Abstract
N6-Methyladenosine (m6A) modification has been implicated in the progression of several cancers. We reveal that during epithelial-mesenchymal transition (EMT), one important step for cancer cell metastasis, m6A modification of mRNAs increases in cancer cells. Deletion of methyltransferase-like 3 (METTL3) down-regulates m6A, impairs the migration, invasion and EMT of cancer cells both in vitro and in vivo. m6A-sequencing and functional studies confirm that Snail, a key transcription factor of EMT, is involved in m6A-regulated EMT. m6A in Snail CDS, but not 3’UTR, triggers polysome-mediated translation of Snail mRNA in cancer cells. Loss and gain functional studies confirm that YTHDF1 mediates m6A-increased translation of Snail mRNA. Moreover, the upregulation of METTL3 and YTHDF1 act as adverse prognosis factors for overall survival (OS) rate of liver cancer patients. Our study highlights the critical roles of m6A on regulation of EMT in cancer cells and translation of Snail during this process.
Subject terms: Cell migration, Metastasis
RNA m6A methylation is known to be dysregulated in many cancers. Here, the authors show that m6A methylation of Snail mRNA regulates its translation with potential effects on epithelial mesenchymal transition.
Introduction
Among the more than 100 modifications in mRNA, N 6-methyladenosine (m6A) has been identified since the 1970s1. m6A modification is the most abundant in eukaryotic mRNAs2, while thousands of RNA transcripts contain m6A modifications with unique distribution patterns as well3. An important report by Jia et al.4 identified the first RNA demethylase, fat mass and obesity-associated protein (FTO), revealing that RNA modification is reversible. Since then, the study of dynamic of m6A modification has greatly expanded5–9. Methyltransferase-like 3 (METTL3), METTL14, and Wilm’s tumor-1-associated protein (WTAP) have been proved to act as m6A methyltransferases (“writers”) in mammalian cells10,11. m6A-binding proteins with YTH domain, including cytoplasmic protein YTHDF1, YTHDF2, YTHDF3, and nuclear protein YTHDC1, have been identified to be the “readers” of m6A and modulate mRNA stability and translation to mediate downstream effects6,7,12,13.
Studies on biological functions of m6A modification in mRNA have arisen in recent years14. It has been reported that m6A modification is involved in various biological processes, including heat-shock response12, ultraviolet-induced DNA damage response15, maternal mRNA clearance5, neuronal functions and sex determination16, cortical neurogenesis17, haematopoietic stem and progenitor cell specification18, and T-cell homeostasis19. Although, m6A modification has been associated with tumorigenesis and progression of cancer cells, the molecular mechanisms involved have not been described yet. Recently, FTO has been shown to be an oncogenic factor in acute myeloid leukemia, which functions as N6-methyladenosine RNA demethylase20. Inhibition of FTO suppresses tumor progression and prolongs lifespan of glioblastoma stem cells (GSC)-grafted mice substantially21. Also, FTO inhibition exerts a broad anti-leukemic activity in vitro and in vivo22. Another identified m6A demethylase, ALKBH5, maintains tumorigenicity of glioblastoma stem-like cells by sustaining FOXM1 expression and cell proliferation program23. ALKBH5-mediated m6A-demethylation of NANOG mRNA is involved in hypoxia-induced breast cancer stem cell phenotype24. Together, these results suggest that m6A modification found in mRNA may regulate the progression of cancer development.
The metastasis of cancer cells is responsible for more than 90% of cancer-associated mortality25, and epithelial–mesenchymal transition (EMT) is considered to be an important step for cancer cell metastasis. During EMT process, epithelial cells lose polarity and gain invasive properties to become mesenchymal stem cells26. Loss of E-cadherin (E-Cad, encoded by the CDH1 gene) has been considered as the most fundamental event in EMT program27. Repression of E-Cad expression, by EMT-inducing transcription factors (EMT-TFs), such as Snail, Slug, Twist, and Zeb, results in promotion of metastasis in cancer cells28. Snail (encoded by SNAI1), acts as a key transcriptional repressor of E-cad expression in EMT, confers tumor cells with cancer stem cell-like traits and promotes drug resistance, tumor recurrence, and metastasis29. Recent evidences indicated that epigenetic modifications regulate progression of EMT and cancer development30. Snail recruits chromatin modifiers, such as lysine-specific demethylase 1 (LSD1)31 and Suv39H1 histone methyltransferases32, and triggers both transient and long-lasting chromatin changes of CDH1 promoter33. However, the precise role of RNA epigenetic factors on progression of EMT has not been reported yet.
This study revealed a significant increase of m6A levels in cancer cells undergoing EMT. However, METTL3-deleted cells were less inducible for EMT. The m6A-seq and m6A-RIP-qPCR results showed that EMT induced elevation of m6A in CDS of SNAI1, the key inducer of EMT, and promoted YTHDF1-mediated Snail translation. Our study revealed that m6A regulated the progression of EMT and translation of Snail.
Results
EMT in cancer cells is regulated by m6A levels of mRNAs
Although EMT can be induced by various extracellular ligands, TGF-β has been considered as the major inducer of this transdifferentiation process of cancer cells34. We treated HeLa and HepG2 cells with 10 ng/ml TGF-β for 3 days. Both TGF-β treated HeLa and HepG2 cells became scattered and adopted the fibroblast-like morphology described for mesenchymal cells (Supplementary Fig. 1A). TGF-β treatments significantly increased wound healing (Supplementary Fig. 1B) and in vitro invasion ability (Supplementary Fig. 1C) of both HeLa and HepG2 cells. Moreover, upregulation of FN1 (fibronectin) and MMP2 mRNA and downregulation of CDH1 (E-Cad) mRNA were observed by qRT-PCR (Supplementary Fig. 1D). These TGF-β-induced changes in expression of EMT markers were further confirmed by western blot analysis (Supplementary Fig. 1E). All these data indicated that cancer cells, treated with TGF-β, were undergoing EMT processes.
We then investigated the variations of m6A levels in mRNAs of cancer cells undergoing EMT. By using LC-MS/MS, we identified that the m6A levels of mRNAs isolated from HeLa and HepG2 cells, treated with TGF-β, were statistically (p < 0.05, t test) more abundant than that of their corresponding control cells (Fig. 1a). The m6A/A levels of mRNA from HeLa and HepG2 cells undergoing EMT increased 20.0% and 14.9%, respectively. This was further confirmed by the results obtained in dot-blot analysis (Supplementary Fig. 2A). Similarly, LC/MS/MS showed that m6A levels of mRNAs isolated from Huh7 and A549 cells, treated with TGF-β, were statistically (p < 0.05, t test) more abundant than that of their corresponding control cells (Supplementary Fig. 2B). Collectively, these data showed that cancer cells undergoing EMT increased m6A levels of mRNAs.
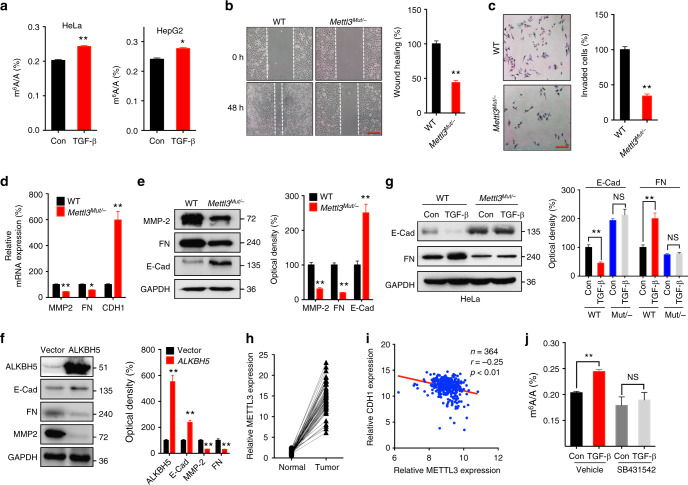
Fig. 1.
EMT in cancer cells is regulated by m6A levels of mRNAs. a HeLa and HepG2 cells were treated with or without 10 ng/ml TGF-β for 3 days, the m6A/A ratio of the total mRNA were determined by LC–MS/MS. b Wound healing of wild-type (control) or Mettl3 Mut/− cells was recorded (left) and quantitatively analyzed (right). c Wild-type or Mettl3 Mut/− cells were allowed to invade for 24 h and tested by CytoSelect™ 24-well Cell Invasion assay kits (8 µm, colorimetric format); d, e mRNA (d) and protein (e) expressions of MMP2, FN, and E-Cad in wild-type and Mettl3 Mut/− HeLa cells were measured by qRT-PCR and western blot analysis, respectively. f HeLa cells were transfected with pcDNA/ALKBH5 or a vector control for 48 h, protein expression was determined by western blot analysis (left) and quantitatively analyzed (right). g Wild-type or Mettl3 Mut/− cells were treated with or without 10 ng/ml TGF-β for 3 days, protein expression was determined by western blot analysis (left) and quantitatively analyzed (right). h The expression of METTL3 in liver cancer and its matched adjacent normal tissues of 50 patients from TCGA database. i Correlation between METTL3 and CDH1 in liver cancer patients (n = 364) from TCGA database. j HeLa cells were pretreated with or without Smad2/3 inhibitor SB431542 (10 μM) and then further treated with 10 ng/ml TGF-β for 3 days, the m6A/A ratio of the total mRNA were determined by LC–MS/MS. Data are presented as means ± SD from three independent experiments. *p < 0.05, **p < 0.01, NS, no significant, by Student’s t test. Red bar = 200 μm
To characterize the roles of m6A in EMT process, we used Mettl3 Mut/− HeLa cells (Supplementary Fig. 2C) generated in our previous study35 by using the CRISPR/Cas9 editing system according to the published protocol35,36. The results showed that Mettl3 Mut/− cells had significantly lower levels of m6A than wild-type cells (Supplementary Fig. 2D), which also confirmed the roles of METTL3 as m6A “writer” of mRNA. We evaluated the EMT-related characteristics of Mettl3 Mut/− cells. The results showed that both wound healing (Fig. 1b) and in vitro invasion abilities (Fig. 1c) of Mettl3 Mut/− HeLa cells were suppressed when compared with wild-type cells. Similarly, sh-Mettl3 or si-Mettl3-mediated knockdown of METTL3 suppressed the in vitro invasion of Huh7 and HepG2 cells, respectively (Supplementary Fig. 2E). The mRNA and protein levels of both MMP2 and FN were downregulated, while E-Cad mRNA and protein levels were upregulated in Mettl3 Mut/− HeLa cells (Fig. 1d, e). In addition, western blot analysis confirmed that METTL3 knockdown decreased MMP2 and FN, while increased E-Cad, in both Huh7 and HepG2 cells (Supplementary Fig. 2F, H). These data suggested that deletion of METTL3 can suppress the EMT of cancer cells.
To verify the role of m6A levels in EMT, we overexpressed ALKBH5, a key m6A demethylase of mammalian mRNA37, in HeLa cells. Our data showed that overexpression of ALKBH5 decreased mRNA of FN and MMP2 in HeLa cells (Supplementary Fig. 2G). Western blot analysis showed that overexpression of ALKBH5 increased E-Cad while it decreased MMP2 and FN in HeLa cells (Fig. 1f). Furthermore, TGF-β-induced downregulation of E-Cad and upregulation of FN were rescued in Mettl3 Mut/− HeLa (Fig. 1g) and Mettl3 knockdown Huh7 (Supplementary Fig. 2H) cells. The clinical roles of METTL3 were further investigated. TCGA data showed that expression of METTL3 in liver cancers was significantly higher than their matched adjacent normal tissues (Fig. 1h). Moreover, the expression of METTL3 was negatively correlated with the CDH1 mRNA in 364 liver cancer patients (Fig. 1i). It has been reported that TGF-β can induce the mRNA methylation in human embryonic stem cells (hESCs) via the interaction between Smad2/3 and the METTL3–METTL14–WTAP complex38. LC/MS/MS showed that the Smad2/3 inhibitor SB431542 (SB) can block TGF-β-induced upregulation of m6A in HeLa cells (Fig. 1j). Together, our data indicated that the total m6A levels in mRNA may regulate EMT progression of cancer cells.
Variations of m6A-regulated genes in cells undergoing EMT
To investigate the variations of m6A modification in specific genes, we mapped m6A methylomes of HeLa cells undergoing EMT (EMT cells) by m6A sequencing (m6A-seq), with independent biological replicates. Principal component analysis (PCA) showed that two repeats of each sample clustered together, suggesting the good reproducibility among the two replicates of each group (Supplementary Fig. 3A). GGAC motif was highly enriched within m6A sites in both control and EMT cells (Fig. 2a). m6A peaks were especially abundant in the vicinity of start and stop codons (Fig. 2b).
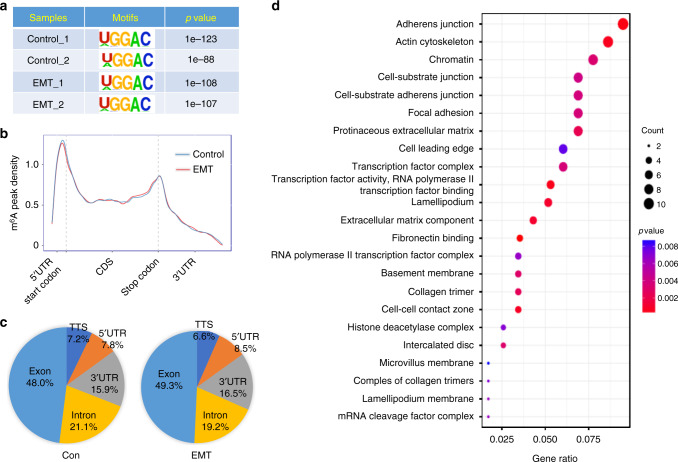
Fig. 2.
Variations of m6A-regulated genes in cells undergoing EMT. a Predominant consensus motif GGAC was detected in both the control and EMT cells in m6A-seq. b Density distribution of m6A peaks across mRNA transcripts. Regions of the 5′ untranslated region (5′UTR), coding region (CDS), and 3′ untranslated region (3′UTR) were split into 100 segments, then percentages of m6A peaks that fall within each segment were determined. c Proportion of m6A peak distribution in the 5′UTR, start codon, CDS, stop codon or 3′UTR region across the entire set of mRNA transcripts. d A cluster profiler identified the enriched gene ontology processes of 128 genes, which showed 1.5-fold m6A expression upregulation in EMT cells compared with control cells
m6A-seq analysis identified 11,687 and 11,785 m6A peaks from 6836 to 7230 m6A-modified transcripts in control and EMT cells, respectively. In EMT cells, 4347 new m6A peaks and 4249 disappearing peaks were observed, while the remaining 7438 peaks were found in both control and EMT cells (Supplementary Fig. 3B). For m6A-regulated genes, m6A-seq identified 569 new modifying genes and 472 genes loss of m6A modification in EMT cells, while the other 2357 genes were found in both control and EMT cells (Supplementary Fig. 3C). The results indicated that EMT cells unique peaks and genes were expected to contain genuine targets. We further analyzed the total m6A distribution patterns of mRNAs according to the m6A-seq results. Similar patterns of the total m6A distribution in control and EMT cells were observed (Fig. 2c), showing that m6A peaks were mainly enriched in exon regions. Furthermore, we investigated the distribution of unique EMT peaks. Compared with the 4249 missing peaks, which displayed identical distribution to total peaks, 4347 unique peaks in EMT cells showed a distinct pattern, in which a relative increase of m6A deposit appeared in both 5′ and 3′ UTR (Supplementary Fig. 3D).
Comparing the control to EMT cells, a total of 128 genes, showing a 1.5-fold m6A change in the expression level, were identified. Gene ontology (GO) enrichment analysis of these 128 genes indicated that a handful of genes were associated to the formation of the adherens junction and actin cytoskeleton in cancer cells undergoing EMT (Fig. 2d). Collectively, the m6A-seq data revealed that m6A modifications occur on a handful of genes related to the cell junction, adhesion, and migration during EMT.
Snail is involved in m6A-regulated EMT in cancer cells
To characterize potential targets involved in m6A-regulated EMT of cancer cells, we identified four genes overlapping between 84 EMT-related genes with key functions (listed in Supplementary Table 1) and the 61 m6A-regulated genes (greater than 2.0-fold m6A change) during EMT (Fig. 3a). Among the four identified genes, Snail has been suggested as an important transcription factor controlling EMT39. Our data confirmed that SNAI1 mRNA was modified by m6A and showed significant enrichment of m6A in its CDS and 3′UTR regions during EMT progression (Fig. 3b, c), which is consistent with the published reports using zebrafish embryonic cells5 and human HEK293T cells40,41. By applying m6A RNA-immunoprecipitation (RIP) qPCR, we verified a 12-fold m6A antibody enriched SNAI1 mRNA (Fig. 3c). During EMT, m6A levels of SNAI1 mRNA were significantly increased, and relative m6A enrichment fold in EMT cells was about 2.3-fold greater than in control cells (Fig. 3c). However, neither m6A pull down enrichment nor m6A upregulation during EMT was observed for the negative candidate gene ZEB1, which is another transcription factor of EMT as well (Supplementary Fig. 4A, B).
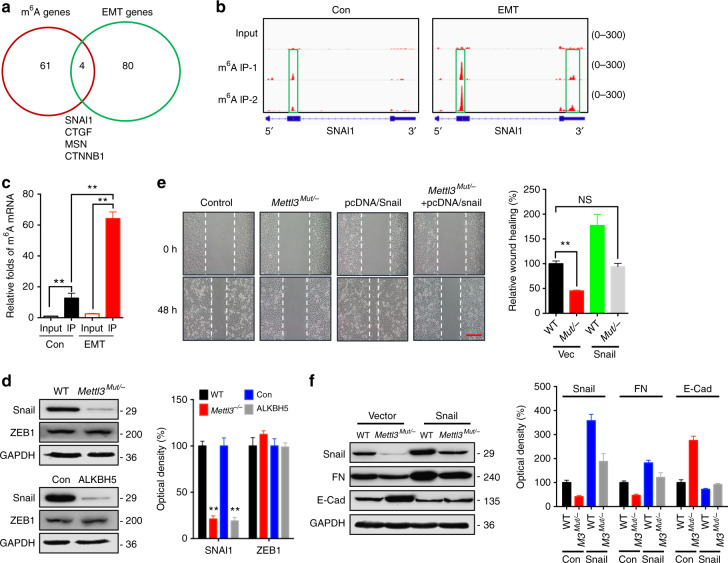
Fig. 3.
Snail is involved in m6A-regulated EMT in cancer cells. a Overlapping of 2.0-fold m6A expression changes in EMT cells and EMT-related functional genes. b m6A peaks were enriched in CDS and 3′UTRs of SNAI1 genes from m6A RIP-seq data. Squares marked increases of m6A peaks in cancer cells undergoing EMT; c m6A RIP-qPCR analysis of SNAI1 mRNA in the control and EMT undergoing HeLa cells. d Protein expression of Snail in Mettl3 Mut/− or AKLBH5 transfected (24 h) HeLa cells and the control. e The wound healing of wild-type or Mettl3 Mut/− HeLa cells transfected with or without pcDNA/Snail for 48 h were recorded (left) and quantitatively analyzed (right). f Wild-type or Mettl3 Mut/− HeLa cells were transfected with or without pcDNA/Snail for 48 h, expression of Snail, FN and E-Cad were measured by western blot analysis. Data are presented as means ± SD from three independent experiments. *p < 0.05, NS, no significant, by Student’s t test. Red bar = 200μm
Next, we investigated protein expression of Snail in Mettl3 Mut/− and ALKBH5 overexpressing HeLa cells. We observed a decrease of Snail protein levels in HeLa (Fig. 3d) and HepG2 (Supplementary Fig. 4C) cells without changes in ZEB1 expression, in both contexts of deletion of METTL3 and overexpression of ALKBH5. To confirm the roles of m6A on Snail expression, we treated wild-type and Mettl3 Mut/− HeLa cells with TGF-β. The results showed that TGF-β-induced expression of Snail was reduced in Mettl3 Mut/− cells (Supplementary Figure 4D), which indicated that m6A participated in TGF-β-induced expression of Snail in cancer cells. Although the promotion roles of Snail in EMT and cancer progression have been well illustrated27,42,43, we further investigated the roles of Snail in m6A-triggered EMT of cancer cells. Overexpression of Snail attenuated the suppression of wound healing via METTL3 deletion in HeLa cells (Fig. 3e). Consistently, overexpression of Snail antagonized the downregulation of FN and upregulation of E-Cad occurred in Mettl3 Mut/− cells (Fig. 3f; Supplementary Fig. 4E). These data suggested that Snail is involved in m6A-regulated EMT in cancer cells.
m6A triggers translation of Snail mRNA in cancer cells
The mechanisms regulating Snail expression through m6A methylation were further investigated. First, we tested the expression of precursor (pre-) and mature (mat-) mRNA of Snail. Surprisingly, expression of both precursor (pre-) and mature (mat-) mRNA of Snail in Mettl3 Mut/− cells were significantly enhanced (Fig. 4a). However, there was no difference on promoter activity in wild-type and Mettl3 Mut/− cells (Supplementary Fig. 5A), suggesting that transcription of Snail was not affected by m6A. By separating the nuclear and cytoplasmic RNAs (Supplementary Fig. 5B), we found that there was no difference on subcellular localization of Snail mRNA between wild-type and Mettl3 Mut/− cells (Fig. 4b). We treated wild-type and Mettl3 Mut/− HeLa cells with Act-D to block transcription. The results showed that half-life of both precursor (Fig. 4c) and mature (Fig. 4d) mRNA in wild-type cells were significantly shorter than in Mettl3 Mut/− cells. It indicated that m6A modification may trigger the splicing of precursor mRNA and the degradation of mature mRNA of Snail in cancer cells. This was confirmed by the results indicating that Snail was a target of YTHDF2 (Supplementary Fig. 5C), which is responsible for m6A-mediated mRNA destabilization of transcripts6. Overexpression of YTHDF2 can decrease the expression and stability of mature Snail mRNA in HeLa cells (Supplementary Fig. 5D).
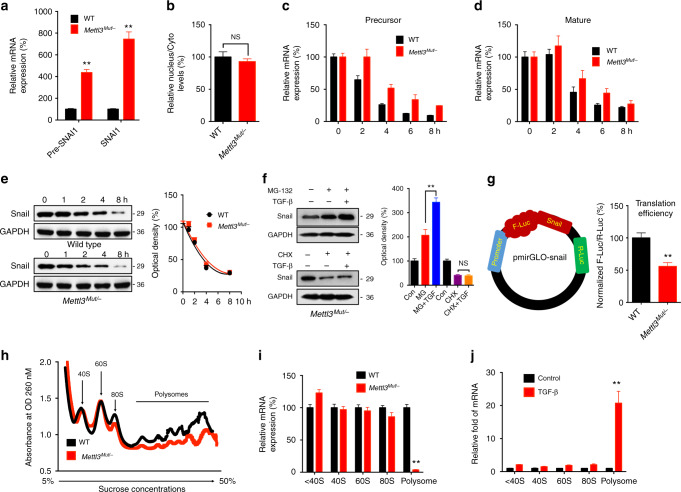
Fig. 4.
m6A triggers translation of Snail mRNA in cancer cells. a Precursor and mature mRNA of SNAI1 in wild-type (WT) and Mettl3 Mut/− cells. b The relative levels of the nuclear versus cytoplasmic SNAI1 mRNA in wild-type (WT) or Mettl3 Mut/− cells. c, d Wild-type (WT) or Mettl3 Mut/− cells were pretreated with Act-D for 90 min, then precursor (c) or mature (d) SNAI1 mRNA were analyzed at indicated times. e Wild-type (WT) or Mettl3 Mut/− cells were treated with CHX for the indicated times, and protein expression of Snail was analyzed by western blot analysis (left) and quantitatively analyzed (right); f Mettl3 Mut/− cells were pretreated with CHX or MG-132 for 6 h and then further treated with or without 10 ng/ml TGF-β for 48 h, the expression of Snail was detected by western blot analysis (left) and quantitatively analyzed (right). g Wild-type (WT) or Mettl3 Mut/− cells were transfected with pmirGLO-Snail reporter for 24 h, and the translation efficiency of Snail is defined as the quotient of reporter protein production (F-luc/R-luc) divided by mRNA abundance7 . h Polysome profiling of wild-type (WT) or Mettl3 Mut/− HeLa cells were analyzed. i Analysis of Snail mRNA in non-ribosome portion (< 40S), 40S, 60S, 80S, and polysome for the Mettl3 Mut/− cells compared to control cells. j Analysis of Snail mRNA in non-ribosome portion (< 40S), 40S, 60S, 80S, and polysome for EMT undergoing HeLa cells compared with control cells. Data are presented as means ± SD from three independent experiments. *p < 0.05, NS, no significant, by Student’s t test
We observed increasing of mature SNAI1 mRNA levels while decreasing of protein levels of Snail in Mettl3 Mut/− cells, then we hypothesized that downregulation of Snail protein in Mettl3 Mut/− cells may be due to a difference on protein stability or translation efficiency. To verify this, both wild-type and Mettl3 Mut/− HeLa cells were treated with protein translation inhibitor cycloheximide (CHX). The results from western blot analysis showed that half-lives of Snail proteins were similar between wild-type and Mettl3 Mut/− HeLa cells (Fig. 4e), suggesting that m6A-induced Snail expression was not related to protein stability.
Furthermore, Mettl3 Mut/− HeLa cells were pretreated with MG132 to inhibit proteasome activity or CHX to block translation and then treated with TGF-β. The data showed that in the presence of CHX, but not MG-132, attenuated TGF-β-induced Snail expression in Mettl3 Mut/− HeLa cells (Fig. 4f). We constructed the pmirGLO-Snail luciferase reporter by ligating Snail CDS to the mutiple cloning site (MCS) site. The dual-luciferase assay showed that translation efficiency of Snail in Mettl3 Mut/− HeLa cells was significantly lower than that in HeLa WT cells (Fig. 4g). It indicated that m6A-induced Snail expression was related to regulation of translation.
Next, we separated the RNA fractions: non-translating fraction (< 40 S), translation initiation fraction (including 40S ribosomes, 60S ribosomes, 80S monosomes, and < 80S) as well as translation active polysomes (> 80S) from wild-type and Mettl3 Mut/− HeLa cells by ribosome profiling (Fig. 4h). Polysome profiling supported the decreases of 80S monosome assembly and polysomes in Mettl3 Mut/− cells (Fig. 4h). qRT-PCR showed that Snail mRNA in translation-active polysomes (> 80S) of Mettl3 Mut/− cells were significantly lower than in wild-type cells (Fig. 4i). In EMT cells, ribosome profiling was similar to that in wild-type cells (Supplementary Fig. 5E). Remarkably, an increase of SNAI1 mRNA in polysomal fractions (> 80S) isolated from cancer cells undergoing EMT was observed (Fig. 4j), indicating that translation of SNAI1 mRNA was improved during EMT process. To further confirm our observations, we targeted cap-independent/dependent translation initiation to investigate whether it was involved in m6A methylation-regulated SNAI1 translation. Both Mettl3 Mut/− and wild-type cells were treated with rapamycin, which inhibits cap-dependent but not cap-independent translation44. However, no obvious differences of Snail1 levels were observed between wild-type and Mettl3 Mut/− cells (Supplementary Fig. 5F). Maintenance of Snail1 expression in the presence of rapamycin was not due to an increase of Snail1 protein stability, as co-treatment of wild-type cells with protein synthesis inhibitor CHX resulted in a rapid disappearance of Snail1 (Supplementary Fig. 5F), suggesting that Snail expression was regulated through cap-independent translation45.
m6A-methylated CDS regulates translation of Snail
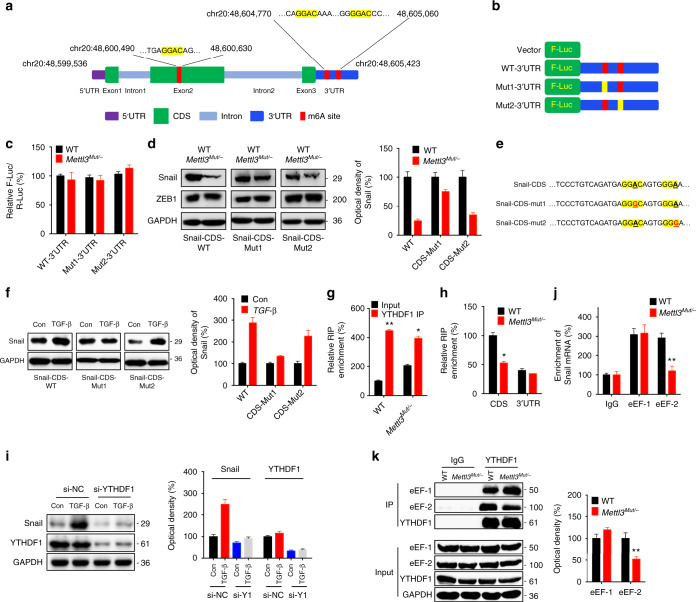
m6A-RIP-seq data showed that m6A peaks in CDS regions ranged from chr20:48,600,490 to 48,600,630, while in 3′UTR regions ranged from chr20: 48,604,770 to 48,605,060 (Fig. 3b). One GGAC motif in CDS and two in 3′UTR of Snail mRNA were identified, as shown in Fig. 5a, which was consistent with positions and amounts of peaks identified by m6A RIP-seq. To characterize m6A methylation in Snail mRNA, we used m6A antibody to immunoprecipitated fragmented RNA isolated from HeLa cells (Supplementary Fig. 6A). qRT-PCR showed that distributions of m6A methylation in Snail mRNA, from high to low, were: CDS, 3′UTR and 5′UTR, in wild-type HeLa cells (Supplementary Fig. 6B). Consistently, the m6A enrichment of Snail CDS was significantly downregulated in Mettl3 Mut/− cells (Supplementary Fig. 6B), suggesting that m6A methylation in CDS regions might be more dynamic than those in 3′UTR.
Fig. 5.
m6A methylated CDS regulates translation of Snail. a Schematic representation of positions of m6A motifs within Snail mRNA. b Schematic representation of mutated (GGAC to GGCC) 3’UTR of pmirGLO vector to investigate the m6A roles on Snail expression. c pmirGLO-3′UTR or pmirGLO-3′UTR-Mut1/2 reporter was transfected into wild-type or Mettl3 Mut/− HeLa cells for 24 h. d pcDNA-Snail-CDS-WT, pcDNA-Snail-mut1/2, or pcDNA/ZEB1 were transfected into HeLa cells for 24 h. Protein expression was measured by western blot analysis (left) and quantitatively analyzed (right). e Schematic representation of mutation in CDS to investigate the m6A roles on Snail expression. f After transfected with pcDNA-Snail-CDS-WT or pcDNA-Snail-mut1/2, HeLa cells were further treated with or without 10 ng/ml TGF-β for 48 h. The expression of Snail was measured by western blot analysis (left) and quantitatively analyzed (right). g YTHDF1 RIP-qPCR analysis of SNAI1 mRNA in wild-type or Mettl3 Mut/− HeLa cells. h Binding of YTHDF1 with the CDS or 3’UTR in wild-type or Mettl3 Mut/− cells were analyzed by YTHDF1 RIP-qPCR using fragmented RNA. i After pre-transfected with siNC or si-YTHDF1 for 12 h, HeLa cells were further treated with or without 10 ng/ml TGF-β for 48 h. Protein expression was measured by western blot analysis (left) and quantitatively analyzed (right). j eEF-1 and eEF-2 RIP-qPCR analysis of SNAI1 mRNA in wild-type or Mettl3 Mut/− HeLa cells. k Binding between YTHDF1 with eEF-1 or eEF-2 in wild-type or Mettl3 Mut/− HeLa cells was checked by immunoprecipitation (left) and quantitatively analyzed (right). Data are presented as means ± SD from three independent experiments. *p < 0.05, NS, no significant, by Student’s t test
To study the potential roles of m6A methylation on the SNAI1 3′UTR region, we generated luciferase reporters containing a firefly luciferase, followed by the wild-type SNAI1 3′UTR, mutant 1 or mutant 2 3′UTR (GGAC to GGCC, Fig. 5b). The SNAI1 3′UTR-reporter luciferase assay showed that Snail 3′UTR from WT-3′UTR, mut1–3′UTR, and mut2–3′UTR had no translational difference between wild-type and Mettl3 Mut/− cells (Fig. 5c). Consistently, co-transfection of ALKBH5 did not alter expression levels of WT-3′UTR, mut1–3′UTR, or mut2–3′UTR in HeLa cells (Supplementary Fig. 6C). This suggested that m6A methylation on 3′UTR might not be involved in the m6A modification-regulated Snail expression.
We then investigated whether m6A methylation in CDS can regulate the expression of Snail. First, we generated an expression construct containing Snail CDS alone. We co-transfected Snail CDS with pcDNA/ZEB1, expression of which was not regulated by m6A methylation, to normalize the transfection efficiency. The results showed that expression of Snail in Mettl3 Mut/− cells remarkably decreased, while expression of ZEB1 was not affected, indicating that m6A methylation in CDS was critically involved in m6A-mediated Snail expression (Fig. 5d). Second, we mutated the potential m6A motif GGAC to GGCC (CDS-mut1). Accordingly, the nearby GGAA was mutated to GGCA for comparison (CDS-mut2, both mutants were located at chr20: 48,600,490–48,600,630, Fig. 5e). Our data showed that Snail level was partially recruited in Mettl3 Mut/− cells overexpressing pcDNA-Snail-CDS-mut1, but not CDS-mut2 (Fig. 5d). HeLa cells transfected with pcDNA-Snail-CDS-WT or pcDNA-Snail-CDS-mut1/mut2 were further treated with TGF-β. Western blot results showed that TGF-β-induced Snail expression of pcDNA-Snail-CDS-mut1 was lower than pcDNA-Snail-CDS-WT or pcDNA-Snail-CDS-mut2 (Fig. 5f). Together, our data indicated that m6A in SNAI1 CDS was the main location for expression regulation.
YTHDF1, acting as a m6A “reader”, recognizes m6A methylated mRNA and promotes the translation of its targets7. In order to verify whether YTHDF1 participates in m6A methylation of SNAI1 mRNA, RIP-qPCR was used to investigate the interaction between SNAI1 mRNA and YTHDF1. Our data showed that YTHDF1 enriched in SNAI1 mRNA remarkably, while this relative enrichment (IP vs. input) was significantly suppressed in Mettl3 Mut/− cells (Fig. 5g). The results of RIP-PCR further revealed that YTHDF1 preferred to interact with the CDS region of Snail mRNA rather than 3′UTR region (Fig. 5h). In Mettl3 Mut/− cells, binding between YTHDF1 with CDS, but not 3′UTR, of SNAI1 mRNA, was significantly decreased in comparison with HeLa cells. To confirm the roles of YTHDF1 in m6A-regulated Snail expression, we knocked down expression of YTHDF1 in HeLa cells (Supplementary Fig. 6D). Our data showed that si-YTHDF1 attenuated TGF-β-induced expression of Snail in HeLa cells (Fig. 5i). Similarly, overexpression of YTHDF1 increased protein expression of Snail in both HeLa and HepG2 cells, while it had no effect on the expression of ZEB1 (Supplementary Fig. 6E).
Since the translation elongation in eukaryotes is carried out with two elongation factors: eEF-1 and eEF-246, we measured the variation of eEF-1- and eEF-2-binding Snail mRNA in Mettl3 Mut/− HeLa cells. Our data showed that the binding between Snail mRNA and eEF-2, but not eEF-1, in Mettl3 Mut/− HeLa cells was significantly less than that in HeLa cells (Fig. 5j). Furthermore, co-IP analysis indicated that the binding between YTHDF1 and eEF-2, while not eEF-1, was significantly decreased in Mettl3 Mut/− HeLa cells compared with that in WT HeLa cells (Fig. 5k). This data suggested that YTHDF1 and eEF-2 are likely responsible for the m6A-induced translation elongation of Snail mRNA in cancer cells.
m6A regulates in vivo cancer progression
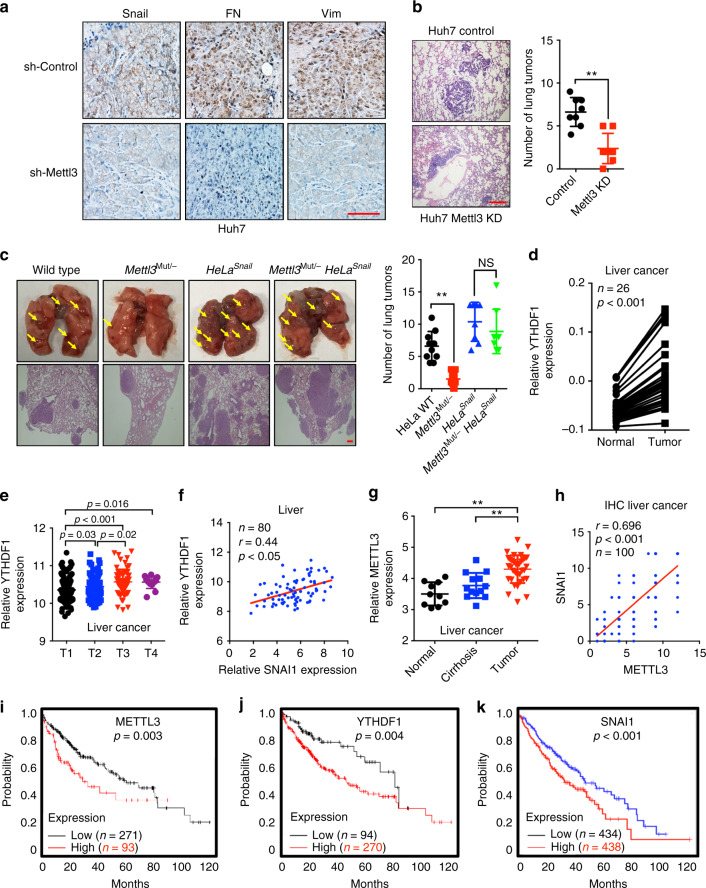
We used three animal cohorts to study the potential effects of m6A methylation on cancer cells in vivo metastasis. First, both sh-control and sh-Mettl3 Huh7 cells were injected subcutaneously into nude female mice. Mice were killed when the tumor volumes were about 100 mm3 for each group. IHC results showed that METTL3 depletion led to a lower level of Snail, FN, and Vim in xenograft tumor tissues (Fig. 6a), which suggested that the knockdown of METTL3 can decrease the EMT potential and Snail expression of Huh7 cells when the tumor volumes were comparable. To further determine the impacts of m6A methylation on in vivo metastasis, both sh-control and sh-Mettl3 Huh7 cells were injected into the BALB/c nude mice, respectively, by tail vein injection to analyze lung colonization. Eight weeks after injection, the experiment was terminated and lungs were analyzed to report the presence of metastatic tumors. As shown in Fig. 6b, the number of lung tumors derived from METTL3 knockdown Huh7 cells was significantly decreased compared with control cells, suggesting that METTL3 deficiency suppressed tumor metastasis in vivo. To investigate whether Snail was involved in m6A regulated in vivo metastasis, HeLa WT, Mettl3 Mut/− HeLa, HeLaSnail, and Mettl3 Mut/− HeLaSnail stable cells were injected into nude mice via intravenous tail vein. In vivo data showed that the number of lung tumors derived from Mettl3 Mut/− HeLa cells was significantly decreased compared with HeLa WT cells; however, overexpression of Snail can attenuate the suppression effect of Mettl3 Mut/− HeLa cells on in vivo lung colonization (Fig. 6c). It suggested that Snail was involved in the in vivo metastasis effect of METTL3.
Fig. 6.
m6A methylation regulates in vivo cancer progression. a IHC (Snail, FN, and Vim)-stained paraffin-embedded sections obtained from sh-control and sh-Mettl3 Huh7 xenografts when the tumor volumes were about 100 mm3 for each group. b The sh-control and sh-Mettl3 Huh7 cells were injected into the nude mice by tail vein injection. Representative images of metastatic lung tumors and the H&E staining results were shown (left), and the number of lung tumors was quantitatively analyzed (right). c HeLa WT, Mettl3 Mut/− HeLa, HeLaSnail, and Mettl3 Mut/− HeLaSnail stable cells were injected into the nude mice by tail vein injection. Representative images of metastatic lung tumors and the H&E staining results were shown (left), and the number of lung tumors was quantitatively analyzed (right). d Expression of YTHDF1 in paired human liver (n = 26) cancer tissues and adjacent normal mucosa tissues from TCGA database. e YTHDF1 expression in liver cancers of T1 (n = 153), T2 (n = 77), T3 (n = 65), and T4 (n = 13) stages from TCGA database. f Correlation between YTHDF1 and SNAI1 in liver cancer patients (n = 80) from TCGA database. g METTL3 expression in HCC tumor tissues (n = 35), liver cirrhosis tissues (n = 13), and normal liver tissues (n = 10) from Oncomine database (Wurmbach liver cancers). h Correlation between Mettl3 and Snail protein in liver cancer patients (100) by use of tissue microarray (CS03–01–008, Alenabio, Xian, Shanxi, China). i, j, k Kaplan–Meier survival curves of OS based on METTL3 (i), YTHDF1 (j), or SNAI1 (k) mRNA expression in HCC patients. The log-rank test was used to compare differences between two groups. Data are presented as means ± SD from three independent experiments. *p < 0.05, **p < 0.01, NS, no significant, by one-way ANOVA with Bonferroni test. Red bar = 200 μm
Our data suggested that knockdown of METTL3 can decrease the expression of Snail and the in vitro invasion and EMT of HepG2 and Huh7 cells (Supplementary Figs. 2E, 2F, 4C), we then questioned the possibility of a link between m6A methylation and clinical development of liver cancer. Increased expression of METTL3 (Fig. 1h) and YTHDF1 (Fig. 6d) was observed in liver cancer tissues as compared with matched adjacent normal tissues. Significant rising expression levels of YTHDF1 from T1 to T4 stage of liver cancer tissues were observed (Fig. 6e), implying an increasing tendency of YTHDF1 expression during malignant transformation. In addition, expression of YTHDF1 was positively correlated with the SNAI1 mRNA in liver (Fig. 6f) cancer patients. Rising expression levels of METTL3 from the normal liver to cirrhosis to liver cancer tissues were observed (Fig. 6g). Considering that Mettl3 induced Snail via translation, we checked their protein expression in 100 cases of liver cancer tissues by use of tissue microarray. IHC analysis confirmed that the expression of Mettl3 was significantly (p < 0.05, t test) positively associated with that of Snail in the measured 100 liver cancers (Fig. 6h). Using the online bioinformatics tool Kaplan–Meier plotter47, we found that liver cancer patients with increased expression of METTL3 (Fig. 6i), YTHDF1 (Fig. 6j), and Snail (Fig. 6k) showed reduced overall survival (OS).
Discussion
Increasing evidences suggested that m6A mRNA modification participates in a number of biological functions and in progression of cancer cells15,20,21. In this study, we demonstrated that m6A can regulate the progression of EMT and expression of Snail. In brief, m6A levels in mRNA of cancer cells undergoing EMT increased significantly compared with control cells. Deletion of METTL3 or overexpression of ALKBH5 suppressed the in vitro migration, invasion, and EMT of cancer cells. m6A-seq and GO analysis showed that genes related to cell migration and adherens junctions are dynamically regulated by m6A during EMT. Particularly, Snail, a key transcription factor of EMT, was m6A methylated in its CDS and 3′UTR regions. Gain and loss of function studies revealed that m6A in CDS of Snail can trigger its translation elongation via interaction with YTHDF1 and eEF-2. Deletion of METTL3 suppressed the in vivo metastasis of cancer cells, which could be attenuated by the overexpression of Snail. Consistently, clinical data showed that tumor tissues contained higher METTL3 and YTHDF1 levels than adjacent normal tissues, which induced worse prognosis for liver cancer patients.
The roles of mRNA modification in controlling the cancer progression have just begun to be studied. FTO, the first characterized m6A demethylase, has been reported to enhance leukemic oncogene-mediated cell transformation and leukemogenesis via regulation of expression of its targets, such as ASB2 and RARA, by reducing their m6A levels20. Another m6A demethylase ALKBH5 can also trigger tumorigenicity of glioma stem-like cells (GSC) by sustaining FOXM1 expression and cell proliferation23. METTL3, which is the methyltransferase of mRNA m6A, exhibits several functions in cancer cells, such as: maintaining myeloid leukemia48, promoting liver cancer progression49, playing essential role in GSC maintenance and radioresistance50, positively regulating proliferation of hematopoietic stem/progenitor cells (HSPCs)51, and triggering the translation in cancer cells52. Contradictorily, METTL3 acts as a tumor suppressor in renal cell carcinoma53. METTL14, which forms a stable heterodimer core complex with METTL3 to function in cellular m6A deposition, can suppress metastatic potential by modulating the primary microRNA 126 processing in an m6A-dependent manner54. In this study, we provided the causative link between mRNA m6A methylation and EMT. We found that levels of m6A in mRNA increased in cancer cells undergoing EMT, while targeted deletion of METTL3 impaired the migration and EMT of cancer cells. GO analysis revealed that modulation of mRNA m6A levels impacted multiple aspects of cancer progression, including migration, self-renewal, and tumorigenesis. Our results described the roles of m6A and METTL3 in cancer progression, and also created the possibility to develop therapeutic strategies against cancer metastasis by targeting m6A modification and its related targets.
m6A modification modulates all stages in the life cycle of RNA, such as RNA processing, nuclear export, and translation modulation13,55. First, splicing regulators and a m6A “reader” HNRNPC could affect the abundance as well as alternative splicing of target mRNAs in a m6A switch-regulated manner56. Second, m6A was found to promote RNA export from the nucleus to cytoplasm, which was evidenced by the fact that METTL3 knockdown delayed mRNA export57, while inhibition of ALKBH5 enhanced mRNA export to the cytoplasm37. Third, m6A modification can trigger mRNA degradation by promoting deadenylation of RNAs through the first characterized m6A “reader” protein YTHDF26. Half-life of Snail mRNA in Mettl3 Mut/− cells was significantly longer than in control cells, which indicated a negative impact of m6A on mRNA stability. FTO mediated repression of ASB2 and RARA stability20 and ALKBH5 modulated FOXM1 expression via m6A modifications in 3′UTR of targets23. However, our luciferase/mutation assays showed that m6A in 3′UTR had no effect on Snail protein expression, suggesting that m6A regulation on mRNA stability is more complicated than expected and could be related to target or location specificity.
We revealed that m6A triggered translation of Snail mRNA through YTHDF1 and eEF-2 binding with methylated mRNA. YTHDF1 interacts with EIF3 to promote the rate-limiting step of translation for m6A-modified mRNAs by increasing their association with polysomes7. Our data showed that YTHDF1 prefers to bind with the CDS of Snail mRNA and then triggers its association with polysome in cancer cells undergoing EMT. The mutation of GGAC motif can partially rescue m6A-regulated translation. Similarly, in METTL3 deletion acute myeloid leukemia (AML) cells, c-MYC, BCL2, and PTEN protein expression were reduced despite a 2–5 log2-fold increase in mRNA expression51. A specific shift of SP1 and SP2 transcripts toward lower-molecular-weight polysomes has been observed in METTL3 depletion cancer cells48. Alternatively, METTL3 can localize to polysomes and functionally associate with translation initiation factors to promote translation in an YTHDF1-independent manner52. In Mettl3 Mut/− cells, the binding between Snail mRNA and eEF-2 or between YTHDF1 and eEF-2 was decreased. eEF-2 is essential for the GTP-dependent translocation of the ribosome along mRNA58. Our results also showed that m6A-regulated Snail translation is cap-independent, which was evidenced by the fact that rapamycin had no effect on Snail expression in wild-type and Mettl3 Mut/− cells. However, to determinate if promoter-bound METTL3 augments translation48 will need further studies.
In summary, we provide compelling in vitro and in vivo evidences demonstrating that m6A can regulate the progression of EMT, cancer metastasis, and Snail translation. Our present research provided the mechanism of regulation of cancer progression by modulation of EMT through RNA m6A modification. It should be noted that the effects of Mettl3-modified Snail mRNA might be heterogeneous in different tumor models, further, Snail is not always associated with the induction of mesenchymal features59,60 and EMT is not necessary for the generation of metastases in some cancers59,61. The roles of m6A in cancer metastasis and Snail expression in other types of cancer need further investigation.
Methods
Cell culture, treatments, and transfection
Human cancer HeLa, HepG2, Huh7, and A549 cells were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA) and maintained in our lab with Dulbecco’s modified Eagle medium (DMEM, GIBCO, Carlsbad, CA, USA) with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin (Invitrogen). Cells were routinely tested for mycoplasma contamination using MycoAlert Mycoplasma Detection Kit. To establish the model of cancer cells undergoing EMT, cells were treated with 10 ng/ml TGF-β for 3 days. To generate Mettl3 Mut/− HeLa cells, the CRISPR-cas9 editing system was used according to the published protocol36. The sh-Control and sh-Mettl3 Huh7 cells were kindly provided by Prof Jun Cui at Sun Yat-sen University. To generate Snail stable overexpression cells, the wild-type and Mettl3 Mut/− HeLa cells were transfected with pcDNA/Snail or vector control and via liposome-mediated transfection. The transfected cells were selected with G418 (700 μg/ml) for 2 weeks. The survived cells were picked out and seeded into 96-well plate for formation of cell clones and further expansion. The expanded monoclonal cell populations were named as HeLa WT, Mettl3 Mut/− HeLa, HeLaSnail, and Mettl3 Mut/− HeLaSnail cells, respectively. The expression of Snail and Mettl3 was checked by western blot analysis. For overexpression of METTL3, Snail, ALKBH5, YTHDF1, or YTHDF2, cells were transfected with plasmids by lipofectamine 2000 according to the manufacturers’ instructions. For YTHDF1 or METTL3 knockdown, three synthesized duplex RNAi oligos targeting human YTHDF1 or METTL3 mRNA sequences from Sigma were used. The most efficient one was selected for following studies. A scrambled duplex RNA oligo (5′-UUCUCCGAACGUGUCACGU) was used as a RNA negative control (si-NC). Lipofectamine RNAiMAX (Invitrogen) was used for siRNA transfection. Working concentration for siRNA was 50 nM.
Wound-healing and in vitro invasion assay
For wound-healing assay, cells were seeded and cultured until a 90% confluent monolayer was formed. Cells were then scratched by a sterile pipette tip and treated as indicated in the text in the FBS-free medium. Cell migration distances into the scratched area were measured in 10 randomly chosen fields under a microscope. Transwell assay was conducted using CytoSelect™ 24-well Cell Invasion assay kits. Briefly, polycarbonate filters (8-μm pore size, Corning) coated with 50% Matrigel (BD bioscience, Bedford, MA) were used to separate upper and lower chambers. In total, 1 × 105 cells in 200 μl of the culture medium (supplemented with 0.1% FBS) were added into the upper chamber, while 600 μl medium supplemented with 10% FBS were added into the lower chamber and served as a chemotactic agent. After incubation, cells invading into the lower chamber were fixed, stained, and counted under the upright microscope (five fields per chamber).
RNA extraction and real-time PCR for gene expression
RNA extraction with Trizol (Invitrogen) and real-time PCR were performed according to the protocol used in our previous study18. Primers of targeted genes were as follow: GAPDH, forward 5′-GTC TCC TCT GAC TTC AAC AGC G-3′ and reverse 5′-ACC ACC CTG TTG CTG TAG CCA A-3′ MMP2, forward 5′-AGC GAG TGG ATG CCG CCT TTA A-3′ and reverse 5′-CAT TCC AGG CAT CTG CGA TGA G-3′ FN1, forward 5′-ACA ACA CCG AGG TGA CTG AGA C-3′ and reverse 5′-GGA CAC AAC GAT GCT TCC TGA G-3′ CDH1, forward 5′-GCC TCC TGA AAA GAG AGT GGA AG-3′ and reverse 5′-TGG CAG TGT CTC TCC AAA TCC G-3′ Pre-Snail, forward 5′-GGG ACA GTT GAA CCC CTC AG-3′ and reverse 5′-GGA GTC AAT GAG GAC CGC AA-3′ (mat-) Snail, forward 5′-TGC CCT CAA GAT GCA CAT CCG A-3′ and reverse 5′-GGG ACA GGA GAA GGG CTT CTC-3′ ZEB1, forward 5′-GGC ATA CAC CTA CTC AAC TAC GG-3′ and reverse 5′-TGG GCG GTG TAG AAT CAG AGT C-3′ METTL3, forward 5′-CTA TCT CCT GGC ACT CGC AAG A-3′ and reverse 5′-GCT TGA ACC GTG CAA CCA CAT C-3’YTHDF1, forward 5′- CAA GCA CAC AAC CTC CAT CTT CG-3′ and reverse 5′-GTA AGA AAC TGG TTC GCC CTC AT-3′. For quantitative RT-PCR, GAPDH was used as endogenous control for the cytoplasmic RNA, while 18S RNA was selected as endogenous control for the nuclear RNA.
Western blot analysis
Western blot analysis was performed as previously described32. The antibodies used in this study were MMP2 (Bioworld, BS1236, 1:500), FN (Novus, NBP1-91258, 1:500), E-Cad (Bioworld, 3195S, 1:500), ALKBH5 (Abcam, ab69325, 1:500), Snail (Bioworld, BS1853, 1:500), ZEB1 (Millipore, ABD53, 1:1000), eEF-1 (Proteintech, 11402–1-AP, 1:500), eEF-2 (Proteintech, 20107–1-AP, 1:500), YTHDF1 (Abcam, ab99080, 1:500), and GAPDH (BOSTER, BM3876, 1:1000). The uncropped gel images are shown in Supplementary Figs 7 and 8.
LC–MS/MS assay
mRNA was purified from the total RNA using oligo dT magnetic beads. About 200 ng of purified mRNAs were incubated with nuclease P1 (0.5 U, Sigma) in 25 -μl reaction system containing 10 mM NH4OAc (pH = 5.3) at 42 °C for 1 h, followed by addition of NH4HCO3 (1 M, 3 μL) and alkaline phosphatase (1 μL, 1 U/μL; Sigma) and incubation at 37 °C for 2 h. After neutralized by 1 μl HCl (3 M), samples were diluted to 50 μl and filtered by 0.22 μm filter (Millipore). All samples (10 μl for each injection) were separated by a C18 column (Agilent) using reverse-phase ultra-performance liquid chromatography and analyzed by an Agilent 6410 QQQ triple-quadrupole LC mass spectrometer using a positive electrospray ionization mode. All nucleosides were quantified by use of retention time and ion mass transitions of 268.0 to 136.0 (A) and 282.1 to 150.0 (m6A). Quantification was calculated using standard curves from standards running in the same batch. Ratio of m6A to A was calculated based on calibration curves.
Dot-blot assay
mRNA was enriched using GenElute messenger RNA (mRNA) Miniprep Kit (Sigma‐Aldrich) and RiboMinus Transcriptome Isolation Kit (human/mouse; ThermoFisher Scientific). After 5 min of denaturation at 70 °C, equal amounts of serial-diluted mRNA were added into an Amersham Hybond N + membrane (GE Healthcare) and cross-linked into auto-cross-linker three times under auto-cross-linking mode. Membrane was blocked with 5% milk in 1× PBST for 1 h at room temperature and then was inducted with anti‐m6A antibody (SySy) overnight (4 °C). Membrane was washed according to the standard protocol. Horseradish peroxidase-conjugated anti-rabbit immunoglobulin G (Cell Signaling Technology) was diluted 1:5000 and incubated with the membranes for 1 h at room temperature. Membranes were developed with a 3,3′‐diaminobenzidine peroxidase substrate kit (Yeasen Biotechnology, Shanghai, China) to detect the signal.
m6A sequencing (m6A-seq) and data analysis
The total polyadenylated RNA was isolated from HeLa cells treated with or without 10 ng/ml TGF-β for 3 days using TRIZOL reagent followed by isolation through FastTrack MAGMaxi mRNA isolation kit (Invitrogen). RNA fragmentation, m6A-seq, and library preparation were performed according to the manufacturer’s instructions and previously published protocol7. NEBNext Ultra Directional RNA Library Prep Kit (New England BioLabs, Ipswich, MA, USA) was used for library preparation. Each experiment was conducted with two biological replicates. m6A-seq data were analyzed according to protocols described before7. Significant peaks with FDR < 0.05 were annotated to RefSeq database (hg19). Sequence motifs were identified by using Homer. Gene expression was calculated by Cufflinks using sequencing reads from input samples. Cuffdiff was used to find DE genes.
m6A-RT-PCR
m6A-RT-PCR was conducted according to previously described protocol with a slight modification20. Briefly, the total RNA was extracted using Trizol and fragmented by RNA fragmentation reagents (Thermo, AM8740) or not. After saving 50 ng of the total RNA as input, the remaining RNAs (2 μg) were used for m6A-immunoprecipitation with m6A antibody (Synaptic Systems) in 500 μL of IP buffer (150 mM NaCl, 0.1% NP-40, 10 mM Tris, pH 7.4, 100 U RNase inhibitor) to obtain m6A pull down portion (m6A IP portion). m6A RNAs were immunoprecipitated with Dynabeads® Protein A (ThermoFisher Scientific) and eluted twice with elution buffer (5 mM Tris-HCL pH 7.5, 1 mM EDTA pH 8.0, 0.05% SDS, 20 mg/ml Proteinase K). m6A IP RNAs were recovered by ethanol precipitation, and RNA concentration was measured with Qubit® RNA HS Assay Kit (ThermoFisher Scientific). Then 2 ng of the total RNA and m6A IP RNA were used as templates in qRT-PCR, as described above. RT-PCR for Snail was conducted using the following primers: 5′UTR, forward 5′-CGG CCT AGC GAG TGG TTC T-3′ and reverse 5′-TCG AGG CAC TGG GGT CG-3′ CDS, forward 5′-GCC AAT TGC CTG GGC CTC C-3′ and reverse 5′-GGC TGG GAG CCT TTC CCA CTG-3′ 3′UTR, forward 5′-TGG ATA CAG CTG CTT TGA GC-3′ and reverse 5′-TGT GAG GGG AGT GGG GTC-3′. Housekeeping gene HPRT1 was chosen as internal control since HPRT1 mRNA did not have m6A peaks from m6A profiling data6.
RIP–RT-PCR
For RNA immunoprecipitation, cells were irradiated twice with 400 mJ/cm2 at 254 nm by Stratalinker on ice and lysed in high salt lysis buffer (300 mM NaCl, 0.2% NP-40, 20 mM Tris-HCl PH 7.6, 0.5 mM DTT, protease inhibitor cocktail (1 tablet/50 ml), and RNase inhibitor (1:200)) at 4 °C for 30 min. After treatment with, or without, 1 U RNase T1 for 15 min at 24 °C, 10% supernatant was collected as input. Remaining supernatant was incubated with anti-YTHDF1 antibody, or IgG-conjugated protein A/G Magnetic Beads in 500 mL 1× IP buffer supplemented with RNase inhibitors at 4 °C overnight. Bound RNAs were immunoprecipitated with beads and treated with 100 µl of elution buffer (5 mM Tris-HCL pH 7.5, 1 mM EDTA pH 8.0, 0.05% SDS, 20 mg/ml Proteinase K) for 2 h at 50 °C, and RNA was recovered with phenol:chloroform extraction followed by ethanol precipitation. IP enrichment ratio of a transcript was calculated as ratio of its amount in IP to that in the input, yielded from same amounts of cells.
RNA stability
To measure RNA stability in HeLa wild-type and Mettl3 Mut/− cells, actinomycin D (Act-D, Catalog #A9415, Sigma, USA) at 5 μg/ml was added to cells. After incubation at the indicated times, cells were collected, and RNA was isolated for real-time PCR. Half-life (t1/2) of precursor and mature Snail mRNA were calculated using ln2/slope and GAPDH was used for normalization.
Protein stability
To measure protein stability, HeLa wild-type and Mettl3 Mut/− cells were treated with cycloheximide (CHX, final concentration 100 μg/ml) during indicated times. The expression of Snail was measured through western blot analysis.
Snail promoter activity assay
Promoter activity of Snail in HeLa wild-type and Mettl3 Mut/− cells was measured according to our previously described protocol62. Briefly, cells were transfected with pGL3-Basic-Snail-luc containing sequence −797/ + 59 of Snail promoter. Transfection efficiency was normalized by co-transfection with pRL-TK. Transcriptional activity was determined by a luminometer, using a dual-luciferase assay kit. The results were displayed as the ratios between the activity of reporter plasmid and pRL-TK.
Luciferase reporter assay
Luciferase assay was performed using reporter lysis buffer (Catalog #E3971, Promega, USA) and luciferase assay reagent according to the manufacturer’s instructions. Briefly, HeLa wild-type and Mettl3 Mut/− cells were transfected with pmiRGLO, pmiRGLO-WT-3′UTR, pmiRGLO-Mut1-3′UTR, or pmiRGLO-Mut2-3′UTR in a 12-well plate. After transfection for 6 h, each cell line was re-seeded into a 96-well plate. After 24 h incubation, cells were analyzed with the Dual-Glo Luciferase Assay system (Promega). Renilla Luciferase (R-luc) was used to normalize firefly luciferase (F-luc) activity to evaluate reporter translation efficiency.
Experimental animals and xenograft models
Female BALB/c nude mice (4-week-old) were purchased from Sun Yat-sen University (Guangzhou, China) Animal Center and raised under pathogen-free conditions. All animal experiments complied with Zhongshan School of Medicine Policy on Care and Use of Laboratory Animals. For subcutaneous transplanted model, sh-control and sh-METTL3 Huh7 cells (5 × 106 per mouse, n = 5 for each group) were diluted in 200 μL of PBS + 200 μL Matrigel (BD Biosciences) and subcutaneously injected into immunodeficient female mice to investigate tumor growth. When tumor volume, in each group, reached ~100 mm3, mice were killed, and tumors were removed and weighed for use in histology and further studies. The tumor volume was calculated using the formula V = 1/2 × larger diameter × (smaller diameter)2.
For in vivo lung metastasis model, mice were injected with sh-control and sh-METTL3 Huh7 cells, or HeLa WT, Mettl3 Mut/− HeLa, HeLaSnail , and Mettl3 Mut/− HeLaSnail stable cells (1 × 106 per mouse, n = 10 for each group). Eight weeks after injection, mice were killed and metastatic lung tumors were analyzed.
Immunohistochemistry (IHC)
Immunohistochemistry (IHC) was performed to measure expression of target protein according to our previous study63.
Kaplan–Meier survival analysis and clinical expression analysis
Kaplan–Meier plotter (http://kmplot.com/analysis/) is a comprehensive online platform that can assess the effect of 54,675 genes on survival based on 10,293 cancer samples64. We used Kaplan–Meier plotter to assess the prognostic value of METTL3, YTHDF1, and SNAI1 expression in patients with liver cancers. mRNA expression of METTL3 and YTHDF1 in cancer tissues and matched adjacent normal tissues of liver cancers were obtained from TCGA (The Cancer Genome Atlas) database. METTL3 expression in HCC tumor tissues (n = 35), liver cirrhosis tissues (n = 13) and normal liver tissues (n = 10) from Oncomine database (Wurmbach liver cancers). mRNA expression of YTHDF1 in liver cancers of T1 (n = 153), T2 (n = 77), T3 (n = 65), and T4 (n = 13) stages from TCGA database.65
Tissue microarray analysis
The protein expression of Mettl3 and Snail in 100 cases of liver cancer patients by use of tissue microarray (CS03–01–008, Alenabio, Xian, Shanxi, China) according to our previous study66. The clinical slides of Mettl3 and Snail were analyzed separately by two pathologists without knowing the patients’ clinical information. The intensity was scored on a scale of 0–3 as negative (0), weak (1), medium (2), or strong (3). The extent of the staining, defined as the percentage of positive stained areas of tumor cells per the whole-tumor area, was scored on a scale of 0 (0%), 1 (1–25%), 2 (26–50%), 3 (51–75%), and 4 (76–100%). An overall protein expression score (overall score range, 0– 12) was calculated by multiplying the intensity and positivity scores. For statistical purposes, the staining score was further categorized as low (0–5), medium (6–8), and high (9–12).
Statistical analyses
Data were reported as mean ± SD from at least three independent experiments unless otherwise specified. Values for capillary tube formation and luciferase activity assays are from three independent experiments that were performed in duplicate. Data were analyzed by two-tailed unpaired Student’s t test between two groups and by one-way ANOVA followed by Bonferroni test for multiple comparison. Statistical analysis was carried out using SPSS 16.0 for Windows. All statistical tests were two sided. A p-value of < 0.05 was considered to be statistically significant. Data are presented as means ± SD from three independent experiments. *p < 0.05, **p < 0.01. NS, no significant.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgements
This research was supported by the National Natural Science Foundation of China (Grant nos. 81673454, 81672608, 31801197, and 81572270), the Guangdong Natural Science Funds for Distinguished Young Scholar (No. 2014A030306025), the Guangdong Province Key Laboratory of Malignant Tumor Epigenetics and Gene Regulation (2017B030314026), and the Fundamental Research Funds for the Central Universities (Sun Yat-sen University) (No. 16ykpy09). We thank the help of Miss Hailing Shi, Mr Phillip Hsu, Dr Ziyang Hao, Dr Boxuan Zhao, Mr Tong Wu, and Mr Lisheng Zhang at University of Chicago for experimental skills.
Author contributions
Conception and design: H.W., C.H., Y.W., J.D. and G.L.; acquisition of the data: X.L., H.W., Y.W., G.C., J. L, F.C.; analysis and interpretation of the data: H.W., G.L. and C.H.; writing, review, and/or revision of the paper: H.W., C.H., J. Li, J.T. and S.L.
Data availability
The high-throughput m6A sequencing data have been deposited in the GEO database under the accession code GSE112795. The data underlying Figs 1h, 1I, 6E~G, and 6I~K referenced during the study are available in a public repository from the TCGA website. All the other data supporting the findings of this study are available within the article and its Supplementary Information files and from the corresponding author upon reasonable request. A reporting summary for this article is available as a Supplementary Information file.
Competing interests
The authors declare no conflict of interest.
Footnotes
This article has been retracted. Please see the retraction notice for more detail: https://doi.org/10.1038/s41467-023-43307-x
This article has been retracted. Please see the retraction notice for more detail: https://doi.org/10.1038/s41467-023-43307-x
Journal peer review information: Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Xinyao Lin, Guoshi Chai, Yingmin Wu.
Supplementary information
Supplementary Information accompanies this paper at 10.1038/s41467-019-09865-9.
References
- 1.Perry JK, Kelley DE. Existence of methylated messenger RNA in mouse L cells. Cell. 1974;1:37–42. doi: 10.1016/0092-8674(74)90153-6. [DOI] [Google Scholar]
- 2.Machnicka MA, et al. MODOMICS: a database of RNA modification pathways–2013 update. Nucleic Acids Res. 2013;41:D262–D267. doi: 10.1093/nar/gks1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dominissini D, et al. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature. 2012;485:201–206. doi: 10.1038/nature11112. [DOI] [PubMed] [Google Scholar]
- 4.Jia G, et al. N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat. Chem. Biol. 2011;7:885–887. doi: 10.1038/nchembio.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao BS, et al. m6A-dependent maternal mRNA clearance facilitates zebrafish maternal-to-zygotic transition. Nature. 2017;542:475–478. doi: 10.1038/nature21355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang X, et al. N6-methyladenosine-dependent regulation of messenger RNA stability. Nature. 2014;505:117–120. doi: 10.1038/nature12730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang X, et al. N(6)-methyladenosine modulates messenger RNA translation efficiency. Cell. 2015;161:1388–1399. doi: 10.1016/j.cell.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fu Y, et al. N6-methyldeoxyadenosine marks active transcription start sites in Chlamydomonas. Cell. 2015;161:879–892. doi: 10.1016/j.cell.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang G, et al. N6-methyladenine DNA modification in Drosophila. Cell. 2015;161:893–906. doi: 10.1016/j.cell.2015.04.018. [DOI] [PubMed] [Google Scholar]
- 10.Liu J, et al. A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat Chem. Biology. 2014;10:93–95. doi: 10.1038/nchembio.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ping XL, et al. Mammalian WTAP is a regulatory subunit of the RNA N6-methyladenosine methyltransferase. Cell Res. 2014;24:177–189. doi: 10.1038/cr.2014.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou J, et al. Dynamic m(6)A mRNA methylation directs translational control of heat shock response. Nature. 2015;526:591–594. doi: 10.1038/nature15377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roundtree IA, Evans ME, Pan T, He C. Dynamic RNA modifications in gene expression regulation. Cell. 2017;169:1187–1200. doi: 10.1016/j.cell.2017.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yue Y, Liu J, He C. RNA N6-methyladenosine methylation in post-transcriptional gene expression regulation. Genes Dev. 2015;29:1343–1355. doi: 10.1101/gad.262766.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiang Y, et al. RNA m6A methylation regulates the ultraviolet-induced DNA damage response. Nature. 2017;543:573–576. doi: 10.1038/nature21671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lence T, et al. m6A modulates neuronal functions and sex determination in Drosophila. Nature. 2016;540:242–247. doi: 10.1038/nature20568. [DOI] [PubMed] [Google Scholar]
- 17.Yoon KJ, et al. Temporal control of mammalian cortical neurogenesis by m(6)A methylation. Cell. 2017;171:877–889. doi: 10.1016/j.cell.2017.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang C, et al. m(6)A modulates haematopoietic stem and progenitor cell specification. Nature. 2017;549:273–276. doi: 10.1038/nature23883. [DOI] [PubMed] [Google Scholar]
- 19.Li HB, et al. m(6)A mRNA methylation controls T cell homeostasis by targeting the IL-7/STAT5/SOCS pathways. Nature. 2017;548:338–342. doi: 10.1038/nature23450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Z, et al. FTO plays an oncogenic role in acute myeloid leukemia as a N6-methyladenosine RNA demethylase. Cancer Cell. 2017;31:127–141. doi: 10.1016/j.ccell.2016.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cui Q, et al. m6A RNA methylation regulates the self-renewal and tumorigenesis of glioblastoma stem cells. Cell Rep. 2017;18:2622–2634. doi: 10.1016/j.celrep.2017.02.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Su R, et al. R-2HG exhibits anti-tumor activity by targeting FTO/m(6)A/MYC/CEBPA signaling. Cell. 2018;172:90–105. doi: 10.1016/j.cell.2017.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang S, et al. m6A demethylase ALKBH5 maintains tumorigenicity of glioblastoma stem-like cells by sustaining FOXM1 expression and cell proliferation program. Cancer Cell. 2017;31:591–606.e596. doi: 10.1016/j.ccell.2017.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang C, et al. Hypoxia induces the breast cancer stem cell phenotype by HIF-dependent and ALKBH5-mediated mϖA-demethylation of NANOG mRNA. Proc. Natl Acad. Sci. USA. 2016;113:E2047–E2056. doi: 10.1073/pnas.1602883113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331:1559–1564. doi: 10.1126/science.1203543. [DOI] [PubMed] [Google Scholar]
- 26.Thiery JP, Acloque H, Huang RYJ, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 27.Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2014;15:178–196. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nieto MA, Huang RY, Jackson RA, Thiery JP. Emt: 2016. Cell. 2016;166:21–45. doi: 10.1016/j.cell.2016.06.028. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y, Shi J, Chai K, Ying X, Zhou BP. The role of Snail in EMT and Tumorigenesis. Curr. Cancer Drug Targets. 2013;13:963–972. doi: 10.2174/15680096113136660102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tam WL, Weinberg RA. The epigenetics of epithelial-mesenchymal plasticity in cancer. Nat. Med. 2013;19:1438–1449. doi: 10.1038/nm.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin YW, et al. The SNAG domain of Snail1 functions as a molecular hook for recruiting lysine-specific demethylase 1. EMBO J. 2010;29:1803–1816. doi: 10.1038/emboj.2010.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dong C, et al. Interaction with Suv39H1 is critical for Snail-mediated E-cadherin repression in breast cancer. Oncogene. 2013;32:1351–1362. doi: 10.1038/onc.2012.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Millanes-Romero A, et al. Regulation of heterochromatin transcription by Snail1/LOXL2 during epithelial-to-mesenchymal transition. Mol. Cell. 2013;52:746–757. doi: 10.1016/j.molcel.2013.10.015. [DOI] [PubMed] [Google Scholar]
- 34.Derynck R, Muthusamy BP, Saeteurn KY. Signaling pathway cooperation in TGF-beta-induced epithelial-mesenchymal transition. Curr. Opin. Cell Biol. 2014;31:56–66. doi: 10.1016/j.ceb.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yue Y, et al. VIRMA mediates preferential m(6)A mRNA methylation in 3’UTR and near stop codon and associates with alternative polyadenylation. Cell Disco. 2018;4:10. doi: 10.1038/s41421-018-0019-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shen B, et al. Generation of gene-modified mice via Cas9/RNA-mediated gene targeting. Cell Res. 2013;23:720–723. doi: 10.1038/cr.2013.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zheng G, et al. ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol. Cell. 2013;49:18–29. doi: 10.1016/j.molcel.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bertero Alessandro, Brown Stephanie, Madrigal Pedro, Osnato Anna, Ortmann Daniel, Yiangou Loukia, Kadiwala Juned, Hubner Nina C., de los Mozos Igor Ruiz, Sadée Christoph, Lenaerts An-Sofie, Nakanoh Shota, Grandy Rodrigo, Farnell Edward, Ule Jernej, Stunnenberg Hendrik G., Mendjan Sasha, Vallier Ludovic. The SMAD2/3 interactome reveals that TGFβ controls m6A mRNA methylation in pluripotency. Nature. 2018;555(7695):256–259. doi: 10.1038/nature25784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Puisieux A, Brabletz T, Caramel J. Oncogenic roles of EMT-inducing transcription factors. Nat. Cell Biol. 2014;16:488–494. doi: 10.1038/ncb2976. [DOI] [PubMed] [Google Scholar]
- 40.Meyer KD, et al. Comprehensive analysis of mRNA methylation reveals enrichment in 3’ UTRs and near stop codons. Cell. 2012;149:1635–1646. doi: 10.1016/j.cell.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schwartz S, et al. Perturbation of m6A writers reveals two distinct classes of mRNA methylation at internal and 5’ sites. Cell Rep. 2014;8:284–296. doi: 10.1016/j.celrep.2014.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee JH, et al. A20 promotes metastasis of aggressive basal-like breast cancers through multi-monoubiquitylation of Snail1. Nat. Cell Biol. 2017;19:1260–1273. doi: 10.1038/ncb3609. [DOI] [PubMed] [Google Scholar]
- 43.Zhang K, et al. The collagen receptor discoidin domain receptor 2 stabilizes SNAIL1 to facilitate breast cancer metastasis. Nat. Cell Biol. 2013;15:677–687. doi: 10.1038/ncb2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beretta L, Gingras AC, Svitkin YV, Hall MN, Sonenberg N. Rapamycin blocks the phosphorylation of 4E-BP1 and inhibits cap-dependent initiation of translation. EMBO J. 1996;15:658–664. doi: 10.1002/j.1460-2075.1996.tb00398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Evdokimova V, et al. Translational activation of snail1 and other developmentally regulated transcription factors by YB-1 promotes an epithelial-mesenchymal transition. Cancer Cell. 2009;15:402–415. doi: 10.1016/j.ccr.2009.03.017. [DOI] [PubMed] [Google Scholar]
- 46.Kapur M, Monaghan CE, Ackerman SL. Regulation of mRNA translation in neurons-A matter of life and death. Neuron. 2017;96:616–637. doi: 10.1016/j.neuron.2017.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Szasz AM, et al. Cross-validation of survival associated biomarkers in gastric cancer using transcriptomic data of 1,065 patients. Oncotarget. 2016;7:49322–49333. doi: 10.18632/oncotarget.10337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barbieri I, et al. Promoter-bound METTL3 maintains myeloid leukaemia by m(6)A-dependent translation control. Nature. 2017;552:126–131. doi: 10.1038/nature24678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen Mengnuo, Wei Lai, Law Cheuk-Ting, Tsang Felice Ho-Ching, Shen Jialing, Cheng Carol Lai-Hung, Tsang Long-Hin, Ho Daniel Wai-Hung, Chiu David Kung-Chun, Lee Joyce Man-Fong, Wong Carmen Chak-Lui, Ng Irene Oi-Lin, Wong Chun-Ming. RNA N6-methyladenosine methyltransferase-like 3 promotes liver cancer progression through YTHDF2-dependent posttranscriptional silencing of SOCS2. Hepatology. 2018;67(6):2254–2270. doi: 10.1002/hep.29683. [DOI] [PubMed] [Google Scholar]
- 50.Visvanathan A, et al. Essential role of METTL3-mediated m(6)A modification in glioma stem-like cells maintenance and radioresistance. Oncogene. 2018;37:522–533. doi: 10.1038/onc.2017.351. [DOI] [PubMed] [Google Scholar]
- 51.Vu LP, et al. The N(6)-methyladenosine (m(6)A)-forming enzyme METTL3 controls myeloid differentiation of normal hematopoietic and leukemia cells. Nat. Med. 2017;23:1369–1376. doi: 10.1038/nm.4416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lin SB, Choe J, Du P, Triboulet R, Gregory RI. The m(6)A methyltransferase METTL3 promotes translation in human cancer cells. Mol. Cell. 2016;62:335–345. doi: 10.1016/j.molcel.2016.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li X, et al. The M6A methyltransferase METTL3: acting as a tumor suppressor in renal cell carcinoma. Oncotarget. 2017;8:96103–96116. doi: 10.18632/oncotarget.21726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ma JZ, et al. METTL14 suppresses the metastatic potential of hepatocellular carcinoma by modulating N(6) -methyladenosine-dependent primary MicroRNA processing. Hepatology. 2017;65:529–543. doi: 10.1002/hep.28885. [DOI] [PubMed] [Google Scholar]
- 55.Zhao BS, Roundtree IA, He C. Post-transcriptional gene regulation by mRNA modifications. Nat. Rev. Mol. Cell Biol. 2017;18:31–42. doi: 10.1038/nrm.2016.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Alarcon CR, et al. HNRNPA2B1 is a mediator of m(6)A-dependent nuclear RNA processing events. Cell. 2015;162:1299–1308. doi: 10.1016/j.cell.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fustin JM, et al. RNA-methylation-dependent RNA Processing controls the speed of the circadian clock. Cell. 2013;155:793–806. doi: 10.1016/j.cell.2013.10.026. [DOI] [PubMed] [Google Scholar]
- 58.Baragana B, et al. A novel multiple-stage antimalarial agent that inhibits protein synthesis (vol 522, pg 315, 2015) Nature. 2016;537:122–122. doi: 10.1038/nature18280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zheng XF, et al. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature. 2015;527:525. doi: 10.1038/nature16064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Krebs AM, et al. The EMT-activator Zeb1 is a key factor for cell plasticity and promotes metastasis in pancreatic cancer. Nat. Cell Biol. 2017;19:518. doi: 10.1038/ncb3513. [DOI] [PubMed] [Google Scholar]
- 61.Fischer KR, et al. Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature. 2015;527:472–476. doi: 10.1038/nature15748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jiang GM, et al. Histone deacetylase inhibitor induction of epithelial-mesenchymal transitions via up-regulation of Snail facilitates cancer progression. Biochim. Biophys. Acta. 2013;1833:663–671. doi: 10.1016/j.bbamcr.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 63.Chen ZJ, et al. Activation of GPER suppresses epithelial mesenchymal transition of triple negative breast cancer cells via NF-kappaB signals. Mol. Oncol. 2016;10:775–788. doi: 10.1016/j.molonc.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gyorffy B, Surowiak P, Budczies J, Lanczky A. Online survival analysis software to assess the prognostic value of biomarkers using transcriptomic data in non-small-cell lung cancer. PloS One. 2013;8:e82241. doi: 10.1371/journal.pone.0082241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vasaikar SV, Straub P, Wang J, Zhang B. LinkedOmics: analyzing multi-omics data within and across 32 cancer types. Nucleic Acids Res. 2018;46:D956–D963. doi: 10.1093/nar/gkx1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu, Q. et al. Epigenetic down regulation of G protein-coupled estrogen receptor (GPER) functions as a tumor suppressor in colorectal cancer. Mol. Cancer16, 87 (2017). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The high-throughput m6A sequencing data have been deposited in the GEO database under the accession code GSE112795. The data underlying Figs 1h, 1I, 6E~G, and 6I~K referenced during the study are available in a public repository from the TCGA website. All the other data supporting the findings of this study are available within the article and its Supplementary Information files and from the corresponding author upon reasonable request. A reporting summary for this article is available as a Supplementary Information file.