Abstract
The neuronal nucleus plays a vital role in information processing, but whether it supports computational functions such as paired-pulse facilitation, comparable to synapses, is unclear. Ca2+-dependent movement of calmodulin (CaM) to the nucleus is highly responsive to Ca2+ entry through L-type channels and promotes activation of the transcription factor CREB (cAMP-responsive element binding protein) through phosphorylation by CaM-sensitive kinases. We characterized key features of this CaM translocation and its possible role in facilitation of nuclear signaling. Nuclear CaM was elevated within 15 s of stimulus onset, preceding the first signs of CREB phosphorylation in hippocampal pyramidal neurons. Depolarization-induced elevation of nuclear CaM also was observed in cerebellar granule cells, neocortical neurons, and dentate gyrus granule cells. Nuclear translocation of CaM was not blocked by disruption of actin filaments or microtubules, or by emptying endoplasmic reticulum Ca2+ stores with thapsigargin. Translocation of fluorescently tagged CaM was prevented by fusing it with the Ca2+/CaM binding peptide M13, suggesting that nuclear CaM accumulation depends on association with endogenous Ca2+/CaM binding proteins. To determine whether increased nuclear [CaM] might influence subsequent nuclear signal processing, we compared responses to two consecutive depolarizing stimuli. After a weak “priming” stimulus that caused CaM translocation, CREB phosphorylation caused by a subsequent stimulus was significantly faster, more sensitive to Ca2+ elevation, and less specifically dependent on Ca2+ influx through L-type channels. CaM translocation not only supports rapid signaling to the nucleus, but also could provide a “memory” for facilitatory effects of repeated neural activity, seen in altered phosphorylated CREB dynamics and Ca2+ channel dependence.
The information-processing repertoire of neurons would be greatly expanded if the nucleus was able to respond to successive stimuli in a supra-additive way. This capability would support recognition of multiple closely spaced inputs beyond simple integration, computations like those synapses perform (e.g., paired-pulse facilitation). Because of the speed required, a plausible candidate for such input pattern decoding is the especially rapid signaling cascade controlling the fast activation kinetics of the key transcription factor CREB (cAMP-responsive element binding protein). This pathway is initiated in hippocampal pyramidal cells by depolarization-induced opening of L-type Ca2+ channels (1–7) and calmodulin (CaM) mobilization to the nucleus (6, 8, 9), supporting activation of CaM kinase kinase (CaMKK) and CaM kinase IV-mediated CREB phosphorylation at Ser-133 (4, 10–23). The effects of the fast CaM kinase pathway can be prolonged sufficiently in vitro and in vivo to control gene expression (10, 20, 23), likely through cooperation with the calcineurin (10, 24) and mitogen-associated protein kinase (MAPK) (22, 25) pathways. But there are few obvious functional advantages for this signaling pathway coupling to gene expression to be so fast in onset (tens of seconds), particularly because only much more prolonged CREB phosphorylation (tens of minutes) suffices for effective gene expression (10, 25). We therefore considered that this pathway may in part be designed to participate in the fast nuclear processing of multiple closely spaced inputs.
What are the likely relevant patterns of neuronal activity in vivo that would serve as the input for the candidate fast nuclear processor? Multielectrode recordings from the hippocampus of awake, freely moving rats have revealed that the dominant cell type in the hippocampus (complex-spike cells corresponding to the pyramidal neurons) is inactive for the vast majority of the time (26). Only when the rat localizes to a cell's place field is activity seen, often consisting of brief clusters of spikes separated by intervals with little appreciable activity (e.g., ≈10-s bursts at 5–10 Hz separated by 45–90 s) (27). These experiments may be biased, if at all, toward recording from the more active cells (because of the necessity of seeking active cells during positioning of the electrodes, and for practical purposes elimination of many common low-frequency firing neurons) (28). The best information at present therefore indicates that the natural state of the average hippocampal pyramidal cell is inactivity (≪1 Hz), insufficient to drive strong nuclear signaling (4), except when the animal is moving in the vicinity of the cell's place field, at which time activity comes in short separated bursts. An appropriate experimental protocol might then involve starting from a physiologically quiescent baseline and providing multiple (e.g., two) spaced mild stimuli.
What could be the signature of altered nuclear signaling that would be detectable experimentally? We might expect larger or smaller responses to the second pulse, by analogy with paired-pulse facilitation and depression. The signaling could also be faster or slower, although it would seem a challenge to make this fast signaling even faster. More interestingly, it is possible that the information content of the signaling pathway from membrane to CREB could be altered. The specific route of Ca2+ entry rather than bulk Ca2+ elevation is in many cases the relevant parameter in nuclear signaling (6, 7, 29), and one recent study has clearly implicated physical coupling between the L-type Ca2+ channel and CaM as part of the mechanism for generating this specificity in phosphorylated CREB (pCREB) formation (7). Signaling to CREB therefore can communicate information about activity of specific membrane channels and because L-type Ca2+ channels respond selectively to certain types of electrical activity (30), signaling to CREB also can carry specific information about electrical activity (4). This specificity could, in principle, also be altered in an activity-dependent way.
One of the most striking characteristics of the fast activity-dependent CREB phosphorylation in hippocampal CA3–CA1 pyramidal neurons is this absolute dependence on L-type Ca2+ channels and N-methyl-d-aspartate receptors (4, 21, 31). When these channels are blocked, other channel types generate similarly robust increases in somatic and nuclear Ca2+ but consistently fail to give any significant CREB phosphorylation (6). This fact demonstrates that fast CREB phosphorylation, in these cells, simply cannot be driven by elevations in bulk nuclear Ca2+ alone. Still, in neocortical neurons in the presence of blockers of N-methyl-d-aspartate receptors and L-type channels a small fast component of pCREB can still be seen (7). These data indicate that, in principle, conditions can exist in which the strict channel-type dependence can be relaxed. It would be of computational significance if CA3–CA1 pyramidal cells could be temporarily converted to this mode by prior activity, as the resulting nuclear events would be more sensitive but less specifically attuned to surface membrane events.
Methods
Neurons were taken from the CA3–CA1 region of the hippocampi of 1- to 2-day-old rat pups as described (4). Cells were similarly cultured from the hippocampal dentate gyrus, cerebellar cortex, and neocortex (6–8 days in vitro for dentate gyrus, 8–10 days in vitro for all other neurons). Because neurons are highly active because of extensive recurrent connections in dissociated culture and both pCREB formation and CaM translocation take ≈2 h to reverse (6), to obtain a physiological baseline neurons were preincubated ≈2 h at room temperature in Tyrode solution containing 129 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 30 mM glucose, 25 mM Hepes, 0.1 mM glycine, 0.001 mM tetrodotoxin (TTX), pH 7.35. Where indicated, solutions also contained nifedipine (5 μM) or D-AP5 (50 μM). Immediately after stimulation with either 20 mM or 90 mM K+ isotonic Tyrode, cells were fixed for ≈30 min with 4% paraformaldehyde in PBS (at 4°C and in 4 mM EGTA except where otherwise indicated). Neurons were then washed twice with PBS containing 100 μM glycine and permeabilized for 5 min in block solution (PBS with 4% goat serum) containing 0.1% Triton X-100. We have also used an overnight permeabilization protocol with 0.4% saponin (Sigma) with comparable results. The cells were then washed and stained for 1–2 h in block solution containing anti-pCREB polyclonal [a marker of CREB activation (32, 33); 1:200] and anti-CaM mAb (1:200) (both from Upstate Biotechnology, Lake Placid, NY). Nuclear fluorescence intensities were determined with confocal digital imaging as described (6). Ca2+ measurements were conducted with fura-2 as described (22). Cyan-fluorescent protein (CFP)–CaM–M13 constructs and mutations were generated by using standard techniques and were transfected by using calcium phosphate as described at in vitro day 7 (6). M13 region mutations were W800A and R812A [amino acid residues corresponding to the smMLCK sequence to prevent Ca2+-CaM binding (34)]. Two successive depolarizing stimuli were used for these experiments, and subsequent CaM localization was determined and quantified by confocal imaging of CFP.
Results
Establishing Experimental Conditions for Studying Fast Signaling.
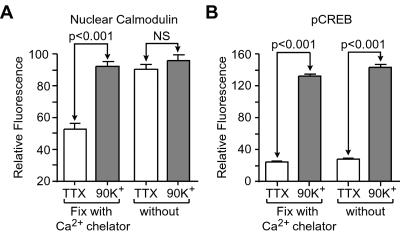
To explore fast nuclear paired-pulse processing, we characterized the onset of the CaM translocation (Figs. 1 and 2). CaM translocation is so fast that fixation conditions may play a role in these experiments (Fig. 1). Previous reports have demonstrated that aldehyde fixation causes an immediate and massive cytosolic Ca2+ increase (35), thus allowing fast Ca2+-dependent processes to proceed during fixation (36). We buffered external Ca2+ in the fixative with 4 mM EGTA to prevent the paraformaldehyde solution from acting as a trigger for CaM translocation. Under these conditions, CaM translocation and CREB phosphorylation were clearly observed in cultured CA3–CA1 hippocampal pyramidal neurons depolarized with 90 mM K+. However, without EGTA in the fixation solution, nuclear CaM was elevated even in the “unstimulated” neurons, occluding the stimulus-evoked increase (Fig. 1). As expected, CREB phosphorylation was unaffected by the composition of the fixation solution, because it is a slower, downstream process. Increasing the temperature of the fixation solution from 4°C to room temperature also increased apparent basal levels of nuclear CaM, particularly when Ca2+ chelators were not present (data not shown).
Figure 1.
Increases in intracellular Ca2+ rapidly induce CaM translocation. (A) A 3-min, 90-mM K+ depolarization significantly increases nuclear CaM in hippocampal pyramidal neurons (P < 0.001). However, the effect is obscured when Ca2+ is not controlled during fixation, as paraformaldehyde itself is a sufficient stimulus to induce CaM translocation. NS, not significant. (B) CREB phosphorylation, a downstream process to CaM translocation, is unaffected by fixation conditions (P < 0.001).
Figure 2.
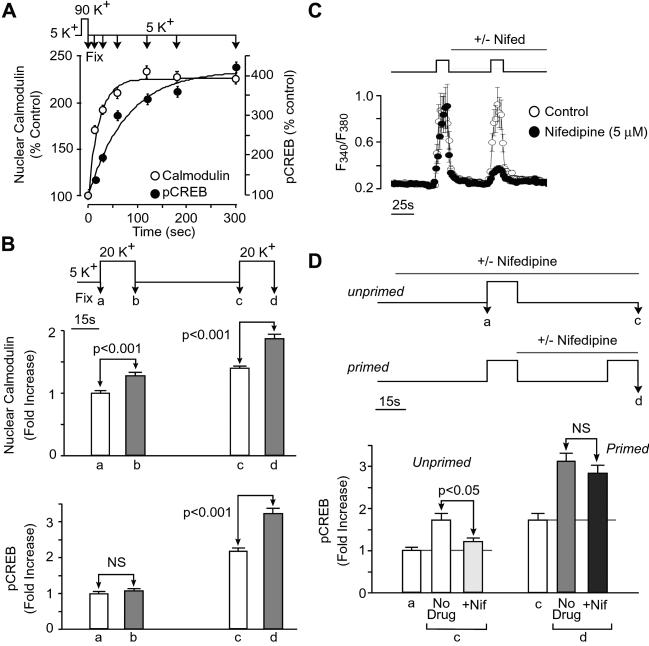
Translocation of CaM into the nucleus potentiates further CREB activation after a subsequent stimulus. (A) Cultured hippocampal neurons were stimulated with 90 mM K+ for 15 s followed by fixation at various time points and then processed for immunocytochemistry using antibodies specific for CaM and 133Ser pCREB. Increases in the nuclear fluorescence for both CaM and pCREB were determined by using confocal microscopy. Significant increases in nuclear CaM (P < 0.001) were observed immediately after the 15-s stimulus, at a time when CREB phosphorylation had not risen significantly (P > 0.05). (B) Paired-pulse facilitation of CREB phosphorylation demonstrated with two 15-s, 20-mM K+ stimuli. In unprimed neurons, as in A, increases in nuclear CaM (P < 0.001), but not CREB phosphorylation (P > 0.05) were detected immediately after a single stimulus. However, after this priming, a significant increase in CREB phosphorylation was found immediately after a second depolarizing stimulus (P < 0.001), indicating a much more rapid activation of CREB, possibly through signaling by previously translocated CaM. (C) Fura-2 measurements of intracellular Ca2+ transients produced by 20 mM K+ pulses (n = 21 cell bodies). The ratio of Ca2+ transients evoked by the first and second 20 K+ challenges was 1.02 ± 0.04. Application of nifedipine indicated that L-type Ca2+ channels were responsible for the bulk of the Ca2+ signal during the second pulse. Similar effects of nifedipine were observed when tested on the first pulse response (data not shown). (D) Effects of blocking L-type Ca2+ channels on increases in pCREB in unprimed and primed neurons. Nifedipine reduced significantly the response to the first stimulus (P < 0.05) but not the second stimulus (P > 0.05). NS, not significant.
Paired-Pulse Facilitation of Nuclear Signaling to CREB.
Using the appropriate Ca2+ chelation during fixation, we next characterized the very early kinetics of this pathway (Fig. 2A). Pyramidal neurons were depolarized with 90 mM K+ for 15 s, then fixed at various time points. Significant increases in nuclear CaM were seen at 15 s, when pCREB had not yet significantly increased (consistent with the idea that CaM translocation can participate in even the fastest CREB activation). We reasoned that because elevation of nuclear CaM slowly reverses over tens of minutes to hours after a single stimulus (6), translocation of CaM after an initial stimulus might influence nuclear signal processing of a subsequent stimulus. To test this hypothesis, based on the reasoning given earlier, we applied a mild paired-pulse protocol using 20 mM K+ and a spacing of 45 s. As found with the stronger stimulus (Fig. 2A), CaM translocation, but not pCREB, was detectable within 15 s of a single weak stimulus (Fig. 2B). However, after this first stimulus, a marked increase in pCREB was observed within 15 s of the onset of a second stimulus. This extremely fast pCREB increment was not attributable to augmented Ca2+ influx during the second stimulus (Fig. 2C). Rather, it appeared to be a novel form of nuclear paired-pulse facilitation whereby one input primes the next.
Relaxed Channel Specificity in the Primed State.
The increase in speed of CREB phosphorylation might be caused by an event such as the reuse of recently translocated CaM and/or associated molecules. If so, the known dependence of fast pCREB formation on specific Ca2+ entry pathways (L-type channels and N-methyl-d-aspartate receptors) also might be changed, because this strict dependence is likely caused by the unique ability of these channel types to mobilize a CaM complex to the nucleus in pyramidal neurons. Accordingly, we compared the effects of the L-type channel inhibitor nifedipine on “unprimed” and “primed” responses. As expected (6), the increment in pCREB associated with the first stimulus was largely ablated by blockade of L-type channels (Fig. 2D, c). In contrast, the rapid primed CREB phosphorylation caused by the second stimulus remained in the presence of nifedipine (Fig. 2D, d); this finding was particularly striking because L-type channel blockade greatly reduced the second Ca2+ transient, leaving only a small residuum of Ca2+ entry caused by other pathways (Fig. 2C). Further, we have found that in primed neurons, as in neocortical neurons (7), rapid CREB phosphorylation can still proceed at reduced levels where both L-type Ca2+ channels and N-methyl-d-aspartate receptors were blocked (P.G.M., unpublished data). From these results we can conclude that priming greatly increased the Ca2+ sensitivity of the CREB phosphorylation and likely broadened its acceptance of Ca2+ sources. This influence of past activity on nuclear signal processing is consistent with a model in which Ca2+ interacts with recently translocated apo-CaM or CaM complexes to activate the fast CaM kinase IV cascade.
Cellular Diversity of CaM Translocation.
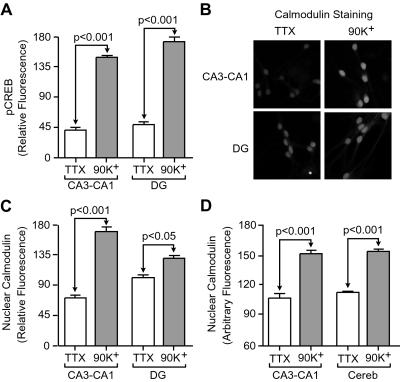
Ca2+-dependent CaM translocation to the nucleus has now been reported by using a variety of techniques in hippocampal pyramidal neurons (6) as well as in cortical neurons (8), sensory ganglion neurons (37), neuroblastoma cells (9), pancreatic acinar cells (38), smooth muscle cells (39, 40), rat basophilic leukemia cells (41), and human embryonic kidney cells (9), indicative of a widely used nuclear signaling pathway. The results in Fig. 2 were obtained with hippocampal pyramidal neurons in cultures from the CA3–CA1 regions. In contrast, little CaM translocation was reported in a study of total hippocampal cultures, likely to include a proportion of dentate granule cells as well as pyramidal neurons (42). Accordingly, we compared dentate gyrus cells with CA3–CA1 pyramidal neurons (Fig. 3 A–C). With similar depolarization-induced CREB phosphorylation (Fig. 3A), CaM translocation in dentate granule cells (Fig. 3 B and C) was less pronounced, albeit significant (P < 0.05). One contributory factor to the difference with CA3–CA1 pyramidal neurons was that the granule cells displayed higher basal levels of nuclear CaM. In contrast, granule cells from cerebellum showed CaM translocation as large as in hippocampal CA3–CA1 neurons, suggesting that variations in the degree of CaM translocation do not strictly depend on cell size. It is interesting to speculate that variations in “basal” levels of nuclear CaM and subsequent variation in CaM translocation dependence of nuclear processes could result from either variations in CaM translocation characteristics in different cell types, or persistent “CaM priming” caused by cells experiencing high recent levels of activity during dissection or in culture. In fact, it appears likely that in some cases Ca2+ entry route specificity can be completely relaxed through one of these mechanisms, as Ca2+ alone was found to generate detectable pCREB in a preparation of partially lysed cells (42).
Figure 3.
Qualitative consistency of CaM translocation after depolarization in various neuronal populations. (A) After a 3-min, 90-mM K+ stimulation, significant increases in CREB phosphorylation were observed in hippocampal neurons taken from region CA3–CA1 (P < 0.001) or dentate gyrus (DG) (P < 0.001). (B and C) In granule cells from the dentate gyrus (DG), significant increases in nuclear CaM also were observed (P < 0.05), although the changes were less pronounced than in CA3–CA1 cells (P < 0.001). (D) Increased nuclear CaM in cerebellar granule cells (Cereb) (P < 0.001), similar in magnitude to that observed in CA3–CA1 pyramidal neurons.
CaM Interacts with a CaM-Binding Molecule for Translocation.
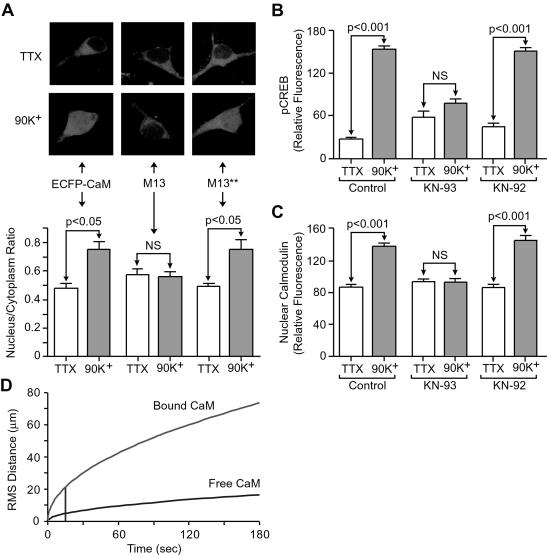
The speed and robustness of CaM translocation raises interesting mechanistic questions. At face value, free diffusion is the simplest explanation, but translocation would be greatly retarded by vigorous CaM buffering within the cytoplasm (39). This finding suggests CaM translocates while complexed with another protein. Such a complex has been previously hypothesized (6) to be of additional theoretical value in stabilizing the Ca2+-bound form of CaM, because thermodynamic cycle analysis dictates that high affinity binding to a Ca2+/CaM-selective protein must greatly increase the stability of Ca2+/CaM and thereby allow the signaling to proceed even in the presence of high affinity Ca2+ buffers. Using various constructs of CaM fused to enhanced CFP (ECFP), we examined whether translocation of CaM would be impaired if Ca2+-dependent interactions were disrupted. In control experiments, ECFP-CaM showed a stimulus-induced increase in nuclear localization (Fig. 4A Left). In contrast, addition of the CaM-binding peptide M13 to the C terminus of the ECFP–CaM eliminated the nuclear increase (Fig. 4A Middle). This inhibition was not found with a modified version of M13 containing mutations at key positions involved in Ca2+-dependent binding to CaM (34) (Fig. 4A Right). Thus, CaM translocation is prevented by a specific interaction with M13 (40), suggesting that normally, Ca2+-dependent association with an endogenous CaM binding protein is necessary to drive nuclear CaM translocation.
Figure 4.
Translocation of CaM into the nucleus depends on its binding to a target protein in a Ca2+-dependent manner. (A) Hippocampal neurons transfected with cDNA encoding for ECFP–CaM exhibit a depolarization-induced nuclear translocation of fluorophore-labeled CaM, indicated by a significant increase in the ratio of nuclear to cytoplasmic fluorescence (P < 0.05). Addition of the Ca2+–CaM binding peptide M13 onto ECFP–CaM prevented translocation. Translocation of ECFP–CaM was restored by mutating the M13 region [W800A, R812A (amino acid residues correspond to the smMLCK sequence)] to prevent Ca2+–CaM binding (34). NS, not significant. (B and C) The CaM kinase inhibitor KN-93 (1 μM) blocked both CREB phosphorylation and CaM translocation (10, 45), suggesting that a CaM kinase (e.g., a CaMKK) may be required for CaM translocation. NS, not significant. (D) How interaction of CaM with a kinase could hasten its translocation to the nucleus. Calculated rms diffusion radius in three dimensions, at room temperature, using a diffusion coefficient (2.5 × 10−9 cm2/s) measured for free CaM in smooth muscle cytoplasm (39). Also plotted is the theoretical corresponding rms radius for the diffusion of a typical large soluble protein (5 × 10−8 cm2/s; e.g., kinase-bound CaM) assuming no binding interactions with cytoplasmic structures, showing greater spread over the same time period.
Among the many possible candidates that may translocate with CaM to the nucleus, we have already excluded certain Ca2+/CaM-dependent kinases (6) and calcineurin (see additional text and Fig. 5, which is published as supporting information on the PNAS web site, www.pnas.org), but ras/MAPK pathway messengers such as the CaM-binding Ras-GRF (43), and isoforms of CaMKK remain viable possibilities. We explored the latter possibility by use of KN-93, a small molecule that specifically binds to and inhibits CaM kinases and CaMKKs solely by interfering with CaM binding (44). Interestingly, KN-93 not only blocked CREB phosphorylation (Fig. 4B) but also completely prevented CaM translocation (Fig. 4C). The inactive homolog KN-92 did neither (10, 45) (Fig. 3C). These data suggest that CaM may require binding to a kinase to move to the nucleus. This signaling pathway, however, does not require an intact cytoskeleton, as disruption of microtubules with nocodozole or actin filaments with cytochalasin D had no effect on CaM translocation or CREB phosphorylation. Intracellular Ca2+ stores were also not required (see additional text and Figs. 6 and 7, which are published as supporting information on the PNAS web site).
Because of the high CaM-binding capacity in cytoplasm (39), formation of a CaM complex would promote CaM translocation to the cell body simply by speeding its diffusion (Fig. 4D). It is interesting to note that based on previous diffusion coefficient measurements in cytoplasm (39), the rms diffusion radius for free CaM would correspond to <5 μm at 15 s; in contrast, a radius of >20 μm can be estimated for a typical soluble protein with no retardation by binding. Thus, on the rapid time scale of CREB phosphorylation, a complex consisting of Ca2+/CaM and another protein could readily reach the cell body, although full translocation into the nucleus may involve an additional nondiffusive, strongly temperature-dependent step (6, 40, 46).
Discussion
A Role for the CaM Pathway in Fast Temporal Processing by the Nucleus.
In hippocampal pyramdical cells, CREB phosphorylation is normally observed only with CaM translocation into the nucleus, a process primarily dependent on activation of L-type calcium channels. However, in the wake of an appropriate priming stimulus, further stimulation causes robust, faster formation of pCREB apparently without the need for further CaM translocation. Further, the restriction for Ca2+ to enter a cell by means of L-type Ca2+ channels for CREB phosphorylation to occur is relaxed. This effect of neuronal priming represents a fast temporal integration of intracellular signals by the nucleus, resulting in altered nuclear signaling (more sensitive and less specific) that could play an important role in the processing underlying memory formation in the hippocampus.
Quantitative Considerations in CaM Signaling.
Because some CaM is constitutively present in the nucleus (6), why might additional CaM be important for controlling nuclear events like CREB phosphorylation? The nuclear CaM kinase cascade of CaMKK and CaM kinase IV steeply depends on CaM, so that even a 2-fold change in free CaM would likely be significant. In turn, stimulus-induced increases in free nuclear CaM are likely to be much greater than 2-fold; because of constitutive interactions with nuclear entities such as histones (47), free CaM represents only a small fraction of total CaM [estimates range from 1/20 to 1/1,000 (48, 49)]. Therefore, simple translocated CaM could greatly stimulate the CaM kinase cascade, during both unprimed and primed responses. Furthermore, if the CaM-binding molecule critical for nuclear CaM translocation is itself a CaMKK, the net effect of the translocated complex on initial CREB phosphorylation would be even more substantial. Needless to say, different cell types in different preparations could show varying dependence on CaM translocation depending on their “basal” free CaM levels, which in turn would likely depend in part on their recent history.
Nature and Significance of CaM Priming.
But what is the priming event itself? Because free nuclear CaM is likely to be limiting, simple persistence of free apo-CaM could greatly enhance the speed and sensitivity (although reduce the specificity) of subsequent responses by responding to nuclear Ca2+, whatever the source. The key priming event also could be an increase in nuclear CaMKK or even the CaMKK-mediated phosphorylation of CaM kinase IV itself. On the other hand, if the CaM-binding molecule in the translocating complex plays a role in MAPK signaling (e.g., Ras-GRF) the priming event also could involve activation of the MAPK pathway to CREB and could underlie reported L-type channel dominance (7, 22) and CaM involvement (7, 22) in slow MAPK signaling to CREB.
A rapidly translocating CaM complex appears to have appropriate quantitative properties to act as a mediator of both rapid nuclear signaling and nuclear memory. Moving Ca2+ sensors from the neighborhood of L-type channels to the nucleus can be thought of as priming the neuron in response to a weak stimulus, altering its response to future stimuli. The consequences for pCREB formation and ultimately, gene expression are both quantitative (sensitizing the system to small Ca2+ signals) and qualitative (allowing participation of a broader range of Ca2+ sources). It appears that the selective communication between L-type channels and CaM not only enables preferential coupling between synaptic depolarizations (as opposed to action potentials) and CREB phosphorylation during an initial round of Ca2+ entry (30), but through priming, also could promote increased recognition of other forms of electrical activity (50) over a subsequent period. It will be of great interest to test for altered primed responses to low frequency or brief electrical stimuli, map out the temporal characteristics (onset and decay) of the priming, determine the types of prepulse stimuli capable of providing the priming, and correlate these findings with the known in vivo patterns of hippocampal pyramidal cell activity.
Supplementary Material
Acknowledgments
We thank Dr. Gang-Yi Wu for participation in the Ca2+ measurements, Takako Mukai for technical assistance, and Harald Reuter for comments on the manuscript. This work was supported by National Institutes of Health Grants MH48108 and GM58234 (to R.W.T.), a SmithKline Beecham postdoctoral fellowship and National Institutes of Health Grant NS41302 (to P.G.M.), and a Medical Scientist Training Program fellowship (to K.D.).
Abbreviations
- CaM
calmodulin
- CaMKK
CaM kinase kinase
- CREB
cAMP-responsive element binding protein
- pCREB
phosphorylated CREB
- MAPK
mitogen-associated protein kinase
- TTX
tetrodotoxin
- CFP
cyan-fluorescent protein
- ECFP
enhanced CFP
References
- 1.Greenberg M E, Thompson M A, Sheng M. J Physiol (Paris) 1992;86:99–108. doi: 10.1016/s0928-4257(05)80013-0. [DOI] [PubMed] [Google Scholar]
- 2.Murphy T H, Worley P F, Baraban J M. Neuron. 1991;7:625–635. doi: 10.1016/0896-6273(91)90375-a. [DOI] [PubMed] [Google Scholar]
- 3.Bading H, Ginty D D, Greenberg M E. Science. 1993;260:181–186. doi: 10.1126/science.8097060. [DOI] [PubMed] [Google Scholar]
- 4.Deisseroth K, Bito H, Tsien R W. Neuron. 1996;16:89–101. doi: 10.1016/s0896-6273(00)80026-4. [DOI] [PubMed] [Google Scholar]
- 5.Impey S, Mark M, Villacres E C, Poser S, Chavkin C, Storm D R. Neuron. 1996;16:973–982. doi: 10.1016/s0896-6273(00)80120-8. [DOI] [PubMed] [Google Scholar]
- 6.Deisseroth K, Heist E K, Tsien R W. Nature (London) 1998;392:198–202. doi: 10.1038/32448. [DOI] [PubMed] [Google Scholar]
- 7.Dolmetsch R E, Pajvani U, Fife K, Spotts J M, Greenberg M E. Science. 2001;294:333–339. doi: 10.1126/science.1063395. [DOI] [PubMed] [Google Scholar]
- 8.Vendrell M, Pujol M J, Tusell J M, Serratosa J. Brain Res Mol Brain Res. 1992;14:285–292. doi: 10.1016/0169-328x(92)90095-s. [DOI] [PubMed] [Google Scholar]
- 9.Wang D, Tolbert L M, Carlson K W, Sadee W. J Neurochem. 2000;74:1418–1425. doi: 10.1046/j.1471-4159.2000.0741418.x. [DOI] [PubMed] [Google Scholar]
- 10.Bito H, Deisseroth K, Tsien R W. Cell. 1996;87:1203–1214. doi: 10.1016/s0092-8674(00)81816-4. [DOI] [PubMed] [Google Scholar]
- 11.Dash P K, Karl K A, Colicos M A, Prywes R, Kandel E R. Proc Natl Acad Sci USA. 1991;88:5061–5065. doi: 10.1073/pnas.88.11.5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sheng M, Thompson M A, Greenberg M E. Science. 1991;252:1427–1430. doi: 10.1126/science.1646483. [DOI] [PubMed] [Google Scholar]
- 13.Enslen H, Tokumitsu H, Soderling T R. Biochem Biophys Res Commun. 1995;207:1038–1043. doi: 10.1006/bbrc.1995.1289. [DOI] [PubMed] [Google Scholar]
- 14.Finkbeiner S, Tavazoie S F, Maloratsky A, Jacobs K M, Harris K M, Greenberg M E. Neuron. 1997;19:1031–1047. doi: 10.1016/s0896-6273(00)80395-5. [DOI] [PubMed] [Google Scholar]
- 15.Shieh P B, Hu S C, Bobb K, Timmusk T, Ghosh A. Neuron. 1998;20:727–740. doi: 10.1016/s0896-6273(00)81011-9. [DOI] [PubMed] [Google Scholar]
- 16.Ahn S, Ginty D D, Linden D J. Neuron. 1999;23:559–568. doi: 10.1016/s0896-6273(00)80808-9. [DOI] [PubMed] [Google Scholar]
- 17.Hardingham G E, Chawla S, Cruzalegui F H, Bading H. Neuron. 1999;22:789–798. doi: 10.1016/s0896-6273(00)80737-0. [DOI] [PubMed] [Google Scholar]
- 18.Soderling T R. Curr Opin Neurobiol. 2000;10:375–380. doi: 10.1016/s0959-4388(00)00090-8. [DOI] [PubMed] [Google Scholar]
- 19.Ribar T J, Rodriguiz R M, Khiroug L, Wetsel W C, Augustine G J, Means A R. J Neurosci. 2000;20:RC107. doi: 10.1523/JNEUROSCI.20-22-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ho N, Liauw J A, Blaeser F, Wei F, Hanissian S, Muglia L M, Wozniak D F, Nardi A, Arvin K L, Holtzman D M, et al. J Neurosci. 2000;20:6459–6472. doi: 10.1523/JNEUROSCI.20-17-06459.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Macias W, Carlson R, Rajadhyaksha A, Barczak A, Konradi C. Brain Res. 2001;890:222–232. doi: 10.1016/s0006-8993(00)03163-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu G Y, Deisseroth K, Tsien R W. Proc Natl Acad Sci USA. 2001;98:2808–2813. doi: 10.1073/pnas.051634198. . (First Published February 20, 2001; 10.1073/pnas.051634198) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kang H, Sun L D, Atkins C M, Soderling T R, Wilson M A, Tonegawa S. Cell. 2001;106:771–783. doi: 10.1016/s0092-8674(01)00497-4. [DOI] [PubMed] [Google Scholar]
- 24.Liu F C, Graybiel A M. Neuron. 1996;17:1133–1144. doi: 10.1016/s0896-6273(00)80245-7. [DOI] [PubMed] [Google Scholar]
- 25.West A E, Chen W G, Dalva M B, Dolmetsch R E, Kornhauser J M, Shaywitz A J, Takasu M A, Tao X, Greenberg M E. Proc Natl Acad Sci USA. 2001;98:11024–11031. doi: 10.1073/pnas.191352298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fenton A A, Muller R U. Proc Natl Acad Sci USA. 1998;95:3182–3187. doi: 10.1073/pnas.95.6.3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dobrunz L E, Stevens C F. Neuron. 1999;22:157–166. doi: 10.1016/s0896-6273(00)80687-x. [DOI] [PubMed] [Google Scholar]
- 28.Gothard K M, Hoffman K L, Battaglia F P, McNaughton B L. J Neurosci. 2001;21:7284–7292. doi: 10.1523/JNEUROSCI.21-18-07284.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ikeda S R. Science. 2001;294:318–319. doi: 10.1126/science.1066160. [DOI] [PubMed] [Google Scholar]
- 30.Mermelstein P G, Bito H, Deisseroth K, Tsien R W. J Neurosci. 2000;20:266–273. doi: 10.1523/JNEUROSCI.20-01-00266.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ginty D D, Kornhauser J M, Thompson M A, Bading H, Mayo K E, Takahashi J S, Greenberg M E. Science. 1993;260:238–241. doi: 10.1126/science.8097062. [DOI] [PubMed] [Google Scholar]
- 32.Gonzalez G A, Montminy M R. Cell. 1989;59:675–680. doi: 10.1016/0092-8674(89)90013-5. [DOI] [PubMed] [Google Scholar]
- 33.Chrivia J C, Kwok R P, Lamb N, Hagiwara M, Montminy M R, Goodman R H. Nature (London) 1993;365:855–859. doi: 10.1038/365855a0. [DOI] [PubMed] [Google Scholar]
- 34.Bagchi I C, Huang Q H, Means A R. J Biol Chem. 1992;267:3024–3029. [PubMed] [Google Scholar]
- 35.Kim K M, Herrera G A, Battarbee H D. Am J Pathol. 1999;154:843–852. doi: 10.1016/S0002-9440(10)65331-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yagi N, Satonaka K, Horio M, Shimogaki H, Tokuda Y, Maeda S. Biotechnol Histochem. 1996;71:123–129. doi: 10.3109/10520299609117148. [DOI] [PubMed] [Google Scholar]
- 37.Milikan J M, Bolsover S R. Pflügers Arch. 2000;439:394–400. doi: 10.1007/s004249900146. [DOI] [PubMed] [Google Scholar]
- 38.Craske M, Takeo T, Gerasimenko O, Vaillant C, Torok K, Petersen O H, Tepikin A V. Proc Natl Acad Sci USA. 1999;96:4426–4431. doi: 10.1073/pnas.96.8.4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luby-Phelps K, Hori M, Phelps J M, Won D. J Biol Chem. 1995;270:21532–21538. doi: 10.1074/jbc.270.37.21532. [DOI] [PubMed] [Google Scholar]
- 40.Liao B, Paschal B M, Luby-Phelps K. Proc Natl Acad Sci USA. 1999;96:6217–6222. doi: 10.1073/pnas.96.11.6217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Teruel M N, Chen W, Persechini A, Meyer T. Curr Biol. 2000;10:86–94. doi: 10.1016/s0960-9822(00)00295-5. [DOI] [PubMed] [Google Scholar]
- 42.Hardingham G E, Arnold F J, Bading H. Nat Neurosci. 2001;4:261–267. doi: 10.1038/85109. [DOI] [PubMed] [Google Scholar]
- 43.Farnsworth C L, Freshney N W, Rosen L B, Ghosh A, Greenberg M E, Feig L A. Nature (London) 1995;376:524–527. doi: 10.1038/376524a0. [DOI] [PubMed] [Google Scholar]
- 44.Rich R C, Schulman H. J Biol Chem. 1998;273:28424–28429. doi: 10.1074/jbc.273.43.28424. [DOI] [PubMed] [Google Scholar]
- 45.Wu G Y, Deisseroth K, Tsien R W. Nat Neurosci. 2001;4:151–158. doi: 10.1038/83976. [DOI] [PubMed] [Google Scholar]
- 46.Pruschy M, Ju Y, Spitz L, Carafoli E, Goldfarb D S. J Cell Biol. 1994;127:1527–1536. doi: 10.1083/jcb.127.6.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Natsukari N, Zhang S P, Nichols R A, Weiss B. Neurochem Int. 1995;26:465–476. doi: 10.1016/0197-0186(94)00156-o. [DOI] [PubMed] [Google Scholar]
- 48.Luby-Phelps K, Taylor D L. Cell Motil Cytoskeleton. 1988;10:28–37. doi: 10.1002/cm.970100107. [DOI] [PubMed] [Google Scholar]
- 49.Romoser V A, Hinkle P M, Persechini A. J Biol Chem. 1997;272:13270–13274. doi: 10.1074/jbc.272.20.13270. [DOI] [PubMed] [Google Scholar]
- 50.Fields R D, Eshete F, Stevens B, Itoh K. J Neurosci. 1997;17:7252–7266. doi: 10.1523/JNEUROSCI.17-19-07252.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.