Key Points
Question
Does adding whole-breast ultrasonography to mammography improve breast cancer screening effectiveness?
Findings
In this cohort study, for women whose breast cancer risk ranged from low to very high, there were significantly higher short-interval follow-up and biopsy recommendation rates with screening mammography plus same-day ultrasonography compared with mammography alone. However, no significant increase in cancer detection or decrease in interval cancer rates was observed.
Meaning
These results suggest that the benefits of supplemental ultrasonography screening may not outweigh associated harms.
This cohort study investigates the performance of screening mammography plus screening ultrasonography compared with screening mammography alone among women at low, intermediate, and high risk for breast cancer.
Abstract
Importance
Whole-breast ultrasonography has been advocated to supplement screening mammography to improve outcomes in women with dense breasts.
Objective
To determine the performance of screening mammography plus screening ultrasonography compared with screening mammography alone in community practice.
Design, Setting, and Participants
Observational cohort study. Two Breast Cancer Surveillance Consortium registries provided prospectively collected data on screening mammography with vs without same-day breast ultrasonography from January 1, 2000, to December 31, 2013. The dates of analysis were March 2014 to December 2018. A total of 6081 screening mammography plus same-day screening ultrasonography examinations in 3386 women were propensity score matched 1:5 to 30 062 screening mammograms without screening ultrasonography in 15 176 women from a sample of 113 293 mammograms. Exclusion criteria included a personal history of breast cancer and self-reported breast symptoms.
Exposures
Screening mammography with vs without screening ultrasonography.
Main Outcomes and Measures
Cancer detection rate and rates of interval cancer, false-positive biopsy recommendation, short-interval follow-up, and positive predictive value of biopsy recommendation were estimated and compared using log binomial regression.
Results
Screening mammography with vs without ultrasonography examinations was performed more often in women with dense breasts (74.3% [n = 4317 of 5810] vs 35.9% [n = 39 928 of 111 306] in the overall sample), in women who were younger than 50 years (49.7% [n = 3022 of 6081] vs 31.7% [n = 16 897 of 112 462]), and in women with a family history of breast cancer (42.9% [n = 2595 of 6055] vs 15.0% [n = 16 897 of 112 462]). While 21.4% (n = 1154 of 5392) of screening ultrasonography examinations were performed in women with high or very high (≥2.50%) Breast Cancer Surveillance Consortium 5-year risk scores, 53.6% (n = 2889 of 5392) had low or average (<1.67%) risk. Comparing mammography plus ultrasonography with mammography alone, the cancer detection rate was similar at 5.4 vs 5.5 per 1000 screens (adjusted relative risk [RR], 1.14; 95% CI, 0.76-1.68), as were interval cancer rates at 1.5 vs 1.9 per 1000 screens (RR, 0.67; 95% CI, 0.33-1.37). The false-positive biopsy rates were significantly higher at 52.0 vs 22.2 per 1000 screens (RR, 2.23; 95% CI, 1.93-2.58), as was short-interval follow-up at 3.9% vs 1.1% (RR, 3.10; 95% CI, 2.60-3.70). The positive predictive value of biopsy recommendation was significantly lower at 9.5% vs 21.4% (RR, 0.50; 95% CI, 0.35-0.71).
Conclusions and Relevance
In a relatively young population of women at low, intermediate, and high breast cancer risk, these results suggest that the benefits of supplemental ultrasonography screening may not outweigh associated harms.
Introduction
Increasing awareness that breast density is a risk factor for developing breast cancer and makes breast cancer more difficult to detect on mammography has led to grassroots efforts to educate women about breast density. In 2009, Connecticut was the first state to pass legislation requiring that all women receiving mammography be directly informed about breast density and that payers cover supplemental ultrasonography screening in women with dense breasts.1 Since then, at least 34 additional states have enacted breast density notification legislation,2 and a federal bill has been introduced.3,4 Seven of these states mandate insurance coverage for screening ultrasonography in women with dense breasts. The laws vary across states, but most require notification if a woman’s mammographic density is either heterogeneously or extremely dense, as determined by a radiologist according to the American College of Radiology’s Breast Imaging Reporting and Data System (BI-RADS).5,6 In addition, notification requirements may include statements that women are at higher breast cancer risk because of their breast density, that breast density may adversely limit the ability of mammography to detect breast cancers, and that women with mammographically dense breasts may want to consider supplemental screening.1,7,8,9 Data from the Breast Cancer Surveillance Consortium (BCSC) indicate that 43% of women undergoing screening mammography aged 40 to 74 years have dense breasts, including 57% of women aged 40 to 44 years.10
Studies of screening ultrasonography have included women with additional risk factors beyond breast density who were at intermediate to high breast cancer risk either due to a personal history of breast cancer or high-risk benign breast lesions or because of genetic susceptibility. In addition, most studies of ultrasonography screening performance have been conducted in academic medical centers.11 A recent systematic review8 of supplemental screening ultrasonography, magnetic resonance imaging (MRI), and digital breast tomosynthesis for women with dense breasts noted that good-quality evidence was sparse, and effects of supplemental screening on breast cancer outcomes remain unclear.
Accurate information on the effectiveness of screening ultrasonography is needed to provide guidance on whether widespread use of screening breast ultrasonography with screening mammography would be a beneficial strategy. We conducted a retrospective analysis of prospectively collected data in 2 BCSC registries to assess use of screening ultrasonography in community practice and to investigate the performance of screening mammography plus ultrasonography compared with screening mammography alone in women across the spectrum of breast cancer risk.
Methods
Study Setting and Data Sources
In this observational cohort study, we included screening mammograms with or without screening ultrasonography performed at breast imaging facilities in 1 of 2 BCSC (http://www.bcsc-research.org) registries12 (Vermont Breast Cancer Surveillance System and San Francisco Mammography Registry), which linked woman-level risk factors and clinical information to information on breast imaging examinations with pathology databases, state tumor registries, and regional Surveillance, Epidemiology, and End Results programs. The BCSC registries and their Statistical Coordinating Center received institutional review board approval for active or passive consenting processes or a waiver of consent to enroll participants, link and pool data, and perform analysis. All procedures were adherent to the Health Insurance Portability and Accountability Act of 1996, and registries and the Statistical Coordinating Center received a Federal Certificate of Confidentiality and other protections for the identities of women, physicians, and facilities.
We identified breast ultrasonography examinations with an indication of screening performed on the same day as a screening mammogram from January 1, 2000, to December 31, 2013. The dates of analysis were March 2014 to December 2018. Exclusion criteria included (1) a personal history of breast cancer, mastectomy, or BI-RADS 6 (known malignant neoplasm) assessment; (2) unilateral examination; and (3) self-reported symptoms (except pain). Data abstractors reviewed radiology reports to confirm the ultrasonography screening indication. For one registry, after abstracting 13.7% (782 of 5728) of reports, 95.7% (748 of 782) were confirmed with a screening indication. Therefore, we assumed the remaining examinations were performed for screening. For the other registry, all reports were abstracted, and 77.9% were confirmed with a screening indication. Reports with indeterminate indication were reviewed by 2 of us (J.M.L. and C.D.L.), with consensus determination of indication. Screening mammograms eligible to be in the matched group were performed at the same facilities, applying the same exclusion criteria as above.
Measures and Definitions
Women completed a questionnaire at each examination to collect information on race/ethnicity, menopausal status,13 history of first-degree relatives (mother, sister, or daughter) with breast cancer, and history of breast biopsy. Prior diagnoses of benign breast disease were collected from pathology databases and grouped into 1 of the following 4 categories: nonproliferative disease, proliferative without atypia, proliferative with atypia, and lobular carcinoma in situ.14 American College of Radiology BI-RADS breast density5,6 was recorded clinically by the interpreting radiologist. The BCSC (version 2.0) 5-year breast cancer risk score was calculated.14
Performance measures and definitions are listed in Table 1. Most women with screening ultrasonography–detected abnormalities received same-day additional imaging, and a single screening report was issued regardless of whether imaging included only screening or both screening and diagnostic views. Therefore, recall rate was based on the end-of-day BI-RADS assessment after any additional workup and was defined as recall for additional imaging that was performed on a different day. A BI-RADS end-of-day assessment of 1 (negative) or 2 (benign) was considered negative, and 0 (needs additional imaging), 3 (probably benign), 4 (suspicious), or 5 (highly suspicious) was positive.
Table 1. Performance Measures and Definitions.
| Performance Measure | Definition |
|---|---|
| Recall rate | Percentage of screening examinations with a positive end-of-day assessment |
| Cancer detection rate | No. of true-positive screens per 1000 screens |
| Interval cancer rate | No. of screening examinations with a negative final assessment and cancer diagnosed within the follow-up period per 1000 screens |
| Cancer rate | No. of screens with cancer within the follow-up period per 1000 screens |
| Biopsy recommendation rate | No. of screening examinations with a positive final assessment per 1000 screens |
| False-positive biopsy recommendation rate | No. of screening examinations with a false-positive final assessment per 1000 screens |
| Positive predictive value of biopsy recommendation (PPV2) | Percentage of category 4 and 5 assessments with a tissue diagnosis of cancer within the follow-up period |
| Short-interval follow-up rate | Percentage of screens with a final category 3 assessment |
| Sensitivity | Percentage of true-positive results among those with cancer within the follow-up period |
| Specificity | Percentage of true-negative results among those without cancer within the follow-up period |
All other performance metrics were based on the final assessment;15 this differed from the end-of-day assessment only for BI-RADS 0 examinations, which were followed up to 90 days for the first nonzero BI-RADS assessment. Final assessment of 4 or 5 was considered positive, and assessments of 1, 2, or 3 were considered negative. Examinations that could not be resolved to a nonzero assessment (5 of 6081 [0.1%] mammography plus ultrasonography and 104 of 30 062 [0.3%] mammography alone) were excluded from calculations of performance metrics using the final assessment.
For each screening examination, women were followed up for 12 months afterward or until the next screening examination, whichever occurred earlier, for breast cancer diagnoses (either ductal carcinoma in situ or invasive adenocarcinoma). True-positive screens (ie, screen-detected cancers) were defined as positive screens with a breast cancer diagnosis. False-positive screens were defined as positive screens without a breast cancer diagnosis. Negative screens were defined as true negative if no cancer was diagnosed and false negative if cancer was diagnosed during the follow-up period. Recall rate, biopsy recommendation rate, and cancer detection rate were also compared between the first mammography plus ultrasonography screening examination in the BCSC and subsequent examinations. We also estimated the following breast cancer outcomes: the median size of invasive cancer, percentage of minimal cancer (defined as ductal carcinoma in situ or invasive carcinoma ≤10 mm), percentage of node-negative invasive cancers, and percentage of American Joint Committee on Cancer stage 0 and I cancers.
Statistical Analysis
We used logistic regression to estimate propensity scores (ie, the probability of screening with mammography plus ultrasonography vs mammography alone) based on BCSC registry, age (linear and quadratic) and year of examination, race/ethnicity, menopausal status, first-degree family history of breast cancer, time since last mammogram, breast density, and prior benign biopsy result. A SAS macro16 was used for 1:5 matching of mammography plus ultrasonography examinations (n = 6081) to mammography alone (n = 30 062) within the same registries and without replacement using the logit of the propensity score with a caliper width of 0.3 SD. Matching was performed separately for each subgroup needed for each performance measure, including all screening examinations for rates per 1000 screens (n = 36 143), screening examinations with cancer for sensitivity (n = 252), screening examinations without cancer for specificity (n = 35 878), and screening examinations with positive final assessment for positive predictive value of biopsy recommendation (PPV2) (n = 2062). We compared the covariate distributions in the mammography plus ultrasonography group and the mammography alone group before vs after matching using the standardized differences of the proportions of each covariate category.17
We assessed the joint distributions of breast density and BCSC 5-year risk in the women receiving mammography plus ultrasonography screening. Unadjusted performance measures were calculated with 95% CIs for the matched groups. We used log binomial regression to estimate relative risks (RRs) comparing performance metrics for mammography plus ultrasonography vs matched screens with mammography alone, including the matched set as a random effect to account for correlation among these examinations and adjusting for characteristics included in the propensity score model to account for potential residual confounding.
Analyses were performed in SAS software (version 9.4; SAS Institute Inc), and figures were produced using Python (version 3.4; Python Software Foundation). All statistical tests were 2-sided, with α = .05 indicating statistical significance.
Results
We identified 6081 mammography plus ultrasonography examinations in 3386 women (Table 2). Compared with women in the overall mammography alone group before matching (n = 113 293), women receiving mammography plus ultrasonography were more likely to be younger than 50 years (49.7% vs 31.7%), be white non-Hispanic (79.4% vs 76.0%), have a first-degree family history of breast cancer (42.9% vs 15.0%), have dense breasts (74.3% vs 35.9%), and have high BCSC 5-year risk scores (≥2.50%) (21.4% vs 6.6%), while 53.6% had low or average risk (<1.67%). Notably, 25.7% of women receiving mammography plus ultrasonography did not have dense breasts.
Table 2. Characteristics of Mammography Plus Ultrasonography Examination Cohort and the Matched Sample and Total Population of Women Receiving Screening Mammography Alonea.
| Variable | Mammography Plus Ultrasonography | Mammography Alone (Matched) | Standardized Mean Difference After Matching | Mammography Alone (Overall) | Standardized Mean Difference Before Matching |
|---|---|---|---|---|---|
| Total | 6081 | 30 062 | NA | 113 293 | NA |
| Age, No. (%), yb | |||||
| 30-39 | 506 (8.3) | 1093 (3.6) | 0.20 | 2800 (2.5) | 0.26 |
| 40-49 | 2516 (41.4) | 12 636 (42.0) | −0.01 | 33 114 (29.2) | 0.26 |
| 50-59 | 2052 (33.7) | 10 477 (34.9) | −0.03 | 32 803 (29.0) | 0.10 |
| 60-69 | 745 (12.3) | 4130 (13.7) | −0.04 | 23 034 (20.3) | −0.22 |
| 70-79 | 212 (3.5) | 1326 (4.4) | −0.05 | 13 857 (12.2) | −0.33 |
| ≥80 | 50 (0.8) | 400 (1.3) | −0.05 | 7685 (6.8) | −0.32 |
| Race/ethnicity, No./total No. (%)b | |||||
| White, non-Hispanic | 2626/3308 (79.4) | 13 330/16 628 (80.2) | −0.02 | 46 107/60 661 (76.0) | 0.08 |
| Black, non-Hispanic | 11/3308 (0.3) | 76/16 628 (0.5) | −0.03 | 654/60 661 (1.1) | −0.10 |
| Asian/Pacific Islander | 396/3308 (12.0) | 1800/16 628 (10.8) | 0.04 | 7681/60 661 (12.7) | −0.02 |
| Hispanic | 213/3308 (6.4) | 1106/16 628 (6.7) | −0.01 | 4685/60 661 (7.7) | −0.05 |
| Mixed/other | 62/3308 (1.9) | 316/16 628 (1.9) | 0.00 | 1534/60 661 (2.5) | −0.04 |
| Menopausal status, No. (%)b | |||||
| Premenopausal | 2074 (34.1) | 11 665 (38.8) | −0.10 | 30 869 (27.2) | 0.15 |
| Postmenopausal | 2723 (44.8) | 12 067 (40.1) | 0.10 | 63 224 (55.8) | −0.22 |
| Surgical/other amenorrhea/unknown | 1284 (21.1) | 6330 (21.1) | 0.00 | 19 200 (16.9) | 0.11 |
| First-degree family history of breast cancer, No./total No. (%)b | |||||
| No | 3460/6055 (57.1) | 21 227/29 915 (71.0) | −0.29 | 95 565/112 462 (85.0) | −0.65 |
| Yes | 2595/6055 (42.9) | 8688/29 915 (29.0) | 0.29 | 16 897/112 462 (15.0) | 0.65 |
| Unknown | 26 | 147 | NA | 831 | NA |
| Year of examination, No. (%)b | |||||
| 2005-2006 | 285 (4.7) | 1784 (5.9) | −0.05 | 16 635 (14.7) | −0.34 |
| 2007-2008 | 1284 (21.1) | 6671 (22.2) | −0.03 | 31 164 (27.5) | −0.15 |
| 2009-2010 | 1999 (32.9) | 9894 (32.9) | 0.00 | 32 216 (28.4) | 0.10 |
| 2011-2013 | 2513 (41.3) | 11 713 (39.0) | 0.05 | 33 278 (29.4) | 0.25 |
| Time since last mammogram, No./total No. (%), yb | |||||
| None | 92/5647 (1.6) | 517/27 958 (1.8) | −0.02 | 4386/106 802 (4.1) | −0.15 |
| 1-2 | 5398/5647 (95.6) | 26 493/27 958 (94.8) | 0.04 | 95 293/106 802 (89.2) | 0.24 |
| ≥3 | 157/5647 (2.8) | 948/27 958 (3.4) | −0.03 | 7123/106 802 (6.7) | −0.18 |
| Unknown | 434 | 2104 | NA | 6491 | NA |
| Breast density, No./total No. (%)b | |||||
| Almost entirely fat | 83/5810 (1.4) | 563/28 930 (1.9) | −0.04 | 8226/111 306 (7.4) | −0.30 |
| Scattered | 1410/5810 (24.3) | 9138/28 930 (31.6) | −0.16 | 63 152/111 306 (56.7) | −0.70 |
| Heterogeneously dense | 3543/5810 (61.0) | 17 126/28 930 (59.2) | 0.04 | 37 074/111 306 (33.3) | 0.58 |
| Extremely dense | 774/5810 (13.3) | 2103/28 930 (7.3) | 0.20 | 2854/111 306 (2.6) | 0.40 |
| Unknown | 271 | 1132 | NA | 1987 | NA |
| Prior benign biopsy result, No. (%)b | |||||
| No prior biopsy | 3045 (50.1) | 18 786 (62.5) | −0.25 | 90 745 (80.1) | −0.66 |
| Biopsy, pathology unknown | 1446 (23.8) | 6696 (22.3) | 0.04 | 15 878 (14.0) | 0.25 |
| Nonproliferative disease | 831 (13.7) | 2956 (9.8) | 0.12 | 4320 (3.8) | 0.36 |
| Proliferative without atypia | 620 (10.2) | 1290 (4.3) | 0.23 | 1808 (1.6) | 0.37 |
| Proliferative with atypia | 109 (1.8) | 287 (1.0) | 0.07 | 418 (0.4) | 0.13 |
| Lobular carcinoma in situ | 30 (0.5) | 47 (0.2) | 0.05 | 124 (0.1) | 0.07 |
| BCSC 5-y risk, No./total No. (%) | |||||
| Low (0%-1.00%) | 1196/5392 (22.2) | 8146/27 931 (29.2) | −0.16 | 37 108/97 393 (38.1) | −0.35 |
| Average (1.00%-1.66%) | 1693/5392 (31.4) | 9573/27 931(34.3) | −0.06 | 37 693/97 393 (38.7) | −0.15 |
| Intermediate (1.67%-2.49%) | 1349/5392 (25.0) | 6317/27 931 (22.6) | 0.06 | 16 127/97 393 (16.6) | 0.21 |
| High (2.50%-3.99%) | 976/5392 (18.1) | 3402/27 931 (12.2) | 0.17 | 5760/97 393 (5.9) | 0.38 |
| Very high (≥3.99%) | 178/5392 (3.3) | 493/27 931 (1.8) | 0.10 | 705/97 393 (0.7) | 0.19 |
| Unknown | 689 | 2131 | NA | 15 900 | NA |
Abbreviations: BCSC, Breast Cancer Surveillance Consortium; NA, not applicable.
For each variable, missing values were not included when calculating the distributions across categories.
Variables controlled for in calculating propensity score.
Mammography plus ultrasonography examinations were matched 1:5 to 30 062 mammography examinations in 15 176 women (Table 2). Before matching, the differences between the covariate distributions in the overall sample were medium to large,18 with the largest absolute standardized differences for scattered breast density (−0.70), no prior biopsy (−0.66), and family history (−0.65). After matching, absolute standardized differences were small,18 with the largest differences for family history (0.29) and no prior biopsy (−0.25). However, after matching, some medium-sized absolute standardized differences remained for the samples used for sensitivity (maximum of 0.50 for no prior biopsy) and PPV (0.46 for family history and 0.43 for no prior biopsy), but differences for specificity sample were small (<0.29). The distribution of propensity scores for mammography plus ultrasonography and mammography alone subgroups demonstrated improved overlap after matching (eAppendix, eFigure 1, and eFigure 2 in the Supplement). However, differences remained for age, menopausal status, family history of breast cancer, year of examination, breast density, benign biopsy result, and BCSC 5-year risk. Therefore, we also adjusted for these characteristics when comparing performance measures.
The Figure shows the joint distribution of BCSC 5-year risk of developing invasive breast cancer and BI-RADS density categories for 5392 mammography plus ultrasonography examinations. While 75.0% (n = 4042) of examinations were performed in women with dense breasts (eTable in the Supplement), only 21.4% (n = 1154) of these examinations were in women with high or very high 5-year risk. Very few women with high or very high risk had nondense breasts (13.2% [152 of 1154]).
Figure. Joint Distributions of BCSC 5-Year Risk by BI-RADS Breast Density Category in 5392 Combined Mammography and Ultrasonography Screening Examinations.
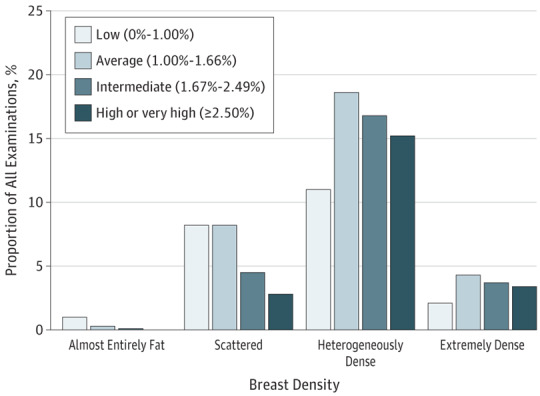
BCSC indicates Breast Cancer Surveillance Consortium; BI-RADS, Breast Imaging Reporting and Data System.
Performance of Combined Mammography and Ultrasound Screening
Compared with mammography alone (Table 3), mammography plus ultrasonography screening was associated with fewer end-of-day assessments for additional imaging (BI-RADS 0, 0.3% vs 17.2%) and lower overall recall rate for additional imaging or biopsy (BI-RADS 0, 3, 4, or 5; 9.9% vs 17.6%; RR, 0.52; 95% CI, 0.48-0.57), indicating that women were less likely to need a second visit to complete diagnostic evaluations. However, with mammography plus ultrasonography, the efficiency of same-day imaging evaluation was offset by an almost doubling of the biopsy recommendation rate (57.4 vs 27.7 per 1000 screens; RR, 2.05; 95% CI, 1.79-2.34). The short-interval follow-up rate for probably benign findings was also significantly increased with mammography plus ultrasonography (3.9% vs 1.1%; RR, 3.10; 95% CI, 2.60-3.70). In addition, the false-positive biopsy recommendation rate more than doubled (52.0 vs 22.2 per 1000 screens; RR, 2.23; 95% CI, 1.93-2.58), with a corresponding decrease in PPV2 of approximately half (9.5% vs 21.4%; RR, 0.50; 95% CI, 0.35-0.71). Increased sensitivity (78.6% vs 73.8%; RR, 1.08; 95% CI, 0.92-1.27) and decreased false-negative rate (1.5 vs 1.9 per 1000 screens; RR, 0.67; 95% CI, 0.33-1.37) were observed with mammography plus ultrasonography, but these differences were not statistically significant. The cancer detection rate was similar (5.4 vs 5.5 per 1000 screens; adjusted RR, 1.14, 95% CI, 0.76-1.68) between groups.
Table 3. Estimated Performance Measures and Results From Log Binomial Regression Analysis.
| Variable | Mammography Plus Ultrasonography | Mammography Alone (Matched) | Relative Risk (95% CI)a |
|---|---|---|---|
| BI-RADS end-of-day assessment, No. (%) | |||
| 0 (Needs additional imaging) | 21 (0.3) | 5159 (17.2) | NA |
| 1 (Negative) | 2689 (44.2) | 23 909 (79.5) | NA |
| 2 (Benign) | 2793 (45.9) | 878 (2.9) | NA |
| 3 (Probably benign) | 234 (3.8) | 64 (0.2) | NA |
| 4 (Suspicious) | 342 (5.6) | 49 (0.2) | NA |
| 5 (Highly suspicious) | 2 (0.0) | 3 (0.0) | NA |
| Total | 6081 (100) | 30 062 (100) | NA |
| Performance based on end-of-day assessment | |||
| Recall rate for additional imaging or biopsy, % (95% CI) | 9.9 (9.1-10.6) | 17.6 (17.1-18.0) | 0.52 (0.48-0.57) |
| End-of-day assessment of 0, 3, 4, 5, No. | 599 | 5275 | NA |
| Total examinations, No. | 6081 | 30 062 | NA |
| Final assessment, No. (%) | |||
| 0 (Needs additional imaging) | 5 (0.1) | 104 (0.3) | NA |
| 1 (Negative) | 2694 (44.3) | 26 848 (89.3) | NA |
| 2 (Benign) | 2798 (46.0) | 1936 (6.4) | NA |
| 3 (Probably benign) | 235 (3.9) | 341 (1.1) | NA |
| 4 (Suspicious) | 347 (5.7) | 792 (2.6) | NA |
| 5 (Highly suspicious) | 2 (0.0) | 41 (0.1) | NA |
| Total | 6081 (100) | 30 062 (100) | NA |
| Performance based on final assessment | |||
| Biopsy recommendation rate per 1000 screens (95% CI) | 57.4 (51.9-63.5) | 27.7 (25.9-29.7) | 2.05 (1.79-2.34) |
| Final assessment of 4, 5, No.b | 349 | 833 | NA |
| Total examinations, No. | 6081 | 30 062 | NA |
| Short-interval imaging follow-up rate (95% CI) | 3.9 (3.4-4.4) | 1.1 (1.0-1.3) | 3.10 (2.60-3.70) |
| Final assessment of 3, No. | 235 | 341 | NA |
| Total examinations, No. | 6081 | 30 062 | NA |
| Sensitivity (95% CI) | 78.6 (67.1-92.0) | 73.8 (68.1-80.0) | 1.08 (0.92-1.27) |
| Final assessment of 4, 5 and cancer, No. | 33 | 155 | NA |
| Total cancers, No. | 42 | 210 | NA |
| Specificity (95% CI) | 94.8 (94.2-95.3) | 97.7 (97.6-97.9) | 0.97 (0.97-0.98) |
| Final assessment of 0, 1, 2, 3 and no cancer, No. | 5719 | 29 169 | NA |
| Noncancers, No. | 6035 | 29 843 | NA |
| PPV2 (95% CI) | 9.5 (6.8-13.1) | 21.4 (19.6-23.5) | 0.50 (0.35-0.71) |
| Final assessment of 4, 5 and cancer | 33 | 367 | NA |
| Biopsy recommended (final assessment of 4, 5) | 349 | 1713 | NA |
| Cancer detection rate per 1000 screens (95% CI) | 5.4 (3.9-7.6) | 5.5 (4.7-6.4) | 1.14 (0.76-1.68) |
| Final assessment of 4, 5 and cancer | 33 | 165 | NA |
| Total examinations | 6081 | 30 062 | NA |
| False-negative rate per 1000 screens (95% CI)c | 1.5 (0.8-2.8) | 1.9 (1.4-2.4) | 0.67 (0.33-1.37) |
| Final assessment of 0, 1, 2, 3 and cancer, No. | 9 | 56 | NA |
| Total examinations, No. | 6081 | 30 062 | |
| Cancer rate per 1000 screens (95% CI) | 6.9 (5.1-9.3) | 7.4 (6.4-8.4) | 0.99 (0.70-1.42) |
| All cancers, No. | 42 | 221 | NA |
| Total examinations, No. | 6081 | 30 062 | NA |
| False-positive biopsy recommendation rate per 1000 (95% CI) | 52.0 (46.7-57.8) | 22.2 (20.6-24.0) | 2.23 (1.93-2.58) |
| Final assessment of 4, 5 and no cancer, No. | 316 | 668 | NA |
| Total examinations, No. | 6081 | 30 062 | NA |
Abbreviations: BI-RADS, Breast Imaging Reporting and Data System; DCIS, ductal carcinoma in situ; NA, not applicable; PPV2, positive predictive value of biopsy recommendation.
Relative risk is from log binomial model adjusted for site, age, menopausal status, first-degree family history of breast cancer, year of examination, prior benign biopsy result, and correlation among women within the same matched set using generalized estimated equations. Sensitivity was adjusted for site and first-degree family history of breast biopsy.
Final assessment of 4, 5 considered a positive examination result and was used to calculate performance measures.
False-negative rate includes both invasive and DCIS.
When recall rate, biopsy recommendation rate, and cancer detection rate were stratified by first vs subsequent mammography plus ultrasonography examinations (n = 2040 and n = 4041, respectively), all rates declined significantly on subsequent examinations. Recall rate decreased from 12.5% to 6.5% (9.9% overall), biopsy recommendation rate decreased from 75 to 49 per 1000 screens (57 per 1000 overall), and cancer detection rate decreased from 10.8 to 2.7 per 1000 screens (5.4 per 1000 overall).
Breast Cancer Characteristics
There were 42 breast cancers among women in the mammography plus ultrasonography cohort and 221 in the matched sample (Table 4). Regardless of screening strategy, most breast cancers were invasive ductal carcinomas, small (≤20 mm), node negative, and estrogen receptor and progesterone receptor positive. Women who underwent mammography plus ultrasonography had a higher proportion of stage 0 noninvasive ductal carcinoma in situ at 47.6% (20 of 42; 95% CI, 32.5%-62.7%) vs 34.8% (77 of 221; 95% CI, 28.6%-41.1%) in matched controls, but this difference was not statistically significant (P = .12).
Table 4. Characteristics of Breast Cancers Occurring Within 1 Year of the Screening Examination Among the Study Groups.
| Variable | No./Total No. (%) | ||
|---|---|---|---|
| Mammography Plus Ultrasonography | Mammography Alone (Matched) | Mammography Alone (Overall) | |
| Total | 249 | 470 | 719 |
| Cancer histology | |||
| Noninvasive (DCIS) | 20/42 (47.6) | 77/221 (34.8) | 249/719 (34.6) |
| Invasive | 22/42 (52.4) | 144/221 (65.2) | 470/719(65.4) |
| Ductal | 19/20 (95.0) | 113/141 (80.1) | 384/458 (83.8) |
| Lobular | 1/20 (5.0) | 20/141 (14.2) | 50/458 (10.9) |
| Mixed | 0/20 | 8/141 (5.7) | 24/458 (5.2) |
| Other/unknown | 2 | 3 | 12 |
| Invasive tumor size, mm | |||
| 1-5 | 2/20 (10.0) | 8/140 (5.7) | 48/454 (10.6) |
| 6-10 | 5/20 (25.0) | 37/140 (26.4) | 116/454 (25.6) |
| 11-15 | 3/20 (15.0) | 25/140 (17.9) | 87/454 (19.2) |
| 16-20 | 3/20 (15.0) | 26/140 (18.6) | 74/454 (16.3) |
| >20 | 7/20 (35.0) | 44/140 (31.4) | 129/454 (28.4) |
| Unknown | 2 | 4 | 16 |
| Minimal cancer | |||
| No | 13/41 (31.7) | 95/210 (45.2) | 290/691 (42.0) |
| Yes | 28/41 (68.3) | 115/210 (54.8) | 401/691 (58.0) |
| Unknown | 1 | 11 | 28 |
| Axillary lymph node status | |||
| Negative | 35/41 (85.4) | 183/219 (83.6) | 620/708 (87.6) |
| Positive | 6/41 (14.6) | 36/219 (16.4) | 88/708 (12.4) |
| Unknown | 1 | 2 | 11 |
| AJCC stage | |||
| 0 | 20/41 (48.8) | 77/219 (35.2) | 249/706 (35.3) |
| I | 11/41 (26.8) | 85/219 (38.8) | 297/706 (42.1) |
| II | 8/41 (19.5) | 43/219 (19.6) | 131/706 (18.6) |
| III | 2/41 (4.9) | 13/219 (5.9) | 24/706 (3.4) |
| IV | 0/41 | 1/219 (0.5) | 5/706 (0.7) |
| Unknown | 1 | 2 | 13 |
| Grade of invasive cancer | |||
| 1 | 8/19 (42.1) | 48/138 (34.8) | 173/448 (38.6) |
| 2 | 8/19 (42.1) | 62/138 (44.9) | 191/448 (42.6) |
| 3 | 3/19 (15.8) | 28/138 (20.3) | 84/448 (18.8) |
| Unknown | 3 | 6 | 22 |
| Hormone receptor status of invasive cancer | |||
| ER+ or PR+ | 17/21 (81.0) | 134/142 (94.4) | 426/457 (93.2) |
| ER− and PR− | 4/21 (19.0) | 8/142 (5.6) | 31/457 (6.8) |
| Unknown | 1 | 2 | 13 |
Abbreviation: AJCC, American Joint Committee on Cancer; DCIS, ductal carcinoma in situ; ER, estrogen receptor; PR, progesterone receptor.
Discussion
As the number of states with breast density notification laws continues to increase,2 postlegislation reports indicate small but significantly increased use of supplemental ultrasonography7,19,20 and MRI19,21 among women with mammographically dense breasts, with a greater observed increase in ultrasonography use compared with MRI. A single study22 of postlegislation outcomes was conducted in Connecticut, where analysis of breast cancers recorded in the Connecticut Surveillance, Epidemiology, and End Results program registry demonstrated a small increase in detection of localized invasive breast cancer but no association with changes in rates of regional or metastatic stages of disease compared with control states without breast density legislation. With increasing use of ultrasonography for supplemental screening, it is critical that its effect on outcomes be evaluated.
Our study found that for every 1000 women screened with mammography plus ultrasonography approximately 5 women would be diagnosed as having breast cancer, while 57 women would receive a recommendation for biopsy, and 52 of these women would have benign, false-positive results at pathology (Table 3). An additional 39 women would receive recommendations for short-interval imaging follow-up of detected findings, and approximately 2 women would be diagnosed as having breast cancer within 1 year of having negative screening results. Our results of increased biopsy recommendation rates and false-positive biopsy recommendation rates and decreased specificity and PPV of supplemental screening ultrasonography are consistent with findings from multiple studies8,23,24,25,26,27,28 conducted in the United States, Europe, and Asia. In a recent meta-analysis27 of screening ultrasonography studies in women with dense breasts, recommendations for further assessment after the addition of ultrasonography to mammography screening approximately doubled, and biopsy recommendation rates increased 2- to 3-fold.
Most other studies have found significant increases in incremental cancer detection rate with mammography plus ultrasonography compared with mammography alone,8,27 and a meta-analysis27 estimated incremental cancer detection at 3.8 per 1000 screens. In contrast, our study using propensity score matching and direct adjustment of confounders demonstrated comparable cancer detection rates between the 2 strategies and a nonsignificant reduction in interval cancer rates with mammography plus ultrasonography screening.
However, incremental cancer detection rates should also be considered in the context of cancer detection rates with mammography alone. The 2 largest studies of screening ultrasonography published to date include (1) a randomized clinical trial (Japan Strategic Anti-Cancer Randomized Trial [J-START]26) of 72 998 women (36 139 women in the mammography plus ultrasonography arm) and (2) a report from an Austrian population–based screening program24 (66 680 women overall and 31 918 women with dense breasts). In the J-START study,26 the cancer detection rate was 3.3 per 1000 screens in the mammography arm and 3.9 per 1000 screens in the mammography plus ultrasonography arm (increase of 0.6 per 1000 screens). In the Austrian study,24 the cancer detection rate with mammography alone was 3.5 per 1000 screens, which increased to 4.0 per 1000 screens when ultrasonography was added. For the subgroup of women with dense breasts, the cancer detection rate with mammography alone was 1.8 per 1000 screens, which increased to 2.4 per 1000 screens when ultrasonography was added. In our study, cancer detection was 5.4 per 1000 screens with mammography plus ultrasonography and 5.5 per 1000 screens with mammography alone (Table 3), with no significant difference detected between the 2 propensity score–matched cohorts.
In the Austrian study,24 with the lowest cancer detection rate in women with dense breasts, the addition of ultrasonography provided the largest increase in sensitivity of 19%, from 62% with mammography alone to 81% with mammography plus ultrasonography. In the J-START study,26 sensitivity in the mammography alone arm was 77% compared with 91% in the mammography plus ultrasonography arm (increase of 14%). Our study demonstrated the smallest, and nonsignificant, increase in sensitivity (6%), from 73.8% with mammography alone to 78.6% with mammography plus ultrasonography, with substantial overlap of 95% CIs (Table 3). The Austrian study24 did not report specificity values. When comparing sensitivity and specificity together, the diagnostic test performance of mammography plus ultrasonography in the J-START study26 was higher (sensitivity of 91% and specificity of 88%) compared with our study (sensitivity of 79% and specificity of 95%). The differences in test performance across studies may reflect variations across study populations, different interpretive thresholds among countries, and the difference between study settings (randomized clinical trial in Japan26 vs population-based screening in Austria24 vs community-based screening in the United States).
In the Austrian study,24 because all women received mammography first and then supplemental ultrasonography, interval cancer rates could not be compared across strategies. The overall interval cancer rate was 0.4 per 1000 screens. Similar to the J-START study,26 our study reported reduction in false-negative rates, from 1.9 per 1000 screens with mammography alone to 1.5 per 1000 screens with mammography plus ultrasonography (absolute difference of 0.4 per 1000 screens) (Table 3). This difference was not significant given our smaller breast cancer sample of 42 cancers, but the 0.5 per 1000 reduction in false-negative cancer rate in the J-START study26 (from 1.0 per 1000 screens with mammography alone to 0.5 per 1000 screens with mammography plus ultrasonography) was statistically significant.
The results presented in this study reflect real-world clinical practice in the United States for women across the spectrum of breast cancer risk who received same-day, supplemental ultrasonography screening, adding information about the incremental performance and outcomes of supplemental screening with ultrasonography compared with mammography alone. We observed that supplemental ultrasonography screening was used not only in women with dense breasts: 25.7% of women receiving it had nondense breasts (Table 2). In our sample of breast imaging examinations with comprehensive capture of cancer outcomes for performance assessment, we found no significant screening benefit as measured by greater sensitivity, increased cancer detection rate, or decreased false-negative rate. Our results may reflect the high proportion of women who were at low or average 5-year risk (53.6%) in our study (Table 2), higher proportion of women with nondense breasts, lower screening ultrasonography sensitivity outside of clinical trial settings, or a combination of these factors. Alternatively, our findings may reflect the relatively small number of cancers in the mammography plus ultrasonography and matched mammography cohorts, with lack of power to detect small differences in measures. Our study had greater than 99% power to detect an incremental cancer detection rate of 3.8 per 1000 as estimated by a recent meta-analysis,27 suggesting that an influence of this magnitude is highly unlikely in the facilities we studied. However, the wide 95% CI around the estimated relative cancer detection rate for mammography plus ultrasonography vs mammography alone is comparable to values from 0.76 to 1.68 (Table 3). Larger studies are needed for more precise estimates.
Limitations
Limitations of this study include lack of information on the experience and expertise of the personnel performing the ultrasonography examinations. We also did not collect information on whether ultrasonography examinations were performed using handheld ultrasound device or automated whole-breast ultrasound devices. The automated whole-breast ultrasound devices are thought to increase consistency of image acquisition and decrease operator dependence, which limits handheld ultrasound examinations.29,30 We also did not fully abstract screening ultrasonography reports in one registry, leaving potential for misclassification of 4.3% of examinations.
The proportions of axillary lymph node–positive breast cancers and false-negative rates were not significantly different for mammography with vs without ultrasonography. A more definitive study, such as a randomized clinical trial conducted in the United States, to evaluate either of these measures, which are thought to correlate with downstream improvement in outcomes for women receiving screening, would require a very large sample size. This is especially true if the primary outcome was reduction in false-negative rate, which would need to be powered to detect a difference of 4 per 10 000 women screened as reported in our study. To date, the only breast cancer guidelines to include supplemental ultrasonography screening are those by the American College of Radiology,31 which support consideration of ultrasonography for women with elevated risk who would quality for but cannot undergo breast MRI and for women with increased breast density “after weighing benefits and risks.”
Conclusions
Our observational cohort study of ultrasonography screening in women across a range of breast cancer risk found modest, nonsignificant benefits and rates of screening harms that were high and consistent with prior reports.8 To apply supplemental ultrasonography screening with greater effectiveness, we suggest that additional efforts are needed to more accurately identify women who will benefit from supplemental screening. We also suggest that development is required of the capacity to deliver high-quality supplemental screening, as well as new interventions to reduce the frequency of screening-related harms.
eAppendix. Supplemental Appendix
eFigure 1. Distribution of Propensity Scores Before and After Propensity Score Matching for the Full Cohort of Women Receiving Mammography and Ultrasound Screening
eFigure 2A. Distribution of Propensity Scores Before and After Propensity Score Matching for Examinations Associated With Breast Cancer Diagnosis Within 1 Year
eFigure 2B. Distribution of Propensity Scores Plots Before and After Propensity Score Matching for Examinations With No Breast Cancer Diagnosis Within 1 Year
eFigure 2C. Distribution of Propensity Scores Before and After Propensity Score Matching for Examinations Associated With Biopsy Recommendations
eTable. Distribution of BI-RADS Breast Density by BCSC 5-Year Risk in 5392 Mammography Plus Ultrasound Examinations
References
- 1.Dehkordy SF, Carlos RC. Dense breast legislation in the United States: state of the states. J Am Coll Radiol. 2013;10(12):899-902. doi: 10.1016/j.jacr.2013.09.007 [DOI] [PubMed] [Google Scholar]
- 2.Are You Dense Advocacy, Inc . State density reporting efforts—because your life matters: 36 state density reporting laws. https://www.areyoudenseadvocacy.org/dense/. Accessed February 12, 2018.
- 3.115th Congress (2017-2018). S.2006 Breast Density and Mammography Reporting Act of 2017. Introduced in Senate (10/25/2017). https://www.congress.gov/bill/115th-congress/senate-bill/2006/text?q=%7B%22search%22%3A%5B%22Health%2C+Education%2C+Labor%2C+and+Pensions%22%5D%7D&r=2. Accessed February 28, 2018.
- 4.115th Congress (2017-2018). H.R.4122 Breast Density and Mammography Reporting Act of 2017. Introduced in House (10/25/2017). https://www.congress.gov/bill/115th-congress/house-bill/4122/text. Accessed February 18, 2018.
- 5.American College of Radiology . American College of Radiology Breast Imaging Reporting and Data System Atlas (BI-RADS Atlas). 4th ed. Reston, VA: American College of Radiology; 2003. [Google Scholar]
- 6.American College of Radiology . American College of Radiology Breast Imaging Reporting and Data System Atlas (BI-RADS Atlas). 5th ed. Reston, VA: American College of Radiology; 2013. [Google Scholar]
- 7.Horný M, Cohen AB, Duszak R Jr, Christiansen CL, Shwartz M, Burgess JF Jr. Dense breast notification laws: impact on downstream imaging after screening mammography [published online January 1, 2018]. Med Care Res Rev. Medline:29347864 [DOI] [PubMed] [Google Scholar]
- 8.Melnikow J, Fenton JJ, Whitlock EP, et al. Supplemental screening for women with dense breasts: a systematic review of the US Preventive Services Task Force. Ann Intern Med. 2016;164(4):268-278. doi: 10.7326/M15-1789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haas JS, Kaplan CP. The divide between breast density notification laws and evidence-based guidelines for breast cancer screening. JAMA Intern Med. 2015;175(9):1439-1440. doi: 10.1001/jamainternmed.2015.3040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sprague BL, Gangnon RE, Burt V, et al. Prevalence of mammographically dense breasts in the United States. J Natl Cancer Inst. 2014;106(10):pii: dju255. doi: 10.1093/jnci/dju255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scheel JR, Lee JM, Sprague BL, Lee CI, Lehman CD. Screening ultrasound as an adjunct to mammography in women with mammographically dense breasts. Am J Obstet Gynecol. 2015;212(1):9-17. doi: 10.1016/j.ajog.2014.06.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ballard-Barbash R, Taplin SH, Yankaskas BC, et al. ; Breast Cancer Surveillance Consortium . Breast Cancer Surveillance Consortium: a national mammography screening and outcomes database. AJR Am J Roentgenol. 1997;169(4):1001-1008. doi: 10.2214/ajr.169.4.9308451 [DOI] [PubMed] [Google Scholar]
- 13.Phipps AI, Ichikawa L, Bowles EJ, et al. Defining menopausal status in epidemiologic studies: a comparison of multiple approaches and their effects on breast cancer rates. Maturitas. 2010;67(1):60-66. doi: 10.1016/j.maturitas.2010.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tice JA, Miglioretti DL, Li CS, Vachon CM, Gard CC, Kerlikowske K. Breast density and benign breast disease: risk assessment to identify women at high risk of breast cancer. J Clin Oncol. 2015;33(28):3137-3143. doi: 10.1200/JCO.2015.60.8869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yankaskas BC, Taplin SH, Ichikawa L, et al. Association between mammography timing and measures of screening performance in the United States. Radiology. 2005;234(2):363-373. doi: 10.1148/radiol.2342040048 [DOI] [PubMed] [Google Scholar]
- 16.Coca-Perraillon M. Local and global optimal propensity score matching (paper 185-2007). In: SAS Global Forum 2007. Cary, NC: SAS Institute Inc; 2007. https://support.sas.com/resources/papers/proceedings/proceedings/forum2007/185-2007.pdf. Accessed March 6, 2017. [Google Scholar]
- 17.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28(25):3083-3107. doi: 10.1002/sim.3697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: Lawrence Erlbaum Associates Inc; 1988. [Google Scholar]
- 19.Sanders LM, King AB, Goodman KS. Impact of the New Jersey breast density law on imaging and intervention volumes and breast cancer diagnosis. J Am Coll Radiol. 2016;13(10):1189-1194. doi: 10.1016/j.jacr.2016.05.005 [DOI] [PubMed] [Google Scholar]
- 20.Sobotka J, Hinrichs C. Breast density legislation: discussion of patient utilization and subsequent direct financial ramifications for insurance providers. J Am Coll Radiol. 2015;12(10):1011-1015. doi: 10.1016/j.jacr.2015.04.015 [DOI] [PubMed] [Google Scholar]
- 21.Chau SL, Alabaster A, Luikart K, Brenman LM, Habel LA. The effect of California’s breast density notification legislation on breast cancer screening. J Prim Care Community Health. 2017;8(2):55-62. doi: 10.1177/2150131916674889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Richman I, Asch SM, Bendavid E, Bhattacharya J, Owens DK. Breast density notification legislation and breast cancer stage at diagnosis: early evidence from the SEER registry. J Gen Intern Med. 2017;32(6):603-609. doi: 10.1007/s11606-016-3904-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berg WA, Blume JD, Cormack JB, et al. ; ACRIN 6666 Investigators . Combined screening with ultrasound and mammography vs mammography alone in women at elevated risk of breast cancer [published correction appears in JAMA. 2010;303(15):1482]. JAMA. 2008;299(18):2151-2163. doi: 10.1001/jama.299.18.2151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buchberger W, Geiger-Gritsch S, Knapp R, Gautsch K, Oberaigner W. Combined screening with mammography and ultrasound in a population-based screening program. Eur J Radiol. 2018;101:24-29. doi: 10.1016/j.ejrad.2018.01.022 [DOI] [PubMed] [Google Scholar]
- 25.Corsetti V, Houssami N, Ghirardi M, et al. Evidence of the effect of adjunct ultrasound screening in women with mammography-negative dense breasts: interval breast cancers at 1 year follow-up. Eur J Cancer. 2011;47(7):1021-1026. doi: 10.1016/j.ejca.2010.12.002 [DOI] [PubMed] [Google Scholar]
- 26.Ohuchi N, Suzuki A, Sobue T, et al. ; J-START Investigator Groups . Sensitivity and specificity of mammography and adjunctive ultrasonography to screen for breast cancer in the Japan Strategic Anti-Cancer Randomized Trial (J-START): a randomised controlled trial. Lancet. 2016;387(10016):341-348. doi: 10.1016/S0140-6736(15)00774-6 [DOI] [PubMed] [Google Scholar]
- 27.Rebolj M, Assi V, Brentnall A, Parmar D, Duffy SW. Addition of ultrasound to mammography in the case of dense breast tissue: systematic review and meta-analysis. Br J Cancer. 2018;118(12):1559-1570. doi: 10.1038/s41416-018-0080-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tagliafico AS, Calabrese M, Mariscotti G, et al. Adjunct screening with tomosynthesis or ultrasound in women with mammography-negative dense breasts: interim report of a prospective comparative trial. J Clin Oncol. 2016;34:1882-1888. doi: 10.1200/JCO.2015.63.4147 [DOI] [PubMed] [Google Scholar]
- 29.Lander MR, Tabár L. Automated 3-D breast ultrasound as a promising adjunctive screening tool for examining dense breast tissue. Semin Roentgenol. 2011;46(4):302-308. doi: 10.1053/j.ro.2011.06.003 [DOI] [PubMed] [Google Scholar]
- 30.Vourtsis A, Kachulis A. The performance of 3D ABUS versus HHUS in the visualisation and BI-RADS characterisation of breast lesions in a large cohort of 1,886 women. Eur Radiol. 2018;28(2):592-601. doi: 10.1007/s00330-017-5011-9 [DOI] [PubMed] [Google Scholar]
- 31.Monticciolo DL, Newell MS, Moy L, Niell B, Monsees B, Sickles EA. Breast cancer screening in women at higher-than-average risk: recommendations from the ACR. J Am Coll Radiol. 2018;15(3, pt A):408-414. doi: 10.1016/j.jacr.2017.11.034 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Supplemental Appendix
eFigure 1. Distribution of Propensity Scores Before and After Propensity Score Matching for the Full Cohort of Women Receiving Mammography and Ultrasound Screening
eFigure 2A. Distribution of Propensity Scores Before and After Propensity Score Matching for Examinations Associated With Breast Cancer Diagnosis Within 1 Year
eFigure 2B. Distribution of Propensity Scores Plots Before and After Propensity Score Matching for Examinations With No Breast Cancer Diagnosis Within 1 Year
eFigure 2C. Distribution of Propensity Scores Before and After Propensity Score Matching for Examinations Associated With Biopsy Recommendations
eTable. Distribution of BI-RADS Breast Density by BCSC 5-Year Risk in 5392 Mammography Plus Ultrasound Examinations


