and Wnt signaling pathways is associated with poor outcomes in triple negative breast cancer. Oncol Lett 14(5):5285–5292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang L, Tang H, Kong Y, Xie X, Chen J, Song C, Liu X, Ye F, Li N, Wang N et al. (2015) LGR5 Promotes Breast Cancer Progression and Maintains Stem-Like Cells Through Activation of Wnt/beta-Catenin Signaling. Stem Cells 33(10):2913–2924 [DOI] [PubMed] [Google Scholar]
- 61.Qureshi A, Pervez S (2010) Allred scoring for ER reporting and it's impact in clearly distinguishing ER negative from ER positive breast cancers. J Pak Med Assoc 60(5):350–353 [PubMed] [Google Scholar]
- 62.Mao Y, Zhang N, Xu J, Ding Z, Zong R, Liu Z (2012) Significance of heterogeneous Twist2 expression in human breast cancers. PLoS One 7(10):e48178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhou S, Sun X, Yu L, Zhou R, Li A, Li M, Yang W (2018) Differential expression and clinical significance of epithelial-mesenchymal transition markers among different histological types of triple-negative breast cancer. J Cancer 9(3):604–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hebbar N, Shrestha-Bhattarai T, Rangnekar VM (2013) Par-4 prevents breast cancer recurrence. Breast Cancer Res 15(5):314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Esparza-Lopez J, Ramos-Elias PA, Castro-Sanchez A, Rocha-Zavaleta L, Escobar-Arriaga E, Zentella-Dehesa A, Leon-Rodriguez E, Medina-Franco H, Ibarra-Sanchez Mde J (2016) Primary breast cancer cell culture yields intra-tumor heterogeneous subpopulations expressing exclusive patterns of receptor tyrosine kinases. BMC Cancer 16(1):740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang Z, Zhang H, Hou J, Niu J, Ma Z, Zhao H, Liu C (2015) Clinical implications of beta-catenin protein expression in breast cancer. Int J Clin Exp Pathol 8(11):14989–14994 [PMC free article] [PubMed] [Google Scholar]
- 67.Heiser LM, Sadanandam A, Kuo WL, Benz SC, Goldstein TC, Ng S, Gibb WJ, Wang NJ, Ziyad S, Tong F et al. (2012) Subtype and pathway specific responses to anticancer compounds in breast cancer. Proc Natl Acad Sci U S A 109(8):2724–2729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bryce NS, Reynolds AB, Koleske AJ, Weaver AM (2013) WAVE2 regulates epithelial morphology and cadherin isoform switching through regulation of Twist and Abl. PLoS One 8(5):e64533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Amin H, Nayak D, Ur Rasool R, Chakraborty S, Kumar A, Yousuf K, Sharma PR, Ahmed Z, Sharma N, Magotra A et al. (2016) Par-4 dependent modulation of cellular beta-catenin by medicinal plant natural product derivative 3-azido Withaferin A. Mol Carcinog 55(5):864–881 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


